Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HARROW HEALTH, INC. | a54103e8vk.htm |
Exhibit 99.1

| Transdel Pharmaceuticals, Inc. Symbol: TDLP.OB October 2009 |

| 2 Safe Harbor Statement The Company cautions you that the statements included in this presentation that are not a description of historical facts are forward-looking statements. These include statements regarding: the Company's interpretation of the results of its Phase 3 clinical trial for Ketotransdel(r); whether the results from the clinical trial, along with any other clinical trials that may be required by the FDA, will be sufficient to support a 505(b)2 New Drug Approval (NDA) submission; the potential indications for use for Ketotransdel(r); the market opportunity for the Company's products; and the Company's ability to complete additional development activities for products utilizing its proprietary transdermal delivery platform. Actual results may differ materially from those set forth in this presentation due to the risks and uncertainties inherent in the Company's business, including, without limitation: the outcome of the final analyses of the data from the Phase 3 clinical trial may vary from the Company's initial conclusions; the FDA may not agree with the Company's interpretation of such results or may challenge the adequacy of the Company's clinical trial design or the execution of the clinical trial; the FDA may continue to require the Company to complete additional clinical trials for Ketotransdel(r) before the Company can submit a 505(b)2 NDA application; the results of any future clinical trials may not be favorable and the Company may never receive regulatory approval for Ketotransdel(r); technological changes or competitive products or pricing may prevent the Company from successfully commercializing its products; and the Company's current need to raise additional funding to complete its product development plans. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q filed with the SEC. Such documents may be read free of charge on the SEC's web site at www.sec.gov. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and the Company undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. |

| 3 Company Overview Specialty pharmaceutical company Focused on the development and commercialization of topically delivered medications Products based on proprietary transdermal delivery technology Lead pain program, Ketotransdel(r), has successfully completed a Phase 3 trial demonstrating significant efficacy and safety Expanding co-development opportunities for other programs |

| 4 Transdel Investment Highlights Positive Phase 3 results for lead pain drug, Ketotransdel Reported statistically significant efficacy results and excellent safety in Phase 3 trial Minimal blood concentrations of ketoprofen in pharmacokinetic studies Large market opportunity for Ketotransdel U.S. market for NSAIDs and Cox-2 inhibitors is in excess of $6 billion per year Partnership/collaboration opportunity Ketotransdel(r) positive data expected to increase partnering interest Discussions underway on co-development opportunities for other programs Novel transdermal delivery platform applicable to a broad range of drugs Proprietary technology provides opportunity to generate additional pipeline candidates using existing or novel drugs Opportunity to leverage technology for cosmeceutical applications (e.g. JH Direct partnership for anti-cellulite product) Highly qualified team with significant industry experience |

| Transdel Transdermal Delivery System (TDS) |

| 6 6 TDS is a nanoparticulate matrix topical cream Transdel's proprietary cream formulation enables active drug to penetrate the skin and reach targeted underlying tissue Components generally regarded as safe (GRAS) Delivery system uses synergistic mechanisms to enhance penetration Thermodynamic stable properties allow solubilization of drug and then promote release of drug Epidermis Dermis Subcut. Tissue Muscle Transdel's Transdermal Delivery System (TDS) |

| 7 TDS platform provides significant benefits Compatible with broad range of drugs and molecular sizes Maximizes solubilization of drugs (lipophilic, hydrophilic and amphiphilic) Broad patented technology platform with issued IP covering composition of matter, methods of use and methods of manufacture Dermatological Antifungals Antibacterials Rosacea Psoriasis Acne Actinic Keratosis Pain Ketotransdel - acute pain & OA Gabapentin Cyclobenzaprine Lidocaine Ketamine Hormones Testosterone Estriol Estradiol Progesterone Insulin Anti-nausea Scopolamine Cosmeceuticals Cellulite Wrinkles/fine lines Varicose veins Hyperpigmentation Nutraceuticals Glucosamine Broad Range Of Opportunities for TDS *Over 500 different drugs are specifically listed in the issued US patent in more than 60 therapeutic areas Transdermal Delivery System (TDS) |

| Ketotransdel(r) |

| Ketotransdel: Product Profile 9 Ketotransdel is comprised of a transdermal cream formulation of ketoprofen, and our proprietary patented Transdel drug delivery system Ketoprofen is a non-steroidal anti-inflammatory drug (NSAID) that has been found to be among the most efficacious topical NSAIDs Delivers 100mg of ketoprofen per 1g of cream Same dosage as oral ketoprofen when used in recommended dosage of 3x per day Completed Phase 1/2 and Phase 3 trials demonstrated: Statistically significant efficacy Excellent safety and tolerability Minimal blood concentrations |
| 10 Pain treatments are the third most-prescribed class of drugs in the U.S. According to market research firm BCC Research the global market for pain relievers was worth $19.1 billion in 2008, and is expected to grow to $32.8 billion by 2013 Market for NSAIDs and Cox-2 inhibitors exceeds $6 billion in the U.S. per year Oral NSAIDs are associated with serious gastro-intestinal, cardiac, renal and liver side effects Withdrawal of Bextra and Vioxx leaves multi-billion dollar market largely replaced by oral NSAIDs Everyday OTC painkillers such as aspirin, acetaminophen (Tylenol) and Advil may result in safety concerns and may not be potent or efficacious for acute musculoskeletal pain of moderate intensity Physicians and patients are continuing to seek alternative options for effective and safe pain medications Ketotransdel, if approved by the FDA, could become the first topical NSAID cream product in the U.S. for acute pain management The U.S. transdermal drug delivery market is projected to increase to $4.5 billion in 2012* *Source: U.S. Transdermal Drug Delivery Markets, Frost and Sullivan, August 2006. Ketotransdel Market Opportunity |

| 11 The withdrawal of Vioxx and Bextra has resulted in a significant decline in COX-2 prescriptions and a significant increase in NSAID prescriptions After an increase in 2005 when the COX-2's were withdrawn, NSAID TRx's have continued to post steady year-over-year growth Since the introduction of Flector Patch and Voltaren Gel in late 2007 / early 2008, prescriptions for these two topical medications have increased substantially In 2009, Voltaren Gel prescriptions have surpassed Flector Patch prescriptions Note: Graphs denote total prescriptions, per IMS data. 2009YTD figures include monthly IMS TRx through September 2009. Total Rx COX2s Vs. NSAIDs Topical NSAID (Diclofenac) TRx Pain Therapeutic Market Dynamics |

| Clinical Design: Randomized, double-blind, placebo controlled Study Population: 364 patients Indication: Acute soft tissue injuries (sprains & strains) Dosing Regimen: Ketoprofen vs. Placebo (Vehicle) cream, 1g three times daily over 7 days Primary Endpoint: Change from baseline in pain intensity on Day 3 (VAS) Secondary Endpoints: Safety assessments, various other efficacy variables Pharmacokinetics in subset of patients Clinical Sites: 26 (USA) 12 Ketotransdel Phase 3 Trial |

| Ketotransdel demonstrated statistically significant higher reduction in pain intensity from baseline in the Per Protocol (PP) analysis and a favorable treatment effect in the Intent-To-Treat (ITT) analysis * p< 0.05 PP (n=252) ITT (n=361) PP population included: Pre-specified minimum use of study medication; Valid Day 3 Pain Assessments; No unallowed drug use. Mean reduction from baseline in mm (100 mm Visual Analogue Scale) mm 13 Change from Baseline in Pain Intensity on Day 3 Primary Efficacy Results |

| Ketotransdel (n= 182) Placebo (n=182) All potentially related Adverse Events (AEs): 15 (4.1%) 7 (3.8%) 8 (4.4%) 14 Ketotransdel demonstrated excellent safety and tolerability Low overall incidence of AEs No related gastrointestinal, cardiac, liver, kidney or other serious AEs Few potentially related adverse events (mainly cutaneous) No clinically relevant changes in blood and urine tests Minimal systemic absorption (mean Cmax 39 ng/mL) consistent with previous findings (approximately 1 - 2 % of oral ketoprofen dose) Safety and Pharmacokinetics |
| Ketotransdel achieved statistical significance in its Primary Efficacy Endpoint in the Per Protocol population (p < 0.05) Ketotransdel demonstrated excellent safety and tolerability No treatment related gastrointestinal, cardiac, liver, kidney or other serious adverse events No clinically relevant changes in blood and urine tests Minimal blood concentrations of ketoprofen in pharmacokinetic study, consistent with previous findings Estimated systemic absorption about 1 - 2 % of a comparable oral ketoprofen dose, consistent with previous findings 15 Conclusions - Ketotransdel Phase 3 |

| 16 Healthy patients with localized pain History of gastrointestinal symptoms / gastrointestinal ulceration History of heart, liver, kidney disease or other serious medical conditions Elderly and Children Intake of multiple drugs for other conditions (minimize drug-to-drug interactions) Corticosteroid use with oral NSAIDs increases adverse risks 15-times Anti-coagulants with oral NSAIDs increase mortality by 12- times Cannot take drugs orally / have swallowing difficulties Patients Who May Benefit From a Topical NSAID |

| 17 Provides alternative to address the significant safety concerns associated with oral COX-2 inhibitors / nonselective oral NSAIDs and acetaminophen Broad technology platform that is patent protected Less invasive delivery Faster - delivers drugs in few minutes with no residue Large and growing market opportunity Void in pain management market due to withdrawal of Vioxx and Bextra Excellent safety profile Potential for multiple indications Rx path with the FDA Ketotransdel Competitive Advantages Ketotransdel(r) could address significant unmet medical need for pain management |

| 18 Finalize registration strategy Continue partnering discussions Discussions underway with several potential sales and marketing partners Ketotransdel Next Steps |
| 19 Lead product - anti-cellulite formulation In June 2009, the Company announced that it entered into a license agreement with JH Direct, LLC for the exclusive worldwide rights to Transdel's anti- cellulite cosmeceutical product which utilizes the Company's Transdel technology Anticipated expansion of cosmetic/cosmeceuticals pipeline to include the following formulations: Anti-aging Hyperpigmentation Varicose veins Pursuing discussions with potential sales and marketing partners for these cosmetic/cosmeceutical products Cosmeceutical Applications of TDS |

| 20 Transdel Management Team Juliet Singh, Ph.D., Chief Executive Officer/President Over 20 years of pharmaceutical management experience: Allergan; Baxter Healthcare; and, Collateral Therapeutics Corporate Officer responsible for Regulatory Affairs & Quality Assurance Managed worldwide regulatory submissions of BOTOX(r) and recombinant factor VIII John Lomoro, Chief Financial Officer Over 15 years of financial experience with public and private organizations, including 5 years with Ernst & Young LLP Director, North America Accounting, Carl Zeiss Vision Inc. Certified Public Accountant Joachim P. H. Schupp, M.D. , Chief Medical Officer Over 24 years of leadership experience in strategic design and execution of international clinical development projects, cross-functional project management and post-marketing surveillance in the pharmaceutical industry Senior management positions at Ciba-Geigy, Novartis, ProSanos Inc., Adventrx Pharmaceuticals Significantly contributed to the successful development , registration and launch of new and life cycle products (Voltaren, Cataflam, Apligraf, Sandoglobulin, Femara, Exjade) |
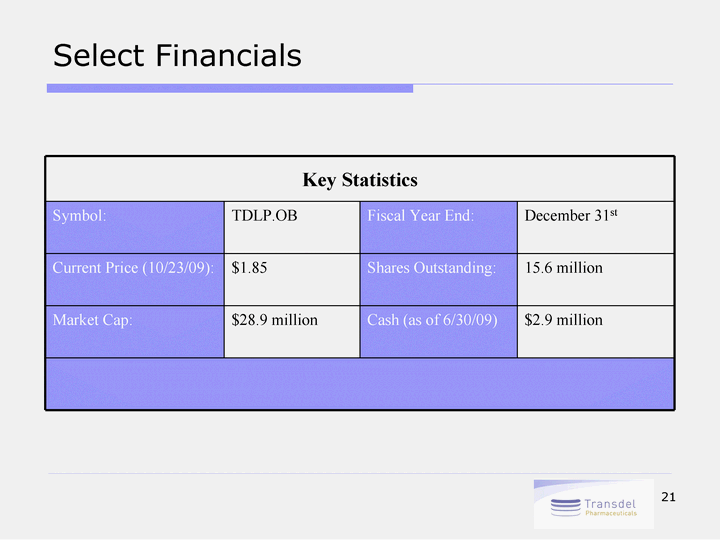
| 21 Select Financials Key Statistics Key Statistics Key Statistics Key Statistics Symbol: TDLP.OB Fiscal Year End: December 31st Current Price (10/23/09): $1.85 Shares Outstanding: 15.6 million Market Cap: $28.9 million Cash (as of 6/30/09) $2.9 million |

| 22 Transdel Investment Highlights Positive Phase 3 results for lead pain drug, Ketotransdel Reported statistically significant efficacy results and excellent safety in Phase 3 trial Minimal blood concentrations of ketoprofen in pharmacokinetic studies Large market opportunity for Ketotransdel U.S. market for NSAIDs and Cox-2 inhibitors is in excess of $6 billion per year Ketotransdel(r) may have advantages over recently launched topical pain medications Partnership/collaboration opportunity Ketotransdel(r) positive data expected to increase partnering interest Discussions underway on co-development opportunities for other programs Novel transdermal delivery platform applicable to a broad range of drugs Proprietary technology provides opportunity to generate additional pipeline candidates using existing or novel drugs Opportunity to leverage technology for cosmeceutical applications (e.g. JH Direct partnership for anti-cellulite product) Highly qualified team with significant industry experience |

| 23 Contact Us Transdel Pharmaceuticals, Inc. (OTC BB: TDLP.OB) 4225 Executive Square Suite 485 La Jolla, CA 92037 Corporate Headquarters Investor Contact John Lomoro Chief Financial Officer (858) 457-5300 johnl@transdelpharma.com Please visit www.transdelpharma.com for more information. |
