Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CATALYST BIOSCIENCES, INC. | d8k.htm |
| EX-99.2 - PRESS RELEASE - CATALYST BIOSCIENCES, INC. | dex992.htm |
 Positive effects
of the nicotinic channel blocker TC-5214 as augmentation treatment in patients with major depressive disorder who are inadequate responders to a first-line SSRI Geoffrey C. Dunbar, M.D. Targacept Exhibit 99.1 |
 Cautionary Note
re: Forward-Looking Statements Cautionary Note re: Forward-Looking Statements This presentation includes “forward-looking statements” made under the provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, without
limitation, statements that are not purely historical in nature regarding: the
progress, scope or duration of the development of TC-5214, such as the size,
design, conduct or objective of any clinical trial of TC-5214, the timing for
initiation or completion of or availability of results from any clinical trial of TC-5214 or for submission or approval of any regulatory filing regarding TC-5214, or the indication(s) for
which TC-5214 may be developed; the benefits that may be derived from TC-5214;
or our plans, expectations, objectives, prospects or future operations, financial
position, revenues, costs or expenses. The words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing,” “scheduled” and similar expressions are intended to identify forward-looking statements. Actual
results, performance or experience may differ materially from those expressed or
implied by any forward-looking statement as a result of various important factors,
including our critical accounting policies and the risks and uncertainties described under the heading “Risk Factors” in our most recent Annual Report on Form 10-K, in our subsequent Quarterly Reports on Form 10-Q and in other
filings that we make with the Securities and Exchange Commission. As a result of
the risks and uncertainties, the results or events indicated by the forward-looking
statements may not occur. All forward-looking statements speak only as of the date
this presentation is made and should not be relied upon as representing our views as of
any date after this presentation is made. We specifically disclaim any obligation
to update any forward-looking statement, except as required by applicable law. |
 Large Unmet
Medical Need for Depressed Patients not Responding Adequately to First-line
Antidepressants Large Unmet Medical Need for Depressed Patients not Responding Adequately to First-line Antidepressants STAR*D study (Funded by NIMH – studied 3,671 “real world” patients) Step 1: Tested effectiveness of first-line treatments for remission Only 36.8 % (QIDS) patients in remission after 12 weeks of treatment with citalopram Step 2: Tested switching vs. augmentation to achieve remission Switch – 27% (QIDS) and 21% (HAM-D) Augment – 35% (QIDS) and 30% (HAM-D) The results suggest that augmentation may be more effective than switching 1 Rush et al., Am. J. Psychiatry 163: 1905-17 (2006) 2 Rush et al., N Engl J Med.
354: 1231–42 (2006) 3 Trivedi et al., N Engl J Med.
354: 1243–52 (2006) 1 1 2 3 1 |
 TC-5214
Profile TC-5214 Profile Mecamylamine demonstrated antidepressant effects as augmentation treatment in inadequate responders to citalopram (2007) TC-5214 is the S-(+) enantiomer of mecamylamine TC-5214 is a nicotinic channel blocker that has unique properties in modulating different forms of the 4 2 NNR TC-5214 has exhibited an overall profile superior to mecamylamine in preclinical models of depression and anxiety TC-5214 |
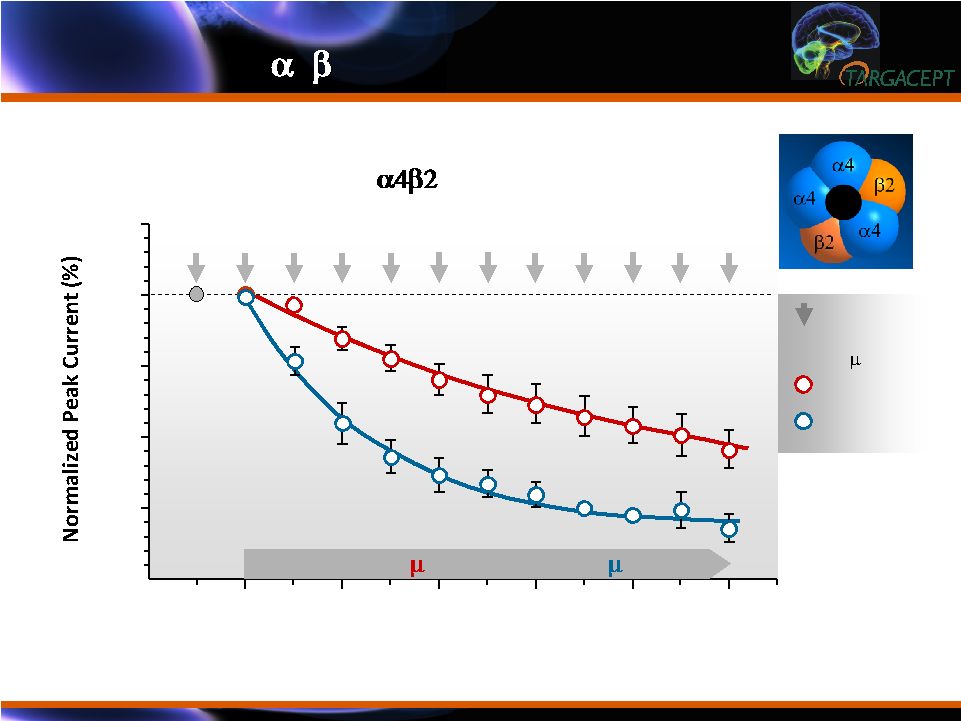 Use-Dependent Effects of Enantiomers on Functional Activation of LS- by Nicotine in SH-EP1 Cells Agonist Applications (@ 30 sec Intervals) 0 10 25 50 75 100 125 TC-5213 (1 M) or TC-5214 (1 M) 2 4 6 8 TC-5214 TC-5213 Nicotine Exposures (10 M, 50 ms) From Fedorov et al., JPET 328: 525-32 (2009) TC-5214 is a More Effective Non-competitive Inhibitor of LS- 4 2 NNRs than TC-5213 TC-5214 is a More Effective Non-competitive Inhibitor of LS- 4 2 NNRs than TC-5213 |
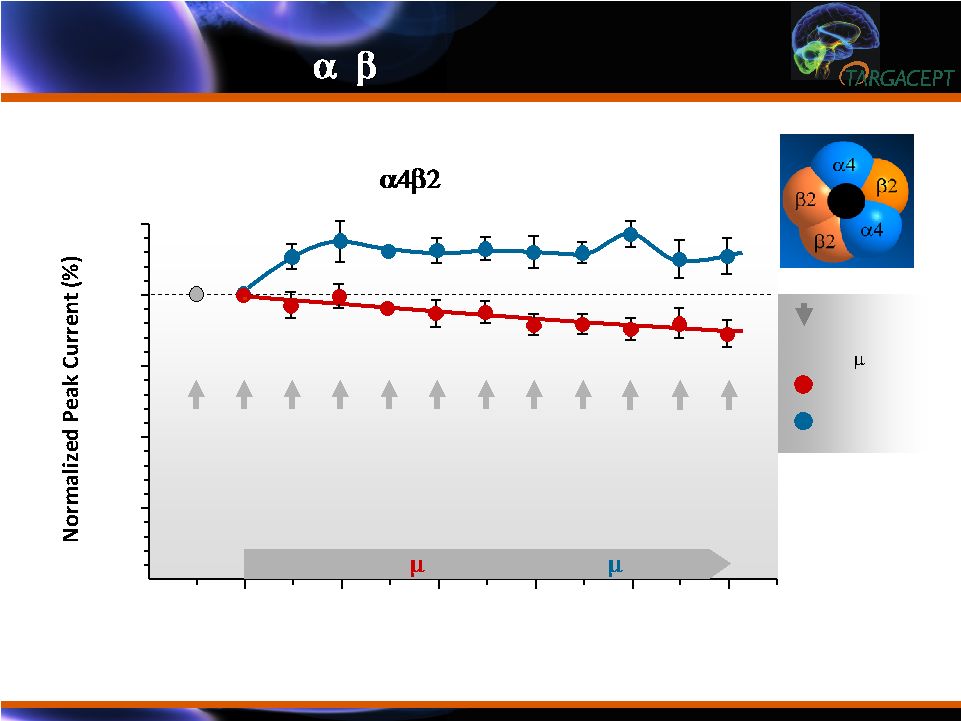 TC-5214
Enhances and TC-5213 Blocks Activation of HS- 4 2 NNRs TC-5214 Enhances and TC-5213 Blocks Activation of HS- 4 2 NNRs Use-Dependent Effects of Enantiomers on Functional Activation of HS- by TC-2559 in SH-EP1 Cells Agonist Applications (@ 30 sec Intervals) 0 10 25 50 75 100 125 TC-5213 (1 M) or TC-5214 (1 M) 2 4 6 8 From Fedorov et al., JPET 328: 525-32 (2009) TC-5214 TC-5213 TC-2559 Exposures (0.5 M, 50 ms) |
 TC-5214
Phase 2b Augmentation Study in Major Depressive Disorder |
 Key Inclusion
Criteria Key Inclusion Criteria Male or female subjects aged 18 – 70 yrs DSM IV criteria for MDD MADRS score > 27; CGI-SI 4 No other uncontrolled medical condition No clinically meaningful abnormalities in biochemistry, vital signs or ECG |
 Trial
Design Trial Design 23 Sites: USA (3) and India (20) Citalopram (20mg then 40 mg) given for 8 weeks Subjects with MADRS change < 50%, MADRS 17 and CGI-SI 4 were considered inadequate responders Randomized to double-blind augmentation with
TC-5214 or placebo (citalopram dose unchanged)
for further 8 weeks TC-5214 given as flexible dose of 2 4 8mg
(1 2 4 mg b.i.d.) depending on tolerability and inadequate response |
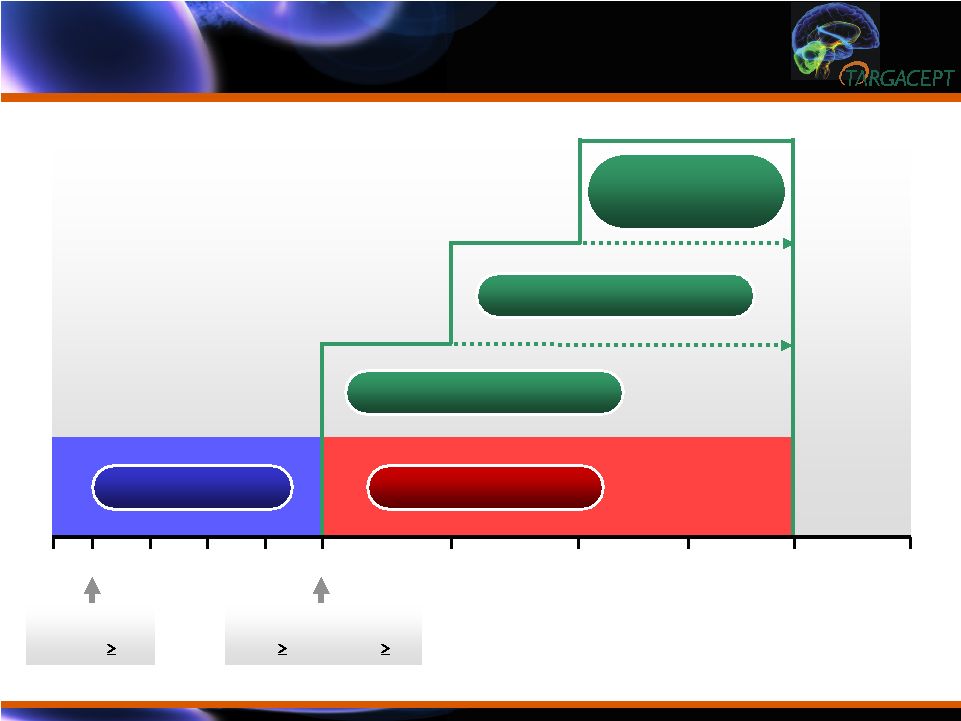 Trial
Design Trial Design 12 18 10 14 6 0 -1 2 mg TC-5214 + Citalopram 2 4 MADRS > 27 CGI(S) 4 HAMD, MADRS, QIDS-SR, CGI(I), SGI-Cog, SDS, SIS 8 MADRS < 50% and 17 CGI(S) 4 4 mg TC-5214 + Citalopram 8 mg TC-5214 + Citalopram Placebo + Citalopram Citalopram alone 16 WEEK |
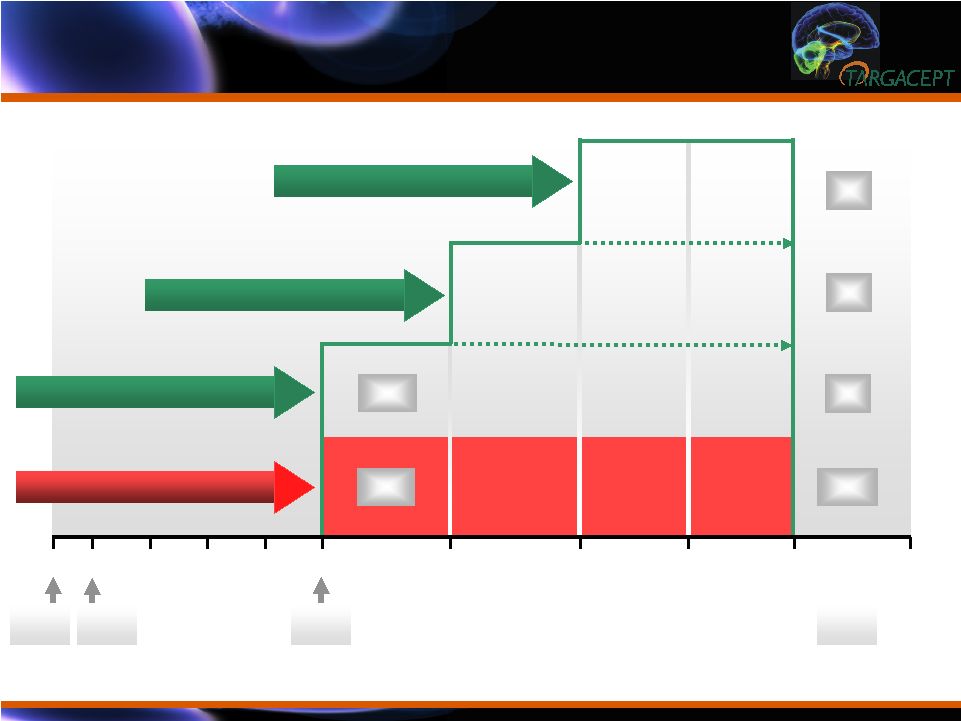 Subject
Progression During Trial Subject Progression During Trial 12 16 10 14 6 0 -1 2 4 707 8 135 112 135 33 30 50 8 mg TC-5214 + Citalopram 4 mg TC-5214 + Citalopram 2 mg TC-5214 + Citalopram Placebo + Citalopram 579 270 225 18 WEEK |
 Baseline
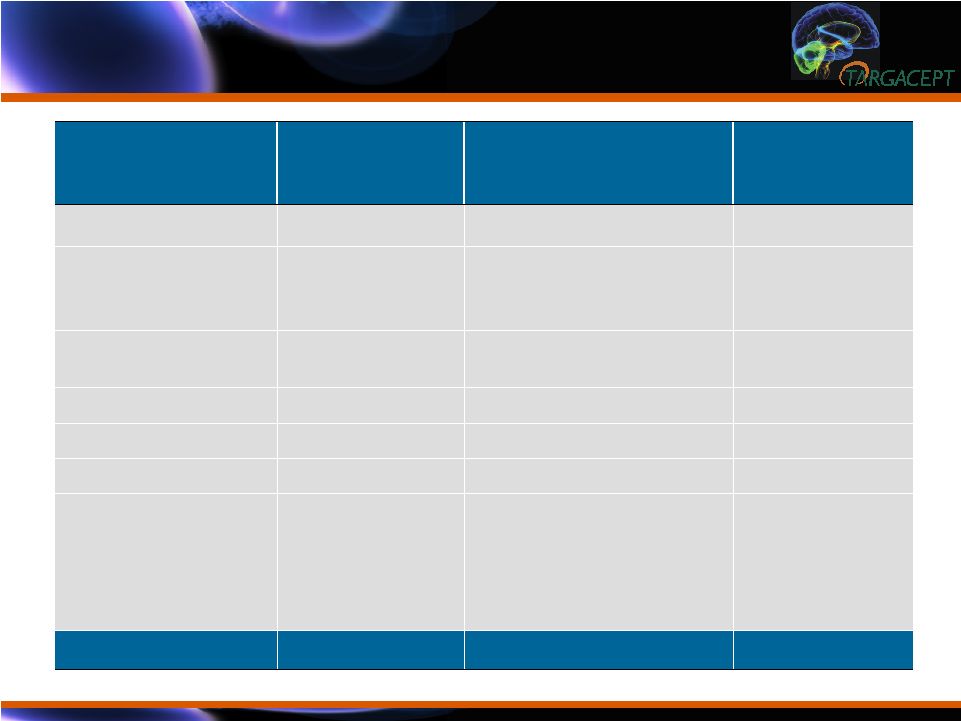
Demographics of Randomized Subjects (N=270) Baseline Demographics of Randomized Subjects (N=270) Variable Placebo + citalopram (N=135) TC-5214 + citalopram (N=135) Total (N=270) Age (Mean) (Yrs) 35.5 35.6 35.55 Asian Hispanic White 126 (93%) 6 (5%) 3 (2%) 125 (93%) 6 (4%) 4 (3%) 251 (93%) 12 (4%) 7 (3%) Male Female 67 (50%) 68 (50%) 66 (49%) 69 (51%) 133 (49%) 137 (51%) Weight (Mean) (kg) 59.9 61.4 60.6 Height (Mean) (cm) 162.9 163.0 162.95 BMI (Mean) (kg/m 2 ) 22.6 23.1 22.8 Education < HS/SS HS/SS College MS/PhD 51 (38%) 53 (39%) 23 (17%) 8 (6%) 56 (41%) 53 (39%) 21 (16%) 5 (4%) 107 (40%) 106 (39%) 44 (16%) 13 (5%) MADRS (sd) 29.8 (5.6) 30.0 (5.25) -- |
 Disposition Placebo + citalopram (N = 132) TC-5214 + citalopram (N = 133) Total (N = 265) *ITT Subjects in Double Blind Phase 132 (100%) 133 (100%) 265 (100%) Completed 112 (85%) 113 (85%) 225 (85%) Prematurely Withdrawn 20 (15%) 20 (15%) 40 (15%) Primary Reason for Withdrawal Adverse event 1 (1%) 1 (1%) 2 (1%) Consent withdrawn 7 (5%) 5 (4%) 12 (5%) Lost to follow up 5 (4%) 8 (6%) 13 (5%) Other 5 (4%) 4 (3%) 9 (3%) Positive drug screening/pregnancy test 1 (1%) 1 (0%) Protocol deviation 1 (1%) 2 (2%) 3 (1%) * 270 Subjects were randomized to drug (135) and placebo (135) but five subjects had no post- baseline assessments making them ineligible to be categorized as ITT as defined in protocol Subject Disposition ITT Population (N = 265) Subject Disposition ITT Population (N = 265) |
 TC-5214
Treatment-Emergent Serious Adverse Events (SAEs) (n=2) TC-5214 Treatment-Emergent Serious Adverse Events (SAEs) (n=2) Not drug related: Heavy menstruation in 51 year old female: End of study Peri-menopausal state 4 weeks of menstrual bleeding and Hb of 8.9 gm/dL on last day of study drug; Hb 5.5 gm/dL on follow-up visit Treated with blood transfusion and hysterectomy Drug related: Seizure – 50 year old male: Day 4 of citalopram + TC-5214 treatment Event not seen by medical personnel and reported inconsistently by relatives Citalopram alone carries 0.3% chance of seizure (1 in 300) TC-5214 is anticonvulsant in pre-clinical models |
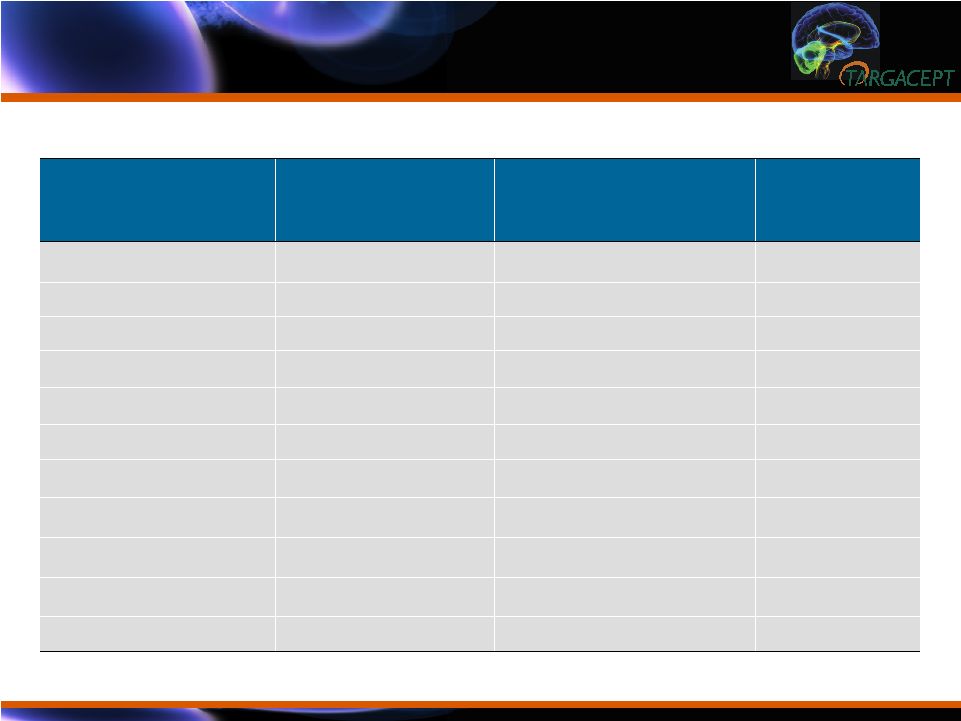 TC-5214 Well
Tolerated:
Most Common Adverse Events TC-5214 Well
Tolerated:
Most Common Adverse Events Adverse Event Placebo + citalopram (N 135) N (%) TC-5214 + citalopram (N = 135) N (%) Total (N = 270) N (%) Subjects w/ AE 43 (32%) 54 (40%) 97 (35%) Headache 4 (3%) 12 (9%) 16 (5%) Constipation 1 (1%) 11 (8%) 12 (4%) Dizziness 4 (3%) 8 (6%) 12 (4%) Pyrexia 4 (3%) 3 (2%) 7 (3%) Asthenia 0 (0%) 6 (4%) 6 (2%) Gastritis 4 (3%) 1 (1%) 5 (2%) Insomnia 2 (1%) 3 (2%) 5 (2%) Nasopharyngitis 3 (2%) 2 (1%) 5 (2%) Somnolence 3 (2%) 1 (1%) 4 (1%) Myalgia 1 (1%) 1 (1%) 2 (1%) |
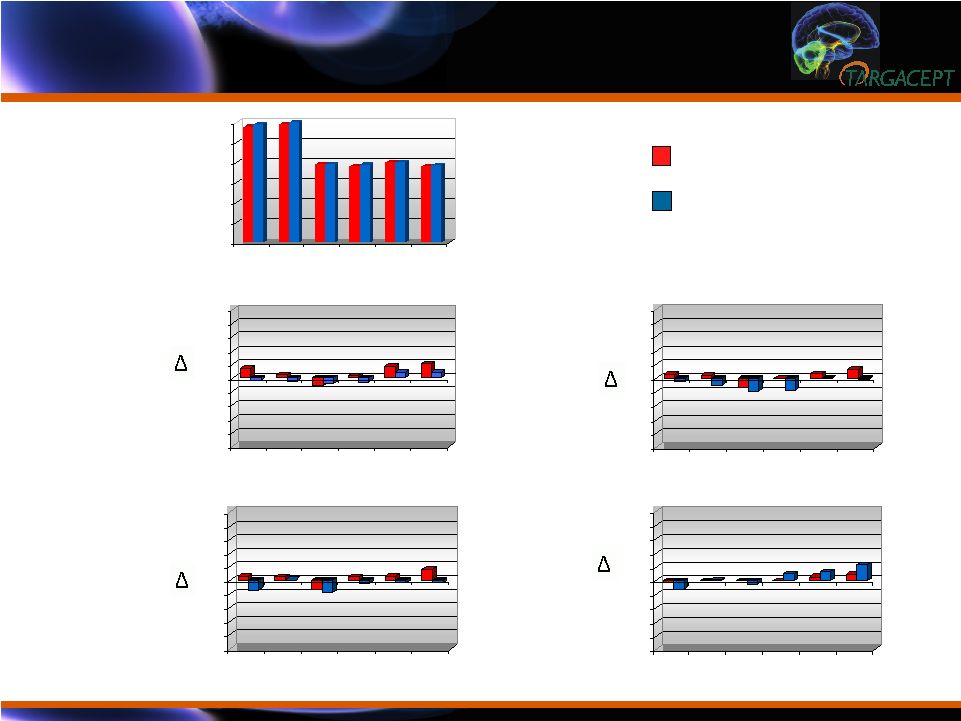 Vital Signs: No
Clinically Meaningful Changes from Baseline Vital Signs: No Clinically Meaningful Changes from Baseline -10 -8 -6 -4 -2 0 2 4 6 8 10 -10 -8 -6 -4 -2 0 2 4 6 8 10 -10 -8 -6 -4 -2 0 2 4 6 8 10 -10 -8 -6 -4 -2 0 2 4 6 8 10 Week 8 (post-dose) Week 12 Week 14 Week 16 (post-dose) S L
S L S L S L
S L S L S L
S L S L S L
S L S L Systolic Diastolic
HR Systolic
Diastolic HR Systolic Diastolic
HR Systolic
Diastolic HR 0 20 40 60 80 100 120 Week 8 Baseline (pre-dose) S L
S L S L Systolic Diastolic
HR Placebo + citalopram TC-5214 + citalopram |
 ECG,
Biochemistry, Hematology and Urine Analysis: No Clinically Meaningful Changes ECG, Biochemistry, Hematology and Urine Analysis: No Clinically Meaningful Changes No clinically meaningful changes on ECG (especially QTc) No clinically meaningful changes in biochemistry Absolute values Change values Shift tables No clinically meaningful changes in hematology Absolute values Change values Shift tables No change in urine analysis |
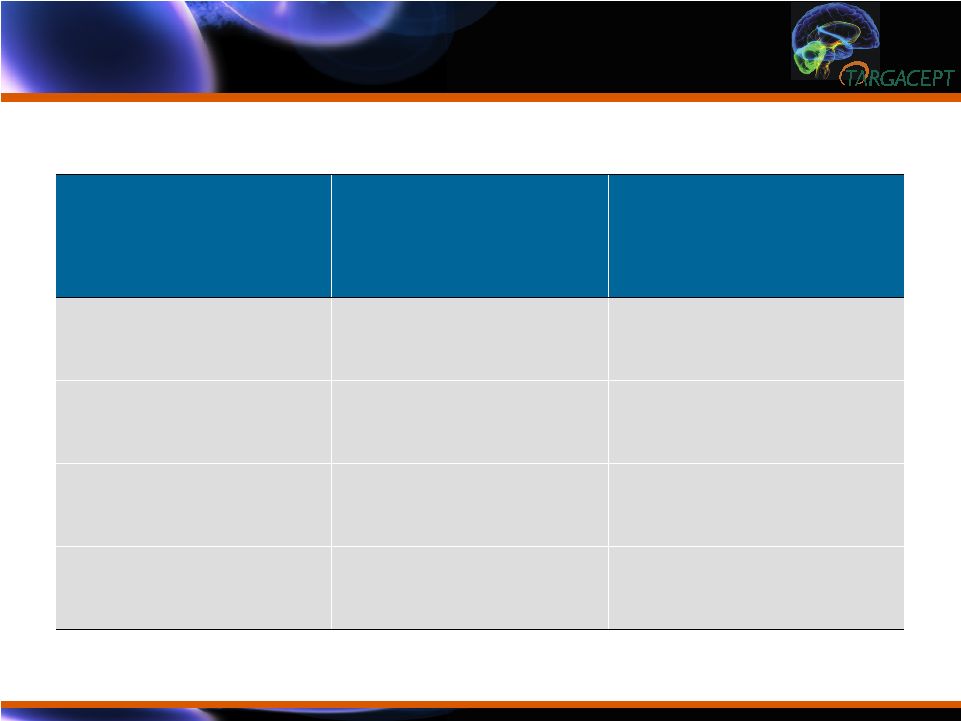 Double Blind
Baseline (Week 8) Scores Double Blind Baseline (Week 8) Scores Baseline Variable Placebo + citalopram N = 132 Mean (sd) TC-5214 + citalopram N = 133 Mean (sd) HAMD-17 Total Score 23.7 (4.68) 23.5 (5.19) CGI-SI Score 4.1 (0.35) 4.3 (0.47) MADRS Total Score 29.8 (5.59) 30.0 (5.25) QIDS-SR Total Score 14.2 (4.31) 14.4 (4.26) |
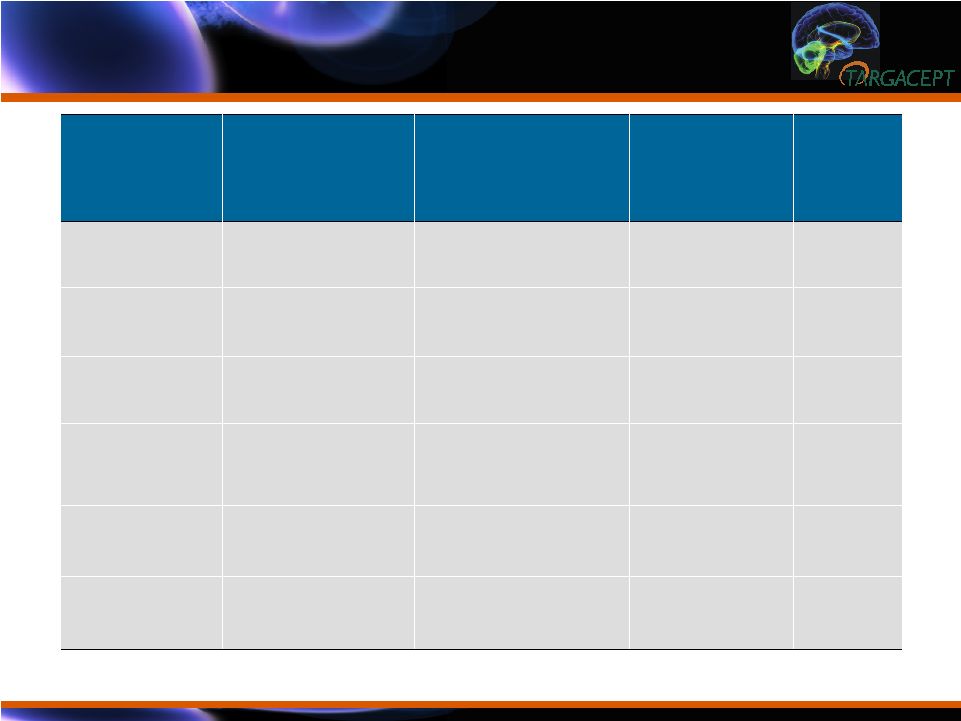 <0.0001 -1.93 (-2.57, -1.29) -4.58 (0.23) -2.65 (0.23) Anxiety/ Somatization (from HAMD-17) Parameter Placebo + citalopram Adj. Mean (SE) (N = 132) TC-5214 + citalopram Adj. Mean (SE) (N = 133) Difference (95% Confidence Interval) P-value HAMD-17 -7.75 (0.62) -13.75 (0.62) -6.0 (-7.72, -4.27) <0.0001 MADRS -9.72 (0.88) -17.26 (0.88) -7.54 (-9.98, -5.10) <0.0001 QIDS-SR -4.28 (0.42) -8.07 (0.41) -3.79 (-4.94, -2.63) <0.0001 CGI-SI -0.91 (0.10) -1.79 (0.10) -0.87 (-1.14, -0.61) <0.0001 CGI-GI 2.70 (0.09) 1.91 (0.09) -0.79 (-1.04, -0.54) <0.0001 HAMD-17 Effect Size = 0.78 TC-5214: Highly Statistically Significant Results Across All Efficacy Endpoints TC-5214: Highly Statistically Significant Results Across All Efficacy Endpoints |
 Parameter
Placebo + citalopram Adj. Mean (SE) (N = 132) TC-5214 + citalopram Adj. Mean (SE) (N = 133) Difference (95% Confidence Interval) P-value Sheehan Disability Scale (SDS) -4.58 (0.53) -9.18 (0.53) -4.60 (-6.08, -3.13) <0.0001 Sheehan Irritability Scale (SIS) -9.45 (1.12) -18.21 (1.11) -8.76 (-11.85, -5.67) <0.0001 SGI- Cognition 8.66 (0.27) 6.52 (0.27) -2.15 (-2.89, -1.41) <0.0001 SGI- Attention 2.85 (0.09) 2.13 (0.09) -0.72 (-0.96, -0.47) <0.0001 SGI-Memory 2.90 (0.09) 2.23 (0.09) -0.67 (-0.93, -0.42) <0.0001 SGI-Speed 2.92 (0.09) 2.16 (0.09) -0.76 (-1.02, -0.50) <0.0001 TC-5214: Highly Statistically Significant Results Across All Efficacy Endpoints TC-5214: Highly Statistically Significant Results Across All Efficacy Endpoints |
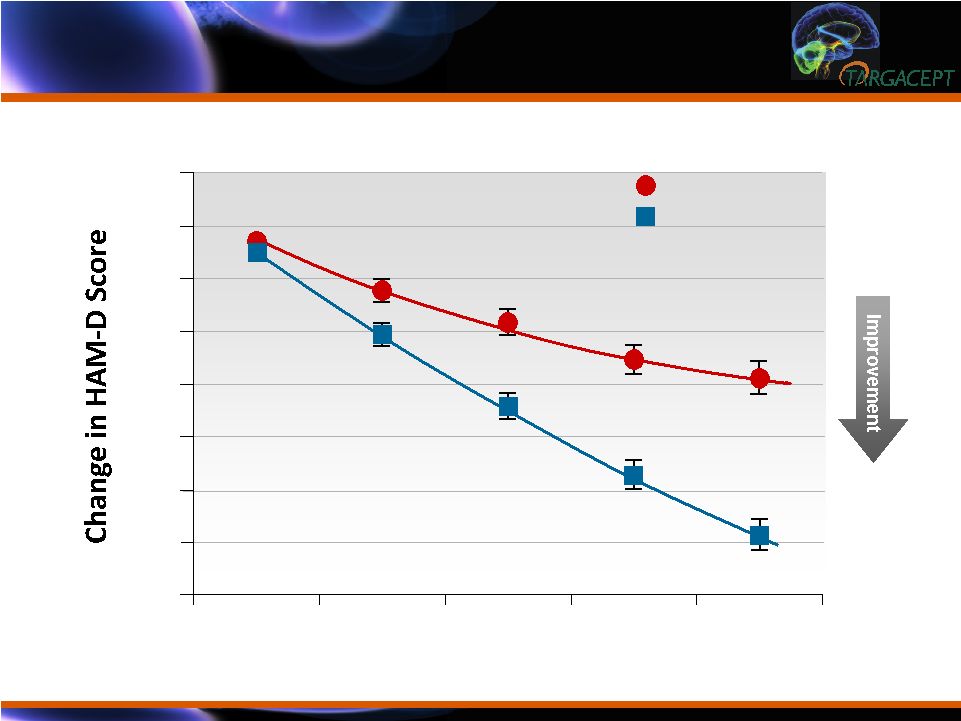 TC-5214:
Early Onset of Effect by Two Weeks and Increasing Improvement over
Duration of Trial TC-5214: Early Onset of Effect by Two Weeks
and Increasing Improvement over Duration of Trial ** ** ** *** Week 9
10 12 14 16 0 -2 -4 -6 -8 -10 -12 -14 -16 ** PBO + cit TC-5214 + cit p < 0.01 P < 0.0001 *** |
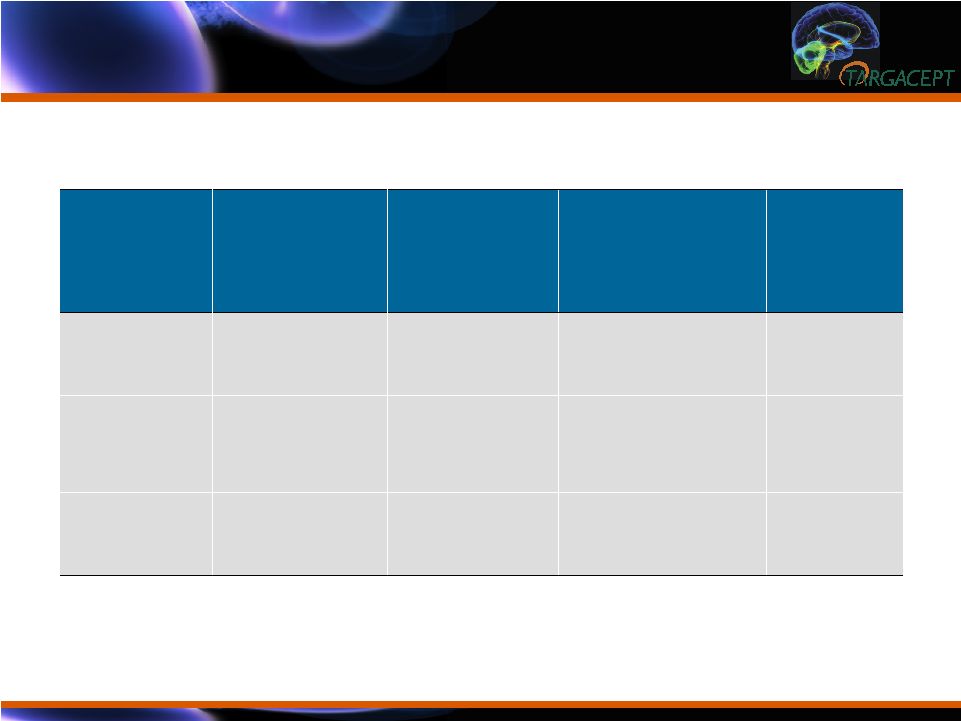 22 -7.75 (0.62) [132] -7.75 (0.62) [132] -7.75 (0.62) [132] Placebo + citalopram Adj. Mean (SE) [n] -14.19 (0.97) [54] -11.78 (1.16) [38] -15.00 (1.11) [41] TC-5214 + citalopram Adj. Mean (SE) [n] End Dose Difference (95% confidence interval) P-value 2mg - 7.25 (-9.75, - 4.74) <0.0001 4mg - 4.02 (-6.60, -1.45) 0.002 8mg - 6.44 (--8.70, - 4.18) <0.0001 HAMD-17 Results by End Dose (PP N=225) HAMD-17 Results by End Dose (PP N=225) |
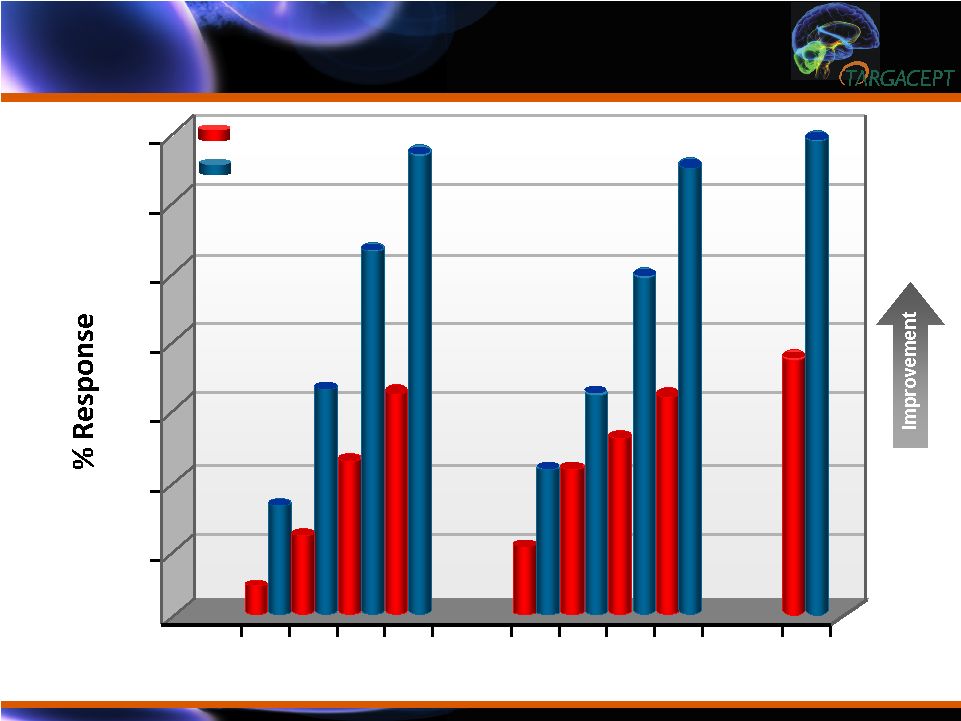 Response Rates
(ITT N=265): Improvement > 50% (HAM-
D; QIDS-SR; MADRS) Response Rates (ITT N=265): Improvement > 50% (HAM-D; QIDS-SR; MADRS) Week 10 12 14 16 10 12 14 16 16 70 60 50 40 30 20 10 ** ** ** HAM-D QIDS-SR MADRS ** * PBO + Cit TC-5214 + Cit ** <0.01 *** <0.0001 * <0.05 ** ** ** *** |
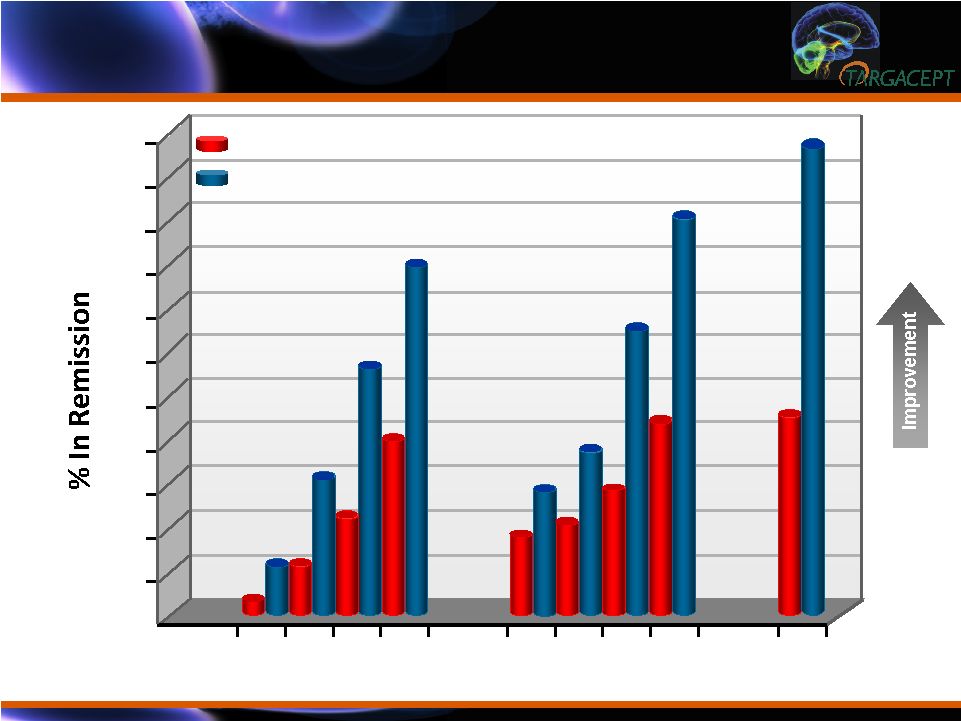 Remission Rates
(ITT N=265): HAM-D = 7 / QIDS-SR = 5 / MADRS = 10 Remission Rates (ITT
N=265): HAM-D = 7 / QIDS-SR = 5 / MADRS = 10 Week 10 12 14 16 10 12 14 16 16 55 50 45 40 35 30 25 20 15 10 5 ** ** ** HAM-D QIDS-SR MADRS *** ** <0.01 *** <0.0001 ** ** PBO + Cit TC-5214 + Cit |
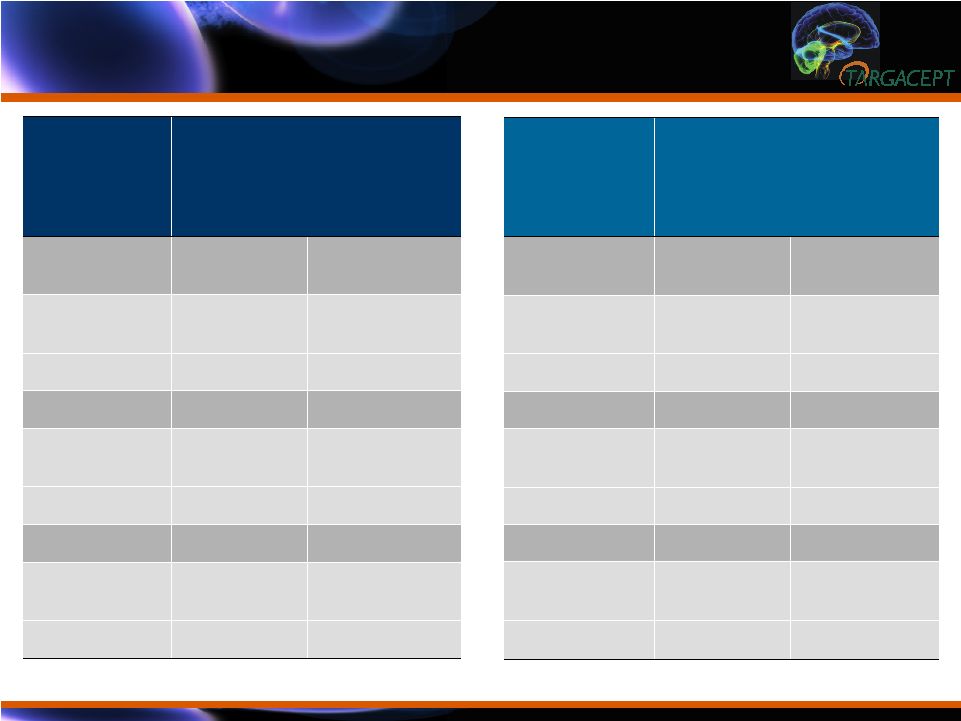 Efficacy Results
in Context: Aripiprazole Augmentation Data Efficacy Results in Context: Aripiprazole Augmentation Data TC-5214 Placebo + Citalopram (n=132) TC-5214 + Citalopram (n=133) MADRS Score -9.82 -17.26 Change from Placebo -7.5 P<0.0001 Remission 21% 52% Change from Placebo -31% P<0.0001 Response 36% 67% Change from Placebo -31% P<0.0001 * From Berman et al., CNS Spectr 14: 197-206 (2009) Aripiprazole * Placebo (n=172) Drug (n=177) MADRS Score -6.4 -10.1 Change from Placebo -3.7 P<0.001 Remission 18.9% 36.8% Change from Placebo -17.9% P<0.001 Response 26.6% 46.6% Change from Placebo -20.0% P<0.001 |
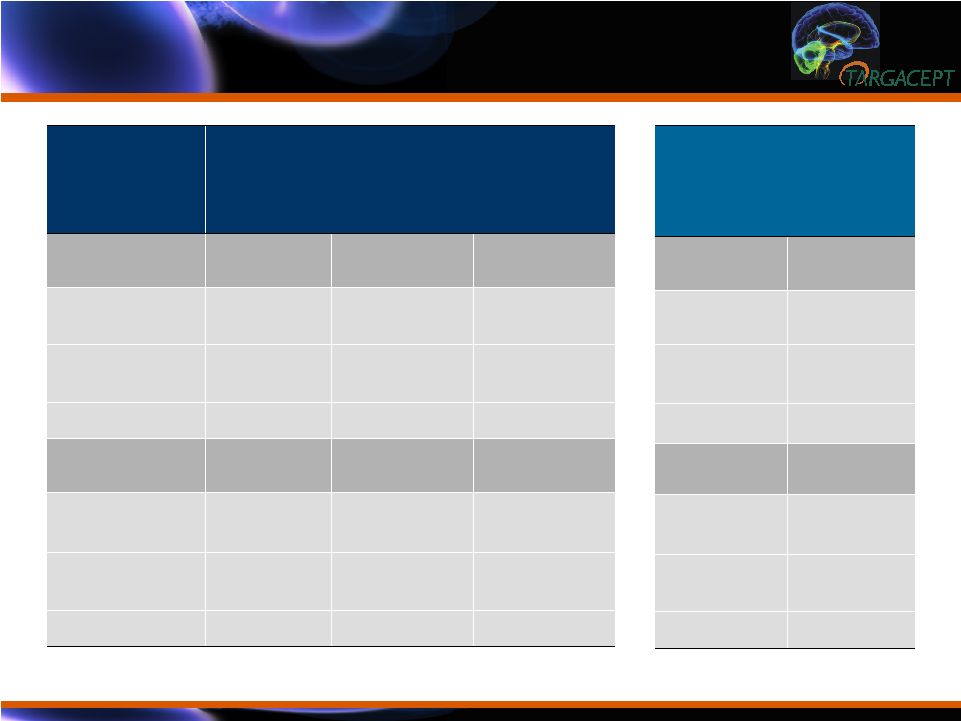 Efficacy Results
in Context: Quetiapine Augmentation Data Efficacy Results in Context: Quetiapine Augmentation Data Quetiapine Phase 3 * Placebo 150 mg 300 mg Study 1 (n=143) (n=143) (n=146) MADRS Score -11.7 -13.6 -14.7 Change from Placebo -1.9 -2.99 p=0.067 P=0.004 Study 2 (n=160) (n=166) (n=161) MADRS Score -12.2 -15.3 -14.9 Change from Placebo -3.05 -2.73 P=0.002 P=0.005 * FDA SBA: Study 1 (U.S.); Study 2 (Europe, S. Africa, N. America, Australia) TC-5214 Placebo + Citalopram TC-5214 + Citalopram (n=132) (n=133) -9.82 -17.26 -7.5 P<0.0001 |
 Summary of
Safety Results Summary of Safety Results Present study provides strong support for a favorable safety and tolerability profile of TC-5214 in depressed subjects Incidence and severity of adverse events reduced compared with earlier mecamylamine trial Constipation (8% vs. 27%) and dizziness (6% vs.
15%) were much reduced Minimal effect of cardiovascular variables including ECG No meaningful changes on biochemistry, hematology or urinary variables |
 Summary of
Efficacy Results Summary of Efficacy Results Present study provides compelling evidence for TC-5214 as a beneficial augmentation treatment for subjects who are inadequate responders to first line SSRI therapy Exceptional agreement between physician and subject assessments Strong effect on symptoms of irritability, anxiety and impaired cognition may have particular therapeutic benefits This study is the second Targacept trial to show antidepressant effects of a nicotinic channel blocker, further supporting this new mechanism of action as a promising approach for treating depression |
 Conclusions Conclusions These clinical results with TC-5214 suggest an efficacy profile as good or better than aripiprazole or quetiapine, with a superior tolerability profile Remission rate as measured by the QIDS of 43% compares favorably with the 35% seen in STAR*D trial These factors combined indicate TC-5214 has the potential to become the augmentation treatment of choice in depression |
 Positive
effects of the nicotinic channel blocker TC-5214 as augmentation treatment in patients with major depressive disorder who are inadequate responders to a first-line SSRI Geoffrey C. Dunbar, M.D. Targacept |
