As
filed with the Securities and Exchange Commission on
November 6, 2009
Registration
No. 333-160833
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Amendment
No. 2 to
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
POLYMEDIX, INC.
(Exact name of registrant as specified in its charter)
| |
|
|
|
|
Delaware
(State or other jurisdiction of
incorporation or organization)
|
|
2834
(Primary Standard Industrial
Classification Code Number)
|
|
27-0125925
(I.R.S. Employer
Identification No.) |
170 N. Radnor-Chester Road, Suite 300
Radnor, Pennsylvania 19087
(484) 598-2400
(Address, including zip code and telephone number, including area code, of Registrant’s principal executive offices)
The Corporation Trust Company
Corporation Trust Center
1209 Orange Street
Wilmington, Delaware
(302) 658-7581
(Name, address, including zip code and telephone number, including area code, of agent for service)
Copies to:
| |
|
|
|
|
Nicholas Landekic
Edward Smith
PolyMedix, Inc.
170 N. Radnor-Chester Road
Suite 300
Radnor, PA 19087
(484) 598-2400
|
|
Jeffrey P. Libson, Esq.
Donald Readlinger, Esq.
Pepper Hamilton LLP
400 Berwyn Park
899 Cassatt Road
Berwyn PA, 19312-1183
(610) 640-7800 |
|
John D. Hogoboom, Esq.
Lowenstein Sandler PC
65 Livingston Avenue
Roseland NJ 07068
(973) 597-2500 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after
this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or
continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box:
o
If this Form is filed to register additional securities for an offering pursuant to Rule
462(b) under the Securities Act, please check the following box and list the Securities Act
registration statement number of the earlier effective registration statement for the same
offering: o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities
Act, check the following box and list the Securities Act registration statement number of the
earlier effective registration statement for the same offering: o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities
Act, check the following box and list the Securities Act registration statement number of the
earlier effective registration statement for the same offering: o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| |
|
|
|
|
|
|
Large accelerated filer o
|
|
Accelerated filer o
|
|
Non-accelerated filer o
|
|
Smaller reporting company þ |
|
|
|
|
(Do not check if a smaller reporting company) |
|
|
CALCULATION OF REGISTRATION FEE
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |
|
|
|
Proposed |
|
|
|
|
| |
|
|
|
maximum |
|
|
|
|
| |
|
|
|
aggregate |
|
|
Amount of |
|
| |
Title of each class of |
|
|
offering |
|
|
registration |
|
| |
securities to be registered |
|
|
price |
|
|
fee (1) |
|
| |
Common Stock |
|
|
|
— |
|
|
|
|
— |
|
|
| |
Warrants to purchase Common Stock |
|
|
|
— |
|
|
|
|
— |
|
|
| |
Common Stock issuable upon exercise of Warrants (2) |
|
|
|
— |
|
|
|
|
— |
|
|
| |
Series C Preferred Stock Purchase Rights (3) |
|
|
|
— |
|
|
|
|
— |
|
|
| |
Total Registration Fee |
|
|
|
$24,062,500 |
|
|
|
|
$1,342.69(4) |
|
|
| |
(1) Calculated pursuant to Rule 457(o) on the basis of the maximum aggregate offering price
of all of the securities to be registered.
(2)
Pursuant to Rule 416, the securities being registered hereunder
include such indeterminant number of additional shares of common
stock as may be issuable upon exercise of warrants registered
hereunder as a result of stock splits, stock dividends, or similar
transactions.
(3) This
registration statement also relates to the rights to purchase shares of Series C Preferred Stock
of the registrant, which, pursuant to the terms of the registrant’s Rights Agreement dated May 12,
2009, will be attached to all shares of common stock issued until the occurrence of certain events
prescribed in the Rights Agreement. The rights will not be exercisable and will be transferred
with and only with shares of our common stock until the occurrence of certain events prescribed in
the Rights Agreement.
(4)
In connection with its filing on Form S-1 on July 28, 2009, the Registrant paid an aggregate filing fee of $1,116 with respect to the
registration of common stock with a proposed maximum aggregate offering price of $20,000,000. Concurrently with the filing of this Amendment No. 2 to the Registration Statement, the Registrant has transmitted $226.69, representing the additional filing fee payable with respect to
the $4,062,500 increase in the proposed maximum aggregate offering price set forth herein.
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE
NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH
SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN
ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF 1933 OR UNTIL THE REGISTRATION STATEMENT
SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(a), MAY
DETERMINE.
The information in this prospectus is not complete and may be changed. We may not sell these
securities until the registration statement filed with the Securities and Exchange Commission is
effective. This prospectus is not an offer to sell these securities and it is not soliciting an
offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED NOVEMBER 6, 2009
PROSPECTUS
UNITS,
EACH CONSISTING OF
SHARE(S) OF COMMON STOCK AND
WARRANT(S) TO PURCHASE
OF
A SHARE OF COMMON STOCK
We
are offering up
to units,
each consisting
of share(s) of our common
stock and warrant(s) to purchase of
a share of our common stock at an exercise price of $
per share, subject to adjustment. The warrants will be exercisable on or after the closing date of this offering through and including close of business on , 20 . The
units will not be certificated and the common stock and warrants will be immediately separable and
will be separately transferable immediately upon issuance.
Our common stock is presently quoted on the OTC Bulletin Board, under the symbol “PYMX.” We do
not intend to apply for listing of the warrants on any securities
exchange. On November 4, 2009, the
last reported sale price of our common stock on the OTC Bulletin
Board was $1.22.
Investing in the offered securities involves risks, including those set forth in the “Risk
Factors” section of this prospectus beginning on page 7 as well as those set forth in any
prospectus supplement.
| |
|
|
|
|
|
|
|
|
| |
|
Per Unit |
|
Total |
Offering Price per Unit |
|
$ |
|
|
|
$ |
|
|
Placement Agent’s Fees |
|
$ |
|
|
|
$ |
|
|
Offering Proceeds before expenses |
|
$ |
|
|
|
$ |
|
|
Merriman
Curhan Ford & Co., Boenning & Scattergood and Noble
Financial Capital Markets have agreed to act as our placement agents in connection with this offering. The placement agents are not purchasing the securities offered by us, and are not required to sell any specific number or
dollar amount of units, but will assist us in this offering on a reasonable “best efforts” basis. We have
agreed to pay the placement agents a cash fee equal to 7% of the gross proceeds of the offering of
units by us and to issue to the placement agents warrants to purchase shares of our common stock equal to
1% of the aggregate number of shares of common stock included in units sold in the offering. The
placement agent warrants will have terms substantially similar to the warrants included in units
offered hereby, except that the placement agent warrants will have a
term of five years from the effective date of the registration
statement of which this prospectus is a part and will otherwise comply with FINRA Rule 5110 (g)(1). We estimate the
total expenses of this offering, excluding the placement agent fees,
will be approximately $300,000.
Because there is no minimum offering amount required as a condition to closing in this offering,
the actual public offering amount, placement agent fees, and proceeds to us, if any, are not
presently determinable and may be substantially less than the total maximum offering amounts set
forth above. See “Plan of Distribution” beginning on page 77 of this prospectus for more
information on this offering and the placement agent arrangements.
The
delivery of the shares and warrants is expected to be made on or
about ______, 2009.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS
APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS
PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Merriman Curhan Ford & Co. Boenning and Scattergood, Inc. Noble Financial Capital Markets
As Placement Agents
The date of this prospectus is , 2009.
TABLE OF CONTENTS
| |
|
|
|
|
| |
|
PAGE |
STATEMENT REGARDING FORWARD LOOKING STATEMENTS |
|
|
1 |
|
PROSPECTUS SUMMARY |
|
|
2 |
|
THE OFFERING |
|
|
7 |
|
RISK FACTORS |
|
|
8 |
|
USE OF PROCEEDS |
|
|
23 |
|
DILUTION |
|
|
24 |
|
OUR BUSINESS |
|
|
25 |
|
EMPLOYEES |
|
|
45 |
|
DESCRIPTION OF PROPERTY |
|
|
45 |
|
LEGAL PROCEEDINGS |
|
|
45 |
|
MANAGEMENT |
|
|
46 |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
|
|
50 |
|
EXECUTIVE COMPENSATION |
|
|
54 |
|
DIRECTOR COMPENSATION |
|
|
57 |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS AND PLAN OF OPERATION |
|
|
59 |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
|
|
67 |
|
CORPORATE GOVERNANCE |
|
|
69 |
|
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS |
|
|
70 |
|
DESCRIPTION OF SECURITIES |
|
|
72 |
|
PLAN OF DISTRIBUTION |
|
|
77 |
|
EXPERTS |
|
|
79 |
|
LEGAL MATTERS |
|
|
79 |
|
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS |
|
|
79 |
|
COMMISSION’S POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES |
|
|
79 |
|
ADDITIONAL INFORMATION |
|
|
79 |
|
You should rely only on the information contained in this prospectus. We have not authorized
anyone to provide you with information different from that contained in this prospectus. We are
offering to sell, and seeking offers to buy, the units only in jurisdictions where such offers and
sales are permitted. The information contained in this prospectus is accurate only as of the date
of this prospectus, regardless of the time and delivery of this prospectus or any sale of units.
STATEMENT REGARDING FORWARD LOOKING STATEMENTS
Some of the statements included in this prospectus and any prospectus supplement contain
forward-looking statements made pursuant to the safe harbor provisions of the Private Securities
Litigation Reform Act of 1995. All statements other than statements of historical facts contained
in this prospectus and any prospectus supplement, including statements regarding our plans,
objectives, goals, strategies, future events, capital expenditures, future results, our competitive
strengths, our business strategy and the trends in our industry are forward-looking statements. The
words “believe,” “may,” “could,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,”
“expect,” “appear,” “future,” “likely,” “probably,” “suggest,” “goal,” “potential” and similar
expressions, as they relate to us, are intended to identify forward-looking statements. All
statements, other than statements of historical fact, included in this prospectus and any
prospectus supplement regarding our financial position, business strategy and plans or objectives
for future operations are forward looking statements.
Forward-looking statements reflect only our current expectations. In any forward-looking
statement, where we express an expectation or belief as to future results or events, such
expectation or belief is expressed in good faith as of the date of such statement and believed to
have a reasonable basis, but the statement of expectation or belief
may not be achieved or accomplished. Our actual results, performance or achievements could differ
materially from those expressed in, or implied by, the forward-looking statements due to a number
of factors, many of which may be unforeseen, including:
| |
Ø |
|
our need for, and the availability of, substantial capital in the future to fund
our operations and planned clinical trials; |
| |
| |
Ø |
|
the conditions in the capital markets and the biopharmaceutical industry that
may make raising capital or entering into strategic arrangements difficult and
expensive; |
| |
| |
Ø |
|
the timing of our product development and evaluation; |
| |
| |
Ø |
|
the timing and magnitude of expenditures we may incur in connection with our
ongoing research and development activities; |
| |
| |
| |
Ø |
|
the results of our preclinical and clinical trials
(including ongoing clinical trials),
including regulatory
approvals; |
| |
| |
| |
Ø |
|
the maintenance of our existing licenses with the University of Pennsylvania and
the University of Massachusetts; |
| |
| |
Ø |
|
the success, timing and financial consequences of our formation of new business
relationships and alliances; and |
| |
| |
| |
Ø |
|
the timing and volume of sales of products for which we may obtain marketing
approval. |
| |
In addition, you should refer to the “Risk Factors” section of this prospectus beginning on
page 8 for a discussion of other factors that may cause our actual results to differ materially
from those implied by our forward-looking statements. As a result of these factors, the forward-looking statements in this prospectus and any prospectus supplement
may not prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate,
the inaccuracy may be material. In light of the significant uncertainties in these forward-looking
statements, you should not regard any forward-looking statement as a representation or warranty by us or any
other person that we will achieve our objectives and plans in any specified time frame, if at all.
Accordingly, you should not place undue reliance on these forward-looking statements. All
subsequent written and oral forward looking statements attributable to us or the persons acting on
our behalf are expressly qualified in their entirety by the applicable cautionary statements. We
undertake no obligation to update any of these forward looking statements, whether as a result of
new information, future events or otherwise, except as required by applicable law or regulation.
PROSPECTUS SUMMARY
The following summary highlights selected information contained in this prospectus. Because it
is a summary, it does not contain all the information you should
consider before investing in the units. Before making any investment decision, you should read the entire prospectus
carefully, including the “Risk Factors” section of this prospectus beginning on page 8, the
financial statements and the notes to the financial statements. Unless stated otherwise, references
in this prospectus to the “Company,” “we,” “us,” or “our” refer to PolyMedix, Inc., a Delaware
corporation.
THE COMPANY
We are a biotechnology company focused on treating life threatening, serious, acute infectious
diseases and cardiovascular disorders with synthetic small molecule compounds that mimic the
activity of large natural protein molecules, compounds referred to as biomimetics. Using our
proprietary computational drug design technology, we have created novel defensin mimetic antibiotic
compounds, heparin antagonist compounds (or “heptagonist”) and other drug compounds intended for
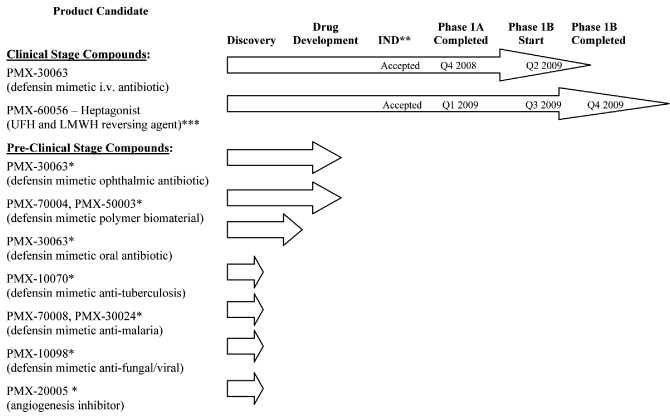
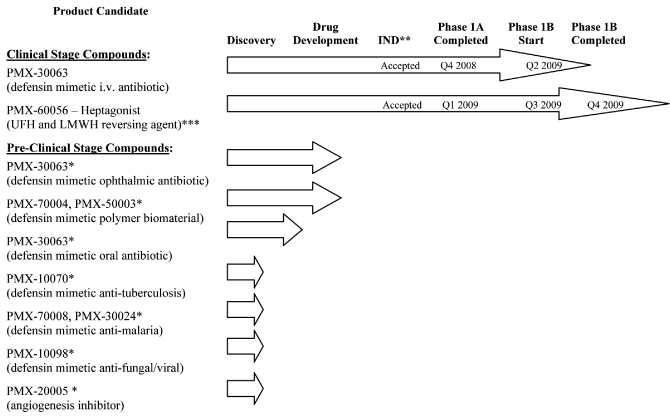
human therapeutic use. The following chart illustrates the current stage of development of our
internally developed pipeline along with planned development timings for our two lead clinical programs:
|
|
|
| * |
|
Program on hold |
| |
| ** |
|
Investigational New Drug Application (or “IND”) or equivalent Canadian Clinical Trial Application (or “CTA”) |
| |
| *** |
|
Unfractionated heparin (“UFH”) and low molecular weight heparin (“LMWH”) |
Clinical Stage Compounds:
Defensin mimetic antibiotics are compounds that have a novel mechanism-of-action that mimics
the antimicrobial activity of host defense proteins, a group of proteins naturally found in most
higher forms of life including humans, which provide the first line of defense against bacterial
infections. We believe that bacteria are less likely to develop resistance to antibiotic products with this
-2-
mechanism-of-action than currently marketed antibiotics. Most antibiotics exert their activity by interacting with a
specific biochemical molecular target or pathway, such as an enzyme. Bacteria can often develop
mutations in these molecular targets in a process called “target mutation”, which renders the
antibiotic drug unable to interact with its intended biochemical target. Additionally, bacteria
can sense that a foreign chemical has entered its cell and can pump it out before it can bind to
its intended biochemical target, through a process called efflux. Both of these factors can cause
bacteria to become drug-resistant. Instead of relying on a specific molecular target that can
mutate, our product candidates target the entire bacterial cell membrane. Our defensin mimetic
antibiotic product candidates destabilize the bacterial cell membrane and cause either leakage of
cellular content or complete disruption of the membrane, which results in bacterial cell death.
Additionally, because our product candidates target the bacterial cell membrane and do not have to
enter the cell, the opportunity for bacterial efflux is reduced. We believe this
mechanism-of-action makes it unlikely for bacteria to develop resistance to our defensin mimetic
antibiotic product candidates.
Worldwide, the hospital antibiotic market is $8 billion, treating approximately seven million
patients per year and the total worldwide antibiotic market is $26 billion, treating approximately
49 million patients per year. According to the Centers for Disease Control and Prevention, or CDC,
bacterial infections are the fourth leading cause of death in the U.S. and are one of the fastest
growing causes of death. Because of the increasing rate of bacterial resistance and the
corresponding death rate, we believe there is a significant medical and market need for a new class
of antibiotics, defensin mimetics, with a novel mechanism of action designed to make it unlikely
for bacteria to develop resistance. Our first product candidate is an i.v. formulation initially
intended for the treatment of complicated skin and soft tissue infections, caused by strains of
Staph bacteria. Future applications may include other infections such as gynecological or
abdominal. We anticipate that the bacterial targets of PMX-30063 will include drug resistant
strains, particularly broadly targeting the various strains of Staph bacteria. In the future, we
also hope to expand indications to potentially include other bacterial strains.
In December 2008, we completed and announced positive results from our single dose escalation
clinical study of healthy volunteers receiving PMX-30063 at various dose levels (Phase 1A). This
ascending single-dose intravenous pharmacokinetic and safety study met the necessary Phase 1 goals
of defining both a limiting single dose and the plasma distribution/elimination kinetics. In this
study, the dose was not limited by any measurable clinical or laboratory parameters. A subjective
syndrome of paresthesias was identified, appearing only at the higher dosages and consisting of
abnormal neuronal sensations (numbness and/or tingling) often likened to dental anesthesia. These
effects were graded as mild to moderate by investigators or subjects, but their reproducibility and
dose-proportionality allowed dose-escalation to be successfully concluded after achieving levels
well in excess of the expected therapeutic range. The effects were temporary and resolved on their
own. The same study provided detailed information on the time course of the drug during and after
dosing. These pharmacokinetics appear favorable for therapeutic use of the drug. The half-time for
elimination from the plasma was approximately 12 to 15 hours, allowing for flexibility in dosing to
obtain optimal peak and trough drug levels.
In
June 2009, we commenced an ascending multi-dose intravenous pharmacokinetic and safety study
of healthy volunteers who will receive 5 intravenous doses of PMX-30063 at various dose levels
(Phase 1B). The primary endpoint for the two Phase 1 studies is a safety assessment, including
identification of dose limiting toxicity. We have conducted these
clinical studies in Canada under a CTA to United States
standards, and plan to submit an IND with the FDA before commencing Phase 2 studies in the United States, and
ultimately seek regulatory approval in the United States.
Heptagonists such as PMX-60056, our second development program, are compounds that bind to and
thereby reverse the anticoagulant effects of both Unfractionated heparin (or UFH) and Low Molecular
Weight Heparins (or LMWH). UFH is a class of commonly used blood clot prevention drugs used in
numerous surgical applications, such as cardiothoracic procedures. LMWH is another class of
commonly used blood clot prevention drugs used in patients under longer-term administration,
because of their ease of administration and longer half-life. Overdoses of UFH or LMWH are
dangerous due to potentially life-threatening bleeding. While widely used, UFH and LMWH have the
possibility of adverse bleeding side effects, which requires frequent monitoring. Protamine is the
only available reversing agent for UFH. It is used as both an antidote in the event of overdose
and, more commonly, in standard treatment following coronary artery bypass grafts in which
post-operative treatment involves administering protamine to counter the action of UFH, which is to
prevent blood clots while a patient is on a heart-lung support machine. While widely used to
reverse UFH, protamine has a number of adverse side effects, including bleeding, difficulty and
complexity in dose titration, allergic reactions, immunogenicity, and abnormal clot
-3-
formation, all of which require frequent monitoring and follow up. As a result, there is a
significant medical and market need for a safer UFH reversing agent. Additionally, there is
currently no approved reversing agent for LMWH.
Worldwide, we estimate that approximately two million extracorporeal blood procedures,
including cardiothoracic procedures, are performed per year that utilize heparin and may require
UFH reversal, and approximately twelve million patients receive LMWH per year for a variety of
uses, including deep vein thrombosis, post heart attack, cancer, and atrial fibrillation. There
have been several published studies which demonstrate that up to 20% of LMWH patients experience
clinically significant bleeding, and up to 4% of cases are considered to be serious or life
threatening. The availability of an antidote to LMWH would allow patients experiencing a LMWH
bleeding event to be pharmaceutically treated. Additionally, the availability of a reversing agent
for LMWH could allow LMWH to be used in cardiothoracic surgical procedures or in patients who may
need to undergo re-operation. Thus, an LMWH antagonist could substantially increase the market and
sales of LMWH drugs.
In March 2009, we successfully completed our first-in-man clinical study with the novel
heparin antagonist drug PMX-60056. This ascending single-dose intravenous pharmacokinetic and
safety study met the necessary Phase 1 goals of defining both a limiting single dose for ten-minute
infusions and also the plasma distribution/elimination kinetics for the drug in the absence of
heparin. This first study has demonstrated that PMX-60056 can be given safely in the absence of
heparin if ten-minute infusions include less than 0.4 mg/kg. The data suggest that the limiting
side effect of hypotension is related to peak plasma drug level.
Analysis of the plasma-level assays indicates that a single-compartment model is
consistent with the observed data, and the plasma elimination half-time is 1.5 to 2.5 minutes in
the dosage range administered.
In October 2009, we completed a second successful clinical study of PMX-60056 (Phase
1B). The clinical study was a pilot proof-of-concept study conducted using a double-blind,
placebo-controlled, crossover design, with six healthy human volunteer subjects. This study
design and low number of subjects were intended to generate meaningful results for a pilot study
at minimal cost. Twenty minutes after administration of a 70 U/kg dose of heparin, which is
sufficient to produce anticoagulant activity, each of the subjects was administered, in a blinded
manner, either a single dose of 0.3 mg/kg of PMX-60056 or a placebo as a 10-minute
intravenous infusion. With the crossover design, each subject was dosed twice, initially with
heparin and either PMX-60056 or a placebo and then, two days after the first dose, with heparin
and whichever (PMX-60056 or a placebo) he did not receive in the first dose. Each subject thus
acted as his own control.
The
primary endpoint of the clinical study was safety, specifically whether blood
pressure decreases would be mitigated by the presence of heparin, and the secondary endpoint
was efficacy, as measured by blood clotting time. The desired outcomes were no clinically
significant decrease in blood pressure, and rapid reversal of activated clotting time (ACT), a
standard bedside blood clotting measurement, and activated partial thromboplastin time (aPTT),
a more accurate laboratory measurement of blood clotting time. These blood clotting times are
machine read to ensure objective measurement.
No
serious adverse events (SAEs) occurred during this Phase 1B clinical study of PMX-60056. No
clinically significant blood pressure effects were observed in this study in the
presence of heparin. There were several non-serious reports of “warmth” and/or “itching” during
and shortly after the infusions, but no such effects were reported significantly beyond the 10-minute infusion time.
In all subjects receiving PMX-60056,
there was a rapid and complete reversal of the
anticoagulant action of heparin, as measured by ACT and aPTT. Each subject’s minimum ACT
and aPTT readings after being dosed with PMX-60056 were at or below the subject’s ACT and
aPTT readings prior to being dosed with heparin. The heparin reversal appeared to occur at or
before the end of the 10-minute infusion of PMX-60056, suggesting that the 0.3 mg/kg dose may
have been in excess of requirements for 70U/kg heparin. Furthermore, the reversal was
permanent: there was no return of anticoagulation during the hours required for heparin’s effects
to decline naturally.
We
believe that our current cash and investment balances, exclusive of any cash to be
generated from this offering or our $10 million Equity Line with Dutchess Equity
Fund, L.P. that we established in May, 2009, which we refer to
as our Equity Line, will be sufficient to fund our planned Phase 1B
study for PMX-30063, and can fund our operations into the fourth quarter of 2010. Due to continued weakness
in capital markets and our understanding that it may be difficult to secure significant additional
financing on favorable terms, we have begun and expect to continue to scale back and delay some of
our planned activities in order to prolong our cash resources. Our current cash and investment
balances are not sufficient to initiate or fund Phase 2 clinical trials or any of the related
development activities for PMX-30063 or PMX-60056. We do, however, anticipate that upon a
successful completion of this offering, a significant portion of the net proceeds will be used for
such activities.
Pre-Clinical Stage Compounds:
While our lead product candidates are our priority, to the extent we obtain targeted grants or
research contracts, or determine an allocation of resources is appropriate, we also intend to
conduct pre-clinical development of our antibiotic compounds for both topical and oral antibiotic
applications. The initial topical application we plan to pursue is an ophthalmic antibiotic for
the treatment of eye infections, and the initial oral application we are investigating is a
non-absorbed antibiotic for the treatment of gastrointestinal infections such as Clostridium
difficile. Provided that we are able to obtain and allocate
sufficient resources, including
financing, we hope to advance pre-clinical development and file additional IND applications for the
ophthalmic topical and non-absorbed oral antibiotic indications.
We are also evaluating our antibiotic compounds for a number of other topical applications,
including use for skin structure infections, oral healthcare applications for treatment of
periodontal disease, a type of gum disease,
-4-
topical treatment for ear infections, topical treatment of fungal infections, topical
treatment of acne, and a variety of non-therapeutic applications in personal care and materials
applications.
SUMMARY FINANCIAL DATA
Because this is only a summary of our financial information, it does not contain all of the
financial information that may be important to you. Therefore, you should carefully read all of
the information in this prospectus, including the financial statements and their explanatory notes
and the section entitled “Management’s Discussion and Analysis of Financial Condition and Results
of Operations,” before making a decision to invest in the units. The information contained in the
following summary is derived from our financial statements for the quarters ended June 30, 2009
and 2008 and the years ended December 31, 2008 and 2007.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Six months ended |
|
Years ended |
| |
|
June 30, |
|
December 31, |
| (in thousands) |
|
2009 |
|
2008 |
|
2008 |
|
2007 |
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant and research revenues |
|
$ |
144 |
|
|
$ |
1,009 |
|
|
$ |
1,066 |
|
|
$ |
1,126 |
|
Operating expenses |
|
|
6,017 |
|
|
|
6,831 |
|
|
|
12,276 |
|
|
|
13,801 |
|
Net loss |
|
|
(5,835 |
) |
|
|
(5,727 |
) |
|
|
(10,986 |
) |
|
|
(12,164 |
) |
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cash and investments |
|
$ |
10,356 |
|
|
$ |
3,479 |
|
|
$ |
15,106 |
|
|
$ |
8,903 |
|
Total assets |
|
|
11,578 |
|
|
|
4,480 |
|
|
|
16,077 |
|
|
|
9,722 |
|
Stockholders’ equity |
|
|
8,155 |
|
|
|
111 |
|
|
|
13,143 |
|
|
|
5,134 |
|
BUSINESS HISTORY
PolyMedix, Inc. (formerly known as BTHC II Acquisition Corp.) was originally organized in the
State of Texas as BTHC II LLC, a public shell company. On September 29, 2004, BTHC II LLC and its
sister companies filed an amended petition under Chapter 11 of the United States Bankruptcy Code.
On November 22, 2004, the court approved BTHC II LLC’s Amended Plan of Reorganization. On March
24, 2005, and in accordance with its Amended Plan of Reorganization, BTHC II LLC changed its state
of organization from Texas to Delaware by merging with and into BTHC II Acquisition Corp., a
Delaware corporation formed solely for the purpose of effecting the reincorporation. After this
merger, BTHC II Acquisition Corp. became the public shell company.
PolyMedix Pharmaceuticals, Inc. (formerly known as PolyMedix, Inc.) was incorporated on August
8, 2002 in the State of Delaware as PolyMedix, Inc. to serve as a biotechnology company focused on
treating infectious diseases. On October 6, 2005, the former BTHC II Acquisition Corp. entered
into an Agreement and Plan of Merger and Reorganization with the former PolyMedix, Inc., pursuant
to which the stockholders of the former PolyMedix, Inc. exchanged their equity ownership interests
in the former PolyMedix, Inc. for an aggregate 94% equity ownership interest in the former BTHC II
Acquisition Corp. Pursuant to the Agreement and Plan of Merger and Reorganization, the former
PolyMedix, Inc. merged with a wholly-owned subsidiary of the former BTHC II Acquisition Corp. and,
as such, became a wholly-owned subsidiary of the former BTHC II Acquisition Corp.
At the time of the merger, the former BTHC II Acquisition Corp. had no operations and no
assets or liabilities. Because the stockholders of the former PolyMedix, Inc. exchanged their
equity ownership interests in the former PolyMedix, Inc. for an aggregate 94% equity ownership
interest in the former BTHC II Acquisition Corp., the former PolyMedix, Inc. was, for accounting
purposes, the surviving entity of the merger. Accordingly, all financial information included in
this prospectus for periods prior to the merger is for the former PolyMedix, Inc.
On February 24, 2006, the former BTHC II Acquisition Corp. changed its corporate name to
PolyMedix, Inc., and the former PolyMedix, Inc. changed its corporate name to PolyMedix
Pharmaceuticals, Inc.
In July, 2008, we sold 21,428,571
units of stock and warrants for aggregate consideration
of approximately $15 million. At closing each unit separated into one share of our common
stock and a warrant to purchase one share of our common stock. The securities sold in the
offering were registered with the Securities and Exchange Commission on Form SB-2, Registration No. 333-146180.
In December, 2007, we sold 2,943,222 units of
stock and warrants for aggregate
consideration of approximately $3.2 million. At closing each unit separated into one share of our
common stock and a warrant to purchase one share of our common stock. The securities sold in
the offering were registered with the Securities and Exchange Commission on Form S-1, Registration No. 333-151084.
The net proceeds from each of the referenced registered offerings were used to support
the commercialization of our current and future product candidates, to fund our research and
development activities, and for general working capital needs.
-5-
Our Principal Executive Offices
Our principal executive offices are located at 170 N. Radnor-Chester Road, Suite 300, Radnor,
Pennsylvania 19087. Our telephone number is (484) 598-2400 and our website address is
www.polymedix.com. Information included or referred to on our website is not a part of this
prospectus.
-6-
THE OFFERING
| |
|
|
Securities Offered:
|
|
Up to units. Each unit
consists of share(s)
of our common stock and
warrant(s) to purchase
of a share of our common stock. The units will not be certificated and the common stock
and warrants will be immediately separable and will be separately
transferable immediately upon issuance. |
|
|
|
Offering Price:
|
|
$ per unit. |
|
|
|
Description of Warrants:
|
|
The warrants will be exercisable
on or after the closing
date of this offering through and
including close of business on
, 20 at an exercise
price of $ per share. |
|
|
|
Common Stock Outstanding Before the
Offering:
|
|
59,845,065 shares |
|
|
|
Common Stock Outstanding After the
Offering:
|
|
shares, which does not include
shares of common stock
issuable upon exercise of the
warrants included in the offered
units or the shares of common
stock issuable upon the exercise
of the placement agent warrants. |
|
|
|
Use of Proceeds:
|
|
We expect to use the proceeds
received from the offering to fund
our research and development
activities, including Phase 2
clinical trials and related
activities for PMX-30063 or
PMX-60056, and for general working
capital needs. |
|
|
|
Risk Factors:
|
|
See “Risk Factors” beginning on
page 8 and the other information
in this prospectus for a
discussion of the factors you
should consider before you decide
to invest in the units. |
The
total number of shares of our common stock outstanding as of
October 30, 2009 is 59,845,065,
and excludes:
| |
Ø |
|
12,000,000 shares of common stock reserved for issuance upon puts to Dutchess
pursuant to our Equity Line; |
| |
| |
Ø |
|
11,023,000 shares of common stock issuable upon exercise of outstanding stock
options at a weighted average exercise price of $1.40 per share; |
| |
| |
Ø |
|
4,119,194 additional shares of common stock reserved for issuance under various
outstanding warrant agreements, at an exercise price of $1.23, as the same may be
adjusted as a consequence of the weighted average anti-dilution provisions
contained in such warrant agreements; |
| |
| |
Ø |
|
2,943,222 additional shares of common stock reserved for issuance under various
outstanding warrant agreements, at an exercise price of $1.10; |
| |
| |
Ø |
|
28,653,495 additional shares of common stock reserved for issuance under the
Series A and Series B Warrants, including placement agent warrants, at an exercise
price of $1.00; and |
| |
| |
| |
Ø |
|
14,570,000 additional shares of common stock reserved for future issuance under
our stock plans. |
| |
Unless otherwise specifically stated, information throughout this prospectus does not assume
the exercise of outstanding options or warrants to purchase shares of our common stock or that we
have drawn down on our Equity Line with Dutchess.
-7-
RISK FACTORS
In addition to the other information included in this prospectus and any prospectus
supplement, the following factors should be carefully considered in
evaluating an investment in the units and our business,
financial position and future prospects. Any of the following risks, either alone or taken
together, could materially and adversely affect an investment in the
units and our business, financial position or future
prospects. If one or more of these or other risks or uncertainties materialize, or if our
underlying assumptions prove to be incorrect, our actual results may vary materially from what we
have projected. There may be additional risks that we do not presently know or that we currently
believe are immaterial which could also materially adversely affect
an investment in the units and our business, financial
position or future prospects.
Risks Related to Our Business
We have incurred losses since inception and anticipate that we will continue to incur losses for
the foreseeable future.
We are a development stage company with a limited operating history. As of June 30, 2009, we
had an accumulated deficit of approximately $42.7 million. We expect to continue to incur significant
and increasing operating losses, in the aggregate and on a per share basis, for the next several
years. These losses, among other things, have had and will continue to have an adverse effect on
our stockholders’ equity, net current assets and working capital.
Because of the numerous risks and uncertainties associated with developing new drugs, we are
unable to predict the extent of any future losses or when we will become profitable, if at all.
Currently, we have no products available for commercial sale, and, to date, we have not generated
any product revenue. We have financed our operations and internal growth primarily through the sale
of equity securities. We have devoted substantially all of our efforts to research and development,
including cGMP manufacturing and cGLP compliant toxicology, safety pharmacology, genotoxicity
studies and clinical studies for our lead clinical product candidates.
If we are unable to meet our needs for additional funding in the future, we will be required to
limit, scale-back or cease operations.
We do not currently have the funding resources necessary to carry out all of our proposed
short and long-term operating activities. We estimate that it will cost $65 to $85 million in
research, drug development and clinical development costs over 30
— 42 months to file an NDA for
PMX-30063 (assuming accelerated approval status is not granted), and a like amount for PMX-60056,
in each case assuming adequate and timely financing. Even if we sell all of the securities subject
to this offering on favorable terms, of which there can be no assurance, we will still need to
obtain additional financing in the future in order to fully fund these product candidates through
the regulatory approval process. Costs of our proposed activities unrelated to our two lead
product candidates would be in addition to these estimates.
We believe that our current cash and investment balances, exclusive of any cash to be
generated from this offering or the Equity Line, will be sufficient
to fund our ongoing Phase 1B
study for PMX-30063, and can fund our operations into the fourth quarter of 2010.
Due to continued weakness in capital markets and our understanding that it may be difficult to
secure significant additional financing on favorable terms, we have begun and expect to continue to
scale back and delay some of our planned activities in order to prolong our cash resources. Our
current cash and investment balances are not sufficient to initiate or fund Phase 2 clinical trials or any of the related
development activities for PMX-30063 or PMX-60056. If we are unable to secure adequate additional
funding during 2009, through this offering or otherwise, we will further delay, scale-back or
eliminate certain of our planned research, drug discovery and development activities and certain
other aspects of our operations and our business until such time as we are successful in securing
adequate additional funding.
Our future capital requirements will depend on many factors, including:
| |
Ø |
|
success of our clinical trials for PMX-30063 and PMX-60056; |
-8-
| |
Ø |
|
continued progress of and increased spending related to our research and
development activities, including our plan to hire additional research and
development employees; |
| |
| |
| |
Ø |
|
the conditions in the capital markets and the biopharmaceutical industry that
make raising capital or entering into strategic arrangements difficult or
expensive; |
| |
| |
| |
Ø |
|
progress with preclinical experiments and clinical trials; |
| |
| |
Ø |
|
ongoing general and administrative expenses related to our being a reporting company; |
| |
| |
Ø |
|
the cost, timing, and results of regulatory reviews and approvals; |
| |
| |
Ø |
|
the maintenance of our existing licenses with the University of Pennsylvania and
the University of Massachusetts; |
| |
| |
| |
Ø |
|
the success, timing, and financial consequences of any future collaborative,
licensing or other arrangements that we may establish; |
| |
| |
| |
Ø |
|
the cost of filing, prosecuting, defending and enforcing any patent claims and
other intellectual property rights; |
| |
| |
| |
Ø |
|
the costs of commercializing any of our product candidates; |
| |
| |
| |
Ø |
|
technological and market developments; |
| |
| |
Ø |
|
the cost of manufacturing development; and |
| |
| |
| |
Ø |
|
timing and level of sales of any products for which we may obtain marketing approval. |
| |
These factors could result in variations from our currently projected operating and liquidity
requirements. Additional funds may not be available when needed, or, if available, we may not be
able to obtain such funds on terms acceptable to us. If adequate funds are unavailable, we may be
required, among other things, to:
| |
Ø |
|
delay, reduce the scope of or eliminate one or more of our research or
development programs; |
| |
| |
Ø |
|
license rights to technologies, product candidates or products at an earlier
stage than otherwise would be desirable or on terms that are less favorable to us
than might otherwise be available; or |
| |
| |
Ø |
|
obtain funds through arrangements that may require us to relinquish rights to
product candidates or products that we would otherwise seek to develop or
commercialize by ourselves. |
Funding, especially on terms acceptable to us, may not be available to meet our future capital
needs because of the deterioration of the credit and capital markets.
Global market and economic conditions have been, and continue to be, disruptive and volatile.
The debt and equity capital markets have been impacted by significant write-offs in the financial
services sector and the re-pricing of credit risk in the broadly syndicated market, among other
things. These events have negatively affected general economic conditions and it is uncertain when
the credit and capital markets will stabilize or improve.
In particular, the cost of raising money in the debt and equity capital markets has increased
substantially while the availability of funds from those markets has diminished significantly.
Also, as a result of concern about the stability of financial markets generally and the solvency of
counterparties specifically, the cost of obtaining money from the credit markets has increased as
many lenders and institutional investors have increased interest rates, enacted tighter lending
standards and reduced and, in some cases, ceased to provide funding to borrowers. Low valuations
and decreased appetite for equity investments, among other factors, may make the equity markets
difficult to access on acceptable terms or unavailable altogether.
-9-
If funding is not available when needed, or is available only on unfavorable terms, meeting
our capital needs or otherwise taking advantage of business opportunities may become challenging,
which could have a material adverse effect on our business plans.
We may not be able to access sufficient funds under our Equity Line with Dutchess Equity Fund, L.P. when needed.
Our ability to put shares to Dutchess and obtain funds under our Equity Line is limited by the terms
and conditions in the Investment Agreement with Dutchess, including restrictions on when we
may exercise our put rights, restrictions on the amount we may put to Dutchess at any one time, which is
determined in part by the trading volume of our common stock, and a limitation on our ability to put
shares to Dutchess to the extent that it would cause Dutchess to beneficially own more than 4.99% of
our outstanding shares. In addition, we do not expect the Equity Line to satisfy all of our funding needs, even if
we are able and choose to take full advantage of the Equity Line.
Development of our proposed product candidates is a lengthy, expensive and uncertain process that
requires investment of substantial amounts of time and money that may not yield viable products
which may cause our business and results of operations to suffer.
We face the risks of failure inherent in developing drugs based on new technologies. None of
our product candidates have received regulatory approval for commercial sale and our product
candidates may never be commercialized. In addition, all of our product candidates are in the
early stages of development and all of our programs except for our two lead product candidates are on hold until we are able to obtain and
allocate sufficient resources, including financing. During the third quarter of 2008, we commenced
clinical trials for the first time with our lead product candidates, PMX-30063 and PMX-60056.
We
commenced an ongoing Phase 1B study for PMX-30063 during the second quarter
of 2009. The progress and results of these and any
future clinical trials or future pre-clinical testing are uncertain, and if our product candidates
do not receive regulatory approvals, our business, operating results, financial condition and cash
flows will be materially adversely affected. Our product candidates are not expected to be
commercially available for several years, if at all.
Our development programs require a significant amount of cash to support the development of
product candidates. We estimate that it will cost $65 to $85 million in research, drug development
and clinical development costs over 30-42 months to file an NDA for PMX-30063 (assuming accelerated
approval status is not granted), and a like amount for PMX-60056, in each case assuming adequate
and timely financing. Even if we sell all of the securities subject to this offering on favorable
terms, we will still need to obtain additional financing in the future. We may not be able to
obtain such additional financing on terms acceptable to us or at all.
In addition, a number of potential drugs have shown promising results in early subject studies, but
failed in subsequent clinical trials and/or failed to obtain necessary regulatory approvals. Data
obtained from such studies are susceptible to varying interpretations, which may delay, limit or prevent
regulatory approval. Regulatory authorities may refuse or delay approval as a result of many other
factors, including changes in regulatory policy during the period of product development.
Completion of clinical trials may take many years. The length of time required varies
substantially according to the type, complexity, novelty and intended use of the product candidate.
The FDA monitors the progress of each phase of testing, and may require the modification,
suspension, or termination of a trial if it is determined to present excessive risks to patients.
The clinical trial process may also be accompanied by substantial delay and expense and the data
generated in these studies ultimately may not be sufficient for marketing approval by the FDA. Our
rate of commencement and completion of clinical trials may be delayed by many factors, including:
| |
Ø |
|
our inability to manufacture sufficient quantities of materials for use in clinical trials; |
| |
| |
Ø |
|
variability in the number and types of patients available for each study; |
| |
| |
Ø |
|
difficulty in maintaining contact with patients after treatment, resulting in incomplete data; |
| |
| |
Ø |
|
unforeseen safety issues or side effects; |
| |
| |
Ø |
|
poor or unanticipated effectiveness of products during the clinical trials; and/or |
| |
| |
Ø |
|
government or regulatory delays. |
Notwithstanding the submission of any requested additional information, the FDA ultimately may
decide that the application does not satisfy the regulatory criteria for approval and refuse to
approve the application by issuing a “not approvable” letter. Furthermore, the FDA may prevent us
from marketing a product candidate under a label for its desired indications or place other
conditions, including restrictive labeling, on distribution as a condition of any approvals, which
may impair commercialization of the product.
-10-
We are a development stage company, which makes it difficult to evaluate our existing business and
business prospects and increases the risk that the value of any investment in our company may
decline.
We are a development stage company and to date, our only revenues have been from research
grants and contracts. We will not be able to generate revenue from product sales or royalties
unless and until we receive regulatory approval and begin commercialization of our product
candidates or otherwise out-license our compounds. We are not certain of when, if ever, that will
occur. Although we intend to introduce new products, we may not do so. Because the market for our
products is relatively new, uncertain and evolving, and because we are a development stage company,
it is difficult to assess or predict the growth rate, if any, and the size of this market. We may
not develop additional products, a market for our products may not develop, and/or our products may
not achieve market acceptance.
If our product candidates are not demonstrated to be sufficiently safe and effective in clinical
trials, they will not receive regulatory approval and we will be unable to commercialize them and
our business and results of operations will suffer.
Our product candidates must satisfy rigorous standards of safety and efficacy before they can
be approved by the FDA and/or other foreign regulatory authorities for commercial use. The FDA and
foreign regulatory authorities have full discretion over this approval process. We will need to
conduct significant additional research, involving testing in animals and in humans, before we can
file applications for product approval. Typically, in the pharmaceutical industry there is a high
rate of attrition for product candidates in pre-clinical testing and clinical trials. Also,
satisfaction of regulatory requirements typically takes many years, is dependent upon the type,
complexity and novelty of the product and requires the expenditure of substantial resources.
Success in pre-clinical testing and early clinical trials does not ensure that later clinical
trials will be successful. For example, a number of companies in the pharmaceutical industry,
including biotechnology companies, have suffered significant setbacks in advanced clinical trials,
even after promising results in earlier trials and in interim analyses. In addition, delays or
rejections may be encountered based upon additional government regulation, including any changes in
FDA policy, during the process of product development, clinical trials and regulatory approvals.
If we are not able to retain our current management and advisory team and attract and retain
qualified scientific, technical and business personnel, our business will suffer.
We are highly dependent on our executive officers and other key management and technical
personnel, including Nicholas Landekic, Richard Scott, Ph.D., Eric McAllister, M.D., Ph.D., Edward
Smith, and Bozena Korczak, Ph.D., as well as key members of our advisory team, including
William DeGrado, Ph.D. and Gregory Tew, Ph.D. The loss of any of them could have a material adverse
effect on our future operations. We presently do not maintain “key person” life insurance policies
on any of our personnel.
Our success is also dependent on our ability to attract, retain and motivate highly trained
technical, marketing, sales and management personnel for the development, maintenance and expansion
of our activities. We may not be able to retain our existing personnel or attract additional
qualified employees. The loss of key personnel or the inability to hire and retain additional
qualified personnel in the future could have a material adverse effect on our business, financial
condition and results of operation.
Our success is dependent on the continuation of certain licensing arrangements and other strategic
relationships with third parties. These arrangements and relationships may not continue and we may
not be successful in entering into other similar arrangements and relationships.
All of our product candidates are licensed from or based upon licenses from either the
University of Pennsylvania or the University of Massachusetts. If either or any of these license
agreements are properly terminated, our ability to advance our current product candidates or
develop new product candidates will be materially adversely affected.
In addition to these licensing arrangements, we rely on the expertise of Dr. William DeGrado,
a Professor of Biochemistry and Biophysics at the University of Pennsylvania, and Dr. Gregory Tew,
an Assistant Professor in the Polymer Science and Engineering Department at the University of
Massachusetts. If our agreements with either
-11-
or both of Drs. DeGrado and Tew, were terminated, our ability to advance our current product
candidates or develop new product candidates may be adversely affected.
We depend, and will continue to depend, on these arrangements, and potentially on other
licensing arrangements and/or strategic relationships with third parties for the research,
development, manufacturing and commercialization of our product candidates. If any of our licenses
or relationships are terminated or breached, we may:
| |
Ø |
|
lose our rights to develop and market our product candidates; |
| |
| |
Ø |
|
lose patent and/or trade secret protection for our product candidates; |
| |
| |
Ø |
|
experience significant delays in the development or commercialization of our
product candidates; |
| |
| |
Ø |
|
not be able to obtain any other licenses on acceptable terms, if at all; and/or |
| |
| |
Ø |
|
incur liability for damages. |
Licensing arrangements and strategic relationships in the pharmaceutical and biotechnology
industries can be very complex, particularly with respect to intellectual property rights. Disputes
may arise in the future regarding ownership rights to technology developed by or with other
parties. These and other possible disagreements between us and third parties with respect to our
licenses or our strategic relationships could lead to delays in the research, development,
manufacture and commercialization of our product candidates. These third parties may also pursue
alternative technologies or product candidates either on their own or in strategic relationships
with others in direct competition with us. These disputes could also result in litigation or
arbitration, both of which are time-consuming and expensive, and could require significant time and
attention from our management.
Our i.v. antibiotic product candidate or any of our other eligible product candidates may not be
granted any of the accelerated development or approval paths by the FDA and, even if any of our
product candidates receive such status, development of the product candidate may not be
accelerated.
We believe that our i.v. antibiotic product candidate, PMX-30063, which is a systemic antibiotic drug,
may be eligible for one of the accelerated development or approval paths under FDA procedures, such
as “fast track,” “priority review” or “accelerated approval.” We have not yet applied for any of
these designations for our i.v. antibiotic product candidate or any of our other product
candidates. Our product candidates may not receive any such consideration. If granted, some of
these paths may help to abbreviate the size and scope of the trials required for submission and
approval of an NDA and/or to shorten the review time of any such filing. If the FDA grants any of
these designations to any of our product candidates, we may then make an application with the FDA
with respect to any further development program and corresponding regulatory strategy.
Even in the event that one of our product candidates is designated for “fast track,” “priority
review” or “accelerated approval” status, such a designation does not necessarily mean a faster
development process or regulatory review process or necessarily confer any advantage with respect
to approval compared to conventional FDA procedures. Any accelerated designation status may be
withdrawn by the FDA if the FDA believes that this designation is no longer supported by emerging
data from our clinical development program or for patient safety reasons. Receiving “fast track,”
“priority review” or “accelerated approval” status from the FDA does not guarantee that we will
qualify for or be able to take advantage of any accelerated development or approval procedures.
Even if the accelerated development or approval procedures are available to us, depending on the
results of clinical trials, we may elect to follow the more traditional approval processes for
strategic and marketing reasons, since drugs approved under “accelerated approval” procedures may
be more likely to be subjected to post-approval Phase 4 clinical studies to provide confirmatory
evidence that they are safe and effective. If we fail to conduct any such required post-approval
studies or if the studies fail to verify that any of our product candidates are safe and effective,
our FDA approval could be revoked. It can be difficult, time-consuming and expensive to enroll
patients in Phase 4 clinical trials because physicians and patients are less likely to participate
in a clinical trial to receive a drug that is already commercially available.
-12-
Even if regulatory authorities approve our product candidates, they may not be commercially
successful.
Our product candidates may not be commercially successful because physicians, government
agencies and other third-party payors may not accept them. Third parties may develop superior
products or have proprietary rights that preclude us from marketing our products. We also expect
that most of our product candidates will be very expensive, if approved. If we do obtain regulatory
approval for any of our product candidates, we will need to achieve patient acceptance and demand
in order to be commercially successful. Patient acceptance of and demand for any product candidates
will depend upon many factors, including but not limited to, the extent, if any, of reimbursement
of drug and treatment costs by government agencies and other third-party payors, pricing, the
safety and effectiveness of alternative products, and the prevalence and severity of side effects
associated with our products. If we do not achieve product acceptance and sufficient demand, we
will not be able to sell our products and our operating results and financial condition will be
materially adversely affected.
We do not currently have sales, marketing or distribution capabilities. If we fail to effectively
sell, market and distribute any product candidate for which we receive regulatory approval, our
business and results of operations will suffer.
If we are unable to create sales, marketing and distribution capabilities or enter into
agreements with third parties to perform these functions, we will not be able to successfully
commercialize any of our product candidates that receive regulatory approval in the future. We
currently have no sales, marketing or distribution capabilities. In order to successfully
commercialize any of our product candidates, we must either internally develop sales, marketing and
distribution capabilities or make arrangements with third parties to perform these services.
If we do not develop a marketing and sales force with technical expertise and supporting
distribution capabilities, we will be unable to market any of our products directly. To promote any
of our products through third parties, we will have to locate acceptable third parties for these
functions and enter into agreements with them on acceptable terms and we may not be able to do so.
In addition, any third-party arrangements we are able to enter into may result in lower revenues
than we could have achieved by directly marketing and selling our products.
We may suffer losses from product liability claims.
Any of our product candidates could cause adverse events to patients, such as immunologic or
allergic reactions. These reactions may not be observed in clinical trials, but may nonetheless
occur after commercialization. If any of these reactions occur, they may render our product
candidates ineffective in some patients and our sales would suffer.
We may be susceptible to product liability lawsuits, from events arising out of the use of
product candidates in clinical studies. If product liability lawsuits are brought against us, we
may incur substantial liabilities and may be required to limit commercialization of our products.
Our business exposes us to potential product liability risks, which are inherent in the testing,
manufacturing, marketing and sale of pharmaceutical products. We may not be able to avoid product
liability claims. Product liability insurance for the pharmaceutical and biotechnology industries
is generally expensive, if available at all. If we are unable to protect against potential product
liability claims, we may be unable to commercialize our product candidates. A successful product
liability claim brought against us in excess of our insurance coverage may cause us to incur
substantial liabilities and, as a result, our business may fail.
Due to our reliance on third-party manufacturers, suppliers and research organizations, we may be
unable to implement our manufacturing, supply and clinical operations strategies, which would
materially harm our business.
If our current and future licensing, manufacturing and supply strategies are unsuccessful,
then we may be unable to complete any future preclinical or clinical trials and/or commercialize
our product candidates in a timely manner, if at all. Completion of any potential future
pre-clinical or clinical trials and/or commercialization of our product candidates will require access to, or
development of, facilities to manufacture a sufficient supply of our product candidates, or the
ability to license them to other companies to perform these functions. We do not have the
resources, facilities or experience to manufacture our product candidates on our own and do not
intend to develop or
-13-
acquire facilities for the manufacture of product candidates for pre-clinical trials, clinical
trials or commercial purposes in the foreseeable future. We intend to continue to license
technology and/or rely on contract manufacturers to produce sufficient quantities of our product
candidates necessary for any pre-clinical or clinical testing we undertake in the future. Such
contract manufacturers may be the sole source of production and may have limited experience at
manufacturing, formulating, analyzing, filling and finishing our types of product candidates.
We also intend to rely on third parties to supply the components that we will need to develop,
test and commercialize all of our product candidates. There may be a limited supply of these
components. We might not be able to enter into agreements that assure us of the availability of
such components in the future from any supplier. Our potential suppliers may not be able to
adequately supply us with the components necessary to successfully conduct our pre-clinical and
clinical trials and/or to commercialize our product candidates. If we cannot acquire an acceptable
supply of components to produce our product candidates, we will not be able to complete
pre-clinical and clinical trials and will not be able to commercialize our product candidates.
In addition, we rely on contract research organizations in conducting clinical trials for our
product candidates. We do not have the resources, facilities or experience to conduct clinical
studies for our product candidates on our own and do not intend to develop or acquire such
resources, facilities or experience in the foreseeable future. The quality, cost and timing of work
performed by our contracted contract research organizations has a significant impact on our
clinical programs and our business.
If we make technology or product acquisitions, we may incur a number of costs, may have integration
difficulties and may experience other risks that could harm our business and results of operations.
All of our product candidates are licensed from, or based upon technologies licensed from,
third parties. We may acquire and/or license additional product candidates and/or technologies in
the future. Any product candidate or technology we license or acquire will likely require
additional development efforts prior to commercial sale, including extensive clinical testing and
approval by the FDA and applicable foreign regulatory authorities, if any. All product candidates
are prone to risks of failure inherent in technology product development, including the possibility
that the product candidate or product developed based on licensed technology will not be shown to
be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot
assure you that any product candidate that we develop based on acquired or licensed technology that
is granted regulatory approval will be manufactured or produced economically, successfully
commercialized or widely accepted in the marketplace. Moreover, integrating any newly acquired
product could be expensive and time-consuming. If we cannot effectively manage these aspects of our
business strategy, our business may not succeed.
Furthermore, proposing, negotiating and implementing an economically viable acquisition or
license can be a lengthy, costly and complex process. Other companies, including those with
substantially greater financial, marketing and sales resources, may compete with us for the
acquisition or license of product candidates and/or technologies. We may not be able to acquire the
rights to alternative product candidates and/or technologies on terms that we find acceptable, or
at all. Our failure to acquire or license alternative products and/or technologies could have a
material adverse effect on our business, prospects and financial condition.
Failure to effectively manage our growth may have a material adverse effect on our business,
results of operations and financial condition.
If we are successful in obtaining sufficient additional financing to commence Phase 2 clinical
trials for one or both of our two lead product candidates, PMX-30063 and PMX-60056, we will need to
expand our operations, including hiring of additional personnel. However, we may not be able to
effectively grow and expand our business. Successful implementation of our business plan will
require management of growth, which will result in an increase in the level of responsibility for
management personnel. To manage growth effectively, we will be required to continue to implement
and improve our operating and financial systems and controls to expand, train and manage our
employee base. The management, systems and controls currently in place or to be implemented may not
be adequate for such growth, and the steps taken to hire personnel and to improve such systems and
controls might not be sufficient. If we are unable to manage our growth effectively, it will have a
material adverse effect on our business, results of operations and financial condition.
-14-
We rely on third parties to provide software necessary to the future success of our business.
Currently, we rely on a non-exclusive license from the University of Pennsylvania to use,
copy, perform, display, distribute, modify and prepare derivative works based on three software
packages, which include a suite of proprietary computational algorithms that we use in the
development, refinement, and testing of our product candidates. If this license agreement is
properly terminated by the University of Pennsylvania, our ability to advance our current product
candidates or develop new product candidates may be adversely affected.
In the future, we expect to rely upon the software programs licensed from the University of
Pennsylvania, as well as software licensed from other third parties, including software that might
be integrated with our internally developed software and used to perform key functions. If we
license such third-party software, it is likely that certain of these licenses may not contain
favorable terms for us, including duration for limited terms, can be renewed only by mutual consent
and may be terminated if we breach the terms of the license and fail to cure the breach within a
specified period of time. Such licenses may not be available to us on commercially reasonable
terms, if at all. The loss of or inability to maintain or obtain licenses on such third party
software could result in the discontinuation of, or delays or reductions in, product shipments
unless and until equivalent technology is identified, licensed and integrated with our software.
Any such discontinuation, delay or reduction would harm our business, results of operations and
financial condition. In addition, financial or other difficulties that may be experienced by such
third-party vendors may have a material adverse effect upon the technologies that may be
incorporated into our products. If such technologies become unavailable, we may not be able to find
suitable alternatives, which could harm our business, operating results, and financial condition.
Our executive officers and directors have the ability to significantly influence matters submitted
to our stockholders for approval.
Our executive officers and directors, in the aggregate, beneficially own shares representing
approximately 14.8% of our common stock. Beneficial ownership includes shares over which an
individual or entity has investment or voting power and includes shares that could be issued upon
the exercise of options and warrants within 60 days after the date of determination. On matters
submitted to our stockholders for approval, holders of our common stock are entitled to one vote
per share. If our executive officers and directors choose to act together, they would have
significant influence over all matters submitted to our stockholders for approval, as well as our
management and affairs. For example, these individuals, if they chose to act together, would have
significant influence on the election of directors and approval of any merger, consolidation or
sale of all or substantially all of our assets. This concentration of voting power could delay or
prevent an acquisition of our company on terms that other stockholders may desire.
Risks Related to our Intellectual Property
The obstacles to procurement and enforcement of our intellectual property and proprietary rights
could harm our competitive position by allowing competitors access to our proprietary technology
and to introduce competing products.
We regard our product candidates as proprietary and rely primarily on a combination of
patents, trademarks, copyrights, and trade secrets and other methods to protect our proprietary
rights. We maintain confidentiality agreements with our employees, consultants and current and
potential affiliates, customers and business partners.
If we fail to secure and then enforce patents and other intellectual property rights
underlying our product candidates and technologies, we may be unable to compete effectively. The
pharmaceutical industry places considerable importance on obtaining patent and trade secret
protection for new technologies, products and processes. Our success will depend, in part, on our
ability, and the ability of our licensors, to obtain and to keep protection for our products and
technologies under the patent laws of the U.S. and other countries, so that we can stop others from
using our inventions. Our success also will depend on our ability to prevent others from using our
trade secrets.
-15-
Our
pending U.S. and foreign patent applications may not result in the issuance of patents to us or may not result in the issuance of patents that will be advantageous to us. If we do not receive patents for these applications or do
not receive adequate protections, our developments will not have any proprietary protection and
other entities will be able to make the products and compete with us. Also, any patents we have
obtained or do obtain may be challenged by reexamination, opposition or other administrative
proceeding, or may be challenged in litigation, and such challenges could result in a determination
that the patent is invalid or unenforceable. In addition, competitors may be able to design
alternative methods or devices that avoid infringement of our patents. To the extent our
intellectual property protection offers inadequate protection, or is found to be invalid, we are
exposed to a greater risk of direct competition. Both the patent application process and the
process of managing patent disputes can be time consuming and expensive and may require significant
time and attention from our management. Furthermore, the laws of some foreign countries may not
protect our intellectual property rights to the same extent as do the laws of the United States.
In addition, the standards that the United States Patent and Trademark Office, or the U.S.
PTO, uses to grant patents can change. Consequently, we may be unable to determine the type and
extent of patent claims that will be issued to us or to our licensors in the future, if any patent
claims are issued at all. In addition, if the U.S. PTO and/or other patent offices where we file
our patent applications increase the fees associated with filing and prosecuting patent
applications we would incur higher expenses and our intellectual property strategy could be
adversely affected.
The confidentiality agreements we require of our employees and those which we enter into with
other parties may not provide adequate protection for our trade secrets, know-how or other
confidential information or prevent any unauthorized use or disclosure or the unlawful development
by others. If any of our confidential intellectual property is disclosed, our business may suffer.
Such agreements may not be enforceable or may not provide meaningful protection for our trade
secrets or other proprietary information in the event of unauthorized use or disclosure or other
breaches of the agreements, and we may not be able to prevent such unauthorized disclosure.
Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken
to prevent such disclosure are, or will be, adequate.
We may have to engage in costly litigation to enforce or protect our proprietary technology, which
may harm our business, results of operations, financial condition and cash flows.
The pharmaceutical field is characterized by a large number of patent filings involving
complex legal and factual questions, and, therefore, we cannot predict with certainty whether any
patents or in-licensed patents will be enforceable. Additionally, we may not be aware of all of the
patents potentially adverse to our interests that may have been issued to others. Should third
parties file patent applications, or be issued patents, claiming technology also claimed by us in
pending applications, we may be required to participate in interference proceedings in the U.S. PTO
to determine priority of invention. We, or our licensors, also could be required to participate in
interference proceedings involving our issued patents and pending applications of another entity.
In the event a competitor infringes upon our patent or other intellectual property rights,
litigation to enforce our intellectual property rights or to defend our patents against challenge,
even if successful, could be expensive and time consuming and could require significant time and
attention from our management. We may not have sufficient resources to enforce our intellectual
property rights or to defend any patents against challenges from others. If our intellectual
property does not provide adequate protection against our competitors’ products, our competitive
position and business could be adversely affected.
Our commercial success depends significantly on our ability to develop and commercialize our
products without infringing the intellectual property rights of third parties.
Our commercial success will depend, in part, on our not infringing the patents or proprietary
rights of third parties. Third parties that believe we are infringing on their rights could bring
actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and
marketing of the affected product or products.
If we become involved in any litigation, it could consume a substantial portion of our
resources, regardless of the outcome of the litigation. If any of these actions are successful, we
could be required, in addition to any potential liability for damages, to obtain a license to
continue to manufacture or market the affected product, in
-16-
which case we may be required to pay substantial royalties or grant cross-licenses to our
patents. However, any such license may not be available on acceptable terms or at all. Ultimately,
we could be prevented from commercializing a product, or forced to cease some aspect of our
business operations, as a result of patent infringement claims, which would harm our business.
We may enter into licensing agreements with third party intellectual property owners for use
of their property in connection with our products in order to ensure that such third party’s rights
are not infringed. Although we are not aware that any of our intended products would materially
infringe the rights of others, a claim of infringement may be asserted against us and any such
assertion may result in costly litigation or may require us to obtain a license in order to make,
use, or sell our products. Third parties may assert infringement claims against us in the future
with respect to current or future products. Any such claims or litigation, with or without merit,
could be costly and a diversion of management’s attention, which could have a material adverse
effect on our business, operating results and financial condition. Adverse determinations in such
claims or litigation could harm our business, operating results and financial condition.
We may be unable to protect the intellectual property rights of the third parties from whom we
license certain of our intellectual property or with whom we have entered into other strategic
relationships, which may harm our business.
We are, and will continue to be, reliant upon such third parties to protect their intellectual
property rights to this technology. Such third parties may determine not to protect the
intellectual property rights that we license from them and we may be unable defend such
intellectual property rights on our own or we may have to undertake costly litigation to defend the
intellectual property rights of such third parties. We may not continue to have proprietary rights
to the intellectual property that we license from such third parties or otherwise have the right to
use through similar strategic relationships. Any loss or limitations on use with respect to our
right to use such intellectual property licensed from third parties or otherwise obtained from
third parties with whom we have entered into strategic relationships could have a material adverse
effect on our business, operating results and financial condition.
Many countries, including certain countries in Europe, have compulsory licensing laws under
which a patent owner may be compelled to grant licenses to third parties. In addition, most
countries limit the enforceability of patents against government agencies or government
contractors. In these countries, the patent owner may be limited to monetary relief and may be
unable to enjoin infringement, which could materially diminish the value of the patent. Compulsory
licensing of life-saving products is also becoming increasingly popular in developing countries,
through either direct legislation or international initiatives. Such compulsory licenses could be
extended to include some of our product candidates, which may limit our potential revenue
opportunities.
International patent protection is particularly uncertain, and if we are involved in opposition
proceedings in foreign countries, we may have to expend substantial sums and management resources.
Patent law outside the U.S. is even more uncertain than in the U.S. and is currently
undergoing review and revision in many countries. Further, the laws of some foreign countries may
not protect our intellectual property rights to the same extent as U.S. laws. For example, certain
countries do not grant patent claims that are related to the treatment of humans. We may
participate in opposition proceedings to determine the validity of our foreign patents or our
competitors’ foreign patents, which could result in substantial costs and diversion of our
management’s efforts.
Risks Related to our Industry
We may experience delays in obtaining required regulatory approvals in the U.S. to market our
proposed product candidates.
Delays in regulatory approval, limitations in regulatory approval and withdrawals of
regulatory approval may have a negative impact on our results of operations. If we experience
significant delays in testing or approvals, our product development costs, or our ability to
license product candidates, will increase. If the FDA grants regulatory approval of a product, this
approval will be limited to those disease states and conditions for which the product has been
demonstrated through clinical trials to be safe and effective. Any product approvals that we
receive
-17-
in the future could also include significant restrictions on the use or marketing of our
products. Product approvals, if granted, can be withdrawn for failure to comply with regulatory
requirements or upon the occurrence of adverse events following commercial introduction of the
products. Failure to comply with applicable FDA or other applicable regulatory requirements may
result in criminal prosecution, civil penalties, recall or seizure of products, total or partial
suspension of production or injunction, as well as other regulatory action against our product
candidates or us. If approvals are withdrawn for a product, or if a product were seized or
recalled, we would be unable to sell or license that product and our revenues would suffer. In
addition, outside the U.S., our ability to market any of our potential products is contingent upon
receiving market application authorizations from the appropriate regulatory authorities and these
foreign regulatory approval processes include all of the risks associated with the FDA approval
process described above.
If competitors develop and market products that offer advantages as compared to our product
candidates, our commercial opportunities will be limited.
Other companies have product candidates in development to treat the conditions we are seeking
to ultimately treat. If these competitors are able to develop products that are more effective,
have fewer side effects, are less expensive or offer other advantages as compared to our product
candidates, our commercial opportunities will be limited. Furthermore, if our competitors
commercialize competing products before we do, then our ability to penetrate the market and sell
our products may be impaired.
Our competitors include fully integrated pharmaceutical companies and biotechnology companies,
universities and public and private research institutions. Many of the organizations competing with
us have substantially greater capital resources, larger research and development staffs and
facilities, greater experience in drug development and in obtaining regulatory approvals, and
greater manufacturing and marketing capabilities than we do. These organizations also compete with
us to:
| |
Ø |
|
attract parties for acquisitions, joint ventures or other collaborations; |
| |
| |
Ø |
|
license proprietary technology that is competitive with the technology we are practicing; |
| |
| |
Ø |
|
attract funding; and |
| |
| |
Ø |
|
attract and hire scientific talent. |
In the antibiotic market, many major pharmaceutical companies, such as GlaxoSmithKline,
Pfizer, Bayer, Merck and Sanofi Aventis have already established significant positions.
Additionally, many smaller companies, such as Cubist Pharmaceuticals, Oscient Therapeutics, NovaBay
Pharmaceuticals, Inc., Ceragenix and Inhibitex either have marketed, or are attempting to enter
this market by developing, novel and more potent antibiotics that are intended to be effective
against drug-resistant bacterial strains. In the UFH antagonist market, protamine is the only
available antidote for and antagonist to UFH and, as such, protamine currently dominates this
market. Because of protamine’s virtual monopoly of the UFH antidote/antagonist market, we believe
that it may be difficult for our future UFH antidote/antagonist products to penetrate this market.
There may be additional competitive products about which we are not aware.
Healthcare reform measures could adversely affect our business.
The business and financial condition of pharmaceutical companies is affected by the efforts of
governmental and third party payors to contain or reduce the costs of healthcare. In the U.S. and
in foreign jurisdictions there have been, and we expect that there will continue to be, a number of
legislative and regulatory proposals aimed at changing the healthcare system. For example, in some
countries other than the U.S., pricing of prescription drugs is subject to government control, and
we expect proposals to implement similar controls in the U.S. to continue. The implementation of
such additional controls could have the effect of reducing the prices that we are able to charge
for any products we develop and sell through these plans. Prescription drug legislation and related
amendments or regulations could also cause third-party payors other than the federal government,
including the states under the Medicaid program, to discontinue coverage for any products we
develop or to lower reimbursement amounts that they pay.
-18-
Further federal, state and foreign healthcare proposals and reforms are likely. While we
cannot predict the legislative or regulatory proposals that will be adopted or what effect those
proposals may have on our business, including the future reimbursement status of any of our product
candidates, the pendency or approval of such proposals could result in a decrease in our stock
price or limit our ability to raise capital or to obtain strategic partnerships or licenses.
Because our activities may involve the use of hazardous materials, we may be subject to claims
relating to improper handling, storage or disposal of these materials that could be time consuming
and costly.
If we use biological and hazardous materials in a manner that causes injury, we may be liable
for damages. Our research and development activities may involve the controlled use of potentially
harmful biological materials as well as hazardous materials, chemicals and various radioactive
compounds. We cannot completely eliminate the risk of accidental contamination or injury from the
use, storage, handling or disposal of these materials. In the event of contamination or injury, we
could be held liable for damages that result, and any liability could exceed our resources. We are
subject to federal, state and local laws and regulations governing the use, storage, handling and
disposal of these materials and specified waste products. The cost of compliance with these laws
and regulations could be significant.
Risks Related to our Capital Stock
Our common stock price is volatile, our stock is highly illiquid, and any investment in our
securities could decline substantially in value.
Our common stock is currently quoted on the OTC Bulletin Board. Trading of our stock through
the OTC Bulletin Board is frequently thin and highly volatile. In light of our small size and
limited resources, as well as the uncertainties and risks that can affect our business and
industry, our stock price is expected to continue to be highly volatile and can be subject to
substantial drops, with or even in the absence of news affecting our business. The following
factors, in addition to the other risk factors described in this prospectus and the
potentially low volume of trades in our common stock, may have a significant impact on the market
price of our common stock, some of which are beyond our control:
• results of pre-clinical studies and clinical trials;
• commercial success of approved products;
• corporate partnerships;
• technological innovations by us or competitors;
• changes in laws and government regulations both in the U.S. and overseas;
• changes in key personnel at our company;
• developments concerning proprietary rights, including patents and litigation matters;
• public perception relating to the commercial value or safety of any of our product
candidates;
• future sales of our common stock;
• future issuance of our common stock causing dilution;
• anticipated or unanticipated changes in our financial performance or future prospects;
• general trends related to the biopharmaceutical and biotechnological industries; and
• general conditions in the stock market.
The stock market in general has recently experienced relatively large price and volume
fluctuations. In particular, the market prices of securities of smaller biotechnology companies
have experienced dramatic fluctuations that often have been unrelated or disproportionate to the
operating results of these companies. Continued market fluctuations could result in extreme
volatility in the price of our common stock, which could cause a decline in its value. You should
also be aware that price volatility may be worse if the trading volume of the common stock remains
limited or declines.
-19-
A decline in the price of our common stock could affect our ability to raise further working
capital and adversely impact our ability to continue operations.
A prolonged decline in the price of our common stock could result in a reduction in the
liquidity of our common stock and a reduction in our ability to raise capital. Because a
significant portion of our operations has been and will continue to be financed through the sale of
equity securities, a decline in the price of our common stock could be especially detrimental to
our liquidity and our operations. Such reductions may force us to reallocate funds from other
planned uses and may have a significant negative effect on our business plans and operations,
including our ability to develop our product candidates and continue our current operations. If our
stock price declines, it may be more difficult to raise additional capital. If we are unable to
raise sufficient capital in the future, and we are unable to generate funds from operations
sufficient to meet our obligations, we will not be able to have the resources to continue our
normal operations.
The market price for our common stock may also be affected by our ability to meet or exceed
expectations of analysts or investors. Any failure to meet these expectations, even if minor, may
have a material adverse effect on the market price of our common stock.
When we issue additional shares, in this offering or in the future, it will likely result in the
dilution of our existing stockholders.
Our certificate of incorporation authorizes the issuance of up to 250,000,000 shares of common
stock with a $0.001 par value and 10,000,000 preferred shares with a par value of $0.001, of which
59,845,065 common shares were issued and outstanding as of October 30, 2009. We may need to increase
our authorized share count in order to issue additional shares of common stock in the future. From
time to time we also will increase the number of shares available for issuance in connection with
our equity compensation plans. As of October 30, 2009, we had
14,570,000 additional shares of common stock reserved for future
issuance under our stock plans. Our board of directors may fix and determine the designations,
rights, preferences or other variations of each class or series within each class of preferred
stock and may choose to issue some or all of such shares to provide additional financing or acquire
more businesses in the future.
Moreover, in the past, we issued warrants and options to acquire shares of common stock. As
of October 30, 2009, we had warrants and options to purchase
an aggregate of 46,738,661 shares of our
common stock outstanding. Warrants to purchase 4,119,194 shares of our common stock have weighted average
anti-dilution protection if we sell certain securities at a price per share less than $1.23 per
share. In addition, our Equity Line with Dutchess contemplates the issuance of up to 12,000,000 shares of our common stock to Dutchess, subject to certain restrictions and obligations.
The issuance of any shares for acquisition, licensing or financing efforts, upon conversion of
any preferred stock or exercise of warrants and options, pursuant to our equity compensation plans,
or otherwise may result in a reduction of the book value and market price of the outstanding shares
of our common stock. If we issue any such additional shares, such issuance will cause a reduction
in the proportionate ownership and voting power of all current stockholders. Further, such
issuance may result in a change of control of our corporation.
Financial Industry Regulatory Authority (FINRA) sales practice requirements may also limit a
stockholder’s ability to buy and sell our common stock.
FINRA has adopted rules that require that in recommending an investment to a customer, a
broker-dealer must have reasonable grounds for believing that the investment is suitable for that
customer. Prior to recommending speculative low priced securities to their non-institutional
customers, broker-dealers must make reasonable efforts to obtain information about the customer’s
financial status, tax status, investment objectives and other information. Under interpretations of
these rules, FINRA believes that there is a high probability that speculative low priced securities
will not be suitable for at least some customers. FINRA requirements make it more difficult for
-20-
broker-dealers to recommend that their customers buy our common stock, which may limit your
ability to buy and sell our stock and have an adverse effect on the market for our shares.
We have never paid dividends on our common stock and do not anticipate paying any in the
foreseeable future.
We have never declared or paid a cash dividend on our common stock and we do not expect to pay
cash dividends in the foreseeable future. If we do have available cash, we intend to use it to grow
our business.
Sales of a substantial number of shares of our common stock into the public market, including
shares of common stock that we may issue to Dutchess pursuant to our Equity Line, may result in
significant downward pressure on the price of our common stock and could affect your ability to
realize the current trading price of our common stock.
We have registered the resale by Dutchess of a maximum of 12,000,000 shares of common stock
that may be issued to Dutchess pursuant to our Equity Line. The common stock to be issued to
Dutchess pursuant to the Investment Agreement with Dutchess will be
sold at a 5% discount to
the volume weighted average price (VWAP) of our common stock during the five consecutive trading
day period beginning on the trading day immediately following the date of delivery of a put notice
by us to Dutchess, subject to certain exceptions. Dutchess has a financial incentive to sell our
common stock upon receiving the shares to realize the profit equal to the difference between the
discounted price and the market price.
Sales of a substantial number of shares of our common stock in the public market, including
shares of common stock that we may issue to Dutchess pursuant to our Equity Line, could cause a
reduction in the market price of our common stock. To the extent stockholders sell shares of common
stock, the price of our common stock may decrease due to the additional shares of common stock in
the market.
Any significant downward pressure on the price of our common stock as stockholders sell their
shares could encourage short sales by our stockholders or others. Any such short sales could place
further downward pressure on the price of our common stock.
Delaware law, our stockholder rights plan and our amended and restated certificate of incorporation
and amended and restated bylaws contain provisions that could delay and discourage takeover
attempts that stockholders may consider favorable.
Certain provisions of our amended and restated certificate of incorporation and our amended
and restated bylaws and applicable provisions of Delaware corporate law may make it more difficult
for or prevent a third party from acquiring control of us or changing our board of directors and
management. These provisions include:
| |
Ø |
|
the ability of our board of directors to issue preferred stock with voting or
other rights or preferences; |
| |
| |
Ø |
|
limitations on the ability of stockholders to amend our charter documents,
including stockholder supermajority voting requirements; |
| |
| |
Ø |
|
requirements that special meetings of our stockholders may only be called by the
chairman of our board of directors, our president, or upon a resolution adopted by,
or an affirmative vote of, a majority of our board of directors; and |
| |
| |
Ø |
|
advance notice procedures our stockholders must comply with in order to nominate
candidates for election to our board of directors or to place stockholders’
proposals on the agenda for consideration at meetings of stockholders. |
We will also be afforded the protections of Section 203 of the Delaware General Corporation
Law, which will prevent us from engaging in a business combination with a person who acquires at
least 15% of our common stock for a period of three years from the date such person acquired such
common stock, unless board or stockholder approval were obtained.
We review these provisions from time to time. Any delay or prevention of a change of control
transaction or changes in our board of directors or management could deter potential acquirers or
prevent the completion of a transaction in which our stockholders could receive a substantial
premium over the then current market price for their shares.
In addition, we have a stockholder rights plan pursuant to which we distributed rights to
purchase shares of our Series C Preferred Stock. The rights become exercisable upon the earlier of
ten business days after a public announcement that a person or group of affiliated or associated
persons has acquired, or obtained the right to acquire, 15% or more of our common stock then
outstanding or ten business days after the commencement of a
-21-
tender or exchange offer that would result in a person or group beneficially owning 15% or
more of our common stock then outstanding. These rights may cause substantial dilution to a person
or group that attempts to acquire us (or which otherwise acquires 15% or more of our common stock)
on terms or in a manner not approved by the Board, except pursuant to an offer conditioned upon the
negation, purchase or redemption of these rights. Accordingly, these rights could delay or
discourage someone from acquiring our business, even if doing so would benefit our stockholders.
Risks Related to this Offering
Our management team will have immediate and broad discretion over the use of the net proceeds from
this offering.
There is no minimum offering amount required as a condition to closing this offering and
therefore net proceeds from this offering will be immediately available to our management to use at
their discretion. We intend to use the net proceeds to fund our research and development
activities, including Phase 2 clinical trials and related activities for PMX-30063 or PMX-60056,
and for general working capital needs. Their judgments may not result in positive returns on your
investment and you will not have an opportunity to evaluate the economic, financial or other
information upon which our management bases its decisions.
You will experience immediate and substantial dilution as a result of this offering and may
experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. After giving
effect to the sale by us of up to units offered in this offering at a public offering
price of $ per unit, and after deducting the underwriting discounts and commissions
and estimated offering expenses payable by us, investors in this offering can expect an immediate
dilution of $ per share, or %, at the public offering price,
assuming no exercise of the warrants. In addition, in the past, we issued options and warrants to
acquire shares of common stock. To the extent these options are ultimately exercised, you will
sustain future dilution. We may also acquire or license other technologies or finance strategic
alliances by issuing equity, which may result in additional dilution to our stockholders.
There is no public market for the warrants to purchase common stock in this offering.
There is no established public trading market for the warrants being offered in this offering,
and we do not expect a market to develop. In addition, we do not intend to apply for listing the
warrants on any securities exchange. Without an active market, the liquidity of the warrants will
be limited.
We will
need additional capital in the future. If additional capital is not available, we may not be able
to continue to operate our business pursuant to our business plan or we may have to discontinue our
operations entirely.
During fiscal 2009, we have used a significant amount of cash to finance
the continued development and testing of our product candidates. If we continue to use cash at this
rate we will need significant additional financing, which we may seek to raise through, among other
things, public and private equity offerings and debt financing. Any equity financings will be
dilutive to existing stockholders, and any debt financings will likely involve covenants
restricting our business activities. Additional financing may not be available on acceptable terms,
or at all.
-22-
USE OF PROCEEDS
We estimate that the net proceeds to us from the sale of units in this offering, assuming
gross proceeds of $ million (which is the amount of gross proceeds received if the offering is fully
subscribed), will be approximately $ million, after deducting the placement agent fees
and estimated expenses of this offering. In addition, if all of the warrants included in the units
offered pursuant to this prospectus are exercised in full for cash, we will receive approximately
an additional $ million in cash. We may not be successful in selling any or all of the
securities offered hereby. Because there is no minimum offering amount required as a condition to
closing in this offering, we may sell less than all of the securities offered hereby, which may
significantly reduce the amount of proceeds received by us. In addition, the warrants included in the units offered pursuant to this prospectus
may not be exercised at all, or if they are exercised, may be exercised on a cash-less basis.
We expect to use any proceeds received from the offering:
| |
Ø |
|
to fund our research and development activities, including Phase 2 clinical
trials and related activities for our lead product candidates, PMX-30063 or
PMX-60056; and |
| |
| |
Ø |
|
for general working capital needs. |
We estimate that it will cost $65 to $85 million in research, drug development and clinical
development costs over 30 – 42 months to file a New Drug Application, or “NDA” for PMX-30063 (assuming accelerated approval
status is not granted), and a like amount for PMX-60056, in each case assuming adequate and timely
financing. Even if we sell all of the securities subject to this offering on favorable terms, of
which there can be no assurance, we will still need to obtain additional financing in the future in
order to fully fund these product candidates through the regulatory approval process. We may seek
such additional financing through public or private equity or debt offerings or other sources,
including collaborative or other arrangements with corporate partners, and through government
grants and contracts.
We will have significant discretion in the use of any net proceeds. Investors will be relying
on the judgment of our management regarding the application of the proceeds of any sale of the
securities.
We anticipate that the net proceeds obtained from this offering (excluding any proceeds we may receive in the future as a result of the exercise of the warrants for cash, if any, that are being issued with the units in this offering) will be used to fund the
following initiatives in order of priority (in thousands):
| |
|
|
|
|
PMX-30063 clinical development |
|
$ |
|
|
PMX-60056 clinical development |
|
$ |
|
|
Other research and development programs |
|
$ |
|
|
General working capital purposes |
|
$ |
|
|
|
|
|
|
Maximum net proceeds of the offering |
|
$ |
|
|
|
|
|
|
We may invest the net proceeds received from this offering temporarily until we use them for
their stated purpose.
-23-
DILUTION
Our reported net tangible book value as of June 30, 2009 was $ , or
$ per share of common stock, based upon shares outstanding as of that date. Net
tangible book value per share is determined by dividing such number of outstanding shares of common
stock into our net tangible book value, which is our total tangible assets less total liabilities.
After giving effect to the sale of the units offered in this offering
at the offering price of $ per unit, at
June 30, 2009 (and excluding shares of common stock issued and
any proceeds received upon exercise of the warrants), after deducting placement agent fees and other estimated
offering expenses payable by us, our net tangible book value at June 30, 2009 would have been
approximately , or $ per share. This represents an immediate
increase in net tangible book value of approximately $ per share to our existing
stockholders, and an immediate dilution of $ per share to investors purchasing
units in the offering.
The following table illustrates the per share dilution to investors purchasing units in the
offering:
| |
|
|
|
|
|
|
|
|
Public offering price per unit |
|
|
|
|
|
$ |
|
|
Net tangible book value per share as of June 30, 2009 |
|
$ |
|
|
|
|
|
|
Increase per share attributable to sale of units to investors |
|
$ |
|
|
|
|
|
|
As adjusted net tangible book value per share after the
offering |
|
|
|
|
|
$ |
|
|
Dilution per share to investors |
|
|
|
|
|
$ |
|
|
Dilution as a percentage of the offering price |
|
|
|
|
|
|
% |
|
The foregoing illustration does not reflect potential dilution from the exercise of
outstanding options or warrants to purchase shares of our common stock, or from puts of shares of
our common stock to Dutchess under our Equity Line.
-24-
OUR BUSINESS
We are a biotechnology company focused on treating life threatening, serious, infectious
diseases and acute cardiovascular disorders with synthetic small molecule compounds that mimic the
activity of large natural protein molecules, compounds referred to as biomimetics. Using our
proprietary computational drug design technology, we have created novel defensin mimetic antibiotic
compounds, heparin antagonist compounds (or “heptagonists”) and other drug compounds intended for
human therapeutic use. The following chart illustrates the current stage of development of our
internally developed pipeline along with planned development timings for our two lead programs that
are in clinical development:
|
|
|
| * |
|
Program on hold |
| |
| ** |
|
Investigational New Drug Application (or “IND”) or
equivalent Canadian Clinical Trial Application (or “CTA”) |
| |
| *** |
|
Unfractionated heparin (“UFH”) and low molecular weight heparin (“LMWH”) |
CLINICAL PROGRAMS
Defensin Mimetic Antibiotic Product Candidates
Market Opportunity
Worldwide, more than seven million patients are hospitalized each year for bacterial
infections, with approximately 49 million total patients being treated for infections annually. In
2002, the estimated number of healthcare associated infections (HAI’s) in U.S. hospitals, adjusted
to include federal facilities, was approximately 1.7 million with 99,000 deaths according to a 2007
Public Health Report issued by the Centers for Disease Control. According to a 2001 article
published in Emerging Infectious Diseases, healthcare-acquired infections cost approximately $4.5
billion annually in the U.S. The same article estimates that drug-resistant bacteria cause more
than 70% of healthcare-acquired infections. A February 2008 report from the Association for
Professionals in
-25-
Infection Control and Epidemiology estimates that 70% of infections may now be resistant to
antibiotics. As drug-resistance continues to spread, the need for potent novel antibiotic drugs
increases.
According to DataMonitor, a provider of healthcare market analysis data, the world’s
antibiotic market was approximately $25.5 billion in 2005 after growing at a compound annual growth
rate of 5.1% between 2001 and 2005 and the hospital antibiotic market across the world’s seven
major markets was $7.9 billion in 2006 (MIDAS sales data, IMS Health, March, 2007). DataMonitor
further estimates that the U.S. market for all antibiotic drugs was approximately $10.8 billion in
2004. The portion of the antibiotic market that we initially intend to target is the North
American acute-care hospital market. This market includes intravenous or subcutaneously infused
products that are administered, or at least prescribed, in the hospital. According to IMS Health,
another leading provider of healthcare market analysis data, the size of this market during the
twelve months ended August 2004 was approximately $3 billion.
Host Defense Proteins
Our small molecule defensin mimetic antibiotic product candidates mimic the activity of host
defense proteins. Host defense proteins are part of the innate immune system. In the human body,
host defense proteins primarily exist in the respiratory tract, the urogenital tract, the
gastrointestinal track and the epidermal tissues under the skin, all locations where microbial
pathogens first enter the human body, and represent a first line of defense against bacterial
attack. Host defense proteins act rapidly against bacteria, unlike other parts of the immune
system that take longer to work.
Host defense proteins use a simple, but effective method for killing bacteria by targeting
bacterial membranes and disrupting them. At low doses, these antimicrobial proteins associate in
membranes causing membrane thinning and formation of transient pores leading to membrane
permeabilization and leakage of cellular ions and metabolites, which results in the killing of the
bacterial cell. At higher doses, they cause generalized disruption of the bilayer structure of the
membrane, leading to the complete breakdown of the bacterial membrane and leakage of cellular
contents, which results in the killing of the bacterial cell.
Antibiotics on the market today generally target specific molecular targets in bacteria and
many must enter the bacteria cell to work. Bacterial cells can become resistant to antibiotics
through:
| |
Ø |
|
genetic mutations that modify the molecular targets themselves, rendering them
invulnerable to the antibiotic in question; or |
| |
| |
Ø |
|
metabolic responses that cause the cell to pump out foreign agents, preventing the
antibiotics from accessing the molecular targets. |
By contrast, host defense proteins physically disrupt the cell from the outside. The
mechanism of action of the host defense proteins makes it difficult for bacteria to develop
resistance because of several reasons:
| |
Ø |
|
they do not have to enter the bacterial cell to work; |
| |
| |
Ø |
|
they act quickly, killing bacteria within minutes of exposure, thereby limiting the
bacterial response time; and |
| |
| |
Ø |
|
in order to develop effective defenses, the bacteria would have to alter the
structure of its cell membrane, which is a highly complex multi-step response that
would likely reduce the ability of the newly mutated bacteria to grow and survive in a
natural environment due to changes in membrane transport of essential nutrients and
wastes. |
It has been documented in many studies published by the American Society of Microbiology and
others that susceptible bacteria do not readily develop resistance to host defense proteins under
experimental conditions where resistance readily develops against conventional antibiotics.
Furthermore, bacteria remain sensitive to the host defense proteins despite hundreds of millions of
years of evolution in which bacteria have been exposed to host defense proteins’ antimicrobial
mechanism of action.
Another favorable attribute of host defense proteins is that they selectively target bacteria
and not mammalian cells, by recognizing the differences in the composition of bacterial and
mammalian cell membranes.
-26-
The outer surface of bacterial cell membranes is more negatively charged than mammalian cells.
Bacterial cell membranes also lack cholesterol, an essential component of all mammalian membranes.
Host defense proteins specifically target membranes that lack cholesterol and have a high degree
of negative electrical charge. Therefore, they selectively attack bacterial cell membranes while
mitigating harm to mammalian cells.
We believe that the host defense proteins provide an attractive mechanistic approach for the
development of a new type of antibiotic therapeutic drug due to their strong antimicrobial
activity, unique mechanism of action for which bacterial resistance appears less likely to develop,
and selective targeting of bacteria but not mammalian cells. Attempts were made in the past by
other companies to develop natural host defense proteins as novel antibiotics. Those products
lacked robust activity in models of systemic infection, and were studied for niche topical
applications, likely as a result of their limited systemic availability. None of them has been
successfully commercialized to date because of difficulties relating to some of the inherent
complexities associated with protein drugs: a lack of systemic bioavailability and stability. For
systemic applications, all protein drugs have a number of limitations, including often not being
bioavailable with either injectable or oral administration, often expensive to produce, often
unstable even with intravenous administration, and at risk of eliciting immunological reactions
which can neutralize their activity.
Our Approach
We have developed PMX-30063, our lead defensin mimetic antibiotic product candidate, and other
novel small molecule defensin mimetic antibiotics that mimic the activity of host defense proteins.
We are seeking to commercialize these defensin mimetic compounds as antibiotics in a variety of
forms to combat drug-resistant bacterial infections. PMX-30063 and our other defensin mimetic
antibiotic product candidates are completely synthetic, which make them easier and less expensive
to produce than proteins. Importantly, our small molecule compounds demonstrate activity in animal
models of systemic infection, activity that was lacking in others’ past attempts to develop natural
host defense proteins as systemic agents. These defensin mimetic antibiotic product candidates may
be developed in a variety of formulations, including injectable, tablet and topical, for a wide
range of antibiotic applications. The results of our preclinical experiments for PMX-30063 and our
other defensin mimetic antibiotic product candidates suggest several advantages compared to other
marketed antibiotics. Such advantages include:
| |
Ø |
|
Broad spectrum of activity, including against resistant bacterial strains. By
acting on bacterial cell membranes, we believe our antibiotics will be effective
against a broad range of Gram-positive and Gram-negative pathogens. In our in vitro
efficacy studies, our defensin mimetic antibiotic product candidates as a class
demonstrated potent antibacterial activity on hundreds of common bacterial pathogens.
To support our initial clinical target of broad treatment of Staphylococcus infections,
we have also demonstrated activity against 148 different strains and species of Staph,
including 89 drug-resistant strains. The antibacterial activity of our defensin
mimetic antibiotic product candidates has also been demonstrated in animal models of
systemic infections. |
| |
| |
Ø |
|
Lower likelihood for the development of drug-resistance. It is very difficult for
bacteria to develop drug resistance to natural antimicrobial host defense proteins
because these proteins act on bacterial membranes rather than on a single, mutable
molecular target, such as an enzyme. Because our defensin mimetic antibiotic product
candidates are designed to have the same mechanism of action as host defense proteins,
we anticipate they will have a similar lower likelihood of developing bacterial
resistance compared to conventional antibiotics. This reduced likelihood of bacterial
resistance has been demonstrated in laboratory serial passage experimental studies. |
| |
| |
Ø |
|
Potentially faster acting than other antibiotics. In time-kill studies, our
defensin mimetic antibiotic product candidates act quickly when bacteria are exposed to
them. We observed bactericidal activity in a matter of minutes after exposure to our
defensin mimetic antibiotic product candidates. In contrast, many currently marketed
drugs can take hours or even several days to show effect. |
PMX-30063 Clinical Development
We have scaled up synthesis of drug substance and manufactured clinical supplies for our lead
antibiotic compound, PMX-30063, under current Good Manufacturing Practice (“cGMP”) conditions. The
current Good Laboratory Practice (“cGLP”) compliant toxicology, safety pharmacology and
genotoxicity studies have been
-27-
completed, which indicate that an effective therapeutic index for PMX-30063 may be achieved.
Our first product candidate is an i.v. formulation initially intended for the treatment of complicated skin and soft
tissue infections, caused by strains of Staph bacteria.
Future applications may include other infections such as gynecological or abdominal. We
anticipate that the bacterial targets of PMX-30063 will include drug resistant strains,
particularly broadly targeting the various strains of Staph bacteria. In the future, we also hope
to expand indications to also potentially include other bacterial strains.
Clinical Studies to Date
In December 2008, we completed and announced positive results from our single dose escalation
clinical study of healthy volunteers receiving PMX-30063 at various dose levels (Phase 1A). This
ascending single-dose intravenous pharmacokinetic and safety study met the necessary Phase 1 goals
of defining both a limiting single dose and the plasma distribution/elimination kinetics. In this
study, the dose was not limited by any measurable clinical or laboratory parameters. A subjective
syndrome of paresthesias was identified, appearing only at the higher dosages and consisting of
abnormal neuronal sensations (numbness and/or tingling) often likened to dental anesthesia. These
effects were graded as mild to moderate by investigators or subjects, but their reproducibility and
dose-proportionality allowed dose-escalation to be successfully concluded after achieving levels
well in excess of the expected therapeutic range. The effects were temporary and resolved on their
own. The same study provided detailed information on the time course of the drug during and after
dosing. These pharmacokinetics appear favorable for therapeutic use of the drug. The half-time for
elimination from the plasma was approximately 12 to 15 hours, allowing for flexibility in dosing to
obtain optimal peak and trough drug levels.
Ongoing
and Planned Clinical Studies
In June 2009, we commenced an ascending multi-dose intravenous
pharmacokinetic and safety study of healthy volunteers who will receive 5 intravenous
doses of PMX-30063 at various dose levels (Phase 1B). The primary endpoint for the two Phase 1 studies is a safety assessment, including
identification of dose limiting toxicity. We have conducted these
clinical studies in Canada under a CTA to United States
standards, and plan to submit an IND with the FDA before commencing Phase 2 studies in the United States, and
ultimately seek regulatory approval in the United States.
After Phase 1, our plan is to investigate and, if appropriate and possible, pursue one of the
accelerated development and/or review processes, which may be granted by the FDA to speed up the
review process for products that address an unmet medical need. These paths include: “fast track,”
which is based on recognition by the FDA of medical need; “priority review,” which provides for a
six-month review time after filing of a New Drug Application (“NDA”) and “accelerated approval,”
under which drugs for serious or life threatening disorders for which there is an unmet medical
need may be approved based on Phase 2 clinical data or surrogate clinical markers, with a
requirement to complete studies and show clinical outcomes. If a compound is granted one of these
designations, it may help to abbreviate the size and scope of the trials required for submission
and approval of an NDA and may help shorten the review time of any such filing. Drugs to treat HIV,
the virus that causes AIDS, are examples of agents that have been approved under “accelerated
approval” provisions. We believe that use of PMX-30063 as an antibiotic would address an unmet medical need;
however, the FDA may not grant PMX-30063 any of these designations that would allow for accelerated
development and/or review status. See the section entitled “Risk Factors” for a further discussion
of the risks associated with our intention to apply for such accelerated development and/or review
status.
If PMX-30063 is not granted accelerated approval status, we estimate that it will cost $65 to
$85 million in research, drug development and clinical
development costs over 30-42 months to file
an NDA for this product candidate. In the event that we are granted “fast track,” “priority
review” or “accelerated development” status by the FDA, it is possible that both the time and cost
to file an NDA could be significantly less than these estimates. The exact extent of these
potential time and cost savings can only be determined based on future discussions with the FDA.
In any case, we do not currently have the funding resources necessary to carry out all of our
proposed short and long-term operating activities. We believe that our current cash and investment
balances, exclusive of any cash to be generated from this offering or the Equity Line, will be
sufficient to fund our ongoing Phase 1B studies for our lead product candidates, including
PMX-30063, and can fund our operations into the fourth quarter of 2010. Due to continued weakness
in capital markets and our understanding that it may be difficult to secure significant additional
financing on favorable terms, we have begun and expect to continue to scale back and delay some of
our planned
-28-
activities in order to prolong our cash resources. Our current cash and investment balances
are not sufficient to initiate or fund Phase 2 clinical trials or any of the related development activities for either of
our lead product candidates, including PMX-30063.
If we are unable to secure adequate additional funding during 2009, through this offering or
otherwise, we will further delay, scale-back or eliminate certain of our planned research, drug
discovery and development activities and certain other aspects of our operations and our business
until such time as we are successful in securing adequate additional funding.
In April 2004, we received a Small Business Innovation Research, or SBIR, grant of $238,000
from the National Institute of Health (NIH) to conduct animal testing of our product candidates.
Research under this grant was completed and, in March 2006, we received a Phase 2 SBIR grant of
$2.9 million over three years in support of our development of an i.v. antibiotic product
candidate.
Preclinical Experiments
Our preclinical research indicates that PMX-30063, our lead defensin mimetic antibiotic
product candidate, has a number of favorable attributes, including relatively low molecular weight,
potent and broad spectrum antibacterial activity, low cytotoxicity, good tolerance in acute
toxicity and repeat dose animal experiments and strong efficacy in animal models of bacterial
infection. Our defensin mimetic antibiotic product candidates have demonstrated efficacy in
preclinical experiments against strains of bacteria that are currently resistant to one or more
classes of currently available antibiotics. Due to the novel mechanism of action of our product
candidates and the results of our preclinical research, we believe it is less likely that
resistance will develop against our defensin mimetic antibiotic product candidates compared with
conventional antibiotic drugs. The results of certain of our preclinical experiments for PMX-30063
are discussed below.
Bacterial Resistance
We employed a serial passage method to measure the potential development of bacterial drug
resistance to PMX-30063. In serial passage experiments, bacteria are repeatedly exposed to
sub-lethal concentrations of a drug, which accelerates the over-growth of mutant forms of bacteria
that are resistant to the action of an antibiotic drug. In our experiments, we compared PMX-30063
with norfloxacin, a widely used broad-spectrum antibiotic drug. Resistance by Staph bacteria,
including MRSA, was readily observed for norfloxacin, whereas no resistance was observed for
PMX-30063. In similar studies performed by a contract research organization, no resistance
developed to PMX-30063, or to our other defensin mimetic antibiotic compounds, when bacterial
strains that were resistant to other antibiotics were used in the test. These results are
consistent with those that have been previously demonstrated with host defense proteins.
Pharmacokinetics
Pharmacokinetics is the study of how a compound behaves in the body. Efficacy studies
determine how effective a compound is for its intended use, in this case, PMX-30063 as an
antibiotic agent. Pharmacokinetic and efficacy studies are performed to establish the most
effective dosage levels. Pharmacokinetic analysis has been completed for PMX-30063 in a mouse
model. In these experiments, blood samples were collected at nine time points over 24 hours and
quantified. One of the important measurements in these blood sample studies is half-life, which
measures the time it takes for half of an administered dose of compound to be cleared from the
body. These measurements guide how often a compound needs to be administered. PMX-30063 generally
exhibited half-lives in the range of 0.81 hours to 2.66 hours in animals, which is comparable to
the half-life in rodents of several antibiotics widely used in the hospital setting.
Proteins and other components in blood can have significant effects on the activities of drug
candidates, such as loss of antimicrobial activity. Our preclinical research suggests that
PMX-30063 is stable and the antimicrobial activity is maintained in the presence of blood serum.
These serum-effect profiles compare very favorably with many marketed antibiotic drugs.
-29-
In Vitro Activity
PMX-30063 has demonstrated potent and selective in vitro antibacterial activity. In our
preclinical experiments, PMX-30063 demonstrated potent activity against both Gram-positive and
Gram-negative bacteria, two general classes of bacteria, as well as fungi. Potent activity is
found against bacteria isolated from human infections including three important hospital pathogens,
MRSA, vancomycin-resistant S. aureus, and vancomycin resistant enterococcus. Additionally,
PMX-30063 was active against Staphylococcal isolates that were no longer susceptible to daptomycin
or linezolid. Overall, our defensin mimetic antibiotic compounds as a class have demonstrated in
the pre-clinical studies antimicrobial activity against a broad spectrum of over 100 strains of
bacteria, including clinical isolates of Gram-positive and Gram-negative bacteria.
In Vitro Metabolism
Metabolism studies seek to determine what happens to a compound after it has been administered
to a body, if it is changed in the body, and how it may be eliminated from the body. PMX-30063 has
been tested for stability in the presence of mouse and human liver hepatocytes to investigate the
extent to which liver metabolism could be a factor upon compound administration. PMX-30063
demonstrated high stability in the presence of liver hepatocytes. PMX-30063 was also stable in
blood plasma from rats, dogs and humans. These data suggest that PMX-30063 should be metabolically
stable.
Animal Efficacy and Toxicology Studies
The following animal efficacy and toxicology data provides important proof-of-concept
information that we believe supports development of PMX-30063 as a novel antibacterial agent for
systemic infections. We have conducted additional efficacy studies in both mice and rats using
intravenous infusions to examine the activity of PMX-30063 in validated models of bacterial
infection to find a dosing regimen that would maximize the tolerability of PMX-30063. Additional
pharmacokinetic, toxicology and safety pharmacology studies were also conducted to evaluate
PMX-30063.
Thigh burden model. The thigh burden model is a widely used and accepted animal model for
evaluating antibacterial activity of antibiotic drugs, such as PMX-30063, in animals. Mice were
inoculated in the thigh muscle with S. aureus and then treated with PMX-30063 by intravenous bolus
administration. PMX-30063 significantly reduced the bacterial burden in thighs compared to the
inoculated control treatment of saline even when first administered one hour after inoculation with
bacteria.
Sepsis model. We also tested the antibacterial efficacy of PMX-30063 in a mouse sepsis model.
In our studies, we infected mice with S. aureus at 100 times the lethal dose through injections
into the body cavity. These animals were then treated with either vancomycin or PMX-30063. All
animals were observed for mortality over seven days following treatment. This is a stringent test
of a compound as it involves a severe infection and the drug must both work quickly, and be able
to reach many body compartments from the bloodstream at sufficiently high concentrations to kill
the infectious bacteria for the animals to survive. When left untreated, the infected mice all
died within one to two days. Mice treated with PMX-30063 demonstrated comparable to superior
survival rates to mice treated with vancomycin. These studies demonstrate that when delivered
intravenously, PMX-30063 can be distributed throughout the body and reach the compartments needed
to effectively treat bacterial infection.
Toxicology Testing. Toxicology studies are done to determine the difference between the dose
at which a compound is effective and the dose at which it demonstrates toxicity. These studies and
measurements are done to determine the ratio between the toxic and effective doses. This ratio is
referred to as the therapeutic index or selectivity ratio. The cGLP compliant toxicology, safety
pharmacology and genotoxicity studies have been completed, which indicate that an effective
therapeutic index for PMX-30063 may be achieved.
Heptagonist Product Candidate
UFH and LMWH are blood clot prevention drugs, which are commonly used in numerous
post-surgical applications as anti-coagulants. Overdoses of UFH or LMWH are dangerous due to
potentially life-threatening
-30-
bleeding. While widely used, UFH and LMWH have the risk of adverse bleeding side effects,
which requires frequent monitoring.
Market Opportunity
Unfractionated Heparin (UFH)
Protamine is the only available reversing agent for UFH. Protamine is used both as an
antidote in the event of overdose, and more commonly in standard treatment as a reversing agent
following coronary artery bypass grafts (CABG, or “bypass” procedures), in which standard practice
involves administering UFH while the patient is on the heart-lung bypass machine to prevent blood
clots from forming in the machine and which could then cause problems when the blood is re-infused
to the patient. However, protamine has many significant potential adverse effects, including:
| |
Ø |
|
unpredictable efficacy, resulting in variable inter-patient activity; |
| |
| |
Ø |
|
anticoagulant activity of protamine can actually increase and worsen the
anticoagulant activity of UFH, and which requires complex dose-titration and
careful administration of often several doses; |
| |
| |
Ø |
|
allergic anaphylactic reactions in some patients due to the fact that protamine
is a biological product derived from fish sperm; |
| |
| |
Ø |
|
inferior fibrokinetics, or clot formation, can result in leaky clots and in more
serious cases rebound bleeding may occur; and |
| |
| |
Ø |
|
immunogenic reactions with antibodies formed after sensitization to protamine in
patients who have previously received protamine can impact efficacy and result in
severe reaction; some patients, such as diabetic patients receiving zinc insulin,
or males who have undergone vasectomy, may have pre-existing antibodies that could
pre-dispose them to sensitivity to protamine. |
As a result, we believe there is a significant medical and market need for a safer UFH
antagonist. Worldwide, we estimate approximately two million extracorporeal blood procedures,
including cardiothoracic procedures, are performed that utilize heparin and may require UFH
reversal, and eight million doses of protamine are used annually (IMS data). We believe our
hepatagonists will be a safer alternative to protamine.
Low Molecular Weight Heparin (LMWH)
The LMWHs are often used for longer term prevention of blood clots (thrombophylaxis), in
indications such as deep vein thrombosis (DVT), in patients who have had heart attacks (post MI),
and in cancer patients. All of these patient groups are at risk of developing potentially
life-threatening blood clots, and are given anti-clotting drugs including LMWHs to prevent
dangerous clot formation. Because of their benefits, LMWHs are being increasingly used, with
worldwide sales of approximately $4 billion in 2005 (according to IMS/RDN Insight), and we estimate
are used by approximately 12 million patients annually.
While having favorable risk/benefit characteristics, a number of clinical studies have shown
that there is a significant incidence of bleeding in patients who receive LMWHs. Most studies
generally show that 1%-4% of patients experience serious, life-threatening bleeding, and up to 20%
may experience clinically significant bleeding. Protamine is not considered reliably effective and
is not approved for reversal of LMWH. In patients who experience bleeding problems while on LMWH,
current care may involve hospitalization, blood transfusions, and surgery, which can be expensive
and unreliable.
LMWHs are not currently used in acute surgical settings, such as cardiothoracic procedures,
because of the lack of a reversing agent. The long half-life of LMWH products of up to 24 hours,
while an advantage for longer-term administration, can be a major disadvantage if a patient has a
major bleeding episode or must be re-operated on shortly after surgery. If clotting time is
prolonged due to systemic LMWH, re-operation may not be possible. There currently are no antidotes
for LMWH on the market. The availability of an antidote to LMWH
-31-
could allow these drugs to be used in cardiothoracic surgical procedures, as well as used in
patients who are currently contraindicated for LMWH or who may need to undergo re-operation. Thus,
an LMWH antagonist could substantially increase the market and sales of LMWH drugs, thereby also
increasing the need and market for a LMWH antagonist such as our heptagonist product candidate.
Product Opportunity
We believe that an antagonist to UFH and LMWH is an attractive product opportunity for the
following reasons:
| |
Ø |
|
Unmet medical need. We believe that a safer antagonist to UFH and an effective
and safe antagonist for LMWH would address a large unmet medical need; |
| |
| |
Ø |
|
Ease of clinical trials. The reversal of the blood clotting effects of UFH and
LMWH provides an effective and easily measured end point for human trials; |
| |
| |
Ø |
|
Attractive companion product for an LMWH. An LMWH-antagonist offers the
possibility to increase market share of a proprietary brand of LMWH and also
provide significant differentiating advantages versus other LMWH products without a
companion antagonist; |
| |
| |
Ø |
|
Predictive preclinical models. The method and laboratory measurement of
clotting time in animals is identical to the methodology needed in human clinical
trials and should be predictive of efficacy; |
| |
| |
Ø |
|
Pricing. If our product candidates are approved by the FDA and marketed, we
believe that such products would have reasonable cost of goods and attractive gross
margins; and |
| |
| |
Ø |
|
Focused acute care markets. Use of UFH and LMWH is concentrated in hospitals,
which could be addressed by a small sales force. |
Our Approach
UFH and LMWH are composed of sulfated polysaccharides, which provide an attractive target for
the structure based design of novel molecules. Based on a model for the three-dimensional
structure of these molecules, we have produced a series of compounds that were designed and
intended to bind to the critical pentasaccharide site found on both UFH and LMWH. In preclinical
experiments, our compounds have demonstrated efficacy at sub-micromolar concentrations, and the
ability to reverse the effect of both UFH and LMWH in whole human blood. Furthermore, we believe
these compounds function with a high degree of specificity. We have synthesized and screened a
number of compounds that demonstrate reversal of both UFH and LMWH resulting in the normalization
of blood clotting time.
Our objectives in this program are to develop a product that:
| |
Ø |
|
is as effective as protamine in reversing the anticoagulant effect of UFH; |
| |
| |
Ø |
|
is safer than protamine; |
| |
| |
Ø |
|
is easy to use; |
| |
| |
Ø |
|
has superior fibrokinetic activity (less deleterious impact on clot formation than protamine); |
| |
| |
Ø |
|
is less sensitizing than protamine; and |
| |
| |
Ø |
|
is effective in reversing the anticoagulant effect of LMWH. |
-32-
We believe that a heptagonist with these attributes could replace protamine for its current
uses, expand the heparin reversal market by introducing a reversing agent for LMWH, provide a new
treatment for patients receiving LMWH and who experience bleeding complications and require
reversal, and further expand the LMWH market by allowing physicians to more widely use LMWH.
PMX-60056 Clinical Development
We have scaled up synthesis of drug substance and manufactured clinical supplies for
PMX-60056, under cGMP conditions. cGLP compliant toxicology, safety pharmacology and genotoxicity
studies have been completed, which indicate that an effective therapeutic index for PMX-60056 may
be achieved.
Clinical Experiments to Date
In March 2009, we successfully completed our first-in-man clinical study with the novel
heparin antagonist drug PMX-60056. This ascending single-dose intravenous pharmacokinetic and
safety study met the necessary Phase 1 goals of defining both a limiting single dose for ten-minute
infusions and also the plasma distribution/elimination kinetics for the drug in the absence of
heparin. This first study has demonstrated that PMX-60056 can be given safely in the absence of
heparin if ten-minute infusions include less than 0.4 mg/kg. The data suggest that the limiting
side effect of hypotension is related to peak plasma drug level, which means that slower infusions
could allow delivery of more drug. To investigate this possibility, we plan to study slower
infusions, of twenty and potentially thirty minutes, in an extension of this study. These longer
infusions are expected to allow higher doses to be given, and will add support for potential
clinical studies for the reversal of LMWH, which may require greater total amounts of drug to be
administered. Analysis of the plasma-level assays indicates that a single-compartment model is
consistent with the observed data, and the plasma elimination half-time is 1.5 to 2.5 minutes in
the dosage range administered.
In October 2009, we completed a second successful clinical study of PMX-60056 (Phase 1B). The
clinical study was a pilot proof-of-concept study conducted using a double-blind,
placebo-controlled, crossover design, with six healthy human volunteer subjects. This study design
and low number of subjects were intended to generate meaningful results for a pilot study at
minimal cost. Twenty minutes after administration of a 70 U/kg dose of heparin, which is sufficient
to produce anticoagulant activity, each of the subjects was administered, in a blinded manner,
either a single dose of 0.3 mg/kg of PMX-60056 or a placebo as a 10-minute intravenous infusion.
With the crossover design, each subject was dosed twice, initially with heparin and either
PMX-60056 or a placebo and then, two days after the first dose, with heparin and whichever
(PMX-60056 or a placebo) he did not receive in the first dose. Each subject thus acted as his own
control.
The primary endpoint of the clinical study was safety, specifically whether blood pressure
decreases would be mitigated by the presence of heparin, and the secondary endpoint was efficacy,
as measured by blood clotting time. The desired outcomes were no clinically significant decrease in
blood pressure, and rapid reversal of activated clotting time (ACT), a standard bedside blood
clotting measurement, and activated partial thromboplastin time (aPTT), a more accurate laboratory
measurement of blood clotting time. These blood clotting times are machine read to ensure objective
measurement.
No serious adverse events (SAEs) occurred during this Phase 1B clinical study of PMX-60056. No
clinically significant blood pressure effects were observed in this study in the presence of
heparin. There were several non-serious reports of “warmth” and/or “itching” during and shortly
after the infusions, but no such effects were reported significantly beyond the 10-minute infusion
time.
In all subjects receiving PMX-60056, there was a rapid and complete reversal of the
anticoagulant action of heparin, as measured by ACT and aPTT. Each subject’s minimum ACT and aPTT
readings after being dosed with PMX-60056 were at or below the subject’s ACT and aPTT readings
prior to being dosed with heparin. The heparin reversal appeared to occur at or before the end of
the 10-minute infusion of PMX-60056, suggesting that the 0.3 mg/kg dose may have been in excess of
requirements for 70U/kg heparin. Furthermore, the reversal was permanent: there was no return of
anticoagulation during the hours required for heparin’s effects to decline naturally.
Ongoing
and Planned Clinical Studies
We estimate that it will cost $65 to $85 million in research, drug development and clinical
development costs over 30-42 months to file an NDA for PMX-60056. We do not currently have the
funding resources necessary to carry out all of our proposed short and long-term operating
activities. We believe that our current cash and investment balances, exclusive of any cash to be
generated from this offering or the Equity Line, will be sufficient
to fund our ongoing Phase 1B
study of PMX-30063, and can fund our operations into the
fourth quarter of 2010. Due to continued weakness in capital markets and our understanding that it
may be difficult to secure significant additional financing on favorable terms, we have begun and
expect to continue to scale back and delay some of our planned activities in order to prolong our
cash resources. Our current cash and investment balances are not sufficient to initiate or fund Phase 2
clinical trials or any of the related development activities for either of our lead product candidates,
including PMX-60056.
If we are unable to secure adequate additional funding during 2009, through this offering or
otherwise, we will further delay, scale-back or eliminate certain of our planned research, drug
discovery and development activities and certain other aspects of our operations and our business
until such time as we are successful in securing adequate additional funding.
In October 2007, we received a Small Business Innovation Research, or SBIR, grant of $100,000
from the National Institute of Health (NIH) to support the development of biomimetic compounds,
such as PMX-60056, as anti-coagulant antagonists. In June 2009, we received a Phase II SBIR grant
in the amount of $923,080, to be disbursed over two years, to support the development of LMWH
anticoagulant reversing agents.
Preclinical Experiments
One laboratory model used to evaluate the activity of an antagonist to a blood clot prevention
drug (UFH or LMWH) is the measurement of time it takes for blood or plasma to clot, with and
without antagonist (PMX-60056),
-33-
in the presence of the blood clot prevention drug. PMX-60056 was able to neutralize the
activity of both UFH and LMWH products, as measured by normalization of clotting time or
normalization of various enzyme activities involved in clot formation. For example, in one
experiment normal untreated blood required 28 seconds to clot. When UFH was added to the human
plasma, clotting time increased to more than 300 seconds. When PMX-60056 was added to plasma that
had been treated with UFH, the clotting time was normalized. PMX-60056 was also able to neutralize
UFH in whole human blood. Normalization of clotting time with PMX-60056 occurred at similar
concentrations as that for protamine, indicating comparable potencies for antagonism of UFH in
human plasma and blood between PMX-60056 and protamine. PMX-60056 also effectively reversed the
anti-coagulant activities of several different clinical preparations of LMWH in human plasma or
whole blood.
In pre-clinical studies, PMX-60056 has been shown to have distinct potential advantages over
protamine in two safety and efficacy-related assays. Protamine significantly impaired the
aggregation of platelets (necessary for normal clot formation) whereas PMX-60056 had little effect
on platelet aggregation. Furthermore, when PMX-60056 reversed the effects of UFH or LMWH it also
restored the normal rate and extent of clot formation. However, when protamine was used to reverse
UFH or the LMWHs, normal rates and extent of clot formation were not achieved. In the selection
of a safe and effective compound to control and prevent bleeding in the presence of an
anti-coagulant, we believe that platelet aggregation and restoration of normal clot formation will
be critically important factors for choosing the preferred agent.
PMX-60056 rapidly restored normal clotting time in UFH-treated rats and dogs. For example,
when rats were administered UFH at an i.v. dose of 75 U/kg, clotting time was greater than 120
seconds. Treatment with PMX-60056 after UFH administration restored clotting time to normal
(approximately 12-15 seconds) within 1 minute after PMX-60056 treatment at an i.v. dose of 2 mg/kg.
In other experiments with UFH-treated rats, normal clotting time was rapidly restored at i.v.
dosages of 0.5 and 1.0 mg/kg of PMX-60056. In dogs treated with 300 U/kg UFH, clotting time was
greater than 120 seconds. Treatment with an i.v. dose of 4 mg/kg PMX-60056 restored normal
clotting time by the first time point examined, which was 5 minutes after compound administration.
Our belief that the safety of PMX-60056 is supported by in vitro hemolysis assays where the
sensitivity, or lysis, of human erythrocytes (red blood cells) was examined in the presence of
increasing concentrations of PMX-60056. In these assays, PMX-60056 was not hemolytic at
concentrations that far exceed the expected therapeutic levels. Single dose toxicity studies in
mice demonstrated that PMX-60056 was well tolerated, indicating that an effective therapeutic
index, which measures the difference between effective doses and toxic doses, may be achieved.
Other safety studies measuring hemodynamic effects (heart rate and blood pressures) in the rat and
cardiovascular responses (EKG, heart rate and blood pressures) in dogs also indicated that an
effective therapeutic index for PMX-60056 may be achieved.
Toxicology Testing. Toxicology studies are done to determine the difference between the dose
at which a compound is effective and the dose at which it demonstrates toxicity. These studies and
measurements are done to determine the ratio between the toxic and effective doses. This ratio is
referred to as the therapeutic index or selectivity ratio. The cGLP compliant toxicology, safety
pharmacology and genotoxicity studies indicated that an effective therapeutic index for PMX-30063
may be achieved.
PRECLINICAL PROGRAMS:
In addition to our clinical programs for PMX-30063 and PMX-60056, we have conducted
preclinical studies for other product candidates that are described below. These preclinical
programs are either currently on hold or are not actively being pursued until such time as we are
able to obtain and allocate sufficient resources, including financing. Further, we may consider
expanding or initiating other development activities as our resources allow.
Defensin Mimetic Antibiotics
To balance the financial rate of return with risk and timing to market, we also intend to
resume pre-clinical development of PMX-30063 and other defensin mimetic antibiotic compounds for
both topical and oral antibiotic applications. These programs are currently on hold until we are
able to obtain and allocate sufficient resources, including financing. The initial topical
application we are pursuing is an ophthalmic topical antibiotic for the
-34-
treatment of eye infections, and the initial oral application is a non-absorbed oral
antibiotic for the treatment of gastrointestinal infections such as Clostridium difficile. With
sufficient resources, we plan to advance pre-clinical development and file additional INDs for the
ophthalmic topical and non-absorbed oral antibiotic indications in the future.
We are also evaluating our defensin mimetic antibiotic compounds for a number of other topical
and local applications, including topical antibiotic use for skin structure infections, oral
healthcare applications for treatment of periodontal disease, a type of gum disease, topical
treatment for ear infections, topical treatment of fungal infections, topical treatment of acne,
and a variety of non-therapeutic applications in personal care and materials applications.
We are also exploring the potential use of our defensin-mimetic antibiotics for the treatment
of other infectious diseases. In particular, recent pre-clinical studies by us and our academic
collaborators have shown activity of certain of our compounds for potential use against
tuberculosis and malaria. We intend to seek grants and other funding sources to continue the
development of compounds for these applications.
Medical Device, Industrial and Consumer Applications
We have conducted preliminary experiments which demonstrate that polymer derivatives of our
compounds, such as PMX-70004 and PMX-50003, which we refer to as our antimicrobial polymers, may be
used as effective antimicrobial agents as additives to materials. In one experiment, we coated
glass slides with a polyurethane plastic film. One of our antimicrobial polymers (0.1%
concentration) was then infiltrated into the polyurethane material using solvent and dried. The
slides were placed in a bacteria-rich nutrient broth for 72 hours to ascertain growth of bacterial
colonies on the submerged surfaces. After 72 hours, uncoated glass slides and slides coated with
polyurethane alone were covered with many bacterial colonies. In contrast, slides coated with
polyurethane film containing our antimicrobial polymer showed no bacterial growth.
Similar experiments have been performed in which solid polyurethane plastic disks were created
with one of our antimicrobial polymers directly mixed into the polyurethane plastic matrix. These
disks were then immersed in bacteria-rich broth for 24 hours. After 24 hours, plain polyurethane
plastic disks were covered with bacteria, whereas disks incorporating our polymer were devoid of
bacterial growth. When the disks treated with our antimicrobial polymer were stored at room
temperature for up to 45 days either dry or submerged in a large excess of water, there was little
to no loss of antibacterial activity. Experiments employing longer storage times are now being
conducted to determine the stability of the polymer-formulated plastics under a variety of
environmental conditions. When our polymers are incorporated into the plastic of intravenous
catheter tubing or the plastics used in sutures and exposed to a high inoculum of bacteria,
significant anti-bacterial activity is observed with the polymer-derivatized catheter and suture
plastics but not with the untreated plastics.
Based on these and other preliminary experiments, we believe that our antimicrobial polymers
can effectively be used as additives to materials, such as various medical devices to prevent
certain healthcare-acquired infection. Additionally, they may be added to paint, plastic or
ceramic materials to create a self-sterilizing environment against certain bacteria in hospitals or
other areas that may benefit from a clean, bacterial resistant environment.
Another potential application of our antimicrobial polymers is to combat Stachybotrys
chartarum, or Black Mold, that causes significant household and commercial building damage in the
U.S. Several of our compounds have shown potent activity against Black Mold as well as other
problematic environmental molds. Polymers can be used as additives to paints, drywall, and other
construction materials to prevent growth of this troublesome and unhealthy fungus.
We do not currently plan to conduct advanced development of any medical device, industrial or
consumer application of our antimicrobial polymers. We intend to focus on generating a limited
core of basic enabling data to support our efforts to license our antimicrobial polymers to
out-licensing partners who will continue research and development efforts in developing marketable
products. We intend to pursue these out-licensing opportunities with minimal use of our resources.
Our antimicrobial polymers and the out-licensing opportunities they present serve as
-35-
a possible avenue to accelerate revenue generation, thereby helping us to fund development of our
more advanced product candidates, including PMX-60056 and PMX-30063.
Biodefense
In recent years, improving our nation’s defense against bioterrorism has become an
increasingly important task. The Department of Homeland Security Appropriation Act signed by
President Bush in October 2003 includes $5.6 billion through 2013 for medical countermeasures
against bioterrorism threats. One of the major components of spending is focused on the
development of antibiotic compounds to treat biowarfare agents, including the highly infectious
bacteria that cause anthrax, tularemia and plague.
Antibiotic activity against anthrax and other biowarfare pathogens, with a mechanism by which
resistance is unlikely to develop, has commercial, medical and national security value.
Preliminary data from our preclinical experiments indicates that our product candidates have potent
activity against biowarfare agents that cause anthrax, tularemia and plague.
As a result of this preliminary data, we are pursuing funding from government sources, such as
the Department of Defense, the Defense Advanced Research Projects Agency and other military and
security agencies to further test and advance our product candidates for indications important to
national security. In November 2004, we received a Phase 1 SBIR grant of $168,000 to support
preliminary research in the biodefense area. In June 2009, we were awarded a $1.6 million contract
with the Defense Threat Reduction Agency to develop new defensin-mimetic antibiotic compounds to
combat biowarfare pathogens. In July 2009, we and our academic collaborators at UMass
received up to a $100,000 contract from the Office of Naval Research to
develop antibacterial compounds againsts pathogens of military
interest. Also in July 2009, we and our academic collaborators at
UMass received up to a $6.6 million grant from the NIH to develop new
compounds for biodefense and emerging food-borne infectious diseases.
In August 2009 we and our academic collaborators at UMass received a $100,000 contract from the Army Research Office to
develop compounds against drug-resistant bacterial biofilms.
Anti-Tuberculosis
Tuberculosis is a highly contagious disease that affects one-third of the world’s population
today. Approximately 2 billion people are infected worldwide, including 10 to 15 million cases in
the United States. There are 8 million newly reported cases each year and 3.1 million people die
from the disease annually. Tuberculosis is the leading cause of death of women, AIDs patients and
the young in the world and there are more deaths from tuberculosis than any other single infectious
disease. Mycobacterium tuberculosis is the primary infectious agent for tuberculosis and drug
resistance has become a paramount issue, accounting for over 50 million infections. The
drug-resistant forms of the disease are significantly more difficult to treat, leading to higher
mortality rates and escalating costs of care. PolyMedix compounds, including PMX-10070, have
demonstrated encouraging in vitro specificity for tuberculosis versus mammalian cells.
Anti-Malaria Agents
Recent pre-clinical studies with PolyMedix’s defensin mimetic antibiotics show encouraging
activity for the potential treatment of the malaria parasite, Plasmodium falciparum, the infectious
agent for the most prevalent and deadly forms of malaria. P. falciparum accounts for 80% of all
human malarial infections and 90% of deaths from such infections. More than 120 million clinical
cases of malaria and between 1 to 1.5 million deaths occur worldwide every year. Current therapies
for malaria are plagued by rapid resistance, which has become endemic in certain regions of the
world. Two of our compounds, PMX-70008 and PMX-30024, have demonstrated encouraging in vitro
specificity for the parasite versus mammalian cells.
Antifungal Agents
Our compounds, such as PMX-10098, have demonstrated promising activity against fungal strains
that often cause human infectious diseases, such as Candida. In preclinical experiments, certain
of our compounds have demonstrated effectiveness at inhibiting fungal growth and, for certain
strains of fungus, effectiveness was achieved at lower concentrations than that of fluconazole, a
commonly used antifungal agent. We intend to continue to investigate the potential of our
compounds as novel treatments for human fungal infections.
As a result of this preliminary data, we are pursuing funding from government sources, such as
the National Institutes of Health to further test and advance our product candidates. In May 2008,
we received a Phase 1 SBIR grant of $126,000 to support preliminary research for antifungal
defensin mimetic applications.
-36-
Angiogenesis Inhibitor
Our angiogenesis inhibitor compounds, such as PMX-20005, have demonstrated promising activity
in in vitro and animal models of inhibition of angiogenesis, the abnormal growth of blood vessels.
An angiogenesis inhibitor may be effective in treating age related macular degeneration (or AMD); a
common form of blindness caused by excessive and abnormal growth of blood vessels in the back of
the eye. With sufficient resources, including financing, we hope to continue to investigate the
potential of our compounds as novel treatments of AMD and other angiogenesis related diseases.
Our Strategy
Our goal is to discover and develop novel agents for the treatment of serious infectious
diseases and cardiovascular disorders using biomimetics. To achieve this objective, we are
implementing the following strategies:
Advance the Development of our Lead Product Candidates. We believe our current financial
resources, exclusive of any cash to be generated from this offering or the Equity Line, provide us
with sufficient funding to support the continued development of two programs through Phase 1B
clinical trials: PMX-30063 as our i.v. antibiotic product candidate and PMX-60056 as our
heptagonist product candidate. To advance other potential indications for our defensin-mimetics,
such as tuberculosis and malaria, or other programs into clinical development, we will require
additional financial resources.
Retain Rights to Market Hospital-Based Products to Hospitals in North America. We have
exclusive commercial rights to all of our current programs and in addition have either exclusive
ownership rights or options to obtain exclusive license rights to any new products that result from
our academic relationships. Our objective is to generate maximum value from sales of our product
candidates if regulatory approval is achieved. To achieve this goal, we intend to build our own
specialty sales force to market hospital-based therapeutics, such as PMX-30063 and PMX-60056, to
hospitals in North America. Additionally, we plan to collaborate with third parties to
commercialize our products in the primary care markets and in international markets.
Pursue Near-Term Revenue Opportunities through Out-Licensing and Partnership Agreements. We
intend to out-license selected product candidates from internal programs that are not part of our
strategic focus. For example, polymer derivatives of our antibiotic product candidates may be used
as additives to medical device, industrial and consumer materials to create self-sterilizing
surfaces and bactericidal products. We are pursuing strategic alliances with leading
pharmaceutical and biotechnology companies to design biomimetic compounds for targets selected by
our partners. Such collaborations could generate multiple sources of revenue, such as up-front
fees, research funding, milestone payments and royalties.
Utilize our Technology to Pursue Additional Drug Development Programs for Other Therapeutic
Areas. Using our computational drug design tools, we have already identified potential product
candidates for other therapeutic areas, including angiogenesis inhibitors. If sufficient resources
become available, we plan to pursue development in these areas through partnerships or on our own
in order to expand our product pipeline.
Strengthen Collaborations with Existing Partners and Enter into Agreements with Potential New
Partners. We have entered into collaborations and agreements with leading academic institutions.
We are actively pursuing additional agreements that would provide for license fees, research
funding and milestone payments and royalties to us from research results and subsequent product
development and commercialization. Through our collaborations with academic institutions, we gain
cost effective access to new technologies and expertise important to the further development of our
technology and products. Our network of academic advisors and collaborators consists of respected
experts in computational chemistry and drug design technology.
Research and Development
We
incurred research and development expenses of $3,240,000 and
4,444,000 for the six month
period ended June 30, 2009 and 2008, respectively, and $7,401,000, $9,328,000 and $3,306,000 for
the years ended December 31, 2008, 2007 and 2006, respectively.
-37-
Proprietary Rights
University of Pennsylvania
In January 2003, we entered into a Patent License Agreement with the University of
Pennsylvania, or Penn. Under the terms of the agreement, we were granted an exclusive, worldwide
royalty-bearing license to use, make and sell products utilizing seven of Penn’s issued patents or
pending patent applications for the life of such patents. These patents and applications cover our
clinical and preclinical stage product candidates, as well as future product candidates utilizing
the licensed patents and applications. The licensed patents and applications include:
| |
Ø |
|
three issued U.S. patents and three pending U.S. patent applications covering the
composition of matter on antimicrobial compounds, including small molecules,
oligomers and polymers. The first issued patent expires in 2017, the second issued
patent expires in 2022, the third issued patent expires in 2023 and the other patents, if issued, will expire at varying
times from 2022 to 2025. There are corresponding foreign applications to one of
the issued U.S. patents and to each of the four pending U.S. patent applications. |
| |
Ø |
|
one U.S. patent application and two corresponding foreign patent applications
covering polycationic compounds and their use for treating cancer. The patent, if
issued, will expire in 2026. |
Penn may terminate the licenses if:
| |
Ø |
|
we are more than 60 days late in paying to Penn royalties, expenses, or any
other undisputed amounts due under the agreement and we do not pay such amounts
within 30 days’ written notice of such delinquency; |
| |
Ø |
|
we become insolvent, enter into bankruptcy or a similar proceeding or call a
meeting of our creditors in order to arrange adjustment of our debts; or |
| |
Ø |
|
we otherwise materially breach the agreement. |
If this license agreement is properly terminated by Penn, we may not be able to execute our
strategy to develop and commercialize our i.v. antibiotic product candidate, our heptagonist
product candidate, our oral antibiotic product candidates, our antimicrobial polymers for
biomaterials applications, or to develop and commercialize future product candidates utilizing the
licensed patents.
We also entered into a Software License Agreement with Penn in May 2003. Under the terms of
the agreement, Penn granted us a non-exclusive, royalty-free license to use three software programs
and an exclusive, royalty-free license to three patent applications relating to such software
programs. The software programs and patents covered by the agreement include a suite of proprietary
computational algorithms that we use in the development, refinement and testing of our product
candidates. The licenses expire contemporaneously with our Patent License Agreement with Penn.
The patents relating to the software licenses, if issued, will expire at varying times in 2023 and
2024. Penn may terminate the agreement if:
| |
Ø |
|
we are more than 60 days late in paying to Penn any undisputed amounts due under
the agreement and we do not pay such amounts within 30 days’ written notice of such
delinquency; |
| |
Ø |
|
we become insolvent, enter into bankruptcy or a similar proceeding or call a
meeting of our creditors in order to arrange adjustment of our debts; or |
| |
Ø |
|
we otherwise materially breach the agreement. |
If this license agreement is properly terminated by Penn, our ability to advance our current
product candidates or develop new product candidates may be adversely affected.
-38-
We also sponsor certain research by Dr. William DeGrado, a Professor of Biochemistry and
Biophysics at Penn. Any discoveries made under this arrangement are intended to be covered by our
Patent License Agreement with Penn or a new license agreement with Penn containing substantially
the same terms. Dr. DeGrado’s current research for us focuses on further development of our
antimicrobial and hepatagonists applications. For these services, we pay Dr. DeGrado a consulting
fee of $7,000 per month. In addition, we have granted stock options in the past to Dr. DeGrado.
Dr. DeGrado also serves as Chairman of our Scientific Advisory Board. Either we or Dr. DeGrado may
terminate his consulting arrangement at any time. If Dr. DeGrado terminates his consulting
arrangement, our ability to advance our antimicrobial and hepatagonists programs may be adversely
affected.
University of Massachusetts
In January 2004, we entered into a Sponsored Research Agreement with the University of
Massachusetts, or UMass. Under the terms of this agreement, as amended, we have agreed to sponsor
certain research of Dr. Gregory Tew, an Assistant Professor in the Polymer Science and Engineering
Department at UMass, through March 2009, and have the exclusive option to license any intellectual
property generated by such research. We may exercise this option by issuing 7,500 shares of our
common stock to UMass for each $100,000 of research conducted by Dr. Tew. During 2007, we issued
12,500 shares to UMass in connection with this agreement. Dr. Tew’s research focuses on methods to
add our antibiotic agents to biomaterials, testing the physical properties of our antibiotic agents
and safety evaluation of our antimicrobial agents. The agreement will terminate if Dr. Tew leaves
UMass or ceases work in the antimicrobial field of research. In addition, UMass may terminate the
agreement including any licenses granted thereunder if we materially breach the agreement,
including by nonpayment of amounts due under the agreement. If the Sponsored Research Agreement or
any license thereunder is properly terminated by UMass, our ability to advance our antimicrobial
program or develop new product candidates may be adversely affected.
In January 2005, we entered into an Exclusive License Agreement with UMass, pursuant to which
UMass granted us an exclusive, worldwide license to use, make and sell products under one U.S.
patent application and seven corresponding foreign patent applications covering polynorborene
co-polymers and methods of use for the life of such patents. The patents, if issued, will expire
in 2025. UMass may terminate the license agreement if we materially breach the agreement,
including by nonpayment of amounts due under the agreement or in the case of a change in our
ownership or control. If the license agreement is properly terminated by UMass, we may not be able
to execute our strategy to develop and commercialize our antimicrobial polymers for biomaterials
applications or to develop and commercialize future product candidates utilizing the licensed
patents.
Our Technology
We initiate the design of our product candidates using our proprietary computational drug
design technology that is licensed from Penn. There are several computational methods used to
assist the drug design process, which includes:
| |
Ø |
|
the ability to model molecular interactions in the presence of solvent, such as
an aqueous environment, rather than in a vacuum, which more closely resembles the
real-life characteristics of how molecules interact with each other; |
| |
Ø |
|
the ability to simulate molecular interactions for hundreds of microseconds
which are time frames much longer than possible with previous molecular dynamics
technologies; and |
| |
Ø |
|
the ability to design compounds which target the bacterial cell membrane,
instead of a biochemical cell target. |
Our innovative and proprietary de novo drug design approach starts with protein targets with
well-understood physical structures and biological activity, and designs small molecule compounds
that mimic or regulate the activity of these targets. This structure-based drug design approach,
as well as the goal of regulating biological activities by mimicking nature itself with synthetic
small molecule compounds, allows us to rationally design novel product candidates in a way that we
believe greatly improves the efficiency of new drug discovery.
-39-
Intellectual Property
We rely on a combination of patents and trade secrets, as well as confidentiality and non-use
agreements to protect our intellectual property. Our patent strategy is designed to facilitate
commercialization of our current and future product candidates; and create barriers to entry. Our
intellectual property portfolio currently consists of three provisional applications, two patent
applications (U.S. and foreign) owned by us, two issued U.S. patents we exclusively license from
Penn and eight U.S. patent applications we exclusively license from Penn and/or UMass (as well as
foreign counterparts thereof). Our patent applications and the ten issued patents and pending
patent applications we exclusively license from Penn and/or UMass include:
| |
Ø |
|
seven U.S. patent applications that have been filed (as well as foreign
counterparts thereof) covering the composition of matter on 11 classes of
antimicrobial compounds, including small molecules, oligomers and polymers. Two
U.S. patents have been granted on two of these applications; |
| |
Ø |
|
one patent application covering the use of claimed oligomers for treating
ophthalmic and otic infections; |
| |
| |
Ø |
|
one patent application covering the use of claimed oligomers for treating mycobacterium; |
| |
| |
Ø |
|
one patent application covering the use of claimed oligomers for treating malarial infections; |
| |
| |
Ø |
|
one patent application covering synthetic mimetics of host defense proteins and their use; |
| |
Ø |
|
one patent application covering the combination of claimed compounds and
polymers with sesquiterpenoid enhancing agents; |
| |
Ø |
|
one patent application covering novel angiogenesis inhibitors with a wide range
of therapeutic uses; |
| |
Ø |
|
one patent application covering the use of claimed oligomers for treating
cancer; and |
| |
Ø |
|
three patent applications covering our proprietary, computational algorithms and
models, such as the coarse grain model and a new force field. These patent
applications do not disclose the specific computer code, which either will be
copyrighted or kept as a proprietary trade secret. One U.S. patent has been granted on one of these applications. |
We expect to continue to expand our intellectual property portfolio with additional filings of
both composition of matter and method of use patent applications. A number of new patent
applications are currently in process.
We attempt to protect our trade secrets by entering into confidentiality agreements with third
parties, employees and consultants. Our employees and consultants also sign agreements requiring
that they assign to us their interests in patents and other intellectual property arising from
their work for us. All employees sign an agreement not to engage in any conflicting employment or
activity during their employment with us and not to disclose or misuse confidential information.
In addition, we have received trademark protection for “PolyMedix.”
COMPETITION
Defensin Mimetic Antibiotic Product Candidate Competition
The pharmaceutical industry is highly competitive. We face significant competition from
pharmaceutical companies and biotechnology companies that are researching and selling products
designed to treat bacterial infections. Many major pharmaceutical companies, such as
GlaxoSmithKline, Pfizer, Bayer, Merck and Sanofi Aventis have already established significant
positions in the antibiotic market. Additionally, many smaller
-40-
companies, such as Cubist Pharmaceuticals, Oscient Therapeutics, Ceragenix, NovaBay
Pharmaceuticals, Inc. and Inhibitex have either marketed antibiotics and/or are attempting to enter
this market by developing novel and more potent antibiotics that are intended to be effective
against drug-resistant bacterial strains.
These companies, as well as potential entrants into the antibiotic market, have longer
operating histories, larger customer or use bases, greater brand recognition and significantly
greater financial, marketing and other resources than we do. Many of these current or potential
competitors can devote substantially greater resources to the development and promotion of their
products than we can.
Additionally, there has been consolidation within the pharmaceutical industry and larger,
well-established and well-financed entities may continue to acquire, invest in or form joint
ventures to gain access to additional technology or products. Any of these trends would increase
the competition we face and could adversely affect our business and operating results.
Heptagonist Product Candidate Competition
There are currently no products available in the marketplace as an antidote for and antagonist
to LMWH, while protamine is the only available antidote for and antagonist to UFH and, as such,
protamine currently dominates this market. Because of protamine’s potentially problematic side
effect profile, we believe that our heptagonist products may penetrate into the UFH antagonist
market.
Government Regulation
Government authorities in the U.S., at the federal, state, and local level, and foreign
countries extensively regulate, among other things, the following areas relating to our product
candidates:
| |
Ø |
|
research and development; |
| |
| |
Ø |
|
testing, manufacture, labeling and distribution; |
| |
| |
Ø |
|
advertising, promotion, sampling and marketing; and |
| |
| |
Ø |
|
import and export. |
All of our product candidates will require regulatory approvals by government agencies prior
to commercialization, but none of our product candidates has received these approvals. In
particular, human therapeutic products are subject to rigorous preclinical and clinical trials to
demonstrate safety and efficacy and other approval procedures of the FDA and similar regulatory
authorities in foreign countries. Various federal, state, local, and foreign statutes and
regulations also govern testing, manufacturing, labeling, distribution, storage and record keeping
related to such products and their promotion and marketing. The process of obtaining these
approvals and the compliance with federal, state, local, and foreign statutes and regulations
require the expenditure of substantial time and financial resources. In addition, the current
regulatory and political environment at the FDA could lead to increased testing and data
requirements that could impact regulatory timelines and costs.
U.S. Government Regulation
In the U.S., the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act and
implementing regulations. If we fail to comply with the applicable requirements at any time during
the product development process, approval process, or after approval, we may become subject to
administrative or judicial sanctions. These sanctions could include:
| |
Ø |
|
refusal to approve pending applications; |
| |
| |
Ø |
|
withdrawals of approvals; |
| |
| |
Ø |
|
clinical holds; |
-41-
| |
Ø |
|
warning letters; |
| |
| |
Ø |
|
product recalls and product seizures; and |
| |
Ø |
|
total or partial suspension of our operations, injunctions, fines, civil
penalties or criminal prosecution. |
Any agency enforcement action could have a material adverse effect on us.
Currently there is a substantial amount of congressional and administration review of the FDA
and the regulatory approval process for drug candidates in the U.S. As a result, there may be
significant changes made to the regulatory approval process in the U.S.
The steps required before a drug may be marketed in the U.S. include:
| |
Ø |
|
preclinical laboratory tests, animal studies and formulation studies; |
| |
Ø |
|
submission to the FDA of an IND, which must become effective before human
clinical trials may begin; |
| |
Ø |
|
execution of adequate and well-controlled clinical trials to establish the
safety and efficacy of the product for each indication for which approval is
sought; |
| |
Ø |
|
submission to the FDA of an NDA, or biologics license application, or BLA; |
| |
Ø |
|
satisfactory completion of an FDA inspection of the manufacturing facility or
facilities at which the product is produced to assess compliance with cGMP; and |
| |
Ø |
|
FDA review and approval of the NDA or BLA, or any supplements thereto,
including, if applicable, a determination of its controlled substance schedule. |
Preclinical studies generally are conducted in laboratory animals to evaluate the potential
safety and activity of a product. Violation of the FDA’s good laboratory practices regulations
can, in some cases, lead to invalidation of the studies, requiring these studies to be replicated.
In the U.S., drug developers submit the results of preclinical trials, together with manufacturing
information and analytical and stability data, to the FDA as part of the IND, which must become
effective before clinical trials can begin in the U.S. An IND becomes effective 30 days after
receipt by the FDA unless before that time the FDA raises concerns or questions about the proposed
clinical trials outlined in the IND. In that case, the IND sponsor and the FDA must resolve any
outstanding FDA concerns or questions before clinical trials can proceed. If these concerns or
questions are unresolved, the FDA may not allow the clinical trials to commence.
Clinical trials involve the administration of the investigational product candidate or
approved products to human subjects under the supervision of qualified investigators. Clinical
trials are conducted under protocols detailing, among other things, the objectives of the study and
the parameters to be used in assessing the safety and the effectiveness of the drug. Typically,
clinical evaluation involves a time-consuming and costly three-phase sequential process, but the
phases may overlap. Each trial must be reviewed, approved and conducted under the auspices of an
independent institutional review board, and each trial must include each patient’s informed
consent. The following sets forth a brief description of the typical phases of clinical trials:
| Phase 1 |
|
Refers typically to closely monitored clinical trials and includes the initial
introduction of an investigational new drug into human patients or healthy volunteer subjects.
Phase 1 clinical trials are designed to determine the safety, metabolism and pharmacologic
actions of a drug in humans, the potential side effects of the product candidates associated
with increasing drug doses and, if possible, to gain early evidence of the product candidate’s
effectiveness. Phase 1 trials also include the study of structure-activity relationships and
mechanism of action in humans, as well as studies in which investigational drugs are used as
research tools to explore biological phenomena |
-42-
| |
|
or disease processes. During Phase 1 clinical trials, sufficient information
about a drug’s pharmacokinetics and pharmacological effects should be obtained
to permit the design of well-controlled, scientifically valid Phase 2 studies.
The total number of subjects and patients included in Phase 1 clinical trials
varies, but is generally in the range of 20 to 80 people. |
| |
| Phase 2 |
|
Refers to controlled clinical trials conducted to
evaluate appropriate dosage and the effectiveness of
a drug for a particular indication or indications in
patients with a disease or condition under study and
to determine the common short-term side effects and
risks associated with the drug. These clinical
trials are typically well controlled, closely
monitored and conducted in a relatively small number
of patients, usually involving no more than several
hundred subjects. Phase 2 studies can be sequenced
as Phase 2a or Phase 2b. |
| |
| Phase 3 |
|
Refers to expanded controlled and uncontrolled
clinical trials. These clinical trials are performed
after preliminary evidence suggesting effectiveness
of a drug has been obtained. Phase 3 clinical trials
are intended to gather additional information about
the effectiveness and safety that is needed to
evaluate the overall benefit-risk relationship of the
drug and to provide an adequate basis for physician
labeling. Phase 3 trials usually include from
several hundred to several thousand subjects. |
| |
| Phase 4 |
|
Refers to trials conducted after approval of a new
drug and which explore approved uses and approved
doses of the product. These trials must also be
approved and conducted under the auspices of an
institutional review board. Phase 4 studies may be
required as a condition of approval. |
Clinical testing may not be completed successfully within any specified time period, if at
all. The FDA closely monitors the progress of each of the first three phases of clinical trials
that are conducted in the U.S. and may, at its discretion, reevaluate, alter, suspend or terminate
the testing based upon the data accumulated to that point and the FDA’s assessment of the
risk/benefit ratio to the patient. The FDA can also provide specific guidance on the acceptability
of protocol design for clinical trials. The FDA or we may suspend or terminate clinical trials at
any time for various reasons, including a finding that the subjects or patients are being exposed
to an unacceptable health risk. The FDA can also request additional clinical trials be conducted
as a condition to product approval. During all clinical trials, physicians monitor the patients to
determine effectiveness and to observe and report any reactions or other safety risks that may
result from use of the drug candidate.
Assuming successful completion of the required clinical trial, drug developers submit the
results of preclinical studies and clinical trials, together with other detailed information
including information on the chemistry, manufacture and control of the product, to the FDA, in the
form of an NDA or BLA, requesting approval to market the product for one or more indications. In
most cases, the NDA/BLA must be accompanied by a substantial user fee. The FDA reviews an NDA/BLA
to determine, among other things, whether a product is safe and effective for its intended use.
Before approving an application, the FDA will inspect the facility or facilities where the
product is manufactured. The FDA will not approve the application unless cGMP compliance is
satisfactory. The FDA will issue an approval letter if it determines that the application,
manufacturing process and manufacturing facilities are acceptable. If the FDA determines that the
application, manufacturing process or manufacturing facilities are not acceptable, it will outline
the deficiencies in the submission and will often request additional testing or information.
Notwithstanding the submission of any requested additional information, the FDA ultimately may
decide that the application does not satisfy the regulatory criteria for approval and refuse to
approve the application by issuing a “not approvable” letter.
The testing and approval process requires substantial time, effort and financial resources,
which may take several years to complete. The FDA may not grant approval on a timely basis, or at
all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary
governmental approvals, which could delay or preclude us from marketing our products. Furthermore,
the FDA may prevent a drug developer from marketing a product under a label for its desired
indications or place other conditions, including restrictive labeling, on distribution as a
condition of any approvals, which may impair commercialization of the product. After approval,
-43-
some types of changes to the approved product, such as adding new indications, manufacturing
changes and additional labeling claims, are subject to further FDA review and approval.
If the FDA approves the NDA/BLA, the drug can be marketed to physicians to prescribe in the
U.S. After approval, the drug developer must comply with a number of post-approval requirements,
including delivering periodic reports to the FDA, submitting descriptions of any adverse reactions
reported, biological product deviation reporting, and complying with drug sampling and distribution
requirements. The holder of an approved NDA/BLA is required to provide updated safety and efficacy
information and to comply with requirements concerning advertising and promotional labeling. Also,
quality control and manufacturing procedures must continue to conform to cGMP after approval. Drug
manufacturers and their subcontractors are required to register their facilities and are subject to
periodic unannounced inspections by the FDA to assess compliance with cGMP, which imposes
procedural, and documentation requirements relating to manufacturing, quality assurance and quality
control. Accordingly, manufacturers must continue to expend time, money and effort in the area of
production and quality control to maintain compliance with cGMP and other aspects of regulatory
compliance. The FDA may require post-market testing and surveillance to monitor the product’s
safety or efficacy, including additional studies to evaluate long-term effects.
In addition to studies requested by the FDA after approval, a drug developer may conduct other
trials and studies to explore use of the approved drug for treatment of new indications, which
require submission of a supplemental or new NDA and FDA approval of the new labeling claims. The
purpose of these trials and studies is to broaden the application and use of the drug and its
acceptance in the medical community.
Foreign Regulation
Whether or not we obtain FDA approval for a product, we must obtain approval of a clinical
trial or product by the comparable regulatory authorities of foreign countries before we can
commence clinical trials or marketing of the product in those countries. The approval process
varies from country to country, and the time may be longer or shorter than that required for FDA
approval. The requirements governing the conduct of clinical trials, product licensing, pricing
and reimbursement also vary greatly from country to country. Although governed by the applicable
country’s regulations, clinical trials conducted outside of the U.S. typically are administered
with the three-phase sequential process that is discussed above under “U.S. Government
Regulation.” However, the foreign equivalent of an IND is generally not a prerequisite to
performing pilot studies or Phase 1 clinical trials.
Furthermore, regulatory approval of prices is required in most countries other than the U.S.
We face the risk that the resulting prices would be insufficient to generate an acceptable return
to us or our collaborators.
Business History
PolyMedix, Inc. (formerly known as BTHC II Acquisition Corp.) was originally organized in the
State of Texas as BTHC II LLC, a public shell company. On September 29, 2004, BTHC II LLC and its
sister companies filed an amended petition under Chapter 11 of the United States Bankruptcy Code.
On November 22, 2004, the court approved BTHC II LLC’s Amended Plan of Reorganization. On March
24, 2005, and in accordance with its Amended Plan of Reorganization, BTHC II LLC changed its state
of organization from Texas to Delaware by merging with and into BTHC II Acquisition Corp., a
Delaware corporation formed solely for the purpose of effecting the reincorporation. After this
merger, BTHC II Acquisition Corp. became the public shell company.
PolyMedix Pharmaceuticals, Inc. (formerly known as PolyMedix, Inc.) was incorporated on August
8, 2002 in the State of Delaware as PolyMedix, Inc. to serve as a biotechnology company focused on
treating infectious diseases. On October 6, 2005, the former BTHC II Acquisition Corp. entered
into an Agreement and Plan of Merger and Reorganization with the former PolyMedix, Inc., pursuant
to which the stockholders of the former PolyMedix, Inc. exchanged their equity ownership interests
in the former PolyMedix, Inc. for an aggregate 94% equity ownership interest in the former BTHC II
Acquisition Corp. Pursuant to the Agreement and Plan of Merger and Reorganization, the former
PolyMedix, Inc. merged with a wholly-owned subsidiary of the former BTHC II Acquisition Corp. and,
as such, became a wholly-owned subsidiary of the former BTHC II Acquisition Corp.
-44-
At the time of the merger, the former BTHC II Acquisition Corp. had no operations and no
assets or liabilities. Because the stockholders of the former PolyMedix, Inc. exchanged their
equity ownership interests in the former PolyMedix, Inc. for an aggregate 94% equity ownership
interest in the former BTHC II Acquisition Corp., the former PolyMedix, Inc. was, for accounting
purposes, the surviving entity of the merger. Accordingly, all financial information included in
this prospectus for periods prior to the merger is for the former PolyMedix, Inc.
On February 24, 2006, the former BTHC II Acquisition Corp. changed its corporate name to
PolyMedix, Inc., and the former PolyMedix, Inc. changed its corporate name to PolyMedix
Pharmaceuticals, Inc.
In July, 2008, we sold 21,428,571 units of stock and warrants for aggregate consideration of
approximately $15 million. At closing each unit separated into one share of our common stock and a
warrant to purchase one share of our common stock. The securities sold in the offering were
registered with the Securities and Exchange Commission on Form SB-2, Registration No. 333-146180.
In December, 2007, we sold 2,943,222 units of stock and warrants for aggregate consideration
of approximately $3.2 million. At closing each unit separated into one share of our common stock
and a warrant to purchase one share of our common stock. The securities sold in the offering were
registered with the Securities and Exchange Commission on Form S-1, Registration No. 333-151084.
The net proceeds from each of the referenced registered offerings were used to support the
commercialization of our current and future product candidates, to fund our research and
development activities, and for general working capital needs.
EMPLOYEES
We
currently have 24 employees, including one M.D. and 11 employees
with Ph.D. degrees. 16
of our employees are focused on research and development and eight are focused on general
administration. We also utilize a number of consultants to assist with research and development
and commercialization activities.
DESCRIPTION OF PROPERTY
We currently lease 24,223 square feet of office and laboratory facilities at 170 N.
Radnor-Chester Road, Suite 300 in Radnor, Pennsylvania, pursuant to a twelve-year lease. All of
our tangible personal property, consisting mainly of computers, office furniture and lab equipment,
is located at our leased office and laboratory facilities and is in good operating condition and
repair (subject to normal wear and tear).
LEGAL PROCEEDINGS
To our knowledge, we are not a party to any pending or threatened material legal proceedings.
To our knowledge, no governmental authority is contemplating commencing a legal proceeding in which
we would be named as a party.
-45-
MANAGEMENT
The following table sets forth the respective names, ages and positions of our executive
officers and directors as of September 30, 2009:
| |
|
|
|
|
| Name |
|
Age |
|
Position(s) |
Nicholas Landekic |
|
51 |
|
President, Chief Executive Officer and Director |
James Gregory Ford |
|
47 |
|
Vice President, Business Development |
Bozena Korczak |
|
57 |
|
Vice President, Drug Development |
R. Eric McAllister, M.D., Ph.D. |
|
67 |
|
Vice President, Clinical Development and Chief Medical Officer |
Richard W. Scott, Ph.D. |
|
56 |
|
Vice President, Research |
Edward F. Smith |
|
38 |
|
Vice President, Finance, Chief Financial Officer and Secretary |
Frank P. Slattery, Jr. |
|
72 |
|
Chairman of the Board of Directors |
Brian Anderson |
|
63 |
|
Director |
Richard W. Bank, M.D. |
|
76 |
|
Director |
Douglas J. Swirsky |
|
39 |
|
Director |
Michael E. Lewis, Ph.D. |
|
58 |
|
Director |
Stefan Loren, Ph.D. |
|
45 |
|
Director |
Shaun F. O’Malley |
|
74 |
|
Director |
Executive Officers and Directors
Nicholas Landekic has served as President, Chief Executive Officer and Director of PolyMedix,
Inc. since November 2005 and of PolyMedix Pharmaceuticals, Inc. where he served in the same
capacity since inception in August 2002. Mr. Landekic has more than 20 years of pharmaceutical
experience. From 2000 to 2002, he was President and Chief Executive Officer of Locus Discovery.
From 1995 to 2000, Mr. Landekic was Senior Vice President of Corporate Development & Investor
Relations at Guilford Pharmaceuticals. From 1991 to 1995, Mr. Landekic served as Senior Director of
Business Development at Cephalon and, from 1988 to 1991, served as Senior Manager for Strategic
Marketing at Bristol-Myers Squibb. He also held positions in Finance and Business Development at
Johnson & Johnson Corporation (McNeil Pharmaceutical) from 1985 to 1988 and positions in the
research laboratories at the Mt. Sinai Medical Center from 1982 to 1983. Mr. Landekic received an
M.B.A. from the State University of New York at Albany, an M.A. in Biology from Indiana University
and a B.S. in Biology from Marist College.
J. Gregory Ford has served as Vice President, Business Development of PolyMedix, Inc. since
December 2008. Mr. Ford has over 20 years of biotechnology and pharmaceutical experience. Prior
to joining PolyMedix, Mr. Ford was Vice President, Business Development and Strategic Planning for
CollaGenex Pharmaceuticals, Inc., from August 2004 until November 2008. Prior to CollaGenex
Pharmaceuticals, Inc., Mr. Ford served as Vice President, Global Business Development of SkyePharma
US Inc. from February 2003 to April 2004. Prior to SkyePharma US Inc., Mr. Ford served in various
positions of increasing responsibility at SkyePharma Canada, Inc. and its predecessor RTP Pharma
Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Mylan Laboratories, Inc., and Mylan
Pharmaceuticals, Inc. Mr. Ford received an M.B.A and a B.S. in Industrial Engineering from West
Virginia University.
Bozena Korczak, Ph.D. has served as Vice President, Drug Development of PolyMedix, Inc. since
November 2007. Dr. Korczak has over 20 years of experience in discovery, pre-clinical and clinical
research at burgeoning biotechnology organizations. Prior to joining PolyMedix, Dr. Korczak was a
consultant for PharmaReach, Ltd., a private drug development consulting company, from October 2005
to November 2007. From
-46-
September 2001 to October 2005, Dr. Korczak was Vice President of Research and Development of
Cytochroma, Inc., a private biotechnology company. Prior to Cytochroma, Inc., Dr. Korczak served
in various positions of increasing responsibility at Glycodesign, Inc., Allelix Biopharmaceutical,
Inc. and Mount Sinai Hospital. She is an author of thirty-five peer review scientific papers and
holds a Ph.D. in biochemistry from the Polish Academy of Science.
R. Eric McAllister, M.D., Ph.D. has served as Vice President, Clinical Development and Chief
Medical Officer of PolyMedix, Inc. since November 2006. Dr. McAllister has over 25 years of
industry and clinical trials experience, most recently from October 2004 to October 2006 as Sr.
Vice President of Clinical Development at CombinatoRx Inc. Prior to that, from May 2002 to October
2004, Dr. McAllister was Vice President, Clinical Research with Sicor Pharmaceuticals, Inc. Dr.
McAllister has held various clinical development positions with TAP Pharmaceuticals, Inc.,
Cholestech Inc., Bristol-Myers Squibb, Co., G.D. Searle and Company, and Syntex Pharmaceuticals
Ltd. Dr. McAllister also spent seven years as a clinical investigator with MedStudies. He has
worked on the development of such major pharmaceutical products as Lupron, Pravachol, Capoten,
Kerlone, Cardene, Calan, Avandia, Actos, Teveten, and Atacand. Dr. McAllister received his M.D.
from Dalhousie University, and D. Phil. (Ph.D.) degree from Oxford University, and was also a
Rhodes Scholar.
Richard W. Scott, Ph.D. has served as Vice President, Research of PolyMedix, Inc. since
November 2005 and of PolyMedix Pharmaceuticals, Inc. since November 2002. Dr. Scott has
approximately 20 years of biopharmaceutical industry experience. Most recently, he was Vice
President of Biology at Cephalon, Inc. where he held positions of increasing responsibility from
1991 to 2001. From 1985 to 1990, Dr. Scott worked at the research laboratories of DuPont and
Company. Dr. Scott has authored more than 45 papers and book chapters, and is named on six
patents. Dr. Scott holds a Ph.D. in Microbiology from the University of Pennsylvania and a B.S. in
Biology from Muhlenberg College.
Edward F. Smith, has served as Vice President, Finance and Chief Financial Officer of
PolyMedix, Inc. since January 2006. Mr. Smith has approximately 15 years of combined
biopharmaceutical industry and financial management experience. From 2000 to 2005, he was
Executive Director of Finance at InKine Pharmaceutical Company, Inc. (acquired by Salix
Pharmaceuticals, Ltd. in 2005). From 1993 to 1999, Mr. Smith held various positions of increasing
responsibility in public accounting, most recently as a manager in the audit practice at Deloitte &
Touche LLP. Mr. Smith is licensed as a Certified Public Accountant in Pennsylvania and holds a
B.S. degree in Business Administration from the University of Hartford.
Frank Slattery, Jr. has served as Chairman of the Board of Directors of PolyMedix, Inc. since
November 2005 and was a co-founder of PolyMedix Pharmaceuticals, Inc. where he served in the same
capacity since inception in August 2002. Frank Slattery is the President and founder of Quintus
Corporation, a consulting company, a position he has held since 1995, and Executive Director and
Chief Executive Officer of the Philadelphia Orchestra, a position he has held since January 2009.
Mr. Slattery was the President, Chief Executive Officer and Director of LFC Financial Corp. from
1969 to 1994. Mr. Slattery was formerly Chairman of the Main Line Health Systems, Inc. and has
founded and currently serves as the Chairman of the Board of Directors of several privately held
companies, including GelMed Inc., Probaris Technologies, Inc., Franklin Fuel Cells, Inc., Knite,
Inc., MinusNine Technologies, Inc. and NanoSelect, Inc. He also currently serves as Vice Chairman
and Trustee of the Jefferson Health Systems and a director of Clarient, Inc., a publicly held
company. Mr. Slattery holds an A.B. from Princeton University and a J.D. from the Law School of
the University of Pennsylvania.
Brian Anderson has served as a Director of PolyMedix, Inc. since January 2008. Since March
2007 he has served as an advisor to companies involved in the health care industry. From January
2006 until March 2007 Mr. Anderson was employed by Alkermes, Inc., in a market development
capacity. Prior to that, from April 2004 to September 2005, Mr. Anderson was Chief Business
officer for MediciNova,Inc., a publicly traded specialty pharmaceutical company. Mr. Anderson was
an advisor to Montridge, Inc., a boutique investor relations firm from September 2002 to January
2004. He was President and CEO of Cognetix, Inc., a biotechnology company, from 1998 to 2002.
Prior to that, from 1995 to 1998, Mr. Anderson was Senior Vice President for Commercial Development
at Indevus (formerly Interneuron) Pharmaceuticals, a publicly traded biopharmaceutical company.
Mr. Anderson has held various senior level positions in sales, marketing and business development
at Bristol-Myers Squibb and Pharmacia (later Pharmacia & Upjohn). Mr. Anderson graduated from the
University of Manitoba where he received his Bachelors degree.
-47-
Richard W. Bank, M.D. has served as a Director of PolyMedix, Inc. since July 2008. Dr. Bank
is currently the President of BioVest Advisors, a consulting company, and has served in such
capacity since July 2006. Dr. Bank has served as Senior Portfolio Manager, Managing Director, and
Senior Vice President of the Liberty View Health Sciences Fund, a division of Liberty View Capital
Management, a Lehman Brothers company, from July 2004 to July 2006. Dr. Bank has served as
President and Managing Director of First-Tier Biotechnology Partners from February 1995 until its
acquisition by Lehman Brothers in July 2004. From February 1995 through April 1996, Dr. Bank
served as President and Secretary of Biomedical Sciences, Incorporated. He has also served as
President and Secretary of BioVest Health Sciences, Incorporated since its organization in April
1996 to July 2004. Dr. Bank was Senior Research Analyst Director/Biotechnology SBC Warburg Dillon
Read from 1998 to 1999. He was also Entrepreneur-In-Residence in Life Sciences for Tucker Anthony
Sutro for 2000 through 2001. Dr. Bank has served as Professor of Medicine at the University of
Southern California and was Medical Commissioner for the 1984 Olympic Games. Dr. Bank received his
B.S. from Washington & Lee University and his M.D. from Finch University of Health Sciences, The
Chicago Medical School.
Michael E. Lewis, Ph.D. has served as a Director of PolyMedix, Inc. since November 2005 and of
PolyMedix Pharmaceuticals, Inc. since its inception. Dr. Lewis has more than 30 years of
scientific experience in academic and government laboratories and in several major pharmaceutical
and biotechnology companies. Since 1994, Dr. Lewis has served as President of BioDiligence
Partners, Inc., where he co-founded three other biotechnology companies, Cara Therapeutics, Inc.,
Arena Pharmaceuticals, Inc. and Adolor Corporation. Dr. Lewis has served as Chief Scientific
Advisor and a director of Cara Therapeutics, Inc. since its inception in 2004. He has also served
as a director of Aeolus Pharmaceuticals, Inc. since 2004. After serving as Chief Scientific
Advisor of Adolor Corporation from 1994 to 1997, Dr. Lewis served as Chief Scientific Advisor of
Arena Pharmaceuticals, Inc. from 1997 to 2003, and as a director from 1997 to 2000. Dr. Lewis is a
co-founder of Cephalon, Inc. and served at Cephalon as Senior Scientist, Director of Pharmacology,
and then as Senior Director of Scientific Affairs from 1988 to 1993. He supervised a molecular
pharmacology research laboratory at the E.I. DuPont Co. from 1985 to 1987, and received
postdoctoral training in pharmacology at the National Institutes of Health, the University of
Michigan, and at the University of Cambridge. He received a Ph.D. from Clark University in 1977.
Stefan D. Loren, Ph.D. has served as a Director of PolyMedix, Inc. since July 2008. Dr. Loren
is currently a Managing Director of Westwicke Partners, a consulting company and a consultant to
MTB Investment Advisors, a family of equity funds, and has held these positions since August, 2008.
Prior to that, Dr. Loren was Analyst/Portfolio Manager with Perceptive Advisors, a health care
hedge fund, from May 2007 to August, 2008 and MTB Investment Advisors, a family of equity funds,
from August 2005 to May 2007. From July 1997 to August 2005, Dr. Loren was a Managing Director in
the healthcare group at Legg Mason. Prior to that, Dr. Loren was a Research Chemist at the
advanced technologies division of Abbott Laboratories and a research fellow at the Scripps Research
Institute. Dr. Loren received his B.A. from the University of California, San Diego, and his Ph.D.
from University of California, Berkeley.
Shaun F. O’Malley has served as a Director of PolyMedix, Inc. since January 2006. Mr.
O’Malley is currently the Chairman Emeritus of Price Waterhouse LLP, a title he has held since July
1995. Prior to 1995, he served as Chairman and Senior Partner of Price Waterhouse LLP. He
currently serves as a member of the Boards of Directors of the Finance Company of Pennsylvania,
Monell Chemical Senses Center and The Philadelphia Contributionship. Mr. O’Malley holds a B.S. in
Economics from the Wharton School of the University of Pennsylvania.
Douglas J. Swirsky has served as a Director of PolyMedix, Inc. since June 30, 2009. Mr.
Swirsky presently serves as Senior Vice President, Chief Financial Officer, Treasurer, and
Corporate Secretary of GenVec, Inc., a biopharmaceutical company, a position he has held since
2006. Prior to joining GenVec, Mr. Swirsky was a Managing Director and the Head of Life Sciences
Investment Banking at Stifel Nicolaus and held the same position at Legg Mason prior to Stifel
Financial’s acquisition of the Legg Mason Capital Markets business in 2005. Mr. Swirsky, a
Certified Public Accountant and a CFA charterholder, has also previously held investment banking
positions at UBS, PaineWebber, and Morgan Stanley. His experience also includes positions in public
accounting and consulting. Mr. Swirsky received his B.S. in Business in Administration from Boston
University and his M.B.A. from the Kellogg School of Management at Northwestern University.
Executive officers of PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc. are appointed by the
board of directors of PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc., respectively. The Chief
Executive Officer,
-48-
Treasurer and Secretary of PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc. are elected
annually by the board of directors of PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc.,
respectively, at its first meeting following the annual meeting of stockholders. Other officers of
PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc. may be appointed by the board of directors of
PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc., respectively, at any meeting. Such officers
shall hold office until his or her successor is elected and qualified, unless a different term is
specified in the resolution electing or appointing such officer, or until such officer’s earlier
death, resignation or removal.
All directors of PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc. are elected at each
annual meeting of the stockholders of the PolyMedix, Inc. and PolyMedix Pharmaceuticals, Inc.,
respectively, to hold office until the next annual meeting of stockholders and until their
successor is elected and qualified, or until such director’s earlier death, resignation or removal.
-49-
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table shows information known to us about beneficial ownership of our common stock by:
| |
Ø |
|
each of our directors and director nominees; |
| |
| |
Ø |
|
each of our Named Executive Officers as identified in the Summary Compensation Table; |
| |
| |
Ø |
|
all of our directors and executive officers as a group; and |
| |
| |
Ø |
|
each person known by us to beneficially own 5% or more of our common stock. |
Beneficial ownership and percentage ownership are determined in accordance with the rules of
the SEC. Under these rules, beneficial ownership generally includes any shares as to which the
individual or entity has sole or shared voting power or investment power and includes any shares
that an individual or entity has the right to acquire beneficial ownership of within 60 days of
October 30, 2009, through the exercise of any option, warrant, conversion privilege or similar right.
In computing the number of shares beneficially owned by a person and the percentage ownership of
that person, shares of our common stock that could be issued upon the exercise of outstanding
options and warrants that are exercisable within 60 days of October 30, 2009 are considered to be
outstanding. These shares, however, are not considered outstanding as of October 30, 2009 when
computing the percentage ownership of each other person.
To our knowledge, except as indicated in the footnotes to the following table and subject to
state community property laws where applicable, all beneficial owners named in this table have sole
voting and investment power with respect to all shares shown as beneficially owned by them.
Percentage of ownership is based on 59,845,065 shares of common stock outstanding as of October 30,
2009.
| |
|
|
|
|
|
|
|
|
| |
|
Amount and Nature of |
|
Percent of |
| |
|
Beneficial Ownership |
|
Common |
| Name and Address of Beneficial Owner (1) |
|
of common stock |
|
Stock |
Directors and Named Executive Officers |
|
|
|
|
|
|
|
|
Nicholas Landekic |
|
|
3,606,833 |
(2) |
|
|
5.8 |
% |
Frank P. Slattery, Jr. |
|
|
2,190,100 |
(3) |
|
|
3.6 |
% |
Richard W. Scott, Ph.D. |
|
|
932,322 |
(4) |
|
|
1.5 |
% |
Michael E. Lewis, Ph.D. |
|
|
810,000 |
(5) |
|
|
1.3 |
% |
R. Eric McAllister, M.D., Ph.D. |
|
|
602,778 |
(6) |
|
|
1.0 |
% |
Edward F. Smith |
|
|
466,528 |
(7) |
|
|
|
* |
Shaun F. O’Malley |
|
|
372,860 |
(8) |
|
|
|
* |
Bozena Korczak, Ph.D. |
|
|
340,278 |
(9) |
|
|
|
* |
Stefan D. Loren, Ph.D. |
|
|
143,000 |
(10) |
|
|
|
* |
Brian Anderson |
|
|
100,000 |
(11) |
|
|
|
* |
Richard W. Bank, M.D. |
|
|
100,000 |
(11) |
|
|
|
* |
Douglas J. Swirsky |
|
|
50,000 |
(11) |
|
|
|
* |
J. Gregory Ford |
|
|
30,000 |
|
|
|
|
* |
| |
All Directors and Executive Officers as a Group (13 persons): |
|
|
9,744,699 |
(12) |
|
|
14.8 |
% |
| |
Five Percent Stockholders (excludes Directors and Executive
Officers set forth above): |
|
|
|
|
|
|
|
|
ACT Capital Management, LLLP
2 Radnor Corporate Center, Suite 111
Radnor, PA 19087 |
|
|
6,095,642 |
(13) |
|
|
9.9 |
% |
Target Capital Management
345 E. 57th Street, 8A
New York, NY 10022 |
|
|
3,306,401 |
(14) |
|
|
5.4 |
% |
-50-
|
|
|
| * |
|
Less than 1%. |
| |
| (1) |
|
Unless otherwise indicated, the address of all individuals and entities listed above is
PolyMedix, Inc., 170 N. Radnor Chester Rd., Suite 300, Radnor, Pennsylvania 19087. |
| |
| (2) |
|
Includes 2,434,833 shares of common stock issuable upon exercise of options. |
| |
| (3) |
|
Includes 330,000 shares of common stock issuable upon exercise of options and 429,000
shares of common stock issuable upon exercise of warrants. The warrants and 477,000 shares of
common stock are held in the name of Kate Partners XX, L.P. (“Kate Partners”). Mr. Slattery
owns a controlling interest in Kate Partners; therefore, he may be deemed to beneficially own
these shares and warrants. Mr. Slattery disclaims beneficial ownership of these securities
except to the extent of his pecuniary interest in Kate Partners. |
| |
| (4) |
|
Includes 394,722 shares of common stock issuable upon exercise of options. |
| |
| (5) |
|
Includes 330,000 shares of common stock issuable upon exercise of options. |
| |
| (6) |
|
Includes 602,778 shares of common stock issuable upon exercise of options. |
| |
| (7) |
|
Includes 466,528 shares of common stock issuable upon exercise of options. |
| |
| (8) |
|
Includes 330,000 shares of common stock issuable upon exercise of options and 21,430
shares of common stock issuable upon exercise of warrants. |
| |
| (9) |
|
Includes 340,278 shares of common stock issuable upon exercise of options. |
| |
| (10) |
|
Includes 100,000 shares of common stock issuable upon exercise of options and 21,500 shares
of common stock issuable upon exercise of warrants. |
| (11) |
|
Includes 100,000 shares of common stock issuable upon exercise of options. |
| |
| (12) |
|
Includes 5,579,139 shares of common stock issuable upon exercise of options and 471,930
shares of common stock issuable upon exercise of warrants. |
| |
| (13) |
|
We understand that Amir L. Ecker and Carol G. Frankenfield are the General Partners of ACT
Capital Management, LLLP and Act Capital Partners, LP and that voting and investment decisions
made on behalf of ACT Capital Management, LLLP are made primarily by its General Partners.
This figure does not include 3,339,958 shares of common stock issuable upon exercise of
outstanding warrants which are not currently exercisable as a result of aggregate holdings
limitations set forth in the warrants. Also includes 66,000 shares of common stock held in
various accounts of which Mr. Ecker personally has sole investment and voting power, 15,000
shares of common stock held by Ms. Frankenfield, 10,000 shares of common stock held jointly by
Ms. Frankenfield and her husband, and 2,100 shares of common stock held by Ms. Frakenfeld’s
husband. Also includes 960,000 shares of common stock acquired upon the conversion of the
Series 2008 Convertible Preferred Stock, as well as 1,180,000 shares of common stock issuable
upon exercise of outstanding Series B Warrants at an exercise price of $1.00 per share. |
| |
| (14) |
|
Includes 409,046 shares of common stock issuable upon exercise of outstanding warrants but
does not include approximately 2,203,097 shares of common stock issuable upon exercise of
outstanding warrants which are not currently exercisable as a result of aggregate holdings
limitations set forth in a subscription agreement by and between PolyMedix, Inc. and Target
Capital Management dated July 15, 2008. Target Capital Management is a sole proprietorship
formed by Stephen A. Springer. |
-51-
EXECUTIVE COMPENSATION
The following summary compensation table sets forth information concerning compensation for
services rendered in all capacities for the years ended December 31, 2008 and 2007 awarded to,
earned by, or paid to: (i) Nicholas Landekic, who served as our President and Chief Executive
Officer (our CEO) during 2008 and 2007, (ii) Edward F. Smith, who served as our Vice President,
Finance, Chief Financial Officer and Corporate Secretary (our CFO) during 2008 and 2007 and (iii)
our three most highly paid executive officers (as determined based on total compensation) other
than our CEO and CFO as of December 31, 2008. These individuals are referred to in this report as
the Named Executive Officers (or “NEOs”).
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Option |
|
All Other |
|
|
| |
|
|
|
Salary |
|
Bonus |
|
Awards |
|
Compensation |
|
Total |
| Name and Principal Position |
|
Year |
|
($) |
|
($) (3) |
|
($) (4) |
|
($) |
|
($) |
| |
Nicholas Landekic |
|
2008 |
|
370,000 |
|
101,750 |
|
393,879 |
(5) |
— |
|
865,629 |
President, Chief Executive |
|
2007 |
|
368,333 |
|
111,000 |
|
249,853 |
(6) |
— |
|
729,186 |
Officer and Director |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Edward F. Smith |
|
2008 |
|
225,000 |
|
56,250 |
|
105,902 |
(7) |
— |
|
387,152 |
Vice President, Finance, Chief |
|
2007 |
|
222,917 |
|
45,000 |
|
83,389 |
(8) |
— |
|
351,306 |
Financial Officer and Secretary |
|
|
|
|
|
|
|
|
|
|
|
|
| |
R. Eric McAllister, M.D., Ph.D. |
|
2008 |
|
280,000 |
|
63,000 |
|
192,112 |
(9) |
— |
|
535,112 |
Vice President, Clinical |
|
2007 |
|
280,000 |
|
42,000 |
|
151,544 |
(10) |
29,329 |
(15) |
502,873 |
Development, Chief Medical
Officer (1) |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Richard W. Scott, Ph.D. |
|
2008 |
|
265,000 |
|
46,375 |
|
33,330 |
(11) |
— |
|
344,705 |
Vice President, Research |
|
2007 |
|
263,750 |
|
26,500 |
|
19,323 |
(12) |
— |
|
309,573 |
| |
Bozena Korczak, Ph.D. |
|
2008 |
|
230,000 |
|
51,750 |
|
48,721 |
(13) |
— |
|
330,471 |
Vice President, Drug Development (2) |
|
2007 |
|
32,436 |
|
53,000 |
|
4,293 |
(14) |
— |
|
89,729 |
| |
| |
|
|
| (1) |
|
Dr. McAllister joined PolyMedix in November 2006. |
| |
| (2) |
|
Dr. Korczak joined PolyMedix in November 2007. |
| |
| (3) |
|
Represents performance bonus awards. The 2007 bonus award was paid in 2008 and the 2008
bonus award was paid in 2009. |
| |
| (4) |
|
This column reflects the dollar amount recognized for financial accounting reporting
purposes, in accordance with SFAS 123(R), pursuant to our equity compensation plans and,
therefore, includes amounts from awards granted in and prior to the applicable fiscal year.
These amounts reflect our accounting expense for these awards, and do not correspond to the
actual value that will be recognized by the Named Executive Officer. The assumptions used in
the calculation of these amounts are described in footnote 6 to our audited financial
statements for the year ended December 31, 2008 and our discussion of stock-based compensation
in our annual report on Form 10-K filed with the Securities and Exchange Commission under
“Management’s Discussion and Analysis Of Financial Condition and Results of
Operations—Critical Accounting Policies and Practices” for the year ended December 31, 2008. |
| |
| (5) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2008 in
connection with option grants to Mr. Landekic to purchase 500,000 shares of common stock on
December 2, 2005, 231,000 shares of |
-52-
|
|
|
| |
|
common stock on February 5, 2007, 600,000 shares of common stock on January 23, 2008, and
1,000,000 shares of common stock on December 30, 2008 pursuant to our 2005 Omnibus Equity
Compensation Plan. |
| |
| (6) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2007 in
connection with option grants to Mr. Landekic to purchase 500,000 shares of common stock on
December 2, 2005 and 231,000 shares of common stock on February 5, 2007 pursuant to our 2005
Omnibus Equity Compensation Plan. |
| |
| (7) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2008 in
connection with option grants to Mr. Smith to purchase 250,000 shares of common stock on
January 2, 2006, 30,000 shares of common stock on February 5, 2007, 125,000 shares of common
stock on January 23, 2008, and 325,000 shares of common stock on December 30, 2008 pursuant to
our 2005 Omnibus Equity Compensation Plan. |
| |
| (8) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2007 in
connection with option grants to Mr. Smith to purchase 250,000 shares of common stock on
January 2, 2006 and 30,000 shares of common stock on February 5, 2007 pursuant to our 2005
Omnibus Equity Compensation Plan. |
| |
| (9) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2008 in
connection with option grants to Dr. McAllister to purchase 400,000 shares of common stock on
November 13, 2006, 200,000 shares of common stock on January 23, 2008, and 500,000 shares of
common stock on December 30, 2008 pursuant to our 2005 Omnibus Equity Compensation Plan. |
| |
| (10) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2007 in
connection with option grants to Dr. McAllister to purchase 400,000 shares of common stock on
November 13, 2006 pursuant to our 2005 Omnibus Equity Compensation Plan. |
| |
| (11) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2008 in
connection with an option grant to Dr. Scott to purchase 40,000 shares of common stock on
February 5, 2006, 50,000 shares of common stock on January 23, 2008, and 225,000 shares of
common stock on December 30, 2008 pursuant to our 2005 Omnibus Equity Compensation Plan. |
| |
| (12) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2007 in
connection with an option grant to Dr. Scott to purchase 40,000 shares of common stock on
February 5, 2006, pursuant to our 2005 Omnibus Equity Compensation Plan. |
| |
| (13) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2008 in
connection with option grants to Dr. Korczak to purchase 250,000 shares of common stock on
November 13, 2007 and 500,000 shares of common stock on December 30, 2008 pursuant to our 2005
Omnibus Equity Compensation Plan. |
| |
| (14) |
|
This amount reflects the compensation expense incurred by us in fiscal year 2007 in
connection with option grants to Dr. Korczak to purchase 250,000 shares of common stock on
November 13, 2007 pursuant to our 2005 Omnibus Equity Compensation Plan. |
| |
| (15) |
|
This amount represents reimbursement of certain relocation expenses to Dr. McAllister as
provided in Dr. McAllister’s offer letter dated October 19, 2006. |
Narrative Disclosure to Summary Compensation Table
In 2008, the Compensation Committee of the Board awarded cash bonuses to each of our NEOs.
These awards are reflected in the column titled “Bonus” in the Summary Compensation Table above.
Such awards were made by the Compensation Committee in its sole discretion at the end of the year
after reviewing the recommendations of our Chief Executive Officer for each NEO other than himself,
individual goals and targets, the performance of each NEO in the applicable fiscal year, evaluating
other components of each NEO’s total compensation package, including the balance of equity to
non-equity compensation, each NEO’s total compensation package relative to executives in benchmark
peer group companies holding similar positions, the report of the Compensation Committee’s
consultant, and our cash position. For benchmarking executive compensation, the Compensation
Committee engaged a compensation consulting firm which assisted in establishing a peer group of
companies, analyzing peer company compensation data and comparing our compensation programs with
the practices of the companies represented in the compensation data reviewed. For 2008, the
Compensation
-53-
Committee’s consulting firm, Compensation Resources, Inc. established a peer group of 35
publicly traded biotechnology companies of similar size to us. The Compensation Committee’s
primary consideration in awarding the cash bonuses for 2008 was to bring each NEO’s total
compensation package in line with the benchmarks established for executives in peer group companies
holding similar positions.
Outstanding Equity Awards
The following table provides information on all stock option awards held by our NEOs as of
December 31, 2008. All outstanding equity awards are in shares of our common stock.
Outstanding Equity Awards at 2008 Fiscal Year-End
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Option Awards (1) |
| |
|
Number |
|
|
|
|
|
|
| |
|
of |
|
Number of Securities |
|
|
|
|
| |
|
Securities Underlying |
|
Underlying |
|
|
|
|
| |
|
Unexercised Options |
|
Unexercised Options |
|
Option Exercise |
|
|
| |
|
(#) |
|
(#) |
|
Price |
|
Option Expiration |
| Name |
|
Exercisable |
|
Unexercisable |
|
($) |
|
Date |
Nicholas Landekic |
|
|
1,000,000 |
(2) |
|
|
— |
|
|
|
1.50 |
|
|
|
08/10/2015 |
|
President, Chief Executive |
|
|
500,000 |
(3) |
|
|
— |
|
|
|
1.50 |
|
|
|
12/01/2015 |
|
Officer and Director |
|
|
141,167 |
(3) |
|
|
89,833 |
|
|
|
2.85 |
|
|
|
02/04/2016 |
|
|
|
|
183,333 |
(3) |
|
|
416,667 |
|
|
|
1.10 |
|
|
|
01/22/2018 |
|
|
|
|
— |
(3) |
|
|
1,000,000 |
|
|
|
1.18 |
|
|
|
12/29/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Edward F. Smith |
|
|
243,056 |
(3) |
|
|
6,944 |
|
|
|
1.50 |
|
|
|
01/01/2016 |
|
Vice President, Finance, Chief |
|
|
18,333 |
(3) |
|
|
11,667 |
|
|
|
2.85 |
|
|
|
02/04/2016 |
|
Financial Officer and Secretary |
|
|
38,194 |
(3) |
|
|
86,806 |
|
|
|
1.10 |
|
|
|
01/22/2018 |
|
|
|
|
— |
(3) |
|
|
325,000 |
|
|
|
1.18 |
|
|
|
12/29/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R. Eric McAllister, M.D., |
|
|
208,333 |
(4) |
|
|
191,667 |
|
|
|
3.50 |
|
|
|
11/12/2016 |
|
Ph.D. |
|
|
61,111 |
(3) |
|
|
138,889 |
|
|
|
1.10 |
|
|
|
01/22/2018 |
|
Vice President, Clinical |
|
|
— |
(3) |
|
|
500,000 |
|
|
|
1.18 |
|
|
|
12/29/2018 |
|
Development, Chief Medical
Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Richard W. Scott, Ph.D. |
|
|
250,000 |
(2) |
|
|
— |
|
|
|
1.50 |
|
|
|
08/10/2015 |
|
Vice President, Research |
|
|
24,444 |
(3) |
|
|
15,556 |
|
|
|
2.85 |
|
|
|
02/04/2016 |
|
|
|
|
15,278 |
(3) |
|
|
34,722 |
|
|
|
1.10 |
|
|
|
01/22/2018 |
|
|
|
|
— |
(3) |
|
|
225,000 |
|
|
|
1.18 |
|
|
|
12/29/2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bozena Korczak, Ph.D. |
|
|
90,278 |
(3) |
|
|
159,722 |
|
|
|
0.98 |
|
|
|
11/12/2017 |
|
Vice President, Drug |
|
|
— |
(3) |
|
|
500,000 |
|
|
|
1.18 |
|
|
|
12/29/2018 |
|
Development |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1) |
|
There are no restricted stock awards outstanding for any of the NEOs. |
| |
| (2) |
|
Grants with an expiration date of August 10, 2015 have a stated term of ten years and vest
50% on the date of grant and 50% on the one-year anniversary of the grant. |
| |
| (3) |
|
Grants with expiration dates of December 1, 2015, January 1, 2016, February 4, 2016, January
22, 2018, November 12, 2017 and December 29, 2018 have a stated term of ten years and vest in
monthly installments over a three-year period beginning after the date of grant. If a “change
in control” (as defined in our 2005 Omnibus Equity Compensation Plan) were to occur, these
options would become immediately exercisable in full. |
| |
| (4) |
|
Grants with expiration dates of November 12, 2016 have a stated term of ten years and vest in
50% on the two-year anniversary of the grant and the remaining 50% vests in monthly
installments over a two-year period |
-54-
|
|
|
| |
|
beginning after the two-year anniversary of the grant. If a “change in control” (as defined in
our 2005 Omnibus Equity Compensation Plan) were to occur, these options would become
immediately exercisable in full. |
Employment Contracts and Termination of Employment and Change-in-Control Arrangements
Nicholas Landekic
We extended the offer of employment to Nicholas Landekic for the position of President and
Chief Executive Officer pursuant to an offer letter dated July 30, 2002, which provided that his
annual salary will be at least $250,000 per year. Mr. Landekic’s base salary was increased to
$350,000 as of January 1, 2006 and further increased to $380,000 as of February 2007. Mr. Landekic
is eligible to receive additional compensation depending upon achievement of performance goals as
established by the Board. As an “at-will” employee, Mr. Landekic’s employment can be terminated by
us or by him, at any time and for any reason. In the event Mr. Landekic is terminated by us other
than for “cause” or other than by reason of his “disability,” or he resigns for “good reason” (each
as defined in his offer letter), Mr. Landekic will be entitled to full vesting of all unvested
stock options and restricted stock previously granted to him and a cash payment equal to two-years
of his then current base salary.
Edward Smith
We extended the offer of employment to Edward Smith for the position of Vice President
Finance, Chief Financial Officer and Corporate Secretary pursuant to an offer letter dated December
5, 2005, which provides that his annual salary will be at least $200,000 per year. Mr. Smith’s
base salary was increased to $225,000 as of February 2007 and increased to $245,000 effective
January 1, 2009. Mr. Smith is eligible to receive additional compensation depending upon
achievement of performance goals as established by the Board. As an “at-will” employee, Mr.
Smith’s employment can be terminated by us or by him, at any time and for any reason. In the event
Mr. Smith is terminated by us other than for “cause” or other than by reason of his “disability”
(each as defined in his offer letter), Mr. Smith will be entitled to full vesting of all unvested
stock options previously granted to him and a cash payment equal to one–year of his then current
base salary.
R. Eric McAllister, M.D., Ph.D.
We extended the offer of employment to R. Eric McAllister, M.D., Ph.D., for the position of
Vice President, Clinical Development and Chief Medical Officer pursuant to an offer letter dated
October 19, 2006, which provides that his annual salary will be at least $280,000 per year. Dr.
McAllister received an initial grant of 400,000 stock options upon joining us, which vested at 50%
on Dr. McAllister’s second anniversary date with the remainder vesting monthly over his third and
fourth years. In addition, Dr. McAllister is eligible to receive a discretionary cash bonus based
on his performance and our performance. Dr. McAllister is eligible to receive additional
compensation depending upon achievement of performance goals as established by the Board. As an
“at-will” employee, Dr. McAllister’s employment can be terminated by us or by him, at any time and
for any reason. In the event Dr. McAllister is terminated by us other than for “cause” or other
than by reason of his “disability” (each as defined in his offer letter), Dr. McAllister will be
entitled to full vesting of all unvested stock options previously granted to him and a cash payment
equal to one–year of his then current base salary. Further, Dr. McAllister received various
relocation benefits.
Richard Scott, Ph.D.
We extended the offer of employment to Richard Scott for the position of Vice President,
Research pursuant to an offer letter dated September 23, 2002, which provides that his annual
salary will be at least $150,000 per year. Dr. Scott’s base salary was increased to $250,000
effective January 1, 2006 and further increased to $265,000 as of February 2007. Dr. Scott is
eligible to receive additional compensation depending upon achievement of performance goals as
established by the Board. As an “at-will” employee, Dr. Scott’s employment can be terminated by us
or by him, at any time and for any reason. In the event Dr. Scott is terminated by us other than
for “cause” or other than by reason of his “disability” (each as defined in his offer letter), Dr.
Scott will be entitled to full vesting of all unvested stock options and restricted stock
previously granted to him and a cash payment equal to one-year of his then current base salary.
Bozena Korczak, Ph.D.
We extended the offer of employment to Bozena Korczak for the position of Vice President
Finance, Drug Development pursuant to an offer letter dated November 5, 2007, which provides that
her annual salary will be at least $230,000 per year. Dr. Korczak’s base salary increased to
$245,000 effective January 1, 2009. Dr. Korczak
-55-
received an initial grant of 250,000 stock options upon joining us, which vest in equal
monthly installments over a three-year period. Dr. Korczak is eligible to receive additional
compensation depending upon achievement of performance goals as established by the Board. As an
“at-will” employee, Dr. Korczak’s employment can be terminated by us or by her, at any time and for
any reason. In the event Dr. Korczak is terminated by us other than for “cause” or other than by
reason of her “disability” (each as defined in her offer letter), Dr. Korczak will be entitled to
full vesting of all unvested stock options previously granted to her and a cash payment equal to
one-year of her then current base salary.
James Gregory Ford
We extended the offer of employment to James Gregory Ford for the position of Vice President
of Business Development pursuant to an offer letter dated November 18, 2008, which provides that
his annual salary will be at least $260,000 per year. Mr. Ford received an initial grant of
400,000 stock options, which vest 50% on the second anniversary of the grant date and the remainder
vest in equal monthly installments thereafter for the third and fourth years from the grant date.
Mr. Ford will be eligible to receive a discretionary cash bonus. For 2009, Mr. Ford may receive a
maximum potential bonus of up to 100% of his base salary for deals in which Mr. Ford was the
primary and lead negotiator and essential to concluding and based on (i) one percent (1%) of cash
revenues actually received by us in 2009 for out-licensing deals, (ii) equity investments actually
received in 2009 made by corporate partners as part of a strategic investment or business
opportunity, and (iii) a 35% bonus payment for in-licensing deals of a marketed or clinical stage
LMWH product, or a to be determined amount for any other clinical stage product. As an “at-will”
employee, Mr. Ford’s employment can be terminated by us or by him, at any time and for any reason.
If Mr. Ford’s employment is terminated as a result of a Qualified Termination (as defined in his
offer letter), then Mr. Ford will be entitled to: (a) continuation of his base salary for one year,
(b) Company payments of COBRA premiums for up to one year following termination, and (c) full
vesting of any unvested stock options. However, if a Qualified Termination occurs within 24 months
of the commencement of Mr. Ford’s employment, 200,000 options of his initial grant will vest in
lieu of the schedule in (c) above.
-56-
DIRECTOR COMPENSATION
The following Director Compensation table sets forth information concerning compensation for
services rendered by our independent directors for fiscal year 2008.
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Fees Earned or |
|
|
|
|
| |
|
Paid in |
|
Option |
|
|
| |
|
Cash |
|
Awards |
|
Total |
| Name |
|
($) |
|
($) (3) |
|
($) |
Frank P. Slattery, Jr. |
|
|
29,000 |
|
|
|
32,100 |
(4) |
|
|
61,100 |
|
Chairman of the Board |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shaun F. O’Malley |
|
|
29,000 |
|
|
|
32,100 |
(4) |
|
|
61,100 |
|
Chairman, Audit Committee |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Brian Anderson |
|
|
20,000 |
|
|
|
63,619 |
(5) |
|
|
83,619 |
|
Chairman, Compensation Committee |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
William N. Kelley, M.D. (1) |
|
|
34,000 |
|
|
|
32,100 |
(4) |
|
|
36,100 |
|
Chairman, Governance Committee |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Richard W. Bank, M.D. |
|
|
9,000 |
|
|
|
33,615 |
(6) |
|
|
42,615 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frank M. DeLape (2) |
|
|
11,000 |
|
|
|
56,869 |
(4) |
|
|
67,869 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael E. Lewis, Ph.D. |
|
|
23,000 |
|
|
|
32,100 |
(4) |
|
|
55,100 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stefan D. Loren, Ph.D. |
|
|
11,000 |
|
|
|
33,615 |
(6) |
|
|
44,615 |
|
|
|
|
| (1) |
|
Dr. Kelley did not stand for re-election to our Board at the Annual Meeting of Stockholders
held on June 30, 2009. |
| |
| (2) |
|
Mr. DeLape retired from the Board of Directors in May 2008. |
| |
| (3) |
|
This column reflects the dollar amount recognized for financial accounting reporting
purposes, in accordance with SFAS 123(R), pursuant to our equity compensation plans and,
therefore, includes amounts from awards granted in and prior to the applicable fiscal year.
These amounts reflect our accounting expense for these awards, and do not correspond to the
actual value that will be recognized by the Named Executive Officer. The assumptions used in
the calculation of these amounts are described in footnote 6 to our audited financial
statements for the year ended December 31, 2008 and our discussion of stock-based compensation
in our annual report on Form 10-K filed with the Securities and Exchange Commission under
“Management’s Discussion and Analysis Of Financial Condition and Results of
Operations—Critical Accounting Policies and Practices” for the year ended December 31, 2008. |
| |
| (4) |
|
Represents the compensation expense incurred by us in fiscal year 2008 in connection with
option grants to purchase 150,000 shares of common stock on September 11, 2007. As of
December 31, 2008, this director holds options to purchase 330,000 shares of common stock. |
| |
| (5) |
|
Represents the compensation expense incurred by us in fiscal year 2008 in connection with the
option grant to purchase 150,000 shares of common stock on January 23, 2008. As of December
31, 2008, this director holds options to purchase 150,000 shares of common stock. |
| |
| (6) |
|
Represents the compensation expense incurred by us in fiscal year 2008 in connection with
option grants to purchase 150,000 shares of common stock on July 31, 2008. As of December 31,
2008, this director holds options to purchase 150,000 shares of common stock. |
-57-
Continuing Education of Directors
We are committed to supporting the continuing education of our directors on relevant matters.
The Governance Committee of the Board will decide on a case-by-case basis the appropriate level and
frequency of support to provide.
Compensation of Directors
Directors who are also our employees receive no additional compensation for serving as a
director or as a member of any Committee of the Board. All non-employee directors receive a fee of
$1,500 and $1,000 per Board meeting and Committee meeting, respectively, and are reimbursed for
expenses incurred in connection with attending Board and Committee meetings. In addition, our
Chairman of the Board receives an annual retainer of $20,000, the Chairman of the Audit Committee
receives an annual retainer of $16,000, the Chairmen of the Compensation and Nominating and
Corporate Governance Committees receive annual retainers of $14,000 and all other non-employee
directors receive an annual retainer of $12,000.
All new non-employee directors receive an initial grant of options to purchase shares of our
common stock upon first becoming a member of the Board. In addition, each non-employee director
may, at the discretion of the Board or Compensation Committee of the Board, receive additional
equity compensation awards. See the Director Compensation Table below for more details.
-58-
MANAGEMENT’S DISCUSSION AND ANALYSIS AND PLAN OF OPERATION
The following discussion and analysis should be read in conjunction with the consolidated
financial statements including the notes thereto. This discussion and analysis may contain
forward-looking statements based upon current expectations that involve risks and uncertainties.
Our actual results may differ materially as a result of various factors, including those set forth
under “Risk Factors” or elsewhere in this prospectus.
Overview
We are a development stage biotechnology company focused on treating life threatening, serious
infectious diseases and acute cardiovascular disorders with synthetic small molecule compounds that
mimic the activity of large natural protein molecules, compounds referred to as biomimetics. Using
our proprietary computational drug design technology, we have created novel defensin mimetic
antibiotic compounds, heparin antagonist compounds and other drug compounds intended for human
therapeutic use. Since 2002, we have been a development stage enterprise, and accordingly, our
operations have been directed primarily toward developing business strategies, raising capital,
research and development activities, conducting pre-clinical testing and human clinical trials of
our product candidates, exploring marketing channels and recruiting personnel.
We have incurred operating losses since inception, have not generated any product sales
revenues and have not achieved profitable operations. Our deficit accumulated during the
development stage through June 30, 2009 aggregated $42.7 million and we expect to continue to incur
substantial losses in future periods. None of our product candidates have received regulatory
approval for commercial sale and our product candidates may never be commercialized. In addition,
all of our product candidates are in the early stages of development and several programs are on
hold pending our receipt of sufficient additional resources. The
progress and results of our current and any future clinical trials or future pre-clinical testing
are uncertain, and if our product candidates do not receive regulatory approvals, our business,
operating results, financial condition and cash flows will be materially adversely affected. Our
development programs require a significant amount of cash to support the development of product
candidates.
We are highly dependent on the success of our research, development and licensing efforts and,
ultimately, upon regulatory approval and market acceptance of our products under development. Our
short and long-term capital requirements depend upon a variety of factors, including market
acceptance for our technologies and product candidates and various other factors. We
believe that our current cash and investment balances, exclusive of any cash to be generated from
this offering or the Equity Line, will be sufficient to fund our ongoing Phase 1B study for
PMX-30063, and can fund our operations into the fourth quarter of 2010. Due to
continued weakness in capital markets and our understanding that it may be difficult to secure
significant additional financing on favorable terms, we have begun and expect to continue to scale
back and delay some of our planned activities in order to prolong our cash resources.
Our
current cash and investment balances are not sufficient to initiate
or fund Phase 2 clinical trials or any of the
related development activities for PMX-30063 or PMX-60056. We do not plan to initiate our Phase 2
development activities for PMX-30063 or PMX-60056 until additional financing is secured, through
this offering or otherwise. We may seek additional funds beyond this offering and the Equity Line,
through equity or debt financing, among other sources. In addition, we may actively seek funds
through government grants and contracts. However, as a result of current conditions in the equity
and debt markets, we may not be able to obtain additional funding on favorable terms, if at all.
In addition, if we choose to apply for government grants and contracts, there is no guarantee of
acceptance of our applications. If we are unable to secure adequate additional funding during
2009, through this offering or otherwise, we will further delay, scale-back or eliminate certain of
our planned research, drug discovery and development activities and certain other aspects of our
operations and our business until such time as we are successful in securing adequate additional
funding. As a result, our business may be materially and adversely affected. In the absence of
adequate additional funding, we believe that we have the ability to scale our operations such that
our current cash and investment balances, exclusive of any cash to be generated from this offering
or the Equity Line, will be sufficient to fund our operations into the fourth quarter of 2010.
Global market and economic conditions have been, and continue to be, disruptive and volatile.
In particular, the cost of raising money in the debt and equity capital markets has increased
substantially while the availability of funds from those markets has diminished significantly. If
funding is not available when needed, or is available only on unfavorable terms, meeting our
capital needs or otherwise taking advantage of business opportunities may become challenging, which
could have a material adverse effect on our business plans.
-59-
The following discussion and analysis provides information that we believe is relevant to an
assessment and understanding of our results of operations for the
three and six-months ended June 30, 2009
and 2008, and financial condition as of June 30, 2009 and December 31, 2008.
Critical Accounting Policies and Practices
The preparation of our Consolidated Financial Statements in conformity with accounting
principles generally accepted in the United States requires management to adopt critical accounting
policies and to make estimates and assumptions that affect the amounts reported in our Consolidated
Financial Statements and accompanying notes. These critical accounting policies and estimates have
been reviewed by our Audit Committee. The principal items in our Consolidated Financial Statements
reflecting critical accounting policies or requiring significant estimates and judgments are as
follows:
Stock-based compensation
From our inception, August 8, 2002, we adopted the fair value recognition provisions of
Statement of Financial Accounting Standards (“SFAS”) No. 123, Accounting for Stock-Based
Compensation and have, since inception, recognized equity compensation expense over the requisite
service period. Beginning January 1, 2006, we adopted SFAS No. 123(R), Share-based Payment using
the modified-prospective transition method. There was no significant impact from switching from
SFAS No. 123 to SFAS No. 123(R). Since inception, we have used the Black-Scholes formula to
estimate the fair value of stock options and have elected to continue to estimate the fair value of
stock options using the Black-Scholes formula. The volatility and expected term assumptions have
the most significant effect on the results obtained from the Black-Scholes option-pricing model. We
have to date assumed that stock options have an expected life of five years, representing about
half of their contractual life, and assumed common stock volatility of between 41% and 75%. Higher
estimates of volatility and expected life of the option increase the value of an option and the
resulting expense. Given the absence of an active market for our common stock in prior periods, the
fair value of our common stock has periodically been estimated using several criteria, including
progress and milestones achieved in our research activities along with the price per share of our
preferred and common stock offerings.
Results of Operations
Three and six-months ended June 30, 2009 compared to three and six-months ended June 30,
2008
Revenues and Expenses
Grant and research revenues were $138,000 and $144,000 for the three and six-month periods
ended June 30, 2009, respectively, compared to $18,000 and $1,009,000 for the three and six-month
periods ended June 30, 2008, respectively. The changes in grant and research revenue for the three
and six-month periods ended June 30, 2009 were the result of the timing of our expenditures and
subsequent reimbursement related to our grant activities.
Research and development expenses were $1,565,000 and $3,240,000 for the three and six-month
periods ended June 30, 2009, respectively, compared to $1,718,000 and $4,444,000 for the three and
six-month periods ended June 30, 2008, respectively. The decreases for the three and six-month
period ending June 30, 2009 were the result of the delay and scale-back of certain research, and
the delay of certain clinical development activities. Pending timely and adequate additional
funding, we expect our research and development costs to increase in the second half of 2009 as a
result of increased staff hiring in connection with the preparation for Phase 2 studies for our
PMX-30063 and PMX-60056 product candidates.
General and administrative expenses were approximately $1,499,000 and $2,777,000 for the three
and six-month periods ended June 30, 2009, respectively, compared to $1,232,000 and $2,387,000 for
the three and six-month periods ended June 30, 2008, respectively. The increase in general and
administrative costs for the three and six month period ending June 30, 2009 is primarily
associated with increased legal fees and increased equity compensation expense.
Interest income and other expenses were $12,000 and $38,000 for the three and six-month
periods ended June 30, 2009, respectively, compared to $28,000 and $95,000 for the three and
six-month periods ended June 30, 2008, respectively. The changes for the three and six months
ending June 30, 2009 were mostly the result of lower interest rates and decreased cash and
investment balances resulting in less interest income.
Historical Cash Flows
Operating Activities. Cash used in operating activities during the six-month period ended June
30, 2009 was approximately $4,594,000, compared to approximately $4,899,000 for the six-month
period ended June 30, 2008. The decrease was primarily due to an increase in accounts payable,
accrued expenses and deferred rent, partially offset by an increase in prepaid expenses and other
current assets.
-60-
Investing Activities. Cash provided by or used in investing activities represents cash paid
for the purchase, and proceeds received from, the maturity of investments and cash paid for
property and equipment. During the six-month periods ended June 30, 2009 and 2008, $3,798,000 and
$1,683,000 was used to purchase investments, respectively. During the six-month periods ended June
30, 2009 and 2008, $9,800,000 and $4,000,000 was received from the maturities of investments,
respectively. During the six-month period ended June 30, 2009, $15,000 was used to purchase
property and equipment.
Financing Activities. To date, we have financed our operating and investing activities
primarily from the proceeds from the sale of equity securities. During the six-month period ended
June 30, 2009 we paid $71,000 in financing costs associated with our May 2009 Equity Line and paid
$72,000 in principal associated with our capital leases. During the six-month period ended June 30,
2008 we paid $496,000 in financing costs associated with our September 2008 private placement of
Series 2008 preferred stock units and paid $64,000 in principal associated with our capital leases.
Year ended December 31, 2008 compared to year ended December 31, 2007
Since inception, our only revenues have been from grants and other research arrangements.
Grant and research revenues were $1,066,000 and $1,126,000 for the years ended December 31, 2008
and 2007, respectively. The decrease in grant and research revenue was attributable to the timing
of expenses and related funds received in connection with our advanced technology grant from the
National Institute of Health, or NIH, in support of our development of our i.v. antibiotic product
candidate and our heptagonist product candidate. We currently have $7,000 remaining available
under these grants.
We incurred research and development expenses of $7,401,000 and $9,328,000 for the years ended
December 31, 2008 and 2007, respectively. The decrease was the result of the delay and scale-back
of certain research and delay of certain clinical development costs during 2008. With the closing
of our third quarter 2008 financing activities, costs to bring both PMX-30063 and PMX-60056 through
Phase 1 development were prioritized and deployed. Research and development costs include $440,000
and $323,000 related to stock-based compensation expense for the years ended December 31, 2008 and
2007, respectively. Pending timely and adequate additional funding, we expect our research and
development costs to increase in the second half of 2009 as a result of increased staff hiring in
connection with the start of preparation for Phase 2 studies for our PMX-30063 and PMX-60056
product candidates.
General and administrative expenses were $4,875,000 and $4,473,000 for the years ended
December 31, 2008 and 2007, respectively. The overall increase was mostly attributable to increased
legal costs and personnel costs. General and administrative costs include $964,000 and $936,000
related to stock-based compensation expense for the years ended December 31, 2008 and 2007,
respectively.
Interest income and other expenses were $224,000 and $511,000 for the years ended December 31,
2008 and 2007, respectively. The decrease was due to decreased interest rates on our cash, cash
equivalent and investment balances.
Historical Cash Flows
Operating Activities. Cash used in operating activities during the year ended December 31,
2008 increased to $10,926,000 as compared to $8,266,000 used for the year ended December 31, 2007.
The increase was primarily due to increased general and administrative expenses and decreased
current liabilities.
Investing Activities. Cash used for investing activities represents cash paid for purchases of
investments and property and equipment, net of maturities of investments. During the year ended
December 31, 2008 and 2007, we purchased $9,553,000 and $9,880,000 of investments, respectively.
During the year ended December 31, 2008 and 2007, maturities of our investments were $5,700,000 and
$12,098,000, respectively. During the years ended December 31, 2008 and 2007, property and
equipment purchases were $0 and $266,000, respectively.
Financing Activities. We have financed our operating and investing activities primarily from
the proceeds from the sale of equity securities. During the years ended December 31, 2008 and 2007,
we received $16,966,000 and $2,558,000, respectively, in net proceeds of such issuances of equity
securities. Additionally, during 2008 and 2007 we received $214,000 and $184,000, respectively
from the exercise of stock options.
-61-
Year ended December 31, 2007 compared to year ended December 31, 2006
Grant and research revenues were $1,126,000 and $821,000 for the years ended December 31, 2007
and 2006, respectively. The increase in grant and research revenue was attributable to funds
received in connection with our advanced technology grant from the National Institute of Health, or
NIH, in support of our development of our i.v. antibiotic product candidate, which commenced in
April 2006.
We incurred research and development expenses of $9,328,000 and $3,306,000 for the years ended
December 31, 2007 and 2006, respectively. The increase was the result of increased headcount and
outside laboratory research costs associated with our preclinical development, GMP compliant
manufacturing and GLP compliant toxicology, safety pharmacology and genotoxicity studies for
PMX-60056 and PMX-30063 planned for 2008. Research and development costs include $323,000 and
$205,000 related to stock-based compensation expense for the years ended December 31, 2007 and
2006, respectively.
General and administrative expenses were $4,473,000 and $4,174,000 for the years ended
December 31, 2007 and 2006, respectively. The increase was the result of facility, investor
relations and legal costs. General and administrative costs include $936,000 and $934,000 related
stock-based compensation expense for the years ended December 31, 2007 and 2006, respectively.
Interest income and other expenses were $511,000 and $693,000 for the years ended December 31,
2007 and 2006, respectively. The decrease was a result of our decreased average cash and investment
balances along with declining interest rates.
Historical Cash Flows
Operating Activities. Cash used in operating activities during the year ended December 31,
2007 increased to $8,266,000 as compared to $4,319,000 used for the year ended December 31, 2006.
The increase is attributed primarily to increased research and development spending and increased
general and administrative expenses.
Investing Activities. Cash used for investing activities represents cash paid for purchases of
investments and property and equipment, net of maturities of investments. During the year ended
December 31, 2007 and 2006, we purchased $9,880,000 and $10,810,000 of investments, respectively.
During the year ended December 31, 2007 and 2006, maturities of our investments were $12,098,000
and $5,000,000, respectively. During the years ended December 31, 2007 and 2006, property and
equipment purchases were $266,000 and $274,000, respectively.
Financing Activities. We have financed our operating and investing activities primarily from
the proceeds from the sale of equity securities. During the years ended December 31, 2007 and 2006,
we received $2,558,000 and $3,720,000, respectively, in net proceeds of such issuances of equity
securities. Additionally, during 2007 we received $184,000 from the exercise of stock options.
Liquidity and Capital Resources
As of June 30, 2009 and December 31, 2008, we had cash and investment balances of approximately
$10,356,000 and $15,106,000, respectively, and total liabilities of approximately $3,423,000 and
$2,934,000, respectively. The decrease in our cash and investment balances was primarily
attributable to cash used to fund ongoing operations.
In May 2009, we entered into an Investment Agreement with Dutchess Equity Fund, L.P., as amended in
July 2009. Pursuant to the Investment Agreement, Dutchess committed to purchase up to $10,000,000
of our common stock, over the course of thirty-six months. We may draw on the facility from time
to time, as and when we determine appropriate in accordance with the terms and conditions of the
Investment Agreement. In each put notice, we will set our minimum acceptable per share purchase
price for the put. The maximum dollar amount that we are entitled to put in any one notice is the
greater of (i) 200% of the average daily volume of our common stock for the three (3) trading days
prior to the date of delivery of the applicable put notice, multiplied by the average of the
closing prices for such trading days or (ii) $250,000. The purchase price shall be set at
ninety-five percent (95%) of the volume weighted average price (VWAP) of our common stock during
the five (5) consecutive trading day period beginning on the trading day immediately following the
date of delivery of the applicable put notice; provided, that any trading day during which the
daily VWAP is 10% above or 10% below the VWAP for the entire period will be omitted from the
calculation, and any trading day having a VWAP below the minimum acceptable price may be omitted
from the calculation. The aggregate number of shares issuable by us and purchasable by Dutchess
under the Investment Agreement is 12,000,000. As of June 30, 2009, we had not sold any shares to
Dutchess in connection with this Equity Line.
The global financial markets have been and continue to be in turmoil, with extreme volatility in
the equity and credit markets and with some financial and other institutions experiencing
significant financial distress. In addition, neither our access to nor the value of our cash
equivalents or short-term investments have been negatively affected by the recent liquidity
problems of financial institutions. Although we have attempted to be prudent in our investment
strategy and in funding our anticipated near term liquidity needs, it is not possible to predict
how the financial market turmoil and the deteriorating economic conditions may affect our financial
position.
These and any future financial institution failures could cause losses to the extent cash amounts
or the values of securities exceed government deposit insurance limits, and could restrict our
access to the public equity and debt markets. In particular, the cost of raising money in the debt
and equity capital markets has increased substantially, while the availability of funds from those
markets has diminished significantly. Also, as a result of concern about the stability of
financial markets generally, and the solvency of counterparties specifically, the cost of obtaining
money from the credit markets has increased as many lenders and institutional investors have
increased interest rates, enacted tighter lending standards and reduced and, in some cases, ceased
to provide funding to borrowers. Low valuations and decreased appetite for equity investments,
among other factors, may make the equity markets difficult to access on acceptable terms or
unavailable altogether.
-62-
We are a development stage company and have not experienced significant revenue generating
activities since our formation. We reached a positive working capital position for the first time
in the fourth quarter of 2005 as a result of our financing activities. We have incurred operating
losses for each year since our inception in 2002. To achieve operating profits, we, alone or with
others, must successfully identify, develop and market product candidates. Our principal
activities, from the beginning of our development stage, have been organizational matters, issuance
of stock, product research and development, fund raising and market research.
In the near-term, we expect to continue to incur significant and increasing operating losses as a
result of the research and development expenses we expect to incur in developing our product
candidates and the general and administrative expenses we incur as a reporting company under the
Securities Exchange Act of 1934, as amended. Additionally, we do not expect to generate any
revenues from sources other than research grants and contracts for the foreseeable future.
We are highly dependent on the success of our research, development and licensing efforts and,
ultimately, upon regulatory approval and market acceptance of our products under development. Our
short and long-term capital requirements depend upon a variety of factors, including the success of
our clinical trials, market acceptance for our
technologies and product candidates and various other factors. We believe that our current cash and
investment balances, exclusive of any cash to be generated from the Equity Line, will be sufficient
to fund our ongoing Phase 1B study for PMX-30063, and can fund our operations into
the fourth quarter of 2010. Due to continued weakness in capital markets and our understanding that
it may be difficult to secure significant additional financing on favorable terms, we have begun
and expect to continue to scale back and delay some of our planned activities in order to prolong
our cash resources.
Our current cash and investment balances are not sufficient to initiate or fund Phase 2
clinical trials or any of the related development activities for PMX-30063 or PMX-60056. As
described above under “Use of Proceeds,” we intend to use a portion of the proceeds of this
offering for Phase 2 development activities for PMX-30063 or PMX-60056. We may seek
additional funds to support our development activities through equity or debt financing, among
other sources. In addition, we may actively seek funds through government grants and contracts.
No assurance can be given that we will be able to obtain additional funding, grants or contracts
on favorable terms, if at all. If we are unable to secure adequate additional funding for our
development activities, we intend to further delay, scale-back or eliminate certain of our planned
research, drug discovery and development activities and certain other aspects of our operations
and our business until such time as we are successful in securing adequate additional funding. As
a result, our business may be materially and adversely affected. We believe that our existing
capital resources will enable us to fund our operations on a reduced scale into the fourth quarter of
2010.
Our short and long-term capital requirements depend upon a variety of factors, including market
acceptance for our technologies and product candidates and various other factors, many of which we
cannot control, including:
| |
Ø |
|
success of our clinical trials for PMX-30063 and PMX-60056; |
| |
| |
Ø |
|
continued progress of and increased spending related to our research and
development activities, including our plan to hire additional research and
development employees; |
| |
| |
Ø |
|
the conditions in the capital markets and the biopharmaceutical industry
that make raising capital or entering into strategic arrangements difficult and
expensive; |
| |
| |
Ø |
|
progress with preclinical experiments and clinical trials; |
| |
| |
Ø |
|
ongoing general and administrative expenses related to our being a reporting company; |
| |
| |
Ø |
|
the cost, timing, and results of regulatory reviews and approvals; |
| |
| |
Ø |
|
the maintenance of our existing licenses with the University of Pennsylvania
and the University of Massachusetts; |
| |
| |
Ø |
|
the success, timing, and financial consequences of any future collaborative,
licensing or other arrangements that we may establish; |
| |
| |
Ø |
|
the cost of filing, prosecuting, defending and enforcing any patent claims
and other intellectual property rights; |
| |
| |
Ø |
|
the costs of commercializing any of our product candidates; |
| |
| |
Ø |
|
technological and market developments; |
| |
| |
Ø |
|
the cost of manufacturing development; and |
-63-
| |
Ø |
|
timing and level of sales of products for which we obtain marketing approval. |
We expect to seek additional funds through equity or debt financing, collaborative or other
arrangements with corporate partners, and from other sources. For instance, we may actively seek
more funding through government grants and contracts.
Most of the grants historically received, including the $923,000 NIH grant to develop
new generation reversing agents for LMWH and the $6.6 million
NIH and $1.6 million Defense Threat Reduction Agency grants for research into
biodefense applications, typically are structured as specific cost-recovery agreements and are,
therefore, not available to fund all aspects of the Company’s product development efforts.
We may not be able to obtain any additional
financing on terms acceptable to us, if at all, or we may not raise as much as we expect. If
adequate additional funds are not available when required, we will have to delay, scale-back or
eliminate certain of our research, drug discovery or development activities or certain other
aspects of our operations and our business will be materially and adversely affected.
We are subject to many risks associated with development-stage businesses, including the
above-discussed risks associated with the ability to raise capital. Please see the section entitled
“Risk Factors” in this prospectus for more information regarding risks associated with our business.
Off-Balance Sheet Arrangements
We do not have any “off-balance sheet arrangements” as defined in applicable SEC regulations.
Commitments and Contingencies
As described above, we believe our current cash and investment balances are adequate to fund
operations, including the following commitments and contingencies,
into the fourth quarter of 2010.
If we are unable to secure adequate additional funding during
2009, through this offering or otherwise, we will
further delay, scale-back or
eliminate certain of our planned research, drug discovery and
development activities and certain other
aspects of our operations and our business until such time as we are successful in securing
adequate additional funding.
Capital Lease
We have entered into lease agreements for laboratory equipment. The initial obligation under
these capital leases was $331,000. The value of the laboratory equipment acquired in connection
with these leases was $398,000 and the depreciation associated with these assets is included along
with that of other owned property and equipment. These equipment leases have terms of up to three
years, at interest rates ranging from 9.5% to 11.5% and contain bargain purchase options. In
connection with these capital leases, we will pay $108,000 during 2009, $4,000 of which will be for
interest. These capital leases end during 2009.
Operating Lease
In June 2006, we entered into a lease agreement for 24,223 square feet of combined office and
laboratory space located in Radnor, Pennsylvania. The initial term of the lease is 12 years.
Payments under the lease commenced on December 1, 2006. Our future minimum lease payments under
this non-cancelable operating lease are as follows (in thousands):
| |
|
|
|
|
| |
|
Operating Leases |
|
2009 |
|
$ |
497 |
|
2010 |
|
$ |
589 |
|
2011 |
|
$ |
667 |
|
2012 |
|
$ |
686 |
|
2013 |
|
$ |
705 |
|
Thereafter |
|
$ |
3,610 |
|
Total minimum lease payments |
|
$ |
6,754 |
|
|
|
|
|
Prior to the commencement of our current operating lease for our Radnor Facility, we leased
approximately 3,500 square feet of combined office and laboratory space on a month-to-month basis
in Philadelphia, Pennsylvania. Rent expense was $598,000, $598,000 and $559,000 and $1,877,000 for
the years ended December 31, 2008, 2007, and 2006, and for the period from August 8, 2002
(Inception) to December 31, 2008, respectively.
-64-
Patent License Agreements
University of Pennsylvania. In January 2003, we entered into a Patent License Agreement with
Penn. Under the terms of the agreement, we were granted an exclusive, worldwide royalty-bearing
license to make and sell products utilizing seven of Penn’s issued or pending patents for the life
of such patents. One issued patent and five patent applications cover the composition of matter on
antimicrobial compounds, including small molecules, oligomers and polymers. One patent application
covers the composition and use of polycationic compounds for treating cancer. If a
change-of-control event occurs, in which we transfer the license to these patents to a third party
or we are acquired by another company, we are required to pay a 3% royalty on the gross sales for
licensed products that are sold as pharmaceuticals and a 1.5% royalty on products sold as coatings
for use in medical devices. We are permitted to sublicense the patents provided that (a) the
sublicensee is prohibited from further licensing of the patents and (b) the sublicensee is subject
to all of the terms of the original license granted to us. In addition, we are required to share
with Penn any consideration we receive from sublicensing our patents to a third party.
University of Massachusetts. In January 2004, we entered into a five-year sponsored research
agreement with UMass. Under the terms of this agreement, we have the exclusive option to license
any intellectual property that may be generated by Dr. Gregory Tew pursuant to research sponsored
under the agreement. We may exercise this option by issuing 7,500 shares of our common stock to
UMass for each $100,000 of research conducted by Dr. Tew. If we exercise this option, we are also
required to reimburse UMass for direct patent costs incurred by it for the patents licensed by us.
During 2007, we issued 12,500 shares to UMass in connection with this agreement. We sponsored
$36,000 and $107,000, $118,000 of Dr. Tew’s research for 2008, 2007 and 2006, respectively.
Other
Agreements with Employees. We have entered into employment agreements with various
executives. These agreements provide for severance arrangements and accelerated vesting of equity
compensation awards in the event that the executive is terminated by us other than for cause or
disability or if the executive resigns for good reason.
Credit Line. In April 2006, we entered into a line of credit agreement with a financial
institution. This line of credit provides for monthly interest-only payments at a variable per
annum rate of 3% plus the 30-day LIBOR rate. The amount available under this line of credit ranges
from 85% to 92% of cash and investments pledged as collateral, based upon the amount and security
type. There is currently no outstanding balance on this line of credit. In June 2006, we entered
into a letter of credit agreement with the same financial institution to secure our payment
obligations under our facility operating lease. This letter of credit is for $1,400,000, expires
on December 1, 2009 and is secured by our credit line.
Recently Issued Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board (or “FASB”) issued Statement of
Financial Accounting Standards (or “SFAS”) No. 157, Fair Value Measurements (“SFAS 157”). SFAS 157
clarifies the definition of fair value, establishes a framework for measuring fair value and
expands disclosures on fair value measurements. SFAS 157 was effective for financial statements
issued for fiscal years beginning after November 15, 2007 and interim periods within those fiscal
years; however, the FASB did provide a one-year deferral for the implementation of SFAS 157 for
nonfinancial assets and liabilities.
SFAS 157 establishes a valuation hierarchy for disclosure of the inputs to valuation used to
measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows. Level
1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs
that are observable for the asset or liability, either directly or indirectly through market
corroboration, for substantially the full term of the financial instrument. Level 3 inputs are
unobservable inputs based on management’s own assumptions used to measure assets and liabilities at
fair value. A financial asset or liability’s classification within the hierarchy is determined
based on the lowest level input that is significant to the fair value measurement. The adoption of
SFAS 157 did not have any impact on our consolidated financial statements.
In February 2007, the FASB issued SFAS No. 159 The Fair Value Option for Financial Assets and
Financial Liabilities (“SFAS 159”). SFAS 159 permits entities to choose to measure many financial
assets and financial liabilities at fair value. Unrealized gains and losses on items for which the
fair value option has been elected are reported in earnings. SFAS 159 was effective for fiscal
years beginning after November 15, 2007. We did not elect the fair value option available under
SFAS 159 for any financial assets or liabilities.
-65-
In June 2007, the FASB ratified the consensus reached in EITF Issue No. 07-03, Accounting for
Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and
Development Activities (Issue No. 07-03). Issue No. 07-03 requires that non-refundable advance
payments for future research and development activities should be deferred and recognized as an
expense as goods are delivered or the related services are performed. Issue No. 07-03 is effective
for fiscal years beginning after December 15, 2007. Issue No. 07-03 was adopted effective January
1, 2008 and did not have a material impact on our consolidated financial statements.
In June 2008, the FASB issued FASB Staff Position No. EITF 03-6-1, Determining Whether
Instruments Granted in Share-Based Payment Transactions Are Participating Securities, (“FSP EITF
03-6-1”). FSP EITF 03-6-1 addresses whether instruments granted in share-based payment transactions
are participating securities prior to vesting and, therefore need to be included in the earnings
allocation in computing earnings per share, or EPS, under the two-class method described in
paragraphs 60 and 61 of FASB Statement No. 128, Earnings per Share (“SFAS No. 128”). FSP EITF
03-6-1 applies to the calculation of EPS under SFAS No. 128 for share-based payment awards with
rights to dividends or dividend equivalents. FSP EITF 03-6-1 clarifies that unvested share-based
payment awards that contain nonforfeitable rights to dividends or dividend equivalents (whether
paid or unpaid) are participating securities and shall be included in the computation of EPS
pursuant to the two class method. FSP EITF 03-6-1 is effective for financial statements issued for
fiscal years beginning after December 15, 2008, and interim periods within those years. All
prior-period EPS data presented must be adjusted retrospectively (including interim financial
statements, summaries of earnings and selected financial data) to conform with the provisions of
FSP EITF 03-6-1. Early adoption is not permitted. We do not expect EITF 03-6-1 to have a material
impact on our consolidated financial statements.
In June 2008, the FASB ratified the consensus reached on EITF Issue No. 07-05, Determining
Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock (“EITF No. 07-05”).
EITF No. 07-05 clarifies the determination of whether an instrument (or an embedded feature) is
indexed to an entity’s own stock, which would qualify as a scope exception under SFAS No. 133,
Accounting for Derivative Instruments and Hedging Activities. EITF No. 07-05 is effective for
financial statements issued for fiscal years beginning after December 15, 2008. Early adoption for
an existing instrument is not permitted. We are currently evaluating the impact of the pending
adoption of EITF No. 07-05 on our consolidated financial statements.
In April 2009, the FASB issued Financial Statement Position (“FSP”) No. FAS 115-2 and FAS
124-2, Recognition and Presentation of Other-Than-Temporary Impairments. FSP No. FAS 115-2 and FAS
124-2 amends current other-than-temporary impairment guidance for debt securities and improves the
presentation and disclosure of other-than-temporary impairments on debt and equity securities in
the financial statements. FSP No. FAS 115-2 and FAS 124-2 are effective for interim and annual
reporting periods ending after June 15, 2009. The adoption of FSP No. FAS 115-2 and FAS 124-2 are
not expected to have a material impact on our consolidated financial statements.
In May 2009, the FASB issued SFAS No. 165, Subsequent Events (“SFAS 165”). SFAS 165
establishes general standards for accounting and disclosure of events occurring subsequent to the
balance sheet date but prior to issuance of the financial statements. SFAS 165 is effective for
interim or annual financial periods ending after June 15, 2009, and shall be applied prospectively.
The adoption of SFAS 165 is not expected to have a material impact on our consolidated financial
statements.
In June 2009, the FASB issued SFAS No. 168, The FASB Accounting Standards Codification and the
Hierarchy of Generally Accepted Accounting Principles — a replacement of FASB Statement No. 162
(“SFAS 168”). SFAS 168 introduces the FASB Accounting Standards Codification that will serve as
the single source of authoritative GAAP. SFAS 168 is effective for financial statements issued for
interim and annual periods ending after September 15, 2009. The adoption of SFAS 168 is not
expected to have a material impact on our consolidated financial statements.
-66-
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Other than compensation agreements and other arrangements with our executive officers and
directors and the transactions described below, during our last three fiscal years, there has not
been, and there is not currently proposed, any transaction or series of similar transactions to
which we were or will be a party in which the amount involved exceeded or will exceed the lesser of
$120,000 or one percent of the average of our total assets at year-end for the last two completed
fiscal years and in which any of our directors, nominees for director, executive officers, holders
of more than five percent of any class of our voting securities or any member of the immediate
family of the foregoing persons had or will have a direct or indirect material interest.
Fordham Financial Management, Inc.
In June 2008, we entered into an Amended and Restated Co-Placement Agent Agreement with
Fordham Financial, a former 5% stockholder of ours, and Carter Securities, LLC. As consideration
for the placement services rendered by the placement agents, including Fordham Financial, in
connection with our July 2008 public offering, during the period from May 2008 to July 2008 Fordham
Financial received:
| |
Ø |
|
a total of $276,000 in commissions, which represented 7% of the gross proceeds
we received for units placed by Fordham Financial; |
| |
Ø |
|
reimbursement of out of pocket expenses in an amount not to exceed $20,000 and
the fees of Fordham Financial’s legal counsel of up to $50,000 plus approved
disbursements; and |
| |
Ø |
|
warrants to purchase 281,578 shares of common stock, which represented 5% of the
aggregate number of units sold in the offering. The warrants are currently
exercisable, have an exercise price of $1.00 per share and expire July 14, 2013. |
In December 2007, Fordham Financial served as a selected dealer in connection with our
December 2007 public offering. In consideration for the services rendered by Fordham Financial in
connection with the December 2007 public offering, Fordham Financial received a total of $30,720 in
commissions, which represented 7% of the gross proceeds we received for units distributed by
Fordham Financial.
Private Placement
On September 23, 2008, we entered into Securities Purchase Agreements with certain
institutional and individual accredited investors, including three of our directors, in a private
placement of $4.25 million of units at $7.00 per unit consisting of (i) one share of our Series
2008 Convertible Preferred Stock and (ii) a Series B Warrant to purchase either shares of our 2008
Convertible Preferred Stock or, if the Series 2008 Convertible Preferred Stock has been converted
into our common stock, shares of our common stock.
In connection with the private placement, Stefan Loren, a director of ours, purchased 2,150
units at an aggregate offering price of $15,050; Frank Slattery Jr., a director of ours, purchased
42,900 units at an aggregate offering price of $300,300; and Shaun O’Malley, a director of ours,
purchased 2,143 units at an aggregate offering price of $15,001.
Pursuant to the terms of the Securities Purchase Agreements, each share of Series 2008
Convertible Preferred Stock automatically converted into 10 shares of common stock on December 10,
2008 upon the effectiveness of an amendment to our certificate of incorporation to increase the
number of shares of common stock authorized for issuance by us to an amount sufficient to cover the
issuance of shares of common stock upon conversion of the 2008 Convertible Preferred Stock and the
issuance of shares of common stock issuable after such conversion upon exercise of all of the
Series B Warrants.
Pursuant to the terms of the Securities Purchase Agreements, we also agreed to file, and
filed, a registration statement on Form S-1 covering the resale of common stock issuable on the
conversion of the 2008 Convertible Preferred Stock and the issuance of common stock issuable upon
exercise of the Series B Warrants.
Each Series B Warrant represents the right to purchase either (a) one share of Series 2008
Convertible Preferred Stock at an exercise price of $10.00 per share or (b) if, at the time the
Series B Warrant is exercised, the Series 2008 Preferred Stock has been converted into shares of
common stock, ten shares of common stock at an exercise price of $1.00 per share. Each Series B
Warrant may be exercised at any time through September 22, 2013. A holder will not have the right
to exercise any portion of a Series B Warrant to the extent that after giving effect to
-67-
such issuance after exercise, the holder (together with the holder’s affiliates), would
beneficially own in excess of 9.99% of the number of shares of the common stock outstanding
immediately after giving effect to such issuance.
-68-
CORPORATE GOVERNANCE
Our Board currently has eight members. William N. Kelley previously served as one of our
directors but elected not to stand for re-election to our Board at the Annual Meeting of
Stockholders held on June 30, 2009. Our Board, upon the recommendation of our Governance Committee,
nominated Douglas Swirsky to stand for election to the Board at the Annual Meeting of Stockholders
held on June 30, 2009 and Mr. Swirsky was elected to our Board at that meeting.
Independence of Directors
In determining the independence of our directors, we apply the definition of “independent
director” provided under the rules of the NYSE Amex (formerly the American Stock Exchange).
Pursuant to the NYSE Amex rules, the Board concluded its annual review of director independence in
May 2009. After considering all relevant facts and circumstances, the Board affirmatively
determined that all of the directors then serving on the Board and those directors nominated for
election at the Annual Meeting of Stockholders to be held on June 30, 2009 are independent pursuant
to the NYSE Amex rules regarding director independence, with the exception of Nicholas Landekic,
who serves as our President and Chief Executive Officer.
Compensation Committee Interlocks and Insider Participation
The current members of the Compensation Committee are Brian Anderson, Richard Bank and Michael
Lewis. Frank DeLape served on the Compensation Committee until his retirement from the Board of
Directors in May 2008, and William Kelley served on the Compensation Committee until his service on
the Board ended June 30, 2009. Except for Mr. DeLape, none of these individuals has ever been an
officer or employee of ours. From August 2005 to November 2005, Mr. DeLape served as the sole
officer as well as director of PolyMedix, Inc., formerly BTHC II Acquisition Corp. In addition,
none of our executive officers serves as a member of the board of directors or compensation
committee of any entity that has one or more executive officers serving as a member of our Board of
Directors or the Compensation Committee.
-69-
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Since August 18, 2006, our common stock has been traded on the OTC Bulletin Board under the
symbol “PYMX”. The market for our common stock is limited and volatile. The following table sets
forth the range of high and low bid quotations or high and low closing prices, as applicable, for
our common stock for each of the periods indicated as reported by the OTC Bulletin Board. The
prices quoted on the OTC Bulletin Board reflect inter-dealer prices, without retail mark-up,
mark-down or commissions. The OTC Bulletin Board prices listed below may not represent actual
transaction prices.
| |
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
| |
|
2009 |
| |
|
High |
|
Low |
First Quarter |
|
$ |
1.24 |
|
|
$ |
0.63 |
|
Second Quarter |
|
$ |
0.98 |
|
|
$ |
0.64 |
|
Third Quarter |
|
|
0.98 |
|
|
|
0.63 |
|
Fourth
Quarter (through November 4, 2009) |
|
|
1.28 |
|
|
|
0.95 |
|
| |
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
| |
|
2008 |
| |
|
High |
|
Low |
First Quarter |
|
$ |
1.90 |
|
|
$ |
0.56 |
|
Second Quarter |
|
$ |
1.05 |
|
|
$ |
0.70 |
|
Third Quarter |
|
$ |
1.05 |
|
|
$ |
0.52 |
|
Fourth Quarter |
|
$ |
1.45 |
|
|
$ |
0.68 |
|
| |
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
| |
|
2007 |
| |
|
High |
|
Low |
First Quarter |
|
$ |
4.00 |
|
|
$ |
1.60 |
|
Second Quarter |
|
$ |
2.35 |
|
|
$ |
1.55 |
|
Third Quarter |
|
$ |
2.10 |
|
|
$ |
0.75 |
|
Fourth Quarter |
|
$ |
2.10 |
|
|
$ |
0.75 |
|
| |
|
|
|
|
|
|
|
|
| |
|
Year Ended December 31, |
| |
|
2006 |
| |
|
High |
|
Low |
Third Quarter |
|
$ |
5.00 |
|
|
$ |
4.46 |
|
Fourth Quarter |
|
$ |
4.60 |
|
|
$ |
2.50 |
|
As
of November 4, 2009, the last reported sales price of our common stock on the OTC
Bulletin Board was $1.22 per share.
Holders of Record
As
of November 4, 2009, there were approximately 700 holders of record of shares of
common stock.
Dividends
Since our reincorporation in Delaware in March 24, 2005, we have not paid or declared any cash
dividends on our common stock. We currently intend to retain any earnings for future growth and,
therefore, do not expect to pay cash dividends on our common stock in the foreseeable future.
-70-
Equity Compensation Plan Information
The following table sets forth, as of December 31, 2008, information concerning equity
compensation plans under which our securities are authorized for issuance. The table does not
reflect grants, awards, exercises, terminations or expirations since that date.
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
(c) |
| |
|
(a) |
|
|
|
|
|
Number of securities |
| |
|
Number of securities |
|
(b) |
|
remaining available |
| |
|
to |
|
Weighted-average |
|
for |
| |
|
be issued upon |
|
exercise price of |
|
future issuance |
| |
|
exercise |
|
outstanding |
|
under equity |
| |
|
of outstanding |
|
options, |
|
compensation plan |
| |
|
options, |
|
warrants and |
|
(excluding securities |
| |
|
warrants and rights |
|
rights |
|
reflected column (a)) |
Equity compensation plans
approved by security holders
(1) |
|
|
8,890,000 |
|
|
$ |
1.41 |
|
|
|
64,500 |
|
Equity compensation plans not
approved by security holders
(2) |
|
|
1,320,000 |
|
|
$ |
1.64 |
|
|
|
— |
|
Total: |
|
|
10,210,000 |
|
|
$ |
1.44 |
|
|
|
64,500 |
|
|
|
|
| (1) |
|
Represents 918,000 and 7,972,000 shares of our common stock issuable upon exercise of
outstanding options granted under our 2002 Equity Compensation Plan and our 2005 Omnibus
Equity Compensation Plan, respectively, as of December 31, 2008, and 64,500 shares of our
common stock available for future issuance under our 2005 Omnibus Equity Compensation Plan as
of December 31, 2008. |
| |
| (2) |
|
An aggregate of 1,320,000 shares of our common stock represent portions of prior grants that
exceeded the aggregate individual grant limit under our 2002 Equity Compensation Plan or 2005
Omnibus Equity Compensation Plan, as applicable, and are considered to have occurred outside
such plans. |
| |
On June 30, 2009, our shareholders approved an amendment and restatement of our 2005 Omnibus
Equity Compensation Plan, which increased the aggregate number of shares of common stock reserved
for issuance under the 2005 Omnibus Equity Compensation Plan from 8,263,306 to 23,582,000.
-71-
DESCRIPTION OF SECURITIES
Capital Stock
We are authorized to issue 250,000,000 shares of common stock, par value $0.001 per share, of
which 59,845,065 shares were issued and outstanding as of June 30, 2009, and 10,000,000 shares of
preferred stock, par value $0.001 per share, of which no shares were issued and outstanding as of
December 31, 2008.
Units
Each
unit will consist of
share(s)
of common stock and warrant(s) to purchase
of a share of common stock. The units will not be certificated and the common stock and
warrants will be immediately separable and will be separately transferable immediately upon
issuance.
Common Stock
Holders of common stock are entitled to one vote per share on all matters to be voted upon by
the stockholders and are not entitled to cumulative voting for the election of directors. Holders
of common stock are entitled to receive ratably such dividends, if any, as may be declared from
time to time by the Board of Directors out of funds legally available therefore subject to the
rights of preferred stockholders. We do not intend to pay any cash dividends to the holders of
common stock and anticipate reinvesting our earnings. In the event of liquidation, dissolution or
winding up of the Company, the holders of common stock are entitled to share ratably in all assets
remaining after payment of liabilities and the preferences of preferred stockholders. Shares of
common stock have no preemptive, conversion or other subscription rights. There are no redemption
or sinking fund provisions applicable to common stock.
Warrants to be Issued as Part of this Offering
The
warrants offered in this offering will be issued in the form that
will be filed as an exhibit to the registration statement of which this
prospectus is a part. You should review a copy of the form of warrant for a
complete description of the terms and conditions applicable to the warrants.
The following is a brief summary of the warrants and is subject in
all respects to the provisions contained in the form of warrant.
Each
warrant represents the right to purchase
of a share of common stock at an
exercise price equal to $ per share,
subject to adjustment as described below. Each
warrant may be exercised on or after the closing date of this offering through and
including the close of business on . The warrant will have a cashless exercise right in the event
that the common stock underlying the warrants are not covered by an effective registration
statement at the time of such exercise.
The exercise price and the number of shares underlying the warrants are subject to appropriate
adjustment in the event of stock splits, stock dividends on our common stock, stock combinations or
similar events affecting our common stock. In addition, in the event we consummate any merger,
consolidation, sale or other reorganization event in which our common stock is converted into or
exchanged for securities, cash or other property or we consummate a sale of substantially all of
our assets, then following such event, the holders of the warrants will be entitled to receive upon
exercise of the warrants the kind and amount of securities, cash or other property which the
holders would have received had they exercised the warrants immediately prior to such
reorganization event.
We may
call the warrants, in whole or in part, if, at any time during the exercise period, the
volume weighted average price of our common stock for ten consecutive trading days exceeds
percent of the exercise price of the warrants. Any warrants which are subject
to a call notice and not exercised by the date that is ______
trading days after the date of the call notice will be cancelled without any consideration
paid to the holder.
No fractional shares of common stock will be issued in connection with the exercise of a
warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the
fractional amount multiplied by the market value of a share of common stock. A warrant may be
transferred by a holder without our consent, upon surrender of the warrant, properly endorsed (by
the holder executing an assignment in the form attached to the warrant). The warrants will not be
listed on any securities exchange or automated quotation system and we do not intend to arrange for
any exchange or quotation system to list or quote the warrants.
The
placement agent warrants will have terms substantially similar to the
warrants included in units offered hereby, except that placement
agent warrants will have a term of five years from the effective date
of the registration statement of which this prospectus is a part and
will otherwise comply with FINRA Rule 5110 (g)(1).
Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, with a par value of $0.001
per share, and the Board of Directors is authorized to create one or more series of preferred
stock, and to designate the rights, privileges, restrictions, preferences and limitations of any
given series of preferred stock. Accordingly, the Board of Directors may, without stockholder
approval, issue shares of preferred stock with dividend, liquidation, conversion, voting or other
rights that could adversely affect the voting power or other rights of the holders of common stock.
-72-
Stockholder Rights Plan
Each outstanding share of our common stock includes an associated preferred stock purchase
right (a “Right”) that will entitle the registered holder to purchase from us one one-thousandth of
a share of Series C Preferred Stock, par value $0.001 per share, at a purchase price of $15.00 per
one one-thousandth of a share, subject to adjustment.
Because of the nature of the dividend, liquidation and voting rights of Series C Preferred
Stock, the value of the one one-thousandth interest in a share of Series C Preferred Stock
purchasable upon exercise of each Right, should approximate the value of one share of our common
stock. The Rights are governed by a Rights Agreement dated as of May 12, 2009 (the “Rights
Agreement”).
Separation and Distribution of Rights; Exercisability. Initially, the Rights will be attached
to all shares of our common stock then outstanding, whether or not certificated, and no separate
Rights certificates will be distributed. The Rights will separate from the common stock upon the
earlier of:
| |
• |
|
10 business days following a public announcement that a person or group of affiliated or
associated persons has acquired, or obtained the right to acquire, beneficial ownership of
15% or more of our shares of common stock then outstanding (“Acquiring Person”) (subject to
certain exceptions set forth in the Rights Agreement), or such earlier date as the Board
becomes aware of the Acquiring Person’s existence; or |
| |
| |
• |
|
10 business days (or some later date as determined by the Board) following the
commencement of a tender or exchange offer that would result in a person or group
beneficially owning 15% or more of our shares of common stock then outstanding (subject to
exceptions as set forth in the Rights Agreement). |
Until the date that the Rights separate from our common stock (the “Distribution Date”), (i)
the Rights will be evidenced by and transferred with, and only with, the shares of our common
stock, (ii) newly issued shares of our common stock, whether or not certificated, will contain a
notation incorporating the Rights Agreement by reference, and (iii) the surrender for transfer of
any shares of our common stock outstanding will also constitute the transfer of the Rights
associated with such shares of our common stock.
The Rights are not exercisable until the Distribution Date and will expire at the close of
business on May 12, 2019, unless earlier redeemed by us as described below.
As soon as practicable after the Distribution Date, Rights certificates will be mailed to the
holders of record of our common stock as of the close of business on the Distribution Date and,
after that, the separate Rights certificates will represent the Rights. Except in connection with
shares of our common stock issued or sold pursuant to the exercise of stock options under any
employee plan or arrangements, or upon the exercise, conversion or exchange of securities issued by
us in the future, or as otherwise determined by the Board, only shares of our common stock issued
prior to the Distribution Date will be issued with Rights.
Flip-in Events. Each holder of a Right (other than the Acquiring Person and any associate or
affiliate thereof) will have the right to receive, upon exercise, shares of our common stock (or,
in some circumstances, cash, property or our other securities) having a value equal to two times
the purchase price of the Right, as the case may be, if:
| |
• |
|
any person becomes an Acquiring Person (except pursuant to specified exceptions); |
| |
| |
• |
|
we are the surviving corporation in a merger with an Acquiring Person and the common
stock is not changed or exchanged; or |
| |
| |
• |
|
during the time that there is an Acquiring Person, a merger, reclassification or similar
event occurs that results in increasing the Acquiring Person’s beneficial ownership of
shares of our common stock by more than 1%. |
Notwithstanding any of the foregoing, following the occurrence of any of the events described
in this paragraph, all Rights that are, or (under some circumstances specified in the Rights
Agreement) were, beneficially owned by any Acquiring Person will be null and void. The events
described in this paragraph are referred to as “Flip-in Events.”
-73-
For example, at a purchase price of $15.00 per Right, each Right not owned by an Acquiring
Person (or by some related parties or transferees) following a Flip-in Event set forth in the
preceding paragraph would entitle its holder to purchase $30.00 worth of our common stock (or other
consideration, as noted above) for $15.00.
Flip-over events. At any time following the earlier of a public announcement that a person has
become an Acquiring Person or the date that a majority of the Board becomes aware of the existence
of an Acquiring Person (in either case, the “Stock Acquisition Date”), each holder of a Right
(except Rights which previously have been voided as set forth above) will have the right to
receive, upon exercise, common stock of an acquiring company having a value equal to two times the
purchase price of the Right if any of the following occur:
| |
• |
|
we enter into a merger in which we are not the surviving corporation; |
| |
| |
• |
|
we are the surviving corporation in a merger pursuant to which all or part of the
outstanding shares of our common stock are changed into or exchanged for stock or other
securities of any other person or cash or any other property; or |
| |
| |
• |
|
more than 50% of our combined assets, cash flow or earning power and our subsidiaries is
sold or transferred (in each case other than some consolidations with, mergers with and
into, or sales of assets, cash flow or earning power by or to our subsidiaries as specified
in the Rights Agreement). |
The events described in this paragraph are referred to as “Flip-over Events.” Flip-in Events
and Flip-over Events are referred to collectively as “Triggering Events.”
Anti-dilution Adjustments; Fractional Shares. The applicable purchase price payable, the
number of shares of Series C Preferred Stock or other securities or property issuable upon the
exercise of the Rights, and the number of applicable Rights outstanding are subject to adjustment
from time to time to prevent dilution, in the event of a stock dividend on, or a subdivision,
combination or reclassification of, Series C Preferred Stock or common stock, or upon the
occurrence of certain other specified dilutive events. No fractional shares of Series C Preferred
Stock are required to be issued (other than fractions which are integral multiples of one
one-thousandth of a share of Series C Preferred Stock) and, in lieu of the issuance of fractional
shares, we may make an adjustment in cash based on the market price of the Series C Preferred Stock
on the trading date immediately prior to the date of exercise.
Dividend, Liquidation and Redemption Rights of the Series C Preferred Stock. Each share of
Series C Preferred Stock will be entitled, when, as and if declared, to a minimum preferential
quarterly dividend payment equal to the greater of $0.001 per share and an aggregate amount of
1,000 times the dividend declared per share of common stock (other than stock dividends payable in
common stock). Upon liquidation, the holders of Series C Preferred Stock will be entitled to the
greater of (1) a minimum preferential liquidation payment of $1,000 per share (plus any accrued but
unpaid dividends) and (2) an aggregate payment equal to 1,000 times the payment to be made per
share of common stock. Each share of Series C Preferred Stock will have 1,000 times the number of
votes each share of the common stock has on matters the respective class is entitled to vote on,
which will be voted together with common stock. Upon any merger, consolidation or other transaction
in which shares of common stock are converted or exchanged, each share of Series C Preferred Stock
will be entitled to receive 1,000 times the amount received per share of common stock. These rights
are protected by customary antidilution provisions.
At any time, or from time to time, the Board may redeem the outstanding shares of Series C
Preferred Stock, in whole but not in part, at a cash price per share equal to 105% of (i) 1,000
(subject to adjustment) times the average market value of common stock plus (ii) all accrued and
unpaid dividends of the Series C Preferred Stock as of the redemption date.
Exchange of the Rights. At any time after the occurrence of a Flip-in Event and prior to the
acquisition by a person or group of 50% or more of the shares of common stock then outstanding, the
Board may, without payment of the purchase price by the holder, exchange the Rights, in whole or in
part, as follows:
| |
• |
|
each Right (other than the Rights owned by the Acquiring Person or group, which will
become void) may be exchanged for one-half the number of shares of common stock, one
one-thousandth of a share of Series C Preferred Stock or shares or other units of other
property for which a Right is exercisable immediately prior to the time of the action of
the Board to exchange the Rights. |
Redemption of the Rights. At any time prior to the earliest of (i) the Stock Acquisition Date,
(ii) the tenth business day (or such later date as may be determined by action of the Board of
Directors prior to such time as any person or group of affiliated or associated persons becomes an
Acquiring Person) after the commencement of, or
-74-
announcement of an intention to commence, a tender offer or exchange offer the consummation of
which would result in any person becoming an Acquiring Person, or (iii) May 12, 2019, we may redeem
all, but not less than all, of the Rights at a price of $0.001 per Right (payable in cash, shares
of our common stock or other consideration deemed appropriate by the Board and subject to
adjustment). Immediately upon the action of the Board ordering redemption of the Rights, the Rights
will terminate and the only right of the holders of these Rights will be to receive the $0.001
redemption price.
No Rights as Stockholder. Until a Right is exercised, the holder will have no rights as a
stockholder of ours, including, without limitation, the right to vote or to receive dividends.
Amendment of the Rights Agreement. Other than those provisions relating to the principal
economic terms of the Rights, any of the provisions of the Rights Agreement may be amended by the
Board at any time during the period in which the Rights are redeemable. At any time when the Rights
are no longer redeemable, the provisions of the Rights Agreement may be amended by the Board only
if the amendment does not adversely affect the interest of holders of Rights (excluding the
interest of any Acquiring Person) or cause the Rights to become redeemable again.
Certain Anti-takeover Effects. The Rights approved by the Board are designed to protect and
maximize the value of our outstanding equity interests in the event of an unsolicited attempt by an
acquirer to take over the Company, in a manner or on terms not approved by the Board. Takeover
attempts frequently include coercive tactics to deprive the Board and its stockholders of a full
opportunity to evaluate an offer in light of our long term prospects. The Rights have been
declared by the Board in order to deter such tactics.
The Rights are not intended to prevent all takeovers of the Company and will not do so. Since,
subject to the restrictions described above, we may redeem the Rights prior to the Distribution
Date, the Rights should not interfere with any merger or business combination approved by the
Board.
The Rights may have the effect of rendering more difficult or discouraging an acquisition of
the Company deemed undesirable by the Board. The Rights may cause substantial dilution to a person
or group that attempts to acquire us (or which otherwise becomes an Acquiring Person) on terms or
in a manner not approved by the Board, except pursuant to an offer conditioned upon the negation,
purchase or redemption of the Rights.
Warrants Issued in Previous Securities Offerings
We issued Series B Warrants to purchase 6,088,340 shares of common stock in connection with
our 2008 Private Placement at an exercise price of $1.00 per share. These warrants expire on
September 22, 2013. In addition, we issued to the placement agents in the 2008 Private Placement
Series B Warrants to purchase 279,583 shares of common stock as partial compensation for services
in connection with the 2008 Private Placement.
We issued Series A Warrants to purchase 21,428,571 shares of common stock, of which 21,214,143
are currently outstanding, in connection with our July 2008 registered direct offering of units at
an exercise price of $1.00 per share. These warrants expire on July 15, 2013. In addition, we
issued to the placement agents and selected dealers in the offering warrants to purchase 1,071,429
shares of common stock as partial compensation for services in connection with the offering. These
warrants are substantially similar to the Series A Warrants and expire July 14, 2013.
We issued warrants to purchase 2,943,222 shares of common stock in connection with our
December 2007 registered direct offering of units at an exercise price of $1.10 per share. These
warrants expire on December 20, 2012.
We issued warrants to purchase 4,119,194 shares of common stock in connection with our 2006
private placement of Series 1 preferred stock to the placement agent of our Series 1 Preferred
Stock. These warrants have an exercise price of $1.23 per share, as adjusted for weighted average
anti-dilution protection. These warrants expire on November 8, 2010 and contain additional
weighted average anti-dilution protection if we issue certain securities at a price per share less
than $1.23.
Anti-Takeover Effects of Delaware Law, our Certificate of Incorporation and our Bylaws
We are subject to Section 203 of the General Corporation Law of the State of Delaware.
Subject to certain exceptions, Section 203 prevents a publicly held Delaware corporation from
engaging in a business combination with any interested stockholder for three years following the
date that the person became an interested stockholder, unless the interested stockholder attained
such status with the approval of our board of directors or unless the business combination is
approved in a prescribed manner. A business combination includes, among other things, a merger or
consolidation involving us and the interested stockholder and the sale of more than 10% of our
assets. In
-75-
general, an interested stockholder is any entity or person beneficially owning 15% or more of
our outstanding voting stock and any entity or person affiliated with or controlling or controlled
by such entity or person.
In addition, our certificate of incorporation and our bylaws provide that directors may be
removed only for cause and only by the affirmative vote of the holders of 75% of our shares of
capital stock present in person or by proxy and entitled to vote. Under our certificate of
incorporation and bylaws, any vacancy on our board of directors, including a vacancy resulting from
an enlargement of our board of directors, may be filled only by vote of a majority of our directors
then in office. The limitations on the ability of our stockholders to remove directors and fill
vacancies could make it more difficult for a third-party to acquire, or discourage a third-party
from seeking to acquire, control of our company.
Our certificate of incorporation and our bylaws provide that any action required or permitted
to be taken by our stockholders at an annual meeting or special meeting of stockholders may only be
taken if it is properly brought before such meeting. Our stockholders may also take any action by
written consent in lieu of a meeting.
Our certificate of incorporation and our bylaws also provide that, except as otherwise
required by law, special meetings of the stockholders can only be called by our Chairman of the
Board, our chief executive officer or our Board. In addition, our bylaws establish an advance
notice procedure for stockholder proposals to be brought before an annual meeting of stockholders,
including proposed nominations of candidates for election to our Board. Stockholders at an annual
meeting may only consider proposals or nominations specified in the notice of meeting or brought
before the meeting by or at the direction of our Board, or by a stockholder of record on the record
date for the meeting who is entitled to vote at the meeting and who has delivered timely written
notice in proper form to our secretary of the stockholder’s intention to bring such business before
the meeting. These provisions could have the effect of delaying until the next stockholder meeting
stockholder actions that are favored by the holders of a majority of our outstanding voting
securities.
The authorization in our certificate of incorporation of undesignated preferred stock makes it
possible for our Board, without obtaining further stockholder approval, to issue preferred stock
with voting rights or other rights or preferences that could impede the success of any attempt to
take control of us.
The General Corporation Law of the State of Delaware provides generally that the affirmative
vote of a majority of the shares entitled to vote on any matter is required to amend a
corporation’s certificate of incorporation or bylaws, unless a corporation’s certificate of
incorporation or bylaws, as the case may be, requires a greater percentage. Our bylaws may be
amended or repealed by a majority vote of our Board. In addition, the affirmative vote of the
holders of at least three-fourths of the votes which all our stockholders would be entitled to cast
in any election of directors is required to amend or repeal or to adopt any provisions inconsistent
with any of the provisions of our certificate of incorporation described in the prior two
paragraphs.
-76-
PLAN OF DISTRIBUTION
Merriman
Curhan Ford & Co., Boenning & Scattergood, Inc. and Noble
Financial Capital Markets have agreed to
act as the exclusive placement agents in connection with this offering subject to the
terms and conditions of a placement agency agreement, dated _____, 2009.
The placement agents may engage selected dealers to assist in the
placement of units.
The placement agents are not purchasing or selling any units offered
by this prospectus, nor are they required to arrange the purchase or
sale of any specific number or dollar amount of the units, but each has
agreed to use its reasonable best efforts to arrange for the sale of all of the
units offered hereby. Therefore, we will enter into purchase agreements directly with
investors in connection with this offering and we may not sell the entire amount of units
offered pursuant to this prospectus.
Any compensation paid by us to the placement agents in connection with the
offering of the securities offered in this prospectus, and any discounts, concessions or
commissions allowed by the placement agents to participating dealers, are set forth
below. In no event will the total amount of compensation paid to any member of The
Financial Industry Regulatory Authority (“FINRA”) upon completion of any offering
exceed 8.0% of the maximum gross proceeds of such offering.
We
have agreed to pay the placement agents a cash fee equal to 7% of the gross proceeds of this
offering and to issue to the placement agents warrants to purchase a number of shares of the our common
stock equal to 1% of the aggregate number of shares of common stock included in units sold in the
offering. The placement agent warrants will have terms substantially similar to the terms of the
warrants included in the units offered hereby, except that the placement agent warrants will comply with
FINRA Rule 5110(g)(1) in that for a period of 180 days after the issuance date of the placement
agent warrants (which shall not be earlier than the closing date of this offering),
neither the placement agent warrants nor any shares of our common stock issued upon exercise of the
placement agent warrants shall be sold, transferred, assigned, pledged, or hypothecated, or be the
subject of any hedging, short sale, derivative, put, or call transaction that would result in the
effective economic disposition of such securities by any person for a period of 180 days
immediately following the date of effectiveness of the registration
statement of which this prospectus is a part or commencement of sales of the offering pursuant
to which the placement agent warrants are being issued, except the transfer of any security:
| |
Ø |
|
by operation of law or by reason of reorganization of the Company; |
| |
Ø |
|
to any FINRA member firm participating in this offering and the officers or
partners thereof, if all securities so transferred remain subject to the lock-up
restriction described above for the remainder of the time period; |
| |
Ø |
|
if the aggregate amount of securities of the Company held by either placement
agent or related person do not exceed 1% of the securities being offered; |
| |
Ø |
|
that is beneficially owned on a pro-rata basis by all equity owners of an
investment fund, provided that no participating member manages or otherwise directs
investments by the fund, and participating members in the aggregate do not own more
than 10% of the equity in the fund; or |
-77-
| |
Ø |
|
the exercise or conversion of any security, if all securities received remain
subject to the lock-up restriction set forth above for the remainder of the time
period. |
The
placement agents will have piggyback registration rights with respect to the shares of
common stock underlying the placement agent warrants. In addition, the warrants will have a
cashless exercise right in the event that an effective registration statement is not available for
the resale of the shares of common stock underlying the placement
agent warrants.
The following table shows the per-unit and total placement agent fee to be paid by us to the
placement agents. This amount is shown assuming all of the units offered pursuant to this
prospectus are sold and issued by us.
| |
|
|
|
|
|
|
|
|
| |
|
Placement Agent |
|
|
| |
|
Fee Per Unit |
|
Total |
|
|
|
$ |
|
|
|
$ |
|
We are offering pursuant to this prospectus up to of our units, but there
can be no assurance that the offering will be fully subscribed. Accordingly, we may sell
substantially less than of our units, in which case our net proceeds would be
substantially reduced and the total placement agent fees may be substantially less than the maximum
total set forth above.
We
have also agreed to reimburse the placement agents for reasonable, customary and documented
out-of-pocket expenses, including, but not limited to, all legal
expenses incurred by the placement
agents for services provided by outside counsel; provided, however, that out-of-pocket expenses
(excluding legal expenses) in excess of $10,000 and legal expenses in excess of $60,000 will
require our approval, such approval not to be unreasonably withheld, and in no event shall the total compensation, including expense reimbursements, received by the placement agents exceed 8% of the gross proceeds of this offering. We estimate that the total
expenses of the offering by us, excluding the placement agent fees,
will be approximately $ . In the event
the offering of securities is not completed, reimbursable expenses
will be limited to out-of-pocket accountable expenses actually
incurred by the placement agents in accordance with FINRA Rule
5110(f)(2)(D).
Our obligation to issue and sell units to the purchasers is subject to the conditions
set forth in the placement agency agreement, which may be waived by us at our
discretion. A purchaser’s obligation to purchase shares is subject to the conditions set
forth in his or her purchase agreement as well, which may also be waived.
We currently anticipate that the sale of the shares of common stock and warrants
offered hereby will be completed on or about _____, 2009. At the closing,
The Depository Trust Company will credit the shares of common stock to the respective
accounts of the investors. We will mail warrants directly to the investors at the respective
addresses set forth in their purchase agreement with us.
We have
agreed to indemnify the placement agents against liabilities under the
Securities Act of 1933, as amended. We have also agreed to contribute to payments the
placement agents may be required to make in respect of such liabilities.
The foregoing does not purport to be a complete statement of the terms and
conditions of the placement agency agreement and purchase agreements. A copy of the
placement agency agreement and the form of purchase agreement with the investors are
included as exhibits to the registration statement of which this prospectus is a part. See
“Additional Information” on page 79.
The
placement agents have informed us that they will not engage in over-allotment, stabilizing
transactions or syndicate covering transactions in connection with this offering.
-78-
EXPERTS
The consolidated financial statements as of December 31, 2008 and 2007, and for each of the
three years in the period ended December 31, 2008, and for the period from August 8, 2002
(inception) to December 31, 2008 included in this prospectus have been audited by Deloitte & Touche
LLP, an independent registered public accounting firm, as stated in their report appearing herein.
Such consolidated financial statements have been so included in reliance upon the report of such
firm given upon their authority as experts in accounting and auditing.
LEGAL MATTERS
Certain legal matters in connection with the securities will be passed upon for us by the law
firm of Pepper Hamilton LLP.
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS
None.
COMMISSION’S POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
We are a Delaware corporation. Article Seventh of our Amended and Restated Certificate of
Incorporation provides to the fullest extent permitted under the Delaware General Corporation Law
(“DGCL”), that our directors or officers will not be personally liable to us or our stockholders
for damages for breach of such director’s or officer’s fiduciary duty. Section 145 of the DGCL
provides that a corporation may indemnify directors, officers, employees and agents against
expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement in
connection with specified actions, suits, or proceedings whether civil, criminal, administrative or
investigative (other than an action by or in the right of the corporation — a “derivative
action”), if they acted in good faith and in a manner they reasonably believed to be in or not
opposed to the best interests of the corporation and, with respect to any criminal action or
proceeding, had no reasonable cause to believe their conduct was unlawful. A similar standard is
applicable in the case of derivative actions, except that indemnification is permitted only for
expenses (including attorneys’ fees) incurred in connection with the defense or settlement of such
action, and the statute requires court approval before there can be any indemnification for
expenses where the person seeking indemnification has been found liable to the corporation. The
statute provides that it is not exclusive of other indemnification that may be granted by a
corporation’s charter, bylaws, disinterested director vote, stockholder vote, agreement, or
otherwise.
Article VIII of our bylaws provides that we shall indemnify and hold harmless, to the fullest
extent permitted by applicable law as it presently exists or may hereafter be amended, any person
who was or is made or is threatened to be made a party or is otherwise involved in any action, suit
or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that
he, or a person for whom he is the legal representative, is or was a director or officer of ours,
or is or was serving at our written request as a director, officer, employee or agent of another
corporation or of a partnership, joint venture, trust, enterprise or nonprofit entity, including
service with respect to employee benefit plans, against all liability and loss suffered and
expenses (including attorneys’ fees as incurred) reasonably incurred by him.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted
to directors, officers or persons controlling us pursuant to the foregoing provisions, or
otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such
indemnification is against public policy as expressed in the Securities Act and is, therefore,
unenforceable.
ADDITIONAL INFORMATION
We have filed with the SEC a Registration Statement on Form S-1 under the Securities Act
covering the sale of the securities offered by this prospectus. This prospectus, which is a part of
the Registration Statement, does not contain all of the information in the Registration Statement
and the exhibits filed with it, portions of which have been omitted as permitted by the SEC rules
and regulations. For further information concerning us and the securities offered by this
prospectus, please refer to the Registration Statement and to the exhibits filed therewith.
The Registration Statement, including all exhibits, may be inspected without charge at the
SEC’s Public Reference Room at the SEC’s principal office at Room 1580, 100 F Street, N.E.,
Washington, D.C. 20549. You may obtain information on the operation of this public reference room
by calling 1-800-SEC-0330. The Registration Statement, including all exhibits and schedules and
amendments, has been filed with the SEC through the Electronic Data Gathering Analysis and
Retrieval system and is available to the public from the SEC’s web site at
http://www.sec.gov.
-79-
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2009 AND
FOR THE QUARTERS ENDED JUNE 30, 2009 AND 2008
AND FOR THE PERIOD
AUGUST 8, 2002 (INCEPTION) TO JUNE 30, 2009
CONTENTS
| |
|
|
| |
|
Page |
Condensed Consolidated Balance Sheets (Unaudited) — as of June 30, 2009 and December 31, 2008
|
|
F-2 |
|
|
|
Condensed
Consolidated Statements Of Operations (Unaudited) — for the
Three and Six-Months Ended
June 30, 2009 and 2008 and for the Period from August 8, 2002 (Inception) to June 30, 2009
|
|
F-3 |
|
|
|
Condensed
Consolidated Statements Of Cash Flows (Unaudited)— for the Six-Months Ended June 30, 2009 and 2008 and for the Period from August 8, 2002 (Inception) to June 30, 2009
|
|
F-4 |
|
|
|
Notes To Condensed Consolidated Financial Statements (Unaudited)
|
|
F-5 |
F-1
PolyMedix, Inc.
(A Development Stage Company)
Condensed Consolidated Balance Sheets
(unaudited)
(in thousands, except per share amounts)
| |
|
|
|
|
|
|
|
|
| |
|
June 30, |
|
|
December 31, |
|
| |
|
2009 |
|
|
2008 |
|
ASSETS
|
Current Assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
8,456 |
|
|
$ |
7,206 |
|
Short-term investments |
|
|
1,900 |
|
|
|
7,900 |
|
Prepaid expenses and other current assets |
|
|
762 |
|
|
|
407 |
|
|
|
|
|
|
|
|
Total current assets |
|
|
11,118 |
|
|
|
15,513 |
|
|
|
|
|
|
|
|
|
|
Property and Equipment: |
|
|
|
|
|
|
|
|
Computers |
|
|
218 |
|
|
|
237 |
|
Office furniture and lab equipment |
|
|
774 |
|
|
|
774 |
|
Accumulated depreciation |
|
|
(532 |
) |
|
|
(447 |
) |
|
|
|
|
|
|
|
Total property and equipment |
|
|
460 |
|
|
|
564 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS |
|
$ |
11,578 |
|
|
$ |
16,077 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
Current Liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,641 |
|
|
$ |
1,072 |
|
Accrued expenses |
|
|
870 |
|
|
|
933 |
|
Short-term portion of capital lease obligation |
|
|
32 |
|
|
|
104 |
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
2,543 |
|
|
|
2,109 |
|
|
|
|
|
|
|
|
|
|
Deferred rent |
|
|
880 |
|
|
|
825 |
|
|
|
|
|
|
|
|
Total liabilities |
|
|
3,423 |
|
|
|
2,934 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity: |
|
|
|
|
|
|
|
|
Preferred Stock ($0.001 par value; 10,000 shares authorized; 0 issued and outstanding at
June 30, 2009 and December 31, 2008) |
|
|
— |
|
|
|
— |
|
Common Stock ($0.001 par value; 250,000 shares authorized; 59,845
issued and outstanding at June 30, 2009 and December 31, 2008) |
|
|
60 |
|
|
|
60 |
|
Additional paid-in capital |
|
|
50,789 |
|
|
|
49,930 |
|
Deficit accumulated during the development stage |
|
|
(42,694 |
) |
|
|
(36,859 |
) |
Unrealized gain on available for sale securities |
|
|
— |
|
|
|
12 |
|
|
|
|
|
|
|
|
Total stockholders’ equity |
|
|
8,155 |
|
|
|
13,143 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’
EQUITY |
|
$ |
11,578 |
|
|
$ |
16,077 |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-2
PolyMedix, Inc.
(A Development Stage Company)
Condensed Consolidated Statements of Operations
(unaudited)
(in thousands, except per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the period |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, 2002 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
|
| |
|
Three-Months Ended June 30, |
|
|
Six-Months Ended June 30, |
|
|
June 30, |
|
| |
|
2009 |
|
|
2008 |
|
|
2009 |
|
|
2008 |
|
|
2009 |
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant and research revenues |
|
$ |
138 |
|
|
$ |
18 |
|
|
$ |
144 |
|
|
$ |
1,009 |
|
|
$ |
3,657 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
|
138 |
|
|
|
18 |
|
|
|
144 |
|
|
|
1,009 |
|
|
|
3,657 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
1,565 |
|
|
|
1,718 |
|
|
|
3,240 |
|
|
|
4,444 |
|
|
|
27,831 |
|
General and administrative |
|
|
1,499 |
|
|
|
1,232 |
|
|
|
2,777 |
|
|
|
2,387 |
|
|
|
20,051 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
3,064 |
|
|
|
2,950 |
|
|
|
6,017 |
|
|
|
6,831 |
|
|
|
47,882 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income and other expenses |
|
|
12 |
|
|
|
28 |
|
|
|
38 |
|
|
|
95 |
|
|
|
1,531 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income |
|
|
12 |
|
|
|
28 |
|
|
|
38 |
|
|
|
95 |
|
|
|
1,531 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(2,914 |
) |
|
$ |
(2,904 |
) |
|
$ |
(5,835 |
) |
|
$ |
(5,727 |
) |
|
$ |
(42,694 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature, conversion inducement and dividends on preferred
stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(11,118 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders |
|
$ |
(2,914 |
) |
|
$ |
(2,904 |
) |
|
$ |
(5,835 |
) |
|
$ |
(5,727 |
) |
|
$ |
(53,812 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share — basic and diluted |
|
$ |
(0.05 |
) |
|
$ |
(0.09 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.18 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding — basic and
diluted |
|
|
59,845 |
|
|
|
32,114 |
|
|
|
59,845 |
|
|
|
32,109 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-3
PolyMedix, Inc.
(A Development Stage Company)
Condensed Consolidated Statements of Cash Flows
(unaudited)
(in thousands)
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
For the period |
|
| |
|
|
|
|
|
|
|
|
|
August 8, 2002 |
|
| |
|
Six-Months Ended June 30, |
|
|
(Inception) to |
|
| |
|
2009 |
|
|
2008 |
|
|
June 30, 2009 |
|
Cash Flows from Operating Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(5,835 |
) |
|
$ |
(5,727 |
) |
|
$ |
(42,694 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
104 |
|
|
|
107 |
|
|
|
627 |
|
Accretion of discount on investment securities |
|
|
(15 |
) |
|
|
(40 |
) |
|
|
(457 |
) |
Stock-based compensation |
|
|
936 |
|
|
|
709 |
|
|
|
6,780 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase in prepaid expenses and other current assets |
|
|
(355 |
) |
|
|
(62 |
) |
|
|
(762 |
) |
Increase in accounts payable, accrued expenses and deferred rent |
|
|
571 |
|
|
|
114 |
|
|
|
3,320 |
|
Loss on disposal of fixed assets |
|
|
— |
|
|
|
— |
|
|
|
23 |
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
|
(4,594 |
) |
|
|
(4,899 |
) |
|
|
(33,163 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows from Investing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for property and equipment |
|
|
(15 |
) |
|
|
— |
|
|
|
(766 |
) |
Purchases of investments |
|
|
(3,798 |
) |
|
|
(1,683 |
) |
|
|
(34,041 |
) |
Proceeds from maturities of investments |
|
|
9,800 |
|
|
|
4,000 |
|
|
|
32,598 |
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities |
|
|
5,987 |
|
|
|
2,317 |
|
|
|
(2,209 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows from Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of stock, net of financing costs |
|
|
(71 |
) |
|
|
(496 |
) |
|
|
43,729 |
|
Proceeds from warrant and stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
398 |
|
Principal payments on capital lease obligations |
|
|
(72 |
) |
|
|
(64 |
) |
|
|
(299 |
) |
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities |
|
|
(143 |
) |
|
|
(560 |
) |
|
|
43,828 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
1,250 |
|
|
|
(3,142 |
) |
|
|
8,456 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents — beginning of period |
|
|
7,206 |
|
|
|
4,936 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents — end of period |
|
$ |
8,456 |
|
|
$ |
1,794 |
|
|
$ |
8,456 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Cash Investing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment acquired under capital lease |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
331 |
|
Capital expenditures acquired on account |
|
$ |
— |
|
|
$ |
30 |
|
|
$ |
15 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Cash Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Conversion inducement and dividends on Series 1 Preferred Stock and Beneficial conversion feature on Series 2008 Preferred Stock |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,118 |
|
Warrants issued to placement agent |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
2,932 |
|
Accrued financing costs |
|
$ |
56 |
|
|
$ |
183 |
|
|
$ |
56 |
|
Capital lease obligation |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
331 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-4
PolyMedix, Inc.
(A Development Stage Company)
Notes to Unaudited Condensed Consolidated Financial Statements
In these condensed consolidated financial statements, “PolyMedix,”
“we,” “us” and “our” refer to PolyMedix, Inc. and its wholly owned subsidiary PolyMedix Pharmaceuticals, Inc., and “Common Stock”
refers to the common stock, par value $0.001 per share of PolyMedix, Inc.
1 — Organization and Business Activities
We are a development stage biotechnology company focused on treating life threatening,
serious infectious diseases and acute cardiovascular disorders with synthetic small molecule compounds that
mimic the activity of large natural protein molecules, compounds referred to as biomimetics. Using
our proprietary computational drug design technology, we have created novel defensin mimetic
antibiotic compounds, heparin antagonist compounds (or “heptagonist”) and other drug compounds
intended for human therapeutic use. Since 2002, we have been a development stage enterprise, and
accordingly, our operations have been directed primarily toward developing business strategies,
raising capital, research and development activities, conducting pre-clinical testing and human
clinical trials of our product candidates, exploring marketing channels and recruiting personnel.
We have incurred operating losses since inception, have not generated any product sales
revenues
and have not achieved profitable operations. Our deficit accumulated during the development stage
through June 30, 2009 aggregated $42,694,000, and we expect to continue to incur substantial losses
in future periods.
None of our product candidates have received regulatory approval for commercial sale
and our product candidates may never be commercialized. In addition, all of our product candidates are in
the early stages of development and several programs are on hold pending receipt of sufficient
additional resources. The progress and results of our current and any future clinical trials or
future pre-clinical testing are uncertain, and if our product candidates do not receive regulatory
approvals, our business, operating results, financial condition and cash flows will be materially
adversely affected. Our development programs require a significant amount of cash to support the
development of product candidates.
We are highly dependent on the success of our research, development and licensing
efforts and, ultimately, upon regulatory approval and market acceptance of our products under development. Our
short and long-term capital requirements depend upon a variety of factors, including market
acceptance for our technologies and product candidates and various other factors. We believe that
our current cash and investment balances, exclusive of any cash to be generated from the $10
million Equity Line with Dutchess Equity Fund, L.P. that we entered into in May, 2009 (the “Equity
Line”), will be sufficient to fund our planned Phase 1 studies for PMX-30063 and PMX-60056, and can
fund our operations into the third quarter of 2010. Due to continued weakness in capital markets
and our understanding that it may be difficult to secure significant additional financing on
favorable terms, we have begun and expect to continue to scale back and delay some of our planned
activities in order to prolong our cash resources.
Our current cash and investment balances are not sufficient to initiate or fund Phase 2
clinical trials or any of the related development activities for PMX-30063 or PMX-60056. We do not plan to
initiate our Phase 2 development activities for PMX-30063 or PMX-60056 until additional financing
is secured. We may seek additional funds through equity or debt financing, among other sources.
In addition, we may actively seek funds through government grants and contracts. However, as a
result of current conditions in the equity and debt markets, we may not be able to obtain
additional funding on favorable terms, if at all. In addition, if we choose to apply for
government grants and contracts, there is no guarantee of approval of our applications. If we are
unable to secure adequate additional funding during 2009 we will further delay, scale-back or
eliminate certain of our planned research, drug discovery and development activities and certain
other aspects of our operations and our business until such time as we are successful in securing
adequate additional funding. As a result, our business may be materially and adversely affected.
In the absence of adequate additional funding, we believe that we have the ability to scale our
operations
F-5
such that our current cash and investment balances will be sufficient to fund our
operations into the third quarter of 2010.
Global market and economic conditions have been, and continue to be, disruptive and
volatile. In particular, the cost of raising money in the debt and equity capital markets has increased
substantially while the availability of funds from those markets has diminished significantly. If
funding is not available when needed, or is available only on unfavorable terms, meeting our
capital needs or otherwise taking advantage of business opportunities may become challenging, which
could have a material adverse effect on our business plans.
Interim Financial Information
The condensed consolidated financial statements include the accounts of PolyMedix, Inc.
and its wholly-owned subsidiary, PolyMedix Pharmaceuticals, Inc. All intercompany accounts and
transactions have been eliminated in consolidation. The information as of June 30, 2009 and for
the periods ended June 30, 2009 and 2008 and the period from August 8, 2002 (Inception) to June 30,
2009 is unaudited but includes all adjustments (consisting only of normal recurring adjustments)
which, in our opinion, are necessary to state fairly the financial information set forth in
accordance with accounting principles generally accepted in the United States of America. The
interim results are not necessarily indicative of the results to be expected for the full fiscal
year. These condensed consolidated financial statements should be read in conjunction with the
audited consolidated financial statements for the year ended December 31, 2008 included in our
annual report on Form 10-K filed with the U.S. Securities and Exchange Commission on March 23,
2009.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally
accepted in the United States of America requires management to make estimates and assumptions that affect
the reported amounts in the financial statements and accompanying notes. Actual results could
differ from those estimates. Significant estimates include the value of our Common Stock, preferred
stock, stock options and warrants.
2 — Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board
(“FASB”) issued Statement of Financial Accounting Standards (“SFAS”) No. 157, “Fair Value Measurements” (“SFAS 157”). SFAS 157
defines fair value, establishes a framework and gives guidance regarding the methods used for measuring
fair value, and expands disclosures about fair value measurements. SFAS 157 is effective for
financial statements issued for fiscal years beginning after November 15, 2007, and interim periods
of those fiscal years. In February 2008, the FASB released a FASB Staff Position (FSP FAS
157-2—Effective Date of FASB Statement No. 157) which delayed, to fiscal years beginning after
November 15, 2008, the effective date of SFAS 157 for all non-financial assets and non-financial
liabilities, except those that are recognized or disclosed at fair value in the financial
statements on a recurring basis (at least annually). Effective January 1, 2008, we adopted SFAS 157
as it applies to our financial instruments. Effective January 1, 2009, we adopted SFAS 157 for our
non-financial assets and non-financial liabilities, without impact to our condensed consolidated
financial statements or related footnotes.
The following table provides the assets and liabilities carried at fair value measured on a
recurring basis as of June 30, 2009 and December 31, 2008 (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Significant other |
|
Significant |
| |
|
|
|
|
|
Quoted prices in |
|
observable |
|
unobservable |
| |
|
Total Carrying |
|
active markets |
|
inputs |
|
inputs |
| |
|
value |
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
June 30, 2009 |
|
$ |
1,900 |
|
|
$ |
— |
|
|
$ |
1,900 |
|
|
$ |
— |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008 |
|
$ |
7,900 |
|
|
$ |
— |
|
|
$ |
7,900 |
|
|
$ |
— |
|
F-6
Our short-term investments, generally fixed income government agency securities, are
measured at
fair value and are derived principally from or corroborated by observable market data by
correlation or other means and are classified within Level 2 of the valuation hierarchy.
In June 2008, the FASB issued FASB Staff Position No. EITF 03-6-1,
“Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities,” (“FSP EITF 03-6-1”). FSP
EITF 03-6-1 addresses whether instruments granted in share-based payment transactions are
participating securities prior to vesting and, therefore need to be included in the earnings
allocation in computing earnings per share, or EPS, under the two-class method described in
paragraphs 60 and 61 of FASB Statement No. 128, “Earnings per Share” (“SFAS No. 128”). FSP EITF
03-6-1 applies to the calculation of EPS under SFAS No. 128 for share-based payment awards with
rights to dividends or dividend equivalents. FSP EITF 03-6-1 clarifies that unvested share-based
payment awards that contain nonforfeitable rights to dividends or dividend equivalents (whether
paid or unpaid) are participating securities and shall be included in the computation of EPS
pursuant to the two class method. FSP EITF 03-6-1 is effective for financial statements issued for
fiscal years beginning after December 15, 2008, and interim periods within those years. All
prior-period EPS data presented must be adjusted retrospectively (including interim financial
statements, summaries of earnings and selected financial data) to conform with the provisions of
FSP EITF 03-6-1. Early adoption is not permitted. The adoption of EITF 03-6-1 did not have an
impact on our consolidated financial statements.
In June 2008, the FASB ratified the consensus reached on EITF Issue
No. 07-05, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock” (“EITF No. 07-05”). EITF
No. 07-05 clarifies the determination of whether an instrument (or an embedded feature) is indexed
to an entity’s own stock, which would qualify as a scope exception under SFAS No. 133, Accounting
for Derivative Instruments and Hedging Activities. EITF No. 07-05 is effective for financial
statements issued for fiscal years beginning after December 15, 2008. Early adoption for an
existing instrument is not permitted. The adoption of EITF No. 07-05 did not have an impact on our
consolidated financial statements.
In April 2009, the FASB issued FSP No. FAS 107-1 and APB 28-1,
“Interim Disclosures about Fair Value of Financial Instruments.” FSP No. FAS 107-1 and APB 28-1 amends current guidance, requiring
that the provisions of FAS 107 and APB 28 be disclosed at interim periods for publicly traded
companies. Due to the short-term nature of our Cash and Cash Equivalents, Short Term Investments
and Accounts Payable, we believe that the respective book values of these accounts approximate
their fair value. FSP No. FAS 107-1 and APB 28-1 is effective for interim reporting periods
ending after June 15, 2009. The adoption of FSP No. FAS 107-1 and APB 28-1 has not had a material
impact on our consolidated financial statements.
In April 2009, the FASB issued Financial Statement Position (“FSP”)
No. FAS 115-2 and FAS 124-2, “Recognition and Presentation of Other-Than-Temporary Impairments.” FSP No. FAS 115-2 and FAS 124-2
amends current other-than-temporary impairment guidance for debt securities and improves the
presentation and disclosure of other-than-temporary impairments on debt and equity securities in
the financial statements. FSP No. FAS 115-2 and FAS 124-2 are effective for interim and annual
reporting periods ending after June 15, 2009. The adoption of FSP No. FAS 115-2 and FAS 124-2 have
not had a material impact on our consolidated financial statements.
In May 2009, the FASB issued SFAS No. 165, “Subsequent
Events” (“SFAS 165”). SFAS 165 establishes general standards of accounting for and disclosure of events that occur after the balance sheet
date but before financial statements are issued or are available to be issued. SFAS 165 is
effective for the quarter ended June 30, 2009. The adoption of SFAS 165 did not have a material
impact on our financial statements. We evaluated all events and transactions that occurred after
June 30, 2009 up through August 7, 2009, the date we issued these financial statements. During this
period, the Company did not have any material recognizable or non-recognizable subsequent events.
In June 2009, the FASB issued SFAS No. 168, “The FASB
Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles — a replacement of FASB Statement No. 162”
(“SFAS 168”). SFAS 168 introduces the FASB Accounting Standards Codification that will serve as
the single source of authoritative GAAP. SFAS 168 is effective for financial statements issued for
interim and annual periods ending after September 15, 2009. The adoption of SFAS 168 is not
expected to have a material impact on our consolidated financial statements.
F-7
3 — Comprehensive Loss
SFAS No. 130, “Reporting Comprehensive Income,” establishes
standards for the reporting of
comprehensive income and its components. Comprehensive income consists of reported net income or
loss and unrealized gains or losses on available for sale securities. The comprehensive loss for
each of the periods presented approximates the net loss in the condensed consolidated statements of
operations.
4 — Short-Term Investments
Short-term investments consist of investment grade fixed income securities with original
maturities of greater than three months. All investments are classified as “available for sale”, and are
considered current assets. As of June 30, 2009, all short-term investments had maturities of less
than one year.
The following summarizes the short-term investments as of June 30, 2009 and
December 31, 2008,
respectively, which were invested solely in U.S. government obligations (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Gross |
|
Gross |
|
|
| |
|
Amortized |
|
Unrealized |
|
Unrealized |
|
|
| |
|
Cost |
|
Gains |
|
Losses |
|
Fair Value |
June 30, 2009 |
|
$ |
1,900 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,900 |
|
| |
December 31, 2008 |
|
$ |
7,888 |
|
|
$ |
12 |
|
|
$ |
— |
|
|
$ |
7,900 |
|
5 — Stock-Based Compensation
We maintain equity compensation plans under which grants of incentive stock options,
nonqualified stock options, stock appreciation rights, stock awards, stock units and other stock-based awards
are granted to employees, non-employee directors and key advisors. Since our inception on August
8, 2002, we have recognized equity compensation expense over the requisite service period using the
Black-Scholes-Merton formula to estimate the fair value of stock options. The following table
summarizes the total stock-based compensation expense included in our unaudited condensed
consolidated statements of operations (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
Three-Months Ended |
|
|
Six-Months Ended |
|
|
August 8, 2002 |
|
| |
|
June 30, |
|
|
June 30, |
|
|
(Inception) to |
|
| |
|
2009 |
|
|
2008 |
|
|
2009 |
|
|
2008 |
|
|
June 30, 2009 |
|
Research and Development |
|
$ |
178 |
|
|
$ |
106 |
|
|
$ |
342 |
|
|
$ |
229 |
|
|
$ |
2,143 |
|
General and administrative |
|
|
309 |
|
|
|
257 |
|
|
|
594 |
|
|
|
480 |
|
|
|
4,637 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stock-based
compensation expense |
|
$ |
487 |
|
|
$ |
363 |
|
|
$ |
936 |
|
|
$ |
709 |
|
|
$ |
6,780 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During the six-months ended June 30, 2009, 514,000 stock options were awarded to
key consultants at a combined grant date fair value of $262,000. There have been no significant changes to the
assumptions used to calculate fair value from those which were disclosed in our 2008 Annual Report
on Form 10-K. As of June 30, 2009, there were 10,724,000 shares of Common Stock issuable upon
exercise of outstanding stock options and 14,869,000 shares of Common Stock available for issuance
of future equity compensation awards in connection with our equity compensation plans. As of June
30, 2009, there was $2,971,000 of total unrecognized compensation cost related to non-vested stock
options, which will be amortized over the weighted average remaining service period of
approximately 1.16 years.
6 — Stockholders’ Equity
Common Stock
We are authorized to issue 250,000,000 shares of Common Stock, with a par value of
$0.001, of which 59,845,065 were issued and outstanding at both June 30, 2009 and December 31, 2008.
In May 2009, we entered into an Investment Agreement (“Investment
Agreement”) with Dutchess Equity Fund, L.P. (“Dutchess”), as amended in July 2009. Pursuant to the Investment Agreement, Dutchess
committed to purchase up to $10,000,000 of our Common Stock, over the course of thirty-six months.
We may draw on the facility from time to time, as and when we determine appropriate in accordance
with the terms and conditions of the Investment Agreement. In each put notice, we will set our
minimum acceptable per share purchase price for the put. The maximum dollar amount that we are
entitled to put in any one notice (“Put”) is the greater of (i) 200% of the average daily volume of
the Common Stock for the three (3) trading days prior to the date of delivery of the applicable put
notice, multiplied by the average of the closing prices for such trading days or (ii) $250,000. An
additional put notice may not be delivered until after the closing of any previous outstanding put
notice. The purchase price shall be set at ninety-five percent (95%) of the volume weighted
average price (VWAP) of our Common Stock during the five (5) consecutive trading day period
beginning on the trading day immediately following the date of delivery of the applicable put
notice; provided, that any trading day during which the daily VWAP is 10% above or 10% below the
VWAP for the entire period will be omitted from the calculation, and any trading day having a VWAP
below the minimum acceptable price may be omitted from the calculation. The aggregate number of
shares issuable by us and purchasable by Dutchess under the Investment Agreement is 12,000,000. As
of June 30, 2009, we had not sold any shares to Dutchess in connection with this Equity Line.
F-8
In December 2008, our then outstanding 608,834 shares of Series 2008
Convertible Preferred Stock (“Series 2008 Preferred Stock”), originally issued in September 2008, automatically converted to
6,088,340 shares of Common Stock. See “— Preferred Stock” section below.
In July 2008, we completed a registered offering of 21,428,000 units. Each unit
consisted of one share of our Common Stock and a Series A Warrant to purchase one share of our Common Stock. Each
unit was sold for $0.70 per unit and each Series A Warrant has an exercise price of $1.00 per
share. We received gross proceeds of $15,000,000 in the registered offering before fees and
expenses. In connection with this registered offering, we incurred commission fees and expenses of
approximately $1,496,000.
Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, with a par value of
$0.001, of which no shares were issued and outstanding at June 30, 2009 and December 31, 2008, respectively.
In May 2009, we adopted a rights plan, which among other things approved a
dividend of a right for each outstanding share of Common Stock to purchase one one-thousandth of a share of Series C
Preferred Stock, par value $0.001 per share, at a purchase price of $15 per share, subject to
adjustment. Subject to limited exceptions, the rights will be exercisable if a person or group
acquires 15% or more of our common stock or announces a tender offer for 15% or more of the common
stock. No shares of Series C Preferred Stock have been issued to date.
In September 2008, we completed a private placement offering of 608,834 units.
Each unit consisted of one share of our Series 2008 Preferred Stock and a Series B Warrant. Each unit was sold for
$7.00 per unit. As explained immediately above under “— Common Stock”, during December 2008, each
share of Series 2008 Preferred Stock automatically converted into 10 shares of Common Stock and the
Series B Warrants became exercisable for Common Stock immediately, and without any action on the
part of the holder of such shares of Series 2008 Preferred Stock or Series B Warrants, upon the
effectiveness of an amendment to our Amended and Restated Certificate of Incorporation, as amended
(the “Certificate of Incorporation”), to increase the number of shares of Common Stock authorized
for issuance by us under the Certificate of Incorporation to an amount sufficient to cover the
issuance of shares of Common Stock upon conversion of the Series 2008 Preferred Stock and the
issuance of shares of Common Stock issuable after such conversion upon exercise of all of the
Series B Warrants. We received gross proceeds of $4,262,000 in the private placement before fees
and expenses. In connection with this private placement, we incurred commission fees and expenses
of approximately $401,000.
Warrants
In connection with the private placement and subsequent conversion of the
Series 2008 Preferred Stock (described above), investors in the private placement received Series B Warrants presently
exercisable for 6,088,340 shares of Common Stock and the placement agent and dealer received Series B Warrants
presently exercisable for 279,583 shares of Common Stock. The Series B Warrants have an exercise price of $1.00 per
share and that expire in July 2013. The fair value of the warrants at the time of issuance was
$3,991,000, which was estimated using the Black-Scholes-Merton option-pricing model with the
following assumptions: no dividends; risk-free interest rate of 3.03%; estimated life of five
years and volatility of 71%.
In connection with the registered offering completed in July 2008 (described
above), we issued Series A Warrants to purchase 21,428,000 shares of Common Stock to investors and Series A Warrants
to purchase 1,071,000 shares of Common Stock to the placement agents and dealer at an exercise
price of $1.00 per share and that expire in September 2013. The fair value of the warrants at the
time of issuance was $11,807,000, which was estimated using the Black-Scholes-Merton option-pricing
model with the following assumptions: no dividends; risk-free interest rate of 3.2% to 3.35%;
estimated life of five years and volatility of 72% to 75%.
In connection with the registered offering completed in December 2007, we issued
warrants to purchase 2,943,000 shares of Common Stock at an exercise price of $1.10 per share. These warrants
expire in December 2012. The fair value of the warrants at the time of issuance was $2,015,000,
which was estimated using the Black-Scholes-Merton option-pricing model with the following
assumptions: no dividends; risk-free interest rate of 3.58%; estimated life of five years and
volatility of 73%.
During 2005 and 2006, we issued warrants to purchase 4,119,000 shares of Common
Stock, as adjusted for weighted average anti-dilution protection, in connection with the private placement of Series 1
preferred stock at an exercise price of $1.23 per share, as adjusted for weighted average
anti-dilution protection. These warrants expire in November 2010 and contain weighted average
anti-dilution protection if we issue certain securities at a price per share less than $1.23 per
share. The fair value of the warrants at the time of issuance was $2,149,000, which was estimated
using the Black-Scholes-Merton option-pricing model with the following assumptions: no dividends;
risk-free interest rate of 4.45%; estimated life of five years and volatility of 60%.
F-9
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2008 AND 2007 AND
FOR THE YEARS ENDED DECEMBER 31, 2008, 2007 AND 2006
AND FOR THE PERIOD
AUGUST 8, 2002 (INCEPTION) TO DECEMBER 31, 2008
CONTENTS
| |
|
|
| |
|
Page |
Report of Independent Registered Public Accounting Firm
|
|
F-11 |
|
|
|
PolyMedix, Inc. Consolidated Balance Sheets as of December 31, 2008 and 2007
|
|
F-12 |
|
|
|
PolyMedix, Inc. Consolidated Statements of Operations for the Years Ended
December 31, 2008, 2007 and 2006, and for the Period from August 8, 2002
(Inception) to December 31, 2008
|
|
F-13 |
|
|
|
PolyMedix, Inc. Consolidated Statements of Changes in Stockholders’ Equity
from August 8, 2002 (Inception) to December 31, 2008
|
|
F-14 |
|
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31,
2008, 2007 and 2006, and for the Period from August 8, 2002 (Inception) to
December 31, 2008
|
|
F-15 |
|
|
|
Notes to Consolidated Financial Statements
|
|
F-16 |
F-10
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
PolyMedix, Inc.
Radnor, Pennsylvania
We have audited the accompanying consolidated balance sheets of PolyMedix, Inc. and its
subsidiary (a development stage company) (the “Company”) as of December 31, 2008 and 2007, and the
related consolidated statements of operations, changes in stockholders’ equity, and cash flows for
each of the three years in the period ended December 31, 2008, and for the period from August 8,
2002 (Inception) to December 31, 2008. These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an opinion on these financial statements
based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting
Oversight Board (United States). Those standards require that we plan and perform the audit to
obtain reasonable assurance about whether the financial statements are free of material
misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its
internal control over financial reporting. Our audits included consideration of internal control
over financial reporting as a basis for designing audit procedures that are appropriate in the
circumstances, but not for the purpose of expressing an opinion on the effectiveness of the
Company’s internal control over financial reporting. Accordingly, we express no such opinion. An
audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in
the financial statements, assessing the accounting principles used and significant estimates made
by management, as well as evaluating the overall financial statement presentation. We believe that
our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material
respects, the financial position of the Company as of December 31, 2008 and 2007, and the results
of its operations and its cash flows for each of the three years in the period ended December 31,
2008, and for the period from August 8, 2002 (Inception) to December 31, 2008, in conformity with
accounting principles generally accepted in the United States of America.
/s/ Deloitte & Touche LLP
Philadelphia, Pennsylvania
March 23, 2009
F-11
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2008 AND 2007
(IN THOUSANDS, EXCEPT PER SHARE AMOUNTS)
| |
|
|
|
|
|
|
|
|
| |
|
2008 |
|
|
2007 |
|
ASSETS |
|
|
|
|
|
|
|
|
Current Assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
7,206 |
|
|
$ |
4,936 |
|
Short-term investments |
|
|
7,900 |
|
|
|
3,967 |
|
Prepaid expenses and other current assets |
|
|
407 |
|
|
|
42 |
|
|
|
|
|
|
|
|
Total current assets |
|
|
15,513 |
|
|
|
8,945 |
|
|
|
|
|
|
|
|
|
|
Property and Equipment: |
|
|
|
|
|
|
|
|
Computers |
|
|
237 |
|
|
|
243 |
|
Office furniture and lab equipment |
|
|
774 |
|
|
|
774 |
|
Accumulated depreciation |
|
|
(447 |
) |
|
|
(240 |
) |
|
|
|
|
|
|
|
Total property and equipment |
|
|
564 |
|
|
|
777 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS |
|
$ |
16,077 |
|
|
$ |
9,722 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,072 |
|
|
$ |
2,341 |
|
Accrued expenses |
|
|
933 |
|
|
|
1,372 |
|
Short-term portion of capital lease obligation |
|
|
104 |
|
|
|
131 |
|
|
|
|
|
|
|
|
Total current liabilities |
|
|
2,109 |
|
|
|
3,844 |
|
|
|
|
|
|
|
|
|
|
Deferred rent and other accrued expenses |
|
|
825 |
|
|
|
640 |
|
Long-term portion of capital lease obligation |
|
|
— |
|
|
|
104 |
|
|
|
|
|
|
|
|
Total liabilities |
|
|
2,934 |
|
|
|
4,588 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity: |
|
|
|
|
|
|
|
|
Preferred Stock ($0.001 par value; 10,000 shares
authorized; 0 and 0 issued and outstanding at
December 31, 2008 and 2007, respectively) |
|
|
— |
|
|
|
— |
|
Common Stock ($0.001 par value; 250,000 shares
authorized; 59,845 and 32,039 issued and
outstanding at December 31, 2008 and 2007,
respectively) |
|
|
60 |
|
|
|
32 |
|
Additional paid-in capital |
|
|
49,930 |
|
|
|
30,973 |
|
Deficit accumulated during the development stage |
|
|
(36,859 |
) |
|
|
(25,873 |
) |
Unrealized gain on available for sale securities |
|
|
12 |
|
|
|
2 |
|
|
|
|
|
|
|
|
Total stockholders’ equity |
|
|
13,143 |
|
|
|
5,134 |
|
|
|
|
|
|
|
|
| |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
$ |
16,077 |
|
|
$ |
9,722 |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-12
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
YEARS ENDED DECEMBER 31, 2008, 2007 AND 2006 AND
PERIOD FROM AUGUST 8, 2002 (INCEPTION) THROUGH DECEMBER 31, 2008
(IN THOUSANDS, EXCEPT per share amounts)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
For the period |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, 2002 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
|
| |
|
Years Ended December 31, |
|
|
December 31, |
|
| |
|
2008 |
|
|
2007 |
|
|
2006 |
|
|
2008 |
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant and research revenues |
|
$ |
1,066 |
|
|
$ |
1,126 |
|
|
$ |
821 |
|
|
$ |
3,513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
|
1,066 |
|
|
|
1,126 |
|
|
|
821 |
|
|
|
3,513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
7,401 |
|
|
|
9,328 |
|
|
|
3,306 |
|
|
|
24,591 |
|
General and administrative |
|
$ |
4,875 |
|
|
|
4,473 |
|
|
|
4,174 |
|
|
|
17,274 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
12,276 |
|
|
|
13,801 |
|
|
|
7,480 |
|
|
|
41,865 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income and other expenses |
|
|
224 |
|
|
|
511 |
|
|
|
693 |
|
|
|
1,493 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other income |
|
|
224 |
|
|
|
511 |
|
|
|
693 |
|
|
|
1,493 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(10,986 |
) |
|
$ |
(12,164 |
) |
|
$ |
(5,966 |
) |
|
$ |
(36,859 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature, conversion
inducement and dividends on preferred stock |
|
|
(5,845 |
) |
|
|
(2,247 |
) |
|
|
(2,899 |
) |
|
|
(11,118 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders |
|
$ |
(16,831 |
) |
|
$ |
(14,411 |
) |
|
$ |
(8,865 |
) |
|
$ |
(47,977 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share — basic and diluted |
|
$ |
(0.40 |
) |
|
$ |
(0.61 |
) |
|
$ |
(0.72 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding -
basic and diluted |
|
|
42,007 |
|
|
|
23,771 |
|
|
|
12,240 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-13
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FROM AUGUST 8, 2002 (INCEPTION) THROUGH DECEMBER 31, 2008
(IN THOUSANDS)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deficit |
|
Unrealized |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
Gain/Loss on |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unearned Stock- |
|
During the |
|
Available for |
Total |
| |
|
Common Stock |
|
Preferred Stock |
|
Additional |
|
Based |
|
Development |
|
Sale |
|
Stockholders’ |
| Description |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Paid-In Capital |
|
Compensation |
|
Stage |
|
Securities |
|
Equity |
Balance at August 8, 2002 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
Issuance of common stock |
|
|
800 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
19 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
20 |
|
Issuance of Series A preferred stock |
|
|
— |
|
|
|
— |
|
|
|
920 |
|
|
|
1 |
|
|
|
229 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
230 |
|
Issuance of restricted common stock grants |
|
|
1,152 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
28 |
|
|
|
(29 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
2 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(162 |
) |
|
|
— |
|
|
|
(162 |
) |
| |
|
|
Balance at December 31, 2002 |
|
|
1,952 |
|
|
$ |
2 |
|
|
|
920 |
|
|
$ |
1 |
|
|
$ |
276 |
|
|
$ |
(27 |
) |
|
$ |
(162 |
) |
|
$ |
— |
|
|
$ |
90 |
|
Issuance of Series A-1 preferred stock |
|
|
— |
|
|
|
— |
|
|
|
1,000 |
|
|
|
1 |
|
|
|
224 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
225 |
|
Issuance of Series A preferred stock |
|
|
— |
|
|
|
— |
|
|
|
497 |
|
|
|
1 |
|
|
|
124 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
125 |
|
Issuance of Series B preferred stock |
|
|
— |
|
|
|
— |
|
|
|
1,328 |
|
|
|
1 |
|
|
|
1,659 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,660 |
|
Issuance of common stock |
|
|
432 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
10 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11 |
|
Issuance of restricted common stock grants |
|
|
227 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5 |
|
|
|
(6 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1 |
) |
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
9 |
|
|
|
— |
|
|
|
— |
|
|
|
9 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,227 |
) |
|
|
— |
|
|
|
(1,227 |
) |
| |
|
|
Balance at December 31, 2003 |
|
|
2,611 |
|
|
$ |
3 |
|
|
|
3,745 |
|
|
$ |
4 |
|
|
$ |
2,298 |
|
|
$ |
(24 |
) |
|
$ |
(1,389 |
) |
|
$ |
— |
|
|
$ |
892 |
|
Issuance of Series B preferred stock |
|
|
— |
|
|
|
— |
|
|
|
3,312 |
|
|
|
3 |
|
|
|
4,097 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,100 |
|
Issuance of common stock grants to non-employees in exchange
for services |
|
|
518 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
336 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
336 |
|
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
8 |
|
|
|
— |
|
|
|
— |
|
|
|
8 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,590 |
) |
|
|
— |
|
|
|
(1,590 |
) |
| |
|
|
Balance at December 31, 2004 |
|
|
3,129 |
|
|
$ |
3 |
|
|
|
7,057 |
|
|
$ |
7 |
|
|
$ |
6,731 |
|
|
$ |
(16 |
) |
|
$ |
(2,979 |
) |
|
$ |
— |
|
|
$ |
3,746 |
|
Proceeds from Series B subscription receivable |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
40 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
40 |
|
Issuance of common stock grants to non-employees in exchange
for services |
|
|
196 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
127 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
127 |
|
Issuance of common stock grants |
|
|
500 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
325 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
326 |
|
Issuance of stock option grants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,855 |
|
|
|
(2,855 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,235 |
|
|
|
— |
|
|
|
— |
|
|
|
1,235 |
|
Conversion of Series A and B preferred stock to common stock |
|
|
7,057 |
|
|
|
7 |
|
|
|
(7,057 |
) |
|
|
(7 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common stock for net assets of PolyMedix, Inc. |
|
|
1,500 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1 |
) |
Issuance of Series 1 preferred stock |
|
|
— |
|
|
|
— |
|
|
|
5,589 |
|
|
|
6 |
|
|
|
14,140 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
14,146 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,764 |
) |
|
|
— |
|
|
|
(4,764 |
) |
| |
|
|
Balance at December 31, 2005 |
|
|
12,382 |
|
|
$ |
12 |
|
|
|
5,589 |
|
|
$ |
6 |
|
|
$ |
24,216 |
|
|
$ |
(1,636 |
) |
|
$ |
(7,743 |
) |
|
$ |
— |
|
|
$ |
14,855 |
|
Reclassification of unearned compensation on nonvested stock
balance upon adoption of SFAS No. 123(R) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,636 |
) |
|
|
1,636 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of Series 1 preferred stock |
|
|
— |
|
|
|
— |
|
|
|
1,466 |
|
|
|
1 |
|
|
|
3,719 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,720 |
|
Issuance of common stock grants to non-employees in exchange
for services |
|
|
30 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
45 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
45 |
|
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,093 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,093 |
|
Conversion of Series 1 preferred stock to common stock |
|
|
68 |
|
|
|
— |
|
|
|
(34 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Dividends paid on Series 1 preferred stock |
|
|
— |
|
|
|
— |
|
|
|
462 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Unrealized loss on available on sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1 |
) |
|
|
(1 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5,966 |
) |
|
|
— |
|
|
|
(5,966 |
) |
| |
|
|
Balance at December 31, 2006 |
|
|
12,480 |
|
|
$ |
12 |
|
|
|
7,483 |
|
|
$ |
7 |
|
|
$ |
27,437 |
|
|
$ |
— |
|
|
$ |
(13,709 |
) |
|
$ |
(1 |
) |
|
$ |
13,746 |
|
Conversion of Series 1 preferred stock to common stock |
|
|
15,944 |
|
|
|
16 |
|
|
|
(7,483 |
) |
|
$ |
(7 |
) |
|
$ |
(9 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
Issuance of common stock grants to non-employees in exchange
for services |
|
|
12 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
12 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
12 |
|
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,189 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,189 |
|
Proceeds from exercise of common stock options |
|
|
122 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
183 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
184 |
|
Issuance of common stock to employees |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
58 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
58 |
|
Issuance of common stock, net of issuance costs |
|
|
3,481 |
|
|
|
3 |
|
|
|
— |
|
|
|
— |
|
|
|
2,103 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,106 |
|
Unrealized gain on available for sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3 |
|
|
|
3 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(12,164 |
) |
|
|
— |
|
|
|
(12,164 |
) |
| |
|
|
Balance at December 31, 2007 |
|
|
32,039 |
|
|
$ |
32 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
30,973 |
|
|
$ |
— |
|
|
$ |
(25,873 |
) |
|
$ |
2 |
|
|
$ |
5,134 |
|
Issuance of Series 2008 preferred stock, net of issuance costs |
|
|
— |
|
|
|
— |
|
|
|
609 |
|
|
|
1 |
|
|
|
3,856 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,857 |
|
Conversion of Series 2008 preferred stock to common stock |
|
|
6,089 |
|
|
|
6 |
|
|
|
(609 |
) |
|
|
(1 |
) |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5 |
|
Amortization of stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,378 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,378 |
|
Proceeds from exercise of common stock options |
|
|
214 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
214 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
214 |
|
Issuance of common stock to employees |
|
|
75 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
26 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
26 |
|
Issuance of common stock, net of issuance costs |
|
|
21,428 |
|
|
|
22 |
|
|
|
— |
|
|
|
— |
|
|
|
13,483 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
13,505 |
|
Unrealized gain on available for sale securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
10 |
|
|
|
10 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(10,986 |
) |
|
|
— |
|
|
|
(10,986 |
) |
| |
|
|
Balance at December 31, 2008 |
|
|
59,845 |
|
|
$ |
60 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
49,930 |
|
|
$ |
— |
|
|
$ |
(36,859 |
) |
|
$ |
12 |
|
|
$ |
13,143 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-14
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2008, 2007 AND 2006 AND
PERIOD FROM AUGUST 8, 2002 (INCEPTION) THROUGH DECEMBER 31, 2008
(IN THOUSANDS)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
For the period |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, 2002 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
|
| |
|
Years Ended December 31, |
|
|
December 31, |
|
| |
|
2008 |
|
|
2007 |
|
|
2006 |
|
|
2008 |
|
Cash Flows from Operating Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(10,986 |
) |
|
$ |
(12,164 |
) |
|
$ |
(5,966 |
) |
|
$ |
(36,859 |
) |
Adjustments to reconcile net loss to net cash used in
operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
213 |
|
|
|
195 |
|
|
|
64 |
|
|
|
523 |
|
Accretion of discount on cash equivalents and investment
securities |
|
|
(70 |
) |
|
|
(254 |
) |
|
|
(118 |
) |
|
|
(442 |
) |
Stock-based compensation |
|
|
1,404 |
|
|
|
1,259 |
|
|
|
1,139 |
|
|
|
5,844 |
|
(Increase) decrease in prepaid expenses and other current
assets |
|
|
(365 |
) |
|
|
18 |
|
|
|
(39 |
) |
|
|
(407 |
) |
Increase (decrease) in accounts payable, accrued expenses
and deferred rent |
|
|
(1,122 |
) |
|
|
2,657 |
|
|
|
601 |
|
|
|
2,785 |
|
Loss on disposal of fixed assets |
|
|
— |
|
|
|
23 |
|
|
|
— |
|
|
|
23 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
|
(10,926 |
) |
|
|
(8,266 |
) |
|
|
(4,319 |
) |
|
|
(28,569 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows from Investing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for property and equipment |
|
|
— |
|
|
|
(266 |
) |
|
|
(274 |
) |
|
|
(751 |
) |
Purchases of investments |
|
|
(9,553 |
) |
|
|
(9,880 |
) |
|
|
(10,810 |
) |
|
|
(30,243 |
) |
Proceeds from maturities of investments |
|
|
5,700 |
|
|
|
12,098 |
|
|
|
5,000 |
|
|
|
22,798 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities |
|
|
(3,853 |
) |
|
|
1,952 |
|
|
|
(6,084 |
) |
|
|
(8,196 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows from Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of stock, net of financing costs |
|
|
16,966 |
|
|
|
2,558 |
|
|
|
3,720 |
|
|
|
43,800 |
|
Proceeds from warrant and stock option exercises |
|
|
214 |
|
|
|
184 |
|
|
|
— |
|
|
|
398 |
|
Principal payments on capital lease obligations |
|
|
(131 |
) |
|
|
(93 |
) |
|
|
(3 |
) |
|
|
(227 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities |
|
|
17,049 |
|
|
|
2,649 |
|
|
|
3,717 |
|
|
|
43,971 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
2,270 |
|
|
|
(3,665 |
) |
|
|
(6,686 |
) |
|
|
7,206 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents — beginning of period |
|
|
4,936 |
|
|
|
8,601 |
|
|
|
15,287 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents — end of period |
|
$ |
7,206 |
|
|
$ |
4,936 |
|
|
$ |
8,601 |
|
|
$ |
7,206 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Cash Investing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment acquired under capital lease |
|
$ |
— |
|
|
$ |
228 |
|
|
$ |
103 |
|
|
$ |
331 |
|
Capital expenditures acquired on account |
|
$ |
— |
|
|
$ |
30 |
|
|
$ |
83 |
|
|
$ |
30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Cash Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion inducement and dividends on Series 1 Preferred
Stock and Beneficial conversion feature on Series 2008
Preferred Stock |
|
$ |
5,845 |
|
|
$ |
2,247 |
|
|
$ |
3,026 |
|
|
$ |
11,118 |
|
Warrants issued to placement agent |
|
$ |
783 |
|
|
$ |
— |
|
|
$ |
446 |
|
|
$ |
2,932 |
|
Accrued financing costs |
|
$ |
51 |
|
|
$ |
452 |
|
|
$ |
— |
|
|
$ |
51 |
|
Capital lease obligation |
|
$ |
— |
|
|
$ |
228 |
|
|
$ |
103 |
|
|
$ |
331 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-15
POLYMEDIX, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2008 AND 2007 AND
FOR THE YEARS ENDED DECEMBER 31, 2008, 2007 AND 2006
AND FOR THE PERIOD
AUGUST 8, 2002 (INCEPTION) TO DECEMBER 31, 2008
In these consolidated financial statements, “PolyMedix,” “we,” “us” and “our” refer to
PolyMedix, Inc. and its wholly owned subsidiary PolyMedix Pharmaceuticals, Inc. and “common stock”
refers to PolyMedix’s common stock, par value $0.001 per share
NOTE 1 — ORGANIZATION AND BUSINESS ACTIVITIES
We are a development stage biotechnology company focused on treating life threatening, serious
infectious diseases and acute cardiovascular disorders with synthetic small molecule compounds that
mimic the activity of large natural protein molecules, compounds referred to as biomimetics. Using
our proprietary computational drug design technology, we have created novel defensin mimetic
antibiotic compounds, heparin antagonist compounds and other drug compounds intended for human
therapeutic use. Since 2002, we have been a development stage enterprise, and accordingly, our
operations have been directed primarily toward developing business strategies, raising capital,
research and development activities, conducting pre-clinical testing and human clinical trials of
our product candidates, exploring marketing channels and recruiting personnel.
We have incurred operating losses since inception, have not generated any product sales
revenues and have not achieved profitable operations. Our deficit accumulated during the
development stage through December 31, 2008 aggregated $36,859,000, and we expect to continue to
incur substantial losses in future periods. None of our product candidates have received
regulatory approval for commercial sale and our product candidates may never be commercialized. In
addition, all of our product candidates are in the early stages of development and several programs
are on hold pending additional financing. The progress and results of our current and any future
clinical trials or future pre-clinical testing are uncertain, and if our product candidates do not
receive regulatory approvals, our business, operating results, financial condition and cash flows
will be materially adversely affected. Our development programs require a significant amount of
cash to support the development of product candidates.
We are highly dependent on the success of our research, development and licensing efforts and,
ultimately, upon regulatory approval and market acceptance of our products under development. Our
short and long-term capital requirements depend upon a variety of factors, including market
acceptance for our technologies and product candidates and various other factors. We anticipate
that in order to achieve our operational objectives, including our plans during 2009 for starting
and completing Phase 1b studies as well as beginning preparation for Phase 2 studies for each of
our PMX-30063 and PMX-60056 product candidates, our expenses and cash requirements will increase
from historical levels and we anticipate the need to raise additional capital during 2009 in order
to fully fund the research and development of our product candidates. We believe that our current
cash and investment balances will fund our planned Phase 1 studies for PMX-30063 and PMX-60056 and
can fund our operations for at least the next twelve months.
Our current cash and investment balances are not sufficient to fund the Phase 2 development of
either of our two lead product candidates. We do not plan to initiate our Phase 2 development
activities until additional financing is secured. We expect to seek additional funds through
equity or debt financing, among other sources. In addition, we may actively seek funds through
government grants and contracts. However, as a result of current conditions in the equity and debt
markets, we may not be able to obtain additional funding on favorable terms, if at all. In
addition, we if we choose to apply for government grants and contracts, there is no guarantee of
acceptance of our applications. If additional capital resources are not obtained by the end of the
second quarter of 2009, we will scale-back, postpone or eliminate certain of our research, drug
discovery or development programs until such additional capital resources have been obtained, and
as a result, our business may be materially and adversely affected. In the absence of adequate
additional funding, we believe that we have the ability to scale our operations such that our
current cash and investment balances can fund our operations into the second half of 2010.
Global market and economic conditions have been, and continue to be, disruptive and volatile.
In particular, the cost of raising money in the debt and equity capital markets has increased
substantially while the availability of funds from those markets has diminished significantly. If
funding is not available when needed, or is available only on unfavorable terms, meeting our
capital needs or otherwise taking advantage of business opportunities may become challenging, which
could have a material adverse effect on our business plans.
F-16
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of PolyMedix, Inc. and its wholly
owned subsidiary PolyMedix Pharmaceuticals, Inc. All intercompany accounts and transactions have
been eliminated in consolidation.
Development Stage Company
We are considered to be in the development stage as defined in Statements of Financial
Accounting Standards (SFAS) No.7, Accounting and Reporting by Development Stage Enterprises. We
have devoted substantially all of our efforts to business planning, research and development,
recruiting management and technical staff, acquiring operating assets and raising capital.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally
accepted in the United States of America requires management to make estimates and assumptions that
affect the reported amounts in the financial statements and accompanying notes. Actual results
could differ from those estimates. Significant estimates include the value of our common stock,
preferred stock, stock options and warrants.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with a maturity of three months or less at
the time of purchase to be cash equivalents. Cash and cash equivalents consist of cash in banks and
money market funds.
Short-Term Investments
Investments purchased with a maturity of more than three months, and which mature less than
twelve months from the balance sheet date, are classified as short-term investments. We generally
hold investments to maturity, however, since we may, from time to time, sell securities to meet
cash requirements, we classify our investments as available-for-sale as defined by Statement of
Financial Accounting Standards (“SFAS”), No. 115, Accounting for Certain Investments in Debt and
Equity Securities” Available-for-sale securities are carried at market value with unrealized gains
and losses reported as a separate component of Stockholders’ Equity.
Property and Equipment
Property and equipment are stated at cost. Depreciation is computed using the straight-line
method over the estimated useful life of the assets; three years for computer equipment and related
software, seven years for office furniture and five years for lab equipment. When property and
equipment are sold or otherwise disposed of, the cost and related accumulated depreciation are
removed from the accounts and the resulting gain or loss is included in operating expenses.
Depreciation expense was $213,000, $195,000, $64,000 and $523,000 for the years ended December 31,
2008, 2007 and 2006 and for the period from August 8, 2002 (Inception) to December 31, 2008
respectively.
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment whenever events or changes in circumstances
indicate that the basis of an asset may not be recoverable. In accordance with SFAS No. 144,
Accounting for the Impairment or Disposal of Long-Lived Assets, impairment is assessed by measuring
the carrying amount of the assets against the estimated undiscounted future cash flows associated
with them. If the expected future cash flows are less than the carrying value, an impairment loss
is recognized for the amount by which the carrying value exceeds the fair value of the assets.
F-17
Grant and Research Revenue
Sponsored grant and research revenues are recognized pursuant to the terms of the related
agreements as work is performed.
Research and Development Expense
Research and development costs are expensed as incurred.
Income Taxes
We recognize deferred tax assets and liabilities for temporary differences between the
financial reporting basis and the tax basis of our assets and liabilities and the expected benefits
of using net operating loss carryforwards. The impact of changes in tax rates and laws on deferred
taxes, if any, applied during the years in which temporary differences are expected to be settled,
is reflected in the consolidated financial statements in the period of enactment. The measurement
of deferred tax assets is reduced, if necessary, if, based on weight of the evidence, it is more
likely than not that some, or all, of the deferred tax assets will not be realized. The effect on
deferred tax assets and liabilities of a change in tax rates is recognized in the period that such
tax rate changes are enacted. At December 31, 2008 and 2007, we have concluded that a full
valuation allowance is necessary for deferred tax assets. (See Note 5).
We adopted the provisions of FIN 48, “Accounting for Uncertainty in Income Taxes — an
Interpretation of FASB Statement No. 109” on January 1, 2007. FIN 48 prescribes the recognition
threshold and measurement attribute for the financial statement recognition and measurement of
uncertain tax positions taken or expected to be taken in a tax return. We had no material amounts
recorded for uncertain tax positions, interest or penalties in the accompanying consolidated
financial statements. See Note 5 for the impact of the adoption of FIN 48.
Loss per Share of Common Stock
We calculate our loss per share under the provisions of SFAS No. 128, Earnings Per Share. SFAS
No. 128 requires a dual presentation of “basic” and “diluted” loss per share on the face of the
income statement. Basic loss per share is computed by dividing loss by the weighted average number
of shares of common stock outstanding during each period. Diluted loss per share includes the
effect, if any, from the potential exercise or conversion of securities, such as unvested
restricted stock, convertible preferred stock, stock options and warrants, which would result in
the issuance of incremental shares of common stock. In computing the basic and diluted net loss per
share allocable to common stockholders the weighted average number of shares remains the same for
both calculations due to the fact that when a net loss exists, dilutive shares are not included in
the calculation. Potentially dilutive securities include unvested restricted stock, convertible
preferred stock, and options and warrants to purchase our common stock. These potentially dilutive
securities are more fully described in Note 6.
Comprehensive Loss
SFAS No. 130, “Reporting Comprehensive Income,” establishes standards for the reporting of
comprehensive income and its components. Comprehensive loss consists of reported net income or loss
and unrealized gains or losses on available for sale securities. The comprehensive loss for each of
the periods presented approximates the net loss in the consolidated statements of operations.
Segment Information
We report segment information in accordance with SFAS No. 131, Disclosure About Segments of an
Enterprise and Related Information. We have one reportable segment operating within the United
States.
Reclassification
Certain prior year amounts have been reclassified to conform to current year presentation.
Fair Value of Financial Instruments and Credit Risk
The carrying amounts reported in the consolidated balance sheets for cash and cash
equivalents, investments, accounts payable and accrued expenses approximate fair value because of
the immediate or short-term maturity of these financial instruments.
F-18
We invest our cash in accordance with a policy objective that seeks to ensure both liquidity
and safety of principal. The policy limits investments to instruments issued by the U.S. government
and commercial institutions with strong investment grade credit ratings and places restrictions on
maturity terms and concentrations by type and issuer.
Stock-Based Compensation
We currently sponsor equity compensation plans. Refer to Note 6. From our inception, August 8,
2002, we applied the fair value recognition provisions of Statement of Financial Accounting
Standards (“SFAS”) No. 123, Accounting for Stock-Based Compensation. Beginning January 1, 2006, we
adopted SFAS No. 123(R), Share-based Payment (“SFAS No. 123(R)”) using the modified-prospective
transition method, and as such, prior periods have not been restated. Compensation expense is
recognized over the requisite service period. The total stock-based compensation expense was
$1,404,000, $1,259,000, $1,139,000, and $5,844,000 for the years ended December 31, 2008, 2007 and
2006 and for the period from August 8, 2002 (Inception) to December 31, 2008, respectively.
Recently Issued and Adopted Accounting Standards
In September 2006, the Financial Accounting Standards Board (or “FASB”) issued Statement of
Financial Accounting Standards (or “SFAS”) No. 157, Fair Value Measurements (“SFAS 157”). SFAS 157
clarifies the definition of fair value, establishes a framework for measuring fair value and
expands disclosures on fair value measurements. SFAS 157 was effective for financial statements
issued for fiscal years beginning after November 15, 2007 and interim periods within those fiscal
years; however, the FASB did provide a one-year deferral for the implementation of SFAS 157 for
other nonfinancial assets and liabilities.
SFAS 157 establishes a valuation hierarchy for disclosure of the inputs to valuation used to
measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows. Level
1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs
that are observable for the asset or liability, either directly or indirectly through market
corroboration, for substantially the full term of the financial instrument. Level 3 inputs are
unobservable inputs based on management’s own assumptions used to measure assets and liabilities at
fair value. A financial asset or liability’s classification within the hierarchy is determined
based on the lowest level input that is significant to the fair value measurement.
The following table provides the assets and liabilities carried at fair value measured on a
recurring basis as of December 31, 2008 (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Fair Value Measurements at December 31, 2008 |
|
| |
|
Total Carrying |
|
|
|
|
|
|
Significant other |
|
|
Significant |
|
| |
|
value as of |
|
|
Quoted prices in |
|
|
observable |
|
|
unobservable |
|
| |
|
December |
|
|
active markets |
|
|
inputs |
|
|
inputs |
|
| |
|
31, 2008 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Short-term investments |
|
$ |
7,900 |
|
|
$ |
— |
|
|
$ |
7,900 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
$ |
7,900 |
|
|
$ |
— |
|
|
$ |
7,900 |
|
|
$ |
— |
|
Our short-term investments, generally fixed income government agency securities, are measured
at fair value using models derived principally from or corroborated by observable market data by
correlation or other means and are classified within Level 2 of the valuation hierarchy. The
adoption of SFAS 157 did not have any impact on our consolidated financial statements.
In February 2007, the FASB issued SFAS No. 159 The Fair Value Option for Financial Assets and
Financial Liabilities (“SFAS 159”). SFAS 159 permits entities to choose to measure many financial
assets and financial liabilities at fair value. Unrealized gains and losses on items for which the
fair value option has been elected are reported in earnings. SFAS 159 was effective for fiscal
years beginning after November 15, 2007. We did not elect the fair value option available under
SFAS 159 for any financial assets or liabilities.
In June 2007, the FASB ratified the consensus reached in EITF Issue No. 07-03, Accounting for
Nonrefundable Advance Payments for Goods or Services Received for Use in Future Research and
Development Activities (Issue No. 07-03). Issue No. 07-03 requires that non-refundable advance
payments for future research and development activities should be deferred and recognized as an
expense as goods are delivered or the related services are performed. Issue No. 07-03 is effective
for fiscal years beginning after December 15, 2007. Issue No.
F-19
07-03 was adopted effective January 1, 2008 and did not have a material impact on our
consolidated financial statements.
In June 2008, the FASB issued FASB Staff Position No. EITF 03-6-1, Determining Whether
Instruments Granted in Share-Based Payment Transactions Are Participating Securities, (“FSP EITF
03-6-1”). FSP EITF 03-6-1 addresses whether instruments granted in share-based payment transactions
are participating securities prior to vesting and, therefore need to be included in the earnings
allocation in computing earnings per share, or EPS, under the two-class method described in
paragraphs 60 and 61 of FASB Statement No. 128, Earnings per Share (“SFAS No. 128”). FSP EITF
03-6-1 applies to the calculation of EPS under SFAS No. 128 for share-based payment awards with
rights to dividends or dividend equivalents. FSP EITF 03-6-1 clarifies that unvested share-based
payment awards that contain nonforfeitable rights to dividends or dividend equivalents (whether
paid or unpaid) are participating securities and shall be included in the computation of EPS
pursuant to the two class method. FSP EITF 03-6-1 is effective for financial statements issued for
fiscal years beginning after December 15, 2008, and interim periods within those years. All
prior-period EPS data presented must be adjusted retrospectively (including interim financial
statements, summaries of earnings and selected financial data) to conform with the provisions of
FSP EITF 03-6-1. Early adoption is not permitted. We do not expect EITF 03-6-1 to have a material
impact on our consolidated financial statements.
In June 2008, the FASB ratified the consensus reached on EITF Issue No. 07-05, Determining
Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock (“EITF No. 07-05”).
EITF No. 07-05 clarifies the determination of whether an instrument (or an embedded feature) is
indexed to an entity’s own stock, which would qualify as a scope exception under SFAS No. 133,
Accounting for Derivative Instruments and Hedging Activities. EITF No. 07-05 is effective for
financial statements issued for fiscal years beginning after December 15, 2008. Early adoption for
an existing instrument is not permitted. We are currently evaluating the impact of the pending
adoption of EITF No. 07-05 on our consolidated financial statements.
NOTE 3 — SHORT-TERM INVESTMENTS
Short-term investments consist of investment grade fixed income securities with original
maturities of greater than three months. All investments are classified as “available for sale”,
and are considered current assets. As of December 31, 2008, all short-term investments had
maturities of less than one-year.
The following summarizes the short-term investments as of December 31, 2008 and December 31,
2007, which were invested solely in U.S. government obligations (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Gross |
|
Gross |
|
|
| |
|
Amortized |
|
Unrealized |
|
Unrealized |
|
|
| |
|
Cost |
|
Gains |
|
Losses |
|
Fair Value |
December 31, 2008 |
|
$ |
7,888 |
|
|
$ |
12 |
|
|
$ |
— |
|
|
$ |
7,900 |
|
December 31, 2007 |
|
$ |
3,965 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
3,967 |
|
NOTE 4 — ACCRUED EXPENSES
At December 31, 2008 and 2007, accrued expenses consisted of the following (in thousands):
| |
|
|
|
|
|
|
|
|
| |
|
2008 |
|
|
2007 |
|
Payroll related costs |
|
$ |
479 |
|
|
$ |
280 |
|
Research and development related costs |
|
|
318 |
|
|
|
827 |
|
Other costs |
|
|
136 |
|
|
|
265 |
|
|
|
|
|
|
|
|
Total accrued expenses |
|
$ |
933 |
|
|
$ |
1,372 |
|
|
|
|
|
|
|
|
NOTE 5 — PROVISION FOR INCOME TAXES
We account for income taxes using the asset and liability approach as required by FASB No.
109. Deferred income taxes are provided for the differences between the tax bases of assets or
liabilities and their reported amounts in the financial statements. This method also requires the
recognition of future tax benefits, such as net operating loss carryforwards and research and
development credits, to the extent that realization of such benefits is more likely than not. At
December 31, 2008 and 2007, deferred tax assets consisted of the following (in thousands):
F-20
| |
|
|
|
|
|
|
|
|
| |
|
2008 |
|
2007 |
Deferred tax assets: |
|
|
|
|
|
|
|
|
Net operating loss carryforward |
|
$ |
11,710 |
|
|
$ |
7,998 |
|
Stock compensation expense |
|
|
1,592 |
|
|
|
1,163 |
|
Contribution carryforward |
|
|
33 |
|
|
|
33 |
|
R & D credit carryforward |
|
|
1,373 |
|
|
|
998 |
|
Depreciation |
|
|
(70 |
) |
|
|
(57 |
) |
Straight-line rent |
|
|
335 |
|
|
|
254 |
|
Other |
|
|
271 |
|
|
|
265 |
|
Gross deferred tax asset |
|
|
15,244 |
|
|
|
10,654 |
|
Valuation allowance |
|
|
(15,244 |
) |
|
|
(10,654 |
) |
Net deferred tax asset |
|
$ |
— |
|
|
$ |
— |
|
We believe there is not sufficient evidence that future taxable income will be adequate to
permit the realization of the future tax deductions embedded in this asset. As such, in accordance
with SFAS No. 109, “Accounting for Income Taxes”, we have established a valuation allowance to
reduce the deferred tax asset since it is more likely than not that the deferred tax asset will not
be realized.
F-21
The difference between the provision for (benefit from) income taxes and the amount computed
by applying the federal statutory income tax rate to income (loss) before provision for (benefit
from) income taxes is explained below (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
2002 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
| |
|
Years ended December 31, |
|
December 31, |
| |
|
2008 |
|
2007 |
|
2006 |
|
2008 |
Loss before provision for income taxes |
|
$ |
(10,986 |
) |
|
$ |
(12,164 |
) |
|
$ |
(5,966 |
) |
|
$ |
(36,859 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax at federal statutory rate |
|
|
(3,735 |
) |
|
|
(4,138 |
) |
|
|
(2,028 |
) |
|
|
(12,533 |
) |
State taxes, net of federal benefit |
|
|
(883 |
) |
|
|
(908 |
) |
|
|
(390 |
) |
|
|
(2,692 |
) |
Research and development credits |
|
|
(290 |
) |
|
|
(348 |
) |
|
|
(159 |
) |
|
|
(959 |
) |
Other permanent differences |
|
|
113 |
|
|
|
74 |
|
|
|
20 |
|
|
|
210 |
|
Other |
|
|
205 |
|
|
|
49 |
|
|
|
476 |
|
|
|
730 |
|
Change in valuation allowance |
|
|
4,590 |
|
|
|
5,271 |
|
|
|
2,081 |
|
|
|
15,244 |
|
Provision for income taxes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
We have available at December 31, 2008, approximately $29,000,000 in unused federal and state
operating loss carryforwards that expire between 2022 and 2028. We also have available at December
31, 2008, approximately $1,400,000 unused federal research and development credit carryforwards
that may provide future tax benefits and expire between 2024 and 2028.
The Tax Reform Act of 1986 contains provisions that may limit the NOL and research and
development credit carryforwards available to be used in any given year upon the occurrence of
certain events, including significant changes in ownership interest. Generally, a change in
ownership of a company of greater than 50% within a three-year period results in an annual
limitation on that company’s ability to utilize its NOL carryforwards and tax credits from the tax
periods prior to the ownership change. A study needs to be performed to determine if we have
undergone a change in ownership, however, we believe that we have undergone an ownership change and
are subject to an annual limitation on the use of our NOL carryforwards pursuant to these
provisions.
In July 2006, the FASB issued FASB Interpretation No. 48, “Accounting for Uncertainty in
Income Taxes,” or FIN 48, which is applicable for fiscal years beginning after December 15, 2006.
FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s
financial statements in accordance with SFAS No. 109, “Accounting for Income Taxes.” FIN 48
prescribes a recognition threshold and measurement for financial statement recognition and
measurement of a tax position reported or expected to be reported on a tax return. FIN 48 also
provides guidance on derecognition, classification, interest and penalties, accounting in interim
periods, disclosure, and transition. On January 1, 2007, we adopted FIN 48. Prior to the adoption
of FIN 48, our policy was to recognize tax benefits of uncertain tax positions unless it was
probable that a position would not be sustained. FIN 48 requires application of a “more likely
than not” threshold to the recognition and derecogntion of tax positions. As a result of the
adoption of FIN 48, no adjustments were made to retained earnings for unrecognized tax benefits.
We file income tax returns in the U.S. federal jurisdiction and Pennsylvania. Our policy is
to record interest and penalties on uncertain tax positions as income tax expense. The tax years
back to 2002 remain open to examination by the major taxing jurisdictions where we file. Net
operating loss and credit carryforwards from earlier periods also remain open to examination by
taxing authorities, and will for a period post utilization. We do not reasonably estimate that the
unrecognized tax benefit will change significantly within the next twelve months. Any changes in
the future would also have no impact on the effective tax rate due to the existence of a full
valuation allowance. As of December 31, 2008, we had no material amounts recorded for uncertain tax
positions, interest or penalties in the accompanying consolidated financial statements.
NOTE 6 — STOCKHOLDERS’ EQUITY
F-22
Common Stock
We are authorized to issue 250,000,000 shares of common stock, with a par value of $0.001, of
which 59,845,000 and 32,039,000 were issued and outstanding at December 31, 2008 and 2007,
respectively.
In December 2008, our then outstanding 608,834 shares of Series 2008 Convertible Preferred
Stock (“Series 2008 Preferred Stock”), originally issued in September 2008, automatically converted
to 6,088,340 shares of common stock. See Preferred Stock section below.
In July 2008, we completed a registered offering of 21,428,000 units. Each unit consisted of
one share of our common stock and a Series A Warrant to purchase one share of our common stock.
Each unit was priced at $0.70 per unit with the exercise price of the Series A Warrants issued at
$1.00 per share. We received gross proceeds of $15,000,000 in the registered offering before fees
and expenses. In connection with this registered offering, we incurred commission fees and
expenses of approximately $1,496,000.
In December 2007, we completed a registered offering of 2,943,000 units. Each unit consisted
of one share of our common stock and a warrant to purchase one share of our common stock. Each
unit was priced at a $1.10 per unit and the exercise price of the warrants issued is also $1.10.
We received gross proceeds of $3,238,000 in the registered offering before fees and expenses. In
connection with this registered offering, we paid commission fees and expenses of $1,132,000.
Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, with a par value of $0.001,
of which no shares were issued and outstanding at December 31, 2008 and 2007, respectively.
In September 2008, we completed a private placement offering of 608,834 units. Each unit
consisted of one share of our 2008 Preferred Stock and a Series B Warrant. Each unit was priced at
a $7.00 per unit. During December 2008, each share of 2008 Preferred Stock automatically converted
into 10 shares of common stock and the Series B Warrants became exercisable for common stock
immediately, and without any action on the part of the holder of such shares of 2008 Preferred
Stock or Series B Warrants, upon the effectiveness of an amendment (the “Certificate Amendment”) to
our Amended and Restated Certificate of Incorporation, as amended (the “Certificate”), to increase
the number of shares of common stock authorized for issuance by us under the Certificate to an
amount sufficient to cover the issuance of shares of common stock upon conversion of the 2008
Preferred Stock and the issuance of shares of common stock issuable after such conversion upon
exercise of all of the Series B Warrants. The conversion of the Series 2008 Preferred Stock
triggered the recognition of a beneficial conversion feature of approximately $5,845,000, which was
included in our net loss in arriving at net loss attributable to common stockholders. We received
gross proceeds of $4,262,000 in the private placement before fees and expenses. In connection with
this private placement, we incurred commission fees and expenses of approximately $401,000.
During the second quarter of 2007, our stockholders voted to approve an amendment to our
Series 1 Preferred Stock certificate of designations to provide for the automatic and immediate
conversion of each share of Series 1 Preferred stock into 2.247 shares of our common stock. The
fair value of the additional shares paid to the then Series 1 Preferred stockholders was
approximately $2,247,000, which has been deducted from our net loss in arriving at net loss
attributable to common stock holders. In addition, notwithstanding the conversion, these former
holders of Series 1 preferred stock maintained their weighted average anti-dilution protection
through December 31, 2007. As a result of the registered offering of common stock in December
2007, which closed during the fourth quarter of 2007 and in satisfaction of the weighted average
anti-dilution protection then in place, we issued to the former holders of Series 1 preferred
stockholders 538,000 shares of common stock.
Warrants
In connection with the private placement and subsequent conversion of the Series 2008
Preferred Stock (described above), investors in the private placement received Series B Warrants
presently exercisable for 6,088,340 shares of common stock and the placement agent and dealer
received Series B Warrants presently exercisable for 279,583 shares of common stock. The Series B
Warrants have an exercise price of $1.00 per share and that expire in July 2013. The December 2008
conversion of the Series 2008 Preferred Stock triggered the recognition of a beneficial conversion
feature of approximately $2,218,000, which was included in our net loss in arriving at net loss
attributable to common stockholders. The fair value of the warrants at the time of issuance was
$3,991,000, which was estimated using the Black-Scholes-Merton option-pricing model with the
following assumptions: no dividends; risk-free interest rate of 3.03%; estimated life of five
years and volatility of 71%.
F-23
In connection with the registered offering completed in July 2008 (described above), we issued
Series A Warrants to purchase 21,428,000 shares of common stock to investors and Series A Warrants
to purchase 1,071,000 shares of common stock to the placement agents and dealer at an exercise
price of $1.00 per share and expire in September 2013. The fair value of the warrants at the time
of issuance was $11,807,000, which was estimated using the Black-Scholes-Merton option-pricing
model with the following assumptions: no dividends; risk-free interest rate of 3.2% to 3.35%;
estimated life of five years and volatility of 72% to 75%.
In connection with the registered offering completed in December 2007 (described above), we
issued warrants to purchase 2,943,000 shares of common stock at an exercise price of $1.10 per
share. These warrants expire in December 2012. The fair value of the warrants at the time of
issuance was $2,015,000, which was estimated using the Black-Scholes-Merton option-pricing model
with the following assumptions: no dividends; risk-free interest rate of 3.58%; estimated life of
five years and volatility of 73%.
During 2005 and 2006, we issued warrants to purchase 4,119,000 shares of common stock in
connection with the private placement of Series 1 preferred stock at an exercise price of $1.23 per
share, as adjusted for weighted average anti-dilution protection. These warrants expire in
November 2010 and contain weighted average anti-dilution protection if we issue certain securities
at a price per share less than $1.23 per share. The fair value of the warrants at the time of
issuance was $2,149,000, which was estimated using the Black-Scholes-Merton option-pricing model
with the following assumptions: no dividends; risk-free interest rate of 4.45%; estimated life of
five years and volatility of 60%.
Stock-Based Compensation
We maintain equity compensation plans under which grants of incentive stock options,
nonqualified stock options, stock appreciation rights, stock awards, stock units and other
stock-based awards are granted to selected employees, non-employee directors and key advisors.
Since our inception on August 8, 2002, we have recognized equity compensation expense over the
requisite service period using the Black-Scholes-Merton formula to estimate the fair value of stock
options. Beginning January 1, 2006, we adopted SFAS No. 123(R) under the modified-prospective
transition approach. The following table summarizes the total stock-based compensation expense
included in our Consolidated Statements of Operations (in thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, 2002 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
|
| |
|
Years Ended December 31, |
|
|
December 31, |
|
| |
|
2008 |
|
|
2007 |
|
|
2006 |
|
|
2008 |
|
Research and development |
|
$ |
440 |
|
|
$ |
323 |
|
|
$ |
205 |
|
|
$ |
1,801 |
|
General and administrative |
|
|
964 |
|
|
|
936 |
|
|
|
934 |
|
|
|
4,043 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stock-based
compensation expense |
|
$ |
1,404 |
|
|
$ |
1,259 |
|
|
$ |
1,139 |
|
|
$ |
5,844 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2008, approximately 4,807,000 stock options were awarded to
non-employee directors, officers, employees and consultants. As of December 31, 2008, there were
10,210,000 shares of common stock issuable upon exercise of outstanding stock options and 565,000
shares of common stock available for issuance of future equity compensation awards in connection
with our equity compensation plans.
Stock Options
As of December 31, 2008, there was approximately $3,645,000 of total unrecognized compensation
cost related to non-vested stock options, which will be amortized over the weighted average
remaining service period of approximately 1.37 years. This expected cost does not include the
impact of any future stock option awards. Options granted are generally exercisable for a period
of up to ten years from the date of grant at an exercise price which is not less than the fair
value on the date of grant and generally vest over terms ranging from immediately to four years. A
summary of the status of our stock options as of December 31, 2008 and changes during the year
ended December 31, 2008 is presented below (in thousands, except per share amounts):
F-24
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Weighted |
|
|
| |
|
|
|
|
|
Weighted |
|
Average |
|
|
| |
|
|
|
|
|
Average |
|
Remaining |
|
Aggregate |
| |
|
|
|
|
|
Exercise Price |
|
Contractual Life |
|
Intrinsic |
| |
|
Options |
|
per share |
|
(in years) |
|
Value |
Outstanding at beginning of period |
|
|
5,421 |
|
|
$ |
1.75 |
|
|
|
|
|
|
|
|
|
Granted |
|
|
4,807 |
|
|
$ |
1.10 |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
Expired |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(18 |
) |
|
$ |
1.65 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at end of period |
|
|
10,210 |
|
|
$ |
1.44 |
|
|
|
8.46 |
|
|
$ |
836 |
|
Exercisable at end of period |
|
|
5,040 |
|
|
$ |
1.61 |
|
|
|
7.39 |
|
|
$ |
189 |
|
The aggregate intrinsic value in the preceding tables represent the total pretax intrinsic
value that would have been received by the option holders had all option holders exercised their
options on the last day of 2008. Intrinsic value is determined by calculating the difference
between the value of our stock on the last day of the year and the exercise price, multiplied by
the number of options.
The following table summarizes the fair value of options granted during the years ended
December 31, 2008, 2007 and 2006 and for the period from August 8, 2002 (Inception) to December 31,
2008, respectively (in thousands, except per share amounts):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, 2002 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
| |
|
Years Ended December 31, |
|
December 31, |
| |
|
2008 |
|
2007 |
|
2006 |
|
2008 |
Fair value of options granted |
|
$ |
3,210 |
|
|
$ |
1,228 |
|
|
$ |
1,475 |
|
|
$ |
8,768 |
|
Fair value of options granted (per share) |
|
$ |
0.67 |
|
|
$ |
0.91 |
|
|
$ |
1.20 |
|
|
$ |
0.81 |
|
The fair value of options granted is amortized over the requisite service period. The fair
value of each option grant is estimated on the date of grant using the Black-Scholes-Merton formula
with the following weighted average assumptions:
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Year Ended |
|
Year Ended |
|
Year Ended |
| |
|
December 31, 2008 |
|
December 31, 2007 |
|
December 31, 2006 |
Range of risk free interest rates |
|
|
1.37% – 3.25% |
|
|
|
3.84% – 4.85% |
|
|
|
4.30% – 4.96% |
|
Dividend yield |
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
Expected volatility |
|
|
69% – 75% |
|
|
|
63% – 71% |
|
|
|
41% – 60% |
|
Expected life of options (in
years) |
|
|
5 |
|
|
|
5 |
|
|
|
5 |
|
Forfeitures |
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
Expected volatility and expected life for the years ended December 31, 2008, 2007 and 2006
were estimated based upon historical activity, when available, and our benchmark analysis of
selected companies. The risk-free interest rate is calculated using the U.S. Treasury yield curves
in effect at the time of grant. We currently estimate that all of our outstanding options will
vest.
F-25
Restricted Common Stock Grants
We have also issued shares of restricted common stock to selected employees, non-employee
directors and key advisors. During the three-year period ended December 31, 2008, there were no
grants of restricted stock As of December 31, 2008, there was no unrecognized compensation cost
related to non-vested restricted stock grants. The restriction period for restricted stock awards
was for a four year vesting period, commencing from the grant date.
A summary of the status of our unvested restricted stock awards as of December 31, 2008, 2007
and 2006 and changes during the years ended December 31, 2008, 2007 and 2006, and for the period
from August 8, 2002 (Inception) to December 31, 2008, respectively, are presented below (in
thousands):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Period from |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
August 8, 2002 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Inception) to |
|
| |
|
Years Ended December 31, |
|
|
December 31, |
|
| |
|
2008 |
|
|
2007 |
|
|
2006 |
|
|
2008 |
|
Restricted common
stock — beginning
of period |
|
|
— |
|
|
|
12 |
|
|
|
291 |
|
|
|
— |
|
Awards Granted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,464 |
|
Awards Vested |
|
|
— |
|
|
|
(12 |
) |
|
|
(279 |
) |
|
|
(1,379 |
) |
Awards Forfeited |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(85 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted common
stock — end of
period |
|
|
— |
|
|
|
— |
|
|
|
12 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The total grant date fair value of these restricted stock awards was $34,000. All restricted
shares awarded have grant date fair values of $0.025 per share.
Common Stock Grants
We also issued common stock grants to selected employees, non-employee directors and key
advisors. For the years ended December 31, 2006, and for the period from August 8, 2002 (Inception)
to December 31, 2008, we issued common stock grants of 30,000 and 1,244,000 shares, respectively.
The fair value and expense recognized from the issuance of common stock grants for the years ended
December 31, 2008, 2007, and 2006 and for the period from August 8, 2002 (Inception) to December
31, 2008, were $0, $0, $45,000, and $834,000, respectively.
Additionally, during January 2008, and December, 2007, in connection with agreements with
certain terminated employees, we granted 50,000 and 25,000 shares, respectively, of common stock as
a one-time termination benefit to certain employees. These shares were issued ratably over the
first five-months following the grant date. During 2007, we also issued 12,500 shares to UMass in
connection with our license agreement. We recognized $26,000 and $70,000, in 2008 and 2007,
respectively, in equity based compensation costs associated with these shares which is included in
research and development costs on our Consolidated Statements of Operations.
NOTE 7 — COMMITMENTS AND CONTINGENCIES
Capital Lease
We have entered into lease agreements for laboratory equipment. The initial obligation under
these capital leases was $331,000. The value of the laboratory equipment acquired in connection
with these leases was $398,000 and the depreciation associated with these assets is included along
with that of other owned property and equipment. These equipment leases have terms of up to three
years, at interest rates ranging from 9.5% to 11.5% and contain bargain purchase options. In
connection with these capital leases, we will pay $108,000 during 2009, $4,000 of which will be for
interest. These capital leases end during 2009.
Operating Lease
In June 2006, we entered into an operating lease agreement for 24,223 square feet of combined
office and laboratory space located in Radnor, Pennsylvania. The initial term of the lease is 12
years. Payments under the lease
F-26
commenced on December 1, 2006. Our annual future minimum lease payments under this
non-cancelable operating lease are as follows (in thousands):
| |
|
|
|
|
| |
|
Operating Leases |
|
2009 |
|
$ |
497 |
|
2010 |
|
|
589 |
|
2011 |
|
|
667 |
|
2012 |
|
|
686 |
|
2013 |
|
|
705 |
|
Thereafter |
|
|
3,610 |
|
|
|
|
|
Total minimum lease payments |
|
$ |
6,754 |
|
|
|
|
|
Prior to the commencement of our current operating lease for our Radnor Facility, we leased
approximately 3,500 square feet of combined office and laboratory space on a month-to-month basis
in Philadelphia, Pennsylvania. Rent expense was $598,000, $598,000 and $559,000 and $1,877,000 for
the years ended December 31, 2008, 2007, and 2006, and for the period from August 8, 2002
(Inception) to December 31, 2008, respectively.
Patent License Agreements
University of Pennsylvania. In January 2003, we entered into a Patent License Agreement with
the University of Pennsylvania, or Penn. Under the terms of the agreement, we were granted an
exclusive, worldwide royalty-bearing license to make and sell products utilizing seven of Penn’s
issued or pending patents for the life of such patents. One issued patent and five patent
applications cover the composition of matter on antimicrobial compounds, including small molecules,
oligomers and polymers. One patent application covers the composition and use of polycationic
compounds for treating cancer. If a change-of-control event occurs, in which we transfer the
license to these patents to a third party, we are acquired by another company, or we conduct an
initial public offering of our securities, we are required to pay a 3% royalty on the gross sales
for licensed products that are sold as pharmaceuticals and a 1.5% royalty on products sold as
coatings for use in medical devices. We are permitted to sublicense the patents provided that (a)
the sublicensee is prohibited from further licensing of the patents and (b) the sublicensee is
subject to all of the terms of the original license granted to us. In addition, we are required to
share with Penn any consideration we receive from sublicensing our patents to a third party.
University of Massachusetts. In January 2004, we entered into a five-year sponsored research
agreement with UMass. Under the terms of this agreement, we have the exclusive option to license
any intellectual property that may be generated by Dr. Gregory Tew pursuant to research sponsored
under the agreement. We may exercise this option by issuing 7,500 shares of our common stock to
UMass for each $100,000 of research conducted by Dr. Tew. If we exercise this option, we are also
required to reimburse UMass for direct patent costs incurred by it for the patents licensed by us.
During 2007, we issued 12,500 shares to UMass in connection with this agreement. We sponsored
$36,000 and $107,000, $118,000 of Dr. Tew’s research for 2008, 2007 and 2006, respectively.
Other
Agreements with Employees. We have entered into employment agreements with various executives.
These agreements provide for severance arrangements and accelerated vesting of equity compensation
awards in the event that the executive is terminated by us other than for cause or disability or if
the executive resigns for good reason.
Credit Line. In April 2006, we entered into a line of credit agreement with a financial
institution. This line of credit provides for monthly interest-only payments at a variable per
annum rate of 3% plus the 30-day LIBOR rate. The amount available under this line of credit ranges
from 85% to 92% of cash and investments pledged as collateral, based upon the amount and security
type. There is currently no outstanding balance on this line of credit. In June 2006, we entered
into a letter of credit agreement with the same financial institution to secure our payment
obligations under our facility operating lease. This letter of credit is for $1,400,000, expires
on December 1, 2009 and is secured by our credit line.
Termination Benefits. During January, 2008, and December, 2007, in connection with agreements
with certain terminated employees, we granted 50,000 and 25,000 shares, respectively, of common
stock as a one-time termination benefit to certain employees. These shares will be issued ratably
over the first five-months following the grant date. We recognized $26,000 and $58,000, in 2008
and 2007, respectively, in equity based compensation costs associated with these shares which is
included in research and development costs on our Consolidated Statements of Operations.
F-27
NOTE 8 — QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
The following tables set forth certain unaudited financial information for each of the
quarters in the years ended December 31, 2008 and 2007. This unaudited quarterly information has
been prepared on the same basis as the audited financial statements and includes all necessary
adjustments, consisting only of normal recurring adjustments that we consider necessary for a fair
presentation of the unaudited quarterly results when read in conjunction with the audited financial
statements and notes. We believe that quarter-to-quarter comparisons of financial results are not
necessarily meaningful and should not be relied upon as an indication of future performance (in
thousands, except per share amounts).
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Q1 |
|
Q2 |
|
Q3 |
|
Q4 |
|
Year |
2008: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant and research revenues |
|
$ |
991 |
|
|
$ |
18 |
|
|
$ |
39 |
|
|
$ |
18 |
|
|
$ |
1,066 |
|
Operating expenses |
|
|
3,881 |
|
|
|
2,950 |
|
|
|
3,055 |
|
|
|
2,390 |
* |
|
|
12,276 |
|
Net loss |
|
|
(2,823 |
) |
|
|
(2,904 |
) |
|
|
(2,955 |
) |
|
|
(2,304 |
) * |
|
|
(10,986 |
) |
Net loss per common share
- basic and diluted |
|
$ |
(0.09 |
) |
|
$ |
(0.09 |
) |
|
$ |
(0.06 |
) |
|
$ |
(0.15 |
) |
|
$ |
(0.40 |
) |
2007: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant and research revenues |
|
$ |
233 |
|
|
$ |
168 |
|
|
$ |
599 |
|
|
$ |
126 |
|
|
$ |
1,126 |
|
Operating expenses |
|
|
2,351 |
|
|
|
3,595 |
|
|
|
3,686 |
|
|
|
4,169 |
** |
|
|
13,801 |
|
Net loss |
|
|
(1,949 |
) |
|
|
(3,284 |
) |
|
|
(2,973 |
) |
|
|
(3,958 |
) ** |
|
|
(12,164 |
) |
Net loss per common share
- basic and diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.24 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.14 |
) |
|
$ |
(0.61 |
) |
|
|
|
| * |
|
includes adjustment of $126,000, which resulted from our reduced estimated bonus accrual |
| |
| ** |
|
includes adjustment of $439,000, which resulted from our reduced estimated bonus accrual |
F-28
PART II — INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than underwriting discounts and
commissions, if any, payable by the Company relating to the sale of common stock being registered.
All amounts are estimates except the SEC registration fee:
| |
|
|
|
|
SEC Registration Fee |
|
$ |
1,116 |
|
Placement Agent Fees and Expenses |
|
$ |
1,400,000 |
|
Printing and Engraving Expenses |
|
$ |
5,000 |
|
Legal Fees and Expenses |
|
$ |
250,000 |
|
Accounting Fees and Expenses |
|
$ |
30,000 |
|
Transfer Agent and Registrar’ s Fees and Expenses |
|
$ |
10,000 |
|
Miscellaneous Expenses |
|
$ |
3,884 |
|
|
|
|
|
Total |
|
$ |
1,700,000 |
|
Item 14. Indemnification of Directors and Officers.
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to
eliminate the personal liability of directors of a corporation to the corporation or its
stockholders for monetary damages for a breach of fiduciary duty as a director, except where the
director breached his duty of loyalty, failed to act in good faith, engaged in intentional
misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock
repurchase in violation of Delaware corporate law or obtained an improper personal benefit. The
Company’s Amended and Restated Certificate of Incorporation, as amended, provides that a director
of the Company shall not be personally liable to it or its stockholders for monetary damages for
any breach of fiduciary duty as a director, except to the extent that such exemption from liability
or limitation thereof is not permitted under the General Corporation Law of the State of Delaware
as currently in effect or as the same may hereafter be amended.
Section 145 of the General Corporation Law of the State of Delaware provides that a
corporation has the power to indemnify a director, officer, employee, or agent of the corporation
and other persons serving at the request of the corporation in related capacities against expenses
(including attorneys’ fees), judgments, fines and amounts paid in settlements actually and
reasonably incurred by the person in connection with an action, suit or proceeding to which he is
or is threatened to be made a party by reason of such position, if such person acted in good faith
and in a manner he reasonably believed to be in or not opposed to the best interests of the
corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his
conduct was unlawful, except that, in the case of actions brought by or in the right of the
corporation, no indemnification shall be made with respect to any claim, issue or matter as to
which such person shall have been adjudged to be liable to the corporation unless and only to the
extent that the Court of Chancery or other adjudicating court determines that, despite the
adjudication of liability but in view of all of the circumstances of the case, such person is
fairly and reasonably entitled to indemnify for such expenses which the Court of Chancery or such
other court shall deem proper.
The Company’s Amended and Restated Certificate of Incorporation provides that the Company will
indemnify and advance expenses upon request to any person who is or was a party or threatened to be
made a party to any threatened, pending or completed action, suit, proceeding or claim, whether
civil, criminal, administrative or investigative, by reason of the fact that he or she is or was or
has agreed to become, a director, officer or employee of the Company or, at the election of the
Company’s board of directors, an agent of the Company or is or was serving at the request of the
Company as a director, officer or employee or, at the election of the Company’s board of directors,
agent of any other corporation, partnership, joint venture, trust or other enterprise against any
and all expenses (including attorneys’ fees), judgments, fines, penalties and amounts paid in
settlement or incurred in connection with the investigation, preparation to defend or defense or
such action, suit, proceeding or claim.
The Company has purchased certain liability insurance for its officers and directors.
II-1
Item 15. Recent Sales of Unregistered Securities.
The following table provides information about the sales of unregistered securities for the
past three years.
| |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Total Offering |
|
|
|
Exemption from |
| Date of Sale |
|
Title of Security |
|
Amount |
|
Price |
|
Purchasers |
|
Registration Claimed |
Sept. 2008
|
|
units consisting of
Series 2008
Convertible
Preferred Stock and
Series B Warrants
to Purchase Capital
Stock
|
|
608,834 units
|
|
$ |
4,261,838 |
|
|
Accredited
investors (8)
|
|
Section 4(2) of the
Securities Act
of 1933 and Rule 506
promulgated
thereunder |
Sept. 2008
|
|
Series B Warrants
to Purchase Capital
Stock
|
|
279,583 shares
|
|
|
— |
(1) |
|
Accredited
investor (8)
|
|
Section 4(2) of the
Securities Act
of 1933 and Rule 506
promulgated
thereunder |
| |
Jan. 2008
|
|
options to purchase
common stock
|
|
220,000 shares
|
|
|
— |
(2) |
|
Directors,
officers, employees
and
consultants
|
|
Section 4(2) of the
Securities Act
of 1933 |
| |
Oct. 2007
|
|
common stock
|
|
12,500 shares
|
|
|
— |
(3) |
|
Accredited
investors (8)
|
|
Section 4(2) of the
Securities Act
of 1933 and Rule 506
promulgated
thereunder |
| |
Dec. 2006
|
|
Series 1 Preferred
Convertible Stock
|
|
462,294 shares
|
|
|
— |
(4) |
|
Accredited
investors (8)
|
|
Section 4(2) of the
Securities Act
of 1933 and Rule 506
promulgated
thereunder |
| |
Nov. 2005 – Dec
2007
|
|
warrant to purchase
common stock
|
|
4,119,194 shares
|
|
|
— |
(5) |
|
Accredited
investor (8)
|
|
Section 4(2) of the
Securities Act
of 1933 and Rule 506
promulgated
thereunder |
Aug. 2005 – Dec.
2006
|
|
common stock and
options to purchase
common stock
|
|
4,305,667 shares
|
|
|
— |
(6) |
|
Directors,
officers,
employees and
consultants
|
|
Rule 701 promulgated
under the
Securities Act of
1933 |
| |
Jan. 2005 – Dec.
2006
|
|
common stock
|
|
596,000 shares
|
|
|
— |
(7) |
|
Directors,
officers,
employees and
consultants
|
|
Section 4(2) of the
Securities Act
of 1933 and Rule 506
promulgated
thereunder |
|
|
|
| (1) |
|
Issued to Emerging Growth Equities Ltd. and Carter Securities, LLC as partial consideration
for placement agent services rendered in connection with the 2008 Private Placement. |
II-2
|
|
|
| (2) |
|
Represents options to purchase common stock issued to our directors, officers, employees and
consultants. We received no cash consideration for the incentive grants. Options issued have
a range of exercise prices between $1.10 and $3.50 per share and expire ten years from their
grant date. |
| |
| (3) |
|
Issued to the University of Massachusetts in exchange for the exclusive right and license to
certain intellectual property owned by the University of Massachusetts. |
| |
| (4) |
|
Represents unregistered securities issued as dividends on Series 1 Preferred Stock. |
| |
| (5) |
|
Issued to Fordham Financial Management, Inc., as partial consideration for placement agent
services rendered in connection with our private placement of Series 1 Preferred Stock,
warrants to purchase 4,119,194 shares of common stock at an exercise price of $1.23 per share,
as adjusted during the for weighted average anti-dilution protection. These warrants expire
on November 8, 2010. |
| |
| (6) |
|
Issued pursuant to the PolyMedix, Inc. 2002 Equity Compensation Plan and the PolyMedix, Inc.
2005 Omnibus Equity Compensation Plan as an incentive to our directors, officers, employees
and consultants. We received no cash consideration for the incentive grants. Options issued
have exercise prices of between $1.50 and $4.10 per share and expire ten years from their
respective grant date. |
| |
| (7) |
|
Represents common and restricted common stock incentive grants to our directors, officers,
employees and consultants. We received no cash consideration for the incentive grants. |
| |
| (8) |
|
As defined in Rule 501 of Regulation D promulgated under the Securities Act. |
II-3
Item 16. Exhibits.
| |
|
|
Exhibit
No. |
|
Description of Exhibit |
| |
1.1*
|
|
Form of Placement Agency Agreement
to be entered into among the Registrant and the placement agents. |
|
|
|
| |
2.1
|
|
Agreement and Plan of Merger and Reorganization dated October 6, 2005, among the Registrant,
PolyMedix Merger Sub, Inc., PolyMedix Pharmaceuticals, Inc. and those stockholders of Registrant
identified on Exhibit A thereto.(1) |
|
|
|
3.1
|
|
Amended and Restated Certificate of Incorporation of Registrant.(1) |
|
|
|
3.2
|
|
Certificate of Designations of Registrant’s Series 1 Convertible Preferred Stock.(1) |
|
|
|
3.3
|
|
Amendment to the Amended and Restated Certificate of Incorporation of Registrant.(1) |
|
|
|
3.4
|
|
Amended and Restated Bylaws of Registrant.(1) |
|
|
|
3.5
|
|
Amendment to Amended and Restated Bylaws of Registrant.(1) |
|
|
|
3.6
|
|
Amendment to the Amended and Restated Certificate of Incorporation of Registrant, as
amended.(2) |
|
|
|
3.7
|
|
Amendment to the Designations of Registrant’s Series 1 Convertible Preferred Stock.(2) |
|
|
|
3.8
|
|
Certificate of Designations of Registrant’s Series 2008 Convertible Preferred Stock. (3) |
|
|
|
3.9
|
|
Certificate of Amendment to Amended and Restated Certificate of Incorporation of Registrant, as
amended. (4) |
|
|
|
3.10
|
|
Certificate of Designation of Registrant’s Series C Preferred Stock.(5) |
|
|
|
4.1
|
|
Form of common stock Purchase Warrant issued to Fordham Financial Management, Inc. on November 8,
2005, December 8, 2005, January 10, 2006 and February 15, 2006.(1) |
|
|
|
4.2
|
|
Form of common stock Purchase Warrant. (6) |
|
|
|
4.3
|
|
Form of Registration Rights Substitution Agreement.(7) |
|
|
|
4.4
|
|
Form of Amendment No. 1 to Registration Rights Substitution Agreement.(8) |
|
|
|
4.5
|
|
Form of Amendment No. 2 to Registration Rights Substitution Agreement.(9) |
|
|
|
4.6
|
|
Form of Series A Warrant Agreement (including Series A Warrant certificate).(10) |
|
|
|
4.7
|
|
Form of Placement Agent Warrant.(11) |
|
|
|
4.8
|
|
Form of Securities Purchase Agreement as executed by the Registrant and the Investors.
(3) |
|
|
|
4.9
|
|
Form of Series B Warrant to Purchase Capital Stock. (3) |
|
|
|
4.10
|
|
Rights Agreement, dated May 12, 2009, between the Registrant and American Stock Transfer & Trust
Company, LLC, as Rights Agent.(5) |
|
|
|
| |
4.11*
|
|
Form of Purchase Agreement between the Registrant and each investor signatory thereto. |
| |
|
|
|
| |
4.12*
|
|
Form of Series C Warrant to
Purchase Common Stock. |
| |
|
|
|
| |
4.13*
|
|
Form of Placement Agent Warrant. |
| |
| |
5.1*
|
|
Legal Opinion of Pepper Hamilton LLP |
|
|
|
10.1
|
|
Patent License Agreement, dated January 3, 2003, between the Registrant and the University of
Pennsylvania.(1) |
|
|
|
10.2
|
|
Letter Agreement, dated December 23, 2003, amending the Patent License Agreement, dated January 3,
2003, between the Registrant and the University of Pennsylvania.(1) |
|
|
|
10.3
|
|
Software License Agreement, dated May 30, 2003, between the Registrant and the University of
Pennsylvania.(1) |
|
|
|
10.4
|
|
Exclusive License Agreement, dated January 2, 2005, between the Registrant and the University of
Massachusetts.(1) |
|
|
|
10.5**
|
|
Employment Agreement, dated July 30, 2002, between Nicholas Landekic and the
Registrant.(1) |
|
|
|
10.6**
|
|
Employment Agreement, dated December 5, 2005, between Edward Smith and the
Registrant.(1) |
|
|
|
10.7**
|
|
Employment Agreement, dated May 21, 2003, between Dawn P. Eringis and the Registrant.(1) |
|
|
|
10.8**
|
|
Employment Agreement, dated March 28, 2003, between Richard Scott, Ph.D. and the
Registrant.(1) |
|
|
|
10.9
|
|
Letter Agreement, dated February 25, 2004, between Dr. William DeGrado and the
Registrant.(1) |
|
|
|
10.10
|
|
Lab/Office Space License Agreement for 3701 Market Street, dated February 22, 2006, between the
Registrant and the University City Science Center.(1) |
|
|
|
10.11
|
|
Pennsylvania Full-Service Lease Agreement for 170 N. Radnor-Chester Road; |
II-4
| |
|
|
Exhibit
No. |
|
Description of Exhibit |
|
|
Suite 300, Radnor, PA
19087, dated May 26, 2006, between the Registrant and the Radnor Properties— SDC,
L.P.(11) |
|
|
|
10.12
|
|
Financial Consulting Agreement, dated November 8, 2005, between the Registrant and Fordham
Financial Management, Inc.(1) |
|
|
|
10.13**
|
|
Amended and Restated 2005 Omnibus Equity Compensation Plan of the Registrant.(12) |
|
|
|
10.14**
|
|
2002 Equity Compensation Plan of the Registrant.(13) |
|
|
|
10.15**
|
|
Form of Incentive Stock Option Agreement.(1) |
|
|
|
10.16**
|
|
Form of Nonqualified Stock Option Agreement.(1) |
|
|
|
10.17
|
|
Sponsored Research Agreement, dated January 5, 2004, between the Registrant and the University of
Massachusetts.(14) |
|
|
|
10.18
|
|
Merrill Lynch Loan Management Account Agreement, dated April 13, 2006, between the Registrant and
Merrill Lynch Bank USA.(11) |
|
|
|
10.19**
|
|
Employment Agreement, dated October 19, 2006, between R. Eric McAllister and the
Registrant.(14) |
|
|
|
10.20**
|
|
Offer Letter to Bozena Korczak, Ph.D.(15) |
|
|
|
10.21
|
|
Form of Amended and Restated Co-Placement Agent Agreement by and among the Registrant, Carter
Securities, LLC and Fordham Financial Management, Inc. (9) |
|
|
|
10.22**
|
|
Offer Letter to J. Gregory Ford.(16) |
|
|
|
10.23
|
|
Investment Agreement, dated May 20, 2009, between the Registrant and Dutchess Equity Fund,
LP.(17) |
|
|
|
10.24
|
|
Amendment No. 1 to Investment Agreement dated July 8, 2009, between the Registrant and Dutchess
Equity Fund, LP.(18) |
|
|
|
10.25
|
|
Registration Rights Agreement, dated May 20, 2009, between the Registrant and Duchess Equity Fund,
LP.(17) |
|
|
|
|
|
|
| |
| |
21
|
|
List of Subsidiaries.(14) |
| |
| |
|
|
|
23.1*
|
|
Consent of Deloitte & Touche LLP |
|
|
|
23.2*
|
|
Consent of Pepper Hamilton, LLP.(19) |
|
|
|
24***
|
|
Power of Attorney.(20) |
|
|
|
| * |
|
Filed herewith. |
| |
| ** |
|
Management contract or compensatory arrangement. |
| |
| *** |
|
Previously filed. |
| |
| (1) |
|
Filed as an Exhibit to the Registration Statement on Form 10-SB (File No. 000-51895) filed on
April 5, 2006 and incorporated herein by reference. |
| |
| (2) |
|
Filed as an Exhibit to the Current Report on Form 8-K filed on June 5, 2007 and incorporated
herein by reference. |
| |
| (3) |
|
Filed as an Exhibit to the Current Report on Form 8-K filed on September 24, 2008 and
incorporated herein by reference. |
| |
| (4) |
|
Filed as an Exhibit to the Current Report on Form 8-K filed on December 12, 2008 and
incorporated herein by reference. |
| |
| (5) |
|
Filed as an Exhibit to the Current Report on From 8-K filed on May 14, 2009 and incorporated
herein by reference. |
| |
| (6) |
|
Filed as an Exhibit to Amendment No. 5 to the Registration Statement on Form SB-2 (File No.
333-146180) on November 30, 2007 and incorporated herein by reference. |
| |
| (7) |
|
Filed as an Exhibit to the Registration Statement on Form SB-2 (File No. 333-146180) on
September 19, 2007 and incorporated herein by reference. |
| |
| (8) |
|
Filed as an Exhibit to Amendment No. 1 to the Registration Statement on Form SB-2 (File No.
333-146180) on October 4, 2007 and incorporated herein by reference. |
| |
| (9) |
|
Filed as an Exhibit to Amendment No. 2 to the Registration Statement on Form SB-2 (File No.
333-146180) on October 16, 2007 and incorporated herein by reference. |
| |
| (10) |
|
Filed as an Exhibit to Amendment No. 1 to the Registration Statement on Form S-1 (File No.
333-151084) and incorporated herein by reference. |
II-5
|
|
|
| (11) |
|
Filed as an Exhibit to Amendment No. 2 to the Registration Statement on Form 10-SB/A (File No.
000-51895) filed on June 19, 2006 and incorporated herein by reference. |
| |
| (12) |
|
Filed as Appendix A to the Registrant’s Definitive Proxy Statement filed on April 10, 2008 and
incorporated herein by reference. |
| |
| (13) |
|
Filed as an Exhibit to the Registration Statement on Form S-8 (File No. 333-139686) and
incorporated herein by reference. |
| |
| (14) |
|
Filed as an Exhibit to the Annual Report on Form 10-KSB filed on March 19, 2007 and
incorporated herein by reference. |
| |
| (15) |
|
Filed as an Exhibit to the Current Report on Form 8-K filed on November 16, 2007 and
incorporated herein by reference. |
| |
| (16) |
|
Filed as an Exhibit to the Current Report on Form 8-K filed on December 4, 2008 and
incorporated herein by reference. |
| |
| (17) |
|
Filed as an Exhibit to the Current Report on Form 8-K filed on May 22, 2009 and incorporated
herein by reference. |
| |
| (18) |
|
Filed as an Exhibit to the Registration Statement on Form S-1 (File No. 333-160470) and
incorporated herein by reference. |
| |
| (19) |
|
Included with Exhibit 5.1. |
| |
| (20) |
|
Included on signature page hereto. |
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
| |
(1) |
|
To file, during any period in which offers or sales are being made, a
post-effective amendment to this registration statement: |
| |
(i) |
|
To include any prospectus required by Section 10(a)(3) of the
Securities Act of 1933, as amended; |
| |
| |
(ii) |
|
To reflect in the prospectus any facts or events arising
after the effective date of this registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate,
represent a fundamental change in the information set forth in the
registration statement. Notwithstanding the foregoing, any increase or
decrease in volume of securities offered (if the total dollar value of
securities offered would not exceed that which was registered) and any
deviation from the low or high end of the estimated maximum offering range may
be reflected in the form of prospectus filed with the SEC pursuant to Rule
424(b) if, in the aggregate, the changes in volume and price represent no more
than a 20 percent change in the maximum aggregate offering price set forth in
the “Calculation of Registration Fee” table in the effective registration
statement; |
| |
| |
(iii) |
|
To include any material information with respect to the plan
of distribution not previously disclosed in the registration statement or any
material change to such information in the registration statement; |
| |
(2) |
|
That, for the purpose of determining any liability under the Securities Act
of 1933, each such post-effective amendment shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such
securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-6
| |
(3) |
|
To remove from registration by means of a post-effective amendment any of the
securities being registered which remain unsold at the termination of the offering. |
| |
(4) |
|
That, for the purpose of determining liability under the Securities Act of
1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a
registration statement relating to an offering, other than registration statements
relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall
be deemed to be part of and included in the registration statement as of the date it
is first used after effectiveness. Provided, however, that no statement made in a
registration statement or prospectus that is part of the registration statement or
made in a document incorporated or deemed incorporated by reference into the
registration statement or prospectus that is part of the registration statement will,
as to a purchaser with a time of contract of sale prior to such first use, supersede
or modify any statement that was made in the registration statement or prospectus that
was part of the registration statement or made in any such document immediately prior
to such date of first use. |
| (b) |
|
Insofar as indemnification for liabilities arising under the Securities Act of 1933, may be
permitted to directors, officers and controlling persons of the registrant pursuant to the
foregoing provisions, or otherwise, the registrant has been advised that, in the opinion of
the SEC, such indemnification is against public policy as expressed in the Securities Act and
is, therefore, unenforceable. In the event that a claim for indemnification against such
liabilities (other than the payment by the registrant of expenses incurred or paid by a
director, officer or controlling person of the registrant in the successful defense of any
action, suit or proceeding) is asserted by such director, officer or controlling person in
connection with the securities being registered, the registrant will, unless in the opinion of
its counsel the matter has been settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such indemnification by them is against public
policy as expressed in the Securities Act and will be governed by the final adjudication of
such issue. |
| (c) |
|
The undersigned registrant hereby undertakes that: |
| |
(1) |
|
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| |
(2) |
|
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-7
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused
this registration statement to be signed on its behalf by the undersigned, in Radnor, Pennsylvania
on November 6, 2009.
| |
|
|
|
|
|
|
| |
|
POLYMEDIX, INC. |
|
|
|
|
|
|
|
|
|
|
|
By:
Name:
|
|
/s/ Nicholas Landekic
Nicholas Landekic
|
|
|
|
|
Title:
|
|
President & Chief Executive Officer |
|
|
|
|
|
|
(principal executive officer) |
|
|
Pursuant to the requirements of the Securities Act of 1933, this registration statement has
been signed by the following persons in the capacities and on the dates indicated.
| |
|
|
|
|
| Name/Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ Nicholas Landekic
Nicholas Landekic
|
|
President, Chief Executive Officer
and Director (principal executive
officer)
|
|
November 6, 2009 |
|
|
|
|
|
/s/ Edward F. Smith
Edward F. Smith
|
|
Chief Financial Officer (principal
financial officer and principal
accounting officer)
|
|
November 6, 2009 |
|
|
|
|
|
|
|
Chairman of the Board of Directors
|
|
November 6, 2009 |
|
|
|
|
|
|
|
Director
|
|
November 6, 2009 |
|
|
|
|
|
|
|
Director
|
|
November 6, 2009 |
|
|
|
|
|
|
|
Director
|
|
November 6, 2009 |
|
|
|
|
|
*
Michael E. Lewis, Ph.D.
|
|
Director
|
|
November 6, 2009 |
|
|
|
|
|
|
|
Director
|
|
November 6, 2009 |
|
|
|
|
|
|
|
Director
|
|
November 6, 2009 |
* By:
Edward F. Smith
Attorney-in-fact
II-8