Attached files
SECURITIES AND EXCHANGE COMMISSION
|
80-0447144 | ||||
___________________
|
Lior Carmeli
Chief Executive Office
Harbor City Research, Inc.
201 N. Charles Street, Suite 3900
Baltimore, MD 21201
Tel: (410) 539-0400 |
|
(Name, Address and Telephone Number of Agent for Service) |
Copy to:
Jerry Gruenbaum, Esq.
LAW OFFICES OF SEC ATTORNEYS, LLC
116 Court Street, Suite 707
New Haven, Connecticut 06510
Tel: (203) 222-9333
Fax: (203) 889-3344
Approximate date of proposed sale to the public: As soon as practical after the effective date of this registration statement.
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
1
If this Form is a post effective amendment filed under Rule 462(c) of the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If this Form is a post effective amendment filed under Rule 462(d) of the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act (Check One):
Large accelerated filer [ ] Accelerated
filer [ ]
Non-accelerated filer [ ] Smaller
reporting company [X]
(Do not check if a smaller reporting company)
(2) There is no current market for the securities and the price at which the shares held by the selling security holders will be sold is unknown. Although the registrant's common stock has a par value of $0.001, the registrant believes that the calculation of $1.00 per share is a bona fide estimate of the offering price in accordance
with Rule 457(a). In the event of a stock split, stock dividend or similar transaction involving our common stock, the number of shares registered shall automatically be increased to cover the additional shares of common stock issuable pursuant to Rule 416 under the Securities Act of 1933, as amended.
(3) Previously paid.
REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF
THE SECURITIES ACT OF 1933, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON DATES AS THE COMMISSION, ACTING UNDER SAID SECTION 8(a), MAY DETERMINE.
2
THE INFORMATION IN THIS PRELIMINARY PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. WE MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE
COMMISSION IS EFFECTIVE. THE SELLING STOCKHOLDERS MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT BECOMES EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
Subject to completion, dated October 16, 2009
NEW AIR, INC.
Up to 1,242,077
Shares of Common Stock held by stockholders
$0.001 par value
Offering Price: $1.00 per share
This prospectus relates to the sale of up to 1,242,077 shares of our common stock by certain existing holders of the securities named on page 31. As of October 16, 2009, 6,476,277 shares
of common stock are issued and outstanding. All costs associated with this registration will be borne by us.
This is our initial public offering. The shares of our common stock are not currently traded: our securities are not listed on any national securities exchange or the Nasdaq Stock market.
Upon the effectiveness of this prospectus: the Selling Shareholders may sell the shares as detailed in
the section entitled "Plan of Distribution."
The information in this prospectus is not complete and may be changed. No securities will be sold until the registration statement filed with the Securities
and Exchange Commission is effective. The prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Each of the selling stockholders may be deemed to be an "underwriter," as such term is defined
in the Securities Act of 1933.
There has been no market for our securities and a public market may not develop, or, if any
market does develop, it may not be sustained. As of October 16, 2009, we have 6,476,277 common shares issued and outstanding. Our common stock is not traded on any exchange or in the over-the-counter market. After
this Registration statement becomes effective, we expect to have a broker dealer file an application with the National Association of Securities Dealers, Inc. for our common stock to be eligible for trading on the OTC
Bulletin Board.
THIS INVESTMENT INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD PURCHASE SECURITIES ONLY IF YOU CAN AFFORD A COMPLETE LOSS.
You should rely only on the information provided in this prospectus or any supplement to this prospectus and information incorporated by reference. We have not authorized anyone else to provide you with different information. Neither the delivery of this prospectus nor any distribution of the shares of common stock
pursuant to this prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
3
Until January ___, 2010 [90 days from the date of effectiveness], all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect
to their unsold allotments.
Subject to Completion, the date of this Prospectus is October 16, 2009.
|
PART I - INFORMATION REQUIRED IN PROSPECTUS |
||||
| 5 | ||||
| 7 | ||||
| 9 | ||||
| 25 | ||||
| 26 | ||||
| 27 | ||||
| 27 | ||||
| 27 | ||||
| 27 | ||||
| 31 | ||||
| 34 | ||||
| 35 | ||||
| 36 | ||||
| 45 | ||||
| 50 | ||||
| 51 | ||||
| 53 | ||||
| 57 | ||||
| 61 | ||||
| 62 | ||||
| 65 | ||||
| 66 | ||||
|
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS |
||||
| 112 | ||||
| 112 | ||||
| 113 | ||||
| 113 | ||||
| 114 | ||||
We have not authorized anyone to provide you with information different from that contained in this prospectus. The Selling Stockholders are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus
is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of common stock.
4
New Air, Inc. (“We” or the “Company”) is a company incorporated in the State of Maryland in July 2009, that was created for and acquired a
company called Baby’s Breath Ltd. an Israeli company founded in 2001 by a group of leading physicians and highly experienced professionals that developed a patented medical devise for the treatment of respiratory diseases.
We, through our subsidiary Baby's Breath are marketing our products mainly in Europe while contemplating the development of new products that are based on the same technology, for wider markets and applications including:
1. Frame and disposable hoods re-engineered for easy replacement of single-use hoods for use in institutions,
2. Product for senior adults,
3. Ultrasonic (extra silent) compressor,
4. A product for use with oxygen in addition to medication.
On September 7, 2009 we acquired Baby’s Breath, Ltd. In a stock for stock trade at a 1:200 ratio whereby we issued 6,280,600 shares of our common stock for 31,403 shares in Baby’s Breath, Ltd. As a consequence, Baby’s Breath, Ltd became a wholly owned subsidiary of our Company and the shareholders of Baby’s
Breath Ltd. Became 96.98% of the total shareholders in our Company.
Risks Related to Our Business
Our business is subject to a number of risks, which you should be aware of before making an investment decision. These risks are disclosed more fully in this section of this prospectus titled “Risk Factors.”
The Business Opportunity
|
1. |
There is a vacuum on the inhalation market: no adequate solution is being currently offered to babies and infants. Although inhalation devices may be purchased in various designs none appropriately tailored to babies and infants’ specific needs. This large unexploited segment represents a high business potential for BabyAir. |
|
2. |
The device market is an expanding one: more than $1.3 billion was spent on ventilators, oxygen therapy systems, and airway management accessories in the United States during 2004. Growing at a healthy compound annual rate of 6.3%, sales of these products are expected to reach
more than $1.9 billion by the year 2010. |
According to a recent Kalorama research report, the US market for home ventilators and home oxygen systems in terms of manufacturers’ revenues totaled $153M in 2007. (All of these products treat a variety of respiratory illnesses including asthma, cystic fibrosis, bronchiolitis, COPD and pneumonia).
|
3. |
The market value is considerable: 10% of adults and 12% of children are diagnosed with asthma in the United States, and the estimated direct health care costs associated with asthma exceed $11.5 billion annually. Albuterol, one of the medicines that relieve asthma attacks, is
the seventh most commonly prescribed drug in the United States where there is about 52 million prescriptions filled for albuterol each year. |
5
The FDA has ruled that as of January 1st 2009 traditional CFC albuterol inhalers be prohibited and replaced by HFA inhalers, which are much more expensive. It is estimated that Americans will spend an additional $1.2 billion a year on three patented inhaler brands
containing the new propellant (Ventolin, ProAir and Proventil) until generic versions reach pharmacies.
We generated minimum revenues to date; we have minimal assets, and have accumulated losses in the amount of ($1,065,386). For the year ending December 31, 2008, we experienced a net loss of ($191,563). In our December
31, 2008 year-end financials, and our quarter end June 30, 2009, our auditor issued an opinion that our financial condition raises substantial doubt as to our ability to continue as a going concern.
Our principal executive office is located at Harbor City Research, Inc., 201 N. Charles Street, Suite 3900, Baltimore, MD 21201 and out telephone number is (401) 539-0400. The office of our subsidiary Baby's Breath is located at Ha'hadas Street, Bldg. #5 North Industrial Area, P.O. Box 42, Or-Akiva, Israel 30600, and our
website address is www.bbreath.com Information contained on our website shall not be deemed to be part of this prospectus.
6
|
1,242,077 shares of common stock being held by certain of our current shareholders. The shares being registered in this prospectus are held by certain shareholders. We issued shares to these shareholders via private placements in exchange for an investment in our Company or as payment for assets or services. | |
|
| |
|
This is our initial registration. There is not currently a market for our common stock. We intend to list our shares on the Over-the-Counter Bulletin Board, or OTCBB, but our stock has not been approved for trading on the OTCBB as of the date of this prospectus. We cannot determine if an active market will
develop for our common stock. Additionally, we cannot determine or predict the price at which our common stock will initially trade. The selling shareholders will sell at a price of $1.00 per share until our shares are quoted on the OTCBB and thereafter at prevailing market prices or privately negotiated prices. | |
7
Selected Financial Information
The selected financial information presented below is derived from and should be read in conjunction with our financial statements and the financial statements of Baby’s Breath, including notes thereto, appearing elsewhere in this prospectus. See "Financial Statements."
|
For six months |
For the year |
|||||||
|
ended |
ended |
|||||||
|
June 30, 2009 |
December 31, 2008 |
|||||||
|
(unaudited) |
(audited) |
|||||||
|
Revenue |
$ |
15,744 |
$ |
43,199 |
||||
|
Expenses: |
||||||||
|
Selling, general and administrative expenses |
78,052 |
188,633 |
||||||
|
Total expenses |
$ |
97,578 |
$ |
234,762 |
||||
|
Number of common shares outstanding |
23,530 |
23,530 |
||||||
|
Net Loss per share |
$ |
(3.72 |
) |
$ |
(8.71 |
) | ||
|
Balance sheet data: |
||||||||
|
June 30, 2009 |
December 31, 2008 |
|||||||
|
(unaudited) |
(audited) |
|||||||
|
Working Capital Deficiency |
$ |
(231,235 |
) |
$ |
(178,137 |
) | ||
|
Total Assets |
$ |
141,928 |
$ |
142,951 |
||||
|
Stockholders' Deficiency |
$ |
(134,064 |
) |
$ |
(71,366 |
) | ||
8
RISK FACTORS RELATING TO OUR FINANCIAL CONDITION
We may not be able to raise sufficient capital or generate adequate revenue to meet our obligations and fund our operating expenses.
We have financed our operations through the sale of stock and through grants received from the Office of the Chief Scientist in Israel (OCS). From 2001 to date we received $931,322 in equity financing and grants from the OCS in the amount of $268,029. As of the date of this prospectus we have approximately US$130,000,
which should enable us to continue operations at our current burn rate for a period of up to 3 months from the date hereof. We need to raise additional capital in order to meet our business plan. Failure to raise adequate capital and generate adequate sales revenues to meet our obligations and develop and sustain our operations could result in reducing or ceasing our operations.
Additionally, even if we do raise sufficient capital and generate revenues to support our operating expenses, there can be no assurances that the revenue will be sufficient to enable us to develop business to a level where it will generate profits and cash flows from operations. These matters raise substantial doubt about our ability to continue as a going concern. Our independent auditors currently included an explanatory paragraph in their report on our financial statements regarding concerns about our ability to continue as a going concern.
We have yet to attain profitable operations.
Because we will need additional financing to fund our activities, our accountants believe there is substantial doubt about our ability to continue as a going concern. Our ability to continue to operate as a going concern is fully dependent upon obtaining sufficient financing to continue our operational activities. The
ability to achieve profitable operations is in direct correlation to our ability to raise sufficient financing. Accordingly, our management believes that our continued existence, future expansion, and ultimate profitability is fully dependent upon raising sufficient proceeds from this offering. It is important to note that even if the appropriate financing is received, there is no guarantee that we will ever be able to operate profitably or derive any significant revenues from its operation.
There is no guarantee that we will ever operate profitably.
We anticipate that we will continue to incur losses and negative cash flow over the next twelve (12) months. There is no guarantee that we will ever operate profitably or even receive positive cash flows from full operations.
Because we expect to incur additional losses, we will require additional financing to sustain our operation. Our Independent Auditors have expressed doubt as to our ability to remain a going concern.
We incurred a net loss of approximately $191,563 and $199,666, for the fiscal years ended December 31, 2008 and 2007, respectively and a net loss of approximately $81,834 for the six months ended June 30, 2009. We have never earned a profit and we anticipate that we will continue to incur losses for at least the next 12 months. We
continue to operate on a negative cash flow basis. We have generated only minimal revenues and are still developing our planned principal operations.
9
We will not receive any proceeds from the sale of the shares offered in this offering. Accordingly, we will rely on pursuing alternative sources to obtain the entire amount of funding needed to fund our operations for the next 12 months. We may need additional funds to continue our operations, and such additional funds may not be available when required at attractive prices or at all. If
we are unable to obtain additional funds at reasonable rates or at all we will be required to substantially curtail our operations and could cease to exist in our current form. Our Independent Public Accounting firm has indicated in their audit opinion, contained in our Financial Statements, that they have substantial doubt about our ability to remain a going concern.
We have financed our operations through the sale of stock and through grants received from the OCS. From 2001 to date we received $931,322 in equity financing and grants from the OCS in the amount of $268,029.
We expect to continue to depend upon outside financing to sustain our operations for at least the next 12 months. Our ability to arrange financing from third parties will depend upon our performance and market conditions. Our inability to raise additional working capital at all or to raise it in a timely manner would
negatively impact our ability to fund our operations, to generate revenues, and to otherwise execute our business plan, leading to the reduction or suspension of our operations and ultimately forcing us to go out of business. Should this occur, the value of any investment in our securities could be adversely affected, and an investor could lose a portion of or even lose their entire investment.
Our shares of common stock are not and may never be quoted on any exchange or listing service.
Our shares of common stock are not and may never be quoted on any exchange or listing service. It may be difficult or impossible for you to sell your shares.
Persons who acquire shares of our common stock will have limited liquidity or opportunity to sell their shares and may not be able to recover any funds that have been invested in our common stock.
The possible sales of shares of common stock by our selling shareholders may have a significant adverse effect on the market price of our common stock should a market develop.
The selling shareholders may sell some or all of their shares immediately after they are registered. In the event that the shareholders sell some or all of their shares, the price of our common stock could decrease significantly. Potential investors may not be interested in purchasing shares of our common stock if
the selling shareholders are selling their shares of common stock. The selling of stock by the shareholders could be interpreted by potential investors as a lack of confidence in us and our ability to develop a stable market for our stock. The price of our common stock could fall if the selling shareholders sell substantial amounts of our common stock. These sales may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that
we deem appropriate because the selling shareholders may offer to sell their shares of common stock to potential investors for less than we do.
We are dependent for our success on a few key executive officers. Our inability to retain those officers would impede our business plan and growth strategies, which would have a negative impact on our business and the value of an investment.
Our success depends on the skills, experience and performance of key members of our management team. We are heavily dependent on the continued services of Yossef De Levie our Chairman; Lior Carmeli our Chief Executive Officer; David Kapon our Chief Financial Officer; and Jacob Bal our Chief Technology Officer. We have entered
into employment or consulting agreements with our CEO, CFO and CTO and we plan to expand the relatively small number of executives when needed. Were we to lose one or more of these key individuals, we would be forced to expend significant time and money in the pursuit of a replacement, which could result in both a delay in the implementation of our business plan and the diversion of limited working capital. We can give you no assurance that we can find satisfactory replacements
for these key individuals at all, or on terms that are not unduly expensive or burdensome to our company. Although we intend to issue stock options or other equity-based compensation to attract and retain employees, such incentives may not be sufficient to attract and retain key personnel.
10
We are dependent for our success on our ability to attract and retain technical personnel, sales and marketing personnel and other skilled management.
Our success depends to a significant degree upon our ability to attract, retain and motivate highly skilled and qualified personnel. Failure to attract and retain necessary technical, sales and marketing personnel and skilled management could adversely affect our business. If we fail to attract, train and retain sufficient
numbers of these highly qualified people, our prospects, business, financial condition and results of operations will be materially and adversely affected.
Lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team has had no public company experience in the United States, which could impair our ability to comply with legal and regulatory requirements such as those imposed by the Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management have had no responsibility for managing a publicly traded
company. Such responsibilities include complying with US federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement and effect programs and policies in an effective and timely manner that adequately respond to such increased legal, regulatory compliance and reporting requirements. Our failure to do so could lead to the imposition of fines and penalties and result in the deterioration of our business.
New rules, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract qualified officers and directors, which could adversely affect the management of our business and our ability to obtain or retain listing of our common stock.
We may be unable to attract and retain qualified officers, directors and members of board committees required to provide for our effective management as a result of the recent and currently proposed changes in the rules and regulations which govern publicly held companies, including, but not limited to, certifications from executive officers
and requirements for financial experts on the board of directors. The perceived increased personal risk associated with these recent changes may deter qualified individuals from accepting these roles. The enactment of the Sarbanes-Oxley Act of 2002 has resulted in the issuance of a series of new rules and regulations and the strengthening of existing rules and regulations by the Securities and Exchange Commission (the “SEC”). Further,
certain of these recent and proposed changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the Company and level of experience in finance and accounting matters. We may have difficulty attracting and retaining directors with the requisite qualifications. If we are unable to attract and retain qualified officers and directors, the management of our business could be adversely affected.
Our internal controls over financial reporting may not be effective, and our independent auditors may not be able to certify as to their effectiveness, which could have a significant and adverse effect on our business.
If we become a publicly traded company, as intended, we will be subject to various regulatory requirements, including the Sarbanes-Oxley Act of 2002. We, like other public companies, would then incur additional expenses and, to a lesser extent, diversion of our management’s time, in our efforts to comply with Section 404
of the Sarbanes-Oxley Act of 2002 regarding internal controls over financial reporting.
We have not yet evaluated our internal controls over financial reporting in order to allow management to report on, and our independent auditors to attest to, our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act of 2002 and the rules and regulations of the SEC, which we collectively refer
to as “Section 404”. We will be required to include our Section 404 management’s assessment of internal control over financial reporting beginning with our second annual report filed after we become publicly registered, and we will be required to include our independent auditor’s attestation on management’s report on internal control over financial reporting beginning with our annual report for
the fiscal year ending December 31, 2010.
11
We intend to comply with the Section 404, Management Assessment of Internal Control over Financial Reporting , beginning with our second annual report filed after we become publicly registered. However, our lack of familiarity with Section 404 may unduly divert management’s time and resources in executing our business
plan. If, in the future, management identifies one or more material weaknesses, or our external auditors are unable to attest that our management’s report is fairly stated or to express an opinion on the effectiveness of our internal controls, this could result in a loss of investor confidence in our financial reports, have an adverse effect on our stock price and/or subject us to sanctions or investigation by regulatory authorities.
RISKS RELATED TO OUR SECURITIES
There is currently no public trading market for our common stock and we cannot assure you that an active public trading market for our common stock will develop or be sustained. Even if a market develops, you may be unable to sell at or near ask prices or at all if you need to sell your
shares to raise money or otherwise desire to liquidate your shares.
There is currently no public trading market for our common stock and no such market may ever develop. While we intend to seek and obtain quotation of our common stock for trading on the OTC Bulletin Board (“OTCBB”) during the third quarter of 2009, there is no assurance
that our application will be approved. An application for quotation on the OTC Bulletin Board must be submitted by one or more market makers who agree to sponsor the security and who demonstrate compliance with SEC Rule 15(c)2-11 before initiating a quote in a security on the OTC Bulletin Board. In order for a security to be eligible for quotation by a market maker on the OTC Bulletin Board, the security must be registered with the SEC and the company must be current in its required filings with the
SEC. There are no listing requirements for the OTC Bulletin Board and accordingly no financial or minimum bid price requirements. We intend to cause a market maker to submit an application for quotation to the OTC Bulletin Board before December 31, 2009.
Even if our application for quotation is approved, the number of institutions or persons interested in purchasing our common stock at or near ask prices at any given time may be relatively small or nonexistent. This situation may be attributable to a number of factors, including the fact that we are a small company that is relatively
unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk averse and may be reluctant to follow a relatively unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, assuming that our common stock is accepted for quotation, there may be
periods of several days or more when trading activity in our shares is minimal or non existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot assure you that an active public trading market for our common stock will develop or be sustained.
Limitations on director and officer liability and indemnification of our officers and directors by us may discourage shareholders from bringing suit against a director.
Our articles of incorporation and bylaws provide, with certain exceptions as permitted by governing state law, that a director or officer shall not be personally liable to us or our shareholders for breach of fiduciary duty as a director, except for acts or omissions which involve intentional misconduct, fraud or knowing violation of law,
or unlawful payments of dividends. These provisions may discourage shareholders from bringing suit against a director for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by shareholders on our behalf against a director. In addition, our articles of incorporation and bylaws provide for mandatory indemnification of directors and officers to the fullest extent permitted by governing state law.
12
We do not expect to pay dividends for the foreseeable future, and we may never pay dividends.
We currently intend to retain any future earnings to the expansion of our business and do not anticipate paying cash dividends in the foreseeable future. Our payment of any future dividends will be at the discretion of our board of directors after taking into account various factors, including but not limited to, our financial
condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. In addition, our ability to pay dividends on our common stock may be limited by state law. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize their investment.
If our common stock is accepted for quotation on the OTC Bulletin Board it may be thinly traded, so you may be unable to sell at or near ask prices or at all if you need to sell your shares to raise money or otherwise desire to liquidate your shares.
If our common stock is accepted for quotation on the OTC Bulletin Board, it may be thinly traded on the OTC Bulletin Board, meaning there has been a low volume of buyers and sellers of the shares. Through this registration statement, we are going public without the typical initial public offering procedures which usually include
a large selling group of broker-dealers who may provide market support after going public. Thus, we will be required to undertake efforts to develop market recognition for us and support for our shares of common stock in the public market. The price and volume for our common stock that will develop cannot be assured. The number of institutions or persons interested in purchasing our common stock at or near ask prices at any given time may be relatively small or non-existent. This
situation may be attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned
and viable. As a consequence, there may be periods of several days, weeks or months when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price.
We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained. In addition to trading on the OTC Bulletin Board, our ultimate intention is to apply for trading on either the Nasdaq Capital Market or the NYSE Alternext U.S. LLC (formerly American Stock Exchange) at
such time that we meet the requirements for listing on those exchanges. We currently do not meet the objective listing criteria for listing on those exchanges and there can be no assurance as to when we will qualify for either of these exchanges or that we will ever qualify for these exchanges.
In order for us to be eligible to trade on the Nasdaq Capital Market, or NYSE Alternext U.S. LLC, we would need, among other things, to qualify with certain requirements in connection with bid and ask prices for our common stock, as well as a minimum value of stockholders equity and market value of our publicly held shares.
Currently, our market capitalization, revenues and stockholders’ equity are insufficient to qualify for these exchanges. We also do not have a sufficient number of shareholders. We would also need to meet the corporate governance and independent director and audit committee standards of Nasdaq and/or the NYSE
Alternext U.S. LLC. We do not satisfy such standards at this time.
If our common stock is accepted for quotation and begins trading on the OTC Bulletin Board, the trading volume we develop may be limited by the fact that many major institutional investment funds, including mutual funds, as well as individual investors follow a policy of not investing in OTC Bulletin Board stocks and certain major brokerage
firms restrict their brokers from recommending OTC Bulletin Board stocks because they are considered speculative, volatile and thinly traded.
13
The application of the “penny stock” rules to our common stock could limit the trading and liquidity of the common stock, adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
If our common stock is accepted for quotation on the OTC Bulletin Board, as long as the trading price of our common stock is below $5 per share, the open-market trading of our common stock will be subject to the “penny stock” rules, unless we otherwise qualify for an exemption from the “penny stock” definition. The
“penny stock” rules impose additional sales practice requirements on certain broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with net assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). These regulations, if they apply, require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the associated
risks. Under these regulations, certain brokers who recommend such securities to persons other than established customers or certain accredited investors must make a special written suitability determination regarding such a purchaser and receive such purchaser’s written agreement to a transaction prior to sale. These regulations may have the effect of limiting the trading activity of our common stock, reducing the liquidity of an investment in our common stock and increasing the transaction
costs for sales and purchases of our common stock as compared to other securities.
The OTC Bulletin Board is a quotation system, not an issuer listing service, market or exchange. Therefore, buying and selling stock on the OTC Bulletin Board is not as efficient as buying and selling stock through an exchange.
The OTC Bulletin Board is a regulated quotation service that displays real-time quotes, last sale prices and volume limitations in over-the-counter securities. Because trades and quotations on the OTC Bulletin Board involve a manual process, the market information for such securities cannot be guaranteed. In addition,
quote information, or even firm quotes, may not be available. The manual execution process may delay order processing and intervening price fluctuations may result in the failure of a limit order to execute or the execution of a market order at a significantly different price. Execution of trades, execution reporting and the delivery of legal trade confirmation may be delayed significantly. Consequently, one may not be able to sell shares of our common stock at the optimum trading prices.
When fewer shares of a security are being traded on the OTC Bulletin Board, volatility of prices may increase and price movement may outpace the ability to deliver accurate quote information. Lower trading volumes in a security may result in a lower likelihood of an individual’s orders being executed, and current prices may differ
significantly from the price one was quoted by the OTC Bulletin Board at the time of the order entry.
Orders for OTC Bulletin Board securities may be canceled or edited like orders for other securities. All requests to change or cancel an order must be submitted to, received and processed by the OTC Bulletin Board. Due to the manual order processing involved in handling OTC Bulletin Board trades, order processing and
reporting may be delayed, and an individual may not be able to cancel or edit his order. Consequently, one may not able to sell shares of common stock at the optimum trading prices.
The dealer’s spread (the difference between the bid and ask prices) may be large and may result in substantial losses to the seller of securities on the OTC Bulletin Board if the common stock or other security must be sold immediately. Further, purchasers of securities may incur an immediate “paper” loss
due to the price spread. Moreover, dealers trading on the OTC Bulletin Board may not have a bid price for securities bought and sold through the OTC Bulletin Board. Due to the foregoing, demand for securities that are traded through the OTC Bulletin Board may be decreased or eliminated.
14
Shares eligible for future sale may adversely affect the market.
From time to time, certain of our shareholders may be eligible to sell all or some of their shares of common stock pursuant to Rule 144, promulgated under the Securities Act of 1933, as amended, subject to certain limitations. In general, pursuant to Rule 144 as in effect as of the date of this prospectus, a shareholder (or shareholders
whose shares are aggregated) who has satisfied the applicable holding period and is not deemed to have been one of our affiliates at the time of sale, or at any time during the three months preceding a sale, may sell their shares of common stock. Any substantial sale, or cumulative sales, of our common stock pursuant to Rule 144 or pursuant to any resale prospectus may have a material adverse effect on the market price of our securities.
We expect volatility in the price of our common stock, which may subject us to securities litigation.
If established, the market for our common stock may be characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against
a company following periods of volatility in the market price of its securities. We may in the future be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
If our stock does trade in a market or exchange, our stock price may be volatile, and you may not be able to resell shares of our common stock at or above the price you paid.
Any investment in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, and all other information contained in this prospectus, before you decide whether to purchase our common stock. The occurrence of any of the following risk factors could harm
our business. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also become important factors that may harm our business. You may lose part or all of your investment due to any of these risks or uncertainties.
A total of 5,234,200 or 80.82% of our outstanding shares following this registration are restricted from immediate resale, but may be sold into the market in the near future. This could cause the market price of our shares to drop significantly, even if our
business is profitable.
After this registration, we will have 6,476,277 shares outstanding. We expect that the remaining 5,234,200 ordinary shares, representing 80.82% of our total outstanding shares following this offering, will become available for resale in the public market as shown in the chart below. As restrictions on resale
end, the market price of our shares could drop significantly if the holders of these restricted shares sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our ordinary shares or other securities.
|
Number of shares |
percentage of total outstanding |
Date of availability for resale into the public market | |||||
| 1,242,077 | 19.18 | % |
Upon the effectiveness of this registration. | ||||
| 5,234,200 | 80.82 | % |
Six months after registration and subject to volume limitation under Rule 144 | ||||
15
Our limited operating history makes evaluation of our business difficult.
We were formed in July 2009, to acquire an Israeli company called Baby’s Breath Ltd. founded in 2001 and to date have generated only minimal revenues. Our ability to implement a successful business plan remains unproven and no assurance can be given that we will ever generate sufficient revenues to sustain our business. We
have a limited operating history which makes it difficult to evaluate our performance. You must consider our prospects in light of these risks, expenses, technical obstacles, difficulties, market penetration rate and delays frequently encountered in connection with the development of new businesses. These factors include uncertainty whether we will be able to:
|
• |
Raise capital; |
|
• |
Develop and implement our business plan in a timely and effective manner; |
|
• |
Be successful in uncertain markets; |
|
• |
Respond effectively to competitive pressures; |
|
• |
Successfully address intellectual property issues of others; |
|
• |
Protect and expand our intellectual property rights; and |
|
• |
Continue to develop and upgrade our products. |
Our business is dependent upon proprietary intellectual property rights, which if we were unable to protect, could have a material adverse effect on our business.
We currently own and may in the future own or license additional patent rights or trade secrets in the U.S., Europe, Asia, Canada and elsewhere in the world that cover certain of our products. We rely on patent laws, and other intellectual property laws, nondisclosure and other contractual provisions and technical measures to
protect our products and intangible assets. These intellectual property rights are important to our ongoing operations and no assurance can be given that any measure we implement will be sufficient to protect our intellectual property rights. We may lose the protection afforded by these rights through patent expirations, legal challenges or governmental action. If we cannot protect our rights, we may lose our competitive advantage or our competitive advantage could be lost if
these patents were found to be invalid in the jurisdictions in which we sell or plan to sell our products. The loss of our intellectual property rights could have a material adverse effect on our business.
If we become subject to intellectual property actions, this could hinder our ability to deliver our products and services and our business could be negatively impacted.
We may be subject to legal or regulatory actions alleging intellectual property infringement or similar claims against us. Companies may apply for or be awarded patents or have other intellectual property rights covering aspects of our technologies or businesses. Moreover, if it is determined that our products infringe
on the intellectual property rights of third parties, we may be prevented from marketing our products. While we are currently not subject to any material intellectual property litigation, any future litigation alleging intellectual property infringement could be costly, particularly in light of our limited resources. Similarly, if we determine that third parties are infringing on our patents or other intellectual property rights, our limited resources may prevent us from litigating or otherwise
taking actions to enforce our rights. Any such litigation or inability to enforce our rights could require us to change our business practices, could potentially hinder or prevent our ability to deliver our products and services, and could result in a negative impact to our business. Expansion of our business via product line enhancements or
16
new product lines to drive increased growth in current or new markets may be inhibited by the intellectual property rights of our competitors and/or suppliers. Our inability to successfully mitigate those factors may significantly reduce our market opportunity and subsequent growth.
We face intense competition and our inability to successfully compete with our competitors who produce inhalers will have a material adverse effect on our results of operation.
Respironics brand holds a very large market share in the sleep apnea segment and is therefore largely identified with it. Respironics’ offering includes a variety of home compressor nebulizer, mobile and disposable versions. DeVilbiss Healthcare presents itself as a leader in the respiratory niche of the home healthcare products market: DeVilbiss
Healthcare design, manufacture and market respiratory medical products that address the respiratory needs of patients in both institutional and homecare settings. The company’s offering ranges from oxygen to sleep therapy, and pulmonary drug delivery devices. Omron Healthcare and Pari Respiratory Equipment also offer variety of portable compressor nebulizers. Cardinal Health maintains a broad portfolio of products and services and offers a wide range of home healthcare nebulizers for children and grown-ups,
all comprising a mouthpiece for inhalation. Westmed Inc. manufactures a complete nebulizer system which is designed to enhance the delivery of aerosolized medication while reducing the apprehension encountered by young patients.
None of the above-mentioned companies provides a truly differentiated offering, in which medication delivery does not involve direct physical contact of the delivery system with the infant/patient.
In terms of the entry barriers: there is an increasing presence of leading manufacturers on all market segments tackling new respiratory therapies and devices. Some of them have really solidified their leadership and gained substantial reputation and as such they may not be
keen to welcome a newcomer offering real and objective added value.
These companies would certainly employ the resources to compete with us if they view them as concrete threat, such as preventing BabyAir registration and compliance with CPT codes (Current Procedural Terminology system, published and maintained by AMA – the American Medical Association www.ama-assn.org).
This is a necessary and tangible milestone for market entry and companies may have the means to influence and bias the CPT Advisory Committee decision to their interest.
The medical industry has historically used a variety of technologies for inhalation drug delivery solutions. Compared to these conventional technologies, our technology is relatively new, and the number of companies using our technology is limited. The commercial success of our product will depend upon the widespread adoption of our technology
as a preferred method by hospitals and patients. In order to be successful, our product must meet the technical and cost requirements for these facilities. Market acceptance will depend on many factors, including:
17
|
• |
the willingness and ability of customers to adopt new technologies; |
|
• |
our ability to convince prospective strategic partners and customers that our technology is an attractive alternative to conventional methods used by the medical industry; |
|
• |
our ability to select and execute agreements with effective distributors and manufacturers representatives to market and sell our product; and |
|
• |
our ability to assure customer use of BabyAir proprietary method. |
Because of these and other factors, our product may not gain market acceptance or become the industry standard for the health care industry. The failure of such companies to purchase our products would have a material adverse effect on our business, results of operations and financial condition.
We rely on local distributors to market and distribute our products in most of the territories.
We rely on distributors for the marketing and distribution of our products. Generally, under our agreements with local distributors, each distributor is granted the right to market our products in a particular country or region, subject to the attainment of minimum sales targets. Our success in generating sales in countries or
regions where we have engaged local distributors depends in part on the efforts of others whom we do not control. If a distributor is terminated by us or goes out of business, it may take us a period of time to locate an alternative distributor and to train its personnel to market our products and our ability to sell our products in that distributor’s country or region could be adversely affected.
If we or our distributors do not obtain and maintain the necessary regulatory approvals in a specific country or region, we will not be able to market and sell our products in that country or region.
We sell our products mainly in the following countries, including Finland, Italy, Spain, Hungary, Germany, Portugal, Poland, Israel, Serbia and in some countries in the Far East. To be able to market and sell our products in a specific country or region, we or our distributors must comply with the regulations of that country
or region. While the regulations of some countries do not impose barriers to marketing and selling our products or only require notification, others require that we or our distributors obtain the approval of a specified regulatory body. These regulations, including the requirements for approvals, and the time required for regulatory review vary from country to country. Obtaining regulatory approvals is expensive and time-consuming, and we cannot be certain that we or our distributors
will receive regulatory approvals in each country or region in which we plan to market our products. If we or our distributors are unable to maintain our authorizations in a particular country or region, we will no longer be able to sell our products in that country or region, and our ability to generate revenues will be materially adversely affected.
Our products require FDA clearance and our business will be subject to intense governmental regulation and scrutiny, both in the U.S. and abroad.
In 2006 Baby’s Breath filed a 510(k) submission with the U.S. Food and Drug Administration (the “FDA”) with respect to a product classification as a Class II non-exempt device. We cannot generate revenues from our product to be used in hospitals without FDA clearance. We received written confirmation
of final FDA clearance in 2006.
The potential production and marketing of some of our products and our ongoing research and development, any pre-clinical testing and clinical trial activities are subject to extensive regulation and review by FDA and other governmental authorities both in the United States and abroad. In addition to testing and approval procedures,
extensive regulations also govern marketing, manufacturing, distribution, labeling, and record keeping. If we do not comply with applicable regulatory requirements, violations could result in warning letters, non-approvals, suspensions of regulatory approvals, civil penalties and criminal fines, product seizures and recalls, operating restrictions, injunctions, and criminal prosecution.
18
Periodically, legislative or regulatory proposals are introduced that could alter the review and approval process relating to medical products. It is possible that the FDA will issue additional regulations further restricting the sale of our present or proposed products. Any change in legislation or regulations that
govern the review and approval process relating to our current and future products could make it more difficult and costly to obtain approval for new products, or to produce, market, and distribute existing products.
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
A federal law commonly known as the Medicare/Medicaid anti-kickback law, and several similar state laws, prohibit payments that are intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of health care products or services. While the federal law applies only
to referrals, products or services for which payment may be made by a federal health care program, state laws often apply regardless of whether federal funds may be involved. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices, such as us, by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians, and other potential purchasers of medical devices. Other federal and state laws
generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or are for items or services that were not provided as claimed. As we may provide some coding and billing advice to purchasers of our products, and since we cannot be sure that the government will regard any billing errors that may be made as inadvertent, these laws are potentially applicable
to us. Anti-kickback and false claims laws prescribe civil and criminal penalties for noncompliance that can be substantial. Even an unsuccessful challenge could cause adverse publicity and be costly to respond to, and thus could have a material adverse effect on our business, results of operations and financial condition.
If we are unable to expand reimbursement coverage from third-party healthcare payors for procedures using the our products, or if reimbursement is insufficient to cover the costs of purchasing or using our products when compared to alternative procedures, demand for our products may not continue to grow.
Demand for our products depends on the eligibility of our products for reimbursement through government-sponsored healthcare payment systems and private third-party payors. In general, the process of obtaining reimbursement coverage approvals has been slower outside of the United States. Reimbursement practices
vary significantly from country to country and within some countries, by region, and we must obtain reimbursement approvals on a country-by-country or region-by-region basis. We may not be able to obtain further approvals in a timely manner or at all. Our business model and marketing strategy will be directly influenced by changes in the reimbursement policy.
Changes in healthcare system policies may make it difficult for physicians, hospitals and other healthcare providers to obtain full reimbursement for the purchase of our products, which could adversely affect demand therefor.
Many healthcare payors have adopted a managed care system in which they contract to provide comprehensive healthcare for a fixed cost per person, irrespective of the amount of care actually provided. Therefore, the amount of reimbursement provided may not be sufficient to encourage physicians to purchase or utilize our products
and solutions. We are unable to predict what changes will be made in the reimbursement policies of third-party payors. We could be adversely affected by changes in reimbursement policies of governmental or private healthcare payors to the extent any such changes affect reimbursement amounts or methods for procedures in which our products are used.
19
Failure to obtain CPT codes for our products may limit our ability to sell our products to clinics and hospitals in the US.
CPTs (Current Procedural Terminology) codes are numbers assigned to every task and service a medical practitioner may provide to a patient including medical, surgical and diagnostic services. They are then used by insurers to determine the amount of reimbursement that a practitioner will receive by an insurer. Since everyone uses the same
codes to mean the same thing, they ensure uniformity. CPT codes are developed, maintained and copyrighted by the AMA (American Medical Association). As the practice of health care changes, new codes are developed for new services, current codes may be revised, and old, unused codes are discarded. Thousands of codes are in use, and they are updated annually.
Our ability to successfully market and distribute our products to hospitals and clinics in the US depends on our ability to obtain CPT codes for our products. Though we intend to pursue procurement of such cods there is no guaranty that we will be successful in our efforts and failure to obtain such codes may have an adverse affect on our
marketing and distribution operations in the US
Our lack of manufacturer could harm our ability to meet demand for our products in a timely manner or within budget.
To date we relied on a single supplier for the production and manufacturing of our BabyAir product. This manufacturer will no longer produce our product in the future. We are currently in negotiation with another manufacturer located in Israel to produce our products for the future. Our lack of
current manufacturer could harm our ability to meet demand for our products in a timely manner or within budget. Out inability to regain production abilities in a timely manner may adversely affect our sales.
None of the marks currently used by us are registered and protected.
None of the following trade names used by us is a registered trademark: BabyAir, WiseAir, and there is no guaranty that we will be able to register such trade names or maintain protection or any rights therein. To the extent other parties will register these trade names as protected marks we may be forced to market our products under different
names which may adversely affect our sale efforts and our financial results. We are not aware of any usage by any third parties of any of the abovementioned marks.
If we are unable to successfully protect our products through the issuance and enforcement of patents and other means of protection, our business could be harmed significantly.
Our ability to prevent competitors from gaining access to our products and solutions is essential to our success. If we fail to protect our intellectual property rights adequately, we may lose an important advantage in the markets in which we compete. Patent, copyright, trade secret laws and ability to trademark our
product in the United States and other jurisdictions, as well as our internal confidentiality procedures and contractual provisions, are at the core of our efforts to protect our proprietary technology and our brand.
Baby's Breath has been granted two patents for BabyAir, which covers the "up side down Nebulizer", the tube that transports the medicine, as well as the hood. The status of patent filing worldwide is presented in the following table.
|
Patent Status September 2009 | |||||||
|
Title |
Country |
Application/Patent no. |
Status | ||||
|
Aerosol Inhalation Interface |
Europe |
01949840.1 |
Renewal June 29, 2010 | ||||
|
Aerosol Inhalation Interface |
Israel |
137185 |
Allowed | ||||
|
Aerosol Inhalation Interface |
United States |
6877509 |
Granted – Renewal October 12, 2012 | ||||
|
Downdraft Nebulizer |
Italy |
ITMI031342A0 |
Renewal December 31, 2009 | ||||
|
Downdraft Nebulizer |
United States |
6883517 |
Granted | ||||
|
Downdraft Nebulizer |
Japan |
2002-506675 |
Request for examination | ||||
|
Downdraft Nebulizer |
Europe |
04078146.1 |
Renewal September 30, 2009 | ||||
20
While we plan to protect our intellectual property with, among other things, patent protection, there can be no assurance that:
|
· |
current or future U.S. or foreign patents applications will be approved; |
|
· |
our issued patents will protect our intellectual property and not be held invalid or unenforceable if challenged by third parties via litigation or administrative proceeding; |
|
· |
we will obtain a favorable outcome if we assert our intellectual property rights against third parties; |
|
· |
we will succeed in protecting our technology adequately in all key jurisdictions in which we or our competitors operate; |
|
· |
the patents of others will not have an adverse effect on our ability to do business; or |
|
· |
others will not independently develop similar or competing products or methods or design around any patents that may be issued to us. |
Because the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our products.
There is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. While we have attempted to ensure that our product does not infringe
other parties’ patents and proprietary rights, our competitors or other parties may assert that our product and the methods it employs may be covered by patents held by them. In addition, because patent applications can take many years to issue, there may be applications now pending of which we are unaware, which may later result in issued patents which our product may infringe. If our products infringe a valid patent, we could be prevented from selling them unless we can obtain a
license or redesign the product to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid any infringement. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and can divert management’s attention from our core business.
Litigation and administrative proceedings are inherently uncertain and divert resources that could be directed towards other business priorities. We may not be able to obtain positive results and may spend considerable resources in our efforts to defend and protect our intellectual property. Furthermore, legal standards
relating to the validity, enforceability, and scope of protection of intellectual property rights are uncertain. Effective patent, trademark, copyright and trade secret protection vary from one jurisdiction to another and may not be attainable in every country in which our products are available. The laws of some foreign countries may not be as protective of intellectual property rights as those in the United States, and mechanisms for enforcement of those rights may be inadequate. Accordingly,
despite our efforts, we may be unable to prevent third parties from infringing upon or misappropriating our intellectual property and utilizing our technology for their competitive advantage. Our failure to obtain trademarks or patents, including with claims of a scope necessary to cover our technology, or the invalidation of our patents, may weaken our competitive position and may adversely affect our revenues and profitability.
Our international operations expose us to the risk of fluctuation in currency exchange rates.
We derive all of our revenues in various countries utilizing various currencies throughout the world. As we expect to begin sales in the United States, we expect that a substantial portion of our revenues in the future will be in U.S. dollars while a significant portion of our expenses will be denominated in Israeli shekels. Our
shekel-denominated expenses consist principally of salaries, building leases and related personnel expenses. We anticipate that a material portion of our expenses will continue to be denominated in shekels. If the U.S. dollar or other world currencies weakens against the shekel, there will be a negative impact on our profit margins. We currently do not hedge our currency exposure through financial instruments. In addition,
if we wish to maintain the dollar-denominated value of our products in non-U.S. markets, devaluation in the local currencies of our customers relative to the U.S. dollar could cause our customers to cancel or decrease orders or default on payment.
21
Fluctuations in our revenues and operating results on a quarterly and annual basis could cause the market price of our common stock to decline.
Our quarterly and annual revenues and operating results are difficult to predict and may fluctuate in the future, from quarter-to-quarter and year-to-year. It is possible that our operating results in some quarters and years will be below market expectations. This may cause the market price of our shares to decline.
Our quarterly and annual operating results are affected by a number of factors, many of which are outside of our control. In particular, we have limited exposure to end customer demand upon which we predict future sales of our solutions.
Additional factors that may affect our quarterly and annual operating results include:
|
· |
the loss of one or more of our customers, or a significant reduction or postponement of orders from our customers; |
|
· |
our customers’ sales outlooks, purchasing patterns and inventory levels based on end-customer demands and general economic conditions; |
|
· |
our ability to successfully develop, introduce and sell new or enhanced products in a timely manner; |
|
· |
product obsolescence and our ability to manage product transitions; |
|
· |
changes in the relative sales mix of our products; |
|
· |
changes in our cost of finished products; |
|
· |
the potential loss of key manufacturer and supplier relationships; and |
Pursuant to the Israeli Tax Authority a tax pre-ruling of August 25 2009 some of our shareholders are subject to limitations with respect to their ability to dispose of their holdings in us. In addition pursuant to the terms of said pre-ruling, we are subject to certain limitations with respect to
our ability to issue additional shares and diluting the holdings of our shareholders in us.
On September 7, 2009, we purchased all the issued and outstanding shares of Baby’s Breath Ltd. from Baby’s Breath shareholders in consideration for 6,280,600 shares of our Common Stock as a result of which Baby’s Breath became our wholly-owned subsidiary. Our acquisition of Baby’s Breath's shares in consideration
for the issuance of shares of common stock of New Air was made pursuant to a tax arrangement and a pre-ruling reached with the Israeli Tax Authorities in accordance with the provisions of Section 104B of the Israeli Tax Ordinance. A translated copy of the Pre-Ruling is attached as an exhibit hereto. The terms of the Pre-Ruling impose certain limitations on the ability of our shareholders that are Israeli residents to dispose of their holdings in New Air, and on the level of dilution to which
such shareholders may be subject in the future in case of additional fund raising by us. The Pre-Ruling also prohibits us from selling our holdings in Baby's Breath for the duration prescribed under law. In order to maintain compliance with the terms of the Pre Ruling the shares of common stock that were issued to those shareholders being parties to the Pre Ruling are held by a trustee appointed by New Air (the “Trustee”, and
are to be held by the Trustee for a period of 24 months following the issuance thereof (the “Restricted Period”). During the Restricted Period those shareholders that are Israeli residents, parties to the Pre Ruling may sell a collective aggregate amount of shares that equals up to 10% of their holdings (i.e. an aggregate amount of 628,060 shares). The shareholders may apportioned their respective right to sell such shares among themselves
such that the total amount of the shares sold by the Israeli resident's shareholders shall not exceed the amount of shares stated above . The Israeli resident's shareholders have agreed that the Israeli resident's shareholders listed on the Selling Shareholders table herein may sell the number of shares included in this registration statement.
22
In recent years new solutions to respiratory ailments, in the form of seasonal vaccines, were put into use.
In recent years new solutions to respiratory ailments, in the form of seasonal vaccines, were put into use. This trend has the potential to decrease the potential market for inhalation solutions. We have no control over trends in the industry and such trends as well as additional trends which may evolve may adversely affect our operational
results.
There is growing requirement for a double sterilization process for medical devices.
Recently, due to recent epidemics (such as the recent swine flue), there is growing requirement for a double sterilization process for medical devices, which is a longer and more expensive process. This can make the use of the BabyAir device more expensive and harder for use in the hospital market.
RISKS RELATING TO OUR LOCATION IN ISRAEL
Conditions in Israel could adversely affect our business.
Our principal offices and research and development facilities are located in Israel. Accordingly, political, economic and military conditions in Israel directly affect our business. Since the State of Israel was
established in 1948, a number of armed conflicts have occurred between Israel and its Arab neighbors. Although Israel has entered into various agreements with Egypt, Jordan and the Palestinian Authority, there has been an increase in unrest and terrorist activity, which began in September 2000 and has continued with varying levels of severity
into 2007. Furthermore, several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel continue or increase. These restrictions may limit materially our ability to sell our solutions to companies in these countries. Additionally, any hostilities involving Israel may have a material adverse effect on our facilities, all or
a portion of our inventory may be damaged, and our ability to deliver products to customers may be materially adversely affected. Our employees in Israel, including executive officers, may be called upon to perform annual military reserve duty and, in emergency circumstances, could be called to active duty. Such disruption could materially adversely affect our business and results of operations.
The government grants we received through Baby's Breath for research and development expenditures restrict our ability to manufacture products and transfer technologies outside of Israel and require us to satisfy specified conditions.
We have received grants from the government of Israel through the Office of the Chief Scientist of the Ministry of Industry, Trade and Labor, for the financing of a portion of our research and development expenditures in Israel. Under Israeli law, royalties on the revenues derived from sales of all of our products are payable
to the Israeli government, generally at the rate of 3.5%, up to the amount of the received grants as adjusted for fluctuation in the U.S. dollar/shekel exchange rate. The amounts received after January 1, 1999, bear interest equal to the 12-month London Interbank Offered Rate applicable to dollar deposits that is published on the first business day of each calendar year. Royalties are paid on our consolidated revenues.
The Government of Israel, through the Office of the Chief Scientist (OCS), encourages Research and Development projects that result in products for export. Through Baby's Breath we received grants of approximately $268,029 from the OCS through December 31, 2005, which were used to fund its initial product development. Pursuant
to the terms of these grants, the Company is obligated to pay royalties to the OCS at the rates of 3.0% and 3.5% of revenues derived from sales of products funded with these grants, up to 100.0% up to 150% of certain grant amounts received in 2005-2007 and 2008 accordingly.
In the event that a project funded by the OCS does not result in the development of a product that generates revenues, the Company would not be obligated to repay the grants the Company received for the product's development. The terms of the grant also require that the know-how derived from the research and development fund by the OCS.
Product may not be transferred to third parties without the approval of the OCS. As of 31 December, 2008, approximately $4,000 was paid in respect of royalties payable to the Chief Scientist.
23
The terms of the grants prohibit us under certain limitations from manufacturing products outside of Israel or transferring intellectual property rights in technologies developed using these grants inside or outside of Israel without special approvals. Even if we receive approval to manufacture our products outside of Israel, we may be required to pay an increased total amount of royalties.
This restriction may impair our ability to outsource manufacturing or engage in similar arrangements for those products or technologies. Know-how developed under an approved research and development program may not be transferred to any third parties, except in certain circumstances and subject to prior approval. In addition, if we fail to comply with any of the conditions and restrictions imposed by Israeli law we may be required to refund any grants previously received together with interest and
penalties, and may be subject to criminal charges.
It may be difficult to enforce a U.S. judgment against us, our officers and directors in Israel or the United States, or to assert U.S. securities laws claims in Israel or serve process on our officers and directors.
All of our executive officers and directors are not residents of the United States, and the majority of our assets and the assets of these persons are located outside the United States. Therefore, it may be difficult for an investor, or any other person or entity, to enforce a U.S. court judgment based upon the civil liability
provisions of the U.S. federal securities laws against us or any of these persons in a U.S. or Israeli court, or to effect service of process upon these persons in the United States. Additionally, it may be difficult for an investor, or any other person or entity, to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws on the grounds that Israel is not the most appropriate forum
in which to bring such a claim. Even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel addressing the matters described above. For
additional Information see “Enforceability of Civil Liabilities.”
ENFORCEABILITY OF CIVIL LIABILITIES
All of our directors and officers reside outside the United States. Service of process upon us and upon our directors and officers may be difficult to obtain within the United States. Furthermore, because substantially all of our assets and all of our directors and officers are located outside the United States, any
judgment obtained in the United States against us or any of our directors and officers may not be collectible within the United States.
It may be difficult to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may
determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law.
Subject to specified time limitations and legal procedures, Israeli courts may enforce a United States judgment in a civil matter which, subject to certain exceptions, is non-appealable, including judgments based upon the civil liability provisions of the Securities Act and the Securities Exchange Act and including a monetary or compensatory
judgment in a non-civil matter, provided that:
· the judgments are obtained after due process before a court of competent jurisdiction, according to the laws of the state in which the judgment is given and the rules of private international law currently prevailing in Israel;
· the prevailing law of the foreign state in which the judgments were rendered allows the enforcement of judgments of Israeli courts;
24
· adequate service of process has been effected and the defendant has had a reasonable opportunity to be heard and to present his or her evidence;
· the judgments are not contrary to public policy, and the enforcement of the civil liabilities set forth in the judgment does not impair the security or sovereignty of the State of Israel;
· the judgments were not obtained by fraud and do not conflict with any other valid judgment in the same matter between the same parties;
· an action between the same parties in the same matter is not pending in any Israeli court at the time the lawsuit is instituted in the foreign court; and
· the obligations under the judgment are enforceable according to the laws of the State of Israel and according to the law of the foreign state in which the relief was granted.
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue
a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange
rates.
The selected pro forma financial information presented below is derived from and should be read in conjunction with our financial statements, the financial statements of Baby’s Breath and the unaudited pro-forma balance sheet including notes thereto, appearing elsewhere in this prospectus. See
"Financial Statements."
|
June 30, 2009 |
||||
|
(unaudited) |
||||
|
Assets: |
||||
|
Cash |
$ |
70,667 |
||
| Other Assets | 127,939 | |||
|
TOTAL ASSETS |
$ |
198,606 |
||
| Liabilities: | ||||
| TOTAL CURRENT LIABILITIES | $ | 276,521 | ||
|
Stockholders' equity: |
||||
|
Common stock, $0.001 par value, 50,000,000 shares authorized, 6,360,000 common shares issued and outstanding. |
6,360 |
|||
|
Additional paid-in capital |
665.488 |
|||
| Accumulated deficit | 314,152 | |||
|
(Deficit) |
(1,065,386 |
) | ||
|
Total stockholders' equity |
(79,386 | ) | ||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
$ |
198,606 |
||
25
This registration statement contains forward-looking statements. We, New Air, Inc., have based these forward-looking statements on our current expectations and projections about future events. Actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors,
including all the risks discussed in “Risk Factors” and elsewhere herein. These statements include but are not limited to:
|
· |
expectations as to our ability to generate positive cash-flow from the sales of our products; |
|
· |
expectations as to the timing of profitability, and the adequacy of our cash balances and cash flow from operations to support our operations or future growth, in general or for specified periods of time; |
|
· |
statements regarding our estimates of future performance, sales, gross margin, expenses (including stock-based compensation expenses) and cost of revenue; |
|
· |
expectations as to raising additional financing; |
|
· |
statements as to the potential or expected expansion in acceptance of our current and future products by the medical community and end users; |
|
· |
statements as to expected increases in sales, operating results and certain expenses, including research and development and sales and marketing expenses; |
|
· |
statements as to anticipated reimbursement from U.S. and non-U.S. healthcare payors for our products; |
|
· |
expectations as to the development of our products and technology, and the timing of enhancements to our products and new product launches; |
|
· |
expectations as to the market opportunities for BabyAir and WiseAir and other future products, as well as our ability to take advantage of those opportunities; |
|
· |
expectations as to the timing and content of future clinical studies and publications; |
|
· |
statements as to our ability to protect our intellectual property and avoid infringing upon others’ intellectual property |
|
· |
statements as to the expected outcome of patent filing proceedings in connection with our intellectual property rights; |
|
· |
expectations as to the receipt and timing of regulatory clearances and approvals, to the extent required, and the anticipated timing of sales of our products in new markets or for new indications; |
|
· |
estimates of the impact of changes in currency exchange rates on our operating results; |
|
· |
expectations as to the adequacy of our inventory of critical components and finished products; |
|
· |
expectations as to the adequacy of our manufacturing facilities and the timing of any expansion; and |
In addition, statements that use the terms “believe,” “expect,” “plan,” “intend,” “estimate,” “anticipate” and any and all similar expressions are intended to identify forward looking statements. All forward looking statements in this prospectus reflect our
current views about future events and are based on assumptions and are subject to risks and uncertainties that could cause our actual results to differ materially from future results expressed or implied by the forward looking statements. Many of these factors are beyond our ability to control or predict. You should not put undue reliance on any forward looking statements.
26
This prospectus relates to the following:
The resale by certain selling security holders of the Company of up to 1,242,077 shares of common stock of which 195,677 shares were issued in reliance upon an exemption from registration under Section 4(2) of the Securities Act and/or Regulation S promulgated thereunder as a transaction not involving a public offering and sold to non US
citizens or residents. (See "Liquidity and Capital Resources" Section)
The selling shareholders may sell their shares of our common stock at a fixed price of $1.00 per share until shares of our common stock are quoted on the OTC Bulletin Board, and thereafter at prevailing market prices or privately negotiated prices. There can be no assurance that we will be able to obtain an OTCBB listing. We
will not receive any proceeds from the resale of common shares by the selling security holders.
Our stock registration is made without the involvement of underwriters or broker-dealers. With the exception of any brokerage fees and commissions which are the obligation of the selling shareholders, we are responsible for the fees, costs and expenses of this offering which are estimated to be approximately
$200,000, inclusive of our legal and accounting fees, printing costs and filing and other miscellaneous fees and expenses.
The $1.00 per share offering price of our common stock was determined arbitrarily by us. There is no relationship whatsoever between this price and our assets, earnings, book value or any other objective criteria of value. We intend to apply to the Over-the-Counter Bulletin Board electronic quotation
service for the trading of our common stock upon our becoming a reporting entity under the Securities Exchange Act of 1934 (the "Exchange Act"). If our common stock becomes so traded and a market for the stock develops, the actual price of stock will be determined by prevailing market prices at the time of sale or by private transactions negotiated by the selling shareholders named in this prospectus. The offering price would thus be
determined by market factors and the independent decisions of the selling shareholders named in this prospectus.
The common stock to be sold by the selling shareholders is common stock that is currently issued and outstanding. Accordingly, there will be no dilution to our existing shareholders.
The selling shareholders named in the table titled 'Selling Shareholders' are offering all of the shares of common stock offered through this prospectus. Some of the selling shareholders who are not US citizens or residents acquired their 195,677 shares of common
stock offered through a private offering that was exempt from registration under Regulation S of the Securities Act of 1933, as amended (the "Securities Act"). None of the selling stockholders are broker-dealers or affiliates of broker-dealers.
27
The persons listed in the following table plan to offer the shares shown opposite their respective names by means of this prospectus. The owners of the shares to be sold by means of this prospectus are referred to as the “selling” shareholders”. These shares may be sold by one or more of the following
methods, without limitations.
|
A block trade in which a broker or dealer so engaged will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
| ||
|
Purchase by a broker or dealer as principal and resale by such broker or dealer for its account pursuant to this prospectus;
| ||
|
Ordinary brokerage transactions and transactions in which the broker solicits purchasers
| ||
|
Face to face transactions between sellers and purchasers without a broker/dealer. |
28
Based upon information available to us as of October 16, 2009, the following table sets forth the names of the selling shareholders, the number of shares owned, the number of shares registered by this prospectus and the number and percent of outstanding shares that the selling shareholders will own after the sale
of the registered shares, assuming all of the shares are sold. The information provided in the table and discussions below has been obtained from the selling shareholders. The selling shareholders may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time or from time to time since the date on which it provided the information regarding the shares beneficially owned, all or a portion of the shares of common stock beneficially owned in
transactions exempt from the registration requirements of the Securities Act of 1933. As used in this prospectus, "selling shareholder" includes donees, pledgees, transferees or other successors-in-interest selling shares received from the named selling shareholder as a gift, pledge, distribution or other non-sale related transfer.
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the Commission under the Securities Exchange Act of 1934. Unless otherwise noted, each person or group identified possesses sole voting and investment power with respect to the shares, subject to community property laws where applicable.
|
Common Shares Owned Prior To this Offering |
Total Number of Shares to Be Offered for Selling Shareholder Account(1) |
Total Shares to be Owned Upon Completion of this Offering |
Percent Owned Upon Completion of this
Offering(2) |
|||||||||||||
|
Prof. David Groshar |
244,400 |
244,400 |
0 |
3.77 |
% | |||||||||||
|
Prof Michaelis Newhouse |
174,000 |
174,000 |
0 |
2.69 |
% | |||||||||||
|
Granot Development Enterprises Ltd. |
543,400 |
543,400 |
0 |
8.39 |
% | |||||||||||
|
Orni Elad |
48,639 |
48,639 |
0 |
0.75 |
% | |||||||||||
|
Boaz Gruener |
50,400 |
50,400 |
0 |
0.78 |
% | |||||||||||
|
Benzion Itamar |
7,220 |
7,220 |
0 |
0.11 |
% | |||||||||||
|
Yehoram Bialik |
10,829 |
10,829 |
0 |
0.17 |
% | |||||||||||
|
Hatan David |
14,707 |
14,707 |
0 |
0.23 |
% | |||||||||||
|
Laron David |
14,707 |
14,707 |
0 |
0.23 |
% | |||||||||||
|
Dagan Yaakov |
14,707 |
14,707 |
0 |
0.23 |
% | |||||||||||
|
Noha Yoel |
7,353 |
7,353 |
0 |
0.11 |
% | |||||||||||
|
Meir Alishkov |
28,877 |
28,877 |
0 |
0.45 |
% | |||||||||||
|
Dekel Yaakov |
14,707 |
14,707 |
0 |
0.23 |
% | |||||||||||
|
Ofman Natan |
14,707 |
14,707 |
0 |
0.23 |
% | |||||||||||
|
Mordechi Zandberg |
7,220 |
7,220 |
0 |
0.11 |
% | |||||||||||
|
Avaska Real Estate Inc. |
2,888 |
2,888 |
0 |
0.04 |
% | |||||||||||
|
Gal Zitun |
14,439 |
14,439 |
0 |
0.22 |
% | |||||||||||
|
Reuven Gutman |
28,877 |
28,877 |
0 |
0.45 |
% | |||||||||||
|
Total |
1,242,077 |
1,242,077 |
0 |
19.18 |
% | |||||||||||
|
This table assumes that each shareholder will sell all of his/her shares available for sale during the effectiveness of the registration statement that includes this prospectus. Shareholders are not required to sell their shares. |
2) The percentage is based on 6,476,277 common shares outstanding as of October 16, 2009.
29
|
1. |
Have not had a material relationship with us other than as a shareholder at any time within the past two years; or |
2. Has never been one of our officers or director; or
3. Are not broker-dealers or affiliates of a broker-dealer
The following table identify each of the Investors and the Founders and the number and percentage of the Company’s common stock held by each:
Investors
|
Name of Investors |
Number of Shares |
Percent of Common Stock Outstanding |
||||
|
Life Support Ltd |
1,690,600 |
26.10 |
% | |||
|
A.M. Maagal Marketing & Business Development Ltd |
92,800 |
1.43 |
% | |||
|
Granot Development Enterprises Ltd. |
543,400 |
8.39 |
% | |||
|
Orni Elad |
48,639 |
0.75 |
% | |||
|
Boaz Gruener |
50,400 |
0.78 |
% | |||
|
Benzion Itamar |
7,220 |
0.11 |
% | |||
|
Yehoram Bialik |
10,829 |
0.17 |
% | |||
|
Hatan David |
14,707 |
0.23 |
% | |||
|
Laron David |
14,707 |
0.23 |
% | |||
|
Dagan Yaakov |
14,707 |
0.23 |
% | |||
|
Noha Yoel |
7,353 |
0.11 |
% | |||
|
Meir Alishkov |
28,877 |
0.45 |
% | |||
|
Dekel Yaakov |
14,707 |
0.23 |
% | |||
|
Ofman Natan |
14,707 |
0.23 |
% | |||
|
Mordechi Zandberg |
7,220 |
0.11 |
% | |||
|
Avaska Real Estate Inc. |
2,888 |
0.04 |
% | |||
|
Gal Zitun |
14,439 |
0.22 |
% | |||
|
Reuven Gutman |
28,877 |
0.45 |
% | |||
|
Ramport Finance |
706,000 |
10.90 |
% | |||
|
Microdel Ltd |
1,706,600 |
26.35 |
% | |||
|
Total |
5,019,677 |
77.51 |
% | |||
Founders
|
Name of Founders |
Number of Shares |
Percent of Common Stock Outstanding |
||||
|
Dr. Israel Amirav |
331,600 |
5.12 | % | |||
|
Prof. David Groshar |
244,400 |
3.77 | % | |||
|
Assaf Halamish |
706,600 |
10.91 | % | |||
|
Prof. Michael Newhouse |
174,000 |
2.69 | % | |||
|
Total |
1,456,600 |
22.49 |
% | |||
30
No underwriters or brokers are involved or are expected to be involved in the distribution. On September 7, 2009 we acquired Baby’s Breath, Ltd. In a stock for stock trade at a 1:200 ratio whereby we issued 6,280,600 shares of our common stock for 31,403 shares in Baby’s Breath, Ltd. As
a consequence, Baby’s Breath, Ltd became a wholly owned subsidiary of our Company and the shareholders of Baby’s Breath Ltd. became 96.98% of the total shareholders in our Company. The remaining 195,677 shares issued and outstanding were purchased from us in a private placement transaction in reliance on Regulation S. A copy of this prospectus will be mailed to each holder of record of New Air's common stock on ____________, 2009. New Air will also
mail copies of this prospectus to brokers and dealers who are known to trade or make a market in New Air's common stock and to other brokers and dealers who may reasonably be expected in the future to trade or make a market in New Air' common stock. However, New Air does not anticipate that an active market for its common stock will develop in the near future, and there can be no assurance that a trading market will develop at any time. See RISK FACTORS – “RISKS ASSOCIATED WITH OWNING NEW
AIR STOCK.”
The selling shareholders will act independently of us in making decisions with respect to the timing, manner and size of each sale. The selling shareholders may sell the shares from time to time:
|
In transactions on the Pink Sheets, the Over-the-Counter Bulletin Board or on any national securities exchange or U.S. inter-dealer system of a registered national securities association on which our common stock may be listed or quoted at the time of sale; | ||
|
In private transactions and transactions otherwise than on these exchanges or systems or in the over-the-counter market; | ||
|
At a price of $1.00 per share for the duration of the offering or until our shares are quoted on the OTC Bulletin Board and thereafter at prevailing market prices or privately negotiated prices; | ||
|
D) |
In negotiated transactions; | |
|
E) |
In a combination of such methods of sale; or | |
|
|
F) |
Any other method permitted by law. |
The selling shareholders may effect such transactions by offering and selling the shares directly to or through securities broker-dealers, and such broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling shareholders and/or the purchasers of the shares for whom such broker-dealers may
act as agent or to whom the selling shareholders may sell as principal, or both, which compensation as to a particular broker-dealer might be in excess of customary commissions.
If our common stock becomes traded on the Over-the-Counter Bulletin Board electronic quotation service, then the sales price to the public will vary according to the selling decisions of each selling shareholder and the market for our stock at the time of resale. In these circumstances, the sales price to the public may be:
|
1. |
The market price of our common stock prevailing at the time of sale; | |
|
2. |
A price related to such prevailing market price of our common stock; or | |
|
3. |
Such other price as the selling shareholders determine from time to time. |
We can provide no assurance that all or any of the common stock offered will be sold by the selling shareholders named in this prospectus.
We are bearing all costs relating to the registration of the common stock. The selling shareholders, however, will pay any commissions or other fees payable to brokers or dealers in connection with any sale of the common stock.
31
On or prior to the effectiveness of the registration statement to which this prospectus is a part, we will advise the selling shareholders that they and any securities broker-dealers or others who may be deemed to be statutory underwriters will be governed by the prospectus delivery requirements under the Securities Act. Under
applicable rules and regulations under the Securities Exchange Act, any person engaged in a distribution of any of the shares may not simultaneously engage in market activities with respect to the common stock for the applicable period under Regulation M prior to the commencement of such distribution. In addition and without limiting the foregoing, the selling shareowners will be governed by the applicable provisions of the Securities and Exchange Act, and the rules and regulations thereunder, including
without limitation Rules 10b-5 and Regulation M, which provisions may limit the timing of purchases and sales of any of the shares by the selling shareholders. All of the foregoing may affect the marketability of our securities.
On or prior to the effectiveness of the registration statement to which this prospectus is a part, we will advise the selling shareholders that the anti-manipulation rules under the Securities Exchange Act may apply to sales of shares in the market and to the activities of the selling security owners and any of their affiliates. We
have informed the selling shareholders that they may not:
|
A) |
Engage in any stabilization activity in connection with any of the shares; |
|
Bid for or purchase any of the shares or any rights to acquire the shares; | |
|
Attempt to induce any person to purchase any of the shares or rights to acquire the shares other than as permitted under the Securities Exchange Act; or | |
|
Effect any sale or distribution of the shares until after the prospectus shall have been appropriately amended or supplemented, if required, to describe the terms of the sale or distribution. |
We have informed the selling shareholders that they must effect all sales of shares in broker's transactions, through broker-dealers acting as agents, in transactions directly with market makers, or in privately negotiated transactions where no broker or other third party, other than the purchaser, is involved. The selling shareholders
may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act. Any commissions paid or any discounts or concessions allowed to any broker-dealers, and any profits received on the resale of shares, may be deemed to be underwriting discounts and commissions under the Securities Act if the broker-dealers purchase shares as principal. In the absence of the registration statement
to which this prospectus is a part, certain of the selling shareholders would be able to sell their shares only pursuant to the limitations of Rule 144 promulgated under the Securities Act.
Penny Stock Regulations
Our shares are "penny stocks" covered by Section 15(g) of the Exchange Act, and Rules 15g-1 through 15g-6 and Rule 15g-9 promulgated thereunder. They impose additional sales practice requirements on broker/dealers who sell our securities to persons other than established customers and accredited investors (generally institutions
with assets in excess of $5,000,000 or individuals with net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouses). While Section 15(g) and Rules 15g-1 through 15g-6 apply to brokers-dealers, they do not apply to us.
32
Blue Sky Restrictions on Resale
If a selling security holder wants to sell shares of our common stock under this registration statement in the United States, the selling security holders will also need to comply with state securities laws, also known as "Blue Sky laws," with regard to secondary sales. All states offer a variety of exemption from registration
for secondary sales. Many states, for example, have an exemption for secondary trading of securities registered under Section 12(g) of the Securities Exchange Act of 1934 or for securities of issuers that publish continuous disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor's. The broker for a selling security holder will be able to advise a selling security holder which states our common stock is exempt from registration
with that state for secondary sales.
Any person who purchases shares of our common stock from a selling security holder under this registration statement who then wants to sell such shares will also have to comply with Blue Sky laws regarding secondary sales. When the registration statement becomes effective, and a selling security holder indicates in which state(s)
he desires to sell his shares, we will be able to identify whether it will need to register or it will rely on an exemption there from.
Transfer Agent
We are currently utilizing the services of Corporate Stock Transfer (www.corporatestock.com), Telephone: 303-282-4800 which serves in the capacity as our transfer agent to have us track and facilitate the transfer of our stock.
33
Admission to Quotation on the OTC Bulletin Board
We intend to apply to have our common stock be quoted on the OTC Bulletin Board. If our securities are not quoted on the OTC Bulletin Board, a security holder may find it more difficult to dispose of, or to obtain accurate quotations as to the market value of our securities. The OTC Bulletin Board differs from
national and regional stock exchanges in that it (1) is not situated in a single location but operates through communication of bids, offers and confirmations between broker-dealers, and (2) securities admitted to quotation are offered by one or more Broker-dealers rather than the "specialist" common to stock exchanges.
To qualify for quotation on the OTC Bulletin Board, an equity security must have one registered broker-dealer, known as the market maker, willing to list bid or sale quotations and to sponsor the company listing. If it meets the qualifications for trading securities on the OTC Bulletin Board our securities will trade on the OTC
Bulletin Board. We may not now or ever qualify for quotation on the OTC Bulletin Board. We currently are working with Spartan Securities Group, Ltd as our market maker to list quotations for our securities.
The Company, a Maryland corporation, is authorized to issue 50,000,000 shares of Common Stock, $0.001 par value. The Company has issued 6,476,277 common shares to the shareholders of the Company. The holders of Common Stock: (i) have equal rights to dividends from funds legally available therefore, ratably when as
and if declared by the Board of Directors of the Company; (ii) are entitled to share ratably in all assets of the Company available for distribution to holders of Common Stock upon liquidation, dissolution, or winding up of the affairs of the Company; (iii) do not have preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions applicable thereto; (iv) are entitled to one non-cumulative vote per share of Common Stock, on all matters which stockholders may vote on at all
meetings of Shareholders; and (v) the holders of Common Stock have no conversion, preemptive or other subscription rights. There is no cumulative voting for the election of directors. There are currently 6,476,277 shares of Common Stock outstanding held by approximately 30 shareholders of record. (See "Principal Shareholders").
There is no provision in our by-laws or other incorporating documents that would delay defer or prevent a change in control of our Company.
Share Purchase Warrants
We have not issued and do not have outstanding any warrants to purchase shares of our common stock.
Share Options Grants
We have an undertaking to grant to our CEO, under a stock option plan to be adopted by us for that purpose, a total of 259,051 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 4% of our outstanding share capital prior to the registration).
In addition we currently have available for additional grant to our employees, subject to the approval of our Board, up to 129,525 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 2% of our outstanding share capital prior to the registration). Unless agreed otherwise by the Board the granted options shall generally be equally vested over a period of 3 years such that at the end of each year 1.33% of the entire amount of granted
options shall become vested.
The options to our Israeli employees may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the shares of Common Stock issued upon their exercise must be deposited with a trustee for at least two years following the end of the calendar year in which the options are granted. Under Section 102
(1) any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or the shares by the trustee to the employee or upon the sale of the options or ordinary shares, (2) gains are subject to capital gains tax of 25%; under the tax rules governing these options, we do not receive a tax deduction in respect of the issuance or exercise of the options.
34
Dividends
We have never paid dividends and do not currently intend to pay dividends on our common stock in the foreseeable future. Instead, we anticipate that any future earnings will be retained for the development of our business. Any future determination relating to dividend policy will be made at the discretion of our board of directors
and will depend on a number of factors, including, but not limited to, our financial condition, operating results, cash needs, growth plans, the terms of any credit agreements that we may be a party to at the time and the Maryland Corporate Code, which provides that dividends are only payable out of surplus or current net profits.
Rule 144 Shares
As of September 15, 2009, no shares currently issued and outstanding could be resold pursuant to Section 144 of the Securities Act. This is because no shares have been held for 2 years in order to satisfy 144(K) and no sufficient public information is available to satisfy the other 144 rules.
A total of 6,476,277 shares of our common stock will be available for resale to the public after six months from the approval of this prospectus, in accordance with the volume and trading limitations of Rule 144 of the Securities Act of 1933.
In general, under Rule 144, as currently in effect, a person who has beneficially owned shares of a company's common stock for at least one year is entitled to sell within any three month period a number of shares that does not exceed the greater of:
|
1. |
one percent of the number of shares of the company's common stock then outstanding, which, in our case, will equal approximately 64,763 shares as of the date of this prospectus, or; |
|
2. |
the average weekly trading volume of the company's common stock during the four calendar weeks preceding the filing of a notice on form 144 with respect to the sale. |
Sales under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about the company.
Under Rule 144(k), a person who is not one of the company's affiliates at any time during the three months preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years, is entitled to sell shares without complying with the manner of sale, public information, volume limitation or notice provisions
of Rule 144.
As of the date of this prospectus, persons who are our affiliates hold 5,234,200 shares of the total shares, which may be sold, at least partially, pursuant to Rule 144(k) after six month from the date of this prospectus.
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis or had, or is to receive,
in connection with the offering, a substantial interest, directly or indirectly, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents, subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
Our officers/directors can be considered promoters of the Company in consideration of their participation and managing of the business of the company since its incorporation.
35
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our Articles and By-laws provide to the fullest extent permitted by law, our directors or officers, former directors and officers, and persons who act at our request as a director or officer of a body corporate of which we are a shareholder or creditor shall be indemnified by us. All our officers and directors have executed such
an agreement with us. We believe that the indemnification provisions in our By-laws are necessary to attract and retain qualified persons as directors and officers. Insofar as indemnification for liabilities arising under the Securities Act of 1933 (the "Act" or "Securities Act") may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions,
or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the Securities Act with the SEC for the securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules which are part of the
registration statement. For additional information about us and our securities, we refer you to the registration statement and the accompanying exhibits and schedules. Statements contained in this prospectus regarding the contents of any contract or any other documents to which we refer are not necessarily complete. In each instance, reference is made to the copy of the contract or document filed as an exhibit to the registration statement, and each statement is qualified in all
respects by that reference. Copies of the registration statement and the accompanying exhibits and schedules may be inspected without charge (and copies may be obtained at prescribed rates) at the public reference facility of the SEC at Room 1024, 100 F Street, N.E. Washington, D.C. 20549.
You can request copies of these documents upon payment of a duplicating fee by writing to the SEC. You may call the SEC at 1-800-SEC-0330 for further information on the operation of its public reference rooms. Our filings, including the registration statement, will also be available to you on the Internet web site
maintained by the SEC at http://www.sec.gov.
We are a newly established US parent company of Baby’s Breath Ltd. founded back in 2001 by a group of entrepreneurs and leading physicians: Dr. Israel Amirav, Dr. David Groshar, Prof. Newhouse, and Asaf Halamish, with a goal of developing innovative inhalation technologies for the treatment of respiratory diseases.
Baby's Breath has completed the development and commercialization of BabyAir, a special inhalation and drug delivery solution for babies under the age of 2 years, who suffer from asthma and other respiratory conditions. The product was developed based on requests that emerged from pediatricians and clinicians in the field and with their
guidance and inputs.
Business Overview
We develop manufacture and market proprietary products for the treatment of respiratory diseases and pulmonary conditions in infants and in elderly people such Asthma, Respiratory Syncytial Virus (RSV), Cystic Fibrosis, Pneumonia, Dyspnea and breathing disorders during sleep.
We currently market and distribute BabyAir, for the treatment of respiratory diseases and pulmonary conditions in infants, and we are in the process of developing WiseAir for the treatment of these conditions in elderly people. We believe that our products provide a patient-friendly solution that addresses a significant market opportunity
and overcomes many of the shortcomings of traditional products for the treatment of such conditions.
Respiratory Diseases – General Overview of the Market
Asthma
Once considered a minor ailment affecting only a few, asthma is now the most common chronic disorder in childhood, affecting an estimated 9 million children under the age of 18 in the US. The prevalence of asthma has progressively increased over the past 15 years. In the United States alone, 30.8 million people – 10.6 percent
of adults and 12.2 percent of children – have been diagnosed with asthma (source: NIEHS – National Institute of Environmental Health Sciences).
36
Respiratory Syncytial Virus (RSV)
Respiratory Syncytial (sin-SISH-uhl) Virus, or RSV, is a respiratory virus that infects the lungs and breathing passages. Respiratory Syncytial Virus (RSV) infection, which manifests primarily as bronchiolitis and/or viral pneumonia, is the leading cause of lower respiratory tract (LRT) infection in infants and young children. In fact,
RSV is considered to be the most common cause of bronchiolitis (inflammation of the small airways in the lung) and pneumonia in children under 1 year of age in the United States. In addition, RSV is more often being recognized as an important cause of respiratory illness in older adults. High-risk adults include those with chronic heart disease, chronic lung disease, or compromised immune systems; the elderly include those 65 or older, particularly if they reside in a long-term care facility or participate in
other senior day-care programs.
Cystic Fibrosis
Cystic fibrosis (CF), is a relatively common hereditary disease that affects the entire body, causing progressive disability and early death. Difficulty breathing is the most common symptom and results from frequent lung infections, which are treated, though not always cured, by antibiotics and other medications.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease, or COPD, refers to a group of diseases that cause airflow blockage and breathing-related problems. It includes emphysema, chronic bronchitis, and in some cases asthma. Approximately, 12 million adults in the United States have been diagnosed with COPD, and another 12 million are believed to be undiagnosed.
COPD is considered to be a leading cause of death, illness, and disability in the United States.
Pneumonia
Pneumonia mainly causes illness in children younger than 2 years old and adults 65 years of age or older. The elderly are especially at risk of getting seriously ill and dying from this condition. Pneumonia is considered the fifth leading cause of death at the age of 65 in the
United States and a third leading cause of death in those over 85.
Dyspnea
Dyspnea is defined as the relentless awareness of shortness of breath. Reported in up to 70% of patients with terminal lung disease, dyspnea has a debilitating effect on function and quality of life. Perhaps in part because the cause of dyspnea has yet to be fully elucidated, its presence in the setting of terminal lung disease can be extremely
difficult to treat.
Acute Pulmonary Thromboembolism (PE)
PE is a major diagnostic dilemma for the clinician and is one of the most common non- or misdiagnosed diseases. Acute pulmonary thromboembolism (PE) is the third most common cause of death in hospital patients. More than 500,000 patients are diagnosed with PE in the United States annually, resulting in approximately 200,000 deaths.
Breathing Disorders During Sleep
Sleep apnea is the most common sleep disorder in terms of mortality and morbidity, especially in middle-age men. Sleep apnea occurs in all age groups and both sexes, but seems to predominate in males (it may be under diagnosed in females) and in African Americans. The Associated Professional Sleep Societies estimates that as many as 20
million Americans have this condition. The conditions associated with sleep apnea are a cascade: apnea, arousal, sleep deprivation, and excessive daytime sleepiness. According to recent studies, at least one in 50 children suffer from obstructive sleep apnea.
37
Respiratory Diseases –Existing Treatments
| Using a Nebulizer - The Most Common Current Treatment for Most Respiratory Conditions
Current treatments of the abovementioned conditions consists mostly of Aerosol therapy. Aerosol therapy consists of a compressor, which generates a controlled airflow to the nebulizer cup, and the medication mist is then inhaled through a mouthpiece or mask. The treatment typically requires up to 20 minutes to administer. It is commonly
used in treating asthma, cystic fibrosis, upper respiratory infection, and other respiratory diseases, and has been even found effective in treating discomfort with morphine for end stage lung diseases. Aerosol therapy however does not adequately meet the infant’s basic need for efficient patient-friendly therapy.
Current Treatment- the Disadvantages of Treating Infants
Treatment is effective only when aerosolized medication delivery systems are used properly. Proper usage is actually being impaired by the presence of the mouthpiece.
The facemask element of the treatment is a major disadvantage. Simply put, babies and children despise the mask and fight from the moment the treatment starts often exhausting themselves in their weak state. Recent studies have stressed the importance of fitting the mask tightly to the child’s face. It was shown that a gap as
small as 0.4 inch between the nebulizer mask and the face can reduce the amount of medicine delivered to the patient by as much as 50%. Part of the dosage is thus wasted, and several repeated inhalation sessions are often required in order to deliver the desired medication dosage. In addition proper distribution of the medication is affected because of the delivery method, and the therapy’s effectiveness is thus eventually jeopardized. |
 |
| In addition, the treatment should be given in an elevated position because of the position of the nebulizer itself. If the nebulizer is laid flat on the child while the child is sleeping, the contents of the nebulizer will spill out. This means waking the child to administer the inhalation, sometimes several times a day and night.
The effectiveness of the treatment is further reduced due to the stress and crying of the infants and children while being forced to wear the masks during the treatments. Several studies have shown that the effect of crying while being treated with a nebulizer causes a significant
reduction in the absorption of the aerosol in the lungs thus reducing the therapeutic value of the drug.
Current Treatment - the Disadvantages of Treating Elderly and Feeble Patients
Another population that cannot use a mouthpiece or a mask with a nebulizer is the elderly and feeble long-term-care patients who suffer from lower respiratory illness and are not able to breathe on their own. Furthermore, older patients, while more constrained in their reaction, also feel constricted and uncomfortable while wearing the
mask. These difficulties ultimately can result in non-compliance due to the extreme discomfort. According to studies conducted by the General Practice Airways Group, of patients admitted to hospital for asthma attacks, 74% could have been prevented with a nebulizer treatment. In short the current delivery method has disadvantages which eventually jeopardize the effectiveness and efficacy of the therapy.
|
 |
38
Competing Products
We consider the following companies as our main competitors in the market in which we operate: Respironics, DeVilbiss Healthcare, Omron Healthcare, Pari Respiratory Equipment, Cardinal Health and Westmed Inc.:
Respironics brand holds a very large market share in the sleep apnea segment and is therefore largely identified with it. Respironics’ offering includes a variety of home compressor nebulizer, mobile and disposable versions. DeVilbiss Healthcare presents itself as a leader in the respiratory niche of the home healthcare products market: DeVilbiss
Healthcare design, manufacture and market respiratory medical products that address the respiratory needs of patients in both institutional and homecare settings. The company’s offering ranges from oxygen to sleep therapy, and pulmonary drug delivery devices. Omron Healthcare and Pari Respiratory Equipment also offer variety of portable compressor nebulizers. Cardinal Health maintains a broad portfolio of products and services and offers a wide range of home healthcare nebulizers for children and grown-ups,
all comprising a mouthpiece for inhalation. Westmed Inc. manufactures a complete nebulizer system which is designed to enhance the delivery of aerosolized medication while reducing the apprehension encountered by young patients.
None of the above-mentioned companies provides a truly differentiated offering, in which medication delivery does not involve direct physical contact of the delivery system with the infant/patient.
Our Products and Their Advantages
| The Product
Our Products have evident unique and sustainable advantages. The main feature of our products and the basis for their differentiation with competitive products is the fact that it is currently the only specifically designed for infants’ device in the market, that consists of a hood and an upside-down nebulizer, which allows for treating
a patient while lying flat on the bed. This increases effectiveness and level of absorption of the administered drugs while it prevents waste of unused drug left in the cup and is better suited for absorbance in infants’ lungs.
● A flexible and collapsible frame,
● A patented hood,
● A patented ‘upside down’ nebulizer for use when lying down,
● Tube adjustable to child/patient’s position. |
 | |
| * Compressor on top is not included. |
|
Child Hood and Frame– Currently, the frame and hood is one construct. The tent is constructed like an umbrella and is designed to be placed over the head and upper torso of the child. The hood is made up of a collapsible plastic frame, which is covered by
transparent plastic sheet. The top and bottom of the hood is open slightly to allow for fresh air. The hood can be decorated with various elements to amuse the child, and can even be equipped with a mobile to further distract the child. When the hood is not in use it can be easily stored in its own bag. Tube - In the center of the Child Hood, affixed to the frame is a corrugated plastic tube with a silicone nipple at the bottom that is designed to easily direct the aerosol to the nose and mouth of the child. If the child wants to turn his head, the parent simply readjusts the
tube without troubling the child.
UDN Nebulizer – The patented down draft nebulizer is designed to deliver aerosol medicine ‘upside down’. This enables delivery of the medication even while the child or infant is lying down or sleeping. The nebulizer is made of polycarbonate and fits on the top of the funnel pipe. The nebulizer is designed to work either with a compressor or be connected to a hospital air
line or oxygen line. |
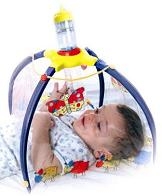 |
39
Compressor – Electrically powered utilizing 115V or 220V is connected to the nebulae by means of an air hose. The compressor provides the correct air/pressure output for proper formation of the aerosol. While the compressor can be bought off the shelf, as there is nothing unique about it, in the long run, it is far more cost effective to manufacture than to license from another company.
During treatment the umbrella like hood is opened over the head of the infant. The medicine is placed in the nebulizer cup and is delivered to the area in front of the nose and mouth of the patient as a nebulized fine aerosol. This system enables the medication to be inhaled by the patient without any physical contact with BabyAir.
Our Advantages
Our products employ a 'hood' technique which does not need to be sealed around the patient’s face for the duration of the treatment for proper function. Our product has currently the only existing nebulizer that is connected to a compressor and can release the aerosol from above. It employs a unique mechanism that filters the droplets
to allow only very small size to exit the nebulizer (droplets size: 2 micron MMD). The unique design of the cup enables almost full use of the drug without leaving an unused portion in the nebulizer.
The main advantages of our products are:
|
§ |
Currently most suitable and practical solution for newborns; |
|
§ |
Direct delivery of inhaled medications to patients in a safe, easy and effective way; |
|
§ |
No physical contact with patients; |
|
§ |
Can be used when infant/patient is awake or asleep; |
|
§ |
High level of patient's comfort and treatment acceptance; |
|
§ |
Sole touch-free solution available; |
|
§ |
Increased mother’s comfort level and peace of mind; |
|
§ |
The product is fully compatible with compressors that can be found on the market. |
|
§ |
Very economic in medication usage, compared to most existing solutions. |
Our Strategy
Our goal is to become a leading manufacturer and seller of products for treatment of respiratory conditions in infants, elderly people and other feeble patients. In order to accomplish this objective, we plan to:
|
· |
Grow Market Awareness to our solution. Our marketing approach is focused on educating physicians and patients about the benefits and efficacy of our system. We believe that we can continue to grow our revenues and maintain strong gross margins by increasing product utilization
rates. |
|
· |
Continue to develop our products. We are seeking to use our technology platform for the development of WiseAir for the treatment of elderly people and feeble patients. We are engaged in research and development activities to develop such product and we intend to pursue regulatory
clearance in the United States and elsewhere to market it. |
|
· |
Establish significant distribution channel. We are now in the midst of negotiations with distributors in over 30 countries (mainly in Europe) with the intention to establish and create solid distribution channels and strengthen our ability to penetrate local markets and significantly enhance our
ability to sell our products worldwide. |
40
|
· |
Pursue reimbursement for our products. We believe that a significant factor in increasing the utilization rates and gaining broader acceptance for our products depends on the level of reimbursement available therefor. In the United States, reimbursement coverage determinations
are made by a large number of third-party payors, including Medicare, Medicaid, private health insurance plans and health maintenance organizations. We intend to devote significant resources to expanding reimbursement coverage for BabyAir and WiseAir. |
|
· |
Enhance our intellectual property portfolio. We intend to continue to invest in research and development and to seek patent protection in key jurisdictions for our products. Using our design experience and clinical data obtained as a result of usage of our product. We believe
that this is the principal method for us to continue to compete with traditional treatments of respiratory conditions. |
Our Marketing Strategy
The following chart demonstrates the marketing strategy we intend to employ in order to increase sales of our products worldwide:
In order to establish a firm brand identity for our products we intend to engage in the following activities:
|
§ |
Participation in clinical trials at Key Opinion Leaders (KOLs) health care sites in the US and Europe and publish peer review articles to create acceptance by the Pediatric community; |
|
§ |
Participation in medical device exhibitions; |
|
§ |
Participation in Scientific meetings focused on respiratory care; |
41
|
§ |
Advertising in medical magazines; |
|
§ |
“Networking” with leading voluntary Associations in the pulmonary and respiratory arena (such as Lung USA or Asthmatic Patients) to potentially exercise lobbying activities at the market entry stage; |
Once the brand has become sufficiently recognized, we intend to launch an advertising and marketing effort directly to the private and institutional sector in conjunction with our distributors.
U.S. Government Regulation
FDA Clearance and Regulation of our Products
In 2006, we received a 510(k) clearance from the FDA to market BabyAir in the United States.
Generally, any new medical device that we wish to commercially distribute in the United States will likely require either 510(k) clearance or premarket application approval from the FDA prior to commercial distribution. 510(k) clearance or amendment to premarket application is also required when a change is made to a legally
marketed device or to expand the product label.
510(k) Clearance Process. Generally, to obtain 510(k) clearance, an applicant must submit a premarket notification demonstrating that the proposed device is substantially equivalent in intended use and in safety and effectiveness to a “predicate
device” – either a previously 510(k) cleared device or a preamendment device for which the FDA has not called for premarket applications. The FDA’s 510(k) clearance process usually takes from four to 12 months, but it can last longer. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could even require a premarket
application approval. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the determination, the agency may retroactively require the manufacturer to seek 510(k) clearance or premarket application approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket application approval is obtained.
De Novo Classification. If the FDA denies 510(k) clearance of a device because it is novel and an adequate predicate device does not exist, the “de novo classification” procedure can be invoked
based upon reasonable assurance that the device is safe and effective for its intended use. This procedure approximates the level of scrutiny in the 510(k) process but may add several months to the clearance process. If the FDA grants the request, the device is permitted to enter commercial distribution in the same manner as if 510(k) clearance had been granted.
Premarket Application Approval Process. If the FDA denies 510(k) clearance for a product, and denies de novo classification, the product must follow the premarket application approval process, which requires proof of the safety and effectiveness of the device to the
FDA’s satisfaction. A premarket application must provide extensive preclinical and clinical trial data and also information about the device and its components regarding, among other things, device design, manufacturing and labeling. After approval of a premarket application, a new premarket application or premarket application supplement is required in the event of a modification to the device, its labeling or its manufacturing process. The premarket application approval
pathway is much more costly, lengthy and uncertain. It generally takes from one to three years or longer.
Anti-Kickback and False Claims Laws.
In the United States, there are federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation
in federal healthcare programs. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices such as us, by limiting the kinds of financial arrangements (including sales programs) we may have with hospitals, physicians and other potential purchasers of the medical devices. Other provisions of state and federal law provide civil and criminal penalties for presenting,
or causing to be presented, to third-party payors for reimbursement, claims that are false or fraudulent, or which are for items or services that were not provided as claimed. Since we may provide coding and billing advice to purchasers of our products, and since we cannot be sure that the government will regard any billing errors as inadvertent, we could be subject to an enforcement proceeding in a case where our advice was deemed to have caused the submission of improper claims.
42
Regulation in Europe
Commercialization of medical devices in member countries of the Europe Union is regulated by directives adopted by the European Union. The European Union presently requires that all medical products bear the CE mark, an international symbol of adherence to quality assurance standards and demonstrated clinical effectiveness. Compliance
with the Medical Device Directive, as certified by a recognized European Competent Authority, permits the manufacturer to affix the CE mark on its products. We currently hold an authorization to affix the CE mark on our BabyAir product.
If we modify our product, we may need to apply for permission to affix the CE mark to it. Additionally, we will need to apply for a CE mark for any new products that we may develop in the future including the WiseAir. We cannot be certain that we will be able to obtain permission to affix the CE mark for new or modified products
or that we will continue to meet the quality and safety standards required to maintain the permissions that we receive. If we are unable to maintain permission to affix the CE mark to our products, we will no longer be able to sell our products in member countries of the European Union.
Regulation in Other Countries
In order for us to market our products in countries other than the United States, we must obtain regulatory approvals and comply with extensive safety and quality regulations in these countries. These regulations, including the requirements for approvals or clearance and the time required for regulatory review, vary from
country to country. Failure to obtain regulatory approval or clearance in any foreign country in which we plan to market our product may harm our ability to generate revenue and harm our business.
In all of the countries in which we are currently selling our products we have either received regulatory approval or been informed that approval is not required. In addition, we are seeking regulatory approval in.
Third Party Reimbursement
Reimbursement in the United States
In the United States, healthcare providers that purchase medical devices generally rely on third-party payors, such as Medicare, Medicaid, private health insurance plans and health maintenance organizations, to reimburse all or a portion of the cost of the devices, as well as any related healthcare services utilizing the devices. FDA
clearance does not necessarily result in coverage and reimbursement by third-party payors.
Coding. Generally, a current procedural terminology, or CPT, code is necessary to facilitate claims for reimbursement. If a procedure is not covered by an appropriate existing code, an application for a new code can be made to the American
Medical Association. This process can be lengthy, however, typically taking two or more years before the new code is effective. In the meantime, claims may be submitted using a miscellaneous CPT code or using a G-code, if one is established by the Department of Health and Human Services’ Centers for Medicare and Medicaid Services, or CMS.
Reimbursement Coverage. A payor’s decision to cover a device or medical procedure is independent of the coding process, although the existence of an appropriate CPT code and APC may assist in obtaining coverage. Generally, payors may
deny coverage if they determine that a procedure was not reasonable or necessary as determined by the payor, was experimental or was used for an unapproved indication. During the past several years, the major third-party payors have substantially revised their reimbursement methodologies in an attempt to contain their healthcare reimbursement costs.
43
Reimbursement Rates. Even if a device or medical procedure is covered, reimbursement rates must be adequate for providers to use it routinely. Reimbursement rates vary depending on the third-party payor and individual insurance plan
involved, the procedure performed and other factors. Medicare reimbursement for inpatient hospital services is based on a fixed amount per admission based on the patient’s specific diagnosis. As a result, any illness to be treated or procedure to be performed in an inpatient setting will be reimbursed only at a prescribed rate set by the government that is known in advance to the hospital. If the treatment cost is less, the hospital is still reimbursed for the entire fixed
amount; if it costs more, the hospital cannot bill the patient for the difference. Many private third-party payors and some state Medicaid programs have adopted similar prospective payment systems. In addition, Medicare has implemented prospective payment systems for some services performed in hospital outpatient departments and skilled nursing facilities as well. The Medicare payment system for outpatient services allows for more separate reimbursement for individual services
than the inpatient system.
Coverage Outside the United States
In countries outside the United States, coverage is obtained from various sources, including governmental authorities, private health insurance plans, and labor unions. In some foreign countries, private insurance systems may also offer payments for some therapies. Although not as prevalent as in the United States,
health maintenance organizations are emerging in certain European countries. Coverage systems in international markets vary significantly by country and, within some countries, by region. Coverage approvals must be obtained on a country-by-country or region-by-region basis.
Our Reimbursement Strategy
We hired a reimbursement consulting company to assess and design the optimal reimbursement strategy to facilitate our sales plan. We intent to prepare and submit an analysis (value story) that demonstrates clinical and economic benefits for the hospital, when using the product for its inpatients, instead of currently available
alternatives. We also intend to employ the resources to make sure BabyAir and WiseAir are compliant with CPT codes. There is also a possibility that existing Coding Systems and Coverage Policies for the patient’s home setting could be immediately utilized, but the expected rates from such coverage may not support our business model. We cannot provide any assurance that we will obtain any additional approvals in a timely manner or at all.
Intellectual property
We were granted two patents for BabyAir, which covers the "up side down Nebulizer", the device that transports the medicine, as well as the hood. In addition we have other applications in the process of examination. The status of our patent filing process worldwide is presented in the following table:
|
Patent Status September 2009 | |||||||
|
Title |
Country |
Application/Patent no. |
Status | ||||
|
Aerosol Inhalation Interface |
Europe |
01949840.1 |
Renewal June 29, 2010 | ||||
|
Aerosol Inhalation Interface |
Israel |
137185 |
Allowed | ||||
|
Aerosol Inhalation Interface |
United States |
6877509 |
Granted – Renewal October 12, 2012 | ||||
|
Downdraft Nebulizer |
Italy |
ITMI031342A0 |
Renewal December 31, 2009 | ||||
|
Downdraft Nebulizer |
United States |
6883517 |
Granted | ||||
|
Downdraft Nebulizer |
Japan |
2002-506675 |
Request for examination | ||||
|
Downdraft Nebulizer |
Europe |
04078146.1 |
Renewal September 30, 2009 | ||||
We currently have not begun registration of BabyAir and WiseAir which we currently use as registered trademarks and we intend to do so in the future.
44
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the notes to those statements included elsewhere in this prospectus. In addition to the historical financial information, the following discussion and analysis contains
forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
Our Company was incorporated in Maryland in July 2009. We incorporated our Company to acquire a company called Baby’s Breath Ltd. an Israeli company founded in 2001 by a group of leading physicians and highly experienced professionals that developed a patented medical devise for the treatment of repertory diseases.
On September 7, 2009 we acquired Baby’s Breath, Ltd. In a stock for stock trade at a 1:200 ratio whereby we issued 6,280,600 shares of our common stock for 31,403 shares in Baby’s Breath, Ltd. As a consequence, Baby’s Breath, Ltd became a wholly owned subsidiary of our Company and the shareholders of Baby’s
Breath Ltd. Became 96.98% of the total shareholders in our Company.
Since inception, we have been unprofitable. We incurred net losses of $191,563 for the fiscal year ended 2008, $199,666 for the fiscal year ended 2007 and a net loss of $81,834 for the six months ended June 30, 2009. As of June 30, 2009, we had an accumulated deficit of $1,065,386. Our limited history of operations makes prediction
of future operating results difficult. We believe that period to period comparisons of our operating results should not be relied on as predictive of our future results.
We are currently in the process of strengthening our distribution channels. We achieved our first billable shipment in 2003 and are hopeful to receive several orders for the U.S. market during the second half of 2009. Since our FDA clearance to sell our FMS product was only received on February 22, 2006 it is too early
to know with a high degree of confidence how quickly, and in what amounts, new orders will develop.
Our future cash requirements and the adequacy of available funds will depend on our ability to sell BabyAir and related products now that FDA final clearance has been obtained. We expect that we will require additional funding to finance operating expenses and to enter the international marketplace.
We have funded our operations to date in accordance with the schedule below:
|
Amounts |
Years |
|||||||
| Initial Share Capital | $ | 5,689 | 2001 | |||||
|
A grant from the Office of Chief Scientist – paid back through royalties from sales of products |
$ | 268,029 | 2001-2005 | |||||
|
Ramport Financial Ltd (investment in equity) |
$ | 150,000 | 2005 | |||||
|
Microdel Ltd (investment in equity) |
$ | 314,152 | 2007-2009 | |||||
|
Life Support Ltd (investment in equity) |
$ | 443,000 | 2004-2007 | |||||
45
Critical Accounting Policies and Estimates and Recent Accounting Developments
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based upon our audited Financial Statements, which have been prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). The preparation of these financial
statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities as of the date of our financial statements, the reported amounts of revenues and expenses during the reporting periods presented, as well as our disclosures of contingent assets and liabilities. On an on-going basis, we evaluate our estimates and assumptions, including, but not limited to, fair value of stock-based compensation, fair value of acquired intangible assets and goodwill,
useful lives of intangible assets and property and equipment, income taxes, and contingencies and litigation.
We base our estimates and assumptions on our historical experience and on various other information available to us at the time that these estimates and assumptions are made. We believe that these estimates and assumptions are reasonable under the circumstances and form the basis for our making judgments about the carrying values
of our assets and liabilities that are not readily apparent from other sources. Actual results and outcomes could differ from our estimates.
Our significant accounting policies are described in the Note titled ‘Summary of Significant Accounting Policie’s, in Notes to Financial Statements of this Form S-1. We believe that the following discussion addresses our critical accounting policies
and reflect those areas that require more significant judgments, and use of estimates and assumptions in the preparation of our Financial Statements.
Results of Operations
Twelve Months Ended December 31, 2008 and 2007
Revenue. The Company’s revenues increased to $43,199 for the year ended December 31, 2008 from $24,013 for the year ended December 31, 2007. Revenues increased primarily due to increased selling to additional countries.
Cost of sales. Cost of sales increased to $40,128 for the year ended December 31, 2008 from $34,988 for for the year ended December 31, 2007. Cost of Sales increased primarily due to an increase in the net revenues primarily due cost of manufacturing and additional
salaries.
General and Administrative expense. General and administrative expense consists of, management salaries, professional fees, consulting fees, travel expense, administrative fees and general office expenses. These expenses also include our operations expense.
General and administrative expense decreased to $112,082 for the year ended December 31, 2008 from $118,307 for the year ended December 31, 2007. General and administrative expense decreased primarily due to decrease in professional services expenses and patent expenses.
46
Sales and Marketing expense. Sales and marketing expense consists of expenses required to sell products through independent reps, attendance at trades shows, product literature and other sales and marketing activities.
Sales and marketing expenses grew to $76,551 in the year ended December 31, 2008 compared to $62,989 in the year ended December 31, 2007 as a result of an increase in marketing and advertising expenses.
Financial expense. financial expense decreased to $6,001. In the year ended December 31, 2008 from $7,395. In the year ended December 31, 2007 primarily due to changes in the $ to NIS exchange rates.
Six months ended June 30, 2009 and 2008
Revenue. The Company’s revenues increased to $15,744 during the six-month period ended June 30, 2009 compared to $6,674 in the six-month period ended June 30, 2008. Revenues increased primarily due to selling to additional countries.
Cost of sale and Gross Profits. Cost of sales increased to $15,121 during the six-month period ended June 30, 2009 compared to $12,522 for six-month period ended June 30, 2008. Gross Profit increased from (5,848) for six-month period ended June 30, 2008 to $623 for the
six-month period ended June 30, 2009 which increase is primarily due to discounting costs and economies of scale.
General and Administrative expense. General and administrative expense primarily consists of, management salaries, professional fees, consulting fees, travel expense, administrative fees and general office expenses.
General and Administrative (G&A) expenses decreased to $38,484 during the six-month period ended June 30, 2009 compared to $48,361 for six-month period ended June 30, 2008. General and administrative expense decreased primarily due to decrease in professional services expense and patent expenses.
Sales and Marketing expense. Sales and marketing expense consists of expenses required to sell products through independent reps, attendance at trades shows, product literature and other sales and marketing activities.
Sales and marketing expenses decreased to $39,568 during the six-month period ended June 30, 2009 compared to $52,827 for six-month period ended June 30, 2008, as a result of a decrease in export expenses, travel expenses and sales commissions.
47
Sales and marketing expense is expected to grow significantly in the next several quarters as we ramp up our sales and marketing activities to establish our products in the marketplace and we commence the payment of commissions to distributors and independent sales representatives.
Financial expense. financial expense decreased to $4,405 during the six-month period ended June 30, 2009 compared to $8,136 for six-month period ended June 30, 2008, as a result of change in the exchange rates.
Liquidity and Capital Resources
We had a cash balance of $14,791 as of December 31, 2008 and $13,989 as of June 30, 2009. Since our inception, we have incurred significant losses. As of December 31, 2008 we had an accumulated deficit of $983,552, and as of June 30, 2009 our accumulated deficit was $1,065,386. We have not achieved profitability and anticipate that we will
continue to incur net losses for the foreseeable future. We expect that our expenses will increase, and as a result we will need to generate significant revenue to achieve profitability.
To date, our operations have been funded through investments in our shareholders equity (totaling in $931,322) and through grants received from the OCS (totaling in $268,029).
As of December 31, 2008, we had accounts payable and accrued expenses of $86,133 and liabilities of $123,640 as of June 30, 2009.
Years Ended December 31, 2008 and 2007
Net cash used in operating activities was $164,796 for 2008 as compared with net cash used of $67,597 for 2007. The increased use of cash was due primarily to an increase in shareholders loans and accrued expenses.
Cash flows used in investing activities was $287 for 2008 as compared to cash used in investing activities of $1,538 for 2007.
Net cash provided by financing activities was $167,881 for 2008 as compared to net cash provided by financing activities of $31,585 for 2007. The increase in 2008 was primarily the result of receipt on amount of shares.
Six months ended June 30, 2009 and 2008
Net cash used in operating activities was $21,800 in the six months ended June 30, 2009 compared to $116,529 net cash used in operating activities in the six months ended June, 2008. The additional cash used in the 2009 period was primarily due accrued expenses.
48
We believe that we have sufficient funds to satisfy our obligations through the third quarter of 2009.
We expect to continue to depend upon outside financing to sustain our operations for at least the next 12 months. Our ability to arrange financing from third parties will depend upon our operating performance and market conditions. Our inability to raise additional working capital at all or to raise it in a timely
manner would negatively impact our ability to fund our operations, to generate revenues, and to otherwise execute our business plan, leading to the reduction or suspension of our operations and ultimately forcing us to go out of business. Should this occur, the value of any investment in our securities could be adversely affected, and an investor could lose a portion of or even lose their entire investment.
Commitments and Contingencies
Effective June 30, 2009, we had bank debts, trade payable, accounts payable and accrued expenses, shareholders loans and accrued severance pay owed.
Our contractual obligations consisted of the following as of June 30, 2009.
|
Payment Due by Period as of June 30 |
||||||||||||||||||||
|
Total |
Less than 1 Year |
1-3 Years |
4-5 Years |
After 5 Years |
||||||||||||||||
|
Short Term Bank Debt |
$ |
8,178 |
$ |
8,178 |
||||||||||||||||
|
Accounts Payable and Accrued Expenses |
123,640 |
123,640 |
||||||||||||||||||
|
Shareholders’ Loans |
142,703 |
142,703 |
||||||||||||||||||
|
Accrued Severance Pay |
1,471 |
$ |
1,471 |
|||||||||||||||||
|
Total Contractual Cash Obligations |
$ |
275,992 |
$ |
274,521 |
$ |
1,471 |
$ |
- |
$ |
- |
||||||||||
49
Amortization of Intangible Assets
Intangible assets currently consist solely of patent costs. These assets are not subject to amortization until the property patented is in production. The assets are reviewed for impairment annually, and impairment losses, if any, are charged to operations when identified. No impairment losses have been
identified by management to date.
Income Tax Expense
The Company provides for deferred taxes using the asset and liability approach. Under this method, deferred tax assets and liabilities are recognized for future tax consequences attributable to operating loss and tax credit carry-forwards, as well as differences between the carrying amounts of existing assets and liabilities
and their respective tax bases. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The major temporary differences are net operating losses. Due to historical losses on the accrual basis, the related tax assets are not recorded in our financial statements.
Stock Options
We have an undertaking to grant to our CEO, under a stock option plan to be adopted by us for that purpose, a total of 259,051 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 4% of our outstanding share capital prior to the registration). In
addition we currently have available for additional grant to our employees, subject to the approval of our Board, up to 129,525 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 2% of our outstanding share capital prior to the registration). Unless agreed otherwise by the Board the granted options shall generally be equally vested over a period of 3 years such that at the end of each year 1.33% of the entire amount of granted options
shall become vested.
The options to our Israeli employees may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the shares of Common Stock issued upon their exercise must be deposited with a trustee for at least two years following the end of the calendar year in which the options are granted. Under Section 102
(1) any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or the shares by the trustee to the employee or upon the sale of the options or ordinary shares, (2) gains are subject to capital gains tax of 25%; under the tax rules governing these options, we do not receive a tax deduction in respect of the issuance or exercise of the options.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet transactions.
Dividend Policy
We follow a policy of retaining earnings, if any, to finance the expansion of our business. We have not paid, and do not expect to declare or pay, cash dividends in the foreseeable future.
There are no pending legal proceedings to which we are a party is or in which to our knowledge any director, officer or affiliate of the Company, any owner of record or beneficially of more than 5% of any class of voting securities of the Company, or security holder is a party adverse to the Company or has a material interest adverse to
the Company. The Company's property is not the subject of any pending legal proceedings.
50
The following table lists, as of October 16, 2009 the number of shares of Common Stock beneficially owned by (i) each person or entity known to our Company to be the beneficial owner of more than 5% of the outstanding common stock; (ii) each officer and director of our Company; and (iii) all
officers and directors as a group. Information relating to beneficial ownership of common stock by our principal shareholders and management is based upon information furnished by each person using "beneficial ownership" concepts under the rules of the Securities and Exchange Commission. Under these rules, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or direct the voting of the security, or investment
power, which includes the power to vote or direct the voting of the security. The person is also deemed to be a beneficial owner of any security of which that person has a right to acquire beneficial ownership within 60 days. Under the Securities and Exchange Commission rules, more than one person may be deemed to be a beneficial owner of the same securities, and a person may be deemed to be a beneficial owner of securities as to which he or she may not have any pecuniary beneficial interest. Except
as noted below, each person has sole voting and investment power.
|
Lior Carmeli, CEO |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
David Kapon, CFO |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Jacob Bal, CTO (3) |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Yossef De-Levie, Director – Chairman of the Board (4) (12) |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Adi Plaschkes, Director (5) |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Dr. Asher Kimchi, Director |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Gilad Golan, Director (6) (11) |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Alon Zifroni, Director (7) |
0 | 0 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Dr. Israel Amirav (13) |
331,600 | 5.12 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Assaf Halamish, Director (8) |
706,600 | 10.91 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Granot Development Enterprises Ltd. (9) |
543,400 | 8.39 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Life Support, Ltd. (5) (6) (10) |
1,690,600 | 26.10 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Ramport Financial (6) (11) |
706,000 | 10.90 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
Microdel, Ltd. (4) (7) (12) |
1,706,600 | 26.35 | % | |||||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
||||||||
|
TOTAL |
5,684,800 | 87.78 | % | |||||
51
|
All directors, named executive officers, and persons owning 5% or more of New Air’s stock have sole voting and investment power with respect to the shares listed. | |
|
Except for our CEO, no director, named executive officer, or persons owning 5% our more of New Air's stock has any rights to acquire any shares from options, warrants, rights, conversion privileges or similar obligations. | |
|
(3) |
Jacob Bal, the Company’s CTO is also the CTO of Microdel Ltd., which owns 1,706,600 shares of New Air, Inc. or 26.35% of the outstanding shares and is its largest single shareholder. Mr. Bal owns none of the outstanding shares of Microdel, Ltd. and is therefore not attributed to owning any of the 1,706,600 common shares
of New Air, Inc. owned by Microdel Ltd. |
|
(4) |
Yossef De Levie, the Company’s Chairman of the Board is also the founder and the Chairman of the Board of Microdel Ltd., which owns 1,706,600 shares of New Air, Inc. or 26.35% of the outstanding shares and is its largest single shareholder. Mr. De Levie owns 83,796 of the outstanding shares or 29.8% of its total outstanding shares
and is therefore attributed to owning all of the 1,706,600 common shares of New Air, Inc. owned by Microdel Ltd. |
|
(5) |
Adi Plaschkes, a member of the Board of Directors is the owner of Life Support Ltd., an Israeli company that develops nuclear, biological and chemical products, which owns 1,690,600 shares of New Air, Inc. or 26.10% of the outstanding shares and is its second largest single shareholder. Mr. Plaschkes owns all of the outstanding
shares of Life Support’s total outstanding shares and is therefore attributed to owning all of the 1,690,600 shares of New Air, Inc. owned by Life Support Ltd. From April 2004 to July 2009 Life Support, Ltd. provided us with management services. |
|
(6) |
Mr. Gilad Golan, a member of the Board of Directors of our Company from 2002 to 2008 was the General Manager of Life Support Ltd., an Israeli company that develops nuclear, biological and chemical products, which owns 1,690,600 shares of New Air, Inc. or 26.10% of the outstanding shares and is its second largest single shareholder. Mr.
Golan owns none of the outstanding shares of Life Support’s total outstanding shares and is therefore not attributed to owning any of the 1,690,600 shares of New Air, Inc. owned by Life Support Ltd. From April 2004 to July 2009 Life Support, Ltd. provided us with management services. In addition, Mr. Golan represents officially the interest of Ramport Financial, a private investor unrelated to any other shareholder. They are located in the Virgin Islands and their mailing address in
Israel is Mr. Zvika Sherf, 18 David Avidan Street, Tel Aviv, Israel 69086. Mr. Golan owns none of the outstanding shares of Ramport Financial and is therefore not attributed to owning any of the 706,000 common shares of New Air, Inc. owned by Ramport Financial. |
|
(7) |
Alon Zifroni, a member of the Board of Directors of our Company is also the CEO of Microdel Ltd., which owns 1,706,600 shares of New Air, Inc. or 26.35% of the outstanding shares and is its largest single shareholder. Mr. Zifroni owns none of the outstanding shares of Microdel, Ltd. and is therefore not attributed to owning any
of the 1,706,600 common shares of New Air, Inc. owned by Microdel Ltd. |
|
(8) |
Mr. Halamish, was one of the founders of Baby’s Breath and from 2001 to 2005, he served as CEO of our subsidiary Baby’s Breath. |
|
(9) |
Granot Development Enterprises Ltd. Registration No. 511664260 ("GAPI") is an Israeli limited liability corporation who received in July 2008 a franchise from the Israeli Office of Chief Scientist ("OCS") to operate as a privatized
technological incubator. GAPI is held 100% by BBOOG Ltd., who is held 75% by OZ M.E.H Ltd Registration No. 513844852 (wholly owned by Ori Katz Oz), and 25% by Mygar Holdings and Information Systems Ltd. Registration No. 511854994 (owned by Granot Cooperative). GAPI is not related to any of the other shareholders. |
|
(10) |
In April 2004 Baby's Breath entered into an investment agreement with Life Support Ltd., one of our shareholders. Adi Plaschkes, a member of the Board of Directors is the owner of Life Support Ltd., an Israeli company that develops nuclear, biological and chemical products which owns 1,690,600 shares of New Air, Inc. or 26.10%
of the outstanding shares and is its second largest single shareholder. Mr. Plaschkes owns all of the outstanding shares of Life Support’s total outstanding shares and is therefore attributed to owning all of the 1,690,600 shares of New Air, Inc. owned by Life Support Ltd. Mr. Gilad Golan, a member of the Board of Directors of our Company from 2002 to 2008 was the General Manager of Life Support Ltd., Mr. Golan owns none of the outstanding shares of Life Support’s
total outstanding shares and is therefore is not attributed to owning any of the 1,690,600 common shares of New Air, Inc. owned by Life Support Ltd. |
|
(11) |
Ramport Financial is a private investor unrelated to any other shareholder. They are located in the Virgin Islands and their mailing address in Israel is Mr. Zvika Sherf, 18 David Avidan Street, Tel Aviv, Israel 69086. Mr. Gilad Golan, our Board member represents officially the interest of Ramport Financial. Mr.
Golan owns none of the outstanding shares of Ramport Financial and is therefore not attributed to owning any of the 706,000 common shares of New Air, Inc. owned by Ramport Financial. |
|
(12) |
Microdel Ltd., owns 1,706,600 shares of New Air, Inc. or 26.35% of the outstanding shares and is its largest single shareholder. Yossef De Levie, the Company’s Chairman of the Board is also the founder and the Chairman of the Board of Microdel Ltd. Mr. De Levie owns 83,796 of the outstanding shares or 29.8 % of its total
outstanding shares and is therefore attributed to owning 1,706,600 of New Air, Inc. |
|
(13) |
Dr. Israel Amirav is one of our founders. |
52
There are no arrangements currently in place which may result in a change of control of New Air.
We believe that all persons named above have full voting and investment power with respect to the shares indicated, unless otherwise noted in the table. Under the rules of the Securities and Exchange Commission, a person (or group of persons) is deemed to be a "beneficial owner" of a security if he or she, directly or indirectly,
has or shares the power to vote or to direct the voting of such security, or the power to dispose of or to direct the disposition of such security. Accordingly, more than one person may be deemed to be a beneficial owner of the same security. A person is also deemed to be a beneficial owner of any security, which that person has the right to acquire within 60 days, such as options or warrants to purchase our common stock.
|
40 |
|||||
|
Ha'hadas Street , Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
40 |
|||||
|
Ha'hadas Street , Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Jacob Bal |
54 |
Chief Technology Officer | |||
|
Ha'hadas Street , Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Yossef De-Levie |
58 |
Director - Chairman of the Board | |||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Adi Plaschkes |
53 |
Director | |||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Dr. Asher Kimchi, MD |
63 |
Director | |||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Gilad Golan |
57 |
Director | |||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Alon Zifroni |
41 |
Director | |||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
|
Assaf Halamish |
58 |
Director | |||
|
Ha'hadas St., Bldg. #5 North Industrial Area, P.O.Box 42, Or-Akiva 30600, Israel |
|||||
There are no family relationships between any of our directors or executive officers. Our executive officers are appointed by our board of directors and serve at the board’s discretion. There is no arrangement or understanding between any of our directors or executive officers and any other person pursuant to
which any director or officer was or is to be selected as a director or officer.
53
Officers
LIOR CARMELI – CHIEF EXECUTIVE OFFICER
Mr. Carmeli, became the Chief Executive Officer of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he served in that capacity since June 17, 2009. From 2006 to 2009 he worked as Division Director & VP Sales & Marketing for Polysack Plastic Ind Ltd, Israel – with sales
of above 100 millions NIS where he was head of the strategic planning process of the division focused on Marketing & Sales, responsible for all the sales & marketing activity in the agricultural division, including four subsidiaries (USA, MX, Spain & Brazil), export form Israel and the Israeli market and where he lead the product development team, agricultural research & the technological R&D. From 1996 to 2006 he worked at various positions with Makhteshim Agan Ind Ltd, among the
five largest industrial companies in Israel & eight largest agrochemical companies worldwide. From 2000 to 2005 he worked as Senior VP & Director of Finance for MAI of North America. NYC ($ 125 mm sales, 40 employees) where he was responsible for Management & leadership of the overall company activity with direct responsibility for the supply chain, Finance, IT & departments. Sales & marketing activity – part of a team leading a company change – “go direct to distributor”. Management
of customer service, logistics, toll manufacturing, warehousing, freight, import, distribution and planning departments. In charge of work plan process, financial reports, cash flow management, web-based expense & sales reporting & analysis, transfer pricing management & custom and tax planning. From 1999 to 2000 he worked as Asian desk sales manager for MAI Israel where he was in charge of regional sales team, involved in negotiation with global and local customers, customer service, work
plans & market development, Focusing on the Japanese and Korean markets. From 1996 to 1999 he worked as Assistant to VP Sales, Marketing and M&A. for MAI Israel where he negotiated global agreements with large customers and suppliers, involved in the work plan process, evaluated acquisitions prospects and was in charge of presentation preparations and investor and public relations. Mr. Carmelli has a BA in Economics & Business Management, Hebrew University, Israel, 1996.
DAVID KAPON – CHIEF FINANCIAL OFFICER
Mr. Kapon, became the Chief Financial Officer of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he served in that capacity since June 2009. Mr. Kapon has 15 years extensive financial management experience. He was the financial manager of Negev Ceramics, a company traded on the TASE (Tel
Aviv Stock Exchange) for six years. Currently he serves as a financial advisor to StarKist Food d'Or. Mr. Kapon also has vast experience in company valuation and business plan preparation of companies in USA, Europe and Israel. Mr. Kapon holds a M.A and B.A in Economics from Tel Aviv University.
JACOB BAL – CHIEF TECHNOLGY OFFICER
Mr. Bal became our Chief Technology Officer with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was their Chief Technology Officer since April 2006. Since 2006 he has been the Chief Technology Officer of Microdel Ltd. a privately owned technology company focusing on finding inventions, and turning
them into a product, including its marketing, where he manages all their projects, and serves on their Idea Section Board as well as manages all their intellectual properties. From 2003 to 2006 he was the Senior Project Manager at NUR Macroprinters, responsible for the management of new foundations of ink plant movement from USA to Israel. From 2002 to 2003 he was the Senior Project Manager at E.G Skarim, Managing 50 people in a railroad and public transportation passengers traveling
behaviors survey where he was responsible for all project aspects: planning, questioners and data entree, reporting, managing daily routines. In 2002 he was the Manager of cellular operations for Elgadfon Ltd., responsible for Testing and installation of cellular Radio Base Station for leading cellular companies, all over the country, Managing 20 people. In 2002 he was also V.P Marketing Sales and business development for Shamrad, developing their business plan local and abroad. From
1996 to 2000 he worked for IBM in various roles including Help-Desk manager and business development manager and Segment Marketing & Business Development Manager: In 1995 he worked at Galcom Communication as Sales and New Technologies Manager. From 1981 to 1995 he worked at Scitex Corporation where from 1990 to1995 he was their Technical Manager International Customer Support. From 196 to 1989 he worked Scitex Corp.
USA as Senior specialist National Customer Support Specialist and from 1984 to 1985 he worked for Scitex Corp. USA, as Manager of Initializing and Operation Repair Lab. From 1981 to 1984 he worked for Scitex Corp. Israel as Team Leader Repair Lab.
54
Board of Directors
YOSSEF DE-LEVIE – DIRECTOR – CHAIRMAN OF THE BOARD
Mr. De Levie, became a member of the Board of Directors of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was a director since February 15, 2008. He is an industrialist, an inventor, and an entrepreneur, with vast experience in executive management, product development and manufacturing. Since
2004 he is associated with Microdel Ltd. a holding company that he founded and is its Chairman of the Board. Microdel Ltd. is a company that is a bridge between inventions and the marketplace. Microdel Idea Center Ltd., a subsidiary of Microdel founded by him in 2005, is an Israeli Idea Bank and incubator platform. Microdel seeks ideas, innovations and patents, to invest in ideas and/or ventures which they believe have great potential, turning them into market-ready and patent
protected products either to be sold or licensed globally via a network of distribution, retail chains, and strategic partners worldwide. From 1996 to 2009, Together with Stef Wertheimer (a leading industrialist in Israel, and founder of Iscar, entrepreneur of a number of industrial parks in Israel), and the inventor Ehud Nagler, he established a start-up that was to become "Tynat Hydro Industries", The company was commercialized in 2000 when the Zvi Yemini group joined the company which has successfully
developed a unique technology for water powered propulsion. As a shareholder and founding member, Mr. De Levie served for a number of years as a member of the Board of Hydro Industries. From 1987 to 2004 he was the CEO of Games and Sports Ltd. – a playground equipment manufacturer – a company he founded in 1987. He was involved in all aspects of product initiation and design, manufacture and marketing. A
leading Israeli and European manufacturer of playground equipment with a range of hundreds of products was partially acquired by Gaon-Holdings in 2002 and sold outright to the Gaon Group in 2004. From 1986 to 2004 he was the owner and manager of a private construction and installation company.
ADI PLASCHKES – DIRECTOR
Mr. Plaschkes became a member of the Board of Directors of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was a director since 2003. Since 2006 he has been the Co-founder & CEO of ActiVein Ltd., an R&D start-up that is developing a dual-action PIV catheter that enables both,
fluid infusion and blood withdrawal from the same vein. From 2002 to 2006, he was the Founder and technical manager of Life Support Ltd., a private company specializing in design and production projects in the NBC and medical devices products. From 1996 to 2002 he was a partner and technical manager of Elad Engineering Ltd., a private company active in R&D, prototyping and production of multidisciplinary projects. From 1989 to 1996 he was the manager of Superweld Ltd., a division
of Supergum group, a manufacturer of chemical protection masks and garments for civil defense and military gear for IMOD and army applications. From 1983 to 1989 he a designer of small arms in the IMI (Israeli Military Industries) last position Team head of the “Negev” Light Machine Gun. The “Negev” is in serve in the IDF from 1995 to the present. Mr. Plasckes has served as a member of the Board in ATX Ltd. an Israeli public company in the textile field
from 1997 to 2001. Since 1998 to the present he serves as a member of the Board of “Neovasc Ltd.” - Medical device field. Cardiologic device development, a company he co-founded. M. Plaschkes graduated in 1982 with a B.Sc. in Mechanical Engineering from Tel Aviv University.
DR. ASHER KIMCHI, MD – DIRECTOR
Dr. Kimchi, became a member of the Board of Directors of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was a director since May 5, 2009. He is an internationally recognized cardiologist. He is an Attending Physician and Vice-Clinical Chief in the Division of Cardiology
at Cedars-Sinai Heart Institute in Los Angeles, California, USA. Dr Kimchi is a Clinical Professor of Medicine in the David Geffen School of Medicine at UCLA. He is also the Founder and Chairman of the International Academy of Cardiology, and is Past-President of the American Heart Association, Los Angeles County Division.
Dr. Kimchi attended and received his medical degree from the Hebrew University, Hadassah Medical School in Jerusalem. He then joined the Israeli Air Force as a Flight Surgeon and subsequently was promoted to be the commander of the Israeli Aero Medical Evacuation Unit, achieving the military rank of Major. He completed his Cardiology
fellowship at Hadassah Medical Center in Jerusalem, before completing a residency in Internal Medicine at the University of California, Davis. At Cedars-Sinai Medical Center, Dr Kimchi concluded two fellowships: Cardiology-Clinical Research, and Critical Care. In 1983 he became Attending Physician in the Division of Cardiology at Cedars-Sinai Medical Center. He is also Clinical Professor of Medicine at UCLA and a fellow of the American College of Cardiology, American College of Physicians and American Heart Association.
Over the past 30 years, Dr. Kimchi has been actively involved in many clinical research projects, dealing predominantly with diagnosis and treatment of coronary artery disease and congestive heart failure. Dr. Kimchi is editor of eight cardiology books and his scientific articles have appeared in many prestigious peer review journals. He also serves on the editorial boards of leading cardiology journals. Dr. Kimchi is the Founder and
Chairman of the International Academy of Cardiology and over the past 20 years has served as chairman of 16 large-scale International Congresses on Heart Failure and Heart Disease. Dr. Kimchi has the distinction of being listed in “America’s Top Physicians” (2002-2006), “America’s Top Cardiologists” (2007-2009) “Best Doctors in America” (2009-2010) and was featured in Los Angeles Magazine in December 2008 as one of “Southern California Super Doctors”
in the field of Cardiology. Dr. Kimchi is a passionate community activist and has been involved in multiple philanthropic projects to fight Heart Disease. He has chaired or co-chaired many successful fundraising events. He has served as co-Chair of Medical Friends of Hadassah, Board Member of the American Friends of Israel’s Heart to Heart Foundation, Board member of the Save-A-Heart Foundation, and Member of the Medical Advisory Board of the Heart Fund of Cedars-Sinai Medical Center. He is currently Board
Member of the American Heart Association, Los Angeles County Division. Dr Kimchi is a member of the Executive Committee of the American College of Cardiology (ACC), California Chapter and Vice-Chairman of the Foundation for the Prevention of Cardiovascular Disease and Stoke of the ACC California Chapter. In 2006, Dr. Asher Kimchi was honored by the American Heart Association and received the prestigious “Passion of the Heart Award” for "distinguished career in cardiology, and lifetime dedication and
leadership in cardiovascular education worldwide.”
55
GILAD GOLAN - DIRECTOR
Mr. Golan, became a member of the Board of Directors of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was a director since April 14, 2004. From 2006 to 2009 he was Baby’s Breath, our subsidiary’s General Manager. From 2008 to 2009 he was the General Manager
of Mpertec Industries Ltd., an Israeli company that develops nuclear, biological and chemical products. From 2002 to 2008 he was the General Manager of Life Support Ltd., an Israeli company that develops nuclear, biological and chemical products. From 1970 to 2002, her served as a Colonel in the IDF, Israel’s military, overseeing the nuclear, biological and chemical industry in Israel, with a market size of three Billion Shekels, working in close contact with Government offices and professional
bodies and was directly in charge of hundreds of soldiers and officers. Mr. Golan has a BA in Political Science and Criminology from Bar Ilan University and a MS in International relations from Haifa University, both in Israel.
ALON ZIFRONI – DIRECTOR
Mr. Zifroni, became a member of the Board of Directors of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was a director since February 15, 2008. Since 2008, he has served as Chief Executive Officer of Microdel Ltd. a privately owned technology company focusing on finding inventions,
and turning them into a product, including its marketing. Microdel manages approximately 15 different ventures simultaneously, and holds 6 subsidiaries. From 2006 to 2007, he was the CEO of AGR, a Start-Up developing financial products, where he Managed the company and its employees, including the development, sales & support teams. Business Development and daily management of the company. From 1999 to 2006 he was the Board Member & Senior Project Manager at Seker Consulting
company where he was served in management and project consulting for IT projects in Israel and world wide. From 1998 to 1999 he was System Administrator at Intel Semiconductors in Kiriat Gat. From 1995 to 1998 he was the Shift Manager at the Kafrit Industries Factory where he managed & supervised the team, including supervision of the daily tasks of creation and control for the quality of the product. Mr. Zifroni has
a B.Sci in Industrial Engineering & Management, focusing on Information System, from Ben Gurion University, Beer Sheva, Israel.
ASSAF HALAMISH – DIRECTOR
Mr. Halamish, became a member of the Board of Directors of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was a director since 2004. He has extensive management experience in the development of new products and was the CEO and co-owner of a medical devices start-up. Previously he
was director and manager of engineering for one of Israel’s largest R&D companies. He has many years of experience in managing research and development projects in Israel and abroad and has served as a business development consultant to several Israeli high-tech companies. Mr. Halamish has special expertise in plastic products for medical applications. Since 2006 he is the Vice President and R&D Manager for Cell Kinetics – Global Park, Lod, Israel, a subsidiary of Medis Technologies (NASDAQ;
MDTL) dedicated to the design, development and marketing of life science and medical device products. The primary product line is a family of next-generation cytometric devices. From 2005 to 2006, he served as COO and VP of Operations for Scent Detection Technologies – Industrial Park, Herzliya, Israel, a start up company in the security sector producing a product for detection of explosives where he was Responsible for transition from development to production, quality assurance, testing and
regulation. From 2001 to 2005, he served as CEO of our subsidiary Baby’s Breath. From 1999 to 2001 he was self employed with Technosaf, Caesarea Industrial Park, Israel, where he was involved in product development and consultation to other firms in the medical field and other technically oriented fields. From 1997 to 1999 he was Head of Engineering, Design and R&D for Aran Research and Development, Caesarea Industrial Park, Israel, the largest R&D company
in Israel. Most of its products are high-tech, specifically in medical technology, irrigation systems, and for the automotive industry. From 1996 to 1997 he was Project Manager for medical products at Influence, Ramat Gan, Israel. From 1992 to 1996 he was Project Manager in the platics, medical and automotive fields for Aran Research and Development, Caesarea Industrial Park, Israel, the largest R&D company in Israel. From 1989 to1992 he was R&D Project
Manager responsible for the development of new technology at Plasson Plastic Products, Kibbutz Maagan Michael, Israel. From 1988 to1989 he was Project Manager at Richco Products, Chicago, Il, USA,a plastic company in the field of electronics. From 1987 to 1988 he was Project Manager at Suncast Corporation, Batavia, Il, USA, an extrusion and injection plastics factory.Mr. Halamish holds a degree in mechanical engineering from the Technion – Israel Institute of Technology (Haifa) and an MBA from
ISEMI, Swinburne, Australia.
Directors’ Compensation
None of our directors received any compensation as a director to date.
Corporate Governance
We currently have six active non-employee members of the board of directors, Yossef De Levie, Adi Plaschkes, Dr. Asher Kimchi, Gilad Golan, Alon Zifroni, and Assaf Halamish are each considered independent directors, as defined in Nasdaq Marketplace Rule 4200.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our executive officers and directors, and persons who beneficially own more than 10.0% of a registered class of our equity securities to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and
annual reports concerning their ownership of our common shares and other equity securities, on Forms 3, 4 and 5 respectively. Since prior to this offering, we did not have a class of equity securities registered pursuant to Section 12 of the Securities Exchange Act of 1934, as amended, we were not required to file such forms with the Securities and Exchange Commission. We do not intend to register a class of our securities on a national securities exchange before this registration statement is effective. Once
we have a class of equity securities registered pursuant to Section 12 of the Securities Exchange Act of 1934, as amended, we intend on filing all such forms in a timely manner and if not, to disclose any untimely filings in accordance with Item 405 Regulation S-K.
56
Code of Ethics
In September 2009, our board of directors adopted a Code of Ethics which is applicable to all of our officers, directors and employees.
Involvement in Certain Legal Proceedings
To our knowledge, our directors, executive officers have not been involved in any of the following events during the past five years and which is material to an evaluation of the ability or the integrity of our directors or executive officers:
|
1. |
any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
|
2. |
any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
|
3. |
being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; and |
|
4. |
being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Audit Committee Financial Expert
None
The following table sets forth the compensation paid by us from inception on July 2009 through September 15, 2009, to our officers and directors. The information includes the dollar value of base salaries, bonus awards and number
of stock options granted, and certain other compensation, if any.
|
Lior Carmeli
Chief Executive Officer (1) |
$0 |
$0 |
$0 |
$0 |
|||||
|
David Kapon
Chief Financial Officer (2) |
$0 |
$0 |
$0 |
$0 |
|||||
|
Yaakov Bal
Chief Technology Officer (3) |
$0 |
$0 |
$0 |
$0 |
|||||
57
There are no other stock option plans, retirement, pension, or profit sharing plans for the benefit of our sole officer and director other than as described herein.
(1) On July 1, 2009 Mr. Carmeli entered into an employment Agreement with Baby’s Breath Ltd, our subsidiary which we assumed in its acquisition on September 7, 2009 when we acquired Baby’s Breath, and Mr. Carmeli became our CEO. Under said Agreement,
his employment commenced on June 17, 2009, with either party can terminate on a 30 days advance notice. For his services Mr. Carmeli is being paid 32,000 NIS which is about $8,000 US per month, being paid 12,000 NIS or about $3,190 US as long as we are not publicly traded with the higher amount and the balance accrued to be paid once we are publicly traded. In addition, Mr. Carmeli receives insurance benefits, an educational fund, vacation pay, a company car, and reimbursement of Company
related expenses.
(2) On July 1, 2009 Mr. Kapon entered into an employment Agreement with Baby’s Breath Ltd, our subsidiary which we assumed in its acquisition on September 7, 2009 when we acquired Baby’s Breath, and Mr. Kapon became our CFO. Under said Agreement, his
employment commenced on June 1, 2009, with either party can terminate on a 60 days advance notice. For his services Mr. Kapon is being paid 15,000 NIS which is about $3,750 US per month based on 90 hours per month work. If Mr. Kapon is required to work additional hours per month, he will receive a proportional additional salary, which is being paid 7,500 NIS or about 1,994 US until December 2009 and as long as we are not publicly traded with the higher amount and the balance accrued from
the date of our filing to be paid once we are publicly traded. In addition, Mr. Kapon receives insurance benefits, an educational fund, vacation pay, half the costs of a company car, and reimbursement of Company related expenses.
(3) On July 1, 2009 Mr. Bal entered into an employment Agreement with Baby’s Breath Ltd, our subsidiary which we assumed in its acquisition on September 7, 2009 when we acquired Baby’s Breath, and Mr. Bal became our CTO. Under said Agreement, his employment
commenced on July 1, 2009, with either party can terminate on a 30 days advance notice. For his services Mr. Bal is being paid 6,000 NIS which is about $1,500 US per month, being paid 2,000 NIS or about $532 US as long as we are not publicly traded with the higher amount to be paid once we are publicly traded. In addition, Mr. Bal receives insurance benefits, an educational fund, vacation pay, half the costs of a company car, and reimbursement of Company related expenses
(4) Mr. Lior Carmeli, our CEO, has a right to receive options exercisable into 4% of our total outstanding shares as of the date hereof which is equal to 259,051 common shares which unless agreed otherwise by the Board shall be exercisable over a three year period as long
as he is employed with us.
Discussion of Compensation
Our board of directors currently evaluates and sets the compensation policies and procedures for our executive officers but as soon as established, this function will be performed by a compensation committee composed solely of independent directors. Except as provided for in the employment agreements described below, annual reviews generally
determine future salary and bonus amounts for our executive officers, as a part of the Company’s compensation procedures.
We have an undertaking to grant to our CEO, under a stock option plan to be adopted by us for that purpose, a total of 259,051 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 4% of our outstanding share capital prior to the registration).
In addition we currently have available for additional grant to our employees, subject to the approval of our Board, up to 129,525 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 2% of our outstanding share capital prior to the registration). Unless agreed otherwise by the Board the granted options shall generally be equally vested over a period of 3 years such that at the end of each year 1.33% of the entire amount of granted
options shall become vested.
The options to our Israeli employees may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the shares of Common Stock issued upon their exercise must be deposited with a trustee for at least two years following the end of the calendar year in which the options are granted. Under Section 102
(1) any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or the shares by the trustee to the employee or upon the sale of the options or ordinary shares, (2) gains are subject to capital gains tax of 25%; under the tax rules governing these options, we do not receive a tax deduction in respect of the issuance or exercise of the options.
58
Family Relationships
Not applicable.
Significant Employees
David Kapon is our CFO since the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he served in that capacity since June 2009.
Jacob Bal is our CTO since the acquisition of Baby’s Breath Ltd., in September 7, 2009, where he was their Chief Technology Officer since April 2006.
We do not have an audit committee financial expert nor do we have an audit committee established at this time.
Auditors; Financial Expert
Our principal PCAOB independent auditors are Schwartz Levitsky Feldman LLP, Toronto, Ontario, Canada, members of HLB international. We do not have an audit committee or nominating committee.
Advisory Board
The name, address, age, of our present Advisory Board is set forth below:
|
Name and Address |
Age |
Position(s) | |||
|
Dr. Avigdor Mandelberg, MD |
56 |
Member Advisory Board | |||
|
Sackler Faculty of Medicine, Tel Aviv University, Ramat Aviv, Israel 69978 |
|||||
|
Dr. David E. Geller, MD |
53 |
Member Advisory Board | |||
|
Florida State University College of Medicine, 1115 West Call St., Tallahassee, Florida 32306 |
|||||
|
Dr. Israel Amirav, MD |
56 |
Member Advisory Board | |||
|
Sieff Government Hospital, P.O. Box 1008, Safed, Israel |
|||||
|
Dr. David Groshar, MD |
52 |
Member Advisory Board | |||
|
Department of Nuclear Medicine, Rabin Medical Center, 39 Jabotinski St., Petah Tikva, Israel 49100 |
|||||
59
Background of Our Officer and Director
DR. AVIGDOR MANDELBERG, MD – MEMBER OF THE ADVISORY BOARD
Dr. Mandelberg, became a member of the Advisory Board of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009. From 1988 to the present he has served as the Director of the Pediatric pulmonary Unit at the E. Wolfson Medical Center, in Holon, Israel. In addition since 1988 to the present, he has served
as Senior-Lecturer in Medicine, Sackler Faculty of Medicine, Tel Aviv University, Pediatric Department, The E. Wolfson Medical Center, Holon, Israel. Since 2005 he holds the title of Senior-Lecturer in Medicine, Sackler Faculty of Medicine, Tel Aviv University. Pediatric Department, The E. Wolfson Medical Center, Holon, Israel. In 1990 Dr. Mandelberg, served was part of the Sackler Faculty of Medicine, Tel Aviv University, in the Postgraduate Course, Family Medicine. He lectures in Pulmonary
Function Tests and Pulmonary medicine. Dr. Mandelberg has extensive clinical experience. From 1977 to 1978 he served in a Rotating Internship, at Sheba Medical Center, and the Sackler Faculty of Medicine, Tel Aviv University. From 1978 to date, he served in the Israeli Military and is in the Reserves as a Flight Surgeon, Israel Medical Corps. From 1983 to 1987 he was a Resident, in the Department of Pediatric Hadassah Hospital, Mount Scopus and Kiryat Hadassah, Hebrew
University, Jerusalem, Israel. In1986 he did a Clinical and Research Fellow, Pediatric Pulmonary Laboratory and Service, Hadassah Hospital, Mount Scopus, Hebrew University, Jerusalem, Israel. In 1990 he received his medical license in Israel as a Specialist in Pulmonary Medicine. From 1990 to1993 he was the Senior Pulmonologist, at The E. Wolfson Medical Center, Holon, Israel. Department of Pulmonary Medicine. From 1992 to 1993he was the physician in charge, Pulmonary
Function Test Lab, The E. Wolfson Medical Center, Holon, Israel, In 1993 he received his medical license in Israel as a Specialist in Pediatric Pulmonary Medicine. In 1994 he did a Clinical and research Fellowship (3 month), Pediatric Pulmonology and Pulmonary unit Hadassah Hospital, Kiryat Hadassah, Hebrew University, Jerusalem, Israel. In 1994 to 1976 he was the Director, Pediatric pulmonary service, The E. Wolfson Medical Center, Holon, Israel. Since 1997-he has served as the
Director, Pediatric pulmonary Unit, The E. Wolfson Medical Center, Holon, Israel. Dr. Mandelberg is a member of the Israel Medical Association. Israel Pediatric Society, Israel Pediatric Pulmonary Medicine Association, Israel Pulmonary Medicine Association, European Respiratory Society, and the American Thoracic Society. From 1984 to 1987 he was an Instructor in Medicine, Pediatric Departments, Hebrew University,
Hadassah Hospital, Mount Scopus and Ein-Carem, Jerusalem, Israel. Active teaching medical students, and lecturing in Pediatric - Pulmonology, Cardiology, and Endocrinology. In 1996, he an Instructor in Medicine, Sackler Faculty of Medicine, Tel Aviv University. Pediatric Department, The E. Wolfson Medical Center, Holon, Israel. In 1986 he served as Instructor for pediatric residents in Pediatric Pulmonology, Hebrew University, Hadassah Hospital, Jerusalem, Israel. Dr.
Mandelberg graduated in 1978 with an M.D. degree from The Hebrew University, Hadassah Medical School, Jerusalem, Israel. In addition in 1990 he has attended Post Graduate courses in Research and Statistics at the Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel. He holds a medical license in the State of Israel.
DR. DAVID E. GELLER, MD – MEMBER OF THE ADVISORY BOARD
Dr. Geller, became a member of the Advisory Board of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009. Since 2007 he is an Associate Clinical Professor at the University of Central Florida School of Medicine, Orlando, Florida. Since 2002 he is a Clinical Assistant Professor, Florida
State University School of Medicine, Tallahassee, Florida. Since 1997 he works as a Pediatric Pulmonologist at Nemours Children Clinic, Orlando, Florida. From 1988 to 1997 he served as Clinical Assistant Professor of Pediatrics at the University of South Florida, All Children’s Hospital, St. Petersburg, Florida. From 1986 to 1988 he served as Assistant Professor of Pediatrics at Loma Linda University Medical Center, Loma Linda, California. From 1985 to 1986 he served as
Clinical Instructor in the Department of Pediatrics, at the University of Arizona Health Science Center, Tucson, Arizona. In addition Dr. Geller, is the Director, Aerosol Research Laboratory, NCC-O 1999 to the present, and the Director, Cystic Fibrosis Center, NCC-O 1997 to the present. He is certified as a Diplomat, American Board of Pediatrics, and as a Diplomat, American Board of Pediatrics Pulmonology Subspecialty. He is a member of the American Thoracic Society, European
Respiratory Society, American Association for Respiratory Care, American Medical Association, International Society for Aerosols in Medicine, Florida Medical Association and the Orange County Medical Society. He is a member of the Florida Institutional Review Board. Nemours Children’s Clinic, 2007 to the present. He was Task Force Member, NIH- National Institute of Childhood Health and Human Development: New Technology and Drug Delivery Systems, 2005 to 2007; Chairman, Clinical Research
Review Committee, NCC-O 1997 to 1998; and Member, North American Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF). Sponsor: Genentech, Inc. 1993 to 1998. Dr. Geller received in 1977 his B.A., Biology, from Washington University, St. Louis, Missouri; in 1980 his M.D., from University of Iowa School of Medicine, Iowa City, Iowa; from 1980 to 1983 his internship and residency in Pediatrics, at UCLA Hospital and Clinics; Los Angeles, California; and from 1983-1986
his Fellowship, Pediatric Pulmonology at the Univ. of Arizona Health Sciences Center; Tucson, Arizona.
DR. ISRAEL AMIRAV, MD – MEMBER OF THE ADVISORY BOARD
Dr. Amirav, became a member of the Advisory Board of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009. Since 2004 he has been the Attending Physician and Consultant in charge of the Pediatric Pulmonary Unit at Sieff Government Hospital, Safed, Israel and as the Attending Physician and Senior
Consultant, in charge of the Pediatric Pulmonary Unit for Kupat Holim National Health System in Northern Israel. In addition since 2005 he teaches as a Lecturer in Continuing Medical Education for Family physicians, at Sherutee Briute Clalit; since 2003 he teaches as a Lecturer: Continuing Medical Education School for pediatricians and family physicians at Sackler School of Medicine, Tel Aviv, Israel; since 1997 he teaches Pediatrics at the Technion School of Medicine, Pediatric Department, Sieff Government Hospital
, Safed, Israel; and since 1997 to the present he teaches Pediatrics Pulmonology at the Technion School of Medicine, 5th and 6th year medical students rotations, 6th year “mini-internship” rotation, Pediatric Department, Sieff Government Hospital , Safed, Israel. In
1995 Dr. Amirav taught as a Visiting consultant in pediatrics at the University of Hawaii, Okinawa, Japan. From 1993 to 1994 he was an Instructor of Pediatrics at the University of Pennsylvania, School of Medicine, and a Junior Faculty in the Division of General Pediatrics at Children’s Hospital of Philadelphia. From 1990 to 1993he worked at Children's Hospital of Philadelphia, Philadelphia, PA, as a Fellow, in the Division of Pulmonary Medicine. From 1989 to 1990 he worked
at Sieff Government Hospital, as a Pediatric Consultant, in charge of the Pediatric Pulmonary Clinic. From 1987 to 1988 he worked at Sieff Government Hospital, Safed, as a Pediatric Resident. In 1987 he worked at Child Health Clinics, Municipality of Jerusalem. From 1985 to 1986 he worked at Hadassah University Hospital, Jerusalem, as a Pediatric Resident. From 1983 to 1984 je worked at J G Stridom Hospital, Johannesburg, as a Fellow in Pulmonary Unit (Asthma Research
Projects, Pediatric Asthma Clinic). Dr. Amirav is a member of the Israel Society of Aerosols in Medicine- Founder and Chairman, the International Society for aerosols in Medicine, the American Academy of Pediatrics, the American thoracic Society and the American College of Chest Physicians. He has received numerous awards including in 1985 "Best Young Investigator" - awarded by South African Pulmonary Society,
1989 Certified with Excellence as a pediatrician by the Israel Medical Association, 1992 Recipient of a Travel Grant Award for the Allergy section of the American Academy of Pediatrics, 1993 the John Caffey Award for best scientific work, The Society for (American) Pediatric Radiology, 1993 Recipient of a Travel Grant Award for the Allergy section of the American Academy of Pediatrics, 1999 Research Award for best paper, 4th International Congress on Pediatric Pulmonology, Nice, France, and 2009 Research Award
for best paper, Israeli Society for Clinical Pediatrics, Tel Aviv. Dr. Amirav graduated from Tel Aviv University Medical School, Faculty of Medicine, with an MD degree.
60
DR. DAVID GROSHAR, MD – MEMBER OF THE ADVISORY BOARD
Dr. Groshar, became a member of the Advisory Board of our Company with the acquisition of Baby’s Breath Ltd., in September 7, 2009. Since 2007 he has been the Head of the Department of Nuclear Medicine at the Rabin Medical Center in Petah Tikva, Israel and since 2005 he has been the Head of the Department of Nuclear Medicine
and PET-CT at the Assuta Medical Center in Tel Aviv, Israel. From 1994 to 2006 he was the Head of the Department of Nuclear Medicine at the Bnai Zion Medical Center in Haifa, Israel. Prior to that in 1991 and 1992, he was doing a Clinical Research Fellowship at Cross Cancer Institute, University of Alberta, in Edmonton, Canada. From 1986 to 1994 he has been the Head of the Division of Nuclear Medicine at the Rebecca Sieff Government Hospital in Safed, Israel. From 1982
to 1986 he did his residency in Nuclear Medicine at the Rambam Medical Center in Haifa, Israel. Dr. Groshar has a long history of teaching medicine. From 2000 to 2007 he was an Associate Professor, Bruce Rappaport Faculty of Medicine at the Technion – Israel Institute of Technology in Haifa, Israel. In 1999 he was a Visiting Associate Professor in the Department of Radiology, at the Stanford Medical Center, Stanford University, in California. From 1993 to 2000
he was a Senior Lecturer, Bruce Rappaport Faculty of Medicine at the Technion – Israel Institute of Technology in Haifa, Israel. From 1988 to 1993 he was a Lecturer, Bruce Rappaport Faculty of Medicine at the Technion – Israel Institute of Technology in Haifa, Israel. Dr. Groshar has received numerous awards in his field of expertise among them; in 2000 he was awarded the Lung and Childhood Young Investigator Award at the 4th International
Congress on Pediatric Pulmonology, in Nice, France. In 1999 he was awarded the Best presentation on Pediatric Urology at 14th Congress of the European Urology Association, in Stockholm, Sweden. In 1994 he was awarded the Most Significant Manuscript of 1993 - The Journal of Nuclear Medicine (USA). In 1991 he was awarded the American Physicians Fellowship Travel Award for Advanced Study Abroad, and the
Israel Medical Association Travel Award for Advanced Study Abroad. From 1982 to 1094 he was awarded the Fellowship Award from the International Atomic Energy Agency, in Vienna, Austria. Dr. Groshar received his MD degree from the Universidade Rio de Janeiro, in Rio de Janeiro, Brazil where he was born.
Advisory Board Compensation
Dr. Israel Amirav, one of our founding shareholders provided us with consulting services in 2005 for which we owe him $18,000. None of our other Advisory Board Members received any compensation to date. Members of the Advisory Board will be paid at their hourly rate whenever their services are needed by us.
Potential Conflicts of Interest
We are not aware of any current or potential conflicts of interest with any of our officers, directors or advisory board members.
Indemnification
Under our Articles of Incorporation and Bylaws, and in accordance with an agreement with each of our officers and directors we agreed to indemnify our officers and directors who is made a party to any proceeding, including a lawsuit, because of his position, if he acted in good faith and in a manner he reasonably
believed to be in our best interest. We may advance expenses incurred in defending a proceeding. To the extent that the officer or director is successful on the merits in a proceeding as to which he is to be indemnified, we must indemnify him against all expenses incurred, including attorney's fees. With respect to a derivative action, indemnity may be made only for expenses actually and reasonably incurred in defending the proceeding, and if the officer or director is judged liable, only
by a court order. The indemnification is intended to be to the fullest extent permitted by the laws of the State of Maryland.
There have been no cash dividends declared on our common stock. Dividends are declared at the sole discretion of our board of directors.
61
Related Party Transactions
The following is a short description of existing material transactions between us, our subsidiary Baby’s Breath, and our shareholders:
Shareholders Rights Agreement of September 2009
On September 7 2009, we have entered into a Shareholders Agreement (the "Shareholders Agreement") with our shareholders. The Shareholders Agreement defines and governs the various rights that our shareholders will have with respect to their holdings in us, until the consummation of
the registration for trading of our shares of common stock under this Registration Statement. The Agreement provides certain rights to those shareholders who hold 5% or more of our outstanding share capital which include a right of first refusal and participation rights of such shareholders in case of a sale of shares by our shareholders, and a right to participate in future issuances of securities by us under a preemptive right. Under the Shareholders Agreement until completion of a registration
of our securities for trade, in the event that parties to the agreement that hold at least 75% of our outstanding share capital accept an offer to sell all of their holdings in us to a third party purchaser (the "Purchaser") and such sale is conditioned upon the sale to the Purchaser of all of our remaining outstanding stock, then all the parties to the Shareholders Agreement shall be required to sell their holdings in us on the same terms and conditions.
The Shareholders Agreement also includes the Shareholders Undertaking (as defined below)
The Shareholders Agreement terminates and ceases to be in effect upon the registration for trade on any stock exchange, OTC system or bulletin board of our issued share capital pursuant to any applicable securities laws and regulations, whether as part of an offering to the public or otherwise.
A copy of the Shareholders Agreement is attached to this Registration Statement and is incorporated by reference in its entirety hereto.
Tax Pre Ruling of August 2009
On September 7, 2009, we purchased all the issued and outstanding shares of Baby’s Breath Ltd. from Baby’s Breath shareholders in consideration for 6,280,600 shares of our Common Stock as a result of which Baby’s Breath became our wholly-owned subsidiary. Our acquisition of Baby’s Breath's shares in consideration
for the issuance of shares of common stock of New Air was made pursuant to a tax arrangement and a pre-ruling reached with the Israeli Tax Authorities in accordance with the provisions of Section 104B of the Israeli Tax Ordinance (the "Pre Ruling"). A translated copy of the Pre-Ruling is attached as an exhibit hereto. The terms of the Pre-Ruling impose certain limitations on the ability of our shareholders that are Israeli residents to dispose
of their holdings in New Air, and on the level of dilution to which such shareholders may be subject in the future in case of additional fund raising by us. The Pre-Ruling also prohibits us from selling our holdings in Baby's Breath for the duration prescribed under law. In order to maintain compliance with the terms of the Pre Ruling the shares of common stock that were issued to those shareholders being parties to the Pre Ruling are held by a trustee appointed by New Air (the “Trustee”,
and are to be held by the Trustee for a period of 24 months following the issuance thereof (the “Restricted Period”). During the Restricted Period those shareholders that are Israeli residents, parties to the Pre Ruling may sell a collective aggregate amount of shares that equals up to 10% of their holdings (i.e. an aggregate amount of 628,060 shares). The shareholders may apportioned their respective right to sell such shares among
themselves such that the total amount of the shares sold by the Israeli resident's shareholders shall not exceed the amount of shares stated above . The Israeli resident's shareholders have agreed that the Israeli resident's shareholders listed on the Selling Shareholders table herein may sell the number of shares included in this registration statement.
A copy of the Tax Pre-Ruling is attached to this Registration and is incorporated by reference in its entirety hereto.
62
Acquisition Agreement of September 2009
On September 7, 2009 in furtherance of the Pre Ruling we have entered into a Stock Purchase and Sale Agreement (the "Acquisition Agreement"), under which we have purchased 31,403 Ordinary Shares, constituting all of the outstanding share capital, of Baby’s Breath from its shareholders,
in return for the issuance thereto of a total of 6,280,600 shares of Common Stock such that for each share of Baby’s Breath purchased by us from a certain shareholder thereof we issued 200 shares of Common Stock to such shareholder. The Agreement includes inter alia provisions which would govern the rights and liabilities of our shareholders until the completion of the registration of our shares for trading under this form S-1.
A copy of the Acquisition is attached to this Registration Statement and is incorporated by reference in its entirety hereto.
Current Shareholders Agreement of July 2009
In July 2009, the Current Shareholders entered into a shareholders agreement to govern their respective rights in Baby’s Breath (the "Current Shareholders Agreement"). The agreement governed inter alia the rights that the shareholders of Baby’s Breath will have
in the interim period until the consummation of the registration of New Air's shares for trading and includes provisions which preserve, to the extent possible and in the event that the offering fails to succeed, their respective rights for distribution on account of their past holdings in Baby’s Breath prior to the completion of the transactions under the Acquisition Agreement.
In the agreement the current shareholders have agreed inter alia to amend certain provisions of the articles of association of Baby’s Breath, to provide Microdel with the right, until the completion of the registration of New Air's shares for trading, to appoint 2 directors to the board of directors of Baby’s Breath and New
Air.
In the Current Shareholders Agreement the Current Shareholders undertaking also undertook towards the Incubator (the "Shareholders Undertaking") that so long as there is a restriction (under law or under the Israeli Tax Ruling obtained in connection with the sale under the Acquisition
Agreement) on the number of shares that can be either: (i) registered for trade as part of the registration so that not all of the shares of the Current Shareholders can be registered for trade, or (ii) sold, then the Current Shareholders shall waive their right to register their relative portion of their shares in New Air for trading, or sell their respective holdings, as applicable, and will allow the Incubator to register all of its shares in New Air for trading, or sell its holdings in New Air, as applicable,
before the registration or sale (as applicable) by any of them of any of their respective holdings in New Air. It was agreed that the rights of the Incubator may be assigned to a third party together with the transfer to such party of Incubator's shares in New Air. However, said rights shall become void in the case where the shares of New Air held by the Incubator are sold to a third party after the Offering.
For additional information please see note 17 in our Financial Statements. A copy of the Current Shareholders Agreement is attached to this Registration Statement and is incorporated by reference in its entirety hereto.
Second Microdel Agreement of June 2009
In June 2009 Microdel entered into a Second Agreement with Baby’s Breath (the "Second Microdel Agreement") which outlined Microdel's liabilities and responsibilities with respect to the provision of additional funding to Baby’s Breath via the incorporation of New Air and
the consummation of a registration for trading of New Air's issued share capital, in consideration for shares to be issued to Microdel in such case. Microdel undertakings in the Second Microdel Agreement included inter alia its obligation to finance Baby's Breath on going operations, and to assume all of the expenses in connection with the registration with the agreement that following the success of the registration (as defined in the Second Microdel Agreement) the Company shall assume the coverage of expenses.
The Second Microdel Agreement also included provisions under which GPI Project Development, Ltd. (the "Incubator”) shall have certain rights in preference over the other existing shareholders of Baby’s Breath or New Air: if a restriction will apply on registering the Incubator’s
shares in New Air for trading and/or on the ability to sell such shares following the registration thereof for trade, then the Incubator’s shares shall be registered for trade at the first opportunity in which New Air's shares of the other shareholders are registered following the termination of the said restriction. In addition each of the following shareholders of New Air: Assaf Halamish, Dr. Israel Amirav, Dr. Michael Neuhaus, Prof. David Grusher, Life Support, Ltd., and Ramport, Ltd. ("Current
Shareholders") undertook towards the Incubator that so long as there is a restriction (under law or under the Israeli Tax Ruling obtained in connection with the sale under the Acquisition Agreement) on the number of shares that can be either: (i) registered for trade as part of the registration so that not all of the shares of the Current Shareholders can be registered for trade, or (ii) sold, then the Current Shareholders shall waive their right to register their relative portion of their shares in New
Air for trading, or sell their respective holdings, as applicable, and will allow the Incubator to register all of its shares in New Air for trading, or sell its holdings in New Air, as applicable, before the registration or sale (as applicable) by any of them of any of their respective holdings in New Air. It was agreed that the rights of the Incubator may be assigned to a third party together with the transfer to such party of Incubator's shares in New Air. However, said rights shall become void
in the case where the shares of New Air held by the Incubator are sold to a third party after the Offering.
For additional information please see note 17in our Financial Statements. A copy of the Second Microdel Agreement is attached to this Registration Statement and is incorporated by reference in its entirety hereto.
63
First Microdel Agreement of April 2007
In April 2007 one of our shareholders, Microdel Ltd., ("Microdel") entered into an Agreement with Baby’s Breath (the "First Microdel Agreement"). The First Microdel Agreement governed the terms upon which Microdel will invest
in Baby's Breath. The Agreement was replaced by the Second Microdel Agreement.
For additional information please see note 17in our Financial Statements. A copy of the First Microdel Agreement is attached to this Registration Statement and is incorporated by reference in its entirety hereto.
Outstanding debts to Life Support, Dr. Amirav and Granot Development Enterprises Ltd.
In April 2004 Baby's Breath entered into an investment agreement with Life Support Ltd., one of our shareholders. As per the terms of the agreement, Life Support provides management services to Baby's Breath. In consideration for such management services Life Support is entitled to a monthly fee. As of October 2007 the monthly fee was set
on $3,000 USD. All monthly fees are currently not paid and are reflected in the financial statements as a loan. The loan is linked to the US dollar, bears no interest and has no maturity date. As of June 30, 2009, the outstanding debt to Life Support is $107,000.
For additional information please see notes_8 and 17_in our Financial Statements. A copy of the Life Support Agreement (as amended) is attached to this Registration Statement and is incorporated by reference in its entirety hereto.
Dr. Israel Amirav, one of our founding shareholders provided us with consulting services in 2005 for which we owe him $18,000.
Granot Development Enterprises Ltd. (GAPI) One of our shareholders provided us with management services in 2002 for which we owe them $17,703.
For additional information please see notes 8 and 17 in our Financial Statements.
Once the registration contemplated under this S-1 form is in effect we intend to fully pay all outstanding debts to our shareholders
Finders Fee Arrangements with A.M. Maagal Marketing & Business Development Ltd.
Our wholly owned subsidiary, Baby's Breath, entered into several agreements with A.M. Maagal Marketing & Business Development Ltd., one of our shareholders ("AM"), entitling AM to fees in case AM is successful in soliciting and introducing investors who will invest in Baby's Breath
share capital, or in soliciting and introducing customers for Bay's Breath products. under said agreements AM will be entitled to receive proceeds that equal 6% of any amount invested (in cash or in kind) in Baby's Breath by an investor introduced by AM, and up to 6% of any proceeds generated by the Company in a transaction between the company and a customer introduced to it by AM. As of June 30, 2009 there is an outstanding debt of US$14,000 to AM on account of compensation due and payable to it in
connection with the introduction to Baby's Breath of Life Support who became an investor and a shareholder of Baby's Breath.
64
Share Option Grants
We have an undertaking to grant to our CEO, under a stock option plan to be adopted by us for that purpose, a total of 259,051 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 4% of our outstanding share capital prior to the registration).
In addition we currently have available for additional grant to our employees, subject to the approval of our Board, up to 129,525 options which will be exercisable into our shares of Common Stock (an amount of shares of Common Stock that constitute approximately 2% of our outstanding share capital prior to the registration). Unless agreed otherwise by the Board the granted options shall generally be equally vested over a period of 3 years such that at the end of each year 1.33% of the entire amount of granted
options shall become vested.
The options to our Israeli employees may be granted under Section 102 of the Israeli Income Tax Ordinance pursuant to which the options or the shares of Common Stock issued upon their exercise must be deposited with a trustee for at least two years following the end of the calendar year in which the options are granted. Under Section 102
(1) any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or the shares by the trustee to the employee or upon the sale of the options or ordinary shares, (2) gains are subject to capital gains tax of 25%; under the tax rules governing these options, we do not receive a tax deduction in respect of the issuance or exercise of the options.
Our Certificate of Formation and Bylaws provide that we shall indemnify our officers or directors against expenses incurred in connection with the defense of any action in which they are made parties by reason of being our officers or directors, except in relation to matters as which such director or officer
shall be adjudged in such action to be liable for negligence or misconduct in the performance of his duty. One of our officers or directors could take the position that this duty on our behalf to indemnify the director or officer may include the duty to indemnify the officer or director for the violation of securities laws. All our officers and directors have signed agreements with us providing for such indemnification.
controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
65
The audited financial statements for the periods ended December 31, 2008 and December 31, 2007 and unaudited financial statements for the periods ended June 30, 2009 and June 30, 2008 are included on the Following pages:
INDEX TO FINANCIAL STATEMENTS
66
NEW AIR INC.
FINANCIAL STATEMENTS
As of August 31, 2009
(In US Dollars)
Audited
NA F-1
67
Schwartz Levitsky Feldman llp
CHARTERED ACCOUNTANTS
LICENSED PUBLIC ACCOUNTANTS
TORONTO ●MONTREAL
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
New Air Inc.
We have audited the accompanying balance sheet of New Air Inc. (“the Company”) as of August 31, 2009 and the related statements of changes in Stockholders’ equity and cash flows for the period from inception (July 10, 2009) to August 31, 2009. These financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of New Air Inc. as of August 31, 2009 and its cash flows for the period ended August 31, 2009 in accordance with generally accepted accounting principles in the United States of America.
The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion
on the effectiveness of the company’s internal controls over financial reporting. Accordingly, we express no such opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 1 of the financial statements, the Company was established specifically for the acquisition of Baby’s Breath Ltd., whose financial statements have negative financial indicators which raises substantial
doubt as to its ability to continue as a going concern. Accordingly, there is a substantial doubt as to its ability to continue as a going concern. Management plans regarding these matters are also described in note 1. The financial statements do not include any adjustment that might result from the outcome of this uncertainty.
| "SCHWARTZ LEVITSKY FELDMAN LLP" |
| Toronto, Ontario, Canada | Chartered Accountants | |
| October 16, 2009 | Licensed Public Accountants |
| 1167 Caledonia Road | ||
|
Toronto, Ontario M6A 2X1 | ||
|
Tel: 416 785 5353 | ||
| Fax: 416 785 5663 | ||
NA F-2
68
(In US Dollars)
|
Note |
As of August 31, |
||||
|
2009 |
|||||
|
Audited |
|||||
| $ | |||||
|
ASSETS |
|||||
|
CURRENT ASSETS: |
|||||
|
Cash and cash equivalents |
56,678 | ||||
|
TOTAL CURRENT ASSETS |
56,678 | ||||
|
TOTAL ASSETS |
56,678 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|||||
|
CURRENT LIABILITIES |
|||||
|
Accrued liabilities |
3 |
2,000 | |||
|
TOTAL LIABILITIES |
2,000 | ||||
|
SUBSEQUENT EVENTS |
4 | |||||
| GOING CONCERN | 1 | |||||
|
STOCKHOLDERS' EQUITY |
||||||
|
Authorized: $ 0.001 par value 50,000,000 common stock; |
||||||
|
Issued: 79,681 common stock |
80 | |||||
|
Additional paid in capital |
54,598 | |||||
|
TOTAL STOCKHOLDERS' EQUITY |
54,678 | |||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
56,678 | |||||
The footnotes are an integral part of these financial statements
NA F-3
69
(In US Dollars)
|
Common stock |
||||||||||||||||
|
Number USD 0.001
par value |
Amount |
Additional paid in capital |
Total stockholder's equity |
|||||||||||||
|
Audited |
$ | $ | ||||||||||||||
|
Issued on incorporation, July 10, 2009 |
- | - | - | - | ||||||||||||
|
Issuance of shares |
79,681 | 80 | 54,598 | 54,678 | ||||||||||||
|
Balance as of August 31, 2009 |
79,681 | 80 | 54,598 | 54,678 | ||||||||||||
The footnotes are an integral part of these financial statements
NA F-4
70
(In US Dollars)
|
Period ended August 31, |
||||
|
2009 |
||||
|
Audited |
||||
| $ | ||||
|
Cash flow from financing activities |
||||
|
Proceed from share issuance (net) |
56,678 | |||
|
Net cash provided by financing activities |
56,678 | |||
|
Increase in cash and cash equivalents |
56,678 | |||
|
Cash and cash equivalents at the beginning of the period |
- | |||
|
Cash and cash equivalents at the end of the period |
56,678 | |||
|
Activity not involving cash flows |
||||
|
Issuance expenses |
2,000 | |||
The footnotes are an integral part of these financial statements
NA F5
71
Notes
Note 1 – Background Information and Going Concern
New Air, Inc. (the “Company”) is a holding company which was incorporated in the State of Maryland on July 10th, 2009, for the purpose of acquiring a company called Baby’s Breath Ltd. an Israeli company founded
in 2001 by a group of leading physicians and highly experienced professionals who developed and are marketing patented medical device for the treatment of respiratory diseases.
On September 7th, 2009 New Air Inc. acquired Baby’s Breath, Ltd. in a stock for stock exchange whereby the Company issued 6,280,600 shares of its common stock for 31,403 shares in Baby’s Breath, Ltd. As a consequence, Baby’s Breath,
Ltd became a wholly owned subsidiary of the Company and the former shareholders of Baby’s Breath Ltd. now control 96.98 percent of the total shares in the Company.
The Company is under going a registration process of its ordinary shares on the Over-The-Counter Bulletin Board (OTCBB), under the form of S-1.
The accompanying finanancial statements have been prepared on a going concern basis which contemplates continuity of operations, realization of assets, liabilities and commitments in the normal course of business. The Company was established specifically for the acquisition of Baby's Breath, Ltd., whose financial statements have negative financial indicators which raises substantial doubt as to its ability
to continue as a going concern. Accordingly there is substantial doubt as to its ability to continue as a going concern. The accompanying financial statements do not reflect any adjustments that may result if the Company is unable to continue as a going concern. The Company plans to acquire sufficient capital by raising funds both internally and externally.
Note 2 – Summary of Significant Accounting Policies
A- Basis of Reporting
The financial statements of the Company have been prepared in accordance with Generally Accepted Accounting Principles in the United States of America (“GAAP”).
B- Use of estimates
The preparation of the financial statements in conformity with U.S. Generally Accepted Accounting Principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. The Company's management believes that the estimates, judgments and assumptions used are reasonable
based upon information available at the time they are made. Actual results could differ from these estimates. Significant estimates included accrual of expenses.
C- Cash and Cash equivalents
Highly liquid investments with maturities of three months or less at the date of purchase are considered to be cash equivalents and are stated at cost, which approximates market value.
Note 2 – Summary of Significant Accounting Policies (continued)
D – Recent Accounting Pronouncements:
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Liabilities, including an amendment of FASB Statement No. 115” (“SFAS No. 159”). SFAS No. 159 permits entities to choose, at specified election dates, to measure many financial instruments and certain other items at fair
value that are not currently required to be measured at fair value. Unrealized gains and losses shall be reported on items for which the fair value option has been elected in earnings at each subsequent reporting date. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provisions of SFAS No. 157 “Fair Value Measurements”
(“SFAS No. 157”). The adoption of FAS No. 159 did not have a material impact on the Company’s financial position or results of operations.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), “Business Combinations” (“FAS 141R”). FAS 141R provides revised guidance on how acquirers recognize and measure the consideration, identifiable assets acquired, liabilities assumed, contingencies,
non-controlling interests and goodwill acquired in a business combination, and expands disclosure requirements surrounding the nature and financial effects of business combinations. Key changes include: acquired in-process research and development will no longer be expensed on acquisition, but capitalized and assessed for impairment where relevant and amortized over its useful life; acquisition costs will be expensed as incurred; restructuring costs will generally be expensed in periods after the acquisition
date; the consideration in shares would be valued at the closing date; and in the event that a deferred tax valuation allowance relating to a business acquisition, including from prior years, is subsequently reduced, the adjustment will be recognized in the statement of income. Early adoption is not permitted. The adoption of SFAS No. 141 did not have a material impact on the Company’s financial position or results of operations.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interest in Consolidated Financial Statements” (“SFAS No. 160”). SFAS No. 160 amends ARB 51 to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies
that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. Before this Statement was issued, limited guidance existed for reporting noncontrolling interests. As a result, considerable diversity in practice existed. So-called minority interests were reported in the consolidated statement of financial position as liabilities or in the mezzanine section between liabilities and equity. This Statement
improves comparability by eliminating that diversity. SFAS No. 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. The Company does not anticipate that SFAS No. 160 will have material impact on its financial statements.
In March 2008, the FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities: an amendment of FASB Statement No. 133" ("SFAS No. 161"). SFAS No. 161, which is effective the first quarter of fiscal 2010 changes the disclosure requirements for derivative instruments and hedging activities. The Company does
not anticipate that this new accounting standard will have an impact on the financial statements.
In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles" (SFAS No. 162). SFAS No. 162 identifies the sources of accounting principles and the framework for selecting principles used in the preparation of financial statements. SFAS No. 162 is effective 60 days following the SEC’s approval
of the Public Company Accounting Oversight Board amendments to AU Section 411, "The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles". The implementation of this standard will not have a material impact on Company’s financial statements.
In May 2008, the FASB issued FASB Statement No. 163, “Accounting for Financial Guarantee Insurance Contracts” (“SFAS No. 163”), which clarifies how FASB Statement No. 60, “Accounting and Reporting by Insurance Enterprises”, applies to financial guarantee insurance contracts issued by insurance enterprises.
The standard is effective for financial statements issued for fiscal years beginning after December 15, 2008, including interim periods in that year. The adoption of SFAS No. 163 did not have a material impact on the Company’s financial position or results of operations.
Note 2 – Summary of Significant Accounting Policies (continued)
D – Recent Accounting Pronouncements (continued):
FASB Statement No. 164, “Not-for-Profit Entities: Mergers and Acquisitions,” governs the information that a not-for-profit entity should provide in its financial reports about a combination with one or more other not-for-profit entities, businesses or nonprofit activities. The adoption of SFAS No. 164 did not have a material impact on the Company’s financial position or
results of operations.
In May 2009, the FASB issued SFAS No.165, “Subsequent Events” (“FAS 165”), which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. The adoption of SFAS No. 165 did not have a material
impact on the Company’s financial position or results of operations.
In June 2009, the FASB issued SFAS 166 “Accounting for Transfers of Financial Assets-an amendment of FASB Statement No. 140”. SFAS 166 requires that a transferor recognize and initially measure at fair value all assets obtained (including a transferor’s beneficial interest) and liabilities incurred as a result of a transfer of financial assets accounted for as a sale. SFAS 166 must be applied as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, for interim periods within that first annual reporting period and for interim and annual reporting periods thereafter. Earlier application is prohibited. This Statement must be applied to transfers occurring on or after the effective date. The Company does not expect SFAS 166 to have a material effect on its financial statements.
In June 2009, the FASB issued SFAS 167 “Amendments to FASB Interpretation No. 46(R)”. SFAS 167 requires an additional reconsideration event when determining whether an entity is a variable interest entity when any changes in facts and circumstances occur such that the holders of the equity investment at risk, as a
group, lose the power from voting rights or similar rights of those investments to direct the activities of the entity that most significantly impact the entity’s economic performance. It also requires ongoing assessments of whether an enterprise is the primary beneficiary of a variable interest entity. SFS 167 shall be effective as of the beginning of each reporting entity’s first annual reporting
period that begins after November 15, 2009, for interim periods within that first annual reporting period, and for interim and annual reporting periods thereafter. Earlier application is prohibited. The Company does not expect SFAS 167 to have a material effect on its financial statements.
In June 2009, the FASB issued SFAS 168 “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles-a replacement of FASB Statement No. 162”. The FASB Accounting Standards Codification will become the source of authoritative U.S. Generally Accepted Accounting Principles
(GAAP) recognized by the FASB to be applied by nongovernmental entities.
Following SFAS 168, the Board will not issue new standards in the form of Statements, FASB Staff Positions, or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates. This Statement is effective for financial statements issued for interim and annual periods ending after September
15, 2009. On the effective date of this Statement, the Codification will supersede all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting
Note 2 – Summary of Significant Accounting Policies (continued)
literature not included in the Codification will become non-authoritative. The Company does not expect SFAS 168 to have a material effect on its financial statements.
E - Comprehensive income:
Comprehensive income, net of related taxes where applicable, includes, in addition to net income: (i) currency translation adjustments; (ii) unrealized holding gain and losses on available-for-sale securities; (iii) gain in respect of derivative instruments designated as a cash flow hedge and (iv) additional minimum pension liability. The
Company has no "other comprehensive income".
Note 3 - Accrued Liabilities
The accrued liabilities of the Company comprise legal and professional fees.
Note 4 - Subsequent events
A - Acquisition agreement of September 2009
On September 7th,2009 the Company has entered into a stock purchase and sale agreement (the "acquisition agreement"), under which the Company issued 6,280,600 shares for 31,403 shares in Baby’s Breath, Ltd. As a consequence Baby’s Breath Ltd became a wholly owned subsidiary of the company and the former shareholders of Baby’s
Breath now control 96.98 % of the total shares in the company. This transaction will be accounted for as a recapitalization with Baby’s Breath deemed to be the acquirer for accounting purposes. The agreement also includes provisions which would govern the rights and liabilities of the Company's shareholders until the completion of the registration of the Company shares for trading.
B – Issuance of shares
Subsequent to the period ended August 31th, 2009, the Company issued 115,996 common stock to private investors for proceed in the amount of $79,500.
C – Subsequent events
Subsequent events have been evaluated on October 16th, 2009.
NA F9
75

BABY'S BREATH LTD
FINANCIAL STATEMENTS
As of December 31, 2008 and 2007 (Audited)
And as of June 30, 2009 and 2008 (Unaudited)
(In US Dollars)
BB F1
76
Schwartz Levitsky Feldman llp
CHARTERED ACCOUNTANTS
LICENSED PUBLIC ACCOUNTANTS
TORONTO ●MONTREAL
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Baby’s Breath Ltd.
We have audited the accompanying balance sheets of Baby’s Breath Ltd. Inc. (A Company incorporated in the State of Israel) as of December 31, 2008 and December 31, 2007 and the related statements of operations and comprehensive loss, changes in stockholders' deficiency and cash flows for the years ended December 31, 2008 and December
31, 2007. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence
supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Baby’s Breath Ltd. Inc. as of December 31, 2008 and December 31, 2007, the results of its operations and its cash flows for the years ended December 31, 2008 and December 31, 2007 in accordance with generally
accepted accounting principles in the United States of America.
The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion
on the effectiveness of the company’s internal controls over financial reporting. Accordingly, we express no such opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 1 of the financial statements, the Company has not generated significant revenue since its incorporation, has accumulated significant losses and further losses are anticipated and has a working capital
deficiency. The Company requires additional funds to meet its obligations and the cost of its operations. These factors raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| "SCHWARTZ LEVITSKY FELDMAN LLP" |
| Toronto, Ontario, Canada | Chartered Accountants | |
| October 16, 2009 | Licensed Public Accountants |
| 1167 Caledonia Road | ||
|
Toronto, Ontario M6A 2X1 | ||
|
Tel: 416 785 5353 | ||
| Fax: 416 785 5663 | ||
BB F2
77

BABY'S BREATH LTD
BALANCE SHEETS
(In US Dollars)
|
Note |
As of June 30, |
As of December 31, |
|||||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
||||||||||||||||
|
Unaudited |
Audited |
||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||
|
ASSETS |
|||||||||||||||||||
|
CURRENT ASSETS: |
|||||||||||||||||||
|
Cash and cash equivalents |
3 | 13,989 | 9,519 | 14,791 | 11,993 | ||||||||||||||
|
Accounts receivable |
4 | 9,718 | 6,420 | 9,824 | 11,798 | ||||||||||||||
|
Inventory |
19,579 | 7,341 | 11,565 | - | |||||||||||||||
|
TOTAL CURRENT ASSETS |
43,286 | 23,280 | 36,180 | 23,791 | |||||||||||||||
|
Property and equipment, net |
5 | 98,642 | 114,685 | 106,771 | 22,617 | ||||||||||||||
|
TOTAL ASSETS |
141,928 | 137,965 | 142,951 | 46,408 | |||||||||||||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIENCY |
|||||||||||||||||||
|
CURRENT LIABILITIES |
|||||||||||||||||||
|
Short term bank debt |
6 | 8,178 | 10,072 | 2,936 | 4,490 | ||||||||||||||
|
Accounts payable and accrued expenses |
7 | 123,640 | 77,012 | 86,133 | 107,554 | ||||||||||||||
|
Stockholders' loans |
8 | 142,703 | 109,698 | 125,248 | 89,039 | ||||||||||||||
|
TOTAL CURRENT LIABILITIES |
274,521 | 196,782 | 214,317 | 201,083 | |||||||||||||||
|
LONG TERM LIABILITIES |
|||||||||||||||||||
|
Accrued severance pay |
1,471 | - | - | - | |||||||||||||||
|
TOTAL LONG TERM LIABILITIES |
1,471 | - | - | - | |||||||||||||||
|
TOTAL LIABILITIES |
275,992 | 196,782 | 214,317 | 201,083 | |||||||||||||||
The footnotes are an integral part of these financial statements.
BB F3
78

BABY'S BREATH LTD
BALANCE SHEETS
(In US Dollars)(continued)
|
Note |
As of June 30, |
As of December 31, |
|||||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
||||||||||||||||
|
Unaudited |
Audited |
||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||
|
RELATED PARTY TRANSACTIONS |
17 | ||||||||||||||||||
|
SUBSEQUENT EVENTS |
18 | ||||||||||||||||||
|
COMMITMENTS AND CONTINGENCIES |
16 | ||||||||||||||||||
|
GOING CONCERN |
1 | ||||||||||||||||||
|
STOCKHOLDERS' DEFICIENCY |
|||||||||||||||||||
|
Share capital |
9 | ||||||||||||||||||
|
Ordinary shares: Series A, B and C ordinary shares: |
5,689 | 5,689 | 5,689 | 5,689 | |||||||||||||||
|
Authorized: $0.2418 (NIS 1) par value -450,000 type A, 448,453 type B and 100,000 type C shares authorized as of December 31, 2007 and 2008 and June 30 2008 and 2009; Issued:11,765 type A, 10,218 type
B and 0 type C shares as of December 31, 2007 and 2008 and June 30, 2008 and 2009; ordinary shares deferred of $0.2418 (NIS 1) par value – 1,547 issued and outstanding as of December 31, 2007 and 2008 and as of June 30 2008 and 2009. |
|||||||||||||||||||
|
Additional paid in capital |
611,481 | 605,219 | 608,101 | 602,664 | |||||||||||||||
|
Deposit on shares |
314,152 | 237,437 | 298,396 | 28,961 | |||||||||||||||
|
Accumulated deficit |
(1,065,386 | ) | (907,162 | ) | (983,552 | ) | (791,989 | ) | |||||||||||
|
TOTAL STOCKHOLDERS' DEFICIENCY |
(134,064 | ) | (58,817 | ) | (71,366 | ) | (154,675 | ) | |||||||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIENCY |
141,928 | 137,965 | 142,951 | 46,408 | |||||||||||||||
The footnotes are an integral part of these financial statements.
BB F4
79

|
Six months ended June 30, |
Year ended December 31, |
||||||||||||||||||
|
Note |
2009 |
2008 |
2008 |
2007 |
|||||||||||||||
|
Unaudited |
Audited |
||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||
|
Revenue |
10 | 15,744 | 6,674 | 43,199 | 24,013 | ||||||||||||||
|
Cost of sales |
11 | 15,121 | 12,522 | 40,128 | 34,988 | ||||||||||||||
|
Gross profit (loss) |
623 | (5,848 | ) | 3,071 | (10,975 | ) | |||||||||||||
|
Selling, general and administrative expenses |
12 | 78,052 | 101,188 | 188,633 | 181,296 | ||||||||||||||
|
Operating loss |
(77,429 | ) | (107,036 | ) | (185,562 | ) | (192,271 | ) | |||||||||||
|
Financial expenses, net |
13 | 4,405 | 8,136 | 6,001 | 7,395 | ||||||||||||||
|
Losses before income taxes |
(81,834 | ) | (115,172 | ) | (191,563 | ) | (199,666 | ) | |||||||||||
|
Income taxes |
15 | - | - | - | - | ||||||||||||||
|
Net loss and comprehensive loss |
(81,834 | ) | (115, 172 | ) | (191,563 | ) | (199,666 | ) | |||||||||||
| Basic and Diluted net loss per share | (3.72 | ) | (5.24 | ) | (8.71 | ) | (9.08 | ) | |||||||||||
|
Weighted average number of shares used in per share calculation |
21,983 | 21,983 | 21,983 | 21,983 | |||||||||||||||
The footnotes are an integral part of these financial statements.
BB F5
80

BABY'S BREATH LTD
(In US Dollars)
| Ordinary shares | ||||||||||||||||||||||||
| Number | Amount ($0.2418 par value) | Additional paid-in-capital | Deposit on shares | Accumulated Deficit | Total stockholder's defiency | |||||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||||||
| Balance as of December 31, 2006 (Audited) | 23,530 | 5,689 | 599,137 | - | (592,323 | ) | 12,503 | |||||||||||||||||
| Deposit on shares | - | - | - | 28,961 | - | 28,961 | ||||||||||||||||||
| Imput interest on stockholders' loan | - | - | 3,527 | - | - | 3,527 | ||||||||||||||||||
| Loss for the year | - | - | - | - | (199,666 | ) | (199,666 | ) | ||||||||||||||||
| Balance as of December 31, 2007 (Audited) | 23,530 | 5,689 | 602,664 | 28,961 | (791,989 | ) | (154,675 | ) | ||||||||||||||||
| Deposit on shares | - | - | - | 269,435 | - | 269,435 | ||||||||||||||||||
| Imput interest on stockholders' loan | - | - | 5,437 | - | - | 5,437 | ||||||||||||||||||
| Loss for the year | - | - | - | - | (191,563 | ) | (191,563 | ) | ||||||||||||||||
| Balance as of December 31, 2008 (Audited) | 23,530 | 5,689 | 608,101 | 298,396 | (983,552 | ) | (71,366 | ) | ||||||||||||||||
| Deposit on shares | - | - | - | 15,756 | - | 15,756 | ||||||||||||||||||
| Imput interest on stockholders' loan | - | - | 3,380 | - | - | 3,380 | ||||||||||||||||||
| Loss for the year | - | - | - | - | (81,834 | ) | (81,834 | ) | ||||||||||||||||
| Balance as of June 30, 2009 (Audited) | 23,530 | 5.689 | 611,481 | 314,152 | (1,065,386 | ) | (134,064 | ) | ||||||||||||||||
The footnotes are an integral part of these financial statements.
BB F6
81

(In US Dollars)
|
Six months ended June 30, |
Year ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Cash flows from operating activities |
||||||||||||||||
|
Net loss |
(81,834 | ) | (115, 172 | ) | (191,563 | ) | (199,666 | ) | ||||||||
|
Adjustments to reconcile net loss to net cash used in operating activities : |
||||||||||||||||
|
Depreciation and Amortization |
8,129 | 7,934 | 16,133 | 6,703 | ||||||||||||
|
Imputed interest on stockholders loan |
3,380 | 2,555 | 5,437 | 3,527 | ||||||||||||
|
Decrease (Increase) in accounts receivable |
106 | 5,378 | 1,974 | (6,027 | ) | |||||||||||
|
Decrease (Increase) in inventory |
(8,014 | ) | (7,341 | ) | (11,565 | ) | 1,596 | |||||||||
|
Increase (Decrease) in accounts payable and accrued expenses |
37,507 | (30,542 | ) | (21,421 | ) | 73,270 | ||||||||||
|
Increase (Decrease) in stockholders loan |
17,455 | 20,659 | 36,209 | 53,000 | ||||||||||||
|
Increase (Decrease) in accrued severance pay |
1,471 | - | - | - | ||||||||||||
|
Net cash used in operating activities |
(21,800 | ) | (116,529 | ) | (164,796 | ) | (67,597 | ) | ||||||||
|
Cash Flow from investing activities |
||||||||||||||||
|
Purchase of property and equipment |
- | - | (287 | ) | (1,538 | ) | ||||||||||
|
Net cash flow from investing activities |
- | - | (287 | ) | (1,538 | ) | ||||||||||
|
Cash flow used in financing activities |
||||||||||||||||
|
Repayment of short term bank debt |
5,242 | 5,582 | (1,554 | ) | 2,624 | |||||||||||
|
Deposit on shares |
15,756 | 108,473 | 169,435 | 28,961 | ||||||||||||
|
Net cash provided by financing activities |
20,998 | 114,055 | 167,881 | 31,585 | ||||||||||||
|
The footnotes are an integral part of these financial statements |
||||||||||||||||
BB F7
82

BABY'S BREATH LTD
(In US Dollars)(continued)
|
Six months ended June 30, |
Year ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Increase (decrease) in cash and cash equivalents |
(802 | ) | (2,474 | ) | 2,798 | (37,550 | ) | |||||||||
|
Cash and cash equivalents at the beginning of the year |
14,791 | 11,993 | 11,993 | 49,543 | ||||||||||||
|
Cash and cash equivalents at the end of the year |
13,989 | 9,519 | 14,791 | 11,993 | ||||||||||||
|
Supplementary disclosure of cash flow information |
||||||||||||||||
|
Cash paid during the year |
||||||||||||||||
|
Income taxes, net of refunds |
- | - | - | |||||||||||||
|
Interest paid |
823 | 1,058 | 1,785 | 1,131 | ||||||||||||
|
Activity not involving cash flows |
||||||||||||||||
|
Issuance of shares in return for purchasing manufacturing moulds |
100,000 | 100,000 | ||||||||||||||
The footnotes are an integral part of these financial statements.
BB F8
83

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 1 – General and Going concern
Baby's Breath, LTD ("The Company") was originally organized as an Israeli limited liability company in 2001. The Company specializes in development, manufacturing and distribution of inhalation technologies under the name BabyAir.
Currently, the Company's principal product includes an inhalation product - marketed under the trade name "BabyAir". BabyAir provides an innovative solution for the delivery of drugs through inhalation without using a facemask for children under the age of two years. The Company has completed the development of BabyAir and has begun marketing
it in Europe. The Company is already working on new products that are based on the same technology, for wider markets and applications. The Company's products are mainly sold from September to December, due to a high demand for inhalation products in that period.
BabyAir has been granted CE certification for medical device class from the European Union (until the year 2014) and has FDA approval in the US. The Company has also a certification for medical device class from the Health Technology and Infrastructure Administration of Israel.
The Company is under going a registration process of its ordinary shares on the Over-The-Counter Bulletin Board (OTCBB), under the form of S-1.
The results of operations for the six-months period ended June 30, 2009 are not necessarily indicative of results to be expected for the full year.
Going concern
The accompanying financial statements have been prepared in conformity with Generally Accepted Accounting Principles in the United States of America (“GAAP”), which contemplates continuation of the Company as a going concern. The Company's total deficit as of December 31,2008 and as of June 30, 2009 is in the amount of $ 983,552
and $1,065,386 respectively. The continuing activity of the Company as a going concern depends on raising money, as mentioned above.
The accompanying financial statements have been prepared on a going concern basis accounting which contemplates continuity of operation, realization of assets, liabilities, and commitments in the normal course of business. As of 31 December 2008 and 2007 and as of June 30, 2009 the Company has a working capital deficit and has incurred
significant losses since inception. Further losses are anticipated which raises substantial doubt as to the Company's ability to continue as a going concern. The accompanying financial statements do not reflect any adjustment that may result if the Company is unable to continue as a going concern. The Company plans to acquire sufficient capital by raising funds both internally and externally.
BB F9
84

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies
A- Basis of Reporting
The financial statements of the Company have been prepared in accordance with Generally Accepted Accounting Principles in the United States of America (“GAAP”).
B- Use of Estimates
The preparation of the financial statements in conformity with U.S. Generally Accepted Accounting Principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. The Company's management believes that the estimates, judgments and assumptions used are reasonable
based upon information available at the time they are made. Actual results could differ from these estimates. Significant estimates were used in the assessment of accrued warranty and to calculate the fair value of its stockholders' loans.
C-Functional Currency
Most of the Company's expenses and revenues are denominated in U.S. Dollars. The Company’s management believes that the U.S. Dollar is the primary currency of the economic environment in which the company operates. Thus, the functional and reporting currency of the Company is the U.S. Dollar.
The company’s books and records are maintained in New Israeli Shekel (NIS).Transactions in U.S. dollars are translated to NIS at the dates those transactions take place. Foreign currency translation gains and losses are included in selling, general and administrative expense. For year end reporting purpose, the financial statements
are remeasured to U.S. dollars using the temporal method. Accordingly exchange adjustments arising in the remeasurement process are included in the determination of income.
D- Cash and Cash equivalents
Highly liquid investments with maturities of three months or less at the date of purchase are considered to be cash equivalents and are stated at cost, which approximates market value.
E- Inventory
Inventory is valued at the lower of cost or market value. Cost is determined using a "first-in first-out" method. The inventory's cost was calculated due to the cost of manufacturing features, including the delivery and transportation costs. Inventories consist only of finished goods.
BB F10
85

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
F- Property and equipment
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is computed on the straight-line method based on the estimated useful lives of the assets. The cost of the manufacturing moulds, which were purchased from a related party, was determined at market value at the purchasing day, (including transportation
and assembly). Annual rates of depreciation (%):
|
Electronic Equipment |
Office furniture and equipment |
Computer and Computer Equipment |
|
15 |
7 - 15 |
33 |
G- Impairment of long lived assets
The Company reviews its assets for impairment in accordance with Statement of Financial Accounting Standards No. 144, "Accounting for the Impairment of Disposal of Long-Lived Assets" ("SFAS No. 144"), whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of an asset
to be held and used is measured by a comparison of the carrying amount of the asset to the future undiscounted cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds the fair value of the asset. The Company has no impairment for the year ended December 31,2008 and 2007 and the periods ended June 30, 2009 and 2008.
H - Revenue Recognition
The Company generates its revenues primarily from the sale of products. Revenue is recognized when title risk and reward for product are transferred to the customer. Revenues are recognized in accordance with Staff Accounting Bulleting ("SAB") No. 104, "Revenue Recognition in Financial Statements", when the following criteria are met: persuasive
evidence of an arrangement exists, delivery of the product has occurred, the selling price to the buyer is fixed or determinable, and collectability is probable. The Customer has no right of retur to company after the date on which products are delivered other than pursuant to warranty obligations. All of the Company’s products sold through agreements with distributors are non-exchangeable, non-refundable, non-returnable
and without any rights of price protection or stock rotation. Revenue is presented net of warranty cost.
BB F11
86

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
The Company generally provides a warranty period of 12 months, at no extra charge. The Company estimates the costs that may be incurred under its basic limited warranty and records a liability in the amount of such costs at the time product revenue is recognized. Factors that affect the Company's warranty liability include the historical
and anticipated rates of warranty claims, and cost per claim. The Company periodically assesses the adequacy of its recorded warranty liability and adjusts the amount as necessary. The Company estimates its warranty accrued as 2% of revenues.
|
Six months ended June 30,
In US Dollars)) |
Year ended December 31, (In US Dollars) |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Balance at the beginning of the period |
864 | 248 | 248 | 248 | ||||||||||||
|
Warranties issued during the period |
170 | 423 | 616 | 116 | ||||||||||||
|
Settlements made during the period |
(719 | ) | (538 | ) | - | (116 | ) | |||||||||
| 315 | 133 | 864 | 248 | |||||||||||||
I- Research and development expenses, net
Research and development expenses include costs of salaries and related expenses, activities related to intellectual property, research materials and suppliers and equipment depreciation. All the research and development expenses less grants which were received prior to fiscal 2007 from the Office of the Chief Scientist Israel (OCS), are
charged to operations.
J- Concentration of credit risk
Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash and cash equivalents and trade receivables. Cash and cash equivalents were deposited with Israelis banks. In general, the exposure of the Company to credit risk relating to trade receivables is immaterial due to the fact
that consideration is usually fully collected before delivery of the goods to the customers. The Company performs ongoing credit evaluations of its customers and to date has not experienced any material losses. An allowance for doubtful accounts is determined upon a specific review with respect to those amounts that the Company has determined to be doubtful of collection. As of December 31, 2008 and 2007 and, June 30, 2009 and 2008, the amount of the allowance is NIL. In certain circumstances, the
Company may require letters of credit, other collateral or additional guarantees. When uncertainty of collectability exists, the Company defers revenue recognition until collection.
BB F12
87

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
K -Fair value of financial instruments
The carrying values of cash and cash equivalents, accounts receivable, short-term bank debt, accounts payable, accrued expenses and stockholders’ loans approximate their fair values because of the short maturity of these instruments.
L-Income taxes
The Company accounts for income taxes in accordance with SFAS No. 109, "Accounting for Income Taxes". This Statement prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between the financial reporting and tax bases of assets and liabilities and for carry
forward losses. Deferred taxes are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that some portion or all of the deferred tax asset will not be realized.
In June 2006, the FASB issued FIN 48, "Accounting for Uncertainty in Income Taxes-an interpretation of FASB Statement No. 109" ("FIN 48"). FIN 48 clarifies the accounting for uncertainty in income taxes recognized under SFAS No. 109. FIN 48 prescribes a recognition threshold and measurement attribute for financial statement recognition
and measurement of a tax position taken or expected to be taken in a tax return and also provides guidance on various related matters such as derecognizing, interest and penalties, and disclosure. The Company adopted FIN 48 on January 1, 2009 in accordance with FSP FIN 48-3. In the initial application there was no material difference between the provisions of SFAS 5 and FIN 48, therefore no adjustment was recorded to the retained earnings. The Company
recognizes interest and penalties, if any, related to unrecognized tax benefits in tax expenses.
M – Severance pay
The Company's liability for severance pay is calculated pursuant to Israel's Severance Pay Law based on the most recent salary of the employees multiplied by the number of years of employment, as of the balance sheet date. Employees are entitled to one month's salary for each year of employment or a portion thereof. Severances pay expense
for the period ended December 31 2008, 2007 and June 30,2008: amounted to NIL and for the period ended June 30, 2009 - amounted to $ 1,471.
N – Comprehensive income
Comprehensive income, net of related taxes where applicable, includes, in addition to net income: (i) currency translation adjustments; (ii) unrealized holding gain and losses on available-for-sale securities; (iii) gain in respect of derivative instruments designated as a cash flow hedge and (iv) additional minimum pension liability. The
Company has no "other comprehensive income".
BB F13
88

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
O – Stock-based compensation
The Company accounts for stock-based compensation to employees in accordance to FASB statement No. 123 (revised 2004), "Share-Based Payment" (SFAS No. 123(R)"). The Company will estimate the fair value of employee stock options using Blak-Scholes valuation model and values restricted stock units ("RSUs) based on the market value of the
underlying shares at the date of grant. The Company will amortize compensation costs using the graded vesting attribution method. There is no vesting at the balance sheet date.
P – Segmented information
The Company manages its business on the basis of one reportable segment. Total revenues are attributed to countries based on the location of the customers. The related data is presented in accordance with SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information” (“SFAS No. 131”).
Q – Shipping and handling costs
Shipping and handling costs are included in selling, general and administration expenses.
R – Earnings per share
The Company presents basic and diluted earnings per share (EPS) data for its common shares. Basic EPS is calculated by dividing the profit or loss attributed to common stockholders of the Company by the weighted average number of common shares outstanding during the period. Diluted EPS is determined by adjusting the profit or
loss attributed to common stockholders and the weighted average number of common shares outstanding for the effects of all potential dilutive common shares, which comprise convertible personanel debentures, restricted shares and optional granted to employees.
S – Recent Accounting Pronouncements:
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Liabilities, including an amendment of FASB Statement No. 115” (“SFAS No. 159”). SFAS No. 159 permits entities to choose, at specified election dates, to measure many financial instruments and certain other items at fair
value that are not currently required to be measured at fair value. Unrealized gains and losses shall be reported on items for which the fair value option has been elected in earnings at each subsequent reporting date. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007. Early adoption is permitted as of the beginning of a fiscal year that begins on or before November 15, 2007, provided the entity also elects to apply the provisions of SFAS No. 157 “Fair Value Measurements”
(“SFAS No. 157”). The adoption of FAS No. 159 did not have a material impact on the Company’s financial position or results of operations.
BB F14
89

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
In December 2007, the FASB issued SFAS No. 141 (revised 2007), “Business Combinations” (“FAS 141R”). FAS 141R provides revised guidance on how acquirers recognize and measure the consideration, identifiable assets acquired, liabilities assumed, contingencies, non-controlling interests
and goodwill acquired in a business combination, and expands disclosure requirements surrounding the nature and financial effects of business combinations. Key changes include: acquired in-process research and development will no longer be expensed on acquisition, but capitalized and assessed for impairment where relevant and amortized over its useful life; acquisition costs will be expensed as incurred; restructuring costs will generally be expensed in periods after the acquisition
date; the consideration in shares would be valued at the closing date; and in the event that a deferred tax valuation allowance relating to a business acquisition, including from prior years, is subsequently reduced, the adjustment will be recognized in the statement of income. Early adoption is not permitted. The adoption of SFAS No. 141 did not have a material impact on the Company’s financial position or results of operations.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interest in Consolidated Financial Statements” (“SFAS No. 160”). SFAS No. 160 amends ARB 51 to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies
that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. Before this Statement was issued, limited guidance existed for reporting noncontrolling interests. As a result, considerable diversity in practice existed. So-called minority interests were reported in the consolidated statement of financial position as liabilities or in the mezzanine section between liabilities and equity. This Statement
improves comparability by eliminating that diversity. SFAS No. 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption is prohibited. The Company does not anticipate that SFAS No. 160 will have material impact on its financial statements.
In March 2008, the FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities: an amendment of FASB Statement No. 133" ("SFAS No. 161"). SFAS No. 161, which is effective the first quarter of fiscal 2010 changes the disclosure requirements for derivative instruments and hedging activities. The Company does
not anticipate that this new accounting standard will have an impact on the financial statements.
In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles" (SFAS No. 162). SFAS No. 162 identifies the sources of accounting principles and the framework for selecting principles used in the preparation of financial statements. SFAS No. 162 is effective 60 days following the SEC’s approval
of the Public Company Accounting Oversight Board amendments to AU Section 411, "The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles". The implementation of this standard will not have a material impact on Company’s financial statements.

BB F15
90

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
In May 2008, the FASB issued FASB Statement No. 163, “Accounting for Financial Guarantee Insurance Contracts” (“SFAS No. 163”), which clarifies how FASB Statement No. 60, “Accounting and Reporting by Insurance Enterprises”, applies to financial guarantee insurance contracts issued by insurance enterprises. The standard is effective for
financial statements issued for fiscal years beginning after December 15, 2008, including interim periods in that year. The adoption of SFAS No. 163 did not have a material impact on the Company’s financial position or results of operations.
FASB Statement No. 164, “Not-for-Profit Entities: Mergers and Acquisitions,” governs the information that a not-for-profit entity should provide in its financial reports about a combination with one or more other not-for-profit entities, businesses or nonprofit activities. The adoption of SFAS No. 164 did not have a material impact on the Company’s financial position or
results of operations.
In May 2009, the FASB issued SFAS No.165, “Subsequent Events” (“FAS 165”), which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. The adoption of SFAS No. 165 did not have a material
impact on the Company’s financial position or results of operations.
In June 2009, the FASB issued SFAS 166 “Accounting for Transfers of Financial Assets-an amendment of FASB Statement No. 140”. SFAS 166 requires that a transferor recognize and initially measure at fair value all assets obtained (including a transferor’s beneficial interest) and liabilities incurred as a result
of a transfer of financial assets accounted for as a sale. SFAS 166 must be applied as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, for interim periods within that first annual reporting period and for interim and annual reporting periods thereafter. Earlier application is prohibited. This Statement must be applied to transfers occurring on or after the effective date. The Company does not expect SFAS 166
to have a material effect on its financial statements.
In June 2009, the FASB issued SFAS 167 “Amendments to FASB Interpretation No. 46(R)”. SFAS 167 requires an additional reconsideration event when determining whether an entity is a variable interest entity when any changes in facts and circumstances occur such that the holders of the equity investment at risk, as a
group, lose the power from voting rights or similar rights of those investments to direct the activities of the entity that most significantly impact the entity’s economic performance. It also requires ongoing assessments of whether an enterprise is the primary beneficiary
BB F16
91

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 2 – Summary of Significant Accounting Policies (continued)
of a variable interest entity. SFS 167 shall be effective as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, for interim periods within that first annual reporting period,
and for interim and annual reporting periods thereafter. Earlier application is prohibited. The Company does not expect SFAS 167 to have a material effect on its financial statements.
In June 2009, the FASB issued SFAS 168 “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles-a replacement of FASB Statement No. 162”. The FASB Accounting Standards Codification will become the source of authoritative U.S. Generally Accepted Accounting Principles
(GAAP) recognized by the FASB to be applied by nongovernmental entities.
Following SFAS 168, the Board will not issue new standards in the form of Statements, FASB Staff Positions, or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates. This Statement is effective for financial statements issued for interim and annual periods ending after September
15, 2009. On the effective date of this Statement, the Codification will supersede all then-existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting
literature not included in the Codification will become non-authoritative. The Company does not expect SFAS 168 to have a material effect on its financial statements.
|
As of June 30, |
As of December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ |
ִִ$ |
$ | $ | |||||||||||||
|
Cash in Banks |
10,863 | 5,873 | 8,588 | 8,817 | ||||||||||||
|
Deposits |
3,126 | 3,646 | 6,203 | 3,176 | ||||||||||||
|
Total |
13,989 | 9,519 | 14,791 | 11,993 | ||||||||||||
BB F17
92

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 4 - Accounts receivable (in US Dollar)
|
As of June 30, |
As of December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ |
ִִ$ |
$ | $ | |||||||||||||
|
Trade receivables |
3,034 | 395 | 3,931 | 6,983 | ||||||||||||
|
Government Institutions |
5,395 | 4,536 | 5,893 | 4,201 | ||||||||||||
|
Other |
1,289 | 1,489 | - | 614 | ||||||||||||
|
Total |
9,718 | 6,420 | 9,824 | 11,798 | ||||||||||||
| Allowance for doubtful accounts | NIL | NIL | NIL | NIL | ||||||||||||
Note 5 - Property and equipment (in US Dollar)
|
As of June 30, |
As of December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ |
ִִ$ |
$ | $ | |||||||||||||
|
Electronic Equipment* |
138,069 | 138,069 | 138,069 | 38,069 | ||||||||||||
|
Office Furniture and Equipment |
1,714 | 1,714 | 1,714 | 1,714 | ||||||||||||
|
Computer and Computer Equipment |
4,156 | 3,871 | 4,156 | 3,869 | ||||||||||||
|
Total |
143,939 | 143,654 | 143,939 | 43,652 | ||||||||||||
|
Less: Accumulated Depreciation |
(45,297 | ) | (28,969 | ) | (37,168 | ) | (21,035 | ) | ||||||||
|
Net |
98,642 | 114,685 | 106,771 | 22,617 | ||||||||||||
| Depreciation charged are as follows | 8,129 | 7,934 | 16,133 | 6,703 | ||||||||||||
*In 2008, the Company purchased manufacturing moulds from a related party in return for shares which have not as yet been issued, in the equivalent amount of $ 100,000. The purchase was valued at market value (which was the most objective measure of value) as of the purchasing date, including installation and delivery (see
note 17 – Related party transactions).
Note 6 - Short term bank debt
The short term bank debt interest rate is 8.7%-10.65% for the year. Interest expenses are as follows: for the period ended June 30, 2009 and 2008: $ 823 and $1,058 respectively and for the year ended December 31, 2008
and 2007 - $1,785 and $1,131 respectively.
The Company's bank credit is guaranteed with a bank guarantee from a related party in the sum of $10,000, and pledges on bank deposits, and cheques and securities which the company may deposit at those banks from time to time. The credit line is due
on demand. There are no other covenants.
BB F18
93

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 7 – Accounts payable and accrued expenses (in US Dollar)
|
As of June 30, |
As of December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ |
ִִ$ |
$ | $ | |||||||||||||
|
Open accounts |
10,353 | 12,847 | 3,318 | 7,237 | ||||||||||||
|
Checks payable |
- | - | 6,602 | 1,809 | ||||||||||||
|
Advance payment from customers |
- | - | - | 8,861 | ||||||||||||
|
Employees |
1,918 | - | 2,077 | 1,595 | ||||||||||||
|
Shareholder's expenses on behalf of the company |
43,028 | - | - | - | ||||||||||||
|
OCS |
2,671 | 1,079 | 2,179 | 735 | ||||||||||||
|
Accrued expenses (mainly legal fees) |
59,094 | 62,197 | 67,174 | 86,833 | ||||||||||||
|
Others (including warranty accrual) |
6,576 | 889 | 4,783 | 484 | ||||||||||||
| 123,640 | 77,012 | 86,133 | 107,554 | |||||||||||||
Note 8- Stockholders' loans
|
As of June 30, |
As of December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
See note a below |
107,000 | 71,000 | 89,000 | 53,000 | ||||||||||||
|
See note b below |
18,000 | 18,000 | 18,000 | 18,000 | ||||||||||||
|
See note c below |
17,703 | 20,698 | 18,248 | 18,039 | ||||||||||||
| 142,703 | 109,698 | 125,248 | 89,039 | |||||||||||||
|
a. |
The Company received a stockholders` loan from Life Support Ltd. ("Life support"). The loan is unsecured and non-interest bearing and is payable on demand. There is no significant difference between its book value and the fair value. |
|
b. |
In November 2005, the Company received from Dr. Amirav (shareholder) a loan in the amount of $18,000. The loan is unsecured and non-interest bearing and is payable on demand. There is no significant difference between its book value and the fair value. |
|
c. |
In 2002 the Company received from CAPI Project Development, Ltd. (GAPI) (Shareholder) a loan linked to NIS. The loan is unsecured and non-interest bearing and is payable on demand. Conditions and redemption have not been determined. There is no significamt difference between its book value and the fair value. |
BB F19
94

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 8- Stockholders' loans (continued)
Imputed interest on those loans has been charged a rate of 5% representing a reasonable estimated of the market rate with a credit to paid in capital.
Note 9 - Share capital
The Company's equity includes ordinary shares from three types A, B and C. Stock A holders are entitled to 60% of all dividends and stock B holders are entitled to 40%, until $500 thousand dollars have been paid to stock A holders. Once stock A holders have received dividends amounting to $500 thousand, stock A holders will not have the
right to receive any additional dividends up until stock B holders have received dividends as follows: Life Support Ltd. received all of their investment proceeds, stockholders' loans and suppliers' credit and Rammport Finance Ltd. received its investment proceeds.
Following the payments of the above amounts, stock A holders will have the right to receive 60% of all further dividends only after due payments are made to stock C holders.
This holds true for a period totaling four years since the above is fulfilled. If within this period, not all of the company's profits are distributed via dividends, then at the first distribution of dividends, A stock holders will receive 60% and B holders 40% after deducting distributions to stock C holders. Following the distribution
as explained above, both A and B stock will be ranked pari passu in regards to dividend distribution. Deferred
stock has no dividend rights. A and B stock holders rank pari passu in regards to voting rights. C stock and deferred stock holders do not have any voting rights.
In March 2007, the Company's board of directors decided to transfer 8,453 shares type B from 10,000 stock held in trust to Life Support, in respect of its investments in the Company. The remaining 1,547 shares are issued but still held in trust. Accordingly, these shares have not been included in the computation of the weighted average
number of shares.
In July 2009 the shareholders of the Company resolved to consolidate all the share capital of the Company into ordinary shares and Company agreed on a new cap table regarding to its stockholders equity (See note 18- Subsequent events).
95

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
The following table presents total revenues in US Dollar classified according to geographical destination for the years ended December 31,2008 and 2007 and periods of six months ended June 30, 2009 and 2008:
|
Period of six months ended June 30, |
Year ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Italy |
- | - | 31,250 | 21,200 | ||||||||||||
|
Spain |
- | - | - | 2,700 | ||||||||||||
|
Hungary |
- | 3,355 | 3,355 | - | ||||||||||||
|
Germany |
- | 2,326 | 2,326 | - | ||||||||||||
|
Portugal |
9,000 | - | - | - | ||||||||||||
|
Poland |
1,911 | - | - | - | ||||||||||||
|
Israel |
3,684 | 993 | 2,909 | - | ||||||||||||
|
Far East |
969 | - | 3,359 | - | ||||||||||||
|
Others |
180 | - | - | 113 | ||||||||||||
|
Total *) |
15,744 | 6,674 | 43,199 | 24,013 | ||||||||||||
*) Revenues are presented net of warranty costs.
The company has only one operational segment.
The company's long-lived assets are located in Israel.
The Company has major customers as follow:
|
Period |
Number of major customers |
Representing % of total revenues |
||||||
|
Period of six months ended 30, June 2009 |
1 | 57 | % | |||||
|
Period of six months ended 30, June 2008 |
1 | 50 | % | |||||
|
Year ended December 31, December 2008 |
1 | 72 | % | |||||
|
Year ended December 31, December 2007 |
1 | 88 | % | |||||
The company is economically dependent on the above customers.
BB F21
96

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 11 – Cost of sales
|
Six months ended June 30, |
Year ended
December 31, |
||||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
||||||||||||||
|
Unaudited |
Audited |
||||||||||||||||
|
Purchases |
$ | $ | $ | $ | |||||||||||||
|
Opening Inventory |
11,565 | - | - | 1,596 | |||||||||||||
|
Purchases |
15,300 | 11,293 | 33,599 | 19,511 | |||||||||||||
|
Closing Inventory |
(19,579 | ) | (7,341 | ) | (11,565 | ) | - | ||||||||||
| 7,286 | 3,952 | 22,034 | 21,107 | ||||||||||||||
|
Direct expenses: |
|||||||||||||||||
|
Insurance |
- | - | 1,862 | 1,010 | |||||||||||||
|
Other |
115 | 1,032 | 914 | 1,884 | |||||||||||||
| 115 | 1,032 | 2,776 | 2,894 | ||||||||||||||
|
Salaries |
- | - | - | 5,284 | |||||||||||||
|
Depreciation |
7,720 | 7,538 | 15,318 | 5,703 | |||||||||||||
| 7,720 | 7,538 | 15,318 | 10,987 | ||||||||||||||
|
Total |
15,121 | 12,522 | 40,128 | 34,988 | |||||||||||||
|
|
* After deduction in respect of inventory, deemed to be obsolete amounting to approximately $7,000, which has been charged to cost of sales for the year ended December 31, 2007. |
BB F22
97

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 12 – Selling, general and administrative expenses (in US Dollar)
|
A- Selling |
||||||||||||||||
|
Six months ended June 30, |
Year ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Sales commissions |
- | 5,835 | 5,541 | 2,147 | ||||||||||||
|
OCS royalties (note 14) |
557 | - | 1,436 | 854 | ||||||||||||
|
Marketing and advertisement |
26,828 | 26,877 | 10,493 | 34,509 | ||||||||||||
|
Export expenses |
- | 1,785 | 3,825 | - | ||||||||||||
|
Travel |
- | 10,088 | 9,180 | 6,773 | ||||||||||||
|
Selling promoting and Others |
12,183 | 8,242 | 46,076 | 18,706 | ||||||||||||
| 39,568 | 52,827 | 76,551 | 62,989 | |||||||||||||
|
B – General and administrative expenses |
||||||||||||||||
|
Management fees |
18,000 | 19,615 | 37,488 | 71,000 | ||||||||||||
|
Rent and office maintenance |
3,953 | 2,318 | 5,022 | 4,024 | ||||||||||||
|
Professional services |
5,348 | 9,457 | 31,543 | 20,205 | ||||||||||||
|
Depreciation |
409 | 396 | 815 | 1,000 | ||||||||||||
|
Patent |
8,536 | 13,661 | 26,757 | 18,556 | ||||||||||||
|
Others |
2,238 | 2,914 | 10,457 | 3,522 | ||||||||||||
| 38,484 | 48,361 | 112,082 | 118,307 | |||||||||||||
|
Total |
78,052 | 101,188 | 188,633 | 181,296 | ||||||||||||
BB F23
98

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 13 – Financial expenses net (in US Dollar)
|
Six months ended June 30, |
Year ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
|
Financing expenses: |
$ | $ | $ | $ | ||||||||||||
|
Exchange differences |
856 | 5,261 | 5,371 | 2,023 | ||||||||||||
|
Imputed interest on stockholder's loan |
3,380 | 2,555 | 5,437 | 3,527 | ||||||||||||
|
Bank's commissions |
181 | 388 | 501 | 2,484 | ||||||||||||
| 4,417 | 8,204 | 11,309 | 8,034 | |||||||||||||
|
Financing income: |
||||||||||||||||
|
Interest |
(12 | ) | (68 | ) | (5,308 | ) | (639 | ) | ||||||||
|
Total |
4,405 | 8,136 | 6,001 | 7,395 | ||||||||||||
Note 14 - Government grants
The Government of Israel, through OCS, encourages Research and Development projects that result in products for export. The Company received grants of approximately $268,029 from the OCS through December 31, 2005, which were used to fund its initial product development. Pursuant to the terms of these grants, repayment is done exclusively
by way of royalties as follows: a rate of 3.0% and 3.5% of revenues derived from sales of products funded with these grants, up to 100%-150% of certain grant amounts received.
In the event that a project funded by the Office of the Chief Scientist does not result in the development of a product that generates revenues, the Company would not be obligated to repay the grants which the Company received for the product's development. The maximum contingency liability for the OCS is $268,029. The terms of the grant
also require that the know-how derived from research and development funded by the OCS may not be transferred to third parties without the approval of the OCS. The Company is required to present to the OCS bi – annual reports in respect of its revenues and the corresponding royalties – as of the balance sheet date of December 31, 2008 and 2007; and for the period of six months ended June 30, 2009 and 2008.
BB F24
99

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 15 – Income on taxes (In US Dollars)
a. The Company has income tax assessments which are considered final through 2003. The Company has accumulated tax losses amounting to 179,000 $ as of December 31, 2008, approximately. Deferred taxes in respect of these losses have not been included in the accompanying Company's balance sheet due to the uncertainty in their realization.
b. The Company is subject to the Israeli income tax regulations. Under these regulations the Company's tax rate is as follows: in 2007: 29%, in 2008: 27%, in 2009: 26% and in 2010: 25%. In 2011: 24%, in 2012: 23%, in 2013: 22%, in 2014: 21%, in 2015: 20%, and from 2016 onwards the tax rate will be 18%.
c. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts deductible for income tax purposes. Significant components of the Company deferred tax assets are as follows in US Dollars:
|
Six months ended June 30, |
Year ended December 31, |
|||||||||||||||
|
2009 |
2008 |
2008 |
2007 |
|||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Tax benefits on losses and allowance* |
(277,000 | ) | (244,934 | ) | (265,559 | ) | (229,678 | ) | ||||||||
|
Valuation allowance |
277,000 | 244,934 | 265,559 | 229,678 | ||||||||||||
| Net deferred tax asset | - | - | - | - | ||||||||||||
(*) Carry forward losses can be utilized with no expiration date.
The valuation allowance for deferred income taxes relates primarily to the uncertainty of utilization of the net operating loss carry forwards, which are dependent on the future profitability of the Company.
Management currently believes that since the Company has a history of losses it is more likely than not that the deferred tax regarding the loss carry forwards and other temporary differences will not be realized in the foreseeable future.
BB F25
100

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 15 – Income on taxes (In US Dollars) (continued)
|
|
Reconciliation of the theoretical tax expenses
|
A reconciliation between the theoretical tax expense, assuming all income is taxed at the statutory tax rate applicable to income of the Company, and the actual tax expense as reported in the statement of operations is as follows in US Dollars:
| Six months ended June 30, | Year ended December 31, | |||||||||||||||
| 2009 | 2008 | 2008 | 2007 | |||||||||||||
| Unaudited | Audited | |||||||||||||||
| $ | $ | $ | $ | |||||||||||||
|
Loss before income taxes, as reported in the statements of operations |
(81,834 | ) | (115,172 | ) | (191,563 | ) | (199,666 | ) | ||||||||
|
Statutory tax rate |
26 | % | 27 | % | 27 | % | 29 | % | ||||||||
|
Theoretical tax recovery |
(21,277 | ) | (31,096 | ) | (51,722 | ) | (57,903 | ) | ||||||||
|
Non deductible expenses |
879 | 689 | 1,468 | 1,023 | ||||||||||||
|
Valuation allowance |
20,398 | 30,407 | 50,254 | 56,880 | ||||||||||||
|
Actual tax expense |
- | - | - | - | ||||||||||||
BB F26
101

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 16- Commitments and contingencies
A. The Company's product lines are distributed to retail channels either through distributors or through direct delivery. The Company generally maintains sufficient inventories to meet customer orders as received. From time to time, the Company may experience back orders that result from variations
in demand for products outside of its control or expectations. The Company has an exclusive distribution agreement in Italy.
B. In April, 2007 the Company entered into a manufacturing agreement with a third party manufacturer “Suko Investment & Manufacturing Limited” (Suko). According to this agreement Suko will manufacture the Baby's Breath products for the Company according to the terms agreed
in the agreement which includes packing and shipping services. In addition, according to the agreement any rejected parts will be replaced by Suko with no charge, up to 3% of total shipment, with QA test as required.
C. Lease commitments:
The Company's facilities are rented under several lease agreements in Israel for periods ending in 2011. Future minimum rental commitments under non-cancelable operating leases for the years ended December 31, are as follows in US Dollars:
|
2009 |
$ | 4,300 | ||
|
2010 |
4,300 | |||
|
2011 |
358 | |||
| $ | 8,958 |
Rent expenses for the year ended 31, 2008 and 2007, were approximately $4,684 and $3,853 respectively. For the six months ended June 30,2009 and 2008 were approximately $2,232 and $2,304, respectively.
D. Repayment of OCS research and development grants:
The Company estimates that the maximum repayments of governments grant under the terms with the OCS are $268,029 (see note 14 - Government grants).
E. Interest on Stockholder Loan:
A founding shareholder has claimed interest on its start-up loans to the Company [see Note 8(c)]. The amount of the interest claimed is approximately $13,000. In management’s opinion, this claim is without merit since the loans are non - interest bearing. Accordingly, no provision has been recorded in the financial
statements for this amount.
BB F27
102

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 17-Related party transactions
Life Support LTD ("Life Support") - In April 2004 the Company entered into an investment agreement with Life Support, one of the Company's stockholders. As per the terms of the agreement, Life Support provides management services to the
Company. In consideration for such management services Life Support is entitled to a monthly fee. As of October 2007 the monthly fee was set at $3,000. All monthly fees are currently not paid and are reflected in the financial statements as a loan which bears no interest, has no maturity date and is payable on demand.
Microdel LTD ("Microdel") - In April 2007 the Company signed an agreement with Microdel, one of the Company's stockholders. According to this agreement the minimum amount to be invested by Microdel is $500,000 up to a maximum of $2,000,000.
The agreement governed the terms upon which Microdel will invest in the Company. The Agreement was replaced by the New Microdel Agreement (see below).
New agreement with Microdel
In June 2009 the Company signed a new agreement with Microdel. According to this agreement Microdel will restrurcture the capital whereby the company will become a wholly- owned subsidiary of a newly incorporated U.S. holding company. Following this transaction, the U.S. holding company expects to seek a listing and offering of its shares
in the U.S.A. where it is expected that the majority of the proceed will come from institutional investors. The Company and Microdel have agreed that the total monies (in cash and monetary equivalent) that Microdel invested in the Company, as of the date upon which the agreement was signed, amounted of $314,000, including $100,000 in moulds whose production was funded by Microdel. In exchange for Microdel’s investment in the Company, Microdel shall be entitled to 375 ordinary shares in the Company, subject
to certain provisions taking into account Microdel's undertaking to assume costs and expenses which were incurred to obtain a listing and offering.
Microdel undertook to assume all of the registration expenses, without exception. Notwithstanding the above, following the success of the registration, the Company shall assume the expenses which were incurred to obtain a listing and offering.
Microdel shall finance the cost of employing the personnel hired to work for the Company. The sum of the amount of funding for the interim period April- November 2009 in the amount of $80,000 (but not including funding for personnel and service providers hired) shall be considered as an investment by Microdel for which the Company
has allocated 8,158 conditional shares to be held in trust with voting rights under restricted circumstances. Upon successful completion of an offering, these shares will be transferred to Microdel.
BB F28
103

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 17-Related party transactions (continued)
In the event the registration process does not result in a successful offering by no later than November 30, 2009, for any reason whatsoever, Microdel shall pay all of the registration expenses, without exception, beyond the $150,000 deposited in a special account; and shall indemnify the Company for any claim brought against it in connection
with undertakings assumed by the Company, at Microdel’s request, to pay sums related to the offering process (beyond the amount held in the special account). The primary agreement will be terminated, the conditional shares will be transferred by the trustee to the current stockholders (except for Microdel) and Microdel shall be entitled to continue to own only the unconditional shares. In the cases that there will be no offering, as described in the agreement, the Company will issue to Microdel
additional shares due to the amounts that Microdel expensed in respect to the offering process, less an amount between the range $55,000-$80,000 or certain offering costs. In addition, the Company will indemnify Microdel up to $55,000 for consulting expenses related to the offering upon termination of the offering Microdel will have no recourse against the Company and its officers.
In certain cases as set forth in the agreement Microdel will be issued additional shares in the Company in case the offering process is terminated In March 2009, Microdel gave to the Company a guarantee for obligation in the amount of $10,000.
Employment agreement:
In July 2009 Baby's Breath entered into an employment agreement with its CEO, under which New Air undertook to grant to the CEO, under a stock option plan to be adopted by us for that purpose, an amount of 4% of our outstanding share capital prior to the registration, which shall be equally vested over a period of 3 years such that
at the end of each year 1.33% of the entire amount of options shall become vested.
BB F29
104

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 17-Related party transactions (continued)
|
Related parties (In US Dollars) |
Balance as of June 30 |
Balance as of December 31 |
||||||||||||||
| 2009 |
2008 |
2008 |
2007 | |||||||||||||
| Unaudited |
Unaudited |
|||||||||||||||
| $ | $ | $ | $ | |||||||||||||
| a. Balances with related parties: | ||||||||||||||||
| Stockholders' loans | 142,703 | 109,698 | 125,248 | 89,039 | ||||||||||||
| Expenses paid on behalf of the company | 43,028 | - | - | - | ||||||||||||
| b Transactions with Related Parties | For six months ended June 30 | For year ended December 31 | ||||||||||||||
| 2009 | 2008 | 2008 | 2007 | |||||||||||||
|
Unaudited |
Audited |
|||||||||||||||
| Administrative expenses | $ | $ | $ | $ | ||||||||||||
| Management fees: | ||||||||||||||||
| Life Support | 18,000 | 18,000 | 36,000 | 53,000 | ||||||||||||
| Dr. Amirav | - | - | - | 18,000 | ||||||||||||
| 18,000 | 18,000 | 36,000 | 71,000 | |||||||||||||
All the balances and transactions mentioned above regarding related parties refer to stockholders. See also comment in note 5 - Property and equipment.
Note 18 - Subsequent events
A. Stockholders rights agreement of September 2009
On September 7th, 2009 the Company has entered into a stockholders` agreement (the "stockholders` agreement") with the Company's stockholders. The stockholders` agreement defines and governs the various rights that the stockholders will have with
respect to their holdings in the Company, until the consummation of the registration for trading of the Company shares of common stock under this registration statement. The agreement provides certain rights for the Company stockholders who hold 5% of the Company outstanding share capital which include a right of first refusal and participation rights of such stockholders in case of a sale of shares by the Company stockholders, and a right to participate in future issuances of securities by the Company under
a preemptive right.
BB F30
105

BABY'S BREATH LTD
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2008 and 2007
Note 18 - Subsequent events (continued)
The stockholders agreement terminates and ceases to be in effect upon the registration for trade on stock exchange, OTCBB. The board of the Company issued share capital pursuant to any applicable securities laws and regulations, whether as part of an offering to the public or otherwise
B. Acquisition agreement of September 2009
On September 7th,2009, all of the shares of the company were acquired by New Air Inc, a U.S. holding company in an exchange of shares. Under the terms of the agreement, New Air Inc. issued 200 of its shares for each share of the company. Following this exchange, the former stockholders of the company now control 96.98% of the total
shares of New Air Inc. The agreement also includes provisions which would govern the rights and liabilities of the Company stockholders until the completion of the registration of the Company shares for trading.
C. Issuance of shares
Subsequent to the period ended June 30th, 2009, the Company issued 9,420 common stock to private investors as follows: 8,533 common stock were allocated to Microdel for various services in addition to the common stock that Microdel already owns (see note 17-
New agreement with Microdel); 423 common stock – were allocated for services that were received after the balance sheet date and valued at $53,600; 464 common stock have been allocated as a capital raising commission to A.M.M Magal, Distribution and Business Development Ltd. (the full commission were already recorded as issuance expenses net of additional paid in capital at the raising date).
D. Subsequent events
Subsequent events have been evaluated up to October 16th, 2009.
BB F31
106
NEW AIR INC.
CONSOLIDATED BALANCE SHEET – PRO - FORMA
As of June 30, 2009
(Unaudited)
(In US Dollars)
|
page |
||||
| PF F2-PF F3 | ||||
| PF F4-PF F5 | ||||
PF F1
107
NEW AIR INC.
CONSOLIDATED BALANCE SHEET – PRO - FORMA
(In US Dollars)
|
As of June 30, 3009 |
|||||||||||||||||
|
Unaudited |
|||||||||||||||||
|
Note |
NEW AIR INC |
BABY'S BREATH LTD |
Adjustments |
Pro-Forma
Consolidated |
|||||||||||||
|
(August 31, 2009) |
(June 30, 2009) |
||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||
|
ASSETS |
|||||||||||||||||
|
CURRENT ASSETS: |
|||||||||||||||||
|
Cash and cash equivalents |
56,678 | 13,989 | - | 70,667 | |||||||||||||
|
Accounts receivable |
- | 9,718 | - | 9,718 | |||||||||||||
|
Inventory |
- | 19,579 | - | 19,579 | |||||||||||||
|
TOTAL CURRENT ASSETS |
56,678 | 43,286 | - | 99,964 | |||||||||||||
|
Property and equipment, net |
- | 98,642 | 98,642 | ||||||||||||||
|
TOTAL ASSETS |
56,678 | 141,928 | - | 198,606 | |||||||||||||
|
LIABILITIES AND STOCKHOLDERS' (DEFICIENCY) EQUITY |
|||||||||||||||||
|
CURRENT LIABILITIES |
|||||||||||||||||
|
Short term bank debt |
- | 8,178 | - | 8,178 | |||||||||||||
|
Accounts payable and accrued expenses |
2,000 | 123,640 | - | 125,640 | |||||||||||||
|
Stockholders' loans |
- | 142,703 | - | 142,703 | |||||||||||||
|
TOTAL CURRENT LIABILITIES |
2,000 | 274,521 | - | 276,521 | |||||||||||||
|
LONG TERM LIABILITIES |
|||||||||||||||||
|
Accrued severance pay |
- | 1,471 | - | 1,471 | |||||||||||||
|
TOTAL LONG TERM LIABILITIES |
- | - | 1,471 | ||||||||||||||
|
TOTAL LIABILITIES |
2,000 | 275,992 | - | 277,992 | |||||||||||||
The footnotes are an integral part of these Pro- Forma financial statements.
PF F 3
108
NEW AIR INC.
CONSOLIDATED BALANCE SHEET – PRO - FORMA (Continued)
(In US Dollars)
|
As of June 30, 2009 |
|||||||||||||||||||
|
Unaudited |
|||||||||||||||||||
| Note |
NEW AIR INC |
BABY'S BREATH LTD |
Adjustments |
Pro-Forma Consolidated |
|||||||||||||||
|
(August 31, 2009) |
(June 30, 2009) |
||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||
|
STOCKHOLDERS' DEFICIENCY (EQUITY) |
|||||||||||||||||||
|
Capital |
|||||||||||||||||||
|
Ordinary shares: |
3 | 80 | 5,689 | 591 | 6,360 | ||||||||||||||
|
Additional paid in capital |
3 | 54,598 | 611,481 | (591 | ) | 665,488 | |||||||||||||
|
Deposit on shares |
- | 314,152 | - | 314,152 | |||||||||||||||
|
Accumulated deficit |
- | (1,065,386 | ) | - | (1,065,386 | ) | |||||||||||||
|
TOTAL STOCKHOLDERS' DEFICIENCY (EQUITY) |
54,678 | (134,064 | ) | - | (79,386 | ) | |||||||||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIENCY (EQUITY) |
56,678 | 141,928 | - | 198,606 | |||||||||||||||
The footnotes are an integral part of these Pro- Forma financial statements.
PF F4
109
NEW AIR INC.
NOTES TO THE UNAUDITED PRO-FORMA CONSOLIDATED BALANCE SHEET
June 30, 2009
Notes
Note 1 – Basis of presentation
This pro-forma consolidated balance sheet has been prepared pursuant to a share exchange agreement dated September 7th, 2009, between New Air Inc ("New Air" or the “Company”), a
holding company which was incorporated in the State of Maryland on July 10th, 2009 and Baby’s Breath Ltd. (“Baby's Breath”), an Israeli company founded in 2001 by a group of leading physicians and highly experienced professionals that developed a patented medical device for the treatment of respiratory diseases.
New Air Inc. acquired Baby’s Breath in a stock for stock exchange at a 1:200 ratio whereby the Company issued 6,280,600 shares of its common stock for 31,403 shares in Baby’s Breath, Ltd. As a consequence, Baby’s Breath became a wholly owned subsidiary of the Company and the stockholders of Baby’s Breath acquired
96.98 percent of the total shares in the Company.
The accompanying Pro-Forma consolidated balance sheet presents the balance sheets of New Air and Baby's Breath as if the acquisition of Baby's Breath by New Air occurred on June 30, 2009 and reflects the balance sheets as of August 31, 2009 (as if New Air was in existence as of June 30, 2009) for New Air and June 30, 2009 for Baby's Breath
respectively. The unaudited pro-forma consolidated balance sheet and notes are presented for information purpose only and do not profess to represent what the balance sheet would have been had the transaction in fact occurred on the date indicated.
For accounting purposes, the transaction is being accounted for as a recapitalization of Baby's Breath with the issuance of shares for the net assets of New Air.
The unaudited consolidated Pro-Forma balance sheet should be read in consideration with the unaudited financial statements of Baby's Breath as at June 30, 2009, the audited financial statements of Baby's Breath as at December 31, 2008, and the unaudited financial statements of New Air as at August 31, 2009. Including the notes thereto and
other information accompanying the registration statement. No income statements are included as New Air had no operation and the income statement of Baby's Breath for the year ended December 31, 2008, and for the six months period ended June 30, 2009, as included in the registration statement would reflect the consolidated operation.
Note 2 – Pro-Forma effect of the share exchange agreement
As per the terms and conditions of the share exchange agreement (SEA) between New Air and Baby's Breath, New Air will acquire all of the 31,403 Baby's Breath outstanding shares from the existing Baby's Breath stockholders by issuing 6,280,600 New Air common stock . Following completion of the transaction, Baby's Breath will become a wholly-owned
subsidiary of New Air. The acquisition of Baby's Breath has been accounted for as a capital transaction using the reverse takeover accounting rules for transactions that do not constitute business combinations for accounting purposes. As
PF F5
110
NEW AIR INC.
NOTES TO THE UNAUDITED PRO-FORMA CONSOLIDATED BALANCE SHEET
June 30, 2009
Note 2 – Pro-Forma effect of the share exchange agreement (continued)
such, Baby's Breath will be the continuing entity for accounting purposes, while the legal shares capital will be that of New Air.
Exchange of common shares
Pursuant to the terms of the SEA, New Air will acquire all of the Baby's Breath outstanding shares from the Baby's Breath stockholders. 6,280,600 New Air common stock will be issued.
The transaction involving New Air, a non operating holding company with nominal net monetary assets, is a capital transaction in substance for Baby's Breath. As a result, this transaction is viewed as the issuance of equity by Baby's
Breath for the net assets in New Air. Accordingly, the unaudited consolidated Pro-Forma balance sheet represents a continuation of Baby's Breath.
New Air's share capital is eliminated on consolidation of Baby's Breath and New Air. Net assets available in New Air are $54,678, which is recorded as additional paid in capital.
Note 3 – Share capital
In accordance with reserve take-over accounting, the Pro-Forma balance sheet is a continuance of Baby's Breath, however, the capital structure of issued and outstanding common shares is that of:
|
a) |
Authorized: |
50,000,000 common stock – $0.001 par value
|
b) |
Issued: |
|
Number of shares |
Amount |
|||||||
| $ | ||||||||
|
Baby's Breath common shares |
31,403 | 5,689 | ||||||
|
Adjustment for reverse take-over (1:200) |
6,249,197 | - | ||||||
|
New Air common stock |
79,681 | 80 | ||||||
|
Adjustment to par value |
- | 591 | ||||||
| 6,360,281 | 6,360 | |||||||
PF F6
111
PART II--INFORMATION NOT REQUIRED IN PROSPECTUS
The following table sets forth the expenses in connection with the issuance and distribution of the securities being registered hereby. All such expenses will be borne by the registrant; none shall be borne by any selling stockholders.
| SEC Registration Fee | $ | 69 | 31 | |
|
|
35,000 | 00 | ||
| TOTAL | $ | 35,069 | 31 | |
The laws in the State of Maryland under the Maryland Corporations and Association Section § 2-418(b) states:
A corporation may indemnify any director made a party to any proceeding by reason of service in that capacity unless it is established that the act or omission of the director was material to the matter giving rise to the proceeding; and was committed in bad faith; or was the result of active and deliberate dishonesty; or the director actually
received an improper personal benefit in money, property, or services; or in the case of any criminal proceeding, the director had reasonable cause to believe that the act or omission was unlawful. It further states that the indemnification may be against judgments, penalties, fines, settlements, and reasonable expenses actually incurred by the director in connection with the proceeding. However, if the proceeding was one by or in the right of the corporation, indemnification may not be
made in respect of any proceeding in which the director shall have been adjudged to be liable to the corporation. The termination of any proceeding by judgment, order, or settlement does not create a presumption that the director did not meet the requisite standard of conduct set forth in this subsection. The termination of any proceeding by conviction, or a plea of nolo contendere or its equivalent, or an entry of an order of probation prior to judgment, creates a rebuttable presumption
that the director did not meet that standard of conduct.
112
A corporation may not indemnify a director or advance expenses under this section for a proceeding brought by that director against the corporation, except for a proceeding brought to enforce indemnification under this section; or if the charter or bylaws of the corporation, a resolution of the board of directors of the corporation, or
an agreement approved by the board of directors of the corporation to which the corporation is a party expressly provide otherwise.
A director may not be indemnified in respect of any proceeding charging improper personal benefit to the director, whether or not involving action in the director's official capacity, in which the director was adjudged to be liable on the basis that personal benefit was improperly received.
Unless limited by the charter, an officer of the corporation shall be indemnified as and to the extent provided for a director and shall be entitled, to the same extent as a director.
Since July 2009 and through September 2009 we issued a total of 195,677 shares of our common stock to investors. All such securities were issued in reliance upon an exemption from registration under Section 4(2) of the Securities Act as a transaction not involving a public offering.
In September 7, 2009 we have issued 6,280,600 shares of our common stock to the shareholders of Baby’s Breath pursuant to the acquisition agreement under which we purchased 100% of the outstanding share capital of Baby’s Breath.
There has been no other issuance of shares.
| 3.2 | Amendments to Articles |
| 14.1 | Code of Ethics |
| 21.1 | Subsidiaries of the Registrant |
|
23.2 |
Consent of SEC ATTORNEYS, LLC (See Exhibit 5.1) |
113
|
To file, during any period in which it offers or sells securities, a post-effective amendment to this registration statement to: |
|
Include any prospectus required by Section 10(a)(3) of the Securities Act; |
|
Reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement; |
|
Include any additional or changed material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
|
For determining liability under the Securities Act, to treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering. |
|
To file a post-effective amendment to remove from registration any of the securities that remains unsold at the end of the offering. |
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the
Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a Director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by
controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
|
For determining any liability under the Securities Act, to treat the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant under Rule 424(b)(1), or (4) or 497(h) under the Securities Act as part of this registration
statement as of the time the Commission declared it effective. |
|
For determining any liability under the Securities Act, to treat each post-effective amendment that contains a form of prospectus as a new registration statement for the securities offered in the registration statement, and that offering of the securities at that time as the initial bona fide offering of those securities. |
114
|
For determining liability of the undersigned registrant under the Securities Act to purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell
the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
|
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
|
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
|
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
|
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
|
Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.
Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in
any such document immediately prior to such date of first use. |
115
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements of filing on Form S-1 and authorized this registration statement to be signed on its behalf by the undersigned, in the Town of New Haven, State of Connecticut on October
16, 2009.
|
NEW AIR, INC.
(Registrant) | ||
|
By:/s/ LIOR CARMELI | ||
|
Lior Carmeli | ||
|
(Principal Executive Officer) | ||
|
By:/s/ DAVID KAPON | ||
|
Chief Financial Officer | ||
|
(Principal Financial and Accounting Officer) |
|
NAME |
TITLE |
||||
|
/s/ LIOR CARMELI |
Lior Carmeli |
Chief Executive Officer (Principal Executive Officer) |
|||
|
/s/ DAVID KAPON |
David Kapon |
Chief Financial Officer (Principal Financial and Accounting Officer) |
|||
|
/s/ JACOB BAL |
Jacob Bal |
Chief Technology Officer |
|||
|
/s/ YOSSEF DE LEVIE |
Yossef De Levie |
Chairman of the Board |
|||
|
/s/ ADI PLASCHKES |
Adi Plaschkes |
Director |
|||
|
/s/ ASHER KIMCHI |
Dr. Asher Kimchi |
Director |
|||
|
/s/GILAD GOLAN |
Gilad Golan |
Director |
|||
|
/s/ALON ZIFRONI |
Alon Zifroni |
Director |
|||
|
/s/ASSAF HALAMISH |
Assaf Halamish |
Director |
116
