Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Orexigen Therapeutics, Inc. | d8k.htm |
 1
28 th Annual JP Morgan Healthcare Conference 28 th Annual JP Morgan Healthcare Conference January 13, 2010 January 13, 2010 Exhibit 99.1 |
 2 Forward-Looking Statements Forward-Looking Statements Orexigen cautions you that statements included in this presentation that are not a description of
historical facts are forward-looking statements. Words such as
"believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify
forward-looking statements. These statements are based on the Company's
current beliefs and expectations. These forward-looking statements include statements regarding the efficacy and safety of Contrave ® and Empatic™, the potential for, and timing of, an NDA submission for Contrave and Empatic,
the commercial and therapeutic potential of Contrave, and the potential to obtain
regulatory approval for, and effectively treat obesity with, Contrave and Empatic.
The inclusion of forward-looking statements should not be regarded as a representation by Orexigen that any of its plans will be achieved. Actual results may differ from those set forth in this
presentation due to the risk and uncertainties inherent in the Orexigen business,
including, without limitation: additional analyses of data from the Contrave Phase 3
trials and any other clinical trials of Contrave or Empatic may produce negative or inconclusive results, or may be inconsistent with previously announced results or previously conducted clinical trials; the FDA may not agree
with the Company’s interpretation of efficacy and safety results; Contrave or
Empatic may not receive regulatory approval on a timely basis or at all, and the FDA may
require Orexigen to complete additional clinical, non-clinical or other requirements prior to the submission or the approval of NDAs for either product candidate; the potential for adverse safety findings relating to Contrave or
Empatic to delay or prevent regulatory approval or commercialization, or result in
product liability claims, including serious adverse events that are not characterized by
clinical investigators as possibly related to Contrave; the third parties on whom Orexigen relies to assist with the development programs for Contrave or Empatic, including clinical investigators, contract
laboratories, clinical research organizations and manufacturing organizations, may not
successfully carry out their contractual duties or obligations or meet expected
deadlines, and the quality or accuracy of the data or materials generated by such third parties may be of insufficient quality to include in the Company’s regulatory submissions; the ability of Orexigen and its
licensors to obtain, maintain and successfully enforce adequate patent and other
intellectual property protection of its product candidates; Orexigen may be unable to
enter a collaboration or partnership relating to Contrave for promotion to broader markets on attractive terms or at all; and other risks described in the Company's filings with the Securities and Exchange Commission.
You are cautioned not to place undue reliance on these forward-looking statements,
which speak only as of the date hereof, and Orexigen undertakes no obligation to revise
or update this presentation to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe
harbor provisions of Section 21E of the Private Securities Litigation Reform Act of
1995. Information included herein is based on the Company’s review and evaluation of the clinical data. All conclusions and determinations contained herein are subject to the
Company’s further analysis of the clinical data. The ultimate determination
of the safety and efficacy of Contrave will be made by the FDA and other relevant regulatory authorities. |
 3 Key Corporate Highlights Key Corporate Highlights Strong Company Seasoned team in place to build for regulatory and commercial success Contrave + Empatic = Obesity franchise Commercial plans to reach target-rich segments, partner for broader markets On track for Contrave NDA filing in 1H 2010 Strong balance sheet; over $90m in cash at 12/31/09 Compelling Products Contrave Addresses FDA guidelines for approval Unique profile may address needs of the diverse obese population Covered by composition of matter IP Upside life cycle opportunities (e.g. smoking cessation without weight gain) Empatic More significant weight loss with different profile has completed Phase II Attractive Markets Complementary to pharma committed to the cardiometabolic space Poised to expand from highly generic, unsatisfied, acute market today to a major therapeutic category |
   4
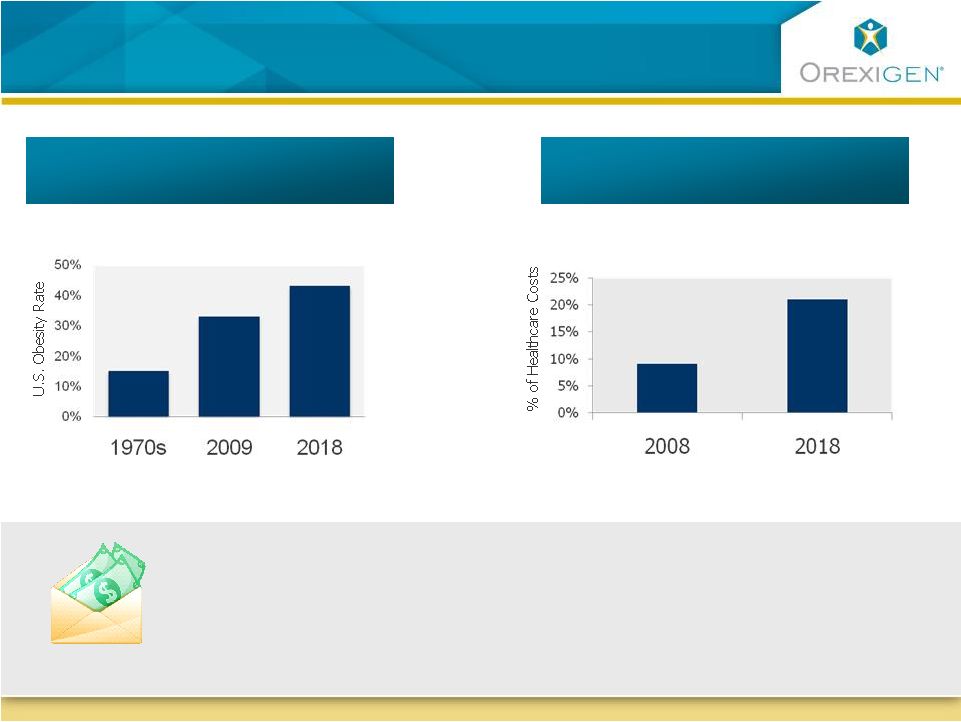
The Obesity Epidemic is Increasing Healthcare Costs to Alarming Levels The Obesity Epidemic is Increasing Healthcare Costs to Alarming Levels 75M 103M 33% 43% 9% 21% Projected US healthcare spend due to obesity 16% US obesity rates $147B $344B The average medical costs for an obese person are 42% higher than for a person of normal weight Sources: Centers for Disease Control and Prevention (CDC); Datamonitor, Pipeline &
Commercial Insight: Obesity, July 2009; The Future Costs of Obesity: National and State
Estimates of the Impact of Obesity on Direct Health Care Expenses, a collaborative report from United Health Foundation, American Public Health Association and Partnership for Prevention based on research by K. Thorpe, November 2009; Health
Affairs, July 2009 . . . Placing increasing cost burden on the healthcare system U.S. obesity rates are projected to continue growing . . . |
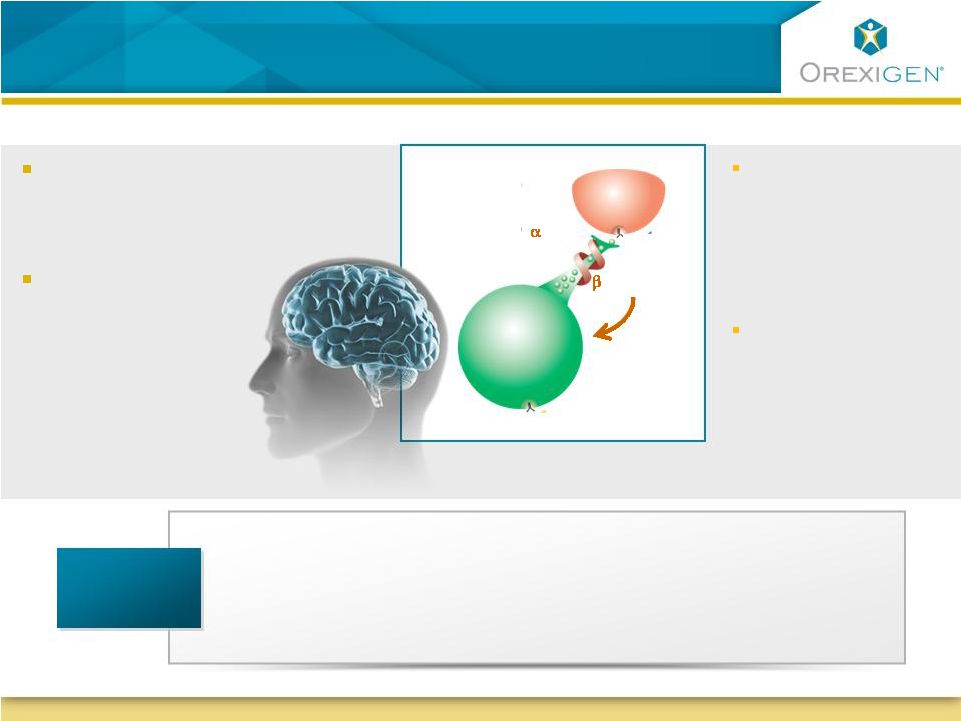 Impact
Physiology: Increase firing of POMC neuron to reduce hunger and raise metabolism Impact Consumption Behavior: Modulate reward system to improve control of eating MC-4 POMC -Endorphin Bupropion Stimulates POMC (proopio- melanocortin) neuron to initiate weight loss Naltrexone Prevents negative feedback loop to sustain weight loss -MSH Novel Scientific Rationale for Contrave Addresses Biology and Behavioral Drivers of Consumption Novel Scientific Rationale for Contrave Addresses Biology and Behavioral Drivers of Consumption Composition of Matter Patent U.S. 7,375,111 Issued – 2025 Methods of Use Patent U.S. 7,462,626 Issued – 2024 Formulations / Packaging / Numerous in Process Additional Claims Patents 5 |
 6
Contrave as First Line Therapy Contrave as First Line Therapy |
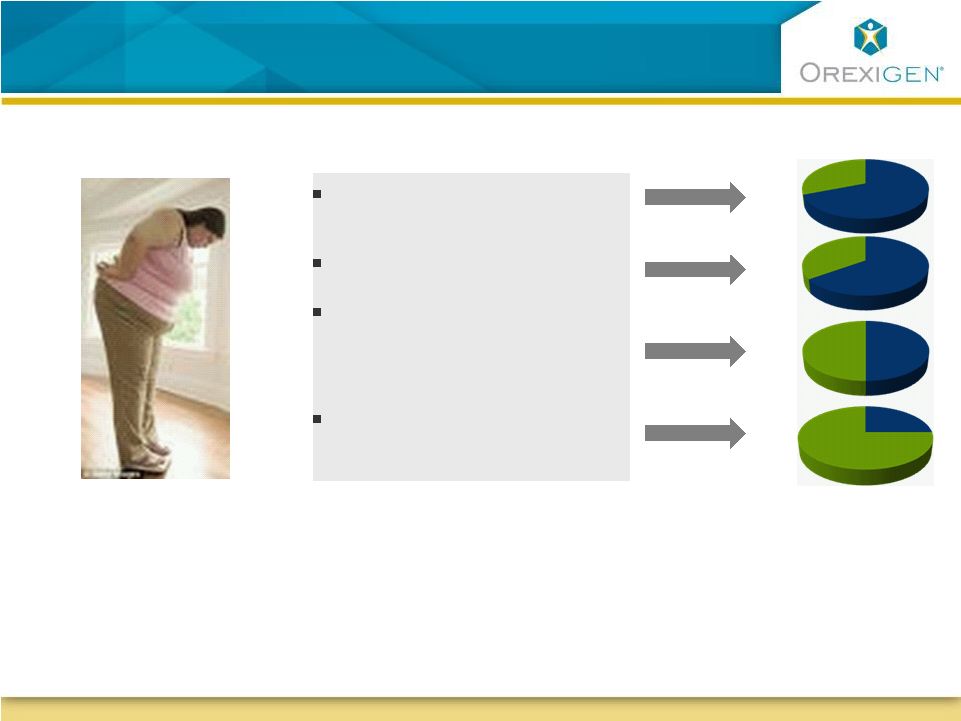 7 Natural Patient Targets for Contrave Natural Patient Targets for Contrave Obese with diabetes 50% 50% 72% 72% Available obese population 26% 26% 36% 36% Obese with dyslipidemia Obese who report uncontrollable food cravings Treatment seeking obese who are female Sources: Orexigen quantitative market research 2008 and 2009 |
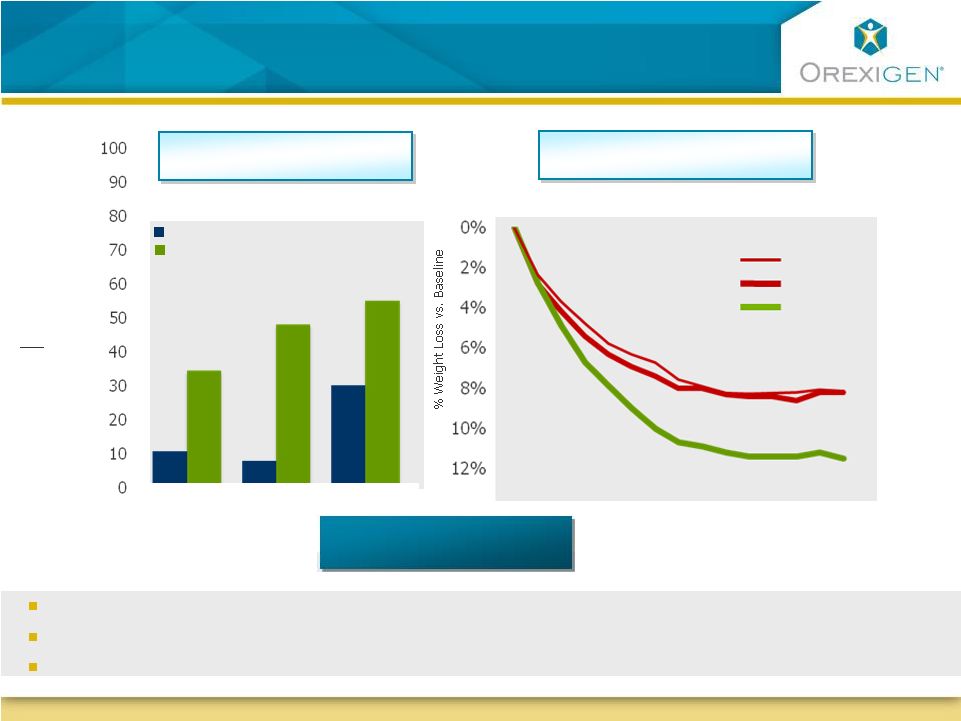 8 Patients on Contrave Lost Meaningful Weight – and Kept it Off Patients on Contrave Lost Meaningful Weight – and Kept it Off Of completers who lost at least 10% of their body weight, they lost on average 34-39 lbs * Most frequent adverse events: Nausea, constipation, vomiting, dizziness, dry mouth Safety profile appears consistent with individual components % % of Patients Losing >10% of Body Weight COR-I COR-II COR-BMOD Week 0 Week 14 Week 28 Week 42 Week 56 8.2% COR-BMOD COR-I COR-II 11.5% Avg loss: 34 lbs Avg loss: 35 lbs Avg loss: 39 lbs * Patients on Contrave32 dose in COR-I, COR-II and COR-BMOD trials Placebo Contrave32 COR Program: Categorical Weight Loss (Completers) COR Program: Observed Case Weight Loss Summary: Summary: |
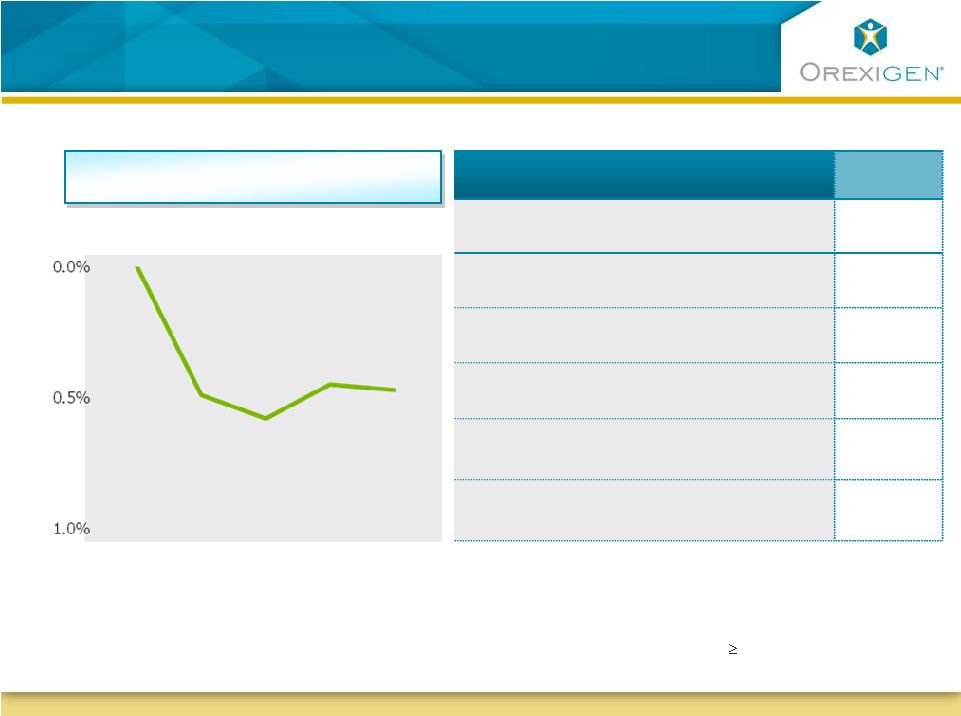 9 Diabetic Patients on Contrave Lost Weight and Improved Glycemic Control Diabetic Patients on Contrave Lost Weight and Improved Glycemic Control Week 0 Placebo- subtracted Change in HbA1c Key Measures (ITT-LOCF) Body Weight Improved ¹ HbA1c (%) Improved ¹ % of Subjects with HbA1c <7.0% Improved ¹ % of patients requiring rescue Rx Improved ¹ Fasting HDL Improved ¹ Fasting triglycerides Improved ¹ Week 14 Week 28 Week 42 Week 56 Placebo-subtracted HbA1c Observed Case Analysis (Contrave32) Data is from COR-Diabetes Phase 3 trial 1 p<0.05 (between Contrave32 and placebo) ITT-LOCF: all randomized patients with 1 post-baseline observation while on study drug |
 10 Contrave Improved Metabolic Parameters in High Risk Subgroups Contrave Improved Metabolic Parameters in High Risk Subgroups Waist Circumference Triglycerides HS-CRP HDL Cholesterol P < 0.05 P < 0.05 Placebo Contrave32 P < 0.05 P < 0.05 P < 0.05 P < 0.05 P < 0.05 P < 0.05 Data is for completers in high-risk subgroups. High risk subgroups defined by ATPIII Guidelines (waist circumference, males >102, females >88 cm; fasting triglycerides, 150 mg/dL, fasting HDL, males <40, females <50 mg/dL, hsCRP, >3 mg/L). HS-CRP based on log transformed data n: 282 279 n: 254 410 n: 82 87 n: 69 135 n: 122 121 n: 117 184 n: 144 261 n: 163 177 |
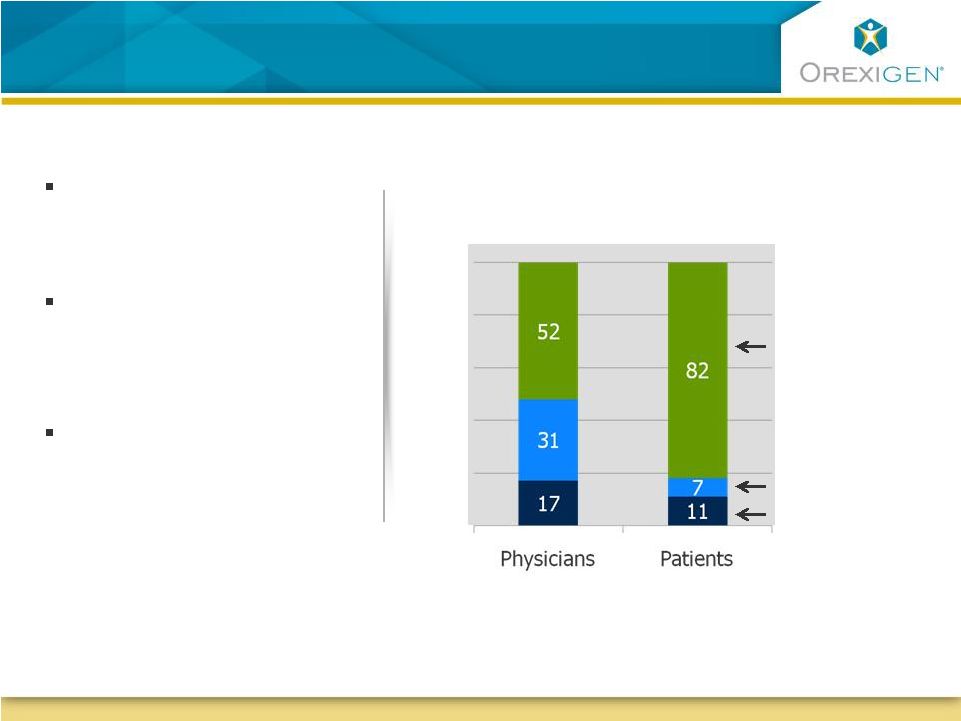 11 Both Physicians and Patients Blame Behavior for Obesity Both Physicians and Patients Blame Behavior for Obesity Patients place much of the blame for their weight on poor eating habits 100 80 60 40 20 Perceived causes of obesity Individual choices, behaviors Genetics/Heredity Medical conditions Half of obese patients self- identify as having eating control issues Half of obese patients acknowledge they consume more calories than they burn Contrave, with two constituents approved for addictive disorders,
may have a unique ability to help obese patients control their eating behavior Source: Orexigen quantitative market research 2008; Harris Interactive, Report on Weight and Obesity in America, June 2007 |
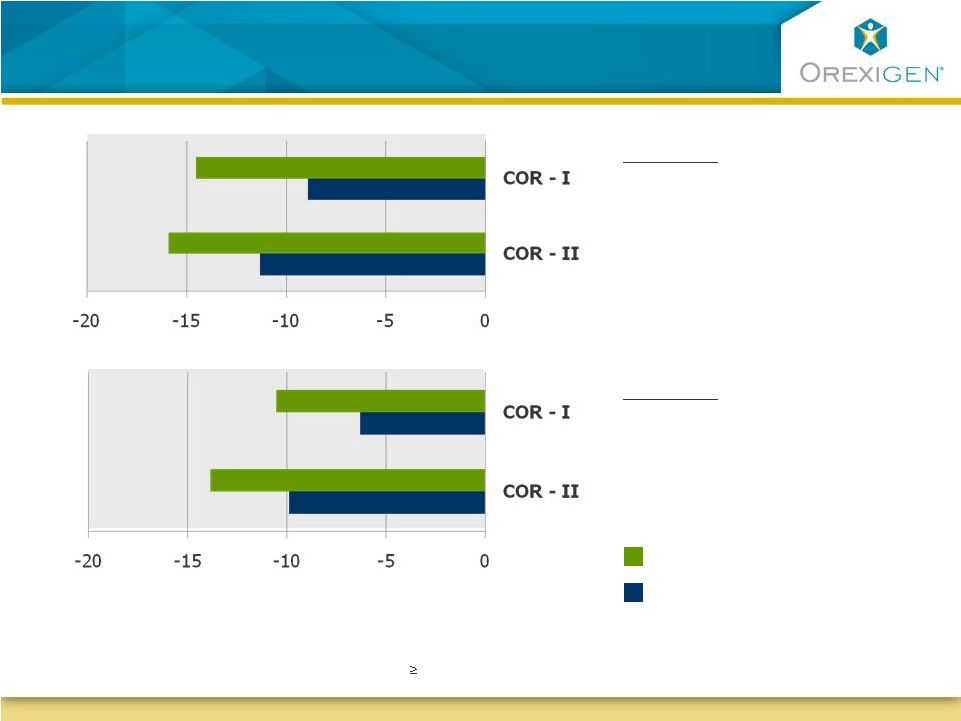 12 Contrave Patients Experienced Statistically Significant Improvements in Eating Control Contrave Patients Experienced Statistically Significant Improvements in Eating Control Question: How difficult has it been to control your eating? Question: How difficult has it been to resist any food cravings? *P<0.05 vs. Placebo; ITT-LOCF (all randomized patients with 1 post-baseline observation while on study drug); results are based on measurements using a 100 mm visual analog scale. N>400 in all groups Less More Contrave32 Placebo |
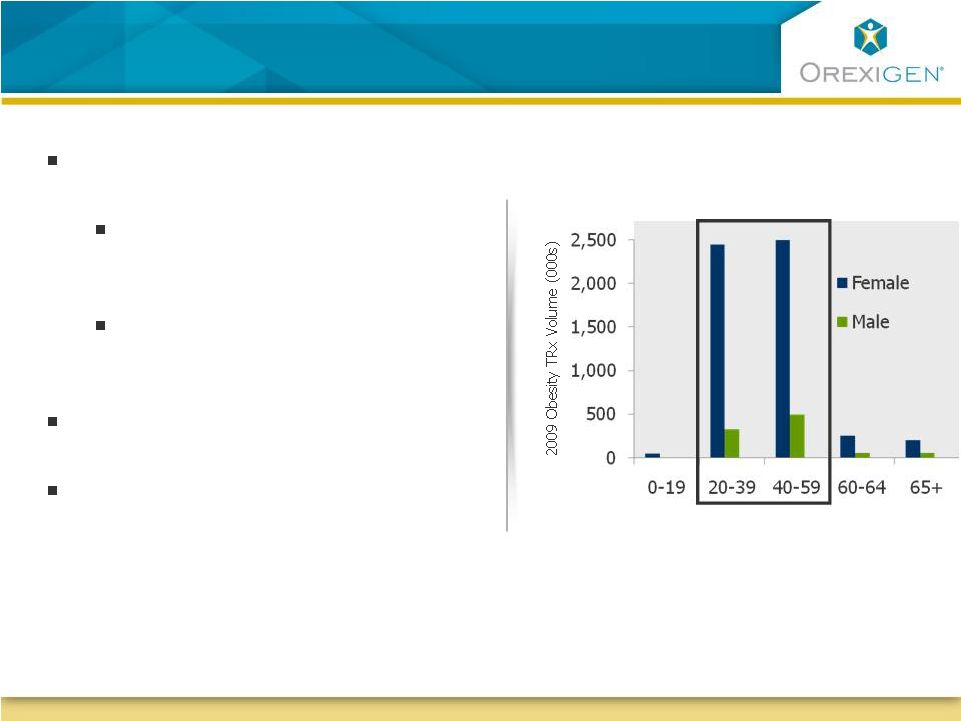 13 Contrave may become the Logical Therapeutic Choice in Women of Child-bearing Age Contrave may become the Logical Therapeutic Choice in Women of Child-bearing Age Source: Orexigen quantitative market research 2008; Food Cravings in Young and Elderly Adults, M. Pelchat,
Appetite, 1997, 28: 103-113; IMS Health,
NPA, National Disease and Therapeutic Index Women are more proactive in
addressing weight 63% of women vs. 40% of men initiated a conversation with physician about weight Women are 2.5x more likely to have tried an obesity Rx than men Research has indicated that women tend to crave food more than men Combinations with anti-convulsants (e.g. Empatic) are expected to carry a Category X pregnancy warning Majority of the obesity RXs today go to women of child-bearing age Age (years) |
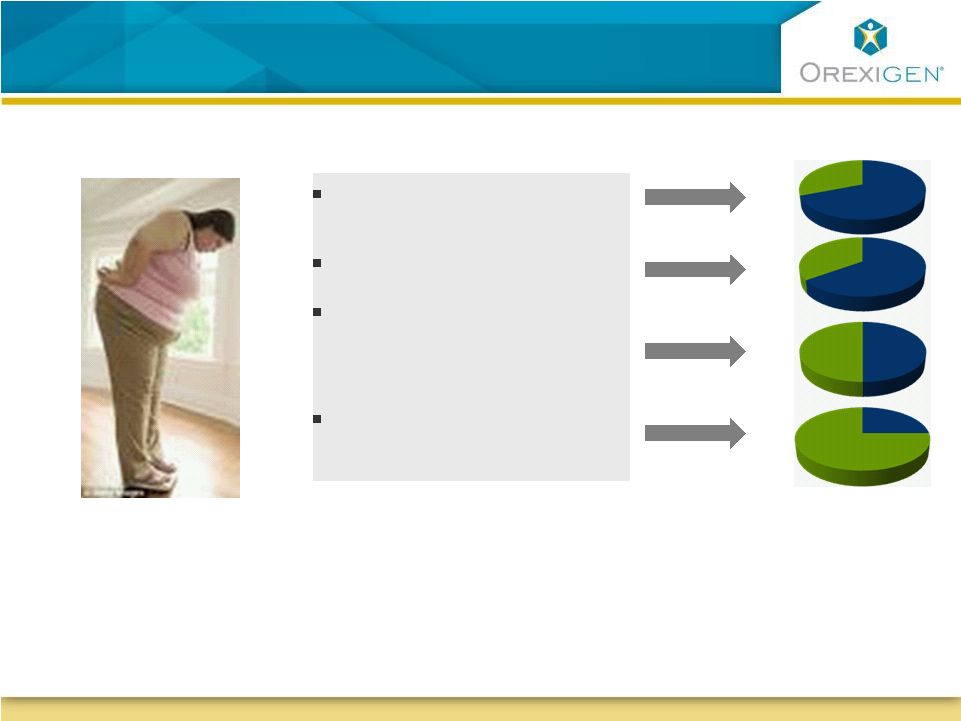 14 Natural Patient Targets for Contrave Natural Patient Targets for Contrave Obese with diabetes 50% 50% 72% 72% Available obese population 26% 26% 36% 36% Obese with dyslipidemia Obese who report uncontrollable food cravings Treatment seeking obese who are female Sources: Orexigen quantitative market research 2008 and 2009 |
 15 Commercialization Considerations Commercialization Considerations |
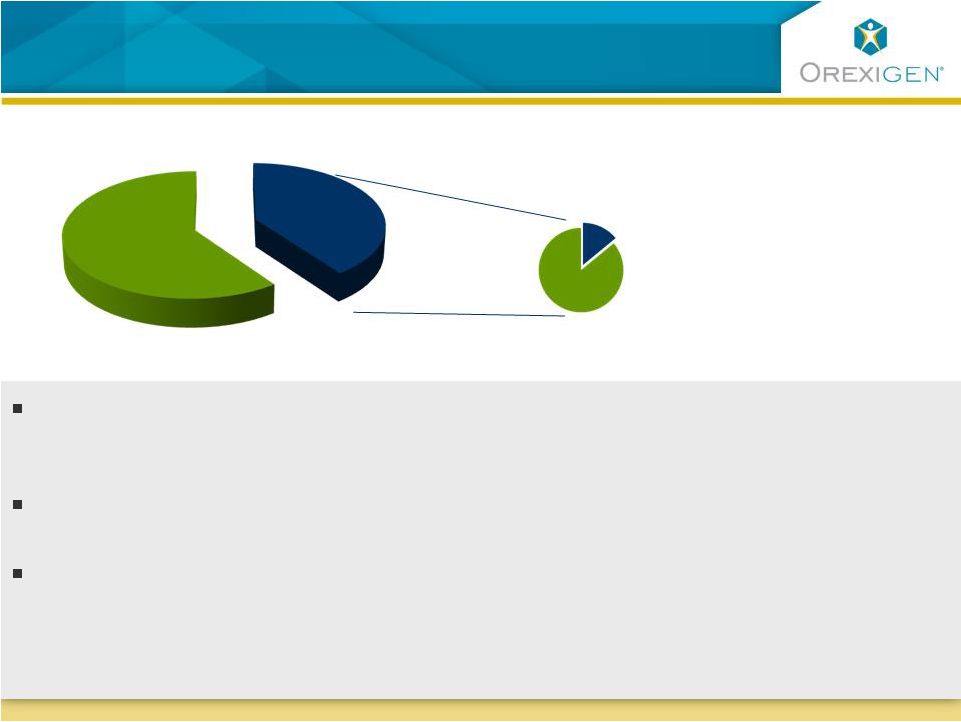 Market is
Dissatisfied with Currently Available Treatments Market is Dissatisfied with Currently Available Treatments Source: CDC; Orexigen quantitative market research 2009 * Meridia, Xenical, phentermine 33% Obese patients 14% 2008 Obesity TRx*=7.8M Market research tells us physicians view current drugs as sub-optimal across the efficacy/safety profile Physicians “strongly agree” that “obesity is a serious health issue in their practices” There is limited promotional spend in obesity today If approved, Contrave could address the unmet need in this dissatisfied market U.S. Population % of Obese Receiving Rx 16 |
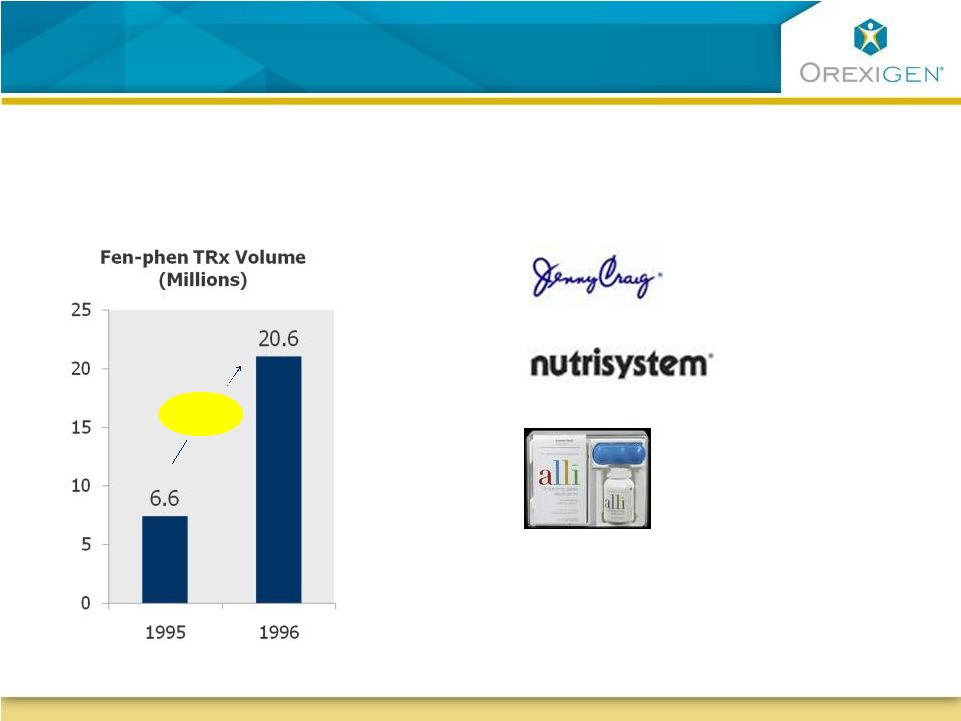 17 There is Clear Demand for an Rx Solution and Consumers Display a Willingness to Pay There is Clear Demand for an Rx Solution and Consumers Display a Willingness to Pay Americans spend nearly $60B/yr out- of-pocket on weight loss products Americans spend nearly $60B/yr out- of-pocket on weight loss products Fen-phen experience demonstrated pent-up demand for therapeutic options Fen-phen experience demonstrated pent-up demand for therapeutic options $60/month $280/month $50 fee plus $500/month for food Nutraceuticals – 1 month supply: Lipofuze: $60 Hydroxycut: $50 3.1x Source: IMS, NPA; Marketdata Enterprises, Inc., Press Release, February 16, 2009; Orexigen
market research 2009 |
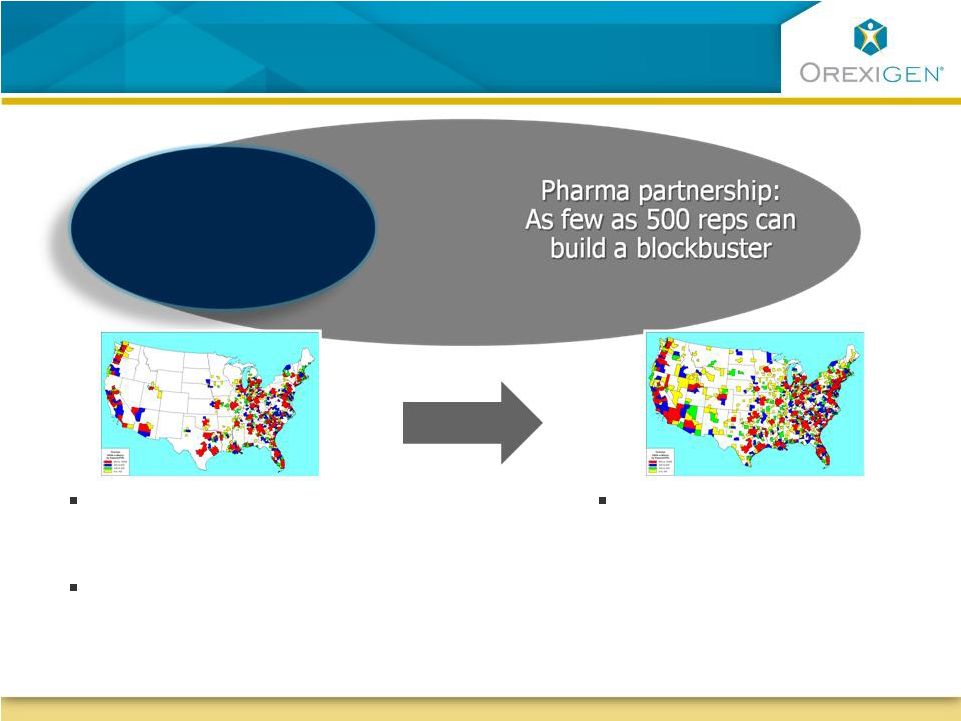 18 Commercial Strategy: Leverage Small Number of Motivated Commercial Strategy: Leverage Small Number of Motivated Obesity Treating Physicians, and Go Broader with a Partner Obesity Treating Physicians, and Go Broader with a Partner 150 rep sales force can reach the top 7 deciles of current obesity prescribers Physicians only treating 14% of their obese patients today due to lack of good options Orexigen obesity specialty sales force: Rapidly grab the low hanging fruit 500 rep sales force can cover top 5 deciles of current prescribers of cardiometabolic risk factor meds Sources: Orexigen quantitative market research 2009 |
 19 Pipeline Opportunities Pipeline Opportunities |
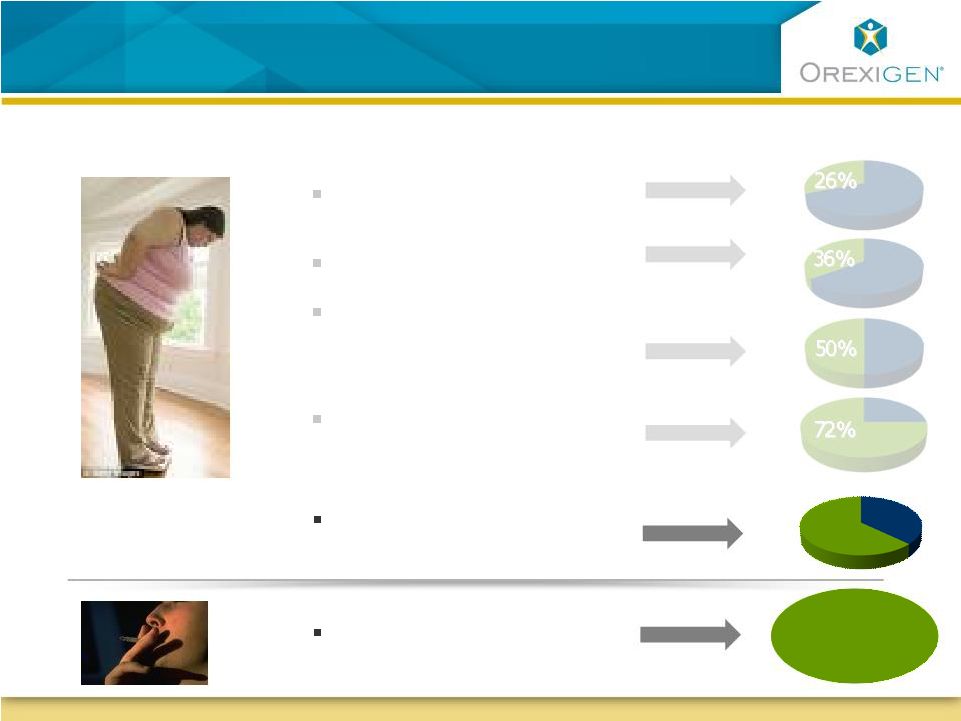 Future
Potential Opportunities for Contrave Future Potential Opportunities for Contrave
Depressed or has depressive symptoms 63% 63% Available obese population Obese with diabetes Obese with dyslipidemia Obese who report uncontrollable food cravings Treatment seeking obese who are female 43.4M smokers in the US Smokers 20 Sources: Orexigen quantitative market research 2009; CDC, MMWR Weekly, Cigarette Smoking Among
Adults – United States 2007, November 14, 2008 |
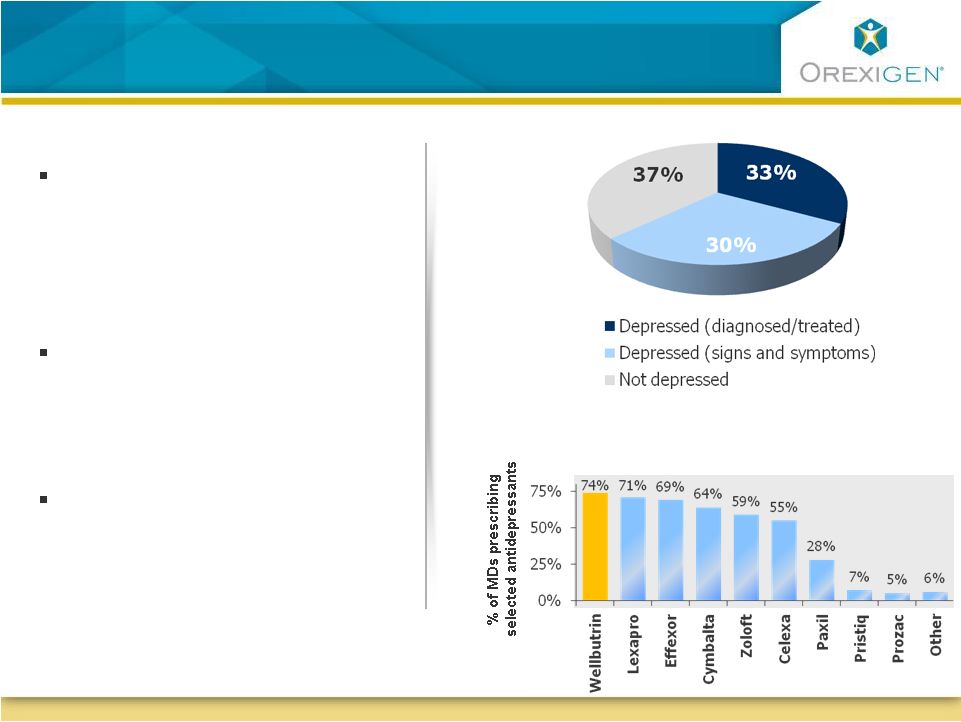 21 Obesity is Associated with an Increased Risk of Depression Obesity is Associated with an Increased Risk of Depression Physicians report that approximately 1/3 of obese patients are taking pharmacologic therapy for depression Another 1/3 of obese patients display signs and symptoms of untreated depression Bupropion is already the antidepressant of choice in the obese population Antidepressants in Obese Population Source: Orexigen quantitative market research 2009 |
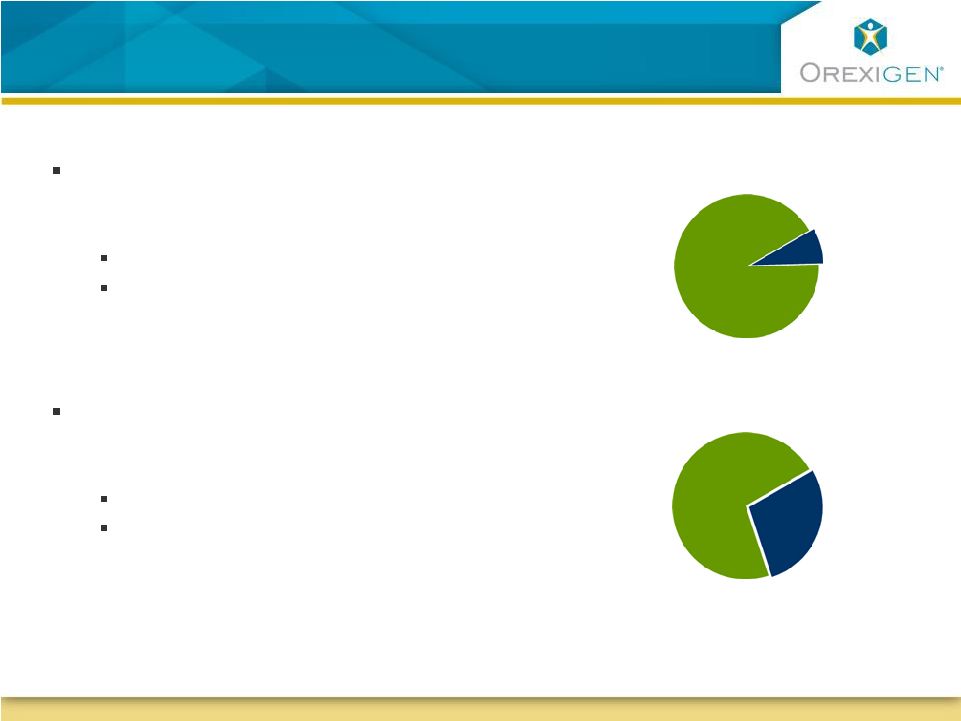 22 Utilization intent measures indicate 92% of physicians would be willing to prescribe Contrave for obesity to patients who: Display depressive symptoms but Are not on an antidepressant Source: Orexigen quantitative market research 2009 Treating Obesity in Patients with Depressive Symptoms may be an Attractive Future Opportunity Treating Obesity in Patients with Depressive Symptoms may be an Attractive Future Opportunity Utilization intent measures indicate 72% of physicians would be willing to prescribe Contrave for obesity to patients who: Display depressive symptoms and Are on an antidepressant Willingness to prescribe Contrave YES 72% YES 92% Willingness to prescribe Contrave |
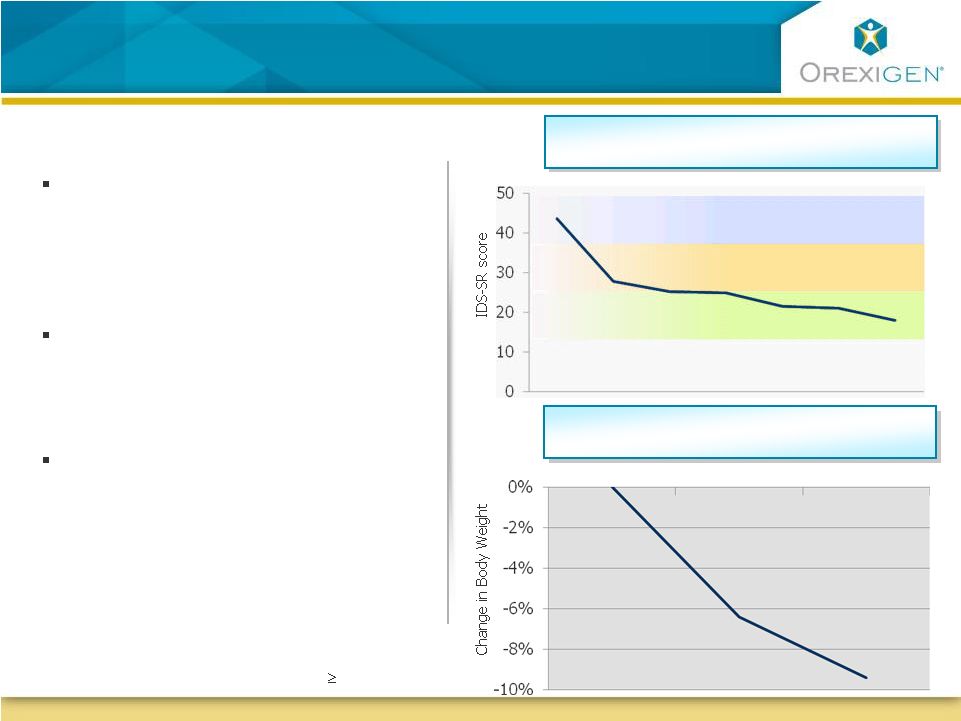 23 Open Label Study: Contrave Reduced Weight and Depressive Symptoms in Obese Depressed Patients Open Label Study: Contrave Reduced Weight and Depressive Symptoms in Obese Depressed Patients Inventory of Depressive Symptoms (Observed Case) Change in Body Weight (Observed Case) Week 0 Week 12 Week 24 In a 24-week trial of obese depressed patients, Contrave significantly improved measures of depression At the same time, patients who completed the trial lost over 9% of their body weight In addition, in a Control of Eating Questionnaire, patients reported decreases in hunger and cravings, increased ability to resist cravings Week 0 Week 12 Week 24 Not depressed Mildly depressed Moderately depressed Severely depressed Trial included 25 obese patients with symptoms of major depression at baseline (IDS-SR total score of 26) |
 24 Smoking Cessation while Reducing Weight Gain Represents an Attractive Follow-on Indication for Contrave Smoking Cessation while Reducing Weight Gain Represents an Attractive Follow-on Indication for Contrave Numerous studies reveal that post-cessation weight gain is a major barrier for smokers to try quitting and a driver of relapse Payors and employers are encouraging people to quit and paying for smoking cessation pharmacotherapy Pfizer’s successful launch of Chantix ® speaks to the underlying demand in the market Chantix has achieved market success despite typical smoking-related weight gain In the pivotal trials for Chantix, patients on Chantix saw 5.0-6.4lb weight gain at 12 weeks Source: CANCER supplement 1994: 74; 2055-2061; Am J Public Health 1991, Vol. 81,
324-327; Orexigen market research 2009; Pfizer, Inc., Press Release, January 26,
2009; JAMA, July 2006, Vol .296, No 1: 47-55 and 56-63 $1,000
$500 $0 |
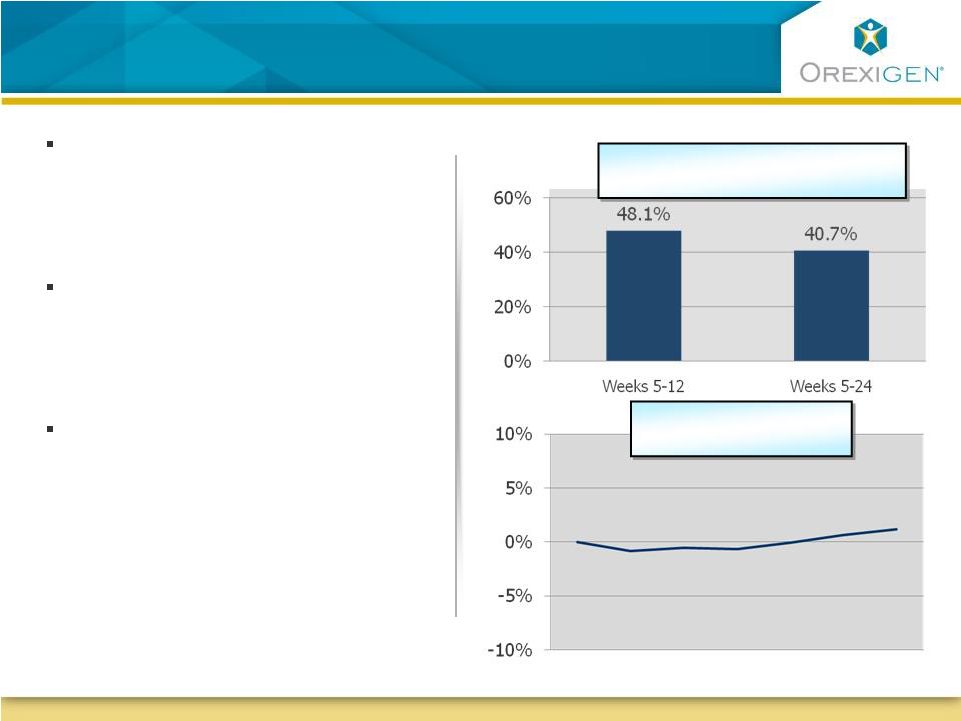 25 Contrave POC Study in Obese Smokers: Compelling Quit Rates without Typical Weight Gain Contrave POC Study in Obese Smokers: Compelling Quit Rates without Typical Weight Gain In a 24-week open label trial, Contrave significantly reduced cigarette use among obese patients trying to quit smoking In contrast to available therapies for smoking cessation, Contrave was not associated with clinically meaningful weight gain In addition, in a Control of Eating Questionnaire, the desire to eat sweet foods was significantly reduced at both 12 and 24 weeks (p<0.05) Week 0 Week 12 Week 24 *Data for continuous abstainers from weeks 5 to 24 Sustained Smoking Cessation Rates % Change in Body Weight* |
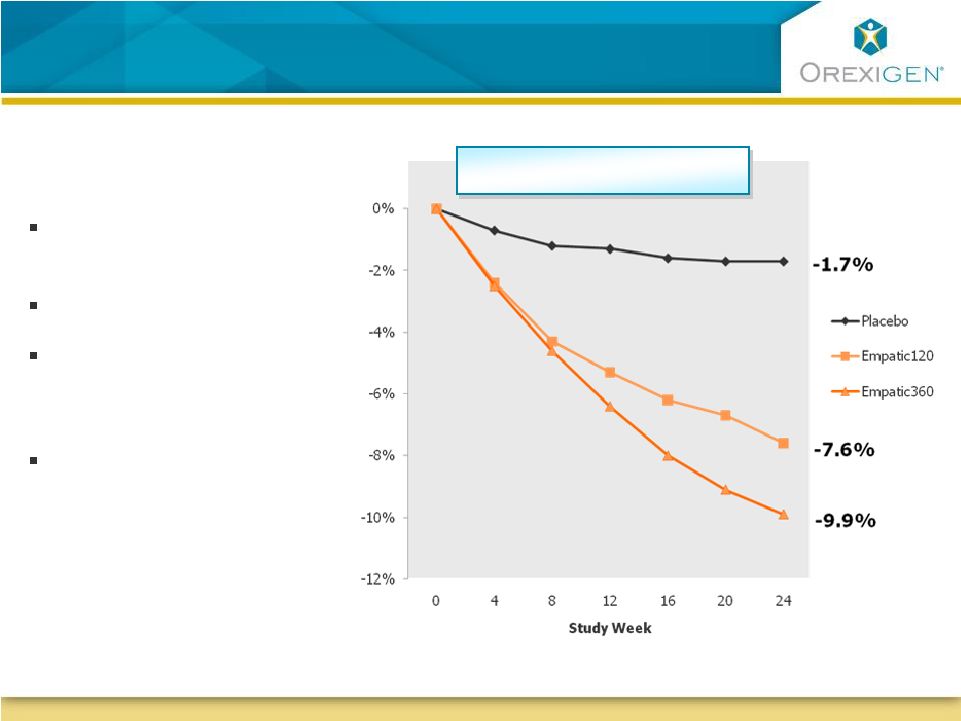 26 Second Obesity Program: Empatic Demonstrates Early and Progressive Weight Loss Second Obesity Program: Empatic Demonstrates Early and Progressive Weight Loss At 24 weeks, Empatic demonstrates: Clinically meaningful weight loss No evidence of plateau Efficacy on both Empatic dosages; could provide dosing flexibility Safety profile consistent with individual constituents; most common adverse events: Headache, insomnia, nausea, constipation, dry mouth Note : All differences between Empatic and placebo highly statistically significant at all time points Observed Case Analysis |
 27 Summary Summary |
 28 Contrave Phase 3 Program Addresses Key Elements of FDA Guidelines for Obesity Products Contrave Phase 3 Program Addresses Key Elements of FDA Guidelines for Obesity Products Key elements of FDA guidance: Total Total Program Program (n=4,536) (n=4,536) Statistical Significance on Co-Primary Weight Loss Endpoints Efficacy Benchmark (5% Categorical Response OR 5% Placebo Subtracted AND p 0.05) Additional Endpoints Efficacy in Obese Patients with Diabetes Sufficient Patient Exposures for Evaluation of Safety and Tolerability (1 year) |
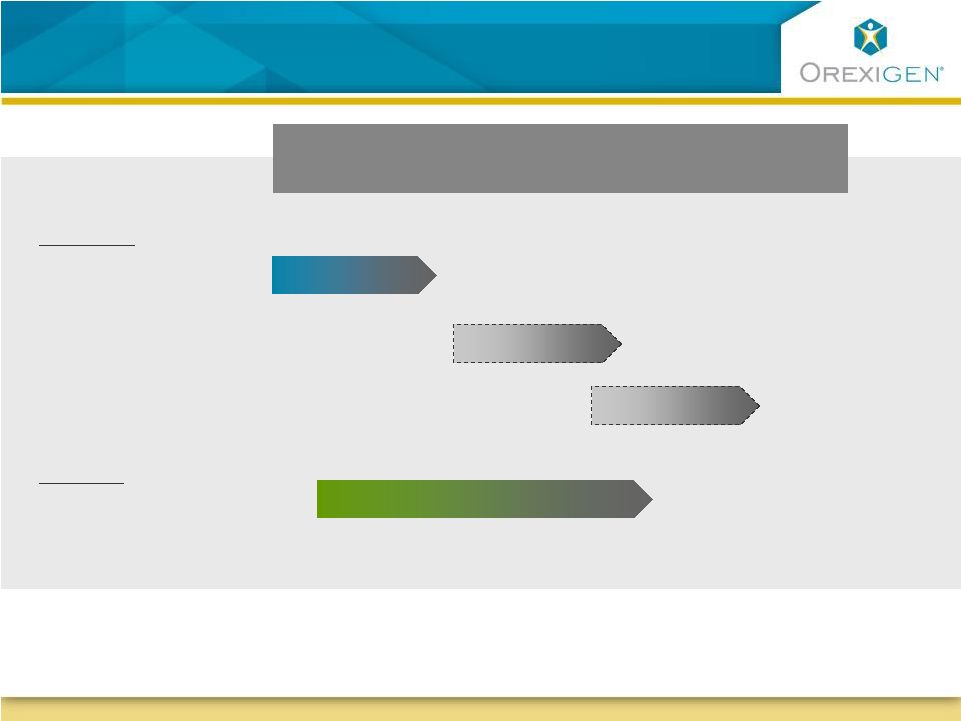 29 Orexigen has a Range of Differentiated Opportunities Orexigen has a Range of Differentiated Opportunities Near-term Opportunities Smoking Cessation Without Weight Gain Phase 3 Pivotal Studies Empatic Treatment of Obesity: NDA Contrave Future Opportunities Treatment in Obese Depressed |
 30 The Orexigen Opportunity The Orexigen Opportunity Obesity: The next big thing Orexigen - Only company with two late stage obesity programs Contrave Profile for First-Line Therapy: Uniquely positioned to meet the needs of the obese patient Empatic - Complementary to Contrave Promotional Execution: Highly efficient specialty and primary care launch scenarios Specialty launch: 150 reps can effectively target the current 14k obesity high prescribers Primary care launch: 500 reps can effectively target the 50k high prescribers of cardiometabolic drugs - a fertile ground for obesity Rx |
