Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HALOZYME THERAPEUTICS, INC. | a54795e8vk.htm |
Exhibit 99.1
| Halozyme Therapeutics Matrix Therapies for Life Jonathan Lim, M.D., President and CEO 28th Annual J.P. Morgan Healthcare Conference San Francisco, January 13, 2010 |

| Safe Harbor The Private Securities Litigation Reform Act of 1995 provides a "safe harbor" for forward-looking statements. All statements made in this presentation that are not statements of historical fact constitute forward-looking statements. The matters referred to in forward-looking statements could be affected by the risks and uncertainties of the Company's business. Such risks inherent to the Company's business will be described in the Company's filings, when they occur, with the Securities and Exchange Commission, as well as in its press releases. The Company's actual results may differ materially from those expressed in or indicated by such forward-looking statements. 2 |
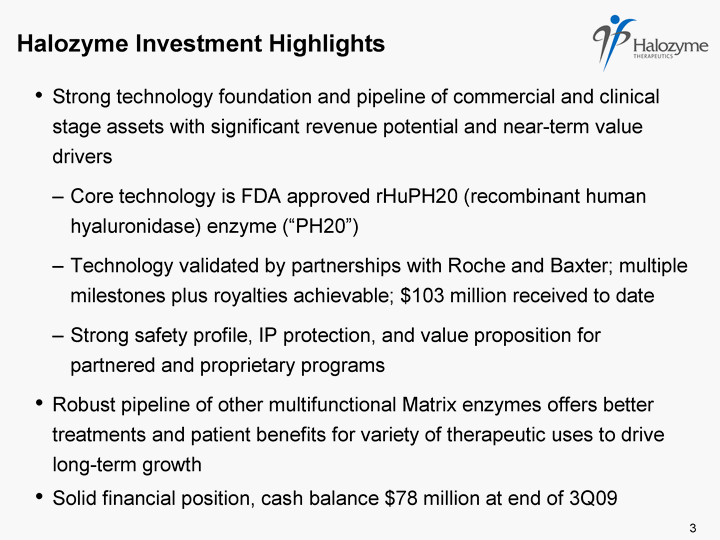
| Halozyme Investment Highlights Strong technology foundation and pipeline of commercial and clinical stage assets with significant revenue potential and near-term value drivers Core technology is FDA approved rHuPH20 (recombinant human hyaluronidase) enzyme ("PH20") Technology validated by partnerships with Roche and Baxter; multiple milestones plus royalties achievable; $103 million received to date Strong safety profile, IP protection, and value proposition for partnered and proprietary programs Robust pipeline of other multifunctional Matrix enzymes offers better treatments and patient benefits for variety of therapeutic uses to drive long-term growth Solid financial position, cash balance $78 million at end of 3Q09 3 |
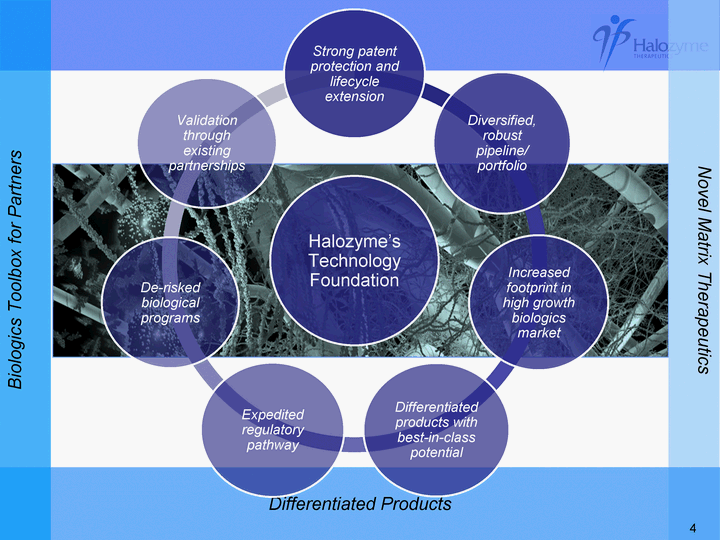
| Novel Matrix Therapeutics Differentiated Products Biologics Toolbox for Partners Halozyme's Technology Foundation Strong patent protection and lifecycle extension Diversified, robust pipeline/ portfolio Increased footprint in high growth biologics market Differentiated products with best-in-class potential Expedited regulatory pathway De-risked biological programs Validation through existing partnerships 4 |
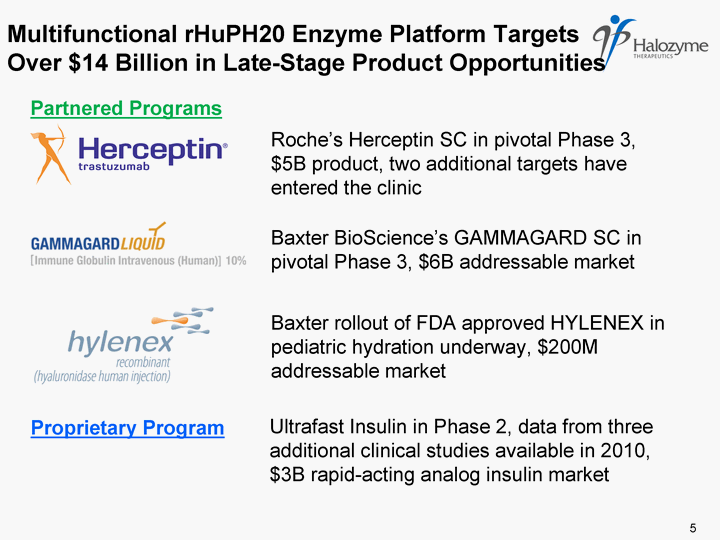
| Multifunctional rHuPH20 Enzyme Platform Targets Over $14 Billion in Late-Stage Product Opportunities Roche's Herceptin SC in pivotal Phase 3, $5B product, two additional targets have entered the clinic Baxter BioScience's GAMMAGARD SC in pivotal Phase 3, $6B addressable market Baxter rollout of FDA approved HYLENEX in pediatric hydration underway, $200M addressable market Ultrafast Insulin in Phase 2, data from three additional clinical studies available in 2010, $3B rapid-acting analog insulin market 5 Partnered Programs Proprietary Program |
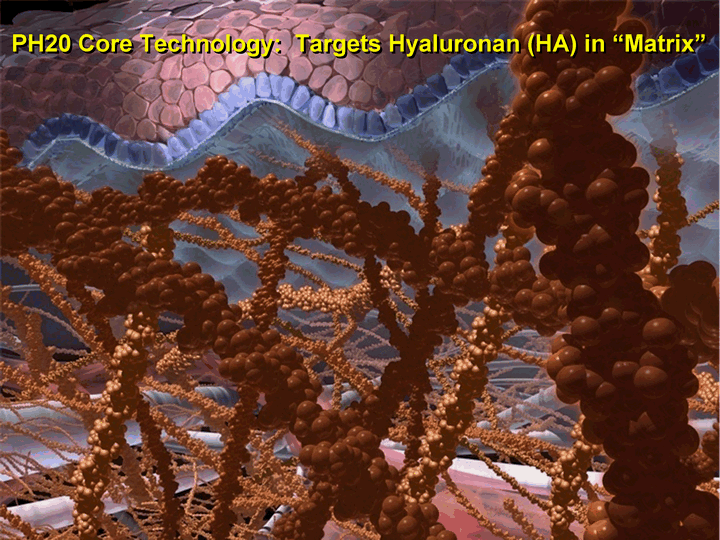
| 6 PH20 Core Technology: Targets Hyaluronan (HA) in "Matrix" |
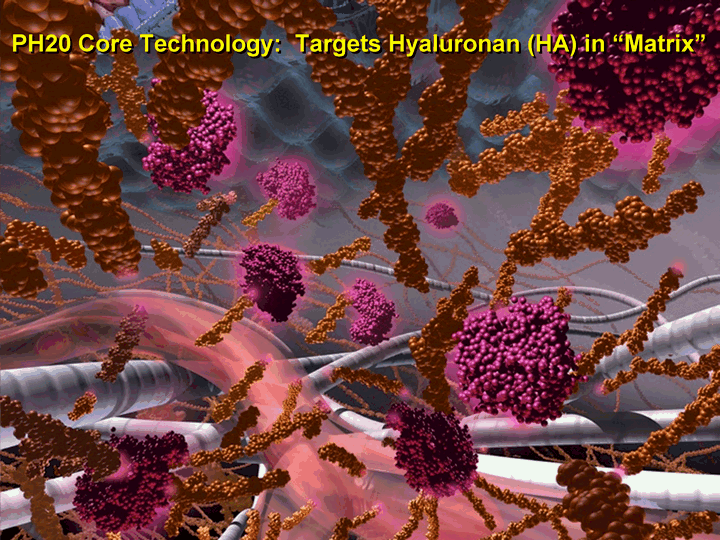
| 7 PH20 Core Technology: Targets Hyaluronan (HA) in "Matrix" |

| 8 PH20 Core Technology: Targets Hyaluronan (HA) in "Matrix" |

| HA reconstitutes within 24 to 48 hours |

| IgG 10mg/kg local Bioavailability = 59 +/- 7% 0.0E+00 2.0E+05 4.0E+05 6.0E+05 8.0E+05 1.0E+06 1.2E+06 1.4E+06 1.6E+06 1.8E+06 2.0E+06 0 10 20 30 40 50 60 70 80 90 100 110 120 130 Time (Hours) Serum Antibody Levels (I-125Counts/ml) in rats IgG 10mg/kg IV Bioavailability = 100% Bioavailability = 94 +/- 7% PH20 with Remicade(r) - IV Like Bioavailability in a Preclinical Model Bookbinder, et al. J Controlled Release. 2006;114:230-1 10 |
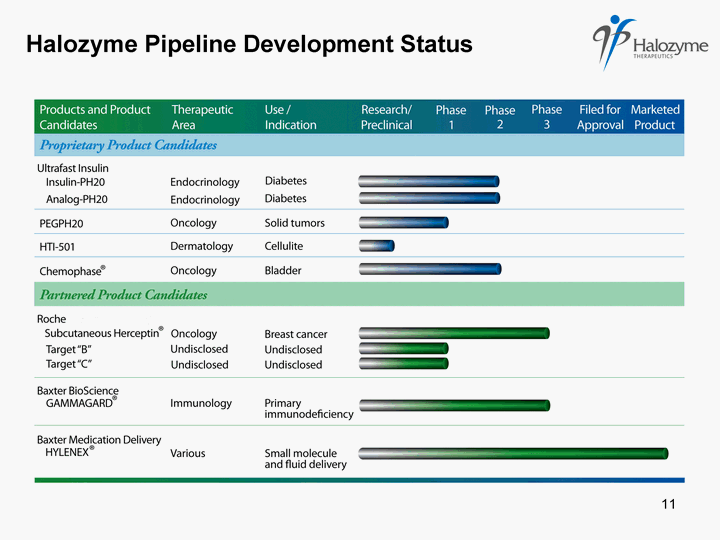
| Halozyme Pipeline Development Status 11 |
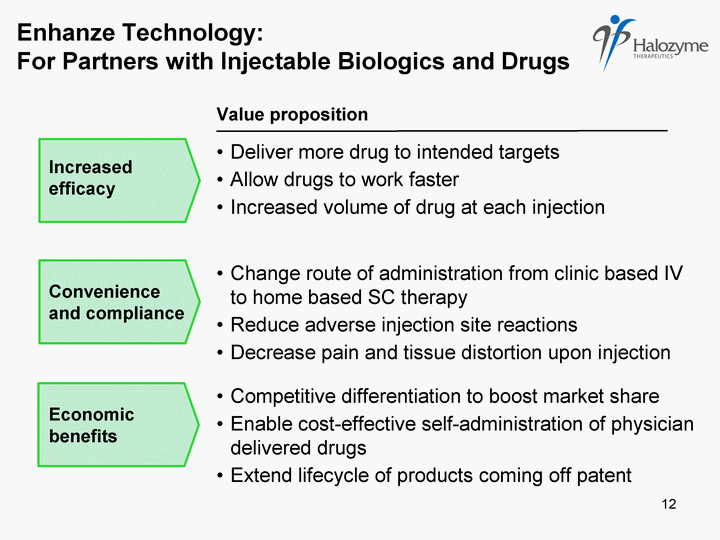
| Change route of administration from clinic based IV to home based SC therapy Reduce adverse injection site reactions Decrease pain and tissue distortion upon injection Convenience and compliance Economic benefits Value proposition Deliver more drug to intended targets Allow drugs to work faster Increased volume of drug at each injection Increased efficacy Enhanze Technology: For Partners with Injectable Biologics and Drugs 12 |
| Existing Alliances Drive Significant Value Over $100 million of alliance related cash received to date Enhanze Technology with Roche (up to 13 biologic targets, 5 currently exclusive) Alliance worth up to $612M ($20M up-front; $111M milestones for first 3 exclusive targets; $470M up-front & milestones for 10 additional targets; $11M equity), plus royalties; $53M received to date Enhanze Technology with Baxter BioScience (GAMMAGARD) Alliance worth up to $47M ($10M up-front; $37M milestones), plus royalties; $10M received to date HYLENEX with Baxter Medication Delivery Alliance worth up to $65M ($10M up-front; $25M milestones; $10M pre- paid royalties; $20M equity), plus royalties; $40M received to date 13 |
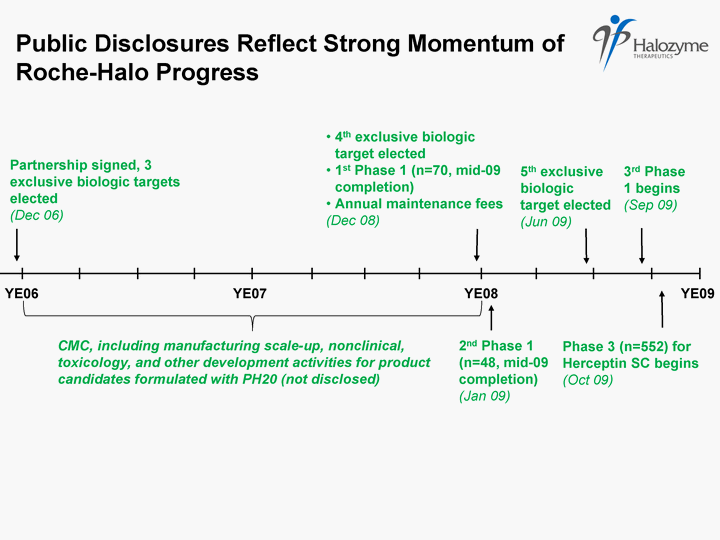
| Partnership signed, 3 exclusive biologic targets elected (Dec 06) YE06 YE07 YE08 YE09 4th exclusive biologic target elected 1st Phase 1 (n=70, mid-09 completion) Annual maintenance fees (Dec 08) 2nd Phase 1 (n=48, mid-09 completion) (Jan 09) 5th exclusive biologic target elected (Jun 09) Phase 3 (n=552) for Herceptin SC begins (Oct 09) CMC, including manufacturing scale-up, nonclinical, toxicology, and other development activities for product candidates formulated with PH20 (not disclosed) Public Disclosures Reflect Strong Momentum of Roche-Halo Progress 3rd Phase 1 begins (Sep 09) |

| Herceptin IV Humanized monoclonal antibody for treatment of HER2-overexpressing breast cancer (BC) Herceptin for 1 year is standard of care in HER2-positive early BC Ongoing clinical trial (HERA) may extend treatment by additional year, results expected in 2012 ~ $5B worldwide sales and growing Herceptin SC Phase 3 pivotal trial began October 2009, N=552, compares SC to IV, Herceptin dosing every three weeks for one year Two primary endpoints - PK serum concentrations (cycles 1-8) and efficacy (pathologic complete response, after cycle 8) Potential for greater patient convenience, simpler administration, faster infusion time, reduced treatment costs, increased compliance Roche Enhanze Technology Alliance Moving Forward - Subcutaneous Herceptin(r) in Phase 3 15 SC = subcutaneous, Herceptin SC = Herceptin formulated with PH20 for subcutaneous administration |

| GAMMAGARD LIQUID - plasma derived immune globulin (IgG) indicated for primary immunodeficiency, approved for IV infusion Growing $1B+ product franchise in ~ $6B worldwide market Current SC IgG limited by low bioavailability (63%) and small injection site volumes results in need for weekly dosing with multiple needles Phase 1/2a trial showed once monthly SC administration with PH20 resulted in bioavailability equal to 92% of IV administration Potential for convenient self-administration once monthly in a single SC injection site Pivotal Phase 3 with ~ 80 patients ongoing, trial completion expected 4Q10 Enhanzed GAMMAGARD - SC Monthly Dosing at Single Site with Halozyme's PH20 16 |
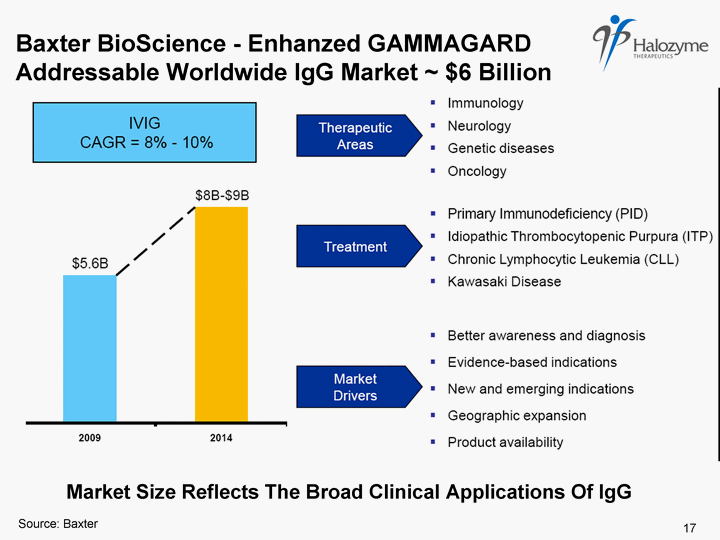
| Baxter BioScience - Enhanzed GAMMAGARD Addressable Worldwide IgG Market ~ $6 Billion Market Size Reflects The Broad Clinical Applications Of IgG Source: Baxter 17 |

| HYLENEX Launched in Pediatric Hydration October 2009 at ACEP Pediatric hydration trial results published in October in peer-reviewed journal PEDIATRICS Label revision 12/09 added clinical studies section with pediatric hydration study description and additional information on pediatric use Perceived favorably by 9/10 healthcare providers compared to IV hydration Potential to benefit over 2 million pediatric patients each year in the U.S. Building a new standard of care for rehydration Two sales forces in place - dedicated HYLENEX specialty team and existing Baxter IV team supported by formulary specialists 90% acceptance rate onto target formularies at numerous pediatric hospitals 18 |
| Proprietary Product Candidates Target the Matrix Ultrafast Insulin - PH20 with regular and rapid-acting analog insulins for diabetes PEGPH20 - Pegylated PH20 targets HA expressing tumors Chemophase - PH20 combination with Mitomycin for bladder cancer HTI-501 - Novel Matrix remodeling enzyme targets collagen for dermatology 19 |
| Goal - To develop best-in-class rapid-acting insulin products that surpass current standards of care Proprietary insulin formulations of novel PH20 permeation enhancing excipient with Rapid-acting analog insulin (Humalog(r), NovoLog(r), Apidra(r)) ? "Analog-PH20" Short-acting regular human insulin (Humulin(r) R) ? "Insulin-PH20" Currently in Phase 2, targeting commercialization by 2014, depending on most attractive product candidate (Analog-PH20 vs. regular Insulin-PH20) or a partnership Halozyme's Ultrafast Insulin Program 20 |
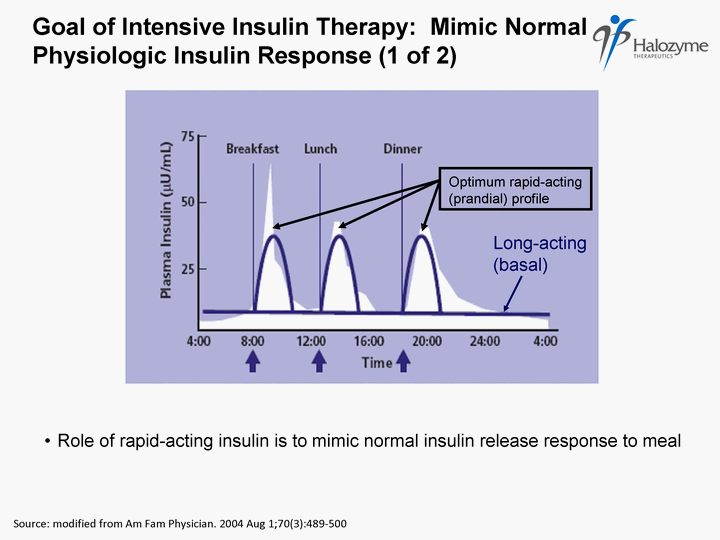
| Goal of Intensive Insulin Therapy: Mimic Normal Physiologic Insulin Response (1 of 2) Source: modified from Am Fam Physician. 2004 Aug 1;70(3):489- 500 Role of rapid-acting insulin is to mimic normal insulin release response to meal Long-acting (basal) Optimum rapid-acting (prandial) profile |
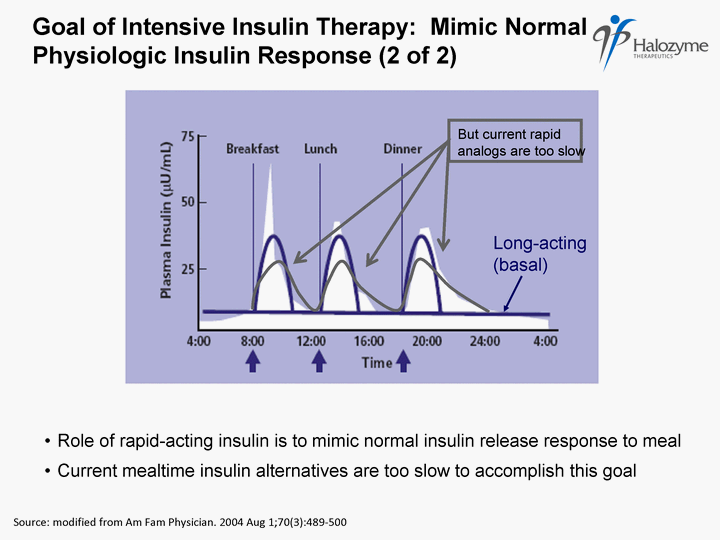
| Goal of Intensive Insulin Therapy: Mimic Normal Physiologic Insulin Response (2 of 2) Source: modified from Am Fam Physician. 2004 Aug 1;70(3):489- 500 Role of rapid-acting insulin is to mimic normal insulin release response to meal Long-acting (basal) Current mealtime insulin alternatives are too slow to accomplish this goal |
| Failure to Mimic Physiologic Insulin Leads to Unmet Needs and Risk of Adverse Outcomes 1 IMS Data, 2008 GFK Roper Patient Survey, N = 2,000 (projectable) Rapid analogs too slow to prevent high blood glucose after a meal Majority of patients report failure to meet ACE target Many patients feel bad when they are hyperglycemic Hypoglycemia still a problem with current products 72% of T1DM and 54% of T2DM patients on insulin reported hypoglycemia in the past 3 months1 In mild form, significant quality of life impact In severe form, can be fatal Intensive insulin therapy and hypoglycemia associated with weight gain Suboptimal glycemic control Inability to control post meal blood sugar impedes diabetes management <30% of all insulin patients meet ADA A1C goal of <7%1 23 |

| 1 Source: IMS 2009 EU =France, Germany , Italy, Spain, UK Sales total includes Humalog, Humulin R, NovoLog, Novolin R, Apidra and equivalent local brands. Excludes mixes and long-acting versions of Humulin and Novolin. Despite Shortcomings, Fast Acting Insulin Sales Are $3B and Growing Rapidly 0.79186431500009 0.956185096000109 1.17782748200013 1.40077801900016 1.66158388700019 2.07601576400024 0.572356658000065 0.627907955000071 0.718925928000082 0.785653765000089 0.852118976000097 0.935964672000106 1.0 1.7 2.1 0.8 U.S. 1.2 1.4 2003 2004 2005 2006 2007 2008 EU $1.4 $1.6 $1.9 $2.2 $2.5 $3.0 21% CAGR 10% CAGR $ Billions Sales in U.S. and EU |
| Phase 2 Meal Study in Type 1 Diabetes 4-way crossover standardized test meal study in type 1 diabetes Objective: compare PK and glucose profiles in response to Humulin R +/- PH20 and Humalog +/- PH20 Primary endpoint: PK AUC0-60 Secondary endpoints: Multiple PK parameters, glycemic excursion Study design Stabilize fasting blood sugar (FBS) with glucose/insulin infusion Up to 3 dose finding visits to optimize post-meal (12 oz. Ensure = 60 gm CHO) glycemic response to lispro-PH20 Using "optimized" dose, repeat test meal with lispro alone Data were presented in 2009 25 |
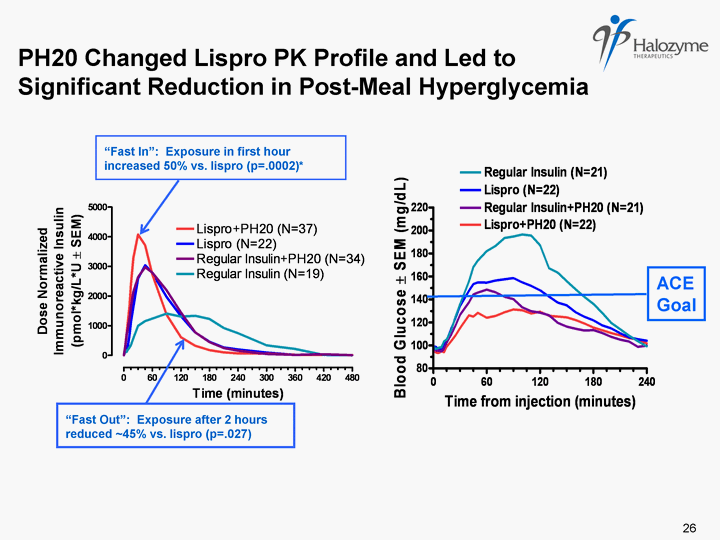
| PH20 Changed Lispro PK Profile and Led to Significant Reduction in Post-Meal Hyperglycemia "Fast In": Exposure in first hour increased 50% vs. lispro (p=.0002)* "Fast Out": Exposure after 2 hours reduced ~45% vs. lispro (p=.027) ACE Goal 26 |
| Phase 2 Type 1 Diabetes Meal Study Conclusions PK results for these preparations confirmed in type 1 patients previous findings from Phase 1 PH20 accelerated insulin absorption compared to insulin alone, combination yielded more physiologic mealtime insulin PK profiles Greater and earlier peak exposure ("fast in") and reduced late exposure ("fast out") compared to insulin alone Faster PK profiles led to significant reductions in postprandial hyperglycemia and were meaningful relative to achieving treatment targets 79% reduction in total hyperglycemic excursion (area > 140 mg/dL) for Lispro-PH20 relative to lispro alone More than twice as many patients met the ACE postprandial glucose goal (2 hr PPG < 140 mg/dL) for Lispro-PH20 (58%) than lispro alone (25%) Lispro-PH20 and Insulin-PH20 were well tolerated 27 |

| Ongoing clinical studies further characterize Ultrafast Insulin Phase 1 euglycemic glucose clamp study comparing 3 marketed fast acting analog insulins +/- PH20 Phase 2 standard meal study in T2DM patients Phase 3 enabling studies Phase 2 3x/day treatment study in T1DM patients compares regular Insulin-PH20 to lispro in a 3 month x 3 month crossover design (initiated 2Q09) Phase 2 3x/day treatment study will compare Analog-PH20 to analog alone (begins 3Q10) Pivotal Phase 3 trials in T1DM and T2DM patients will compare PH20 product candidate to analog insulin Partner or proceed to Phase 3 with most attractive product candidate(s) (Analog-PH20 and/or regular Insulin-PH20) Efficient Development Strategy to Characterize Ultrafast Insulin Value Proposition 28 |

| Less mealtime hyperglycemia More patients meet established goals for postprandial hyperglycemia Accelerated insulin exposure (i.e., fast in) results in early glucose lowering effects and reduced post-meal hyperglycemia Fast in, fast out profile is ideal for insulin pump applications Ultrafast Insulin Could Have Best-in-Class Product Profile vs. Standard of Care Analogs Less hypoglycemia Post-meal hyperglycemic excursions may be controlled with lower doses of insulin with PH20, which would reduce hypoglycemia risk In addition, reduced late post-meal exposure (i.e., fast out) may result in fewer hypoglycemic events Fewer hypoglycemic events and lower insulin doses could result in less weight gain (less self-medicating snacking), especially in type 2 patients Potential for improved outcomes Better mealtime glucose control and reduced hypoglycemia may allow more patients to reach A1C treatment goals 29 |
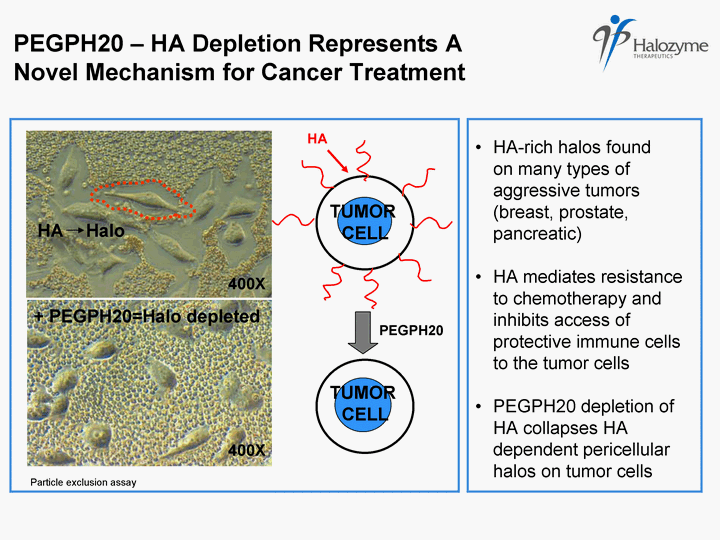
| HA-rich halos found on many types of aggressive tumors (breast, prostate, pancreatic) HA mediates resistance to chemotherapy and inhibits access of protective immune cells to the tumor cells PEGPH20 depletion of HA collapses HA dependent pericellular halos on tumor cells HA TUMOR CELL + PEGPH20=Halo depleted Halo TUMOR CELL PEGPH20 - HA Depletion Represents A Novel Mechanism for Cancer Treatment PEGPH20 HA 400X 400X Particle exclusion assay |
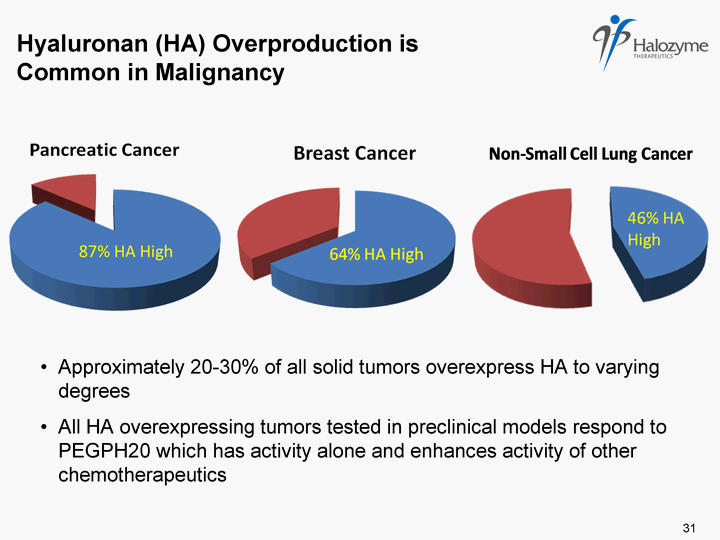
| Hyaluronan (HA) Overproduction is Common in Malignancy 87% HA High 46% HA High Approximately 20-30% of all solid tumors overexpress HA to varying degrees All HA overexpressing tumors tested in preclinical models respond to PEGPH20 which has activity alone and enhances activity of other chemotherapeutics 31 |
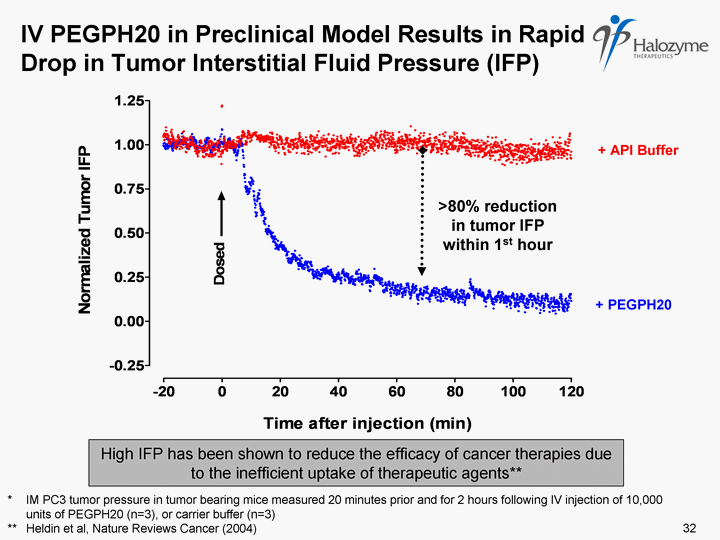
| IV PEGPH20 in Preclinical Model Results in Rapid Drop in Tumor Interstitial Fluid Pressure (IFP) * IM PC3 tumor pressure in tumor bearing mice measured 20 minutes prior and for 2 hours following IV injection of 10,000 units of PEGPH20 (n=3), or carrier buffer (n=3) ** Heldin et al, Nature Reviews Cancer (2004) + API Buffer + PEGPH20 >80% reduction in tumor IFP within 1st hour 32 High IFP has been shown to reduce the efficacy of cancer therapies due to the inefficient uptake of therapeutic agents** |

| PEGPH20 Demonstrated Antitumor Activity Alone and When Combined with Docetaxel IV PEGPH20 monotherapy in HA-positive PC3 prostate tumors Combined dosing of PEGPH20 with docetaxel resulted in synergistic anti- tumor activity (survival improvement of 225% for PEGPH20 + docetaxel vs. 59% for docetaxel alone) IV PEGPH20 + Docetaxel combination therapy in HA-positive PC3 prostate tumors Extended dosing duration of PEGPH20 alone revealed significant single agent antitumor activity Kadhim et al., Proceedings AACR; 2009 = Dose 33 |
| PEGPH20 Program Summary Hyaluronan is a major component of the tumor matrix PEGPH20 disrupts tumor matrix via unique mechanism of action and may enhance cancer cell sensitivity to chemotherapeutics Multicenter, open-label, dose evaluation trial of IV administered PEGPH20 in advanced cancer patients underway Additional studies to further assess dosing, safety, and antitumor activity of PEGPH20 alone and in combination with other chemotherapeutics are being planned 34 |
| Milestones for 2010 Presentation of PEGPH20 preclinical data, 2Q10 Presentations of new Ultrafast Insulin data: Phase 1 comparison of three analogs +/-PH20 euglycemic clamp study, 2Q10 Phase 2 standard meal study in T2DM patients, 2Q10 Phase 2 treatment study 3x/day in T1DM patients, regular Insulin-PH20 vs. lispro, 3Q10 Initiation of Analog-PH20 Phase 2 3x/day treatment study, 3Q10 Preliminary data from PEGPH20 Phase 1 clinical trial, 2H10 Completion of Phase 3 GAMMAGARD clinical trial by Baxter in primary immunodeficiency, 4Q10 Potential for additional milestones from the Roche partnership (due to the initiation of clinical trials or other activities) 35 |

| Halozyme's Programs Driving Significant Value for Shareholders Roche's Herceptin SC in pivotal Phase 3, $5B product, two additional targets have entered the clinic Baxter BioScience's GAMMAGARD SC in pivotal Phase 3, $6B addressable market Baxter rollout of FDA approved HYLENEX in pediatric hydration underway, $200M addressable market Ultrafast Insulin in Phase 2, data from three additional clinical studies available in 2010, $3B rapid-acting analog insulin market PEGPH20 in Phase 1 for solid tumors, multi-billion dollar market opportunity 36 Partnered Programs Proprietary Programs |
| Halozyme Therapeutics - Supplementary Slides J.P. Morgan Healthcare Conference January 13, 2010 37 |
| Herceptin(r) - For Treatment of HER2-Overexpressing Breast Cancer Breast Cancer Most common cancer among women worldwide More than 1M new cases of breast cancer (BC) diagnosed annually worldwide 20-25% of women with breast cancer are HER2-positive Herceptin Humanized monoclonal antibody that targets and blocks the HER2 receptor Approved for adjuvant treatment of HER2-overexpressing BC and metastatic BC Herceptin for 1 year is standard of care in HER2-positive early BC, HERA 2 year results expected in 2012 Herceptin until progression is standard of care in HER2-positive metastatic breast cancer Improves response rates, disease-free survival and overall survival as monotherapy or in combination with chemotherapy Has been used to treat > 650,000 women with HER2-positive BC worldwide Sources: www.herceptin.com, World Health Organization, http://www.who.int/cancer/detection/breastcancer/en/, Ferlay J, et al., GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No.5, Version 2.0. IARCPress, Lyon, 2004. |
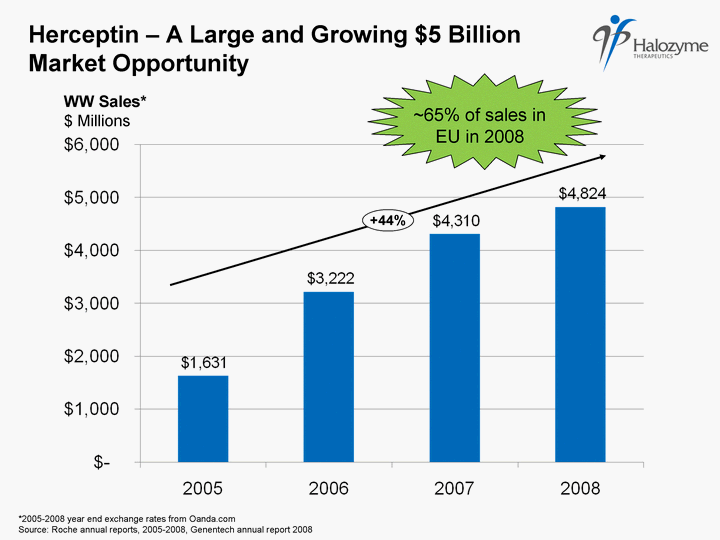
| % Herceptin - A Large and Growing $5 Billion Market Opportunity +44% ~65% of sales in EU in 2008 *2005-2008 year end exchange rates from Oanda.com Source: Roche annual reports, 2005-2008, Genentech annual report 2008 WW Sales* $ Millions |
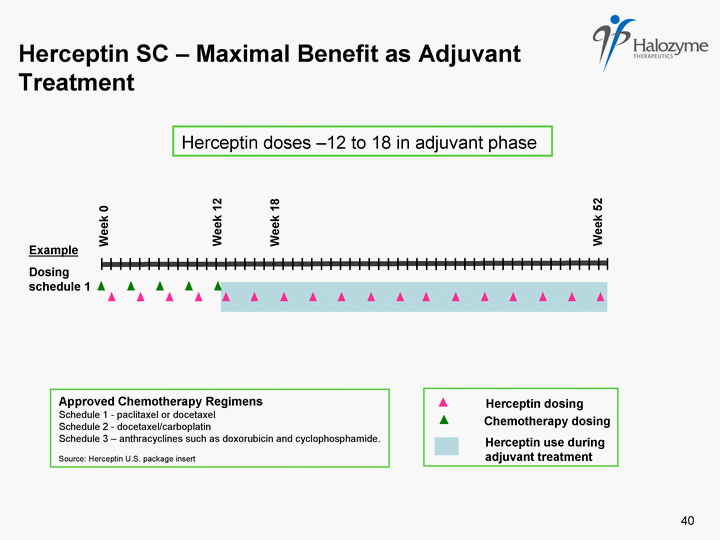
| Herceptin dosing Approved Chemotherapy Regimens Schedule 1 - paclitaxel or docetaxel Schedule 2 - docetaxel/carboplatin Schedule 3 - anthracyclines such as doxorubicin and cyclophosphamide. Source: Herceptin U.S. package insert 40 Chemotherapy dosing Herceptin use during adjuvant treatment Herceptin SC - Maximal Benefit as Adjuvant Treatment Dosing schedule 1 Week 0 Week 18 Week 52 Week 12 Herceptin doses -12 to 18 in adjuvant phase Example |
| Herceptin SC - Phase 3 Clinical Trial Design Registration trial began October 2009, N = 552, women with HER2- positive early breast cancer, HANNAH Pre-operative treatment with 8 cycles of chemotherapy (docetaxel followed by 5-FU/epirubicin/cyclophosphamide) concurrent with IV or SC Herceptin, followed by 10 cycles of Herceptin SC or IV for 10 cycles after surgery, q 3 weeks Surgery between cycles 8 and 9 Primary endpoints Trastuzumab serum concentration, comparing SC vs. IV pre-surgery Pathologic complete response post-surgery Secondary endpoints and follow up for 24 months after last dose of Herceptin European filing targeted in 2012 (as stated by Roche, October 15, 2009, analyst conference call) |
| Potential Advantages of Herceptin SC vs. Herceptin IV Greater convenience - patients can be treated at physician office or at home Simpler administration - ready-to-use pre-filled administration device, not an infusion-bag prepared by pharmacist Faster infusion time - expected to be <5 minutes vs. 30-90 minutes for IV Increased compliance - travel to a hospital or infusion center for treatment not necessary; especially important for extended maintenance treatment Reduced treatment costs for Herceptin - outside of hospital and infusion center setting, so more efficient use of healthcare resources Less pain and discomfort at the injection site - SC easier to administer, smaller needle size 42 |
