Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
|
x |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the quarterly period ended September 30, 2009 |
or
|
o |
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from __________________ to __________________ |
|
|
|
Commission file number: 000-32581 |
LOTUS PHARMACEUTICALS, INC.
(Name of registrant as specified in its charter)
|
NEVADA |
|
20-0507918 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
16 Cheng Zhuang Road, Feng Tai District, Beijing100071 People’s Republic of China |
|
N/A |
|
(Address of principal executive offices) |
|
(Zip Code) |
86-10-63899868
(Registrant’s telephone number, including area code)
N/A
(Former name, former address and former fiscal year, if changed since last report)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o |
|
Accelerated filer o |
|
Non-accelerated filer o (Do not check if smaller reporting company) |
|
Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
Yes o No x
Indicated the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date, 46,181,332 shares of common stock are issued and outstanding as of November 12, 2009.
TABLE OF CONTENTS
|
|
|
|
|
Page No. |
|
PART I. - FINANCIAL INFORMATION | ||||
|
Item 1. |
|
Financial Statements. |
|
4 |
|
|
|
Consolidated Balance Sheets as of September 30, 2009 (Unaudited) and December 31, 2008 |
|
4 |
|
|
|
Consolidated Statements of Income and Comprehensive Income for the Three and Nine Months Ended September 30, 2009 and 2008 (unaudited) |
|
5 |
|
|
|
Consolidated Statements of Cash Flows for the Nine Months Ended September 30, 2009 and 2008 (unaudited) |
|
6 |
|
|
|
Notes to Unaudited Consolidated Financial Statements |
|
7 |
|
Item 2. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
|
33 |
|
Item 3. |
|
Quantitative and Qualitative Disclosures About Market Risk. |
|
48 |
|
Item 4T |
|
Controls and Procedures. |
|
48 |
|
PART II - OTHER INFORMATION | ||||
|
Item 1. |
|
Legal Proceedings. |
|
49 |
|
Item 1A. |
|
Risk Factors. |
|
49 |
|
Item 2. |
|
Unregistered Sales of Equity Securities and Use of Proceeds. |
|
49 |
|
Item 3. |
|
Defaults Upon Senior Securities. |
|
50 |
|
Item 4. |
|
Submission of Matters to a Vote of Security Holders. |
|
50 |
|
Item 5. |
|
Other Information. |
|
50 |
|
Item 6. |
|
Exhibits. |
|
51 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
Certain statements in this report contain or may contain forward-looking statements that are subject to known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements were based on various factors and were derived utilizing numerous assumptions and other factors that could cause our actual results to differ materially from those in the forward-looking statements. These factors include, but are not limited to, our ability to enforce the Contractual Arrangements, Lotus East’s strategic initiatives, economic, political and market conditions and fluctuations, U.S. and Chinese government and industry regulation, interest rate risk, U.S., Chinese and global competition, and other factors. Most of these factors are difficult to predict accurately and are generally beyond our control. You should consider the areas of risk described in connection with any forward-looking statements that may be made herein. Readers are cautioned not to place substantial reliance on these forward-looking statements and readers should carefully review this report in its entirety together with our Annual Report on Form 10-K for the year ended December 31, 2008 as filed with the SEC, including the risks described in Item 1A. Risk Factors. Except for our ongoing obligations to disclose material information under the Federal securities laws, we undertake no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. These forward-looking statements speak only as of the date of this report, and you should not rely on these statements without also considering the risks and uncertainties associated with these statements and our business.
OTHER PERTINENT INFORMATION
We maintain a web site at www.lotuspharma.com. Information on this web site is not a part of this report.
CERTAIN DEFINED TERMS USED IN THIS REPORT
Unless specifically set forth to the contrary, when used in this report the terms:
|
|
• |
“Lotus,” “we,” “us,” “our,” the “Company,” and similar terms refer to Lotus Pharmaceuticals, Inc., a Nevada corporation formerly known as S.E. Asia Trading Company, Inc., and its subsidiary, |
|
|
|
|
|
|
• |
“Lotus International” refers to Lotus Pharmaceutical International, Inc., a Nevada corporation and a subsidiary of Lotus, |
|
|
• |
“Lotus Century” refers to Lotus Century Pharmaceutical (Beijing) Technology co., Ltd., a wholly foreign-owned enterprise (WFOE) Chinese company which is a subsidiary of Lotus, |
|
|
|
|
|
|
• |
“Liang Fang” refers to Beijing Liang Fang Pharmaceutical Co., Ltd., a Chinese limited liability company formed on June 21, 2000 and an affiliate of En Ze Jia Shi, |
|
|
|
|
|
|
• |
“En Ze Jia Shi” refers to Beijing En Ze Jia Shi Pharmaceutical Co., Ltd., a Chinese limited liability company formed on September 17, 1999 and an affiliate of Liang Fang, |
|
|
|
|
|
|
• |
“Lotus East” collectively refers to Liang Fang and En Ze Jia Shi, |
|
|
|
|
|
|
• |
“Consulting Services Agreements” refers to the Consulting Services Agreements dated September 20, 2006 between Lotus and Lotus East. |
|
|
|
|
|
|
• |
“Operating Agreements” refers to the Operating Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
|
|
|
• |
“Equity Pledge Agreements” refers to the Equity Pledge Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
|
|
|
• |
“Option Agreements” refers to the Option Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
|
|
|
• |
“Proxy Agreements” refers to the Proxy Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
|
|
|
• |
“Contractual Arrangements” collectively refers to the Consulting Services Agreements, Operating Agreements, Equity Pledge Agreements, Option Agreements and the Proxy Agreements, |
|
|
• |
“SFDA” refers to The State Food and Drug Administration,
|
|
|
• |
“China” or the “PRC” refers to the People’s Republic of China, and |
|
|
|
|
|
|
• |
“RMB” refers to the renminbi which is the currency of mainland PRC of which the yuan is the principal currency. |
PART 1. - FINANCIAL INFORMATION
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
|
|
|
September 30, 2009 |
|
December 31,2008 |
| ||
|
|
|
(Unaudited) |
|
|
| ||
|
ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
|
|
Cash |
|
$ |
2,561,489 |
|
$ |
1,278,808 |
|
|
Accounts receivable, net of allowance for doubtful accounts |
|
|
1,765,390 |
|
|
6,132,912 |
|
|
Other receivable |
|
|
16,131 |
|
|
15,757 |
|
|
Other receivable-related party |
|
|
— |
|
|
2,027,954 |
|
|
Inventories, net of reserve for obsolete inventory |
|
|
2,994,995 |
|
|
3,787,802 |
|
|
Prepaid expenses and other assets |
|
|
123,695 |
|
|
121,274 |
|
|
Deferred debt costs |
|
|
153,024 |
|
|
398,067 |
|
|
|
|
|
|
|
|
|
|
|
Total Current Assets |
|
|
7,614,724 |
|
|
13,762,574 |
|
|
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT - Net |
|
|
22,872,484 |
|
|
7,554,817 |
|
|
|
|
|
|
|
|
|
|
|
OTHER ASSETS |
|
|
|
|
|
|
|
|
Deposit and Installments on intangible assets |
|
|
41,924,067 |
|
|
41,093,053 |
|
|
Intangible assets, net of accumulated amortization |
|
|
8,429,005 |
|
|
1,231,730 |
|
|
Deferred debt costs |
|
|
— |
|
|
66,344 |
|
|
|
|
|
|
|
|
|
|
|
Total Assets |
|
$ |
80,840,280 |
|
$ |
63,708,518 |
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses |
|
$ |
477,619 |
|
$ |
895,283 |
|
|
Other payables |
|
|
1,305,565 |
|
|
1,274,882 |
|
|
Taxes payable |
|
|
6,751,037 |
|
|
5,015,908 |
|
|
Unearned revenue |
|
|
630,475 |
|
|
565,629 |
|
|
Due to related parties |
|
|
1,968,158 |
|
|
1,588,689 |
|
|
Series A convertible redeemable preferred stock, $.001 par value; 10,000,000 shares authorized; 5,748,271 and 5,747,118 shares issued and outstanding at September 30, 2009 and December 31, 2008, respectively, net |
|
|
4,534,129 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
Total Current Liabilities |
|
|
15,666,983 |
|
|
9,340,391 |
|
|
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES: |
|
|
|
|
|
|
|
|
Due to related parties |
|
|
329,063 |
|
|
525,225 |
|
|
Notes payable - related parties |
|
|
5,068,726 |
|
|
5,056,451 |
|
|
Series A convertible redeemable preferred stock, $.001 par value; 10,000,000 shares authorized; 5,748,271 and 5,747,118 shares issued and outstanding at September 30, 2009 and December 31, 2008, respectively, net |
|
|
— |
|
|
3,652,341 |
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
|
21,064,772 |
|
|
18,574,408 |
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY: |
|
|
|
|
|
|
|
|
Common stock ($.001 par value; 200,000,000 shares authorized; 44,239,095 and 42,420,239 shares issued and outstanding on September 30, 2009 and December 31, 2008, respectively) |
|
|
44,239 |
|
|
42,420 |
|
|
Additional paid-in capital |
|
|
12,318,895 |
|
|
11,554,381 |
|
|
Statutory reserves |
|
|
5,296,804 |
|
|
3,750,529 |
|
|
Retained earnings |
|
|
37,755,694 |
|
|
25,557,537 |
|
|
Accumulated other comprehensive income |
|
|
4,359,876 |
|
|
4,229,243 |
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ Equity |
|
|
59,775,508 |
|
|
45,134,110 |
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
|
$ |
80,840,280 |
|
$ |
63,708,518 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements
4
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF INCOME AND OTHER COMPREHENSIVE INCOME
(UNAUDITED)
|
|
|
For the Three Months Ended |
|
For the Nine Months Ended |
| ||||||||
|
|
|
September 30, |
|
September 30, |
| ||||||||
|
|
|
2009 |
|
2008 |
|
2009 |
|
2008 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET REVENUES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wholesale |
|
$ |
11,606,288 |
|
$ |
12,191,037 |
|
$ |
31,220,411 |
|
$ |
34,863,680 |
|
|
Retail |
|
|
2,708,071 |
|
|
2,831,030 |
|
|
7,604,678 |
|
|
8,492,107 |
|
|
Other revenues |
|
|
198,345 |
|
|
1,681,817 |
|
|
1,143,631 |
|
|
4,442,886 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Net Revenues |
|
|
14,512,704 |
|
|
16,703,884 |
|
|
39,968,720 |
|
|
47,798,673 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF SALES |
|
|
5,993,176 |
|
|
8,219,358 |
|
|
16,922,500 |
|
|
25,684,501 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
|
8,519,528 |
|
|
8,484,526 |
|
|
23,046,220 |
|
|
22,114,172 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling expenses |
|
|
1,895,901 |
|
|
4,293,704 |
|
|
5,398,935 |
|
|
11,291,590 |
|
|
Research and development |
|
|
— |
|
|
12,448 |
|
|
— |
|
|
1,193,916 |
|
|
General and administrative |
|
|
610,519 |
|
|
420,716 |
|
|
2,126,828 |
|
|
1,589,176 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
|
2,506,420 |
|
|
4,726,868 |
|
|
7,525,763 |
|
|
14,074,682 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME FROM OPERATIONS |
|
|
6,013,108 |
|
|
3,757,658 |
|
|
15,520,457 |
|
|
8,039,490 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Debt issuance costs |
|
|
(112,355 |
) |
|
(99,516 |
) |
|
(311,388 |
) |
|
(261,919 |
) |
|
Registration rights penalty |
|
|
— |
|
|
— |
|
|
— |
|
|
(650 |
) |
|
Interest income |
|
|
1,295 |
|
|
9,032 |
|
|
47,407 |
|
|
11,620 |
|
|
Interest expense |
|
|
(436,481 |
) |
|
(453,498 |
) |
|
(1,355,129 |
) |
|
(1,399,507 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Other Income (Expense) |
|
|
(547,541 |
) |
|
(543,982 |
) |
|
(1,619,110 |
) |
|
(1,650,456 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME BEFORE INCOME TAXES |
|
|
5,465,567 |
|
|
3,213,676 |
|
|
13,901,347 |
|
|
6,389,034 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME TAXES |
|
|
74,770 |
|
|
— |
|
|
156,915 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME |
|
$ |
5,390,797 |
|
$ |
3,213,676 |
|
$ |
13,744,432 |
|
$ |
6,389,034 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME |
|
|
5,390,797 |
|
|
3,213,676 |
|
|
13,744,432 |
|
|
6,389,034 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized foreign currency translation gain |
|
|
65,626 |
|
|
127,833 |
|
|
130,633 |
|
|
2,173,475 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME |
|
$ |
5,456,423 |
|
$ |
3,341,509 |
|
$ |
13,875,065 |
|
$ |
8,562,509 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME PER COMMON SHARE: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
0.12 |
|
$ |
0.08 |
|
$ |
0.32 |
|
$ |
0.15 |
|
|
Diluted |
|
$ |
0.11 |
|
$ |
0.07 |
|
$ |
0.28 |
|
$ |
0.13 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
43,997,079 |
|
|
42,420,239 |
|
|
43,527,746 |
|
|
42,269,997 |
|
|
Diluted |
|
|
49,745,350 |
|
|
48,167,357 |
|
|
49,186,167 |
|
|
48,017,115 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements
5
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
|
|
|
For the Nine Months Ended |
| ||||
|
|
|
September 30, |
| ||||
|
|
|
2009 |
|
2008 |
| ||
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
Net income |
|
$ |
13,744,432 |
|
$ |
6,389,034 |
|
|
Adjustments to reconcile net income from operations to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
1,081,953 |
|
|
469,455 |
|
|
Amortization of deferred debt issuance costs |
|
|
311,388 |
|
|
261,545 |
|
|
Amortization of debt discount |
|
|
— |
|
|
208,355 |
|
|
Amortization of discount on convertible redeemable preferred stock |
|
|
880,788 |
|
|
592,966 |
|
|
Amortization of prepaid expense attributable to warrants |
|
|
14,849 |
|
|
— |
|
|
Stock-based compensation |
|
|
113,834 |
|
|
392,341 |
|
|
Warrants revaluation |
|
|
— |
|
|
74,593 |
|
|
Decrease in allowance for doubtful accounts and sales returns |
|
|
— |
|
|
(490,310 |
) |
|
Recognition of unearned revenue |
|
|
(594,738 |
) |
|
— |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
4,379,267 |
|
|
8,244,225 |
|
|
Inventories |
|
|
801,428 |
|
|
(4,880,418 |
) |
|
Prepaid expenses and other current assets |
|
|
2,018,565 |
|
|
1,080,576 |
|
|
Accounts payable and accrued expenses |
|
|
230,951 |
|
|
1,032,245 |
|
|
Other current payables |
|
|
(287,905 |
) |
|
— |
|
|
Taxes payable |
|
|
1,721,716 |
|
|
2,637,331 |
|
|
Unearned revenue |
|
|
658,165 |
|
|
26,139 |
|
|
Due to related parties |
|
|
178,047 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES |
|
|
25,252,740 |
|
|
16,038,077 |
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
Deposits on patent right |
|
|
— |
|
|
(2,857,608 |
) |
|
Deposits on land use right |
|
|
— |
|
|
(16,768,445 |
) |
|
Payments on intangible assets |
|
|
(8,622,567 |
) |
|
(3,429 |
) |
|
Purchase of property and equipment |
|
|
(15,352,107 |
) |
|
(1,430,894 |
) |
|
|
|
|
|
|
|
|
|
|
NET CASH USED IN INVESTING ACTIVITIES |
|
|
(23,974,674 |
) |
|
(21,060,376 |
) |
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Repayment of convertible debt |
|
|
— |
|
|
(2,520,000 |
) |
|
Proceeds from sale of convertible redeemable stocks |
|
|
— |
|
|
5,000,000 |
|
|
Payment of debt issuance costs |
|
|
— |
|
|
(468,568 |
) |
|
Proceeds from related party advances |
|
|
— |
|
|
860,916 |
|
|
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY FINANCING ACTIVITIES |
|
|
— |
|
|
2,872,348 |
|
|
|
|
|
|
|
|
|
|
|
EFFECT OF EXCHANGE RATE ON CASH |
|
|
4,615 |
|
|
248,764 |
|
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH |
|
|
1,282,681 |
|
|
(1,901,187 |
) |
|
|
|
|
|
|
|
|
|
|
CASH - beginning of period |
|
|
1,278,808 |
|
|
4,557,957 |
|
|
|
|
|
|
|
|
|
|
|
CASH - end of period |
|
$ |
2,561,489 |
|
$ |
2,656,770 |
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
Cash paid for: |
|
|
|
|
|
|
|
|
Interest |
|
$ |
— |
|
$ |
103,250 |
|
|
Income taxes |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
Non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
Warrants issued for prepaid financing costs and consulting service |
|
$ |
— |
|
$ |
505,752 |
|
|
Common stock issued for prior services |
|
$ |
249,000 |
|
$ |
318,551 |
|
|
Common stock issued for current and future services |
|
$ |
118,333 |
|
$ |
— |
|
|
Common stock issued for conversion of convertible debt |
|
$ |
— |
|
$ |
250,000 |
|
|
Common stock issued for conversion of convertible redeemable preferred stock |
|
$ |
399,000 |
|
$ |
— |
|
|
Debt discount for grant of warrants and beneficial conversion feature |
|
$ |
— |
|
$ |
2,033,025 |
|
|
Preferred stock issued for dividend payable |
|
$ |
400,000 |
|
$ |
— |
|
The accompanying notes are an integral part of these condensed consolidated financial statements
6
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
Lotus Pharmaceuticals, Inc. (“Lotus” or the “Company”), formerly S.E. Asia Trading Company, Inc. (“SEAA”), was incorporated on January 28, 2004 under the laws of the State of Nevada. SEAA operated as a retailer of jewelry, framed art and home accessories. In December 2006, SEAA changed its name to Lotus Pharmaceuticals, Inc.
On September 28, 2006, pursuant to a Share Exchange Agreement with Lotus Pharmaceutical International, Inc. (“Lotus International”), the Company acquired all of the outstanding common stock of Lotus International from the Lotus International shareholders in exchange for newly-issued stock of the Company (“Stock Exchange”). Lotus International became a wholly-owned subsidiary of the Company and Lotus International’s shareholders became the owners of the majority of the Company’s voting stock. The acquisition of Lotus International by the Company was accounted for as a reverse merger on a post-merger basis; the former shareholders of Lotus International hold a majority of the outstanding common stock of the Company on a voting and fully-diluted basis. As a result, Lotus International is deemed to be the acquirer for accounting purposes.
Lotus International was incorporated under the laws the State of Nevada on August 28, 2006 to develop and market pharmaceutical products in the People’s Republic of China (“PRC” or “China”). PRC law currently has limits on foreign ownership of certain companies. To comply with these foreign ownership restrictions, Lotus International operates its pharmaceutical business in China through Beijing Liang Fang Pharmaceutical Co., Ltd. (“Liang Fang”) and an affiliate of Liang Fang, Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. (“En Ze Jia Shi”), both of which are pharmaceutical companies headquartered in the PRC and organized under the laws of the PRC (hereinafter, referred to together as “Lotus East”). Lotus International controlled Lotus East through various contracts with Lotus East and its shareholders in September 2006, pursuant to which Lotus International should provide technology consulting and other general business operation services to Lotus East. Lotus International also has the ability to substantially influence Lotus East’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval. As a result, Lotus International is considered the primary beneficiary of Lotus East.
Accordingly, the consolidated financial statements include the accounts of Lotus, Lotus International, and Lotus East.
In September 2006, Lotus International entered into the following contractual arrangements:
Operating Agreement. Pursuant to the operating agreement among Lotus, Lotus East and the shareholders of Lotus East, (collectively “Lotus East’s Shareholders”), Lotus provides guidance and instructions on Lotus East’s daily operations, financial management and employment issues. The shareholders of Lotus East must designate the candidates recommended by Lotus as their representatives on Lotus East’s Board of Directors. Lotus has the right to appoint senior executives of Lotus East. In addition, Lotus agreed to guarantee Lotus East’s performance under any agreements or arrangements relating to Lotus East’s business arrangements with any third party. Lotus East, in return, agreed to pledge its accounts receivable and all of its assets to Lotus. Moreover, Lotus East agreed that without the prior consent of Lotus, Lotus East would not engage in any transaction that could materially affect the assets, liabilities, rights or operations of Lotus East, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party. The term of this agreement is ten (10) years from September 6, 2006 and may be extended only upon Lotus’s written confirmation prior to the expiration of the this agreement, with the extended term to be mutually agreed upon by the parties.
Consulting Services Agreement. Pursuant to the exclusive consulting services agreements between Lotus and Lotus East, Lotus has the exclusive right to provide to Lotus East general pharmaceutical business operations services as well as consulting services related to the technological research and development of pharmaceutical products as well as general business operation advice and strategic planning (the “Services”). Under this agreement, Lotus owns the intellectual property rights developed or discovered through research and development, in the course of providing the Services, or derived from the provision of the Services. Lotus East is required to pay a quarterly consulting service fees in Renminbi (“RMB”), the functional currency of the PRC, to Lotus that is equal to Lotus East’s profits, as defined, for such quarter.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Organization (continued)
The company’s structure is commonly used to allow foreign investors to invest operating businesses in China. Our holding company Lotus Pharmaceuticals and its subsidiary in the US have no operations. All of our operations are conducted through our two controlled entities (called “Lotus East”) in China. A set of contractual agreements provide the holding company with effective voting and management control over Lotus East in Beijing, In fact, the management of the holding entity is the same as the one of Lotus East. The board of Lotus Pharmaceuticals has decided that income generated by the operating entities are retained within China for operating purpose.
Equity Pledge Agreement. Under the equity pledge agreement between the shareholders of Lotus East and Lotus, the shareholders of Lotus East pledged all of their equity interests in Lotus East to Lotus to guarantee Lotus East’s performance of its obligations under the technology consulting agreement. If Lotus East or Lotus East’s Shareholders breaches its respective contractual obligations, Lotus, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Lotus East’s Shareholders also agreed that upon occurrence of any event of default, Lotus shall be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of Lotus East’s Shareholders to carry out the security provisions of the equity pledge agreement and take any action and execute any instrument that Lotus may deem necessary or advisable to accomplish the purposes of the equity pledge agreement. The shareholders of Lotus East agreed not to dispose of the pledged equity interests or take any actions that would prejudice Lotus’ interest. The equity pledge agreement will expire two (2) years after Lotus East’s obligations under the exclusive consulting services agreements have been fulfilled.
Option Agreement. Under the option agreement between the shareholders of Lotus East and Lotus, the shareholders of Lotus East irrevocably granted Lotus or its designated person an exclusive option to purchase, to the extent permitted under PRC law, all or part of the equity interests in Lotus East for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable PRC law. Lotus or its designated person has sole discretion to decide when to exercise the option, whether in part or in full. The term of this agreement is 10 years from September 6, 2006 and may be extended prior to its expiration by written agreement of the parties.
Proxy Agreement. Pursuant to the proxy agreement among Lotus and Lotus East’s Shareholders, Lotus East’s Shareholders agreed to irrevocably grant a person to be designated by Lotus with the right to exercise Lotus East’s Shareholders’ voting rights and their other rights, including the attendance at and the voting of Lotus East’s Shareholders’ shares at the shareholders’ meetings (or by written consent in lieu of such meetings) in accordance with applicable laws and its Article of Association, including but not limited to the rights to sell or transfer all or any of his equity interests of Lotus East, and appoint and vote for the directors and Chairman as the authorized representative of the shareholders of Lotus East. The term of this Proxy Agreement is ten (10) years from September 6, 2006 and may be extended prior to its expiration by written agreement of the parties.
Liang Fang is a Chinese limited liability company and was formed under laws of the People’s Republic of China on June 21, 2000. Liang Fang is engaged in the production, trade and retailing of pharmaceuticals. Further, Liang Fang is focused on developing innovative medicines and investing strategic growth to address various medical needs for patients worldwide. Liang Fang’s operations are based in Beijing, China.
As of September 30, 2009, Liang Fang owns and operates 10 drug stores throughout Beijing, China. These drugstores sell Western and traditional Chinese medicines, and medical treatment accessories.
Liang Fang’s affiliate, En Ze Jia Shi is a Chinese limited liability company and was formed under laws of the People’s Republic of China on September 17, 1999. En Ze Jia Shi is the sole manufacturer for Liang Fang and maintains facilities for the production of medicines, patented Chinese medicine, as well as the research and production of other new medicines.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Organization (continued)
As a result of the management agreements between Lotus International and Lotus East, Lotus East was deemed to be the acquirer of Lotus International for accounting purposes. Accordingly, the financial statement data presented are those of Lotus East for all periods prior to the Company’s acquisition of Lotus International as of September 28, 2006, and the financial statements of the consolidated companies from the acquisition date forward.
On May 29, 2007, the Company formed a new entity, Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. (‘‘Lotus Century’’), a wholly foreign-owned enterprise (“WFOE”) organized under the laws of the Peoples’ Republic of China. Lotus Century is a Chinese limited liability company and a wholly-owned subsidiary of Lotus. Lotus Century intends to be engaged in development of innovative medicines, medical technology consulting and outsourcing services, and related training services.
Basis of presentation; management’s responsibility for preparation of financial statements
Management acknowledges its responsibility for the preparation of the accompanying interim consolidated financial statements which reflect all adjustments, consisting of normal recurring adjustments, considered necessary in its opinion for a fair statement of its consolidated financial position and the results of its operations for the interim period presented.
The interim consolidated financial statements included herein have been prepared by the Company, pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). Certain information and footnote disclosures
normally included in an annual financial statement prepared in accordance with generally accepted accounting principles in the United States (“ US GAAP”) have been condensed or omitted pursuant to such rules and regulations. In the opinion of management, the interim consolidated financial statements reflect all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of the statement of the results for the interim periods presented. These interim consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto, as well as the accompanying Management’s Discussion and Analysis of Financial Condition and Results of Operations for the year ended December 31, 2008 included in its Annual Report on Form 10-K. Interim financial results are not necessarily indicative of the results that may be expected for a full year.
The Company has adopted ASC 810 “Consolidation of Variable Interest Entities” (“ASC 810”), an Interpretation of Accounting Research Bulletin No. 51. ASC 810 requires a Variable Interest Entity (VIE) to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns. VIEs are those entities in which the Company, through contractual arrangements, bears the risks of, and enjoys the rewards normally associated with ownership of the entities, and therefore the Company is the primary beneficiary of these entities. As a VIE, Lotus East’s revenues are included in the Company’s total revenues, its income from operations is consolidated with the Company’s, and the Company’s net income includes all of Lotus East’s net income.
The accompanying unaudited consolidated financial statements are prepared in accordance with US GAAP. The consolidated statements include the accounts of Lotus Pharmaceuticals, Inc. and its wholly-owned subsidiaries, Lotus and Lotus Century and variable interest entities under its control (Liang Fang and En Ze Jia Shi). All significant inter-company balances and transactions have been eliminated.
Use of estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. Significant estimates in 2009 and 2008 include the allowance for doubtful accounts, the allowance for obsolete inventory, the useful life of property and equipment and intangible assets, fair value of warrants and beneficial conversion features related to the convertible preferred stock and fair value of warrants granted.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair value of financial instruments
The Company adopted ASC 820, Fair Value Measurements and Disclosures. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, accounts receivable, accounts payable and accrued expenses, convertible debt, customer advances, and amounts due to related parties approximate their fair market value based on the short-term maturity of these instruments. The Company did not identify any assets or liabilities that are required to be presented on the consolidated balance sheets at fair value in accordance with ASC 820.
Cash and cash equivalents
For purposes of the consolidated statements of cash flows, the Company considers all highly liquid instruments purchased with a maturity of three months or less and money market accounts to be cash equivalents. The Company maintains cash and cash equivalents with various financial institutions mainly in the PRC and the United States. Balances in the United States are insured up to $250,000 at each bank. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. Non-performance by these institutions could expose the Company to losses for amounts in excess of insured balances.
Accounts receivable
The Company records accounts receivable, net of an allowance for doubtful accounts and sales returns. The Company maintains allowances for doubtful accounts for estimated losses. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, customer’s historical payment history, its current credit-worthiness and current economic trends. The amount of the provision, if any is recognized in the consolidated statement of operations within “General and Administrative Expenses”. Accounts are written off after exhaustive efforts at collection. Because we have good relationship with our customers and our collection representative make great efforts to collect our outstanding receivable, the majority age of the balance of our accounts receivable are less than three months. Based on a review of its outstanding balances, the Company did not consider it necessary to record any allowance for doubtful accounts during the nine months ended September 30, 2009 and 2008.
Inventories
Inventories, consisting of raw materials, work-in-process and finished goods related to the Company’s products are stated at the lower of cost or market utilizing the moving average method. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the market value. These reserves are recorded based on estimates and reflected in cost of sales. The Company did not consider it necessary to record any inventory reserve during the nine months ended September 30, 2009 and 2008.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Property and equipment
Property and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition.
The construction-in-progress which consists of factories and office buildings under construction in China was included in property and equipment. No provision for depreciation is made on construction-in-progress until such time as the relevant assets are completed and ready for their intended use.
Impairment of long-lived assets
In accordance with ASC 360, “Accounting for the Impairment or Disposal of Long-Lived Assets”, the Company periodically reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. The Company did not consider it necessary to record any impairment charges during the nine months ended September 30, 2009 and 2008.
Income taxes
The Company is governed by the Income Tax Law of the People’s Republic of China and the United States. Income taxes are accounted for under ASC 740, “Accounting for Income Taxes,” which is an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s financial statements or tax returns.
In July 2006, the ASC 740, “Accounting for Uncertainty in Income Taxes,” which clarifies the accounting and disclosure for uncertain tax positions. This interpretation is effective for fiscal years beginning after December 15, 2006, and the Company has implemented this interpretation as of July 1, 2007. ASC 740 prescribes a recognition threshold and measurement attribute for recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC 740 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Under ASC 740, evaluation of a tax position is a two-step process. The first step is to determine whether it is more likely than not that a tax position will be sustained upon examination, including the resolution of any related appeals or litigation based on the technical merits of that position. The second step is to measure a tax position that meets the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized in the first subsequent financial reporting period in which the threshold is no longer met.
The adoption of ASC 740 on July 1, 2007 had no effect on the Company’s consolidated financial statements.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Value added tax
The Company is subject to value added tax (“VAT”) for manufacturing products and business tax for services provided. The applicable VAT rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). Under the commercial practice of the PRC, the Company paid VAT based on tax invoices issued. The tax invoices may be issued subsequent to the date on which revenue is recognized, and there may be a considerable delay between the date on which the revenue is recognized and the date on which the tax invoice is issued. In the event that the PRC tax authorities dispute the date on which revenue is recognized for tax purposes, the PRC tax office has the right to assess a penalty, which can range from zero to five times the amount of the taxes which are determined to be late or deficient, and will be charged to operations in the period if and when a determination is been made by the taxing authorities that a penalty is due.
Revenue recognition
Product sales
Product sales are generally recognized when title to the product has transferred to customers in accordance with the terms of the sale. The Company recognizes revenue in accordance with the SEC Staff Accounting Bulletin (SAB) No. 101, “Revenue Recognition in Financial Statements “as amended by SAB No. 104 and 605, “SAB 104 states that revenue should not be recognized until it is realized or realizable and earned. In general, the Company records revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
ASC 605 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if the seller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated.
The Company’s net product revenues represent total product revenues less allowances for returns.
Allowance for returns
The Company accounts for sales returns in accordance with ASC 605, Revenue Recognition When Right of Return Exists, by establishing an accrual in an amount equal to its estimate of sales recorded for which the related products are expected to be returned. The Company determines the estimate of the sales return accrual primarily based on historical experience regarding sales returns, but also by considering other factors that could impact sales returns. These factors include levels of inventory in the distribution channel, estimated shelf life, product discontinuances, and price changes of competitive products, introductions of generic products and introductions of competitive new products. In general, for wholesale sales, the Company provides credit for product returns that are returned six months prior to and up to six months after the product expiration date. Upon sale, the Company estimates an allowance for future product returns. The Company provides additional reserves for contemporaneous events that were not known and knowable at the time of shipment. In order to reasonably estimate future returns, the Company analyzed both quantitative and qualitative information including, but not limited to, actual return rates, the level of product manufactured by the Company, the level of product in the distribution channel, expected shelf life of the product, current and projected product demand, the introduction of new or generic products that may erode current demand, and general economic and industry wide indicators. The Company also utilizes the guidance provided in SAB 104 in establishing its return estimates. Historically, approximately 49% of our total revenues consist of sales of four principal products and product returns from these principal products, as well as the Company’s other products, have been immaterial. Accordingly, based upon the Company’s experience, it historically does not record a reserve at the time of sale and there have been no accounting entries related to its product return policy which have reduced its gross revenues or had any material impact on its financial statements.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue recognition (continued)
Other revenues
Other revenues consist of (i) leasing revenues received for the lease of retail space to various retail merchants; (ii) advertising revenues from the lease of counter space at the Company’s retail locations; (iii) leasing revenue from the lease of retail space to licensed medical practitioners; (iv) revenues received by the Company for research and development projects and lab testing jobs conducted on behalf of third party companies, and; (v) revenues received for performing third party contract manufacturing projects. In connection with third-party manufacturing, the customer supplies the raw materials and we are paid a fee for manufacturing their product and revenue is recognized at the completion of the manufacturing job. The Company recognizes revenues from leasing of space and advertising revenues as earned from contracting third parties. The Company recognizes revenues upon performance of any research or lab testing jobs. Revenues received in advance are reflected as deferred revenue on the accompanying balance sheet. Additionally, the Company receives income from the sale of developed drug formulas. Income from the sale of drug formulas are recognized upon performance of all of the Company’s obligations under the respective sales contract and are included in other income on the accompanying consolidated statement of operations.
Concentrations of credit risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and trade accounts receivable. Substantially, all of the Company’s cash is maintained with state-owned banks within the People’s Republic of China of which no deposits are covered by insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts. A significant portion of the Company’s sales are credit sales which are primarily to customers whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivables is limited due to generally short payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
Unearned Revenue
Unearned revenue consists of prepayments from customers for merchandise that had not yet been shipped. The Company will recognize the deposits as revenue as customers take delivery of the goods, in accordance with its revenue recognition policy. At September 30, 2009 and December 31, 2008, we have unearned revenue of $630,475 and $565,629, respectively.
Stock-based compensation
Stock-based compensation is accounted for under ASC 718, “Share-Based Payment.” ASC 718 requires recognition in the financial statements of the cost of employee and director services received in exchange for an award of equity instruments over the period the employee or director is required to perform the services in exchange for the award (presumptively the vesting period). ASC 718 also requires measurement of the cost of employee and director services received in exchange for an award based on the grant-date fair value of the award. The Company accounts for non-employee share-based awards in accordance with EITF No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquisition, or in Conjunction with Selling, Goods or Services.”
Shipping costs
Shipping costs are expensed as incurred. Shipping costs were included in selling expenses and amounted to $292 and $208,997 for the nine months ended September 30, 2009 and 2008, respectively.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Employee benefits
The Company’s operations and employees are all located in the PRC. The Company makes mandatory contributions to the PRC government’s health, retirement benefit and unemployment funds in accordance with the relevant Chinese social security laws, which is approximately 20% of salaries. The costs of these payments are charged to the same accounts as the related salary costs in the same period as the related salary costs and are not material.
Advertising
Advertising is expensed as incurred. Advertising expenses were included in selling expenses and amounted to $46,439 and $175,086 for the nine months ended September 30, 2009 and 2008, respectively.
Research and development
Research and development costs are expensed as incurred. These costs primarily consist of cost of material used and salaries paid for the development of the Company’s products and fees paid to third parties. For the nine months ended September 30, 2009, the Company did not have any research and development expense. For the nine months ended September 30, 2008, the Company expensed $1,193,916 as research and development expense.
Foreign currency translation
The reporting currency of the Company is the U.S. dollar. The functional currency of the Company is the local currency, the Chinese Renminbi (“RMB”). Results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
All of the Company’s revenue transactions are transacted in the functional currency. The Company does not enter any material transaction in foreign currencies and accordingly, transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Asset and liability accounts on September 30, 2009 and December 31, 2008 were translated at 6.8376 RMB to $1.00 USD and at 6.8542 RMB to $1.00 USD, respectively. Equity accounts were stated at their historical rate. The average translation rates applied to income statements for the nine months ended September 30, 2009 and 2008 were 6.84251 RMB and 6.99886 RMB to $1.00 USD, respectively. In accordance with Statement of Financial Accounting Standards No. 95, “Statement of Cash Flows,” cash flows from the Company’s operations is calculated based upon the local currencies using the average translation rate. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Earnings per Share
Basic earnings per share is computed by dividing net income available to common shareholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is computed by dividing net income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. Potentially dilutive common shares consist of common shares issuable upon the conversion of series A preferred stock (using the if-converted method) and common stock warrants (using the treasury stock method). The following table presents a reconciliation of basic and diluted net income per share:
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Earnings per Share (continued)
|
|
|
For the Three Months Ended September 30, | ||||
|
|
|
2009 |
|
2008 | ||
|
|
|
(Unaudited) |
|
(Unaudited) | ||
|
Net income for basic and diluted earnings per share |
|
$ |
5,390,797 |
|
$ |
3,213,676 |
|
Weighted average shares outstanding – basic |
|
|
43,997,079 |
|
|
42,420,239 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
Unexercised warrants |
|
|
- |
|
|
- |
|
Convertible debentures |
|
|
5,748,271 |
|
|
5,747,118 |
|
Weighted average shares outstanding– diluted |
|
|
49,745,350 |
|
|
48,167,357 |
|
Earnings per share – basic |
|
$ |
0.12 |
|
$ |
0.08 |
|
Earnings per share – diluted |
|
$ |
0.11 |
|
$ |
0.07 |
|
|
|
For the Nine months Ended September 30, | ||||
|
|
|
2009 |
|
2008 | ||
|
|
|
(Unaudited) |
|
(Unaudited) | ||
|
Net income for basic and diluted earnings per share |
|
$ |
13,744,432 |
|
$ |
6,389,034 |
|
Weighted average shares outstanding – basic |
|
|
43,527,746 |
|
|
42,269,997 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
Unexercised warrants |
|
|
- |
|
|
- |
|
Convertible debentures |
|
|
5,658,422 |
|
|
5,747,118 |
|
Weighted average shares outstanding– diluted |
|
|
49,186,167 |
|
|
48,017,115 |
|
Earnings per share – basic |
|
$ |
0.32 |
|
$ |
0.15 |
|
Earnings per share – diluted |
|
$ |
0.28 |
|
$ |
0.13 |
As of September 30, 2009 and 2008, a total of 5,166,999 outstanding warrants have not been included in the calculation of diluted earnings per share in order to avoid any anti-dilutive effect. The average closing market price of all outstanding warrants of the Company for the nine months ended September 30, 2009 and 2008 and for the three months ended September 30, 2009 and 2008 were lower than the exercise price of all outstanding warrants. Because of that, the Company assumes that none of the outstanding warrants during that period would have been exercised and therefore none were included in the computation of the diluted earnings per share for the nine months ended September 30, 2009 and 2008 and for the three months ended September 30, 2009 and 2008. Accordingly, the Company has excluded any effect of outstanding warrants with anti-dilutive effect.
Accumulated other comprehensive income
The Company follows ASC 220 “Reporting Comprehensive Income” to recognize the elements of comprehensive income. Comprehensive income is comprised of net income and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. For the Company, accumulated other comprehensive income consisted of unrealized gains on foreign currency translation adjustments from the translation of financial statements from Chinese RMB to US dollars. For the nine months ended September 30, 2009 and 2008, unrealized foreign currency translation gain was $130,633 and $2,173,475, respectively. For us, comprehensive income for the nine months ended September 30, 2009 and 2008 included net income and unrealized gains from foreign currency translation adjustments.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Subsequent Events
For purposes of determining whether a post-balance sheet event should be evaluated to determine whether it has an effect on the financial statements for the period ending September 30, 2009, subsequent events were evaluated by the Company as of November 12, 2009, the date on which the unaudited consolidated financial statements at and for the quarter ended September 30, 2009, were available to be issued.
Recent Accounting Pronouncements
In June 2009, FASB established Accounting Standards Codification TM (“Codification”) as the single source of authoritative accounting principles recognized by the FASB in the preparation of financial statements in conformity with the GAAP. The Codification will supersede all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting literature not included in the Codification will become non-authoritative. The Codification is effective for financial statements issued for interim and annual periods ending after September 15, 2009. Adoption of the Codification is not expected to have a material impact on the Company’s results of operations or financial position.
In June 2009, FASB updated the accounting standards related to the consolidation of variable interest entities (“VIEs”). The standard amends current consolidation guidance and requires additional disclosures about an enterprise’s involvement in VIEs. The standard shall be effective as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, for interim periods within the first annual reporting period, and for interim and annual reporting periods thereafter. Earlier application is prohibited. The Company does not expect the adoption to have a material impact on the Company’s results of operations or financial position.
In May 2009, FASB issued FAS No. 165, “Subsequent Events,” which was subsequently codified within ASC 855, “Subsequent Events”. The standard establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. An entity should apply the requirements of ASC 855 to interim or annual financial periods ending after June 15, 2009. Adoption of this standard does not have a material impact on the Company’s results of operations or financial position.
In April 2009, the FASB updated the accounting standards to provide guidance on estimating the fair value of a financial asset or liability when the trade volume and level of activity for the asset or liability have significantly decreased relative to historical levels. The standard requires entities to disclose the inputs and valuation techniques used to measure fair value and any changes in valuation inputs or techniques. In addition, debt and equity securities as defined by GAAP shall be disclosed by major category. This standard is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009, and is to be applied prospectively. The adoption did not have a material effect on the Company’s results of operations and financial condition.
In April 2009, the FASB updated the accounting standards for the recognition and presentation of other-than-temporary impairments. The standard amends existing guidance on other-than-temporary impairments for debt securities and requires that the credit portion of other-than-temporary impairments be recorded in earnings and the noncredit portion of losses be recorded in other comprehensive income. The standard requires separate presentation of both the credit and noncredit portions of other-than-temporary impairments on the financial statements and additional disclosures. This standard is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. At the date of adoption, the portion of previously recognized other-than-temporary impairments that represent the noncredit related loss component shall be recognized as a cumulative effect of adoption with an adjustment to the opening balance of retained earnings with a corresponding adjustment to accumulated other comprehensive income (loss). The adoption of this standard did not have a material effect on the preparation of the Company’s consolidated financial statements.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 1 - ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recent Accounting Pronouncements (continued)
In August 2009, the FASB updated the accounting standards to provide additional guidance on estimating the fair value of a liability in a hypothetical transaction where the liability is transferred to a market participant. The standard is effective for the first reporting period, including interim periods, beginning after issuance. ACC does not expect the adoption to have a material effect on ACC’s consolidated results of operations and financial condition.
Reclassifications
Certain prior period amounts have been reclassified to conform to the current period presentation. These reclassifications have no effect on the previously reported net income and cash flows.
NOTE 2 - ACCOUNTS RECEIVABLE
On September 30, 2009 and December 31, 2008, accounts receivable consisted of the following:
|
|
|
September 30, 2009 |
|
December 31, 2008 |
|
Accounts receivable |
$ |
1,765,390 |
$ |
6,132,912 |
|
Less: allowance for sales returns |
|
- |
|
- |
|
Less: allowance for doubtful accounts |
|
- |
|
- |
|
|
$ |
1,765,390 |
$ |
6,132,912 |
NOTE 3 - INVENTORIES
On September 30, 2009 and December 31, 2008, inventories consisted of the following:
|
|
|
September 30, 2009 |
|
December 31, 2008 |
|
Raw materials |
$ |
2,549,961 |
$ |
2,884,092 |
|
Work in process |
|
- |
|
- |
|
Packaging materials |
|
14,540 |
|
16,100 |
|
Finished goods |
|
430,494 |
|
887,610 |
|
|
|
2,994,995 |
|
3,787,802 |
|
Less: reserve for obsolete inventory |
|
- |
|
- |
|
|
$ |
2,994,995 |
$ |
3,787,802 |
NOTE 4 - PROPERTY AND EQUIPMENT
On September 30, 2009 and December 31, 2008, property and equipment consist of the following:
|
|
Useful Life |
|
September 30, 2009 |
|
December 31, 2008 |
|
Office equipment and furniture |
3-8 Years |
$ |
244,476 |
$ |
228,673 |
|
Manufacturing equipment |
10 – 15 Years |
|
5,589,163 |
|
5,585,793 |
|
Building and building improvements |
20 – 40 Years |
|
3,143,778 |
|
3,136,164 |
|
Construction in progress |
|
|
16,858,401 |
|
1,181,757 |
|
|
|
|
25,835,818 |
|
10,132,387 |
|
Less: accumulated depreciation |
|
|
(2,963,334) |
|
(2,577,570) |
|
|
|
$ |
22,872,484 |
$ |
7,554,817 |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 4 - PROPERTY AND EQUIPMENT (Continued)
On September 30, 2009, construction in progress amounted to $16,858,401, representing (i) construction for a new manufacturing plant of approximately $7.02 million located in Cha Ha Er Industrial Park in Inner Mongolia, China, and (ii) payment for construction of a new building of approximately $ 9.84 million in Beijing, China. The amount for the new plant in Inner Mongolia includes costs for road, paving, water well, water reservoir, pump station, switchboard room, distribution room materials, equipment installation engineering (such as transformation boxes, related corollary equipments and various cables etc.,), filter plant, flameproof equipment, fire control pond and pump, civil defense engineering and underground long corridor project, such as electrical, drainage system, heating, water pipes constructions and payment for design service. The amount for the new construction building in Beijing includes payments for design service and additional payments for land. The new building will be set up on the land of 6700 square meters where Beijing En Ze Ji Shi currently is located. The permit to upgrade to industrial use of this parcel of land from the previous green-use was granted by Beijing Land Planning Bureau after the Company fully paid the required land value increment taxes in the fiscal 2009. Currently, the Company’s administration office, sales office, R&D center and production base are widely separated in various districts of Beijing. After finishing the construction of the new building, we will relocate above mentioned operating units into one concentrated area, which would help us run our business more efficiently. Upon completion of the construction in progress, the assets will be classified to be its respective property and equipment category.
For the nine months ended September 30, 2009 and 2008, depreciation expense amounted to $379,234 and $356,556, of which $359,654 and $346,151 was included in cost of sales, respectively.
NOTE 5 - DEPOSIT ON PATENT LICENSE AND INSTALLMENTS ON INTANGIBLE ASSETS
Deposit and Installments on a Chinese Class I drug patent-Laevo-Bambutero
Pursuant to the technology transfer agreement the Company entered into in April 2008 (See note 12), the Company made a deposit to acquire a Chinese Class I drug patent. Accordingly, the Company recorded $2,925,003 (RMB 20 million) as deposit on patent as of September 30, 2009.
Also, the Company has arranged an installment payment plan on the Chinese Class I drug patent to obtain the patent according to the signed contract. Therefore, the Company made $5,411,255 (RMB 37 million) as installment payments on the intangible assets as of September 30, 2009. The Company will need to make additional installment payments of approximately $1.6 million (RMB 11 million) to obtain the patent.
Deposit and Installments on a Chinese Class I drug patent-Laevo-Bambutero (continued)
In addition, we expect to incur approximately $11.7 million (RMB 80 million) related to the clinical trial evaluations for our Laevo-Bambutero drug in next 36 months, the application of which was accepted by the Chinese SFDA in July 10, 2008
Installments on land use right
As of September 30, 2009, the Company paid a total of $32,710,308 (RMB 223.66 million) for the land use rights to the Company’s new plant site in the Cha Ha Er Industrial Park ( See note 12), located in Inner Mongolia. The amount has been recorded as an installment payment on intangible assets on the Company’s balance sheet as of September 30, 2009. The installment payment is refundable if the Chinese local government would not grant its land use rights certificate.
Installments on Gliclazide-Controlled Release Tablets
Pursuant to the new drug patent transfer agreement the Company entered into in February 2009 (See note 12 for Gliclazide-Controlled Release Tablets), the Company has made the first installment to the transferor to obtain the patent. Hence, the Company recorded $877,501 (RMB 6 million) as installment payment on intangible assets as of September 30, 2009. In order to acquire the patent, the Company needs to make additional installments of approximately $439,000 (RMB 3 million).
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 5 - DEPOSIT ON PATENT LICENSE AND INSTALLMENTS ON INTANGIBLE ASSETS (Continued)
In addition, we expect to incur approximately $293,000 million (RMB 2 million) related to our Gliclazide-Controlled Release Tablets that was recently accepted by the Chinese SFDA for medicine registration application in the next 12 months.
NOTE 6 - INTANGIBLE ASSETS
The Company purchased manufacturing rights for two approved drugs. The manufacturing rights issued are in connection with the Company’s products Valsartan and Brimonidine. The manufacturing rights for Valsartan became effective in November 2000 and had a life of 6.5 years, which expired in 2007. The manufacturing rights for Brimonidine became effective on August 27, 2005 and expired in August 2009.
On October 9, 2006, the Company entered into a five-year loan agreement and a contract with Wu Lan Cha Bu Emergency Hospital (“Wu Lan”), whereby the Company agreed to lend Wu Lan approximately $4 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. In exchange for the loan, Wu Lan agreed to grant the Company an exclusive right to supply all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. In October 2006, the Company’s chief executive officer, Mr. Liu Zhongyi (hereafter, “Mr. Liu”), lent this loan to Wu Lan on behalf of the Company. On October 21, 2006, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Mr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus. Since Mr. Liu accepted the assignment with all the risks and obligations but no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Mr. Liu compensation for an aggregate of approximately $1.3 million (RMB 9 million) in five (5) equal annual installments of approximately $263,000 (RMB 1.8 million) started from October 21, 2006. Accordingly, the Company recorded an intangible asset of approximately $1.3 million (RMB 9 million) related to the exclusive rights to provide all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. The Company will amortize this exclusive right over a term of 20 years.
The Company entered into an intellectual rights transfer contract with Beijing Yipuan Bio-Medical Technology Co., Ltd. in December 2008 to acquire the drug Yipubishan. According to a clinical research report issued by Beijing Union Medical College Hospital Center for Clinical Pharmacology, Yipubishan is a highly effective and stable octreotide acetate injection solution. The drug was begun to be used to treat the symptoms of gastric ulcers and hemorrhages of the upper digestive tract since 2004. The intellectual property right is valued at a fixed amount of RMB 54 million (approximately $7.9 million). We have paid the transfer fee in full for the intellectual property right to Yipuan as of June 30, 2009. The intellectual property right has a term of 10 years and will not expire until December 31, 2018. The Company amortizes the intellectual property right over the term of the intellectual property right.
On September 30, 2009 and December 31, 2008, intangible assets consist of the following:
|
|
|
September 30, 2009 |
|
December 31, 2008 |
|
Manufacturing rights |
$ |
1,267,404 |
$ |
1,264,334 |
|
Revenue rights |
|
1,316,251 |
|
1,313,064 |
|
Intellectual rights |
|
7,897,508 |
|
- |
|
Software |
|
10,822 |
|
10,796 |
|
|
|
10,491,985 |
|
2,588,194 |
|
Less: accumulated amortization |
|
(2,062,980) |
|
(1,356,464) |
|
|
|
|
|
|
|
|
$ |
8,429,005 |
$ |
1,231,730 |
Amortization expense amounted to approximately $702,719 and $112,900 for the nine months ended September 30, 2009 and 2008, respectively.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 6 - INTANGIBLE ASSETS (Continued)
The projected amortization expense attributed to future periods is as follows:
|
Period ending September 30: |
|
|
Expense |
|
2010 |
|
$ |
858,196 |
|
2011 |
|
|
857,806 |
|
2012 |
|
|
855,685 |
|
2013 |
|
|
855,563 |
|
Thereafter |
|
|
5,001,755 |
|
|
|
|
|
|
Total |
|
$ |
8,429,005 |
NOTE 7 - RELATED PARTY TRANSACTIONS
Notes payable – related parties
Notes payable - related parties consisted of the following on September 30, 2009 and December 31, 2008:
|
|
|
September 30, |
|
December 31, |
|
Note to Song Guoan, father of Song Zheng Hong, director and spouse of Liu Zhong Yi, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.75% and 4.75% on September 30, 2009 and December 31, 2008, respectively), and unsecured |
$ |
763,744 |
$ |
761,894 |
|
|
|
|
|
|
|
Note to Zheng Gui Xin, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.75% and 4.75% on September 30, 2009 and December 31, 2008, respectively), and unsecured |
|
1,653,358 |
|
1,649,354 |
|
|
|
|
|
|
|
Note to Ma Zhao Zhao, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.75% and 4.75% on September 30, 2009 and December 31, 2008, respectively), and unsecured |
|
662,166 |
|
660,562 |
|
|
|
|
|
|
|
Note to Liu Zhong Yi, Chief Executive Officer and director, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.75% and 4.75% on September 30, 2009 and December 31, 2008, respectively), and unsecured |
|
1,409,507 |
|
1,406,094 |
|
|
|
|
|
|
|
Note to Song Zheng Hong, director and spouse of the Company Chief Executive Officer, Liu Zhong Yi, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.75% and 4.75% on September 30, 2009 and December 31, 2008, respectively), and unsecured |
|
579,952 |
|
578,547 |
|
Total notes payable – related parties, long term |
$ |
5,068,727 |
$ |
5,056,451 |
For the nine months ended September 30, 2009 and 2008, interest expense related to these related loans amounted to $178,047 and $233,704, respectively.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 7 - RELATED PARTY TRANSACTIONS (Continued)
Due to related parties
The chief executive officer of the Company and his spouse and several employees of the Company, from time to time, provided advances to the Company for working capital purpose. During the nine months ended September 30, 2009 and 2008, the Company did not repay any of these advances. On September 30, 2009 and December 31, 2008, the Company had a payable to the chief executive officer and his spouse and other employees at an amount of $720,280 and $718,536, respectively. These advances are short-term in nature and non-interest bearing.
On October 9, 2006, the Company entered into a five-year loan agreement and a contract with Wu Lan, whereby the Company agreed to lend Wu Lan approximately $4 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. In exchange for the loan, Wu Lan agreed to grant the Company an exclusive right to supply all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. In October 2006, the Company’s chief executive officer, Mr. Liu, lent this loan to Wu Lan on behalf of the Company. On October 21, 2006, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Mr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus. Since Mr. Liu accepted the assignment with all the risks and obligations but had no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Mr. Liu compensation for an aggregate of approximately $1.3 million (RMB 9 million) in 5 equal annual installments of approximately $263,000 (RMB 1.8 million) started from October 21, 2006. Accordingly, the Company recorded an intangible asset of approximately $1.3 million (RMB 9 million) related to the exclusive rights to provide all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. The Company will amortize this exclusive right over a term of 20 years.
For the nine months ended September 30, 2009 and for the year ended December 31, 2008, the Company did not pay anything to Mr. Liu for the liability incurred by the assignment in the agreement mentioned above. On September 30, 2009 and December 31, 2008, amounts due under this assignment agreement were $1,033,532 and $1,031,028, of which $329,063 and $525,225 were included in long-term liabilities and has been included in due to related parties on the accompanying balance sheets, respectively.
On September 30, 2009 and December 31, 2008, the Company has recorded accrued interest relating to notes payable - related parties of $543,409 and $364,350, respectively, which have been included in due to related parties on the accompanying balance sheets.
For the nine months ended September 30, 2009 and for the year ended December 31, 2008, a summary of activities in due to related parties is as follows:
|
|
|
Assignment fee |
|
Working capital |
|
Accrued |
|
Total |
|
Balance - December 31, 2007 |
$ |
966,198 |
$ |
34,072 |
$ |
61,208 |
$ |
1,061,478 |
|
Additions |
|
- |
|
682,179 |
|
299,036 |
|
981,215 |
|
Payments made |
|
- |
|
- |
|
- |
|
- |
|
Foreign currency fluctuations |
|
64,830 |
|
2,285 |
|
4,106 |
|
71,221 |
|
Balance - December 31, 2008 |
$ |
1,031,028 |
$ |
718,536 |
$ |
364,350 |
$ |
2,113,914 |
|
Additions |
|
- |
|
- |
|
178,175 |
|
178,175 |
|
Payments made |
|
- |
|
- |
|
- |
|
- |
|
Foreign currency fluctuations |
|
2,504 |
|
1,744 |
|
884 |
|
5,132 |
|
Balance - September 30, 2009 |
$ |
1,033,532 |
$ |
720,280 |
$ |
543,409 |
$ |
2,297,221 |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 8 - CONVERTIBLE REDEEMABLE PREFERRED STOCK
On February 25, 2008 (“Closing Date”), the Company sold, pursuant to a Convertible Redeemable Preferred Share and Warrant Purchase Agreement (the “Purchase Agreement”) by and among the Company, Dr. Liu Zhongyi and Mrs. Song Zhenghong (the “Founders”), and accredited investors (each a “Purchaser” and collectively, the “Purchasers”), 5,747,118 shares of the Company’s series A convertible redeemable preferred stock (the “Preferred Stock”) and warrants (the “Warrants”) to purchase 2,873,553 shares of the Company’s common stock, in a private placement (the “February 2008 Private Placement”) pursuant to Regulation D under the Securities Act of 1933, for an aggregate purchase price of $5 million (the “Transaction”). Net proceeds, exclusive of expenses of the transaction were $4.6 million in cash, after the Company paid fees of approximately $469,000 in cash, of which $400,000 was paid to Maxim Group, LLC, the placement agent for the Transaction. The Company recorded approximately $796,000 fee, of which $327,565 was related to the value of the warrants granted to the placement agent, as a deferred debt cost and amortized approximately $ 311,388 and $232,205 of the deferred cost during the nine months ended September 30, 2009 and 2008, respectively. The convertible redeemable preferred stock is deemed debt due to the mandatory redeemable feature of the preferred stocks.
The Company used $2,576,556 of the net proceeds of the Transaction to repay in full all of its outstanding principal obligations including accrued interest under the 14% Secured Convertible Notes due February 2008, and the Company used the remainder of the net proceeds for working capital and general corporate purposes.
Each of these Preferred Shares is convertible into one share of the Company’s common stock (as adjusted for stock splits, stock dividends, reclassification and the like), pays an 8% dividend annually, payable in additional Convertible Preferred Shares and also pays any dividend to be paid on the common shares on an as-converted basis. Until May 25, 2010, the Preferred Shares may be redeemed at the option of the Purchasers at the redemption price of $0.87 per share (as adjusted for stock splits, stock dividends, reclassification and the like), and no other capital stock of the Company may be redeemable prior to the Preferred Shares. Holders of Preferred Shares may not convert Preferred Shares to common shares if the conversion would result in the holder beneficially owning more than 4.99% of the Company’s outstanding common shares. That limitation may be waived by a holder of Preferred Shares on not less than 61 days written notice to the Company.
The Warrants have an initial exercise price of $1.20 (subject to adjustment pursuant to the terms of the warrants), are exercisable for a period of five (5) years and may not be exercised if exercise would result in the holder beneficially owning more than 4.99% of the Company’s outstanding common shares. That limitation may be waived by a holder of the warrants by sending a written notice to the Company in not less than 61 days.
Other key provisions of the February 2008 Private Placement:
|
|
• |
The Preferred Shares vote with the common stock on an as converted basis, except that the Preferred Shares are not entitled to vote for directors. The Company is prohibited from taking certain corporate actions, including, without limitation, issuing securities senior to the Preferred Shares, selling substantially all assets, repurchasing securities and declaring or paying dividends, without the approval of the holders of a majority of the Preferred Shares then outstanding. |
|
|
|
|
|
|
• |
The Company agreed to undertake to file a resale registration statement within 60 days following the Closing Date registering the maximum number of shares common stock issuable upon conversion of the Preferred Shares and exercise of the warrants allowable under applicable federal securities regulations. If the Company was informed by the SEC that there were no comments to the registration statement, then the registration statement was required to be declared effective within five (5) business days thereafter or on the 60th day after the filing date, whichever is sooner. If the SEC issued comments to the registration statement, then the registration statement was required to be declared effective by the 120th day after it was filed. If the registration statement was not declared effective by the applicable date, the Company would be subject to liquidated damages, equal to 1% of the total conversion price and exercise price for the common stock being registered under the registration statement, for every 30-day period following the date that the registration statement should have been effective, prorated for any period less than 30 days, until either all of common shares registered under the registration statement have been sold or all such common shares may be sold in any three (3) month period pursuant to Rule 144 promulgated under the Securities Act, whichever is earlier. The Company must also pay the liquidated damages if sales cannot be made pursuant to the registration statement for any reason (excepting market conditions). The maximum amount of liquidated damages is $500,000. The Company filed the resale registration statement on May 13, 2008 and received comments from the SEC. The SEC declared the resale registration statement effective on July 25, 2008. |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 8 - CONVERTIBLE REDEEMABLE PREFERRED STOCK (Continued)
|
|
• |
The Founders own aggregate 7,500,000 shares of the Company’s common stock owned (the “Escrow Shares”). Portions of the Escrow Shares are being held in escrow subject to the Company meeting certain net income targets in fiscal years 2007, 2008 and 2009. The $8.5 million net income target for 2007 was achieved; as was 95% of $13.8 million in net income target for 2008. The net income target for 2009 is 95% of $17.5 million of net income, both after eliminating the effect of non-cash charges associated with the Transaction and adjusting for differences in the exchange rate between Chinese Renminbi and US dollars used in the Company’s financial statements and an exchange rate of RMB 7.30 to USD 1.00. A portion of the Escrow Shares will be transferred to the Purchasers if the Company does not meet the earning targets, and released back to the Founders if the Company does; another portion of the Escrow Shares is being held in escrow subject to the Company listing on the NASDAQ Stock Market within 18 months following the Closing Date. These Escrow Shares will be transferred to the Purchasers if the listing is not completed within that time period, and released back to the Founders if it is. In addition, two-thirds of the Escrow Shares are held in escrow to ensure that the Purchasers receive their full redemption payments if they choose to redeem their Preferred Shares. If a Purchaser receives less than the full redemption amount for each Preferred Share being redeemed, the Purchaser will receive a number of Escrow Shares to make up the difference, based on the then-current market price of the common shares. Following the end of the redemption period, these Escrow Shares, less those transferred to any Purchasers that redeemed their Preferred Shares, will be released back to the Founders. |
In connection with the issuance of the Preferred Shares and Warrants, the Company recorded a total debt discount of $2,310,263 to be amortized over the term of the Purchase Agreement. For the nine months ended September 30, 2009 and 2008, amortization of debt discount amounted to $ 880,788 and $592,966, respectively, have been included in interest expense.
The Company classified the series A Convertible redeemable preferred stock as liability because of its mandatory redeemable feature according to ASC 480.
On February 25, 2009, the Company issued 459,772 additional shares of Series A Preferred Stock to the holders of Series A Preferred Stock for the mandatory 8% annual dividends.
In May 2009, a Series A convertible redeemable preferred stockholder converted 57,471 shares of preferred stock to 57,471 shares of our common stock.
In June 2009, another Series A convertible redeemable preferred stockholder converted 114,942 shares of preferred stock to 114,942 shares of our common stock.
In September 2009, a few of Series A convertible redeemable preferred stockholder converted 286,206 shares of preferred stock to 286,206 shares of our common stock.
The series A convertible redeemable preferred stock on September 30, 2009 and December 31, 2008 is as follows:
|
|
|
September 30, 2009 |
|
December 31, 2008 |
|
|
|
(Unaudited) |
|
(Audited) |
|
Series A convertible redeemable preferred stock |
$ |
5,001,000 |
$ |
5,000,000 |
|
Less: unamortized discount |
|
(466,871) |
|
(1,347,659) |
|
Series A convertible redeemable preferred stock, net |
$ |
4,534,129 |
$ |
3,652,341 |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 9 - TAXES PAYABLE
Value Added Tax Payable
The Company is subject to VAT for manufacturing products. The applicable VAT tax rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). Under the commercial practice of the PRC, the Company paid VAT based on tax invoices issued. The tax invoices may be issued subsequent to the date on which revenue is recognized, and there may be a considerable delay between the date on which the revenue is recognized and the date on which the tax invoice is issued. In the event that the PRC tax authorities dispute the date of which revenue is recognized for tax purposes, the PRC tax office has the right to assess a penalty, which can range from zero to five times the amount of the taxes which are determined to be late or deficient. According to the PRC tax laws, any potential tax penalty payable on late or deficient payments of this tax could be between zero and five times the amount of the late or deficient tax payable, and will be expensed as a period expense if and when a determination has been made by the taxing authorities that a penalty is due. On September 30, 2009 and December 31, 2008, the Company had accrued VAT taxes payable of $5,937,332 and $5,015,908, respectively.
Income tax payable
On March 16, 2007, the National People’s Congress of China passed the new Enterprise Income Tax Law (“EIT Law”), and on November 28, 2007, the State Council of China passed the Implementing Rules for the EIT Law (“Implementing Rules”) which took effect on January 1, 2008. The EIT Law and Implementing Rules impose a unified EIT rate of 25.0% on all domestic-invested enterprises and Foreign Interest Enterprises (“FIEs”), unless they qualify under certain limited exceptions. Therefore, nearly all FIEs are subject to the new tax rate alongside other domestic businesses rather than benefiting from the Foreign Enterprise Income Tax (“FEIT”), and its associated preferential tax treatments, beginning on January 1, 2008. However, we got income tax exemption for taxable income in fiscal year 2008 from National Taxation Bureau of P.R.C. located in Fengtai District, Beijing, P.R.C. on January 22, 2008.
In addition to the changes to the current tax structure, under the EIT Law, an enterprise established outside of China with “de facto management bodies” within China is considered a resident enterprise and will normally be subject to an EIT of 25.0% on its global income. The Implementing Rules define the term “de facto management bodies” as “an establishment that exercises, in substance, overall management and control over the production, business, personnel, accounting, etc., of a Chinese enterprise.” If the PRC tax authorities subsequently determine that the Company should be classified as a resident enterprise, then the organization’s global income will be subject to PRC income tax of 25.0%. Beijing Liang Fang was subject to 25% income tax rate since January 1, 2009. However, located in Inner Mongolia, Liang Fang’s branch got income tax exemption for its fiscal 2009 taxable income from Cha You Qian Qi government situated in Inner Mongolia, P.R.C. on June 3, 2008.
The following table reconciles the statutory rates to the Company’s effective tax rate for the nine months ended September 30, 2009 and 2008:
|
|
2009 |
|
2008 |
|
US statutory rates |
34% |
|
34% |
|
Foreign income not recognized in the US |
(34%) |
|
(34%) |
|
China Statutory rates |
25% |
|
25% |
|
China income tax exemption |
(23.99%) |
|
(25%) |
|
Effective income tax rate |
1.01% |
|
0% |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 9 - TAXES PAYABLE (continued)
On September 30, 2009 and December 31, 2008, taxes payable are as follows:
|
|
|
September 30, 2009 |
|
December 31, 2008 |
|
|
|
(Unaudited) |
|
(Audited) |
|
Value added taxes |
$ |
5,937,332 |
$ |
5,015,908 |
|
Income taxes |
|
157,027 |
|
- |
|
Other taxes |
|
656,678 |
|
- |
|
Total |
$ |
6,751,037 |
$ |
5,015,908 |
NOTE 10 - SHAREHOLDERS’ EQUITY
Common Stock
In February 2009, the Company issued 842,308 shares of common stock to Mr. Liu Zhongyi, Chairman and CEO of the Company, for his services rendered through December 31, 2008 as the Company’s chief executive officer. The stock was valued at the fair value of $0.26 per share on the grant date.
In February 2009, the Company issued 67,308 shares of common stock to Adam Wasserman (former CFO) using a fair value of $0.26 per share on the grant date for his services rendered through December 31, 2008 as the Company’s chief financial officer.
In February 2009, the Company issued 48,077 shares of common stock to Mel Rothberg using a fair value of $0.26 per share on the grant date for his services as an independent director from January 1, 2008 to April 15, 2008 and consulting services rendered through November 30, 2008.
In May 2009, the Company issued 57,471 shares of its common stock to a Series A convertible redeemable preferred stockholder in connection with the conversion of 57,471 shares of Series A Preferred Stock.
In June 2009, the Company issued 114,942 shares of its common stock to a Series A convertible redeemable preferred stockholder in connection with the conversion of 114,942 shares of Series A Preferred Stock.
In June 2009, the Company issued 251,668 shares of common stock to Mr. Liu Zhongyi, Chairman and CEO of the Company, for his services rendered from January 1, 2009 to May 31, 2009 as the Company’s chief executive officer. The stock was valued at the fair value of $0.30 per share on the grant date.
In June 2009, the Company issued 90,876 shares of common stock to Adam Wasserman for services rendered from January 1, 2009 to April 30, 2009 as the Company’s former chief financial officer and services rendered from May 1, 2009 to May 31, 2009 as the Company’s consultant. The stock was valued at the fair value of $0.28 per share on the grant date.
In June 2009, the Company issued in aggregate 60,000 shares of common stock to the six directors of the Company for services rendered and to be rendered from January 1, 2009 to December 31, 2009 as the Company’s Board of Directors’ members. The Company valued these common shares at the fair value on the grant date at $0.30 per share or an aggregate of $18,000. In connection with issuance of these shares, the Company recorded prepaid stock-based expenses of $18,000. As of September 30, 2009, the Company recorded amortization on prepaid stock-based expenses of $13,500 related to those issuances.
In September 2009, the Company issued in aggregate 286,206 shares of common stock to two Series A convertible redeemable preferred stockholders in connection with the conversion of 286,206 shares of Series A Preferred Stock.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 10 - SHAREHOLDERS’ EQUITY (Continued)
Statutory Surplus Reserves
The Company is required to make appropriations to reserve funds, comprising the statutory surplus reserve and discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve is required to be at least 10% of the after tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entities’ registered capital. Appropriations to the discretionary surplus reserve are made at the discretion of the Board of Directors.
The statutory surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of shares currently held by them, provided that the remaining statutory surplus reserve balance after such issue is not less than 25% of the registered capital.
The discretionary surplus reserve may be used to acquire fixed assets or to increase the working capital to expend on production and operation of the business. The Company’s Board of Directors decided not to make an appropriation to this reserve for the fiscal year 2009.
Pursuant to the Company’s articles of incorporation, the Company is to appropriate 10% of its net profits as statutory surplus reserve. For the nine months ended September 30, 2009, statutory surplus reserve activity was as follows:
|
|
|
Statutory Surplus Reserve |
|
Balance – December 31, 2008 |
$ |
3,750,529 |
|
Addition to statutory reserves |
|
1,546,275 |
|
Balance – September 30, 2009 |
$ |
5,296,804 |
Stock warrants
Stock warrants issued, terminated/forfeited, exercised and outstanding during the nine months ended September 30, 2009 (unaudited) are as follows:
|
|
|
Shares |
|
Average Exercise price per share |
| ||
|
Warrants outstanding December 31, 2007 |
|
|
1,500,000 |
|
$ |
0.87 |
|
|
Warrants issued |
|
|
3,726,999 |
|
|
1.21 |
|
|
Warrants terminated/forfeited |
|
|
- |
|
|
- |
|
|
Warrants exercised |
|
|
(60,000) |
|
|
0.87 |
|
|
Warrants outstanding, December 31, 2008 |
|
|
5,166,999 |
|
|
1.13 |
|
|
Warrants granted |
|
|
- |
|
|
- |
|
|
Warrants expired/forfeited |
|
|
- |
|
|
- |
|
|
Warrants exercised |
|
|
- |
|
|
- |
|
|
Warrants outstanding, September 30, 2009 |
|
|
5,166,999 |
|
$ |
1.13 |
|
The following table summarizes the shares of the Company’s common stock issuable upon exercise of warrants outstanding on September 30, 2009:
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 10 - SHAREHOLDERS’ EQUITY (Continued)
Stock warrants (continued)
|
Warrants Outstanding |
|
Warrants Exercisable | |||||||||
|
|
Range of Exercise Price |
|
Number Outstanding on September 30, 2009 |
|
Weighted Average Remaining Contractual Life (Years) |
|
Weighted Average Exercise Price |
|
Number Exercisable on September 30, 2009 |
|
Weighted Average Exercise Price |
|
$ |
0.87 |
|
1,440,000 |
|
2.37 |
$ |
0.87 |
|
1,440,000 |
$ |
0.87 |
|
|
1.20 |
|
2,873,553 |
|
3.41 |
|
1.20 |
|
2,873,553 |
|
1.20 |
|
|
1.21 |
|
603,446 |
|
3.41 |
|
1.21 |
|
603,446 |
|
1.21 |
|
$ |
1.50 |
|
250,000 |
|
2.57 |
|
1.50 |
|
250,000 |
|
1.50 |
|
|
|
|
5,166,999 |
|
3.07 |
$ |
1.13 |
|
5,166,999 |
$ |
1.13 |
NOTE 11 - SEGMENT INFORMATION
The following information is presented in accordance with ASC 280, Disclosure about Segments of an Enterprise and Related Information. In the nine months ended September 30, 2009 and 2008, the Company operated in two reportable business segments: (1) the manufacture and distribution of pharmaceutical products and examination of other companies’ products and (2) the retailing of traditional and Chinese medicines and supplies through ten drug stores located in Beijing China and other ancillary revenues generated from retail location such as advertising income and rental income. The Company’s reportable segments are strategic business units that offer different products. They are managed separately based on the fundamental differences in their operations.
Information with respect to these reportable business segments for the three months ended September 30, 2009 and 2008 is as follows:
|
For the three months ended September 30, 2009 |
|
Wholesale and third-party manufacturing and examination |
|
Retail operations |
|
Rent and advertising |
|
Unallocated |
|
Total |
|
Net revenues |
$ |
11,606,345 |
|
2,708,071 |
|
198,288 |
|
- |
|
14,512,704 |
|
Cost of sales |
|
4,051,221 |
|
1,931,050 |
|
10,905 |
|
- |
|
5,993,176 |
|
Operating expenses |
|
- |
|
- |
|
- |
|
2,506,420 |
|
2,506,420 |
|
Other expense (income) |
|
- |
|
- |
|
- |
|
547,541 |
|
547,541 |
|
Income tax |
|
- |
|
- |
|
- |
|
74,770 |
|
74,770 |
|
Net income |
$ |
7,555,124 |
|
777,021 |
|
187,383 |
|
(3,128,731) |
|
5,390,797 |
|
For the three months ended September 30, 2008 |
|
Wholesale and third-party manufacturing and examination |
|
Retail operations |
|
Rent and advertising |
|
Unallocated |
|
Total |
|
Net revenues |
$ |
13,655,881 |
|
2,831,030 |
|
216,973 |
|
- |
|
16,703,884 |
|
Cost of sales |
|
6,520,740 |
|
1,698,618 |
|
- |
|
- |
|
8,219,358 |
|
Operating expenses |
|
- |
|
- |
|
- |
|
4,726,868 |
|
4,726,868 |
|
Other expense (income) |
|
- |
|
- |
|
- |
|
543,982 |
|
543,982 |
|
Income tax |
|
- |
|
- |
|
- |
|
- |
|
- |
|
Net income |
$ |
7,135,141 |
|
1,132,412 |
|
216,973 |
|
(5,270,850) |
|
3,213,676 |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 11 - SEGMENT INFORMATION (Continued)
Information with respect to these reportable business segments for the nine months ended September 30, 2009 and 2008 is as follows:
|
For the nine months ended September 30, 2009 |
|
Wholesale and third-party manufacturing and examination |
|
Retail operations |
|
Rent and advertising |
|
Unallocated |
|
Total |
|
Net revenues |
$ |
31,769,304 |
|
7,604,678 |
|
594,738 |
|
- |
|
39,968,720 |
|
Cost of sales |
|
11,452,209 |
|
5,437,580 |
|
32,711 |
|
- |
|
16,922,500 |
|
Operating expenses |
|
- |
|
- |
|
- |
|
7,525,763 |
|
7,525,763 |
|
Other expense (income) |
|
- |
|
- |
|
- |
|
1,619,110 |
|
1,619,110 |
|
Income tax |
|
- |
|
- |
|
- |
|
156,915 |
|
156,915 |
|
Net income |
$ |
20,317,095 |
|
2,167,098 |
|
562,027 |
|
(9,301,788) |
|
13,744,432 |
|
For the nine months ended September 30, 2008 |
|
Wholesale and third-party manufacturing and examination |
|
Retail operations |
|
Rent and advertising |
|
Unallocated |
|
Total |
|
Net revenues |
$ |
38,725,099 |
|
8,492,107 |
|
581,467 |
|
- |
|
47,798,673 |
|
Cost of sales |
|
20,589,237 |
|
5,095,264 |
|
- |
|
- |
|
25,684,501 |
|
Operating expenses |
|
- |
|
- |
|
- |
|
14,074,682 |
|
14,074,682 |
|
Other expense (income) |
|
- |
|
- |
|
- |
|
1,650,456 |
|
1,650,456 |
|
Income tax |
|
- |
|
- |
|
- |
|
- |
|
- |
|
Net income |
$ |
18,135,862 |
|
3,396,843 |
|
581,467 |
|
(15,725,138) |
|
6,389,034 |
The Company does not allocate selling expenses, general and administrative expenses and income tax to its reportable segments, because these activities are managed at a corporate level.
Asset information by reportable segment is not reported to or reviewed by the chief operating decision maker and, therefore, the Company has not disclosed asset information for each reportable segment. Substantially all of the Company’s assets are located in China.
NOTE 12 - COMMITMENTS AND CONTINGENCIES
Technology Transfer Agreement
In April 2008, one of the Company’s affiliates, En Ze Jia Shi, entered into a Technology Transfer Agreement with Dong Guan Kai Fa Biological Medicine LTD (“Dong Guan”) pursuant to which Dong Guan agreed to transfer the technology material, new medicine research and rights to the Chinese patent of the anti-asthma new medicine R-BM to En Ze Jia Shi on an exclusive basis in exchange for a transfer technology fee of approximately $7.02 million (RMB 48 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia Shi is obligated to:
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 12 - COMMITMENTS AND CONTINGENCIES (Continued)
Technology Transfer Agreement (continued)
|
|
• |
complete the filing with the SFDA of the medicine’s clinical research ratification document, |
|
|
|
|
|
|
• |
complete the clinical research, |
|
|
|
|
|
|
• |
complete the medicine’s trial production, and |
|
|
|
|
|
|
• |
provide raw materials and formulation related documentation and apply for the new medicine certification and production approval. |
In addition to the payment of the technology transfer fee, En Ze Jia Shi is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at $11.7 million (RMB 80 million). Lotus East intends to use its working capital to fund the project costs.
Dong Guan is responsible for preparing and transferring the clinical research and application documents as well as assisting En Ze Jia Shi in the completing the clinical research and applying for the new medicine certification and production approval documents.
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
|
|
• |
Approximately $1.46 million (RMB 10 million) is due by the 15th business day following the receipt of the processing notice of the receipt of the clinical application and all related material from SFDA is received, |
|
|
|
|
|
|
• |
Approximately $1.17 million (RMB 8 million) is due by the 10th business day after the receipt of the medicine’s clinical ratification document, |
|
|
|
|
|
|
• |
Approximately $1.46 million (RMB 10 million) is due by the 15th business day after the medicine’s Phase I clinical study is completed and ratification from the SFDA is obtained, and |
|
|
|
|
|
|
• |
Approximately $2.93 million (RMB 20 million) is due by the 10th business day after the medicine’s Phase II clinical study is completed and ratification from the SFDA is obtained. |
En Ze Jia Shi paid Dong Guan a deposit of approximately $2.93 million (RMB 20 million) in April 2008 which is to be returned to En Ze Jia Shi within 10 days after the transfer technology fee is fully paid. In the event Dong Guan should be unable to timely return the deposit, it will pay En Ze Jia Shi a late fee and En Ze Jia Shi is entitled to damages for Dong Guan’s failure to timely return the deposit.
The intellectual property arising from the agreement will be jointly shared by the parties. In addition, En Ze Jia Shi has guaranteed that both parties must jointly apply for related government grants prior to when the new medicine is marketed. Upon receipt of the government grants En Ze Jia Shi guaranteed that the grant monies will be shared equally by both parties. As of September 30, 2009, the Company has not received any government grant. The agreement can be terminated by Dong Guan if En Ze Jia Shi should fail to make any of the aforedescribed payments in which event the patent rights would revert to Dong Guan and it is entitled to transfer the project rights to a third party.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 12 - COMMITMENTS AND CONTINGENCIES (Continued)
New Manufacturing Facility
In June 2008, one of the Company’s affiliates, Liang Fang, entered into an agreement with Cha You Qian Qi Economy Commission, a governmental agency (“Cha You”) related to the construction of a pharmaceutical plant in Cha You’s Cha Ha Er Industrial Garden District in Inner Mongolia. The new facility, which will be comprised approximately 40,000 square meters situated on 600 MU of land (approximately 400,200 square meters), will be used to expand Liang Fang’s current manufacturing capacity. The Company was subsequently granted the right to expand the land use right area to 1,000 MU (approximately 667,000 square meters). The new facility, which will manufacture medical injection products, including 0.9% physiological saline injection, hydroxyethyl starch 130/0.4 injection and hydroxyethyl starch 200/0.5 injection, Qiang Yi Ji starch, a medical corn starch commonly known as O-2-hydeoxyethyl starch, dextran and additional pharmaceuticals, will require a total investment of RMB 623.66 million, or approximately $91 million. The construction began on the project in August 2008 and the Company anticipates that it will take between 12 to 30 months to complete the construction. As of September 30, 2009, the Company has incurred approximately $39.7 million related to this project and the amount has been included as construction in progress of approximately $7 million and installments on intangible assets of approximately $32.7 million in the consolidated financial statements.
Included in the total cost of the project is land cost of approximately $32.7 million (RMB 223.66 million) which was paid in full to Cha You government. Other components of the project include construction costs of approximately $17.5 million (RMB 120 million) costs associated with the various production lines estimated at approximately $33.6 million (RMB 230 million) and working capital of approximately $7.3 million (RMB 50 million).
Liang Fang intends to use its present working capital together with bank loans and government grants to fund the project. The funds are required to be invested over the next 15 months under a specified schedule ending in December 2010. As of September 30, 2009, Liang Fang has paid approximately $36.3 million (approximately RMB 248.5 million) of the total investment. Liang Fang, however, has not secured either the bank loans or government grants and does not have sufficient working capital to complete this project without securing substantial funds from those third party sources.
Under the terms of the agreement, Cha You agreed to abate fees associated with water resources, waste and other relative supplies for a period of 30 years and agreed to ensure that the land use tax to be paid by Liang Fang after it begins normal production will be at the lowest tax rate imposed for five years. Once the project is completed, for a period of eight years the local reserved portion of the imposed corporation income tax will be returned to Liang Fang.
New Drug Patent Transfer Agreement
In February 2009, one of our affiliates, En Ze Jia Shi, entered into a New Drug Patent Transfer Agreement with Beijing Huicheng Ruixiang Pharmaceutical Technology Co. LTD (“Huicheng”) pursuant to which Huicheng agreed to transfer the patent technology and related research materials about the Chinese drug of Gliclazide-Controlled Released Tablets to En Ze Jia Shi on an exclusive basis in exchange for a transfer patent fee of approximately $1.3million (RMB 9 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia Shi is obligated to:
|
|
• |
Finishing other needed related technical materials of this new medicine and providing the legal invoice for raw materials and purchase agreement etc., |
|
|
• |
Providing enough raw materials, of which amount should ensure Huicheng to successfully prepare new medicine of 100,000 dosage units, and standard samples for experiments and research, |
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 12 - COMMITMENTS AND CONTINGENCIES (Continued)
New Drug Patent Transfer Agreement (continued)
|
|
• |
Providing the choice basis and quality standards of the package materials, |
|
|
• |
Paying for organization, seal, signature, field-exam, registration and related fees including registration and examination fees, registration evaluation fees etc. for the new medicine registration materials |
|
|
• |
Completing clinical research and paying related fees if the new medicine is required for clinical research, |
|
|
• |
Making payment to Huicheng according to specific schedule mentioned in the agreement. |
In addition to the payment of the patent transfer fee, En Ze Jia Shi is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at proximately $293,000 million (RMB 2 million). Lotus East intends to use its working capital to fund the project costs.
Huicheng Ruixiang Pharmaceutical Technology Co. Ltd. is obligated to
|
|
• |
Provide the patent of the new medicine, |
|
|
|
|
|
|
• |
Provide the related technical materials and the original records of the experiments which satisfy the requirement of the fifth classified chemical drug registration by Drug Registration Administration Method (2005) issued by Chinese SFDA. The Huicheng has to provide above mentioned materials to En Ze Jia Shi in 30 days after it received first installment payment and qualified documents from En Ze Jia Shi, |
|
|
|
|
|
|
• |
Provide the new medicine registration samples with 100,000 dosage units, |
|
|
|
|
|
|
• |
Supplement and improve the technical materials according to the requirement of the Evaluation Center of Chinese State Drug Administration, |
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
|
|
• |
Approximately $0.9 million (RMB 6 million) is due by the 90th business day following the receipt of the Notice of China Accepted Patent and Notice of Medicine Registration Application, |
|
|
|
|
|
|
• |
Approximately $0.2 million (RMB 1.5 million) is due by the 30th business day after the receipt of the medicine’s clinical ratification document, |
|
|
|
|
|
|
• |
Approximately $0.2 million (RMB1.5 million) is due by the 30th business day after the production ratification from the SFDA is obtained. |
En Ze Jia Shi made the first installment of approximately $0.9 million (RMB 6 million) to Huicheng in May 2009 which is to be returned to En Ze Jia Shi if it can not obtain the production ratification from the SFDA due to any fault caused by Huicheng.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
NOTE 13 - OPERATING RISK
(a) Country risk
Currently, the Company’s revenues are primarily derived from the sale of pharmaceutical products to customers in P.R.C. The Company hopes to expand its operations to countries outside the PRC, however, such expansion has not been commenced and there are no assurances that the Company will be able to achieve such an expansion successfully. Therefore, a downturn or stagnation in the economic environment of the PRC could have a material adverse effect on the Company’s financial condition.
(b) Products risk
In addition to competing with other manufacturers of pharmaceutical product offerings, the Company competes with larger Chinese and International companies who may have greater funds available for expansion, marketing, research and development and the ability to attract more qualified personnel. These Chinese companies may be able to offer products at a lower price. There can be no assurance that the Company will remain competitive.
(c) Political risk
Currently, PRC is in a period of growth and is openly promoting business development in order to bring more business into PRC. Additionally, PRC allows a Chinese corporation to be owned by a United States corporation. If the laws or regulations are changed by the PRC government, the Company’s ability to operate the PRC subsidiaries and controlled entities could be affected.
NOTE 14 - SUBSEQUENT EVENTS
During the period from October 1, 2009 to November 12, 2009, the Company issued in aggregate 692,237 shares of common stock to various Series A convertible redeemable preferred stockholders in connection with the conversion of 692,237 shares of Series A preferred stock.
In October 2009, the Company issued 18,390 shares of Series A convertible redeemable preferred stock to Silver Rock I, Ltd. to pay off accrued and unpaid preferred dividend since Silver Rock I, Ltd. converted all of its convertible preferred stock at that moment.
In October 22, 2009, the Company issued 1,250,000 restricted shares of its common stock par value of $0.001 (to Shihong Zhai pursuant to a Consulting Agreement dated May 6, 2007 by and between the Company and Shihong Zhai as compensation for his consulting services for a period of two (2) years from May 6, 2007 to May 6, 2009.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
OVERVIEW
We are a Nevada corporation incorporated on January 28, 2004. On September 28, 2006, pursuant to a Share Exchange Agreement with Lotus International, we acquired all of the outstanding common stock of Lotus International from the its shareholders in exchange for 40,041,600 newly-issued stock of the Company.
We operate, control and beneficially own the pharmaceutical businesses in China through Lotus International, which in turn controls Lotus East, Liang Fang and En Ze Jia Shi, through various Contractual Arrangements entered into in September 2006. Both Liang Fang and En Ze Jia Shi are pharmaceutical companies headquartered in the PRC and organized under the laws of the PRC. Other than the Contractual Arrangements with Lotus East, we do not have any business or operations. Pursuant to the Contractual Arrangements, we are the primary beneficiary of Lotus East, we provide business consulting and other general business operation services to Lotus East., and we have the ability to (i) control the daily operations and financial affairs of Lotus East, (ii) appoint each of their senior executives, and (iii) approve all matters requiring stockholder approval. In addition, the creditors of Lotus East do not have recourse to any assets we may have.
On May 29, 2007, through Lotus International, we formed Lotus Century, a WFOE organized under the laws of the Peoples’ Republic of China. Lotus Century is primarily engaged in development of innovative medicines, medical technology consulting and outsourcing services, and related training services.
PRC law currently places certain limitations on foreign ownership of Chinese companies. To comply with these foreign ownership restrictions, we operate our business in China through the Contractual Arrangements with Lotus East. The contractual relationship among the above companies as follows:
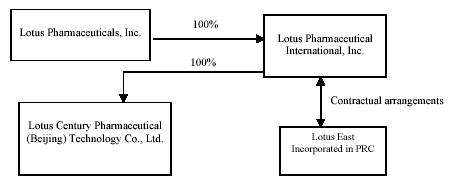
Based in Beijing (Lang Fang) and Inner Mongolia (En Ze Jia Shi), China, Lotus East is engaged in the production, trade and retailing of pharmaceuticals, focusing on the development of innovative medicines and investing in strategic growth to address various medical needs. Lotus East owns and operates 10 drug stores throughout Beijing and China sells Western and traditional Chinese medications, leases medical treatment facilities to licensed physicians, and generates revenues from the leasing of retail space to third party vendors and the leasing of advertising locations at its retail stores.
When used in this section, and except as may be set forth otherwise, the terms “we,” “us,” “ours,” and similar terms includes Lotus and its subsidiary Lotus International as well as Lotus East.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses, fair valuation of stock related to stock-based compensation and income taxes. We based our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
REVENUE RECOGNITION
Product sales are generally recognized when title to the product has transferred to customers in accordance with the terms of the sale. The Company recognizes revenue in accordance with the SEC Staff Accounting Bulletin (SAB) No. 101, “Revenue Recognition in Financial Statements” as amended by SAB No. 104 (together, “SAB 104”), and ASC 605 “Revenue Recognition When Right of Return Exists.”SAB 104 states that revenue should not be recognized until it is realized or realizable and earned. In general, the Company records revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
ASC 605 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if the seller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated.
The Company’s net product revenues represent total product revenues less allowances for returns.
PRODUCT RETURNS
The Company accounts for sales returns in accordance with Statements of ASC 605, Revenue Recognition When Right of Return Exists, by establishing an accrual in an amount equal to its estimate of sales recorded for which the related products are expected to be returned. The Company determines the estimate of the sales return accrual primarily based on historical experience regarding sales returns, but also by considering other factors that could impact sales returns. These factors include levels of inventory in the distribution channel, estimated shelf life, product discontinuances, and price changes of competitive products, introductions of generic products and introductions of competitive new products. In general, for wholesale sales, the Company provides credit for product returns that are returned six months prior to and up to six months after the product expiration date. Upon sale, the Company estimates an allowance for future product returns. The Company provides additional reserves for contemporaneous events that were not known and knowable at the time of shipment. In order to reasonably estimate future returns, the Company analyzed both quantitative and qualitative information including, but not limited to, actual return rates, the level of product manufactured by the Company, the level of product in the distribution channel, expected shelf life of the product, current and projected product demand, the introduction of new or generic products that may erode current demand, and general economic and industry wide indicators. The Company also utilizes the guidance provided in SAB 104 in establishing its return estimates.
OTHER REVENUE
Other revenues consist of (i) rental income received for the lease of retail space to various retail merchants; (ii) advertising revenues from the lease of counter space at our retail locations; (iii) rental income from the lease of retail space to licensed medical practitioners; and (iv) revenues received by us for research and development projects. We recognize revenues upon performance of such funded research. We recognize revenues from leasing of space as earned from contracting third parties. Revenues received in advance are reflected as deferred revenue on the accompanying balance sheets. Additionally, we receive income from the sale of developed drug formulas. Income from the sale of drug formulas are recognized upon performance of all of our obligations under the respective sales contract and are included in other income on the accompanying consolidated statement of operations.
ACCOUNTS RECEIVABLE
Accounts receivable are carried at original invoice amount less an estimate made for doubtful receivables based on a review of all outstanding amounts on a monthly basis. Management judgment and estimates are made in connection with establishing the allowance for doubtful accounts. Specifically, we analyze the aging of accounts receivable balances, historical bad debts, customer concentrations, customer credit-worthiness, current economic trends and changes in our customer payment terms. Significant changes in customer concentration or payment terms, deterioration of customer credit-worthiness or weakening in economic trends could have a significant impact on the collectability of receivables and our operating results. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
INVENTORIES
Inventories are stated at the lower of cost or market with cost determined under the weighted-average method. Inventory consists of finished capsules, liquids, finished oral suspension powder and other western and traditional Chinese medicines and medical equipment. At least on a quarterly basis, we review our inventory levels and write down inventory that has become obsolete or has a cost basis in excess of its expected net realizable value or is in excess of expected requirements. Inventory levels are evaluated by management relative to product demand, remaining shelf life, future marketing plans and other factors, and reserves for obsolete and slow-moving inventories are recorded for amounts which may not be realizable.
PROPERTY AND EQUIPMENT
Property and equipment are stated at cost less accumulated depreciation. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition.
In accordance with ASC 360, “Accounting for the Impairment or Disposal of Long-Lived Assets”, we examine the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable. We recognize an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value.
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets. The useful lives for property and equipment are as follows:
|
Buildings and leasehold improvement |
|
|
20 to 40 years |
|
|
Manufacturing equipment |
|
|
10 to 15 years |
|
|
Office equipment and furniture |
|
|
5 to 8 years |
|
RESEARCH AND DEVELOPMENT
Research and development costs are expensed as incurred. These costs primarily consist of cost of material used and salaries paid for the development of our products and fees paid to third parties. We had no research and development expenses during the nine months ended September 30, 2009.
INCOME TAXES
Taxes are calculated in accordance with taxation principles currently effective in the United States and PRC. We account for income taxes using the liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as income in the period that includes the enactment date. A valuation allowance is provided for the amount of deferred tax assets that, based on available evidence, are not expected to be realized.
RECENT ACCOUNTING PRONOUNCEMENTS
In June 2009, FASB established Accounting Standards Codification TM (“Codification”) as the single source of authoritative accounting principles recognized by the FASB in the preparation of financial statements in conformity with the GAAP. The Codification will supersede all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting literature not included in the Codification will become non-authoritative. The Codification is effective for financial statements issued for interim and annual periods ending after September 15, 2009. Adoption of the Codification is not expected to have a material impact on the Company’s results of operations or financial position.
In June 2009, FASB updated the accounting standards related to the consolidation of variable interest entities (“VIEs”). The standard amends current consolidation guidance and requires additional disclosures about an enterprise’s involvement in VIEs. The standard shall be effective as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, for interim periods within the first annual reporting period, and for interim and annual reporting periods thereafter. Earlier application is prohibited. The Company does not expect the adoption to have a material impact on the Company’s results of operations or financial position.
In May 2009, FASB issued FAS No. 165, “Subsequent Events,” which was subsequently codified within ASC 855, “Subsequent Events”. The standard establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. An entity should apply the requirements of ASC 855 to interim or annual financial periods ending after June 15, 2009. Adoption of this standard does not have a material impact on the Company’s results of operations or financial position.
In April 2009, the FASB updated the accounting standards to provide guidance on estimating the fair value of a financial asset or liability when the trade volume and level of activity for the asset or liability have significantly decreased relative to historical levels. The standard requires entities to disclose the inputs and valuation techniques used to measure fair value and any changes in valuation inputs or techniques. In addition, debt and equity securities as defined by GAAP shall be disclosed by major category. This standard is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009, and is to be applied prospectively. The adoption did not have a material effect on the Company’s results of operations and financial condition.
In April 2009, the FASB updated the accounting standards for the recognition and presentation of other-than-temporary impairments. The standard amends existing guidance on other-than-temporary impairments for debt securities and requires that the credit portion of other-than-temporary impairments be recorded in earnings and the noncredit portion of losses be recorded in other comprehensive income. The standard requires separate presentation of both the credit and noncredit portions of other-than-temporary impairments on the financial statements and additional disclosures. This standard is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. At the date of adoption, the portion of previously recognized other-than-temporary impairments that represent the noncredit related loss component shall be recognized as a cumulative effect of adoption with an adjustment to the opening balance of retained earnings with a corresponding adjustment to accumulated other comprehensive income (loss). The adoption of this standard did not have a material effect on the preparation of the Company’s consolidated financial statements.
In August 2009, the FASB updated the accounting standards to provide additional guidance on estimating the fair value of a liability in a hypothetical transaction where the liability is transferred to a market participant. The standard is effective for the first reporting period, including interim periods, beginning after issuance. ACC does not expect the adoption to have a material effect on ACC’s consolidated results of operations and financial condition.
CURRENCY EXCHANE RATES
Our results of operations and cash flow are translated at average exchange rates during the period, and assets and liabilities are translated at the unified exchange rate as quoted by the People’s Bank of China at the end of the period. Translation adjustments resulting from this process are included in accumulated other comprehensive income in our statement of shareholders’ equity. We have not used any forward contracts, currency options or borrowings to hedge our exposure to foreign currency exchange risk. We cannot predict the impact of future exchange rate fluctuations on our results of operations and may incur net foreign currency losses in the future.
Our financial statements are expressed in U.S. dollars but the functional currency of our operating subsidiary is China RMB. The value of your investment in our stock will be affected by the foreign exchange rate between U.S. dollars and China RMB. To the extent we hold assets denominated in U.S. dollars, any appreciation of the RMB against the U.S. dollar could result in a change to our statement of operations and a reduction in the value of our U.S. dollar denominated assets. On the other hand, a decline in the value of RMB against the U.S. dollar could reduce the U.S. dollar equivalent amounts of our financial results, the value of your investment in our company and the dividends we may pay in the future, if any, all of which may have a material adverse effect on the price of our stock.
RESULTS OF OPERATIONS
For the Nine Months and Three Months Ended September 30, 2009 and 2008
Nine Months Ended September 30, 2009 Compared to Nine Months Ended September 30, 2008
Total Net Revenues
Total revenues for the nine months ended September 30, 2009 were $39,968,720 as compared to total revenues of $47,798,673 for the nine months ended September 30, 2008, a decrease of $7,829,953 or approximately 16.38%. For the nine months ended September 30, 2009 and 2008, net revenues consisted of the following:
|
|
|
2009 |
|
2008 |
| ||
|
Wholesale |
|
$ |
31,220,411 |
|
$ |
34,863,680 |
|
|
Retail |
|
|
7,604,678 |
|
|
8,492,107 |
|
|
Other revenues |
|
|
1,143,631 |
|
|
4,442,886 |
|
|
Total net revenues |
|
$ |
39,968,720 |
|
$ |
47,798,673 |
|
|
|
• |
For the nine months ended September 30, 2009, wholesale revenues decreased by $3,643,269 or 10.45%. Although our wholesale quantities for the nine months ended September 30, 2009 increased by approximately 39% compared to the wholesale quantities for the nine months ended September 30, 2008, the decrease in the total wholesale revenue was primarily attributed to the decrease, by approximately 42%, of the average unit price sold for our wholesale drugs for the nine months ended September 30, 2009 compared to the average unit price sold for our wholesale drugs for the corresponding period in 2008. In order to maintain a long term growth and incentivize our distributors, we lowered our average wholesale drugs price in a planned way. We expect our wholesale revenue will maintain at its current level with minimal growth in the remaining part of fiscal 2009. |
|
|
|
|
|
|
• |
For the nine months ended September 30, 2009, retail revenues decreased by $887,429 or 10.45%. The decrease is primarily attributed to the slowdown in economy growth. The slowdown in the economy growth resulted in decreased purchase of non-essential health supplements. The Company also experienced a reduction in the sale of higher priced drugs in 2009. As a result, the Company generated less per store revenue. We expect our retail revenue will slightly increase in the remaining part of fiscal 2009 because of the coming of flu season. |
|
|
|
|
|
|
• |
For the nine months ended September 30, 2009, other revenues decreased by $3,299,255 or 74.26%. The decrease in other revenues is attributed to the following: |
|
|
|
2009 |
|
2008 |
| ||
|
Leasing revenues |
|
$ |
594,738 |
|
$ |
581,238 |
|
|
Third-party manufacturing |
|
|
513,957 |
|
|
3,505,225 |
|
|
Advertising revenues |
|
|
- |
|
|
229 |
|
|
Research and development and lab testing services |
|
|
34,936 |
|
|
356,194 |
|
|
Total other revenues |
|
$ |
1,143,631 |
|
$ |
4,442,886 |
|
|
|
• |
We sublease certain portion of our retail stores and counter spaces to various other vendors and generate leasing revenue. The increase was primarily due to leasing a more counter spaces to third parties as a result of higher demand for our counter spaces and the favorable RMB currency appreciation which converted our leasing revenue in RMB into higher US dollar amounts. We expect the lease revenues will maintain at its current level with minimal growth in the remaining of fiscal 2009. |
|
|
|
|
|
|
• |
For third-party manufacturing, customers supply the raw materials and we are paid a fee for manufacturing their products. We had less large manufacturing contracts during the nine months ended September 30, 2009 as the demand for the third-party manufacturing decreased as compared to the corresponding period in 2008. In addition, several of our old large third party manufacturing customers have built their own facilities and started manufacturing products on their own in 2009 and we were unable to find new customers to replace them. We anticipate the third-party manufacturing revenue will continue to decrease for the last quarter of fiscal 2009 since we do not expect to retain new customers with large third party manufacturing contracts. |
|
|
|
|
|
|
• |
We receive advertising fee for the lease of counter and other space at our retail locations in Beijing. We did not have any advertising revenue during the nine months ended September 30, 2009. Because the local government tightens the advertising rules and regulations in the pharmaceutical industry, we do not anticipate signing any advertisement contracts in a near future. |
|
|
|
|
|
|
• |
We performed research and development (“R&D”) and lab testing projects for various third parties and performed drug testing and analysis. We preformed less R&D testing services and drug testing and analysis work for third parties during the nine months ended September 30, 2009 as compared to the corresponding period in 2008. Some of our prior customers we provided such service for started to do similar R&D and lab testing in their own labs at a lower price and decided not to use our services in 2009. We expect the revenue from R&D and lab testing will continue to decrease in the remaining part of fiscal 2009. |
Cost of Sales
Cost of sales for our retail includes packing materials, purchased third-party manufactured finished good and related taxes. Cost of sales for our wholesale and other revenues includes direct materials, direct labor fees, manufacturing overhead such as indirect materials, indirect labor fees, utilities and depreciation indirectly related to product production, and related taxes. For the nine months ended September 30, 2009, our total cost of sales amounted to $16,922,500 or approximately 42% of total revenues as compared to cost of sales of $25,684,501 or approximately 54% of total revenues for the nine months ended September 30, 2008. The decrease in cost of sales as a percentage of total revenue was primarily due to better purchase pricing management for raw material and third party manufactured finished goods and more efficient control in labor fees.
Gross Profit
Gross profit for the nine months ended September 30, 2009 was $23,046,220 or 58% of total revenues, as compared to $22,114,172 or 46% of revenues for the nine months ended September 30, 2008. The increase in gross profit margin was attributed to the decrease in cost of sales as a percentage of revenue. The decrease in cost of sales as a percentage of revenue was primarily contributed to better managed raw material and third party manufactured finished goods purchase and more efficient control in labor fees. We expect our gross profit margin to remain in its current level with slight growth in the future.
Operating Expenses
Total operating expenses for the nine months ended September 30, 2009 were $7,525,763, a decrease of $6,548,919 or 46.53%, from total operating expenses in the nine months ended September 30, 2008 of $14,074,682. This decrease included the following:
For the nine months ended September 30, 2009, selling expenses amounted to $5,398,935 as compared to $11,291,590 for the nine months ended September 30, 2008, a decrease of $5,892,655 or 52.19%. For the nine months ended September 30, 2009, we decreased the average unit price distributed to our distributors for our wholesale drugs compared to the average unit price distributed to our distributors for our wholesale drugs for the nine months ended September 30, 2008. For our long term growth perspective and the purpose to incentivize our distributors, we lowered our average price distributed to our distributors in a planned way. Therefore, our distributors can get more profits from our lowered distribution price for the wholesale drugs. Thus, we started to transfer a portion of our profits to our distributors instead of paying them commission. As a result, our selling expenses decreased significantly. We expect our selling expenses will maintain at its current level with minimal growth in the remaining part of fiscal 2009.
For the nine months ended September 30, 2009, we do not have any research and development costs. Therefore, compared to $1,193,916 for the nine months ended September 30, 2008, there is a decrease of $1,193,916 or 100% from the comparable period in 2008. In late 2007, we entered into several research and development agreements with third parties for the design, research and development of designated pharmaceutical projects. We fulfilled these research and development agreements by June 30, 2008. We did not enter into any new research and development agreement in the second half of fiscal 2008 and during the nine months ended September 30, 2009. As a result, expenses related to research and development projects for the nine months ended September 30, 2009 was zero.
For the nine months ended September 30, 2009, general and administrative expenses were $2,126,828 as compared to $1,589,176 for the nine months ended September 30, 2008, an increase of $537,652 or 33.83% as summarized below:
|
|
Nine Months Ended September 30, | |||
|
|
|
2009 |
|
2008 |
|
|
|
(Unaudited) |
|
(Unaudited) |
|
Salaries and related benefits |
$ |
464,234 |
$ |
965,682 |
|
Amortization and depreciation expenses |
|
722,298 |
|
123,305 |
|
Rent |
|
226,414 |
|
133,336 |
|
Travel and entertainment |
|
233,150 |
|
49,609 |
|
Professional fees |
|
174,834 |
|
329,211 |
|
Bad debt recovery |
|
- |
|
(490,310) |
|
Other |
|
305,898 |
|
478,343 |
|
Total |
$ |
2,126,828 |
$ |
1,589,176 |
The changes in these expenses for the nine months ended September 30, 2009 as compared to the nine months ended September 30, 2008 included the following:
|
|
• |
Salaries and related benefits decreased $501,448 or 51.93% primarily due to a decrease in salaries and bonuses paid to administrative personnel. As a result of our continuous efforts to control corporate spending, the salaries and related benefits cost decreased accordingly. |
|
|
|
|
|
|
• |
Amortization of our intangible assets and depreciation on our fixed assets increased by $598,993 or approximately 485.78%, which is primarily attributable to the purchase of and amortization on new intangible assets in our fiscal 2009 and the purchase of and depreciation on additional fixed assets in our fiscal 2009. |
|
|
|
|
|
|
• |
Rent increased by $93,078 or approximately 69.81%, which is primarily attributable to the RMB currency appreciation which converted our rent expenses in RMB into higher US dollar amounts and the slight increase in rent rate for the nine months ended September 30, 2009 compared to the rent rate for the nine months ended September 30, 2008. |
|
|
|
|
|
|
• |
Travel and entertainment expenses increased by $183,541 or 369.98%, which is primarily attributed to both increased spending in business travel in order to manage our business more efficiently and increased entertainment expenditure to enhance our visibility. |
|
|
|
|
|
|
• |
Professional fees decreased $154,377 or 46.89%, which is primarily attributed to the decrease in fees related to our attorney’s service and decrease in payments for auditors’ service and investor relations service. |
|
|
|
|
|
|
• |
We did not have any bad debt recovery for the nine months ended September 30, 2009 while we had $490,310 bad debt recovery for the nine months ended September 30, 2008. In the nine months ended September 30, 2008, we made many efforts in our collection in order to expedite the progress of outstanding accounts receivable. As a result, we collected a lot of outstanding accounts receivable which were written off as bad debts. From then on, we tried our best to keep our accounts receivable aging in three months. Therefore, our bad debt recovery for the nine months ended September 30, 2009 was zero. |
|
|
|
|
|
|
• |
Other general and administrative expenses including postage, office expenses, other management fees, officers’ car insurance, meeting expenses, etc., decreased by $172,445 or approximately 36.05%. The decrease is primarily attributed to decreased office expenses as a result of our tightening in non-selling expenses. |
Income from Operations
We reported income from operations of $15,520,457 for the nine months ended September 30, 2009 as compared to income from operations of $8,039,490 for the nine months ended September 30, 2008, an increase of $7,480,967 or approximately 93.05%.
Other Expense
For the nine months ended September 30, 2009, total other expense amounted to $1,619,110 as compared to other expense of $1,650,456 for the nine months ended September 30, 2008, a decrease of $31,346 or 1.90%. This slight change is primarily attributed to:
|
|
• |
For the nine months ended September 30, 2009, we recorded debt issuance costs of $311,388 compared to $261,919 for the nine months ended September 30, 2008, an increase of $49,469 or approximately 18.89%, due to the debt issuance costs on our February 2008 funding for which we began our amortization in March 2008. |
|
|
|
|
|
|
• |
For the nine months ended September 30, 2009, we recorded interest income of $47,407 as compared to $11,620 for the nine months ended September 30, 2008, an increase of $35,787 or 307.98%, which is primarily attributable to the interest paid by an employee who advanced it from us for a few months and repaid the principal amount and accrued interest in full soon. |
|
|
|
|
|
|
• |
For the nine months ended September 30, 2009, interest expense was $1,355,129 as compared to $1,399,507 for the nine months ended September 30, 2008, a decrease of $44,378 or approximately 3.17%. For the nine months ended September 30, 2008, we recorded a revaluation expense for previously issued warrants in connection with our February 2008 private placement financing for which there was no comparable expense in the comparable period of fiscal 2009. As a result, our interest expense decreased. |
Income Taxes
For the nine months ended September 30, 2009, our income tax expense was $156,915. We did not need to accrue and pay any income taxes in fiscal 2008, because we got income tax exemption from National Taxation Bureau of PRC located in Fengtai District, Beijing, PRC on January 22, 2008.
Net Income
As a result of these factors, we reported net income of $13,744,432 for the nine months ended September 30, 2009 as compared to net income of $6,389,034 for the nine months ended September 30, 2008. This translates to basic and diluted net income per common share of $0.32, $0.28 and $0.15, $0.13 for the nine months ended September 30, 2009 and 2008, respectively.
Other Comprehensive Income
The functional currency of Lotus East operating in the PRC is the RMB. The financial statements of Lotus East are translated into U.S. dollars using period end rates of exchange for assets and liabilities, and average rates of exchange (for the period) for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. As a result of these translations, which are a non-cash adjustment, we reported a foreign currency translation gain of $130,633 for the nine months ended September 30, 2009 as compared to $2,173,475 for comparable period in 2008. This non-cash gain increased our reported comprehensive income.
Comprehensive Income
As a result of our foreign currency translation gains, we had comprehensive income for the nine months ended September 30, 2009 of $13,875,065, compared with $8,562,509 for the nine months ended September 30, 2008.
Three Months ended September 30, 2009 compared to three months ended September 30, 2008
Total Net Revenues
Total revenues for the three months ended September 30, 2009 were $14,512,704 as compared to total revenues of $16,703,884 for the three months ended September 30, 2008, a decrease of $2,191,180 or approximately 13.12%. For the three months ended September 30, 2009 and 2008, net revenues consisted of the following:
|
|
|
2009 |
|
2008 |
|
|
|
(Unaudited) |
|
(Unaudited) |
|
Wholesale |
$ |
11,606,288 |
$ |
12,191,037 |
|
Retail |
|
2,708,071 |
|
2,831,030 |
|
Other revenues |
|
198,345 |
|
1,681,817 |
|
Total |
$ |
14,512,704 |
$ |
16,703,884 |
|
|
• |
For the three months ended September 30, 2009, wholesale revenues decreased by $584,749 or 4.80%. For the three months ended September 30, 2009, while our wholesale quantities increased by approximately 33% compared to the wholesale quantities for the three months ended September 30, 2008, the decrease in the total wholesale revenue was primarily attributed to the decrease, by approximately 43%, of the average unit price sold for our wholesale drugs compared to the average unit price sold for our wholesale drugs for the three months ended September 30, 2008. In order to maintain a long term growth and incentivize our distributors,, we lowered our average wholesale drugs price in a planned way. We expect our wholesale revenue will maintain at its current level with minimal growth in the remaining part of fiscal 2009. |
|
|
|
|
|
|
• |
For the three months ended September 30, 2009, retail revenues decreased by $122,959 or 4.34%. The decrease was primarily attributed to the slowdown in economy growth. The slowdown in the economy growth resulted in less non-essential health supplements being purchased. The Company also experienced a reduction in the sale of higher priced drugs in the third quarter of fiscal 2009. As a result, the Company generated less per store revenue. We expect our retail revenue to increase in the remaining part of fiscal 2009. |
|
|
|
|
|
|
• |
For the three months ended September 30, 2009, other revenues decreased by $1,483,472 or 88.21%. The significant decrease in other revenues was attributed to the following: |
|
|
|
2009 |
|
2008 |
|
|
|
(Unaudited) |
|
(Unaudited) |
|
Leasing revenues |
$ |
198,345 |
$ |
216,959 |
|
Third-party manufacturing |
|
- |
|
1,375,233 |
|
Research and development and lab testing services |
|
- |
|
89,625 |
|
Total other revenues |
$ |
198,345 |
$ |
1,681,817 |
|
|
• |
We sublease certain portion of our retail stores and counter spaces to various other vendors and generate leasing revenue. The slight decrease in leasing revenues for the three months ended September, 2009 compared to the leasing revenues for the three months ended September 30, 2008 was primarily due to leasing less counter spaces to third parties as a result of lower demand of our counter spaces. We expect the lease revenues to maintain at its current level with minimal growth in the remaining of 2009. | |
|
|
|
|
|
|
|
• |
For third-party manufacturing, we are paid a fee for manufacturing customer’s products with the raw materials supplied by them. We did not have any manufacturing contracts during the three months ended September 30, 2009. As a result, our third-party manufacturing revenue for the three months ended September 30, 2009 was zero. We do not expect to have new customers with large third party manufacturing contracts in the remaining part of fiscal 2009. |
|
|
|
|
|
|
|
|
• |
We performed research and development and lab testing projects for various third parties and performed drug testing and analysis. We did not provide any R&D testing services and drug testing and analysis work for any third parties during the three months ended September 30, 2009. Accordingly, our revenue from research and development and lab testing services for the three months ended September 30, 2009 was zero. We do not anticipate having any new customers with large R&D and lab testing contracts in the near future. |
|
Cost of Sales
Cost of sales for retail includes packing materials and purchased third-party manufactured finished goods and related taxes. Cost of sales for wholesale and other revenues includes direct materials, direct labor fees, manufacture overhead such as indirect materials, indirect labor fees, utilities and depreciation etc., and related taxes. For the three months ended September 30, 2009, cost of sales amounted to $5,993,176 or approximately 41 % of total net revenues as compared to cost of sales of $8,219,358 or approximately 49% of total net revenues for the three months ended September 30, 2008. The decrease in cost of sales as a percentage of revenue was primarily contributed to better managed raw material and third party manufactured finished goods purchase and more efficient manufacture overhead controls.
Gross Profit
Gross profit for the three months ended September 30, 2009 was $8,519,528 or 59% of total net revenues, as compared to $8,484,526 or 51% of total net revenues for the three months ended September 30, 2008. The increase in gross profit margin was primarily contributed to our better managed raw materials, the purchase of third party manufactured finished goods and more efficient manufacture overhead controls. We expect our gross profit margin to remain in its current level with slight growth in the remaining period of fiscal 2009.
Operating Expenses
Total operating expenses for the three months ended September 30, 2009 were $2,506,420, a decrease of $2,220,448 or 46.98% from total operating expenses in the three months ended September 30, 2008 of $4,726,868. The significant decrease included the following:
For the three months ended September 30, 2009, selling expenses amounted to $1,895,901 as compared to $4,293,704 for the three months ended September 30, 2008, a decrease of $2,397,803 or 55.84% from the comparable period in fiscal 2008. For the three months ended September 30, 2009, we decreased the average unit price distributed to our distributors for our wholesale drugs compared to the average unit price distributed to our distributors for our wholesale drugs for the three months ended September 30, 2008. For our long term growth perspective and the purpose to incentivize our distributors, we lowered our average price distributed to our distributors in a planned way. Therefore, our distributors can get more profits from our lowered distribution price for the wholesale drugs. In addition, we started to transfer portion of our profits instead of paying commission to our distributors. As a result, our selling expenses decreased significantly. We expect our selling expenses to maintain at its current level with minimal growth in the remaining part of fiscal 2009.
For the three months ended September 30, 2009, we did not have any research and development expenses. So, as compared to $12,448 for the three months ended September 30, 2008, a decrease of $12,448 or 100% from the comparable period in 2008. In late 2007, we entered into several research and development agreements with third parties for the design, research and development of designated pharmaceutical projects. We fulfilled these research and development agreements by June 30, 2008. We did not enter into any new research and development agreement in the second half of fiscal 2008 and during the nine months ended September 30, 2009. As a result, expenses related to research and development projects for the three months ended September 30, 2009 was zero.
For the three months ended September 30, 2009, general and administrative expenses were $610,519 as compared to $420,716 for the three months ended September 30, 2008, an increase of $189,803 or 45.11% from the comparable period in fiscal 2008. These changes were summarized below:
|
|
Three Months Ended September 30, | |||
|
|
|
2009 |
|
2008 |
|
|
|
(Unaudited) |
|
(Unaudited) |
|
Salaries and related benefits |
$ |
140,148 |
$ |
421,228 |
|
Amortization and depreciation expenses |
|
236,675 |
|
42,185 |
|
Rent |
|
75,523 |
|
54,387 |
|
Travel and entertainment |
|
16,274 |
|
12,570 |
|
Professional fees |
|
52,043 |
|
143,593 |
|
Bad debt recovery |
|
- |
|
(490,310) |
|
Other |
|
89,856 |
|
237,063 |
|
Total |
$ |
610,519 |
$ |
420,716 |
The changes in these expenses from the three months ended September 30, 2009 as compared to the three months ended September 30, 2008 included the following:
|
|
• |
Salaries and related benefits decreased $281,080 or 66.73%, which is primarily due to a decrease in salaries and bonuses paid to administrative personnel. We continue our efforts to control corporate spending. As a result, the salaries and related benefits costs decreased. |
|
|
|
|
|
|
• |
Depreciation on our fixed assets and amortization of our intangible assets increased by $194,490 or approximately 461.04%, which was primarily attributed to the purchase of and amortization on new intangible assets in our fiscal 2009 and the purchase of and depreciation on additional fixed assets in our fiscal 2009. |
|
|
|
|
|
|
• |
Rent increased by $21,136 or approximately 38.86%, which is primarily due to slight increase in rent rate and the RMB currency appreciation which converted our rent expense in RMB into higher US dollar amounts. |
|
|
|
|
|
|
• |
Travel and entertainment expenses increased by $3,704 or 29.47% which is primarily attributed to the RMB currency appreciation which converted our travel and entertainment expenses in RMB into higher US dollar amounts. |
|
|
|
|
|
|
• |
Professional fees decreased $91,550 or 63.76%, which was primarily attributed to the decrease in compensations paid to our attorney and decrease in payments for auditors’ services and investor relations service. |
|
|
|
|
|
|
• |
For the three months ended September 30, 2009, we did not have any bad debt recovery while for the three months ended September 30, 2008, we had bad debt recovery of $490,310. In the third quarter of fiscal 2008, we made our best efforts to collect our outstanding accounts receivable which were written off as bad debts. From then on, we tried our best to keep our accounts receivable aging in 90 days. |
|
|
|
|
|
|
• |
Other general and administrative expenses, which includes postage, office expenses, other small amount and miscellaneous management fees, officers’ car insurance, meeting expenses, etc., decreased by $147,207 or approximately 62.10%, which was primarily attributed to the increased efforts to control our spending. As a result, our other general and administrative expenses decreased. |
Income from Operations
We reported income from operations of $6,013,108 for the three months ended September 30, 2009 as compared to income from operations of $3,757,658 for the three months ended September 30, 2008, an increase of $2,255,450 or approximately 60.02%.
Other Expense
For the three months ended September 30, 2009, total other expense amounted to $547,541 as compared to other expense of $543,982 for the three months ended September 30, 2008, an increase of $3,559 or 0.65% from the comparable period in 2008. These changes were primarily attributed to:
|
|
• |
For the three months ended September 30, 2009, we recorded debt issuance costs of $112,355 as compared to $99,516 for the three months ended September 30, 2008, an increase of $12,839 or 12.90% which was primarily attributed to the additional amortization of debt issuance costs for our converted debt during the three months ended September 30, 2009. |
|
|
|
|
|
|
• |
For the three months ended September 30, 2009, we recorded interest income of $1,295 as compared to $9,032 for the three months ended September 30, 2008, a decrease of $7,737 or 85.66% which was primarily attributed to the incremental interest born by our bank deposit. |
|
|
|
|
|
|
• |
For the three months ended September 30, 2009, interest expense was $436,481 as compared to $453,498 for the three months ended September 30, 2008, a decrease of $17,017 or 3.75% which was primarily attributed to the conversion of convertible redeemable debt for which we did not need to accrue or pay any interest after conversion. |
Income Taxes
For the three months ended September 30, 2009, our income tax expense was $74,770. We did not need to accrue and pay any income tax in our fiscal 2008, because we got income tax exemption from National Taxation Bureau of PRC located in Fengtai District, Beijing, P.R.C. on January 22, 2008.
Net Income
As a result of these factors, we reported net income of $5,390,797 for the three months ended September 30, 2009 as compared to net income of $3,213,676 for the three months ended September 30, 2008. This translates to basic and diluted net income per common share of $0.12, $0.11 and $0.08, $0.07 for the three months ended September 30, 2009 and 2008, respectively.
Other Comprehensive Income
The functional currency of Lotus East operating in the PRC is the Chinese Yuan or Renminbi (“RMB”). The financial statements of Lotus East are translated to U.S. dollars using period end rates of exchange for assets and liabilities, and average rates of exchange (for the period) for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. As a result of these translations, which are a non cash adjustment, we reported a foreign currency translation gain of $65,626 for the three months ended September 30, 2009 as compared to $127,833 for comparable period in 2008. This non-cash gain had the effect of increasing our reported comprehensive income.
Comprehensive Income
For the three months ended September 30, 2009, comprehensive income was $5,456,423, as compared with comprehensive income of $3,341,509 for the three months ended September 30, 2008.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, fulfill its obligations and otherwise operate on an ongoing basis.
On September 30, 2009 and December 31, 2008, we had a cash balance of $2,561,489 and $1,278,808, respectively. These funds are distributed in financial institutions located in following countries:
|
|
|
September 30, 2009 |
|
December 31, 2008 |
|
China |
$ |
2,561,459 |
$ |
1,277,649 |
|
USA |
|
30 |
|
1,159 |
|
Total |
$ |
2,561,489 |
$ |
1,278,808 |
Our working capital position decreased $12,474,442 from $4,422,183 on December 31, 2008 to $ (8,052,259) on September 30, 2009. This decrease in working capital is primarily attributed to a decrease in accounts receivable net of allowance for doubtful accounts of approximately $4.37 million, a decrease in other receivable- related party of approximately $2.03 million, a decrease in inventories, net of reserve for obsolete inventories of approximately $0.79 million, a decrease in deferred debt issuance costs (current portion) of approximately $0.25 million, an increase in taxes payable of approximately $1.74 million, an increase in unearned revenue of approximately $0.06 million, an increase in due to related parties (current portion) of approximately $0.38 million and an increase in Series A convertible redeemable preferred stock (current portion) of approximately $4.53 million offset by a decrease in accounts payable and accrued expenses of approximately $0.42 million.
On September 30, 2009, our accounts receivable, net of allowance for doubtful accounts, was $1,765,390 as compared to $6,132,912 on December 31, 2008, a decrease of $4,367,522. The decrease was primarily due to our strong collection effort which significantly improved our accounts receivable aging days. We expect our accounts receivable will maintain at the current level.
On September 30, 2009, our other receivable- related party was $0 as compared to $2,027,954 on December 31, 2008, a decrease of $2,027,954. The decrease was attributed to one loan made to our CEO to register a subsidiary in Inner Mongolia, China. In January 2009, our CEO repaid it in full to the Company, because the Company decided to set up a branch office instead of a subsidiary, thus, the registered capital was not required.
On September 30, 2009, our inventories of raw materials, work in progress, packaging materials, finished goods and reserve for obsolete inventories totaled $2,994,995, as compared to $3,787,802 on December 31, 2008, a decrease of $792,807. This change includes a decrease of $334,131 in raw materials and a decrease of $1,560 in packing materials and a decrease in finished goods of $457,116. We expect our inventory will maintain at its current level in the remaining period of fiscal 2009.
On September 30, 2009, our prepaid expenses and other assets was $123,695 as compared to $121,274 on December 31, 2008, an increase of $2,421. The increase was primarily attributed to the favorable RMB currency appreciation which converted our prepaid expenses and other assets in RMB into higher US dollar amounts.
On September 30, 2009, we have deferred debt costs of $153,024 as compared to $464,411 on December 31, 2008, a decrease of $ 311,387. In the February 2008 financing, the Company paid fees of approximately $469,000 in cash and granted warrants to placement agent for the transaction. The value of the warrants granted to the placement agent was $327,565. Accordingly, we recorded a total deferred debt cost of $796,133 and started to amortize it in March 2008. The decrease in deferred debt costs was primarily attributed to the amortization for our deferred debt cost in the nine months ended September 30, 2009.
On September 30, 2009, we have a property and equipment, net of accumulated depreciation, of $22,872,484 as compared to $7,554,817 on December 31, 2008, an increase of $15,317,667. The increase was primly attributed to the increased payments and payables for our construction-in-progress of Inner Mongolia facility and Beijing office building (See note 4) offset by the 2009 depreciation on our fixed assets.
On September 30, 2009, we have a deposit on patent of $2,925,003 as compared to $2,917,919 on December 31, 2008, an increase of $7,084. We made the deposit on patent in order to acquire a new Chinese Class I anti-asthma medicine drug patent in accordance with a technology transfer agreement the Company entered into in April 2008. The increase is attributed to the favorable RMB currency appreciation which converted our deposit on patent in RMB into higher US dollar amounts. On September 30, 2009, we have installments on intangible assets of $38,999,064 as compared to $38,175,134 on December 31, 2008, an increase of $823,930. The increase was primarily attributed to our payments to acquire (i) a patent of a new Chinese Class I anti-asthma medicine drug in accordance with a technology transfer agreement the Company entered into in April 2008 and (ii) another patent of a new Chinese drug which cures Hormone-Diabetes according to a new drug patent transfer agreement the Company entered into in February 2009.
On September 30, 2009, we have intangible assets, net of accumulated amortization, of $8,429,005 as compared to $1,231,730 on December 31, 2008, an increase of $7,197,275. The increase was primarily attributed to the purchase of a new drug certificate and intellectual property right for which we paid in full in fiscal 2009.
On September 30, 2009, we have an accounts payable and accrued expenses of $477,619 as compared to $895,283 on December 31, 2008, a decrease of $417,664. The decrease was primarily attributed to the decrease in accrued compensations for our CEO and board directors and the increase in payments made for accounts payable.
On September 30, 2009, we have other payables of $1,305,565 as compared to $1,274,882 on December 31, 2008, an increase of $30,683. The increase in other payables is primarily due to the increased payables for our construction-in-progress.
On September 30, 2009, we have a taxes payable of $6,751,037 as compared to $5,015,908 on December 31, 2008, an increase of $1,735,129. The increase in the taxes payable is primarily due to the increase in our revenues for which we accrued related taxes.
On September 30, 2009, we have unearned revenue of $630,475 as compared to $565,629 on December 31, 2008, an increase of $64,846. The increase was primly attributed to the RMB currency appreciation which converted our unearned revenue in RMB into higher US dollar amounts.
Our balance sheet dated September 30, 2009 also reflects a balance due to related parties of $2,297,221, which was a working capital advance made to us by our president, vice-president and an officer of the Company and a Board member as well as an amount payable of approximately $1 million related to an assignment agreement as discussed elsewhere in this report. These advances are non-interest bearing and are due on demand. We are currently planning to repay these balances as soon as operating cash become available.
On September 30, 2009, we have a series A convertible redeemable preferred stock of $4,534,129 as compared to $3,652,341 on December 31, 2008, an increase of $881,788. The increase was primarily attributed to the amortization of discount on convertible redeemable preferred stock of $880,788 and the issued additional convertible redeemable preferred stock of $400,000 as dividend and the conversion of convertible series A convertible redeemable preferred stock of $399,000.
Our balance sheet dated September 30, 2009 also reflects notes payable to related parties of approximately $5 million due on December 30, 2015 which is a working capital loan made to us on December 31, 2005 by the Company’s Chief Executive Officer, two employees of the Company and a Board member. These loans bear the interest based on a floating annual interest rate, which is 80% bank interest rate of the day and are unsecured. During the nine months ended September 30, 2009, we did not repay any portion of these loan balances.
The changes in asset and liabilities discussed above is based on a comparison of amounts on our balance sheets on September 30, 2009 and December 31, 2008 and does not necessarily reflect changes in assets and liabilities reflected on our cash flow statement, for which we use the average foreign exchange rate during the period to calculate these changes.
Net cash provided by operating activities for the nine months ended September 30, 2009 was $25,252,740 as compared to net cash provided by operating activities of $16,038,077 for the nine months ended September 30, 2008. For the nine months ended September 30, 2009, net cash provided by operating activities was primarily attributed to a decrease in accounts receivable of $4,379,267, decrease in inventories of $801,428, decrease in prepaid expenses and other current assets of $2,018,565, increase in accounts payable and accrued expense of $230,951, increase in taxes payable of $1,721,716, increase in unearned revenue of $658,165 and increase in due to related parties of $178,047 and the add back of net income of $13,744,432, depreciation and amortization of $1,081,953, amortization of deferred debt issuance costs of $311,388, amortization of discount on convertible redeemable preferred stock of $880,788, amortization of prepaid expense attributable to warrants of $14,849 and stock-based compensation of $113,834 offset by recognition of unearned revenue of $594,738 and decrease in other current payables of $287,905. For the nine months ended September 30, 2008, net cash provided by operating activities was primarily attributed to decrease in accounts receivable of $8,244,225, decrease in prepaid and other current assets of $1,080,576, increase in accounts payable and accrued expenses of $1,032,245, increase in value-added and service taxes payable of 2,637,331, increase in unearned revenue of $26,139, and the add back of net income of $6,389,034, depreciation and amortization of $469,455, amortization of debt discount of $208,355, amortization of debt issuance costs of $261,545, stock based compensation of $392,341, the amortization of discount on convertible redeemable preferred stock of $592,966 and warrant revaluation of $74,593 offset by an increase in inventories of $4,880,418 and a decrease in allowance for doubtful accounts and sales returns of $490,310.
Net cash used in investing activities for the nine months ended September 30, 2009 amounted to $23,974,674. For the nine months ended September 30, 2009, net cash used in investing activities was attributed to the payment for installments on intangible assets of $8,622,567 and the purchase of property and equipment of $15,352,107. For the nine months ended September 30, 2008, net cash used in investing activities was primarily attributed to the deposit on patent right of $2,857,608, the deposit on land use right of $16,768,445 and the purchase of property and equipment of $1,430,894.
Net cash provided by financing activities was $0 for the nine months ended September 30, 2009 since we did not have any financing activities during the nine months ended September 30, 2009. Net cash provided by financing activities was $2,872,348 for the nine months ended September 30, 2008 and was attributed to the receipt of net proceeds of $5,000,000 from our private financing and proceeds from related party advances of $860,916 offset by payments on convertible debt of $2,520,000 and debt issuance costs of $468,568.
We reported a net increase in cash for the nine months ended September 30, 2009 of $1,282,681 as compared to a net decrease in cash of $1,901,187 for the nine months ended September 30, 2008.
In 2007, we made advanced approximately $2 million to Lotus East, which was used as its working capital. We obtained the funds to make the loan out of the net proceeds received from the sale of $3 million principal amount our convertible debt in February 2008. These advances are unsecured and interest free. During the first quarter of 2008, we used a portion of the proceeds from the sale of Preferred Shares to satisfy these notes, lent Lotus East an additional $1.6 million and retained the balance to fund our operating expenses. Our holding company Lotus Pharmaceuticals and its subsidiary in the US have no operations. All of our operations are conducted through our two controlled entities (called “Lotus East”) in China. A set of contractual agreements provide the holding company with effective voting and management control over Lotus East in Beijing. In fact, the management of the holding entity is the same as the one in Lotus East. The board of Lotus Pharmaceuticals has decided that incomes generated by the operating entities are retained within China for operating purpose.
Lotus East has historically funded its capital expenditures from its working capital. Lotus East believes this capital is sufficient for its current needs. Lotus East has contractual commitments for approximately $56.9 million related to a Technology Transfer Agreement and the construction of the new manufacturing facility and a New Drug Patent Transfer Agreement. While it intends to fund the costs with its existing working capital associated with the Technology Transfer Agreement and the New Drug Patent Transfer Agreement and a portion of the construction of the new manufacturing facility, it is dependent upon the continued growth of its operations and prompt payment of outstanding accounts receivables by its customers to ensure that it has sufficient cash for these commitments. In addition, its ability to fully fund the costs associated with the new manufacturing facility is materially dependent upon its ability to secured bank financing and/or government grants. As the banking industry is tightening the credit/lending policy, we expect the bank financing will become more challenging and the time to obtain the bank funding might be longer than expected. Although the Chinese government has recently announced an economic simulation plan, there is no guarantee that we will be awarded the government grant successfully. Since it has no firm commitments for either, while its management believes the company will be successful in securing the necessary funding through its increasing revenue, faster collections on receivables, and continuance discussions with various commercial banks. There are no assurances that the funding will be available in the amounts or at the time required to meet Liang Fang’s commitment. In the event Lotus East is not successful in obtaining the funds it needs for the Technology Transfer Agreement and the New Drug Patent Transfer Agreement, it is possible that it could default under the terms of the agreement and forfeit any funds paid to date. If Lotus East fails to obtain all of the funding necessary to complete the construction of the new facility, which is estimated to be approximately $51 million, it could get back approximately $36.3 million spent to date, including the approximately $32.7 million for the installments on the land use rights, which is refundable if the Chinese local government would not grant it land use rights certificate.
However, the ability of Lotus East to raise any significant capital to expand their operations is limited. We believe that it is in our best long term interests to assist Lotus East in their growth plans. Accordingly, it is likely that we will seek to raise working capital to Lotus East for these projects as well as providing working capital necessary for its ongoing operations and obligations. No assurances can be given that we will be successful in obtaining additional capital, or that such capital will be available in terms acceptable to our company, particularly given the current conditions in the capital markets.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We have certain fixed contractual obligations and commitments that include future estimated payments. Changes in our business needs, cancellation provisions, changing interest rates, and other factors may result in actual payments differing from the estimates. We cannot provide certainty regarding the timing and amounts of payments. We have presented below a summary of the most significant assumptions used in our determination of amounts presented in the tables, in order to assist in the review of this information within the context of our consolidated financial position, results of operations, and cash flows. The total of contractual obligations and commitments does not include any payments made by us.
The following tables summarize our contractual obligations as of September 30, 2009, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
|
|
Payments due by period | |||||||||
|
Contractual obligations: |
|
Total |
|
Less than |
|
1-3 |
|
3-5 |
|
5+ |
|
Series A convertible redeemable preferred stock |
$ |
4,534,129 |
$ |
4,534,129 |
$ |
- |
$ |
- |
$ |
- |
|
Related parties indebtedness |
$ |
7,365,947 |
$ |
1,968,158 |
$ |
329,063 |
$ |
- |
$ |
5,068,726 |
|
Technology purchase obligations |
$ |
1,608,752 |
$ |
1,608,752 |
$ |
- |
$ |
- |
$ |
- |
|
New drug patent purchase obligations |
$ |
438,750 |
$ |
219,375 |
$ |
219,375 |
$ |
- |
$ |
- |
|
Construction obligations |
$ |
54,865,596 |
$ |
13,915,555 |
$ |
40,950,041 |
$ |
- |
$ |
- |
|
Total contractual obligations |
$ |
68,813,174 |
$ |
22,245,969 |
$ |
41,498,479 |
$ |
- |
$ |
5,068,726 |
Off-balance Sheet Arrangements
As of the date of this report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors, however, we have agreed to guarantee loans for Lotus East, if required. As of the date of this report, we have not entered into any guarantee arrangements with Lotus East. The term “off-balance sheet arrangement” generally means any transaction, agreement or other contractual arrangement to which an entity unconsolidated with us is a party, under which we have: (i) any obligation arising under a guarantee contract, derivative instrument or variable interest; or (ii) a retained or contingent interest in assets transferred to such entity or similar arrangement that serves as credit, liquidity or market risk support for such assets.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable for a smaller reporting company.
Item 4T. Controls and Procedures.
Our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and fraud. A control system, no matter how well conceived and operated, can provide only reasonable, but no absolute, assurance that the objectives of a control system are met. Further, any control system reflects limitations on resources, and the benefits of a control system must be considered relative to its costs. These limitations also include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of a control. A design of a control system is also based upon certain assumptions about potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
We maintain “disclosure controls and procedures” as such term is defined in Rule 13a-15(e) under the Securities Exchange Act of 1934. In designing and evaluating our disclosure controls and procedures, our management recognized that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
As required by Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, our management has carried out an evaluation, with the participation and under the supervision of our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of September 30, 2009. As discussed in more detail below, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures are ineffective as of September 30, 2009, due to material weaknesses that we identified in internal control over financial reporting in our annual report on Form 10-K for the fiscal year ended December 31, 2008.
Remediation Measures of Material Weaknesses
We have implemented, or plan to implement, the measures described below under the supervision and guidance of our management to remediate the control deficiencies identified in the Form 10-K and to strengthen our internal controls over financial reporting. Key elements of the remediation effort include, but are not limited to, the following initiatives, which have been implemented, or are in the process of implementation, as of the date of filing of this interim report:
|
|
1. |
We have increased efforts to enforce internal control procedures. We have also reorganized the structure of our China financial department and clarified the responsibilities of each key personnel in order to increase communications and accountability. |
|
|
|
|
|
|
2. |
We have recruited and will continue to bring in additional qualified financial personnel for the accounting department to further strengthen our China financial reporting function. |
|
|
|
|
|
|
3. |
We continually review and improve our standardization of our monthly and quarterly data collection, analysis, and reconciliation procedures. To further improve the timeliness of data collection, we are selecting and will install new point of sale systems and enterprise resource planning systems for our wholesale and retail operations. |
|
|
|
|
|
|
4. |
We plan on significantly increasing the level of communication and interaction among our China management, independent auditors, our directors of the Board, and other external advisors. |
|
|
|
|
|
|
5. |
We are in the process of searching for qualified internal control consultants to help us be in compliance with internal control obligations, including Section 404. We also plan to dedicate sufficient resources to implement required internal control procedures. |
We believe that the foregoing steps will remediate the material weaknesses identified above, and we will continue to monitor the effectiveness of these steps and make any changes that our management deems appropriate. Due to the nature of these material weaknesses in our internal control over financial reporting, there is more than a remote likelihood that misstatements which could be material to our annual or interim financial statements could occur that would not be prevented or detected.
Changes in Internal Control over Financial Reporting
Except as described above, there have been no changes in our internal control over financial reporting during our first half fiscal year that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
None.
Item 1A. Risk Factors.
Not applicable to smaller reporting company.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
In February 2009, the Company issued 842,308 shares of common stock to Mr. Liu Zhongyi, Chairman and CEO of the Company, for his services rendered through December 31, 2008 as the Company’s chief executive officer. The stock was valued at the fair value of $0.26 per share on the grant date.
In February 2009, the Company issued 67,308 shares of common stock to Adam Wasserman (former CFO) using a fair value of $0.26 per share on the grant date for his services rendered through December 31, 2008 as the Company’s chief financial officer.
In February 2009, the Company issued 48,077 shares of common stock to Mel Rothberg using a fair value of $0.26 per share on the grant date for his services as an independent director from January 1, 2008 to April 15, 2008 and consulting services rendered through November 30, 2008.
In May 2009, the Company issued 57,471 shares of its common stock to a Series A convertible redeemable preferred stockholder in connection with the conversion of 57,471 shares of Series A Preferred Stock.
In June 2009, the Company issued 114,942 shares of its common stock to a Series A convertible redeemable preferred stockholder in connection with the conversion of 114,942 shares of Series A Preferred Stock.
In June 2009, the Company issued 251,668 shares of common stock to Mr. Liu Zhongyi, Chairman and CEO of the Company, for his services rendered from January 1, 2009 to May 31, 2009 as the Company’s chief executive officer. The stock was valued at the fair value of $0.30 per share on the grant date.
In June 2009, the Company issued 90,876 shares of common stock to Adam Wasserman for services rendered from January 1, 2009 to April 30, 2009 as the Company’s former chief financial officer and services rendered from May 1, 2009 to May 31, 2009 as the Company’s consultant. The stock was valued at the fair value of $0.28 per share on the grant date.
In June 2009, the Company issued in aggregate 60,000 shares of common stock to the six directors of the Company for services rendered and to be rendered from January 1, 2009 to December 31, 2009 as the Company’s Board of Directors’ members. The Company valued these common shares at the fair value on the grant date at $0.30 per share or an aggregate of $18,000. In connection with issuance of these shares, the Company recorded prepaid stock-based expenses of $18,000.
In September 2009, the Company issued in aggregate 286,206 shares of common stock to two Series A convertible redeemable preferred stockholders in connection with the conversion of 286,206 shares of Series A Preferred Stock.
During the period from October 1, 2009 to November 12, 2009, the Company issued in aggregate 692,237 shares of common stock to various Series A convertible redeemable preferred stockholders in connection with the conversion of 692,237 shares of Series A preferred stock.
In October 2009, the Company issued 18,390 shares of Series A convertible redeemable preferred stock to Silver Rock I, Ltd. to pay off accrued and unpaid preferred dividend since Silver Rock I, Ltd. converted all of its convertible preferred stock at that moment.
In October 22, 2009, the Company issued 1,250,000 restricted shares of its common stock par value of $0.001 (to Shihong Zhai pursuant to a Consulting Agreement dated May 6, 2007 by and between the Company and Shihong Zhai as compensation for his consulting services for a period of two (2) years from May 6, 2007 to May 6, 2009.
These issuances were exempt from registration under the Securities Act in reliance on an exemption provided by Section 4(2) of that Act.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Submissions of Matters to a Vote of Security Holders.
None.
Item 5. Other Information.
None.
Item 6. Exhibits.
|
No. |
|
Description |
|
31.1 |
|
Rule 13a-14(a)/ 15d-14(a) Certification of Chief Executive Officer |
|
|
|
|
|
31.2 |
|
Rule 13a-14(a)/ 15d-14(a) Certification of Chief Financial Officer |
|
|
|
|
|
32.1 |
|
Section 1350 Certification of Chief Executive Officer |
|
|
|
|
|
32.2 |
|
Section 1350 Certification of Chief Financial Officer |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
Lotus Pharmaceuticals, Inc. | |
|
|
|
|
|
Date: November 12, 2009 |
By: |
/s/ Zhongyi Liu |
|
|
Zhongyi Liu | |
|
|
Chief Executive Officer and President, principal executive officer | |
|
|
|
|
|
|
|
|
|
Date: November 12, 2009 |
By: |
/s/ Yan Zeng |
|
|
Yan Zeng | |
|
|
Chief Financial Officer, principal financial and accounting officer | |
