Attached files
| file | filename |
|---|---|
| 8-K - VIROPHARMA INCORPORATED -- FORM 8-K - VIROPHARMA INC | d8k.htm |
| EX-99.1 - PRESS RELEASE - VIROPHARMA INC | dex991.htm |
 ViroPharma
Incorporated ViroPharma Incorporated Delivering Innovative Treatments for Life-threatening Diseases Delivering Innovative Treatments for Life-threatening Diseases Exhibit 99.2 |
  This presentation contains certain forward-looking statements that involve
risks and uncertainties including statements regarding our
financial guidance for 2009 and 2010, our ability to continue to
execute a successful launch of Cinryze, our ability to achieve peak year sales projections, our
ability to complete manufacturing scale up procedures,
including PCP and industrial scale, and receive regulatory approvals in the time frames
anticipated, interpretations of market research data, our ability to
develop life cycle management plans for Cinryze, including
designing and commencing clinical studies for additional
indications, and pursuing regulatory approvals in additional
territories. Actual results and events may differ
significantly from results and events discussed in forward- looking statements. Factors that might cause or contribute to such
differences include, but are not limited to, those discussed in
“Risk Factors” in our 2008 10K as well as in our 10Q-s for the periods ended March 31, 2009, June 30, 2009 and September 30, 2009 filed with the
Securities and Exchange Commission. There may be additional risks
that the Company does not presently know or that the Company
currently believes are immaterial which could cause actual
results to differ from those contained these forward looking
statements. The statements in this presentation are made as
of the date of this presentation and subsequent events may cause
our assessment to change. ViroPharma does not assume any
responsibility for updating any forward-looking statements. |
    ViroPharma Incorporated We are Focused Solely on We are Focused Solely on Improving Patients’ Improving Patients’ Lives Lives |
 |
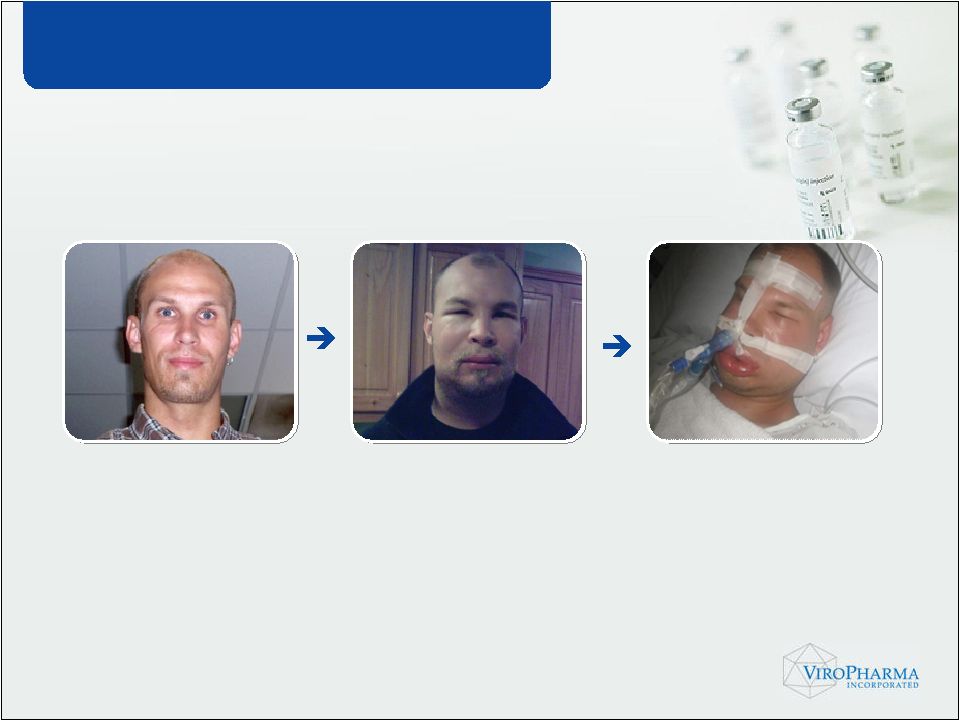
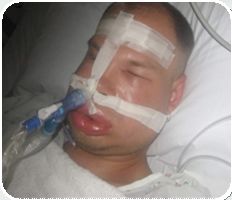
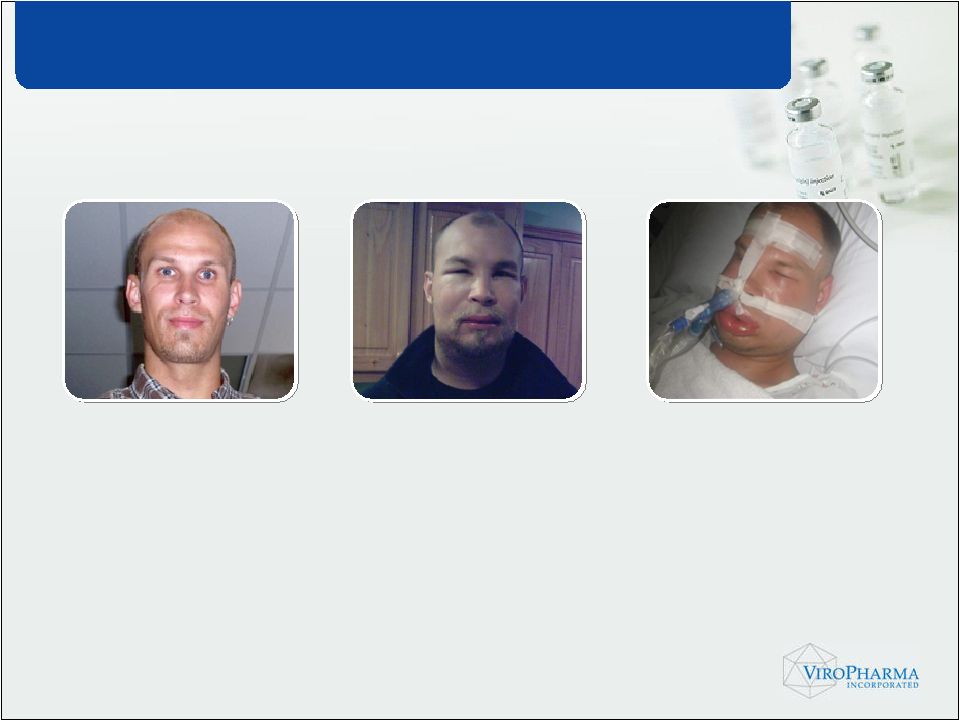   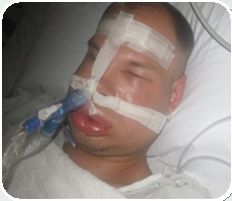 • Inherited deficiency or dysfunction in C1 Inhibitor (C1-INH) gene • Attacks of intense and painful swelling • Up to 40% mortality, if left untreated HAE – A Life-threatening Disorder |
 • Only C1-INH indicated for routine prophylaxis • Addresses the underlying cause of the disease • Proven to reduce frequency, number and severity of HAE attacks • Self-Administration unique to Cinryze A Prophylactic Option CINRYZE ™ (C1 Esterase Inhibitor [human]) |
 Prophylaxis vs.
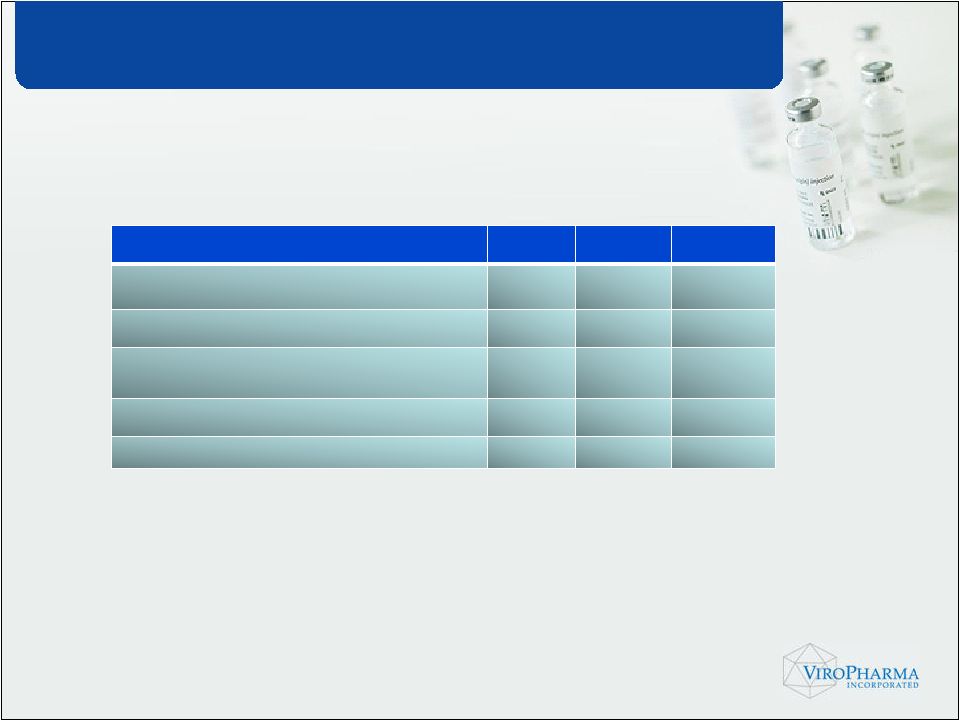
Acute Treatment 2009 2010 2011 Prevalent Population i.e. unique individuals in the US who have the disease 11,059 11,154 11,250 Estimate of diagnosed patients 2,544 2,789 3,150 Number of HAE patients who would receive prescription drug therapy 1,628 1,840 2,142 Potential Prophylactic Drug Treated patients (78%) 1,270 1,436 1,671 Potential Acute Drug Treated patients (21%) 342 386 450 US Population 2009: 331,809,237* * US population and annual growth rate based on US Census Data. ** Based on ViroPharma Market Research Conducted in 2009 |
 • Individualized case management • Benefits coverage investigations • Reimbursement assistance • Single point of contact for ongoing support and follow up Patient Access Made Easy CINRYZESolutions™ |
 • Over 400 patients on commercial Cinryze at year end 2009 • Average dosing of 1.7 per week • Significant number of additional patients enrolled into CinryzeSolutions • 218 plans covering at least one patient Impressive U.S. Launch |
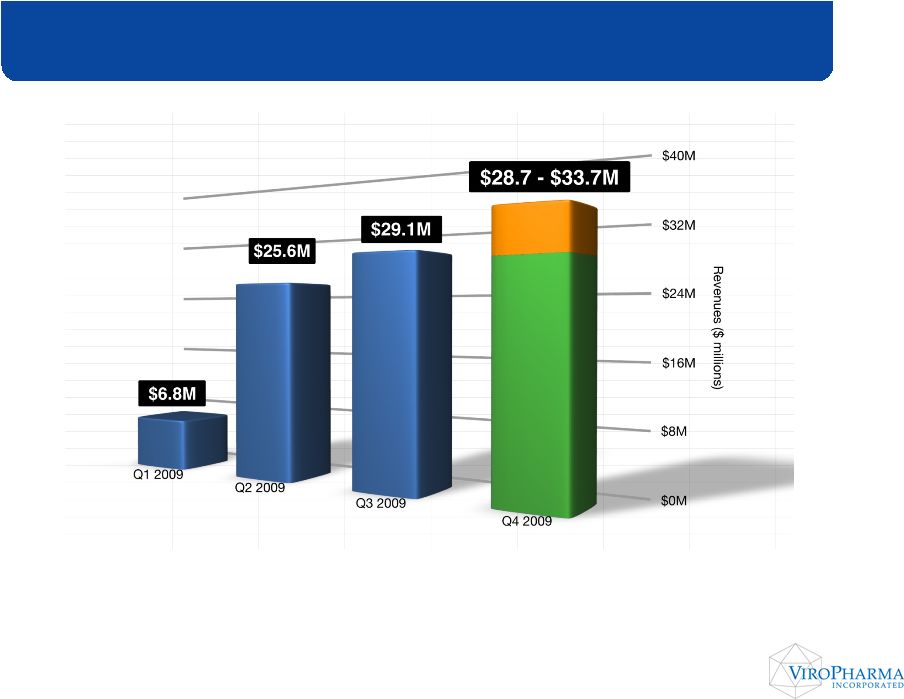 *
Q4 range denotes 2009 Cinryze Net Sales guidance provided on Monday, January 11, 2010 Quarterly Cinryze Revenue Growth |
 Scale up
Progressing to Plan PCP Industrial Scale Equipment In Place Additional Shift Staffed Personnel Trained Begin Production Finished Product (Q2) Equipment In Place Equipment Validated Conformance Lots Made Pre-Filing FDA Meeting 3-Month Stability FDA Filing (Q3) Finished Product (EOY) |
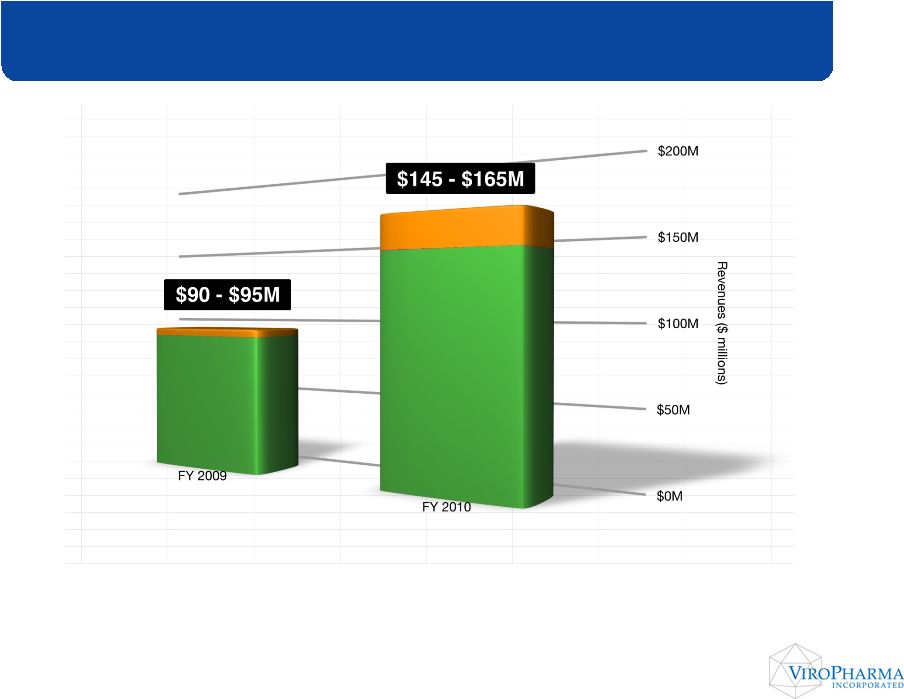 *
2009 Cinryze Net Sales expected to be near the higher end of the provided
range of $90-$95 million * 2010 Cinryze Net Sales Guidance provided January 11, 2010 Annual Cinryze Revenue Growth Estimated Peak Year Sales $350 - Estimated Peak Year Sales $350 - $450M $450M |
 • FDA advisory panel conducted in 2009 • Panel supported Q1/Q2 sameness requirement • Continued vigilance into the process • Authorized generic ready for release upon approval of 3 party generic product Vancocin… rd |
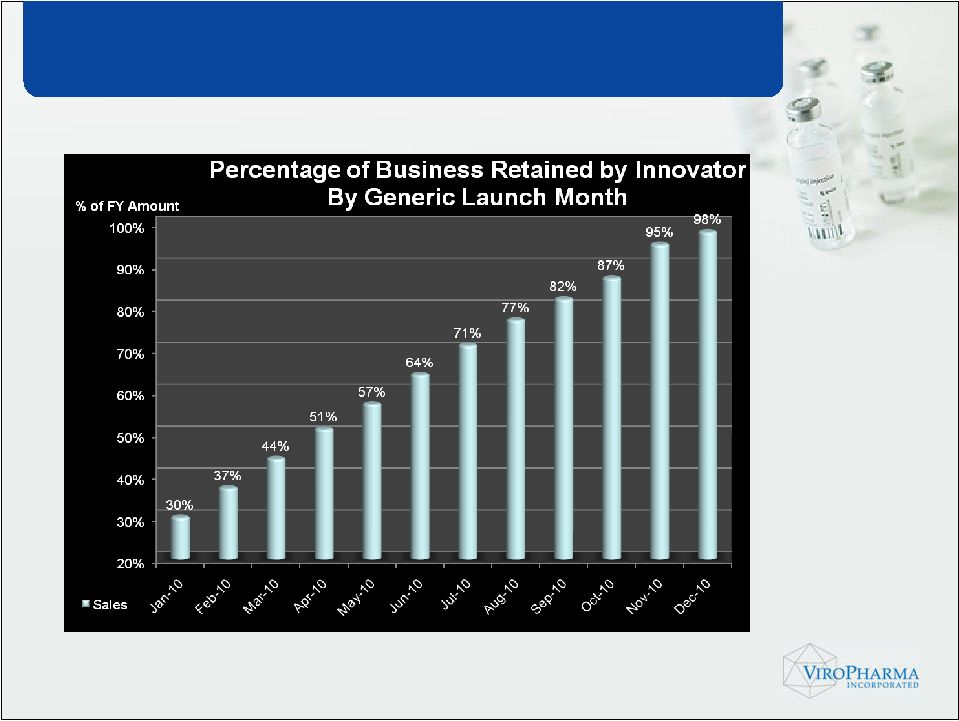 One Way to
Consider Generic Impact * Assumes greater than 3 generic entrants
|
 • Total Products Revenue of $223M • Cinryze Net Sales of $61.3M • Reported Adjusted Net Income of $76M • Increased Cash Position to $289M Strong Financial Results Financial Results through Q3 2009 Working Capital of $358 of $358 Million * Reconciliation of Adjusted Net Income to GAAP Net Income can be found on Form 8-K Filed October 28, 2009 |
 • Cinryze as a Platform for Growth • Business Development • Non-toxigenic C. difficile (NTCD) Additional Growth Opportunities |
 • Expanded Territorial and Indication Rights • Orphan drug status in EU • Submitted PIP to EMEA • Plan to submit MAA in second half of 2010 • Analyst Day event planned for March • Global Expansion • Formulation Development • Additional Indications Broadening the Cinryze Opportunity |
 • Treating serious or life threatening illnesses • High unmet medical needs • Limited commercial infrastructure • Providing both top and bottom line growth Adding to the Business We are a Biopharmaceutical Company Focused On: Focused Business Development as Another Engine for Growth |
 |
 ViroPharma
Incorporated ViroPharma Incorporated Delivering Innovative Treatments for Life-threatening Diseases Delivering Innovative Treatments for Life-threatening Diseases
|
