Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d8k.htm |
| EX-99.2 - PRESS RELEASE - INFINITY PHARMACEUTICALS, INC. | dex992.htm |
 Innovation. Evolution. Impact. Exhibit 99.1 |
 Innovation. Evolution. Impact. 2 Safe Harbor Statement 2 2 • This presentation contains forward-looking statements within the meaning of The Private Securities
Litigation Reform Act of 1995. •
These statements involve risks and uncertainties that could cause actual results to be
materially different from historical results or from any future results expressed or implied by
such forward-looking statements. • Such forward-looking statements include statements regarding
the therapeutic potential of Infinity’s Hedgehog pathway, FAAH, and Hsp90 chaperone
inhibitors; future clinical trial activity for IPI-504, IPI-493, IPI-926 and IPI-940; the presentation of clinical data for IPI-504, IPI-493 and IPI-926; estimates of 2010 financial performance; the continuation of the Purdue/Mundipharma alliance;
and the expectation that Infinity will have capital to support its current operating plan into
2013. • Such forward-looking statements are subject to numerous factors, risks and uncertainties that may cause
actual events or results to differ materially from the company's current expectations. For
example, there can be no guarantee that Infinity’s strategic alliance with Purdue/Mundipharma will continue for its expected term or that these entities will fund Infinity’s
programs as agreed, or that any product candidate Infinity is developing will successfully
complete necessary preclinical and clinical development phases. Further, there can be no guarantee that any positive developments in Infinity’s product portfolio will result in stock price
appreciation. Infinity’s expectations could also be affected by risks and uncertainties
relating to: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S.
Food and Drug Administration and other regulatory authorities, investigational review boards at
clinical trial sites, and publication review bodies; Infinity's ability to enroll patients in its
clinical trials; unplanned cash requirements and expenditures, including in connection with business development activities; market acceptance of any products Infinity or its partners may successfully develop; and Infinity's ability
to obtain, maintain and enforce patent and other intellectual property protection for any product
candidate it is developing. •
These and other risks which may impact management's expectations are described in greater
detail under the caption "Risk Factors" included in Infinity's quarterly report on Form
10-Q filed with the U.S. Securities and Exchange Commission in November 2009. • Further, any forward-looking statements contained in this presentation speak only as of the date hereof,
and Infinity expressly disclaims any obligation to update any forward-looking statements,
whether as a result of new information, future events or otherwise. • All trademarks used in this presentation are the property of their respective owners. • Our Internet website is http://www.infi.com. We regularly use our website to post information regarding our
business, product development programs and governance. We encourage investors to use
www.infi.com, particularly the information in the section entitled “Investors/Media,” as
a source of information about Infinity. References to www.infi.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.infi.com into this presentation by reference.
|
 Infinity: Innovation. Evolution. Impact. 3 Proven drug developers and company builders Financial Strength Cash runway into 2013 Ownership of Valuable Product Rights Establishing commercial infrastructure in US Fourth poised to enter in early 2010 Innovation. Evolution. Impact. Diverse Pipeline of Broadly Applicable Targeted Therapies Integrated Team Three candidates in the clinic; |
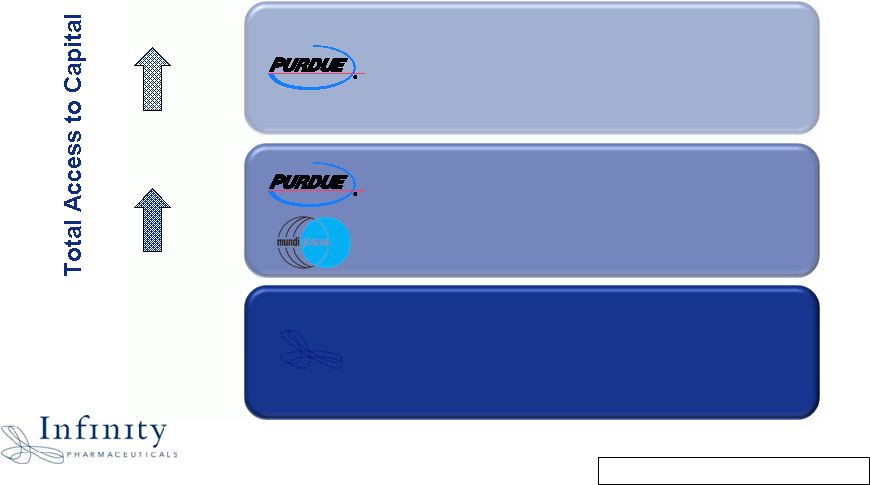 Innovation. Evolution. Impact. Financial Strength to Create Value 4 * Unaudited ~$330M to aggressively invest in pipeline and access external opportunities ~$330M ~$130M ~$130M in Cash & Investments at 12/31/09* ~$280M $150M in Committed R&D Funding through 2011 $50M Line of Credit |
 Innovation. Evolution. Impact. Financial Strength: Cash Runway into 2013 • ~$330 million to aggressively invest in pipeline and access external opportunities • Projected 2010 cash burn of $25M - $35M • Anticipate year-end cash and investments balance of $95M - $105M – Based on current operating plan; excludes $50M line of credit from Purdue • Low share base (~26 million shares outstanding) 5 |
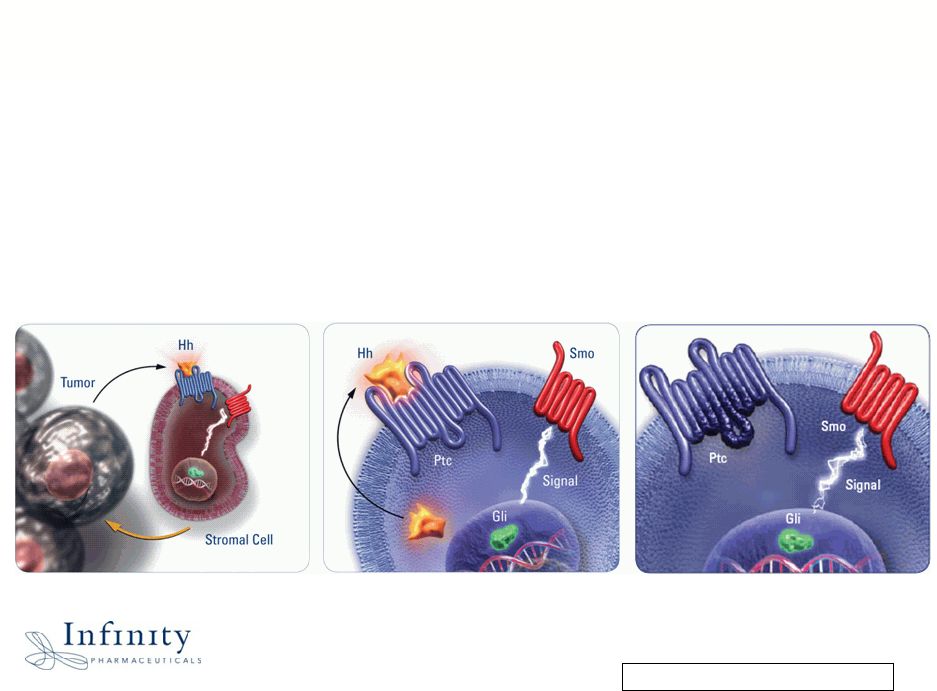 Innovation. Evolution. Impact. 6 IPI-926: Significant Anti-Cancer Opportunity by Inhibiting Malignant Activation of the Hedgehog Pathway Signal to progenitor cell Signal to tumor microenvironment Signal to tumor cell |
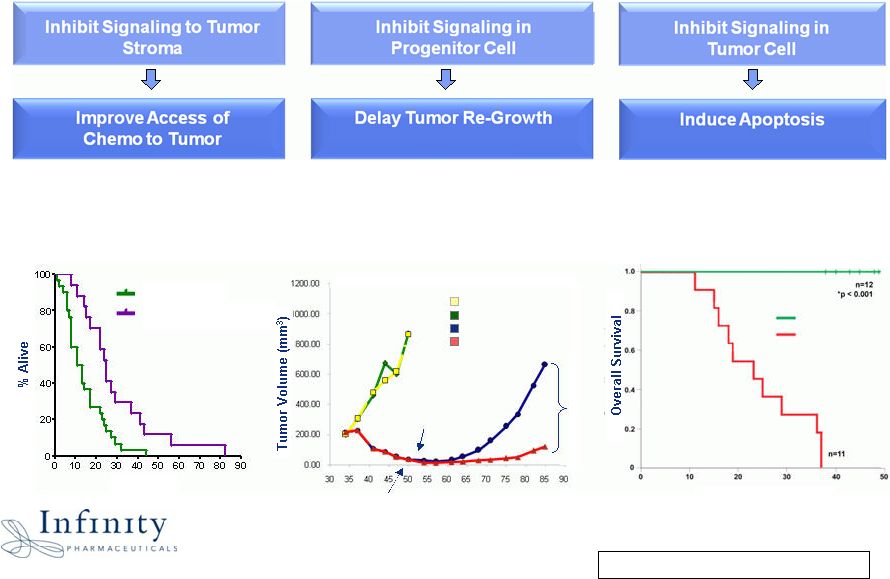 Innovation. Evolution. Impact. IPI-926: Inhibiting Multiple Modes of
Malignant Activation to Prolong Survival 7 1 Olive et al., 21 May 2009 Science IPI-926 significantly delays tumor re-growth post-chemo in SCLC model IPI-926 + Gemcitabine doubles median survival in pancreatic cancer model IPI-926 results in 100% survival in medulloblastoma model Controls IPI-926 + Gemcitabine Days 2 Travaglione et al., 2008 AACR 3 Olson et al., 2009 AACR Days IPI-926 Vehicle Days Post Implant Mice randomized +/- IPI-926 Last day of E/P treatment E/P - IPI-926 E/P -Vehicle Vehicle IPI-926 82% 1 2 3 |
 Innovation. Evolution. Impact. Advanced BCC, Medulloblastoma IPI-926: Targeting a Broad Range of
Difficult to Treat Cancers
8 IPI-926 in Phase 1 study in advanced solid tumors; Phase 2 development anticipated to begin in 2010
Pancreatic cancer SCLC, ovarian cancer, NSCLC, and heme malignancies |
 Innovation. Evolution. Impact. 9 IPI-940: Combating the Magnitude of Neuropathic Pain by Inhibiting Fatty Acid Amide Hydrolase (FAAH) FAA H IPI-940 (FAAH Inhibitor) |
 Innovation. Evolution. Impact. IPI-940: Novel Agent Enabling the Body’s Natural Analgesia • Novel, oral agent potentiating the magnitude and duration of body’s natural analgesia – Designed to avoid common side effects (e.g., drowsiness) • IND filed in December 2009 • Ph 1 study in normal, healthy volunteers planned for early 2010 10 |
 Innovation. Evolution. Impact. IPI-940: Targeting Broad Areas of Unmet Need • Neuropathic Pain – Postherpetic neuralgia – HIV pain – Peripheral diabetic neuropathy – Chemotherapy-induced neuropathy – Trigeminal neuralgia – Fibromyalgia • Osteoarthritic pain • Inflammatory Bowel Disease • Anxiety & Depression • Inflammatory Conditions 11 |
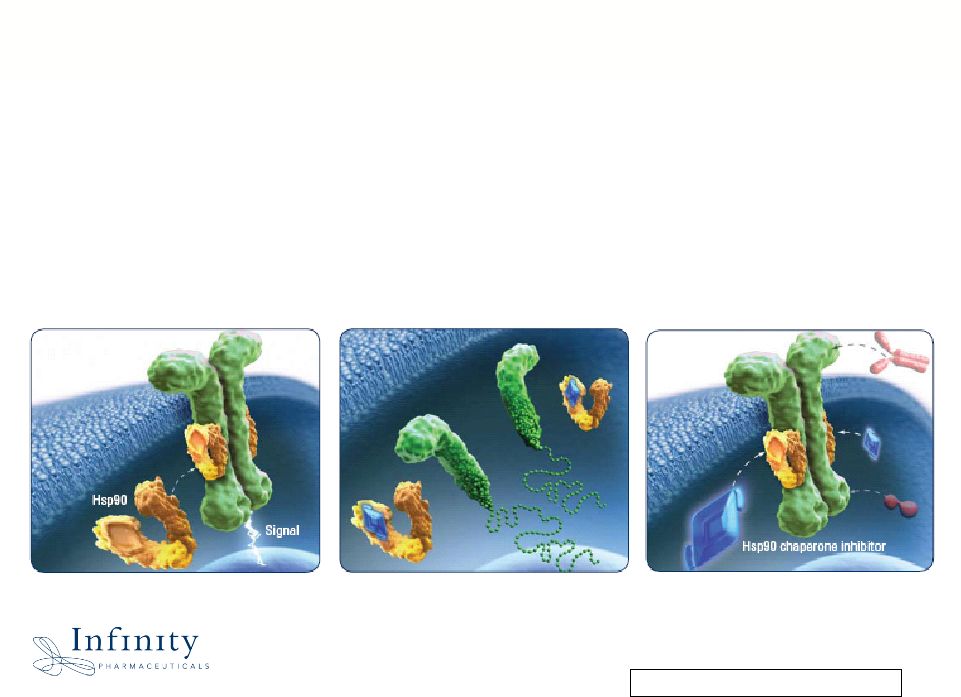 Innovation. Evolution. Impact. 12 Hsp90 chaperone stabilizes oncoproteins IPI-504 and IPI-493 combinable with best available therapies Inhibiting Hsp90 degrades oncoproteins, stopping tumor growth IPI-504 and IPI-493: Broadly Attacking
Oncoproteins through Hsp90 Chaperone Inhibition |
 Innovation. Evolution. Impact. Hsp90: Focused Development of Two Distinct Product Candidates • IPI-504 (i.v.): Establish therapeutic window and identify appropriate
patient populations – Ongoing Ph 2 development in HER2 breast cancer and NSCLC – Potential paths forward in breast and lung cancers, liposarcoma, and broad range of other cancer reliant on oncoproteins • IPI-493 (oral): Determine optimal Ph 2 dose and schedule – Ongoing Ph 1 study in solid tumors – Additional Ph 1 in hematological malignancies expected to start early 2010 13 + |
 Innovation. Evolution. Impact. Key 2010 Development Objectives • IPI-926 (Hedgehog Pathway Inhibitor) – Phase 1 data in solid tumors – Phase 2 initiation • IPI-940 (FAAH Inhibitor) – Phase 1 completion • IPI-504 (Hsp90 Chaperone Inhibitor) – Phase 2 data in NSCLC and initiate further clinical development – Phase 2 data in HER2 breast cancer – Phase 2 initiation in liposarcoma • IPI-493 (Hsp90 Chaperone Inhibitor) – Phase 1 data in solid tumors – Phase 1 initiation and data in hematological malignancies 14 + |
 Innovation.
Evolution. Impact. • U.S. commercialization rights to entire oncology portfolio – Building commercial infrastructure • Significant financial participation ex-U.S. – Ex-U.S. royalty up to 20% by Mundipharma for oncology programs – Global royalty up to 20% by Purdue for FAAH program • Operational capabilities and financial strength to access external strategic opportunities • Low share base (~26 million shares outstanding) Delivering Patient Benefit and Creating Shareholder Value 15 |
 Infinity: Innovation. Evolution. Impact. 16 Proven drug developers and company builders Financial Strength Cash runway into 2013 Ownership of Valuable Product Rights Fourth poised to enter in early 2010 Innovation. Evolution. Impact. Diverse Pipeline of Broadly Applicable Targeted Therapies Integrated Team Three candidates in the clinic; Establishing commercial infrastructure in US |
