Attached files
| file | filename |
|---|---|
| EX-31.2 - ADVANCED MEDICAL INSTITUTE INC. | v166069_ex31-2.htm |
| EX-32.1 - ADVANCED MEDICAL INSTITUTE INC. | v166069_ex32-1.htm |
| EX-31.1 - ADVANCED MEDICAL INSTITUTE INC. | v166069_ex31-1.htm |
| EX-32.2 - ADVANCED MEDICAL INSTITUTE INC. | v166069_ex32-2.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
|
x
|
ANNUAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
fiscal year ended June 30,
2009
or
|
o
|
TRANSITION
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
transition period from _____________ to ________________
Commission
file number 000-29531
ADVANCED
MEDICAL INSTITUTE INC.
(Exact
name of registrant as specified in its charter)
|
NEVADA
|
88-0409144
|
|
|
(State
or other jurisdiction of
incorporation
or organization)
|
(I.R.S.
Employer Identification No.)
|
|
|
Level
4, 80 William Street
East
Sydney, NSW Australia
|
2011
|
|
|
(Address
of principal executive offices)
|
(Zip
Code)
|
|
Registrant’s
telephone number, including area code: (61)
2-9640-5253
Securities
registered pursuant to Section 12(b) of the Act: None
Securities
registered pursuant to Section 12(g) of the Act:
$0.001 Common
Stock
(Title of
class)
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes o No x
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Exchange
Act. Yes o No x
Indicate
by check mark whether the registrant (1) has filed all reports required by
Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was
required to file such reports), and (2) has been subject to such filing
requirements for the past 90 days. Yes x
No o
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes o
No o
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§229.405 of this chapter) is not contained herein, and will not
be contained, to the best of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K. o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company. See
definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act.
|
Large
Accelerated Filer ¨
|
Accelerated
Filer ¨
|
Non-Accelerated
Filer ¨ (Do
not check if a smaller reporting company)
|
Smaller
Reporting Company x
|
Indicate by check mark whether the
registrant is a shell company (as defined in Rule 12b-2 of the Exchange
Act). Yes o No x
The
aggregate market value of the voting stock held by non-affiliates of the
Registrant as of December 31, 2008 was $2,954,521.
The
number of shares outstanding of the registrant’s common stock at $.001 par value
as of November 13, 2009 was 53,507,450.
DOCUMENTS
INCORPORATED BY REFERENCE
None.
ADVANCED
MEDICAL INSTITUTE INC.
Annual
Report on Form 10-K for the Year Ended June 30, 2009
|
Part I
|
Page
|
|
|
Item
1. Description of Business
|
3
|
|
|
Item
1A. Risk Factors
|
8
|
|
|
Item
1B. Unresolved Staff Comments
|
17
|
|
|
Item
2. Properties
|
17
|
|
|
Item
3. Legal Proceedings
|
19
|
|
|
Item
4. Submission of Matters to Vote of Security
Holders
|
19
|
|
|
Part II
|
Page
|
|
|
Item
5. Market for Registrant's Common Equity, Related
Stockholder Matters and Issuer Purchases of Equity
Securities
|
20
|
|
|
Item
6. Selected Financial Data
|
20
|
|
|
Item
7. Management’s Discussion and Analysis of Financial Condition
and Results of Operations
|
20
|
|
|
Item
7A. Quantitative and Qualitative Disclosures about Market
Risk
|
31
|
|
|
Item
8. Financial Statements and Supplementary Data
|
F-1
|
|
|
Item
9. Changes in and Disagreements With Accountants on Accounting
and Financial Disclosure
|
31
|
|
|
Item
9A(T). Controls and Procedures
|
32
|
|
|
Item
9B. Other Information
|
33
|
|
|
Part III
|
Page
|
|
|
Item
10. Directors, Executive Officers and Corporate
Governance
|
33
|
|
|
Item
11. Executive Compensation
|
36
|
|
|
Item
12. Security Ownership of Certain Beneficial Owners and
Management and Related Stockholder Matters
|
38
|
|
|
Item
13. Certain Relationships and Related Transactions, and
Director Independence
|
39
|
|
|
Item
14. Principal Accounting Fees and Services
|
39
|
|
|
Part IV
|
Page
|
|
|
Item
15. Exhibits, Financial Statement Schedules
|
40
|
|
|
Signatures
|
|
41
|
2
PART
I
This
report contains certain forward-looking statements and information relating to
Advanced Medical Institute, Inc. (“AMI” or the “Company”)) that are based on the
beliefs and assumptions made by AMI's management, as well as on information
currently available to the management. When used in this document, the words
"anticipate", "believe", "estimate", and "expect" and similar expressions, are
intended to identify forward-looking statements. Such statements reflect the
current views of AMI with respect to future events and are subject to certain
risks, uncertainties and assumptions. Should one or more of these risks or
uncertainties materialize, or should underlying assumptions prove incorrect,
actual results may vary materially from those described herein as anticipated,
believed, estimated or expected. Certain of these risks and uncertainties are
discussed in this report under the caption "Risk Factors" in Item 1A. AMI does
not intend to update these forward-looking statements.
|
Item
1.
|
Description
of Business.
|
History
We were
originally incorporated under the name of Hawksdale Financial Visions, Inc. on
December 6, 1996 under the laws of the State of Nevada. We were
involved in the business of timeshares, but became dormant on March 31, 1997,
and until January 28, 2005, we were a “blank check” company with nominal assets.
On October 15, 2004, we changed our name to “Advanced Medical Institute
Inc.”
On March
21, 2005, we completed a Share Exchange Agreement with Advanced Medical
Institute Pty Limited, a privately owned Australian company (“AMI Australia”),
whereby AMI Australia became our wholly owned subsidiary.
On
November 17, 2005 we entered a Share Exchange Agreement with PE Patent Holdco
Pty Limited, a privately owned Australian company (“PE Patent Holdco”), whereby
PE Patent Holdco became our wholly-owned subsidiary.
On
September 8, 2006, we entered a Share Exchange Agreement with Worldwide PE
Patent Holdco Pty Limited (ACN 117 157 727), a privately owned Australian
company (“Worldwide PE”), whereby Worldwide PE became our wholly owned
subsidiary.
Business
Overview
AMI is a
service provider company, which arranges for patients with sexual dysfunction
and prostate problems in Australia, New Zealand and the United Kingdom to be
provided with medical services, pharmaceuticals and associated clinical support
services.
For the
year ended June 30, 2009, AMI’s revenues were approximately $53.4
million.
Principal Products and
Services
AMI
provides a variety of treatment programs to its customers, via its call center
and clinics, for the treatment of sexual dysfunction and prostate problems. A
patient is diagnosed by telephone or in person at a clinic, in either case, by a
licensed physician, who sends a prescription directly to a compounding pharmacy
under contract with AMI to prepare the formulation. The prescription
is delivered to the patient, or the patient may pick up the prescription at the
clinic. The patient pays for treatments for a specified treatment
period, during which the formulations may be varied to best suit the patient’s
needs.
3
Our
physicians prescribe varying combinations or dosages of medications for erectile
dysfunction (predominantly phentalomine, apomorphine or a combination of them),
premature ejaculation (predominantly clomipramine) and prostate problems
(mixture of medicinal herbs). Our compound formulations have not been
subject to any clinical trial, but may lawfully be prescribed on an individual
prescription (“off-label”) basis in each country in which we
operate.
The
effectiveness of AMI’s treatment programs depend highly on the delivery
system. These include:
(a) injections,
nasal spray, lozenges, tablets and gels for the treatment of erectile
dysfunction;
(b) injections,
nasal spray, lozenges and gels for the treatment of premature
ejaculation;
(c) topical
gels for the treatment of female sexual arousal dysfunction; and
(d) an
oral elixir for the treatment of prostate problems.
New Products and
Services
Since 2003, AMI’s subsidiary,
Intelligent Medical Technologies Pty Limited (“IMT”) has been developing an
ultrasonic nebulizer which will deliver drugs to the lungs. The
group’s intention is to use this delivery system to administer its compound
formulations. In order to utilize this delivery system IMT needs to
obtain regulatory approval of the nebulizer as a medical device. IMT
has been working on this process for the last four years, however IMT has not
yet completed such process.
Sales and
Marketing
For the
year ended June 30, 2009, the Company spent $22.4 million on advertising,
including commercials (radio, television and newspapers) and billboards. We
maintain a website, http://www.amiaustralia.com.au/, where a potential patient
can submit medical history and information concerning the problem for which
treatment is sought, prefatory to being contacted by AMI.
AMI’s
erectile dysfunction treatment programs predominantly focus on men aged 40 and
over, whereas AMI’s premature ejaculation treatment programs are targeted at men
aged 18 and over.
Distribution
AMI currently operates a centralized
call center and 20 clinics throughout Australia and New Zealand. AMI
has recently established two clinics in the United Kingdom to service UK
patients. AMI’s products and services are only available by prescription and are
sold on an “off-label” basis. AMI Australia’s treatment programs are
generally available in the same manner through its medical clinics and its
over-the-phone sales and marketing program.
4
Intellectual
Property
The Company’s intellectual property
consists of an Australian innovation patent for AMI’s premature ejaculation
treatment programs (Australian Innovation Patent No 2005100183, which is due to
expire on July 9, 2012), an Australian standard patent application (Australian
standard patent application No. 2004222783), a New Zealand patent for its
premature ejaculation treatment programs (New Zealand patent 544484), an Indian
patent for its premature ejaculation treatment programs (patent
00283/KOL-NP/2006) and patent applications for its premature ejaculation
treatment programs in most industrialized countries. The innovation
patent, titled “Treatment of Premature Ejaculation” relates to various methods
of treatment delivery via nasal (mucosal) inhalation and topical application of
certain formulations which are used in AMI’s treatment programs for premature
ejaculation. The patents and associated formulations are integral to
AMI’s premature ejaculation treatment programs. AMI’s nasal spray
erectile dysfunction treatment programs are not patent
protected. This technology was developed by AMI and its founder, Dr
Jack Vaisman.
AMI’s
wholly-owned subsidiary, IMT, was granted the exclusive worldwide right and
license from Sheiman Ultrasonic Research Foundation Pty Limited to exploit and
sub-license certain inventions, patents and other intellectual property in
relation to certain ultrasonic nebulizer technology (including Australian Patent
No’s 693064 and 753817, European Patent No’s 0 705 145 and 1 071 479 and US
Patent No’s 5,908,158 and 6,379,616) within the field of the treatment of sexual
dysfunction in men and women (including impotence, premature ejaculation and the
treatment of female sexual arousal disorders). The patents relating
to the initial concepts underpinning the technology expire in October 2013, with
the remaining patents relating to extensions of the device expiring around
2023.
IMT applied for seven provisional
worldwide patents in July 2005 and February 2006. In September 2006,
IMT filed an international (PCT) patent application relating to its Nebuliser
device based on aspects of these provisional applications. The international
examination of the PCT application was successful, resulting in a clear
International Preliminary Report on Patentability. This PCT application
has now entered the National Phase in the following jurisdictions: Australia,
Canada, China, Europe, India, Japan, New Zealand and the United States, and
these national phase patent applications are currently awaiting examination in
the respective patent offices. Further, several Australian design
applications, and a United States design patent application have been made
for IMT’s device, the subject of the international (PCT) patent
application. The Australian Design applications are now registered, and
the United States Design patent application is currently under
examination.
AMI
applied for a broad based provisional patent relating to the technology
underpinning its topical gels on July 29, 2008. This application has
yet to be examined and is at an early stage.
Research and
Development
AMI is continuously researching and
developing new methods of treatment in relation to the treatment of sexual
dysfunction in men and women, including impotence, premature ejaculation,
reduced male libido and female sexual arousal disorders.
AMI’s Chief Executive Officer, Dr Jack
Vaisman, spends 25-35% of his time each year researching treatment
methods. In addition, the Company has engaged Dr Jim Rowe (a leading
Australian chemist) to assist the Company to research treatment options, and the
Company has also engaged various other personnel and consultants to assist the
Company in its research programs, either on a part time or project specific
basis, including research assistants, patent attorneys and legal
advisors.
5
Competition
We
compete with rival treatments such as Viagra, Cialis and Levitra in the erectile
dysfunction field. AMI does not currently have any major competitors
in the premature ejaculation market in Australia and New Zealand (its
competitors are individual doctors providing other pharmaceutical treatments on
an individual prescription or “off-label” basis, as well as individual doctors
and psychologists providing counseling services to patients).
AMI’s
delivery methods have the following advantages over the oral delivery methods of
its competitors:
|
|
·
|
Elimination
of gastric and hepatic drug degradation by avoiding metabolism and
gastro-internal tract;
|
|
|
·
|
Faster
entrance into the bloodstream; and
|
|
|
·
|
Fewer
side effects due to the potential reduced dosage of the
drug.
|
Competition
is intense in the erectile dysfunction segment of our business and includes many
competitors that have greater revenue, more customers and higher levels of brand
recognition than the Company. The Company has smaller competitors in the
premature ejaculation segment of our business that have similar business models
to the Company’s business. The efficacy, safety, patients’ and
customers’ ease of use and cost effectiveness of our products are important
factors for success in our principal businesses. Many of our
competitors have substantially greater financial and other resources, larger
research and development staff and more experience in the regulatory approval
process. Moreover, potential competitors have or may have
intellectual property or other rights that conflict with patents, license
agreements and other intellectual property rights covering our
technologies.
6
Significant Subsidiaries of
the Company and AMI Australia
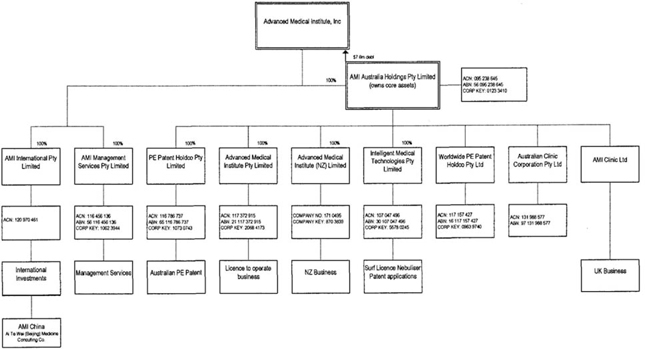
The Company’s subsidiaries are AMI
Australia, AMI International Pty Limited and AMI Management Services Pty
Limited.
AMI Australia’s subsidiaries are
Advanced Medical Institute Pty Limited, PE Patent Holdco, Worldwide PE, Advanced
Medical Institute (NZ) Limited, IMT and AMI Clinic Limited.
On June 30, 2009, AMI discontinued
operations of AMI China.
AMI International Pty Limited was
established to hold the Group’s shareholdings in the Chinese company established
to conduct operations in that jurisdiction.
AMI Management Services Pty Limited
provides treasury and management services to AMI Australia and its
subsidiaries.
Advanced Medical Institute (NZ) Limited
conducts the group’s business in New Zealand.
AMI
Clinic Limited conducts the group’s business in UK.
Ai Te Wei (Beijing) Medicine Consulting
Company conducts the group’s business in China.
7
During September 2006, AMI commenced a
not-for-profit division of the Company, AMI-SCI, which provides treatment
options to men who have sustained a spinal cord injury. Details regarding this
unit’s services and operations are located at rocketlaunch.com.au.
Employees
As of
June 30, 2009, AMI had a total staff of approximately 256 people, of which 184
are full-time and 72 are part-time, working in the areas of sales and marketing,
customer support, product development and back office functions. None of the
Company’s employees or staff are members of a union or labor
organization.
Governmental
Regulation
The core government regulations
affecting the business relate to pharmaceutical prescription related
regulations, doctor related regulations and advertising related
regulations. As set out above, each of our treatments is provided on
an individual prescription (“off-label”) basis. Our treatments are
not available from retail pharmacies on an over the counter basis and our
treatments are not marketed under a brand name. It is illegal to mass
produce our medications, and our medications must be prepared in a regulated
facility to a specified standard.
Telephone consultation by doctors is
legal in Australia and the United Kingdom but is illegal in other
markets. Telephone consultation is a core component of our
business.
Our advertisements and marketing is
direct to consumers and therefore required to comply with consumer based
legislation. This legislation prohibits false and misleading
statements from being made in our advertising (amongst other things). Government
regulation also restricts the manner in which our advertising may be undertaken
due to our treatments containing prescription based medications.
Reports to Security
Holders
Annual reports. We deliver
annual reports containing audited financial statements to security
holders.
Periodic reports and other
information. We file annual and quarterly reports, current reports, proxy
statements, and information statements with the SEC.
Availability of Filings. You
may read and copy any materials we file with the SEC at the SEC’s Public
Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may obtain
information on the operation of the Public Reference Room by calling the SEC at
1-800-SEC-0330. Additionally, the SEC maintains an Internet site (http://www.sec.gov)
that contains reports and proxy and information statements and other information
regarding issuers that file electronically with the SEC.
|
Item 1A.
|
Risk
Factors.
|
In
addition to the other information in this Annual Report, the following factors
should be considered carefully in evaluating the Company’s business and
prospects. THE FOLLOWING MATTERS MAY HAVE A MATERIAL ADVERSE EFFECT
ON THE BUSINESS, FINANCIAL CONDITION, LIQUIDITY, RESULTS OF OPERATIONS OR
PROSPECTS, FINANCIAL OR OTHERWISE, OF THE COMPANY. REFERENCE TO THIS CAUTIONARY
STATEMENT IN THE CONTEXT OF A FORWARD-LOOKING STATEMENT OR STATEMENTS SHALL BE
DEEMED TO BE A STATEMENT THAT ANY ONE OR MORE OF THE FOLLOWING FACTORS MAY CAUSE
ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE IN SUCH FORWARD-LOOKING STATEMENT
OR STATEMENTS. We are subject to, among others, the following
risks:
8
Risks Related to our
Business
Our
business may be adversely affected by competition in the market for the
treatment of sexual dysfunction.
Our
business is operating in a competitive market in the erectile dysfunction (“ED”)
segment, and our competitors have significantly greater resources. It
is likely that new competitors will emerge in the short to medium term which may
have an adverse impact on our business.
While we
do not have any significant current known competitors to our premature
ejaculation (“PE”) business in Australia, New Zealand or the United Kingdom it
is likely that competitors will emerge in the short to medium term which may
have an adverse impact on our business. There are various PE
treatments under development internationally which may enter the Australian
market in the short to medium term and which could impact on our ability to
expand our PE business internationally.
An
inability to respond quickly and effectively to new trends could adversely
impact our competitive position.
Any
failure to maintain the level of our technological capabilities or to respond
effectively to technological changes could adversely affect our ability to
retain existing business and secure new business. We will need to
constantly seek out new products and develop new solutions to maintain in our
portfolio. If we are unable to keep current with new trends, our
competitors’ services, technologies or products may render us noncompetitive and
our services and products obsolete.
Potential
impact of current litigation
We may be
adversely affected as a result of current matters in litigation due to legal
costs being incurred or judgments being given against us.
We may be
subject to adverse publicity, costs orders and damages if we lose the legal
actions we are currently involved in.
Increases
in staffing costs could adversely affect our business.
Our
business is labor intensive and we are highly dependent on the provision of
services by highly qualified personnel. These resources are scarce
and we may face competition for these services which could result in increased
expenses for the business. These labor expenses constitute a
significant component of our overall cost of doing business and increases in
these expenses may adversely impact our business.
Increases
in advertising expenses and/or decreases in the effectiveness of our advertising
could result in higher expenses with no corresponding increase in
revenue.
Our
current business model is significantly reliant upon advertising and changes to
the costs associated with that advertising or a decrease in the effectiveness of
that advertising could have a significant adverse impact on the revenue and/or
profitability of our business.
9
Increases
in cost for pharmaceutical compounds and other chemicals could adversely affect
our business.
We
arrange for patients to be provided with medications. Significant
increases in the price of medications or other chemicals used to make the
medications could increase the overall expense of doing business and reduce its
profitability.
We
are subject to risks associated with joint ventures and third party
agreements.
Development
of IMT’s technology is also dependent on contractual arrangements with various
consultants. In addition, AMI Australia has third party contractor
agreements with its doctors and compounding pharmacies. We may incur
significant costs if issues or disputes arise in relation to those arrangements
and joint ventures and our future operation, growth and expansion is dependent
on the success of those arrangements.
We
may be subject to product liability claims which could negatively impact our
profitability.
We
arrange for our patients to be treated with pharmaceutical products, which
involve risks such as product contamination or spoilage, product tampering and
other adulteration of pharmaceutical products. We may be subject to
liability if the consumption of any of our products causes injury, illness or
death. A significant product liability judgment against us may
negatively impact our profitability for a period of time depending on product
availability, competitive reaction and consumer attitudes. Even if a
product liability claim is unsuccessful or is not fully pursued, any negative
publicity surrounding such assertion that products provided through our
treatment programs caused illness or injury could adversely affect our
reputation with existing and potential customers and including irreparable harm
to our corporate and brand image.
We
have limited business liability insurance coverage.
We have
limited business liability insurance coverage for our operations. Any
loss due to business disruption, litigation or natural disaster may exceed or
may be excluded from the insurance coverage we have and could result in
substantial expenses and a diversion of resources. We do not have
liability insurance to cover any operations outside of Australia, New Zealand or
the United Kingdom and likely would need to put such insurance in place in the
event that we commence operations outside these geographic
areas.
We
have and may in the future experience negative results from our plans for
expansion.
We may,
in the future, acquire new Australian clinic facilities and may expand our
business beyond Australia, New Zealand, China and the United
Kingdom. Entering into any expansion transaction entails many risks,
any of which could have a negative impact on our business, including: (a)
diversion of management’s attention from other business concerns; (b) failure to
integrate the acquired company with our existing business; (c) additional
operating expenses not offset by additional revenue; and (d) dilution of our
stock as a result of issuing equity securities. Any expansion
globally also entails additional risks relating to operating our business in
environments with different legal and regulatory systems and business customs
than those in Australia, New Zealand and the United Kingdom, incurring
additional costs in locating and retaining those professionals with expertise in
these area. There is also the likelihood that significant start up
costs will be incurred and that it may take time to achieve successful market
penetration in new markets. We may discover that global operations
are less profitable than existing operations and that losses may be
incurred. There is a risk that our operations in those and other
international areas will continue to be unprofitable.
10
We
may be unable to implement our acquisition or expansion strategy and as a
result, we may be less successful in the future.
We may
not be able to identify and acquire companies meeting our acquisition criteria
on terms acceptable to us. Additionally, financing, if needed, to
complete acquisitions may not always be available on satisfactory
terms. Further, our acquisition strategy presents a number of risks
to us, including adverse effects on our earnings after each acquisition,
diversion of management’s attention from our core business, failure to retain
key acquired personnel and other risks from unanticipated events or liabilities
arising after each acquisition. Some or all of these risks could have
a material adverse effect on our business, financial condition and results of
operations.
We
may be adversely affected should certain patents expire or if our intellectual
property becomes widely available.
We are
currently exploiting or planning to exploit patented technology in our existing
businesses and intend to continue to exploit such technology if we expand
further internationally. Our ability to do business in a similar
manner may be adversely affected by the expiration of those patents and if our
competitors utilize such technology to compete with our businesses.
Some of
our patent applications have yet to be approved by the relevant regulatory
body. The inability to obtain patent protection for our technology or
misappropriation of our intellectual property could adversely affect our
competitive position.
Our
success depends on our medication formulations and internally developed
know-how, and related intellectual properties. Management regards
this intellectual property as proprietary and has and will continue to attempt
to protect such intellectual property by seeking patents, copyrights or
trademarks (as appropriate), and by invoking trade secret laws and entering into
confidentiality and nondisclosure agreements with third
parties. Despite these precautions, it may be possible for a third
party to obtain, misappropriate and use our services or intellectual
property.
We may
need to resort to litigation in the future to enforce or to protect our
intellectual property rights. In addition, our intellectual property
and patents may be claimed to conflict with or infringe upon the patent,
trademark or other proprietary rights of third parties. If this
occurred, we would need to defend ourselves against such challenges, and such
defenses could result in substantial costs and the diversion of resources which
could materially harm our business.
Our
products may infringe upon the intellectual property rights of others, which
could cause damages that may increase our costs and negatively affect our
profitability.
Third
parties may in the future assert against us misappropriation claims or claims
that we have infringed a patent, copyright, trademark or other proprietary right
belonging to them. Any claim, even if not meritorious, could result
in the expenditure of significant financial and managerial resources and could
negatively affect our profitability.
11
Our
ongoing research and development activities are, and the production and
marketing of our pharmaceutical products and services and medical devices
derived therefrom will be, subject to regulation by numerous governmental
authorities in Australia and elsewhere, principally the Therapeutics Goods
Administration, or “TGA” and the functional equivalent bodies in the United
States, Europe, Asia and elsewhere.
Under our
current business model, our medical formulations are able to be provided through
a compounding pharmacy in Australia on an individual prescription
basis. We may need to make variations to our business model to
commence operations in certain jurisdictions outside Australia, New Zealand and
the United Kingdom and there may be other jurisdictions where we would need to
fully comply with clinical trial requirements.
Our
nebulizer medical device is currently undergoing an extensive regulatory
approval process mandated by the TGA prior to marketing and use in Australia
and, to the extent that our nebulizer is marketed internationally, it will
likely have to go through additional regulatory approval by foreign regulatory
agencies. This review process can take considerable time and require
substantial expense. We must obtain regulatory approval from the respective
agencies in countries outside of Australia. We can make no guarantees
that such approvals will be granted.
Delays in
obtaining regulatory approvals would adversely affect the development and
commercialization of our nebulizer device. We cannot be certain that
we will be able to obtain the clearances and approvals necessary for
manufacturing and marketing our nebulizer device.
We
may have future capital needs for which we will need to access additional
financing. Additional financing could dilute our current
stockholders’ equity interests.
We
currently anticipate that our available funds and resources, including sales,
will be sufficient to meet our anticipated needs for working capital and capital
expenditures for the next twelve months within Australia and New
Zealand. We also anticipate that we have sufficient funds to continue
to operate in the United Kingdom on the present scale, however there is a
risk that our budgets may be incorrect and that such finance may prove
inadequate or that we may decide to expand more quickly and into other markets
and that we may need to raise additional funds in the future in order to fund
more research and development and more rapid expansion and to develop new or
enhanced products.
If
additional funds are raised through the issuance of equity or debt securities,
our current stockholders may experience dilution and any such securities may
have rights, preferences or privileges senior to those of the rights of our
common stock.
There can
be no assurance that additional financing will be available on terms favorable
to us, or at all. If adequate funds are not available or not
available on acceptable terms, we may not be able to fund our expansion, promote
our brand name as we desire, take advantage of unanticipated acquisition
opportunities, develop or enhance products or respond to competitive
pressures. Any such inability could have a material adverse effect on
our business, results of operations and financial condition.
12
We
may not be able to retain our key management or contractors.
We are
highly dependent on the services of Dr. Jacov (Jack) Vaisman, Mr. Dilip Shrestha
and Mr. Forhad (Tony) Khan, as well as the other principal members of our
management and scientific staff and the services of Doyle Corporate Pty Limited
and its principal Mr. Richard Doyle. The loss of one or more of such
persons could substantially impair ongoing operations. Our success
depends in large part upon our ability to attract and retain highly qualified
personnel. We compete in our hiring efforts with other pharmaceutical
and medical treatment companies and we may have to pay higher salaries to
attract and retain personnel.
Much of
our intellectual property has been devised and developed by Dr. Vaisman and the
loss or impairment of his services could significantly affect our ability to
fully exploit our intellectual property or affect our ability to develop new
intellectual property.
Risks related to our common
stock
Our
stock price is highly volatile.
Our stock
price has fluctuated significantly. There is a significant risk that
the market price of our common stock could decrease in the future in response to
any of the following factors, some of which are beyond our control:
|
|
·
|
variations
in our quarterly operating results;
|
|
|
·
|
general
economic slowdowns;
|
|
|
·
|
changes
in market valuations of similar
companies;
|
|
|
·
|
sales
of large blocks of our common
stock;
|
|
|
·
|
announcements
by us or our competitors of significant contracts, acquisitions, strategic
partnerships, joint ventures or capital commitments;
and
|
|
|
·
|
fluctuations
in stock market prices and volumes, which are particularly common among
highly volatile securities of internationally-based
companies.
|
Our
common stock is subject to the “penny stock” rules.
Our
common stock is subject to the United States Securities and Exchange
Commission’s penny stock rules, broker-dealers may experience difficulty in
completing customer transactions and trading activity in our securities may be
adversely affected.
Due to
the fact that we have net tangible assets of USD5,000,000 or less and our common
stock has a market price per share of less than USD5.00, transactions in our
common stock may be subject to the “penny stock” rules promulgated under the
Securities Exchange Act of 1934. Under these rules, broker-dealers
who recommend such securities to persons other than institutional accredited
investors:
|
|
·
|
must
make a special written suitability determination for the
purchaser;
|
|
|
·
|
must
receive the purchaser’s written agreement to a transaction prior to
sale;
|
|
|
·
|
must
provide the purchaser with risk disclosure documents which identify
certain risks associated with investing in “penny stocks” and which
describe the market for these “penny stocks” as well as a purchaser’s
legal remedies; and
|
|
|
·
|
must
obtain a signed and dated acknowledgment from the purchaser demonstrating
that the purchaser has actually received the required risk disclosure
document before a transaction in a “penny stock” can be
completed.
|
13
If our
common stock becomes subject to these rules, broker-dealers may find it
difficult to effectuate customer transactions and trading activity in our
securities may be adversely affected. As a result, the market price
of our securities may be depressed, and you may find it more difficult to sell
our securities.
Efforts
to comply with recently enacted changes in securities laws and regulations have
required substantial financial and personnel resources and we still may fail to
comply.
As
directed by the Sarbanes-Oxley Act of 2002, the Securities and Exchange
Commission adopted rules requiring public companies to include a report of
management on our internal controls over financial reporting in their annual
reports on Form 10-K. In addition, the public accounting firm
auditing our financial statements must attest to and report on management’s
assessment of the effectiveness of our internal controls over financial
reporting.
The
formal process of evaluating our internal controls over financial reporting,
which has required the devotion of substantial financial and personnel
resources, is not complete. Given the status of our efforts, coupled
with the fact that guidance from regulatory authorities in the area of internal
controls continues to evolve, uncertainty exists regarding our ability to comply
by applicable deadlines.
Risks associated with doing
business in Australia and other foreign countries
We
are subject to the risks associated with doing business in Australia and
Asia.
As most
of our current operations are conducted in Australia, New Zealand and Asia we
are subject to special considerations and significant risks not typically
associated with companies operating in North America and Western
Europe. These include risks associated with, among others, the
political, economic and legal environments and foreign currency
exchange. Our results may be adversely affected by changes in the
political and social conditions in Australia, New Zealand and Asia, and by
changes in governmental policies with respect to laws and regulations,
anti-inflationary measures, currency conversion and remittance abroad, and rates
and methods of taxation, among other things.
We
are subject to risks associated with the domicile, place of incorporation and
place of residence of AMI Australia and our directors and officers.
AMI
Australia is incorporated in Australia. All of our executive officers
and directors are non-residents of the United States. Therefore, it
may be more difficult for an investor, or any other person or entity, to enforce
a U.S. court judgment based upon the civil liability provisions of the U.S.
federal securities laws in an Australian court against us or any of those
persons or to effect service of process upon these persons in the United States
than it would be if these persons were resident in the United
States.
Additionally,
it may be difficult for an investor, or any other person or entity, to enforce
civil liabilities under U.S. federal securities laws in original actions
instituted in Australia. It may be difficult to enforce a judgment in
the United States against us and most of our officers and directors or to assert
U.S. securities laws claims in Australia or serve process on most of our
officers and directors.
14
We
may experience an impact of the United States Foreign Corrupt Practices Act on
our Business.
We are
subject to the United States Foreign Corrupt Practices Act, which generally
prohibits United States companies from engaging in bribery or other prohibited
payments to foreign officials for the purpose of obtaining or retaining
business. Foreign companies, including some that may compete with us,
are not subject to these prohibitions. Compliance with the Foreign
Corrupt Practices Act could adversely impact our competitive position and
failure to comply could subject us to penalties and other adverse
consequences. We have attempted to implement safeguards to prevent
and discourage non-compliant conduct by our employees and agents. We
can make no assurance, however, that our employees or other agents will not
engage in such conduct for which we might be held responsible. If our
employees or other agents are found to have engaged in such practices, we could
suffer severe penalties and other consequences that may have a material adverse
affect on our business, financial condition and results of
operations.
We
may experience an impact due to Australian, New Zealand or United Kingdom
regulation of the pharmaceutical and/or medical services industry.
We may
suffer an adverse impact to our business if the manner in which our services are
provided is required to change due to changes in the relevant regulatory regimes
applicable to those services. This could render our current business
model unlawful or render it uneconomic, adversely impacting our profitability
and prospects. The Australian government is in the process of
undertaking a review of the impotency industry in Australia and a committee of
the Australian federal parliament is scheduled to release a report containing
recommendations relating to the ongoing regulation of this industry in November
or December 2009. This report may recommend changes to the current
regulation of this industry and the Company’s business.
We
may experience a risk of revenue reduction due to a saturation of the Australian
Market.
Australia
is a country with a population of approximately 22 million
people. There are a limited number of people who are candidates for
our treatments. We are subject to increased risks or over saturation
of market share as we continue to treat more patients in the Australian
market.
Effects
of international sales.
We intend
to market our current and future services internationally. A number
of risks are inherent in international transactions. In order for us
to market our services in the U.S., Europe, Canada, Asia and certain other
foreign jurisdictions, we may have to obtain required regulatory approvals or
clearances and otherwise comply with extensive regulations regarding safety,
manufacturing processes and quality and advertising rules and
regulations. There can be no assurance that we will be able to obtain
or maintain regulatory approvals or clearances in such countries or that it will
not be required to incur significant costs in obtaining or maintaining its
foreign regulatory approvals or clearances.
Fluctuations
in currency exchange rates may adversely affect the demand for our services by
increasing the price of our services in the currency of the countries in which
the services are marketed.
Our
consolidated financial statements are presented in Australian and U.S.
dollars. Fluctuations in the rates of exchange between U.S. dollar
and other foreign currencies may negatively impact our financial condition and
results of operations. As we expand our presence into international
markets, we expect the percentage of both our revenues and expenditures
denominated in non-Australian dollars to increase. For the
foreseeable future, we expect our expenditures to be predominantly denominated
in Australian dollars, resulting primarily from our activities in Australia, and
expect capital expenditures to be denominated in Australian
dollars. Our expansion into the United Kingdom will also expose us to
currency fluctuations relating to UK Pounds.
15
We
are subject to a risk that our current patent applications may not be
granted.
We have
pending certain patents relating to the treatment of premature
ejaculation. We can make no guarantee that these patents will be
granted and, if the patents are rejected, the intellectual property underlying
these patents will then reside within the public domain and we would not have
any patent protection from competition using its intellectual
property. While the Company believes that formulation secrets not
contained in the patent applications will create a barrier to entry, there can
be no guarantee that such a barrier will prove sufficient. In the
event that such competition was to arise, it could adversely affect our market
share, pricing power, profitability and prospects as a going
concern.
We
are subject to risks associated with our method of patient consultation. We may
experience risks due to dispensing medication through prescriptions written by
our doctors after our over-the-phone consultations.
We retain
doctors to consult with patients over the phone and to write prescriptions on
the basis of these telephone consultations. Telephone diagnosis and
prescription is widely considered, in many countries including Australia,
appropriate for some ailments but not for others. Telephone diagnosis
and prescription written as a result of such consultation may be deemed
unsuitable either in general or for those treatments that we provide and as a
result, we have assumed this risk in its current business model. If
over-the-phone diagnosis and prescriptions were prohibited, we could be subject
to a substantial increase in costs due to the need for full-time physicians at
each clinic and, further, could result in lost sales as a result of potential
customers being unable or unwilling to see a doctor in
person. Furthermore, such a requirement could significantly impair
our future profitability and prospects as a going concern.
Our
current business model may not be profitable in international
markets.
We have
limited experience in operating outside of Australia and New Zealand, and
failure to achieve our overseas expansion strategy may have an adverse effect on
our business growth in the future. Our future growth depends, to a
considerable extent, on our ability to expand our customer base in both the
domestic and overseas markets. We have limited experience with
foreign regulatory environments and market practices, and cannot guarantee that
we will be able to penetrate any overseas market. In connection with
our initial efforts to expand overseas, we may encounter many obstacles,
including cultural and linguistic differences, difficulties in keeping abreast
of market, business and technical developments in foreign jurisdictions, and
political and social disturbances. Failure in the development of
overseas markets may have an adverse effect on our business growth in the
future.
We
may not achieve the widespread brand recognition necessary to succeed outside of
Australia.
We
believe it is imperative to our long term success that we obtain significant
market share for our services before other competitors enter the
market. Therefore, we must quickly establish recognition of our
brand. This will require us to must make substantial expenditures on
product development, strategic relationships and marketing initiatives in new
markets. In addition, we must devote significant resources to ensure
that our customers are provided with a high quality product and a high level of
customer service. Many of our potential competitors may have
substantially greater financial, marketing and other resources and potentially
greater access to content and distribution channels than us. We
cannot be certain that we will have sufficient resources to achieve the early
and widespread brand recognition we believe necessary to realize commercial
acceptance of our services.
16
Regulations
in international markets relating to advertising could adversely affect our
business.
Our
business model is dependent upon advertising and marketing. Our
current model may not be able to be enacted internationally due to regulatory
requirements in the relevant jurisdictions. In certain countries
(including Australia), advertisers, advertising agencies and companies that
engage in advertising activities are liable for the accuracy of the content of
advertisements and they have to ensure that the advertised products, activities
and services are in full compliance with applicable law. We do not
plan to do business in countries in which the advertising of our services and/or
pharmaceuticals in the manner we advertise in Australia would be
illegal. If we are found to be in breach of advertising law
requirements, we could be subject to liability and penalties under the respected
laws of such country. These penalties may include fines, confiscation
of profits, order to cease the dissemination of the advertisement and orders to
publish an advertisement correcting the misleading information. Any
such action by any relevant regulatory body, whether or not it is ultimately
successful, could distract management attention and have a material adverse
effect on our business and financial condition or results of
operations.
Regulations
in international markets relating to pharmaceuticals could adversely affect our
business.
Our
business model is dependant upon the distribution of pharmaceutical
prescriptions. Our current model may not be enacted
internationally. In certain countries, we may be subject to state or
national laws governing the distribution and compounding of
pharmaceuticals. We may be required to expend substantial unforeseen
resources to comply with such regulations. Failure to comply with
such regulations could cause a significant disruption in our business
domestically and internationally as it could distract management attention and
have a material adverse effect on our business and financial condition or
results of operations.
Inability
to hire experienced and capable employees or executives in non-Australian
markets could adversely affect our business in such markets.
We rely
heavily on the performance of our officers and key employees in
Australia. Any international expansion will be dependent on our
ability to retain and motivate qualified personnel in those markets, especially
in management. If we do not succeed in attracting new qualified
employees, our business could suffer significantly. Competition for
qualified personnel is intense in certain markets, and we may be unable
identify, attract, hire, train, assimilate, or retain a sufficient number of
highly skilled managerial, marketing and technical personnel necessary to
implement our business plan outside of Australia.
|
Item
1B.
|
Unresolved
Staff Comments.
|
|
|
Not
applicable.
|
|
Item
2.
|
Properties.
|
As of
June 30, 2009, AMI had leasehold interests in 19 Australian properties and one
New Zealand property, being the 20 clinics used to operate the business in New
Zealand and Australia. There are no encumbrances over AMI’s interest
in these properties. All of these properties are rented and none of
these properties are owned. AMI’s principal executive office is
located at Level 4, Terrace Tower, 80 William Street, East Sydney,New South
Wales, Australia. AMI’s principal executive office houses AMI’s
corporate head office, its centralized call center and some of AMI’s licensed
doctors, nursing staff and sales and administration staff.
17
AMI’s
clinics and sales offices are located at:
New
South Wales
AMI
Direct, East Sydney, Sydney, New South Wales
Bondi
Junction, Sydney, New South Wales
Dubbo,
Regional New South Wales
Hurstville,
Sydney, New South Wales
Newcastle,
Regional New South Wales
Parramatta,
Sydney, New South Wales
Sydney
City, Sydney, New South Wales
Wollongong,
Regional New South Wales
Queensland
Bundall,
Gold Coast, Queensland
Cairns,
Regional Queensland
Kallangur,
Queensland
Townsville,
Regional Queensland
Upper Mt
Gravatt, South Brisbane, Queensland
South
Australia
Adelaide,
South Australia
Victoria
St Kilda,
Melbourne, Victoria
Mitcham,
Melbourne, Victoria
Dandenong,
Melbourne, Victoria
Western
Australia
Perth,
Western Australia (2 clinics)
New
Zealand
Auckland,
North Island, New Zealand
United
Kingdom
London,
England
Twickenham,
England
In
addition, the Company presently utilizes office space of our registered agent
representative in the State of Nevada at 6767 Tropicana Avenue, Suite 207, Las
Vegas, Nevada 89103 as our US registered office.
18
|
Item 3.
|
Legal
Proceedings.
|
As of
June 30, 2009, there was no litigation pending or threatened by or against the
Company or any of its direct or indirect subsidiaries other than AMI Australia.
AMI Australia currently is involved in the following litigation and
administrative matters:
On
October 25, 2006, Bade Medical Institute Pty Limited, Mr. David Wade, Mr. Buddy
Beani and others (collectively “Bade”) applied for Trade Mark No. 114322021 in
respect of the words “AMI Nasal Spray.” AMI Australia lodged an objection to the
registration of that application with IP Australia on June 21, 2007 and that
application was subsequently refused. On December 13, 2007, AMI
Australia commenced proceedings in the Federal Court of Australia Sydney
Registry against Bade alleging Bade was infringing upon AMI Australia’s
trademarks and other intellectual property rights. On December 19, 2007, Bade
consented to orders that Bade transfer ownership of certain domain names and
telephone numbers to AMI Australia and consented to orders that Bade would not
use certain names which contained the word AMI. Bade appear to have
ceased operation since the orders were obtained and has transferred the required
names to AMI Australia. AMI subsequently applied for final orders and damages
against Bade and final hearing of this matter concluded on September 2,
2009. The court has indicated that judgment will be released
shortly.
On June
12, 2009, AMI Australia obtained an interlocutory injunction from the Supreme
Court of New South Wales preventing Fairfax Media Publications Limited
(“Fairfax”) from publishing information which AMI Australia alleges was
disclosed to Fairfax in breach of a duty of confidence owed to AMI
Australia. Final hearing of this matter commenced on October 26, 2009
but was unable to be concluded by October 28, 2009, the final scheduled date for
the hearing of the matter. Final hearing of the matter is now
scheduled for February 24 and 25, 2010 and AMI Australia’s interlocutory
injunction has been extended until that date.
|
Item 4.
|
Submission
of Matters to Vote of Security
Holders.
|
Not
applicable.
19
PART
II
|
Item
5.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters
and Issuer Purchases of Equity
Securities.
|
Our
Common Stock is listed on the OTC Bulletin Board under the symbol
“AVMD.OB”. The following sets forth, for the periods indicated, the
high and low bid price for our Common Stock as reported for the prior two fiscal
years. The quotations set forth below may reflect inter-dealer
prices, without retail mark-up, mark-down or commissions and may not necessarily
represent actual transactions.
|
Quarter Ending:
|
High
|
Low
|
||||||
|
September
30, 2007
|
$ | 0.20 | $ | 0.10 | ||||
|
December
31, 2007
|
$ | 0.40 | $ | 0.10 | ||||
|
March
31, 2008
|
$ | 0.39 | $ | 0.15 | ||||
|
June
30, 2008
|
$ | 0.35 | $ | 0.15 | ||||
|
September
30, 2008
|
$ | 0.35 | $ | 0.17 | ||||
|
December
31, 2008
|
$ | 0.17 | $ | 0.07 | ||||
|
March
31, 2009
|
$ | 0.07 | $ | 0.02 | ||||
|
June
30, 2009
|
$ | 0.19 | $ | 0.04 | ||||
As of
November 11, 2009 there were 328 shareholders of record of our common
stock.
We have
not paid any stock dividends or cash dividends to date and have no plans to pay
any stock or cash dividends in the immediate future.
We do not
have any equity compensation plans.
|
Item 6.
|
Selected
Financial Data.
|
Not
applicable.
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operation.
|
Special Note on Forward-Looking
Statements. Some of the statements contained in this annual report on
Form 10-K that are not historical facts are “forward-looking statements” which
can be identified by the use of terminology such as “estimates,” “projects,”
“plans,” “believes,” “expects,” “anticipates,” “intends,” or the negative or
other variations, or by discussions of strategy that involve risks and
uncertainties. We urge you to be cautious of the forward-looking statements,
that such statements, which are contained in this annual report Form 10-K,
reflect our current beliefs with respect to future events and involve known and
unknown risks, uncertainties and other factors affecting operations, market
growth, services, products and licenses. No assurances can be given regarding
the achievement of future results, as actual results may differ materially as a
result of the risks we face, and actual events may differ from the assumptions
underlying the statements that have been made regarding anticipated events.
Factors that may cause actual results, performance or achievements, or industry
results, to differ materially from those contemplated by such forward-looking
statements include without limitation:
20
|
|
·
|
Our
ability to attract and retain management, and to integrate and maintain
technical information and management information
systems;
|
|
|
·
|
Our
ability to raise capital when needed and on acceptable terms and
conditions;
|
|
|
·
|
The
intensity of competition; and
|
|
|
·
|
General
economic conditions.
|
All
written and oral forward-looking statements made in connection with this annual
report on Form 10-K that are attributable to us or persons acting on our behalf
are expressly qualified in their entirety by these cautionary statements. Given
the uncertainties that surround such statements, you are cautioned not to place
undue reliance on such forward-looking statements.
Information
regarding market and industry statistics contained in this report is included
based on information available to us that we believe is accurate. It is
generally based on academic and other publications that are not produced for
purposes of securities offerings or economic analysis. We have not reviewed or
included data from all sources, and we cannot assure you of the accuracy or
completeness of the data included in this report. Forecasts and other
forward-looking information obtained from these sources are subject to the same
qualifications and the additional uncertainties accompanying any estimates of
future market size, revenue and market acceptance of products and services. We
have no obligation to update forward-looking information to reflect actual
results or changes in assumptions or other factors that could affect those
statements. See “Risk Factors” for a more detailed discussion of uncertainties
and risks that may have an impact on future results.
Overview
Overall, the Company was disappointed
with its financial and operating performance in the year ended June 30,
2009.
On the positive side, total gross
revenue in the year ended June 30, 2009 was $53,351,946 compared to $51,847,992
in the year ended June 30, 2008, an increase of $1,503,954 or 2.9%.
Revenue in the Company’s premature
ejaculation (“PE”) treatment programs has increased by $5,766,406 or 24.0% to
$29,800,571 and revenue in the Company’s erectile dysfunction (“ED”) treatment
programs has decreased by $3,711,921 or 15.3% to $20,590,812 in the year ended
June 30, 2009, compared to the year ended June 30, 2008.
The Company also generated $955,353 in
revenue from its prostate programs in the year ended June 30, 2009, compared to
$3,566,629 in the year ended June 30, 2008, a decrease of 73.2%.
21
Revenue in our Australian operations
was $41,801,703 in the year ended June 30, 2009, compared to $50,572,311 during
the year ended June 30, 2008, a decrease of $8,770,608 or 17.3%. This
decrease was primarily attributable to an increase in the average program length
and the depreciation in the Australian dollar against the US dollar during the
12 months. Revenue in Australian dollars was A$58,370,389 in the year
ended June 30, 2009, compared to A$57,603,350 in the year ended June 30, 2008,
an increase of $767,039 or 1.3%.
Revenue in our UK operations was
$10,575,461 in the year ended June 30, 2009 compared to $Nil during the year
ended June 30, 2008. Our UK operation started its business activities
in September 2008.
As set out above, the Company’s
Australian revenue increased slightly during the year ended June 30, 2009, with
a substantial increase in revenue in its premature ejaculation market segment
and a substantial decrease in its erectile dysfunction and prostate market
segments. The decrease in the Company’s prostate market segment is
due to AMI Australia substantially decreasing its focus and advertising on that
market segment. At the same time, the Company achieved more than
$10.5 million in revenue in the United Kingdom from a standing
start.
Less positively, the Company incurred
substantial losses during the year ended June 30, 2009. Fortunately,
more than half of these losses were due to non-cash amortization and impairment
charges which have not affected the cash position of the Company. In
addition, a further significant portion of these losses are due to translation
of the Company’s results from Australian dollars to US
dollars. Notwithstanding these non-cash related impacts, management
was disappointed with the performance of the Company during 2009.
Cost of revenue increased to
$12,957,681 in the year ended June 30, 2009 compared to $11,597,159 in the year
ended June 30, 2008. This increase is primarily attributable to an
increase in the medication cost incurred by AMI Australia and the Company’s UK
operation as well as start up costs associated with the Company’s UK
operations. As a percentage of revenue, cost of revenue was 24.3% in
the year ended June 30, 2009 compared to 22.4% in the year ended June 30,
2008.
Gross profit was $40,394,265 in the
year ended June 30, 2009 compared to $40,250,833 in the year ended June 30,
2008. As a percentage of revenue, gross profit decreased to 75.7% in
the year ended June 30, 2009 from 77.6% in the year ended June 30,
2008. Once again, the 1.9% decrease in the gross profit percentage is
primarily attributable to the high percentage of cost of revenue in our UK
operation
Selling, general and administrative
expenses were $46,616,853 in the year ended June 30, 2009 compared to
$36,801,510 in the year ended June 30, 2008. As a percentage of
revenue, selling, general and administrative expenses increased to 87.4% in the
year ended June 30, 2009 from 71.0% in the year ended June 30,
2008. The increase in the year ended June 30, 2009 is primarily
attributable to the establishment of the Company’s UK operations as well as the
expenditure on ineffective advertising in Australia in January and February
2009.
Net income (loss) before income tax was
($12,105,376) in the year ended June 30, 2009 compared to $2,252,458 in the year
ended June 30, 2008. The decrease is primarily attributable to the
impairment on patents that were held by Worldwide PE Patent Holdco Pty Limited
amounting to $5,279,462 (a non-cash item), the increase in selling, general and
administrative expenses and the establishment of the UK operation.
Net income (loss) was ($9,344,989) in
the year ended June 30, 2009 compared to $994,779 in the year ended June 30,
2008. This decrease is mainly attributable to the impairment on the
patent and formulation rights owned by Worldwide PE Patent Holdco Pty Limited,
the increase in selling, general and administrative expenses and the
establishment of the UK operations.
22
The Company was disappointed with the
operating performance of its Australian operations and has taken steps to
address unnecessary expenditures. The Company believes that the
operating performance of its Australian operations since the end of the
financial year has substantially improved.
The Company expended substantial funds
during 2009 in endeavouring to establish its operations in the United
Kingdom. The Company is pleased to have generated substantial revenue
from its United Kingdom operations in its initial year of operations and is
hopeful that it will generate a profit from those operations during
2010.
As of June 30, 2009, the Company had
total liabilities of $29,668,233 and a positive net worth of
$18,110,615. As of June 30, 2008, the Company had total liabilities
of $21,887,563 and a positive net worth of $33,474,159.
As at June 30, 2009 our total current
assets were $26,670,960, our total current liabilities were $25,222,649 and our
net current assets were $1,448,311.
The Company’s aggregate cash balances
as at June 30, 2009 were $295,245, a substantial decrease on last
year. The core reason for this decrease is the substantial
expenditure incurred by the Company in establishing its UK
operations. The Company also substantially increased its third party
borrowings in order to undertake this expansion.
Economic conditions were difficult for
the Company over the past 12 months. This is partly due to the global
financial crisis but is also due to some ineffective advertising earlier this
year as well as the costs associated with establishing the Company’s UK
operations.
Management has taken steps to address
operating performance and the business has improved since July
2009. As a result of these changes in operating performance, we
forecast that we will be able to generate sufficient funds from our business in
order to fund our operations in the ordinary course during the next 12
months.
The Company intends to focus its
activities over the next 12 months on its profitable Australian operations as
well as its new United Kingdom operations.
The core factors for the Company’s
success over the next 12 months will be to ensure that the Company’s new United
Kingdom operations are successfully established as well as ensuring that its
core Australian operations continue to operate profitably.
Critical
Accounting Policies
The
accounting and reporting policies of the Company conform to accounting
principles generally accepted in the United States of America. The
following are descriptions of the more significant policies:
Basis of
Accounting
The
accompanying financial statements are prepared on an accrual
basis.
23
Principles of
Consolidation
The
consolidated financial statements include the accounts of the Company and its
wholly owned subsidiaries, AMI Australia Holdings Pty Limited, AMI Management
Services Pty Limited (“AMI MS”) and AMI International Pty Limited (“AMI
International”) and their direct and indirect wholly-owned subsidiaries:
Advanced Medical Institute Pty Ltd, PE Patent Holdco Pty Limited (“PE”),
Advanced Medical Institute (NZ) Limited (“AMI NZ”), Intelligent Medical
Technologies Pty Ltd (“IMT”), Ai Te Wei (Beijing) Medicine Consulting Company,
AMI Japan Kabushiki Gaisya (75% owned) and AMI Clinic Limited (“AMI
UK”). All significant intercompany accounts and transactions have
been eliminated upon consolidation.
Cash
Equivalents
For
purposes of the statement of cash flows, the Company considers all highly liquid
investments with maturities of three months or less to be cash
equivalents.
Use of
Estimates
The
preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect certain reported amounts and disclosures. Accordingly, actual
results could differ from those estimates.
Revenue
Recognition
Sales are
reported as deferred income when the sales contracts are executed and the term
of the contract exceeds three months. Up to three months of
medication is delivered to the patient upon the signing of a
contract. Generally the terms of the treatment contracts are up to
one year, but they can be for longer periods of time. The deferred
income arising from the contracts that exceed three months is then amortized, on
a straight line basis, into income during the approximated composite remaining
medication delivery period. This approximated composite is an
estimate that may vary from period to period.
Income
Tax
Income
taxes have been provided based upon the tax laws and rates in the countries in
which operations are conducted and income is earned. The income tax
rates imposed by the taxing authorities vary. Taxable income may vary
from pre-tax income for financial accounting purposes. There is no
expected relationship between the provision for income taxes and income before
income taxes because the countries have different taxation rules, which vary not
only to nominal rates but also in terms of available deductions, credits and
other benefits. Deferred tax assets and liabilities are recognized
for the anticipated future tax effects of temporary differences between the
financial statement basis and the tax basis of the Company’s assets and
liabilities using the applicable tax rates in effect at year end as prescribed
by SFAS 109 (ASC 740) “Accounting for Income
Taxes.”
Inventories
Inventories
are valued at the lower of cost (determined on a first-in first-out basis) or
market value. Management compares the cost of inventories with the
market value and allowance is made for writing down our inventories to market
value, if lower. As of June 30, 2009 and 2008 inventory mainly
consisted of raw materials.
24
Property and
Equipment
Equipment
placed in service is depreciated over the estimated useful lives of the assets
using the diminishing value depreciation method.
Property
and equipment are carried at the lesser of cost and written down
value. Expenditures for maintenance and repairs are expenses as
incurred and expenditures for major renewals and betterments are
capitalized. Assets retired or sold are removed from the property
accounts, with gains or losses on disposal included in income.
Exchange Gain
(Loss)
During
the year ended June 30, 2009 and 2008, the transactions of AMI Australia were
denominated in foreign currency and were recorded in Australian Dollars (AUD) at
the rates of exchange in effect when the transactions occur. Exchange gains and
losses are recognized for the different foreign exchange rates applied when the
foreign currency assets and liabilities are settled.
Foreign
Currency
As of
June 30, 2009, the accounts of AMI Australia and its subsidiaries were
maintained and its financial statements were expressed in the local currency for
the jurisdiction in which the entity operated. Such financial
statements were translated into the functional currency in Australian Dollars
(AUD) and thereafter to reporting currency in U.S. Dollars (USD) in accordance
with Statement of Financial Accounts Standards (“SFAS”) No. 52 (ASC 830),
“Foreign Currency Translation,” with the AUD as the functional currency.
According to the Statement, all assets and liabilities were translated at the
current exchange rate, stockholders’ equity (deficit) is translated at the
historical rates and income statement items are translated at the average
exchange rate for the period. The resulting translation adjustments are reported
under other comprehensive income in accordance with SFAS No. 130 (ASC 220),
“Reporting Comprehensive Income” as a component of shareholders’ equity
(deficit).
Research and Development
Costs
Research
and development costs are charged against income from ordinary activities before
income tax as incurred.
Intangible
assets
The
Company applies the criteria specified in SFAS No. 141 (ASC 805), “Business
Combinations” to determine whether an intangible asset should be recognized
separately from goodwill. Intangible assets acquired through business
acquisitions are recognized as assets separate from goodwill if they satisfy
either the “contractual-legal” or “separability” criterion. Per SFAS 142 (ASC
350), intangible assets with definite lives are amortized over their estimated
useful life and reviewed for impairment in accordance with SFAS No. 144
(ASC 360), “Accounting for the Impairment or Disposal of Long-lived Assets.”
Intangible assets, such as purchased technology, trademark, customer list, user
base and non-compete agreements, arising from the acquisitions of subsidiaries
and variable interest entities are recognized and measured at fair value upon
acquisition. Intangible assets are amortized over their estimated useful lives
from one to ten years. The Company reviews the amortization methods and
estimated useful lives of intangible assets at least annually or when events or
changes in circumstances indicate that it might be impaired. The recoverability
of an intangible asset to be held and used is evaluated by comparing the
carrying amount of the intangible asset to its future net undiscounted cash
flows. If the intangible asset is considered to be impaired, the impairment loss
is measured as the amount by which the carrying amount of the intangible asset
exceeds the fair value of the intangible asset, calculated using a discounted
future cash flow analysis. The Company uses estimates and judgments in its
impairment tests, and if different estimates or judgments had been utilized, the
timing or the amount of the impairment charges could be
different.
25
Goodwill,
trademarks, patents and other intangible assets determined to have indefinite
useful lives are not amortized. We test such trademarks and other
intangible assets with indefinite useful lives for impairment annually, or more
frequently if events or circumstances indicate that an asset might be
impaired. Goodwill, trademarks, patents and other intangible assets
determined to have definite lives are amortized over their estimated useful
lives or the life of the trademark, patent and other intangible asset, whichever
is less.
Long-Lived
Assets
Effective
January 1, 2002, the Company adopted Statement of Financial Accounting Standards
No. 144 (ASC 360), “Accounting for the Impairment or Disposal of Long-Lived
Assets” (“SFAS 144”), which addresses financial accounting and reporting for the
impairment or disposal of long-lived assets and supersedes SFAS No. 121,
“Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to
be Disposed Of,” and the accounting and reporting provisions of APB Opinion No.
30, “Reporting the Results of Operations for a Disposal of a Segment of a
Business.” The Company periodically evaluates the carrying value of long-lived
assets to be held and used in accordance with SFAS 144 (ASC 360). SFAS 144 (ASC
360) requires impairment losses to be recorded on long-lived assets used in
operations when indicators of impairment are present and the undiscounted cash
flows estimated to be generated by those assets are less than the assets’
carrying amounts. In that event, a loss is recognized based on the amount by
which the carrying amount exceeds the fair market value of the long-lived
assets. Loss on long-lived assets to be disposed of is determined in a similar
manner, except that fair market values are reduced for the cost of
disposal.
Segment
Reporting
Statement
of Financial Accounting Standards No. 131 (“SFAS 131”) (ASC 280), “Disclosure
About Segments of an Enterprise and Related Information” requires use of the
“management approach” model for segment reporting. The management approach model
is based on the way a company’s management organizes segments within the company
for making operating decisions and assessing performance. Reportable segments
are based on products and services, geography, legal structure, management
structure, or any other manner in which management disaggregates a company. SFAS
131 (ASC 280) has no effect on the Company’s consolidated financial statements
as the Company consists of one reportable business segment.
Basic and Diluted Earnings
Per Share
Earnings
per share is calculated in accordance with the Statement of financial accounting
standards No. 128 (SFAS No. 128) (ASC 260), “Earnings per share.” SFAS No. 128
superseded Accounting Principles Board Opinion No.15 (APB 15). Net loss per
share for all periods presented has been restated to reflect the adoption of
SFAS No. 128. Basic net loss per share is based upon the weighted average number
of common shares outstanding. Diluted net loss per share is based on the
assumption that all dilutive convertible shares and stock options were converted
or exercised. Dilution is computed by applying the treasury stock method. Under
this method, options and warrants are assumed to be exercised at the beginning
of the period (or at the time of issuance, if later), and as if funds obtained
thereby were used to purchase common stock at the average market price during
the period.
26
Currency
Conversion
As of
June 30, 2009 and 2008, the accounts of AMI Australia were maintained, and its
financial statements were expressed, in Australian Dollars
(AUD). Such financial statements were translated into US Dollars
(USD) in accordance with Statement of Financial Accounts Standards (“SFAS”) No.
52 (ASC 830), “Foreign Currency Translation,” with the AUD as the functional
currency. According to the Statement, all assets and liabilities were
translated at the exchange rate (AUD1 = USD0.80480) as of June 30, 2009,
stockholder’s equity are translated at the historical rates and income statement
items are translated at the weighted-average exchange rate for the
period. The resulting translation adjustments are reported under
other comprehensive income in accordance with SFAS No. 130 (ASC 220), “Reporting
Comprehensive Income.”
The
following table sets forth, for the periods indicated, certain operating
information expressed as a percentage of revenue:
Result of
operations:
|
Year
ended
June
30,
|
||||||||
|
2009
|
2008
|
|||||||
|
Revenue
|
100 | % | 100 | % | ||||
|
Cost
of Revenue
|
24.3 | % | 22.4 | % | ||||
|
Gross
Profit
|
75.7 | % | 77.6 | % | ||||
|
Selling
General and administrative expenses
|
87.4 | % | 71.0 | % | ||||
|
Impairment
loss
|
9.9 | % | - | |||||
|
Other
income and expenses
|
(0.4 | )% | (0.2 | )% | ||||
|
Discontinued
operation
|
(0.7 | )% | (2.1 | )% | ||||
|
Income
before income tax
|
(22.7 | )% | 4.3 | % | ||||
|
Income
tax expenses
|
(5.2 | )% | 2.4 | % | ||||
|
Net
Income
|
(17.5 | )% | 1.9 | % | ||||
Liquidity
and Capital Resources
As of
June 30, 2009, we had total liabilities of $29,668,233, including unearned
revenue of $8,003,468, and we had a positive net worth of
$18,110,615. As of June 30, 2008, we had total liabilities of
$21,887,563 including unearned revenue of $6,922,800 and a positive net worth of
$33,474,159. As at June 30, 2009 our total current assets were
$26,670,960, our total current liabilities were $25,222,649 and our net current
assets were $1,448,311.
27
Our
aggregate cash balances as at June 30, 2009 were $295,245. While
economic conditions have been difficult over the past 12 months, management has
taken steps to address operating performance, and the business has improved
since July 2009. As a result of these changes in operating
performance, we forecast that we will be able to generate sufficient funds from
our business in order to fund our operations in the ordinary course during the
next 12 months. In September 2008 management expanded our business
into the United Kingdom.
In the
event that we continue to expand our business, we may need to raise debt or
equity funding in order to undertake such expansion. However, there
can be no assurance that we can or will obtain sufficient funds from operations
or from additional financings on terms acceptable to us. If we are
unable to obtain sufficient additional financing, we may not be able to expand
our operations as considered or we could be required to reduce spending and
operations.
The
Company borrowed A$3 million from ANZ Nominees Limited in its capacity as
custodian of the Professional Pensions PST in September 2006. The
outstanding balance of this loan was fully repaid in December 2008 using funds
from the St George facility discussed below.
The
Company borrowed A$2.4 million from St George Bank Limited in December 2008
comprising a A$1.9 million term facility and a A$500,000 overdraft facility. The
loan is secured by a first ranking security interest in all of AMI Australia’s
assets and undertakings (including its existing equity interests in PE Patent
Holdco Pty Limited, Intelligent Medical Technologies Pty Limited and Advanced
Medical Institute (NZ) Limited). The term facility is a commercial bill
acceptance/discount facility whereby the Company regularly discounts bills with
the bank at the prevailing interest rate plus a margin of 1.65%. The average
all-up cost of this facility during the period was 5.12%. On each rollover the
interest rate is reset. The loan is due for repayment in
December 2011, however the Company is required to make principal repayments of
A$50,000 per month during each month of the term. As at June 30,
2009, the total outstanding under the facility was A$2,177,056.99.
The
Company borrowed a further A$1 million from Secare Health Centre Pty Limited
(“Secare”) in December 2008. Secare is controlled by the company’s
chief executive officer and founder, Jack Vaisman and the Company has
subsequently borrowed a further A$3,581,489 from Secare. The loan is secured by
a second ranking security interest in all of AMI Australia’s assets and
undertakings (including its existing equity interests in PE Patent Holdco Pty
Limited, Intelligent Medical Technologies Pty Limited and Advanced Medical
Institute (NZ) Limited). The loan attracts an interest cost of 10% per
annum. No repayment of this loan has been made up to June 30,
2009. The loan is due for repayment in December 2011.
The loans
referred to above were provided to repay the ANZ facility, to fund expansion
into the United Kingdom and to meet operational expenses.
YEAR
ENDED JUNE 30, 2009 COMPARED TO YEAR ENDED JUNE 30, 2008
REVENUE. Revenue was
$53,351,946 in the year ended June 30, 2009 compared to $51,847,992 in the year
ended June 30, 2008, an increase of $1,503,954 or 2.9%. The increase
in revenue in the twelve-month period is primarily attributable to (1) expansion
of the business into the United Kingdom; (2) increased advertising campaigns in
the premature ejaculation segment; (3) increased brand name recognition; and (4)
effectiveness of our products.
28
We
recognize all expenses on the date they are incurred regardless of whether they
relate to recognized or unearned revenue whereas unearned revenue (which is
generated by current expenses) is unable to be recognized in the current
period.
By
contrast, our unearned revenue increased by $1,080,668 to $8,003,468 for the
year ended June 30, 2009, compared to the year ended June 30,
2008. The increase in unearned revenue in the twelve-month period is
primarily attributed to the increase in weighted average program length of
treatment programs during the year ended June 20, 2009.
Revenue
in AMI Australia’s premature ejaculation (“PE”) treatment programs has increased
by $5,766,406 or 24.0% to $29,800,571, and revenue in AMI Australia’s erectile
dysfunction (“ED”) treatment programs has decreased by $3,711,921 or 15.3% to
$20,590,812 in the year ended June 30, 2009, compared to the year ended June 30,
2008. AMI Australia also generated $955,353 in revenue from its
prostate programs in the year ended June 30, 2009, compared to $3,566,629 in the
year ended June 30, 2008, a decrease of 73.2%. This decrease is due
to AMI Australia substantially decreasing its focus and advertising in this
market segment.
Revenue
in our Australian operations was $41,801,703 in the year ended June 30, 2009
compared to $50,572,311 during the year ended June 30, 2008, a decrease of
$8,770,608 or 17.3%. The decrease in revenue is primarily
attributable to the increase in average program length (higher unearned revenue)
and the depreciation in the Australian dollar against the US dollar from the
average rate of A$:US$ 1:0.89646 for the year ended June 30, 2008 to
A$:US$ 1:0.74803 for the year ended June 30, 2009. Revenue
including unearned revenue in Australian dollars was A$58,370,389 in the year
ended June 30, 2009, compared to A$57,603,350 in the year ended June 30, 2008,
an increase of $767,039 or 1.3%. We attribute this increase to the
following factors: (1) increased advertising campaigns in the premature
ejaculation segment; (2) increased brand name recognition; and (3) effectiveness
of our products.
Revenue
in our UK operations was $10,575,461 in the year ended June 30, 2009 compared to
$Nil during the year ended June 30, 2008. Our UK operation started
its business activities in September 2008.
COST OF
REVENUE. Cost of revenue
increased to $12,957,681 in the year ended June 30, 2009 compared to $11,597,159
in the year ended June 30, 2008. This increase is primarily
attributable to an increase in the medication cost incurred by AMI Australia and
AMI’s UK operation as well as start up costs associated with AMI’s UK
operations. As a percentage of revenue, cost of revenue was 24.3% in
the year ended June 30, 2009 compared to 22.4% in the year ended June 30,
2008. The increase in the cost of revenue percentage by 1.90% is
primarily attributable to the establishment of the UK operations. The Company’s
UK operations started its business activities in September 2008. The
initial stage of UK operation led to a high percentage of cost of revenue due to
the operation being a start up business.
GROSS
PROFIT. Gross profit was $40,394,265 in the year ended June 30, 2009
compared to $40,250,833 in the year ended June 30, 2008. As a
percentage of revenue, gross profit decreased to 75.7% in the year ended June
30, 2009 from 77.6% in the year ended June 30, 2008. The 1.9%
decrease in the gross profit percentage is primarily attributable to the high
percentage of cost of revenue in our UK operation
29
Gross
profit in our Australian operations was $32,142,990 in the year ended June 30,
2009 compared to $39,067,551 in the year ended June 30, 2008. This
decrease in primarily attributable to the increase in average program length
(higher unearned revenue) and the depreciation in the Australian dollar against
the US dollar from the average rate of A$:US$ 1:0.89646 for the year ended
June 30, 2008 to A$:US$ 1:0.74803 for the year ended June 30,
2009. Gross profit including unearned revenue in Australian dollars
was A$45,458,188 in the year ended June 30, 2009, compared to A$44,769,804 in
the year ended June 30, 2008, an increase of A$688,384 or 1.5%. This
increase is mainly attributable to an increase in revenue.
Gross
profit in our UK operations was $7,586,441 in the year ended June 30, 2009
compared to a gross profit of $Nil in the year ended June 30,
2008. Our UK operation started its business activities in September
2008.
SELLING, GENERAL
AND ADMINISTRATIVE EXPENSES. Selling, general and
administrative expenses were $46,616,853 in the year ended June 30, 2009
compared to $36,801,510 in the year ended June 30, 2008. As a
percentage of revenue, selling, general and administrative expenses increased to
87.4% in the year ended June 30, 2009 from 71.0% in the year ended June 30,
2008. The increase in the year ended June 30, 2009 is primarily
attributable to the establishment of UK operations as well as the expenditure on
ineffective advertising in Australia in January and February 2009, which
expenditure has now been ceased. The Company’s UK operations started its
business activities in September 2008. The initial stage of UK
operations led to a high percentage of selling, general and administrative
expenses compared to revenue due to the operation being a start up
business.
OTHER INCOME AND
EXPENSES. Other income and
expenses were $(197,219) in the year ended June 30, 2009 compared to $(113,391)
in the year ended June 30, 2008. As a percentage of revenues other
income and expenses increased to (0.4)% in the year ended June 30, 2009 from
(0.2)% in the year ended June 30, 2008. The percentage change is not
material.
NET (LOSS) INCOME
BEFORE INCOME TAX AND INCOME TAX EXPENSES. Net income before
income tax was ($12,105,376) in the year ended June 30, 2009 compared to
$2,252,458 in the year ended June 30, 2008. The decrease is primarily
attributable to the impairment on patents that were held by Worldwide PE Patent
Holdco Pty Limited amounting to $5,279,462, the increase in selling, general and
administrative expenses and the establishment of the UK operation.
Net
(loss) income before income tax in our Australian operations was ($9,101,273) in
the year ended June 30, 2009 compared to $4,312,262 in the year ended June 30,
2008. This decrease is mainly attributable to an impairment on the
patent and formulation rights owned by Worldwide PE Patent Holdco amounting to
$5,279,462, and an increase in advertising expenses in Australia.
Net loss
before income tax in our UK operations was ($2,017,595) in the year ended June
30, 2009 compared to a net loss $Nil in the year ended June 30,
2008. Our UK operation started its business activities in September
2008.
30
Income
tax expenses (benefit) were ($2,760,387) in the year ended June 30, 2009
compared to $1,257,679 in the year ended June 30, 2008. This decrease
is attributable to the decreasing profitability of the Company’s business. As a
percentage of gross income, income tax expense decreased from 2.4% to (5.2)%
which is attributable to the increase in selling, general and administrative
expenses and the establishment of the UK operations.
NET INCOME
(LOSS). Net income was
($9,344,989) in the year ended June 30, 2009 compared to $994,779 in the year
ended June 30, 2008. This decrease is mainly attributable to the
impairment on the patent and formulation rights owned by Worldwide PE Patent
Holdco Pty Limited, the increase in selling, general and administrative expenses
and the establishment of the UK operations.
Net
(loss) income in our Australian operations was ($6,340,886) in the year ended
June 30, 2009 compared to $3,054,583 in the year ended June 30,
2008. This decrease is mainly attributable to the impairment on the
patent and formulation rights owned by Worldwide PE Patent Holdco and the
increase in selling, general and administrative expenses.
Net loss
in our UK operations was ($2,017,595) in the year ended June 30, 2009 compared
to $Nil in the year ended June 30, 2008. Our UK operation started its
business activities in September 2008.
|
Item 7A.
|
Quantitative
and Qualitative Disclosures About Market
Risk.
|
Not
applicable.
|
Item
8.
|
Financial
Statements and Supplementary Data.
|
Our consolidated financial statements
and the notes thereto begin on page F-1 of this Annual Report.
|
Item
9.
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure.
|
Not
applicable.
31
|
Item
9A(T).
|
Controls
and Procedures.
|
(a) Evaluation of Disclosure Controls and
Procedures
Under the supervision and with the participation of our management, including
our Chief Executive Officer and Chief Financial Officer, we conducted an
evaluation of our disclosure controls and procedures, as such term is defined in
Exchange Act Rules 13a-15(e) and 15d-15(e), as of the end of the period covered
by this Annual Report (“Evaluation Date”). Based on such
evaluation, our Chief Executive Officer and Chief Financial Officer have
concluded that, as of the Evaluation Date, our disclosure controls and
procedures are effective to ensure that information required to be disclosed
by us in our Exchange Act reports is recorded, processed, summarized, and
reported within the time periods specified in the SEC’s rules and forms, and
that such information is accumulated and communicated to our management,
including our Chief Executive Officer and Chief Financial Officer, as
appropriate, to allow timely decisions regarding required disclosure.
(b) Managements Report on Internal Control Over
Financial Reporting
The management of the Company is responsible for establishing and maintaining
adequate internal control over financial reporting, as such term is defined in
Exchange Act Rules 13a-15(f) and 15d-15(f). The Company’s internal control over
financial reporting is a process designed under the supervision of the Company’s
Chief Executive Officer and Chief Financial Officer, and effected by the Board
of Directors, management and other personnel, to provide reasonable assurance
regarding the reliability of financial reporting and the preparation of the
Company’s financial statements for external purposes in accordance with U.S.
generally accepted accounting principles (GAAP).
As of June 30, 2009, management assessed the effectiveness of the Company’s
internal control over financial reporting based on the criteria for effective
internal control over financial reporting established in Internal
Control--Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission (“COSO”) and SEC guidance on conducting
such assessments. Based on that evaluation, they concluded that, during the
period covered by this report, such internal controls and procedures were not
effective to detect the inappropriate application of US GAAP rules as more fully
described below. This was due to deficiencies that existed in the design or
operation of our internal control over financial reporting that adversely
affected our internal controls and that may be considered to be material
weaknesses.
The matters involving controls over financial reporting that the Company’s
management considered to be material weaknesses under the standards of the
Public Company Accounting Oversight Board were : (1) lack of a functioning audit
committee and lack of majority of outside directors on the Company’s board of
directors, resulting in ineffective oversight in the establishment and
monitoring of required internal controls and procedures, and, (2) inadequate
segregation of duties consistent with control objectives. The aforementioned
material weaknesses were identified by the Company’s Chief Financial Officer in
connection with the audit of our financial statements as of June 30, 2009 and
communicated to our management.
Management believes that the material weaknesses set forth in items (1) and (2)
above did not have an effect on the Company’s financial results. However,
management believes that although its disclosure controls and procedures are
effective, the lack of a functioning audit committee and lack of a majority of
outside directors on the Company’s board of directors could result in
ineffective oversight in the establishment and monitoring of required internal
controls and procedures. Management’s goals are to have a functional audit
committee and a majority of outside directors on the Company’s board of
directors when funds are available.
This Annual Report does not include an attestation report of the Company’s
registered public accounting firm regarding internal control over financial
reporting. Management’s report was not subject to attestation by the Company’s
registered public accounting firm pursuant to temporary rules of the Securities
and Exchange Commission that permit the Company to provide only management’s
report in this Annual Report.
32
(c)
Changes in Internal Controls.
No change in
our internal control over financial reporting (as defined in Rules 13a-15 or
15d-15 under the 1934 Act) occurred during the quarter ended June 30, 2009, that
has materially affected, or is reasonably likely to materially affect, our
internal control over financial reporting.
|
Item 9B.
|
Other
Information.
|
None.
PART
III
|
Item
10.
|
Directors,
Executive Officers and Corporate
Governance.
|
33
Set forth below are the names of the
directors, executive officers and key employees of the Company as of November
13, 2009.
|
Name
|
Age
|
Position
|
||
|
Jacov
(Jack) Vaisman
|
62
|
Chief
Executive Officer, President and Director
|
||
|
Dilip
Shrestha
|
31
|
Chief
Financial Officer
|
||
|
Forhad
(Tony) Khan
|
46
|
Executive
Vice President, Secretary and Director
|
||
|
Anatoly
Fanshil
|
57
|
Director
|
||
|
Spiro
Baramilis
|
|
51
|
|
Director
|
Executive officers of the Company are
appointed at the discretion of the Board of Directors with no fixed term. There
are no family relationships between or among any of the executive officers or
directors of the Company.
There are no agreements or
understandings for any officer or director of the Company to resign at the
request of another person and none of the officers or directors is acting on
behalf of or will act at the direction of any other person.
DR. JACOV (JACK) VAISMAN, CHIEF
EXECUTIVE OFFICER, PRESIDENT AND DIRECTOR – Dr. Vaisman has served as the
Company’s Chief Executive Officer, President and as a Director since March 21,
2005. Dr. Vaisman is a pioneer in the sexual dysfunction business in
Australia. Since 2001, he has served as the managing director and
chief executive officer of AMI Australia. He is responsible for the
overall management and strategic direction of AMI Australia's impotency
operations with the support of AMI Australia's chief operations officer, Tony
Khan, and AMI's chief financial officer, Dilip Shrestha. Dr. Vaisman is also
currently a consultant to and shareholder in Yayasan On Clinic, which operates a
similar business in Indonesia. From 1993 through 2001, Dr Vaisman was the
founder and director of On Clinic International in Australia, a predecessor of
AMI Australia. The holder of a Bachelor of Medicine, a Master of
Gynecology and a PhD in Medical Science, Dr Vaisman has more than 35 years of
experience and expertise in the field of sexual health care provision and was
recently granted an innovation patent in the field of premature ejaculation
treatment by the Australian patent office.
DILIP SHRESTHA, CHIEF FINANCIAL OFFICER
– Mr. Shrestha was appointed as the Company’s Chief Financial Officer on April
7, 2005. Mr. Shrestha has also served as the chief financial officer of AMI
Australia since July 1, 2005. From 2001 to July 1, 2005, Mr. Shrestha
was the Financial Controller of AMI Australia. Prior to that he was
an Assistant Accountant with Australian Momentum Health Pty Ltd, one of two
medical establishments leading in providing impotency treatment in Australia and
a predecessor company to AMI Australia, a position he held from 1999 until
2001. Mr. Shrestha holds a Bachelor of Business Accounting, Master of
E-Commerce and a Graduate Diploma of Information System and E-Commerce, and is a
fully qualified certified practicing accountant in Australia.
FORHAD (TONY) KHAN, EXECUTIVE VICE
PRESIDENT, SECRETARY AND DIRECTOR- – Mr. Khan has served as the Company’s
Executive Vice President since July 30, 2005, he has been the Company’s
Secretary and a Director since March 21, 2005. Mr. Khan has also
served as chief operating officer of AMI Australia since July 1,
2005. From 2001 to July 1, 2005, Mr. Khan was the General Manager of
AMI Australia. Prior to joining AMI Australia, Mr. Khan was the
Director and sole operator of Australian Momentum Health (“AMH”), one of two
medical establishments leading in providing impotency treatment in
Australia. Mr. Khan was Sales Manager and then Operations Manager for
On Clinic International prior to establishing AMH. Mr. Khan holds a
Masters degree in Commerce and Accounting and has been involved in the industry
for over 10 years.
34
ANATOLY FANSHIL, DIRECTOR - From 1994
through the present, Mr. Fanshil has held the position of director of Australian
Overseas Shipping Services Pty Limited. From 1991 to 1994, Mr.
Fanshil was an engineering service manager for Australian Ocean
Lines. From 1972 through 1991, Mr. Fanshil was employed by the Black
Sea Shipping Company as an electrical engineer and also had oversight over the
entire company for the repairs of various ships anchored in the seaports of
Finland, Greece, Italy and Germany. Mr. Fanshil holds a degree in
electrical engineering.
SPIRO BARAMILIS, DIRECTOR - From 1992
through 2004, Mr. Baramilis has been the owner and Managing Director of
Australian International Marine Services Pty Limited, a company which provides
supplies to marine vessels. From 1981 through 1992, Mr. Baramilis was
employed by Nautilus Trading PTY LTD (and its predecessor company, Baramilis
Marine PTY LTD), a company that supplies vessels with provisions and technical
supplies. Mr. Baramilis attended Sydney Technical College and was
educated in the field of mechanical engineering.
Section 16(a) Beneficial Ownership
Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), requires the executive officers and directors of the Company and every
person who is directly or indirectly the beneficial owner of more than 10% of
any class of security of the Company to file reports of ownership and changes in
ownership with the Securities and Exchange Commission. Such persons also
are required to furnish our company with copies of all Section 16(a) forms they
file. Based solely on our review of copies of such forms received by us,
we believe that during the fiscal year ended June 30, 2009, all of the executive
officers and directors of the Company and every person who is directly or
indirectly the beneficial owner of more than 10% of any class of security of the
Company complied with the filing requirements of Section 16(a) of the Exchange
Act, except Anatoly Fanshil who did not timely file four Form 4s disclosing
seven reportable transactions.
Code of Ethics
We have adopted a Code of Business Conduct and Ethics that applies to our
directors, officers and employees, including our Chief Executive Officer and
Chief Financial Officer. The Code was filed as an exhibit to our Annual Report
on Form 10-KSB for the year ended June 30, 2005. A written copy of the Code will
be provided upon request at no charge by writing to our Chief Financial Officer,
Level 1, 204-218 Botany Road, Alexandria, NSW, Australia and a copy is also
available for free on our Internet website (http://www.avmd.com.au).
Corporate Governance
The Board of Directors has an Audit Committee, Compensation Committee and a
Nominating and Corporate Governance Committee.
There have been no changes to the procedures by which the stockholders of the
Company may recommend nominees to the Board of Directors since the filing of the
Company’s Definitive Proxy Statement on July 30, 2008 for its Annual Meeting of
Stockholders, which was held on August 19, 2008.
Audit Committee
The Board of Directors has adopted an Audit Committee Charter. The Board of
Directors had appointed each of Anatoly Fanshil and Spiro Baramilis to the Audit
Committee. Spiro Baramilis meets the independence requirements and standards
currently established by the SEC. An internal review by the Board of Directors
has determined that Anatoly Fanshil does not meet the independence requirements
established by the SEC, however he currently serves as a member of the Audit
Committee because the Board of Directors has deemed it in the best interest of
the Company. In addition, the Board of Directors has not yet designated a member
to serve on the Audit Committee as an “audit committee financial expert” within
the meaning of the rules and regulations of the SEC because they have not found
a qualified independent individual who meets the requirements for the position.
The Company is currently in the process of meeting with independent persons who
have the qualifications to serve as a director and on the Audit Committee as the
“audit committee financial expert” for the Audit Committee.
The Audit Committee assists the Board by overseeing the performance of the
independent auditors and the quality and integrity of our internal accounting,
auditing and financial reporting practices. The Audit Committee is responsible
for retaining (subject to stockholder ratification) and, as necessary,
terminating, the independent auditors, annually reviews the qualifications,
performance and independence of the independent auditors and the audit plan,
fees and audit results, and pre-approves audit and non-audit services to be
performed by the auditors and related fees.
35
Compensation Committee
At a board of directors meeting on April 30, 2005, the Board of Directors
approved a Compensation Committee and adopted a Compensation Committee Charter.
At the meeting no director was elected to serve on the Compensation Committee
and as a result all compensation decisions are currently made by unanimous
consent of all independent directors of the Company.
The Compensation Committee’s charter states that it is the responsibility of the
Compensation Committee to make recommendations to the Board of Directors with
respect to all forms of compensation paid to our executive officers and to such
other officers as directed by the Board and any other compensation matters as
from time to time directed by the Board.
Nominating and Corporate Governance
Committee
At a board of directors meeting on April 30, 2005, the Board of Directors
approved a Nominating and Corporate Governance Committee, adopted a Nominating
and Corporate Governance Committee Charter. At the meeting no director was
elected to serve on the Nominating Committee and as a result all director
nominees are currently made by unanimous consent of all independent directors of
the Company.
The Board of Directors accepts director nominations made by stockholders. The
Board of Directors may consider those factors it deems appropriate in evaluating
director nominees, including judgment, skill, diversity, strength of character,
experience with businesses and organizations comparable in size or scope to the
Company, experience and skill relative to other Board members, and specialized
knowledge or experience. Depending upon the current needs of the Board, certain
factors may be weighed more or less heavily. In considering candidates for the
Board, they evaluate the entirety of each candidate’s credentials and do not
have any specific minimum qualifications that must be met by a nominee. They
will consider candidates from any reasonable source, including current Board
members, stockholders, professional search firms or other persons. They will not
evaluate candidates differently based on who has made the recommendation.
|
Item
11.
|
Executive
Compensation.
|
The
following table sets forth the compensation paid or accrued by us to our Chief
Executive Officer and President and Chief Operating Officer and each of our
other officers whose compensation exceeded $100,000 for each of the Company’s
last three completed fiscal years.
Summary
Compensation Table
|
Name
&
Principal
Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive
Plan
Compensation
($)
|
Change
in
Pension
Value
and
Non-
Qualified
Deferred
Compensation
Earnings
($)
|
All
Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||||||
|
Jack
Vaisman, Chief Executive Officer
|
2009
|
$ | 287,915 | N/A | N/A- | N/A | N/A— | N/A | $ | 126,949 | $ | 414,864 | ||||||||||||||||||||||
|
2008
|
$ | 361,853 | N/A | N/A- | N/A | N/A— | N/A | $ | 129,268 | $ | 491,121 | |||||||||||||||||||||||
|
2007
|
$ | 226,966 | N/A | N/A | N/A | N/A | N/A | 95,615 | $ | 322,581 | ||||||||||||||||||||||||
|
Dilip
Shrestha, Chief Financial Officer
|
2009
|
$ | 189,884 | N/A | N/A | N/A | N/A | N/A | 38,335 | $ | 228,219 | |||||||||||||||||||||||
|
2008
|
$ | 210,629 | N/A | N/A | N/A | N/A | N/A | 50,460 | $ | 261,089 | ||||||||||||||||||||||||
|
2007
|
$ | 126,054 | N/A | N/A | N/A | N/A | N/A | 9,862 | $ | 135,916 | ||||||||||||||||||||||||
|
Tony
Khan, Executive Vice President
|
2009
|
$ | 189,884 | N/A | N/A | N/A | N/A | N/A | 33,099 | $ | 222,983 | |||||||||||||||||||||||
|
2008
|
$ | 240,221 | N/A | N/A | N/A | N/A | N/A | 34,693 | $ | 274,914 | ||||||||||||||||||||||||
|
2007
|
$ | 160,737 | N/A | N/A | N/A | N/A | N/A | 9,971 | $ | 170,708 | ||||||||||||||||||||||||
Outstanding
Equity Awards at Fiscal Year-End
There
were no grants of options to purchase our common stock to the named executive
officers at June 30, 2009.
36
Director
Compensation
None of
the directors have received compensation for their respective services rendered
to the Company for the year ended June 30, 2009.
Employee
Benefit Plans
The
Company has no employee benefit plans.
Employee
contracts and termination of Employment and Change-in-Control
As of November 13, 2009, none of our
officers and/or directors received any compensation for their respective
services rendered to the Company, nor have they received such compensation in
the past. However, compensation to officers is paid pursuant to
employment contracts with our wholly-owned subsidiary, AMI Australia Holdings
Pty Limited (“AMI Australia”). We have not adopted any retirement, pension,
profit sharing, stock option or insurance programs or other similar programs for
the benefit of our directors, officers and/or employees. None of our
executive officers or directors owned any securities exercisable for or
convertible into our Common Stock as of November 13, 2009.
On August 29, 2005, AMI Australia
executed an employment agreement with Dr. Jacov (Jack) Vaisman, the Company’s
Chief Executive Officer and President and AMI Australia’s Chief Executive
Officer. The employment agreement is effective as of July 1, 2005 and
continues in perpetuity unless earlier terminated in accordance with the terms
of the employment agreement. The Board of Directors of the Company
approved the employment agreement by unanimous written consent on August 29,
2005. Pursuant to his employment agreement, Dr. Vaisman is entitled to receive
an annual base salary of AU$350,000, approximately US$340,480, subject to
increases as shall be determined by the Company’s board of directors and
management of AMI Australia. In addition, the annual salary of Dr.
Vaisman shall be supplemented by a bonus based on the Company achieving certain
profit targets during prescribed periods. In addition to these base
and bonus salary components, AMI Australia will also contribute retirement
benefits (superannuation) for Dr. Vaisman in accordance with Australian legal
requirements.
On June 21, 2005, AMI Australia
executed employment agreements with Forhad (Tony) Khan, the Company’s Executive
Vice President and Secretary and AMI Australia’s Chief Operating Officer, and
Dilip Shrestha, the Company’s and AMI Australia’s Chief Financial
Officer. Each of these employment agreements is effective as of July
1, 2005 and continues in perpetuity unless earlier terminated in accordance with
the terms of the respective employment agreements. The Board of
Directors of the Company approved the employment agreements by unanimous written
consent on July 30, 2005.
Pursuant to his employment agreement,
Mr. Khan is entitled to receive an annual base salary of AU$250,000,
approximately US$233,472, subject to increases as shall be determined by the
Company’s board of directors and management of AMI Australia. In
addition, the annual salary of Mr. Khan shall be supplemented by a bonus based
on the Company achieving certain profit targets during prescribed periods. In
addition to these base and bonus salary components, AMI Australia will also
contribute retirement benefits (superannuation) for Mr. Khan in accordance with
Australian legal requirements.
Pursuant to his employment agreement,
Mr. Shrestha is entitled to receive an annual base salary of AU$250,000,
approximately US$233,472, subject to increases as shall be determined by the
Company’s board of directors and management of AMI Australia. In
addition, the annual salary of Mr. Shrestha shall be supplemented by a bonus
based on the Company achieving certain profit targets during prescribed
periods. In addition to these base and bonus salary components, AMI
Australia will also contribute retirement benefits (superannuation) for Mr.
Shrestha in accordance with Australian legal requirements.
There were no agreements in place for
payments to any officers in the event of a change-in-control of the
Company.
Compensation Committee Interlocks and
Insider Participation
Spiro Baramilis, as an independent director of the Company, performed the
functions of the Company’s Compensation Committee during the fiscal year ended
June 30, 2009. No member of the Compensation Committee has a relationship
that would constitute an interlocking relationship with executive officers or
directors of the Company or another entity.
Compensation Committee Report
(1)
The members of the Board have reviewed and discussed the discussion and analysis
of the Company’s compensation which appears above with management, and, based on
such review and discussion, the members of the Board recommended to the
Company’s Board of Directors that the above disclosure be included in this
Annual Report on Form 10-K.
Jacov (Jack) Vaisman
Dilip Shrestha
Forhad (Tony) Khan
Anatoly Fanshil
Spiro Baramilis
(1) The material in the above Compensation
Committee reports is not soliciting material, is not deemed filed with the SEC
and is not incorporated by reference in any filing of the Company under the
Securities Act of 1933, or the Securities Exchange Act of 1934, whether made
before or after the date of this Form 10-K and irrespective of any general
incorporation language in such filing.
37
|
Item 12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters.
|
The following table sets forth as of
November 13, 2009 the number of shares of our Common Stock beneficially owned by
(i) each person who is known by us to be the beneficial owner of more than five
percent of the Company’s Common Stock; (ii) each director and nominee for
election to the Board of Directors; (iii) each of the named executive officers
in the Summary Compensation Table; and (iv) all directors and executive officers
as a group. Unless otherwise indicated, the stockholders listed in the table
have sole voting and investment power with respect to the shares
indicated.
|
Name
and Address of Beneficial Owner
|
Shares
Beneficially Owned (1)
|
Percent
of Class
|
||||||
|
Jacov
(Jack) Vaisman
Unit
131
18-34
Waverley Street
Bondi
Junction NSW 2022 Australia
|
10,250,000 | 19.16 | % | |||||
|
Forhad
(Tony) Khan
Suite
4, Level 1
26/3
Wolesly Grove
Zetland
NSW 2017 Australia
|
250,000 | * | ||||||
|
Dilip
Shrestha
18/30
Mary Street
Lidcambe
NSW 2141 Australia
|
0 | * | ||||||
|
Anatoly
Fanshil
22/2
Ocean Street
Bondi
Junction NSW 2026 Australia
|
517,000 | * | ||||||
|
Spiro
Baramilis
12
Merton Street
Kogarah
Bay NSW 2217 Australia
|
0 | * | ||||||
|
Geissler
Holdings Limited
1
Raffles Place #21-01 OUB Centre
Singapore
048616
|
8,850,000 | (2) | 16.54 | % | ||||
|
RRD
Investments Pty Limited
65
Prospect Road
Summer
Hills, NSW 2130
Australia
|
3,400,000 | (3) | 6.35 | % | ||||
|
All
Directors and Officers (5 people)
|
11,017,000 | 20.59 | % | |||||
_______
* Less
than one percent.
(1) Beneficial
ownership is determined in accordance with the rules of the Securities and
Exchange Commission and generally includes voting or investment power with
respect to the shares shown. Except as indicated by footnote and subject to
community property laws where applicable, to our knowledge, the stockholders
named in the table have sole voting and investment power with respect to all
common stock shares shown as beneficially owned by them. A person is
deemed to be the beneficial owner of securities that can be acquired by such
person within 60 days upon the exercise of options, warrants or convertible
securities (in any case, the “Currently Exercisable Options”). Each beneficial
owner’s percentage ownership is determined by assuming that the Currently
Exercisable Options that are held by such person (but not those held by any
other person) have been exercised and converted. The persons named in the table
have sole voting and investment power with respect to all shares of common
stock.
(2) Information
obtained from Schedule 13D filed with the SEC on September 14,
2006.
(3) Information
obtained from Schedule 13D filed with the SEC on September 14,
2006.
38
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence.
|
The
Company borrowed A$1.0 million from Secare Health Centre Pty Limited (“Secare”)
in November 2008. Secare is controlled by the company’s chief
executive officer and founder, Jack Vaisman. The Company subsequently borrowed a
further A$3,881,489 from Secare. The loan is secured by a second ranking
security interest in all of AMI Australia’s assets and undertakings (including
its existing equity interests in PE Patent Holdco Pty Limited, Intelligent
Medical Technologies Pty Limited and Advanced Medical Institute (NZ) Limited).
The loan attracts an interest cost of 10% per annum. No repayment of
this loan has been made up to June 30, 2009. The loan is due for
repayment in December 2011.
There were no other related party
transactions exceeding $120,000 during the year ended June 30, 2008 and
2009.
Spiro Baramilis meets
the independence requirements and standards currently established by the
SEC.
|
Item
14.
|
Principal
Accounting Fees and Services.
|
The firm of Kabani & Company, Inc.
has served as our independent auditors since March 29, 2006 when it replaced
Lichter, Yu & Associates. The Board of Directors has appointed
Kabani & Company, Inc. to continue as our independent auditors for the
fiscal year ending June 30, 2010.
AUDIT
FEES FOR FISCAL YEAR ENDED JUNE 30, 2009
Audit
fees consist of fees billed for professional services rendered for the audit of
our financial statements and the review or audit of the interim
statements. The total fees billed for Kabani & Company, Inc. for
the fiscal year ended June 30, 2009 was $69,500 and applicable for the fiscal
year ended June 30, 2008 was $46,950.
AUDIT
RELATED FEES FOR FISCAL YEAR ENDED JUNE 30, 2009
Audit
fees consist of fees billed for professional services rendered for the audit of
our financial statements and the review or audit of the interim
statements. The total fees billed for Kabani & Company, Inc.
during the fiscal year ended June 30, 2009 was $0.
TAX
FEES FOR FISCAL YEAR ENDED JUNE 30, 2009
There were no tax fees billed by Kabani
& Company, Inc. for the fiscal year ended June 30, 2009.
ALL
OTHER FEES FOR FISCAL YEAR ENDED JUNE 30, 2009
There were no other fees billed by
Kabani & Company, Inc. for the fiscal year ended June 30, 2009.
39
PART
IV
|
Item
15.
|
Exhibits
Financial Statement Schedules.
|
(a) The
following are filed with this report:
(1) The
financial statements listed on the Financial Statement’s Table of
Contents
(2) Not
applicable
(3) The
exhibits referred to below, which include the following managerial contracts or
compensatory plans or arrangements:
|
|
·
|
Employment
Agreement between AMI Australia and Forhad (Tony) Khan dated July 30,
2005
|
|
|
·
|
Employment
Agreement between AMI Australia and Dilip Shrestha dated July 30,
2005
|
|
|
·
|
Employment
Agreement between AMI Australia and Jacov (Jack) Vaisman dated July 30,
2005
|
(b) The
exhibits listed on the Exhibit Index are filed as part of this
report.
(c) Not
applicable.
40
SIGNATURES
Pursuant
to the requirements of Section 12 of the Securities Exchange Act of 1934, the
Company has duly caused this Form 10-K to be signed on its behalf by the
undersigned, thereunto duly authorized.
|
ADVANCED
MEDICAL INSTITUTE INC.
|
||
|
Date: November
13, 2009
|
By:
|
/s/ Jacov (Jack) Vaisman
|
|
Jacov
(Jack) Vaisman
|
||
|
Chief
Executive Officer and President
|
||
|
(Principal
Executive Officer)
|
||
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons on behalf of the registrant and in the
capacities and on the dates indicated.
|
Date:
November 13, 2009
|
By:
|
/s/ Jacov (Jack) Vaisman
|
|
Jacov
(Jack) Vaisman
|
||
|
Chief
Executive Officer and President
|
||
|
(Principal
Executive Officer)
|
||
|
Date:
November 13, 2009
|
By:
|
/s/ Dilip Shrestha
|
|
Dilip
Shrestha
|
||
|
Chief
Financial Officer
|
||
|
(Principal
Financial Officer and Principal Accounting Officer)
|
||
|
Date:
November 13, 2009
|
By:
|
/s/ Forhad (Tony) Kahn
|
|
Forhad
(Tony) Kahn
|
||
|
Executive
Vice President and Secretary
|
||
|
Date:
November 13, 2009
|
By:
|
/s/ Anatoly Fanshil
|
|
Anatoly
Fanshil
|
||
|
Director
|
||
|
Date:
November 13, 2009
|
By:
|
/s/ Spiro Baramilis
|
|
Spiro
Baramilis
|
||
|
Director
|
41
EXHIBIT
INDEX
|
Exhibit
Number
|
Description
|
|
|
2.1
|
Share
Exchange Agreement, dated January 28, 2005 (incorporated herewith by
reference to Exhibit 2.1 to Advanced Medical Institute Inc.’s Current
Report on Form 8-K dated March 21, 2005 and filed with the Securities and
Exchange Commission on March 28, 2005).
|
|
|
2.2
|
Share
Exchange Agreement, dated November 16, 2005 (incorporated herewith by
reference to Exhibit 2.2 to Advanced Medical Institute Inc.’s Current
Report on Form 8-K dated November 16, 2005 and filed with the Securities
and Exchange Commission on November 17, 2005).
|
|
|
2.3
|
Share
Exchange Agreement, dated April 18, 2006 (incorporated herewith by
reference to Exhibit 2.2 to Advanced Medical Institute Inc.’s Current
Report on Form 8-K dated April 18, 2006 and filed with the Securities and
Exchange Commission on April 19, 2006).
|
|
|
3.1
|
Certificate
of Incorporation of Advanced Medical Institute Inc. (incorporated herewith
by reference to Exhibit 3.1 to Advanced Medical Institute Inc.’s
Registration Statement on Form 10-SB filed with the Securities and
Exchange Commission on February 16, 2000).
|
|
|
3.2
|
By-laws
of Advanced Medical Institute Inc. (incorporated herewith by reference to
Exhibit 3.2 to Advanced Medical Institute Inc.’s Registration Statement on
Form 10-SB filed with the Securities and Exchange Commission on February
16, 2000).
|
|
|
10.1
|
Subscription
Agreement, dated June 29, 2005 (incorporated herewith by
reference to Exhibit 10.1 to Advanced Medical Institute Inc.’s Current
Report on Form 8-K dated June 29, 2005 and filed with the Securities and
Exchange Commission on June 30, 2005).
|
|
|
10.2
|
Employment
Agreement between AMI Australia and Forhad (Tony) Khan (incorporated
herewith by reference to Exhibit 10.2 to Advanced Medical Institute Inc.’s
Current Report on Form 8-K dated July 30, 2005 and filed with the
Securities and Exchange Commission on August 1, 2005).
|
|
|
10.3
|
Employment
Agreement between AMI Australia and Dilip Shrestha (incorporated herewith
by reference to Exhibit 10.3 to Advanced Medical Institute Inc.’s Current
Report on Form 8-K dated July 30, 2005 and filed with the Securities and
Exchange Commission on August 1,
2005).
|
42
|
10.4
|
Employment
Agreement between AMI Australia and Jacov (Jack) Vaisman (incorporated
herewith by reference to Exhibit 10.4 to Advanced Medical Institute Inc.’s
Current Report on Form 8-K dated August 29, 2005 and filed with the
Securities and Exchange Commission on August 30, 2005).
|
|
|
10.5
|
Consultant
Agreement between AMI Australia and Doyle Corporate Pty Limited
(incorporated herewith by reference to Exhibit 10.1 to Advanced Medical
Institute Inc.’s Current Report on Form 8-K dated April 24, 2006 and filed
with the Securities and Exchange Commission on April 24,
2006).
|
|
|
10.6
|
Share
Exchange Agreement dated as of September 8, 2006 (incorporated herewith by
reference to Exhibit 10.1 to Advanced Medical Institute Inc.’s Current
Report on Form 8-K dated September 8, 2006 and filed with the Securities
and Exchange Commission on September 11, 2006).
|
|
|
10.7
|
Loan
Agreement between AMI Australia and ANZ Nominees Limited as Custodian for
the Professional Pension PST – Super dated September 8, 2006 (incorporated
herewith by reference to Exhibit 10.2 to Advanced Medical Institute Inc.’s
Current Report on Form 8-K dated September 8, 2006 and filed with the
Securities and Exchange Commission on September 11,
2006).
|
|
|
10.8
|
Loan
Agreement between AMI Australia and ANZ Nominees Limited as Custodian for
the Professional Pension PST – Pension dated September 8, 2006
(incorporated herewith by reference to Exhibit 10.3 to Advanced Medical
Institute Inc.’s Current Report on Form 8-K dated September 8, 2006 and
filed with the Securities and Exchange Commission on September 11,
2006).
|
|
|
10.9
|
Fixed
and Floating Charge Agreement between AMI Australia and ANZ Nominees
Limited as Custodian for the Professional Pension PST – Super dated
September 8, 2006 (incorporated herewith by reference to Exhibit 10.4 to
Advanced Medical Institute Inc.’s Current Report on Form 8-K dated
September 8, 2006 and filed with the Securities and Exchange Commission on
September 11, 2006).
|
|
|
10.10
|
Fixed
and Floating Charge Agreement between AMI Australia and ANZ Nominees
Limited as Custodian for the Professional Pension PST – Pension dated
September 8, 2006 (incorporated herewith by reference to Exhibit 10.5 to
Advanced Medical Institute Inc.’s Current Report on Form 8-K dated
September 8, 2006 and filed with the Securities and Exchange Commission on
September 11, 2006).
|
|
|
10.11
|
Consulting
Agreement between the Company and the Heartcheck Group dated February 12,
2007 (incorporated herewith by reference to Exhibit 10.11 to Advanced
Medical Institute Inc.’s Annual Report on Form 10-KSB filed with the
Securities and Exchange Commission on October 15,
2007).
|
43
|
10.12
|
Option
Agreement between Worldwide PE Patent Holdco Pty Limited and AMI Group
Limited dated May 2, 2007 (incorporated herewith by reference to Exhibit
10.1 to Advanced Medical Institute Inc.’s Current Report on Form 8-K dated
May 2, 2007 and filed with the Securities and Exchange Commission on May
3, 2007).
|
|
|
10.13
|
Share
Sale Agreement, dated as of April 30, 2008 (incorporated herewith by
reference to Exhibit 10.13 to Advanced Medical Institute Inc.’s Annual
Report on Form 10-KSB, filed with the Securities and Exchange Commission
on October 15, 2007).
|
|
| 10.14 |
Facility
Agreement with St. George Bank Limited, dated October 22, 2008
(incorporated herewith by reference to Exhibit 10.1 to Advanced Medical
Institute Inc.’s Quarterly Report on Form 10-Q, filed with the Securities
and Exchange Commission on May 15, 2009).
|
|
|
10.15
|
Amendment to Facility Agreement with St. George Bank Limited, dated November 21, 2008 (incorporated herewith by reference to Exhibit 10.2 to Advanced Medical Institute Inc.’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission on May 15, 2009). | |
|
10.16
|
Loan
Agreement with Secare Health Centre Pty Limited, dated December 4, 2008
(incorporated herewith by reference to Exhibit 10.3 to Advanced Medical
Institute Inc.’s Quarterly Report on Form 10-Q, filed with the Securities
and Exchange Commission on May 15, 2009).
|
|
|
14.1
|
Code
of Ethics (incorporated herewith by reference to Exhibit 14.1 to Advanced
Medical Institute Inc.’s Annual Report on Form 10-KSB for the period ended
December 31, 2004 filed with the Securities and Exchange Commission on May
31, 2005).
|
|
|
31.1
|
Certification
of Chief Executive Officer pursuant to Rule 13A-14(A)/15D-14(A) of the
Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002.*
|
|
|
31.2
|
Certification
of the Principal Financial Officer pursuant to Rule 13A-14(A)/15D-14(A) of
the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of
the Sarbanes-Oxley Act of 2002.*
|
|
|
32.1
|
Certification
of Chief Executive Officer Pursuant to 18 U.S.C. 1350 (Section 906 of the
Sarbanes-Oxley Act of 2002).*
|
|
|
32.2
|
|
Certification
of the Principal Financial Officer Pursuant to 18 U.S.C. 1350 (Section 906
of the Sarbanes-Oxley Act of
2002).*
|
*Filed
herewith
44
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
FINANCIAL
REPORT
FOR
THE YEARS ENDED JUNE 30, 2009 AND 2008
F-1
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
FINANCIAL
STATEMENTS
TABLE
OF CONTENTS
|
REPORTS
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
F-3
|
|
FINANCIAL
STATEMENTS
|
|
|
Consolidated
Balance Sheets as of June 30, 2009 and 2008
|
F-4
|
|
Consolidated
Statements of Operations for the years ended June 30, 2009 and
2008
|
F-5
|
|
Consolidated
Statements of Stockholders’ Equity for the years ended June 30, 2009 and
2008
|
F-6
|
|
Consolidated
Statements of Cash Flows for the years ended June 30, 2009 and
2008
|
F-7
|
|
Notes
to Consolidated Financial Statements
|
F-9
|
F-2
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors and
Stockholders
of Advanced Medical Institute, Inc.
We have
audited the accompanying consolidated balance sheets of Advanced Medical
Institute, Inc. and its subsidiaries (the “Company”) as of June 30, 2009 and
2008, and the related consolidated statements of operations, stockholders’
equity, and cash flows for the years ended June 30, 2009 and
2008. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on
these financial statements based on our audits.
We
conducted our audits of these statements in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those standards
require that we plan and perform the audit to obtain reasonable assurance about
whether the financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by management, as well
as evaluating the overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Advanced Medical Institute, Inc.
and its subsidiaries as of June 30, 2009 and 2008, and the results of its
operations and its cash flows for the years ended June 30, 2009 and 2008 in
conformity with accounting principles generally accepted in the United States of
America.
The
Company's financial statements are prepared using the generally accepted
accounting principles applicable to a going concern, which contemplates the
realization of assets and liquidation of liabilities in the normal course of
business. The Company has accumulated deficit of $7,046,257 as of June 30, 2009
and a net loss of $9,344,989 for the year ended June 30, 2009. Theses factors,
as discussed in Note 1 to the financial statements raises substantial doubt
about the Company's ability to continue as a going concern. Management's plans
in regard to the matter are also described in Note 1. The statements do not
include any adjustments.
/s/
Kabani & Company, Inc.
CERTIFIED
PUBLIC ACCOUNTANTS
Los
Angeles, California
November
13, 2009
F-3
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
CONSOLIDATED
BALANCE SHEETS
|
June 30, 2009
|
June 30, 2008
|
|||||||||||
|
Notes
|
||||||||||||
|
CURRENT
ASSETS
|
||||||||||||
|
Cash
& cash equivalent
|
$ | 295,245 | $ | 3,092,611 | ||||||||
|
Receivables,
net
|
24,021,361 | 18,196,304 | ||||||||||
|
Receivables
due from related parties
|
11
|
428,525 | 751,034 | |||||||||
|
Inventory
|
911,405 | 468,950 | ||||||||||
|
Other
assets
|
3
|
1,014,424 | 657,388 | |||||||||
|
TOTAL
CURRENT ASSETS
|
26,670,960 | 23,166,287 | ||||||||||
|
NON-CURRENT
ASSETS
|
||||||||||||
|
Security
deposits
|
181,584 | 124,569 | ||||||||||
|
Property,
plant and equipment, net
|
4
|
1,630,979 | 1,283,879 | |||||||||
|
Net
assets held for disposition
|
10
|
289,977 | 505,647 | |||||||||
|
Deferred
tax assets
|
12
|
678,270 | - | |||||||||
|
Intangible
assets, net
|
2
|
18,327,078 | 30,281,340 | |||||||||
|
TOTAL
NON-CURRENT ASSETS
|
21,107,888 | 32,195,435 | ||||||||||
|
TOTAL
ASSETS
|
47,778,848 | $ | 55,361,722 | |||||||||
|
CURRENT
LIABILITIES
|
||||||||||||
|
Unearned
revenue
|
$ | 8,003,468 | $ | 6,922,800 | ||||||||
|
Accounts
payables & accrued expenses
|
6
|
15,852,436 | 9,874,446 | |||||||||
|
Payables
due to related parties
|
278,737 | - | ||||||||||
|
Interest
bearing liabilities – current
|
5
|
1,083,908 | 422,715 | |||||||||
|
Liabilities
related to discontinued operation
|
4,100 | 385 | ||||||||||
|
Income
taxes payable
|
- | 820,289 | ||||||||||
|
TOTAL
CURRENT LIABILITIES
|
25,222,649 | 18,040,635 | ||||||||||
|
NON-CURRENT
LIABILITIES
|
||||||||||||
|
Interest
bearing liabilities – non current
|
5
|
642,453 | 1,523,716 | |||||||||
|
Payables
due to related parties
|
3,803,131 | - | ||||||||||
|
Deferred
tax liabilities
|
- | 2,323,212 | ||||||||||
|
TOTAL
NON-CURRENT LIABILITIES
|
4,445,584 | 3,846,928 | ||||||||||
|
TOTAL
LIABILITIES
|
29,668,233 | 21,887,563 | ||||||||||
|
COMMITMENTS
& CONTINGENCIES
|
- | - | ||||||||||
|
STOCKHOLDERS’
EQUITY
|
||||||||||||
|
Common
stock, par value $0.001 per share,
|
||||||||||||
|
90,000,000
shares authorized,
|
||||||||||||
|
53,507,450
issued and outstanding as of June 30, 2009 and 2008
|
53,507 | 53,507 | ||||||||||
|
Additional
paid in capital
|
24,149,420 | 24,149,420 | ||||||||||
|
Other
comprehensive income
|
14
|
953,945 | 6,972,500 | |||||||||
|
(Accumulated
losses) Retained earnings
|
(7,046,257 | ) | 2,298,732 | |||||||||
|
TOTAL
STOCKHOLDERS’ EQUITY
|
18,110,615 | 33,474,159 | ||||||||||
|
TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$ | 47,778,848 | $ | 55,361,722 | ||||||||
See
accompanying notes to the financial statements.
F-4
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF OPERATIONS
FOR
THE YEARS ENDED JUNE 30, 2009 AND 2008
|
2009
|
2008
|
|||||||
|
NET
REVENUES
|
$ | 53,351,946 | 51,847,992 | |||||
|
COST
OF REVENUES
|
12,957,681 | 11,597,159 | ||||||
|
GROSS
PROFIT
|
40,394,265 | 40,250,833 | ||||||
|
SELLING,
GENERAL AND ADMINISTRATIVE EXPENSES
|
46,616,853 | 36,801,510 | ||||||
|
Impairment
loss
|
5,279,462 | - | ||||||
|
TOTAL
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
|
51,896,315 | 36,801,510 | ||||||
|
OPERATING
INCOME
|
(11,502,050 | ) | 3,449,323 | |||||
|
OTHER
INCOME AND EXPENSE
|
||||||||
|
Bank
interest
|
52,828 | 92,499 | ||||||
|
Sundry
income
|
19,818 | 23,779 | ||||||
|
Management
fee income
|
- | 33,813 | ||||||
|
Interest
expense
|
(269,865 | ) | (263,482 | ) | ||||
|
TOTAL
OTHER INCOME AND EXPENSE
|
(197,219 | ) | (113,391 | ) | ||||
|
INCOME/(LOSS)
FROM CONTINUING OPERATIONS BEFORE INCOME TAXES
|
(11,699,269 | ) | 3,335,932 | |||||
|
INCOME
TAX BENEFIT (EXPENSE)
|
2,760,387 | (1,257,679 | ) | |||||
|
INCOME/(LOSS)
FROM CONTINUING OPERATIONS
|
(8,938,882 | ) | 2,078,253 | |||||
|
LOSS
FROM DISCONTINUED OPERATIONS
|
(406,107 | ) | ( 1,083,474 | ) | ||||
|
NET
INCOME/(LOSS)
|
(9,344,989 | ) | 994,779 | |||||
|
Other
Comprehensive item – Foreign currency translation
income/(loss)
|
(6,018,555 | ) | 3,800,994 | |||||
|
Net
Comprehensive Income/(Loss)
|
$ | (15,363,544 | ) | $ | 4,795,773 | |||
|
Earnings/(Loss)
per share, continued operations
|
$ | (0.16 | ) | $ | 0.03 | |||
|
Loss
per share, discontinued operations
|
(0.01 | ) | (0.01 | ) | ||||
|
Earnings/(Loss)
per share, basic & diluted
|
$ | (0.17 | ) | $ | 0.02 | |||
|
Weighted
average number of shares outstanding, basic
|
53,507,450 | 53,507,450 | ||||||
See
accompanying notes to the financial statements.
F-5
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY
FOR
THE YEARS ENDED JUNE 30, 2009 AND 2008
|
Additional
|
Other
|
|||||||||||||||||||||||
|
Common Stock
|
Paid-In
|
Comprehensive
|
Retained
|
|||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Income (Loss)
|
Earnings
|
Total
|
|||||||||||||||||||
|
Balance
June 30, 2007
|
53,507,450 | 53,507 | 24,149,420 | 3,171,506 | 1,303,953 | 28,678,386 | ||||||||||||||||||
|
Other
comprehensive income
|
- | - | - | 3,800,994 | - | 3,800,994 | ||||||||||||||||||
|
Net
income, June 30, 2008
|
- | - | - | - | 994,779 | 994,779 | ||||||||||||||||||
|
Balance
June 30, 2008
|
53,507,450 | 53,507 | 24,149,420 | 6,972,500 | 2,298,732 | 33,474,159 | ||||||||||||||||||
|
Other
comprehensive income
|
- | - | - | (6,018,555 | ) | - | (6,018,555 | ) | ||||||||||||||||
|
Net
loss, June 30, 2009
|
- | - | - | - | (9,344,989 | ) | (9,344,989 | ) | ||||||||||||||||
|
Balance
June 30, 2009
|
53,507,450 | $ | 53,507 | $ | 24,149,420 | $ | 953,945 | $ | (7,046,257 | ) | $ | 18,110,615 | ||||||||||||
See
accompanying notes to the financial statements.
F-6
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CASH FLOWS
FOR
THE YEARS ENDED JUNE 30, 2009 AND 2008
|
2009
|
2008
|
|||||||
|
Cash
Flows from Operating Activities
|
||||||||
|
Receipts
from customers
|
$ | 42,496,872 | $ | 43,664,868 | ||||
|
Interest
received
|
52,827 | 89,200 | ||||||
|
Interest
paid
|
(673,730 | ) | (263,482 | ) | ||||
|
Payment
to suppliers & employees
|
(47,355,193 | ) | (39,105,267 | ) | ||||
|
Income
tax paid
|
(96,945 | ) | (214,786 | ) | ||||
|
Net
cash (used in) provided by operating activities of continuing
operations
|
(5,576,169 | ) | 4,170,533 | |||||
|
Net
cash provided by (used in)
discontinuing operation
|
81,147 | (609,639 | ) | |||||
|
Net
cash (used in) provided by operating activities
|
(5,495,022 | ) | 3,560,894 | |||||
|
Cash
Flows from Investing Activities
|
||||||||
|
Loan
to related entities
|
(212,035 | ) | - | |||||
|
Proceeds
from sales of investment
|
239,370 | - | ||||||
|
Payment
for property, plant & equipment
|
(378,305 | ) | (338,501 | ) | ||||
|
Purchase
of intangible assets
|
(152,159 | ) | (93,819 | ) | ||||
|
Net
cash used in investing activities
|
(503,129 | ) | (432,320 | ) | ||||
|
Cash
Flows From Financing Activities
|
||||||||
|
Proceeds
from borrowings
|
4,975,492 | - | ||||||
|
Repayment
of borrowings
|
(1,615,939 | ) | (869,468 | ) | ||||
|
Net
cash (used in) provided by financing activities
|
3,359,553 | (869,468 | ) | |||||
|
Net
increase in cash and cash equivalent
|
(2,638,598 | ) | 2,259,106 | |||||
|
Effect
of exchange rate changes on cash and cash equivalent
|
(158,768 | ) | 128,953 | |||||
|
Cash
& cash equivalent at beginning of year
|
3,092,611 | 704,552 | ||||||
|
Cash
& cash equivalent at end of year
|
$ | 295,245 | $ | 3,092,611 | ||||
|
NON-CASH
INVESTING & FINANCING ACTIVITY:
|
||||||||
|
1)
Assets acquired under capital leases $353,088
|
||||||||
See
accompanying notes to the financial statements.
F-7
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
STATEMENTS
OF CASH FLOWS
FOR
THE YEARS ENDED JUNE 30, 2009 AND 2008
|
June
30, 2009
|
June
30, 2008
|
|||||||
|
Reconciliation
of Cash
|
||||||||
|
Cash
at the end of financial year as shown in the Statement of Cash Flows is
reconciled to the related items in the Statement of Financial Position as
follows:
|
||||||||
|
Cash
|
$ | 295,245 | $ | 3,092,611 | ||||
|
Reconciliation
of Cash Flow from Operations with Profit from Ordinary Activities after
Income Tax
|
||||||||
|
Net
(Loss) Income
|
(9,344,989 | ) | 994,779 | |||||
|
Adjustments
to reconcile net (loss) income to net cash provided by operating
activities:
|
||||||||
|
Depreciation
and amortization expense
|
1,627,210 | 1,787,437 | ||||||
|
Provision
for doubtful debt
|
4,737,659 | 4,917,980 | ||||||
|
Impairment
loss
|
5,279,462 | - | ||||||
|
Gain
on disposal of investment
|
(119,677 | ) | - | |||||
|
Changes
in Assets and Liabilities
|
||||||||
|
Increase
in current inventories
|
(482,280 | ) | (152,377 | ) | ||||
|
Increase
in receivables
|
(13,270,978 | ) | (9,202,808 | ) | ||||
|
Increase
in deferred tax assets/liabilities
|
(2,579,005 | ) | 580,143 | |||||
|
Increase
in unearned revenue
|
2,053,093 | 1,066,787 | ||||||
|
Increase
in payables
|
6,981,933 | 3,315,337 | ||||||
|
(Decrease)
Increase in rental deposit received
|
(71,863 | ) | 2,959 | |||||
|
Increase
in provisions for compensated absences
|
251,494 | 397,768 | ||||||
|
(Decrease)
Increase in income tax payable
|
(638,228 | ) | 462,528 | |||||
|
Net
cash (used in) provided by operating activities of continuing
operations
|
(5,576,169 | ) | 4,170,533 | |||||
|
Net
cash provided by (used in) operating activities of discontinued
operation
|
81,147 | (609,639 | ) | |||||
|
Cash
Flows (Used in) Provided by Operating Activities
|
$ | (5,495,022 | ) | $ | 3,560,894 | |||
See
accompanying notes to the financial statements.
F-8
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE
30, 2009 AND 2008
|
1.
|
NATURE
OF BUSINESS
|
We were
originally incorporated under the name of Hawksdale Financial Visions, Inc. on
December 6, 1996 under the laws of the State of Nevada. We were
involved in the business of timeshares, but became dormant on March 31, 1997,
and until January 28, 2005, we were a “blank check” company with nominal assets.
On October 15, 2004, we changed our name to “Advanced Medical Institute
Inc.”
On March
21, 2005, we completed a Share Exchange Agreement with Advanced Medical
Institute Pty Limited, a privately owned Australian company (“AMI Australia”),
whereby AMI Australia became our wholly owned subsidiary.
On
November 17, 2005 we entered a Share Exchange Agreement with PE Patent Holdco
Pty Limited, a privately owned Australian company (“PE Patent Holdco”), whereby
PE Patent Holdco became our wholly-owned subsidiary.
On
September 8, 2006, we entered a Share Exchange Agreement with Worldwide PE
Patent Holdco Pty Limited (ACN 117 157 727), a privately owned Australian
company (“Worldwide PE”), whereby Worldwide PE became our wholly owned
subsidiary.
Business
Overview
|
|
AMI
is a service provider company, which arranges for patients with sexual
dysfunction and prostate problems in Australia, New Zealand, China and the
United Kingdom to be provided with medical services, pharmaceuticals and
associated clinical support
services.
|
|
|
On
September 15, 2008, we announced expansion of our operations to the United
Kingdom where we opened up our treatment centers, contracts with
independent doctors and pharmacies and begun airing infomercials
throughout the country.
|
|
|
For
the year ended June 30, 2009, AMI’s revenues were approximately $53.4
million.
|
Principal Products and
Services
|
|
AMI
provides a variety of treatment programs to its customers, via its call
center and clinics, for the treatment of sexual dysfunction and prostate
problems. A patient is diagnosed by telephone or in person at a clinic, in
either case, by a licensed physician, who sends a prescription directly to
a compounding pharmacy under contract with AMI to prepare the
formulation. The prescription is delivered to the patient, or
the patient may pick up the prescription at the clinic. The
patient pays for treatments for a specified treatment period, during which
the formulations may be varied to best suit the patient’s
needs.
|
|
|
Our
physicians prescribe varying combinations or dosages of medications for
erectile dysfunction (predominantly phentalomine, apomorphine or a
combination of them), premature ejaculation (predominantly clomipramine)
and prostate problems (mixture of medicinal herbs). Our
compound formulations have not been subject to any clinical trial, but may
lawfully be prescribed on an individual prescription (“off-label”) basis
in each country in which we
operate.
|
|
|
The
effectiveness of AMI’s treatment programs depend highly on the delivery
system. These include:
|
|
(a)
|
injections,
nasal spray, lozenges, tablets and gels for the treatment of erectile
dysfunction;
|
F-9
|
(b)
|
injections,
nasal spray, lozenges and gels for the treatment of premature
ejaculation;
|
|
(c)
|
topical
gels for the treatment of female sexual arousal dysfunction;
and
|
|
(d)
|
an
oral elixir for the treatment of prostate
problems.
|
New Products and
Services
|
|
On
July 29, 2008, AMI announced the development of new transdermal gel used
to treat premature ejaculation and erectile dysfunction in men as well as
sexual arousal dysfunction in
women.
|
|
|
Since
2003, AMI’s subsidiary, Intelligent Medical Technologies Pty Limited
(“IMT”) has been developing an ultrasonic nebulizer which will deliver
drugs to the lungs. The group’s intention is to use this
delivery system to administer its compound formulations. In
order to utilize this delivery system IMT needs to obtain regulatory
approval of the nebulizer as a medical device. IMT has been
working on this process for the last 3 years however IMT has not yet
completed such process.
|
Distribution
|
|
AMI
currently operates a centralized call center and 20 clinics offices
throughout Australia and New Zealand. AMI has an arrangement
with two hospitals in Beijing where Chinese patients are treated, and AMI
has recently established 2 clinics in the United Kingdom to service UK
patients. AMI’s products and services are only available by prescription
and are sold on an “off-label” basis. AMI Australia’s treatment
programs are generally available in the same manner through its medical
clinics and its over-the-phone sales and marketing
program.
|
Subsidiaries of the
Company
Following are the
significant Subsidiaries of the Company and AMI Australia
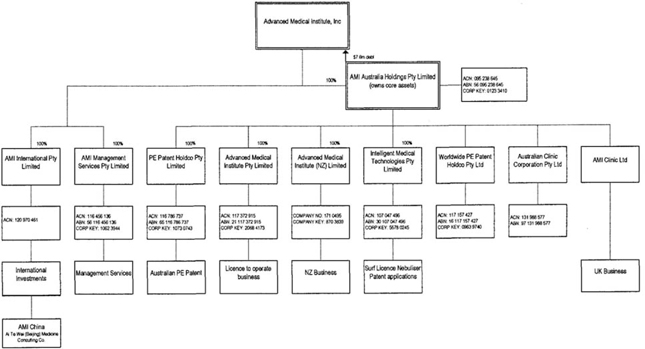
F-10
|
|
The
Company’s subsidiaries are AMI Australia, AMI International Pty Limited
and AMI Management Services Pty
Limited.
|
|
|
AMI
Australia’s subsidiaries are Advanced Medical Institute Pty Limited, PE
Patent Holdco, Worldwide PE, Advanced Medical Institute (NZ) Limited, IMT
and AMI Clinic Limited.
|
|
|
During
the second half of the financial year, AMI discontinued operations of AMI
China.
|
|
|
On
February 4, 2008, AMI discontinued operations of AMI Japan Kabushiki
Gaisya .
|
|
|
AMI
International Pty Limited was established to hold the Group’s
shareholdings in the Chinese company established to conduct operations in
that jurisdiction.
|
|
|
AMI
Management Services Pty Limited provides treasury and management services
to AMI Australia and its
subsidiaries.
|
|
|
Advanced
Medical Institute (NZ) Limited conducts the group’s business in New
Zealand.
|
|
|
Ai
Te Wei (Beijing) Medicine Consulting Company conducts the group’s business
in China.
|
|
|
During
September 2006, AMI commenced a not-for-profit division of the Company,
AMI-SCI that provides treatment options to men who have sustained a spinal
cord injury.
|
Managements
Plans
The
Company has incurred significant net losses, cash outflows from operations and
has working capital of $9,344,989, $5,495,022 and $1,448,311, respectively,
for the year ended June 30, 2009. This factor raises doubt about the
Company’s ability to continue as a going concern. The ability to continue
as a going concern will be dependent on its ability of the Company to reduce
operating expenses, generate sufficient cash flows from operations and to obtain
additional financing to meet its obligations on a timely basis.
In July
2009, as part of its efforts to conserve its existing capital resources, the
Company implemented steps to reduce operating expenses, and will monitor its
liquidity closely and will take further steps, if need, to further reduce
operating cost to preserve operating capital. The Company also may need to
raise additional capital through the sale of debt or equity. The Company
believes that with these steps, it should have sufficient cash to fund its
activities in the near future
F-11
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
JUNE
30, 2009
|
2.
|
SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES
|
The
accounting and reporting policies of the Company conform to accounting
principles generally accepted in the United States of America. The
following are descriptions of the more significant policies:
Principles of
Consolidation
The
consolidated financial statements include the accounts of the Company and its
wholly owned subsidiaries, AMI Australia Holdings Pty Limited, AMI Management
Services Pty Limited (“AMI MS”) and AMI International Pty Limited (“AMI
International”) and their direct and indirect wholly-owned subsidiaries:
Advanced Medical Institute Pty Ltd, PE Patent Holdco Pty Limited (“PE”),
Advanced Medical Institute (NZ) Limited (“AMI NZ”) and Intelligent Medical
Technologies Pty Ltd (“IMT”), Worldwide PE Patent Holdco Pty Ltd and AMI Clinic
Limited. All significant inter-company accounts and transactions have been
eliminated upon consolidation.
The
Company also re-classed assets of its 75% owned subsidiary AMI Japan and wholly
own subsidiary AMI China, which were discontinued during the reporting period,
to ‘Assets held for disposition’.
Concentration of Credit
Risk
Financial
instruments that potentially subject the Company to concentrations of credit
risk are cash, accounts receivable and other receivables arising from its normal
business activities. The Company places its cash in what it believes to be
credit-worthy financial institutions. The Company has a diversified customer
base. The Company controls credit risk related to accounts receivable through
credit approvals, credit limits and monitoring procedures. The Company routinely
assesses the financial strength of its customers and, based upon factors
surrounding the credit risk, establishes an allowance, if required, for
uncollectible accounts and, as a consequence, believes that its accounts
receivable credit risk exposure beyond such allowance is limited.
Cash
Equivalents
For
purposes of the statement of cash flows, the Company considers all highly liquid
investments with maturities of three months or less to be cash
equivalents.
Use of
Estimates
The
preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect certain reported amounts and disclosures. Accordingly, actual results
could differ from those estimates.
Revenue
Recognition
Sales are
reported as deferred income when the sales contracts are executed and the term
of the contract exceeds three months. Up to three months of
medication is delivered to the patient upon the signing of a
contract. Generally the term of the sales contracts are up to one
year, but they can be for longer periods of time. The deferred income
arising from the contracts that exceed three months is then amortized, on a
straight line basis, into income during the approximated composite remaining
medication delivery period. This approximated composite is an
estimate that may vary from period to period.
F-12
Unearned
Revenue
Unearned
revenue arises from programs that exceed three months. The average
program length purchased by each individual patient is approximately 4 ½ months.
The Company made its estimate of unearned revenue based on the average program
length less three months for which medication is provided and revenue is
recognized.
Advertising
Expense
Advertising
costs are charged to expense as they are incurred. Advertising expense is $22.4
million in financial year 2009 and $18.0 million in financial year
2008.
Income
Tax
Income
taxes have been provided based upon the tax laws and rates in the countries in
which operations are conducted and income is earned. The income tax
rates imposed by the taxing authorities vary. Taxable income may vary
from pre-tax income for financial accounting purposes. There is no
expected relationship between the provision for income taxes and income before
income taxes because the countries have different taxation rules, which vary not
only to nominal rates but also in terms of available deductions, credits and
other benefits. Deferred tax assets and liabilities are recognized
for the anticipated future tax effects of temporary differences between the
financial statement basis and the tax basis of the Company’s assets and
liabilities using the applicable tax rates in effect at year end as prescribed
by SFAS 109 (ASC 740) “Accounting for Income Taxes.”
Accounts
Receivable
Accounts
receivable are sales for the year, net of sales refund, cancellation, unearned
revenue and provision for doubtful debt. The Company estimates
provision for doubtful debts based on the historical results.
Inventories
Inventories
are valued at the lower of cost (determined on a first-in first-out basis) or
market. Management compares the cost of inventories with the market
value and allowance is made for writing down our inventories to market value, if
lower. As of June 30, 2009 inventory mainly consisted of raw
materials.
Property and
Equipment
Property
and Equipment placed in service is depreciated over the estimated useful lives
of the assets using the diminishing value depreciation method.
Property
and equipment are carried at the lesser of cost and written down
value. Expenditures for maintenance and repairs are expenses as
incurred and expenditures for major renewals and betterments are
capitalized. Assets retired or sold are removed from the property
accounts, with gains or losses on disposal included in income.
F-13
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENT
JUNE
30, 2009
2. SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Employee
Entitlements
Contributions
are made to an employee superannuation fund and are charged as expenses when
incurred.
Exchange Gain
(Loss)
During
the fiscal year ended June 30, 2009 and 2008, the transactions of AMI Australia
were denominated in foreign currency and were recorded in Australian Dollars
(AUD) at the rates of exchange in effect when the transactions occur. Exchange
gains and losses are recognized for the different foreign exchange rates applied
when the foreign currency assets and liabilities are settled.
Foreign
Currency
As of
June 30, 2009, the accounts of AMI Australia and its subsidiaries were
maintained and its financial statements were expressed in the local currency for
the jurisdiction in which the entity operated. Such financial
statements were translated into the functional currency in Australian Dollars
(AUD) and thereafter to reporting currency in U.S. Dollars (USD) in accordance
with Statement of Financial Accounts Standards (“SFAS”) No. 52 (ASC 830),
“Foreign Currency Translation,” with the AUD as the functional currency.
According to the Statement, all assets and liabilities were translated at the
current exchange rate, stockholders’ equity (deficit) is translated at the
historical rates and income statement items are translated at the average
exchange rate for the period. The resulting translation adjustments are reported
under other comprehensive income in accordance with SFAS No. 130 (ASC 220),
“Reporting Comprehensive Income” as a component of shareholders’ equity
(deficit).
Research and Development
Costs
Research
and development costs are charged against income from ordinary activities before
income tax as incurred. Research and development costs are $0.3 million in
financial year 2009 and $0.2 million in financial year 2008.
F-14
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENT
JUNE
30, 2009
2. SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Intangible
assets
The
Company applies the criteria specified in SFAS No. 141 (ASC 805), “Business
Combinations” to determine whether an intangible asset should be recognized
separately from goodwill. Intangible assets acquired through business
acquisitions are recognized as assets separate from goodwill if they satisfy
either the “contractual-legal” or “separability” criterion. Per SFAS 142 (ASC
350), intangible assets with definite lives are amortized over their estimated
useful life and reviewed for impairment in accordance with SFAS No. 144
(ASC 360), “Accounting for the Impairment or Disposal of Long-lived Assets.”
Intangible assets, such as purchased technology, trademark, customer list, user
base and non-compete agreements, arising from the acquisitions of subsidiaries
and variable interest entities are recognized and measured at fair value upon
acquisition. Intangible assets are amortized over their estimated useful lives
from one to ten years. The Company reviews the amortization methods and
estimated useful lives of intangible assets at least annually or when events or
changes in circumstances indicate that it might be impaired. The recoverability
of an intangible asset to be held and used is evaluated by comparing the
carrying amount of the intangible asset to its future net undiscounted cash
flows. If the intangible asset is considered to be impaired, the impairment loss
is measured as the amount by which the carrying amount of the intangible asset
exceeds the fair value of the intangible asset, calculated using a discounted
future cash flow analysis. The Company uses estimates and judgments in its
impairment tests, and if different estimates or judgments had been utilized, the
timing or the amount of the impairment charges could be different.
Goodwill,
trademarks, patents and other intangible assets determined to have indefinite
useful lives are not amortized. We test such trademarks and other
intangible assets with indefinite useful lives for impairment annually, or more
frequently if events or circumstances indicate that an asset might be
impaired. Goodwill, trademarks, patents and other intangible assets
determined to have definite lives are amortized over their estimated useful
lives or the life of the trademark, patent and other intangible asset, whichever
is less.
Impairment
is determined by assessing the recoverable amount of the cash-generating unit
(group of cash-generating units), to which the intangible asset relates. When
the recoverable amount of the cash-generating unit (group of cash-generating
units) is less than the carrying amount, an impairment loss is
recognised.
At June
30, 2009, intangibles consist of the following:
|
Intangibles
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Impairment
Loss
|
Net
|
||||||||||||
|
Amortized
intangibles:
|
||||||||||||||||
|
Australian
Patents
|
$ | 5,314,538 | $ | (1,029,765 | ) | $ | - | $ | 4,284,773 | |||||||
|
Worldwide
Patents
|
4,023,996 | (634,373 | ) | (1,378,100 | ) | 2,011,523 | ||||||||||
|
Intellectual
Properties
|
16,314,189 | (2,487,941 | ) | (4,961,166 | ) | 8,865,082 | ||||||||||
|
Computer
Software
|
476,830 | (317,947 | ) | - | 158,883 | |||||||||||
|
Unamortized
intangibles:
|
||||||||||||||||
|
Intellectual
Properties
|
3,006,817 | - | - | 3,006,817 | ||||||||||||
| $ | 29,136,370 | $ | (4,470,026 | ) | $ | (6,339,266 | ) | $ | 18,327,078 | |||||||
The
Company assigned an 18 year life to the patents that are held by Worldwide PE
Patent, a 19 year life to the patents that are held by PE
Patent.
F-15
For
intellectual property, the company assigned an 18 year life to the intellectual
property that arose from Worldwide PE Patent, a 19 year life to the intellectual
property that arose from PE Patent, and indefinite life to the intellectual
property that arose from IMT.
Computer
software was assigned as having a 3 year life.
Amortization
expense from continuing operation for the year ended June 30, 2009 and 2008 was
$1,361,727 and $1,618,431 respectively. The Company expects the
amortization expenses for the next five years to be as
follows:
|
Year
Ending June 30,
|
Annual
Amount
|
|||
|
2010
|
$ | 1,362,000 | ||
|
2011
|
$ | 1,362,000 | ||
|
2012
|
$ | 1,362,000 | ||
|
2013
|
$ | 1,362,000 | ||
|
2014
|
$ | 1,362,000 | ||
Long-Lived
Assets
Effective
January 1, 2002, the Company adopted Statement of Financial Accounting Standards
No. 144 (ASC 360), “Accounting for the Impairment or Disposal of Long-Lived
Assets” (“SFAS 144”), which addresses financial accounting and reporting for the
impairment or disposal of long-lived assets and supersedes SFAS No. 121,
“Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to
be Disposed Of,” and the accounting and reporting provisions of APB Opinion No.
30, “Reporting the Results of Operations for a Disposal of a Segment of a
Business.” The Company periodically evaluates the carrying value of long-lived
assets to be held and used in accordance with SFAS 144 (ASC 360). SFAS 144 (ASC
360) requires impairment losses to be recorded on long-lived assets used in
operations when indicators of impairment are present and the undiscounted cash
flows estimated to be generated by those assets are less than the assets’
carrying amounts. In that event, a loss is recognized based on the amount by
which the carrying amount exceeds the fair market value of the long-lived
assets. Loss on long-lived assets to be disposed of is determined in a similar
manner, except that fair market values are reduced for the cost of disposal.
Based on its review, the Company believes that, as of June 30, 2009 there were
no significant impairments of its long-lived assets.
Segment
Reporting
Statement
of Financial Accounting Standards No. 131 (“SFAS 131”) (ASC 280), “Disclosure
About Segments of an Enterprise and Related Information” requires use of the
“management approach” model for segment reporting. The management approach model
is based on the way a company’s management organizes segments within the company
for making operating decisions and assessing performance. Reportable segments
are based on products and services, geography, legal structure, management
structure, or any other manner in which management disaggregates a company. SFAS
131 (ASC 280) has no effect on the Company’s consolidated financial statements
as the Company consists of one reportable business segment.
F-16
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENT
JUNE
30, 2009
2. SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Basic and Diluted Earnings
Per Share
Earnings
per share is calculated in accordance with the Statement of financial accounting
standards No. 128 (SFAS No. 128) (ASC 260), “Earnings per share.”. Net loss per
share for all periods presented has been restated to reflect the adoption of
SFAS No. 128 (ASC 260). Basic net loss per share is based upon the weighted
average number of common shares outstanding. Diluted net loss per share is based
on the assumption that all dilutive convertible shares and stock options were
converted or exercised. Dilution is computed by applying the treasury stock
method. Under this method, options and warrants are assumed to be exercised at
the beginning of the period (or at the time of issuance, if later), and as if
funds obtained thereby were used to purchase common stock at the average market
price during the period.
F-17
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENT
JUNE
30, 2009
2. SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recent
Pronouncements
In
December 2007, the FASB issued SFAS No. 160 (ASC 810), “Noncontrolling Interests
in Consolidated Financial Statements-An Amendment of ARB No. 51.” The objective
of this statement is to establish new accounting and reporting standards for the
Noncontrolling interest in a subsidiary and for the deconsolidation of a
subsidiary. Statement 160 is effective for fiscal years, and interim periods
within those fiscal years, beginning on or after December 15, 2008. The Company
does not expect the adoption of SFAS No. 160 to have a material impact on the
consolidated financial statements.
In March,
2008, the “FASB” issued SFAS No. 161 (ASC 815), “Disclosures about Derivative
Instruments and Hedging Activities.” The new standard is intended to improve
financial reporting about derivative instruments and hedging activities by
requiring enhanced disclosures to enable investors to better understand their
effects on an entity’s financial position, financial performance, and cash
flows. It is effective for financial statements issued for fiscal years and
interim periods beginning after November 15, 2008, with early application
encouraged. The new standard also improves transparency about the location and
amounts of derivative instruments in an entity’s financial statements; how
derivative instruments and related hedged items are accounted for under
Statement 133; and how derivative instruments and related hedged items affect
its financial position, financial performance, and cash flows. FASB Statement
No. 161 (ASC 815) achieves these improvements by requiring disclosure of the
fair values of derivative instruments and their gains and losses in a tabular
format. It also provides more information about an entity’s liquidity by
requiring disclosure of derivative features that are credit risk-related.
Finally, it requires cross-referencing within footnotes to enable financial
statement users to locate important. Based on current conditions, the Company
does not expect the adoption of SFAS 161 (ASC 815) to have a significant impact
on its results of operations or financial position.
In May
2008, FASB issued SFASB No. 163 (ASC 944), “Accounting for Financial Guarantee
Insurance Contracts-an interpretation of FASB Statement No. 60.” The scope of
the statement is limited to financial guarantee insurance (and reinsurance)
contracts. The pronouncement is effective for fiscal years beginning after
December 31, 2008. The Company does not believe this pronouncement will impact
its financial statements.
In June
2009, the FASB issued SFASB No.167, Consolidation of Variable Interest
Entities, which changes the consolidation rules as they relate to
variable interest entities. Specifically, the new standard makes significant
changes to the model for determining who should consolidate a variable interest
entity, and also addresses how often this assessment should be performed. This
standard will be effective for us on July 1, 2010. We do not expect the adoption
will have a material impact on our consolidated financial
statements.
In August
2009, the FASB issued Accounting Standards Update (“ASU”) 2009-05, which amends
ASC Topic 820, Measuring
Liabilities at Fair Value, which provides additional guidance on the
measurement of liabilities at fair value. Specifically, when a quoted price
in an active market for the identical liability is not available, the new
standard requires that the fair value of a liability be measured using one or
more of the valuation techniques that should maximize the use of relevant
observable inputs and minimize the use of unobservable inputs. In addition,
an entity is not required to include a separate input or adjustment to other
inputs relating to the existence of a restriction that prevents the transfer of
a liability. This standard will be effective for us on October 1, 2009. We
do not expect the adoption will have a material impact on our consolidated
financial statements.
In
October 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-13, which
amends ASC Topic 605, Revenue
Recognition, to require companies to allocate revenue in multiple-element
arrangements based on an element’s estimated selling price if vendor-specific or
other third-party evidence of value is not available. ASU 2009-13 is effective
beginning July 1, 2010. Earlier application is permitted. The Company is
currently evaluating both the timing and the impact of the pending adoption of
the ASU on its consolidated financial statements.
F-18
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENT
JUNE
30, 2009
3. OTHER
ASSETS
Following
is the summary of other assets as of June 30, 2009
|
June
30, 2009
|
June
30, 2008
|
|||||||
|
Bank
guarantees
|
$ | 206,863 | $ | 112,256 | ||||
|
Advances
and prepayments
|
42,640 | 23,220 | ||||||
|
Other
receivables
|
9,134 | 67,305 | ||||||
|
Other
debtors
|
755,787 | 454,607 | ||||||
|
Total
|
$ | 1,014,424 | $ | 657,388 | ||||
4. PROPERTY, PLANT AND
EQUIPMENT
|
June
30, 2009
|
June
30, 2008
|
|||||||
|
Leasehold
improvements at cost
|
$ | 590,908 | $ | 557,408 | ||||
|
Less:
Accumulated depreciation
|
62,258 | 118,234 | ||||||
| 528,650 | 439,174 | |||||||
|
Motor
Vehicles
|
75,235 | 89,884 | ||||||
|
Less:
Accumulated Depreciation
|
38,966 | 36,316 | ||||||
| 36,269 | 53,568 | |||||||
|
Office
Furniture & Equipment
|
1,217,911 | 757,542 | ||||||
|
Less:
Accumulated Depreciation
|
289,911 | 162,810 | ||||||
| 928,000 | 594,732 | |||||||
|
Computer
Hardware – at Cost
|
413,800 | 462,013 | ||||||
|
Less:
Accumulated Depreciation
|
315,635 | 318,141 | ||||||
| 98,165 | 143,872 | |||||||
|
Low
Value Pooled Fixed Assets
|
173,057 | 188,491 | ||||||
|
Less:
Accumulated Depreciation
|
133,162 | 135,958 | ||||||
| 39,895 | 52,533 | |||||||
|
Total
Property, Plant and Equipment
|
$ | 1,630,979 | $ | 1,283,879 | ||||
Depreciation
expenses were $265,483 and $172,222 for the fiscal years ended June 30, 2009 and
2008, respectively.
F-19
Included
in property and equipment is approximately $489,814 of assets, which are leased
under non-cancelable leases and accounted for as capital leases, which expire
through October 2013. The accumulated depreciation included in the property and
equipment for these leases is approximately $102,469.
F-20
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENT
JUNE
30, 2009
5. INTEREST
BEARING LIABILITIES
|
June
30, 2009
|
June
30, 2008
|
|||||||
|
Current,
as of the period ended June 30:
|
||||||||
|
Capitalized
lease liability
|
$ | 165,533 | $ | 45,037 | ||||
|
Secured
loan 2008
|
918,375 | 377,678 | ||||||
| $ | 1,083,908 | $ | 422,715 | |||||
|
Non-current,
due during the year ended June 30:
|
||||||||
|
Capitalized
lease liability
|
$ | 212,720 | $ | 26,061 | ||||
|
Secured
Loan
|
429,733 | 1,497,655 | ||||||
|
Total
non-current
|
$ | 642,453 | $ | 1,523,716 | ||||
|
|
The
interest bearing liabilities require monthly payments of principal and
interest at a per annum interest rate ranging from 7.4% to
13.8%. Obligations under the notes are secured by the financed
assets included in property and
equipment.
|
|
|
Interest
expenses were $269,865 and $263,479 for the fiscal years ended June 30,
2009 and 2008, respectively.
|
Obligations
under the notes are secured by the financed assets included in property and
equipment. The secured loan is secured over all of AMI Australia’s assets and
undertakings. The principal of the loan is due to mature in September
2009.
Obligation
under capital lease will expire during the period from September 2010 to April
2013.
6. ACCOUNTS
PAYABLE AND ACCRUED EXPENSES
Accounts
payable and accrued expenses as of June 30, 2009 and 2008 are
summarized as follows:
|
Amount
|
||||||||
|
June
30, 2009
|
June
30, 2008
|
|||||||
|
Bank
overdraft
|
$ | 481,319 | $ | - | ||||
|
Accounts
payable
|
8,642,972 | 4,195,940 | ||||||
|
Accrued
salaries
|
480,436 | 611,049 | ||||||
|
Accrued
professional fees
|
60,360 | 62,305 | ||||||
|
Accrued
legal fee
|
- | 89,083 | ||||||
|
Other
current liabilities
|
1,087,589 | 290,800 | ||||||
|
Accrued
DDR medication cost
|
1,259,512 | 1,204,760 | ||||||
|
Accrued
DDR sales commission
|
1,377,818 | 1,204,760 | ||||||
|
Accrued
DDR collection fee
|
1,377,818 | 1,204,760 | ||||||
|
Provision
for patient refunds
|
120,720 | 182,685 | ||||||
|
Accrued
compensated absences
|
963,892 | 828,304 | ||||||
|
Total
|
$ | 15,852,436 | $ | 9,874,446 | ||||
F-21
7. LAWSUITS
As of
June 30, 2009, there was no litigation pending or threatened by or against the
Company or any of its direct or indirect subsidiaries other than AMI Australia.
AMI Australia currently is involved in the following litigation and
administrative matters:
On
October 25, 2006, Bade Medical Institute Pty Limited, Mr. David Wade, Mr. Buddy
Beani and others (collectively “Bade”) applied for Trade Mark No. 114322021 in
respect of the words “AMI Nasal Spray.” AMI Australia lodged an objection to the
registration of that application with IP Australia on June 21, 2007 and that
application was subsequently refused. On December 13, 2007, AMI
Australia commenced proceedings in the Federal Court of Australia Sydney
Registry against Bade alleging Bade was infringing upon AMI Australia’s
trademarks and other intellectual property rights. On December 19, 2007, Bade
consented to orders that Bade transfer ownership of certain domain names and
telephone numbers to AMI Australia and consented to orders that Bade would not
use certain names which contained the word AMI. Bade appear to have
ceased operation since the orders were obtained and has transferred the required
names to AMI Australia. AMI subsequently applied for final orders and damages
against Bade and final hearing of this matter concluded on September 2,
2009. The court has indicated that judgment will be released
shortly.
On June
12, 2009, AMI Australia obtained an interlocutory injunction from the Supreme
Court of New South Wales preventing Fairfax Media Publications Limited
(“Fairfax”) from publishing information which AMI Australia alleges was
disclosed to Fairfax in breach of a duty of confidence owed to AMI
Australia. Final hearing of this matter commenced on October 26, 2009
but was unable to be concluded by October 28, 2009, the final scheduled date for
the hearing of the matter. Final hearing of the matter is now
scheduled for February 24 and 25, 2010 and AMI Australia’s interlocutory
injunction has been extended until that date.
F-22
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE FINANCIAL STATEMENT
JUNE
30, 2009
8. LEASE
COMMITMENTS
The
Company is party to long-term, non-cancelable operating lease agreements for
administrative offices and clinic locations. The future aggregate
minimum annual lease payments arising from these lease agreements are as
follows:
|
June
30, 2009
|
||||
|
Due
during the period ended June 30:
|
||||
|
2010
|
$ | 566,563 | ||
|
2011
|
440,783 | |||
|
2012
|
355,830 | |||
|
2013
|
301,973 | |||
|
2014
|
272,463 | |||
|
2015
|
272,463 | |||
|
2016
|
136,232 | |||
| $ | 2,346,307 | |||
Total
rent expense under these operating leases was approximately $1,239,402 and
$1,085,779 during the years ended June 30, 2009 and 2008,
respectively.
9. CONCENTRATIONS
Sales
Markets – 75% of the Company’s revenues arise from Australian
customers.
Suppliers
- Although the Company has access to a variety of suppliers, the majority of the
Company’s products that are obtained for resale are purchased from a limited
number of suppliers.
Concentration
of Credit Risk - Financial instruments that potentially subject the Company to
credit risk consist of uninsured cash deposited in the bank and large
receivables that are sometimes due from a limited number of
debtors. The Company extends credit to a variety of related
parties.
10. DISCONTINUED OPERATION/ASSETS HELD
FOR DISPOSITION
AMI
Japan Kabushiki Gaisya
AMI Japan
Kabushiki Gaisya (“AMI Japan”) was an indirect 75% owned subsidiary of the
Company. AMI Japan had formed an alliance with a Japanese party with expertise
in marketing in the Japanese market and two financial investors. AMI Japan
commenced operations on October 1, 2006.
On or
about February 4, 2008, the operation of AMI Japan was wound down and
discontinued due to unsatisfied operating result.
Loss from
the discontinued operation of AMI Japan during the period ended June 30, 2009
was US$358,033.
The
capital contributed to AMI Japan of US$212,020 has been classified as assets
pending sale on the accompanying consolidated balance sheet as of June 30,
2009.
F-23
Following
is the summary of net assets held as of June 30:
|
June 30,
2009
|
June 30,
2008
|
|||||||
|
Assets
:-
|
||||||||
|
Cash
and cash equivalents
|
$ | 1,704 | $ | 34,288 | ||||
|
Other
current assets
|
84,005 | 75,693 | ||||||
|
Property,
Plant & Equipment, net
|
126,552 | 117,950 | ||||||
|
Intangible
assets
|
3,837 | 8,643 | ||||||
|
Total
Assets
|
216,098 | 236,574 | ||||||
|
Liabilities
:-
|
||||||||
|
Accounts
payable and accrued expense
|
4,078 | 849 | ||||||
|
Total
Liabilities
|
4,078 | 849 | ||||||
|
Net
Assets Held for disposal
|
$ | 212,020 | $ | 235,725 | ||||
The
components of loss from operations related to the entity held for disposal for
the year ended June 30, 2009 are shown below.
|
June 30,
2009
|
June 30,
2008
|
|||||||
|
Net
sales
|
$ | 164,845 | $ | 77,092 | ||||
|
Operating
expenses
|
||||||||
|
Selling,
general and administrative
|
540,825 | 553,425 | ||||||
|
Total
operating expenses
|
540,825 | 553,425 | ||||||
|
Loss
from operations
|
(375,980 | ) | (476,333 | ) | ||||
|
Non-operating
income (expenses) :-
|
||||||||
|
Other
income
|
17,904 | 218 | ||||||
|
Interest
income
|
43 | 93 | ||||||
|
Net
Loss before income tax
|
(358,033 | ) | (476,022 | ) | ||||
|
Provision
for Income tax
|
- | - | ||||||
|
Net
loss from entity held for disposal
|
$ | (358,033 | ) | $ | (476,022 | ) | ||
AMI
China
AMI China
was a fully owned subsidiary of the Company.
During
the second half of the financial year, the operation of AMI China was wound down
and discontinued due to unsatisfied operating result.
Loss from
the discontinued operation of AMI China during the period ended June 30, 2009
was US$167,752.
The
capital contributed to AMI Japan of US$78,378 has been classified as assets
pending sale on the accompanying consolidated balance sheet as of June 30,
2009.
F-24
Following
is the summary of net assets held as of June 30:
|
June 30,
2009
|
June 30,
2008
|
|||||||
|
Assets
:-
|
||||||||
|
Cash
and cash equivalents
|
$ | 13,121 | $ | 34,418 | ||||
|
Other
current assets
|
39,532 | 44,964 | ||||||
|
Property,
Plant & Equipment, net
|
21,226 | 30,328 | ||||||
|
Total
Assets
|
73,879 | 109,710 | ||||||
|
Liabilities
:-
|
||||||||
|
Accounts
payable and accrued expense
|
22 | 386 | ||||||
|
Total
Liabilities
|
22 | 386 | ||||||
|
Net
Assets Held for disposal
|
$ | 73,857 | $ | 109,324 | ||||
The
components of loss from operations related to the entity held for disposal for
the year ended June 30, 2009 are shown below.
|
June 30,
2009
|
June 30,
2008
|
|||||||
|
Net
sales
|
$ | 5,116 | $ | 42,057 | ||||
|
Operating
expenses
|
||||||||
|
Selling,
general and administrative
|
172,868 | 492,892 | ||||||
|
Total
operating expenses
|
172,868 | 492,892 | ||||||
|
Net
Loss before income tax
|
(167,752 | ) | (450,825 | ) | ||||
|
Provision
for Income tax
|
- | - | ||||||
|
Net
loss from entity held for disposal
|
$ | (167,752 | ) | $ | (450,825 | ) | ||
F-25
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE FINANCIAL STATEMENT
JUNE
30, 2009
11. RELATED
PARTY TRANSACTIONS
|
June
30, 2009
|
||||
|
Prostate
Health Clinic Pty. Ltd. (an AU co.)
|
||||
|
Relationship:
Under common management control by Jack Vaisman
|
||||
|
Receivable
from this related party
|
1,719 | |||
|
Loan
to affiliate in Indonesia
|
426,806 | |||
|
Receivable
due from related parties - current
|
428,525 | |||
|
Peter
Riley
|
||||
|
Relationship: Director
of AMI UK
|
||||
|
Payable
to this related party
|
73,513 | |||
|
Jacov
Vaisman
|
||||
|
Relationship: Director
of AMI Inc.
|
||||
|
Payable
to this related party
|
173,032 | |||
|
Tony
Khan
|
||||
|
Relationship: Executive
Vice President of AMI Inc.
|
||||
|
Payable
to this related party
|
32,192 | |||
|
Payables
due to related parties - current
|
278,737 | |||
|
Secare
Health Centre Pty Limited
|
||||
|
Relationship:
Under common management control by Jack Vaisman
|
||||
|
Payables
to this related party – non-current
|
3,803,131 | |||
Total
receivables due from related parties included in the Company’s balance sheet was
$428,525 and $751,034 as of June 30, 2009 and 2008.
Total
payables due to related parties included in the Company’s balance sheet was
$4,081,868 and $Nil as of June 30, 2009 and 2008.
Receivables
from related parties are unsecured, interest-free and are due on
demand.
Payables
to related parties are as follows:
The
Company originally borrowed A$1.0 million from Secare Health Centre Pty Limited
(“Secare”) in November 2008. Secare is controlled by the company’s
chief executive officer and founder, Jack Vaisman. The Company subsequently
borrowed a further A$3,881,489 from Secare. The loan is secured by a second
ranking security interest in all of AMI Australia’s assets and undertakings
(including its existing equity interests in PE Patent Holdco Pty Limited,
Intelligent Medical Technologies Pty Limited and Advanced Medical Institute (NZ)
Limited). The loan attracts an interest cost of 10% per annum. No
repayment of this loan has been made up to June 30, 2009. The loan is
due for repayment in December 2011.
F-26
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE FINANCIAL STATEMENT
JUNE
30, 2009
12. INCOME
TAXES
Total
Federal and State income tax expense for the fiscal years ended June 30, 2009
and 2008 amounted to ($2,760,387) and $1,257,679, respectively. For the fiscal
years ended June 30, 2009 and 2008 there is a difference of 1% between the
Australian federal statutory tax rate and the effective tax
rate. This difference is due to the domicile of operating profits and
losses in the Group as well as the impact of timing and past operating profits
(losses) on these matters.
|
June 30, 2009
|
U.S.
|
State
|
International
|
Total
|
||||||||||||
|
Current
|
$ | 0 | $ | 0 | $ | (3,560,106 | ) | $ | (3,560,106 | ) | ||||||
|
Deferred
|
$ | 0 | $ | 0 | $ | 799,719 | $ | 799,719 | ||||||||
|
Total
|
$ | 0 | $ | 0 | $ | (2,760,387 | ) | $ | (2,760,387 | ) | ||||||
|
June
30, 2008
|
U.S.
|
State
|
International
|
Total
|
||||||||||||
|
Current
|
$ | 0 | $ | 0 | $ | 821,610 | $ | 821,610 | ||||||||
|
Deferred
|
$ | 0 | $ | 0 | $ | 436,069 | $ | 436,069 | ||||||||
|
Total
|
$ | 0 | $ | 0 | $ | 1,257,679 | $ | 1,257,679 | ||||||||
|
Reconciliation
of the differences between the statutory U.S. Federal income tax rate and
the effective rate is as follows:
|
||||||||||||||||
|
June
30,
2009
|
June
30,
2008
|
|||||||||||||||
|
Federal
statutory tax rate
|
34 | % | 34 | % | ||||||||||||
|
Increase
(decrease) in rate resulting from:
|
||||||||||||||||
|
Non
US income taxed at different rates
|
(1 | )% | (1 | )% | ||||||||||||
| 33 | % | 33 | % | |||||||||||||
|
Temporary
Difference
|
(9 | ) | 5 | % | ||||||||||||
|
Effective
Tax Rate
|
24 | % | 38 | % | ||||||||||||
F-27
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE FINANCIAL STATEMENT
JUNE
30, 2009
Deferred
tax asset arises due to the following temporary differences.
|
June
30,
2009
|
Future
tax
rate
%
|
Deferred
tax
liability
|
||||||||||
|
Owing
to patients via ACFC
|
(28,861,821 | ) | 30 | % | (8,658,546 | ) | ||||||
|
Provision
DDR cancellation
|
16,788,933 | 30 | % | 5,036,680 | ||||||||
|
Provision
un-dispensed DDR medications
|
1,259,512 | 30 | % | 377,854 | ||||||||
|
Accrued
ACFC collection and commission
|
2,755,635 | 30 | % | 826,691 | ||||||||
|
Provision
for patient refund
|
120,720 | 30 | % | 36,216 | ||||||||
|
Unutilized
tax losses
|
5,663,851 | 30 | % | 1,699,155 | ||||||||
|
Amortization
of patents
|
(2,083,987 | ) | 30 | % | (625,196 | ) | ||||||
|
Impairment
loss
|
5,680,134 | 30 | % | 1,704,040 | ||||||||
|
Other
miscellaneous
|
937,920
|
30 | % |
281,376
|
||||||||
|
Total
|
2,260,900
|
30 | % | $ |
678,270
|
|||||||
F-28
ADVANCED
MEDICAL INSTITUTE INC.
AND
SUBSIDIARIES
NOTES
TO THE FINANCIAL STATEMENT
JUNE
30, 2009
13. STOCK
On
October 28, 2004 the officers and directors of the Company surrendered for
cancellation 1,450,000 shares of common stock.
On
November 8, 2004 the Company issued a stock dividend of 15 shares for each share
outstanding on November 8, 2004.
All prior
year information has been adjusted to reflect the stock cancellation and the
stock dividend.
On May
25, 2005 the Company’s Articles of Incorporation were amended to increase the
number of authorized shares of the capital stock of the Company to
100,000,000. The Company designated 90,000,000 shares as Common Stock
and 10,000,000 shares as Preferred Stock.
On June
29, 2005 the Company entered into a subscription agreement with certain non-U.S.
persons pursuant to which the Company issued an aggregate of 6,122,450 shares of
common stock for aggregate gross proceeds of $762,000.
On
November 17, 2005, the Company entered into a share exchange agreement with PE
Patent Holdco Pty Limited (“PE”), pursuant to which the Company acquired all of
the issued and outstanding shares of stock of PE in exchange for the issuance in
the aggregate of 5,000,000, or 13.85%, of the Company’s issued shares of Common
Stock to the shareholders of PE.
On April
18, 2006, the Company entered into a share exchange agreement with certain
non-US persons pursuant to which the Company acquired the remaining 7% of
Intelligent Medical Technologies Pty Limited (“IMT”) which was previously held
by those persons in exchange for 1,260,000 or 3.37% of the Company’s issued
shares of Common Stock.
On
September 8, 2006, the Company
entered into a share exchange Agreement with
Worldwide PE Patent Holdco Pty Limited (ACN 117 157 727), a privately owned
Australian company (“Worldwide PE”), and Worldwide PE’s shareholders pursuant to
which the Company acquired all of the issued and outstanding shares of stock of
Worldwide PE in exchange for the payment of A$3 million (approximately $2.25
million) and the issuance in the aggregate of 16,125,000 shares of the Company’s
common stock valued at $1.00 per share.
14. OTHER
COMPREHENSIVE INCOME
Balances
of related after-tax components comprising accumulated other comprehensive
income (loss), included in stockholders’ equity, at June 30, 2009are as
follows:
|
Accumulated
Other
Comprehensive
Income
|
||||
|
Balance
at June 30, 2007
|
$ | 3,171,506 | ||
|
Change
for 2008
|
3,800,994 | |||
|
Balance
at June 30, 2008
|
$ | 6,972,500 | ||
|
Change
for 2009
|
(6,018,555 | ) | ||
|
Balance
at June 30, 2009
|
$ | 953,945 | ||
F-29
15
– Segment Information
The
following tables summarize segment information for the years ended:
|
June 30,
|
June 30,
|
|||||||
|
2009
|
2008
|
|||||||
|
Total revenues
|
||||||||
|
AMI Australia
|
41,801,703 | 50,572,311 | ||||||
|
AMI New Zealand
|
974,782 | 1,275,681 | ||||||
|
AMI UK
|
10,575,461 | - | ||||||
| 53,351,946 | 51,847,992 | |||||||
|
Income (Loss) from operations
|
||||||||
|
AMI Inc.
|
(176,594 | ) | (227,270 | ) | ||||
|
AMI Australia
|
(9,017,724 | ) | 4,292,129 | |||||
|
AMI New Zealand
|
(290,137 | ) | (615,536 | ) | ||||
|
AMI UK
|
(2,017,595 | ) | - | |||||
| (11,502,050 | ) | 3,449,323 | ||||||
|
Income tax (expense) benefit
|
||||||||
|
AMI Australia
|
2,760,387 | (1,257,679 | ) | |||||
|
AMI New Zealand
|
- | - | ||||||
|
AMI UK
|
- | - | ||||||
|
Others
|
- | - | ||||||
| 2,760,387 | (1,257,679 | ) | ||||||
|
Net income (loss)
|
||||||||
|
AMI Inc.
|
(176,594 | ) | (227,271 | ) | ||||
|
AMI Australia
|
(6,340,886 | ) | 3,054,583 | |||||
|
AMI New Zealand
|
(284,129 | (615,443 | ) | |||||
|
AMI
UK
|
(2,017,595 | - | ||||||
|
Discontinued
operation
|
(525,785 | (1,217,090 | ) | |||||
| (9,344,989 | ) | 994,779 | ||||||
|
Provision
for depreciation and amortization
|
||||||||
|
AMI
Australia
|
1,535,715 | 1,787,437 | ||||||
|
AMI
New Zealand
|
7,033 | - | ||||||
|
AMI
UK
|
84,462 | - | ||||||
| 1,627,210 | 1,787,437 | |||||||
|
Total
Assets
|
||||||||
|
AMI
Australia
|
44,531,003 | 54,148,005 | ||||||
|
AMI
New Zealand
|
436,457 | 708,070 | ||||||
|
AMI
UK
|
2,521,411 | - | ||||||
|
Discontinued
operation
|
289,977 | 505,647 | ||||||
| 47,778,848 | 55,361,722 | |||||||
F-30
