Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d8k.htm |
 NASDAQ: INFI December 2009 Infinity Innovative. Independent. Inspired. Exhibit 99.1 |
 Innovative. Independent. Inspired. 2 Safe Harbor Statement • This presentation contains forward-looking statements within the meaning of The Private
Securities Litigation Reform Act of 1995. • These statements involve risks and uncertainties that could cause actual results to be
materially different from historical results or from any future results
expressed or implied by such forward-looking statements. •
Such forward-looking statements include statements regarding future clinical
trial activity for IPI-504 and IPI-493; the presentation of clinical
data for IPI-504 and IPI-493; the filing of an IND for IPI-940; estimates of 2009 financial performance; the continuation of the Purdue/Mundipharma alliance and the exercise of warrants held by Purdue, and the expectation that Infinity will have capital to support its current operating plan into 2013. • Such forward-looking statements are subject to numerous factors, risks and
uncertainties that may cause actual events or results to differ materially
from the company's current expectations. For example, there can be no guarantee that Infinity’s strategic alliance with Purdue/Mundipharma will continue for its expected term or that these entities will fund Infinity’s programs as agreed, or that any product candidate Infinity is developing will successfully complete necessary preclinical
and clinical development phases. In addition, Infinity’s expectations
could be affected by risks and uncertainties relating to: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and
future studies; the content and timing of decisions made by the U.S. Food and
Drug Administration and other regulatory authorities, investigational review boards at clinical trial sites, and publication review bodies; Infinity's ability to enroll patients in its
clinical trials; unplanned cash requirements and expenditures; market
acceptance of any products Infinity or its partners may successfully develop; and Infinity's ability to obtain, maintain and enforce patent and other intellectual property protection for any product
candidate it is developing. • These and other risks which may impact management's expectations are described in greater detail under the caption "Risk Factors" included in Infinity's quarterly report on Form 10-Q filed with the U.S. Securities
and Exchange Commission in November 2009. • Further, any forward-looking statements contained in this presentation speak only as
of the date hereof, and Infinity expressly disclaims any obligation to update
any forward-looking statements, whether as a result of new information, future events or otherwise. • All trademarks used in this presentation are the property of their respective
owners. • Our Internet website is http://www.infi.com. We regularly use our website to post information regarding our business, product development programs and governance. We encourage investors to use www.infi.com,
particularly the information in the section entitled “Investors/Media,” as a source of information about Infinity. References to www.infi.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.infi.com into this presentation by reference. 2 2 |
 Infinity: Uniquely positioned for success Full ownership of industry-leading Hsp90 chaperone inhibitors U.S. commercialization rights to all oncology programs Worldwide royalties up to 20% on neuropathic pain program 3 Financial strength Value-creating asset ownership Innovative pipeline Cash and funding to aggressively invest in programs to key clinical inflection points Phase 2: Hsp90 chaperone inhibition Phase 1: Hedgehog pathway inhibition Near IND: Fatty acid amide hydrolase (FAAH) inhibition World-class discovery organization Innovative. Independent. Inspired. |
 Innovative. Independent. Inspired. Financial strength today to aggressively invest in pipeline • $140 million in cash & equivalents as of September 30,
2009 • Purdue / Mundipharma collaboration – Access to $50 million line of credit (interest at
prime; payment due 2019) – Up to $162.5 million in committed R&D funding
through 2011 ($12.5M in 4Q2009, up to $65M in 2010, up to $85M in 2011);
options to extend through 2013 • ~ 26 million shares outstanding – Up to $200 million in potential proceeds from Purdue exercisable warrants (6 million shares at exercise price of $15 to $40 per share) 4 |
 Innovative. Independent. Inspired. 5 Financial Strength: Runway to Clinical Inflection Points 2009 Key objectives • Advance Hsp90 trials • Advance Hedgehog trial • IND for FAAH inhibitor program Total investment $80 – 90M $157M 2009 starting cash* 2009 ending cash $127 – 137M *$127M on 12/31/08, plus $30M Purdue equity investment 1/7/09 ** After $12.5M residual funding from AstraZeneca Net cash burn (Purdue funds non-Hsp90) ($20 - 30M)** 5 2010-2012 • Advance late-stage Hsp90 program • Initiate addt’l Hh and FAAH studies • Strategically enhance pipeline $375 – 425M ($140 – 170M) Additional sources of cash $50M Purdue line of credit • Up to $200M Purdue warrants • Partner Hsp90 ex-U.S. |
 Innovative. Independent. Inspired. 6 6 Infinity: Developing Breakthrough
Therapeutics 6 Discovery Preclinical Phase 1 Phase 2 Phase 3 Hsp90 Chaperone Inhibitors Hsp90 IV: IPI-504 (retaspimycin hydrochloride) NSCLC HER2+ mBC Solid Tumors Liposarcoma Hsp90 oral: IPI-493 Solid Tumors Hedgehog Pathway: IPI-926 Solid Tumors FAAH: IPI-940 Bcl-2/Bcl-xL* Discovery Programs *Transitioned to Novartis Pipeline at November 2009 Herceptin® Combination Taxotere® Combination Expected to Begin Early 2010 |
 Innovative. Independent. Inspired. 7 Hsp90 Chaperone Inhibition Program: New angle of attacking oncoproteins |
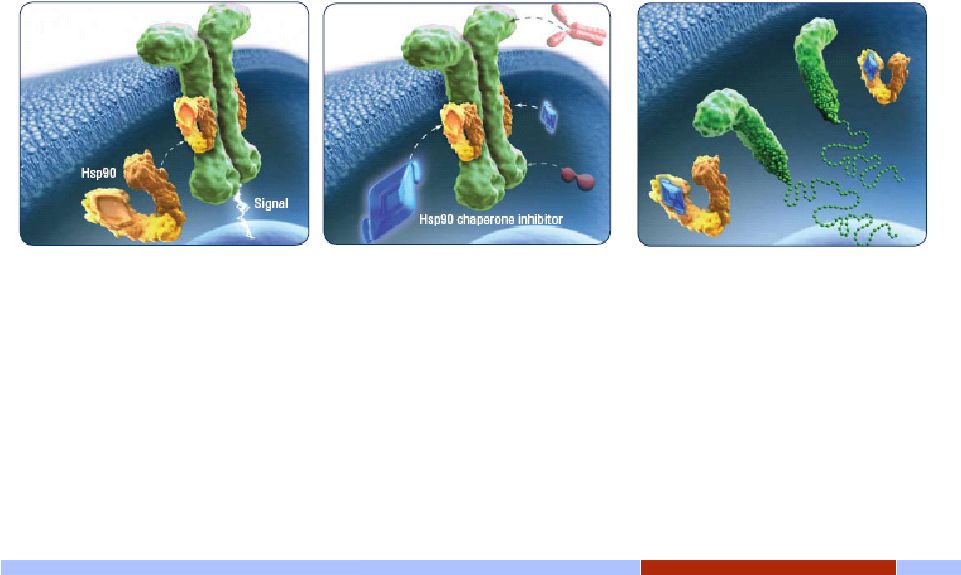 Innovative. Independent. Inspired. 8 8 Hsp90 chaperone inhibition: A new angle for targeting oncoproteins 8 • Hsp90 chaperone stabilizes oncoprotein, enabling growth signaling and tumor cell proliferation • Inhibit Hsp90 and oncoproteins become unstable, unfolded, and unable to signal • Unfolded oncoproteins will then be degraded rapidly, arresting tumor cell growth • Currently, targeted therapies inhibit oncoproteins in distinctly different ways that do not affect the critical function of Hsp90 |
 Hsp90
Chaperone Inhibition: Broad medical & commercial potential 9 Breast cancer Additional tumors Address substantial oncology market with a new treatment approach Combine Hsp90 chaperone therapy with existing standards of care to delay recurrence Serve as late-line therapy where tumors have become refractory to other drugs Lung cancer Innovative. Independent. Inspired. IPI-504 IV (retaspimycin hydrochloride) IPI-493 oral |
 Innovative. Independent. Inspired. 10 10 Infinity’s Hsp90 chaperone inhibition program Broad medical & commercial potential 10 Lung cancer Non-small cell 180,000 US incidence • IPI-504 Ph 2 in NSCLC (ASCO 2009) 14% ORR in wtEGFR patients • IPI-504 Ph 1b combo with Taxotere ® (ASCO 2009) Generally well-tolerated; combinable • Molecular characterization ongoing • Ph 2 data and further clinical development expected 2010 INDICATION TRIALS & DATA CATALYTS |
 Innovative. Independent. Inspired. 11 11 Infinity’s Hsp90 chaperone inhibition program Broad medical & commercial potential 11 Lung cancer Non-small cell 180,000 US incidence • IPI-504 Ph 2 in NSCLC (ASCO 2009) 14% ORR in wtEGFR patients • IPI-504 Ph 1b combo with Taxotere ® (ASCO 2009) Generally well-tolerated; combinable • Molecular characterization ongoing • Ph 2 data and further clinical development expected 2010 Breast cancer HER2+ 45,000 US incidence • IPI-504 Ph 2 in HER2+ mBC combination with Herceptin ® Demonstrated potency and tumor growth inhibition in preclinical models • Clinical data expected 2010 INDICATION TRIALS & DATA CATALYTS |
 Innovative. Independent. Inspired. 12 12 Infinity’s Hsp90 chaperone inhibition program Broad medical & commercial
potential 12 Lung cancer Non-small cell 180,000 US incidence • IPI-504 Ph 2 in NSCLC (ASCO 2009) 14% ORR in wtEGFR patients • IPI-504 Ph 1b combo with Taxotere ® (ASCO 2009) Generally well-tolerated; combinable • Molecular characterization ongoing • Ph 2 data and further clinical development expected 2010 Breast cancer HER2+ 45,000 US incidence • IPI-504 Ph 2 in HER2+ mBC combination with Herceptin ® Demonstrated potency and tumor growth inhibition in preclinical models • Clinical data expected 2010 Additional tumors • IPI-493 oral Ph 1 study in solid tumors • IPI-504 Ph 2 in dedifferentiated liposarcoma expected to begin early 2010 • Ph 1 data and further IPI-493 clinical development expected 2010 • RING data to be presented at ASCO GI INDICATION TRIALS & DATA CATALYTS |
 Innovative. Independent. Inspired. 13 Hedgehog Program |
  Innovative. Independent. Inspired. 14 The Hedgehog pathway: A promising new focus for targeted drug therapy 14 14 • The Hedgehog (Hh) pathway is not active in most adult cells • Smoothened (Smo) -- which can promote growth of certain cancers – is held in an inactive state by Patched (Ptc) • Gli transcription factors do not enter the nucleus to promote transcription Malignant activation of the Hedgehog pathway may be implicated in many insidious cancers including pancreatic, ovarian, small cell lung, breast, brain,
and basal cell (skin) cancers |
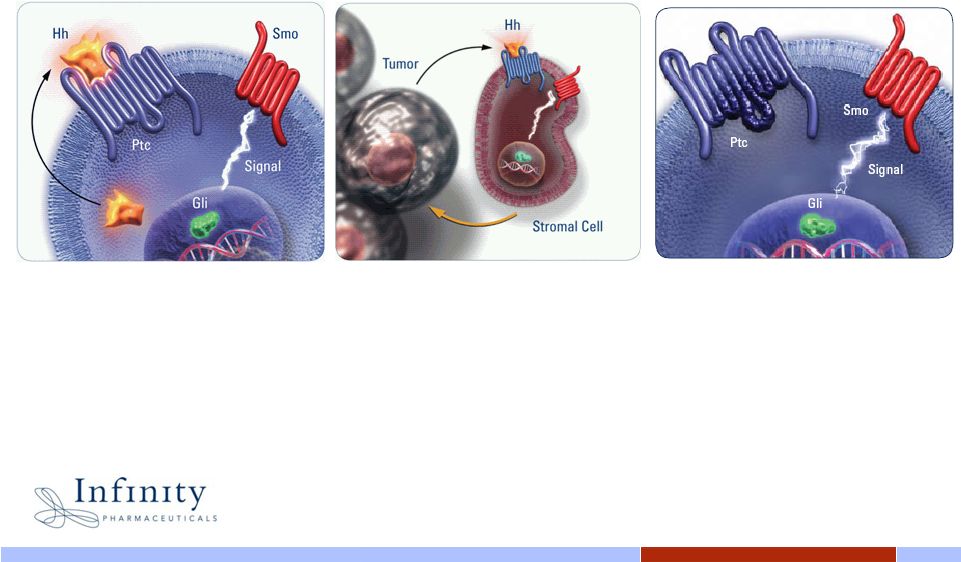 Innovative. Independent. Inspired. 15 The role of Smoothened in malignant activation of the Hedgehog pathway 15 15 • Tumor derived Hh ligands act through Smo to initiate a signaling cascade through Gli • Gli dependent transcription of genes can now promote tumor survival and growth • Tumor derived Hh ligands act through Smo on stromal cells in the tumor microenvironment • Stromal cells in the microenvironment provide support for growth and survival of tumor cells • Hh signaling activated by genetic mutation • Loss-of-function mutations in Ptc allow Smo to initiate a signaling cascade in the absence of Hh ligand, leading to tumor survival and growth Ligand-Dependent Signaling Ligand-Independent Signaling |
 Innovative. Independent. Inspired. 16 Infinity’s IPI-926 inhibits Smoothened 16 16 • IPI-926 inhibits Smo, preventing the signaling cascade that otherwise leads to tumor cell growth and survival IPI-926 IPI-926 in ongoing
Ph 1 clinical trial |
 Innovative. Independent. Inspired. 17 17 Significant anti-tumor activity with IPI-926 in small cell lung cancer model Days Post Implant 5 weeks total of IPI-926 follow-up treatment; 40 mg/kg, PO QD Travaglione et al., 2008 AACR |
 Innovative. Independent. Inspired. 18 IPI-926 + Gemcitabine provides a survival benefit in pancreatic cancer model IPI-926 depletes tumor stroma
through down-regulation of Hh signaling, resulting in increased drug delivery to the tumor Olive et al., 2009 Science 0 15 30 45 60 75 90 0 20 40 60 80 100 Days of Treatment Vehicle Gem IPI-926 IPI-926/Gem Model resembling human pancreatic cancer responds poorly to gemcitabine due to lack of tumor perfusion |
 Innovative. Independent. Inspired. Compelling potential path forward for Phase 2 development in 2010 • Recent Science article highlights promising role of IPI-926 in chemo-resistant pancreatic cancer model • Evidence for Hh inhibition as maintenance therapy in chemo-responsive tumor models, including SCLC and ovarian • IPI-926 advancing through Phase 1 study 19 |
 Innovative. Independent. Inspired. 20 Early Pipeline: Fatty Acid Amide Hydrolase (FAAH) |
 Innovative. Independent. Inspired. Fatty acid amide hydrolase (FAAH): 21 21 Next Generation Innovation: IPI-940 for neuropathic pain 21 Advance IPI-940 to IND by year-end 2009
FAAH inhibition increases the duration of anandamide’s analgesic effect, prolonging pain relief at the site of release CB1 Anandamide |
 Innovative. Independent. Inspired. 22 22 Infinity: Developing Breakthrough Therapeutics 22 Discovery Preclinical Phase 1 Phase 2 Phase 3 Hsp90 Chaperone Inhibitors Hsp90 IV: IPI-504 (retaspimycin hydrochloride) NSCLC HER2+ mBC Solid Tumors Liposarcoma Hsp90 oral: IPI-493 Solid Tumors Hedgehog Pathway: IPI-926 Solid Tumors FAAH: IPI-940 Bcl-2/Bcl-xL* Discovery Programs *Transitioned to Novartis Pipeline at November 2009 Herceptin® Combination Taxotere® Combination Expected to Begin Early 2010 |
 Innovative. Independent. Inspired. 23 23 • Report IPI-504 NSCLC Ph 2 data, mid-2009 • Report IPI-504 Ph 1b Taxotere® combo data, mid-2009 • Advance oral IPI-493 Ph 1 • Initiate additional trials (including HER2+ mBC) 23 2009 R&D Milestones Hsp90: IPI-504 and IPI-493 oral • Advance IPI-940 to IND FAAH: IPI-940 • Report IPI-926 preclinical data, multiple tumor models, mid-2009 Hedgehog: IPI-926 |
 Innovative. Independent. Inspired. 24 24 Business and Commercial Objectives 24 • Build market presence – Infinity to commercialize all oncology products in U.S. – Potential to partner Hsp90 for ex-U.S. marketing – Purdue/Mundipharma ex-U.S. marketing • Identify strategic opportunities to enhance profile • Maintain strong financial profile – Opportunities for additional capital including Purdue warrants, Hsp90 partnering |
 Innovative. Independent. Inspired. Infinity: Uniquely positioned for success Full ownership of industry-leading Hsp90 chaperone inhibitors U.S. commercialization rights to all oncology programs Worldwide royalties up to 20% on neuropathic pain program 25 Financial strength Value-creating asset ownership Innovative pipeline Cash and funding to aggressively invest in programs to key clinical inflection points Phase 2: Hsp90 chaperone inhibition Phase 1: Hedgehog pathway inhibition Near IND: Fatty acid amide hydrolase (FAAH) inhibition World-class discovery organization |
 Innovative. Independent. Inspired. 26 26 Infinity Innovative. Independent. Inspired. 26 |
