Attached files
| file | filename |
|---|---|
| EX-4.4 - BIOCUREX INC | c58919_ex4-4.htm |
| EX-23.1 - BIOCUREX INC | c58919_ex23-1.htm |
As filed with the Securities and Exchange Commission on December 31, 2009
Registration No. 333-162345
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT No. 7
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
BIOCUREX, INC.
(f/k/a Whispering Oaks International, Inc.)
(Exact name of registrant as specified in its charter)
|
|
|
|
|
Texas |
3841 |
75-2742601 |
||
(State or other jurisdiction of incorporation) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
7080 River Road
Suite 215
Richmond, British Columbia V6X 1X5
Canada
(866) 884-8669
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Dr. Ricardo Moro-Vidal
Chief Executive Officer
7080 River Road
Suite 215
Richmond, British Columbia V6X 1X5
Canada
(866) 884-8669
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Please send copies of all communications to:
|
|
|
Joel J. Goldschmidt, Esq. |
Mark A. von Bergen, Esq. |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”), check the following box. R
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
|
|
|
|
|
|
|
Large accelerated filer £ |
Accelerated filer £ |
Non-accelerated filer £ |
Smaller reporting company R |
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES
THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF 1933 OR UNTIL THIS REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE
SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(a), MAY DETERMINE.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction
where the offer or sale is not permitted. SUBJECT TO COMPLETION, DATED DECEMBER 31, 2009 PRELIMINARY PROSPECTUS 1,200,000 Units We are offering, on a firm commitment basis, 1,200,000 units consisting of an aggregate of 84,000,000 shares of common stock and 84,000,000 redeemable common stock purchase warrants which we refer to in this prospectus as the redeemable warrants. Each unit will consist of 70 shares of our
common stock and 70 redeemable warrants. The units will not trade separately, they will not be listed on any exchange or quoted on any market and no certificates will be issued evidencing the units. The shares of common stock and the redeemable warrants comprising the units will be issued and
quoted separately on the Over-the-Counter Bulletin Board. We expect that the units will be offered at a price within a range of $5.60 to $7.60 per unit. Our common stock is currently quoted on the Bulletin Board under the symbol BOCX. We expect the redeemable warrants to be quoted on the
Bulletin Board under the symbol BOCXW. On December 30, 2009 the last sales price as quoted on the Bulletin Board was $0.115. Each redeemable warrant included in the units entitles its holder to purchase one share of our common stock at an exercise price equal to 150% of the unit offering price divided by the number of shares included in a unit. The redeemable warrants are exercisable at any time until their expiration
date, five years after the effective date of the registration statement of which this prospectus is a part. We may redeem some or all of the redeemable warrants at a price of $0.003 per warrant by giving the holders not less than 30 days’ notice at any time the common stock closes at 200% of the unit
offering price divided by the number of shares included in a unit for five consecutive trading days. INVESTING IN THESE SECURITIES INVOLVES SIGNIFICANT RISKS. WE ARE CONSIDERED TO BE IN UNSOUND FINANCIAL CONDITION. YOU SHOULD PURCHASE OUR SECURITIES ONLY IF YOU CAN AFFORD A COMPLETE LOSS OF YOUR INVESTMENT.
SEE “RISK FACTORS” BEGINNING ON PAGE 8. NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY
REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Per Unit
Total Public offering price
$
$
Underwriting discount
$
$ Proceeds to us, before expenses
$
$ We have also agreed to pay Paulson Investment Company, Inc., the representative of the underwriters of this offering, a non-accountable expense allowance equal to 3% of the total public offering price for the units offered by this prospectus and issue to Paulson a warrant to purchase 120,000 units,
identical to the units offered by this prospectus, having an exercise price per unit equal to 120% of the unit public offering price. We have also granted the underwriters a 45-day option to purchase up to an additional 180,000 units to cover over-allotments. PAULSON INVESTMENT COMPANY, INC. The date of this prospectus is , 2010
each consisting of
70 shares of common stock and 70 common stock purchase warrants
or an aggregate of
84,000,000 shares and 84,000,000 warrants
Figure 1 This chart reflects our analysis of human clinical samples of Serum-RECAF test from subjects without cancer and from patients with diagnosed cancers or benign conditions related to cancer diagnosis. See page 36. We have not sought or received FDA
or other regulatory approvals for the marketing and sale of any of our products in the United States or any other jurisdiction. The clinical samples on which the chart below is based are not related in any way to the clinical trials required to obtain
regulatory approval. Neither we nor, to our knowledge, any of our licensees have commenced conducting the clinical trial required to obtain regulatory approval for our products. In addition, some of our test results have not been independently verified
by any laboratory or other research facility. Figure 2 A proposed rendering of the POC rapid device test prototype currently being tested. See pages 38-39.

TABLE OF CONTENTS
Page
(ii
)
3
5
6
8
19
20
20
21
22
23 MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
24
31
46
52
53
54
58
60
63
63
63
64 DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION OF SECURITIES ACT LIABILITIES
64 SUITABILITY STANDARDS Notice to California investors: Each purchaser of securities in California must meet one of the following suitability standards: (1) annual gross income of at least $65,000, plus liquid net worth (exclusive of home, home furnishings and automobiles) of at least $250,000; or (2) liquid net worth (exclusive
of home, home furnishings and automobiles) of at least $500,000, regardless of annual gross income. Notice for New Jersey investors: Offers and sales in this offering in New Jersey may only be made to accredited investors as defined in Rule 501(a) of Regulation D under the Securities Act of 1933. Under Rule 501(a), to be an accredited investor, an individual must have: (1) net worth or joint net
worth with the individual’s spouse of more than $1,000,000 or (b) income of more than $200,000 in each of the two most recent years or joint income with the individual’s spouse of more than $300,000 in each of those years and a reasonable expectation of reaching the same income level in the current
year. Other standards apply to investors who are not individuals. There will be no secondary sales of the securities to persons who are not accredited investors for 90 days after the date of the offering in New Jersey by the underwriters and selected dealers. Notice to North Dakota investors: Each purchaser of securities in North Dakota must meet one of the following suitability standards: (1) a minimum annual gross income of $70,000 and a minimum net worth of $70,000 exclusive of automobile, home and home furnishings; or (2) a minimum net worth
of $250,000, exclusive of automobiles, home and home furnishings. Notice to Oregon investors: Each purchaser of securities in Oregon must meet one of the following suitability standards: (1) a minimum annual gross income of $100,000 and a minimum net worth of $100,000 exclusive of automobile, home, and home furnishings; or (2) a minimum net worth of
$350,000, exclusive of automobile, home, and home furnishings. Notice to Washington investors: Each purchaser of securities in Washington must meet one of the following suitability standards: (1) a minimum annual gross income of $70,000 and a minimum net worth of $70,000 exclusive of automobile, home and home furnishings; or (2) a minimum net worth of
$250,000, exclusive of automobiles, home and home furnishings. (i)
You should rely only on the information contained in this prospectus. Neither we nor the representative of the underwriters have authorized anyone to provide you with information different from or in addition to that contained in this prospectus. You should not rely on any information that is different or
inconsistent with the information contained in this prospectus. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus. This prospectus is not an offer to sell any securities other than the units, the shares of common stock and redeemable warrants that comprise the units and the shares of common stock issuable upon exercise of the redeemable warrants, all of which securities are offered hereby. This prospectus is not an
offer to sell securities in any circumstances in which such an offer is unlawful. We are not making an offer of these securities in any state where the offer is not permitted. No action is being taken in any jurisdiction outside the United States to permit a public offering of the securities covered by this prospectus,
or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to those
jurisdictions. FORWARD-LOOKING STATEMENTS Except for statements of historical fact, some information in this document contains “forward-looking statements” that involve substantial risks and uncertainties. You can identify these forward-looking statements by words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,”
“should,” “will,” “would” or similar words. The statements that contain these or similar words should be read carefully because these statements discuss our future expectations, contain projections of our future results of operations or of our financial position, or state other forward-looking information.
We believe that it is important to communicate our future expectations to our investors. However, there may be events in the future that we are not able to accurately predict or control. Further, we urge you to be cautious of the forward-looking statements which are contained in this registration
statement because they involve risks, uncertainties and other factors affecting our operations, market growth, service, products and licenses. The factors listed in the sections captioned “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and
“Business,” as well as other cautionary language in this registration statement and events in the future may cause our actual results and achievements, whether expressed or implied, to differ materially from the expectations we describe in our forward-looking statements. The occurrence of any of the
events described as risk factors or other future events could have a material adverse effect on our business, results of operations and financial position. Since our common stock is considered a “penny stock” we are ineligible to rely on the safe harbor for forward-looking statements provided in Section
27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”). The pronouns “we,” “our,” “us” and the like used herein refer to BioCurex, Inc., f/k/a Whispering Oaks International, Inc. We own the trademark RECAF™, when used alone or in conjunction with any other term or word. Other brand names or trademarks appearing in this prospectus are the property of their respective owners. (ii)
This summary highlights key information contained elsewhere in this prospectus and is qualified in its entirety by the more detailed information and financial statements included elsewhere in this prospectus. This summary does not contain all of the information that is important to you. You should read the
entire prospectus, including “Risk Factors,” our consolidated financial statements and the related notes thereto and condensed consolidated financial statements and the related notes thereto, and the other documents to which this prospectus refers, before making an investment decision. Overview We are a development stage company focusing on developing and commercializing products for the early detection, diagnosis and monitoring the recurrence of cancer. We have developed and evaluated in clinical trials a blood test that can detect the presence of cancer in humans and animals using a
new cancer marker named RECAF. We developed and own, royalty-free, the proprietary technology related to the RECAF marker, with patents granted in the United States, Europe and China and patents pending in other major worldwide markets. RECAF is a molecule found on most cancer cells, including breast, colon, prostate and lung cancers, but not on normal cells. RECAF can be used in blood tests to determine if a patient has cancer. The blood test can be formatted for use on automated instrumentation typically found in large clinical
and hospital laboratories or manually. It can also be formatted as a single use rapid test for point-of-care (POC) use in physicians’ offices, urgent care facilities and at the bedside. Once approved by the U.S. Food and Drug Administration (FDA), the tests could be used in general screening or in high
risk patients to determine if an individual has cancer. Unlike other notable cancer markers, such as CEA and PSA, which only detect the presence of a specific cancer type (CEA for colon cancer and PSA for prostate cancer), RECAF is found on most types of cancer and, therefore, could have much broader use than most other cancer markers in
development or currently in use. Moreover, unlike existing cancer markers, RECAF has been shown to detect early stages of breast and prostate cancers when the likelihood of cure is highest. We have granted Abbott Laboratories (Abbott) and Inverness Medical Switzerland GmbH (Inverness) semi-exclusive licenses to use the RECAF tests on blood samples processed through automated diagnostic equipment typically found only in large clinical or hospital laboratories and non-exclusive
licenses for other test formats and we have retained rights for manual tests not processed in automatic equipment, POC tests for the physicians’ office and all other single-format potential uses and all test formats used for veterinary applications. Under the respective terms of these licenses, we can grant
one additional similar semi-exclusive license for automated testing. The Abbott license has been amended to relieve them of research and development responsibilities and, to our knowledge, they have not taken any steps towards commercializing our technology. Inverness has been conducting research
and development trying to adapt our technology to their diagnostic platform. However, to our knowledge, they have not reached the stage where they are prepared to enter into clinical trials in order to obtain FDA approval or to commercialize our technology or any related products. Our principal objectives for the first twelve months after completion of this offering will be the following:
•
grant one additional semi-exclusive license for testing blood samples using automated testing equipment; • commercialize veterinary applications of RECAF testing technology not requiring regulatory approvals; • finish developing a POC rapid format test for the doctor’s office and bedside use; • conduct clinical trials and seek FDA approval for marketing of the POC rapid format test; and • commercialize manual testing formats, principally in large cities in foreign countries where further regulatory clearance is not required. 3
We cannot assure you that we can successfully achieve any of these objectives. Company History We were incorporated in Texas in December 1997. In February 2001, we acquired a U.S. patent, several foreign patents and other intellectual property related to the early detection of cancer from Lagostar Trading S.A., a Uruguayan company. In March 2001, we acquired a Canadian patent
application and proprietary technology related to RECAF technology from Curex Technologies, Inc., now known as Pacific BioSciences Research Centre, Inc. (PBRC). PBRC was incorporated in November 1996 under the Business Corporation Act of British Columbia, Canada. PBRC is owned by Dr.
Ricardo Moro-Vidal, our chief executive officer and a member of our board of directors. On October 27, 2009, we changed our legal name to BioCurex, Inc., the name under which we have conducted business since March 2001. Corporate Information Our principal executive offices are located at 7080 River Road, Suite 215, Richmond, British Columbia, Canada V6X 1X5, and our telephone number is 866-884-8669. Our website is located at www.biocurex.com. None of the information on our website is part of this prospectus. 4
Securities offered:
1,200,000 units, each of which will consist of 70 shares of our common stock and 70 redeemable warrants, or an aggregate of 84,000,000 shares and 84,000,000 redeemable warrants. The units will not trade separately, they will not be listed on any exchange or
quoted on any market and no certificates will be issued evidencing the units. The common stock and the redeemable warrants comprising the units will be issued and quoted separately.
Securities outstanding after this offering: Common stock: 157,062,205 shares based on 73,062,205 shares issued and outstanding as of December 31, 2009. Redeemable warrants:
84,000,000, each to purchase one share of our common stock at an exercise price equal to 150% of the unit offering price divided by the number of shares included in a unit. The redeemable warrants are exercisable at any time until their expiration date, five
years after the effective date of the registration statement of which this prospectus is a part. We may redeem some or all of the redeemable warrants at a price of $0.003 per warrant by giving the holders not less than 30 days’ notice at any time the common
stock closes, as quoted on the Bulletin Board, at 200% of the unit offering price divided by the number of shares included in a unit for five consecutive trading days.
Use of proceeds:
For working capital and general corporate purposes, including obtaining FDA approval for and commercializing various applications of our testing technology, sales and marketing expenses and repayment of indebtedness.
Bulletin Board symbol: Common stock:
BOCX
Proposed Bulletin Board symbol: Redeemable warrants:
BOCXW
Risk factors:
Investing in these securities involves a high degree of risk. As an investor you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section. The number of shares of common stock to be outstanding after this offering does not include any of the following:
•
approximately 17,000,000 shares of common stock underlying warrants outstanding at September 30, 2009 having a weighted average exercise price of $0.14 per share;
• approximately 13,500,000 shares of common stock issuable upon conversion in full of our amended secured convertible notes in the aggregate outstanding principal amount of $1.75 million as of December 31, 2009, assuming a conversion price of $0.13 per share, which number of shares does not
include shares issuable if the amount converted includes accrued interest and which number of shares will be reduced to approximately 3.0 million shares after this offering if the holders of the amended secured convertible notes accept a prepayment out of the net proceeds of this offering reducing
the balance due on those notes to $390,000;
5
<R>
</R>
• 84,000,000 shares of common stock underlying the redeemable warrants; • 8,400,000 shares of common stock included in the units underlying the representative’s warrant; • 8,400,000 shares of common stock underlying the warrants included in the units underlying the representative’s warrant; • 28,500,000 shares of common stock issuable upon exercise of options that we intend to grant to our senior executives and directors upon completion of this offering (see below);
• 9,857,723 shares of common stock reserved for future issuance under our Non-Qualified Stock Option Plan, of which 5,987,057 shares are issuable upon exercise of options outstanding at December 31, 2009 having an exercise price of $0.001 per share; and • 1,606,275 shares of common stock that may be issued under our Stock Bonus Plan.
On the closing date of this offering, we will grant options to our senior executives and directors. These options will have an exercise price equal to the greater of (i) the closing market price of a share of our common stock on the date of grant or (ii) the unit offering price divided by the number of
shares in a unit. As currently contemplated, these options will be granted as follows:
Grantee
Options Ricardo Moro-Vidal
15,000,000 Denis Burger
10,000,000 Phil Gold
1,000,000 Jim Walsh
1,000,000 Antonia Bold-de-Haughton
1,000,000 Gladys Chan
500,000 Except as otherwise indicated, all information in this prospectus assumes no exercise by the representative of the underwriters’ over-allotment option to purchase up to an additional 180,000 units. Statement of Operations Data:
Nine Months Ended
Years Ended
2008
2009
2007
2008 Revenue
$
1,000,000
$
—
$
50,000
$
1,000,000 General and administrative expense
770,927
607,360
1,260,865
928,845 Research and development expense
513,959
432,814
662,944
675,302 Other operating expenses
362,355
356,651
341,821
419,179 Total operating expenses
1,647,241
1,396,825
2,265,630
2,023,326 Loss from operations
(647,241
)
(1,396,825
)
(2,215,630
)
(1,023,326
) Other expense, net
(1,636,700
)
(423,001
)
(1,138,689
)
(3,090,659
) Net loss
$
(2,283,941
)
$
(1,819,826
)
$
(3,354,319
)
$
(4,113,985
) Total comprehensive loss
$
(2,255,122
)
$
(1,804,297
)
$
(3,469,380
)
$
(4,087,325
) Balance Sheet Data:
At September 30, 2009
Actual
Pro Forma, Cash
$
364,020
$
5,360,420 Working capital (deficit)
$
(996,112
)
$
4,236,511 Total assets
$
1,149,540
$
5,923,894 Total liabilities
$
2,980,684
$
1,490,637 Stockholders’ equity (deficit)
$
(1,831,144
)
$
4,433,257 6
September 30,
December 31,
As Adjusted
As
adjusted information gives effect to the following: (i) the issuance of 2,785,714
shares of common stock subscribed for on or before September 30, 2009 and
issued subsequent to that date; (ii) the issuance of an aggregate of 1,483,516
shares of common stock in October, and December 2009 to the holders of our
amended secured convertible notes in connection with the exercise of their
conversion of $200,000 principal amount of such notes; (iii) the issuance
of 300,000 shares of common stock in December 2009 to settle an account payable
for services; (iv) a rescission of the exercise of options cancelling the
issuance of 3,648,947 shares in September 2009; (v) the receipt of approximately
$6.8 million of net proceeds from this offering assuming a public offering
price of $7.00 per unit, within the range set forth on the cover of this
prospectus; and (vi) the repayment of approximately $1.8 million
of indebtedness out of the net proceeds of this offering. Subsequent to September 30, 2009, we issued an additional 4,569,230 shares. Of these shares, 2,785,714 shares were subscribed for before that date and were reflected on the balance sheet as common stock subscriptions. We had not issued those shares as of that date and, therefore, they are not
included in the number of shares outstanding at that date. In addition, 714,286, shares were issued in October 2009 in connection with the conversion of $100,000 principal amount of the amended secured convertible notes (at a conversion price of $0.14) and 769,230 shares were issued in December 2009
in connection with the conversion of another $100,000 principal amount of those notes (at a conversion price of $0.13). Finally, in December 2009, we issued 300,000 shares under our Stock Bonus Plan to settle an account payable for services. We have given pro forma effect to these issuances in order to
be consistent with the disclosure elsewhere in this prospectus. As stated in this prospectus, the number of shares outstanding both immediately before and immediately after the offering includes those 4,569,230 shares and the outstanding principal balance on the amended secured convertible notes reflects
the October and December 2009 conversions. We believe that the repayment of $1.8 million of indebtness is material. The repayment of debt is a required use of proceeds under the various debt instruments. The total amount of proceeds that will be used to repay debt represents the largest single use of proceeds constitutes the largest single use
of proceeds, approximately 26.6%, other than working capital and general corporate purposes. However, unlike working capital and general corporate purposes, the impact of the repayment of debt will be immediate as opposed to being spread out over time. Also, as a result of that repayment, we will
have significantly reduced our total indebtedness and our total liabilities significantly. 7
An investment in our securities involves a high degree of risk. You should carefully consider the risks described below and the other information in this registration statement, including our financial statements and the notes to those statements, before you purchase any of our securities. The risks and
uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us, or that we currently deem immaterial, could negatively impact our business, results of operations or financial condition in the future. If any of the following risks and uncertainties develop
into actual events, our business, results of operations or financial condition could be adversely affected. In those cases, the trading price of our securities could decline, and you may lose all or part of your investment. Risks Related to Our Business Auditors have doubt as to our ability to continue in business. In their report on our December 31, 2008 financial statements, our auditors expressed substantial doubt as to our ability to continue as a going concern. A going concern qualification could impair our ability to finance our operations through the sale of debt or equity securities. Our ability to continue
as a going concern will depend, in large part, on our ability to obtain additional financing and generate positive cash flow from operations, neither of which is certain. If we are unable to achieve these goals, our business would be jeopardized and we may not be able to continue operations. We have limited operations and a history of losses and expect to incur additional losses in the future. Historically, we have funded our operations through the private sale of securities and with private loans. To date, we have raised approximately $3.5 million in proceeds from private offerings of our common stock, and an additional $1.1 million in proceeds as the result of the exercise of privately
placed options and warrants. However, we have never earned a profit. For the nine-month period ended September 30, 2009, we reported an operating loss of $1.4 million and a net loss of $1.8 million. As of September 30, 2009, our accumulated deficit was $18.9 million and our stockholders’ deficit was
$1.8 million. We expect to incur additional losses for an indefinite period. To date, we have had limited revenues and no revenues from the sale of the products and services that we are currently focused on developing. We cannot assure that our products and services will be successfully commercialized
or that we will ever earn a profit. We recently entered into a loan modification agreement with the holders of our secured notes pursuant to which they waived defaults caused by our failure to make certain principal payments when due. We may be unable to repay the amended notes when due. Under the terms of the original secured convertible notes issued in June 2007, we were obligated to make certain principal payments on May 1, 2009, which we failed to do. As a result, these notes were characterized as short-term debt, rather than long-term debt, on our June 30, 2009 balance sheet
and our working capital deficit as of that date was $3.6 million. As of August 31, 2009, we entered into a Loan Modification Agreement with the holders of the original secured convertible notes under which they waived those defaults and we agreed to issue amended secured convertible notes in the
aggregate principal amount of $2.15 million in exchange for the original secured convertible notes. The amended secured convertible notes bear interest at the same rate as the original secured convertible notes, prime plus 2.75%, mature on December 31, 2012 and had an initial conversion price of $0.14
per share. If a default under the amended secured convertible notes should occur prior to the maturity date, the holders would be able to require us to pay the entire outstanding balance then due. We cannot assure you that we will not be in default under the amended secured convertible notes or that
we will be able to repay those notes when they mature. If we are unable to repay the principal amount of those notes when due, the holders, as secured creditors, would be able to force the sale of our assets, including the intangible assets that are at the 8
core of our business in order to repay the notes. In such case, we would no longer be able to continue operations and you could lose your entire investment. To date, we have generated limited revenues. Our future success depends on our ability to begin generating revenues on a regular and continuing basis. Since inception, we have generated aggregate revenues of only $1.5 million all of which were license fees or transfer fees related to material, technology and related services. Our future success depends on our ability to begin generating revenues on a regular and continuing basis and to properly
manage our costs. Our business strategy contemplates that we will derive revenue from licensing our technology and from sales of our products and services. Our ability to generate these revenues depends on a number of factors, some of which are outside our control. These factors include the following:
•
our ability to obtain necessary government and regulatory approvals; • our ability to successfully complete all the research and development work on the various test formats and applications of RECAF technology; • our ability to successfully commercialize the various test formats and applications of our RECAF technology; • our ability to protect our intellectual property; • the success of our sales and marketing efforts; • our ability to maintain our competitive advantages; • new developments in the area of cancer detections and the efficacy of competing technologies; • market acceptance of our products and services; and • our ability to raise additional capital as and when needed and on acceptable terms. We cannot assure you that we will be able to meet any of these challenges or that we will be able to generate any revenues. If we do not generate any revenues, you may lose your entire investment. If we are unable to raise additional capital, we may be unable to continue operating. The process to obtain regulatory approval of our products involves significant costs. In addition, the costs associated with our proposed research, development and marketing activities may be substantially higher than our estimated costs of these activities. We will probably need additional capital to
fully implement our growth strategy, which includes obtaining the necessary regulatory approvals to commercialize various applications of our RECAF technology, sales and marketing programs and continued research and development activities, in addition to our general and administrative expenses. If we
are unable to raise additional capital, we may be forced to delay or postpone the regulatory approval process and our research, development and marketing activities. Further, any capital raised through the issuance of additional equity will have a dilutive impact on other stockholders and could have a
negative effect on the market price of our common stock. We have granted semi-exclusive licenses relating to the development, sale and distribution of our products, and our dependence on these licensees exposes us to significant commercialization and development risks. In April 2005 and December 2007, we entered into a semi-exclusive license agreement with Abbott and Inverness, respectively, under which we granted each a semi-exclusive license to use RECAF tests on blood samples processed in automated equipment typically found only in large clinical and
hospital laboratories and a non-exclusive license for other test formats. Under the license agreements, we are entitled to annual minimum royalty payments, sublicense fees and royalties based on net sales. The Abbott license agreement also gives Abbott the right to grant sublicenses for these activities
and for engaging in commercial sales of our products to third parties upon payment to us of sublicense fees and also gives Abbott the right, though not the obligation, to 9
obtain the necessary regulatory approvals to exploit the RECAF technology covered by the license. To date, except for license fees and transfer related to materials, technology and related services, we have not received any payments from Abbott or Inverness as a result of their exploitation of the
licenses. The royalty payments we receive from the sale of our products under the Abbott and Inverness license agreements depend heavily on their efforts. Each of Abbott and Inverness has significant discretion in determining the efforts and resources it applies to obtaining regulatory approval and to the
development and sale of our products, and each of them likely has a significant number of other research commitments competing for its resources. Furthermore, regardless of the effort and resources they invest, Abbott and Inverness may not be effective in developing or marketing our products. In
addition, Abbott and Inverness may have corporate agendas which may not be consistent with our best interests. A disagreement between us and Abbott or Inverness could lead to lengthy and expensive dispute resolution proceedings as well as to extensive financial and operational consequences to us,
and have a material adverse effect on our business, results of operations and financial condition. If our relationship with Abbott or Inverness were to terminate, we may not be able to enter into another semi-exclusive license agreement with a company with similar resources to develop and commercialize our products and perform these functions on acceptable terms or at all. As a result, we
could experience delays in our ability to distribute and commercialize our products and increased expenses, all of which would have a material adverse affect on our business, results of operations and financial condition. Any failure to obtain or any delay in obtaining required regulatory approvals may adversely affect our ability to successfully license our products or market any products we may develop. The cancer marker RECAF tests that we have developed for human and veterinarian applications are subject to oversight by regulatory authorities in the United States and in other countries, including, without limitation, the FDA and U.S. Department of Agriculture (USDA). We believe that all of
our products are classified as Type I or II Medical Devices by the FDA. As such these medical devices do not come under the more rigorous approval guidelines as Type III Medical Devices (e.g., HIV test kits) or the arduous Phase I, II, and III clinical trial process that is required for approval of drugs.
Type I and II Medical Device approval falls under the category referred to as a 510k application and after submission of supporting data to the FDA is subject to a 90-day review process. Marketing clearance for medical devices in the veterinary market in the United States is regulated by the USDA and
that process typically takes approximately one year after submission of appropriate data. We have not initiated the approval process for our products with the FDA or with the USDA for marketing clearance in the United States. Among other requirements, FDA marketing clearance and approval of the facilities used to manufacture our cancer marker RECAF tests will be required before any of these tests may be marketed in the United States. A similar regulatory process will be required by European regulatory authorities
before our cancer tests can be marketed in Europe. As with the FDA review process, there are numerous risks associated with the review of medical devices by foreign regulatory agencies. The foreign regulatory agencies may request additional data to demonstrate the clinical safety and efficacy of a
product. We rely and will continue to rely on third parties, including Abbott and Inverness, to assist us in managing and monitoring all of our preclinical studies and clinical trials. To our knowledge, our licensees, Abbott and Inverness, have not initiated any steps toward regulatory approval of our products
for marketing clearance in the United States. If any of these parties terminate their relationship with us, the development of the products covered by those agreements could be substantially delayed. In addition, these third parties may not successfully carry out their contractual obligations, meet expected
deadlines or follow regulatory requirements, including clinical, laboratory and manufacturing guidelines. Our reliance on these third parties may result in delays in completing, or in failing to complete, these trials if they fail to perform with the speed and competency we expect. Further, if any of these
parties fail to perform their obligations under our agreements with 10
them in the manner specified in those agreements and in the trial design, the FDA may not accept the data generated by those trials, which would increase the cost of and the development time for that product candidate. If clinical testing of our product candidates is compromised for any of the above-
mentioned reasons, we will be unable to meet our anticipated development or commercialization timelines, which would have a material adverse effect on our business. Although FDA marketing clearance may not be required for certain foreign markets, we believe that FDA clearance for our RECAF cancer marker tests would add credibility when negotiating with overseas distributors. Failure to obtain FDA marketing clearance in the United States may limit our
ability to successfully market our products even where regulatory approvals are not required. Delays or rejection in obtaining FDA marketing clearance may also be encountered based upon changes in applicable law or regulatory policy during the period of regulatory review. Any failure to obtain, or any delay in obtaining, marketing clearance would adversely affect our ability to license or
market our products. Moreover, even if FDA marketing clearance is granted, such approval may include significant limitations on indicated uses for which the product could be marketed. Both before and after marketing clearance is obtained, a product and its manufacturer are subject to comprehensive regulatory oversight. Violations of regulatory requirements at any stage of the process may result in adverse consequences, including the FDA’s delay in approving or refusing to
approve a product for marketing, withdrawal of an approved product from the market and/or the imposition of criminal penalties against the manufacturer. In addition, later discovery of previously unknown problems relating to a marketed product may result in restrictions on such product or
manufacturer including withdrawal of the product from the market. We cannot assure you that we will receive the required clearances in order to be able to market any of our products. If we and our third-party suppliers do not maintain high standards of manufacturing in accordance with applicable manufacturing regulations, our development and commercialization activities could suffer significant interruptions or delays. We and any third-party suppliers on which we currently or may in the future rely must continuously adhere to applicable manufacturing regulations. In complying with these regulations, we and our third-party suppliers must expend significant time, money and effort in the areas of design and
development, testing, production, record-keeping and quality control to assure that our products meet applicable specifications and other regulatory requirements. The failure to comply with these requirements could result in an enforcement action against us, including the seizure of products and shutting
down of production. Any of these third-party suppliers and we also may be subject to audits by the FDA and other regulatory agencies. If any of our third-party suppliers or we fail to comply with applicable manufacturing regulations, our ability to develop and commercialize our products could suffer
significant interruptions. If our products do not achieve market acceptance, we will be unable to generate significant revenues from them. The commercial success of our products will depend primarily on convincing health care providers and veterinarians to adopt and use our products. To accomplish this, we, together with our licensees and any other marketing or distribution collaborators, will have to convince members of the medical
and veterinary communities of the benefits of our products through, for example, published papers, presentations at scientific conferences and additional clinical data. Medical providers and veterinarians will not use our products unless we can demonstrate that they consistently produce results comparable
or superior to existing products and have acceptable safety profiles and costs. If we are not successful in these efforts, market acceptance of our products could be limited. Even if we demonstrate the effectiveness of our products, medical practitioners may still use other products. If our products do not
achieve broad market acceptance, we will be unable to generate significant revenues from them, which would have a material adverse effect on our business, cash flows and results of operations. 11
We may not achieve or maintain a competitive position in our industry and future technological developments may result in our proprietary technologies becoming uneconomical or obsolete. The field in which we are involved is undergoing rapid and significant technological change. Our ability to successfully commercialize various applications of our cancer detection technology will depend on our ability to maintain our technological advantage. We cannot assure you that we will achieve
or maintain such a competitive position or that other technological developments will not cause our proprietary technologies to become uneconomical or obsolete. Many of our potential competitors, including large multi-national pharmaceutical companies, well-capitalized biotechnology companies, and
privately and publicly financed research facilities, have significantly greater financial resources than we do. Our revenues and profits will be adversely impacted if we cannot compete successfully with new or existing products or technologies. Our patents might not protect our technology from competitors. Certain aspects of our technologies are covered by a United States patent and a number of foreign patents. In addition, we have a number of patents pending in foreign countries. There is no assurance that the applications still pending or which may be filed in the future will result in the issuance of
additional patents. In addition, our patents expire in 2014 and 2015, and there is no assurance that we will be able to successfully renew them. Furthermore, there is no assurance as to the breadth and degree of protection the issued patents might afford us. We may not be able to prevent
misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the United States. Thus, any patents that we own may not provide commercially meaningful protection from competition. Disputes may arise between us and others as to the scope,
validity and ownership rights of these or other patents. Any defense of the patents could prove costly and time consuming and we cannot assure you that we will be in a position, or will deem it advisable, to carry on such a defense. Our patents may not contain claims that are sufficiently broad to
prevent others from practicing our technologies or developing competing products. Competitors may be able to use technologies in competing products that perform substantially the same as our technologies but avoid infringing our patent claims. Under these circumstances, our patents would be of little
commercial value to us. Other private and public concerns may have filed applications for, or may have been issued, patents and are expected to obtain additional patents and other proprietary rights to technology potentially useful or necessary to us. The scope and validity of any of these patents, if any,
are presently unknown. For many of the technologies we employ in our business, we rely on maintaining competitively sensitive know-how and other information as trade secrets, which may not sufficiently protect this information. Disclosure of this information could impair our competitive position. As to many technical aspects of our business, we have concluded that competitively sensitive information is either not patentable or that for competitive reasons it is not commercially advantageous to seek patent protection. In these circumstances, we seek to protect this know-how and other
proprietary information by maintaining it in confidence as a trade secret. To maintain the confidentiality of our trade secrets, we generally enter into confidentiality agreements with our employees, consultants and collaborators when their relationship with us commences. These agreements require that all
confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. However, we may not obtain these agreements in all circumstances, and individuals with whom we
have these agreements may not comply with the terms of these agreements. The disclosure of our trade secrets would impair our competitive position. Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. Further, to the extent that our
employees, consultants or contractors use trade secret technology or know-how owned by others in their work for us, disputes may arise as to the ownership of related inventions. 12
We may incur significant liability if we infringe the patents and other proprietary rights of third parties. In the event that our technologies infringe or violate the patent or other proprietary rights of third parties, we may be prevented from pursuing product development, manufacturing or commercialization of our products that utilize such technologies. There may be patents held by others of which we
are unaware that contain claims that our products or operations infringe. In addition, given the complexities and uncertainties of patent laws, there may be patents of which we know that we may ultimately be held to infringe, particularly if the claims of the patent are determined to be broader than we
believe them to be. If a third party claims that we infringe its patents, any of the following may occur:
•
we may become liable for substantial damages for past infringement if a court decides that our technologies infringe upon a competitor’s patent; • a court may prohibit us from selling or licensing our product without a license from the patent holder, which may not be available on commercially acceptable terms or at all, or which may require us to pay substantial royalties or grant cross-licenses to our patents; and • we may have to redesign our product so that it does not infringe upon others’ patent rights, which may not be possible or could require substantial funds or time. In addition, employees, consultants, contractors and others may use the trade secret information of others in their work for us or disclose our trade secret information to others. Either of these events could lead to disputes over the ownership of inventions derived from that information or expose us
to potential damages or other penalties. If any of these events occurs, our business will likely suffer and the market price of our common stock decline. We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights. There has been substantial litigation and other proceedings regarding patent and intellectual property rights in the biotechnology industry. We may be forced to defend claims of infringement brought by our competitors and others, and we may institute litigation against others who we believe are
infringing our intellectual property rights. The outcome of intellectual property litigation is subject to substantial uncertainties and may, for example, turn on the interpretation of claim language by the court, which may not be to our advantage, or on the testimony of experts as to technical facts upon
which experts may reasonably disagree. Our involvement in intellectual property litigation could result in significant expense to us. Some of our competitors have considerable resources available to them and a strong economic incentive to undertake substantial efforts to stop or delay us from
commercializing products. We, on the other hand, are a relatively small company with comparatively few resources available to us to engage in costly and protracted litigation. Moreover, regardless of the outcome, intellectual property litigation against or by us could significantly disrupt our development
and commercialization efforts, divert our management’s attention and quickly consume our financial resources. In addition, if third parties file patent applications or issue patents claiming technology that is also claimed by us in pending applications, we may be required to participate in interference proceedings with the U.S. Patent and Trademark Office or in other proceedings outside the United States,
including oppositions, to determine priority of invention or patentability. Interference or oppositions could adversely affect our patent rights. Even if we are successful in these proceedings, we may incur substantial costs, and the time and attention of our management and scientific personnel will be
diverted in pursuit of these proceedings. 13
If product liability lawsuits are brought against us, we might incur substantial liabilities and could be required to limit the commercialization of our products. Although we are not presently generating revenues from the sale of products, our short-term objectives include commercializing various applications of our RECAF testing technology, which may involve the sale of test kits. If our products do not function properly, we may be exposed to the risk of
product liability claims. We may even be subject to claims against us despite the fact that the injury is due to the actions of others, such as manufacturers with whom we contract to make the test kits. Any product liability litigation would consume substantial amounts of our financial and managerial
resources and might result in adverse publicity, regardless of the ultimate outcome of the litigation. We do not currently maintain clinical trial insurance or product liability insurance and we may never obtain such insurance. In any event, liability insurance is subject to deductibles and coverage limitations
and may not provide adequate coverage against potential claims or losses. A successful product liability claim brought against us could cause us to incur substantial costs and liabilities. Our future success depends on the continued availability of our chief executive officer, Dr. Ricardo Moro-Vidal, and other scientific and technical personnel. The loss of Dr. Moro-Vidal’s services or those of other technical personnel could have a significant adverse impact on our business. The success of our business depends to a great extent on the efforts and abilities of our senior executive officer, Dr. Ricardo Moro-Vidal. Dr. Moro-Vidal discovered the RECAF molecule and has done or supervised most of the research that has led to the development of our various cancer detection
tests. In addition, Dr. Moro-Vidal has most of the unpatented technical know-how that is crucial to our research and development efforts. We do not plan to obtain a key-person life insurance policy on Dr. Moro-Vidal. We have entered into a four-year employment agreement with Dr. Moro-Vidal,
terminating in December 31, 2013, which will contain confidentiality and non-compete covenants. Nevertheless, we cannot assure you that Dr. Moro-Vidal will continue to work for us or that the non-compete and confidentiality provisions of his employment agreement will be enforceable. If Dr. Moro-
Vidal terminates his employment relationship with us, it could be difficult to find a replacement with comparable skill and knowledge. In addition, the pool of individuals with relevant experience in biotechnology is limited and retaining and training personnel with the skills necessary to continue our
research and development activities would be challenging, costly and time-consuming. The loss of any of our scientific or technical personnel could significantly delay the achievement of our goals and materially and adversely affect our business, financial condition and results of operations. We depend on the continued availability of research and development services provided by PBRC. The loss of these services could adversely affect us. All of the research regarding RECAF is performed by PBRC, which is wholly-owned by Dr. Moro-Vidal. We have entered into a contract with PBRC pursuant to which PBRC will continue to perform research and development as well as diagnostic services for us. The agreement has an initial term
that expires on December 31, 2013 and we have the right to extend the agreement for two additional four-year terms. If PBRC elects to discontinue its relationship with us, we may be unable to find another firm with the same technical expertise, which could impair our ability to develop our technology
and materially and adversely affect our business, financial condition and results of operations. We may expand our operations internationally in the future, particularly in China and India, and would be subject to the political systems, economic conditions, government programs and tax structures of those countries. To the extent that we expand our operations internationally in the future, particularly in China and India, the political and economic conditions of those countries may directly affect our operations. In addition, any government programs in which we participate or any tax benefits we may derive in
those countries may not be continued at favorable levels or at all. Further, we are a 14
U.S. entity and our executive officers and assets are located in Canada. It may be difficult for us to assert legal claims in actions instituted in foreign jurisdictions. Foreign courts may refuse to hear our legal claims because they may not be the most appropriate forums in which to bring such a claim. Even
if a foreign court agrees to hear a claim, it may determine that the law of the jurisdiction in which the foreign court resides, and not Canadian or U.S. law, is applicable to the claim. Further, if Canadian or U.S. law is found to be applicable, the content of applicable Canadian or U.S. law must be proved
as a fact, which can be a time-consuming and costly process, and certain matters of procedure would still be governed by the law of the jurisdiction in which the foreign court resides. As a result of the difficulty associated with enforcing a judgment against us, you may not be able to collect any damages
awarded by either a U.S., Canadian or other foreign court. We have not yet completed the assessment of our internal controls over financial reporting required by the Sarbanes-Oxley Act of 2002 and the cost of compliance could be significant. We are in the process of completing an assessment of our disclosure controls and procedures and our internal controls over financial reporting as required by Section 404 of the Sarbanes-Oxley Act of 2002. The purpose of the assessment is to confirm that our systems, procedures and controls
regarding financial reporting and disclosure comply with the rules applicable to public companies, including (1) assuring that our systems, controls and procedures satisfy the requirements of the framework to which we have chosen to adhere and (2) developing a system of gathering and maintaining
evidence to support management’s assessment of disclosure controls and procedures and internal controls over financial reporting. In addition, our independent registered public accounting firm must provide an attestation report regarding our assessment by March 31, 2011. The costs of the assessment and
obtaining an attestation report from an independent public accounting firm regarding our assessment may be significant. Moreover, if there is a “material weakness” in our systems, controls and/or procedures, there may be additional costs to rectify those weaknesses. A finding of a “material weakness”
could also damage our reputation and, if we cannot address those weaknesses in a timely and efficient manner, could potentially subject us to administrative action by the SEC and result in the imposition of monetary penalties. Risks Related to Ownership of Our Securities and this Offering The public offering price of the securities sold in this offering may not reflect our true fair market value. The public offering price of the securities sold in this offering has been determined by negotiation between us and the representative and does not necessarily bear any direct relationship to our assets, results of operations, financial condition, book value or any other recognized criterion of value and,
therefore, might not be indicative of prices that will prevail in the trading market. We cannot assure you that the price of the securities sold in this offering will not decline immediately after the offering is completed. The market price of our common stock may decline as a result of this offering. As a result of this offering, the number of shares of our common stock outstanding will increase substantially without an immediate increase in potential net income. To the extent that the market price of our common stock reflects a multiple of discounted projected earnings per share, the share price
may decline. The market price for our common stock is volatile and the price at which you may be able to sell any of the securities purchased in this offering might be lower than the offering price. The market price of our common stock, as well as the securities of other biotechnology companies, has historically been highly volatile, and the market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies.
Factors such as fluctuations in our operating results, announcements of technological innovations or new products by us or our competitors, governmental regulation, developments in patent or other proprietary rights, public concern as to the safety of products developed by us or other biotechnology and
pharmaceutical companies, and general market 15
conditions may have a significant effect on the future market price of our publicly traded or quoted securities. There is, at present, only a limited market for our common stock and no market for the redeemable warrants and there is no assurance that an active trading market for either or both of these securities will develop. Trades of our common stock are subject to Rule 15g-9 promulgated by the Securities and Exchange Commission (the SEC) under the Exchange Act, which imposes certain requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited
investors. For transactions covered by the rule, broker/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction prior to sale. The SEC also has other rules that regulate broker/dealer practices in connection with
transactions in “penny stocks.” Penny stocks generally are equity securities with a price of less than $5.00 (other than securities listed on a national securities exchange, provided that current price and volume information with respect to transactions in that security is provided by the exchange or system).
The penny stock rules require a broker/dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document prepared by the SEC that provides information about penny stocks and the nature and level of risks in the penny stock market.
The broker/dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker/dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid
and offer quotations, and the broker/dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. These disclosure requirements have the
effect of reducing the level of trading activity for our common stock. As a result of the foregoing, investors may find it difficult to sell their shares. We lack analyst coverage. We do not have research analysts reviewing our performance or our securities on an ongoing basis. Therefore, we do not have an independent review of our performance or the value of our common stock relative to other public companies. You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future. If you purchase securities in this offering, you will experience immediate and substantial dilution insofar as the public offering price of a unit will be substantially greater than the tangible book value attributable to the shares of our common stock included in a unit after giving effect to this offering.
In the past, we have issued options and warrants to acquire shares of common stock at prices significantly below the imputed public offering price of a share of our common stock (assuming no value is attributed to the redeemable warrants included in the units). To the extent these options and warrants
are ultimately exercised, you will incur further dilution. We have broad discretion in the use of the proceeds from this offering and our use of the offering proceeds may not yield a favorable return on your investment. We expect to use proceeds from this offering for working capital and general corporate purposes including obtaining FDA approval and for commercializing various applications of our technology and sales and marketing expenses relating thereto. However, we have broad discretion over how these
proceeds will be used and could spend the proceeds in ways with which you may not agree. We may not apply the proceeds of this offering effectively or in a manner that yields a favorable or any return, and consequently, this could result in financial losses that could have a material adverse effect on
our business, cause the price of our common stock to decline or delay the development of our product candidates. 16
Future sales, or the potential sale, of a substantial number of shares of our common stock could cause the trading price of our securities to decline and could impair our ability to raise capital through subsequent equity offerings. Sales of a substantial number of shares of our common stock in the public markets, or the perception that these sales may occur, could cause the market price of our securities to decline and could materially impair our ability to raise capital through the sale of additional equity securities. After
taking into account the 84,000,000 shares included in the units offered hereby, we will have 157,062,205 shares of common stock issued and outstanding immediately after this offering based on 73,062,205 shares issued and outstanding as of December 31, 2009. In addition, we may issue up to an additional 196,500,000 shares of our common stock as follows:
•
84,000,000 shares upon exercise of the redeemable warrants; • 25,200,000 shares upon exercise of the underwriters’ over-allotment option to purchase 180,000 units (including the shares underlying the redeemable warrants included in such units); • 16,800,000 shares upon exercise of the representative’s warrant to purchase 120,000 units (including the shares underlying the warrants included in those units);
• 9,857,723 shares under our Non-Qualified Stock Option Plan, of which 5,987,057 are issuable upon exercise of options outstanding at December 31, 2009 having an exercise price of $0.001 per share;
• 28,500,000 shares upon exercise of options that we intend to grant to our senior executives and directors upon completion of this offering, which will have an exercise price equal to the result obtained by dividing the unit public offering price by the number of shares of common stock included in a
unit;
• 1,606,275 shares under our Stock Bonus Plan;
• approximately 17,000,000 shares upon exercise of warrants outstanding at September 30, 2009 having a weighted average exercise price of $0.14 per share; and
• approximately 13,500,000 shares upon conversion in full of our amended secured convertible notes in the aggregate outstanding principal amount of $1.75 million as of December 31, 2009, assuming a conversion price of $0.13 per share, which number of shares does not include shares issuable if the
amount converted includes accrued interest and which number of shares will be reduced to approximately 3.0 million shares after this offering if the holders of the amended secured convertible notes accept a prepayment out of the net proceeds of this offering reducing the balance due on those
notes to $390,000. Of the foregoing shares, approximately 137,500,000 either will be covered by the registration statement of which this prospectus is a part or are covered by another effective registration statement and, thus, upon issuance, will be freely tradable subject only to the resale restrictions set forth in Rule
144, promulgated under the Securities Act, applicable to affiliates, and subject to the further caveat that shares cannot be issued unless the applicable registration statement is effective at the time of issuance. In addition, the shares issuable upon conversion of the principal amount of the amended secured
convertible notes will be freely tradeable under Rule 144. For additional information regarding Rule 144, see page 58 of this prospectus. Finally, we intend to file a registration statement on Form S-8 as soon as practicable after completion of this offering covering the 28,500,000 shares that will be
reserved for issuance upon exercise of the options we intend to grant to our senior executives and directors upon completion of this offering. We do not anticipate paying dividends in the foreseeable future. This could make our stock less attractive to potential investors. We anticipate that we will retain all future earnings and other cash resources for the future operation and development of our business, and we do not intend to declare or pay any cash dividends in the foreseeable future. Future payment of cash dividends will be at the discretion of our board of
directors after taking into account many factors, including our operating results, financial condition and capital requirements. Corporations that pay dividends may be viewed as a better investment than corporations that do not. 17
Certain provisions of Texas law and our organizational documents could delay or discourage takeover attempts that stockholders may consider favorable. Our articles of incorporation and bylaws include provisions that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors. These provisions may make it more difficult to remove directors and management or could have the effect of delaying,
deferring or preventing a future takeover or a change in control, unless the takeover or change in control is approved by our board of directors, even though the transaction might offer our stockholders an opportunity to sell their shares at a price above the current market price. In addition, we are
subject to the provisions of the Texas Business Combination Law (Articles 13.01 through 13.08 of the Texas Business Corporation Act), which generally prohibit us from engaging in any business combination with certain persons who own 20% or more of our outstanding voting stock without the approval
or our board of directors. These provisions could make it difficult for a third party to acquire us, or for members of our board of directors to be replaced, even if doing so would be beneficial to our stockholders. Any delay or prevention of a change of control transaction or changes in our board of
directors or management could deter potential acquirers or prevent the completion of a transaction in which our stockholders could receive a substantial premium over the then current market price for their shares. As a result, these provisions may adversely affect the price of our common stock. 18
Based on an assumed public offering price of $7.00 per unit, within the range set forth on the cover of this prospectus, we estimate that the net proceeds from this offering will be approximately $6.8 million after deducting the (i) underwriting discounts and commissions ($0.70 per unit, or $840,000 in
the aggregate), (ii) representative’s non-accountable expense allowance (approximately $252,000) and (iii) estimated offering expenses (approximately $500,000), or approximately $7.9 million if the underwriters’ over-allotment option is exercised in full. If the redeemable warrants are exercised, we will
receive an additional $12.6 million in gross proceeds ($14.5 million if the redeemable warrants underlying the underwriters’ over-allotment option are exercised). We have agreed to pay Paulson a warrant solicitation fee equal to 5% of the exercise price of each redeemable warrant exercised more than
one year after the date of the prospectus if Paulson solicits the exercise. We currently expect to use our net proceeds from this offering as follows:
Purpose
Amount
Percentage Develop and obtain marketing approval of our POC test format
$
1,000,000
14.7
% Repayment, in part, of our amended secured convertible notes(1)(2)
$
1,360,000
20.0
% Repayment, in part, of our 10% unsecured notes(3)
$
450,000
6.6
% Fund ongoing research and development activities relating to the RECAF marker
$
200,000
2.9
% Fund marketing expenses relating to commercializing the manual format of our serum testing technology
$
150,000
2.2
% Fund marketing expenses relating to the veterinary applications of our serum testing technology
$
100,000
1.5
% Fund working capital and other general corporate purposes, including interest on our outstanding indebtedness, accounting and legal expenses, facilities expenses and personnel expenses(2)
$
3,540,000
52.1
% TOTAL
$
6,800,000
100.0
%
(1) The amended secured convertible notes are due and payable in full on December 31, 2012 and bear interest at a floating rate of interest equal to the prime rate plus 2.75%. Based on the current prime rate of 3.25%, the amended secured notes are currently accruing interest at the rate of 6% per
annum. As of December 31, 2009, the aggregate outstanding principal balance of the amended secured convertible notes was $1.75 million. Assuming no further reductions in principal as a result of conversion or repayments, after this offering the aggregate outstanding principal balance due on these
notes will be $390,000. We may offer to repay the entire amount due on these notes as well as the notes described in the last sentence of note 3 below, in which case the amount of net proceeds allocated to working capital and general corporate purposes will be reduced by $515,000. (2) After this offering, there will remain approximately $390,000 outstanding on the amended secured convertible notes and $125,000 of unsecured notes. (3) In September 2009, we issued unsecured notes in the aggregate principal amount of $575,000. The net proceeds from the sale of the unsecured notes, after payment of all expenses relating to their issuance, were used to pay expenses relating to this offering and for working capital and general
corporate purposes. These notes bear interest at 10% per annum. Notes having an aggregate original principal amount of $450,000 are due and payable in full out of the net proceeds of this offering. The remaining notes, in an aggregate principal amount of $125,000, are due and payable on the earlier
of (a) January 31, 2013 and (b) 30 days after all amounts due with respect to the amended secured convertible notes have been paid in full or fully “cash collateralized.”
The amounts and timing of our actual expenditures will depend on numerous factors, including current global economic conditions and internal cash flows. We will have broad discretion in the 19
application of the net proceeds. Pending the uses described above, we intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities. In the event the estimated net proceeds of this offering is less than $6.8 million, the amount allocated to working capital and general
corporate purposes will be reduced. We anticipate that the net proceeds from this offering together with our existing cash balances and our anticipated cash flow from operations will allow us to sufficiently manage our cash flow needs for the next twelve months. Our existing cash balances include the net proceeds from the sale of our
10% unsecured promissory notes in the original aggregate principal amount of $575,000 ($520,000 after the payment of placement agent fees), which were used to pay the expenses related to that offering, expenses related to this offering and for working capital and general corporate purposes. We have never declared or paid cash dividends on our capital stock and do not anticipate declaring or paying cash dividends on our common stock in the foreseeable future. Payments of future dividends on our common stock, if any, will be at the discretion of our board of directors after taking into
account various factors, including our financial condition, operating results, current and anticipated cash needs, plans for expansion and other factors that our board of directors may deem relevant. Our common stock is quoted on the Bulletin Board under the symbol BOCX. The following table sets forth the high and low bid prices for our common stock reported on the Bulletin Board for the periods indicated below. These quotations reflect inter-dealer prices, without retail mark-up, mark-
down or commission and may not necessarily represent actual transactions.
Price Range Per Share
High ($)
Low ($) Year Ending December 31, 2009 First Quarter
0.18
0.06 Second Quarter
0.11
0.04 Third Quarter
0.29
0.07 Fourth Quarter (through December 30)
0.21
0.10 Year Ended December 31, 2008 First Quarter
0.75
0.53 Second Quarter
0.65
0.44 Third Quarter
0.43
0.17 Fourth Quarter
0.25
0.14 Year Ended December 31, 2007 First Quarter
0.71
0.57 Second Quarter
0.67
0.56 Third Quarter
0.82
0.46 Fourth Quarter
0.70
0.55 On December 30, 2009, the last reported sales price for a share of our common stock, as reported on the Bulletin Board, was $0.115. Holders As of December 31, 2009, there were 138 holders of record of our common stock. 20
The
following table sets forth our actual capitalization as of December 31, 2008
and as of September 30, 2009 on an actual basis and on an as adjusted
basis. As adjusted information gives effect to the following; (i) the issuance
of 2,785,714 shares of common stock subscribed for on or before September
30, 2009 and issued subsequent to that date; (ii) the issuance of an aggregate
of 1,483,516 shares of common stock in October and December 2009 to the holders
of our amended secured convertible notes in connection with the exercise
of their conversion of $200,000 principal amount of such notes; (iii) the
issuance of 300,000 shares of common stock in December 2009 to settle an
account payable for services; (iv) a rescission of the exercise of options
cancelling the issuance of 3,648,947 shares in September 2009; (v) the receipt
of approximately $6.8 million of net proceeds from this offering assuming
a public offering price of $7.00 per unit, within of the range set forth
on the cover of this prospectus; and (vi) the repayment of approximately
$1.8 million of indebtedness out of the net proceeds of this offering. You should read this table in conjunction with the sections of this prospectus captioned “Use of Proceeds” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as the financial statements and related notes included elsewhere in this prospectus.
December 31,
September 30,
Actual
Actual
Pro Forma, Debt(1)
$
2,020,186
$
1,858,309
$
400,613
Common stock, $0.001 par value: 125,000,000 shares authorized actual and 450,000,000 shares authorized as adjusted; 43,713,399 shares issued and outstanding actual at December 31, 2008; and 72,141,921 and 157,062,205 shares issued and outstanding actual and
as adjusted, respectively, at September 30, 2009
43,713
72,142
157,062 Additional paid-in capital
15,178,205
16,975,387
23,927,351 Common stock subscribed
40,050
122,500
— Accumulated other comprehensive (loss)
(15,529
)
—
— Accumulated deficit
(114,175
)
(114,175
)
(114,175
) Deficit accumulated during development stage
(17,067,172
)
(18,886,998
)
(19,536,981
) Total stockholders’ equity (deficit)
$
(1,934,908
)
(1,831,144
)
4,433,257 Total capitalization
$
85,278
$
27,165
$
4,833,870
(1)
Includes loans payable, convertible notes payable and the short- and long-term portion of the secured convertible notes. Does not include accounts payable, accrued liabilities or amounts due to related parties.
Subsequent to September 30, 2009, we issued an additional 4,569,230 shares. Of these shares, 2,785,714 shares were subscribed for before that date and were reflected on the balance sheet as common stock subscriptions. We had not issued those shares as of that date and, therefore, they are not
included in the number of shares outstanding at that date. In addition, 714,286, was issued in October 2009 in connection with the conversion of $100,000 principal amount of the amended secured convertible notes (at a conversion price of $0.14) and 769,230 shares were issued in December 2009 in
connection with the conversion of another $100,000 principal amount of those notes (at a conversion price of $0.13). Finally, in December 2009, we issued 300,000 shares under our Stock Bonus Plan to settle an account payable for services. We have given pro forma effect to these issuances in order to be
consistent with the disclosure elsewhere in this prospectus. As stated in this prospectus, the number of shares outstanding both immediately before and immediately after the offering includes those 4,569,230 shares and the outstanding principal balance on the amended secured convertible notes reflects the
October and December 2009 conversions. We believe that the repayment of $1.8 million of indebtness is material. The repayment of debt is a required use of proceeds under the various debt instruments. The total amount of proceeds that will be used to repay debt represents the largest single use of proceeds constitutes the largest single 21
2008
2009
As Adjusted
use of proceeds, approximately 26.6%, other than working capital and general corporate purposes. However, unlike working capital and general corporate purposes, the impact of the repayment of debt will be immediate as opposed to being spread out over time. Also, as a result of that repayment, we
will have significantly reduced our total indebtedness and our total liabilities significantly. If you purchase units in this offering, your interest will be diluted to the extent of the excess of the public offering price per share of common stock over the as adjusted negative net tangible book value per share of common stock after this offering. Negative net tangible book value per share
represents the amount of our total tangible assets reduced by the amount of our total liabilities, divided by the total number of shares of common stock outstanding. For purposes of the dilution computation and the following tables, we have allocated the full purchase price of a unit to the shares of
common stock included in the unit and none to the redeemable warrants. At September 30, 2009, we had a negative net tangible book value of approximately $2,586,664, or approximately $0.035 per share based on 73,062,205 shares issued and outstanding on a pro forma basis. After taking into account the estimated net proceeds from this offering of $6.8 million, and the
issuance of 84,000,000 shares in this offering, our pro forma as adjusted net tangible book value at September 30, 2009 would have been approximately $3,869,783, or $0.025 per share. This represents an immediate increase in book value of $0.060 per share to existing stockholders and immediate dilution
of $0.075 per share, or 75%, to the new investors who purchase units in this offering. The following table illustrates this per share dilution: Assumed public offering price per share(1)
$
0.100 Net tangible book value per share at September 30, 2009
$
(0.035
)
Increase in net tangible book value per share attributable to new investors
0.060 Net tangible book value per share after the offering
$
0.025 Dilution per share to new investors
$
0.075
(1)
Based on a public offering price of $7.00 per unit, within the range set forth on the cover of this prospectus, divided by the number of shares included in a unit.
22
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA The selected financial data set forth below as of and for the two years ended December 31, 2007 and 2008 have been derived from our audited consolidated financial statements, which have been audited and reported upon by Manning Elliott, Chartered Accountants, an independent registered public
accounting firm, and are included elsewhere in this prospectus. The selected financial data as of September 30, 2009 and for the nine months ended September 30, 2008 and 2009 are derived from our unaudited interim consolidated financial statements and include, in the opinion of management, all
adjustments (consisting only of normal recurring adjustments) necessary to present fairly the data for such periods. The results of operations for the nine-month period ended September 30, 2009 do not necessarily indicate the results that may be expected for any future period. You should read this information in conjunction with our audited consolidated financial statements and related notes, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other financial information appearing elsewhere in this prospectus. Consolidated Statement of Operations:
Nine Months Ended
Years Ended
2008
2009
2007
2008 Revenue
$
1,000,000
$
—
$
50,000
$
1,000,000 General and administrative
770,927
607,360
1,260,865
928,845 Amortization
26,898
30,661
27,896
37,758 Professional and consulting fees
335,457
325,990
313,925
381,421 Research and development
513,959
513,959
662,944
675,302 Total operating expenses
1,647,241
1,396,825
2,265,630
2,023,326 Loss from operations
(647,241
)
(1,396,825
)
(2,215,630
)
(1,023,326
) Other expense, net
(1,636,700
)
(423,001
)
(1,138,689
)
(3,090,659
) Net loss
$
(2,283,941
)
$
(1,819,826
)
$
(3,354,319
)
$
(4,113,985
) Unrealized gain (loss) on investment securities
$
28,819
$
15,529
$
(115,061
)
$
26,660 Total comprehensive loss
$
(2,255,122
)
$
(1,804,297
)
$
(3,469,380
)
$
(4,087,325
) Consolidated Balance Sheet Data:
As of
As of
2009
2007
2008 Cash
$
364,020
$
1,372,598
$
45,625 Working capital (deficit)
$
(996,112
)
202,252
(1,566,901
) Total assets
$
1,149,540
2,475,402
972,594 Total liabilities
$
2,980,684
1,642,872
2,907,482 Stockholders’ equity (deficit)
$
(1,831,144
)
832,530
(1,934,908
) 23
September 30,
December 31,
September
30,
December 31,
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION We are a development stage company focusing on developing and commercializing products for the early detection, diagnosis and monitoring the recurrence of cancer. We have developed and evaluated in clinical trials a blood test that can detect the presence of cancer in humans and animals using a
new cancer marker named RECAF. We developed and own, royalty free, the proprietary technology related to the RECAF marker, with patents granted in the United States, Europe and China and pending in other major worldwide markets. RECAF is a molecule found on most cancer cells, including breast, colon, prostate and lung cancers, but not on normal cells. RECAF can be used in blood tests to determine if a patient has cancer. The blood test can be formatted for use on automated instrumentation typically found in large clinical
and hospital laboratories or manually. It can also be formatted as a POC single use rapid test for use in physicians’ offices, urgent care facilities and at the bedside. Once approved by the FDA, the tests could be used in general screening or in high risk patients to determine if an individual has cancer. Unlike other notable cancer markers, such as CEA and PSA, which only detect the presence of a specific cancer type (CEA for colon cancer and PSA for prostate cancer), RECAF is found on most types of cancer and, therefore, could have much broader use than most of the previous cancer
markers in development or currently in use. Moreover, unlike existing cancer markers, RECAF has been shown to detect early stages of breast and prostate cancers when the likelihood of cure is highest. We have granted Abbott and Inverness, two large diagnostic equipment manufacturers, semi-exclusive licenses to use the RECAF tests on blood samples processed through automated diagnostic equipment and non-exclusive licenses for other test formats and we have retained rights for manual tests
not processed in automatic equipment, POC tests for the physicians’ office and including all other single format potential uses and all test formats used for veterinary applications. Under the terms of these licenses, we can grant one additional similar semi-exclusive license for automated testing. The Abbott license has been amended to relieve them of research and development responsibilities and, to our knowledge, they have not taken any steps towards commercializing our technology. Inverness has been conducting research and development trying to adapt our technology to their
diagnostic platform. However, to our knowledge, they have not yet reached the stage where they are prepared to enter into clinical trials in order to obtain FDA approval or to commercialize our technology or any related products. We have previously developed the following tests, which are no longer the focus of our growth plans, for the detection of cancer in tissue or cells based on RECAF technology:
•
Histo-RECAF—a tissue-based cancer detection test that involves staining cancer cells, thereby allowing a pathologist to easily view the cancer cells with the use of a microscope; and • Cryo-RECAF—a cell-based cancer detection test that can be used by pathologists during surgery to determine whether cancer cells are benign or malignant. Our principal objectives for the first twelve months after completion of this offering will be the following:
•
grant one additional semi-exclusive license for testing blood samples using automated testing equipment; • commercialize veterinary applications of RECAF testing technology not requiring regulatory approvals; • finish developing a POC rapid format test for the doctor’s office, bedside and veterinary use; • conduct clinical trials and seek FDA approval for marketing of the POC rapid format test; and 24
• commercialize manual testing formats, principally large cities in foreign countries where further regulatory clearance is not required. We cannot assure you that we can successfully achieve any of these objectives. All research involving RECAF is conducted on our behalf by PBRC, which is owned by Dr. Ricardo Moro-Vidal, our chief executive officer and a member of our board of directors. We expect that PBRC will also function as a testing laboratory for the veterinarian market once it is developed. We
have entered into an agreement with PBRC under which we will pay PBRC fees for research and development and general and administrative expenses. The material terms of the agreement include the following:
•
The balance that we owed to PBRC at September 30, 2009, approximately $390,000, plus all accrued and unpaid interest, will be due and payable on December 31, 2014, unless earlier terminated by us without cause or by PBRC as a result of our breach of our monthly payment obligation, in which
instances all amounts due PBRC will become immediately due and payable. • The amount due will accrue interest at a rate equal to the prime rate. Interest will be payable monthly. • We will pay PBRC monthly for its services in an amount that is equal to all costs incurred by PBRC in connection with services it provides to us (the “Costs”) plus a 15% cost adjustment. Such Costs will not include any salary paid by PBRC to Dr. Moro-Vidal. • To the extent the cost adjustment in any month exceeds $20,834, such excess will reduce the amount owed by us to PBRC. • PBRC will not be allowed to provide services to any person or entity other than us unless its average monthly Costs for any three consecutive months are less than its total expense for salaries and consulting fees for that three-month period. However, we will be allowed to use other laboratories
together with or in lieu of PBRC. In addition, we will have the right to terminate the agreement with PBRC at any time upon 90 days prior written notice. • PBRC has assigned to us all of its right, title and interest in and to all intellectual property developed or to be developed, including, but not limited to, know-how, processes, data and research results and all tangible property relating to RECAF. • The initial term of the agreement expires December 31, 2013 and we have the right to extend the agreement for two additional four-year terms. • If we terminate the agreement for any reason other than on account of a default by PBRC, then (i) we must pay PBRC a cancellation payment in an amount equal to 15% of the costs incurred by PBRC for the six months preceding such termination, (ii) we must give PBRC a perpetual non-
exclusive license to our RECAF technology and (iii) PBRC may thereafter perform services for any person or entity. Liquidity and Capital Resources We do not have any lines of credit with banks or other financial institutions or any other traditional financing arrangements. We will need additional capital until we are able to generate significant revenues to cover our expenditures. Since January 2003, we have been able to finance our operations through the private sale of securities and from borrowings from private lenders. Our sources and (uses) of cash during the nine months ended September 30, 2008 and 2009 were as follows:
2008
2009 Cash provided (used in) operations
$
(309,530
)
$
(248,256
) Patent costs
$
(174,711
)
$
(63,760
) Sale of investment securities
$
19,564
$
12,608 Advances (loans) from related parties
$
(126,041
)
$
63,185 Repayment of convertible notes
$
(725,000
)
$
(36,250
) Sale of common stock, net of offering costs
$
234,933
$
(304,814
) Proceeds from loans payable
—
$
575,000 25
Convertible Notes and Warrants By agreement dated as of June 25, 2007, we sold secured convertible notes and warrants to two private investors for $3,000,000. These notes bore interest annually at a rate of prime (as adjusted monthly on the first business day of each month) plus 2.75% per year. These notes were due and payable
on June 25, 2010 and were secured by substantially all of our assets. Interest on these notes was payable monthly and was due the first day of every month. We were also required to make monthly payments of $100,000 towards the principal amount of the notes. If we failed to make any interest or
principal payment when due, the notes would become immediately due and payable. At the holder’s option, the notes were convertible into shares of our common stock at a conversion price of $0.60. We failed to make certain required principal payments on the notes when due, resulting in a default. As of August 31, 2009, we entered into a Loan Modification Agreement with the holders of those notes. Under this agreement, the defaults were waived the original secured convertible notes were
exchanged for amended secured convertible notes having the following terms:
•
All interest due on the original notes through June 30, 2009 was added to the outstanding principal balance of the amended and, a result, the aggregate principal amount of the amended notes was determined to be $2.15 million. • The interest rate remains at prime (as adjusted monthly on the first business day of each month) plus 2.75% per year and accrues from July 1, 2009 and is payable in arrears on the first day of each month. • The notes are secured by a lien on all of our assets, including our patents and other technology. • The maturity date is December 31, 2012 and, except as provided below, no principal payments are due on the notes prior to the maturity date. • The conversion price is $0.14 per share. The maximum number of shares that we are obligated to issue upon the exercise of the holder’s conversion right may not exceed 4.99% of the total number of shares outstanding after giving effect to the conversion of the notes. • Our failure to close a firm commitment underwritten registered public offering of our common stock (including a sale of units which units include shares of our common stock) for gross proceeds of at least $3,000,000 on or before February 19, 2010 (a “Qualified Offering”) will result in a default. • Upon the closing of a Qualified Offering, we must offer to prepay the outstanding principal of the amended notes in an amount equal to the greater of 20% of the net proceeds we receive from such Qualified Offering or $600,000. • If the underwriter(s) of the Qualified Offering elect to exercise their option to cover over-allotments, we must offer to prepay the principal amount of the amended notes in an amount equal to 50% of the gross proceeds we receive from such transaction. • If we sell any equity or debt securities other than in a Qualified Offering, we must offer to prepay the principal amount of the amended notes in an amount equal to 30% of the gross proceeds we receive from such transaction. • If we receive any revenue from licensing arrangements, we must offer to prepay the principal amount of the amended notes in an amount equal to 10% of such revenues. • The holders of the notes have ten business days to decide whether or not to accept any such offer of prepayment. • We may not prepay the amended notes. However, we may elect to deposit with an escrow agent an amount, in cash, sufficient to pay all of the then outstanding principal and interest accruing to the maturity date of the amended notes, in which event all of the covenants in the notes (except for the
conversion rights) will be terminated and the holders will release their security interest in all of our property, except for cash collateral. 26
On September 17, September 30, October 23 and December 23, 2009, the holders of the amended secured convertible notes elected to convert an aggregate of $400,000 of the principal amount of those notes into an aggregate of 2,912,088 shares of our common stock, reducing the outstanding
principal amount of the secured notes to $1.75 million. Unsecured Notes In September 2009, we sold an aggregate of (i) 8,214,292 shares of our common stock and (ii) $575,000 principal amount of unsecured promissory notes. As of September 30, 2009, 6,428,578 of those shares had been issued. The balance of the shares, 1,785,715, was issued in November, 2009. The gross
proceeds to us from the sale of these securities were $575,000 and the net proceeds after the payment of fees to the placement agent were $520,000. The notes bear interest at a rate of 10% per annum. Notes having an aggregate principal amount of $450,000 are due and payable on the earlier of (i)
August 31, 2010 or (ii) the completion of an equity offering in which the gross proceeds are at least $3 million. The balance of the notes are due and payable on the earlier of (i) January 31, 2013 or (ii) 30 days after all amounts due with respect to the amended secured convertible notes have been paid
in full or fully “cash collaterized.” Operating Requirements We anticipate that our operating requirements for the twelve months period following the completion of this offering will be as follows: Research, development and production of our diagnostic products
$
1,000,000 General and administrative expenses
750,000 Marketing and investor communications
150,000 Business development
200,000 Payment of interest on amended senior convertible notes and unsecured promissory notes
150,000 Payment of outstanding liabilities
250,000
$
2,500,000 Our most significant capital requirements are general research and development and administrative expenses. General and administrative expenses, exclusive of depreciation, amortization and other expenses not requiring the use of cash (such as the costs associated with issuing stock and options for
services), average approximately $60,000 per month. Our research and development expenses vary, depending upon available capital. When more capital is available to us, research and development expenses increase. Conversely, research and development expenses decline when less capital is available. We may not be successful in obtaining additional capital in the future. If we are unable to raise the capital we need, our research and development activities will be curtailed or delayed and our operations will be reduced to a level which can be funded with the capital available to us. Segment Reporting Our business is managed and financial results are reported as one segment. We focus on consolidated results to make strategic and tactical decisions. In addition, important functions, such as marketing and research and development, are made on a company-wide basis. Evaluation of Financial Performance We evaluate our financial performance based on revenues; gross profit and gross margin; selling/research and development/administrative expenses; and cash flow. We use earnings before interest, taxes, depreciation and amortization as a performance measure of operational cash flows. 27
Results of Operations The following table compares our statement of operations data for the nine months ended September 30, 2008 and 2009 and for the years ended December 31, 2007 and 2008. The trends suggested by this table may not be indicative of future operating results, which will depend on various factors
including our ability to commercialize various products and the success we have with those efforts and whether we are successful in obtaining a third semi-exclusive license for Serum-RECAF.
Nine Months Ended
Years Ended
2008
2009
2007
2008 Revenue
$
1,000,000
$
—
$
50,000
$
1,000,000 General and administrative
770,927
607,360
1,260,865
928,845 Amortization
26,898
30,661
27,896
37,758 Professional and consulting fees
335,457
325,990
313,925
381,421 Research and development
513,959
432,814
662,944
675,302 Total operating expenses
1,647,241
1,396,825
2,265,630
2,023,326 Loss from operations
(647,241
)
(1,396,825
)
(2,215,630
)
(1,023,326
) Other expense, net
(1,636,700
)
(423,001
)
(1,138,689
)
(3,090,659
) Net loss
$
(2,283,941
)
$
(1,819,826
)
$
(3,354,319
)
$
(4,113,985
) Unrealized gain (loss) on investment securities
$
28,819
$
15,529
$
(115,061
)
$
26,660 Total comprehensive loss
$
(2,255,122
)
$
(1,804,297
)
$
(3,469,380
)
$
(4,087,325
) Nine months ended September 30, 2008 and 2009 Operating expenses for the nine months ended September 30, 2009 were approximately 15.2% lower than those for the comparable 2008 period. The decrease was due to the fact that we had to curtail our activities because we had no revenue in the 2009 period. Material changes of items in our Statement of Operations for the nine months ended September 30, 2009, as compared to the same period in the prior year, are as follows: Revenue. During the year ended December 31, 2008, we received aggregate payments of $1 million from Inverness under the license agreement. We recognized one-half of these payments as revenue in the first quarter and one-half as revenue in the second quarter. The payments were in
consideration for the transfer of various materials and technology and for providing assistance to Inverness in connection with such transfers in order to enable Inverness to produce RECAF material on its own in sufficient quantities to support its own research and development and other
commercialization efforts, defined in the license agreement as a “Successful Transfer.” A Successful Transfer was completed over the six month period ended June 30, 2008. We had no revenue in the 2009 period. General and administrative expenses. The decrease from 2008 to 2009 was primarily attributable to lower stock-based compensation expenses, as a result of the resignation of one of our senior executive officers and directors, and lower public relations expenses. Professional and consulting fees. In addition to the amount reflected in the table above, in the 2009 period we also incurred professional fees in connection with preparing and negotiating the terms of the loan modification agreement with the holders of our secured convertible notes, negotiating the
terms of our unsecured promissory notes and preparing the agreements and documents in connection with the offer and sale of those notes and preparing and filing the registration statement in connection with this offering. As of September 30, 2009, $227,000 of professional fees was included in deferred
financing costs, of which $89,450 was attributable to this offering. Research and development expenses. Research and development expenses were lower in the 2009 period as a result of decreased use of chemicals and laboratory supplies. 28
September 30,
December 31,
Other expense, net. This category includes net interest expense, amortization of debt issuance costs and accrued discounts on our convertible notes. While the accrued discount amount decreased, the amortization of debt issuance cost and interest expense increased resulting in an overall increase in this
category. The increase in interest expense was primarily the result of the fact that we were in default of the notes for failing to make payments when due and interest was calculated at the default rate of 18%. On August 31, 2009, we entered into a loan modification agreement with the holders of the
original secured convertible notes. We deemed the terms of the amended secured convertible notes to be substantially different and treated the original secured convertible notes extinguished and exchanged for the amended secured convertible notes. As such, we recorded a gain on extinguishment of debt
of $969,538. Years ended December 31, 2007 and 2008 Revenues for the year ended December 31, 2008 were higher than revenues for the prior year and operating expenses for the year ended December 31, 2008 were lower than those for the year ended December 31, 2007. However, our net loss increased as a result of costs associated with the original
secured convertible notes. Material changes of items in our Statement of Operations for the year ended December 31, 2008, compared to the year ended December 31, 2007, are as follows: Revenue. As discussed above, during the year ended December 31, 2008, we received aggregate payments of $1 million from Inverness under the license agreement, which we recognized in the first and the second quarter of the year. None of that revenue was recognized in 2007 because no transfers
to or services on behalf of Inverness occurred in 2007 and we were not yet able to accurately estimate the period over which we would be required to provide transfers and assistance in order to complete a Successful Transfer. Revenues for 2007 reflected the minimum royalty payment under the Abbott
license. General and administrative expenses. General and administrative expenses decreased in 2008 from 2007 primarily as a result of lower stock-based compensation expenses and lower public relations expenses. Professional and consulting fees. Professional and consulting fees for 2008 increased compared to 2007 primarily as a result of the fee for a research report. Research and development expenses. Research and development expenses were consistent year-to-year. Other expense, net. This category increased significantly as a result of the sale of our convertible notes at the end of June 2007. Thus, for 2008 we recorded a full year of net interest expense, amortization of debt issuance costs and accrued discounts on our convertible notes compared to only half a
year in 2007. Recent Accounting Pronouncements In June 2009, the FASB issued guidance now codified as FASB ASC Topic 105, Generally Accepted Accounting Principles, as the single source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in
conformity with U.S. GAAP, aside from those issued by the SEC. ASC 105 does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all authoritative literature related to a particular topic in one place. The adoption of ASC 105 did not
have a material impact on our consolidated financial statements, but did eliminate all references to pre-codification standards. In May 2009, FASB issued ASC 855, Subsequent Events, which establishes general standards of for the evaluation, recognition and disclosure of events and transactions that occur after the balance sheet date. Although there is new terminology, the standard is based on the same principles as those
that currently exist in the auditing standards. The standard, which includes a new required disclosure of the date through which an entity has evaluated subsequent events, is effective for interim or 29
annual periods ending after June 15, 2009. The adoption of ASC 855-10 did not have a material effect on our consolidated financial statements. Refer to Note 14. We have implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and we do not believe that there are any other new accounting pronouncements that have been issued that
might have a material impact on its financial position or results of operations. Critical Accounting Policies Certain accounting policies are particularly important to the portrayal of our financial position and results of operations and require the application of significant judgments by management. As a result, the consolidated financial statements are subject to an inherent degree of uncertainty. In applying
those policies, management uses its judgment to determine the appropriate assumptions to be used in the determination of certain estimates. These estimates are based on our historical experience, terms of existing contracts, observance of trends in the industry and information available from outside
sources, as appropriate. Our significant accounting policies include: Revenue Recognition. We recognize revenue in accordance with Securities and Exchange Commission Staff Accounting Bulletin No. 104, “Revenue Recognition in Financial Statements” and Staff Accounting Bulletin Topic 13A3(f), “Nonrefundable Up-front Fees.” In accordance with SAB 104,
revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service is performed, and collectability is reasonably assured. In accordance with SAB Topic 13A3(f), we determine, under the facts and circumstances, whether up-front fees under a
license agreement are in exchange for products delivered or services performed that represent the culmination of a separate earnings process and therefore should be recognized over the period that such fees are earned. Registration Payment Arrangements. We account for registration rights arrangements and related liquidated damages provisions under FASB ASC 815-40, Derivatives and Hedging—Contracts in Entity’s own Entity, which addresses an issuer’s accounting for registration payment arrangements. ASC 815-40
defines a registration payment arrangement as an arrangement where the issuer i) will endeavor to file a registration statement for the resale of financial instruments, have the registration statement declared effective, or maintain its effectiveness and ii) transfer consideration to the counterparty if the
registration statement is not declared effective or its effectiveness is not maintained. ASC 815-40 requires the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of
a financial instrument or other agreement, to be separately recognized and measured in accordance with ASC 450, Contingencies. Long-lived Assets. In accordance with ASC 360, “Property Plant and Equipment”, the carrying value of intangible assets and other long-lived assets is reviewed on a regular basis for the existence of facts or circumstances that may suggest impairment. We recognize impairment when the sum of the
expected undiscounted future cash flows is less than the carrying amount of the asset. Impairment losses, if any, are measured as the excess of the carrying amount of the asset over its estimated fair value. Investments. Investments consist of equity securities classified as “available-for-sale” securities under FASB ASC 320, “Investments—Debt and Equity Securities,” and are reported at fair value. Accordingly, unrealized gains and losses on these investments are reflected as other comprehensive income in
stockholders’ equity. Stock-based Compensation. We record stock-based compensation in accordance with ASC 718, “Compensation—Stock Based Compensation,” using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for
based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. 30
Overview We are a development stage company focusing on developing and commercializing products for the early detection, diagnosis and monitoring the recurrence of cancer. We have developed a blood test that can detect the presence of cancer in humans and animals using a new cancer marker named
RECAF. We developed and own, royalty-free, the proprietary technology related to the RECAF marker, with patents granted in the United States, Europe and China and pending in other major worldwide markets. RECAF is a molecule found on most cancer cells, including breast, colon, prostate and lung cancers, but not on normal cells. RECAF can be used in blood tests to determine if a patient has cancer. The blood test can be formatted for use on automated instrumentation typically found in large clinical
and hospital laboratories or manually. It can also be formatted as a point-of-care (POC) single use rapid test for use in physicians’ offices, urgent care facilities and at the bedside. Once approved by the FDA, the tests could be used in general screening or in high risk patients to determine if an individual
has cancer. It could also be used to detect recurrence of cancer in patients after therapy. Unlike other notable cancer markers that only detect the presence of a specific cancer type (CEA for colon cancer and PSA for prostate cancer), RECAF is found on most types of cancer and, therefore, could have much broader use than most other cancer markers in development or currently in
use. Moreover, unlike existing cancer markers, RECAF has been shown to detect early stages of breast and prostate cancers when the likelihood of cure is highest. We have granted Abbott and Inverness, two large diagnostic equipment manufacturers, semi-exclusive licenses to use the RECAF tests on blood samples processed in automatic equipment typically found only in large clinical/hospital laboratories and non-exclusive licenses for other test formats. Under
the terms of these licenses, we can grant one additional similar semi-exclusive license for automated testing and we have retained rights for manual tests not processed in automatic equipment, POC rapid tests for the physicians’ office, including all other single-format potential uses and all test formats
used for veterinary applications. The Abbott license has been amended to relieve them of research and development responsibilities and, to our knowledge, they have not taken any steps towards commercializing our technology. Inverness has been conducting research and development trying to adapt our
technology to their diagnostic platform. However, to our knowledge, they have not yet reached the stage where they are prepared to enter into clinical trials in order to obtain FDA approval or to commercialize our technology or any related products. Our principal objectives for the first twelve months after completion of this offering will be the following:
•
grant one additional semi-exclusive license for testing blood samples using automated testing equipment; • commercialize veterinary applications not requiring regulatory approvals; • finish developing a POC rapid format test for the doctor’s office, bedside and veterinary use; • conduct clinical trials and seek FDA approval for marketing of the POC rapid format test; and • commercialize manual testing formats, principally in large cities in foreign countries where further regulatory clearance is not required. Cancer Cancer is a term used for diseases in which abnormal cells divide without control and are able to invade other healthy tissue. Cancer cells spread to other parts of the body through the blood and lymph systems. There are more than 100 different types of cancers which are named for the organ or
type of cell in which they appear—e.g., lung cancer, colon cancer, breast cancer, prostate cancer, liver cancer and stomach cancer. 31
The American Cancer Society has estimated that there were over 12.3 million new cancer diagnoses worldwide and roughly 7.6 million deaths during 2007, of which nearly 40% were in developed nations. Although the United States has reported declining cancer-related deaths for the past few years,
the World Health Organization estimates that worldwide there will likely be approximately 16 million new cancer diagnoses annually by the year 2020, with roughly 10 million related deaths each year. Over the next 20 years, the global incidence of cancer is projected to increase by 50%. We believe that
the growing numbers of people developing and living with cancer will continue to increase the demand for cancer diagnostic products. In particular, two diagnostic areas that have significant unmet need are the early detection of primary cancer and early detection of recurrence after therapy. Market Dynamics The oncology market is one of the largest pharmaceutical markets. The global cancer market is forecast to grow at an average annual growth rate of 5.49% to $53.1 billion in 2009, up from $38.5 billion in 2003. Overall costs of cancer in 2008 were estimated to be $228.1 billion, composed of $93.2
billion for direct medical costs (total of all health expenditures), $18.8 billion for indirect morbidity costs (cost of lost productivity due to illness), and $116.1 billion for indirect mortality costs (cost of lost productivity due to premature death). Worldwide Cancer Diagnostics Market As of 2005, the global market for laboratory-based diagnostic tests exceeded $25 billion annually, with molecular diagnostic testing growing by approximately 20% each year and forecast to reach over $5 billion by 2009. Within this larger diagnostics market, cancer testing is anticipated to experience
some of the most robust growth over the next three to five years, having recently exceeded $1 billion in annual sales. We believe that the primary drivers for sales of diagnostic products for cancer markers are performance, price, service and marketing. At present, the five largest markets for these
products are the United States, Europe, Japan, China and India. Need for Improved Early Detection Methods Cancer that is detected early has the best prognosis. If cancer is diagnosed early in the disease process, before it spreads (metastasizes) to surrounding tissue, physicians are more likely to be able to successfully treat the patient and the likelihood of survival can be significantly increased. Surgical
removal of malignant tumors is much less effective once cancer cells have invaded additional locations, many of which are undetectable. While advances in early detection have improved the prognosis of many cancers, prostate, lung, and breast cancers are still among the most commonly diagnosed and the most fatal cancers. For example, among both men and women, lung cancer is the number one cause of cancer-related death, which
is believed to be due to the lack of early detection methods. By the time of diagnosis, only approximately 16% of lung cancer patients have tumors that are still in an early stage. For these patients, the five-year survival rate is 50% versus 15% when more advanced tumors are also included. If breast
cancer is caught and treated at its earliest stages, patients have five-year survival rates between 81% and 100%. However, if the cancer progresses to Stage IV before it is diagnosed, a patient’s likelihood of survival at five years is only 20%. Cancer Markers Cancer markers are a group of proteins, hormones, enzymes, receptors and other cellular products that are over expressed (produced in higher than normal amounts) by malignant cells. Cancer markers are usually normal cellular constituents that are present at very low levels in the blood of healthy
persons. If the substance in question is produced by the cancer, its levels will be increased in blood or other body fluids or in the tissue of origin. Detecting a cancer marker in higher-than-normal amounts in the body may signify the presence of a malignancy. For some indications, the expressed amount of a particular marker can also signal 32
the disease’s stage (i.e., how far the cancer has progressed). For instance, a common cancer marker for liver cancer, alpha-fetoprotein (“AFP”), not only signals the potential presence of liver cancer, but can also indicate the size of the tumor. However, it is important to note that AFP’s sensitivity as a
cancer marker is only approximately 60%, meaning that roughly 40% of patients with liver cancer do not have an elevated AFP. (In oncology, sensitivity is the ability of a test to detect cancer. If all cancer patients test positive for having cancer with a particular test, the test’s sensitivity would be 100%.
Specificity measures how well the test detects healthy individuals, i.e., whether it produces false positives, that is, falsely identifies patients as having cancer when they do not. If a test does not return any false positives, it has 100% specificity.) Cancer Markers in Clinical Use
Markers
Associated Cancers
Alpha-fetoprotein (“AFP”)
Testicular cancer, Liver cancer
CA-125
Ovarian cancer, Endometrial cancer
Carcinoembryonic antigen (“CEA”)
Colorectal cancer
Prostate specific antigen (“PSA”)
Prostate cancer
Human chorionic gonadotropin (“hCG”)
Testicular cancer, Choriocarcinoma
Nuclear matrix protein (“NMP22”)
Bladder cancer After testing for a cancer marker, further identifying the cells that express the marker may enable a definitive diagnosis. Oncologists measure marker levels to assess a patient’s response to treatment, evaluate appropriate future treatments, and check for signs that the cancer may be recurring. If, after
treatment, marker levels have decreased from the level at diagnosis, it may indicate that the cancer is responding favorably to the treatment. Conversely, if marker levels rise, the oncologist may consider an alternative therapy option, as the tumor is probably not responding to treatment. Depending upon
the patient and the cancer, these follow-up tests may be continued for life, occurring as frequently as every two to three months. Limitations of Current Cancer Markers We believe that validation of new cancer markers is one of the most important goals in cancer research. The National Cancer Institute (NCI) emphasized the need for finding new markers for prostate cancer as well as identifying markers for hard-to-detect cancers, such as those in the ovary and
pancreas. In addition, the NCI specifically listed validating cancer markers for disease prognosis, metastasis, treatment response, and progression as one of its future strategies. The continuing need for enhanced cancer diagnostic markers is partly due to the limitations of current markers. Although there has been significant historical research into cancer diagnostics, we believe that few cancer markers have been accepted into clinical use. Moreover, markers are not used today as the sole method to diagnose cancer due to several factors that limit the capabilities of current cancer
markers to accurately diagnose the disease. These limitations have prevented cancer marker tests from functioning as wholly effective screens for many cancers. We believe that a cancer marker that is expressed on all cancer cells regardless of type would be an effective screening tool.
•
Currently available markers are not 100% specific to a particular type of cancer, indicating that other non-cancerous conditions can also cause an increase in certain cancer markers. For example, elevated levels of the prostate-specific antigen (PSA), a marker for prostate cancer, do not always signal a
malignant condition. The NCI reports that only 25% to 35% of men that express higher-than-normal amounts of PSA in the blood actually have prostate cancer. The remaining 65% to 75% of men have benign prostate conditions, such as inflammation, which also cause an increase in PSA levels. If the minimum PSA value is increased (where men would have to show even higher levels of the marker in order to enable detection by a PSA test), the PSA could be considered to be more accurate, as more men will likely be correctly identified as having prostate cancer and not a benign
condition. However, for many of these men, waiting for their PSA levels to 33
increase to an amount detectable by a more stringent test also prevents early detection of the prostate cancer. If the PSA cut-off value is increased, over 50% of men may not be diagnosed with prostate cancer until after their tumor has spread beyond the prostate gland, significantly decreasing the
likelihood of successful treatment. As a result, there is still an unmet need for a clinically effective diagnostic technique for the early detection of prostate cancer. • Many markers are also restricted to only certain cancers. For example, the PSA test can help detect prostate cancer, but would not be used to screen for breast cancer. • The same marker is not always expressed on every patient’s cancer even if it is related to the same organ. For instance, Genentech’s cancer drug, Herceptin, treats metastatic breast cancer that is positive for human epidermal growth factor receptor 2 (HER2). However, HER2 over-expression occurs in
only approximately 25% of women with breast cancer. • The detection of “normal” levels of a cancer marker can occasionally be ambiguous. For some cancer markers (such as CA-125, which is more prevalent in ovarian cancer cells than in other cells), even individuals without the cancer can demonstrate varying levels of the marker. In some cases, CA-
125 expression depends on age and gender, with women younger than 50 having higher amounts of this protein in their bodies than women over 50 or men. Like other markers, benign conditions, including infections and endometriosis, can also cause elevated CA-125 levels. As a result, the
classification of a normal value is difficult. MedlinePlus, a service of the U.S. National Library of Medicine and the National Institutes of Health (NIH), reports that perceived normal CA-125 levels vary depending on which laboratory is administering the test. Consequently, CA-125 tests are more
effectively used to monitor the progression of ovarian cancer and the patient’s response to treatment, rather than to diagnose the cancer in an otherwise healthy individual. In addition, in the early stages of cancer, many patients express relatively low levels of known cancer markers, evading detection by current cancer marker tests. As a result, even widespread markers—such as carcinoembryonic antigen (CEA), which can be found in patients with a variety of
cancers—are not effective at detecting occult (hidden) cancers. The CEA assay, discovered by Dr. Phil Gold, a member of our board of directors, was one of the first successful blood tests to enter general clinical use. Types of Cancer Testing Cancer testing encompasses a wide variety of products and technologies, including the following: (1) assays for cancer markers; (2) imaging, such as mammography (a breast X-ray to detect tumors); (3) clinical chemistry assays that detect changes in normal physiological parameters; and (4) cytological
and histological tests. Each of these procedures is used for at least one of three tasks—screening, diagnosis/monitoring, or imaging—each of which is briefly described below. Screening. Cancer screening entails performing regular tests on people who have no symptoms. Mammograms, Papanicolaou (Pap) smears, and PSA tests are all examples of cancer screens. These tests can reveal hidden diseases, but need further corroboration, such as a tissue biopsy, to provide a
final diagnosis. Most cancer marker tests do not have high enough measures of sensitivity or specificity to be considered useful as a cancer screen. Even the PSA test, which is routinely used to screen men for prostate cancer, is still debated as to its usefulness in older males. Diagnosis/Monitoring. Cancer markers are primarily used for diagnostic and monitoring purposes. While typically markers alone are not used to diagnose a disease, they do help determine if cancer is likely. They also help monitor the cancer’s progression, response to treatment, and potential for
recurrence. To test for a marker, a sample of the patient’s tissue, blood or other body fluid is sent to a laboratory where the detection of the marker is determined. Imaging. In healthcare, imaging is the process by which physicians obtain pictures of the body’s interior. Oncologists use imaging as a noninvasive method to help see tumors and detect occult metastatic cancer. Special dyes are often administered to enable organs to show up better on film. We
believe that there are two primary unmet needs in imaging at present: (1) the existence of a 34
marker test that can detect cancerous cells before the disease clinically manifests itself; and (2) the presence of a marker to identify secondary cancer after the primary treatment has begun. Cancer testing is dominated by serum-based cancer markers, including CEA, PSA, CA-125, bladder tumor antigen (BTA), and TruQuant BR (for monitoring breast cancer). In 2003, worldwide sales of these serum assays were approximately $860 million. We estimate that there are over 100 million
serum screening tests performed each year. However, most of the assays are specific to a particular cancer and suffer from poor sensitivity and specificity. As an example, assay sales for CEA, a relatively insensitive assay for colorectal cancer, are estimated to be over $300 million annually. In The
Nation’s Investment in Cancer Research: A Plan and Budget Proposal for Fiscal Year 2008, the NCI emphasized the need for improved markers for prostate cancer as well as the development of more markers for hard-to-detect cancers. In addition, the NCI specifically listed validating cancer markers for
disease prognosis, metastasis, treatment response, and progression as one of its future strategies. Reasons for Growth of Cancer Diagnostics The following factors may affect the size and growth of the worldwide cancer diagnostic market: Demographic shifts due to an aging population. The United Nations has documented a rapidly aging population worldwide. In developed countries, the number of individuals over 60 years old exceeded the number of children under 15 years old for the first time in 1998. While risk factors for cancer
include tobacco and alcohol use, diet, and sun exposure, one of the most significant factors is age. For example, more than 65% of all prostate cancers occur in men over the age of 65, and overall, approximately 77% of all cancers are diagnosed in individuals over the age of 55. Increased focus on early detection and diagnostics. According to the NCI, 85% of cancer patients are treated in community-based, private practice oncology settings. Accordingly, global expansion of cancer marker technologies may be fueled by an increased marketing of new diagnostic tests to
physicians. In addition, as a growing number of people are considered to be at high risk for developing cancer, diagnostic tests may also be administered more frequently. Reimbursement, third-party payers and financing for companies developing diagnostics. In the United States, the costs of a variety of medical procedures, including diagnostic laboratory tests, are covered by both federal and private insurance plans. We believe the reimbursement policies of healthcare
providers will drive increased usage of cancer marker tests and that reimbursement amounts will reflect the usefulness of the tests—the more accurate the test, the higher the reimbursement amount. On that basis, a RECAF-based test, which has broad applicability and is highly accurate, should command a
relatively high reimbursement amount. Due to cost containment practices of managed care organizations as well as federal healthcare programs, certain testing technologies may be used more selectively by medical providers. We estimate that reducing healthcare expenses could lead to the reduction or the
elimination of cancer markers with low associated sensitivities and specificities. We want to market RECAF as a high value-added test with widespread utility and significant predictive value that will meet applicable cost containment guidelines. Funding for basic and disease-related research. The NIH invests over $28 billion annually in medical research, of which an estimated $5.5 billion was spent on cancer research in particular during fiscal year 2008, which ended September 30, 2008. Additionally, R&D spending is increasing, with the top
100 biotechnology companies having spent approximately $14.8 billion on R&D during 2006, up from $12.6 billion in 2005 and $11.2 billion in 2004. An increased focus on lowering healthcare spending via improved diagnostic testing and patient monitoring that can reduce the costs of misdiagnosis. In 2006, U.S. healthcare expenditures totaled approximately $2.2 trillion, and are forecasted to reach $2.5 trillion in 2009 and $4.4 trillion by 2018. For
2005, healthcare accounted for 16.0% of the gross domestic product in the United States compared to 10.9% in Switzerland, 10.7% in Germany, 9.7% in Canada, and 9.5% in France. U.S. healthcare premiums increased by 8.8% between 2004 and 2005. The largest cause of this increase was a greater
utilization of services, accounting for approximately 43.0% of the rise in premiums. 35
This growing utilization is attributable to new medical treatments, more intensive diagnostic testing (i.e., defensive medicine), an aging population, which requires more medical attention, and progressively unhealthy lifestyles. As a result of rising costs, we believe that there is a demand for more cost-effective approaches to disease management, specifically for cancer, as well as for emphasis on screening and accurate diagnostic testing to facilitate early detection of potentially costly, severe afflictions. Likewise, a poll
conducted by the Harvard School of Public Health in June 2009 found that 54.0% of respondents felt that high costs were one of the most important healthcare issues for the government to address. We also estimate that up to 20.0% of all diagnostic tests may eventually be performed in non-laboratory
settings, such as by patients or non-medical professionals. Our Technology We believe that our RECAF technology offers an improved detection, diagnostic, and monitoring solution for patients with cancer. The RECAF Cancer Marker Based on our research, which has been confirmed by Abbott and jointly presented at an international cancer conference, RECAF appears to be a cancer marker for multiple types of cancer. Every cancerous tissue that we have tested has expressed RECAF. It is expressed on over 90% of cancer
samples that we have studied thus far, including breast, lung, stomach, colon, ovarian and prostate cancer samples. To our knowledge, there is no other cancer marker that has the same universal presence as RECAF. As such, we believe that RECAF could replace many currently available cancer markers
that are targeted to only one type of cancer, as well as offer a useful diagnostic tool for cancers where there is not yet thought to be an effective marker, such a lung and breast cancer. The chart depicted below is based on our analysis of human clinical samples of Serum-RECAF tests from subjects without cancer and from patients with diagnosed cancers or benign conditions related to cancer diagnosis. Each symbol in the chart represents the analysis of a patient’s blood sample
measured by our RECAF test as expressed in RECAF units on the left vertical axis. Symbols above the horizontal lines indicate the presence of cancer at either a 95% and 99% confidence level. Symbols below the vertical lines indicate normal levels of RECAF and therefore indicate that the patient does
not have cancer. Samples from non cancer patients that appear above the horizontal cutoff lines would be false positives. Samples from cancer patients that appear below the horizontal cutoff lines would be false negatives or patients with cancer that are missed by the test. Overall these data indicate a
very low level of false positive results (specificity) and a very high level of reliability of detecting cancer in cancer patients (sensitivity). 36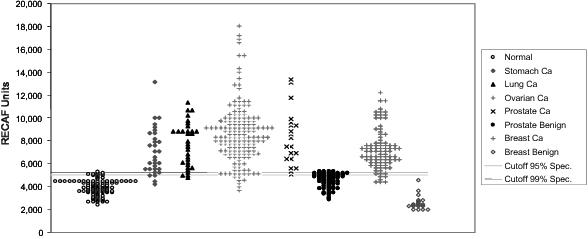
RECAF is a molecule that is present on cancer cells but is not detected in significant levels on healthy cells or benign tumor cells. This characteristic enables RECAF to more accurately detect cancer than many current tumor markers, as RECAF is less likely to report a false positive result. RECAF is a receptor for AFP (Alpha-fetoprotein), which is a marker for liver and testicular cancer that was discovered in 1963 by Dr. Garri Abelev, a member of our scientific advisory board. RECAF is present on the cell surface and binds and takes up circulating AFP. Both AFP and RECAF first
emerge in the fetus, but disappear by birth. AFP binds small molecules, such as lipids, and transports them into fetal cells when taken up by the receptor for AFP. Once a fetal organ or tissue reaches its maturity, it no longer takes up AFP or expresses RECAF. After birth, RECAF is only known to exist
in a cancerous state, where tissues re-express the ability to take up AFP via the RECAF receptor. The expression of RECAF is related to rapid tissue growth, which is characteristic of both cancer and fetal development. RECAF is classified as an oncofetal antigen due to its presence on both fetal tissues
that incorporate AFP and on malignant tissues in later life. We believe that RECAF has potential as a universal cancer marker for the following reasons:
•
Current serum markers are deficient in terms of sensitivity and specificity, creating a need for enhanced markers. • Current markers for breast and lung cancers (one of the most fatal cancers) are not very accurate and therefore not widely used. These types of cancers are among the best detected by RECAF. • Routine RECAF testing after cancer therapy may be able to detect recurrence earlier and more economically than other technologies in current use. We believe that having one cancer marker to monitor all patients is a great advantage for the clinical laboratory. • There is not yet a universal cancer marker. Oncologists use different tests for each cancer. Moreover, we believe only a few of the cancer markers used today are very useful. Our intent is to develop RECAF as a universal cancer marker, potentially capable of detecting many cancers with high
sensitivity and specificity. Product Pipeline All of our product candidates are based on the RECAF technology. The RECAF molecule is expressed on the cell surface of cancer cells and, because tumors are highly vascularized, it is shed into the blood stream and other bodily fluids. As a result, we can detect the marker using blood, or serum,
as the test sample. Since 2004, we have performed over 120,000 tests on more than 4,000 serum samples. Results of these studies have shown that our serum-based assay, Serum-RECAF, has between 80% and 90% sensitivity for a variety of cancers, with 95% specificity for lung, breast, prostate, stomach,
and ovarian cancers among others. Moreover, these tests demonstrate that RECAF technology performs better than competing technologies at detecting prostate cancers and at discriminating between malignant and benign lesions. RECAF technology detected 92% of cervical cancer with 95.7% specificity in a study involving 25 cervical cancer samples and 69 normal samples. In contrast, the Pap test, which is widely used to detect cervical abnormalities, has an estimated sensitivity for high-grade lesions of only 55% to 80%.
Further, we compared 73 colon cancer samples to 352 normal samples and found that our RECAF blood test had a sensitivity of 74% with 95% specificity. When the specificity was improved to 100%, the test was still able to identify over 71% of the colon cancers. Data suggest an average sensitivity for
RECAF of 90% across all cancers when the specificity is 95%. Serum-RECAF can be effectively used to initially screen patients who present symptoms of cancer as well as to monitor patients for recurrence who have already been treated for cancer. We believe that our Serum-RECAF assay performs better than many current technologies at detecting prostate
cancer as well as at discriminating between malignant and benign tumors. Accordingly, Serum-RECAF may have the potential to become a standardized blood test widely available in clinical laboratories due to its detection capabilities and ease of use. If successfully developed and submitted to the FDA
for clearance, we believe that Serum-RECAF will be considered a Class II Medical Device, which is important in the pathway to regulatory approval. Future variations of this 37
product could include the ability to test other body fluids, such as saliva, vaginal fluids, and urine, for RECAF. RECAF Product Formats There are three basic formats for RECAF technology: (i) automated testing in large clinical and hospital laboratories; (ii) non-automated, or manual, testing by clinicians in smaller laboratory settings and where expensive automated instrumentation is not available or not practical; and (iii) point-of-
care (“POC”), rapid test formats for physicians’ offices, urgent care facilities, or the bedside. These formats may be used to detect cancer in patients and for veterinarian use. Automated Format Our initial business strategy was to license the automated testing format on a semi-exclusive basis to three licensees. We have granted two of the three semi-exclusive licenses for this testing format–one to Abbott and one to Inverness. Under the agreements with Abbott and Inverness, we are allowed
to grant one more semi-exclusive license for the automated format. In early 2007, we completed converting our blood based Serum-RECAF test to colorimetric format (“flash chemiluminescense”) to make it more practical for laboratory use required by our licensees and to improve sensitivity. This format improves detection of smaller, earlier stage tumors and
magnifies the measured difference in RECAF serum values between cancer and normal patients. The test results found that RECAF had 80% to 90% sensitivity for a variety of cancers, with 95% specificity for lung, breast, stomach and ovarian cancers in particular. Manual Format We have developed prototype RECAF test kits and materials for small laboratories where automated instrumentation is not available or not practical. These manual formats have the same sensitivity and specificity as the formats that use automated instrumentation. We plan to finish development of
these kits and to place them in a few laboratories in major metropolitan cities in China, India and Mexico where those laboratories can market and run the RECAF tests without further government regulatory requirements. To initiate this, we have formed a wholly owned subsidiary in China. The subsidiary, named “Biocurex China Co., Ltd.” will be used to assemble, market, and distribute our RECAF tests in China. The critical reagents will be shipped from North America for quality control purposes. Our first market
is Shanghai, where we are represented by a clinical oncologist who will collect the samples and administer the tests in-house. Once we are operational in Shanghai, we will expand to other large population centers in China such as Beijing and Tianjin. If the model is successful, we then plan to replicate it
in other countries in Asia, Latin America and Eastern Europe. In the United States, once the FDA has approved the automated testing format, we will be able to apply for 510k approval for the manual testing format on an expedited basis. Point-of-Care, Rapid Test As another segment of our current strategy, we have developed a prototype blood-based POC rapid test for cancer detection or follow-up in physicians’ offices and urgent care facilities. This format may also be useful in third-world countries or areas with large rural populations where access to even
small clinical laboratories is not available. Our POC rapid test device, a rendering of which appears below, will be similar to a common pregnancy test kit. The rapid test cartridge will contain a small strip that is coated with the indicator molecules to detect RECAF in a blood sample. These types of tests are used for a variety of applications
in diagnostic medicine, and they can be efficiently developed from the prototype data that we currently have. We intend to contract with an experienced developer of POC tests to fully develop our POC rapid test. We have selected QuantRx Inc. to run a feasibility study for our POC test on their fully
developed RapidSense POC platform. This platform was selected since it avoids 38
the many patent issues surrounding POC test formats, has already been developed for another POC cancer marker, and has an instrument reader available for it. We anticipate that our POC rapid test will require the development of a small, portable instrument to read the intensity of the colorimetric endpoint line in order to alleviate the variability of eye measurements. We will likely need to eliminate operator variability to be eligible for certain medical
reimbursements. When a patient enters a physician’s office with a specific symptom or concern where cancer is suspected, the physician could administer the rapid test to receive a preliminary indication as to the presence of elevated RECAF levels in the blood. We anticipate that such a cancer test could be used as
easily and as routinely as a blood sugar or cholesterol reading is now used as part of a blood test. The more detailed Serum-RECAF laboratory test would be used to confirm the rapid test result as is common now for most of the rapid tests used in the infectious disease setting. License Agreements We have licensed aspects of our RECAF technology on a semi-exclusive and on a non-exclusive basis to Abbott, a worldwide leader in diagnostics, and Inverness, a global supplier of in vitro diagnostic products. Abbott License In March 2005, we entered into a worldwide, semi-exclusive licensing agreement with Abbott to commercialize Serum-RECAF. Manual and POC RECAF test formats are licensed on a non-exclusive basis. Thus, we may commercialize and license manual tests to as many licensees as we deem
appropriate. Under the license agreement, as amended, Abbott has the right, but not the obligation, to commercialize or perform further research and development on the RECAF technology. Abbott paid us an upfront licensing fee of $200,000 and will pay us royalties on any RECAF products it sells
during the term of the license. In April 2008, Abbott and we amended the license agreement. The amendment relieved Abbott of future obligations to perform further research and development with respect to the RECAF technology as well as the obligation to pay annual minimum royalties. At any
time, at its option, Abbott may resume research and development work and commercialize products incorporating the RECAF technology in accordance with the license agreement. In consideration for this modification, we will receive a more favorable royalty rate on any RECAF products that may be
sold by Abbott. We have the right to terminate the license at any time, if following notice from us, Abbott and we do not agree within 90 days to new due diligence obligations for the commercialization of any products using the RECAF technology. Since this agreement was amended, Abbott has not
conducted any research and development regarding RECAF technology or, to our knowledge, taken any other steps toward commercializing our technology. Finally, Abbott has the right to grant sublicenses to third parties. 39
Inverness License In December 2007, we entered into a second semi-exclusive, worldwide licensing agreement for our Serum-RECAF technology. This agreement allows Inverness to commercialize products using the Serum-RECAF technology in exchange for paying an upfront fee and periodic royalty payments. In
addition, Inverness is responsible for obtaining FDA approvals, and managing manufacturing, marketing, and distribution for clinical laboratory testing. The manual and POC rapid tests, as well as other applications of RECAF, are licensed on a non-exclusive basis. Inverness paid us a $1 million up-front
fee for RECAF technology and material and assistance that would enable it to produce RECAF material on its own. Inverness has been conducting research and development on our technology and may have successfully adapted our technology to their diagnostic platform. The agreement with Inverness
provides for periodic exchanges of information between the parties. Our policy is to tell them as much as we can on the technical side. Inverness, on the other hand, has been reluctant to share with us their intentions or progress on their general business strategy including manufacturing,
commercialization, regulatory approval and marketing. Inverness has advised us of their intention to implement the RECAF test in a particular format (called Triage), which is not based on classic and widely known assay formats but rather on their proprietary platform. We believe their reluctance is
caused in large part on their concern to protect the intellectual property relating to their proprietary diagnostic platform. Our last communication with Inverness took place in June 2009 and, at the time, they indicated that our assay was working in their facilities and generating results consistent with ours.
However, they did not share those results with us or indicate what diagnostic platform they used. Inverness has also informed us that they had become self-sufficient and independent in generating the critical reagents necessary to produce the test. We do not know what Inverness intends to do next. If
they have been successful in adapting our technology to their diagnostic platform, the logical next step for them would be to initiate clinical trials for the purpose of obtaining regulatory approvals, whether in the United States or elsewhere, but we have not had been able to confirm whether that is the
case. Our license agreement with Inverness does not provide for any development or product milestones. Under the license agreement, the annual minimum royalty of $150,000 began to accrue on December 4, 2009 and will continue until December 4 following the first commercial sale by Inverness of a
product using RECAF technology to a third party. Thereafter, for the balance of the annual minimum royalty term, which ends on the later of the expiration of all the RECAF patents or when Inverness ceases to manufacture and distribute any products based on RECAF technology, Inverness is
obligated to pay a higher minimum royalty. The Inverness license agreement does not provide when or how the annual minimum will be paid. Presumably, that will be determined based on subsequent discussions with Inverness. Additional Licensing Opportunities Under the license agreements with Abbott and Inverness we are free to grant one additional semi-exclusive license regarding Serum-RECAF and pursue unlimited licensing opportunities with respect to all other applications of our RECAF technology and test formats, including manual and POC rapid
tests and veterinary applications. Further, our RECAF technology has additional applications that could be licensed, including imaging functions and therapeutic uses. Ultimately, we seek to license out specific aspects of our technology, striving to achieve a significant market share by selecting licensees
that can support this goal. We believe that this licensing strategy will be the most effective way to expand our market share. Business Strategy Our RECAF technology has possibilities in a wide variety of applications in the fields of human and veterinary medicine. Our strategy is to continue to focus on obtaining non-exclusive licensing agreements for various application of RECAF technology while developing other applications ourselves.
Specifically, with the net proceeds of this offering we intend to pursue the following:
•
grant one additional semi-exclusive license for testing blood samples using automated testing equipment;
40
• commercialize veterinary applications of RECAF testing technology not requiring regulatory approvals; • finish developing a POC rapid format test for the doctor’s office and bedside use; • conduct clinical trials and seek FDA approval for marketing of the POC rapid format test; and • commercialize manual testing formats, principally in large cities in foreign countries where further regulatory clearance is not required. Licensing To date our primary business strategy has been to license our Serum-RECAF technology under semi-exclusive limited license agreements. With this strategy, instead of having to allocate all of our funding in an attempt to commercialize one product, we select licensees that have strategic advantages
over us when it comes to commercialization (e.g., our licenses with Abbott and Inverness). As part of this strategy, we provide all the assistance that we can to our licensees; however, the licensees are responsible for obtaining regulatory approvals and bringing the products to market. Under our existing
semi-exclusive licenses with Abbott and Inverness, we are allowed to enter into one additional semi-exclusive license for Serum-RECAF. These licenses only cover the automated testing format for Serum-RECAF in a clinical and hospital laboratory settings. They cover the use of Serum-RECAF in
connection with other test formats and other applications of our technology on a non-exclusive basis. Market distribution channels for a diagnostic test kit typically entail accessing the automated diagnostic platforms of one or more of the larger diagnostic companies, such as Abbott, F. Hoffmann-La Roche or Bayer AG. These companies provide automated diagnostic instruments that are capable of
processing a variety of laboratory tests. Some instruments can process 1,200 clinical chemistry and 200 immunoassay tests each hour. Through licensing, we seek to place our cancer assays, such as Serum-RECAF, on the instrument menu of these diagnostic platforms. Point-of Care Rapid Tests We anticipate that a POC rapid cancer test could be used in the future as easily and as routinely as a blood sugar or cholesterol reading is now part of a blood test. When a patient enters a physician’s office with a specific symptom or concern where cancer is suspected, the physician could administer
the rapid test to receive a preliminary indication as to the presence of elevated RECAF levels in the blood. The more detailed Serum-RECAF laboratory test would be used to confirm the rapid test result as is common now for most of the rapid tests used in the infectious disease setting. We recently presented preliminary results with our prototype rapid test to an international cancer congress. Data indicated solid discrimination between cancer and healthy cells and correlated with results from our Serum-RECAF. With the N.N. Blokhin Cancer Research Center in Moscow, Russia, we
studied RECAF as a rapid test for cancer detection. Results found that RECAF could detect 80.4% of ovarian cancers in Stages I to III with an 88% specificity. This study tested 64 normal, non-cancerous samples and 51 ovarian cancer serum samples, which included 25 Stage I or II cancers and 26 Stage
III cancers. We believe that these results signify a potential breakthrough that could simplify cancer detection. When applied to early stage ovarian cancer, our prototype POC demonstrated better performance than a CA-125 blood test, a tumor marker often found in higher-than-normal amounts in the
blood of women with ovarian cancer. We believe that the POC tests will not cannibalize the clinical laboratory markets since POC tests are routinely confirmed by the slightly more accurate clinical laboratory tests. We believe that the widespread use of POC RECAF tests will actually promote the use of the clinical laboratory RECAF
tests. We estimate that there are approximately 250,000 physicians in the United States who would use these POC tests. One test per doctor per week would yield 13 million rapid tests per year. We 41
expect final development, clinical testing, FDA registration and Medicare approval to take approximately 18 months. We may license this test for distribution, contract with a distribution network or use a contract sales force for marketing and sales of this test. Veterinary Applications Basic research shows that RECAF is a highly conserved (common and essentially identical) molecule in humans and animals. We confirmed in our laboratory with samples provided from three different sources that our RECAF test detects malignancy in dogs and cats. The test, which we call Pet-
RECAF, correctly detected 85% of the cancers at the standard specificity value of 95. These figures are consistent with those obtained on human patients. Initially our focus will be directed to dogs and cats. We believe we can begin marketing this application quickly because it does not require any laboratory testing of blood samples for governmental or regulatory approvals and we have completed the developmental testing. We will market this
application under a separate brand name. We plan to pursue a dual-channel revenue generation strategy. In some markets we will license our technology to clinical labs who will conduct the testing and in other markets PBRC will do the testing in our own contract laboratory. Our POC rapid test is also
being developed for the veterinary market and may be available for commercialization before it is available for human use. We will market our product directly to end users, such as veterinarians and animal protection societies, and through distributors. Cancer in Household Pets Cancer is the number one cause of death among dogs and cats in the United States, Europe, and Japan. Recent studies have shown that more than 50% of all dogs ultimately die of cancer, and some breeds, like golden retrievers and boxers, have cancer rates that are even higher. However, cancer is
also the most curable of all chronic diseases in pets. To help improve detection, specialists encourage veterinarians to include cancer screenings in their wellness exams for pets of all ages. Expenditures on Household Pets and Market Size There are approximately 75 million household dogs in the United States and on average dog owners spend $219 on veterinary visits annually. There are approximately 88 million household cats in the United States and their owners spend an average of $175 a year on routine veterinary visits. Dog-
owning households that spent $1,000 or more in a year jumped from 2.2 percent in 1996 to 8.4 percent in 2006. Dogs averaged 1.5 visits to the veterinarian during 2006, and cats averaged 0.7 visits to the vet in the same year. We have studied the market in British Columbia and, based on our findings, we believe that the potential market for testing dogs and cats in British Columbia may be $5.0 million per year. We found that in British Columbia at least 120,000 routine blood-screening tests are carried out every year in
dogs and cats, at a cost of $35-$40 per test to the veterinarian. This does not include tests conducted by veterinary hospitals that have their own in-house laboratories. We believe that the addition of a screening test for cancer for $50 is reasonable to both owners and veterinarians and may be
incorporated in routine annual checkups. In addition to screening, animals already diagnosed and treated for cancer can be monitored for the disease with the Pet-RECAF test. We estimate that each animal diagnosed with cancer could be tested 3-4 times over its lifespan. Market Strategy Our marketing strategy for our Pet-RECAF test is based on the following assumptions:
There is little or no need for regulatory approval related to our initial plans and, therefore, we can begin marketing our Pet-RECAF product immediately. • The costs involved to commercialize Pet-RECAF are manageable. We plan to begin in British Columbia where we can commercialize the application ourselves and then expand into other markets as we establish ourselves. In essence, the local market becomes 42
•
a testing ground to trim and assess the logistics related to this enterprise. Blotted blood samples collected by veterinarians will be shipped to PBRC for testing. Our marketing efforts will target both veterinarians, who have to recommend Pet-RECAF to the pet owners, and the pet owners themselves. For
marketing purposes, we have reserved the Internet domain OncoPet.net. Sales and Marketing We do not plan to build our own sales force for any of our RECAF formats for human use. Sales and marketing for our automated laboratory testing format will be done primarily by our licensees. Manual laboratory test kits and materials will be marketed by our partner laboratories. Once we have
achieved FDA clearance in the United States for our POC rapid test, we plan to contract with medical device distributors and/or a contract sales force for marketing and sales. Our RECAF tests for the veterinarian market will be marketed initially by us and by distributors of veterinarian products. We have so far received inquires from approximately 20 veterinary distributors. When our POC rapid test is approved for animal use on a commercial basis, we will either license
it for distribution or use a contract sales force for distribution. Suppliers and Manufacturing/Production For the Serum-RECAF products licensed on a semi-exclusive basis, our licensees are responsible for manufacturing. We plan to contract with OEMs for all of the products that are not covered by our license agreements. Research and Development Our research and development efforts are all related to improving our RECAF technology for detection, diagnosis and follow-up of cancer. We continually focus on improving our various RECAF test formats leading to filing of additional patents to protect our technology. Since the basic research on
our RECAF cancer marker is complete, most of our continuing work will be in the development area rather than in research. The clinical data from our studies and the validation from independent data from our licensees, Abbott and Inverness, support our contention that we are in the final development
stages rather than at the research stage. Patents Our patents, currently registered in over 20 countries, cover over 40 claims and relate to methods for diagnosis and treatment of cancer using the RECAF cancer marker. Our U.S. patent expires in 2014 and our patents in Australia, Russia and China expire in 2015. Our U.S. patent (“Detection of
cancer using antibodies to the AFP receptor”) includes 17 claims and protects technologies using Serum-RECAF kits. The patent also entails in vitro applications for diagnosis, screening, and follow-up of cancer and leukemia. At present, we are working toward the submission of additional patent
applications related to RECAF that potentially could provide us with protection for an additional 20 years. In March 2008, the European Patent Office granted our patent claims for cancer diagnostic serum tests based on the RECAF marker. These patents will also expire in 2015. This development is particularly beneficial as granted patent claims can generate a higher royalty than pending claims per our
existing license agreements. In addition, we believe that the European healthcare and medical insurance systems are more familiar and supportive of cancer markers than are other locales. As a result, we anticipate that regulatory approval for diagnostic tests in Europe could be easier and faster than in
the United States. Due to the complexity of RECAF technology, we believe that our proprietary know-how for developing the technology and working with the RECAF family of molecules is critical and extends beyond patented information. Accordingly, we include know-how in our licensing packages in order to
obtain royalties in countries where we do not have patent protection. 43
Competition Given the nature of our product and the fact that it works well in combination with existing cancer markers, it is difficult to separate competitors from potential partners/clients/licensees. We have found that we can combine RECAF with a second marker (e.g., CEA for colorectal cancer samples, PSA for prostate cancer samples and CA125 for ovarian samples), thus increasing the overall performance. For example, combining CEA with RECAF results in 91% sensitivity and 100%
specificity, which is extremely important for screening purposes. From a marketing point of view, the possibility of combining existing and widely used tests with ours offers obvious advantages in terms of acceptance, market penetration time and pricing. The latter is of particular interest for licensees who
are already commercializing other markers because the enhanced performance allows them to increase the price of the other marker, which is usually low due to competition and lack of patent protection. Under our existing semi-exclusive license agreements with Abbott and Inverness, we receive, as a
royalty, a portion of the additional price on any other marker sold in conjunction with RECAF. Our potential competitors include large pharmaceutical and medical device companies who develop, market, and sell diagnostic products such as cancer detection kits, instruments and reagents used in clinical laboratories to measure serum cancer markers. Such companies include F. Hoffman-La
Roche Ltd., Dako A/S, DIANON Systems (an affiliate of Lab Corp. of America Holdings), Miraculins Inc. and Ortho-Clinical Diagnostics, Inc. (an affiliate of Johnson & Johnson Co.). In addition, potential competition may come from smaller companies, research facilities and government-funded
organizations that seek to discover improved cancer markers or that are developing new screening and diagnostic tests and tools for patients and animals. To our knowledge, no existing cancer markers can detect the range of cancers that can be detected by RECAF with similar sensitivity and specificity.
Potential competitors in the veterinary market include Idexx and Abaxis but they are also potential licensees. At this point in time, we believe that our competitive position in the cancer detection market is strong for a number of reasons including the following:
•
Inherent Advantages of the RECAF marker. As previously discussed, the RECAF marker has several advantages over all other known cancer markers including its ability (i) to detect all of the major cancers and likely the less ubiquitous ones as well, (ii) to detect them in early stages, where 80-90%
can be cured and (iii) to function as a diagnostic and follow-up tool. In addition, and based upon studies we have conducted, we believe that for certain types of cancer, its serum-based screening assays is more accurate than the screening assays of our competitors. • Strategic relationships. Our license agreements with Abbott and Inverness provide us with access to major testing laboratories. In addition, Abbot and Inverness have agreed to bear the cost of obtaining FDA approval for our serum-detection technology and, once obtained, will market our testing
technology to laboratories, healthcare providers and consumers. At the same time, our license agreements with Abbot and Inverness give us the flexibility to exploit other applications of the technology. • Funding. While many of our existing and potential competitors are large pharmaceutical companies with large research and development budgets and government-funded research facilities, the large capital investment required to identify and prove the efficacy of a cancer marker may act as a
deterrent. On the other hand, most of the research into verifying the RECAF marker has been completed. Government Regulation Drugs, pharmaceutical products, medical devices and other related products are regulated in the United States under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act, and the laws of certain states. The FDA exercises significant regulatory control over the clinical
investigation, manufacture and marketing of pharmaceutical and biological products. 44
Medical device regulation is based on classification of the device into three classes, I, II or III. Class III medical devices are regulated much like drugs, whereas Class I and II devices have less stringent data requirements than drugs and do not require the rigorous clinical trials that the FDA requires
for drugs. Products submitted to the FDA for clearance as medical devices can refer to the safety and effectiveness data of medical devices which perform similar functions as other products and which the FDA has already cleared. As long as a medical device submitted to the FDA has the same clinical
use as a medical device previously cleared by the FDA, the medical device submitted will normally receive FDA clearance provided data proving substantial equivalence to the other approved medical devices and verification of claims is provided to the FDA. This type of FDA submission is referred to as
a 510k submission and is routinely handled by the FDA within a 90-day timeframe. We expect that all of our RECAF diagnostic products will be classified as Class II medical devices. Under our existing license agreements, the licensees are responsible for obtaining the necessary regulatory approvals. However, we cannot assure you that any of our licensees will be successful in obtaining additional clearances or approvals from any regulatory authority with respect to our cancer
detection kits or its serum screening assay. The lack of regulatory approval for our products will prevent the sale of these products. Delays in obtaining regulatory approval or the failure to obtain regulatory approval in one or more countries will have a material adverse impact on our operations. Employees As of December 31, 2009, we had three employees, all of whom are in administrative positions. All of our research and development and other technical activities and administrative services are performed for us by PBRC, which is owned by Dr. Moro-Vidal, our founder and chief executive officer
and a member of our board of directors. All of our employees are also employees of PBRC. As of December 31, 2009, PBRC had seven full-time employees/consultants. Our relationship with PBRC employees and with our employees is good. Offices Our executive offices are located at 7080 River Road, Suite 215 Richmond, British Columbia, and consist of 5,000 square feet of space. We rent on a month-to-month basis for $4,895 per month from PBRC. Legal Proceedings We are not a party to any legal proceedings. 45
The following table contains information with respect to our directors and executive officers. To the best of our knowledge, none of our directors or executive officers has an arrangement or understanding with any other person pursuant to which he or she was selected as a director or officer. There
are no family relationships between any of our directors or executive officers. Directors serve for one-year terms and are elected annually by our stockholders. Our executive officers are appointed by and serve at the pleasure of the board of directors.
Name
Age
Position
Ricardo Moro-Vidal, M.D.
57
President, Chief Executive Officer and Director
Gladys Chan
36
Chief Financial Officer
Antonia Bold-de-Haughton
59
Secretary and Director
Denis Burger, Ph.D.
66
Executive Chairman and Director
Phil Gold, M.D.
70
Director
Jim Walsh, Ph.D.
51
Director Ricardo Moro-Vidal, M.D. has been an officer and director since March 2001. Since 1996, Dr. Moro-Vidal has been the president of PBRC, formerly named Curex Technologies Inc., where he developed the RECAF cancer marker concept. From 1980 to 1985, Dr. Moro-Vidal worked in cancer
research at the French National Cancer Institute near Paris, France. From late 1985 to 1988, he worked at the University of Alberta, Edmonton on onco-developmental biology. From 1989 to 1996, he was engaged in various entrepreneurial ventures relating to diagnostics and instrumentation. Dr. Moro-
Vidal received his medical degree from the University of the Republic of Uruguay in 1979. Denis R. Burger, Ph.D. was appointed a director and our executive chairman in September 2009. Prior to joining us, he had been managing director of Sovereign Ventures, LLC, a biotech investing and consulting firm, since 1991. He was chairman and chief executive officer of AVI BioPharma, Inc.,
a drug development company using gene targeted therapeutics, from 1997 to 2007 and founding chairman of Epitope, Inc., a developer of diagnostic products, from 1981 to 1990. He is currently a director of Trinity Biotech PLC, a diagnostic products developer, and Lorus Therapeutics, a cancer
therapeutics company. Earlier in his career, he was a Professor of Microbiology and Immunology, an Associate Professor of Surgery and the Director of the Histocompatibility Testing Laboratory at Oregon Health Sciences University. He holds a B.A. degree in Bacteriology and Immunology from the
University of California at Berkeley, a M.S. and Ph.D. in Microbiology and Immunology from the University of Arizona, Tucson. Gladys Chan joined us in July 2005 as comptroller and was promoted to chief financial officer in October 2009. Prior to joining us, from September 2004 to June 2005, Ms. Chan served as senior accountant at DTI Dental Technologies Inc. She is a Certified General Accountant in Canada, qualified
in August 2004, and holds a Bachelor degree in Art from the University of Tunghai, Taiwan. Antonia Bold-De-Haughton has served as our corporate administrator since our inception. In October 2009, she was appointed to the Board and corporate secretary. From March 2006 to February 2008, she was also the chief financial officer of Douglas Lake Minerals Inc. (OTCBB: DLKM). Ms.
Haughton has over 20 years of experience in administration and management, is a commercial arbitrator and was educated at the University of Oxford, England and the University of British Columbia. Phil Gold, C.C., O.Q., M.D., Ph.D. has been a director since March 2001. He has been employed by McGill University and/or its affiliate, Montreal General Hospital, in one or more capacities since 1968. Currently, he is the Douglas G. Cameron Professor of Medicine, and Professor of Physiology
and Oncology, at McGill University and the Executive Director of the Clinical Research Centre of the McGill University Health Centre. In the past he has served as Chairman of the Department of Medicine at McGill and Physician-in-Chief at the Montreal General Hospital. From 1978 to 1980, Dr.
Gold was Director of the McGill Cancer Centre in Montreal, Quebec. From 1980 to 1984, he was Physician-in-Chief of the Montreal General Hospital. From 1985 to 1990, he served as Chairman of the Department of Medicine at McGill University in Montreal. Dr. Gold’s 46
early research led to the discovery and definition of the Carcinoembryonic Antigen (CEA), the blood test most frequently used in the diagnosis and management of patients with cancer. He has been elected to numerous prestigious organizations and has been the recipient of such outstanding awards as
the Gairdner Foundation Annual International Award, the Isaak Walton Killam Award in Medicine of the Canada Council, the National Cancer Institute of Canada R.M. Taylor Medal, the Heath Medal of the MD Anderson Hospital, the Inaugural Ernest C. Manning Foundation Award, the Johann-
Georg-Zimmerman Prize for Cancer Research, Medizinische Hochschule, Germany, the ISOBM Abbott Award (Japan), the Award of the Academy of International Dental Studies, and the Queen Elizabeth II Jubilee Medal. He has been elected to membership in the Royal Society of Canada, the
American Society for Clinical Investigation, the Association of American Physicians, and Mastership in the American College of Physicians. His contributions to teaching have been recognized by an award as a Teacher of Distinction from his Faculty of Medicine. He has been honored by his country, his
province his city, and his university by appointment as a Companion of the Order of Canada, an Officer of l’Ordre National du Québec, a member of the Academy of Great Montrealers; and a the recipient of the Gold Medal of the McGill University Graduate Society, respectively. He has been the Sir
Arthur Sims Traveling Professor to the British Commonwealth, and has served as a member of the Executive, and Chair of the Burroughs Wellcome Fund. In 2006, the Phil Gold Chair in Medicine was inaugurated at McGill University and the first incumbent was selected in 2009. Dr. Gold received a B.
Sc. in 1957 and a M.Sc. in 1961 in Physiology from McGill University. He received his MDCM in 1961 and his Ph.D. in 1965 from McGill University as well. Jim Walsh, Ph.D. was appointed a director in September 2009. Dr. Walsh has been the chief executive officer of Biosensia Ltd., a point of care diagnostics company, since 2008 and Interim Chief Executive Officer of Stokes Bio Ltd., a company specializing in the area of molecular diagnostics, since
2006. Dr. Walsh has also been a non-executive director of Trinity Biotech Plc (NASDAQ: TRIB), an Irish diagnostics company, since 1996 and a non-executive director of PuriCore Plc. (LSE: PURI), a U.S.-based healthcare company, since 2006. Dr. Walsh has also been investment advisor to Bank of
Ireland Kernel Capital Partners since 2007. From 1990 to 1995, Dr. Walsh was managing director of Cambridge Diagnostics Ltd., a wholly owned subsidiary of Inverness Medical Innovations Inc. (AMEX: IMA). From 1988 to 1990, Dr. Walsh worked with Fleming GmbH as R&D Manager. Dr. Walsh is a
graduate of the National University of Ireland and holds a Doctorate in Inorganic Chemistry and Post Doctorate qualifications in Immunochemistry. Family Relationships None of our directors or executive officers is related by blood, marriage or adoption. Board Composition Under our bylaws, as amended, the number of directors at any one time may not be less than one or more than nine. The maximum number of directors at any one time may be increased by a vote of a majority of the directors then serving. Currently, the board of directors consists of five members –
Dr. Ricardo Moro-Vidal, Dr. Denis Burger, Dr. Phil Gold, Dr. Jim Walsh and Antonia Bold-de-Haughton. None of Dr. Moro-Vidal, Dr. Burger or Ms. Bold-de-Haughton is “independent” within the meaning of that term as defined by the rules and regulations of the SEC and the various stock exchanges.
On the other hand, Dr. Gold and Dr. Walsh qualify as independent directors. Since we are not listed on a national securities exchange, we are not required to maintain a board of directors, a majority of the members of which are independent. Executive Compensation The table below sets forth the compensation earned by (i) our chief executive officer and (ii) each other executive officer who received total compensation in excess of $100,000 for the fiscal years ended December 31, 2008 and 2007. We refer to these individuals collectively as our named executive
officers. 47
SUMMARY COMPENSATION TABLE Name and Principal Position
Year
Salary
Option Awards
Total Ricardo Moro-Vidal, M.D.
2008
—
(2)
255,000
(3)
255,000 Chief executive officer
2007
—
(2)
260,000
(3)
260,000 Gerald Wittenberg, M.D.
2008
—
255,000
(5)
255,000 Principal financial officer,
2007
—
260,000
(5)
260,000 secretary and treasurer (4)
(1)
Calculated in accordance with SFAS No. 123(R). (2) Dr. Moro-Vidal does not receive any cash compensation from us directly. We pay PBRC, a company owned by Dr. Moro-Vidal, to conduct all research on our behalf, and Dr. Moro-Vidal receives a salary from PBRC. In 2008 and 2007, Dr. Moro-Vidal received salaries of $120,000 and $135,000,
respectively, from PBRC. (3) At year end options were fully vested and exercisable at $0.001 per share. Options were exercised on August 27, 2009. (4) Dr. Wittenberg resigned as an executive officer and director on February 17, 2009. (5) Options were fully vested and exercisable at $0.001 per share. Options were exercised on February 24, 2009. Outstanding Equity Awards at December 31, 2008 The following table includes certain information with respect to the value of all outstanding equity awards to our named executive officers at December 31, 2008. OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
Name
OPTIONS AWARDS
Number of
Equity
Option
Option Ricardo Moro-Vidal, M.D.
260,000
(3)
0.001
01/31/2010
230,000
(3)
0.001
02/28/2010
255,000
(3)
0.001
02/28/2010
1,578,947
(3)
0.001
03/19/2011
225,000
(3)
0.001
01/31/2012
450,000
(3)
0.001
03/31/2012
650,000
(3)
0.001
03/31/2014 Gerald Wittenberg, M.D.
260,000
(4)
0.001
01/31/2010
230,000
(4)
0.001
02/28/2010
255,000
(4)
0.001
02/28/2010
1,275,000
(1)
0.08
01/15/2010
252,278
(1)
0.05
12/31/2011
(1)
Warrants not granted pursuant to our Non-Qualified Stock Option Plan. (2) Granted pursuant to our Non-Qualified Stock Option Plan. (3) Options exercised August 27, 2009. (4) Options exercised February 24, 2009. 48
($)
($)(1)
($)
Securities
Underlying
Unexercised
Options
Exercisable
(#)(1)
Incentive
Plan Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
(#)(2)
Exercise
Price
($)
Expiration
Date
Employment Arrangements with Named Executive Officers During the fiscal years ended December 31, 2008 and 2007, we compensated our two named executive officers, Dr. Moro-Vidal and Dr. Wittenberg, with option grants. The number of options granted each year was determined by dividing $225,000 by the average closing price of our common stock
for the two weeks prior to the date options were awarded, which was in March of each year. The exercise price of the options was set at $0.001 per share. As of September 15, 2009, we entered into an employment agreement with Denis R. Burger, Ph.D., our executive chairman. The employment agreement expires on December 31, 2013. If we do not renew the employment agreement, we must pay Dr. Burger twelve months severance pay. Under the
employment agreement, Dr. Burger is responsible for performing such duties as assigned to him from time to time by our board of directors. Dr. Burger is also required to devote his best efforts to our service throughout the term of the agreement, including devoting at least 40 hours per month to our
affairs. In return for his services, Dr. Burger will receive an initial annual base compensation of $100,000 and reimbursement for all expenses reasonably incurred by him in discharging his duties and is entitled to participate in any applicable benefit plans. Dr. Burger may also receive a bonus at the
discretion of the board of directors. Our employment agreement with Dr. Burger may be terminated voluntarily by Dr. Burger upon sixty days written notice. We may terminate the employment agreement upon thirty days written notice, in which event we must pay Dr. Burger eighteen months severance
pay. As of October 1, 2009, we entered into an employment agreement with Dr. Ricardo Moro-Vidal, our chief executive officer. The employment agreement expires on December 31, 2013. If we do not renew the employment agreement, we must pay Dr. Moro-Vidal twelve months severance pay. Under
the employment agreement, Dr. Moro-Vidal is responsible for performing such duties as assigned to him from time to time by our board of directors. Dr. Moro-Vidal is also required to devote his best efforts to our service throughout the term of the agreement, on a full-time basis except to the extent his
services are required by PBRC. In return for his services, Dr. Moro-Vidal will receive an initial annual base compensation of $250,000 and reimbursement for all expenses reasonably incurred by him in discharging his duties and is entitled to participate in any applicable benefit plans. We will receive a
credit against Dr. Moro-Vidal’s annual base compensation for any “profit” paid to PBRC under our services agreement with PBRC. Dr. Moro-Vidal may also receive a bonus at the discretion of the board of directors. Our employment agreement with Dr. Moro-Vidal may be terminated voluntarily by
him upon sixty days written notice. We may terminate the employment agreement upon thirty days written notice, in which event we must pay Dr. Moro-Vidal eighteen months severance pay. Equity Compensation Plan Information We adopted a Non-Qualified Stock Option Plan and a Stock Bonus Plan. A summary description of each plan follows. Non-Qualified Stock Option Plan The Non-Qualified Stock Option Plan authorizes the issuance of shares of our common stock to persons who exercise options or warrants granted pursuant to the Option Plan. Our employees, directors, officers, consultants and advisors are eligible to be granted options or warrants pursuant to the
option plan, provided however, that bona fide services must be rendered by such consultants or advisors and such services must not be in connection with the offer or sale of securities in a capital-raising transaction. The exercise price of the option or warrant is determined by our board of directors. At the discretion of the board of directors, any option granted pursuant to the option plan may include installment exercise terms such that the option becomes fully exercisable in a series of cumulating portions. The board of directors may also accelerate the date upon which any option is first
exercisable. Options are generally non-transferable except upon the death of the option holder. 49
Stock Bonus Plan Under the Stock Bonus Plan, our employees, directors, officers, consultants and advisors are eligible to receive a grant of shares of our common stock, provided however, that bona fide services must be rendered by consultants or advisors and such services must not be in connection with the offer or
sale of securities in a capital-raising transaction. Shares issued pursuant to the bonus plan will generally not be transferable until the person receiving the shares satisfies the vesting requirements imposed by the board of directors when the shares were issued. Other Information Regarding the Option Plan and Bonus Plan The option plan and bonus plan (collectively, the Plans) are administered by our board of directors. Our board of directors is vested with the authority to interpret the provisions of the Plans and supervise the administration of the Plans. In addition, the board of directors is empowered to: (i) select
those persons to whom shares or options are to be granted; (ii) determine the number of shares subject to each grant of a stock bonus or an option; and (iii) determine when, and upon what conditions, shares or options granted under the Plans will vest or otherwise be subject to forfeiture or cancellation. Any shares issued pursuant to the bonus plan and any options granted pursuant to the option plan will be forfeited if the “vesting” schedule established by the board of directors at the time of the grant is not met. For this purpose, vesting means the period during which the employee must remain
our employee or the period of time a non-employee must provide services to us. At the discretion of the board of directors, payment for the shares of common stock underlying options may be paid through the delivery of shares of our common stock having an aggregate fair market value equal to the
option price, provided such shares have been owned by the option holder for at least one year prior to such exercise. A combination of cash and shares of common stock may also be permitted at the discretion of the board of directors. Our board of directors may at any time, and from time to time, amend, terminate, or suspend one or both Plans in any manner they deem appropriate, provided that such amendment, termination or suspension will not adversely affect rights or obligations with respect to shares or options previously
granted. The board of directors may not make any amendment which would materially modify the eligibility requirements for the Plans or materially increase in any other way the benefits accruing to employees who are eligible to participate in the Plans, without stockholder approval. The following table provides information as of December 31, 2008 with respect to the Plans: Plan category
Number of securities
Weighted-average
Number of securities Equity compensation plans approved by security holders
—
—
— Equity compensation plans not approved by security holders: Non-Qualified Stock Option Plan
3,890,000
$
0.001
2,587,723 Stock Bonus Plan
n/a
n/a
1,796,411 Total
3,890,000
$
0.001
4,384,134 Director Compensation The table below sets forth the compensation earned by our non-employee director for the fiscal year ended December 31, 2008. Each year, we grant to our non-employee directors five-year options to 50
to be issued upon
exercise of
outstanding options,
warrants and rights
(a)
exercise price of
outstanding options,
warrants and rights
(b)
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected
in column (a))
(c)
purchase 60,000 shares of our common stock at $0.001 per share. Each year, we grant to our employee directors five-year options to purchase 650,000 shares of our common stock at $0.001 per share. Name
Fees Earned
Stock Awards
Option
All other
Total Phil Gold, M.D.
—
—
60,000
(2)
—
60,000
(1)
Calculated in accordance with SFAS No. 123(R). (2) Options vested immediately on the date of grant and are exercisable to purchase 60,000 shares of our common stock at $0.001 per share. At December 31, 2008, all of Mr. Gold’s options remained outstanding. 2009 Management Options On the closing date of this offering, we will grant options to our directors and senior executives. The material terms of the options will include the following:
•
The options will constitute non-qualified options for income tax purposes. • The options will not be transferable. • One-third of the options will vest 90 days after the grant date, one-third will vest on the first anniversary of the grant date and the balance will vest on the second anniversary of the grant date. • The options will expire and no longer be exercisable after the tenth anniversary of the grant date. • The exercise price of the options will be equal to the greater of (i) the closing market price of a share of our common stock on the date of grant or (ii) the unit offering price divided by the number of shares in a unit. • The options will be exercisable for cash or, at our discretion, through the delivery of shares of our common stock having a fair market value equal to the aggregate exercise price of the options being exercised. • If, before the second anniversary date of the grant date, any option holder’s employment terminates for any reason other than death or disability or an option holder ceases to be a director for any reason other than death or disability, any unvested options will be immediately forfeited. Any vested
options will continue to be exercisable until the termination date. In the event of death or disability, the vesting of any unvested options will accelerate. As currently contemplated, these options will be granted as follows:
Grantee
Options Ricardo Moro-Vidal
15,000,000 Denis Burger
10,000,000 Phil Gold
1,000,000 Jim Walsh
1,000,000 Antonia Bold-de-Haughton
1,000,000 Gladys Chan
500,000 51
or Paid in
Cash
($)
($)(1)
Awards
($)(1)
Compensation
($)
($)
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS PBRC conducts all research and development relating to our RECAF technology and also provides us with a variety of administrative services. Dr. Ricardo Moro-Vidal, our chief executive officer, owns 100% of PBRC. PBRC conducts research for us in accordance with research protocols and expenditures
approved on a monthly basis by Dr. Moro-Vidal and, until February 2009, Dr. Gerald Wittenberg, who was an officer and director until then. During the years ended December 31, 2006, 2007 and 2008, PBRC billed us $749,406, $871,329 and $916,909, respectively, for research and development and
administrative services. The amounts billed to us represent the costs incurred by PBRC plus 15%. PBRC pays Dr. Moro-Vidal a salary from the amounts it collects from us. For the years ended December 31, 2006, 2007 and 2008, PBRC paid Dr. Moro-Vidal salaries of $103,000, $135,000 and $120,000,
respectively. As of September 30, 2009, we owed PBRC $390,000. We have entered into an agreement with PBRC, dated as of October 1, 2009, the material terms of which are as follows:
•
The balance owed to PBRC at September 30, 2009, approximately $390,000, plus all accrued and unpaid interest, will be due and payable on December 31, 2014, unless earlier terminated by us without cause or by PBRC as a result of our breach of our monthly payment obligation, in which
instances all amounts due PBRC will become immediately due and payable. • The amount due will accrue interest at a rate equal to the prime rate. Interest will be payable monthly. • We will pay PBRC monthly for its services in an amount that is equal to all costs incurred by PBRC in connection with services it provides to us (the “Costs”) plus a 15% cost adjustment. Such Costs will not include any salary paid by PBRC to Dr. Moro-Vidal. • To the extent the cost adjustment in any month exceeds $20,834, such excess will reduce the amount owed by us to PBRC. • PBRC will not be allowed to provide services to any person or entity other than us unless its average monthly Costs for any three consecutive months are less than its total expense for salaries and consulting fees for that three-month period. However, we will be allowed to use other laboratories
together with or in lieu of PBRC. In addition, we will have the right to terminate the agreement with PBRC at any time upon 90 days prior written notice. • PBRC has assigned to us all of its right, title and interest in and to all intellectual property developed or to be developed, including, but not limited to, know-how, processes, data and research results and all tangible property relating to RECAF. • The initial term of the agreement expires December 31, 2013 and we have the right to extend the agreement for two additional four-year terms. • If we terminate the agreement for any reason other than on account of a default by PBRC, then (i) we must pay PBRC a cancellation payment in an amount equal to 15% of the costs incurred by PBRC for the six months preceding such termination, (ii) we must give PBRC a perpetual non-
exclusive license to our RECAF technology and (iii) PBRC may thereafter perform services for any person or entity. Our Articles of Incorporation provide that any contract or other transaction between us and one or more of our directors, or between us and any firm of which one or more of our directors are members or employees, or in which they are interested, or between us and any corporation or association
of which one or more of our directors are stockholders, members, directors, officers or employees, or in which they are interested, shall be valid for all purposes, notwithstanding the presence of the director or directors at the meeting of our board of directors that acts upon, or in reference to, the
contract or transaction, and notwithstanding his or their participation in the action, if the facts of such interest shall be disclosed or known to the board of directors and the board of directors shall, nevertheless, authorize or ratify the contract or transaction, the interested director or directors to be
counted in determining whether a quorum is present and to be entitled to vote on such authorization of ratification. 52
The following table provides information as of December 31, 2009 regarding beneficial ownership of our common stock by: (i) each person known to us who beneficially owns more than five percent of our common stock; (ii) each of our directors; (iii) each of our named executive officers; and (iv) all
of our directors and executive officers as a group. Name of
Number of Shares
Percent of Class(2)
Before
After Executive Officers: Ricardo Moro-Vidal, M.D.
3,648,947
(3)(4)
4.8
%
2.3
% Denis Burger, Ph.D.
857,286
(4)
1.2
%
* Gladys Chan
161,400
(4)(5)
*
* Antonia Bold-de-Haughton
220,800
(4)(5)
*
* Non-Employee Directors: Jim Walsh, Ph.D.
714,286
*
* Phil Gold, M.D.
884,210
(3)(4)
1.2
%
* All directors and executive officers as a group (6 persons)
6,486,929
(6)
8.3
%
4.0
%
*
Less than 1% (1) According to the rules and regulations of the SEC, shares that a person has a right to acquire within 60 days of the date of this prospectus are deemed to be beneficially owned by such person and are deemed to be outstanding only for the purpose of computing the percentage ownership of that
person. Except as otherwise indicated, and subject to applicable community property and similar laws, each of the persons named has sole voting and investment power with respect to the shares shown as beneficially owned.
(2) Based on 73,062,205 shares issued and outstanding immediately before this offering and 157,062,205 shares issued and outstanding immediately after the offering. (3) Reflects
shares underlying options currently exercisable that have an exercise price
of $0.001 per share. (4) Does not include shares underlying options that we intend to grant on the closing date of this offering, none of which will be exercisable until 90 days after this offering. The options will have an exercise price equal to the greater of (i) the offering price of the units divided by the number of shares
included in a unit or (ii) the closing market price of a share of our common stock on the date of the grant. (5) Reflects shares underlying options, having an exercise price of $0.001 per share, which are first exercisable March 1, 2010. (6) Includes 4,915,357 shares of common stock underlying options currently exercisable that have an exercise price of $0.001 per share. Does not include an aggregate 28,500,000 shares of common stock underlying options described in note 4 above.
53
Beneficial Owner
Beneficially
Owned(1)
Offering
Offering
(4)
The following description of our securities and the relevant provisions of our Articles of Incorporation and bylaws are summaries and are qualified by reference to these documents, which are attached as exhibits to this registration statement. Units The units will not trade separately, they will not be listed on any exchange or quoted on any market, and no certificates will be issued evidencing the units. The shares of common stock and the redeemable warrants comprising the units will be issued and quoted separately. Common Stock Our authorized capital stock consists of 450,000,000 shares of common stock, par value $0.001 per share. Our common stock is quoted on the Bulletin Board under the trading symbol BOCX. As of December 31, 2009, 73,062,205 shares of our common stock were outstanding, and were held of record by approximately 138 holders. Holders of common stock are entitled to one vote per share on matters
submitted to a vote of stockholders. The vote of the holders of a majority of the shares entitled to vote at any meeting of stockholders at which a quorum is present shall be the act of that stockholders’ meeting, unless the vote of a greater number is required by law. Holders of common stock do not
have cumulative voting rights, preemptive rights or conversion rights. Holders of common stock are entitled to receive dividends as may be declared by our board of directors at any regular or special meeting and may be paid in cash, property, or in shares of common stock. Such declaration and payment
is at the discretion of the board of directors. Upon our liquidation, dissolution or winding up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities. Redeemable Warrants General. The redeemable warrants issued in this offering may be exercised after they are issued until the expiration date of [ ] (five years from the effective date of the registration statement of which this prospectus is a part). Each redeemable warrant entitles the holder to purchase one share
of common stock at an exercise price of $[ ] per share (150% of the unit offering price divided by the number of shares included in a unit). This exercise price will be adjusted if specific events, summarized below, occur. The redeemable warrants will be callable by us on thirty days’ written notice
for $0.003 per warrant at any time our common stock closes, as reported on the Bulletin Board, at 200% of the unit offering price divided by the number of shares included in a unit for five consecutive trading days. A holder of a redeemable warrant will not be deemed a holder of the underlying stock
for any purpose until the redeemable warrant is exercised. Exercise. The holders of the redeemable warrants may exercise them only if an appropriate registration statement is then in effect. To exercise a redeemable warrant, the holder must deliver to our warrant agent the warrant certificate on or before the expiration date, with the form on the reverse
side of the certificate executed as indicated, accompanied by payment of the full exercise price for the number of redeemable warrants being exercised. Fractional shares of common stock will not be issued upon exercise of the redeemable warrants. Adjustments in Certain Events. We will make adjustments to the terms of the redeemable warrants if certain events occur. If we distribute to our stockholders additional shares of common stock through a dividend or distribution, or if we effect a stock split of our common stock, we will
proportionately increase the total number of shares of common stock purchasable on exercise of a redeemable warrant so that the holder of a redeemable warrant thereafter exercised will be entitled to receive the number of shares of common stock the holder would have owned or received after such
event if the redeemable warrant holder had exercised the redeemable warrant before the event causing the adjustment. The aggregate exercise price of the redeemable warrant will remain the same in that circumstance, but the effective purchase price per share of common stock purchasable 54
upon exercise of the redeemable warrant will be proportionately reduced because a greater number of shares of common stock will then be purchasable upon exercise of the adjusted redeemable warrant. If, however, we effect a reverse stock split or take other corporate action that decreases our
outstanding common stock, we will instead proportionately decrease the total number of shares of common stock purchasable on exercise of a public warrant so that the holder of a public warrant thereafter exercised will be entitled to receive the number of shares of common stock the holder would have
owned or received after such event if the public warrant holder had exercised the public warrant before the event causing the adjustment. The aggregate exercise price of the public warrant will remain the same in that circumstance, but the effective purchase price per share of common stock purchasable
upon exercise of the public warrant will be proportionately increased because a lesser number of shares of common stock will then be purchasable upon exercise of the adjusted public warrant. In the event of a capital reorganization or reclassification of our common stock, the redeemable warrants will be adjusted so that thereafter each warrant holder will be entitled to receive upon exercise the same number and kind of securities that such holder would have received if the redeemable
warrant had been exercised before the capital reorganization or reclassification of our common stock. If we merge or consolidate with another corporation, we will make provisions so that warrant holders will be entitled to receive upon exercise of a redeemable warrant the kind and number of securities, cash or other property that would have been received as a result of the transaction by a person
who was our stockholder immediately before the transaction and who owned the same number of shares of common stock for which the redeemable warrant was exercisable immediately before the transaction. No adjustment to the redeemable warrants will be made, however, if a merger or consolidation
does not result in any reclassification or change in our outstanding common stock. Representative’s Warrants In connection with this offering, we are issuing a warrant to Paulson Investment Company, the representative of the underwriters, to purchase up to 120,000 units at an exercise price of $[ ] per unit, subject to certain adjustments. Each unit consists of 70 shares of common stock and 70
redeemable warrants. The representative’s warrant may be exercised at any time during the four-year period beginning one year after the effective date of the registration statement of which this prospectus is a part. Other Warrants On June 29, 2007, we sold secured convertible notes, plus warrants, to two private investors for $3,000,000. The warrants allow the two investors to purchase up to 3,500,000 shares of our common stock at a price of $0.25 per share, subject to certain adjustments. The warrants are currently exercisable
and expire in June 2012. On December 31, 2002, we issued a warrant to purchase up to 252,278 shares of our common stock at a price of $0.05 per share, subject to certain adjustments, as consideration for a $283,061 loan. The warrant is currently exercisable and expires in December 2011. On January 15, 2003, we issued a
warrant to purchase up to 1,275,000 shares of our common stock at a price of $0.08 per share, subject to certain adjustments, as consideration for a $120,000 loan. The warrant is currently exercisable and expires in January 2010. Authorized but Unissued Shares The authorized but unissued shares of common stock are available for future issuance without stockholder approval. These additional shares may be used for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans.
The existence of authorized but unissued shares could hinder or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise. 55
Anti-Takeover Provisions The following items enhance the likelihood of continuity and stability in the composition of the board of directors and in the policies formulated by the board of directors and discourage certain types of transactions that may involve an actual or threatened change of control of us. These items also
reduce our vulnerability to an unsolicited proposal for a takeover of us that does not contemplate the acquisition of all of our outstanding shares or an unsolicited proposal for the restructuring of sale of all or part of us. These items, however, could discourage potential acquisition proposals and could
delay or prevent a change of control of us. They may also have the effect of preventing changes in our management. Special Meetings of Stockholders Our bylaws provide that special meetings of the stockholders, for any purpose or purposes, unless otherwise prescribed by statute or our bylaws, may be called by the chairman of the board, president, the board of directors, or the holders of not less than 25% of all of our outstanding shares entitled
to vote at the meeting. Board of Directors Our bylaws provide that the entire board of directors or any director may be removed from office, either with or without cause, at any special meeting of stockholders by the affirmative vote of a majority in number of shares of the stockholders present in person or by proxy at such meeting and
entitled to vote for the election of such director or directors if notice of intention to act upon the question of removing such director shall have been stated as one of the purposes for the calling of such meeting and such meeting shall have been called in accordance with our bylaws. Further, any vacancy occurring in the board of directors may be filled by election at an annual or special meeting of stockholders called for that purpose or may be filled by the affirmative vote of a majority of the remaining directors, though less than a quorum of the board of directors. Any
directorship to be filled by reason of an increase in the number of directors may be filled be election at an annual or special meeting of stockholders called for that purpose of may be filled by the board of directors for a term of office continuing only until the next election of one or more directors by
the stockholders; provided that the board of directors may not fill more than two such directorships during the period between any two successive annual meetings of stockholders. Amending Bylaws Our articles of incorporation provide that except to the extent such power may be modified or divested by action of the stockholders representing a majority of the issued and outstanding shares of our common stock taken at a regular or special meeting of the stockholders, the power to adopt, alter,
amend or repeal our bylaws shall be vested in the board of directors. Our bylaws provide that our bylaws may be altered, amended, or repealed either by unanimous written consent of the board of directors, or at any meeting of the board of directors at which a quorum is present, by the affirmative vote of a majority of the directors present at such meeting, provided
note of the proposed alteration, amendment, or repeal is contained in the notice of such meeting. Amending Articles of Incorporation The Texas Business Corporation Act provides generally that the affirmative vote of two-thirds of the shares entitled to vote on any matter is required to amend a corporation’s articles of incorporation, unless the corporation’s articles of incorporation requires a lower percentage, but not less than a
majority. Our Articles of Incorporation require only a majority vote to amend our articles of incorporation. 56
Texas Anti-Takeover Statute We are subject to the provisions of the Texas Business Combination Law (Articles 13.01 through 13.08 of the Texas Business Corporation Act), which provide that a Texas corporation may not engage in certain business combinations, including mergers, consolidations and asset sales, with a person, or
an affiliate or associate of such person, who is an “affiliated stockholder” (generally defined as the holder of 20% or more of the corporation’s voting shares) for a period of three years from the date such person became an affiliated stockholder unless: (1) the business combination or purchase or
acquisition of shares made by the Affiliated Stockholder was approved by the board of directors of the corporation before the affiliated stockholder became an affiliated stockholder or (2) the business combination was approved by the affirmative vote of the holders of at least two-thirds of the
outstanding voting shares of the corporation not beneficially owned by the affiliated stockholder, at a meeting of stockholders called for that purpose (and not by written consent), not less than six months after the affiliated stockholder became an affiliated stockholder. State and British Columbia Securities Laws The securities issued in this offering will only be offered and sold in states where the securities have been registered or qualified in accordance with applicable state law or where an exemption from the registration or qualification requirements is available. In addition, because we are headquartered
and conduct operations in British Columbia, we are required to comply with the securities laws of the Province of British Columbia, Canada. Transfer Agent and Registrar The transfer agent and registrar for our common stock is Securities Transfer Corporation, 2591 Dallas Parkway, Suite 102, Frisco, Texas 75034. 57
SHARES ELIGIBLE FOR FUTURE SALE Our authorized capital consists of 450,000,000 shares of common stock. After this offering, we will have 157,062,205 shares of common stock issued and outstanding (169,662,205 if the over-allotment option is exercised in full) and approximately 171,300,000 shares of common stock reserved for future
issuance (183,900,000 if the over-allotment option is exercised in full). If the holders of our amended secured convertible notes accept the prepayment out of the net proceeds of this offering, the number of shares reserved for future issuance will be reduced by 10,500,000 shares. Of the shares of common
stock to be outstanding immediately after this offering, approximately 128,000,000 will be freely tradable without restriction under the Securities Act, unless they are held by “affiliates,” as that term is defined in Rule 144 of the Securities Act (“Rule 144”). The 84,000,000 shares of common stock
underlying the redeemable warrants issued in this offering will also be freely tradable after exercise of the redeemable warrants, except for shares held by our affiliates. Our officers and directors, owning in the aggregate 1,571,572 of the issued and outstanding shares of common stock as well as options
and warrants to purchase an additional 4,915,357 shares of our common stock (not taking into account the 28,500,000 options we intend to grant on the closing date of this offering), have executed lock-up agreements with Paulson Investment Company, the representative of the underwriters, providing that
they will not offer, sell, contract to sell or otherwise dispose of any shares of our common stock or any securities that are convertible into shares of our common stock for a period of 90 days after the date of the completion of this offering without the prior written consent of Paulson. Rule 144 Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144. Rule 144, as currently in effect, provides that non-affiliates that have held restricted securities of a reporting company for at least six months and have not had
an affiliate relationship with us during the preceding three months may sell their securities without restriction or limitation, other than that Rule 144’s public information requirements must be satisfied during the six months following satisfaction of the six-month holding period requirement. Affiliates who
have held restricted securities for at least six months may sell such restricted securities in accordance with the conditions of Rule 144, including the public information requirement, the volume limitations, manner of sale provisions and notice requirements. In particular, an affiliate who has beneficially
owned shares of our common stock for at least six months would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
•
1% of the number of shares of our common stock then outstanding, which will equal approximately [ ] shares immediately after this offering; or • the average weekly trading volume of our common stock on the Bulletin Board during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. Options, Warrants and Restricted Stock Upon completion of this offering, subject to the lock-up agreements with the representative of the underwriters, all of the restricted shares will be eligible for sale under Rule 144 assuming the holders of any such shares comply or have complied with the requirements of Rule 144, including the
holding period requirement. In addition, we have reserved approximately 196,500,000 shares of our common stock for issuance upon the exercise of outstanding warrants and options as follows:
•
approximately 17,000,000 shares issuable upon exercise of warrants outstanding at September 30, 2009 having a weighted average exercise price of $0.14 per share; • 28,500,000 shares issuable upon exercise of options that we intend to grant to our senior executives and directors upon completion of this offering, which will have an exercise price equal to the result obtained by dividing the unit public offering price by the number of shares of common stock
included in a unit;
• 1,606,275 shares reserved or future issuance under our Stock Bonus Plan;
58
• 9,857,723 shares reserved for future issuance under our Non-Qualified Option Plan, of which 5,987,057 shares are issuable upon exercise of options outstanding at December 31, 2009 having an exercise price of $0.001 per share; • approximately 13,500,000 shares issuable upon conversion in full of our amended secured convertible notes in the aggregate outstanding principal amount of $1.75 million as of December 31, 2009, assuming a conversion price of $0.14 per share, which number of shares does not include shares issuable
if the amount converted includes interest and which number of shares will be reduced to approximately 3.0 million shares after this offering if the holders of the amended secured convertible notes accept a prepayment out of the net proceeds of this offering reducing the balance due on those notes
to $390,000;
• 84,000,000 shares issuable upon exercise of the redeemable warrants included in the units sold in this offering; • 25,200,000 shares reserved for issuance in connection with the exercise of the underwriters’ over-allotment option (including the shares underlying the redeemable warrants underlying the units covered by that option); and • 16,800,000 shares issuable upon exercise of the representative’s warrant (including the shares issuable upon exercise of the redeemable warrants included in the units underlying the representative’s warrant. Of the foregoing shares, approximately 137,500,000 either will be covered by the registration statement of which this prospectus is a part or are covered by another effective registration statement and, thus, upon issuance, will be freely tradable subject only to the resale restrictions set forth in Rule
144, promulgated under the Securities Act, applicable to affiliates, and subject to the further caveat that shares cannot be issued unless the applicable registration statement is effective at the time of issuance. In addition, the shares issuable upon conversion of the principal amount of the amended secured
convertible notes will be freely tradeable under Rule 144. Finally, we intend to file a registration statement on Form S-8 as soon as practicable after completion of this offering covering the 28,500,000 shares that will be reserved for issuance upon exercise of the options we intend to grant to our senior
executives and directors upon completion of this offering. 59
Paulson Investment Company, Inc. is acting as the representative of the several underwriters of this offering. We have entered into an underwriting agreement with Paulson, as representative of the several underwriters, with respect to the units being offered. In connection with this offering and
subject to certain terms and conditions, each of the underwriters named below has severally agreed to purchase, and we have agreed to sell, the number of units set forth opposite the name of each underwriter. Underwriters
Number of Units Paulson Investment Company
[ ] [ ]
[ ] Total
1,200,000 The underwriting agreement provides that the underwriters are obligated to purchase all of the units offered by this prospectus other than those covered by the over-allotment option, if any units are purchased. The underwriters are offering the units when, as and if issued to and accepted by them,
subject to a number of conditions, including:
•
receipt by the underwriters of an auditor’s letter and officer’s certificate; • no stop order suspending the effectiveness of the registration statement in effect and no proceedings for such purpose instituted or threatened; • approval of legal matters by counsel for the underwriters, including the validity of the units; and • the underwriters’ right to reject orders in whole or in part. Paulson has advised us that the underwriters propose to offer the units to the public at the offering price set forth on the cover page of this prospectus and to selected dealers at that price less a concession of not more than $[ ] per unit. The underwriters and selected dealers may re-allow a
concession to other dealers, including the underwriters, of not more than $[ ] per unit. After completion of the public offering of the units, the offering price, the concessions to selected dealers and the reallowance to their dealers may be changed by the underwriters. The underwriters have informed us that they do not expect to confirm sales of the units offered by this prospectus on a discretionary basis. We have been advised by Paulson that the underwriters intend to make a market in our securities but that they are not obligated to do so and may discontinue making a market at any time without notice. In connection with the offering, the underwriters or certain of the securities dealers, may distribute prospectuses electronically. Over-allotment Option Pursuant to the underwriting agreement, we have granted the underwriters an option, exercisable for 45 days from the effective date of the registration statement of which this prospectus is a part, to purchase up to an additional 180,000 units on the same terms as the other units being purchased by
the underwriters from us. The underwriters may exercise the option solely to cover over-allotments, if any, in the sale of the units that the underwriters have agreed to purchase. If the over-allotment option is exercised in full, the total public offering price, underwriting discount and proceeds to us before
offering expenses will be $[ ], $[ ] and $[ ], respectively. Stabilization The rules of the SEC generally prohibit the underwriters from trading in our securities on the open market during this offering. However, the underwriters are allowed to engage in some open market transactions and other activities during this offering that may cause the market price of our
securities to be above or below that which would otherwise prevail in the open market. These 60
activities may include stabilization, short sales and over-allotments, syndicate covering transactions and penalty bids:
•
Stabilizing transactions consist of bids or purchases made by the representative for the purpose of preventing or slowing a decline in the market price of our securities while this offering is in progress. • Short sales and over-allotments occur when the representative, on behalf of the underwriting syndicate, sells more of our shares than it purchases from us in this offering. To cover the resulting short position, the representative may exercise the over-allotment option described above or may engage
in syndicate covering transactions. There is no contractual limit on the size of any syndicate covering transaction. The underwriters will make available a prospectus in connection with any such short sales. Purchasers of shares sold short by the underwriters are entitled to the same remedies under
the federal securities laws as any other purchaser of shares covered by the registration statement. • Syndicate covering transactions are bids for or purchases of our securities on the open market by the managing underwriter on behalf of the underwriters in order to reduce a short position incurred by the representative. • Penalty bids permit the managing underwriter to reclaim a selling concession from a syndicate member when the shares originally sold by the syndicate member are purchased in a syndicate covering transaction to cover syndicate short positions. If the underwriters commence these activities, they may discontinue them at any time without notice. The underwriters may carry out these transactions on the Bulletin Board or otherwise. Indemnification The underwriting agreement provides for indemnification between us and the underwriters against specified liabilities, including liabilities under the Securities Act, and for contribution by us and the underwriters to payments that may be required to be made with respect to those liabilities. We have
been advised that, in the opinion of the SEC, indemnification for liabilities under the Securities Act is against public policy as expressed in the Securities Act and is therefore unenforceable. Underwriters’ Compensation We have agreed to sell the units to the underwriters at the offering price of $[ ] per unit, which represents the public offering price of the units set forth on the cover page of this prospectus less the 10% underwriting discount. The underwriting agreement also provides that Paulson, as
representative, will be paid a non-accountable expense allowance equal to 3% of the gross proceeds from the sale of the units offered by this prospectus, excluding any units purchased on exercise of the over-allotment option. We are not required to pay, or reimburse the underwriters for, the legal fees
incurred by the underwriters in connection with this offering. The underwriting agreement also grants the underwriter, for a period of 36 months from the effective date of the registration statement of which this prospectus is a part, the right of first refusal to act as lead underwriter for any and all of our
future public and private equity and debt offerings with gross proceeds of up to $20 million or less, including the offerings by any successor to or subsidiary of ours, excluding ordinary course of business financings such as bank lines of credit, accounts receivable and factoring. On completion of this offering, we will issue to Paulson warrants to purchase up to 120,000 units at a price of per share equal to 120% of the offering price of the units. These warrants will be exercisable at any time during the four-year period beginning one year after the effective date of the
registration statement of which this prospectus is part. However, in compliance with the lock-up restrictions set forth in FINRA Rule 5110(g)(1), neither these warrants nor the underlying securities may be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale,
derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of one year immediately following the date of effectiveness or commencement of sales of the offering, except to any member participating in the offering and the 61
officers or partners thereof, and only if all securities so transferred remain subject to the one year lock-up restriction for the remainder of the lock-up period. The holder of these warrants will have, in that capacity, no voting, dividend or other stockholder rights. Any profit realized on the sale of the securities issuable upon exercise of these warrants may be deemed to be additional underwriting compensation. These warrants and the underlying securities
are being registered pursuant to the registration statement of which this prospectus is a part. During the term of these warrants, the holder thereof is given the opportunity to profit from a rise in the market price of our common stock. We may find it more difficult to raise additional equity capital while
these warrants are outstanding. At any time at which these warrants are likely to be exercised, we may be able to obtain additional equity capital on more favorable terms. The following table summarizes the underwriting discount we will pay to the underwriters and the non-accountable expense allowance we will pay to the representative of the underwriters. These amounts are shown assuming both no exercise and full exercise of the underwriters’ over-allotment
option.
Per Share
Total
Without
With Underwriting discount
$
$
$
Non-accountable expense allowance
$
$
$ Warrant Solicitation Fee We have engaged Paulson, on a non-exclusive basis, as our agent for the solicitation of the exercise of the redeemable warrants. To the extent not inconsistent with the guidelines of the Financial Industry Regulatory Authority and the rules and regulations of the SEC, we have agreed to pay the
underwriter for bona fide services rendered a commission equal to 5% of the exercise price for each warrant exercised more than one year after the date of the registration statement of which this prospectus is a part if the exercise was solicited by Paulson. No compensation will be paid to the
underwriter upon the exercise of the warrants if:
•
the market price of the underlying shares of common stock is lower than the exercise price; • the holder of the warrants has not confirmed in writing that the underwriter solicited his, her or its exercise; • the warrants are held in a discretionary account, unless prior specific written approval for the exercise is received from the holder; • the warrants are exercised in an unsolicited transaction; or • the arrangement to pay the commission is not disclosed in the prospectus provided to warrant holders at the time of exercise. Lock-Up Agreements Our officers and directors have agreed that for a period of 90 days from the date this registration statement becomes effective they will not sell, contract to sell, grant any option for the sale or otherwise dispose of any of our equity securities, or any securities convertible into or exercisable or
exchangeable for our equity securities, other than through existing Rule 10b5-1 trading plans, intra-family transfers or transfers to trusts for estate planning purposes, without the consent of Paulson, which consent will not be unreasonably withheld. Paulson may consent to an early release from the 90-day
lock-up period if, in its opinion, the market for the common stock would not be adversely affected by sales and in cases of an officer’s or director’s financial emergency. We are unaware that any of our officers or directors intends to ask for consent to dispose of any of our equity securities during the
lock-up period. 62
Over-Allotment
Over-Allotment
Determination of Offering Price The public offering price of the units offered by this prospectus and the exercise price of the redeemable warrants have been determined by negotiation between us and the underwriters. Among the factors considered in determining the public offering price of the units and the exercise price of the
redeemable warrants were:
•
the current price of our common stock at the time of pricing; • our history and our prospects; • the industry in which we operate; • the status and development prospects for our products and proposed products; • the previous experience of our executive officers; and • the general condition of the securities markets at the time of this offering. The offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the shares included in the units. That price is subject to change as a result of market conditions and other factors, and we cannot assure you that the units, or the common stock
and redeemable warrants contained in the units, can be resold at or above the public offering price. The validity of the securities offered in this prospectus is being passed upon for us by Morse, Zelnick, Rose & Lander, LLP, New York, New York. The partners of Morse, Zelnick own an aggregate of 2,357,143 million shares. In addition, upon completion of this offering we will issue an additional
300,000 shares to the partners of Morse, Zelnick, Rose & Lander, LLP for legal services in connection with this offering. Certain legal matters will be passed upon for the underwriter by Holland & Knight LLP, Portland, Oregon. Our consolidated balance sheets as of December 31, 2008 and 2007 and the related consolidated statements of operations, cash flows and stockholders’ equity (deficit) for the year then ended and accumulated for the period from January 1, 2001 to December 31, 2008, have been audited by Manning
Elliott LLP Chartered Accountants, an independent registered public accounting firm, as set forth in their report appearing herein and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing. WHERE YOU CAN FIND ADDITIONAL INFORMATION In connection with the securities offered by this prospectus, we have filed a registration statement on Form S-1 under the Securities Act with the SEC. This prospectus, filed as part of the registration statement, does not contain all of the information included in the registration statement and the
accompanying exhibits. For further information with respect to our securities and us, you should refer to the registration statement and the accompanying exhibits. Statements contained in this prospectus regarding the contents of any contract or any other document are not necessarily complete, and you
should refer to the copy of the contract or other document filed as an exhibit to the registration statement, each statement being qualified in all respects by the actual contents of the contract or other document referred to. You may inspect a copy of the registration statement and the accompanying
exhibits without charge at the SEC’s public reference facilities, 100 F Street, N.E., Washington, D.C. 20549, and you may obtain copies of all or any part of the registration statement from those offices for a fee. You may obtain information on the operation of the Public Reference Room by calling the
SEC at 1-800-SEC-0330. The SEC maintains a website that contains registration statements, reports, proxy and information statements and other information regarding registrants that file electronically. The address of the website is www.sec.gov. 63
We are subject to the information requirements of the Exchange Act that require us to file reports, proxy statements and other information with the SEC. Such reports, proxy statements and other information may be inspected at the public reference facilities of the SEC at the address set forth
above, and copies of such material may be obtained from the Public Reference Section of the SEC at prescribed rates. Because we file documents electronically with the SEC, you may also obtain this information by visiting the SEC’s website at www.sec.gov. Texas law generally permits a corporation to provide indemnification if the individual: (i) acted in good faith; and (ii) reasonably believed that, in the case of conduct in an official capacity, such conduct was in the corporation’s best interests and, in all other cases, that such conduct was at least not
opposed to the corporation’s best interests. In a criminal proceeding, the person must have had no reasonable cause to believe the person’s conduct was unlawful. Under our articles of incorporation and bylaws, in the event a director, officer or employee is made a party or threatened to be made a party
by reason of service as a director, officer or employee of ours (or by reason of service as a director, officer, employee or agent of another entity at our request), then we shall indemnify that individual against judgments, penalties, fines, settlements and reasonable expenses (including attorneys’ fees)
actually incurred by the person in connection with the defense of the action if the person acted in good faith and, in the case of conduct in the person’s official capacity, in a manner the person reasonably believed to be in our best interests or, in all other cases, in a manner that was at least not opposed
to our best interests. Notwithstanding the foregoing, no indemnification shall be made if the person is adjudged to be liable to us, or if the person is adjudged liable on the basis that the person derived an improper personal benefit. DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION OF Insofar as indemnification for liabilities arising under the Securities Act may be permitted for directors, officers or control persons pursuant to applicable state law, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act
and is therefore unenforceable. 64
SECURITIES ACT LIABILITIES
INDEX TO FINANCIAL STATEMENTS BIOCUREX, INC.
Page Unaudited Consolidated Financial Statements: Consolidated Balance Sheets at September 30, 2009 and December 31, 2008
F-2
F-3
F-4
F-5 Audited Financial Statements:
F-26
F-27
F-28
F-29
F-30
F-38 F-1
BIOCUREX, INC.
September 30,
December 31,
(unaudited) ASSETS Current Assets Cash
364,020
45,625 Investment securities (Note 3)
—
18,014 Prepaid expenses and other
30,000
137,672 Notes receivable, net (Note 4)
—
2,666 Total Current Assets
394,020
203,977 Deferred financing costs (Note 6, 8 (b) and 14 (b))
275,475
321,651 Patents (Note 5)
480,045
446,946 Total Assets
1,149,540
972,574 LIABILITIES AND STOCKHOLDERS’ DEFICIT Current Liabilities Accounts payable
359,419
174,400 Accrued liabilities
366,572
377,627 Loans payable (Note 6 (a))
233,872
— Due to related parties (Note 7)
396,384
335,269 Convertible notes payable (Note 8 (a))
33,885
194,828 Current portion of convertible debt (Note 8 (b))
—
688,754
1,390,132
1,770,878 Loans payable (Note 6 (b))
62,772
— Convertible debt (Note 8 (b))
1,527,780
1,136,604
2,980,684
2,907,482 Commitments and Contingencies (Notes 1, and 13) Stockholders’ Deficit Common stock Authorized: 125,000,000 shares, par value $0.001 Issued and outstanding: 72,141,921 and 43,713,399 respectively
72,142
43,713 Additional paid-in capital
16,975,387
15,178,205 Common stock subscribed(Notes 6 (b), 9 (x) (aa))
122,500
40,050 Accumulated other comprehensive loss
—
(15,529
) Accumulated deficit
(114,175
)
(114,175
) Deficit accumulated during the development stage
(18,886,998
)
(17,067,172
) Stockholders’ Deficit
(1,831,144
)
(1,934,908
) Total Liabilities and Stockholders’ Deficit
1,149,540
972,574 The accompanying notes are an integral part of these consolidated financial statements F-2
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
CONSOLIDATED BALANCE SHEETS
(Expressed in U.S. dollars)
2009
$
2008
$
BIOCUREX, INC.
Three Months Ended
Nine Months Ended
Accumulated
2009
2008
2009
2008 Revenue
—
—
—
1,000,000
1,464,456 Operating Expenses Amortization
10,641
10,294
30,661
26,898
204,677 General and administrative (Note 6(a))
140,371
253,394
607,360
770,927
5,823,067 Professional and consulting fees
176,554
74,910
325,990
335,457
5,123,445 Research and development (Note 6(a))
199,586
151,066
432,814
513,959
4,172,651 Total Operating Expenses
527,152
489,664
1,396,825
1,647,241
15,323,840 Loss From Operations
(527,152
)
(489,664
)
(1,396,825
)
(647,241
)
(13,859,384
) Other Income (Expense) Accretion of discounts on debt
(63,691
)
(193,300
)
(193,333
)
(910,272
)
(3,134,489
) Amortization of debt issue costs
(13,473
)
(53,608
)
(335,123
)
(160,825
)
(656,773
) Gain (loss) sale of equity investment securities
—
(163
)
(20,935
)
6,253
147,990 Interest expense
(112,345
)
(235,534
)
(826,423
)
(533,405
)
(1,700,634
) Interest income
—
—
—
8,164
383,679 Gain (loss) on issuance of shares
13,916
(18,240
)
(16,725
)
(46,615
)
(96,393
) Gain on extinguishments of convertible debt
969,538
—
969,538
—
96,626 Loss on impairment interest of patent cost
—
—
—
—
(67,620
) Total Other Income (Expense)
793,945
(500,845
)
(423,001
)
(1,636,700
)
(5,027,614
) Net Income (Loss) for the Period
266,793
(990,509
)
(1,819,826
)
(2,283,941
)
(18,886,998
) Other Comprehensive Income (Loss) Unrealized gain (loss) on investment securities
—
(20,155
)
15,529
28,819
— Total Comprehensive Income (Loss)
266,793
(1,010,664
)
(1,804,297
)
(2,255,122
)
(18,886,998
) Net Loss Per Share—Basic and Diluted
0.00
(0.02
)
(0.04
)
(0.05
) Weighted Average Shares Outstanding
58,174,000
43,207,000
50,697,000
42,812,500 The accompanying notes are an integral part of these consolidated financial statements F-3
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(Expressed in U.S. dollars)
(unaudited)
September 30,
September 30,
During the
Development
Stage
January 1, 2001
to September 30,
2009
$
$
$
$
$
BIOCUREX, INC.
Nine Months Ended
Accumulated During
2009
2008 Operating Activities: Net loss for the period
(1,819,826
)
(2,283,941
)
(18,886,998
) Adjustments to reconcile net loss to net cash used in operating activities: Accretion of discounts on debt
193,333
910,272
3,134,489 Allowance for uncollectible notes receivable
—
—
98,129 Amortization
30,661
26,898
204,677 Amortization of debt issue costs
335,123
160,825
656,773 Loss (gain) on extinguishments of debt
(969,538
)
—
(96,626
) Loss (gain) on sale of investment securities
20,935
(6,253
)
(253,065
) Loss from impairment of patents
—
—
67,620 Loss on issuance of shares
16,725
46,615
96,393 Stock-based compensation
821,069
588,327
5,707,533 Changes in operating assets and liabilities: Notes and interest receivable
—
—
(6,296
) Prepaid expenses and other
107,670
105,616
43,365 Accounts payable
302,626
152,194
1,588,238 Accrued liabilities
712,966
(10,083
)
979,794 Deferred revenue
—
—
(162,000
) Subscriptions receivable
—
—
(100,682
) Net Cash Used in Operating Activities
(248,256
)
(309,530
)
(6,928,656
) Investing Activities: Net Proceeds from notes receivable
—
—
1,171 Patent costs
(63,760
)
(174,711
)
(547,873
) Proceeds from sale of investment securities
12,608
19,564
451,123 Net Cash Used in Investing Activities
(51,152
)
(155,147
)
(95,579
) Financing Activities: Due to related parties
63,185
(126,041
)
464,605 Proceeds from loans payable
575,000
—
575,000 Proceeds from convertible debt
—
—
3,639,743 Repayment on convertible debt
(36,250
)
(725,000
)
(1,214,250
) Debt issue costs
(288,946
)
—
(821,446
) Proceeds from private placements of common stock and share subscriptions received
315,000
218,900
3,501,472 Proceeds from the exercise of stock options and warrants
3,649
16,033
1,148,103 Share issuance costs
(13,835
)
—
(147,523
) Net Cash Provided by (Used in) Financing Activities
617,803
(616,108
)
7,145,704 Net Increase (Decrease) in Cash
318,395
(1,080,785
)
121,469 Cash—Beginning of Period
45,625
1,372,598
242,551 Cash—End of Period
364,020
291,813
364,020 Non-cash Investing and Financing Activities: Share issued to settle debt
131,665
140,500
952,679 Note payable converted into common shares
360,945
175,000
1,394,021 Supplemental Disclosures: Interest paid
32,966
346,102
598,331 Income taxes
—
—
— The accompanying notes are an integral part of these consolidated financial statements F-4
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Expressed in U.S. dollars)
(unaudited)
September 30,
The Development Stage
January 1, 2001
to September 30, 2009
$
$
$
BIOCUREX, INC. 1. NATURE OF BUSINESS AND CONTINUANCE OF OPERATIONS BioCurex, Inc. (f/k/a Whispering Oaks International, Inc.) (the “Company”) was incorporated on December 8, 1997, under the laws of the State of Texas. During the first quarter of 2001, the Company ceased its business activities relating to the acquisition and sale of thoroughbred racehorses when a
change of majority control occurred. On February 21, 2001, the Company acquired intellectual properties and patents relating to cancer diagnostics and therapeutics. The Company is now in the business of developing, producing, marketing and licensing cancer diagnostic kits and is currently considered a
development stage enterprise as defined by Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 915, Development Stage Entities. On October 31, 2008, the Company incorporated BioCurex China Co., Ltd. (“Biocurex China”), a wholly-owned subsidiary in China. The consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company does
not have sufficient cash nor does it have an established source of revenue to cover its ongoing costs of operations. As at September 30, 2009, the Company has a working capital deficiency of $996,112 and has accumulated losses of $18,886,998 since the inception of the development stage. These factors
raise substantial doubt about the Company’s ability to continue as a going concern. These financial statements do not include any adjustments that might result from the outcome of this uncertainty. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES Basis of Presentation These consolidated financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States, and are expressed in U.S. dollars. These consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary,
Biocurex China. The Company’s fiscal year-end is December 31. Interim Financial Statements The interim unaudited financial statements have been prepared in accordance with accounting principles generally accepted in the United States for interim financial information and with the instructions for Securities and Exchange Commission (“SEC”) Form 10-Q. They do not include all of the
information and footnotes required by generally accepted accounting principles for complete financial statements. Therefore, these financial statements should be read in conjunction with the Company’s audited financial statements and notes thereto for the year ended December 31, 2008, included in the
Company’s Annual Report on Form 10-K filed on March 31, 2009 with the SEC. The financial statements included herein are unaudited; however, they contain all normal recurring accruals and adjustments that, in the opinion of management, are necessary to present fairly the Company’s financial position as at September 30, 2009, and the results of its operations and cash flows
for the nine months ended September 30, 2009 and 2008. The results of operations for the nine months ended September 30, 2009 are not necessarily indicative of the results to be expected for future quarters or the full year. F-5
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Use of Estimates The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the
financial statements and the reported amounts of revenues and expenses during the periods. The Company regularly evaluates estimates and assumptions related to allowance for doubtful accounts, valuation of patent costs, stock-based compensation, and deferred income tax asset valuation allowances. The
Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and
expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be
affected. Cash and Cash Equivalents The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents. Marketable Securities The Company defines marketable securities as income yielding securities that can be readily converted into cash. Examples of marketable securities include U.S. Treasury and agency obligations, commercial paper, corporate notes and bonds, time deposits, foreign notes and certificates of deposit. The
Company accounts for its investment in debt and equity instruments under FASB ASC 320, Investments—Debt and Equity Securities. We follow the guidance provided by ASC 320 to assess whether our investments with unrealized loss positions are other than temporarily impaired. Realized gains and losses
and declines in value judged to be other than temporary are determined based on the specific identification method and are reported in other income (expense). Management determines the appropriate classification of such securities at the time of purchase and re-evaluates such classification as of each
balance sheet date. Registration Payment Arrangements The Company accounts for registration rights arrangements and related liquidated damages provisions under FASB ASC 815-40, Derivatives and Hedging—Contracts in Entity’s own Entity, which addresses an issuer’s accounting for registration payment arrangements. ASC 815-40 defines a registration
payment arrangement as an arrangement where the issuer i) will endeavor to file a registration statement for the resale of financial instruments, have the registration statement declared effective, or maintain its effectiveness and ii) transfer consideration to the counterparty if the registration statement is
not declared effective or its effectiveness is not maintained. ASC 815-40 requires the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or F-6
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Registration Payment Arrangements (continued) included as a provision of a financial instrument or other agreement, to be separately recognized and measured in accordance with ASC 450, Contingencies. Research and Development Costs Research and development costs are charged to operations as incurred. Foreign Currency Translation The Company’s functional and reporting currency is the United States dollar. Monetary assets and liabilities denominated in foreign currencies are translated to United States dollars in accordance with ASC 830, Foreign Currency Translation Matters, using the exchange rate prevailing at the balance
sheet date. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income. Foreign currency transactions are primarily undertaken in Canadian dollars and Chinese Renminbi. Revenue Recognition The Company recognizes revenue in accordance with ASC 605, Revenue Recognition. Revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service is performed, and collectability is reasonably assured. The Company’s revenue consists of
license fees related to the licensing of its RECAF™ technology. Long-lived Assets In accordance with ASC 360, Property Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not
limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses
combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment
loss is recognized when the carrying amount is not recoverable and exceeds fair value. Fair Value of Financial Instruments Our financial instruments consist principally of cash and cash equivalents and short-term marketable securities, and accounts payable. Marketable securities consist of time deposits longer than three months and are classified as held to maturity securities. Pursuant to ASC 820, Fair Value F-7
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Fair Value of Financial Instruments (continued) Measurements and Disclosures and ASC 825, Financial Instruments, the fair value of our cash equivalents and marketable securities is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. We believe that the recorded values of all of our other financial
instruments approximate their current fair values because of their nature and respective relatively short maturity dates or durations. The Company’s financial instruments consist principally of cash, accounts payable, loans payable, notes payable, convertible debt and amounts due to related parties. Income Taxes The Company accounts for income taxes using the asset and liability method in accordance with ASC 740, Income Taxes. The asset and liability method provides that deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the
financial reporting and tax bases of assets and liabilities, and for operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using the currently enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company records a valuation
allowance to reduce deferred tax assets to the amount that is believed more likely than not to be realized. Comprehensive Income ASC 220, Comprehensive Income, establishes standards for the reporting and display of comprehensive income and its components in the financial statements. As at September 30, 2009 and 2008, the Company’s only component of comprehensive income was unrealized holding gains and losses on
available-for-sale investment securities. Basic and Diluted Net Loss per Share The Company computes net loss per share in accordance with ASC 260 Earnings Per Share which requires presentation of basic earnings per share and diluted earnings per share (“Diluted EPS”). The computation of basic earnings per share is computed by dividing income available to common
stockholders by the weighted-average number of outstanding common shares during the period. Diluted earnings per share give effect to all potentially dilutive common shares outstanding during the period. The computation of diluted EPS does not assume conversion, exercise or contingent exercise of
securities that would have an anti-dilutive effect on earnings. Components of basic and diluted earnings per share for the three and nine months ended September 30, 2009 and 2008 were as follows: F-8
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Basic and Diluted Net Loss per Share (continued)
Three Months Ended
Nine Months Ended
September 30,
September 30,
September 30,
September 30, Net income (loss) available to common shareholders
266,795
(990,509
)
(1,819,826
)
(2,283,941
) Reduction of interest expense due to conversion of notes
32,966
—
—
— Adjusted net income (loss) (A)
299,761
(990,509
)
(1,819,826
)
(2,283,941
) Weighted average outstanding shares of common stock—Basic (B)
58,174,000
43,207,000
50,697,000
42,812,500 Dilutive securities—Diluted (C)
33,643,000
—
—
—
91,817,000
43,207,000
50,697,000
42,812,500 Earnings per share: Basic (A/B)
0.00
(0.02
)
(0.04
)
(0.05
) Diluted (A/C)
0.00
(0.02
)
(0.04
)
(0.05
) Stock-based Compensation The Company records stock-based compensation in accordance with ASC 718, Compensation—Stock Based Compensation, using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Recent Accounting Pronouncements In June 2009, the FASB issued guidance now codified as FASB ASC Topic 105, Generally Accepted Accounting Principles, as the single source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in
conformity with U.S. GAAP, aside from those issued by the SEC. ASC 105 does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all authoritative literature related to a particular topic in one place. The adoption of ASC 105 did not
have a material impact on the Company’s consolidated financial statements, but did eliminate all references to pre-codification standards In May 2009, FASB issued ASC 855, Subsequent Events, which establishes general standards of for the evaluation, recognition and disclosure of events and transactions that occur after the balance sheet date. Although there is new terminology, the standard is based on the same principles as those
that currently exist in the auditing standards. The standard, which includes a new required disclosure of the date through which an entity has evaluated subsequent events, is effective for interim or annual periods ending after June 15, 2009. The adoption of ASC 855-10 did not have a material effect on
the Company’s consolidated financial statements. Refer to Note 14. F-9
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
2009
$
2008
$
2009
$
2008
$
BIOCUREX, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Recent Accounting Pronouncements (continued) The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have
been issued that might have a material impact on its financial position or results of operations. Reclassifications Certain reclassifications have been made to the prior period’s financial statements to conform to the current period’s presentation. 3. INVESTMENT SECURITIES In November 2002, the Company entered into a licensing agreement (the “Agreement”) with a third party whereby it licensed part of its technology in exchange for cash and 600,000 shares of the third party’s publicly traded common stock that had a fair value of $162,000. The 600,000 shares of
common stock were classified as “available for sale” in accordance with ASC 320 and reported at fair value. During the nine months ended September 30, 2009, the Company sold the remaining 124,235 shares for proceeds of $12,608, resulting in a realized loss of $20,935. 4. NOTES RECEIVABLE
September 30,
December 31, Note receivable including interest at prime plus 4%
73,489
73,489 Notes receivables from employees
15,497
35,497 Less: allowance for doubtful accounts
(88,986
)
(106,320
) Total
—
2,666 Notes receivable from various employees are pursuant to stock options exercised and are non-interest bearing and due on demand. 5. PATENTS Patents relate to developing the method for diagnostic and treatment of cancer using a new cancer marker called “RECAF.” These patents are presently registered in 23 countries with ongoing registrations currently being conducted. Patents are stated at cost and have a definite life. Once the
Company receives patent approval, amortization is calculated using the straight-line method over the remaining life of the patents. F-10
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
2009
$
2008
$
BIOCUREX, INC. 5. PATENTS (continued) A schedule of the patents is as follows:
September 30,
December 31, Patents
684,721
688,581 Less: Accumulated amortization
(204,676
)
(174,015
) Loss on impairment of patent cost
—
(67,620
) Net Carrying Value
480,045
446,946 Amortization expense totaled $30,661 and $26,898 for the nine months ended September 30, 2009 and 2008, respectively. The estimated future amortization expense is as follows:
$ 2009
10,220 2010
40,882 2011
40,882 2012
40,882 2013
40,882 Thereafter
306,297
480,045 6. LOANS PAYABLE
a)
On September 10, 2009, the Company completed a private placement financing in which it sold 17 promissory notes in the aggregate principal amount of $450,000 and 6,428,578 shares of its common stock for an aggregate purchase price of $450,000. The promissory notes bear interest at a rate of 10% per annum. Both interest and principal are payable on August 31, 2010. However, if the Company sells any capital stock and receives gross proceeds of at least $3 million from such sale prior to August 31, 2010, it must prepay the principal under
the notes from such proceeds. As of September 30, 2009, the Company accrued interest payable in the amount of $2,589 (2008—$nil). The aggregate purchase price for the units was allocated equally between the notes and shares contained in each unit. The relative fair value assigned to the shares totaled $225,000. These amounts were recorded as a notes discount and will be amortized as interest expense over the term of the
promissory notes. For the nine months ended September 30, 2009, the Company recorded $8,872 (2008—$nil) of accretion expense related to these promissory notes. b) On September 21, 2009, the Company completed a private placement financing in which it sold 3 promissory notes in the aggregate principal amount of $125,000 and 1,785,715 shares of its common stock for an aggregate purchase price of $125,000. The promissory notes bear interest at a rate of 10% per annum. Both interest and principal are payable on January 31, 2013. However, if the Company sells any capital stock and receives gross proceeds of at least $3 million from such sale prior to August 31, 2010, it must prepay F-11
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
2009
$
2008
$
BIOCUREX, INC. 6. LOANS PAYABLE (continued)
the principal under the notes from such proceeds. As of September 30, 2009, the Company accrued interest payable in the amount of $342 (2008 - $nil) and charged into interest expense. The aggregate purchase price for the units was allocated equally between the notes and shares contained in each unit. The relative fair value assigned to the shares totalled $62,500. At September 30, 2009, the Company has not issued the 1,785,715 shares and the relative fair value of $62,500 is
recorded in common stock subscribed. These amounts were recorded as a notes discount and will be amortized as interest expense over the term of the promissory notes. For the nine months ended September 30, 2009, the Company recorded $272 (2008—$nil) of accretion expense related to these
promissory notes. The Company incurred $118,612 in debt issue costs for the promissory notes described in Note 6(a) and (b). The debt issue costs are being expensed over the term of the promissory notes. During the nine months ended September 30, 2009, the Company expensed $8,181 (2008—$nil) of the debt issue
costs related to these promissory notes. 7. RELATED PARTY TRANSACTIONS
September 30,
December 31, Due to Pacific BioSciences Research Centre Inc. (a)
391,454
328,269 Due to a former officers (b)
4,930
7,000
396,384
335,269
a)
The Company’s research and development is performed by Pacific BioSciences Research Centre (“Pacific”). Pacific is 100% owned by the President of the Company. During the nine months ended September 30, 2009 and 2008, Pacific performed research and development for the Company valued
at $432,614 and $435,217, respectively. Pacific also provided administrative services during the nine months ended September 30, 2009 and 2008, valued at $138,147 and $275,494, respectively. During the nine months ended September 30, 2009, and 2008, Pacific charged interest of $7,980 and $6,548, respectively, calculated at bank prime
rate on the monthly balance owed. The amount due to Pacific is unsecured and due on demand. b) The amounts owing to a former officer are unsecured, non-interest bearing and due on demand. c) During the nine month period ended September 30, 2009, the Company granted 2,263,157 (2008—570,000) stock options to two directors at a below market exercise price of $0.001 per share. d) In September 2009, the Company issued 143,000 shares of common stock at a fair value of $10,000 to a new director for management services rendered. F-12
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
2009
$
2008
$
BIOCUREX, INC. 8. CONVERTIBLE NOTES AND DEBT
a)
The Company received funds during 2003 relating to ten convertible notes payable totaling $529,743, bearing interest at 5% and due on demand. One of the notes payable in the amount of $53,000 was repaid in April 2003. A gain of $33,584 was recorded on the date of repurchase of the
convertible debenture as determined through the calculation of the intrinsic value of the beneficial conversion feature on the date of extinguishment. Under the convertibility terms of the notes payable, the principal, plus accrued interest, can be converted immediately, at the option of the holder,
either in whole, or in part, into fully paid common shares of the Company. The conversion price per share is equal to the lesser of the stated price (ranging between $0.05 and $0.23) or 75% of the average closing bid prices for the five trading days ending on the trading day immediately before the
date of the conversion. In conjunction with the issuance of the notes, the Company issued 2,434,088 warrants to the note holders entitling them to purchase 2,434,088 shares of common stock at exercise prices between $0.08 and $0.38. The warrants expired two years after the issuance date. In accordance with ASC 470-20, Debt—Debt with Conversion and Other Options, the proceeds were allocated between the debt and warrants based on their relative fair values. The value assigned to the warrants totaled $274,601 and was expensed immediately due to the notes being due on demand.
The fair values were determined using the Black-Scholes option pricing model using the following weighted average assumptions: average risk-free interest rate of 1.49%; expected life of two years; expected volatility of 473%; and no expected dividends. In addition to the shares to be received upon
conversion, the note holder will also receive an equal number of warrants to purchase shares at 110% of the conversion price amount. The beneficial conversion feature was calculated under ASC 470-20, and equaled $255,142. Due to the notes being due on demand, the discount was expensed in fiscal 2003. In February 2005, a note in the amount of $143,370 was converted into 955,800 units, consisting of one common share at $0.15 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.17 per share expiring on March 9,
2010. In accordance with ASC 470-20, the Company recognized $67,829 for the intrinsic value of the embedded conversion option. In July 2006, a note in the amount of $61,890 was converted into 343,833 units, consisting of one common share at $0.18 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.20 per share expiring on July 7, 2011. In
accordance with ASC 470-20, the Company recognized $29,506 for the intrinsic value of the embedded conversion option. In July 2006, a note in the amount of $11,655 was converted into 233,092 units, consisting of one common share at $0.05 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.055 per share expiring on July 7, 2011.
In accordance with ASC 470-20, the Company recognized $5,565 for the intrinsic value of the embedded conversion option. In July 2006, a note in the amount of $65,000 was converted into 590,909 units, consisting of one common share at $0.11 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.12 per share expiring F-13
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 8. CONVERTIBLE NOTES AND DEBT (continued)
on July 19, 2011. In accordance with ASC 470-20, the Company recognized $30,089 for the intrinsic value of the embedded conversion option. In August 2009, four notes in the amount of $160,945 were converted into 2,204,730 units, consisting of one common share at $0.073 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.08 per share expiring on
August 26, 2014. In accordance with ASC 470-20, the Company recognized $71,389 for the intrinsic value of the embedded conversion option. The effective interest rate of the remaining convertible notes at September 30, 2009 is 335%. b) On July 7, 2007, the Company received proceeds of $3,000,000 from the issuance of convertible notes (the “Notes”), plus share purchase warrants, to two private investors. The share purchase warrants allow the holders to purchase up to 3,500,000 shares of the Company’s common stock at a price of
$0.60 per share expiring September 25, 2012. The Notes bear interest annually at a rate of prime (as adjusted monthly on the first business day of each month) plus 2.75% per year. The Notes are due and payable on June 25, 2010 and are secured by substantially all of the Company’s assets.
Interest is payable monthly with the first interest payment due on August 1, 2007. Beginning on November 1, 2007, the Company is required to make monthly payments of $100,000 towards the principal amount of the Notes. If the Company fails to make any interest or principal payment when due,
the Notes will become immediately due and payable. At the holders’ option the Notes are convertible into shares of the Company’s common stock at a conversion price of $0.60 per share. The Company may elect to pay the monthly redemption amounts and accrued interest with shares of its
common stock, which will be determined by dividing the amount to be paid by the lesser of the conversion price then in effect or 80% of the weighted average price of the Company’s common stock for the ten trading days preceding the payment date. In order to make principal or interest payments with shares of its common stock certain conditions must be met, including the condition that the number of shares to be issued in payment of principal or interest cannot exceed 25% of the total shares traded for the ten trading days prior to the
payment date. The Company agreed to file a Form SB-2 Registration Statement (“SB-2”) with the U.S. Securities and Exchange Commission in order that the shares of common stock issuable upon the conversion of the Notes or the exercise of the share purchase warrants may be resold in the
public market. The Company was required to file the SB-2 no later than July 30, 2007 (filed), to cause the SB-2 to become effective by November 26, 2007, and to keep the SB-2 continuously effective until the shares covered by the SB-2 have been sold or can be sold pursuant to Rule 144(k). In the event the closing price of the Company’s common stock is $1.20 or greater for ten consecutive trading days, the holders will be required to exercise the 3,500,000 share purchase warrants within ten days notice by the Company. Following the exercise of the share purchase warrants, the
Company will issue to the holders 3,500,000 new share purchase warrants, which will entitle the holders to purchase 1,750,000 shares of common stock. Two share purchase warrants will be exercisable at a price of $1.20 per share at any time prior to the later of June 25, 2012 or three years from the
date the new share purchase warrants are issued. F-14
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 8. CONVERTIBLE NOTES AND DEBT (continued) In accordance with ASC 470-20, the proceeds were allocated between the debt and warrants based on their relative fair values. The relative fair value assigned to the share purchase warrants totaled $1,426,381 and was determined using the Black-Scholes option pricing model using the following
weighted average assumptions: average risk-free interest rate of 4.76%; expected life of five years; expected volatility of 176%; and no expected dividends. These amounts were recorded as a debt discount and will be amortized as interest expense over the term of the convertible debentures. The
effective interest rate at December 31, 2008 is 406%. For the year ended December 31, 2008, the Company recorded $976,064 (2007—$791,092) of accretion expense related to the convertible debt. On August 18, 2008, the Company agreed to re-price the 3,500,000 share purchase warrants to an exercise price of $0.25 per share. In accordance with ASC 718, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental interest expense is
measured as the excess, if any, of the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental interest expense of $192,264 for these modified purchase warrants. On November 26, 2008, the Company received notification from the note holders which modified the terms of the Notes. Pursuant to the notification the interest and principal payments payable in December 2008 and all subsequent principal and interest payments were deferred until May 1, 2009.
In addition the principal amount outstanding was increased by $255,000 to $1,955,000. In accordance with ASC 470-60, Debt—Troubled Debt Restructurings by Debtors, the Company determined that the creditor did not grant a concession even though the payments were deferred as the total amount owing by the Company was increased. As at November 26, 2008, prior to the
modification of the convertible notes, the carrying value of the convertible notes was $613,738. The remaining unaccreted discount of $304,467 related to the convertible notes was charged to operations in the year ended of 2008. In accordance with ASC 470-20, the Company determined there was no beneficial conversion feature on the modified convertible notes. The Company recorded a discount of $130,298 which was equal to the difference of the face value of the new note and the present value of the revised cash
flows. The effective interest rate of the new notes was 6.56%. The Company incurred $717,668 in debt issue costs for these convertible debentures. The debt issue costs will be expensed over the term of the convertible debt. During the nine months ended September 30, 2009, the Company expensed $326,942 (2008—$160,825) of the debt issue costs related to the
convertible debt. On May 1, 2009, as a result of the Company defaulting on paying interest and principal repayment, the Company expensed the remaining discount of $69,412 and deferred financing fees of $214,434 relating to the Notes. On June 4, 2009, the Company repaid $36,250 to the debt holders and the
amount was applied to the principal. As at June 30, 2009, the carrying value of the convertible notes was $1,918,750. As a result of the default on repayment, the Company accrued a mandatory prepayment amount of $479,688 at 25% of the outstanding principal, interest in the amount of $232,324 at F-15
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 8. CONVERTIBLE NOTES AND DEBT (continued)
18% retroactive from November 1, 2008 and late fee of $12,009 at 18% on the unpaid interest. These amounts owing are included in accrued liabilities. The Company entered into a loan modification agreement, dated as of August 31, 2009, with the holders of the Notes. Pursuant to the agreement, the defaults were waived and the terms of the Notes were amended as follows:
–
The maturity date of the Notes was extended to December 31, 2012 and no principal payments are due on the Notes prior to the maturity date. – All interest due on the Notes through June 30, 2009 was added to the outstanding principal balance and as a result the aggregate principal amount of the notes at June 30, 2009 was $2,150,000. – The interest rate on the Notes remains at prime (as adjusted monthly) plus 2.75% per annum and accrued from July 1, 2009 and is payable in arrears on the first day of each month – The conversion price was reset at $0.14 per share.
The present value of the cash flows under the terms of the July 1, 2009 debt instrument was greater than 10% different from the November 26, 2008 debt instrument. As a result, in accordance with ASC 470-50, Debt—Modifications and Extinguishments, the Company deemed the terms of the
amendment to be substantially different and treated the November 26, 2008 convertible notes extinguished and exchanged for new convertible notes. The fair value of the July 1, 2009 Notes of $1,673,243 was recorded at July 1, 2009. The Company recorded a gain on extinguishment of debt of
$969,538. In accordance with ASC 470-20, for the three months period ended September 30, 2009 the Company determined there was no intrinsic value to the conversion feature and thus no beneficial conversion feature. The Company recorded a discount of $476,757 which equals to the difference of the face
value of the new note and the present value of the revised cash flows. During the three months period ended September 30, 2009, the debt holders converted $200,000 into 1,428,572 shares at $0.14 per share. The Company recorded interest expense of $43,450 related to the amounts converted. For the nine months ended September 30, 2009, the Company recorded $140,739 (2008—$910,972) of accretion expense related to the convertible debt. The effective interest rate of the remaining convertible notes at September 30, 2009 is 7.57%. 9. COMMON STOCK
a)
In January 2009, the Company issued 150,000 shares of common stock at a fair value of $36,000 to settle debt. b) In January 2009, the Company issued 267,000 units at $0.15 per unit for common share subscriptions totaling $40,050 received in December 2008. Each unit consisted of one share of common stock and one half share purchase warrant entitling the holder to purchase one share of common stock at
an exercise price of $0.30 per share expiring on November 30, 2010. c) In January 2009, the Company issued 31,250 shares of common stock at a fair value of $5,000 to settle debt. F-16
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 9. COMMON STOCK (continued) d) In January 2009, the Company issued 56,000 shares of common stock at a fair value of $8,960 to an employee for services rendered in December 2008 and January 2009. e) In February 2009, the Company issued 639,142 shares of common stock at a fair value of $89,480 to eight employees and one consultant for services provided from February to March 2009. f) In March 2009, an employee returned 33,333 shares with a fair value of $2,666, to settle $20,000 amount owing to the Company. The Company recorded $17,333 of bad debt expense in the fiscal year ended December 31, 2008. g) In April 2009, the Company issued 900,000 units at $0.05 per unit for common share for proceeds of $45,000. Each unit consisted of one share of common stock and one purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on
April 5, 2011. h) In April 2009, the Company issued 125,000 shares of common stock at a fair value of $10,000 to settle debt. i) In April 2009, the Company issued 2,070,000 shares of common stock to a former director for the exercise of 1,620,000 options and 450,000 warrants at $0.001 per share. A total of $2,070 was reduced from the outstanding balance of amounts owing to related parties. See note 7(b). j) In April 2009, the Company issued 418,060 shares of common stock at a fair value of $33,863 to eight employees and one consultant for services provided in April 2009. k) In April 2009, the Company issued 307,892 units at $0.13 per unit for common share subscriptions totaling $40,000 received in January 2009. Each unit consisted of one share of common stock and one purchase warrant entitling the holder to purchase one share of common stock at an exercise price
of $0.17 per share expiring on January 2, 2011. l) In April 2009, the Company issued 250,000 shares of common stock at a fair value of $22,500 to settle debt. m) In May 2009, the Company issued 200,000 shares of common stock to an investor relations company for consulting services at a fair value of $10,000. n) In May 2009, the Company issued 350,750 shares of common stock at a fair value of $28,060 to six employees for services provided in May 2009. o) In May 2009, the Company issued 2,000,000 shares of common stock at $0.05 per share for proceeds of $100,000. Each unit consisted of one share of common stock and purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on
April 1, 2012. The Company paid a commission of $10,000 in connection with this private placement. p) In May 2009, the Company issued 268,730 shares of common stock at a fair value of $21,498 to five employees as bonus. q) In June 2009, the Company issued 300,000 shares of common stock to an investor relations company for their consulting services at a fair value of $26,700. F-17
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 9. COMMON STOCK (continued) r) In June 2009, the Company issued 816,932 shares of common stock at a fair value of $44,931 to five employees for services provided in June 2009. s) In July 2009, the Company issued 125,000 shares of common stock at a fair value of $10,000 to settle debt. t) In July 2009, the Company issued 500,000 shares of common stock at a fair value of $37,500 to settle debt. u) In August 2009, the Company issued 379,452 shares of common stock at a fair value of $31,115 to five employees for services provided in August 2009. v) In August 2009, the Company issued 125,000 shares of common stock at a fair value of $10,000 for settle debt. w) In August 2009, the Company issued 1,000,000 units at $0.05 per unit for common shares subscriptions totaling $50,000 received in June 2009. Each unit consisted of one share of common stock and purchase warrant entitling the holder to purchase one share of common stock at an exercise price of
$0.11 per share expiring on June 15, 2011. The Company recorded a commission of $2,500 that was paid in July 2009 in connection with this private placement. x) In August 2009, the Company received shares subscriptions of 500,000 units at $0.05 per unit for proceeds of $25,000. Each unit consisted of one share of common stock and purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring
on August 17, 2011. y) In September 2009, the Company issued 300,000 shares of common stock to an investor relations company for consulting services at a fair value of $24,600. z) In September 2009, the Company issued 143,000 shares of common stock to a director for management services at a fair value of $10,000.
aa)
In September 2009, the Company received shares subscriptions of 500,000 units at $0.07 per unit for proceeds of $35,000. Each unit consisted of one share of common stock and purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share
expiring on September 3, 2011. bb) In September 2009, four notes in the amount of $160,945 were converted into 2,204,730 units, consisting of one common share at $0.073 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.08 per share expiring on
August 26, 2014. cc) In September 2009, the Company issued 3,648,947 shares of common stock to a director for the exercise of 3,648,947 options at $0.001 per share for gross proceeds of $3,649. dd) In September 2009, the Company issued 2,000,000 shares of common stock at a fair value of $70,000 for services. ee) In September 2009, the Company issued 92,500 shares of common stock at a fair value of $3,332 to settle debt. F-18
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 9. COMMON STOCK (continued) ff) In September 2009, the Company issued 535,320 shares of common stock at a fair value of $36,402 to five employees for services provided in September 2009. gg) In September 2009, the Company issued 6,428,578 shares pursuant to the promissory notes described in Note 6. The aggregate purchase price of $450,000 for the units was allocated equally between the notes and shares contained in each unit. The relative fair value assigned to the shares totaled
$225,000. hh) In September 2009, the Company issued 1,428,572 shares to a convertible debt holder for the debt conversion of $200,000. ii) In September 2009, the Company issued 400,000 units at $0.05 per unit for proceeds of $20,000. Each unit consisted of one share of common stock and purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on August 18, 2011. 10. STOCK-BASED COMPENSATION Stock Bonus Plan Under the Company’s Stock Bonus Plan, employees, directors, officers, consultants and advisors are eligible to receive a grant of the Company’s shares, provided that bona fide services are rendered by consultants or advisors and such services must not be in connection with the offer or sale of
securities in a capital-raising transaction. On April 23, 2009, the Company increased the number of shares issuable pursuant to this plan from 5,500,000 shares to 10,500,000 shares with 1,906,275 common shares available for future issuance as of September 30, 2009. Non-Qualified Stock Option Plan The Company’s Non-Qualified Stock Option Plan authorizes the issuance of common shares to persons that exercise stock options granted. The Company’s employees, directors, officers, consultants and advisors are eligible to be granted stock options pursuant to this plan, provided that bona fide
services are rendered by such consultants or advisors and such services must not be in connection with the offer or sale of securities in a capital-raising transaction. The stock option exercise price is determined by a committee and cannot be less than $0.001. On April 23, 2009, the Company increased the number of shares issuable pursuant to this plan from 12,500,000 shares to 17,500,000 shares with 3,870,666 common shares available for future issuance as of September 30, 2009. F-19
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 10. STOCK-BASED COMPENSATION (continued) A summary of the changes in the Company’s stock options is presented below:
Number of
Weighted
Weighted
Aggregate Outstanding, December 31, 2007
3,666,666
0.03 Granted
570,000
0.001 Exercised
(33,333
)
0.001 Expired
(313,333
)
0.367 Outstanding, December 31, 2008
3,890,000
0.001
2.99
774,110 Granted
3,717,057
0.001 Exercised
(5,268,947
)
0.001 Outstanding, September 30, 2009
2,338,110
0.001
2.88
418,522 Exercisable, September 30, 2009
884,210
0.001
1.28
158,274 During the nine months period ended September 30, 2009, the Company granted 3,717,057 stock options at a fair value of $349,837 to directors, employees and consultants at a below market exercise price of $0.001 per share. The fair value for stock options granted was estimated at the date of grant using the Black-Scholes option-pricing model and the weighted average fair value of stock options granted during the nine months period ended September 30, 2009 and 2008 were $0.09 and $0.64 per share, respectively. The weighted average assumptions used are as follows:
Nine Months Ended
September 30,
September 30, Expected dividend yield
0
%
0
% Risk-free interest rate
1.73
%
1.97
% Expected volatility
120
%
63
% Expected option life (in years)
2.78
2.0 As at September 30, 2008, there was $102,189 of unrecognized compensation costs related to non-vested share-based compensation arrangements granted which are expected to be recognized over a weighted-average period of five months. The total fair value of shares vested during the nine months
ended September 30, 2009 and 2008 were $247,648 and $373,294, respectively. F-20
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
Shares
Average
Exercise Price
$
Average
Remaining
Contractual Life
(Years)
Intrinsic Value
$
2009
2008
BIOCUREX, INC. 10. STOCK-BASED COMPENSATION (continued) A summary of the status of the Company’s non-vested options as of September 30, 2009, and changes during the period of September 30, 2008, is presented below:
Non-vested shares
Number of
Weighted Average Non-vested at December 31, 2008
—
— Granted
3,717,057
0.001 Vested
(2,263,157
)
0.001 Non-vested at September 30, 2009
1,453,900
0.001 11. SHARE PURCHASE WARRANTS
Number
Weighted Average Balance, December 31, 2007
11,890,672
0.47 Issued
473,500
0.48 Exercised
(84,210
)
0.19 Expired
(505,000
)
0.81 Balance, December 31, 2008
11,774,962
0.35 Issued
7,812,422
0.10 Exercised
(450,000
)
0.001 Expired
(2,184,573
)
0.77 Balance, September 30, 2009
16,952,811
0.14 In January 2009, the Company extended the term of 2,455,000 share purchase warrants. In accordance with ASC 718, Compensation—Stock Compensation, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is
measured as the excess, if any, of the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $3,349 for these modified share purchase warrants. In April 2009, the Company extended the term and modified the exercising price of 1,000,000 share purchase warrants. In accordance with ASC 718, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is measured
as the excess, if any, of the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $63,074 for these modified share purchase warrants. F-21
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
Options
Grant Date
Fair Value
$
Exercise Price
$
BIOCUREX, INC. 11. SHARE PURCHASE WARRANTS (continued) As at September 30, 2009, the following share purchase warrants were outstanding:
Warrants
Exercise Price
Expiration Date 1,275,000
0.08
January 15, 2010 955,800
0.17
March 09, 2010 115,000
0.65
May 01, 2010 541,666
0.12
October 31, 2010 199,311
0.17
November 11, 2010 133,500
0.30
November 30, 2010 307,692
0.17
January 02, 2011 900,000
0.11
April 5, 2011 1,000,000
0.11
June 15, 2011 233,092
0.06
July 07, 2011 343,833
0.20
July 07, 2011 590,909
0.12
July 19, 2011 500,000
0.11
August 17, 2011 400,000
0.11
August 18, 2011 500,000
0.11
September 3, 2011 252,278
0.05
December 31, 2011 2,000,000
0.11
April 1, 2012 1,000,000
0.25
April 30, 2012 3,500,000
0.25
June 27, 2012 2,204,730
0.08
August 26, 2014 16,952,811
12. FAIR VALUE MEASUREMENTS ASC 825 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. In determining fair value for assets and liabilities required or permitted to be recorded at fair value, the
Company considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability. Fair Value Hierarchy ASC 825 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is
significant to the fair value measurement. ASC 825 establishes three levels of inputs that may be used to measure fair value. F-22
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
$
BIOCUREX, INC. 12. FAIR VALUE MEASUREMENTS (continued) Level 1 Level 1 applies to assets and liabilities for which there are quoted prices in active markets for identical assets or liabilities. Valuations are based on quoted prices that are readily and regularly available in an active market and do not entail a significant degree of judgment. Level 2 Level 2 applies to assets and liabilities for which there are other than Level 1 observable inputs such as quoted prices for similar assets or liabilities in active markets, quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets), or
model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. Level 2 instruments require more management judgment and subjectivity as compared to Level 1 instruments. For instance: Determining which instruments are most similar to the instrument being priced requires management to identify a sample of similar securities based on the coupon rates, maturity, issuer, credit rating and instrument type, and subjectively select an individual security or multiple securities that are
deemed most similar to the security being priced; and Determining whether a market is considered active requires management judgment. Level 3 Level 3 applies to assets and liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. The determination of fair value for Level 3 instruments requires the most management judgment and
subjectivity. Pursuant to ASC 825, the fair value of the cash equivalents is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. Marketable securities and convertible notes are valued based on “Level 2” inputs, consisting of quoted prices in less active
markets. The Company believes that the recorded values of all of the other financial instruments approximate their current fair values because of their nature and respective relatively short maturity dates or durations. F-23
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
BIOCUREX, INC. 12. FAIR VALUE MEASUREMENTS (continued) Assets and liabilities measured at fair value on a recurring basis were presented on the Company’s consolidated balance sheet as of September 30, 2009 as follows:
Fair Value Measurements Using
Quoted Prices in
Significant
Significant
Balance as of Assets: Cash equivalents
$
364,020
$
—
$
—
$
364,020 Total assets measured at fair value
$
364,020
$
—
$
—
$
364,020 Liabilities: Loan payable
$
—
$
296,644
$
—
$
296,644 Convertible notes
—
1,527,780
—
1,527,780 Total liabilities measured at fair value
$
—
$
1,824,424
$
—
$
1,824,424 13. COMMITMENTS AND CONTINGENCIES
a)
On April 4, 2006, the Company entered into a consulting agreement with a term of nine months for consideration of 75,000 common shares. As of September 30, 2009, the Company has issued 37,500 common shares and 37,500 common shares are still owed to the consultant. b) On April 10, 2006, the Company entered into a consulting agreement with a term of one year for consideration of 75,000 common shares. As of September 30, 2009, the Company has issued 37,500 common shares and 37,500 common shares are still owed to the consultant. 14. SUBSEQUENT EVENTS In preparing the accompanying unaudited consolidated financial statements, the Company has reviewed, as determined necessary by the Company’s management, events that have occurred after September 30, 2009, up until the issuance of the financial statements, which occurred on November 12,
2009. During this period, the Company did not have any material recognizable subsequent events other than as disclosed below:
a)
In October 2009, the Company issued 500,000 units that it sold in August 2009. See note 9(x). Each unit consisted of one share of common stock and purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on August 17, 2011. b) In October 2009, the convertible debt holders elected to convert $100,000 of the principal amount of these notes into 714,286 shares of common stock. See Note 8(b). c) On October 5, 2009, the Company filed a registration statement for a proposed offering of 1,200,000 units. As set forth in the registration statement, the unit offering price is expected to be approximately $5.00 and each unit will consist of an equal number of the Company’s common shares and
share purchase warrants. The number of the Company’s common shares and share purchase warrants included in each unit will be based on the market price of a F-24
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
Active Markets
For Identical
Instruments
(Level 1)
Other
Observable
Inputs
(Level 2)
Unobservable
Inputs
(Level 3)
September 30, 2009
BIOCUREX, INC. 14. SUBSEQUENT EVENTS (continued)
share of the Company’s common stock at the time of offering. At September 30, 2009, $89,540 of costs associated with this offering were included in deferred financing costs.
On October 27, 2009, the Company’s shareholders appoved a legal name change to BioCurex, Inc. and increased the Company’s authorized shares to 450,000,000. e) On November 11, 2009, the Company issued an aggregate of 1,785,715 shares of common stock to three investors. These shares were sold in September 2009. See Note 6(b). F-25
(formerly, WHISPERING OAKS INTERNATIONAL, INC.)
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in U.S. dollars)
NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(unaudited)
d)
Report of Independent Registered Public Accounting Firm To the Directors and Stockholders We have audited the accompanying balance sheets of Whispering Oaks International, Inc. (A Development Stage Company) as of December 31, 2008 and 2007, and the related statements of operations, cash flows and stockholders’ equity (deficit) for the years then ended and accumulated for the
period from January 1, 2001 to December 31, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. An audit includes consideration of internal control over
financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of internal control over financial reporting. An audit also includes assessing the accounting principles used and significant estimates
made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion. In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Whispering Oaks International, Inc. (A Development Stage Company) as of December 31, 2008 and 2007, and the results of its operations and its cash flows for the years then
ended and accumulated for the period from January 1, 2001 to December 31, 2008, in conformity with accounting principles generally accepted in the United States. The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has a working capital deficiency and has incurred significant operating losses since inception. These factors raise
substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also discussed in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. /s/ “Manning Elliott LLP” CHARTERED ACCOUNTANTS Vancouver, Canada March 25, 2009 F-26
Whispering Oaks International, Inc. (dba Biocurex, Inc.)
(A Development Stage Company)
WHISPERING OAKS INTERNATIONAL, INC.
December 31,
December 31, ASSETS Current Assets Cash
45,625
1,372,598 Investment securities (Note 3)
18,014
61,366 Prepaid expenses and other (Notes 6(a) and 8(a))
137,672
109,045 Notes receivable, net (Note 4)
2,666
35,497 Total Current Assets
203,977
1,578,506 Deferred financing costs (Note 7 (b))
321,651
536,084 Patents (Note 5)
446,946
360,812 Total Assets
972,574
2,475,402 LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) Current Liabilities Accounts payable
174,400
105,505 Accrued liabilities
377,627
359,854 Due to related parties (Note 6)
335,269
344,355 Convertible notes payable (Note 7 (a))
194,828
194,828 Current portion of convertible debt (Note 7 (b))
688,754
371,712
1,770,878
1,376,254 Convertible debt (Note 7 (b))
1,136,604
266,618
2,907,482
1,642,872 Commitments and Contingencies (Notes 1, and 11) Stockholders’ Equity (Deficit) Common stock Authorized: 125,000,000 shares, par value $0.001 Issued and outstanding: 43,713,399 and 42,143,275 respectively
43,713
42,143 Additional paid-in capital
15,178,205
13,899,938 Common stock subscribed
40,050
— Accumulated other comprehensive loss
(15,529
)
(42,189
) Accumulated deficit
(114,175
)
(114,175
) Deficit accumulated during the development stage
(17,067,172
)
(12,953,187
) Stockholders’ Equity (Deficit)
(1,934,908
)
832,530 Total Liabilities and Stockholders’ Equity (Deficit)
972,574
2,475,402 The accompanying notes are an integral part of these financial statements F-27
(dba BIOCUREX, INC.)
(A Development Stage Company)
BALANCE SHEETS
(Expressed in U.S. dollars)
2008
$
2007
$
WHISPERING OAKS INTERNATIONAL, INC.
Year Ended
Accumulated
2008
2007 Revenue
1,000,000
50,000
1,464,456 Operating Expenses Amortization
37,758
27,896
174,015 General and administrative (Note 6(a))
928,845
1,260,865
5,215,709 Professional and consulting fees
381,421
313,925
4,797,456 Research and development (Note 6(a))
675,302
662,944
3,739,837 Total Operating Expenses
2,023,326
2,265,630
13,927,017 Loss From Operations
(1,023,326
)
(2,215,630
)
(12,462,561
) Other Income (Expense) Accretion of discounts on convertible debt
(1,280,531
)
(791,092
)
(2,941,154
) Amortization of debt issue costs
(214,434
)
(107,217
)
(321,650
) Gain (loss) sale of equity investment securities
(16,389
)
—
168,926 Interest expense
(569,982
)
(242,628
)
(874,212
) Interest income
8,164
12,956
383,679 Loss on extinguishments of convertible debt
(906,496
)
—
(872,912
) Loss on impairment of patent cost
(67,620
)
—
(67,620
) Loss on issuance of shares
(43,371
)
(10,708
)
(79,668
) Total Other Expense
(3,090,659
)
(1,138,689
)
(4,604,611
) Net Loss for the Period
(4,113,985
)
(3,354,319
)
(17,067,172
) Other Comprehensive Income (Loss) Unrealized gain (loss) on investment securities
26,660
(115,061
)
(15,529
) Total Comprehensive Loss
(4,087,325
)
(3,469,380
)
(17,082,701
) Net Loss Per Share—Basic and Diluted
(0.10
)
(0.08
) Weighted Average Shares Outstanding
42,917,000
41,127,000 The accompanying notes are an integral part of these financial statements F-28
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENTS OF OPERATIONS
(Expressed in U.S. dollars)
December 31,
During the
Development
Stage
January 1, 2001
to December 31,
2008
$
$
$
WHISPERING OAKS INTERNATIONAL, INC.
Year Ended
Accumulated During
2008
2007 Operating Activities: Net loss for the period
(4,113,985
)
(3,354,319
)
(17,067,172
) Adjustments to reconcile net loss to net cash used in operating activities: Accretion of discounts on convertible debt
1,280,531
791,092
2,941,154 Allowance for uncollectible notes receivable
32,831
—
98,129 Amortization
37,758
27,896
174,015 Amortization of debt issue costs
214,433
107,217
321,650 Loss on extinguishments of debt
906,496
—
872,912 Loss (gain) on sale of investment securities
16,389
—
(274,000
) Loss from impairment of patents
67,620
—
67,620 Loss on issuance of shares
43,371
10,708
79,668 Stock-based compensation
729,402
666,650
4,886,464 Changes in operating assets and liabilities: Notes and interest receivable
—
—
(6,296
) Prepaid expenses and other
(28,627
)
5,767
(64,305
) Accounts payable
166,027
95,082
1,285,615 Accrued liabilities
17,775
139,968
266,828 Deferred revenue
—
—
(162,000
) Subscriptions receivable
—
—
(100,682
) Net Cash Used in Operating Activities
(629,979
)
(1,509,939
)
(6,680,400
) Investing Activities: Net Proceeds from notes receivable
—
—
1,711 Patent costs
(191,512
)
(59,704
)
(484,113
) Proceeds from sale of investment securities
53,621
—
438,515 Net Cash Used in Investing Activities
(137,891
)
(59,704
)
(44,427
) Financing Activities: Due to related parties
(9,086
)
(14,408
)
401,420 Proceeds from convertible debt
—
3,000,000
3,639,743 Repayment on convertible debt
(825,000
)
(300,000
)
(1,178,000
) Debt issue costs
—
(532,500
)
(532,500
) Proceeds from private placements of common stock and share subscriptions received
258,950
124,750
3,186,472 Proceeds from the exercise of stock options and warrants
16,033
85,333
1,144,454 Share issuance costs
—
(11,188
)
(133,688
) Net Cash (Used in) Provided by Financing Activities
(559,103
)
2,351,987
6,527,901 Net (Decrease) Increase in Cash
(1,326,973
)
782,344
(196,926
) Cash—Beginning of Year
1,372,598
590,254
242,551 Cash—End of Year
45,625
1,372,598
45,625 Non-cash Investing and Financing Activities: Share issued to settle debt
140,500
118,000
818,347 Note payable converted into common shares
175,000
—
1,033,076 Supplemental Disclosures: Interest paid
359,453
205,133
565,365 Income taxes
—
—
— The accompanying notes are an integral part of these financial statements F-29
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENTS OF CASH FLOWS
(Expressed in U.S. dollars)
December 31,
The Development Stage
January 1, 2001
to December 31, 2008
$
$
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount Balance at January 1, 2001
8,225,022
8,225
46,775
—
—
—
—
(114,175
)
—
(59,175
) Capital contributed relating
to the forgiveness of
advances payable
—
—
59,175
—
—
—
—
—
—
59,175 Issuance of common stock at
$2.00 per share for patents
and intellectual properties
(February 2001)
1,950,000
1,950
(1,950
)
—
—
—
—
—
—
— Issuance of common stock at
$1.51 per share in
settlement of convertible
notes payable (May 2001)
1,544,404
1,545
464,616
—
—
—
—
—
—
466,161 Issuance of common stock for
cash: October 2001—$1.25
per share
52,000
52
65,000
—
—
—
—
—
—
65,052 December 2001—$0.97 per
share
32,260
32
31,406
—
—
—
—
—
—
31,438 Issuance of common stock at
$2.00 per share for services
rendered (December 2001)
11,000
11
21,989
—
—
—
—
—
—
22,000 Issuance of warrants
—
—
175,000
—
—
—
—
—
—
175,000 Cumulative foreign currency
translation adjustment
—
—
—
—
—
—
28,213
—
—
28,213 Net loss for the year
—
—
—
—
—
—
—
—
(1,089,464
)
(1,089,464
) Balance at
11,814,686
11,815
862,011
—
—
—
28,213
(114,175
)
(1,089,464
)
(301,600
) Issuance of common stock at
$0.75 per share (January
2002)
105,313
105
78,880
—
—
—
—
—
—
78,985 Issuance of common stock at
$0.10 per share to settle
convertible notes payable
(December 2002)
1,100,000
1,100
108,900
—
—
—
—
—
—
110,000 Issuance of common stock for
services rendered April
2002—$0.64 per share
77,149
77
49,062
—
—
—
—
—
—
49,139 July 2002—$1.25 per share
7,400
8
9,207
—
—
—
—
—
—
9,215 Issuance of common stock for
consulting services at $0.05
per share (November 2002)
2,300,000
2,300
112,700
—
—
(115,000
)
—
—
—
— Issuance of common stock to
settle accounts payable at
$0.08 per share (December
2002)
929,244
929
74,181
—
—
—
—
—
—
75,110 Fair value of stock options
granted
—
—
21,042
—
—
—
—
—
—
21,042 Fair value of warrants issued
—
—
207,188
—
—
—
—
—
—
207,188 Reclassification of warrants
and options to liability
—
—
(529,785
)
—
—
—
—
—
—
(529,785
) Reclassification of warrant
liability to equity
—
—
71,675
—
—
—
—
—
—
71,675 Beneficial conversion feature
of convertible debt
—
—
99,800
—
—
—
—
—
—
99,800 Cumulative foreign currency
translation adjustment
—
—
—
—
—
—
(28,213
)
—
—
(28,213
) Net loss for the year
—
—
—
—
—
—
—
—
(646,771
)
(646,771
) Balance—December 31, 2002
16,333,792
16,334
1,164,861
—
—
(115,000
)
—
(114,175
)
(1,736,235
)
(784,215
) The accompanying notes are an integral part of these financial statements F-30
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income
(Loss)
$
Deficit
$
Accumulated
During
the
Development
Stage
$
Equity
(Deficit)
$
#
$
(February 2001)
December 31, 2001
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount Balance—December 31, 2002
16,333,792
16,334
1,164,861
—
—
(115,000
)
—
(114,175
)
(1,736,235
)
(784,215
) Issuance of common stock for
cash: January 2003—$0.07 per
share
900,543
900
62,137
—
—
—
—
—
—
63,037 November 2003—$0.21 per
share
288,095
288
60,195
—
—
—
—
—
—
60,483 Issuance of common stock
pursuant to exercise of
stock options: March 2003—$0.07 per share
1,560,000
1,560
107,640
—
—
—
—
—
—
109,200 May 2003—$0.16 per share
1,000,000
1,000
159,000
—
—
—
—
—
—
160,000 June 2003—$0.17 per share
305,822
306
51,594
—
—
—
—
—
—
51,900 November 2003—$0.001 per
share
450,000
450
—
—
—
—
—
—
—
450 March 2003—$0.07 per share
135,000
135
9,315
—
—
—
—
—
—
9,450 June 2003—$0.17 per share
294,118
294
49,706
—
—
—
—
—
—
50,000 October 2003—$0.18 per
share
277,777
278
49,722
—
—
—
—
—
—
50,000 November 2003—$0.24 per
share
104,167
104
24,896
—
—
—
—
—
—
25,000 Issuance of common stock for
services: March 2003—$0.40 per share
156,250
156
62,344
—
—
—
—
—
—
62,500 October 2003—$0.16 per
share
1,000,000
1,000
159,000
—
—
(160,000
)
—
—
—
— Fair value of stock options
granted
—
—
841,349
—
—
—
—
—
—
841,349 Amortization of deferred
compensation
—
—
—
—
—
141,667
—
—
—
141,667 Fair value of warrants issued
—
—
274,601
—
—
—
—
—
—
274,601 Fair value of beneficial
conversion feature related
to convertible notes
—
—
255,142
—
—
—
—
—
—
255,142 Fair value of warrants issued
for loan provided
—
—
99,778
—
—
—
—
—
—
99,778 Reacquisition value of
beneficial conversion
feature
—
—
(33,584
)
—
—
—
—
—
—
(33,584
) Unrealized gain on
investment securities
—
—
—
—
—
—
48,000
—
—
48,000 Net loss for the year
—
—
—
—
—
—
—
—
(2,618,955
)
(2,618,955
) Balance—December 31, 2003
24,983,564
24,983
3,741,470
—
—
(133,333
)
48,000
(114,175
)
(4,355,190
)
(788,245
) Issuance of common stock for
cash: January 2004—$0.19 per share
100,000
100
18,900
—
—
—
—
—
—
19,000 March 2004—$0.15 per share
633,334
633
94,367
—
—
—
—
—
—
95,000 March 2004—$0.19 per share
315,790
316
59,684
—
—
—
—
—
—
60,000 July 2004—$0.50 per share
500,000
500
249,500
—
—
—
—
—
—
250,000 The accompanying notes are an integral part of these financial statements F-31
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income
(Loss)
$
Deficit
$
Accumulated
During
the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount July 2004—$0.60 per share
33,333
33
19,967
—
—
—
—
—
—
20,000 Dec 2004—$0.47 per share
320,600
321
150,361
—
(150,682
)
—
—
—
—
— Issuance of common stock for services: February 2004—$0.22 per share
142,928
143
31,301
—
—
—
—
—
—
31,444 March 2004—$0.23 per share
25,000
25
5,725
—
—
—
—
—
—
5,750 July 2004—$0.91 per share
200,000
200
181,800
—
—
—
—
—
—
182,000 October 2004—$0.72 per share
60,000
60
43,140
—
—
—
—
—
—
43,200 December 2004—$0.63 per share
79,616
80
50,078
—
—
—
—
—
—
50,158 Issuance of common stock pursuant to the exercise of stock options for cash: March 2004—$0.14 per share
40,000
40
5,560
—
—
—
—
—
—
5,600 March 2004—$0.22 per share
200,000
200
43,800
—
—
—
—
—
—
44,000 April 2004—$0.14 per share
65,000
65
9,035
—
—
—
—
—
—
9,100 April 2004—$0.001 per share
150,000
150
—
—
—
—
—
—
—
150 July 2004—$0.14 per share
125,000
125
17,375
—
—
—
—
—
—
17,500 July 2004—$0.07 per share
25,000
25
1,725
—
—
—
—
—
—
1,725 July 2004—$0.001 per share
200,000
200
—
—
—
—
—
—
—
200 September 2004—$0.07 per share
20,000
20
1,380
—
—
—
—
—
—
1,400 October 2004—$0.73 per share
128,000
128
93,312
—
—
—
—
—
—
93,440 Fair value of stock options granted
—
—
419,204
—
—
—
—
—
—
419,204 Issuance of common stock pursuant to the exercise of warrants for cash: June 2004—$0.07 per share
628,571
629
43,371
—
—
—
—
—
—
44,000 June 2004—$0.19 per share
105,263
105
19,895
—
—
—
—
—
—
20,000 July 2004—$0.05 per share
30,000
30
1,470
—
—
—
—
—
—
1,500 July 2004—$0.30 per share
153,945
154
46,030
—
—
—
—
—
—
46,184 August 2004—$0.21 per share
338,095
338
70,662
—
—
—
—
—
—
71,000 September 2004—$0.07 per share
271,972
272
18,766
—
—
—
—
—
—
19,038 September 2004—$0.001 per share
200,000
200
—
—
—
—
—
—
—
200 Issuance of common stock pursuant to the exercise of warrants for cash: December 2004—$0.08 per share
145,683
146
11,509
—
—
—
—
—
—
11,655 The accompanying notes are an integral part of these financial statements F-32
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income (Loss)
$
Deficit
$
Accumulated
During the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount December 2004—$0.05 per share
337,313
337
16,528
—
—
—
—
—
—
16,865 December 2004—$0.30 per share
206,300
206
61,684
—
—
—
—
—
—
61,890 Amortization of deferred compensation
—
—
—
—
—
106,499
—
—
—
106,499 Unrealized gain on investment securities
—
—
—
—
—
—
174,000
—
—
174,000 Net loss for the year
—
—
—
—
—
—
—
—
(1,406,455
)
(1,406,455
) Balance—December 31, 2004
30,764,307
30,764
5,527,599
—
(150,682
)
(26,834
)
222,000
(114,175
)
(5,761,645
)
(272,973
) Issuance of common stock for services: February 2005—$0.71 per share
15,492
15
10,985
—
—
—
—
—
—
11,000 March 2005—$0.90 per share
30,000
30
26,970
—
—
—
—
—
—
27,000 May 2005—$1.26 per share
15,000
15
18,885
—
—
—
—
—
—
18,900 July 2005—$1.00 per share
70,000
70
72,930
—
—
—
—
—
—
73,000 December 2005—$0.89 per share
25,000
25
22,225
—
—
—
—
—
—
22,250 Issuance of common stock for cash: May 2005—$1.00 per share
25,000
25
24,975
—
—
—
—
—
—
25,000 June 2005—$1.00 per share
135,000
135
134,865
—
—
—
—
—
—
135,000 June 2005—$1.10 per share
4,545
5
4,995
—
—
—
—
—
—
5,000 Issuance of common stock pursuant to the exercise of stock options for notes receivable: February 2005—$0.60 per share
209,000
209
125,191
—
—
—
—
—
—
125,400 April 2005—$0.60 per share
5,000
5
7,495
—
—
—
—
—
—
7,500 Fair value of stock options granted
—
—
384,500
—
—
—
—
—
—
384,500 Issuance of common stock pursuant to the exercise of stock options for cash: March 2005—$0.001 per share
1,750,000
1,750
—
—
—
—
—
—
—
1,750 March 2005—$0.07 per share
25,000
25
1,725
—
—
—
—
—
—
1,750 December 2005—$0.001 per share (cancellation)
(1,750,000
)
(1,750
)
—
—
—
—
—
—
—
(1,750
) Issuance of common stock pursuant to the exercise of warrants for cash: January 2005—$0.30 per share
26,305
26
7,865
—
—
—
—
—
—
7,891 January 2005—$0.38 per share
65,789
66
24,934
—
—
—
—
—
—
25,000 The accompanying notes are an integral part of these financial statements F-33
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income (Loss)
$
Deficit
$
Accumulated
During the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount March 2005—$0.21 per share
50,000
50
10,450
—
—
—
—
—
—
10,500 March 2005—$0.001 per share
450,000
450
—
—
—
—
—
—
—
450 June 2005—$0.21 per share
682,714
683
142,687
—
—
—
—
—
—
143,370 Issuance of common stock pursuant to the exercise of warrants for cash: June 2005—$0.10 per share
600,000
600
59,400
—
—
—
—
—
—
60,000 August 2005—$0.75 per share
77,266
77
57,873
—
—
—
—
—
—
57,950 December 2005—$0.001 per share (cancellation)
(450,000
)
(450
)
—
—
—
—
—
—
—
(450
) Issuance of common stock pursuant to the cashless exercise of warrants: February 2005 (139,474 warrants)
70,643
71
(71
)
—
—
—
—
—
—
— March 2005 (272,903 warrants)
213,576
213
(213
)
—
—
—
—
—
—
— Issuance of common stock pursuant to the conversion of notes payable (February 2005)
955,800
956
142,414
—
—
—
—
—
—
143,370 February 2005, fair value of warrants issued on conversion of note payable
—
—
67,829
—
—
—
—
—
—
67,829 December 2005, fair value of warrants issued for services
—
—
222,587
—
—
—
—
—
—
222,587 Proceeds from stock subscriptions receivable
—
—
—
—
150,682
—
—
—
—
150,682 Proceeds from common shares subscribed pursuant to warrants exercised
—
—
—
85,962
—
—
—
—
—
85,962 Amortization of deferred compensation
—
—
—
—
—
26,834
—
—
—
26,834 Unrealized loss on investment securities
—
—
—
—
—
—
(18,000
)
—
—
(18,000
) Net loss for the year
—
—
—
—
—
—
—
—
(1,755,930
)
(1,755,930
) Balance—December 31, 2005
34,065,437
34,065
7,099,095
85,962
—
—
204,000
(114,175
)
(7,517,575
)
(208,628
) Issuance of common stock for services: June 2006—$1.50 per share
25,000
25
37,475
—
—
—
—
—
—
37,500 July 2006—$0.72 per share
37,500
38
26,962
—
—
—
—
—
—
27,000 July 2006—$0.77 per share
37,500
38
28,837
—
—
—
—
—
—
28,875 September 2006—$0.80 per share
100,000
100
79,900
—
—
—
—
—
—
80,000 October 2006—$0.75 per share
225,000
225
168,525
—
—
—
—
—
—
168,750 The accompanying notes are an integral part of these financial statements F-34
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income (Loss)
$
Deficit
$
Accumulated
During the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount November 2006—$0.86 per share
50,000
50
42,950
—
—
—
—
—
—
43,000 Issuance of common stock for debt settlement: January 2006—$0.78 per share
200,000
200
155,800
—
—
—
—
—
—
156,000 January 2006—$0.83 per share
6,250
6
5,181
—
—
—
—
—
—
5,187 February 2006—$0.73 per share
6,850
6
4,994
—
—
—
—
—
—
5,000 June 2006—$0.95 per share
90,000
90
85,410
—
—
—
—
—
—
85,500 September 2006—$0.55 per share
15,000
15
8,235
—
—
—
—
—
—
8,250 September 2006—$0.80 per share
200,000
200
159,800
—
—
—
—
—
—
160,000 October 2006—$0.72 per share
90,000
90
64,710
—
—
—
—
—
—
64,800 Issuance of common stock for cash: April 2006—$0.50 per share
150,000
150
74,850
—
—
—
—
—
—
75,000 July 2006—$0.50 per share
150,000
150
74,850
—
—
—
—
—
—
75,000 July 2006—$0.70 per share
110,000
110
76,890
—
—
—
—
—
—
77,000 September 2006—$0.50 per share
460,000
460
229,540
—
—
—
—
—
—
230,000 October 2006—$0.50 per share
1,995,000
1,995
995,505
—
—
—
—
—
—
997,500 Share issuance costs
—
—
(122,500
)
—
—
—
—
—
—
(122,500
) Issuance of common stock pursuant to the exercise of stock options (December 2006) $0.001 per share
25,000
25
—
—
—
—
—
—
—
25 Fair value of stock options granted
—
—
375,457
—
—
—
—
—
—
375,457 Fair value of stock options modified
—
—
68,067
—
—
—
—
—
—
68,067 Issuance of common stock pursuant to the exercise of warrants for cash: January 2006—$0.10 per share
500,000
500
49,500
(50,000
)
—
—
—
—
—
— January 2006—$0.05 per share
719,244
719
35,243
(35,962
)
—
—
—
—
—
— Issuance of common stock pursuant to the conversion of notes payable (September 2006)
1,167,834
1,168
137,377
—
—
—
—
—
—
138,545 September 2006, fair value of warrants issued on conversion of note payable
—
—
65,160
—
—
—
—
—
—
65,160 Unrealized loss on investment securities
—
—
—
—
—
—
(131,128
)
—
—
(131,128
) Net loss for the year
—
—
—
—
—
—
—
—
(2,081,293
)
(2,081,293
) Balance, December 31, 2006
40,425,615
40,425
10,027,813
—
—
—
72,872
(114,175
)
(9,598,868
)
428,067 The accompanying notes are an integral part of these financial statements F-35
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income (Loss)
$
Deficit
$
Accumulated
During the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount Balance, December 31, 2006
40,425,615
40,425
10,027,813
—
—
—
72,872
(114,175
)
(9,598,868
)
428,067 Issuance of common stock for services: January 2007—$0.62 per share
135,000
135
83,565
—
—
—
—
—
—
83,700 August 2007—$0.63 per share
15,873
16
9,984
—
—
—
—
—
—
10,000 August 2007—$0.56 per share
17,857
18
9,982
—
—
—
—
—
—
10,000 December 2007—$0.72 per share
57,142
57
41,085
—
—
—
—
—
—
41,142 December 2007—$0.62 per share
10,488
10
6,492
—
—
—
—
—
—
6,502 December 2007—$0.53 per share
223,000
223
117,967
—
—
—
—
—
—
118,190 Issuance of common stock for debt settlement: May 2007—$0.65 per share
100,000
100
55,900
—
—
—
—
—
—
56,000 July 2007—$0.62 per share
100,000
100
61,900
—
—
—
—
—
—
62,000 Issuance of common stock for cash: June 2007—$0.45 per share
220,000
220
98,780
—
—
—
—
—
—
99,000 May 2007—$0.43 per share
23,256
23
9,977
—
—
—
—
—
—
10,000 April 2007—$0.45 per share
35,000
35
15,715
—
—
—
—
—
—
15,750 Share issuance costs
—
—
(11,188
)
—
—
—
—
—
—
(11,188
) Fair value of stock options granted
—
—
412,545
—
—
—
—
—
—
412,545 Issuance of common stock pursuant to the exercise of warrants for cash: March 2007—$0.15 per share
266,667
267
39,733
—
—
—
—
—
—
40,000 March 2007—$0.17 per share
266,667
267
45,067
—
—
—
—
—
—
45,334 Fair value of warrants issued
—
—
22,106
—
—
—
—
—
—
22,106 Issuance of common stock pursuant to the cashless exercise of warrants (December 2007)
246,710
247
(247
)
—
—
—
—
—
—
— Fair value of warrants issued with convertible debt
—
1,426,381
—
—
—
—
—
—
—
1,426,381 Intrinsic value of beneficial conversion feature on convertible debt
—
—
1,426,381
—
—
—
—
—
—
1,426,381 Unrealized loss on investment securities
—
—
—
—
—
—
(115,061
)
—
—
(115,061
) Net loss for the year
—
—
—
—
—
—
—
—
(3,354,319
)
(3,354,319
) Balance, December 31, 2007
42,143,275
42,143
13,899,938
—
—
—
(42,189
)
(114,175
)
(12,953,187
)
832,530 The accompanying notes are an integral part of these financial statements F-36
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income (Loss)
$
Deficit
$
Accumulated
During the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC.
Additional
Common
Stock
Deferred
Other
Accumulated
Deficit
Stockholders’
Common Stock
Shares
Amount Balance, December 31, 2007
42,143,275
42,143
13,899,938
—
—
—
(42,189
)
(114,175
)
(12,953,187
)
832,530 Issuance of common stock for services: December 2008—$0.17 per share
36,000
36
6,084
—
—
—
—
—
—
6,120 December 2008—$0.15 per share
469,914
470
70,017
—
—
—
—
—
—
70,487 Issuance of common stock for debt settlement: January 2008—$0.53 per share
100,000
100
52,900
—
—
—
—
—
—
53,000 April 2008—$0.70 per share
125,000
125
87,375
—
—
—
—
—
—
87,500 Issuance of common stock for cash: March 2008—$0.60 per share
200,000
200
119,800
—
—
—
—
—
—
120,000 June 2008—$0.43 per share
230,000
230
98,670
—
—
—
—
—
—
98,900 Exercise of stock options at $0.001 per share
33,333
33
—
—
—
—
—
—
—
33 Fair value of stock options granted
—
—
372,848
—
—
—
—
—
—
372,848 July 2008, fair value of warrants issued for services
—
—
27,150
—
—
—
—
—
—
27,150 Exercise of warrants at $0.19 per share
84,210
84
15,916
—
—
—
—
—
—
16,000 Fair value of warrants / options modified
—
—
252,799
—
—
—
—
—
—
252,799 Notes payable converted into common shares at $0.60 per share
291,667
292
174,708
—
—
—
—
—
—
175,000 Common stock subscribed—$0.15 per share
—
—
—
40,050
—
—
—
—
—
40,050 Unrealized loss on investment securities
—
—
—
—
—
—
26,660
—
—
26,660 Net loss for the year
—
—
—
—
—
—
—
—
(4,113,985
)
(4,113,985
) Balance, December 31, 2008
43,713,399
43,713
15,178,205
40,050
—
—
(15,529
)
(114,175
)
(17,067,172
)
(1,934,908
) The accompanying notes are an integral part of these financial statements F-37
(dba BIOCUREX, INC.)
(A Development Stage Company)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM JANUARY 1, 2001 (INCEPTION OF DEVELOPMENT STAGE) TO DECEMBER 31, 2008
(Expressed in U.S. dollars)
Paid-in
Capital
$
Stock
Subscribed
$
Subscriptions
Receivable
$
Compensation
$
Comprehensive
Income (Loss)
$
Deficit
$
Accumulated
During the
Development
Stage
$
Equity
(Deficit)
$
#
$
WHISPERING OAKS INTERNATIONAL, INC. 1. NATURE OF OPERATIONS AND CONTINUANCE OF BUSINESS Whispering Oaks International, Inc. (dba BioCurex, Inc.) (the “Company”) was incorporated on December 8, 1997, under the laws of the State of Texas. During the first quarter of 2001, the Company ceased its business activities relating to the acquisition and sale of thoroughbred racehorses when a
change of majority control occurred. On February 21, 2001, the Company acquired intellectual properties and patents relating to cancer diagnostics and therapeutics. The Company is now in the business of developing, producing, marketing and licensing cancer diagnostic kits and is currently considered a
development stage enterprise as defined by Statement of Financial Accounting Standards (“SFAS”) No. 7 “Accounting and Reporting by Development Stage Enterprises”. The financial statements are prepared in conformity with accounting principles generally accepted in the United States of America applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company does not have
sufficient cash nor does it have an established source of revenue to cover its ongoing costs of operations. As of December 31, 2008 the Company has a working capital deficiency of $1,566,901 and accumulated losses of $17,067,172 since the inception of the development stage. These factors raise
substantial doubt about the Company’s ability to continue as a going concern. These financial statements do not include any adjustments that might result from the outcome of this uncertainty. Management’s anticipates expenditures of $3,429,000 over the next twelve months. Management is currently seeking additional financing through the sale of equity and from borrowings from private lenders to cover its operating expenses and restructuring its convertible notes payable into common
stock. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES Use of Estimates The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the
financial statements and the reported amounts of revenues and expenses during the periods. The Company regularly evaluates estimates and assumptions related to allowance for doubtful accounts, valuation of patent costs, valuation of convertible debt, stock-based compensation, and deferred income tax
asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities
and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future
results of operations will be affected. Cash and Cash Equivalents The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents. F-38
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Registration Payment Arrangements The Company accounts for registration rights arrangements and related liquidated damages provisions under EITF 00-19-2 “Accounting for Registration Payment Arrangements” (“EITF 00-19-2”), which addresses an issuer’s accounting for registration payment arrangements. EITF 00-19-2 defines a
registration payment arrangement as an arrangement where the issuer i) will endeavor to file a registration statement for the resale of financial instruments, have the registration statement declared effective, or maintain its effectiveness and ii) transfer consideration to the counterparty if the registration
statement is not declared effective or its effectiveness is not maintained. EITF 00-19-2 requires the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, to be separately recognized and
measured in accordance with Financial Accounting Standards Board (“FASB”) No. 5, “Accounting for Contingencies” and FASB Interpretation No. 14 “Reasonable Estimation of the Amount of a Loss”. Foreign Currency Translation The Company’s functional and reporting currency is the United States dollar. Monetary assets and liabilities denominated in foreign currencies are translated to United States dollars in accordance with SFAS No. 52 “Foreign Currency Translation” using the exchange rate prevailing at the balance
sheet date. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income. Foreign currency transactions are primarily undertaken in Canadian dollars. Revenue Recognition The Company recognizes revenue in accordance with Securities and Exchange Commission Staff Accounting Bulletin No. 104, “Revenue Recognition in Financial Statements” and Staff Accounting Bulletin Topic 13A3(f), “Nonrefundable Up-front Fees.” In accordance with SAB104, revenue is
recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service is performed, and collectibility is reasonably assured. The Company’s revenue consists of license fees related to the licensing of its RECAF™ technology. Currently, there is one license
agreement. Long-lived Assets In accordance with SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, the carrying value of intangible assets and other long-lived assets is reviewed on a regular basis for the existence of facts or circumstances that may suggest impairment. The Company recognizes an
impairment when the sum of the expected undiscounted future cash flows is less than the carrying amount of the asset. Impairment losses, if any, are measured as the excess of the carrying amount of the asset over its estimated fair value. F-39
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Research and Development Costs Research and development costs are charged to operations as incurred. Financial Instruments/Concentrations The fair values of cash, investment securities, notes receivable, accounts payable, due to related parties and convertible notes payable, and convertible debt were estimated to approximate their carrying values due to the immediate or short-term maturity of these financial instruments. The Company’s operations are in Canada, which results in exposure to market risks from changes in foreign currency rates. The financial risk to the Company’s operations results from fluctuations in foreign exchange rates and the degree of volatility of these rates. Currently, the Company does not
use derivative instruments to reduce its exposure to foreign currency risk. Financial instruments that potentially subject the Company to credit risk consist principally of cash. Cash was deposited with a high quality credit institution. For the years ended December 31, 2008 and 2007, revenue from a single customer represented 100% of total revenue. Income Taxes The Company accounts for income taxes using the asset and liability method in accordance with SFAS No. 109, “Accounting for Income Taxes”. The asset and liability method provides that deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary
differences between the financial reporting and tax bases of assets and liabilities, and for operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using the currently enacted tax rates and laws that will be in effect when the differences are expected to reverse. The
Company records a valuation allowance to reduce deferred tax assets to the amount that is believed more likely than not to be realized. Investment Securities The Company reports investments in debt and marketable equity securities at fair value based on quoted market prices or, if quoted prices are not available, discounted expected cash flows using market rates commensurate with credit quality and maturity of the investment. All investment securities
are designated as available-for-sale with unrealized gains and losses included in comprehensive income. The Company regularly reviews investment securities for impairment based on criteria that include the extent to which the investment’s carrying value exceeds its related market value, the duration of
the market decline, the Company’s ability to hold to recovery and the financial strength and specific prospects of the issuer of the security. Unrealized losses that are other than temporary are included in the determination of income. Realized gains and losses are accounted for on the specific
identification method. Comprehensive Income SFAS No. 130, “Reporting Comprehensive Income,” establishes standards for the reporting and display of comprehensive income and its components in the financial statements. As at December F-40
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) 31, 2008 and 2007, the Company’s only component of comprehensive income was unrealized gains and losses on available-for-sale investment securities. Earnings Per Share The Company computes net loss per share in accordance with SFAS No. 128, “Earnings Per Share,” which requires presentation of basic earnings per share and diluted earnings per share (“Diluted EPS”). The computation of basic earnings per share is computed by dividing earnings available to
common stockholders by the weighted-average number of outstanding common shares during the period. Diluted earnings per share give effect to all potentially dilutive common shares outstanding during the period. The computation of Diluted EPS does not assume conversion, exercise or contingent
exercise of securities that would have an anti-dilutive effect on earnings. As of December 31, 2008 and 2007, the Company had approximately 20,650,000 and 22,709,000 respectively, of anti-dilutive securities, including options, warrants and equity instruments related to convertible notes payable. Stock-based Compensation The Company records stock-based compensation in accordance with SFAS No. 123R “Share Based Payments”, using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the
consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Reclassifications Certain reclassifications have been made to the prior period’s financial statements to conform to the current period’s presentation. Recent Accounting Pronouncements In June 2008, the Financial Accounting Standards Board (“FASB”) issued FASB Staff Position No. EITF No. 03-6-1, “Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities” (“FSP EITF No. 03-6-1”). According to FSP EITF No. 03-6-1,
unvested share-based payment awards that contain nonforfeitable rights to dividends or dividend equivalents are considered participating securities under SFAS No. 128. As such, they should be included in the computation of basic earnings per share (“EPS”) using the two-class method. FSP EITF No. 03-
6-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, as well as interim periods within those years. Once effective, all prior-period EPS data presented must be adjusted retrospectively. The Company is currently evaluating the impact of the adoption of EITF No.
03-6-1 on its financial position and results of operations. In May 2008, the Financial Accounting Standards Board (“FASB”) issued SFAS No. 163, “Accounting for Financial Guarantee Insurance Contracts—An interpretation of FASB Statement No. 60”. SFAS No. 163 requires that an insurance enterprise recognize a claim liability prior to an event of default
when there is evidence that credit deterioration has occurred in an insured financial obligation. It also clarifies how Statement 60 applies to financial guarantee insurance contracts, F-41
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) Recent Accounting Pronouncements (continued) including the recognition and measurement to be used to account for premium revenue and claim liabilities, and requires expanded disclosures about financial guarantee insurance contracts. It is effective for financial statements issued for fiscal years beginning after December 15, 2008, except for some
disclosures about the insurance enterprise’s risk- management activities. SFAS No. 163 requires that disclosures about the risk-management activities of the insurance enterprise be effective for the first period beginning after issuance. Except for those disclosures, earlier application is not permitted. The
adoption of this statement is not expected to have a material effect on the Company’s financial statements. In May 2008, the FASB issued SFAS No. 162, “The Hierarchy of Generally Accepted Accounting Principles”. SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of nongovernmental entities
that are presented in conformity with generally accepted accounting principles in the United States. It is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411, “The Meaning of Present Fairly in Conformity With Generally
Accepted Accounting Principles”. The adoption of this statement is not expected to have a material effect on the Company’s financial statements. In March 2008, the FASB issued SFAS No. 161, “Disclosures about Derivative Instruments and Hedging Activities—an amendment to FASB Statement No. 133”. SFAS No. 161 is intended to improve financial standards for derivative instruments and hedging activities by requiring enhanced disclosures
to enable investors to better understand their effects on an entity’s financial position, financial performance, and cash flows. Entities are required to provide enhanced disclosures about: (a) how and why an entity uses derivative instruments; (b) how derivative instruments and related hedged items are
accounted for under Statement No. 133 and its related interpretations; and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance, and cash flows. It is effective for financial statements issued for fiscal years beginning after November 15, 2008,
with early adoption encouraged. The adoption of this statement is not expected to have a material effect on the Company’s financial statements. 3. INVESTMENT SECURITIES In November 2002, the Company entered into a licensing agreement (“the Agreement”) with a third party whereby it licensed part of its technology in exchange for cash and 600,000 shares of the third party’s publicly traded common stock that had a fair value of $162,000. The 600,000 shares of
common stock are classified as “available for sale” in accordance with SFAS No. 115 and are reported at fair value. During the year of 2008, the Company sold 259,300 shares for proceeds of $53,621, resulting in a realized loss of $16,389. As of December 31, 2008 the Company has 124,235 shares and the
fair market value of these shares was $18,014. F-42
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 4. NOTES RECEIVABLE
December 31,
December 31, Note receivable including interest at prime plus 4%
73,489
73,489 Notes receivables from employees
35,497
35,497 Less: allowance for doubtful accounts
(106,320
)
(73,489
) Total
2,666
35,497 Notes receivable from various employees are pursuant to stock options exercised and are non-interest bearing and due on demand. 5. PATENTS Patents relate to developing the method for diagnostic and treatment of cancer using a new cancer marker called “RECAF.” These patents are presently registered in 23 countries with ongoing registrations currently being conducted. Patents are stated at cost and have a definite life. Once the
Company receives patent approval, amortization is calculated using the straight-line method over the remaining life of the patents. Patent costs of $191,512 and $59,704 were capitalized during the years ended December 31, 2008 and 2007, respectively. As of December 31, 2008, the management decided to recognize an impairment loss on patent costs incurred in Japan as the respective patent was allowed to lapse. The Company has recorded an impairment loss of $67,620 in the year ended December 31, 2008. A schedule of the patents is as follows:
December 31,
2008
2007 Patents
688,581
497,069 Less: Accumulated amortization
(174,015
)
(136,257
) Loss on impairment of patent cost
(67,620
)
— Net Carrying Value
446,946
360,812 Amortization expense totaled $37,758 and $27,896 for the years ended December 31, 2008 and 2007, respectively. The estimated future amortization expense is as follows:
$ 2009
37,758 2010
37,758 2011
37,758 2012
37,758 2013
37,758 Thereafter
258,156
446,946 F-43
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
2008
$
2007
$
$
$
WHISPERING OAKS INTERNATIONAL, INC. 6. RELATED PARTY TRANSACTIONS
December 31,
2008
2007 Due to Pacific BioSciences Research Centre Inc. (a)
328,269
337,355 Due to officers (b)
7,000
7,000
335,269
344,355
a)
The Company’s research and development is performed by Pacific BioSciences Research Centre (“Pacific”). Pacific is 100% owned by the President of the Company. During the years ended December 31, 2008 and 2007, Pacific performed research and development for the Company valued at
$674,326 and $651,009, respectively. Pacific also provided general and administrative services during the years ended December 31, 2008 and 2007, valued at $242,583 and $220,320, respectively. Included in these amounts were rent expense of $57,098 and $59,444, respectively. During the year ended December 31, 2008, Pacific charged
interest of $7,241 (2007—$15,667), calculated at bank prime rate on the monthly balance owed. The amount due to Pacific is unsecured and due on demand. A total of $100,000 was paid to Pacific for prepaid expenses as at December 31, 2008. b) The amounts owing to officers are unsecured, non-interest bearing and due on demand. c) During the year ended December 31, 2008, the Company granted 570,000 (2007—580,000) stock options to three directors at a below market exercise price of $0.001 per share. d) On August 28, 2008, the Company extended the term of 1,300,000 fully vested stock options for two directors. The Company recognized an incremental compensation cost of $446 for these modified stock options. e) On December 3, 2008, the Company extended the term of 925,000 fully vested stock options for three directors. The Company recognized an incremental compensation cost of $43 for these modified stock options. f) On December 3, 2008, the Company extended the term of 1,977,278 share purchase warrants for one director. The Company recognized an incremental compensation cost of $34,902 for these modified share purchase warrants. 7. CONVERTIBLE DEBT
a)
The Company received funds during 2003 relating to ten convertible notes payable totaling $529,743, bearing interest at 5% and due on demand. One of the notes payable in the amount of $53,000 was repaid in April 2003. A gain of $33,584 was recorded on the date of repurchase of the
convertible debenture as determined through the calculation of the intrinsic value of the beneficial conversion feature on the date of extinguishment. Under the convertibility terms of the notes payable, the principal, plus accrued interest, can be converted immediately, at the option of the holder,
either in whole, or in part, into fully paid common shares of the Company. The conversion price per share is equal to the lesser of the stated price (ranging between $0.05 and $0.23) or 75% of the average closing bid prices for the five trading days ending on the trading day immediately before the
date of the conversion. In conjunction with the issuance of the notes, the Company issued 2,434,088 warrants to the note holders entitling them to purchase 2,434,088 shares of common stock at exercise prices between $0.08 and $0.38. The warrants expired two years after the issuance date.
F-44
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
$
$
WHISPERING OAKS INTERNATIONAL, INC. 7. CONVERTIBLE DEBT (continued)
In accordance with EITF 00-27 “Application of Issue No. 98-5 to Certain Convertible Instruments” and EITF 98-5 “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios”, the proceeds were allocated between the debt and warrants
based on their relative fair values. The value assigned to the warrants totaled $274,601 and was expensed immediately due to the notes being due on demand. The fair values were determined using the Black-Scholes option pricing model using the following weighted average assumptions: average
risk-free interest rate of 1.49%; expected life of two years; expected volatility of 473%; and no expected dividends. In addition to the shares to be received upon conversion, the note holder will also receive an equal number of warrants to purchase shares at 110% of the conversion price amount.
The beneficial conversion feature was calculated under EITF 00-27, and equaled $255,142. Due to the notes being due on demand, the discount was expensed in fiscal 2003. The convertibility feature expires five years after the date of the Agreement. In February 2005, a note in the amount of $143,370 was converted into 955,800 units, consisting of one common share at $0.15 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.17 per share expiring on March 9,
2010. In accordance with EITF 00-27, the Company recognized $67,829 for the intrinsic value of the embedded conversion option. In July 2006, a note in the amount of $61,890 was converted into 343,833 units, consisting of one common share at $0.18 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.20 per share expiring on July 7, 2011. In
accordance with EITF 00-27, the Company recognized $29,506 for the intrinsic value of the embedded conversion option. In July 2006, a note in the amount of $11,655 was converted into 233,092 units, consisting of one common share at $0.05 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.055 per share expiring on July 7, 2011.
In accordance with EITF 00-27, the Company recognized $5,565 for the intrinsic value of the embedded conversion option. In July 2006, a note in the amount of $65,000 was converted into 590,909 units, consisting of one common share at $0.11 per share and one common share purchase warrant entitling the holder to acquire an additional common share at an exercise price of $0.12 per share expiring on July 19, 2011.
In accordance with EITF 00-27, the Company recognized $30,089 for the intrinsic value of the embedded conversion option. The effective interest rate of the remaining convertible notes at December 31, 2008 is 335%. b) On July 7, 2007, the Company received proceeds of $3,000,000 from the issuance of convertible notes (the “Notes”), plus share purchase warrants, to two private investors. The share purchase warrants allow the holders to purchase up to 3,500,000 shares of the Company’s common stock at a price of
$0.60 per share expiring June 25, 2012. The Notes bear interest annually at a rate of prime (as adjusted monthly on the first business day of each month) plus 2.75% per year. The Notes are due and payable on June 25, 2010 and are secured by substantially all of the Company’s assets. Interest is
payable monthly with the first interest payment due on August 1, 2007. Beginning on November 1, 2007, the Company is required to make monthly payments of $100,000 towards the principal amount of the Notes. If F-45
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 7. CONVERTIBLE DEBT (continued)
the Company fails to make any interest or principal payment when due, the Notes will become immediately due and payable. At the holders’ option the Notes are convertible into shares of the Company’s common stock at a conversion price of $0.60 per share. The Company may elect to pay the
monthly redemption amounts and accrued interest with shares of its common stock, which will be determined by dividing the amount to be paid by the lesser of the conversion price then in effect or 80% of the weighted average price of the Company’s common stock for the ten trading days
preceding the payment date. In order to make principal or interest payments with shares of its common stock certain conditions must be met, including the condition that the number of shares to be issued in payment of principal or interest cannot exceed 25% of the total shares traded for the ten
trading days prior to the payment date. The Company agreed to file a Form SB-2 Registration Statement (“SB-2”) with the U.S. Securities and Exchange Commission in order that the shares of common stock issuable upon the conversion of the Notes or the exercise of the share purchase warrants
may be resold in the public market. The Company was required to file the SB-2 no later than July 30, 2007 (filed), to cause the SB-2 to become effective by November 26, 2007, and to keep the SB-2 continuously effective until the shares covered by the SB-2 have been sold or can be sold pursuant
to Rule 144(k). In the event the closing price of the Company’s common stock is $1.20 or greater for ten consecutive trading days, the holders will be required to exercise the 3,500,000 share purchase warrants within ten days notice by the Company. Following the exercise of the share purchase warrants, the
Company will issue to the holders 3,500,000 new share purchase warrants, which will entitle the holders to purchase 1,750,000 shares of common stock. Two share purchase warrants will be exercisable at a price of $1.20 per share at any time prior to the later of June 25, 2012 or three years from the
date the new share purchase warrants are issued. In accordance with EITF 00-27 and EITF 98-5, the proceeds were allocated between the debt and warrants based on their relative fair values. The relative fair value assigned to the share purchase warrants totaled $1,426,381 and was determined using the Black-Scholes option pricing model using
the following weighted average assumptions: average risk-free interest rate of 4.76%; expected life of five years; expected volatility of 176%; and no expected dividends. These amounts were recorded as a debt discount and will be amortized as interest expense over the term of the convertible
debentures. The effective interest rate at December 31, 2008 is 406%. For the year ended December 31, 2008, the Company recorded $976,064 (2007—$791,092) of accretion expense related to the convertible debt. On August 18, 2008, the Company agreed to re-price the 3,500,000 share purchase warrants to an exercise price of $0.25 per share. In accordance with SFAS No. 123R, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental interest
expense is measured as the excess, if any, of the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental interest expense of $192,264 for these modified purchase
warrants. On November 26, 2008, the Company received notification from the note holders which modified the terms of the Notes. Pursuant to the notification the interest and principal payments payable in December 2008 and all subsequent principal and interest payments were F-46
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 7. CONVERTIBLE DEBT (continued)
deferred until May 1, 2009. In addition the principal amount outstanding was increased by $255,000 to $1,955,000. In accordance with EITF 02-04 “Determining Whether a Debtor’s Modification or Exchange of Debt Instruments in within the Scope of FASB Statement No. 15” the Company determined that the creditor did not grant a concession even though the payments were deferred as the total amount owing
by the Company was increased. As at November 26, 2008, prior to the modification of the convertible notes, the carrying value of the convertible notes was $613,738. The remaining unaccreted discount of $304,467 related to the convertible notes was charged to operations. In accordance with EITF 98-5 “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios”, the Company determined there was no beneficial conversion feature on the modified convertible notes. The Company recorded a discount of
$130,298 which was equal to the difference of the face value of the new note and the present value of the revised cash flows. The effective interest rate of the new notes is 6.56%. The Company incurred $643,301 in debt issue costs for these convertible debentures. The debt issue costs will be expensed over the term of the convertible debt. During the year ended December 31, 2008, the Company expensed $214,434 (2007—$107,217) of the debt issue costs related to the
convertible debt. 8. COMMON STOCK For the year ended December 31, 2008:
a)
In December 2008, the Company issued 505,914 shares of common stock at a fair value of $76,607 to eight employees and one consultant for services provided from December to January 2009, a total of $37,672 was recorded as prepaid expenses as at December 31, 2008. b) In December 2008, the Company received stock subscriptions of 267,000 shares of common stock at $0.15 per share for proceeds of $40,050. Each unit consisted of one share of common stock and one half share purchase warrant entitling the holder to purchase one share of common stock at an
exercise price of $0.30 per share expiring on November 30, 2010. c) In June 2008, the Company issued 230,000 shares of common stock at $0.43 per share for proceeds of $98,900. Each unit consisted of one share of common stock and one half share purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.65 per share
expiring on May 1, 2010. d) In June, 2008, the Company issued 42,105 shares of common stock pursuant to the exercise of share purchase warrants for proceeds of $8,000. e) In April, 2008, the Company issued 33,333 shares of common stock pursuant to the exercise of 33,333 stock options for proceeds of $33. f) In April, 2008, the Company issued 42,105 shares of common stock pursuant to the exercise of share purchase warrants for proceeds of $8,000. g) In April, 2008, the Company issued 125,000 shares of common stock at a fair value of $87,500 to settle debt. F-47
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 8. COMMON STOCK (continued)
h)
In March 2008, the Company issued 291,667 shares of common stock at $0.60 per share for the conversion of debt of $175,000. i) In March 2008, the Company issued 200,000 shares of common stock at $0.60 per share for proceeds of $120,000. j) In January 2008, the Company issued 100,000 shares of common stock at a fair value of $53,000 to settle debt. For the year ended December 31, 2007:
a)
In December 2007, the Company issued 57,142 shares of common stock to a consultant at a fair value of $41,142 for consulting services, of which $6,857 was expensed and $34,285 was recorded as prepaid expenses as at December 31, 2007. b) In December 2007, the Company issued 10,488 shares of common stock to a consultant at a fair value of $6,502 for consulting services of which $3,251 was expensed and $3,251 was recorded as a prepaid expense as at December 31, 2007. c) In December 2007, the Company issued 223,000 shares of common stock at a fair value at $118,190 to eight employees and one consultant for performance bonuses. d) In December 2007, the Company issued 246,710 shares of common stock pursuant to the exercise of 250,000 warrants. This exercise was based on the cashless exercise provision of the warrants. e) In August 2007, the Company issued 33,730 shares of common stock to a consultant at a fair value of $20,000 for consulting services. f) In July 2007, the Company issued 100,000 shares of common stock at a fair value of $62,000 to settle debt. g) In June 2007, the Company issued 100,000 shares of common stock at a fair value of $56,000 to settle debt. h) In June 2007, the Company issued 533,334 shares of common stock for proceeds of $85,334 pursuant to the exercise of share purchase warrants. i) In June 2007, the Company issued 220,000 units at $0.45 per unit for proceeds of $99,000. Each unit consisted of one share of common stock and one share purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.66 per share expiring on April 9, 2009.
The Company paid a commission of $9,900 in connection with this private placement. j) In May 2007, the Company issued 23,256 units at $0.43 per unit for proceeds of $10,000. Each unit consisted of one share of common stock and one share purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.68 per share expiring on May 31, 2009.
The Company paid a commission of $500 in connection with this private placement. k) In April 2007, the Company issued 35,000 units at $0.45 per unit for proceeds of $15,750. Each unit consisted of one share of common stock and one share purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.66 per share expiring on April 30,
2009. The Company paid a commission of $788 in connection with this private placement. l) In January 2007, the Company issued 135,000 shares of common stock at a fair value at $83,700 to eight employees for performance bonuses. F-48
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 9. SHARE PURCHASE WARRANTS A summary of the changes in the Company’s share purchase warrants is presented below:
Number
Weighted Average Balance, December 31, 2006
9,154,416
0.46 Issued
3,878,256
0.60 Exercised
(783,334
)
0.11 Expired
(358,666
)
2.46 Balance, December 31, 2007
11,890,672
0.47 Issued
473,500
0.48 Exercised
(84,210
)
0.19 Expired
(505,000
)
0.81 Balance, December 31, 2008
11,774,962
0.35 As at December 31, 2008, the following share purchase warrants were outstanding:
Warrants
Exercise
Expiration Date 2,455,000
0.90
February 01, 2009 126,317
0.19
March 31, 2009 220,000
0.66
April 06, 2009 35,000
0.66
April 30, 2009 23,256
0.68
May 31, 2009 225,000
0.50
July 15, 2009 100,000
0.60
July 17, 2009 1,275,000
0.08
January 15, 2010 955,800
0.17
March 09, 2010 115,000
0.65
May 01, 2010 541,666
0.12
October 31, 2010 199,311
0.17
November 11, 2010 133,500
0.30
November 30, 2010 233,092
0.06
July 07, 2011 343,833
0.20
July 07, 2011 590,909
0.12
July 19, 2011 252,278
0.05
December 31, 2011 450,000
0.00
March 31, 2012 3,500,000
0.25
June 27, 2012 11,774,962 In January 2008, the Company extended the term of 1,275,000 share purchase warrants. In accordance with SFAS No. 123R, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is measured as the excess, if any, of
the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $2,675 for these modified share purchase warrants. F-49
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
Exercise Price
$
Price
$
WHISPERING OAKS INTERNATIONAL, INC. 9. SHARE PURCHASE WARRANTS (continued) In July 2008, a total of 225,000 warrants were issued to a consulting firm at an exercise price of $0.50 per share expiring on July 15, 2009. The fair value of warrants of $27,150 was charged to operations. The fair value was determined using the Black-Scholes option pricing model using the following
assumptions: risk-free interest rate of 2.35%, expected life of 1 year, expected volatility of 66% and no expected dividends. The grant date fair value of these warrants was $0.36 per warrant. In August 2008, the Company extended the term of 3,185,977 share purchase warrants. In accordance with SFAS No. 123R, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is measured as the excess, if any, of
the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $15,710 for these modified share purchase warrants. In December 2008, the Company extended the term of 4,432,278 share purchase warrants. In accordance with SFAS No. 123R, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is measured as the excess, if any, of
the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $41,660 for these modified share purchase warrants. 10. STOCK OPTIONS Stock Bonus Plan Under the Company’s Stock Bonus Plan, employees, directors, officers, consultants and advisors are eligible to receive a grant of the Company’s shares, provided that bona fide services are rendered by consultants or advisors and such services must not be in connection with the offer or sale of
securities in a capital-raising transaction. A total of 5,500,000 common shares are reserved pursuant to this Plan, with 1,769,411 (2007—2,500,325) common shares available for future issuance as of December 31, 2008. Non-Qualified Stock Option Plan The Company’s Non-Qualified Stock Option Plan authorizes the issuance of common shares to persons that exercise stock options granted pursuant to this Plan. The Company’s employees, directors, officers, consultants and advisors are eligible to be granted stock options pursuant to this Plan,
provided that bona fide services are rendered by such consultants or advisors and such services must not be in connection with the offer or sale of securities in a capital-raising transaction. The stock option exercise price is determined by a committee and cannot be less than $0.001. A total of 12,500,000
common shares are reserved pursuant to this Plan, with 2,587,723 (2007—2,844,390) common shares available for future issuance as of December 31, 2008. On January 31, 2007, the Company granted 580,000 stock options to three directors at a below market exercise price of $0.001 per share. On May 30, 2007, the Company granted 180,000 stock options to a consultant at an exercise price of $0.60 per share expiring on May 30, 2009. On September 15, 2007, the Company granted 66,666 stock options to a consultant at an exercise price of $0.60 per share expiring on September 15, 2010. F-50
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
WHISPERING OAKS INTERNATIONAL, INC. 10. STOCK OPTIONS (continued) Non-Qualified Stock Option Plan (continued) On February 14, 2008, the Company granted 570,000 stock options to three directors at a below market exercise price of $0.001 per share. On August 28, 2008, the Company extended the term of 1,300,000 fully vested stock options for two directors. In accordance with SFAS No. 123R, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is measured as
the excess, if any, of the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $446 for these modified stock options. On December 3, 2008, the Company extended the term of 925,000 fully vested stock options for three directors. In accordance with SFAS No. 123R, modifications to the terms of an award are treated as an exchange of the original award for a new award. Incremental compensation cost is measured
as the excess, if any, of the fair value of the original award immediately before its terms are modified, measured based on the share price and other pertinent factors at that date. The Company recognized an incremental compensation cost of $44 for these modified stock options. A summary of the changes in the Company’s stock options is presented below:
Number of
Weighted
Weighted
Aggregate Outstanding, December 31, 2006
3,065,000
0.05 Granted
826,666
0.13 Expired
(225,000
)
0.64 Outstanding, December 31, 2007
3,666,666
0.03 Granted
570,000
0.001 Exercised
(33,333
)
0.001 Expired
(313,333
)
0.367 Outstanding, December 31, 2008
3,890,000
0.001
2.99
774,110 Exercisable, December 31, 2008
3,890,000
0.001
2.99
774,110 The fair value for stock options granted was estimated at the date of grant using the Black-Scholes option-pricing model and the weighted average fair value of stock options granted during the year ended December 31, 2008 and 2007 were $0.64 and $0.51 per share, respectively. The weighted average assumptions used are as follows:
December 31,
2008
2007 Expected dividend yield
0
%
0
% Risk-free interest rate
1.97
%
4.84
% Expected volatility
63
%
89
% Expected option life (in years)
2.0
2.78 F-51
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
Shares
Average
Exercise Price
$
Average
Remaining
Contractual Life
(Years)
Intrinsic Value
$
WHISPERING OAKS INTERNATIONAL, INC. 10. STOCK OPTIONS (continued) Non-Qualified Stock Option Plan (continued) As at December 31, 2008, there was $nil (2007—$14,447)) of total unrecognized compensation costs related to non-vested share-based compensation arrangements. The total fair value of shares vested during the year ended December 31, 2008 and 2007 were $364,252 and $412,545, respectively. A summary of the status of the Company’s non-vested shares as of December 31, 2008, and changes during the period of December 31, 2008, is presented below:
Number of
Weighted Average Non-vested at December 31, 2006
—
— Granted
180,000
0.19 Vested
(105,000
)
0.19 Non-vested at December 31, 2007
75,000
0.19 Granted
570,000
0.001 Forfeited
(30,000
)
0.19 Vested
(615,000
)
0.19 Non-vested at December 31, 2008
—
— 11. COMMITMENTS
a)
On April 4, 2006, the Company entered into a consulting agreement with a term of six months for consideration of 75,000 common shares. As of December 31, 2008, the Company has issued 37,500 common shares and 37,500 common shares are still owed to the consultant. b) On April 10, 2006, the Company entered into a consulting agreement with a term of one year for consideration of 75,000 common shares. As of December 31, 2008, the Company has issued 37,500 common shares and 37,500 common shares are still owed to the consultant. 12. INCOME TAXES The Company has adopted the provisions of SFAS 109, “Accounting for Income Taxes”. Pursuant to SFAS 109 the Company is required to compute tax asset benefits for net operating losses carried forward. The potential benefit of net operating losses have not been recognized in the consolidated
financial statements because the Company cannot be assured that it is more likely than not that it will utilize the net operating losses carried forward in future years. The Company has incurred operating losses of approximately $10,572,264 which, if unutilized, will expire through to 2028. Future tax benefits, which may arise as a result of these losses, have not been recognized in these consolidated financial statements, and have been offset by a valuation
allowance. The following table lists the fiscal year in which the loss was incurred and the expiration date of the loss. F-52
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
Shares
Grant Date
Fair Value
$
WHISPERING OAKS INTERNATIONAL, INC. 12. INCOME TAXES (continued)
Net Loss
Expiration Date 1999
$
89,948
2019 2000
24,052
2020 2001
793,976
2021 2002
231,928
2022 2003
1,120,379
2023 2004
1,400,412
2024 2005
1,645,391
2025 2006
1,888,080
2026 2007
2,327,750
2027 2008
1,050,348
2028
$
10,572,264 The Company is subject to United States federal and state income taxes at an approximate rate of 35%. The reconciliation of the provision for income taxes at the United States federal statutory rate compared to the Company’s income tax expense as reported is as follows:
Year Ended
Year Ended Income tax recovery at statutory rate
1,439,895
1,140,468 Convertible debt interest
(448,186
)
(346,138
) Loss on convertible debt modification
(317,274
)
— Stock based compensation
(255,291
)
— Other
(19,255
)
(2,895
) Change in tax rates
95,219
— Valuation allowance change
(495,108
)
(791,435
) Provision for income taxes
—
— The significant components of deferred income tax assets and liabilities as at December 31, 2008 and 2007 are as follows:
December 31,
December 31, Net operating loss carryforward
3,700,292
3,237,452 Intangible assets
32,268
— Valuation allowance
(3,732,560
)
(3,237,452
) Net deferred income tax asset
—
— 13. SUBSEQUENT EVENTS
a)
On January 2, 2009, the Company received stock subscriptions of 307,692 shares of common stock at $0.13 per share for proceeds of $40,000. Each unit consisted of one share of common stock and one purchase warrant entitling the holder to purchase one share of common stock at an exercise price
of $0.17 per share expiring on January 2, 2011.
F-53
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
December 31,
2008
$
December 31,
2007
$
2008
$
2007
$
WHISPERING OAKS INTERNATIONAL, INC. 13. SUBSEQUENT EVENTS (continued)
On January 8, 2009, the Company issued 150,000 shares of common stock at a fair value of $36,000 to settle debt. c) On January 8 2009, the Company issued 267,000 units at $0.15 per unit for common share subscriptions totaling $40,050 received in December 2008. Each unit consisted of one share of common stock and one half share purchase warrant entitling the holder to purchase one share of common stock at
an exercise price of $0.30 per share expiring on November 30, 2010. d) On January 9, 2009, the Company issued 31,250 shares of common stock at a fair value of $5,000 to settle debt. e) On January 9, 2008, the Company issued 56,000 shares of common stock at a fair value of $8,960 to an employee for services rendered in December 2008 and January 2009. f) On January 21, 2009, the Company advanced $50,000 to Biocurex China, in order to complete the incorporation procedures and comply with Chinese statutory regulations. g) On February 20, 2009, the Company issued 639,142 shares of common stock at a fair value of $89,480 to eight employees and one consultant for services provided from February to March 2009. h) On March 3, 2009, an employee returned 33,333 shares with a fair value of $2,666, to settle an amount owing to the Company in the amount of $20,000. A total of $17,333 was recorded as bad debt expense in the fiscal year ended December 31, 2008. i) On March 17, 2009, the Company granted 2,263,157 stock options to two directors at a below market exercise price of $0.001 per share. F-54
(dba BIOCUREX, INC.)
(A Development Stage Company)
NOTES TO THE FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2008 AND 2007
(Expressed in U.S. dollars)
b)
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus or any prospectus supplement. This prospectus is not an offer of these securities in any jurisdiction where an offer
and sale is not permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. 1,200,000 Units Prospectus Paulson Investment Company, Inc. , 2010
each consisting of
70 shares of common stock and 70 common stock purchase warrants
or an aggregate of
84,000,000 shares and 84,000,000 warrants
PART II INFORMATION NOT REQUIRED IN PROSPECTUS Item 13. Other Expenses of Issuance and Distribution SEC registration fee
$
1,769 FINRA filing fee
$
3,670 Accounting fees and expenses
$
50,000
* Printing and engraving expenses
$
85,000
* Legal fees and expenses
$
250,000
* State Blue Sky fees and expenses
$
75,000
* Miscellaneous and “Road Show” expenses
$
34,561
* Total
$
500,000
*
*
Estimates.
Item 14. Indemnification of Directors and Officers Article 2.02-1 of the Texas Business Corporation Act (the “TBCA”) and our bylaws provide that any of our directors or officers may be indemnified against judgments, penalties, fines, settlements and reasonable expenses actually incurred by him in connection with or in defending any action, suit or
proceeding in which he was, is, or is threatened to be made a named defendant by reason of his position as director or officer, provided that he conducted himself in good faith and reasonably believed that, in the case of conduct in his official capacity as a director or officer of the corporation, such
conduct was in the corporation’s best interests; and, in all other cases, that such conduct was at least not opposed to the corporation’s best interests. In the case of a criminal proceeding, a director or officer may be indemnified only if he had no reasonable cause to believe his conduct was unlawful. If a
director or officer is wholly successful, on the merits or otherwise, in connection with such a proceeding, such indemnification is mandatory. In addition, our articles of incorporation provide that we may indemnify any person made a party to any action, suit or proceeding, whether civil or criminal, by reason of the fact that he or she, his or her testator, or intestate, is or was a director, officer, or employee of us, or of any corporation,
which he or she served in such capacity at our request, against the reasonable expenses, including attorney’s fees, actually and reasonably incurred by him or her in connection with the defense of the action, suit or proceeding or in connection with any appeal in it. This right to indemnification conferred
shall not restrict our power to make any other type of indemnification permitted by law. Under our bylaws, we may also indemnify and advance expenses to persons who are not or were not officers, employees, or agents of us but who are or were serving at our request as a director, officer, partner,
venturer, proprietor, trustee, employee, agent, or similar functionary of another foreign or domestic corporation, partnership, joint venture, sole proprietorship, trust, employee benefit plan, or other enterprise to the same extent that we may indemnify and advance expenses to directors and officers under
our bylaws. Further, our articles of incorporation and bylaws provide that no person shall be liable to us for any loss or damage suffered by us on account of any action taken or omitted to be taken by him as a director, officer or employee of us in good faith, it, in the exercise of ordinary care, this person (A)
replied upon our financial statements represented to be correct by our President or our officer having charge of our books of account, or stated in a written report by an independent public or certified public accountant or firm of such accountants fairly to reflect the financial condition of us, or
considered the assets to be of their book value, or (B) relied upon the written opinion of any attorney hired by or representing us. However, a director may not be indemnified under our bylaws for obligations resulting from a proceeding: (A) in which the person is found liable on the basis that personal benefit was improperly received by him, whether or not the benefit resulted from an action taken in the person’s II-1
official capacity; or (B) in which the person is found liable to us. Further, a person may be indemnified under our bylaws against judgments, penalties, fines, settlements and reasonable expenses actually incurred by the person in connection with the proceeding, but if the proceeding was brought by us or
on our behalf, the indemnification is limited to reasonable expenses actually incurred by the person in connection with the proceeding. Under our bylaws, a determination of indemnification must be made: (A) by a majority vote of a quorum consisting of directors who at the time of the vote are not named defendants or respondents in the proceeding, (B) if such a quorum cannot be obtained, by a majority vote of a committee of
the board of directors, designated to act in the matter by a majority vote of all directors, consisting exclusively of directors who at the time of the vote are not named defendants or respondents in the proceeding, (C) by special legal counsel selected by the board of directors or a committee of the board
of directors by vote as set forth in (A) or (B), or, if such a quorum cannot be obtained and such a committee cannot be established, by a majority vote of all directors; or (D) by the stockholders in a vote that excludes the shares held by the directors who are named defendants or respondents in the
proceeding. Additionally, authorization of indemnification and determination as to reasonableness of expenses must be made in the same manner as the determination that indemnification is permissible, except that if the determination that indemnification is permissible is made by special legal counsel,
authorization of indemnification and determination as to reasonableness of expenses must be made in the manner specified by (C), for the selection of special legal counsel. A provision contained in our articles of incorporation, our bylaws, a resolution of stockholders or directors, or an agreement that
makes mandatory the indemnification permitted under our bylaws shall be deemed to constitute authorization of indemnification in the manner required even though such provision may not have been adopted or authorized in the same manner as the determination that indemnification is permissible. These limitations of liability, indemnification and expense advancements may discourage a stockholder from bringing a lawsuit against directors for breach of their fiduciary duties. The provisions may also reduce the likelihood of derivative litigation against directors and officers, even though an
action, if successful, might benefit us and our stockholders. A stockholder’s investment may be adversely affected to the extent we pay the costs of defense or settlement and damage awards against directors and officers pursuant to these limitations of liability and indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is
against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment of expenses incurred or paid by a director, officer or controlling person in a successful defense of any action, suit or
proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to the court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. Insurance. Article 2.02-1(R) of the TBCA, our articles of incorporation and our bylaws authorize us to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of us or who is or was serving at our request as a director, officer, partner, venturer,
proprietor, trustee, employee, agent or similar functionary of another foreign or domestic corporation, employee benefit plan, other enterprise or other entity, against any liability asserted against him and incurred by him in such a capacity or arising out of his status as such a person, whether or not we
would have the power to indemnify him against that liability under Article 2.02-1. We do not maintain directors and officers liability insurance, which covers our directors and officers against certain claims or liabilities arising our of the performance of their duties. II-2
Item 15. Recent Sales of Unregistered Securities.
In October 2009, we issued an aggregate of 714,286 shares of common stock to the two holders of our amended secured convertible notes on the conversion of $100,000 of principal of those notes. 2. In September 2009, we issued an aggregate of 1,428,576 shares of common stock to the holders (2) of our amended secured convertible notes on the conversion of $200,000 of principal of those notes. 3. In September 2009, we issued $450,000 aggregate principal amount of our 10% unsecured promissory notes due August 31, 2010 and an aggregate of 6,428,578 shares of common stock to 17 investors, all of whom qualified as “accredited investors”. 4. In September 2009, we sold $125,000 aggregate principal amount of our 10% unsecured promissory notes due January 31, 2013 and an aggregate of 1,785,714 shares of common stock to three investors, all of whom qualified as “accredited investors”. 5. In September 2009, we sold 400,000 shares to a private investor for $20,000. 6. In August 2009, we issued 143,000 shares of our common stock to a consultant for services. 7. In August 2009 we issued 2,204,730 shares of our common stock to note holders upon the conversion of a total principle balance of $160,945. 8. In August 2009 we issued 2,000,000 shares of our common stock to one person for services previously rendered. The shares were valued at $70,000. 9. In August 2009 we issued our amended secured convertible notes, in the aggregate principal amount of $2.15 million, to the original holders of our secured convertible notes issued in June 2007. The amended secured convertible notes were issued in exchange for the original secured convertible notes
issued in June 2007 to two accredited investors. (See paragraph 19 below.) The new notes were exempt securities under Section 3(a)(9) of the Securities Act and the transaction was exempt under Sections 4(2) and 4(6) of the Securities Act and Regulation D promulgated thereunder. 10. In June 2009, we sold 1,000,000 units at $0.05 per unit for gross proceeds of $50,000. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on June 15, 2011. We recorded a
commission of $2,500 that was paid in July 2009 in connection with this private placement. 11. In June 2009, we issued 300,000 shares of common stock to an investor relations company for their consulting services at a fair value of $26,700. 12. In May 2009, we issued 2,000,000 units at $0.05 per unit for gross proceeds of $100,000. Each unit consisted of one share of common stock and one warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on April 1, 2012. We paid a
commission of $10,000 in connection with this private placement. 13. In April 2009, we issued 900,000 units at $0.05 per unit for gross proceeds of $45,000. Each unit consisted of one share of common stock and one purchase warrant entitling the holder to purchase one share of common stock at an exercise price of $0.11 per share expiring on April 5, 2011. 14. In April 2009, we issued 307,892 units at $0.13 per unit for gross proceeds of $40,000. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.17 per share expiring on January 2, 2011. 15. In January 2009, we issued 267,000 units at $0.15 per unit for gross proceeds of $40,050. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one-half share of common stock at an exercise price of $0.30 per share expiring on November 30, 2010. 16. In December 2008, we sold 267,000 units at $0.15 per share for gross proceeds of $40,050. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase II-3
1.
one-half share of common stock at an exercise price of $0.30 per share expiring on November 30, 2010. 17. In June 2008, we issued 230,000 units at $0.43 per unit for gross proceeds of $98,900. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.65 per share expiring on May 1, 2010. 18. In June, 2008, we issued 42,105 shares of common stock pursuant to the exercise warrants, realizing gross proceeds of 8,000. 19. In April, 2008, we issued 42,105 shares of common stock pursuant to the exercise of warrants, realizing proceeds of $8,000. 20. In March 2008, we issued 200,000 shares of common stock at $0.60 per share, realizing gross proceeds of $120,000. 21. In December 2007, we issued 246,710 shares of common stock pursuant to the exercise of 250,000 warrants. This exercise was based on the cashless exercise provision of the warrants. 22. In June 2007, we issued secured convertible notes, in the aggregate principal amount of $3,000,000, and warrants to purchase 3,500,000 shares of our common stock to two accredited investors in a private placement transaction. The total consideration received was $3,000,000. 23. In June 2007, we issued 533,334 shares of common stock pursuant to the exercise of warrants, realizing proceeds of $85,334. 24. In June 2007, we issued 220,000 units at $0.45 per unit, realizing gross proceeds of $99,000. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.66 per share expiring on April 9, 2009. We paid a
commission of $9,900 in connection with this private placement. 25. In May 2007, we issued 23,256 units at $0.43 per unit for proceeds of $10,000. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.68 per share expiring on May 31, 2009. We paid a commission of
$500 in connection with this private placement. 26. In April 2007, we issued 35,000 units at $0.45 per unit for proceeds of $15,750. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.66 per share expiring on April 30, 2009. We paid a commission of
$788 in connection with this private placement. 27. In October 2006, we issued 1,995,000 units at $0.50 per unit for proceeds of $997,500. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.90 per share expiring on September 1, 2008. We paid
commissions of $122,500 in connection with this private placement. 28. In September 2006, we issued 460,000 units at $0.50 per unit for proceeds of $230,000. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.90 per share expiring on September 1, 2008. 29. In July 2006, we issued 150,000 units at a $0.50 per unit for proceeds of $75,000. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.80 per share expiring on April 15, 2008. 30. In July 2006, we issued 110,000 shares of common stock and warrants to purchase 55,000 shares of common stock at $1.00 per share for an aggregate purchase price of $77,000. The warrants expired on April 15, 2008. 31. In July 2006, a note payable in the amount of $61,890 was converted into 343,833 units. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.20 per share expiring on July 7, 2011. 32. In July 2006, a note payable in the amount of $11,655 was converted into 233,092 units. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.055 per share expiring on July 7, 2011. II-4
33. In July 2006, a note payable in the amount of $65,000 was converted into 590,909 units. Each unit consisted of one share of common stock and a warrant entitling the holder to purchase one share of common stock at an exercise price of $0.12 per share expiring on July 19, 2011. 34. In April 2006, we issued 150,000 units at $0.50 per unit for proceeds of $75,000. Each unit consisted of one common share and a warrant entitling the holder to purchase one common share at an exercise price of $0.80 per share expiring on April 15, 2008. 35. In January 2006, we issued 1,219,244 shares of common stock pursuant to the exercise of share purchase warrants, realizing gross proceeds of $85,962.
36. In
December 2009, we issued an aggregate of 769,230 shares of common stock to
the two holders of our amended secured convertible notes on the conversion
of $100,000 of principal of those notes. The offer and issuance of the securities described in paragraphs 1, 2, 5-8 and 10-36 above were exempt from the registration requirements of the Securities Act pursuant to Sections 4(2) and/or 4(6) thereof, and the offer and issuance of the securities described in paragraphs 3 and 4 above were
exempt from the registration requirements of the Securities Act pursuant to Regulation D promulgated thereunder. Each security issued pursuant to the exemption set forth in section 4(2) or 4(6) of the Act or Regulation D thereunder were appropriately legended. Item 16. Exhibits and Financial Statement Schedules.
(a)
The following exhibits are filed as part of this Registration Statement:
Exhibit No.
Description
1.1
Form of Underwriting Agreement**
3.1
Articles of Incorporation and amendments thereto (1)
3.2
Bylaws, as amended (2)
4.1
Specimen Common Stock Certificate**
4.2
Specimen Redeemable Warrant Certificate (see Exhibit A to Exhibit 4.4)
4.3
Form of Representative’s Warrant**
4.4 Form of Redeemable Warrant Agreement
5.1
Form of Opinion of Morse, Zelnick, Rose & Lander, LLP (revised)**
10.1
Non-Qualified Stock Option Plan (3)
10.2
Stock Bonus Plan (4)
10.3(a)
License Agreement with Abbott Laboratories (5)
10.3(b)
Amendment to Semi-Exclusive License Agreement**
10.3(c)
Second Amendment to Semi-Exclusive License Agreement (5)
10.4
License Agreement with Inverness Medical Switzerland GmbH (portions of Exhibit 10.4 have been omitted pursuant to a request for confidential treatment)**
10.5
Agreement with Pacific BioScience Research Centre**
10.6
Employment Agreement with Dr. Ricardo Moro-Vidal**
10.7
Employment Agreement with Denis Burger, Ph.D.**
10.8
Final form of Loan Modification Agreement dated August 31, 2009 (6)
10.9
Final form of the Bridge Unit Purchase and Investor Subscription Agreement dated September 10, 2009 (7)
10.10
Final form of Promissory Note (8)
21.1
Subsidiaries of the Registrant**
23.1
Consent of Manning Elliott Chartered Accountants, independent registered public accounting firm
23.2 Consent of Morse, Zelnick, Rose & Lander, LLP (included in Exhibit 5.1)**
24.1
Power of Attorney (included on page II-9)**
(1)
The original Articles of Incorporation are incorporated by reference to Exhibit 3.1 of our registration statement on Form 10-SB, filed with the SEC on August 5, 1999 and the amendment to the Articles of Incorporation is incorporated by reference to Exhibit 3.1 to a Current Report on Form 8-K
filed on October 30, 2009.
II-5
(2) Incorporated by reference to Exhibit 3.2 of our registration statement on Form 10-SB, filed with the SEC on August 5, 1999 and to Exhibit 3.1 to a Current Report on Form 8-K filed with the SEC on September 10, 2009. (3) Incorporated by reference to Exhibit 4.1 of our registration statement on Form S-8, filed with the SEC on April 23, 2009. (4) Incorporated by reference to Exhibit 4.2 of our registration statement on Form S-8, filed with the SEC on April 23, 2009. (5) The original license agreement is incorporated by reference to Exhibit 10.4 of Amendment No. 2 of our registration statement on Form SB-2, filed with the SEC on November 2, 2007 and the second amendment to the licensing agreement is incorporated by reference to Exhibit 10 to a Current Report
on Form 8-K/A filed on August 15, 2008. Portions of Exhibits 10.3(a) and 10.3(c) have been omitted pursuant to a request for confidential treatment. (6) Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K/A filed with the SEC on September 10, 2009. (7) Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K filed with the SEC on September 16, 2009. (8) Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K filed with the SEC on September 16, 2009. ** Previously filed Item 17. Undertakings. We hereby undertake:
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; (ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement.
Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration
statement. (iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof. (3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. (4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i)
If the registrant is relying on Rule 430B:
II-6
(1)
(i)
Each prospectus filed by the registrant pursuant to Rule 424(b)(3)shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and (B) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of
the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As
provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the
offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated
by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was
part of the registration statement or made in any such document immediately prior to such effective date; or
(ii)
If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of
and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference
into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration
statement or made in any such document immediately prior to such date of first use.
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration
statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell
such securities to such purchaser:
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; (ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; (iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and (iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. II-7
(A)
(5)
(i)
(6)
If the registrant requests acceleration of the effective date of the registration statement pursuant to Rule 461 under the Securities Act, and
Any provision or arrangement exists whereby the registrant may indemnify a director, officer or controlling person of the registrant against liabilities arising under the Securities Act, or (ii) The underwriting agreement contains a provision whereby the registrant indemnifies the underwriter or controlling persons of the underwriter against such liabilities and a director, officer or controlling person of the registrant is such an underwriter or controlling person thereof or a member
of any firm which is such an underwriter, and (iii) The benefits of such indemnification are not waived by such persons: Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and
Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or
controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by
controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(7)
That, in a registration statement permitted by Rule 430A under the Securities Act of 1933:
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or
(4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. (ii) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof. II-8
(i)
(i)
SIGNATURES Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Amendment No. 7 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Richmond, Province of British Columbia, Canada, on the 31st day of
December 2009. BIOCUREX, INC.
By: /s/ DR. RICARDO
MORO-VIDAL Dr. Ricardo Moro-Vidal Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 7 to the Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Name
Position
Date
/S/
DR.
RICARDO
MORO-VIDAL Dr. Ricardo Moro-Vidal President, Chief Executive Officer, and Director (Principal Executive Officer) December 31, 2009 /S/ GLADYS
CHAN Gladys Chan Chief Financial Officer (Principal Accounting and Financial Officer) December 31, 2009 /S/ ANTONIA
BOLD-DE-HAUGHTON Antonia Bold-de-Haughton Secretary and Director December 31, 2009 * Dr. Phil Gold Director December 31, 2009 * Denis Burger, Ph.D. Director December 31, 2009 * Jim Walsh, Ph.D. Director December 31, 2009 *By: /s/ DR. RICARDO
MORO-VIDAL as Attorney-in-Fact December 31, 2009 II-9
(f/k/a Whispering Oaks International, Inc.)
Chief Executive Officer
Exhibit Index
Exhibit No.
Description
1.1
Form of Underwriting Agreement**
3.1
Articles of Incorporation and amendments thereto (1)
3.2
Bylaws, as amended (2)
4.1
Specimen Common Stock Certificate**
4.2
Specimen Redeemable Warrant Certificate (see Exhibit A to Exhibit 4.4)
4.3
Form of Representative’s Warrant**
4.4 Form of Redeemable Warrant Agreement
5.1
Form of Opinion of Morse, Zelnick, Rose & Lander, LLP (revised)**
10.1
Non-Qualified Stock Option Plan (3)
10.2
Stock Bonus Plan (4)
10.3(a)
License Agreement with Abbott Laboratories (5)
10.3(b)
Amendment to Semi-Exclusive License Agreement**
10.3(c)
Second Amendment to Semi-Exclusive License Agreement (5)
10.4
License Agreement with Inverness Medical Switzerland GmbH (portions of Exhibit 10.4 have been omitted pursuant to a request for confidential treatment)**
10.5
Agreement with Pacific BioSciences Research Centre**
10.6
Employment Agreement with Dr. Ricardo Moro-Vidal**
10.7
Employment Agreement with Denis Burger, Ph.D.**
10.8
Final form of Loan Modification Agreement dated August 31, 2009 (7)
10.9
Final form of the Bridge Unit Purchase and Investor Subscription Agreement dated September 10, 2009 (8)
10.10
Final form of Promissory Note (9)
21.1
Subsidiaries of the Registrant**
23.1
Consent of Manning Elliott Chartered Accountants, independent registered public accounting firm
23.2 Consent of Morse, Zelnick, Rose & Lander, LLP (included in Exhibit 5.1)**
24.1
Power of Attorney (included on page II-9)**
(1)
The original Articles of Incorporation are incorporated by reference to Exhibit 3.1 of our registration statement on Form 10-SB, filed with the SEC on August 5, 1999 and the amendment to the Articles of Incorporation is incorporated by reference to Exhibit 3.1 to a Current Report on Form 8-K
filed on October 30, 2009. (2) Incorporated by reference to Exhibit 3.2 of our registration statement on Form 10-SB, filed with the SEC on August 5, 1999 and to Exhibit 3.1 to a Current Report on Form 8-K filed with the SEC on September 10, 2009. (3) Incorporated by reference to Exhibit 4.1 of our registration statement on Form S-8, filed with the SEC on April 23, 2009. (4) Incorporated by reference to Exhibit 4.2 of our registration statement on Form S-8, filed with the SEC on April 23, 2009. (5) The original license agreement is incorporated by reference to Exhibit 10.4 of Amendment No. 2 of our registration statement on Form SB-2, filed with the SEC on November 2, 2007 and the second amendment to the licensing agreement is incorporated by reference to Exhibit 10 to a Current Report
on Form 8-K/A filed on August 15, 2008. Portions of Exhibits 10.3(a) and 10.3(c) have been omitted pursuant to a request for confidential treatment. (6) Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K/A filed with the SEC on September 10, 2009. (7) Incorporated by reference to Exhibit 10.1 of our Current Report on Form 8-K filed with the SEC on September 16, 2009. (8) Incorporated by reference to Exhibit 10.2 of our Current Report on Form 8-K filed with the SEC on September 16, 2009. ** Previously filed.
