Attached files
| file | filename |
|---|---|
| EX-31.2 - EFT Holdings, Inc. | v163076_ex31-2.htm |
| EX-32.2 - EFT Holdings, Inc. | v163076_ex32-2.htm |
| EX-31.1 - EFT Holdings, Inc. | v163076_ex31-1.htm |
| EX-32.1 - EFT Holdings, Inc. | v163076_ex32-1.htm |
UNITED
STATES SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K/A
(Amendment
No. 2)
(Mark
One)
|
x
|
ANNUAL
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
fiscal year ended: March 31, 2009
|
¨
|
TRANSITION
REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the
transition period from ________ to ________
EFT
BIOTECH HOLDINGS, INC.
(Exact
name of registrant as specified in its charter)
Commission
File No. 001-34222
|
Nevada
(State
or other Jurisdiction of
Incorporation
or Organization)
|
20-1211204
(I.R.S.
Employer
Identification
No.)
|
|
929 Radecki
Court
City
of Industry, CA
|
91748
|
|
(Address
of Principal Executive Offices)
|
(Zip
Code)
|
Registrant's Telephone
Number: (626) 581 -
0388
With
Copies to:
Virginia
K Sourlis, Esq.
The
Sourlis Law Firm
2 Bridge
Avenue
The
Galleria
Red Bank,
New Jersey 07701
Telephone:
(732) 530-9007
Securities
registered pursuant to - Section 12(b) of the Act:
|
Title
of each class
|
Name
of each exchange on which
registered
|
|
|
N/A
|
N/A
|
Securities
registered pursuant to Section 12 (g) of the Act:
Common
Stock, par value $0.00001 per share
(Title of
Class)
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act.
¨ Yes x No
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act.
¨ Yes x No
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days.
x Yes ¨ No
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
¨ Yes ¨ No
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not
be contained, to the best of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K.
x Yes ¨ No
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
the definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act.
|
Large
accelerated filer
|
¨
|
Accelerated
filer
|
¨
|
||
|
Non-accelerated
filer
|
¨
|
Smaller
reporting company
|
x
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Act).
¨ Yes x No
State the
aggregate market value of the voting and non-voting common equity held by
non-affiliates computed by reference to the price at which the common equity was
last sold, or the average bid and asked price of such common equity, as of the
last business day of the registrant’s most recently completed first fiscal
quarter. $71,187,018 based on the last sales price of the
Registrant’s common stock on June 30, 2009 of $3.75.
(APPLICABLE
ONLY TO CORPORATE REGISTRANTS)
Indicate
the number of shares outstanding of each of the registrant’s classes of common
stock, as of the latest practicable date.
As of
October 16, 2009, there are 75,983,205 shares of common stock issued and
outstanding.
DOCUMENTS
INCORPORATED BY REFERENCE:
None
|
Item:
|
Page No.:
|
||
|
PART
I
|
|||
|
Item
1.
|
Business.
|
3
|
|
|
Item
1A.
|
Risk
Factors.
|
13
|
|
|
Item
1B.
|
Unresolved
Staff Comments.
|
19
|
|
|
Item
2.
|
Properties.
|
19
|
|
|
Item
3.
|
Legal
Proceedings.
|
20
|
|
|
Item
4.
|
Submission
of Matters to a Vote of Security Holders.
|
20
|
|
|
PART
II
|
|||
|
Item
5.
|
Market
for Registrant’s Common Equity, Related Stockholders
Matters
|
20
|
|
|
and
Issuer Purchases of Equity Securities.
|
|||
|
Item
6.
|
Selected
Financial Data.
|
22
|
|
|
Item
7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations.
|
22
|
|
|
Item
7A.
|
Quantitative
and Qualitative Disclosures About Market Risk.
|
29
|
|
|
Item
8.
|
Financial
Statements and Supplementary Data.
|
29
|
|
|
Item
9.
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure.
|
30
|
|
|
Item
9A(T).
|
Controls
and Procedures.
|
30
|
|
|
Item
9B.
|
Other
Information.
|
31
|
|
|
PART
III
|
|||
|
Item
10.
|
Directors,
Executive Officers and Corporate Governance.
|
31
|
|
|
Item
11.
|
Executive
Compensation.
|
33
|
|
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and Management
|
36
|
|
|
and
Related Stockholders Matters.
|
|||
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence.
|
37
|
|
|
Item
14.
|
Principal
Accountant Fees and Services.
|
38
|
|
|
|
|||
|
PART
IV
|
|||
|
Item
15.
|
Exhibits
and Financial Statement Schedules.
|
39
|
|
|
SIGNATURES
|
39
|
||
2
PART
I
FORWARD-LOOKING
STATEMENTS
Certain
statements made in this Annual Report on Form 10-K are “forward-looking
statements” (within the meaning of the Private Securities Litigation Reform Act
of 1995) regarding the plans and objectives of management for future operations.
Such statements involve known and unknown risks, uncertainties and other factors
that may cause actual results, performance or achievements of THE Registrant to
be materially different from any future results, performance or achievements
expressed or implied by such forward-looking statements. The forward-looking
statements included herein are based on current expectations that involve
numerous risks and uncertainties. The Registrant’s plans and objectives are
based, in part, on assumptions involving the continued expansion of business.
Assumptions relating to the foregoing involve judgments with respect to, among
other things, future economic, competitive and market conditions and future
business decisions, all of which are difficult or impossible to predict
accurately and many of which are beyond the control of the Registrant. Although
the Registrant believes its assumptions underlying the forward-looking
statements are reasonable, any of the assumptions could prove inaccurate and,
therefore, there can be no assurance the forward-looking statements included in
this Report will prove to be accurate. In light of the significant uncertainties
inherent in the forward-looking statements included herein, the inclusion of
such information should not be regarded as a representation by the Registrant or
any other person that the objectives and plans of the Registrant will be
achieved.
PART
I
Explanatory
Note
EFT
BioTech Holdings, Inc. (the “Company”) is filing this Amendment No. 2 to
its Annual Report on Form 10-K for the fiscal year ended March 31, 2009 in
response to a letter, dated September 30, 2009, received by the Company from the
Securities and Exchange Commission. The letter contained comments
regarding the Company’s Amendment No. 4 to its Form 10 (File No.: 000-53730)
filed with the SEC on September 3, 2009 and to the Company’s Amendment No. 1 to
its Form 10-K for the fiscal year ended March 31, 2009 also filed with the SEC
on September 3, 2009.
3
ITEM
1. BUSINESS.
General
The
Registrant together with its subsidiaries is an e-Business company designed
around the concept of “Business-to-Customer,” meaning products are sold directly
to individuals, rather than the traditional “Business to Business” model where
products are sold to distributors who then sell to individuals, using our
website, www.eftb.us. The contents of our website are not
incorporated by reference herein. The Registrant is a holding
company and conducts its business through its operating
subsidiaries. See “Organizational History” below. The
information in this registration statement concerning the Registrant’s business
and operations pertains to the operating subsidiaries. Terms such as the
“Company,” “EFT,” “we,” “us,” “our” and similar phrases pertain to the
activities of the operating subsidiaries unless otherwise noted.
We offer
25 different nutritional products, some of which are oral sprays; 18 different
personal care products; an environmentally protective automotive product, an
environmentally friendly house cleaner and flip top portable drinking container
which contains a filter to remove impurities from the water. See
“Products” below for a more detailed description of our products.
We market
and sell our products through an internet platform which consists of us selling
our products directly to customers through our website. Once a
customer purchases our products he or she becomes an “Affiliate” by being
recommended by another Affiliate. Currently, a majority of our
Affiliates are located in China and Hong Kong.
To become
an Affiliate, a customer must make a minimum purchase of $300 plus $30 for
shipping and handling fees. After that point, the new Affiliate is not required
to make any additional purchases. Our Affiliates only purchase because they want
to. Affiliates are not required to pay membership fees, buy products, resell
products, recruit others, attend meetings or report to us. Free educational
classes are offered to our Affiliates where they can learn more about our
products and how to use them. Affiliate rewards are issued in the form of a
reward card. Rewards are credited in U.S. Dollars and can be withdrawn in local
currency at automated teller machines (ATM’s) in the country of the
Affiliate. By using this method, we eliminate cumbersome accounting
chores such as issuing checks and reconciling bank statements. This method helps
us to keep our accounting staff smaller than it would be if we used a check
payment method, thereby saving operating expenses.
The
Company generally does not have a return policy. The Company does, however,
provide a warranty for its products. If a customer receives defective products,
we will send replacement products. Historically, the company warranty provisions
have not been material. The specific warranty terms and conditions vary
depending upon the product sold, but generally include replacement over a period
of six months.
Customers
who originally enrolled in the Affiliate program (the “EFT Program”) shared this
program with friends and relatives in China. From this, our Chinese
business grew. Customers can join the EFT program only by being
recommended by another Affiliate and by making a purchase through our website.
To purchase products, customers order on line and send payment for the
order to an off-shore account. Currently, the Company has no sales
activities in the United States. EFT International Ltd. (“EFT International”) an
off-shore subsidiary, will verify receipt of payment and notify the appropriate
distribution center to ship the products. The Affiliate then
receives the products for personal use.
As of
March 31, 2009, we had approximately 980,000Affiliates enrolled in the EFT
Program. When a customer joins the EFT Program, the customer is given a
membership ID number.
We also
have a commission plan. The Company's commission plan is calculated
on every nine new orders that are placed under an Affiliate’s identification
number. When an Affiliate places an order, he/she is required to provide us with
identification number of the Affiliate which referred him/her to
us. Each Affiliate is recognized to have both a right and left sales
side. The commissions that each Affiliate earns are calculated on the
accumulation of nine new orders placed on these two sides with a minimum of
three orders placed on one side in order to generate the commission. Commissions
are paid on a weekly basis and are calculated on total new sales generated each
week.
Full
payment is required in U.S. Dollars prior to shipment of the products
purchased. At period end, we recognize cash received where orders
have not been shipped as a liability. We report unshipped orders as a
liability under unearned revenues. Revenue is recognized at the date
of shipment to customers when a formal arrangement exists, the price is fixed or
determinable, the delivery is completed, no other significant obligations of the
Company exist and collectibility is reasonably assured. Payments received before
all of the relevant criteria for revenue recognition are satisfied are recorded
as unearned revenue. Cash consideration given by the Company to its
Affiliates is considered to be a reduction of the selling prices of the
Company's products, thus, is recorded as a reduction of revenue. Customers may
return defective merchandise for an exchange or refund.
EFT does
not own any manufacturing facilities. Our products are manufactured
by third party vendors, and are packaged under the EFT brand. EFT
packages clearly state the country of manufacture which currently is the United
States in most cases. We do not have any long-term supply contracts
or agreements with any merchants to produce or market our products at this time.
We order our products directly from vendors, on an “as-needed” or “expected
need” basis.
4
The
Registrant’s Common Stock is currently trading on the OTC Pink Sheets under the
ticker symbol “EFTB.”As of the year ended March 31, 2009, there were
75,983,205 shares of Common Stock outstanding, 53,300,000 of which
(approximately 70%) are beneficially held or controlled by the executive
officers and directors of the Company.
Organizational
History
EFT
BioTech Holdings, Inc., (formerly HumWare Media Corporation, GRG, Inc.,
Ghiglieri Corporation, Karat Productions, Inc.) was incorporated in the state of
Nevada on March 19, 1992 (“EFT Holdings”). HumWare’s stock had been
trading on the Pink Sheets and as a result EFT Holdings is a public company
trading on the Pink Sheets.
On
November 7, 2007, HumWare Media Corporation changed its name to EFT BioTech
Holdings, Inc. and effected a reverse stock split of 20,000 shares of common
stock for 1 share of common stock, which resulted in a decrease in the total
amount of common shares then issued and outstanding.
On
November 18, 2007, EFT Holdings issued an aggregate of 53,300,000 shares of its
Common Stock in connection with a share exchange with EFT BioTech, Inc. (“EFT
BioTech”), a Nevada corporation formed on September 18, 2007, pursuant to which
EFT Holdings acquired 100% of the issued and outstanding shares of EFT BioTech
in consideration for such 53,300,000 shares, representing 70.15% of EFT Holdings
capital stock on a fully-diluted basis. See Item 4, “Security
Ownership of Certain Beneficial Owners and Management” herein for a description
of the current holders of such 53,300,000 common shares.
Upon the
consummation of the merger, EFT BioTech became a wholly-owned subsidiary of EFT
Holdings. The Registrant is a holding company and conducts its
business through the operations of the subsidiaries of EFT Limited, a British
Virgin Islands corporation (“EFT Limited”). EFT Limited has four
wholly-owned subsidiaries: EFT (HK), Inc., Top Capital International Limited,
EFT, Ltd. and EFT International Ltd.
Excalibur
International Marine Corporation
Due to
the recent changes in policy between Mainland China and Taiwan, an opportunity
was recognized to take advantage of direct sailings for cargo and passengers
through the Taiwan Strait. EFT identified Excalibur International
Marina Corporation (“Excalibur”), a shipping company located in Taiwan, as a
viable entity to participate with in this business opportunity. In
order to expedite the purchase of a new vessel, EFT’s Board of Directors
approved a non-interest bearing, unsecured loan to facilitate this
purchase.
On July
28, 2008, the Registrant loaned $19,193,000 to Excalibur. The purpose
of this loan was to provide Excalibur funds to purchase a vessel ($17,628,283)
and working capital ($1,564,717). This loan was still outstanding with balance
of $1,564,717 as of June 30, 2009. At the time of the transaction,
Excalibur was not a related party nor did any of the Company or any of its
officers or directors have any relationship with Excalibur or any of its
officers and directors.
On
September 23, 2008, the Registrant signed a loan agreement with Excalibur to
lend $2,000,000 at an interest rate of 3.75% per month with a term of no more
than 60 days. At the end of the 60 days term, the term of the loan was extended
for six months. On December 25, 2008, the Company extended this loan to May 25,
2009. On May 25, 2009, the Company extended this loan to Excalibur for another
six months and decreased the interest rate to 12.5% per annum. The
loan was used to fund operations.
On
October 20, 2008, EFT Investment Co., Ltd. was formed as a
wholly-owned subsidiary of EFT BioTech Holdings, Inc. EFT
Investment Co., Ltd was formed in Taiwan. On October 25, 2008, EFT Investment
Co., Ltd. completed the acquisition of 585,677,500 shares of common stock of
Excalibur; representing approximately 49% shares of issued and outstanding
shares of Excalibur, for an aggregate purchase price of USD $19,193,000. Prior
to the acquisition of Excalibur, Excalibur was not a related person under Item
404 of Regulation S-K.
On
November 24, 2008, the Registrant signed an additional loan agreement with
Excalibur, a then related party, pursuant to which the Registrant loaned
Excalibur $500,000 at the interest rate of 3.75% per month with a term of 30
days with an extension of six months. On December 25, 2008, the
Company extended the loan to May 25, 2009. On May 25, 2009, the
Company extended this loan for another six months and decreased the interest
rate to 12.5% per annum. The loan was used to fund operations.
On May
13, 2009, the Company signed another loan agreement denominated in U.S. dollars
with Excalibur to lend Excalibur $600,000 at interest rate of 12.5% per annum
with a maturity date of November 13, 2009. The loan was used to fund
operations.
On June
28, 2009, Excalibur completed its inaugural passenger voyage across the Taiwan
Strait.
5
Below is
our corporate chart:
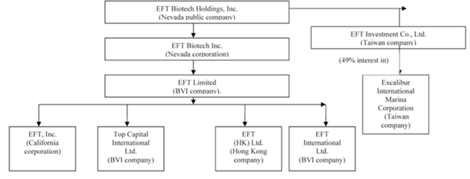
EFT
Limited (BVI) has four wholly-owned subsidiaries: EFT, Inc., a California
company formed on January 1, 2003, Top Capital International, Ltd. (BVI), a BVI
company formed on May 22, 2002, EFT (HK), Ltd., a Hong Kong (“HK”) company
formed on November 1, 2006 and EFT International Ltd. (BVI), a BVI company
formed on April 20, 2005, which it acquired all on November 14, 2007. EFT
International Ltd. is the operating company that generates substantially all of
the company’s net income. As EFT Limited (BVI) and the four companies being
acquired were under common control, this acquisition also represents a
reorganization of entities under common control.
Products
Nutritional
Products:
Our
nutritional products are non-pharmaceutical nutritional
products. They are ingestible through oral liquids, oral sprays,
tablets and tea. Our oral sprays are delivered through very fine mist
sprayed directly into the mouth. Our containers used to deliver our nutritional
products are small, compact and easy to carry.
Our
products are all natural, made from pure ingredients, and are designed to
address specific goals of the user such as strengthening the immune system,
assisting in weight loss, helping to overcome a sore throat and fighting off
colds. Each product has been formulated to address specific need, symptom and
condition. We make no claims as to the products curing any medical condition, or
preventing any medical ailment. Our products have not been tested and/or
approved by the FDA, as with all non-prescription products.
We
currently offer 25 different nutritional products for various
purposes:
|
1.
|
Zeolite
Plus:
|
An oral
liquid designed to detoxify the body, support immune system strength and
normalize pH in the body.
|
2.
|
2006 Celprotect
I:
|
Ingestible
tablets designed to eliminate toxins and viruses (e.g., cold sores) and promote
energy.
|
3.
|
2007 Celprotect II Bullet
Points:
|
An oral
liquid designed to stimulate cellular metabolism, neutralize toxins, assist in
avoiding food poisoning, balance cell life and boost energy.
|
4.
|
2006 – 2007 Celprotect
I:
|
A kit
containing 2006 Celprotect I and 2006 - 2007 Celprotect II.
|
5.
|
CardioSupport:
|
An oral
spray designed to promote heart health.
|
6.
|
Colloidal
Silver:
|
An oral
liquid designed to combat bacterial, fungal and viral infections.
|
7.
|
Colostrum:
|
An oral
spray designed to promote anti-aging, weight loss and immune system
support.
6
|
8.
|
Deer Antler Velvet
Plus:
|
An oral
spray designed to promote white blood cell count and to help the body handle
stress and promote recovery from the effects of injury and fatigue.
|
9.
|
Essential
90+:
|
An oral
spray designed to promote overall health.
|
10.
|
GlucoBalance:
|
An oral
spray designed to maintain proper levels of blood sugar for good
health.
7
|
11.
|
Liver
Support:
|
An oral
spray designed to cleanse the liver and rebuild damaged tissue.
|
12.
|
Memory
Plusb:
|
An oral
spray designed to overcome the natural processes associated with aging and
enhance healthy cognitive ability.
|
13.
|
MSM (Methylsulfonymethane):
|
An oral
spray designed to rebuild connective tissue and joints.
|
14.
|
Perform
Plus:
|
An oral
spray designed to promote endurance, performance and increased
libido.
|
15.
|
Re-Live
Again:
|
An oral
spray designed to increase the release of Human Growth Hormone within the body
to increase energy and endurance.
|
16.
|
ReishiPlus:
|
An oral
spray designed to help lower blood pressure and decrease elevated cholesterol
and triglyceride levels and support the immune system.
|
17.
|
Rooibos
Tea:
|
A popular
South African tea believed to promote anti-aging and immune system
health.
|
18.
|
Slim’n
Easy:
|
An oral
spray designed to promote and sustain weight loss.
|
19.
|
Slumber
Plus:
|
An oral
spray designed to aid sleep.
|
20.
|
Spray-EEZE:
|
An oral
spray designed to alleviate colds and sore throats.
|
21.
|
Super
Hydro-Oxy:
|
An oral
liquid designed to revitalize and detoxify the human body.
|
22.
|
Super
Re-Vitalizer:
|
An oral
spray designed to promote overall health.
|
23.
|
Super
Silica:
|
An oral
liquid designed to support bones, arteries, connective tissue, healthy hair,
skin and nails.
|
24.
|
Super
Cal:
|
An oral
spray designed to promote bone health.
|
25.
|
Vision
Plus:
|
An oral
spray designed to nourish the eyes.
8
Personal
Care Products:
We
currently offer the following 18 different Personal Care products;
|
1.
|
Bust Cream: An herbal
cream containing natural ingredients for the purpose of stimulating the
development of the breast tissue and tightening and firming of the
breast.
|
|
2.
|
Daily Eye
Treatment: A soothing and hydrating eye cream for the
purpose of reducing puffiness, fine lines and the effects of stress and
fatigue.
|
|
3.
|
Lip gloss: A
long lasting moisturizing lipstick.
|
|
4.
|
Pressed Mineral
Powder: A multi-functional face power containing zinc,
Vitamins A and E and green tea
extract.
|
|
5.
|
Fountain of Youth: A
daily skin care regimen including a synergistic blend of 10 oriental herbs
for the purpose of skin brightening, cleaning, and anti-wrinkle
effects.
|
|
6.
|
Gold Cream: A
topical cream containing colloidal gold for the purpose of relieving pain
associated with arthritis, stiff and swollen joints, sprains, strains,
muscle spasms, bursitis and
tendonitis.
|
|
7.
|
Instant Whitening Cream:
A cream for the purpose of brightening overall complexion, lightening age
spots, liver spots and sun damaged
skin.
|
|
8.
|
Lifting Masque: A 20
minute masque for the purpose of reducing the visible signs of aging while
lifting, tightening, and refining the pores of the
skin.
|
|
9.
|
Perfume set: A floral
fragrance perfume.
|
|
10.
|
Nia 3 Plus 1 Lash &
Line:
Mascara and eyeliner package containing two items in
each tube: dark brown mascara and navy blue mascara
in one tube and black mascara and black eyeliner in the other
tube.
|
|
11.
|
Nia Concealer: A light
colored concealer for the purpose of providing coverage for any skin
imperfection as in darkness around the eyes, blemishes and to even out
skin tones.
|
|
12.
|
Nia Eye Color: A palette
of four color-coordinated eye shadows: Pearl grey, Soft pink, Cranberry
and Charcoal.
|
|
13.
|
Nia Face and Body
Powder: A jar containing face and body powder and a powder
puff.
|
|
14.
|
Nia Lip Magic: A lip
gloss. Colors include Celebration Red with Pink shimmer and Plum Raisin
with Peach shimmer.
|
|
15.
|
Progesterone Cream: A
non-pharmaceutical cream containing natural ingredients for menopausal and
postmenopausal women.
|
|
16.
|
Rooibos Tea Cream: A
skin cream containing Alpha-Hydroxy acids, antioxidant, Vitamin B, Vitamin
C and Vitamin E , Zinc, Potassium, Calcium, Copper and
DHEA.
|
9
|
17.
|
The Collection: A makeup
kit containing Face Primer, Silk Whipped Foundation, Wet/Dry Powder,
Eye Shadow, Black Eye Pencil, Pressed Shimmer Powder, Shimmer
Blush, Long Lasting Lipstick, Lip Gloss Palate, Cream Lipstick,
and Coordinating Lip Pencils.
|
|
18.
|
Travel Kits. An
Anti-Aging Skin Care Travel Kit containing products designed for balancing
skin tone, increasing hydration, diminishing lines and wrinkles and
restoring resiliency.
|
Automotive
Additive Products:
We
currently offer the following one automotive product:
10
Fast Team Plus: A fuel
additive that acts as a lubricant and cleaning compound and has been found to
significantly improve gas mileage and performance and reduce smog in all
gasoline powered engines.
Environmentally
Friendly Home Cleaning Product:
Natural Clean:
A 100%
biodegradable multi-purpose cleaning solution that aids in the clean-up and
removal of a number of different stains and spills including grease, tar,
crayons, pet stains, soap film, blood, ink and make-up. Natural Clean is
non-toxic, non-caustic, non-pollutant, non-flammable and non-rusting and can be
used for cleaning kitchens, baths and cars as well being used as an insect
repellant when applied on skin or clothing.
Other:
Flip-Top Portable
Filter:
A
24-ounce drinking container in a portable tote and featuring a filtration
system.
Distribution
of Our Products
Our
products are sold exclusively on the Internet. Customer orders are
filled using the following general process:
|
·
|
To
purchase products, customers order on line and send payment for the order
to an off-shore account. EFT International will verify receipt
of payment and notify the appropriate distribution center to ship the
products. Currently, orders are filled primarily through
our subsidiary EFT (HK) Ltd., located in Hong Kong and we do not have any
sales in the United States. We are currently in the process of
establishing operations in other locations around the world, specifically
Europe, Thailand, Vietnam and South America, from which products may also
be shipped if we determine there is sufficient
demand.
|
|
·
|
Once
orders are placed on-line, EFT International will notify EFT (HK) Ltd.
that payment was received. EFT (HK) Ltd. will notify IFC
(defined below) how much of any particular type of product will be
needed. In most cases, products ordered are shipped directly
from our third party vendor to the distribution center in Hong
Kong. In some cases, however, products are shipped to
California rather than directly to the distribution center in Hong
Kong. As a result some inventory may be maintained
in California but only for a short period of time, generally not to exceed
three months. Any products received in California are subsequently shipped
to Hong Kong for distribution. Vendors are paid for their
products by EFT International.
|
The
product formulations, delivery systems (spray), packages, packaging design and
labels are proprietary to EFT. There are several manufacturers who
produce these formulated products owned by EFT. We do not own any
manufacturing facilities.
It would
be difficult and prohibitively expensive for a competitor to duplicate the
process without a ready market to sell hundreds of thousands of products into,
therefore, we do not copyright or patent our products. To date, we
have not encountered any competitor who has products similar to ours.
Additionally, we are not fully dependent upon any one manufacturer supplier for
100% of any single product.
Significant
Vendors
The
vendors that supply the Company’s formulated products are currently located in
the United States. None of our vendors account for a significant portion of our
business and can be replaced. In December of 2008, we contracted with
Industry Fulfillment Co., Inc. (“IFC”), a California corporation, to provide
quality control on products ordered from vendors beginning in January
2009. IFC tracks the quantity and progress on delivery of these
orders. In the future products may be purchased from vendors located
outside the United States. There are no commitments or
manufacturing agreements with any of our current
vendors. We order products on an “as needed” or an
“expected need” basis.
Sources
and Availability of Raw Materials
Raw
materials used in the manufacture of our products by third parties are readily
available to the manufacturers of our products. We are not
a party to any agreement for the purchase or delivery of such raw
materials.
Significant
Customers and Dependence on One or More Customers
None of
our customers or Affiliates account for a significant portion of our
business. We do not currently depend on any one or more customers or
Affiliates for the purchase of our products.
11
Competition
The
nutritional supplement and cosmetic e-business markets have and continue to
become increasingly competitive and are rapidly evolving. In
addition, the internet online commerce market is rapidly evolving and intensely
competitive. Barriers to entry are minimal and current and new competitors can
launch new websites at a relatively low cost. Continued
advancement in technology and increasing access to that technology is paving the
way for growth in the internet consumer industry. We believe that we are
well-positioned within the Asian consumer market with our current marketing plan
of supplying American merchandise brands to Asian consumers and that our
exposure to both the Asian and American cultures gives us a competitive
advantage. We also face competition for consumers from retailers, duty-free
retailers, specialty stores, department stores and specialty and general
merchandise catalogs, many of which have greater financial and marketing
resources than we have.
Government
Regulation
Food
& Drug Administration
Currently,
pre-market government approval is not necessary for any of our products and none
of our products are otherwise subject to governmental regulation. The
FDA may in the future determine to regulate our nutritional products. If certain
of our products are deemed to be drugs or biologics, we will be required to
conduct clinical trials to demonstrate the safety and efficacy of these products
in order to continue to market and sell them.
Personal
Identifiable Information
The
collection of data and processing of transactions through our systems require us
to receive and store a large volume of personally identifiable data. We are
subject to various consumer protection laws relating to the collection, use,
retention, security and transfer of personally identifiable information about
our users, especially for financial information. In many states, there is
currently great uncertainty whether or how existing laws governing issues such
as property ownership, sales and other taxes, apply to the Internet and
commercial online services. New laws in this area have been passed by several
jurisdictions, and other jurisdictions are considering imposing additional
restrictions. The interpretation and application of these laws are in a state of
flux. These laws may be interpreted and applied inconsistently from country to
country and our current data protection policies and practices may not be
consistent with those interpretations and applications. Complying with these
varying requirements could cause us to incur substantial costs or require us to
change our business practices in a manner adverse to our business. Any failure,
or perceived failure, by us to comply with any regulatory requirements or orders
or other federal, state or international privacy or consumer protection-related
laws and regulations could result in proceedings or actions against us by
governmental entities or others, subject us to significant penalties and
negative publicity and adversely affect us. In addition, we are subject to the
possibility of security breaches, which themselves may result in a violation of
these laws.
Seasonality
Our
business is not seasonal in nature.
Intellectual
Property
We do not
currently hold any patents or trademarks, nor are we a party to any licenses,
franchises, concessions, royalty agreements or labor contracts except as
disclosed herein. The Company uses the “EFT” name, a trademark owned by EFT
Assets Limited and licensed by EFT Assets Limited to the Company. EFT
Limited is required to pay an annual royalty fee equal to a percentage of the
Company’s gross sales for the previous fiscal year. The percentage is
5% for the first $30 million in gross sales, 4% for the $10 million in gross
sales in excess of $30 million, 3% for the $10 million in gross sales in excess
of $40 million and up to $50 million; 2% for the $10 million in gross sales in
excess of $50 million and up to $60 million; and 1% for the $10
million in gross sales in excess of $60 million.
Research
and Development Activities
We have
not and do not engage in any research and development activities nor do we
contemplate spending any time on such activities in the foreseeable
future. On an as needed basis we may outsource research and
development of a new product.
Environmental
Laws
Our
products are biodegradable and are not impacted by federal, state or local
environmental laws.
Employees
As of the
date of this Annual Report, we have 5 full-time employees at the executive
offices of the Registrant in the City of Industry, California and the remaining
9 at our Kowloon, Hong Kong office. The number of employees was
reduced in the City of Industry office because EFT, Inc. is no longer a
fulfillment or procurement center and in the Kowloon office as a result of
decreased sales generally. We adjust the number of employees
from time to time as necessary to meet the needs of the Company.
None of
our employees are represented by a collective bargaining agreement. There are no
pending labor-related legal actions against us filed with any state or federal
agency. We believe our employee relations are good.
12
Available
Information
We filed
the original Form 10 with the SEC on December 10, 2008 and the Registration
Statement is effective by operation of law as of February 9,
2009. Since the effectiveness date of the Form 10, we have been
required to file annual, quarterly and other required reports and forms with the
SEC under the Securities Exchange Act of 1934, as amended. This Form
10-K, and our other reports and other information may be inspected and copied at
the public reference facilities maintained by the SEC at 100 F Street, N.E.,
Washington, D.C. 20549. The public may obtain information on the operation of
the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the
SEC maintains an Internet website that contains reports, proxy and information
statements and other information regarding registrants that file electronically.
The address of the website is http://www.sec.gov.
ITEM
1A. RISK FACTORS
Risk
Factors
Investing
in our securities involves risk. You should carefully consider all of
the information contained in or incorporated by reference into this report and,
in particular, the risks described below before investing in our
securities. If any of the following risks actually occur, our
business, financial condition or results of operations could be materially
harmed and you may lose part or all of your investment.
Risks
Related to Our Business
Current
economic conditions may adversely affect our industry, business and results of
operations and could cause the market value of our common stock to
decline.
The
global economy is currently undergoing a period of unprecedented volatility, and
the future economic environment may continue to be less favorable than that of
recent years. This has led, and could further lead, to reduced consumer spending
in the foreseeable future, and may include consumer spending on nutritional and
beauty products and other discretionary items. In addition, reduced
consumer spending may drive us and our competitors to decrease prices. These
conditions may adversely affect our industry, business and results of operations
and may cause the market value of our common stock to decline.
FINRA
might not approve of Buckman, Buckman & Reid, Inc’s application to act as a
market maker for our common stock on the OTC Bulletin Board.
After we
have been informed by the SEC that they no longer have any further comments
regarding our Form 10, Buckman, Buckman & Reid, Inc., will file an
application with FINRA for authorization to act as a market maker of our common
stock on the OTC Bulletin Board. Buckman, Buckman & Reid,
Inc. served as the placement agent of our Units in the Regulation S Offering
which commenced in January of 2008 and expired on October 25, 2008. No assurance
can be made that their application will be approved by FINRA. If
their application is not approved, we will remain quoted on the OTC Pink Sheets
which could make our common stock less attractive for potential investments and
our company less attractive for any deals. This could decrease the
value of our common stock.
We
regularly maintain cash balances at a commercial bank in excess of the Federal
Deposit Insurance Corporation insurance limit of $250,000.
We
regularly maintain cash balances at a commercial bank in excess of the Federal
Deposit Insurance Corporation (FDIC) insurance limit of $250,000. If the
financial position and/or liquidity of the bank were to become impaired, our
financial position and the results of our operations could be negatively
affected to the extent of account balances held at the financial institution in
excess of the federally insured limit.
The
extent of our sourcing and manufacturing may adversely affect our business,
financial condition and results of operations.
All of
our products are currently manufactured in the United States and a majority of
them are sold to customers in Hong Kong and China. As a result of the
magnitude of this sourcing and shipping, our respective businesses are subject
to the following risks:
|
·
|
political
and economic instability in foreign countries, including heightened
terrorism and other security concerns, which could subject imported or
exported goods to additional or more frequent inspections, leading to
delays in deliveries or impoundment of goods, or to an increase in
transportation costs of raw materials or finished
product;
|
|
·
|
the
imposition of regulations and quotas relating to exports and imports,
including quotas imposed by bilateral agreements between the United States
from where we source our products and foreign countries, including
China;
|
|
·
|
the
imposition of duties, taxes and other charges on exports and
imports;
|
13
|
·
|
significant
fluctuation of the value of the U.S. dollar against the Hong Kong Dollar,
Chinese Yuan and other foreign
currencies;
|
|
·
|
restrictions
on the transfer of funds to or from foreign countries;
and
|
|
·
|
violations
by foreign contractors of labor and wage standards and resulting adverse
publicity.
|
We
operate on very tight delivery schedules and, if there are delays and expected
delivery dates cannot be met, it could negatively affect our
profitability.
If there
is a delay in the delivery of goods and delivery schedules cannot be met, then
our Affiliates and retail customers may cancel orders with us which
would impact our gross profits and therefore, our profitability. We may also
incur extra costs to meet delivery dates, which would also reduce our company’s
profitability.
We
face intense competition and any failure to timely implement our business plan
could diminish or suspend our development and possibly cease our
operations.
From time
to time in the Business to Consumer (B2C) e-commerce business, competitors,
typically catalog and other online retailers, will attempt to secure contracts
with various merchandise brands to offer merchandise to their consumers. We also
face competition for consumers from retailers, duty-free retailers, specialty
stores, department stores and specialty and general merchandise catalogs, many
of which have greater financial and marketing resources than we have. The
internet online commerce market is rapidly evolving and intensely competitive.
Barriers to entry are minimal and current and new competitors can launch new
websites at a relatively low cost. Many competitors in this area have greater
financial, technical and marketing resources than our Company. Continued
advancement in technology and increasing access to that technology is paving the
way for growth in the internet consumer industry. In addition, the nutritional
supplement and cosmetic e-business markets have and continue to become
increasingly competitive and are rapidly evolving. We believe that we
are well-positioned within the Asian consumer market with our current marketing
plan of supplying American merchandise brands to Asian consumers and that our
exposure to both the Asian and American cultures gives us a competitive
advantage but there can be no assurance that we will maintain our competitive
edge or that we will continue to provide only American made
merchandise.
Consumers’
concerns about purchasing items through the Internet as well as external or
internal infrastructure system failures could negatively impact our e-commerce
sales or cause us to incur additional costs.
The
e-commerce business is vulnerable to consumer privacy concerns relating to
purchasing items over the Internet, security breaches, and failures of Internet
infrastructure and communications systems. If consumer confidence in making
purchases over the Internet declines as a result of privacy or other concerns,
e-commerce net sales could decline. We may be required to incur increased costs
to address or remedy any system failures or security breaches.
We
are subject to laws governing our customers’ personal identifiable information.
The processing, storage and use of personal data could give rise to liabilities
as a result of governmental regulation, conflicting legal requirements or
differing views of personal privacy rights.
The
collection of data and processing of transactions through our systems require us
to receive and store a large volume of personally identifiable data. We are
subject to various consumer protection laws relating to the collection, use,
retention, security and transfer of personally identifiable information about
our users, especially for financial information. In many states, there is
currently great uncertainty whether or how existing laws governing issues such
as property ownership, sales and other taxes, apply to the Internet and
commercial online services. The interpretation and application of these laws are
in a state of flux. These laws may be interpreted and applied inconsistently
from country to country and our current data protection policies and practices
may not be consistent with those interpretations and applications. . We might
become exposed to potential liabilities with respect to the data that we
collect, manage and processes, and may incur legal costs if our information
security policies and procedures are not effective or if we are required to
defend our respective methods of collection, processing and storage
of personal data. Complying with these varying requirements could cause us to
incur substantial costs or require us to change our business practices in a
manner adverse to our business. Any failure, or perceived failure, by us to
comply with any regulatory requirements or orders or other federal, state or
international privacy or consumer protection-related laws and regulations could
result in proceedings or actions against us by governmental entities or others,
subject us to significant penalties and negative publicity and adversely affect
us. In addition, we are subject to the possibility of security breaches, which
themselves may result in a violation of these laws. Future investigations,
lawsuits or adverse publicity relating to our methods of handling personal data
could adversely affect our business, financial condition and results of
operations due to the costs and negative market reaction relating to such
developments.
14
Our
business depends on the ability to source merchandise in a timely and
cost-effective manner.
Our
business depends on being able to find qualified vendors and access products in
a timely and efficient manner. All of the vendors must comply with applicable
laws. Political or financial instability, changes in U.S. and foreign laws and
regulations affecting the importation and taxation of goods, including duties,
tariffs and quotas, or changes in the enforcement of those laws and regulations,
as well as currency exchange rates, transport capacity and costs and other
factors relating to foreign trade and the inability to access suitable
merchandise on acceptable terms could adversely impact our results of
operations.
We
face significant inventory risks.
We are
exposed to significant inventory risks that may adversely affect our operating
results as a result of new product launches, rapid changes in product cycles,
changes in consumer tastes with respect to our products and other factors. We
must accurately predict these trends and avoid overstocking or under-stocking
products. All of our products are supplied by third parties which we order
generally on an “as needed” basis. However, based on ordering trends
we do stock certain items that we believe will be in demand so that they are
available for immediate shipping. In recent months we have mitigated
decreases in sales by lowering our levels of inventory to preserve cash on hand.
Demand for products, however, can change significantly between the time
inventory is ordered and the date of sale. In addition, when we begin selling a
new product, it may be difficult to establish vendor relationships, determine
appropriate product selection, and accurately forecast product demand. The
acquisition of certain types of inventory, or inventory from certain sources,
may require significant lead-time and prepayment, and such inventory may not be
returnable. Although we have significantly reduced inventory levels and
primarily order products on an as needed basis, any one of the inventory risk
factors set forth above may adversely affect our operating results.
We
depend on third parties to manufacture all of the products we sell, and we don’t
have any contracts with any of the manufacturers of our products. If we are
unable to maintain these manufacturing relationships or enter into additional or
different arrangements, we may fail to meet customer demand and our net sales
and profitability may suffer as a result.
All of
our products are manufactured by third parties. We don’t have any contracts with
any of the manufacturers of our products. The fact that we do not have contracts
with our third-party manufacturers means that they could cease manufacturing
these products for us at any time and for any reason. In addition, our
third-party manufacturers are not restricted from manufacturing our competitors'
products. Our inability to secure adequate and timely supplies of
merchandise would harm inventory levels, net sales and gross profit, and
ultimately our results of operations.
Our
manufacturers may increase the cost of the products we purchase from
them.
If our
manufacturers increase their costs, our margins would suffer unless we were able
to pass along these increased costs to our customers. We may not be able to
develop relationships with new vendors and manufacturers at the same prices or
at all, and even if we do establish such relationships, such new vendors and
manufacturers might not allocate sufficient capacity to us to meet our
requirements. Furthermore, if we increase our product orders significantly from
the amounts we have historically ordered from our manufacturers, our
manufacturers might be unable to meet this increased demand.
Our
third-party manufacturers may not continue to produce products that are
consistent with our standards or applicable regulatory requirements, which could
harm our brand, cause customer dissatisfaction and require us to find
alternative suppliers of our products.
Our
third-party manufacturers may not maintain adequate controls with respect to
product specifications and quality and may not continue to produce products that
are consistent with our quality standards. If we are forced to rely on products
of inferior quality, then our customer satisfaction and brand reputation would
likely suffer, which would lead to reduced net sales. In addition, we may be
required to find new third-party manufacturers to supply our products. There can
be no assurance that we would be successful in finding third-party manufacturers
that make products meeting our standards of quality.
Future
increases in the price of gasoline may cut into our margins and if we are unable
to pass those costs to our customers, our profit margins will
decrease.
We pay
for the shipment of goods from our vendors. The recent worldwide
prices of gas have significantly and rapidly fluctuated in the recent
past. Increased fuel prices increase our costs of sales which
decrease our profit margins. Future and sustained increases in the
price of gasoline will decrease our profit margins to the extent we are unable
to foresee them and pass on any increased costs to our customers.
We
are subject to the risks of doing business abroad.
Some of
our products originate from abroad (e.g., our teas originate from South Africa)
and all of our Affiliates are currently located in China and Hong
Kong. As such, we are subject to the usual risks of doing business
abroad, including currency fluctuations, political or labor instability and
potential import restrictions, duties and tariffs. We do not maintain insurance
for the potential lost profits due to such disruptions. Political or economic
instability in China or Hong Kong or elsewhere could cause substantial
disruption in our business. This could materially adversely affect our financial
condition and results of operations. Heightened terrorism security concerns
could subject exported goods to additional, more frequent or more thorough
inspections. This could delay deliveries or increase costs, which could
adversely impact our results of operations. In addition, since we negotiate our
purchase orders with customers in United States dollars, the value of the United
States dollar against local currencies could impact our cost in dollars of
production from these manufacturers. We are not currently engaged in any hedging
activities to protect against these currency risks. If there is downward
pressure on the value of the dollar, our customers’ purchase prices for our
products could increase. We may not be able to offset an increase in production
costs with a price increase to our customers.
15
Fluctuations
in the price, availability and quality of materials used in our products could
have a material adverse effect on our cost of goods sold and our ability to meet
our customers’ demands.
Fluctuations
in the price, availability and quality of the materials used in the manufacture
of our products by third parties could have a material adverse effect on the
cost of such products to us or our ability to meet our customers’ demands. We
may not be able to pass on all or any portion of higher material prices to our
customers.
The
regulatory status of our products could change, and we may be required to
conduct clinical trials to establish efficacy and safety or cease to market
these products.
The Food
and Drug Administration, or FDA, does not have a pre-market approval system for
our products. However, the FDA may in the future determine to regulate our
products or the ingredients included in our products as drugs or biologics. If
certain of our products are deemed to be drugs or biologics we would be required
to conduct clinical trials to demonstrate the safety and efficacy of these
products in order to continue to market and sell them. In such event, we may not
have sufficient resources to conduct any required clinical trials, and we may
not be able to establish sufficient efficacy or safety data to resume the sale
of these products. Any inquiries by the FDA or any foreign regulatory
authorities into the regulatory status of our products and any related
interruption in the marketing and sale of these products could severely damage
our brand reputation and image in the marketplace, as well as our relationships
with customers, which would harm our business, prospects, financial condition
and results of operations.
The
failure to upgrade information technology systems as necessary could have an
adverse effect on our operations.
Some of
our information technology systems, which are primarily utilized to manage
information necessary to price and ship products and to generate reports
that report each customer’s order are dated and are comprised of
multiple applications, rather than one overarching state-of-the-art system.
Modifications involve replacing legacy systems with successor systems, making
changes to legacy systems or acquiring new systems with new functionality. If we
are unable to effectively implement these systems and update them where
necessary, this could have a material adverse effect on our business, financial
condition and results of operations.
We
are highly dependent on our current management.
Our
success is significantly dependent upon our management team. Our success is
particularly dependent upon Mr. Jack Qin, our Chairman and CEO, Ms. Sharon Tang,
our Chief Financial Officer, and Mr. George Curry, Chief Marketing Officer and
Director. The loss of any of them could have an adverse effect on us. If we were
to lose the services of our officers and directors, we may experience
difficulties in effectively implementing our business plan.
16
Dragon
Win beneficially owns 52,099,000 shares of common stock thereby controlling
68.57% of our issued and outstanding common stock as of the date of this Annual
Report.
As of the
date of this annual report, Dragon Win Management, Ltd., a British Virgin
Islands company (“Dragon Win”) owns 52,099,000 shares of our common stock,
thereby representing approximately 68.57% of our issued and outstanding common
stock. The board of directors of Dragon Win has voting and
dispositive control of the Registrant’s common stock held by Dragon
Win. Due to the fact that Dragon Win owns a majority of our issued
and outstanding common stock, the board of directors of Dragon Win can thus
approve or reject all matters on which the Registrant needs approval by not less
than a majority of stockholders, including mergers, acquisitions, sales of
assets, amending the Registrant’s Certificate of Incorporation, electing the
Registrant’s Board of Directors, and appointing the Registrant’s officers. This
might make the Company less attractive for strategic partners or tender offers
which consequently might artificially suppress the value of the Registrant’s
common stock.
Our
Preferred Stock may be used to avoid a change in control of the
Registrant.
Our
Certificate of Incorporation authorizes the issuance of 25,000,000 shares of
preferred stock with designations, rights and preferences determined from time
to time by the Board of Directors. As of the date of this Form 10-K, there are
no shares of preferred stock outstanding. However, the Board of Directors is
empowered, without stockholder approval, to issue preferred stock with dividend,
liquidation, conversion, voting or other rights that could be used to avoid a
change of control of the Registrant and which could suppress the value of our
common stock.
We
may not be able to manage our growth effectively.
We must
continually implement and improve our products and/or services, operations,
operating procedures and quality controls on a timely basis, as well as expand,
train, motivate and manage our work force in order to accommodate anticipated
growth and compete effectively in our market segment. Successful implementation
of our strategy also requires that we establish and manage a competent,
dedicated work force and employ additional key employees in corporate
management, product design, client service and sales. We can give no assurance
that our personnel, systems, procedures and controls will be adequate to support
our existing and future operations. If we fail to implement and improve these
operations, there could be a material, adverse effect on our business, operating
results and financial condition.
Our
business is concentrated in Hong Kong and China, making our operations sensitive
to economic fluctuations.
All of
our offered products are marketed outside of the U.S., mostly in Hong Kong and
China. Should we be unable to further diversify our markets, we may be subject
to economic fluctuations within Hong Kong and China. If our business
does not succeed, an investor could lose all or part of his
investment.
If
we do not meet our expansion strategy, we may not achieve our anticipated
results.
Our
business strategy is designed to expand the sales of our products and services
internationally. Our ability to implement our plans will depend primarily on the
ability to attract new customers. To implement this strategy the Registrant in
March of 2009 retained the services of Aero Strategic Advisory, a division of
Aero Financial, a global consulting and financial services
firm, Based in Las Vegas, Nevada, Aero Strategic Advisory will assist
the Registrant in a number of capacities, including corporate communications,
handling of investor inquires, dissemination of news, business development and
other services. We can give you no assurance that any of our
expansion plans will be successful or that we will be able to establish
additional favorable relationships for the marketing and sales of products and
services. If we are unable to expand our business, our business operations could
be adversely affected.
A
dispute concerning the infringement or misappropriation of our proprietary
rights or the proprietary rights of others could be time consuming and costly,
and an unfavorable outcome could harm our business.
We may be
exposed to future litigation by third parties based on claims that our programs
infringe the intellectual property rights of others. If we become involved in
litigation, it could consume a substantial portion of our managerial and
financial resources, regardless of whether we win or lose. We may not be able to
afford the costs of litigation. Any legal action against us or our collaborators
could lead to:
|
·
|
payment
of damages, potentially treble damages, if we are found to have willfully
infringed a party’s patent rights;
|
|
·
|
injunctive
or other equitable relief that may effectively block our ability to
further develop, commercialize and sell products;
or
|
|
·
|
we
or our collaborators having to enter into license arrangements that may
not be available on commercially acceptable terms, if at
all.
|
17
As a
result, we could be prevented from commercializing current or future
products.
We
have agreed to indemnify our officers and directors to the fullest extent
permitted under Nevada law.
Our
Certificate of Incorporation contains a provision eliminating the personal
liability of officers and directors to the extent allowed under the law of the
State of Nevada. Under such provision, stockholder(s) may only prosecute an
action against an officer and/or a director if the stockholder(s) can show acts
or omissions which involve intentional misconduct, fraud or a knowing violation
of law or the unlawful payment of distributions.
We
may make acquisitions and strategic investments, which will involve numerous
risks. We may not be able to address these risks without substantial expense,
delay or other operational or financial problems.
Although
we have a limited history of making acquisitions or strategic investments, we
may acquire or make investments in related businesses or products in the future.
Acquisitions or investments involve various risks, such as:
|
·
|
higher
than expected acquisition and integration
costs;
|
|
·
|
the
difficulty of integrating the operations and personnel of the acquired
business;
|
|
·
|
the
potential disruption of our ongoing business, including the diversion of
management time and attention;
|
|
·
|
the
possible inability to obtain the desired financial and strategic benefits
from the acquisition or investment;
|
|
·
|
assumption
of unanticipated liabilities;
|
|
·
|
incurrence
of substantial debt or dilutive issuances of securities to pay for
acquisitions;
|
|
·
|
impairment
in relationships with key suppliers and personnel of any acquired
businesses due to changes in management and
ownership;
|
|
·
|
the
loss of key employees of an acquired business;
and
|
|
·
|
the
possibility of our entering markets in which we have limited prior
experience.
|
Future
acquisitions and investments could also result in substantial cash expenditures,
potentially dilutive issuance of our equity securities, our incurring of
additional debt and contingent liabilities, and amortization expenses related to
other assets that could adversely affect our business, operating results and
financial condition.
We
are subject to SEC regulations relating to low-priced penny-stocks.
Our
Common Stock is currently traded on the OTC Pink Sheets under the ticker symbol
“EFTB.” As of the year ended March 31, 2009, there are 75,983,205
shares of common stock issued and outstanding. Our common stock has recently
been trading under $5.00 per share. The Securities and Exchange Commission has
adopted regulations concerning low-priced (or “penny”) stocks. The regulations
generally define “penny stock” to be any equity security that has a market price
less than $5.00 per share, subject to certain exceptions. Due to the fact that
our stock is trading under $5.00, our stock is currently classified as a penny
stock.
The penny
stock regulations require that broker-dealers, who recommend penny stocks to
persons other than institutional accredited investors make a special suitability
determination for the purchaser, receive the purchaser’s written agreement to
the transaction prior to the sale and provide the purchaser with risk disclosure
documents that identify risks associated with investing in penny stocks.
Furthermore, the broker-dealer must obtain a signed and dated acknowledgment
from the purchaser demonstrating that the purchaser has actually received the
required risk disclosure document before effecting a transaction in penny stock.
These requirements have historically resulted in reducing the level of trading
activity in securities that become subject to the penny stock
rules.
The
additional burdens imposed upon broker-dealers by these penny stock requirements
may discourage broker-dealers from effecting transactions in our common stock,
which could severely limit the market liquidity of our common stock and our
shareholders’ ability to sell our common stock in the secondary
market.
18
Penny
stocks may trade infrequently, which means that it may be difficult to sell
penny stock shares once you own them. Because it may be difficult to find
quotations for certain penny stocks, they may be impossible to accurately
price. Investors in
penny stocks and in the common stock of the Registrant should be prepared for
the possibility that they may lose their whole investment.
Our
stock price has been thinly traded but may become highly volatile in the
future.
Our
common stock trades on the OTC Pink Sheets and there has historically been a
very low volume of transactions. However, the market price of our common stock
may become highly volatile and be subject to wide fluctuations in response to
factors such as the following, some of which are beyond our
control:
|
•
|
Quarterly
variations in our operating
results;
|
|
•
|
Operating
results that vary from the expectations of securities analysts and
investors;
|
|
•
|
Changes
in expectations as to our future financial performance, including
financial estimates by securities analysts and
investors;
|
|
•
|
Reaction
to our earnings releases and conference calls, or presentations by
executives at investor and industry
conferences;
|
|
•
|
Changes
in our capital structure;
|
|
•
|
Changes
in market valuations of other internet or online service
companies;
|
|
•
|
Announcements
of innovations or new services by us or our
competitors;
|
|
•
|
Announcements
by us or our competitors of significant contracts, acquisitions, strategic
partnerships, joint ventures or capital
commitments;
|
|
•
|
Lack
of success in the expansion of our business
operations;
|
|
•
|
Announcements
by third parties of significant claims or proceedings against us or
adverse developments in pending
proceedings;
|
|
•
|
Additions
or departures of key personnel;
|
|
•
|
Rumors
or public speculation about any of the above factors;
and
|
|
•
|
Market
and volume fluctuations in the stock markets in
general.
|
These
market fluctuations may also materially and adversely affect the market price of
our Common Stock.
ITEM
1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM
2. PROPERTIES.
The
principal executive office of our operating company, EFT Limited, consists of
6,500 square feet located at Langham Office Tower, 8 Argyle Street, Suite 3706,
Kowloon, Hong Kong SAR which is leased from a third party pursuant to a five
year lease commencing on March 31, 2007. The lease expires on March 31, 2012.
Pursuant to the lease, there is no rent for the first two years. Commencing on
the third year of the lease, the monthly rent is $50,000 USD starting the
beginning of the third year and we expense the 5-year total rent evenly over the
life of the lease. There is no affiliation between any of our officers and
directors with the landlord for these premises.
We also
lease, through EFT, Inc., a 10,268 square foot facility center in the City of
Industry in California for $10,063 per month pursuant to a lease, dated August
1, 2005, with Lee & Lee. This lease expires on July 31, 2009. There is no
affiliation between any of our officers and directors with the landlord for
these premises.
19
The
Company also rents storage space for its sales division in Hong Kong. The lease
provides for monthly lease payments approximating $1,135 USD starting on May 8,
2008 and expires on May 7, 2010.
We
believe our properties are sufficient for our current operations.
ITEM
3. LEGAL PROCEEDINGS.
We are
not a party to nor are any of our properties the subject of any material pending
legal proceedings. We have not been threatened with nor
have any knowledge of any potential claims or legal actions that would have a
material adverse impact on our financial position, operations or potential
performance. There are no material proceedings to which any director,
officer or affiliate of the Registrant or any of its subsidiaries, any owner of
record or beneficially of more than five percent of any class of voting
securities of the Registrant, or any associate of any such director, officer,
affiliate of the Registrant, or security holder is a party adverse to the
Registrant or has a material interest adverse to the Registrant.
ITEM
4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
None.
PART
II
ITEM
5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND
ISSUER PURCHASES OF EQUITY SECURITIES.
Our
Common Stock
As of the
date of this Annual Report, our common stock is listed for trading on the Pink
OTC Markets Inc. (the “Pink Sheets”) under the ticker symbol
“EFTB.” As of the date of this Annual Report, there are 75,983,205
shares of common stock issued and outstanding, 7,793,165 (10.13%) of the issued
and outstanding shares of common stock of which are held by
non-affiliates. . Our common stock has recently been trading under $5.00
per share. Stocks traded on the Pink Sheets are usually thinly traded, highly
volatile, and not followed by analysts. Investors in our common stock may
experience a loss or liquidity problem with their share holdings.
Upon the
effectiveness of our registration statement on Form 10, and notification by the
SEC that they have no further comments, Buckman, Buckman & Reid, Inc., a
registered broker/dealer, intends to file an application with FINRA for
authorization to act as a market maker of our common stock on the OTC Bulletin
Board. Buckman, Buckman & Reid, Inc. served as the placement
agent of our Units in the Regulation S Offering which commenced in January of,
2008 and expired on October 25, 2008.
The
holders of the Registrant’s common stock are entitled to one vote per share. The
common stockholders do not have preemptive rights to purchase, subscribe for, or
otherwise acquire any shares of common stock.
The
ability of individual stockholders to trade their shares in a particular state
may be subject to various rules and regulations of that state. A number of
states require that a Registrant's securities be registered in their state or
appropriately exempted from registration before the securities are permitted to
trade in that state. Presently, the Registrant has no plans to
register its securities in any particular state.
The
Securities and Exchange Commission has adopted regulations concerning low-priced
(or “penny”) stocks. The regulations generally define “penny stock” to be any
equity security that has a market price less than $5.00 per share, subject to
certain exceptions. Due to the fact that our stock is trading under $5.00, our
stock is currently classified as a penny stock.
The penny
stock regulations require that broker-dealers, who recommend penny stocks to
persons other than institutional accredited investors make a special suitability
determination for the purchaser, receive the purchaser’s written agreement to
the transaction prior to the sale and provide the purchaser with risk disclosure
documents that identify risks associated with investing in penny stocks.
Furthermore, the broker-dealer must obtain a signed and dated acknowledgment
from the purchaser demonstrating that the purchaser has actually received the
required risk disclosure document before effecting a transaction in penny stock.
These requirements have historically resulted in reducing the level of trading
activity in securities that become subject to the penny stock
rules.
The
additional burdens imposed upon broker-dealers by these penny stock requirements
may discourage broker-dealers from effecting transactions in our common stock,
which could severely limit the market liquidity of our common stock and our
shareholders’ ability to sell our common stock in the secondary
market.
Penny
stocks may trade infrequently, which means that it may be difficult to sell
penny stock shares once you own them. Because it may be difficult to find
quotations for certain penny stocks, they may be impossible to accurately
price. Investors in
penny stocks should be prepared for the possibility that they may lose their
whole investment.
20
The
Registrant’s fiscal year end is March 31st. The range of high and low
bid information for our common stock on the Pink Sheets for each quarterly
period within the two most recent fiscal years is set forth below. Such
quotations reflect inter-dealer prices, without retail mark-up, mark-down or
commission and may not necessarily represent actual transactions. There was no
active trading market for our common stock during the period reflected
below:
|
Fiscal Period
|
Low Bid Price
|
High Bid Price
|
||||||
|
2009
|
||||||||
|
1st
Quarter as of June 30, 2009
|
$ | 3.50 | $ | 3.75 | ||||
|
2008
|
||||||||
|
4th
Quarter Ended March 31, 2009
|
$ | 2.75 | $ | 2.75 | ||||
|
3rd
Quarter Ended December 31, 2008
|
$ | 3.70 | $ | 3.80 | ||||
|
2nd
Quarter Ended September 30, 2008
|
$ | 3.90 | $ | 3.95 | ||||
|
1st
Quarter Ended June 30, 2008
|
$ | 5.25 | $ | 5.25 | ||||
|
2007
|
||||||||
|
4th
Quarter Ended March 31, 2008
|
$ | 5.45 | $ | 5.50 | ||||
|
3rd
Quarter Ended December 31, 2007
|
$ | 4.15 | $ | 4.50 | ||||
|
2nd
Quarter Ended September 30, 2007
|
N/A | N/A | ||||||
|
1st
Quarter Ended June 30, 2007
|
N/A | N/A | ||||||
Dividend
Policy
The
Company did not record a dividend for the fiscal year-ended March 31,
2009. During the fiscal year-ended March 31, 2008, the Company paid
$18.5 million in dividends to the former owners of EFT BioTech.
We
currently intend to retain all future earnings for use in the operation and
expansion of our business and do not anticipate paying any cash dividends on
common stock in the foreseeable future. Any future dividends will be at the
discretion of the board of directors, after taking into account various factors,
including among others, operations, current and anticipated cash needs and
expansion plans, the income tax laws then in effect, the requirements of Nevada
law, and any restrictions that may be imposed by our future credit
arrangements.
Record
Holders
As of
July 13, 2009, there were 29,821 record holders of our common
stock.
Transfer
Agent
Our
transfer agent
Standard
Registrar and Transfer Company, Inc.
12528
South 1840 East
Draper,
UT 84020
Phone: (801)
571-8844
Fax: (801)
571-2551
Email: investors@amsrcorp.com
21
ITEM
6. SELECTED FINANCIAL DATA.
N/A
ITEM
7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS
OF OPERATIONS.
You
should read the following discussion together with "Selected Historical
Financial Data" and our consolidated financial statements and the related notes
included elsewhere in this Annual Report. This discussion contains
forward-looking statements, which involve risks and uncertainties. Our actual
results may differ materially from those we currently anticipate as a result of
many factors, including the factors we describe under "Risk Factors” and
elsewhere in this Annual Report.
Forward-Looking
Statements
Some of
the information in this section contains forward-looking statements that involve
substantial risks and uncertainties. You can identify these statements by
forward-looking words such as "may," "will," "expect," "anticipate," "believe,"
"estimate" and "continue," or similar words. You should read statements that
contain these words carefully because they:
|
·
|
discuss
our future expectations;
|
|
·
|
contain
projections of our future results of operations or of our financial
condition; and
|
|
·
|
state
other "forward-looking"
information.
|
We
believe it is important to communicate our expectations. However, there may be
events in the future that we are not able to accurately predict or over which we
have no control. Our actual results and the timing of certain events could
differ materially from those anticipated in these forward-looking statements as
a result of certain factors, including those set forth under "Risk Factors,"
"Business" and elsewhere in this Annual Report. See "Risk Factors."
Unless
stated otherwise, the words “we,” “us,” “our,” “the Company” or “EFT BioTech” in
this Annual Report collectively refers to EFT BioTech Holdings, Inc. and its
subsidiaries.
Industry
Trends
We
believe that the Business to Customer business is robust and that consumers have
become more confident in ordering products, like ours, over the internet.
However, the nutritional supplement and cosmetic e-business markets have and
continue to become increasingly competitive and are rapidly evolving. Barriers
to entry are minimal and current and new competitors can launch new websites at
a relatively low cost. Many competitors in this area have greater financial,
technical and marketing resources than our Company. Continued advancement in
technology and increasing access to that technology is paving the way for growth
in direct marketing. We also face competition for consumers from retailers,
duty-free retailers, specialty stores, department stores and specialty and
general merchandise catalogs, many of which have greater financial and marketing
resources than we have. Notwithstanding the foregoing, we believe that we are
well-positioned within the Asian consumer market with our current plan of
supplying American merchandise brands to consumers and that our exposure to both
the Asian and American cultures gives us a competitive advantage. There can be
no assurance that we will maintain our competitive edge or that we will continue
to provide only American made merchandise.
However,
the global economy is currently undergoing a period of unprecedented volatility,
and the future economic environment may continue to be less favorable than that
of recent years. This has led, and could further lead, to reduced consumer
spending in the foreseeable future, and this may include spending on nutritional
and beauty products and other discretionary items, like our products. In
addition, reduced consumer spending may drive us and our competitors to decrease
prices. These conditions may adversely affect our revenues and
profits.
Our
long-term plan is to use funds from the private placement and revenues earned
for investments and acquisitions to allow us to grow our existing business
operations and to enter into additional territories. To date, we have not
located any acquisition targets nor do we have any commitments for capital
expenditures, other than Excalibur. We believe that due to the current global
economic recession, there might be material opportunities for us to acquire
smaller companies at discount prices. There can be no assurances however that we
will be successful in doing so. Our expansion will rely to a great degree on
global economic conditions and perceived future changes. Until such time, we
intend to retain our cash reserves to fund our operations.
22
RESULTS
OF OPERATIONS
The
Fiscal Year Ended March 31, 2009 Compared to the Fiscal Year Ended March 31,
2008
Assets. At
March 31, 2009, we had $68,666,322 in total assets, compared to $57,427,420 at
March 31 2008. This was primarily due to an increase in cash and cash
equivalents to $38,181,837 at March 31, 2009 from $15,165,620 at March 31, 2008
following our Regulation S Private Placement. Our inventories also increased to
$3,908,629 at March 31, 2009 from $2,619,429 at March 31, 2008 due to
anticipated sales increases. At March 31, 2009, our restricted cash decreased to
$0 from $37,845,432 at March 31, 2008 because of release from restriction.
Restricted cash consisted of $37 million of funds received during March of 2008
from investors for the Regulation S Private Placement Offering and held in
escrow until closing.
Liabilities.
At March 31, 2009, our Total Liabilities were $12,276,962, compared to
$55,687,992 at March 31, 2008. Liabilities consist of Accounts Payable; Other
Liabilities; Unearned Revenue; Deposits from Investors; and Income Tax Payable.
Accounts payable increased to $3,610,195 at March 31, 2009 from $804,041 at
March 31, 2008 primarily due to due to increases in freight expenses incurred
from decreased sales. Other liabilities consist of commissions (Affiliate
rewards) payable, payroll liabilities and other liabilities, and decreased to
$6,675,552 at March 31, 2009 from $12,787,714 at March 31, 2008 because of
decreased commissions (Affiliate rewards) payable and payroll payable. Unearned
revenue consists of customer deposits for unshipped products, and decreased to
$1,991,215 at March 31, 2009 from $3,945,805 at March 31, 2008 due to decreased
orders. Deposits from investors decreased to $0 at March 31, 2009 from
$37,845,432 at March 31, 2008 due to our Regulation S Private Placement Offering
being completed in year 2008. Income tax payable decreased to $0 at March 31,
2009 from $305,000 at March 31, 2008 due to recognition of a tax calculation
method according to the treasury regulations.
Stockholders’
Equity (Deficit). Our Stockholders’ Equity (Deficit) increased to
$56,389,360 at March 31, 2009 from $1,739,428 at March 31, 2008. This increase
was primarily due to an increase in Additional Paid in Capital to $52,854,891 at
March 31, 2009 from $6,552 at March 31, 2008 and in Retained Earnings to
$4,023,992 at March 31, 2009 from $1,895,330 at March 31, 2008.
Revenues. Our Revenues decreased to
$12,846,809 for the fiscal year ended March 31, 2009 from $30,249,302 for the
fiscal year ended March 31, 2008 because of decreased sales. Our decrease in
sales can be attributable to several factors including the worldwide recession
and several natural disasters in China in 2009. Also, in July 2008, we increased
our initial cost to our customers in order for them to become Affiliates from
$250 to $300. This increase in the initial cost might have contributed to a
decrease in sales as customers believe that such an increase is prohibitively
expensive for their budgets. Our products are discretionary items and in light
of the worldwide recession, consumers have been decreasing their purchases of
discretionary items. Also, in 1
st quarter of 2008, we discontinued two (2) of our
environmental home care products and, in 4 th quarter of 2008,
we have added four (4) nutritional products. Consumers might have decreased
their purchases of our products due to the change in the products we
offer.
Shipping
Charges . Shipping Charges decreased to $5,657,625 for the fiscal year
ended March 31, 2009 from $10,110,360 for the fiscal year ended March 31, 2008
due to decreased sales.
Costs of Goods
Sold. Costs of Goods Sold decreased to $5,780,447 for the fiscal year
ended March 31, 2009 from $11,423,852 for the fiscal year March 31, 2008. Costs
of Goods Sold consist of merchandise purchases from vendors and decreased
because of decreased sales.
Shipping
Costs . Shipping Costs decreased to $2,204,502 for the fiscal year ended
March 31, 2009 from $ $4,467,140 for the fiscal year ended March 31, 2008.
Shipping Costs consist of freight charges to our Hong Kong facility and
decreased because of decreased sales.
Gross
Profits . Gross Profits decreased to $10,519,486 for the fiscal year
ended March 31, 2009 from $24,468,670 for the fiscal year ended March 31, 2008.
Our gross profit margin for the fiscal year ended March 31, 2009 decreased to
57% from 61% for the fiscal year ended March 31, 2008. Gross Profits and gross
profit margins decreased because of decreased sales in year 2009. Also, during
the year 2009, we held many promotions by giving out free products to boost our
sale. As a result, our cost of goods sold was correspondingly higher in year
2009.
Selling, General
and Administrative Expenses . Selling, General and Administrative
Expenses increased to $8,929,162 for the fiscal year ended March 31, 2009 from
$3,693,369 for the fiscal year ended March 31, 2008. Selling, General and
Administrative Expenses consist of advertising and corporate administrative
expenses, and increased because of professional fees incurred in connection with
our Regulation S Private Placement Offering and royalty fees accrued for
trademark at the year end.
Interest
Income . Interest Income increased to $1,246,433 for the fiscal year
ended March 31, 2009 from $275,538 for the fiscal year ended March 31, 2008.
Interest Income consists of bank deposit interest, interest income from the
outstanding loans and increased because of cash balance increases due to our
Regulation S Private Placement Offering.
Foreign Exchange
Loss . Foreign Exchange Gain increased to $723,357 for the fiscal year
ended March 31, 2009 from $(4,248) for the fiscal year ended March 31, 2008.
Foreign Exchange Gain increased because of gains on foreign exchange
rates.
Other Income
(Net). Other Income (Net) increased to $634,635 for the fiscal year ended
March 31, 2009 from $54,904 for the fiscal year ended March 31, 2008. Other
Income (Net) consists of fees received for educational training classes and
increased due to additional classes held.
23
LIQUIDITY
AND CAPITAL RESOURCES
As
reflected in the accompanying consolidated financial statements, at March 31,
2009, the Company had $38,181,837 cash on hand and a stockholders’ equity of
$56,389,360 . To date, we have funded our operations primarily from sales to our
Affiliates and through private equity financings. While we believe in the
viability of our strategy to improve sales volume and in our ability to raise
additional funds, there can be no assurances to that effect.
At March
31, 2009, we had $68,666,322 in total assets, compared to $57,427,420 at March
31, 2008. This was primarily due to our Regulation S Private Placement and
outstanding loans. Our inventories also increased to $3,908,629 at March 31,
2009 from $2,619,429 at March 31, 2008 due to anticipated sales increase. Our
increase in investments was due to an equity investment in Excalibur to
$17,129,314 at March 31, 2009 from $0 at March 31, 2008, and related party Notes
Receivable of $5,961,717 at March 31, 2009 compare to $0 at March 31, 2008 was
due to loans made to Excalibur and Yeuh-Chi Liu. At March 31, 2009 we had
$508,746 invested in mutual funds, compared to $835,965 at March 31, 2008. Our
prepaid expenses increased to $2,551,298 at March 31, 2009 from $793,760 at
March 31, 2008.
Our
products are sensitive to business and personal discretionary spending levels
and tend to decline or grow more slowly during economic downturns, including
downturns in any of our major markets. The current worldwide recession is
expected to adversely affect our sales and liquidity for the foreseeable future.
Although we have mitigated decreases in sales by lowering our levels of
inventory to preserve cash on hand, we do not know when the recession will
subside and when consumer spending will increase from its current depressed
levels. Even if consumer spending increases, we are not sure when consumer
spending will increase for our products which will affect our liquidity. We
believe we have enough capital to fund our operations during the next 12
months.
Our
Revenues decreased to $12,846,809 for the fiscal year ended March 31, 2009 from
$30,249,302 for the fiscal year ended March 31, 2008 due to decreased sales. In
July 2008, we increased our initial cost to our customers in order for them to
become Affiliates from $250 to $300. This increase in the initial cost might
have contributed to a decrease in sales as customers believe that such an
increase is prohibitively expensive for their budgets. Our products are
discretionary items and in light of the worldwide recession, consumers have been
decreasing their purchases of discretionary items. Also, in 1 st quarter of ,
2008, we discontinued two (2) of our environmental home care products and , in
4 th quarter of
2008, we have added four (4) nutritional products. Consumers might have
decreased their purchases of our products due to the change in the products we
offer.
Accounts
Payable increased to $3,610,195 at March 31, 2009 from $804,041 at March 31,
2008 due to the trademark royalty fee accrued at the year end. Other Liabilities
consist of commissions (Affiliate rewards) payable, payroll liabilities and
other liabilities, and decreased to $6,657,552 at March 31, 2009 from
$12,787,714 at March 31, 2008 because of decreases in commissions (Affiliate
rewards) payable as a result of decreases in sales. Unearned Revenues consist of
customer deposits for unshipped products, and decreased to $1,991,215 at March
31, 2009 from $3,945,805 at March 31, 2008 because of decreased sales. Deposits
from Investors consist of net proceeds from our Regulation S Private Placement
Offering of Units, and decreased to $0 at March 31, 2009 from $37,845,432 at
March 31, 2008 because of the expiration of the Regulation S Offering. Income
Tax Payables consist of Federal Tax Provision, and decreased to $0 at March 31,
2009 from $305,000 at March 31, 2008 because of recognition of a tax calculation
method according to the treasury regulation.
Our
stockholders’ equity increased to $56,389,360 at March 31, 2009 from $1,739,428
at March 31, 2008. This increase was primarily due to an increase in retained
earnings to $4,023,992 at March 31, 2009 from $1,895,330 at March 31, 2008 and
proceeds from our Regulation S Private Placement of $51,149,412 at December 31,
2008.
In
January of 2008, we commenced a private placement of Units exclusively to
non-U.S. residents at a purchase price of $3.80 per Unit under the exemption
from the registration requirements of the Securities Act of 1933, as amended,
afforded the Registrant under Regulation S thereunder due to the fact that
offers and sales were only made to non U.S. residents. The offering was
conducted on a best-efforts basis. The original offering was for up to
10,000,000 Units but was oversubscribed and increased to 14,890,040 Units
pursuant to the terms of the Private Placement Memorandum.
Each Unit
consisted of one share of Common Stock and one Redeemable Common Stock Purchase
Warrant (a “Warrant”). Each Warrant is exercisable to purchase one share of
Common Stock at $3.80 per share until the second anniversary date of the date of
issuance. The Warrants are redeemable by the Registrant, on a pro rata basis, at
a purchase price of $0.0001 per share within 30 days from the tenth (10th)
consecutive trading day that the Registrant’s common stock trades on the OTCBB
or any public securities market within the U.S. at a closing sales price, or the
average of the closing bid and asked price, of at least $11.
Moneys
received from investors were held in an escrow account by Buckman, Buckman &
Reid, Inc., the placement agent, pending the payment of attorneys’ fees and
placement agent fees and considered “restricted cash.” The cash was released
from escrow once such payments were made and following each of five closings:
three in July of 2008, one in August of 2008 and one in October of 2008. The
cash was then available for lending or operating purposes. The related Units
were issued following each closing. Until such release from escrow “restricted
cash” was accounted for as an asset and a liability. Following the release from
escrow and until the completion of the offering in October 2008 proceeds
received from the offering were considered “deposits from investors” and
accounted for as a liability in accordance with GAAP. The private placement
ended on October 25, 2008 and the Registrant sold an aggregate of 14,890,040
Units for net proceeds of $51,149,412 consisting of a total of 14,890,040 shares
of Common Stock and 14,890,040 Warrants. As of the date hereof, none of the
warrants have been exercised or redeemed.
24
TABULAR
DISCLOSURE OF CONTRACTUAL OBLIGATIONS
|
Payments
due by period
|
||||||||||||||||||||
|
Total
|
Less than
1 year
|
1-3 years
|
3-5
years
|
More
than 5
years
|
||||||||||||||||
|
Long-Term
Debt Obligations
|
- | - | - | - | ||||||||||||||||
|
Capital
Lease Obligations
|
- | - | - | - | ||||||||||||||||
|
Operating
Lease Obligations (1)
|
$ | 413,875 | $ | 721,135 | - | - | ||||||||||||||
|
Purchase
Obligations
|
- | - | - | - | ||||||||||||||||
|
Other
Long-Term Liabilities Reflected on the Registrant's Balance Sheet under
GAAP
|
- | - | - | - | ||||||||||||||||
|
Total
|
$ | 413,875 | $ | 721,135 | - | - | ||||||||||||||
|
(1)
|
Operating
Lease
|
The
Company leases office space in the US under an operating lease agreement. The
lease provides for monthly lease payments approximating $10,063 and expires on
July 31, 2009. Future minimum lease payments under the operating leases as of
March 31, 2009 approximate the following:
|
Year Ending March 31,
|
||||
|
2010,
four months
|
$
|
40,250
|
||
The
Company rents office space for its sales division in Hong Kong. The lease
provides for free lease in the first two years and a monthly lease payments
approximating $50,000 USD starting the beginning of the third year and expires
on March 31, 2012. Expensing the 5-year total rent evenly over the life of the
lease, the future minimum lease payments under the operating lease are as
follows:
|
Year Ending March 31,
|
||||
|
2010
|
$
|
360,000
|
||
|
2011
|
360,000
|
|||
|
2012
|
360,000
|
|||
The
Company rents storage space for its sales division in Hong Kong. The lease
provides for monthly lease payments approximating $1,135 USD starting on May 8,
2008 and expires on May 7, 2010. Future minimum lease payments under the
operating leases as of March 31, 2009 approximate the following:
|
Year Ending March 31,
|
||||
|
2010
|
$
|
13,625
|
||
|
2011
|
1,135
|
|||
Rent
expenses for the year ended March 31, 2009 and March 31, 2008 were approximately
$494,487 and $487,896, respectively.
Excalibur
International Marine Corporation
Due to
the recent changes in policy between Mainland China and Taiwan, an opportunity
was recognized to take advantage of direct sailings for cargo and passengers
through the Taiwan Strait. EFT identified Excalibur International Marina
Corporation (“Excalibur”), a shipping company located in Taiwan, as a viable
entity to participate with in this business opportunity. In order to expedite
the purchase of a new vessel, EFT’s Board of Directors approved a non-interest
bearing, unsecured loan to facilitate this purchase. On July 28, 2008, the
Registrant loaned $19,193,000 to Excalibur. This loan was still outstanding with
balance of $1,564,717 March 31, 2009. At the time of the transaction, Excalibur
was not a related party nor did any of the Company or any of its officers or
directors have any relationship with Excalibur or any of its officers and
directors.
25
On
September 23, 2008, the Registrant signed a loan agreement with Excalibur to
lend $2,000,000 at an interest rate of 3.75% per month with a term of no more
than 60 days. At the end of the 60 days term, the term of the loan was extended
for six months. On November 23, 2008, the Company extended this loan to May 25,
2009. On May 25, 2009, the Company extended this loan to Excalibur for another
six months and decreased the interest rate to 12.5% per annum.
On
October 20, 2008, EFT Investment Co., Ltd. was formed as a wholly-owned
subsidiary of EFT BioTech Holdings, Inc. EFT Investment Co., Ltd was formed in
Taiwan. On October 25, 2008, EFT Investment Co., Ltd. completed the acquisition
of 585,677,500 shares of common stock of Excalibur; representing approximately
49% shares of issued and outstanding shares of Excalibur, for an aggregate
purchase price of USD $19,193,000. Prior to the acquisition of Excalibur,
Excalibur was not a related person under Item 404 of Regulation
S-K.
On
November 25, 2008, the Registrant signed an additional loan agreement with
Excalibur, a related party, pursuant to which the Registrant loaned Excalibur
$500,000 at the interest rate of 3.75% per month with a term of 30 days with an
extension of six months. On December 25, 2008, the Company extended the loan to
May 25, 2009. On May 25, 2009, the Company extended this loan for another six
months and decreased the interest rate to 12.5% per annum.
Note
Receivable – Related party
The Board
of Directors approved two non-interest bearing unsecured demand loans in the
amount of U.S. $330,000 and $1,567,000 respectively on July 14, 2008 and July
25, 2008 to Yeuh-Chi Liu, a vendor and a member of the board of
directors of Excalibur. As of the date hereof the full principal amount remains
outstanding.
26
Off-Balance
Sheet Arrangements
The
Registrant does not have any off-balance sheet arrangements that have or are
reasonably likely to have a current or future effect on the Registrant’s
financial condition, changes in financial condition, revenues or expenses,
results of operations, liquidity, capital expenditures or capital resources that
is material to investors.
Quantitative
and Qualitative Disclosures about Market Risk
For our
fiscal year ended March 31, 2009, 100% of our total sales consisted of sales
outside of the United States, with less than 0% of total sales denominated in
currencies other than the United States dollar. In addition, from time to time
we execute intercompany loans with our foreign subsidiaries that are denominated
in foreign currencies.
We are
exposed to foreign currency risks that arise from normal business operations.
These risks include the translation of local currency balances of our Company
and foreign subsidiaries, intercompany loans with foreign subsidiaries and
transactions denominated in foreign currencies. It is our policy not to enter
into derivative financial instruments for speculative purposes. We do not hedge
our exposure to the translation of reported results of our foreign subsidiaries
from local currency to United States dollars. A 10% adverse change in the
underlying foreign currency exchange rates would not be significant to our
financial condition or results of operations.
Critical
Accounting Policies
The
Registrant’s financial statements are based on the selection and application of
significant accounting policies, which require management to make significant
estimates and assumptions. The Registrant believes that the following are some
of the more critical judgment areas in the application of the Registrant’s
accounting policies that currently affect the Registrant’s financial condition
and results of operations.
Cash
& Cash Equivalents
Cash and
cash equivalents include cash on hand and cash in time deposits, and
certificates. The Company maintains its accounts in various banks and several
which exceed the federally insured limit.
Inventories
Inventories
are valued at the lower of cost or market. Product cost includes completed
merchandise and is accounted for using the first-in, first-out basis. The
Company has two warehouses, one in City of Industry, CA and the other one in
Kowloon, HK. On a quarterly basis, the Company reviews inventory levels in each
country for estimated obsolescence or unmarketable items, as compared to future
demand requirements and the shelf life of the various products. Based on this
review, the Company records inventory write-downs when costs exceed expected net
realizable value. Historically, the Company estimates of the obsolete or
unmarketable items have been insignificant.
SFAS No.
151, “Inventory Costs,” (“SFAS 151”) clarifies that abnormal amounts of idle
facility expense, freight, handling costs, and wasted material (spoilage) should
be recognized as period charges, rather than as an inventory value. This
standard also requires the allocation of fixed production overheads to inventory
based on the normal capacity of the production facilities. The Registrant’s
existing accounting policy for inventory valuation is generally consistent with
this guidance, and therefore, the adoption of SFAS 151 did not have a
significant impact on the Registrant’s 2008 and 2009 financial
results.
Notes
Receivables from Related Parties
Notes
receivable consists of receivables from the Registrant’s loans to Excalibur,
Taiwan, and Yeuh-Chi Liu, a related party. As of March 31, 2009, outstanding
loans to Excalibur totaled $ 4.06 million and to Yeuh-Chi Liu $1.89 million. The
Registrant periodically reviews notes receivables for reliability and
collectability, and recent account activities. If the Registrant’s estimates
regarding collectability are inaccurate or an unforeseen matter is to occur, the
Registrant may be exposed to a write-offs or bad debts. As of March 31, 2009,
the Registrant does not have an allowance for bad debts.
Investment
The
Registrant accounts for equity investments in entities in which it exercises
significant influence but does not own a majority equity interest in or have
control using the equity method. The Registrant evaluates its equity investments
for impairment whenever events and changes in business circumstances indicate
the carrying amount of the equity investment may not be fully recoverable. On
October 25, 2008, the Registrant, through its wholly-owned subsidiary, EFT
Investment Co. Ltd., invested $19,193,000 in Excalibur International Marine
Corporation for 49% of its ownership. The Registrant recorded this investment
using the equity method because of its significant influence over the
entity.
27
Unearned
Revenues
Unearned
Revenues consist of cash amounts received in advance for goods and services to
be delivered at a future date. The Registrant records the cash from customers as
a liability until the products are delivered.
Revenue
The
Registrant receives payment by cash only for orders from customers or
Affiliates. Cash consideration given by the Registrant to its sales Affiliates
is considered to be a reduction of the selling prices of the Company’s products,
thus, is recorded as a reduction of revenue. Sales revenue are recorded when the
merchandise delivery is completed.
Foreign
Currency Translation
The
Company’s functional currency is the U.S. dollar and its operation in Hong Kong
uses Hong Kong dollar (HKD) as its functional currency. An entity’s functional
currency is the currency of the primary economic environment in which the entity
operates. Management must use judgment in determining an entity’s functional
currency, assessing economic factors including cash flow, sales price, sales
market, expense, financing and inter-company transactions and arrangements.
Impact from exchange rate changes related to transactions denominated in
currencies other than the functional currency is recorded as a gain and loss in
the statements of operations, while impact from exchange rate changes related to
translating a foreign entity’s financial statements from the functional currency
to its reporting currency, the U.S. dollar, is disclosed and accumulated in a
separate component under the equity section of the balance sheets. Different
judgments or assumptions resulting in a change of functional currency may
materially impact the Registrant’s financial position and results of
operations.
Income
Taxes
The
Registrant uses the asset and liability method of accounting for income taxes.
Under this method, income tax expense is recognized for the amount of taxes
payable or refundable for the current year. In addition, deferred tax assets and
liabilities are recognized for the expected future tax consequences of temporary
differences between the financial reporting and tax bases of assets and
liabilities and for operating losses and tax credit carry-forwards. Management
must make assumptions, judgments and estimates to determine the current
provision for income taxes and the deferred tax assets and liabilities and any
valuation allowance to be recorded against a deferred tax asset. Management’s
judgments, assumptions and estimates relative to the current provision for
income tax take into account current tax laws, management’s interpretation of
current tax laws and possible outcomes of current and future audits conducted by
foreign and domestic tax authorities. Changes in tax law or management’s
interpretation of tax laws and the resolution of current and future tax audits
could significantly impact the amounts provided for income taxes in the
financial statements. Management’s assumptions, judgments and estimates relative
to the value of a deferred tax asset take into account predictions of the amount
and category of future taxable income, such as income from operations. Actual
operating results and the underlying amount and category of income in future
years could render management’s current assumptions, judgments and estimates of
recoverable net deferred taxes inaccurate. Any of the assumptions, judgments and
estimates mentioned above could cause our actual income tax obligations to
differ from the estimates, thus materially impacting the financial position and
results of operations.
RECENT
ACCOUNTING PRONOUNCEMENTS
The FASB
issued SFAS No. 168, The FASB
Accounting Standards Codification and the Hierarchy of Generally Accepted
Accounting Principles, on June 29, 2009 and, in doing so,
authorized the Codification as the sole source for authoritative U.S. GAAP. SFAS
No. 168 will be effective for financial statements issued for reporting periods
that end after September 15, 2009. Once it's effective, it will supersede all
accounting standards in U.S. GAAP, aside from those issued by the SEC. SFAS No.
168 replaces SFAS No. 162 to establish a new hierarchy of GAAP sources for
non-governmental entities under the FASB Accounting Standards Codification. The
Company will evaluate the impact of SFAS No. 168 upon its
effectiveness.
SFAS No.
167 amends FASB Interpretation (FIN) No. 46(R), Consolidation of Variable Interest
Entities, by altering how a company determines when an entity that is
insufficiently capitalized or not controlled through voting should be
consolidated, the FASB said. A company has to determine whether it should
provide consolidated reporting of an entity based upon the entity's purpose and
design and the parent company's ability to direct the entity's
actions.
The
standards will be effective at the start of the first fiscal year beginning
after November 15, 2009, which will mean January 2010 for companies that are on
calendar years. The guidance will have to be applied for first-quarter filings.
The Company will comply with the disclosure requirements of this statement when
and if it acquires a variable interest entity, upon its
effectiveness.
28
FAS No.
166 revises SFAS No. 140,
Accounting for Transfers and Servicing of Financial Assets and Extinguishments
of Liabilities, and will require entities to provide more
information about sales of securitized financial assets and similar
transactions, particularly if the seller retains some risk to the assets, the
FASB said. The statement eliminates the concept of a qualifying special-purpose
entity, changes the requirements for the derecognition of financial assets, and
calls upon sellers of the assets to make additional disclosures about them. The
Company does not believe implementation of FAS No. 166 will have a material
impact on its financial statements.
On May
28, 2009 the FASB announced the issuance of SFAS 165, Subsequent Events . SFAS 165
should not result in significant changes in the subsequent events that an entity
reports. Rather, SFAS 165 introduces the concept of financial statements being
available to be issued. Financial statements are considered available to be
issued when they are complete in a form and format that complies with generally
accepted accounting principles (GAAP) and all approvals necessary for issuance
have been obtained. The Company believes that SFAS No. 165 will have an effect
on its financial statements and will comply upon effectiveness
ITEM
7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not
applicable.
ITEM
8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
INDEX TO CONSOLIDATED FINANCIAL
STATEMENTS
|
Page(s)
|
||
|
Audited
Consolidated Financial Statements
|
||
|
Consolidated
Balance Sheets
|
F-2
|
|
|
Consolidated
Statements of Operations and Other Comprehensive Income
|
F-3
|
|
|
Consolidated
Statements of Changes in Stockholders’ Equity
|
F-4
|
|
|
Consolidated
Statements of Cash Flows
|
F-5
|
|
|
Notes
to Consolidated Financial Statements
|
F-6
|
29

|
Douglas
W. Child, CPA
Marty D. Van Wagoner, CPA
J.
Russ Bradshaw, CPA
William
R. Denney, CPA
Russell
E. Anderson, CPA
Scott
L. Farnes
1284 W. Flint Meadow Dr. #D
Kaysville,
Utah 84037
Telephone
801.927.1337
Facsimile
801.927.1344
5296
S. Commerce Dr. #300
Salt
Lake City, Utah 84107
Telephone
801.281.4700
Facsimile
801.281.4701
Suite
A, 5/F
Max
Share Centre
373
King’s Road
North
Point, Hong Kong
Telephone
852.21.555.333
Facsimile
852.21.165.222
www.cpaone.net
|
Report
of Independent Registered Public Accounting Firm
To
the Board of Directors and Audit Committee
EFT
Biotech Holdings, Inc.
City
of Industry, California
We
have audited the consolidated balance sheets of EFT Biotech Holdings, Inc.
(the Company) as of March 31, 2009 and 2008, and the related consolidated
statements of operations and other comprehensive income, stockholders’
equity and cash flows for the years then ended. These
consolidated financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on
these consolidated financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public
Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audits to obtain reasonable
assurance about whether the consolidated financial statements are free of
material misstatement. The company is not required to have, nor
were we engaged to perform, an audit of its internal control over
financial reporting. Our audit included consideration of
internal control over financial reporting as a basis for designing audit
procedures that are appropriate in the circumstances, but not for the
purpose of expressing an opinion on the effectiveness of the company’s
internal control over financial reporting. Accordingly, we
express no such opinion. An audit also includes examining, on a
test basis, evidence supporting the amounts and disclosures in the
consolidated financial statements, assessing the accounting principles
used and significant estimates made by management, as well as evaluating
the overall consolidated financial statement presentation. We
believe that our audits provide a reasonable basis for our
opinion.
In
our opinion, the consolidated financial statements referred to above
present fairly, in all material respects, the consolidated financial
position of EFT Biotech Holdings, Inc. as of March 31, 2009 and 2008, and
the consolidated results of its operations and its cash flows for the
years ended, in conformity with accounting principles generally accepted
in the United States of America.
/s/
Child, Van Wagoner & Bradshaw, PLLC
Child,
Van Wagoner & Bradshaw, PLLC
Salt
Lake City, Utah
July
15, 2009
|
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Balance Sheets
|
As of March 31,
|
||||||||
|
2009
|
2008-Restated
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 38,181,837 | $ | 15,165,620 | ||||
|
Inventories
|
3,908,629 | 2,619,429 | ||||||
|
Available
for sale securities
|
508,746 | 835,965 | ||||||
|
Prepaid
expenses
|
2,551,298 | 793,760 | ||||||
|
Short-term
note receivables – related party
|
4,064,717 | - | ||||||
|
Total
current assets
|
49,215,227 | 19,414,774 | ||||||
|
Property
and equipment, net
|
360,156 | 140,106 | ||||||
|
Other
receivables
|
33,504 | - | ||||||
|
Investments
|
17,129,314 | - | ||||||
|
Restricted
cash
|
- | 37,845,432 | ||||||
|
Loan
to related party
|
1,897,000 | - | ||||||
|
Security
deposit
|
31,121 | 27,108 | ||||||
|
Total
assets
|
$ | 68,666,322 | $ | 57,427,420 | ||||
|
LIABILITIES
AND STOCKHOLDERS' EQUITY (DEFICIT)
|
||||||||
|
Current
liabilities
|
||||||||
|
Accounts
payable and accrued expenses
|
$ | 3,610,195 | $ | 804,041 | ||||
|
Other
liabilities
|
6,675,552 | 12,787,714 | ||||||
|
Unearned
revenues
|
1,991,215 | 3,945,805 | ||||||
|
Deposits
from investors
|
- | 37,845,432 | ||||||
|
Income
tax payable
|
- | 305,000 | ||||||
|
Total
current liabilities
|
12,276,962 | 55,687,992 | ||||||
|
Stockholders'
equity
|
||||||||
|
Preferred
stock, $.001 par value, 25,000,000 shares authorized, none issued and
outstanding
|
- | - | ||||||
|
Common
stock, $0.00001 par value, 4,975,000,000 authorized, 75,983,205 and
61,022,414 shares issued and outstanding at March 31, 2009 and
2008
|
760 | 610 | ||||||
|
Additional
paid in capital
|
52,854,891 | 6,552 | ||||||
|
Retained
earnings
|
4,023,992 | 1,895,330 | ||||||
|
Accumulated
other comprehensive loss
|
(490,283 | ) | (163,064 | ) | ||||
|
Total
stockholders' equity
|
56,389,360 | 1,739,428 | ||||||
|
Total
liabilities and stockholders' equity
|
$ | 68,666,322 | $ | 57,427,420 | ||||
The
accompanying notes are an integral part of these consolidated financial
statements.
F-2
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Statements of Operations and Other Comprehensive Income
|
Year Ended
|
||||||||
|
March 31, 2009
|
March 31, 2008 - Restated
|
|||||||
|
Sales
revenues, net
|
$ | 12,846,809 | $ | 30,249,302 | ||||
|
Shipping
charge
|
5,657,625 | 10,110,360 | ||||||
| 18,504,434 | 40,359,662 | |||||||
|
Cost
of goods sold
|
5,780,447 | 11,423,852 | ||||||
|
Shipping
cost
|
2,204,502 | 4,467,140 | ||||||
| 7,984,949 | 15,890,992 | |||||||
|
Gross
profit
|
10,519,485 | 24,468,670 | ||||||
|
Selling,
general and administrative expenses
|
8,929,162 | 3,693,369 | ||||||
|
Net
operating income
|
1,590,323 | 20,775,301 | ||||||
|
Other
income (expense)
|
||||||||
|
Interest
income
|
1,246,433 | 275,538 | ||||||
|
Investment
loss
|
(2,063,686 | ) | - | |||||
|
Foreign
exchange income
|
723,357 | (4,248 | ) | |||||
|
Other
income, net
|
634,635 | 54,904 | ||||||
|
Total
other income
|
540,739 | 326,194 | ||||||
|
Net
income before income taxes
|
2,131,062 | 21,101,495 | ||||||
|
Income
taxes
|
2,400 | 305,800 | ||||||
|
Net
income
|
$ | 2,128,662 | $ | 20,795,695 | ||||
|
Unrealized
loss on available for sale securities
|
(327,219 | ) | (163,064 | ) | ||||
|
Comprehensive
income
|
$ | 1,801,443 | $ | 20,632,631 | ||||
|
Net
income per common share
|
||||||||
|
Basic
and diluted
|
$ | 0.03 | $ | 0.37 | ||||
|
Weighted
average common shares outstanding
|
||||||||
|
Basic
and diluted
|
66,637,448 | 55,350,545 | ||||||
The
accompanying notes are an integral part of these consolidated financial
statements.
F-3
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Statements of Changes in Stockholders’ Equity
|
Accumulated
|
||||||||||||||||||||||||
|
Additional
|
Other
|
Total
|
||||||||||||||||||||||
|
Common Stock
|
Paid-in
|
Retained
|
Comprehensive
|
Stockholders'
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Earnings
|
Income (Loss)
|
Equity
|
|||||||||||||||||||
|
BALANCE,
,MARCH 31, 2007
|
52,099,000
|
$
|
521
|
$
|
4,479
|
$
|
(374,188
|
)
|
$
|
-
|
$
|
(369,188
|
)
|
|||||||||||
|
Issuance
of common stock for services
|
1,201,000
|
12
|
2,150
|
-
|
-
|
2,162
|
||||||||||||||||||
|
Shares
effectively issued to former shareholders as part of the
recapitalization
|
7,722,414
|
77
|
(77
|
)
|
-
|
-
|
-
|
|||||||||||||||||
|
Net
income
|
-
|
-
|
-
|
20,795,695
|
-
|
20,795,695
|
||||||||||||||||||
|
Dividend
paid
|
-
|
-
|
-
|
(18,526,177
|
)
|
-
|
(18,526,177
|
)
|
||||||||||||||||
|
Unrealized
loss on available for sale securities
|
-
|
-
|
-
|
-
|
(163,064
|
)
|
(163,064
|
)
|
||||||||||||||||
|
BALANCE,
MARCH 31, 2008 - restated
|
61,022,414
|
610
|
6,552
|
1,895,330
|
(163,064
|
)
|
1,739,428
|
|||||||||||||||||
|
Shares
effectively issued to former shareholders as part of the
recapitalization
|
66,667
|
1
|
(1
|
)
|
-
|
-
|
-
|
|||||||||||||||||
|
Shares
issued for service
|
4,084
|
-
|
16,731
|
-
|
-
|
16,731
|
||||||||||||||||||
|
Shares
issued pursuant to private placement offering-common stock, net of
operating costs
|
14,890,040
|
149
|
43,919,414
|
-
|
43,919,563
|
|||||||||||||||||||
|
Fair
value of warrants issued with common stock
|
8,912,195
|
8,912,195
|
||||||||||||||||||||||
|
Net
income
|
2,128,662
|
2,128,662
|
||||||||||||||||||||||
|
Unrealized
loss on available for sale securities
|
-
|
-
|
-
|
-
|
(327,219
|
)
|
(327,219
|
)
|
||||||||||||||||
|
BALANCE,
MARCH 31, 2009
|
75,983,205
|
$
|
760
|
$
|
52,854,891
|
$
|
4,023,992
|
$
|
(490,283
|
)
|
$
|
56,389,360
|
||||||||||||
The
accompanying notes are an integral part of these consolidated financial
statements.
F-4
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Statements of Cash Flows
|
Year Ended
|
||||||||
|
March 31, 2009
|
March 31, 2008
|
|||||||
|
Cash
flows from operating activities:
|
||||||||
|
Net
income
|
$
|
2,128,662
|
$
|
20,795,695
|
||||
|
Adjustments
to reconcile net income to net cash
|
||||||||
|
provided
by (used in) operating activities:
|
||||||||
|
Depreciation
and amortization
|
51,514
|
38,340
|
||||||
|
Loss
on investment-equity method
|
2,063,686
|
-
|
||||||
|
Warranty
liability
|
(33,924
|
)
|
36,912
|
|||||
|
Stock
based compensation
|
16,731
|
2,162
|
||||||
|
Changes
in operating assets and liabilities:
|
||||||||
|
Inventories
|
(1,289,200
|
)
|
(833,670
|
)
|
||||
|
Prepaid
expenses and other current assets
|
(1,761,551
|
)
|
(411,560
|
)
|
||||
|
Accounts
payable and accrued liabilities
|
2,806,154
|
497,625
|
||||||
|
Other
liabilities
|
(6,078,238
|
)
|
12,372,996
|
|||||
|
Unearned
revenues
|
(1,954,590
|
)
|
1,434,470
|
|||||
|
Income
tax payable
|
(305,000
|
)
|
305,000
|
|||||
|
Net
cash provided by operating activities
|
(4,355,756
|
)
|
34,237,970
|
|||||
|
Cash
flows from investing activities:
|
||||||||
|
Additions
to fixed assets
|
(305,068
|
)
|
(101,706
|
)
|
||||
|
Note
receivables – related party
|
(5,961,717
|
)
|
-
|
|||||
|
Investment
|
(19,193,000
|
)
|
-
|
|||||
|
Purchase
of available for sale securities
|
-
|
(999,029
|
)
|
|||||
|
Net
cash (used in) investing activities
|
(25,459,785
|
)
|
(1,100,735
|
)
|
||||
|
Cash
flows from financing activities:
|
||||||||
|
Restricted
cash
|
-
|
(37,845,432
|
)
|
|||||
|
Proceeds
from investor deposits
|
-
|
37,845,432
|
||||||
|
Proceeds
from issuance of stock and warrants
|
52,831,758
|
-
|
||||||
|
Payment
of dividends
|
-
|
(18,526,177
|
)
|
|||||
|
Net
cash provided by (used in) financing activities
|
52,831,758
|
(18,526,177
|
)
|
|||||
|
Net
increase (decrease) in cash
|
23,016,217
|
14,611,058
|
||||||
|
Cash,
beginning of period
|
15,165,620
|
554,562
|
||||||
|
Cash,
end of period
|
$
|
38,181,837
|
$
|
15,165,620
|
||||
|
Supplemental
disclosures of cash flow information:
|
||||||||
|
Interest
paid in cash
|
$
|
-
|
$
|
-
|
||||
|
Income
taxes paid in cash
|
$
|
2,400
|
$
|
800
|
||||
|
Non-cash
investing and financing activities:
|
||||||||
|
Unrealized
loss on available for sale securities
|
$
|
327,219
|
$
|
163,064
|
||||
|
Fixed
assets sold with receivable
|
$
|
33,504
|
$
|
-
|
||||
|
Release
of cash from restriction
|
$
|
37,845,432
|
$
|
-
|
||||
The
accompanying notes are an integral part of these consolidated financial
statements.
F-5
Note
1 - ORGANIZATION
EFT
Biotech Holdings, Inc. (“EFT Holdings” or “the Company”), formerly HumWare Media
Corporation, GRG, Inc., Ghiglieri Corporation, Karat Productions, Inc., was
incorporated in the State of Nevada on March 19, 1992.
On
November 18, 2007, the Company issued an aggregate of 53,300,000 shares of its
common stock in connection with a share exchange with the stockholders of EFT
BioTech, Inc. (“EFT BioTech”), a Nevada Corporation formed on September 18, 2007
(the “Transaction”), pursuant to which EFT BioTech became a wholly-owned
subsidiary of the Company. The 53,300,000 common shares issued included
52,099,000 to pre-capitalization shareholders and 1,201,000 to four directors
and officers of EFT BioTech, and represented approximately 87.34% of the
Company’s common stock outstanding after the Transaction. Consequently, the
stockholders of EFT BioTech, Inc. own a majority of the Company's common stock
immediately following the Transaction, therefore, the Transaction is being
accounted for as a "reverse acquisition", and EFT BioTech is deemed to be the
accounting acquirer in the reverse acquisition. As EFT Holdings was a
non-operating public shell corporation that acquired an operating company,
this Transaction is treated as a capital transaction where the acquiring
corporation issued stock for the net monetary assets of the shell corporation,
accompanied by a recapitalization. The accounting is similar in form to a
reverse acquisition, except that goodwill or other intangibles are not
recorded. All references to EFT BioTech common stock have been
restated to reflect the equivalent numbers of EFT Holdings common
shares.
At its
formation on September 18, 2007, EFT BioTech acquired EFT Limited, a British
Virgin Islands company (“BVI”) formed on August 22, 2007, pursuant to which EFT
Limited (BVI) became a wholly-owned subsidiary of EFT BioTech. Since
both EFT BioTech and EFT Limited (BVI) were under common control, this
acquisition represents a reorganization of entities under common
control.
EFT
Limited (BVI) has four wholly-owned subsidiaries: EFT, Inc., a California
company formed on January 1, 2003, Top Capital International, Ltd. (BVI), a BVI
company formed on May 22, 2002, EFT (HK), Ltd., a Hong Kong (“HK”) company
formed on November 1, 2006 and EFT International Ltd. (BVI), a BVI company
formed on April 20, 2005, which it acquired all on November 14, 2007. EFT
International Ltd. is the operating company that generates substantially all of
the company’s net income. As EFT Limited (BVI) and the four companies being
acquired were under common control, this acquisition also represents a
reorganization of entities under common control.
These
reorganizations of entities under common control resulted in changes in the
legal organization of these predecessors to EFT BioTech but did not result in
changes in the reporting entity.
On
October 20, 2008, EFT Investment Co., Ltd., a Taiwan company, was formed as a
wholly-owned subsidiary of EFT Biotech Holding, Inc.
The
Company, through its subsidiaries, is engaged in the E-Business designed around
the concept of Business-to-customer using the World Wide Web as its “storefront”
and business platform to market, sell and distribute 46 American brand products
consisting of 25 nutritional products, 18 personal care products, 1 automotive
fuel additives, 1 home product and a portable drinking container.
Note
2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
1.
Basis of Presentation
The
accompanying consolidated financial statements have been prepared in conformity
with accounting principles generally accepted in the United States of
America.
Principles
of Consolidation
The
consolidated financial statements include the accounts of the Company and its
wholly-owned subsidiaries. All inter-company accounts and transactions have been
eliminated in consolidation.
Foreign
Currency
The
Company’s reporting currency is the U.S. dollar. The Company’s operation in Hong
Kong uses Hong Kong dollar (HKD) as its functional currency. The financial
statements of the subsidiary are translated into U.S. Dollars (USD) in
accordance with Statement of Financial Accounting Standards (SFAS) No. 52,
Foreign Currency Translation. According to the Statement, all assets and
liabilities were translated at the year end current exchange rate,
stockholders equity items are translated at the historical rates and income
statement items are translated at the average exchange rate for the year. The
resulting translation adjustments are reported under other comprehensive income
in accordance with SFAS No. 130, Reporting Comprehensive Income as a Component
of Stockholders Equity. Foreign exchange transaction gains and losses are
reflected in the income statement. During the years 2009 and 2008
there have been immaterial currency fluctuations between HKD and
USD.
F-6
Use of
Estimates
The
preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect the reported amounts of assets and liabilities and disclosure of
contingent assets and liabilities at the date of the financial statements and
the reported amounts of revenues and expenses during the reporting period.
Actual results could differ from those estimates.
Cash and
Cash Equivalents
Cash and
cash equivalents include cash on hand and cash in time deposits, certificates of
deposit and all highly liquid debt instruments with original maturities of three
months or less. The Company maintains its accounts in banks, several of which
exceed the federally insured limit. In aggregate, approximately $35.5 million
were above the federally insured limit. Management believes the Company is not
exposed to any significant credit risk on those accounts.
Available
for sale securities
The
Company’s investments in publicly traded equity securities are classified as
available-for-sale and are reported at fair value (based on quoted prices and
market prices) using the specific identification method. Unrealized gains and
losses, net of taxes, are reported as a component of stockholders’ equity.
Realized gains and losses on investments are included in investment and other
income, net when realized. Any impairment loss to reduce an investment’s
carrying amount to its fair market value is recognized in income when a decline
in the fair market value of an individual security below its cost or carrying
value is determined to be other than temporary.
Inventories
Inventories
are valued at the lower of cost (determined on a first-in, first-out basis) or
market. The Management compares the cost of inventories with the market value
and allowance is made for writing down the inventories to market value, if
lower. Inventory consists of high tech nutritional,
cosmetic, automotive maintenance and environmentally safe products.
Property
and equipment
Property
and equipment are stated at cost less accumulated depreciation. Expenditures for
maintenance and repairs are charged to earnings as incurred; additions, renewals
and betterments are capitalized. When property and equipment are retired or
otherwise disposed of, the related cost and accumulated depreciation are removed
from the respective accounts, and any gain or loss is included in operations.
Depreciation of property and equipment is provided using the straight-line
method for substantially all assets with estimated lives of:
|
Machinery
& equipment
|
3
years
|
|
Computers
& office equipment
|
3
years
|
|
Automobile
|
5
years
|
For the
years ended March 31, 2009 and 2008, depreciation expenses were $51,514 and
$38,340, respectively.
Long-Lived
Assets
Effective
January 1, 2002, the Company adopted Statement of Financial Accounting Standards
No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets (SFAS
144), which addresses financial accounting and reporting for the impairment or
disposal of long-lived assets and supersedes SFAS No. 121, Accounting for the
Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of, and
the accounting and reporting provisions of APB Opinion No. 30, Reporting the
Results of Operations for a Disposal of a Segment of a Business. The Company
periodically evaluates the carrying value of long-lived assets to be held and
used in accordance with SFAS 144. SFAS 144 requires impairment losses to be
recorded on long-lived assets used in operations when indicators of impairment
are present and the undiscounted cash flows estimated to be generated by those
assets are less than the asset’s carrying amounts. In that event, a loss is
recognized based on the amount by which the carrying amount exceeds the fair
market value of the long-lived assets. Loss on long-lived assets to be disposed
of is determined in a similar manner, except that fair market values are reduced
for the cost of disposal. Based on its review, the Company believes that, as of
March 31, 2009 there were no significant impairments of its long-lived
assets.
Fair
Value of Financial Instruments
Statement
of Financial Accounting Standards No. 107, “Disclosures about Fair Value of
Financial Instruments”, requires that the Company discloses estimated fair
values of financial instruments. The carrying amounts reported in the statements
of financial position for current assets and current liabilities qualifying as
financial instruments are a reasonable estimate of fair value due to the
short-term maturity of these instruments.
F-7
Fair
Value Measurements
Effective
April 1, 2008, the Company adopted Financial Accounting Standards Board (“FASB”)
Statement of Financial Accounting Standards (“SFAS”) No. 157, “ Fair Value Measurements ”,
which defines fair value, establishes guidelines for measuring fair value and
expands disclosures regarding fair value measurements. SFAS No. 157 does
not require any new fair value measurements, but rather eliminates
inconsistencies in guidance found in various other accounting pronouncements.
The adoption of SFAS No. 157 did not have a material effect on the
Company’s financial condition or operating results.
Refer to
Note 4, “Fair Value Measurements” for additional information on the adoption of
SFAS No. 157.
Stock-Based
Compensation
In
December 2004, the FASB issued FASB Statement No. 123R ("SFAS 123R"),
"Share-Based Payment, an Amendment of FASB Statement No. 123". SFAS 123R
requires companies to recognize in the statement of operations the grant date
fair value of stock options and other equity-based compensation issued to
employees. The Company adopted SFAS 123R on April 1, 2006.
Stocks
issued to officers or employees
On
November 18, 2007 in conjunction with the reverse acquisition, the Company
granted its officers an aggregate 1,201,000 shares of fully vested stock with no
future requisite service requirement. The share compensation cost is measured at
grant date, based on estimated fair value of the award which is $0.0018 per
share.
The
amount of compensation was included as a period compensation
expense. For the years ended March 31, 2009 and 2008, the stock-based
compensation for shares awarded were to employees was $0 and $2,162,
respectively.
During
the years 2009 and 2008, the Company has not issued any stock options or
warrants to employees nor are there any outstanding warrants or options during
the year ended March 31, 2008, therefore pro forma disclosures are not
required.
Stock
issued for service
The
company accounts for equity instruments issued in exchange for the receipts of
goods or service from other than employees in accordance with SFAS No.123 (R)
and the conclusions reached by the Emerging Issues Task Force (“EITF”) in Issue
No. 96-18. Costs are measured at the estimated fair market value of
the consideration received or the estimated fair value of the equity instruments
issued, whichever is more reliably measurable. The value of equity
instruments issued for consideration other than employee services is determined
on the earliest of performance commitment or completion of performance by the
provider of goods or service as defined by EITF 96-18.
During
November 2008, we issued 4,084 shares of common stock in exchange of service we
received.
For
the years ended March 31, 2009 and 2008, the stock-based compensation for shares
issued to non-employees was $16,731 and $0, respectively.
Revenue
Recognition
The
Company’s revenue recognition policy is in accordance with the requirements of
Staff Accounting Bulletin (“SAB”) No. 104, Revenue
Recognition, (“SAB 104”), EITF 01-09, Accounting for
Consideration Given by a Vendor to a Customer (Including a Reseller of the
Vendor’s Products) (“EITF 01-09”) and other applicable
revenue recognition guidance and interpretations. Sales revenue is recognized at
the date of shipment to customers when a formal arrangement exists, the price is
fixed or determinable, the delivery is completed, no other significant
obligations of the Company exist and collectibility is reasonably assured.
Payments received before all of the relevant criteria for revenue recognition
are satisfied are recorded as unearned revenue. Cash consideration
given by the Company to its sales affiliates is considered to be a reduction of
the selling prices of the Company's products, thus, is recorded as a reduction
of revenue.
Warranty
The
Company generally does not provide customers with right of return except for
defective products which is within six month warranty period from date of
sales. Historically, the company warranty provisions have not been
material. The specific warranty terms and conditions vary depending upon
the product sold, but generally include replacement over a period of six months.
Factors that affect the Company’s warranty liability include the number of
products currently under warranty, historical and anticipated rates of warranty
claims on those products, and cost per claim to satisfy the warranty obligation.
The anticipated rate of warranty claims is the primary factor impacting the
estimated warranty obligation. Warranty claims are relatively predictable based
on our historical experience. Warranty reserves are included in other
liabilities and the provision for warranty accruals is included in cost of goods
sold in the consolidated statement of Operations and Other Comprehensive Income.
Management reviews the adequacy of warranty reserves each reporting period based
on historical experience and management's estimate of the costs to remediate the
claims and adjusts the provisions accordingly.
F-8
Currently,
the Company estimates its warranty expense as follows:
|
Products
sold for
|
|
|
0-2
months
|
2%
of cost
|
|
3-4
months
|
1.5%
of cost
|
|
5-6
months
|
1%
of
cost
|
The
Company records warranty liabilities at the time of sale for the estimated costs
that may be incurred under its limited warranty. The specific warranty terms and
conditions vary depending upon the product sold, but generally include
replacement over a period of six months. Factors that affect the Company’s
warranty liability include the number of products currently under warranty,
historical and anticipated rates of warranty claims on those products, and cost
per claim to satisfy the warranty obligation. The anticipated rate of warranty
claims is the primary factor impacting the estimated warranty obligation. The
other factors are less significant due to the fact that the warranty period is
only six months and replacement is generally already in stock or available
at a pre-determined price. Warranty claims are relatively predictable based on
historical experience of failure rates. If actual results differ from the
estimates, the Company revises its estimated warranty liability.
Currently,
the Company estimates its warranty expense as follows:
|
Products
sold for
|
|
|
0-2
months
|
2%
of cost
|
|
3-4
months
|
1.5%
of cost
|
|
5-6
months
|
1%
of cost
|
Shipping
Costs
The
Company’s shipping costs are included in cost of sales in the accompanying
Consolidated Statements of Operations and Other Comprehensive Income for all
periods presented.
Unearned
Revenues
Unearned
Revenues consist of cash amounts received in advance for goods and services to
be delivered at a future date. The Registrant records the cash from customers as
a liability until the products are delivered.
2.
Advertising
Advertising
expenses consist primarily of costs of promotion for corporate image and product
marketing and costs of direct advertising. The Company expenses all advertising
costs as incurred. For the years ended March 31, 2009 and 2008, advertising
expenses were $89,283 and $1,540, respectively.
Consultant
Fee
On
January 1, 2009, EFT International Ltd, a wholly-owned subsidiary of EFT BioTech
Holdings, Inc., entered a contract with ZR Public Relation Consultant Ltd. (the
Consultant), which provides public relation consulting services in Asia.
In consideration of the services rendered by the Consultant, EFT International
Ltd agrees to pay 5% of total commission payout for each fiscal year. For
the year ended March 31, 2009, consultant expense for EFT International Ltd was
$1,771,944.
Income
Taxes
The
Company utilizes SFAS No. 109, Accounting for Income Taxes, which requires the
recognition of deferred tax assets and liabilities for the expected future tax
consequences of events that have been included in the financial statements or
tax returns. Under this method, deferred income taxes are recognized for the tax
consequences in future years of differences between the tax bases of assets and
liabilities and their financial reporting amounts at each period end based on
enacted tax laws and statutory tax rates applicable to the periods in which the
differences are expected to affect taxable income. Valuation allowances are
established, when necessary, to reduce deferred tax assets to the amount
expected to be realized.
The
Company adopted the provisions of FASB Interpretation No. 48, Accounting for
Uncertainty in Income Taxes (“FIN 48”), on April 1, 2007 The Interpretation
addresses the determination of whether tax benefits claimed or expected to be
claimed on a tax return should be recorded in the financial statements. Under
FIN 48, we may recognize the tax benefit from an uncertain tax position only if
it is more likely than not that the tax position will be sustained on
examination by the taxing authorities, based on the technical merits of the
position. The tax benefits recognized in the financial statements from such a
position should be measured based on the largest benefit that has a greater than
fifty percent likelihood of being realized upon ultimate settlement. FIN 48 also
provides guidance on derecognition, classification, interest and penalties on
income taxes, accounting in interim periods and requires increased
disclosures.
Net
Earnings per Share
Basic net
earnings per common share of stock is calculated by dividing net
income available to common stockholders by the weighted-average of
common shares outstanding during each period.
F-9
Diluted
net income per common share is calculated by dividing adjusted net
income by the weighted-average of common shares outstanding,
including the effect of other dilutive securities. The Company’s potentially
dilutive securities consist of in-the-money outstanding warrants to purchase the
Company’s common stock. Diluted net income per common share does not give effect
to dilutive securities as their effect would be anti-dilutive.
The
treasury stock method is used to measure the dilutive impact of stock options
and warrants. Under this method, options and warrants are assumed to be
exercised at the beginning of the period (or at the time of issuance, if later),
and as if funds obtained thereby were used to purchase common stock at the
average market price during the period. For the year ended March 31,
2009 and 2008, stock warrants of 14,890,000 and zero shares have no effect on
the diluted calculation because they are not in-the-money.
|
For the Years ended
March 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Historical
Numerator:
|
||||||||
|
Net
Income
|
$
|
2,128,662
|
$
|
20,795,695
|
||||
|
Denominator:
|
||||||||
|
Weighted-average
shares used for basic net income per share
|
66,637,448
|
55,350,545
|
||||||
|
Effect
of common stock equivalents
|
-
|
-
|
||||||
|
Weighted-average
shares used for diluted net income per share
|
66,637,448
|
55,350,545
|
||||||
|
Basic
and diluted net income per share
|
$
|
0.03
|
$
|
0.37
|
||||
|
Diluted
net income per share
|
$
|
0.03
|
$
|
0.37
|
||||
Comprehensive
income
Comprehensive
income is defined as the change in equity of a company during a period from
transactions and other events and circumstances excluding transactions resulting
from investments from owners and distributions to owners. For the Company,
comprehensive income for the periods presented is comprised of net income and
unrealized loss on marketable securities classified as
available-for-sale.
F-10
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit
risk are cash, accounts receivable and other receivables arising from its normal
business activities. The Company places its cash in what it believes to be
credit-worthy financial institutions, but several of its bank accounts exceed
the federally insured limit. The Company’s accounts receivable is constantly at
a marginal to zero dollar ($0) level and its revenues are derived from orders
place by consumers located anywhere in the world over the Company’s designated
internet portal. The Company maintains a zero dollar ($0) allowance for doubtful
accounts and authorizes credits based upon its historical “sound and quality”
after sales customer services provided to affiliates and customers.
Historically, such customer services have been maintained in accordance with the
management expectations. The Company routinely assesses the credits authorized
to its customers and, based upon factors surrounding the credit risk,
establishes an allowance, if required, for uncollectible accounts and, as a
consequence, believes that its accounts receivable credit risk exposure beyond
such allowance is limited.
Segment
Reporting
Statement
of Financial Accounting Standards No. 131 (SFAS 131), “Disclosure about Segments
of an Enterprise and Related Information” requires use of the management
approach model for segment reporting. The management approach model is based on
the way a company's management organizes segments within the company for making
operating decisions and assessing performance. Reportable segments are based on
products and services, geography, legal structure, management structure, or any
other manner in which management disaggregates a company. Since management does
not disaggregate Company data, the Company has determined that only one segment
exists. Accordingly, no segment reporting is provided. The Company’s maintains 5
product categories; nutritional products, personal care products, automotive
fuel additives, home products and portable drinking containers. The
Company’s current “in-house” information systems do not have the ability to
compile revenues from external customers for each product category.
Recent
accounting pronouncements
The FASB
issued SFAS No. 168, The FASB
Accounting Standards Codification and the Hierarchy of Generally Accepted
Accounting Principles, on June 29, 2009 and, in
doing so, authorized the Codification as the sole source for authoritative U.S.
GAAP. SFAS No. 168 will be effective for financial statements
issued for reporting periods that end after September 15, 2009. Once
it's effective, it will supersede all accounting standards in U.S. GAAP, aside
from those issued by the SEC. SFAS No. 168 replaces SFAS No. 162 to
establish a new hierarchy of GAAP sources for non-governmental entities under
the FASB Accounting Standards Codification. The Company will evaluate the impact
of SFAS No. 168 upon its effectiveness.
SFAS No.
167 amends FASB Interpretation (FIN) No. 46(R), Consolidation of Variable Interest
Entities, by altering how a company determines when an entity that is
insufficiently capitalized or not controlled through voting should be
consolidated, the FASB said. A company has to determine whether it should
provide consolidated reporting of an entity based upon the entity's purpose and
design and the parent company's ability to direct the entity's
actions.
The
standards will be effective at the start of the first fiscal year beginning
after November 15, 2009, which will mean January 2010 for companies that are on
calendar years. The guidance will have to be applied for first-quarter filings.
The Company will comply with the disclosure requirements of this statement when
and if it acquires a variable interest entity, upon its
effectiveness.
FAS
No. 166 revises SFAS No. 140, Accounting for Transfers and
Servicing of Financial Assets and Extinguishments of
Liabilities, and will require entities to provide more
information about sales of securitized financial assets and similar
transactions, particularly if the seller retains some risk to the assets, the
FASB said. The statement eliminates the concept of a qualifying special-purpose
entity, changes the requirements for the derecognition of financial assets, and
calls upon sellers of the assets to make additional disclosures about
them. The Company does not believe implementation of FAS No.
166 will have a material impact on its financial statements.
On May
28, 2009 the FASB announced the issuance of SFAS 165, Subsequent Events . SFAS 165
should not result in significant changes in the subsequent events that an entity
reports. Rather, SFAS 165 introduces the concept of financial statements being
available to be issued. Financial statements are considered available to be
issued when they are complete in a form and format that complies with generally
accepted accounting principles (GAAP) and all approvals necessary for issuance
have been obtained. The Company believes that SFAS No. 165 will have
an effect on its financial statements and will comply upon
effectiveness.
Note
3 - FINANCIAL INSTRUMENTS
The
following table summarizes unrealized gains and losses related to the Company’s
investments in marketable securities designated as available-for-sale. The fair
value of available for sale securities has been estimated based on quoted market
prices, which the Company currently believes are indicative of fair value. The
Company’s available for sale securities are mainly on equity securities mutual
funds.
F-11
|
March 31, 2009
|
March 31, 2008
|
|||||||||||||||||||||||
|
Fair Value
|
Cost
|
Unrealized
(Loss)
|
Fair
Value
|
Cost
|
Unrealized
(Loss)
|
|||||||||||||||||||
|
Available
for sale securities
|
$
|
508,746
|
$
|
999,029
|
$
|
(490,283
|
)
|
$
|
835,965
|
$
|
999,029
|
$
|
(163,064
|
)
|
||||||||||
|
Total
|
$
|
508,746
|
$
|
999,029
|
$
|
(490,283
|
)
|
$
|
835,965
|
$
|
999,029
|
$
|
(163,064
|
)
|
||||||||||
Note
4 - FAIR VALUE MEASUREMENTS
On April
1, 2008, the Company adopted the effective portions of SFAS 157. In
February 2008 the FASB issued FSP FAS 157-2, “Effective Date of FASB
Statement No. 157”, which provides a one year deferral of the effective
date of SFAS No. 157 for all nonfinancial assets and nonfinancial
liabilities, except those that are recognized or disclosed at fair value in the
financial statements on a recurring basis (at least annually). Therefore, the
Company adopted the provisions of SFAS 157 with respect to only financial
assets and liabilities.
SFAS 157
defines fair value, establishes a framework for measuring fair value and
enhances disclosure requirements for fair value measurements. This statement
does not require any new fair value measurements. SFAS 157 defines fair
value as the price that would be received to sell an asset or paid to transfer a
liability in an orderly transaction between market participants at the
measurement date. As such, fair value is a market-based measurement that should
be determined based on assumptions that market participants would use in pricing
an asset or a liability. As a basis for considering such assumptions, SFAS 157
establishes a three-tier value hierarchy, which prioritizes the inputs used in
the valuation methodologies in measuring fair value:
Level 1—Observable
inputs that reflect quoted prices (unadjusted) for identical assets or
liabilities in active markets.
Level 2—Include other
inputs that are directly or indirectly observable in the
marketplace.
Level 3—Unobservable
inputs which are supported by little or no market activity.
The fair
value hierarchy also requires an entity to maximize the use of observable inputs
and minimize the use of unobservable inputs when measuring fair
value.
In
accordance with SFAS 157, the Company measures its available for sale
securities at fair value. The available for sale securities are classified
within Level 1. This is because the available for sale securities are valued
using quoted market prices.
Assets
and liabilities measured at fair value are summarized below.
|
December 31, 2008
|
||||||||||||||||
|
Level 1
|
||||||||||||||||
|
Quoted Prices
|
Level 2
|
|||||||||||||||
|
in Active
|
Significant
|
Level 3
|
||||||||||||||
|
Markets for
|
Other
|
Significant
|
||||||||||||||
|
Identical
|
Observable
|
Unobservable
|
||||||||||||||
|
Assets
|
Inputs
|
Inputs
|
Total
|
|||||||||||||
|
Available
for sale securities
|
$
|
508,746
|
$
|
-
|
$
|
-
|
$
|
508,746
|
||||||||
|
Total
assets measured at fair value
|
$
|
508,746
|
$
|
-
|
$
|
-
|
$
|
508,746
|
||||||||
Note
5 - NOTE RECEIVABLES, RELATED PARTY
Short-Term
On June
30, 2008, the Company signed a loan agreement denominated in U.S. dollars with
Excalibur International Marine Corporation (“Excalibur”) to lend $19,193,000 at
no interest with a term of five month. On November 14, 2008, the
Company has received $17,628,283 from Excalibur. At the end of the five month
term, the term of the loan was extended for another nine months. This loan still
has an outstanding balance of $1,564,717 at year end of March 31,
2009.
On
September 23, 2008, the Company signed a loan agreement denominated in U.S.
dollars with Excalibur International Marine Corporation (“Excalibur”) to lend
$2,000,000 at interest rate of 3.75% per month with a term of no more than 60
days. At the end of the 60-day term, the term of the loan was
extended for six months. On May 25, 2009, the Company extended this loan to
Excalibur for another six months and lowered the interest rate to 12.5% per
annum.
On
November 24, 2008, the Company signed another loan agreement denominated in U.S.
dollars with Excalibur to lend $500,000 at interest rate of 3.75% per month with
a term of no more than 30 days. At the end of the 30-day term, the
term of the loan was extended for six months. On May 25, 2009, the Company
extended this loan to Excalibur for another six months and lowered the interest
rate to 12.5% per annum.
F-12
Long-Term
The Board
of Directors approved two non-interest bearing unsecured demand loans in the
amount of U.S. $330,000 and $1,567,000 respectively on July 14,
2008 and July 25, 2008 to Yeuh-Chi Liu, a vendor and a
member of the board of directors of Excalibur. The loan amount in
$1,567,000 is collateralized with 3.97% ownership of Excalibur International
Marine Corp. As of the date hereof the full principal amount remains
outstanding.
Note
6 – OTHER RECEIVABLE
EFT USA,
Inc. (EFT), a wholly-owned subsidiary of EFT BioTech Holdings, Inc., sold
certain business properties, including computers and an auto to Industrial
Fulfillment Co. (IFC) in the amount of $33,504, its net book value as other
receivable, pursuant to an asset purchase agreement. The sale of the
assets under this agreement constitutes a complete transfer of all of its
rights, title and interest with respect to the assets.
Note
7 – PROPERTY AND EQUIPMENT
Property
and equipment consist of the following:
|
March 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Automobile
|
$
|
154,724
|
$
|
176,384
|
||||
|
Furniture
and fixture
|
12,278
|
-
|
||||||
|
Computer
equipment
|
26,373
|
22,068
|
||||||
|
Machinery
and equipment
|
6,405
|
15,959
|
||||||
|
Leasehold
improvement
|
262,679
|
-
|
||||||
|
462,459
|
214,411
|
|||||||
|
Less:
Accumulated depreciation
|
(102,303
|
)
|
(74,305
|
)
|
||||
|
$
|
360,156
|
$
|
140,106
|
|||||
Note
8 – INVESTMENT
On
October 25, 2008, the Company through its wholly-owned subsidiary, EFT
Investment Co. Ltd, a Taiwan company formed on October 20 2008, completed the
acquisition of 48.81% of equity interest of Excalibur for approximately
$19,193,000. The equity method has been used for this
investment. The Company’s investment in Excalibur was $17,129,314 due
to Excalibur has $4,222,808 net loss for year ended 2008.
Investment
consists of:
|
|
March 31,
|
March 31,
|
||||||
|
|
2009
|
2008
|
||||||
|
48.81%
equity interest (a)
|
$
|
$17,129,314
|
$
|
-
|
||||
|
$
|
$17,129,314
|
-
|
||||||
(a)
On October 20, 2008, EFT Investment Co., Ltd. was formed as a
wholly-owned subsidiary of EFT BioTech Holdings, Inc. EFT
Investment Co., Ltd was formed in Taiwan. On October 25, 2008, EFT Investment
Co., Ltd. completed the acquisition of 585,677,500 shares of common stock of
Excalibur; representing approximately 49% shares of issued and outstanding
shares of Excalibur, for an aggregate purchase price of USD $19,193,000. Prior
to the acquisition of Excalibur, Excalibur was not a related person under Item
404 of regulation S-K. The equity method has been used for this investment
for the year ended March 31, 2009.
The
following table shows the summary of income statement for Excalibur
International Corp. for the year ended March 31, 2009:
|
Excalibur International Marine Corp
|
||||||||
|
|
Year Ended March 31,
|
|||||||
|
|
2009
|
2008
|
||||||
|
Exchange
rate
|
33
|
-
|
||||||
|
Revenue
|
$
|
10,775
|
$
|
-
|
||||
|
Gross
profit
|
$
|
(239,403)
|
$
|
-
|
||||
|
Income
from continuing operations
|
$
|
(4,222,808)
|
$
|
-
|
||||
|
Net
income
|
$
|
(4,222,808)
|
$
|
-
|
||||
|
EFT
48.81% investment loss
|
$
|
(2,063,686)
|
$
|
-
|
||||
F-13
The
following table provides the summary of balance sheet information for Excalibur
International Marine Corp. as of March 31, 2009 and March 31, 2008:
|
Excalibur International Marine Corp
|
||||||||||||||||
|
|
March 31, 2009
|
March 31, 2008
|
||||||||||||||
|
NT$
|
USD
|
NT$
|
USD
|
|||||||||||||
|
Total
assets
|
1,289,432,107
|
39,073,700
|
-
|
-
|
||||||||||||
|
Total
liabilities
|
204,417,971
|
6,194,484
|
-
|
-
|
||||||||||||
|
Net
assets
|
1,085,014,136
|
32,879,216
|
-
|
-
|
||||||||||||
|
EFT
48.81% ownership
|
529,595,400
|
16,048,345
|
-
|
-
|
||||||||||||
|
Ending
balance of investment account
|
17,129,314
|
-
|
||||||||||||||
|
Difference/Premium
|
(1,080,969)
|
-
|
||||||||||||||
The
premium of $1,080,969 was mainly the excess we paid to purchase of the 48.81% of
ownership in Excalibur as of March 31, 2009.
The
following is the shareholder’s list of Excalibur International Marine Corp as of
March 31, 2009:
|
Excalibur International Marine Corp. Shareholders’
List
|
|||||||||
|
Shareholders’ Name
|
# of shares
|
%
|
|||||||
|
1
|
EFT
Investment Co. Ltd
|
585,677,500
|
48.81
|
%
|
|||||
|
2
|
Lu,
TsoChun
|
100,000,000
|
8.33
|
%
|
|||||
|
3
|
Chiao,
Jen-Ho
|
82,000,000
|
6.83
|
%
|
|||||
|
5
|
Lin,
Ming-i
|
51,700,000
|
4.31
|
%
|
|||||
|
4
|
Ms.
Ku
|
50,000,000
|
4.17
|
%
|
|||||
|
6
|
Yeuh-Chi
Liu
|
47,660,000
|
3.97
|
%
|
|||||
|
7
|
Steve
Hsiao
|
46,392,500
|
3.87
|
%
|
|||||
|
8
|
Wen
Investment
|
40,000,000
|
3.33
|
%
|
|||||
|
Others
(*)
|
196,570,000
|
16.38%
|
|||||||
|
Total
|
1,200,000,000
|
100
|
%
|
||||||
*: 56
individuals, none exceeds 2.5% interest in Excalibur International Marine
Corp.
Currently, the Company does not comply
with Rule 8-04 of Regulation S-X. However, the Company is using its
best efforts to get audited financial statements from Excalibur to comply with
Rule 8-04 of Regulation S-X.
Note
9 – OTHER LIABILITIES
Other
liabilities consist of the following:
|
March 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Commission
payable
|
$
|
5,977,969
|
$
|
12,028,644
|
||||
|
Payroll
liabilities
|
645,900
|
671,409
|
||||||
|
Warranty
liability
|
51,683
|
85,608
|
||||||
|
Other
|
-
|
2,053
|
||||||
|
$
|
6,675,552
|
$
|
12,787,714
|
|||||
Note
10 – STOCKHOLDERS’ EQUITY
Common
stock
As of
March 31, 2009 the Company has 4,975,000,000 shares of common stock authorized
and 75,983,205 shares issued and outstanding at par value $0.00001 per
share. 7,793,165 shares are held by non-affiliates and the remaining
68,190,040 are held by affiliates.
F-14
For the
year ended March 31, 2009, the Company issued 14,960,791 shares of Common
Stock including 14,890,040 shares issued upon the completion of a private
placement of its common stock to non-resident aliens at a purchase price of
$3.80 per unit, for a unit consisting of one share of common stock and one
common stock redeemable purchase warrant.
Warrants
Each
warrant underlying the unit offered in the private placement is immediately
exercisable in whole or in part and from time to time, to purchase one share of
common stock at $3.80 per share until the second anniversary date of the date of
issuance.
The
Company shall have the right, not the obligation to redeem the outstanding
warrants, on a pro rata basis, at a purchase price of $0.00001 per warrant
within thirty (30) days from the tenth (10 th ) consecutive trading day
that the closing sales price, or the average of the closing bid and asked price
in the event that the Company’s common stock trades on the OTC or any public
securities market within the U.S., is at least Eleven Dollars ($11.00) per
share.
As the
only settlement option for the warrants is physical settlement, in which the
party designated in the contract as the buyer delivers the full stated amount of
cash to the seller, and the seller delivers the full stated number of shares to
the buyer, the Company accounted for the warrants as permanent equity and
recorded it in additional paid in capital.
Dividend
For the
years ended March 31, 2009 and 2008, approximately $0 and $18.5 million
dividends were paid to the stockholders of EFT BioTech before the
merger.
Deposits
from investors and restricted cash
In March
2008, The Company received $37,845,432 deposits related to a future private
placement of its common stock to non-resident aliens at a purchase price of
$3.80 per unit, for a unit consisting of one share of common stock and one
common stock purchase warrant. The deposits are held in an escrow account and
are refundable anytime before stock subscription agreement is executed. As of
March 31, 2008 we had not executed any of the stock subscription
agreement.
Note
11 - INCOME TAXES
The
Company was incorporated in the United States of America (“US”) and has
operations in three tax jurisdictions - the United States of America, the Hong
Kong Special Administrative Region (“HK SAR”) and the BVI. The Company generated
substantially all of its net income from its BVI operations for the years ended
March 31, 2009 and 2008 which are not subject to any tax provision
according to BVI tax law. The Company’s HK SAR subsidiaries had no taxable
income in the respective periods. The deferred tax assets for the Company’s US
operations and HK SAR subsidiaries were immaterial at March 31, 2009 and
2008.
The
income tax expenses consist of the following:
|
Years Ended March 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Current:
|
||||||||
|
Domestic
|
$
|
2,400
|
$
|
305,800
|
||||
|
Foreign
|
-
|
-
|
||||||
|
Deferred
|
-
|
-
|
||||||
|
Income
tax expenses
|
$
|
2,400
|
$
|
305,800
|
||||
A
reconciliation of income taxes, with the amount computed by applying the
statutory federal income tax rate (37% for the years ended March 31, 2009
and 2008) to income before income taxes for the years ended March 31, 2009
and 2008, is as follows:
|
Years Ended March 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Income
tax at U.S. statutory rate
|
$
|
788,493
|
$
|
7,807,553
|
||||
|
State
tax
|
2,400
|
800
|
||||||
|
Indefinitely
invested earnings of foreign subsidiaries
|
(819,633
|
)
|
(7,516,351
|
)
|
||||
|
Nondeductible
expenses
|
31,140
|
13,798
|
||||||
|
$
|
2,400
|
$
|
305,800
|
|||||
|
Effective
tax rate
|
0
|
%
|
1
|
%
|
||||
The
Company’s effective tax rate decreased for the year ended March 31, 2009,
compared to the same period of 2008, due to, after the re-capitalization, a
higher proportion of its operating profits is subject to income
tax.
F-15
Uncertain
Tax Positions
As a
result of the implementation of Interpretation 48 on April 1, 2007, the Company
recognized no material adjustments to liabilities or stockholders’ equity.
Interest associated with unrecognized tax benefits are classified as income tax
and penalties are classified in selling, general and administrative expenses in
the statements of operations. The adoption of FIN 48 did not have a material
impact on the Company’s financial statements.
For the
years ended March 31, 2009 and 2008, the Company had no unrecognized tax
benefits and related interest and penalties expenses. Currently, the
Company is not subject to examination by major tax jurisdictions.
Note
12 - WARRANTY LIABILITY
The
Company records warranty liabilities at the time of sale for the estimated costs
that may be incurred under its limited warranty. Changes in warranty liability
for standard warranties which are included in current liabilities on the
Company’s Consolidated Balance Sheets are presented in the following
tables:
|
Years Ended
|
||||||||
|
March 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Warranty
liability at beginning of year
|
$
|
85,608
|
$
|
48,696
|
||||
|
Costs
accrued
|
(33,924
|
)
|
36,912
|
|||||
|
Service
obligations honored
|
-
|
-
|
||||||
|
Warranty
liability at end of year
|
$
|
51,684
|
$
|
85,608
|
||||
|
Current
portion
|
$
|
51,684
|
$
|
85,608
|
||||
|
Non-current
portion
|
-
|
-
|
||||||
|
Warranty
liability at end of year
|
$
|
51,684
|
$
|
85,608
|
||||
Note
13 - COMMITMENT
Operating
Lease
The
Company leases office space in the US under an operating lease
agreement. The lease provides for monthly lease payments
approximating $10,063 and expires on July 31, 2009. Future minimum lease
payments under the operating leases as of March 31, 2009 approximate the
following:
|
Year
Ending March 31,
|
||||
|
2010,
four months
|
$
|
40,250
|
||
The
Company rents office space for its sales division in Hong Kong. The
lease provides for free lease in the first two years and a monthly lease
payments approximating $50,000 USD starting the beginning of the third year and
expires on March 31, 2012. Expensing the 5-year total rent evenly
over the life of the lease, the future minimum lease payments under the
operating lease are as follows:
|
Year
Ending March 31,
|
||||
|
2010
|
$
|
360,000
|
||
|
2011
|
360,000
|
|||
|
2012
|
360,000
|
|||
The
Company rents storage space for its sales division in Hong Kong. The
lease provides for monthly lease payments approximating $1,135 USD starting on
May 8, 2008 and expires on May 7, 2010. Future minimum lease payments
under the operating leases as of March 31, 2009 approximate the
following:
|
Year
Ending March 31,
|
||||
|
2010
|
$
|
13,625
|
||
|
2011
|
1,135
|
|||
Rent
expenses for the year ended March 31, 2009 and March 31, 2008 were approximately
$494,487 and $487,896, respectively.
F-16
Note
14 - RESTATEMENT
Subsequent
to the issuance of the consolidated financial statements for the years ended
March 31, 2008 and 2007, the Company determined that certain errors existed in
the Consolidated Balance Sheets, Consolidated Statements of Operations and
Comprehensive Income and Consolidated Statements of Changes in Stockholders’
Equity with respect to the correct par value and amount of shares issued during
the respective periods. The changes to the previously discussed
consolidated financial statements had no impact on the Company’s net
income.
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Balance Sheets
|
As
of March 31,
|
||||||||
|
2008
|
2008
|
|||||||
|
restated
|
original
|
|||||||
|
ASSETS
|
||||||||
|
Current
assets
|
||||||||
|
Cash
and cash equivalents
|
$
|
15,165,620
|
$
|
15,165,620
|
||||
|
Inventories
|
2,619,429
|
2,619,429
|
||||||
|
Available
for sale securities
|
835,965
|
835,965
|
||||||
|
Prepaid
expenses
|
793,760
|
793,760
|
||||||
|
Total
current assets
|
19,414,774
|
19,414,774
|
||||||
|
Property,
plant and equipment, net
|
140,106
|
140,106
|
||||||
|
Restricted
cash
|
37,845,432
|
37,845,432
|
||||||
|
Security
deposit
|
27,108
|
27,108
|
||||||
|
Total
assets
|
$
|
57,427,420
|
$
|
57,427,420
|
||||
|
LIABILITIES
AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current
liabilities
|
||||||||
|
Accounts
payable and accrued expenses
|
$
|
804,041
|
$
|
804,041
|
||||
|
Other
liabilities
|
12,787,714
|
12,787,714
|
||||||
|
Unearned
revenues
|
3,945,805
|
3,945,805
|
||||||
|
Deposits
from investors
|
37,845,432
|
37,845,432
|
||||||
|
Income
tax payable
|
305,000
|
305,000
|
||||||
|
Total
current liabilities
|
55,687,992
|
55,687,992
|
||||||
|
Stockholders'
equity (deficit) Preferred stock, $0.001 par value, 25,000,000 shares
authorized, none issued and outstanding
|
-
|
-
|
||||||
|
Common
stock, $0.00001 par value, 4,975,000 authorized, 61,022,414 and
52,099,000 shares issued and outstanding at March 31, 2008 and
2007
|
610
|
6,102
|
||||||
|
Additional
paid in capital
|
6,552
|
1,060
|
||||||
|
Retained
earnings (deficit)
|
1,895,330
|
1,895,330
|
||||||
|
Accumulated
other comprehensive loss
|
(163,064
|
)
|
(163,064
|
)
|
||||
|
Total
stockholders' equity
|
1,739,428
|
1,739,428
|
||||||
|
Total
liabilities and stockholders' equity
|
$
|
57,427,420
|
$
|
57,427,420
|
||||
F-17
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Statements of Operations and Other Comprehensive Income
|
Year
Ended
|
||||||||
|
March
31, 2008
|
March
31, 2008
|
|||||||
|
restated
|
original
|
|||||||
|
Sales
revenues, net
|
$
|
30,249,302
|
$
|
30,249,302
|
||||
|
Shipping
charge
|
10,110,360
|
10,110,360
|
||||||
|
40,359,662
|
40,359,662
|
|||||||
|
Cost
of goods sold
|
11,423,852
|
11,423,852
|
||||||
|
Shipping
costs
|
4,467,140
|
4,467,140
|
||||||
|
15,890,992
|
15,890,992
|
|||||||
|
Gross
profit
|
24,468,670
|
24,468,670
|
||||||
|
Selling,
general and administrative expenses
|
3,693,369
|
3,693,369
|
||||||
|
Net
operating income
|
20,775,301
|
20,775,301
|
||||||
|
Other
income (expense)
|
||||||||
|
Interest
income
|
275,538
|
275,538
|
||||||
|
Foreign
exchange loss
|
(4,248
|
)
|
(4,248
|
)
|
||||
|
Other
expense, net
|
54,904
|
54,904
|
||||||
|
Total
other income
|
326,194
|
326,194
|
||||||
|
Net
income before income taxes
|
21,101,495
|
21,101,495
|
||||||
|
Income
taxes
|
305,800
|
305,800
|
||||||
|
Net
income
|
$
|
20,795,695
|
$
|
20,795,695
|
||||
|
Unrealized
loss on available for sale securities
|
(163,064
|
)
|
(163,064
|
)
|
||||
|
Comprehensive
income
|
$
|
20,632,631
|
$
|
20,632,631
|
||||
|
Net
income per common shares Basic and diluted
|
$
|
0.37
|
$
|
0.34
|
||||
|
Weighted
average common shares outstanding Basic and diluted
|
55,350,545
|
60,277,531
|
||||||
F-18
EFT
BIOTECH HOLDINGS, INC.
Consolidated
Statements of Changes in Stockholders' Equity (Deficit)
|
Accumulated
|
Accumulated
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Additional
|
Additional
|
Other
|
Other
|
Total
|
Total
|
|||||||||||||||||||||||||||||||||||||||||||
|
Paid-in
|
Paid-in
|
Retained
|
Retained
|
Comprehensive
|
Comprehensive
|
Stockholders'
|
Stockholders'
|
|||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Shares
|
Amount
|
Amount
|
Capital
|
Capital
|
Earnings
|
Earnings
|
Income (loss)
|
Income (loss)
|
Equity (Deficit)
|
Equity (Deficit)
|
|||||||||||||||||||||||||||||||||||||
|
restated
|
original
|
restated
|
original
|
restated
|
original
|
restated
|
original
|
restated
|
original
|
restated
|
original
|
|||||||||||||||||||||||||||||||||||||
|
BALANCE,
APRIL 1, 2006
|
52,099,000
|
59,821,414
|
$
|
521
|
$
|
5,982
|
$
|
4,479
|
$
|
(982
|
)
|
$
|
1,563
|
$
|
1,563
|
$
|
-
|
$
|
-
|
$
|
6,563
|
$
|
6,563
|
|||||||||||||||||||||||||
|
Net
income
|
-
|
-
|
-
|
-
|
-
|
-
|
10,063,293
|
10,063,293
|
-
|
-
|
10,063,293
|
10,063,293
|
||||||||||||||||||||||||||||||||||||
|
Dividend
paid
|
-
|
-
|
-
|
-
|
-
|
-
|
(10,439,044
|
)
|
(10,439,044
|
)
|
-
|
-
|
(10,439,044
|
)
|
(10,439,044
|
)
|
||||||||||||||||||||||||||||||||
|
BALANCE,
MARCH 31, 2007
|
52,099,000
|
59,821,414
|
521
|
5,982
|
4,479
|
(982
|
)
|
(374,188
|
)
|
(374,188
|
)
|
-
|
-
|
(369,188
|
)
|
(369,188
|
)
|
|||||||||||||||||||||||||||||||
|
Issuance
of common stock for services
|
1,201,000
|
1,201,000
|
12
|
120
|
2,150
|
2,042
|
-
|
-
|
-
|
-
|
2,162
|
2,162
|
||||||||||||||||||||||||||||||||||||
|
Shares
effectively issued to former shareholders as part of the
recapitalization
|
7,722,414
|
-
|
77
|
-
|
(77
|
)
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||||||||||||||||
|
Net
income
|
-
|
-
|
-
|
-
|
-
|
-
|
20,795,695
|
20,795,695
|
-
|
-
|
20,795,695
|
20,795,695
|
||||||||||||||||||||||||||||||||||||
|
Dividend
paid
|
-
|
-
|
-
|
-
|
-
|
-
|
(18,526,177
|
)
|
(18,526,177
|
)
|
-
|
-
|
(18,526,177
|
)
|
(18,526,177
|
)
|
||||||||||||||||||||||||||||||||
|
Unrealized
loss on available for sale securities
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(163,064
|
)
|
(163,064
|
)
|
(163,064
|
)
|
(163,064
|
)
|
||||||||||||||||||||||||||||||||
|
BALANCE,
MARCH 31, 2008
|
61,022,414
|
61,022,414
|
$
|
610
|
$
|
6,102
|
$
|
6,552
|
$
|
1,060
|
$
|
1,895,330
|
$
|
1,895,330
|
$
|
(163,064
|
)
|
$
|
(163,064
|
)
|
$
|
1,739,428
|
$
|
1,739,428
|
||||||||||||||||||||||||
F-19
ITEM
9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE.
We
dismissed Weinberg & Company, P.A. (“Weinberg”) as our independent
accountants on May 4, 2008. We had engaged Weinberg to audit our financial
statements. On May 4, 2008, we engaged Child, Van Wagoner & Bradshaw,
PLLC (“CVB”) as our independent accountants to audit our financial statements
for the fiscal year ended March 31, 2008 and the subsequent fiscal years ending
March 31 st and
to review our unaudited reports for the interim periods. The change in
accountants was approved by our board of directors on May 4, 2008. We do
not have an audit committee due to the size of our board of
directors
For the
period of their engagement, there were no disagreements with Weinberg on any
matters of accounting principles or practices, financial statement disclosure,
or auditing scope or procedure which, if not resolved to Weinberg’s
satisfaction, would have caused Weinberg to make reference to the subject matter
in connection with its reports; and there were no reportable events as defined
in Item 304(a)(1)(iv) of Regulation S-K. During the period of the Company’s
engagement of Weinberg, Weinberg did not issue any reports on the Company’s
audited financial statements.
Prior to
its engagement, neither the Company nor anyone on its behalf has consulted CVB,
regarding (i) either: the application of accounting principles to a specific
completed or contemplated transaction, or the type of audit opinion that might
be rendered on the Registrant’s financial statements; as such, no written or
oral advice was provided, and none was an important factor considered by the
Registrant in reaching a decision as to the accounting, auditing or financial
reporting issues; or (ii) any matter that was a subject of a disagreement or
reportable event, as there were none.
We
provided Weinberg with a copy of this disclosure on April 17, 2009, requesting
Weinberg to furnish us with a letter addressed to the Securities and Exchange
Commission stating whether it agrees with the statements made by us, and, if
not, stating the respects in which it does not agree. A copy of the letter
furnished by Weinberg in response to that request is filed as Exhibit 16.1 to
Amendment No. 2 to the Company’s Form 10 filed with the SEC on April 21,
2009.
ITEM
9A(T). CONTROLS AND PROCEDURES.
Evaluation
of Disclosure Controls and Procedures
In
connection with the preparation of this annual report, an evaluation was carried
out by the Registrant’s management, with the participation of the principal
executive officer and the principal financial officer, of the effectiveness of
the Registrant’s disclosure controls and procedures (as defined in Rules
13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (“Exchange
Act”)) as of March 31, 2009. Disclosure controls and procedures are designed to
ensure that information required to be disclosed in reports filed or submitted
under the Exchange Act is recorded, processed, summarized, and reported within
the time periods specified in the Commission’s rules and forms, and that such
information is accumulated and communicated to management, including the chief
executive officer and the chief financial officer, to allow timely decisions
regarding required disclosures.
Based on
that evaluation, the Registrant’s management concluded, as of the end of the
period covered by this report, that the Registrant’s disclosure controls and
procedures were effective in recording, processing, summarizing, and reporting
information required to be disclosed, within the time periods specified in the
Commission’s rules and forms, and that such information was accumulated and
communicated to management, including the principal executive officer and the
principal financial officer, to allow timely decisions regarding required
disclosures.
Management’s
Report on Internal Control over Financial Reporting
The
management of the Registrant is responsible for establishing and maintaining
adequate internal control over financial reporting. The Registrant’s internal
control over financial reporting is a process, under the supervision of the
principal executive officer and the principal financial officer, designed to
provide reasonable assurance regarding the reliability of financial reporting
and the preparation of the Registrant’s financial statements for external
purposes in accordance with United States generally accepted accounting
principles (GAAP). Internal control over financial reporting includes those
policies and procedures that:
|
●
|
Pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of the Registrant’s
assets;
|
30
|
|
●
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of the financial statements in accordance with generally
accepted accounting principles, and that receipts and expenditures are
being made only in accordance with authorizations of management and the
board of directors; and
|
|
|
●
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use, or disposition of the Registrant’s assets
that could have a material effect on the financial
statements.
|
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions or that the degree of compliance
with the policies or procedures may deteriorate.
The
Registrant’s management conducted an assessment of the effectiveness of our
internal control over financial reporting as of March 31, 2009, based on
criteria established in Internal Control – Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission. Our
management has concluded that our internal control over financial reporting is
effective based on these criteria.
Changes
in Internal Controls over Financial Reporting
During
the fourth quarter ended on March 31, 2009, there has been no change in internal
control over financial reporting that has materially affected, or is reasonably
likely to materially affect our internal control over financial
reporting.
Attestation
Report of the Registered Public Accounting Firm
This
annual report does not include an attestation report of our independent
registered public accounting firm regarding internal control over financial
reporting. We were not required to have, nor have we, engaged our independent
registered public accounting firm to perform an audit of internal control over
financial reporting pursuant to the rules of the Commission that permit us to
provide only management’s report in this annual report.
ITEM
9B. OTHER INFORMATION.
None
PART
III
ITEM
10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Set forth
below is information regarding the current directors and executive officers of
EFT BioTech Holdings, Inc. The directors are elected annually by stockholders.
The executive officers serve at the pleasure of the board of
directors.
|
Name:
|
Age:
|
Title:
|
Director
Since:
|
|||
|
Jack
Jie Qin
|
49
|
President,
Chief Executive Officer and Chairman (Principal Executive
Officer)
|
November
2007
|
|||
|
Sharon
Tang
|
51
|
Chief
Financial Officer (Principal Financial and Accounting
Officer)
|
—
|
|||
|
George
W. Curry
|
64
|
Chief
Marketing Officer and Director
|
November
2007
|
|||
|
Jerry
B. Lewin
|
54
|
Director
|
August
5, 2009
|
|||
|
Visman
Chow
|
54
|
Director
|
August
5, 2009
|
|||
|
Norman
Ko
|
45
|
Director
|
August
5, 2009
|
Biographies
Jack
Jie Qin
Mr. Qin
has been serving as our President, Chief Executive Officer and Chairman of the
Board of Directors since November 2007. Since January 2004, Mr. Qin has
been serving as the President of EFT BioTech, Inc. From July 1998 to
December 2002, Mr. Qin serviced as the President of eFastTeam International,
Inc. located in Los Angeles, California. From June 1992 to December 1997,
he served as the President of LA Import & Export Company located in Los
Angeles, California. In May 1991, Mr. Qin earned an MBA from Emporia State
University located in Kansas. In May 1982, Mr. Qin graduated from Jiangxi
Engineering Institute located in Nanchang, China with a major in Mechanic
Engineering.
31
Sharon
Tang
Sharon
Tang has been serving as our Chief Financial Officer since June 2008. From
April 2007 to June 2008, she served as the Chief Financial Officer of Advanced
Battery Technologies, Inc. (NASDAQCM: ABAT) located in New York City.
From May 2006 to April 2007, Ms. Tang served as a Managing Director of First
Federal Group of Companies, Inc. located in New York City. From February 2006 to
May 2006, she served as a Vice President of Crucible Capital Group, Inc. located
in New York City. From April 1998 to February 2006, she served as a Financial
Advisor at Smith Barney, Citigroup in New York City. Ms. Tang’s professional
experience also includes serving as an Associate Engineer with The Research
Institute of Petroleum Exploration and Development, Ministry of Petroleum in
Beijing, China from December 1983 to July 1986 and Assistant Professor at the
Peking Business College in Beijing, China from January 1983 to December 1983.
Ms. Tang holds a MBA from Baruch College in New York City (June
2005), Master of Science in Chemical Engineering from the University of
Rochester in New York, NY (1988) and a Bachelor of Science in Chemistry from
Peking University in Beijing, China (1982) Ms. Tang holds Series 7 and 63
licenses from FINRA.
George
W. Curry
Mr. Curry
has been serving as our Chief Marketing Officer and as a Director since November
2007. From 1996 to October 2007, Mr. Curry served as a sales representative of
Mayor Pharmaceutical Labs, Inc. where he marketed products directly to the
public and recruited and trained additional sales people. He also served
as a motivational speaker at company training seminars throughout the U.S.
From 1992 to 1995, Mr. Curry owned Continental Limited, an import export
business focused on the clothing industry. In 1968, Mr. Curry earned a Bachelor
in Business Administration (BBA) from the University of North Texas with a major
in Marketing.
Jerry
B. Lewin
During
the past five years, Mr. Lewin has been serving as the Senior Vice President of
Field Operations North America for Hyatt Hotel Corporation. Mr. Lewin is
responsible for, and oversees the operation of 23 Hyatt Hotels throughout the
East Coast and Canada including the Flag Ship Hotel, Grand Hyatt New York in
Mid-Town Manhattan. Mr. Lewin also serves on several boards and foundations
including the New York City Hotel Association and the New York Law Enforcement
Foundation.
Visman
Chow
Since
1993, Mr. Chow has been serving as the Chief Lending Officer and board member at
Universal Bank. From 1979 to 1983, Mr. Chow was with Union Bank where he managed
a commercial real estate portfolio of approximately $50 million. Mr. Chow then
went on to become President of Unieast Financial Corporation prior to his
current position.
Norman
Ko
Since
2007, Mr. Ko has been a partner with Smith Mandel and Associates, LLP. He
provides audit and assurance services to private clients in various industry
groups along with SEC audit preparation and tax planning. Mr. Ko also has
experience in areas including due diligence, mergers and acquisition and
business reorganizations.
Legal/Disciplinary
History
None of
our executive officers or directors has been the subject of:
|
1.
|
A
conviction in a criminal proceeding or named as a defendant in a pending
criminal proceeding (excluding traffic violations and other minor
offenses);
|
|
|
|
|
2.
|
The
entry of an order, judgment or decree, not subsequently reversed,
suspended or vacated, by a court of competent jurisdiction that
permanently or temporarily enjoined, barred, suspended or otherwise
limited such person’s involvement in any type of business, securities,
commodities, or banking
activities;
|
|
3.
|
A
finding or judgment by a court of competent jurisdiction (in a civil
action), the Securities and Exchange Commission, the Commodity Futures
Trading Commission, or a state securities regulator of a violation of
federal or state securities or commodities law, which finding or judgment
has not been reversed, suspended, or vacated;
or
|
|
4.
|
The
entry of an order by a self-regulatory organization that permanently or
temporarily barred, suspended or otherwise limited such party’s
involvement in any type of business or securities
activities.
|
Family/Certain
Relationships
There are
no family relationships among or between any of the Registrant’s officers
and directors.
Employment
Agreements
We
currently do not have any employment agreements with any officer or director
other than with Ms. Sharon Tang.
32
Ms.
Sharon Tang serves as our Chief Financial Officer pursuant to an Employment
Agreement, dated May 1, 2008. The employment agreement commenced on June
1, 2008 and expires on June 1, 2013, unless terminated earlier by either party
pursuant to the terms of the employment agreement. Ms. Tang’s
compensation is $120,000 per year payable in equal monthly installments with 10%
annual increases for each subsequent year. She is also entitled to common
stock of the Registrant as determined by the Registrant’s board of
directors.
COMPLIANCE
WITH SECTION 16(a) OF THE SECURITIES EXCHANGE ACT OF 1934
Section
16(a) of the Exchange Act requires the Registrant’s directors and officers, and
persons who beneficially own more than 10% of a registered class of the
Registrant’s equity securities, to file reports of beneficial ownership and
changes in beneficial ownership of the Registrant’s securities with the SEC on
Forms 3, 4 and 5. Officers, directors and greater than 10% stockholders are
required by SEC regulation to furnish the Registrant with copies of all Section
16(a) forms they file.
Jack Jie
Qin, our President, CEO and Chairman; Sharon Tang, our Chief Financial Officer;
and George Curry, our Chief Marketing Officer, have not filed Forms 3, 4 and 5
to date. They intend to file them in the near future. Our 5%
stockholders have not filed a Form 3 to date.
ITEM
11. EXECUTIVE COMPENSATION.
Compensation
Discussion and Analysis
The goal
of our named executive officers’ compensation levels is the same as our goal for
operating the Company – to create long-term value for our stockholders.
Toward this goal, we establish compensation levels based on our named executive
officers’ relevant experience and leadership experience and skills. We also
consider the named executive officers’ ability and likelihood of contributing to
our Company’s growth and success. We also take into account comparable
salary ranges at similar companies in order to attract and retain our named
executive officers.
We do not
have a formula or benchmark or necessarily react to short-term changes in
business performance when reviewing our named executive officers’
salaries. We consider their past contributions to the Company, ability to
work cohesively with our management team and our expectations regarding their
future performance. Executive officers have an active role in our
determination of their compensation levels and we consider such executive
officers’ opinions and expectations.
To date,
we have not adopted a stock option plan or other incentive plan. We intend
to adopt a plan in the future to further align our management’s interest with
our stockholders’ interest.
Other
than our employment with Ms. Sharon Tang, our Chief Financial Officer, we do not
have any employment agreements with our executive officers. Ms. Tang’s
employment agreement does not contain a change in control or severance
provisions. Our executive officers serve at the will of the Board which enables
the Company to terminate their employment at their discretion. This is
consistent with the Company’s performance-based employment and compensation
philosophy.
The table
below summarizes the compensation we have paid our Named Executive Officers in
the last two fiscal years.
SUMMARY
COMPENSATION TABLE
|
Name and
Principal Position
|
Fiscal
Year
Ended
March 31,
|
Salary
|
Bonus
|
Stock
Awards
|
Option
Awards
|
Non-
Equity
Plan
Comp
|
Non-
Equity
Incentive
Comp
|
Non-
Qualified
Comp
Earnings
|
Other
Comp
|
Total
|
||||||||||||||||||||||||||||
|
Jack
Jie Qin (President, CEO
and Chairman)
|
2009
|
$ | 300,000 | (1) | $ | 0 | $ | 1 | (2) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 300,001 | |||||||||||||||||
|
(Principal
Executive Officer)
|
2008
|
$ | 300,000 | (1) | $ | 0 | $ | 1 | (2) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 300,001 | |||||||||||||||||
|
Sharon
Tang (Chief
Financial Officer)
|
2009
|
$ | 120,000 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 120,000 | |||||||||||||||||||
|
(Principal
Financial and Accounting Officer)(3)
|
2008
|
$ | 120,000 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 120,000 | |||||||||||||||||||
|
Dr.
Joseph B. Williams
|
2009
|
$ | 100,000 | $ | 0 | $ | 300 | (5) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 100,300 | ||||||||||||||||||
|
(Former
Chief Administrative Officer, Secretary and Director (8)
|
2008
|
$ | 100,000 | $ | 0 | $ | 300 | (5) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 100,300 | ||||||||||||||||||
|
George
Curry
|
2009
|
$ | 0 | $ | 0 | $ | 300 | () | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 300 | ||||||||||||||||||
|
(Chief
Marketing Officer)
|
2008
|
$ | 0 | $ | 0 | $ | 300 | () | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 300 | ||||||||||||||||||
|
Jun
Qin Liu
|
2009
|
$ | 120,000 | $ | 0 | $ | 300 | (5) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 120,300 | ||||||||||||||||||
|
(Former
Operations Manager and Director)(6)
|
2008
|
$ | 120,000 | $ | 0 | $ | 300 | (5) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 120,300 | ||||||||||||||||||
|
Tony
So
|
2009
|
$ | 100,000 | $ | 0 | $ | 300 | (4) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 100,000 | ||||||||||||||||||
|
(Former
Treasurer) (7)(8)
|
2008
|
$ | 100,000 | $ | 0 | $ | 300 | (4) | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 100,000 | ||||||||||||||||||
33
Notes:
|
(1)
|
Accrued
compensation
|
|
(2)
|
1,000
shares at $0.0018 per share. Share price is based on estimate fair market
price on the grant date.
|
|
(3)
|
Ms.
Tang commenced serving as the Company’s Chief Financial Officer on June 1,
2008.
|
|
(4)
|
Dr.
Williams served as our Chief Administrative Officer and Secretary from
June 2008 to February 6, 2009 and as a Director from November 2007 to
February 6, 2009. Dr. Williams served as our Chief Financial Officer
(Principal Financial Officer) from February 2008 to June 2008.
Before his employment with the Registrant, Mr. Williams served as a
consultant for the Registrant for seven months in the fiscal year ended
March 31, 2008.
|
|
(5)
|
300,000
shares at $0.001 per share. Share price is based on estimated fair market
price on the grant date.
|
|
(6)
|
Ms.
Liu resigned as the Registrant’s Operations Manager and a Director
effective December 2, 2008.
|
|
(7)
|
Tony
So resigned from the Registrant in September
2008.
|
|
(8)
|
From
June 1, 2007 to December 31, 2007, we retained Dr. Williams and Mr. So as
consultants at an annual salary of $120,000 each. As of
January 1, 2008, Dr. Williams and Mr. So became officers of the Registrant
at a monthly salary of $10,000 each. Mr. So resigned in September of
2008 and Dr. Williams resigned in February of
2009.
|
Board
Committees
In light
of the current size of the Board of Directors, the Registrant’s Board of
Directors has not established a standing Audit Committee,
Compensation/Nominating Committee or any other committee but intends to do so in
the future. Currently, our Board serves as the Audit Committee and
Compensation/Nominating Committee and none of the directors are deemed to be
“independent.” The Registrant has not appointed separately standing
committees because the Registrant desires to keep overhead expenses to a
minimum.
Financial
Expert
Our Board
of Directors has designated Mr. Norman Ko as a “financial expert” as that term
is defined in Item 407 of the Sarbanes Oxley Act of 2002.
Director
Compensation
Directors
are reimbursed for their out-of-pocket expenses incurred in connection with
attending board meetings. In the fiscal year ended March 31, 2009, the
Registrant reimbursed the directors an aggregate of $0 for such
expenses.
Code
of Ethics
We
currently have a Code of Ethics for our directors and principal executive
officers.
Code
of Business Conduct
The
Company has adopted a Code of Business Conduct which is followed in connection
with all Related Party Transactions (as defined below) with Related Persons (as
defined in Rule 404 of Regulation S-K). For these purposes, a “Related Person
Transaction” is a financial transaction, arrangement or relationship, or any
series of similar transactions, between the Company and an entity in which any
Related Person will have a direct or indirect material interest, other
than:
34
1. Transactions
available to all employees generally.
2. Transactions
involving less than $25,000 annually ($6,250 quarterly) when aggregated with all
similar transactions.
This
includes any transactions requiring disclosure under item 404 of Regulation S-K
under the Securities and Exchange Act of 1934,as amended.
Approval
Procedures:
|
1.
|
A
proposed Related Person Transaction shall be brought before the Board of
Directors. The Board must be informed of (a) the Related Person’s
relationship or interest, including all conflicts of interest that may
exist or otherwise arise on account of the Related Person Transaction, and
(b) the material facts of the proposed Related Person
Transaction.
|
|
2.
|
The
Board shall determine whether to approve a Related Person Transaction
after considering the following factors, as deemed relevant by the
Board:
|
• Whether
the transaction is on terms comparable to those that could be obtained in
arms-length dealing with an unrelated third party;
• Whether
there are business reasons to enter into the Related Person
Transaction;
• Whether
the Related Person Transaction could impair the independence of a director, if
applicable;
• Whether
the Related Person Transaction would present an improper conflict of interest,
taking into account the size of the transaction, the overall financial position
of the executive officer, Director or Director Nominee, the direct or indirect
nature of the interest of the executive officer, Director or Director Nominee in
the transaction, the ongoing nature of any proposed relationships or any other
factors the Board deems relevant.
|
3.
|
The
Board will approve such transactions to be entered into by the Company,
including the ratification of such transactions if applicable. At
subsequent meetings, management shall update the Board as to any material
changes to those proposed
transactions.
|
|
4.
|
The
Board shall also periodically review and assess ongoing relationships with
Related Persons to assure compliance with the Board guidelines and
directives and to ensure that such Related Person Transaction remains fair
to the Company.
|
|
5.
|
Any
member of the Board who has an interest in the transaction under
consideration will abstain from voting, but may participate in the
discussion if invited to do so by the Chair of the
Board.
|
|
6.
|
These
procedures generally should be used to approve Related Person Transactions
in advance of the transaction being entered into. On occasion, however, it
may be advisable to commence a Related Person Transaction before the Board
has evaluated it, or a transaction may commence before it is discovered
there is a Related Person. Accordingly, in such instances, notwithstanding
the above, management should consult with the Chair of the Board to
determine the appropriate course of action, which may include subsequent
ratification by the Board.
|
Disclosure:
Related
Person Transactions are to be disclosed in the Company’s applicable filings as
required by the Securities Act of 1933 and the Securities Exchange Act of 1934
and related rules.
Options
To date
the Registrant has not issued any options to its executive officers, directors
or employees.
35
ITEM
12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND
RELATED STOCKHOLDER MATTERS.
The
following table sets forth information regarding the beneficial ownership of
outstanding Common Stock as of the date of this Amendment No. 2 (i) each of our
directors and executive officers, (ii) all directors and executive officers as a
group, and (iii) each owner of more than 5% of our Common Stock (5% owners).
Except as set forth in the footnotes to this table, the business address of each
director and executive officer listed is c/o EFT BioTech Holdings, Inc., 929
Radecki Ct., City of Industry, CA 91789.
|
Name of Beneficial Owners
|
Number of
Shares
Beneficially
Owned
|
Percent of
Shares
Outstanding
|
||||||
| (1 | ) | (2 | ) | |||||
|
Jack
Jie Qin
—President,
Chief Executive Officer and Chairman
(Principal
Executive Officer)
|
1,000 | * | ||||||
|
Sharon
Tang
—Chief
Financial Officer
(Principal
Financial Officer)
|
0 | — | ||||||
|
George
W. Curry
—Chief
Marketing Director
|
300,000 | * | ||||||
|
Jerry
B. Lewin
—Director
|
0 | — | ||||||
|
Visman
Chow
—Director
|
0 | — | ||||||
|
Norman
Ko
—Director
|
0 | — | ||||||
|
Dr.
Joseph B. Williams
—Former
Chief Administrative Officer and Director (3)
|
300,000 | * | ||||||
|
Tony
So
—Former
Treasurer (4)
|
300,000 | * | ||||||
|
Jun
Qin Liu
—Former
Operations Manager and Director (5)
|
300,000 | * | ||||||
|
Dragon
Win Management, Ltd. (6)
Palm
Grove Houses,
P.O.
Box 438
Road
Town, Tortola
British
Virgin Islands
|
52,099,000 | 68.57 | % | |||||
|
All
Current and Former Officers and Directors as a group (7
persons)
|
1,201,000 | 1.58 | % | |||||
*
Represents less than 1%.
36
|
(1)
|
As
used herein, the term beneficial ownership with respect to a security is
defined by Rule 13d-3 under the Securities Exchange Act of 1934, as
amended, as consisting of sole or shared voting power (including the power
to vote or direct the vote) and/or sole or shared investment power
(including the power to dispose or direct the disposition of) with respect
to the security through any contract, arrangement, understanding,
relationship or otherwise, including a right to acquire such power(s)
during the next 60 days. Unless otherwise noted, beneficial ownership
consists of sole ownership, voting and investment
rights.
|
|
(2)
|
Based
on 75,983,205 shares of common stock issued and outstanding as of the date
of this Amendment No. 2.
|
|
(3)
|
Dr.
Williams served as our Chief Administrative Officer and Secretary from
June 2008 to February 6, 2009 and as a Director from November 2007 to
February 6, 2009. Dr. Williams served as our Chief Financial Officer
(Principal Financial Officer) from February 2008 to June 2008.
Before his employment with the Registrant, Mr. Williams served as a
consultant for the Registrant for seven months in the fiscal year ended
March 31, 2008.
|
|
(4)
|
Tony
So resigned from the Registrant in September
2008.
|
|
(5)
|
Ms.
Jin Qin Liu resigned from the Registrant on December 2,
2008.
|
|
(6)
|
On
or around November, 2007, the owner of Top Capital International Limited,
EFT International Limited, and EFT (HK) Limited (collectively, the
“Offshore Operating Entities”), reached an agreement in principle with the
Registrant to transfer, sell and assign, with exception of certain assets,
the entire business operations of these Offshore Operating Entities to EFT
Limited, in exchange for shares of common stock of the Registrant
following the share exchange between the Registrant and EFT BioTech, the
parent of EFT Limited. In consideration for the ownership transfer of the
Offshore Operating Entities, 52,099,000 shares of common stock were issued
to Dragon Win Management Limited, a British Virgin Islands company.
The board of directors of Dragon Win has voting and dispositive control of
the Registrant’s common stock held by Dragon Win. Ning-Sheng Cai and
Xiao-Bao Hu are currently the two directors of Dragon
Win.
|
|
(7)
|
Wallace
Gaikus and Peter Lau have voting and dispositive control of Greenstone
Holdings, Inc.
|
ITEM
13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE.
The
Registrant’s Code of Business Conducts states that the Registrant shall not,
directly or indirectly, including through any subsidiary, make or maintain any
new extension of credit or arrange for the extension of credit in the form of a
personal loan to or for any director or executive officer (or equivalent
thereof) of the Registrant. This prohibition includes corporate guarantees but
excludes loans under the Registrant’s 401(k) plan, if any, reimbursable travel
and similar expenses incurred while performing executive responsibilities,
reimbursable relocation expenses, use of company vehicles for business purposes,
and credit and charge cards used only in connection with business and limited
ancillary personal purposes (e.g., personal items included in hotel room
charges) settled within a reasonable time period (e.g., monthly).
Our Board
of Directors review and vote on all proposed transactions involving a related
person, including our officers and directors and any affiliates thereof or their
respective family members, if any. The interested director, if any, does
not vote on any such matter(s). In its determination, the Board
deliberates whether the proposed transaction is in the best interests of the
Registrant and its stockholders and whether the proposed transaction is as fair
and equitable as it would be with non-related party on an “arm’s length
basis.”
Loan
to Excalibur Marine Exploration
As
disclosed in this Form 10, the Company has loaned amounts to Excalibur, a
related party. On October 25, 2008, EFT Investment Co., Ltd. completed the
acquisition of 58,567,750 shares of common stock of Excalibur, representing
approximately 49% shares of issued and outstanding shares of Excalibur, for an
aggregate purchase price of USD $19,193,000. Prior to the acquisition of
Excalibur, the Company did not have any relationship with Excalibur nor was it
deemed to be a related party.
37
Note
Receivable
The Board
of Directors approved two non-interest bearing unsecured demand loans in the
amount of U.S. $330,000 and $1,567,000 respectively on July 14,
2008 and July 25, 2008 to Yeuh-Chi Liu, a vendor and a
member of the board of directors of Excalibur. The loan amount in
$1,567,000 is collateralized with 3.97% ownership of Excalibur International
Marine Corp. As of the date hereof the full principal amount remains
outstanding.
Director
Independence
Jerry B.
Lewin, Visman Chow and Norman Ko are deemed to be independent
directors.
ITEM
14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
(1)
Audit Fees
The
aggregate fees billed for each of the last two fiscal years for professional
services rendered by the principal accountant for our audit of annual financial
statements and review of financial statements included in our quarterly
reports or services that are normally provided by the accountant in
connection with statutory and regulatory filings or engagements for those fiscal
years were:
|
For Fiscal Year Ended March 31,
|
Amount
|
Auditor
|
|||
|
2009
|
$
|
0
|
Weinberg
& Company, P.A.
|
||
|
2009
|
$
|
84,500
|
Child,
Van Wagoner & Bradshaw, PLLC
|
||
|
2008
|
$
|
135,000
|
Child,
Van Wagoner & Bradshaw,
PLLC
|
||
(2)
Audit-Related Fees
The
aggregate fees billed in each of the last two fiscal years for assurance and
related services by the principal accountants that are reasonably related to the
performance of the audit or review of our financial statements and are not
reported in the preceding paragraph:
|
For Fiscal Year Ended March 31,
|
Amount
|
Auditor
|
|||
|
2009
|
$
|
0
|
Weinberg
& Company, P.A.
|
||
|
2009
|
$
|
25,500
|
Child,
Van Wagoner & Bradshaw, PLLC
|
||
|
2008
|
$
|
23,500
|
Child,
Van Wagoner & Bradshaw,
PLLC
|
||
(3)
Tax Fees
The
aggregate fees billed in each of the last two fiscal years for professional
services rendered by the principal accountant for tax compliance, tax advice,
and tax planning were:
|
For Fiscal Year Ended March 31,
|
Amount
|
Auditor
|
|||
|
2009
|
$
|
0
|
Weinberg
& Company, P.A.
|
||
|
2009
|
$
|
0
|
Child,
Van Wagoner & Bradshaw, PLLC
|
||
|
2008
|
$
|
0
|
Child,
Van Wagoner & Bradshaw,
PLLC
|
||
(4)
All Other Fees
The
aggregate fees billed in each of the last two fiscal years for the products and
services provided by the principal accountant, other than the services reported
in paragraphs (1), (2), and (3) was:
|
For Fiscal Year Ended March 31,
|
Amount
|
Auditor
|
|||
|
2009
|
$
|
0
|
Weinberg
& Company, P.A.
|
||
|
2009
|
$
|
0
|
Child,
Van Wagoner & Bradshaw, PLLC
|
||
|
2008
|
$
|
18,953
|
Child,
Van Wagoner & Bradshaw,
PLLC
|
||
38
PART
IV
ITEM
15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
|
Exhibit No.:
|
Description:
|
|
|
3.1(1)
|
Articles
of Incorporation of GRG, Inc. (now EFT BioTech Holdings,
Inc.).
|
|
|
3.1.1(1)
|
Articles
of Merger filed December 28, 2004 between HumWare Media Corporation, World
Wide Golf Web, Inc. and GRG, Inc.
|
|
|
3.1.2(1)
|
Certificate
of Amendment, effective November 7, 2007, to the Articles of
Incorporation of HumWare Media Corporation
|
|
|
3.2(3)
|
By-laws
|
|
|
4.1(1)
|
Form
of Common Stock Certificate
|
|
|
4.2(1)
|
Form
of Warrant to purchase one share of Common Stock for a purchase price of
$3.80 per share until the second anniversary date of the date of
issuance
|
|
|
10.1(3)
|
Share
Exchange Agreement, dated as of the 1st day of November, 2007, by and
among EFT BioTech Holdings, Inc. (formerly HumWare Media Corporation), a
Nevada corporation; certain EFT Shareholders and EFT BioTech Corporation,
a Nevada corporation
|
|
|
10.2(2)
|
Subscription
Agreement for Units in connection with the Registrant’s Regulation S
Private Placement
|
|
|
10.3(3)
|
Employment
Agreement, dated May 10, 2008, between EFT BioTech Holdings, Inc. and
Sharon Tang
|
|
|
10.4(5)
|
$500,000Loan
Agreement (the “Agreement”), dated November 24, 2008 , between the EFT
Biotech Holdings, Inc. (as the Lender), EFT Investment Co., LTD., and
Excalibur International Marine Corporation (as the
Borrower).
|
|
|
10.5(5)
|
First
Extension of $500,000 Loan, dated December
25, 2008
|
|
|
10.6(5)
|
Second
Extension of $500,000 Loan, dated May 25, 2009
|
|
|
10.7(6)
|
$2,000,000
Loan Agreement (the “Agreement”) and promissory note, dated September 23,
2008, between the EFT Biotech Holdings, Inc. (as the Lender), EFT
Investment Co., LTD., and Excalibur International Marine Corporation (as
the Borrower).
|
|
|
10.8(5)
|
First
Extension of $2,000,000 Loan, dated November 25, 2008
|
|
|
10.9(5)
|
Second
Extension of $2,000,000 Loan, dated May 25, 2009
|
|
|
10.10(6)
|
$600,000
Loan Agreement, dated May 13, 2009, between the EFT Biotech Holdings, Inc.
(as the Lender), EFT Investment Co., LTD., and Excalibur International
Marine Corporation (as the Borrower).
|
|
|
10.11(6)
|
Addendum
to $600,000 Loan Agreement, dated May 13, 2009, between the EFT Biotech
Holdings, Inc. (as the Lender), EFT Investment Co., LTD., and Excalibur
International Marine Corporation (as the Borrower).
|
|
|
10.12
(7)
|
$330,000
Loan Agreement, dated July 14, 2008, between EFT
BioTech Holdings, Inc. (Lender) and Yeuh-Chi Liu
(Borrower)
|
|
|
10.13
(7)
|
Addendum
to $330,000 Loan Agreement, dated July 15, 2008, between BioTech Holdings,
Inc. and Yeuh-Chi Liu
|
|
|
10.14
(7)
|
$1,567,000
Loan Agreement, dated July 25, 2008, between BioTech Holdings, Inc.
(Lender) and Yeuh-Chi Liu (Borrower)
|
|
|
14.1(3)
|
Code
of Ethics
|
|
|
14.2(6)
|
Amended
Code of Business Conduct
|
|
|
16.1(4)
|
Letter
of Weinberg & Company P.A., dated April 21, 2009
|
|
|
31.1
|
Certification
by Jack Jie Qin, Principal Executive Officer of EFT BioTech Holdings,
Inc., pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act
of 1934, as amended.
|
|
|
31.2
|
Certification
by Sharon Tang, Principal Financial and Accounting Officer of EFT BioTech
Holdings, Inc., pursuant to Rule 13a-14(a)/15d-14(a) of the Securities
Exchange Act of 1934, as amended.
|
|
|
32.1
|
Certification
by Jack Jie Qin, Principal Executive Officer of EFT BioTech
Holdings, Inc., pursuant to 18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.2
|
Certification
by Sharon Tang, Principal Financial and Accounting Officer of EFT BioTech
Holdings, Inc., pursuant to 18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of
2002
|
Notes:
|
(1)
|
Filed
as an exhibit to Form 10 (File No.: 001-34222) filed with the SEC on
December 10, 2008 and incorporated by reference
herein.
|
|
(2)
|
Filed
as an exhibit to Form 10-Q for the quarter ended December 31, 2008 (File
No.: 001-34222) filed with the SEC on February 13, 2009 and incorporated
by reference herein.
|
|
(3)
|
Filed
as an Exhibit to Amendment No. 1 to Form 10 (File No.: 001-34222) filed
with the SEC on April 13, 2009 and incorporated by reference
herein.
|
|
(4)
|
Filed
as an Exhibit to Amendment No. 2 to Form 10 (File No.: 001-34222) filed
with the SEC on April 21, 2009 and incorporated by reference
herein.
|
|
(5)
|
Filed
as an Exhibit to Form 10-K (File No.: 001-34222) filed with the SEC on
July 17, 2009 and incorporated by reference
herein.
|
|
(6)
|
Filed
as an Exhibit to Amendment No. 4to Form 10 (File No.: 000-53730) filed
with the SEC on September 3, 2009 and incorporated by reference
herein.
|
|
(7)
|
Filed
as an Exhibit to Amendment No. 5 to Form 10 (File No.: 000-53730) and
incorporated by reference herein.
|
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
|
Date:
October 19, 2009
|
EFT
BIOTECH HOLDINGS, INC.
|
|
|
By:
|
/s/
Jack Jie Qin
|
|
|
Name:
Jack Jie Qin
Title:
President, Chief Executive Officer and Chairman
(Principal
Executive Officer)
|
In
accordance with the requirements of the Securities Act of 1933, this
registration statement was signed by the following persons in the capacities and
on the dates stated.
|
Signature
|
Title
|
Date
|
||
|
/s/
Jack Jie Qin
|
October
19, 2009
|
|||
|
Jack
Jie Qin
|
President,
Chief Executive Officer and Chairman (Principal Executive
Officer)
|
|||
|
/s/
Sharon Tang
|
Chief
Financial Officer
(Principal
Financial and Accounting Officer)
|
October
19, 2009
|
||
|
Sharon
Tang
|
||||
|
/s/
George W. Curry
|
Chief
Marketing Officer and Director
|
October
19, 2009
|
||
|
George
W. Curry
|
||||
|
Jerry
B. Lewin
|
Director
|
|||
|
/s/
Visman Chow
|
||||
|
Visman
Chow
|
Director
|
October
19, 2009
|
||
|
/s/
Norman Ko
|
||||
|
Norman
Ko
|
Dirctor
|
October
19, 2009
|
39
