Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a10-1537_28k.htm |
Exhibit 99.1
|
|
Nasdaq: AMAG San Francisco January 2010 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to, statements regarding the potential size of the overall US CKD market, the potential size of the U.S. non-dialysis CKD market, our expected fourth quarter 2009 Feraheme® revenues (including the amount attributable to our previously deferred product revenues), our expectation that utilization of previously deferred Feraheme launch incentive program revenues will increase, our expectation that we will not record any new deferred revenue during the fourth quarter of 2009, the expected timing of the filing of our marketing authorization application for Feraheme with the EMEA, our plan to conduct one additional Feraheme clinical study in Europe and the expected timing of that trial, our plan to conduct pediatric studies in the U.S. and the expected timing of these studies, and the intended timing of initiation of our planned study of Feraheme for the treatment of iron deficiency anemia regardless of the underlying cause are forward looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward looking statements. Such risks and uncertainties include: (1) uncertainties regarding our ability to manufacture Feraheme, (2) the fact that we have limited experience developing and commercializing a pharmaceutical product on our own, particularly outside of the U.S., (3) uncertainties regarding our ability to successfully compete in the intravenous iron replacement market, (4) uncertainties regarding our ability to ensure favorable coverage, pricing and reimbursement for Feraheme, (5) uncertainties relating to our patents and proprietary rights, and (6) other risks identified in our Securities and Exchange Commission filings, including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2009. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. |
|
|
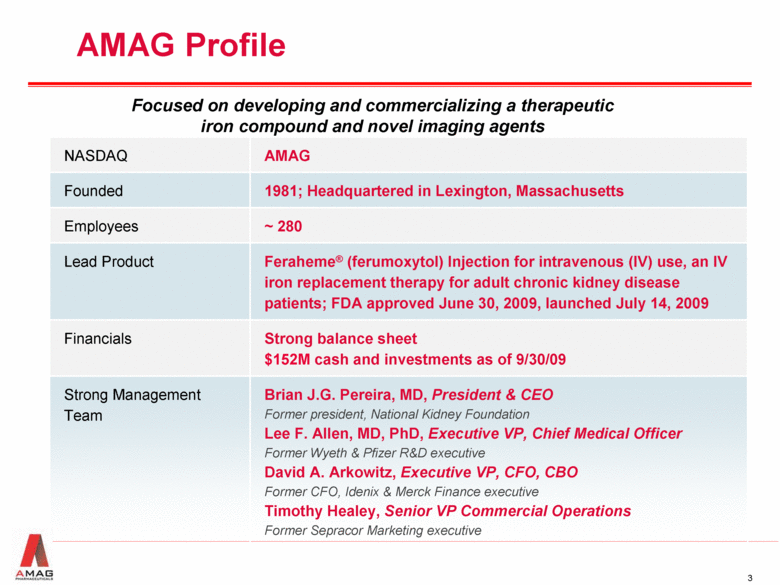
AMAG Profile Focused on developing and commercializing a therapeutic iron compound and novel imaging agents NASDAQ AMAG Founded 1981; Headquartered in Lexington, Massachusetts Employees ~ 280 Lead Product Feraheme® (ferumoxytol) Injection for intravenous (IV) use, an IV iron replacement therapy for adult chronic kidney disease patients; FDA approved June 30, 2009, launched July 14, 2009 Financials Strong balance sheet $152M cash and investments as of 9/30/09 Strong Management Team Brian J.G. Pereira, MD, President & CEO Former president, National Kidney Foundation Lee F. Allen, MD, PhD, Executive VP, Chief Medical Officer Former Wyeth & Pfizer R&D executive David A. Arkowitz, Executive VP, CFO, CBO Former CFO, Idenix & Merck Finance executive Timothy Healey, Senior VP Commercial Operations Former Sepracor Marketing executive |
|
|
2009: A Transformational Year for AMAG AMAG has become a commercial biopharmaceutical company Approval and launch of Feraheme® provides a foundation for growth Broad US chronic kidney disease (CKD) iron deficiency anemia (IDA) label provides an opportunity to participate in a market with a billion dollar potential Expanding development to treat IDA in other patient populations Ex-US strategic opportunity for growth Studying as imaging agent for vascular enhanced magnetic resonance imaging (VE-MRI) in patients with suspected peripheral arterial disease; “fast track” designation received |
|
|
MARKET OPPORTUNITY Feraheme® for the Treatment of IDA in CKD Patients |
|
|
Attractive Label Provides Access To The Entire CKD IDA Market Feraheme® indicated for the treatment of iron deficiency anemia in adult patients with CKD Recommended dose is an initial 510 mg IV injection followed by a second 510 mg IV injection 3 to 8 days later The recommended dose may be readministered to patients with persistent or recurrent iron deficiency anemia Feraheme is available for IV injection in single use vials. Each vial contains 510 mg of elemental iron in 17 mL each mL of Feraheme has low bleomycin-detectable iron |
|
|
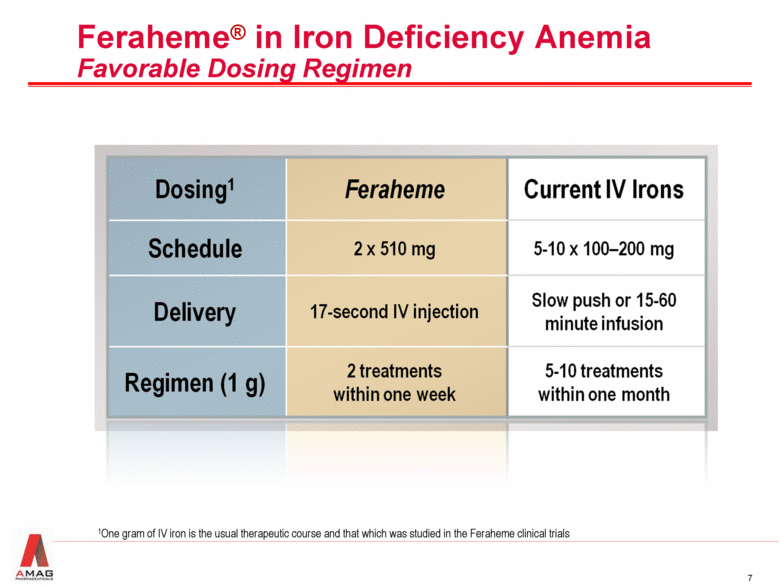
Feraheme® in Iron Deficiency Anemia Favorable Dosing Regimen 1One gram of IV iron is the usual therapeutic course and that which was studied in the Feraheme clinical trials |
|
|
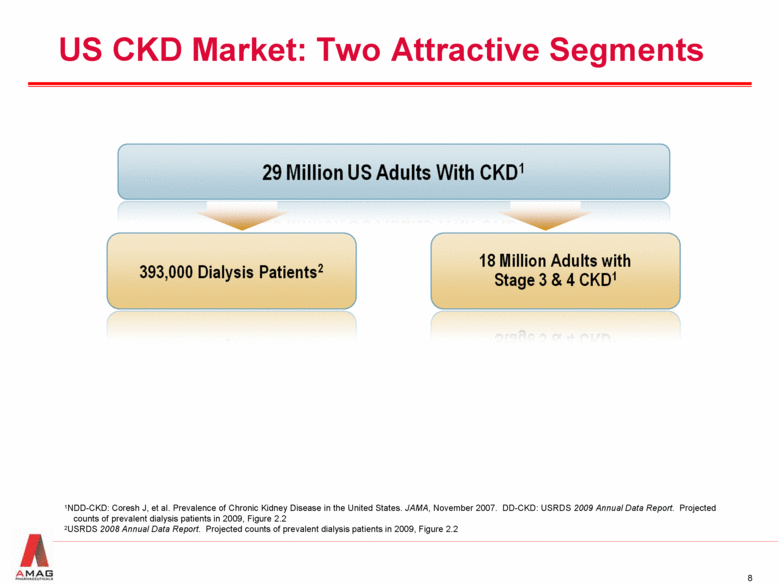
US CKD Market: Two Attractive Segments 1NDD-CKD: Coresh J, et al. Prevalence of Chronic Kidney Disease in the United States. JAMA, November 2007. DD-CKD: USRDS 2009 Annual Data Report. Projected counts of prevalent dialysis patients in 2009, Figure 2.2 2USRDS 2008 Annual Data Report. Projected counts of prevalent dialysis patients in 2009, Figure 2.2 |
|
|
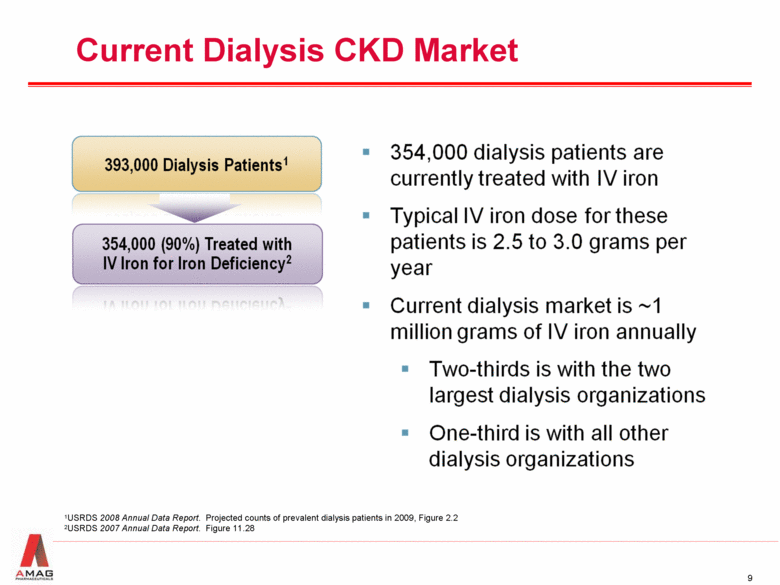
Current Dialysis CKD Market 1USRDS 2008 Annual Data Report. Projected counts of prevalent dialysis patients in 2009, Figure 2.2 2USRDS 2007 Annual Data Report. Figure 11.28 |
|
|
Dialysis Strategy and Sales Cycle Encourage use through education and with contractual incentives 3Q09 launch incentive program accelerated adoption by several dialysis organizations Pilot programs provide an opportunity to test adoption, train personnel and modify procedures Many dialysis organizations have initiated or completed pilots, including each launch incentive program customer Once pilots are successfully completed, sites can fully convert Several organizations have begun or completed full conversion Product-specific Q-code, effective January 1, 2010, is expected to automate and expedite reimbursement |
|
|
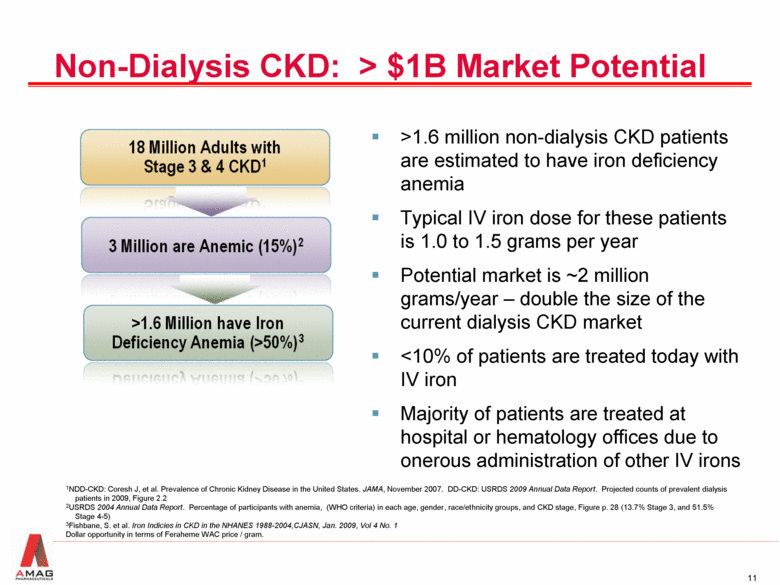
Non-Dialysis CKD: > $1B Market Potential 1NDD-CKD: Coresh J, et al. Prevalence of Chronic Kidney Disease in the United States. JAMA, November 2007. DD-CKD: USRDS 2009 Annual Data Report. Projected counts of prevalent dialysis patients in 2009, Figure 2.2 2USRDS 2004 Annual Data Report. Percentage of participants with anemia, (WHO criteria) in each age, gender, race/ethnicity groups, and CKD stage, Figure p. 28 (13.7% Stage 3, and 51.5% Stage 4-5) 3Fishbane, S. et al. Iron Indicies in CKD in the NHANES 1988-2004,CJASN, Jan. 2009, Vol 4 No. 1 Dollar opportunity in terms of Feraheme WAC price / gram. >1.6 million non-dialysis CKD patients are estimated to have iron deficiency anemia Typical IV iron dose for these patients is 1.0 to 1.5 grams per year Potential market is ~2 million grams/year – double the size of the current dialysis CKD market <10% of patients are treated today with IV iron Majority of patients are treated at hospital or hematology offices due to onerous administration of other IV irons |
|
|
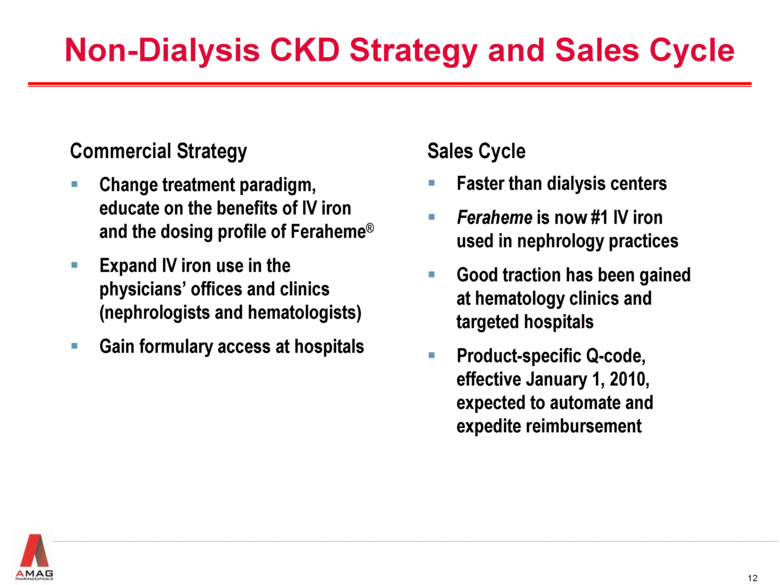
Non-Dialysis CKD Strategy and Sales Cycle Commercial Strategy Change treatment paradigm, educate on the benefits of IV iron and the dosing profile of Feraheme® Expand IV iron use in the physicians’ offices and clinics (nephrologists and hematologists) Gain formulary access at hospitals Sales Cycle Faster than dialysis centers Feraheme is now #1 IV iron used in nephrology practices Good traction has been gained at hematology clinics and targeted hospitals Product-specific Q-code, effective January 1, 2010, expected to automate and expedite reimbursement |
|
|
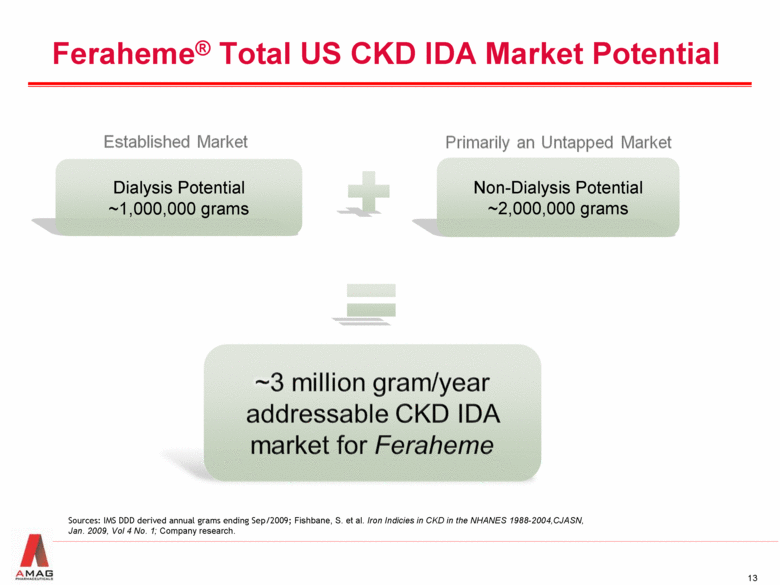
Feraheme® Total US CKD IDA Market Potential Non-Dialysis Potential ~2,000,000 grams Dialysis Potential ~1,000,000 grams Sources: IMS DDD derived annual grams ending Sep/2009; Fishbane, S. et al. Iron Indicies in CKD in the NHANES 1988-2004,CJASN, Jan. 2009, Vol 4 No. 1; Company research. |
|
|
COMMERCIAL LAUNCH Feraheme® for the Treatment of IDA in CKD Patients |
|
|
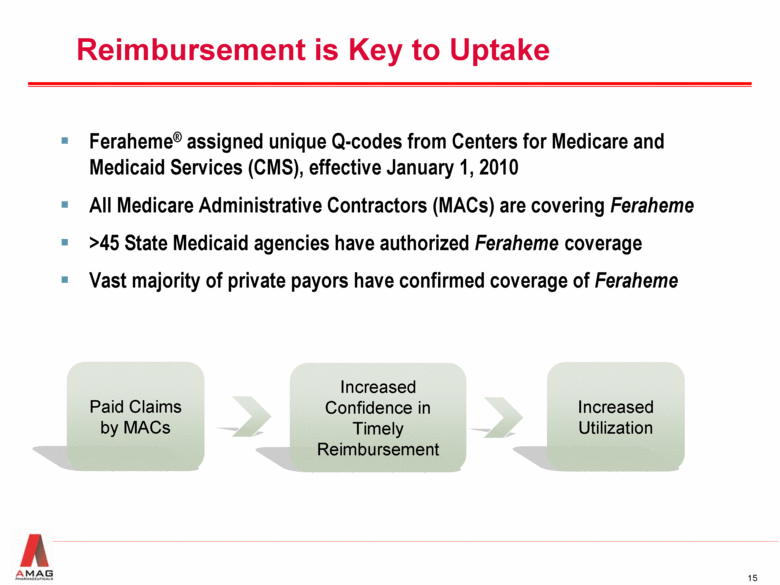
Reimbursement is Key to Uptake Feraheme® assigned unique Q-codes from Centers for Medicare and Medicaid Services (CMS), effective January 1, 2010 All Medicare Administrative Contractors (MACs) are covering Feraheme >45 State Medicaid agencies have authorized Feraheme coverage Vast majority of private payors have confirmed coverage of Feraheme Paid Claims by MACs Increased Confidence in Timely Reimbursement Increased Utilization |
|
|
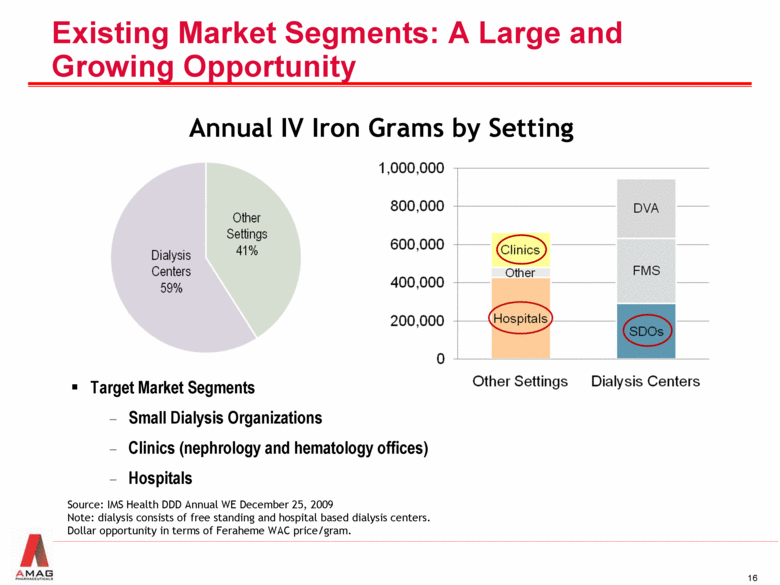
Existing Market Segments: A Large and Growing Opportunity IV Iron Sales by Setting of Care (Annual Grams) Annual IV Iron Grams by Setting Source: IMS Health DDD Annual WE December 25, 2009 Note: dialysis consists of free standing and hospital based dialysis centers. Dollar opportunity in terms of Feraheme WAC price/gram. Target Market Segments Small Dialysis Organizations Clinics (nephrology and hematology offices) Hospitals |
|
|
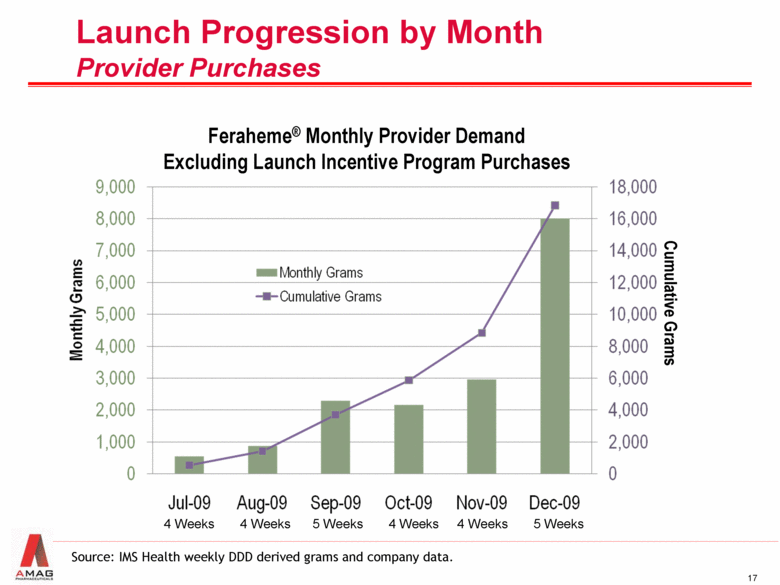
Launch Progression by Month Provider Purchases Feraheme® Monthly Provider Demand Excluding Launch Incentive Program Purchases Cumulative Grams 4 Weeks 4 Weeks 5 Weeks 4 Weeks 4 Weeks 5 Weeks Source: IMS Health weekly DDD derived grams and company data. |
|
|
Launch Momentum Building $12 - $13 million in estimated 4Q09 Feraheme® net product revenues Includes approximately $1 million of $11.5 million previously deferred product revenues originally recorded in 3Q09 Each launch incentive program customer has initiated a pilot and begun to use Feraheme Majority of payments are due early in 2010 Utilization should increase going forward No new deferred revenues expected during 4Q09 Estimated 4 to 5 weeks of inventory currently at wholesalers/distributers, based on December 2009 demand data |
|
|
Launch Momentum Building (cont.) ~60% of Feraheme® provider demand through December 2009 was non-dialysis CKD; ~40% was dialysis >1,000 customers have purchased Feraheme Collectively, these customers have used more than 200,000 grams of all forms of IV iron over past 52 weeks1 >60% of customers have purchased Feraheme on a repeat basis 1IMS DDD derived annual grams ending 12/25/2009. |
|
|
POSITIONED FOR GROWTH AMAG Pharmaceuticals |
|
|
Expanding the International Reach of Feraheme® Canadian marketing application filed in 4Q09 for treatment of IDA in CKD patients Chinese registrational trial application filed by AMAG partner (3SBio) in 4Q09 EU regulatory filing (MAA) by mid-2010 planned for the treatment of IDA in CKD patients One clinical study planned vs. an IV iron comparator Study to be conducted concurrent with the EMEA’s review of the MAA Approval received from the EMEA for the Pediatric Investigation Plan Actively engaged in discussions with potential commercial partners for Feraheme in the EU |
|
|
Expanding the Label of Feraheme® Therapeutic Broad iron deficiency anemia (IDA) registrational program, including patients with abnormal uterine bleeding and cancer; initiation of phase III program targeted by mid-20101 Pediatric protocols finalized and submitted to meet post-approval Pediatric Research Equity Act requirement; initiation of studies targeted for 2010 Diagnostic Ferumoxytol is being developed as an imaging agent for vascular-enhanced magnetic resonance imaging (VE-MRI) Phase II trial for patients with suspected peripheral arterial disease is now more than 50 percent enrolled 1Study design and timelines are subject to completion of FDA discussions and final protocol review |
|
|
AMAG: Positioned for Growth Large and growing IV iron market Feraheme® provides multiple commercial and development opportunities to address unmet medical needs Intravenous iron replacement therapy for iron deficiency anemia (IDA) in chronic kidney disease (CKD) that provides patients and physicians with a new option and treatment paradigm Additional programs in development to treat IDA in patient populations other than CKD Fast track designation as an imaging agent for vascular enhanced magnetic resonance imaging (VE-MRI) to evaluate peripheral arterial disease Active business development and licensing efforts Seeking to supplement portfolio with renal (or related) therapies Actively engaged in discussions with potential commercial partners for Feraheme in the EU |
|
|
Nasdaq: AMAG Investor Relations Contact: 617-498-3303 |