Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ev3 Inc. | c55450e8vk.htm |
Exhibit 99.1

| ev3 Investor Presentation Robert Palmisano, President & CEO |

| Forward-Looking Statements Statements contained in this presentation that relate to future, not past, events are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Forward-looking statements often can be identified by words such as "expect," "anticipate," "intend," "will," "may," "believe," "could," "continue," "future," "estimate," "outlook," "guidance," or the negative of these words or other words of similar meaning. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause ev3's actual results to be materially different than those expressed in or implied by ev3's forward-looking statements. For ev3, particular uncertainties and risks include, among others, ev3's future operating results and financial performance, fluctuations in foreign currency exchange rates, the effect of the current global economic crisis, ev3's ability to implement, fund and achieve sustainable cost savings measures that will better align its operating expenses with its anticipated net sales levels and reallocate resources to better support growth initiatives, the timing of regulatory approvals and introduction of new products, market acceptance of new products, success of clinical testing, availability of third party reimbursement, impact of competitive products and pricing, the effect of regulatory actions and the cost and effect of changes in tax and other legislation. More detailed information on these and additional factors that could affect ev3's actual results are described in ev3's filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q. Except as required by law, ev3 undertakes no obligation to publicly update its forward-looking statements. |

| Use of Non-GAAP Financial Measures ev3 uses certain non-GAAP financial measures in this presentation. ev3 uses non-GAAP financial measures as supplemental measures of performance and believes these measures provide useful information to investors in evaluating our operations, period over period. However, non-GAAP financial measures have limitations as analytical tools, and should not be considered in isolation or as a substitute for ev3's financial results prepared in accordance with GAAP. In addition, investors should note that any non- GAAP financial measures ev3 uses may not be the same non-GAAP financial measures, and may not be calculated in the same manner, as that of other companies. We have posted a reconciliation of our non-GAAP financial measures to the most directly comparable GAAP financial measures on our website at www.ev3.net. |

| The ev3 Story Setting The global medical device market for peripheral vascular and neurovascular diseases is >$2.5B and growing The Company ev3 is a leading global medical device company focused on breakthrough endovascular treatments The Strategy Executing a focused plan to: grow at or above the market improve operating leverage sustain improvement in profitability generate cash There is clear evidence the plan is working |

| Gaining Market Share in Both Segments 1 YTD Q3 2009 product revenues * 2010 - Internal estimates (PV market includes stents, atherectomy and PTA balloons only; NV market includes coils, flow diversion, liquid embolics, access devices and ischemic stroke therapy) Business WW Market Size/Growth Market Share Size: ~$1.6B* Growth: ~7% Size: ~$946M* Growth: ~15% 2007 2009E East 0.09 0.14 2007 2009E East 0.18 0.21 Peripheral Vascular Disease (PV) 64% of revenues1 Neurovascular Disease (NV) 36% of revenues1 |

| A Record of Setting and Meeting Goals Goal Result Status Grow at/above market rates Expand gross margins Achieve & sustain profitability Generate cash Integrate/stabilize FoxHollow acquisition Add breakthrough growth platforms * Q3'09 vs. Q3'08 Ongoing 1,000+ basis pt. improvement* Profitable from Q2'09; Ongoing $81M cash balance end of Q3'09 (includes $26.2M Q2'09 Chestnut payment) Integrated/stabilized Acquired Chestnut Medical Q2'09 |

| Two Growth Platforms Neurovascular Business 1 Peripheral Vascular Business 2 |

| Strong sales growth record - 2X market ev3 Neurovascular: Building a Global Leader Competitive strengths #2 globally Strong international footprint Over 65 countries Constant product innovation ? leadership #1 in liquid embolics 8 new launches in 2009 Launching breakthrough products 2 new markets > flow diversion, ischemic stroke 06 07 08 East 81 104 132 28% CAGR |

| Future Growth Drivers Grow base business - next generation products AXIUM(tm) MicroFx (PGLA, Nylon) New Access Products Apollo(tm) Micro-Catheter for Onyx(r) Liquid Embolic Breakthrough products Pipeline(tm) ? $350M market potential Solitaire(tm) ? untapped ischemic stroke market Clinical trials 4 underway for Solitaire(tm), AXIUM(tm) and Pipeline(tm) (2) Goal: Continue to outgrow a growth market ev3 Neurovascular Sales YTD Q3'09 28% growth* * Versus YTD Q3'08; Constant currency. For reconciliations, see our website at www.ev3.net. |
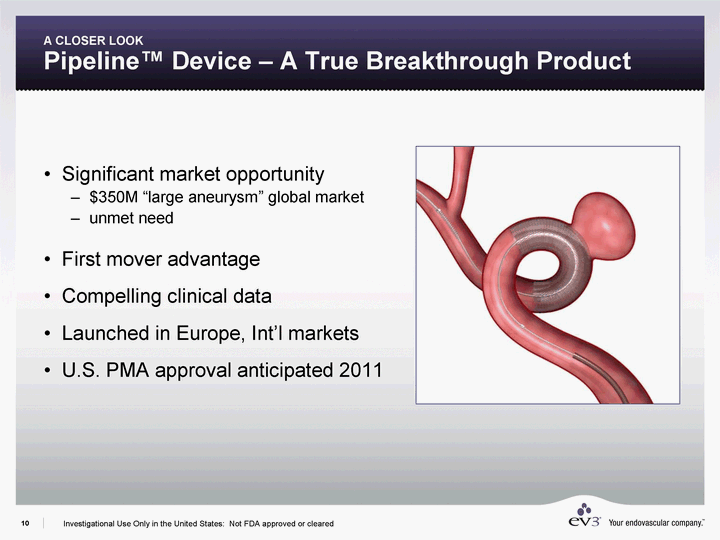
| A CLOSER LOOK Pipeline(tm) Device - A True Breakthrough Product Significant market opportunity $350M "large aneurysm" global market unmet need First mover advantage Compelling clinical data Launched in Europe, Int'l markets U.S. PMA approval anticipated 2011 Investigational Use Only in the United States: Not FDA approved or cleared |

| 49%2 ev3 estimates; Worldwide aneurysm procedures suitable for endovascular treatment in 2013; excludes ruptured aneurysms Assumed treatable with flow diversion device (large & giant, wide-neck, or nonsaccular aneurysms) Significant Market Opportunity for Pipeline(tm) Solution Potential Endovascular Procedures1 85,000 Procedures 42,000 ASP $8,200-$8,500 Avg. Implants/Procedure 1.5 Market Opportunity Investigational Use Only in the United States: Not FDA approved or cleared Global Market Opportunity by 2013 ~$350M |

| A CLOSER LOOK Pipeline(tm) Device - Offers Significant Advantages Investigational Use Only in the United States: Not FDA approved or cleared Pipeline(tm) Device Treats giant aneurysms effectively YES Low recurrence YES Faster procedure time YES Treats root cause of aneurysms - parent artery YES |

| PIPELINE(tm) EMBOLIZATION DEVICE (PED): How It Works Step 1: Insert guidewire and microcatheter Step 2: Deliver PIPELINE(tm) through microcatheter Step 3: Extract microcatheter and expand PIPELINE(tm) Step 4: Withdraw delivery wire Investigational Use Only in the United States: Not FDA approved or cleared |

| PIPELINE(tm) CASE EXAMPLE: Standalone Treatment of Aneurysm Investigational Use Only in the United States: Not FDA approved or cleared Before Post treatment 1 month follow up After No recurrence Treated with Pipeline(tm) Aneurysm |

| A CLOSER LOOK Solitaire(tm) FR: Breakthrough Product for Ischemic Stroke Attractive market significant unmet clinical need U.S. stroke incidence 795,000 people annually; 87% are ischemic* Growing opportunity for endovascular device-based therapies Solitaire(tm) FR Revascularization Device unique design - potential to restore flow and remove thrombus CE Mark July '09: European limited launch underway US SWIFT IDE study expected to begin 1H'10 * Source: American Heart Association Solitaire(tm) FR is not available for use in the United States |

| Initial angiogram of occlusion in left middle cerebral artery (taken at 8:53pm) After deploying Solitaire(tm) FR and retrieving the clot, flow is restored to the left middle cerebral artery (taken at 9:08pm) SOLITAIRE(tm) FR CASE EXAMPLE: Ischemic Clot Removed and Flow Restored - in 15 Minutes After Before Solitaire(tm) FR is not available for use in the United States |

| Two Growth Platforms Neurovascular business 1 Peripheral Vascular Business 2 |
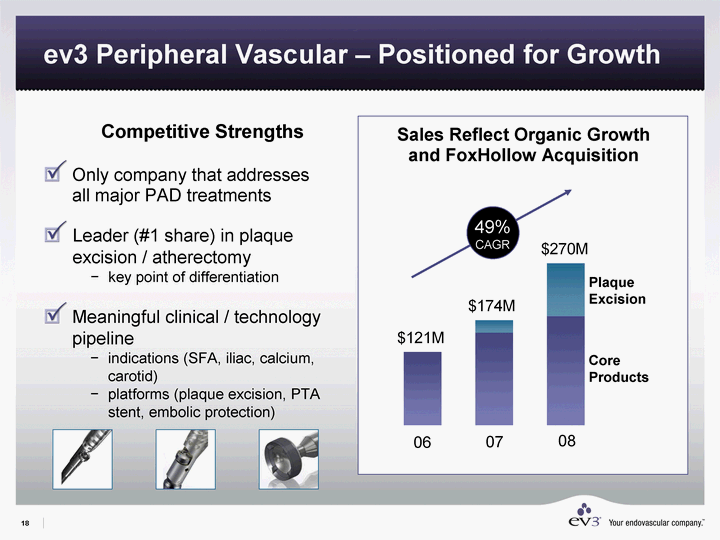
| ev3 Peripheral Vascular - Positioned for Growth Competitive Strengths Only company that addresses all major PAD treatments Leader (#1 share) in plaque excision / atherectomy key point of differentiation Meaningful clinical / technology pipeline indications (SFA, iliac, calcium, carotid) platforms (plaque excision, PTA stent, embolic protection) Sales Reflect Organic Growth and FoxHollow Acquisition 49% CAGR 06 07 8 East 121 153 181 21 89 06 07 08 Core Products Plaque Excision |

| Annualized Net Sales Per Territory Q3'09 up 29% vs. Q3'08 Key Drivers Plaque Excision Clinical Specialists GPO/IDN penetration Market development DEFINITIVE clinical data Neuro business already > $2M per territory Peripheral Vascular Sales Force Productivity in U.S. Continues to Improve Q3 08 Q1 09 Q3 09 Goal East 1.4 1.6 1.8 2 |

| Future Growth Drivers Grow base business Global PTA Balloon launch Expanded Plaque Excision tool kit TurboHawk(tm) above-the-knee TrailBlazer(tm) catheter New products TurboHawk below-the-knee 2H'10 EverFlex+ 4Q'10 Next Gen Plaque Excision 2H'11 Clinical studies 4 studies underway to drive growth and indications Key to accelerated growth ev3 Peripheral Vascular sales YTD Q3'09 13% growth* * Versus YTD Q3'08, excluding atherectomy; Constant currency. For reconciliations, see our website at www.ev3.net. |

| A CLOSER LOOK Hawk is a Key Differentiator - Keeps Getting Better New TurboHawk(tm) - plaque excision above-the-knee cuts through difficult plaque and calcium faster easier for physicians to use Launched in U.S. for endovascular use incremental opportunity to treat mild to moderately calcified lesions below-the-knee device planned in 2H'10 DEFINITIVE Ca++ study for heavily calcified lesions Hawk pulls through core ev3 products preserves future treatment options leverages full product portfolio |

| TurboHawk(tm) CASE EXAMPLE: Calcified SFA Occlusion Pre-TurboHawk(tm) Post-TurboHawk(tm) Calcified SFA occlusion Treated with TurboHawk(tm) Calcified debris removed from SFA |

| A CLOSER LOOK AT DEFINITIVE TRIAL SERIES: A Key Catalyst to Accelerate Atherectomy Growth Broader usage by Peripheral Endovascular Specialists Core User Group: "Hawkers" Establish plaque excision as a front-line therapy 3 trials, enroll 600-900 patients TRIAL SERIES Clinical Data |

| International Revenue* ev3 International: Continued Sales Momentum Competitive strengths Strong share & growth in EU Robust emerging market presence Brazil, China, Russia, India, Korea, Japan International product launches NV: Pipeline(tm), Solitaire(tm) PV: TurboHawk(tm), ev3-PTA balloons 06 07 08 East 81 107 147 34% CAGR * Translated to U.S. dollars at the applicable or estimated foreign exchange rate for the periods presented Leading international commercial organization - 65+ countries leveraging broad portfolio to capitalize on regional opportunities |

| Successful launch of Pipeline(tm), Solitaire(tm) Reimbursement for SilverHawk(r) International Markets will Fuel Growth and Expansion Across Both Businesses Market development (flow diversion, stroke, atherectomy) Grow neurovascular market share; develop peripheral vascular market Direct sales Government-sponsored stroke program Continued market expansion Expand coil market share Business development and local partnerships Hybrid distribution model Training initiatives Further penetration of Axium(tm), launch of MicroFx coils All EU China Russia Brazil Singapore & Korea |

| FINANCIAL SUMMARY: Continuing to Improve Performance Net Product Sales* $100,018 $105,656 $100,395 $109,086 $112,838 Gross Profit $68,747 $71,316 $69,407 $78,608 $84,230 Gross Margin 64.2% 67.2% 69.1% 72.1% 74.6% Net Income (loss) $(7,310) $(291,120) $(1,809) $23,989 $6,736 Adjusted EPS per Diluted Share** $0.04 $0.08 $0.07 $0.14 $0.17 GAAP Diluted EPS (loss) $(0.07) $(2.78) $(0.02) $0.23 $0.06 Q3 2008 Q4 2008 Q1 2009 Q2 2009 Q3 2009 $000's except EPS Adjusted Net Income** $3,850 $7,948 $7,070 $14,614 $19,435 * Excluding Merck research collaboration revenue of $7M for Q308 and $469K for Q408. ** All quarters presented are adjusted for amortization and non-cash stock-based compensation. Q4 2008 is also adjusted for non-cash goodwill and other intangible asset impairment charges. Q1 2009 is also adjusted for a non-cash FoxHollow lease reserve adjustment and a realized gain on divestiture of investments. Q2 2009 is also adjusted for charges relating to the change in fair value of contingent consideration and a non-cash tax benefit resulting from the acquisition of Chestnut. Q3 2009 is also adjusted for charges relating to the change in fair value of contingent consideration. For reconciliations, see our website at www.ev3.net. |

| Key Growth Drivers Long growth runway 3. Next gen plaque excision 1. Commercial execution 2. Flow diversion 5. International 6. Clinical studies 7. Selective M&A 4. Ischemic stroke |

| IN SUMMARY: WHY INVEST IN ev3 ev3: Leading the Way Investing in 8 clinical studies to expand usage Powerful Growth Drivers Robust pipeline of novel products - 20 launches by 2012 Leveraging business model improving gross margin expanding operating income Pipeline(tm) - a game changing product Profitable, generating cash A Transformed Company Stabilized atherectomy business New growth platforms both businesses Launched innovative products |

