Attached files
| file | filename |
|---|---|
| EX-5.1 - OPINION - MMRGlobal, Inc. | exh5-1.htm |
| EX-23.1 - CONSENT - MMRGlobal, Inc. | exh23-1.htm |
As filed with the Securities
and Exchange Commission on October 13, 2009
Registration No. 333-________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
![]()
FORM S-1
![]()
MMR INFORMATION SYSTEMS, INC.
|
Delaware (State or other jurisdiction of incorporation or organization) |
7389 (Primary Standard Industrial Classification Code Number) |
33-0892797 (I.R.S. Employer Identification Number) |
468 Camden Drive, Suite 200
Beverly Hills, California 90210
(310) 476-7002
(Address, including zip code, and telephone number, including
area code, of Registrant's principal executive offices)
![]()
Robert H. Lorsch
Chief Executive Officer
MMR Information Systems, Inc.
468 Camden Drive, Suite 200
Beverly Hills, California 90210
(310) 476-7002
area code, of agent for service)
![]()
Copies to:
Mark L. Skaist, Esq.
Stradling Yocca Carlson & Rauth,
a Professional Corporation
660 Newport Center Drive, Suite 1600
Newport Beach, California 92660
(949) 725-4100
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated file, or a smaller
reporting company. See the definitions of "large accelerated filer," "accelerated file" and "smaller reporting
company" in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o |
Accelerated filer o |
Non-accelerated filer o
|
Smaller reporting company x |
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered |
Amount to |
Proposed Maximum |
Proposed Maximum |
Amount of |
|||||
|
Common Stock, $0.001 par value per share |
35,975,049 |
$0.08 |
$2,878,004 |
$160.60 |
![]()
(1) Pursuant to Rule 416 under the Securities Act, the shares being registered hereunder include such indeterminate number of shares of common stock as may be issuable with respect to the shares being registered hereunder as a result of stock splits, stock dividends or similar transactions.
(2) Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(c) of the Securities Act of 1933, using the average of the high and low prices as reported on the OTC Bulletin Board on October 8, 2009.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission acting pursuant to said section 8(a) may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities
and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. Subject to Completion, Dated October 13, 2009 Prospectus

35,975,049 Shares
Common Stock
This prospectus relates to the offer and resale of up to 35,975,049 shares of our common stock, par value $0.001 per share, by the selling stockholder, Dutchess Equity Fund, L.P., or "Dutchess". Of such shares, (i) Dutchess has agreed to purchase 35,744,249 pursuant to the investment agreement dated September 15, 2009, between Dutchess and us, and (ii) 230,800 shares were issued to Dutchess in partial consideration for the preparation of the documents for its investment. Subject to the terms and conditions of such investment agreement, which is referred to in this prospectus as the "Investment Agreement," we have the right to put up to $8.0 million in shares of our common stock to Dutchess. This arrangement is sometimes referred to as an "Equity Line." For more information on the selling stockholder, please see the section of this prospectus entitled "Selling Stockholder".
We will not receive any proceeds from the resale of these shares of common stock offered by Dutchess. We will, however, receive proceeds from the sale of shares to Dutchess pursuant to the Equity Line. When we put an amount of shares to Dutchess, the per share purchase price that Dutchess will pay to us in respect of such put will be determined in accordance with a formula set forth in the Investment Agreement. Generally, in respect of each put, Dutchess will pay us a per share purchase price equal to ninety-four percent (94%) of the lowest closing best bid of the Company's common stock during the five consecutive trading day period beginning on the trading day immediately following the date of delivery of the applicable put notice.
Dutchess may sell the shares of common stock from time to time at the prevailing market price on the Over-the Counter (OTC) Bulletin Board, or on an exchange if our shares of common stock become listed for trading on such an exchange, or in negotiated transactions. Dutchess is an "underwriter" within the meaning of the Securities Act of 1933, as amended (the "Securities Act") in connection with the resale of our common stock under the Equity Line. For more information, please see the section of this prospectus entitled "Plan of Distribution".
Our common stock is quoted on the OTC Bulletin Board under the symbol "MMRF". The last reported sale price of our common stock on the OTC Bulletin Board on October 9, 2009 was $0.08 per share.
Investing in the offered securities involves a high degree of risk, including those risks set forth in the "Risk Factors" section of this prospectus, as well as those set forth in any prospectus supplement.
We will be responsible for all fees and expenses incurred in connection with the preparation and filing of this registration statement, provided, however, we will not be required to pay any underwriters' discounts or commissions relating to the securities covered by the registration statement.
You should read this prospectus and any prospectus supplement carefully before you decide to invest. You should not assume that the information in this prospectus is accurate as of any date other than the date on the front of this document.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
![]()
The date of this prospectus is October __, 2009
TABLE OF CONTENTS
|
|
Page |
|
|
|
iii |
|
|
|
1 |
|
|
|
8 |
|
|
|
16 |
|
|
|
17 |
|
|
|
18 |
|
|
|
19 |
|
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
|
34 |
|
|
49 |
|
|
|
51 |
|
|
|
59 |
|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
|
61 |
|
|
63 |
|
|
|
65 |
|
|
|
68 |
|
|
|
70 |
|
|
|
70 |
|
|
|
70 |
|
|
|
70 |
|
|
|
F-1 |
![]()
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission. You should rely only on the information contained in this prospectus or to which we have referred you. We have not authorized anyone to provide you with information or to make any representation on behalf of the Company that is different from that contained in this prospectus. You should not rely on any unauthorized information or representation. This prospectus is an offer to sell only the securities offered by this prospectus under circumstances and in jurisdictions where it is lawful to do so. The information in this prospectus is accurate only as of the date of this
i
prospectus, regardless of the date of delivery of this prospectus or of any sales of these securities. Our business, financial condition, results of operations and prospects may have changed since the date of this prospectus. This prospectus may be used only in jurisdictions where it is legal to sell these securities.
![]()
ii
CAUTIONARY STATEMENT REGARDING FORWARD LOOKING STATEMENTS
Some of the statements contained or incorporated by reference in this prospectus are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act") and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), and we intend that such forward-looking statements be subject to the safe harbors created thereby. These statements are based on the current expectations, forecasts, and assumptions of our management and are subject to various risks and uncertainties that could cause our actual results to differ materially from those expressed or implied by the forward-looking statements. Forward-looking statements are sometimes identified by language such as "believe," "may," "could," "estimate," "continue," "anticipate," "intend," "should," "plan," "expect," "appear," "future," "likely," "probably," "suggest," "goal," "potential" and similar expressions and may also include references to plans, strategies, objectives, and anticipated future performance as well as other statements that are not strictly historical in nature. The risks, uncertainties, and other factors that could cause our actual results to differ materially from those expressed or implied in this prospectus include, but are not limited to, those noted under the caption "Risk Factors" beginning on page 8 of this prospectus. Readers should carefully review this information as well the risks and other uncertainties described in other filings we may make after the date of this prospectus with the Securities and Exchange Commission.
Readers are cautioned not to place undue reliance on forward-looking statements. They reflect opinions, assumptions, and estimates only as of the date they were made, and we undertake no obligation to publicly update or revise any forward-looking statements in this prospectus, whether as a result of new information, future events or circumstances, or otherwise.
![]()
iii
This summary highlights the information contained elsewhere in this prospectus. Because this is only a summary, it does not contain all of the information that you should consider before buying shares of our common stock. You should read the entire prospectus and any prospectus supplements carefully, especially the sections entitled "Caution Regarding Forward Looking Statements," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations," together with our financial statements and the related notes included elsewhere in this prospectus and in any prospectus supplements related thereto, before deciding to purchase shares of our common stock.
MMR Information Systems, Inc.
Depending upon the context, the terms "MMR Information Systems," "Company," "we," "our" and "us," refers to either MMR Information Systems, Inc. alone, or MMR Information Systems, Inc. and its subsidiaries collectively.
Organizational History
We were incorporated in Delaware in 2000 under the name "Favrille, Inc." and previously operated as a biopharmaceutical company focused on the development and commercialization of targeted immunotherapies for the treatment of cancer and other diseases of the immune system. In May 2008, our ongoing Phase 3 registration trial for our lead product candidate failed to show a statistically significant improvement in the treatment of patients with follicular B-cell non-Hodgkin's lymphoma, and accordingly, we determined to sell all of our equipment and other personal property in an auction. On September 9, 2008, this auction was consummated and we received $3.2 million in net proceeds from the sale of the assets. With the disposition of all of our equipment and other personal property, we ceased to engage in any operations and became a "shell company" as such term is defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the "Exchange Act".
Agreement and Plan of Merger and Reorganization
On January 27, 2009, we consummated a reverse subsidiary merger with what was then called MyMedicalRecords.com, Inc., a Delaware corporation, or MMRCI, pursuant to which the holders of MMRCI equity prior to the merger, on a fully diluted basis, owned or had the right to acquire approximately 60.3% of our equity, the holders of our equity prior to the merger, on a fully diluted basis, owned approximately 33.2% of our equity, and certain beneficiaries (including our former officers, former directors and their affiliates, and certain trade creditors) under a creditor plan (that we refer to from time to time as the "Creditor Plan") had the right to own up to approximately 6.5% of our equity. We refer to this business combination as the "Merger" from time to time in this prospectus. In connection with the Merger, we changed our name from "Favrille, Inc." to "MMR Information Systems, Inc.", MMRCI changed its name to "MyMedicalRecords, Inc." and became our wholly owned subsidiary, and we ceased being a "shell company" as such term is defined in Rule 12b-2 of the Exchange Act. We refer to MyMedicalRecords, Inc. as "MMR" from time to time in this prospectus. MMR was incorporated in Delaware in 2005 and is headquartered in Los Angeles, California.
The Merger is being accounted for as a reverse acquisition in accordance with U.S. generally accepted accounting principles. Under this method of accounting, we were treated as the "acquired" company for financial reporting purposes. This determination was primarily based on the fact that MMR's former shareholders hold a majority of the equity of the consolidated company, MMR's operations comprise the ongoing operations of the consolidated entity and MMR's senior management and director designees have assumed control of the consolidated company.
Our Company and Business Subsequent to the Merger
As of the closing date of the Merger on January 27, 2009, we adopted MMR's business of empowering consumers to manage the important records in their life, whether paper-based or digital, and by doing so, to better control and organize their lives overall. We do this through web-based products that facilitate consumer access to
1
medical records and vital documents (such as living wills, birth certificates, insurance policies and important financial records). The following description of our business relates to our current business and operations.
Our principal product, "MyMedicalRecords," is an easy-to-use, secure web-based Personal Health Record system, or PHR, which allows documents, images and voice mail messages to be transmitted in and out of our proprietary system using a variety of methods, including fax and file upload. Our platform converts documents it receives by fax into a Portable Digital Format, or PDF, file. These files and any other uploaded files are stored in the consumer's personal account, which is secured by a unique user ID and password combination. A notification is sent to up to three user e-mail addresses (including to e-mail enabled cell phones) whenever a new record is received. Users can then access their files via the Internet and take advantage of an easy-to-use, customized filing system that allows them to categorize, annotate and file their records for easy access, organization and retrieval. Users can then print, download or e-mail their records from their account, giving our customers greater control over their own personal health and other information, which they can share with health care providers and others as needed. We are currently selling our MyMedicalRecords PHR product primarily through corporations as an employee benefit and to affinity groups as a "value-added" service for their members, as well as directly to retail customers, although we plan to sell through additional sales channels in the future.
In the second half of 2007, we launched our "MyESafeDepositBox" service, which is geared towards small businesses, the financial services, insurance and legal service industries. MyESafeDepositBox is based on the same technology and architecture as our MyMedicalRecords PHR product. However, rather than focusing on medical records, MyESafeDepositBox is designed to provide secure on-line storage for vital financial, legal and insurance documents. MyESafeDepositBox may be used as a virtual on-line "safe" and could serve as an essential part of any household's or business's disaster preparedness plan.
We are now also in the final stages of developing a new product, "MyMedicalRecords Pro" (MMRPro), which we believe will provide physician offices with a powerful and cost-effective solution to the costly and time-consuming problem of digitizing paper-based medical records. When complete, our MMRPro service is expected to combine the scanning of medical records at a physician's office with 24/7 access to these records for both doctors and patients through separate secure web-based portals. We recognize that the critical nature of personal health information requires that these product advances be implemented with the utmost care to protect the privacy and confidentiality of our customers' data. Although we are not a covered entity under the Health Insurance Portability and Accountability Act of 1996, commonly referred to as HIPAA, we consider it important to take into account privacy and security standards and requirements of HIPAA when implementing our products and services and we believe that we meet and/or exceed current HIPAA standards.
We plan to continue to take advantage of the burgeoning consumer health information market and leverage recent and anticipated federal legislation, including by adapting the technology supporting our products to be able to take advantage of government funds in the 2009 economic stimulus package. We believe that as medical costs and insurance costs increase, while benefits decrease, healthcare consumers will require greater control over their personal (and their family's) health and health information. Our MyMedicalRecords PHR product is designed to enable healthcare consumers to store their important medical records and data in one central and secure place where they can manage those records and control how they are accessed and shared. While every healthcare consumer in the U.S. is a potential user of our MyMedicalRecords PHR product, we believe that the product has most appeal to these particular market niches:
- the chronically ill and their families who share information among many doctors;
- consumers with "senior" parents who, in their role as caregivers, want to be sure they have current medical information readily available to react quickly in case of parental illness;
- consumers with newborns who will be able to build a complete medical file for the newborn, which can last the newborn's entire life;
- travelers and businesspeople working overseas who want to ensure that they always have access to their medical records in the event of an emergency; and
2
- corporations seeking to offer their employees a valuable benefit (we believe those with expatriate employees would be particularly interested in our product).
Because of the similarity in functionality between our MyMedicalRecords PHR product and our MyESafeDepositBox product, we market these products through many of the same channels. Our marketing strategy for our MyMedicalRecords PHR product and MyESafeDepositBox product focuses primarily on the following channels:
- to corporate accounts, as an added employee benefit for their employees or bundled or co-branded with their product offerings to their customers;
- through affinity groups (such as alumni organizations and other membership organizations) and discount health benefit membership groups to their members; and
- direct to consumers as a retail subscription product, on-line and through hospitals and doctors.
Our MyMedicalRecords Pro product is designed to combine onsite scanning services with the storage and accessibility features of our MyMedicalRecords PHR product. We plan to market our MyMedicalRecords Pro product to doctors, particularly solo and small group practitioners, who are looking for an easy and inexpensive way to digitize patient medical files and give patients up-to-date access to those records.
For a complete description of our business, please see the section entitled "Our Business".
Summary Financial Data
Because this is only a summary of our financial information, it does not contain all of the financial information that may be important to you. Therefore, you should carefully read all of the information in this prospectus and any prospectus supplement, including the financial statements and their explanatory notes and the section entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations," before making a decision to invest in our common stock. The information contained in the following summary are derived from our financial statements for the quarters ended June 30, 2009 and 2008 and the years ended December 31, 2008 and 2007.
3
For accounting purposes, the Merger was treated as a reverse acquisition with MMR being the accounting acquirer. Accordingly, the historical financial results prior to the Merger are those of MMR and replace our historical financial results as we existed prior to the Merger. Our results of operations are included in MMR's financial results beginning on January 27, 2009.
|
|
|
Six months |
|
Fiscal years |
|||||||||
|
|
|
2009 |
|
2008 |
|
2008 |
|
2007 |
|
|
|
|
|
|
|
|
(unaudited) |
|
|
|||||||||
|
Consolidated Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
$ 307,885 |
|
$ 174,060 |
|
$ 451,355 |
|
$216,090 |
|
|
|
|
|
|
Cost of revenues |
|
209,137 |
|
211,047 |
|
409,900 |
|
319,447 |
|
|
|
|
|
|
Gross profit (loss) |
|
98,748 |
|
(36,987 |
) |
41,455 |
|
(103,357 |
) |
|
|
|
|
|
Total operating expenses |
|
3,371,019 |
|
1,406,717 |
|
3,165,256 |
|
2,914,530 |
|
|
|
|
|
|
Loss from operations |
|
(3,272,271 |
) |
(1,443,704 |
) |
(3,123,801 |
) |
(3,017,887 |
) |
|
|
|
|
|
Net Loss |
|
$ (4,197,446 |
) |
$ (1,686,125 |
) |
$ (3,522,476 |
) |
$(3,126,406 |
) |
|
|
|
|
|
Net loss available to common |
|
$ (0.04) |
|
$ (0.03) |
|
$ (0.28) |
|
$ (0.31) |
|
|
|
|
|
|
Weighted average common shares |
|
117,357,033 |
|
65,683,009 |
|
12,572,308 |
|
10,139,631 |
|
|
|
|
|
|
|
|
As of |
|
As of December 31, |
|
||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|
|||||||||
|
Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ 2,605 |
|
$ 75,779 |
|
$ 11,450 |
|
|
|
|
|
|
|
|
Total assets |
|
742,080 |
|
464,654 |
|
852,623 |
|
|
|
|
|
|
|
|
Total stockholders' deficit |
|
( 5,326,111 |
) |
( 2,788,406 |
) |
(350,701) |
|
|
|
|
|
|
|
Our Principal Executive Offices
Our principal executive offices are located at 468 Camden Drive, Suite 200, Beverly Hills, California 90210. Our telephone number is (310) 476-7002 and our website address is www.mmrinformationsystems.com. Information included or referred to on our website is not a part of this prospectus.
Market Data and Industry Information
We obtained the market data and industry information contained in this prospectus from internal surveys, estimates, reports and studies, as appropriate, as well as from market research, publicly available information and industry publications. Although we believe our internal surveys, estimates, reports, studies and market research, as well as industry publications are reliable, we have not independently verified such information, and as such, we do not make any representation as to its accuracy.
4
Summary of the Offering
This prospectus relates to the resale of up to 35,975,049 shares of our common stock by Dutchess. The Investment Agreement with Dutchess provides that Dutchess is committed to purchase up to $8,000,000 of our common stock over the course of 60 months. We may draw on the facility from time to time, as and when we determine appropriate in accordance with the terms and conditions of the Investment Agreement. A maximum of 100 million shares may be issued under the Equity Line, at per share prices set at ninety-four percent (94%) of the lowest closing best bid of our common stock during the five (5) consecutive trading day period beginning on the trading day immediately following the date of delivery of the applicable put notice (such five-day period, the "Pricing Period").
The Investment Agreement is further described below under the heading, "Investment Agreement".
|
Shares of common stock offered by us |
None. |
|
|
|
|
Shares of common stock offered by the |
(i) 230,800 shares were issued to Dutchess as a document preparation fee for the Equity Line and are outstanding upon the effective date of the registration statement to which this prospectus relates; and (ii) 35,744,249 shares are issuable to Dutchess pursuant to the terms of the Investment Agreement. |
|
|
|
|
Offering Price |
To be determined by the prevailing market price for the shares at the time of the sale or in negotiated transactions. |
|
|
|
|
Use of proceeds |
We will not receive any proceeds from the sale of shares by the selling stockholder. However, we will receive proceeds from the Equity Line. See "Use of Proceeds." We intend to use such proceeds for working capital, reduction of indebtedness, acquisitions and other general corporate purposes. |
|
|
|
|
Risk Factors |
An investment in our common stock is speculative and involves substantial risks. You should read the "Risk Factors" section of this prospectus for a discussion of certain factors to consider carefully before deciding to invest in shares of our common stock. |
|
|
|
|
Plan of Distribution |
The shares of common stock covered by this prospectus may be sold by the selling stockholder in the manner described under "Plan of Distribution." |
|
|
|
|
OTC Bulletin Board Symbol |
"MMRF" |
Investment Agreement
We entered into the Investment Agreement with Dutchess on September 15, 2009. Pursuant to the Investment Agreement, Dutchess committed to purchase up to $8,000,000 of our common stock, over the course of 60 months. The aggregate number of shares issuable by us and purchasable by Dutchess under the Investment Agreement is 100,000,000.
5
We may draw on the facility from time to time, as and when we determine appropriate in accordance with the terms and conditions of the Investment Agreement. The maximum amount that we are entitled to put in any one notice is the greater of (i) 200% of the average daily volume (U.S. market only) of the common stock for the three (3) trading days prior to the date of delivery of the applicable put notice, multiplied by the average of the closing prices for such trading days or (ii) $150,000. The purchase price shall be set at ninety-four percent (94%) of the lowest closing best bid of our common stock during the Pricing Period. However, if, on any trading day during a Pricing Period, the daily volume weighted average price of the common stock is lower than the floor price specified by us in the put notice, then we reserve the right, but not the obligation, to withdraw that portion of the put amount for each such trading day during the Pricing Period, with only the balance of such put amount above the minimum acceptable price being put to Dutchess. There are put restrictions applied on days between the put notice date and the closing date with respect to that particular put. During such time, we are not entitled to deliver another put notice.
There are circumstances under which we will not be entitled to put shares to Dutchess, including the following:
• we will not be entitled to put shares to Dutchess unless there is an effective registration statement under the Securities Act to cover the resale of the shares by Dutchess;
• we will not be entitled to put shares to Dutchess unless our common stock continues to be quoted on the OTC Bulletin Board, or becomes listed on a national securities exchange;
• we will not be entitled to put shares to Dutchess to the extent that such shares would cause Dutchess's beneficial ownership to exceed 4.99% of our outstanding shares; and
• we will not be entitled to put shares to Dutchess prior to the closing date of the preceding put.
The Investment Agreement further provides that the Company and Dutchess are each entitled to customary indemnification from the other for any losses or liabilities we or it suffers as a result of any breach by the other of any provisions of the Investment Agreement or our registration rights agreement with Dutchess, or as a result of any lawsuit brought by a third-party arising out of or resulting from the other party's execution, delivery, performance or enforcement of the Investment Agreement or the registration rights agreement.
The Investment Agreement also contains representations and warranties of each of the parties. The assertions embodied in those representations and warranties were made for purposes of the Investment Agreement and are subject to qualifications and limitations agreed to by the parties in connection with negotiating the terms of the Investment Agreement. In addition, certain representations and warranties were made as of a specific date, may be subject to a contractual standard of materiality different from what a stockholder or investor might view as material, or may have been used for purposes of allocating risk between the respective parties rather than establishing matters as facts.
In connection with the preparation of the Investment Agreement and the registration rights agreement, we paid Dutchess a document preparation fee in the amount of $5,000, and issued Dutchess 230,800 shares of common stock, which equaled $30,000 worth of common stock based on the closing sales price of our common stock on August 13, 2009, the execution date of the non-binding term sheet with Dutchess.
6
Registration Rights Agreement
Pursuant to the terms of a Registration Rights Agreement, dated September 15, 2009, between Dutchess and us, we are obligated to file one or more registration statements with the SEC to register the resale by Dutchess of shares of common stock issued or issuable under the Investment Agreement. We are obligated to file with the SEC an initial registration statement on Form S-1 of which this prospectus forms a part by October 15, 2009, covering the resale of a portion of the 100,000,000 shares of common stock equal to one-third (1/3) of our current public float (where "public float" shall be derived by subtracting the number of shares of common stock held by our officers, directors and "affiliates" (as such term is defined in Rule 144(a)(1) of the 1933 Act) from the total number of shares of our common stock then outstanding). We are obligated to use all commercially reasonable efforts to have the initial registration statement declared effective by the SEC within 120 days after the closing date. After the later of (i) sixty (60) days after the time that Dutchess shall have resold substantially all of the shares registered for resale under the initial registration statement, or (ii) six (6) months after the effective date of the initial registration statement, we are obligated to register for resale another portion of the 100,000,000 equal to one-third (1/3) of our then outstanding public float. This registration process will continue until such time as all 100,000,000 shares of common stock issuable under the Investment Agreement have been registered for resale on effective registration statements. In no event will we be obligated to register for resale more than 100,000,000 shares of common stock.
7
Your investment in our common stock involves a high degree of risk. You should consider the risks described below and the other information contained in this prospectus carefully before deciding to invest in our common stock. If any of the following risks actually occur, our business, financial condition and operating results could be harmed. As a result, the trading price of our common stock could decline, and you could lose a part or all of your investment.
Risks Related to Our Business
It is anticipated that we will continue to incur losses and negative cash flow from operations for the foreseeable future and may not be able to continue as a going concern.
Following the Merger, we are still a company with a limited operating history although we have generated minimal revenue. While our new business includes personal health care record and storage products that are currently available, we are nevertheless still at an early stage in developing a business model that will enable us to generate significant revenue from the use of our products. Prior to the Merger, MMR financed its operations primarily through private placements of MMR capital stock and from secured loans from The RHL Group, Inc., a wholly-owned affiliate of MMR's founder and Chief Executive Officer, and our current Chairman, President and Chief Executive Officer, Robert H. Lorsch. Although we expect to continue to receive financing from The RHL Group, we have also continued to incur losses from operations and need additional sources of financing to fund our operations until we develop a profitable business. Even with additional funds from The RHL Group, there is no assurance that we will be able to generate sufficient revenue and working capital to fund our operations and create a sustainable going concern. As a result, it is expected that we will continue to incur operating losses for the foreseeable future.
If we fail to obtain additional financing, we will be unable to fund our operations.
We expect that the cash used in our operations will increase for the next several years. Although we generate some cash from our operations, we currently need to obtain financing not only to fund our operations, but also in order to meet the obligations for installment payments under our creditor plan (including to our former Chief Executive Officer and former Chief Financial Officer) of $853,000, as well as the obligations under the subordinated secured indebtedness owed to The RHL Group (which note payable had a balance of $ 1,516,049 at June 30, 2009). We will also need financing in order to fund operations until we can become cash flow positive, which is not expected to occur in the foreseeable future.
Other than to meet the creditor plan obligations, our future funding requirements will depend on many factors, including:
- The pace of market adoption of current and future products;
- The length of sales cycles and implementation efforts for major corporate accounts;
- The launch of new products; and
- The build up of a sales and service delivery organization.
If we are required to raise additional capital in the future such additional financing may not be available on favorable terms, or at all, or may be dilutive to our existing stockholders. If additional debt financing is raised in the future, we may be required to grant lenders a security interest in all or a portion of our assets. In addition, any such debt financing may involve restrictive covenants, including limitations on our ability to incur additional debt, limitations on our ability to acquire or assign intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business.
8
Future additional funding may not be available on acceptable terms, or at all. If we are unable to raise additional capital when required or on acceptable terms, then we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our products. There can be no assurance that we will be successful in generating cash from operations, reducing expenses as planned, selling assts, or raising additional capital and these matters raise substantial doubt about our ability to continue as a going concern.
We have been, and may continue to be, unable to generate sufficient cash flow to service our debt obligations.
Our ability to make payments on our indebtedness and to fund our operations, working capital and capital expenditures, depends on our ability to generate cash in the future, which is subject to our ability to execute on our business plan, and also to general economic, industry, financial, competitive, operating, and other factors that are beyond our control.
MMR has a secured credit facility with The RHL Group, with all outstanding amounts due thereunder being guaranteed by us. On August 18, 2009, we entered into a Waiver Agreement with The RHL Group, pursuant to which The RHL Group waived MMR's continuing payment default under the credit facility until August 31, 2009, which waiver period will continue automatically until The RHL Group notifies MMR otherwise. Should The RHL Group notify us that the waiver period has been terminated and, as of that time, we continue to be in payment default under the credit facility, The RHL Group would have the right to call any outstanding debt immediately due and payable, as well as exercise any other rights it has as a secured creditor under the credit facility and applicable law. As of June 30, 2009, the aggregate principal amount owed under The RHL Group credit facility was approximately $1,516,049.
As of June 30, 2009, we owed an aggregate of approximately $853,000 in principal amount to certain of our former employees and creditors, as evidenced by promissory notes issued to the same in connection with the Merger. We are obligated to make 18 monthly payments to such employees and creditors beginning August 2, 2009. We have not yet made any of the payments due and payable to such employees and creditors, and thus they are entitled to exercise such rights as may be available to them under applicable law.
We cannot assure you that our business will generate sufficient cash flow from operations or that future borrowings will be available to us in an amount sufficient to enable us to pay amounts due on our existing indebtedness or to fund our other liquidity needs. Thus, we may need to refinance all or a portion of our indebtedness on or before maturity. Our ability to refinance our indebtedness or obtain additional financing will depend on, among other things:
- our financial condition at the time;
- restrictions in our outstanding debt instruments; and
- other factors, including the condition of the financial markets.
As a result, we may not be able to refinance any of our indebtedness on commercially reasonable terms, or at all. If we do not generate sufficient cash flow from operations, and additional borrowings or refinancings or other proceeds are not available to us, we may not have sufficient cash to enable us to meet all of our obligations, thereby potentially rendering us insolvent.
The level of our indebtedness could adversely affect our financial condition.
We have significant debt service obligations. As of June 30, 2009, the aggregate principal amount owed under all of our outstanding debt was approximately $2.5 million. The level of our indebtedness could have important consequences. For example, it could:
- increase our vulnerability to adverse economic and industry conditions;
- require us to dedicate a substantial portion of our cash flow from operations to the payment of our indebtedness, thereby reducing the availability of cash to fund working capital and capital expenditures and for other general corporate purposes;
9
- restrict us from making strategic acquisitions, acquiring new content or exploring other business opportunities;
- limit our ability to obtain financing for working capital, capital expenditures, general corporate purposes or acquisitions;
- place us at a disadvantage compared to our competitors that have less indebtedness; and
- limit our flexibility in planning for, or reacting to, changes in our business and industry.
We are a company with limited operating history and currently have only a small management team and staff, which could limit our ability to effectively seize market opportunities and grow our business.
Our operations are subject to all of the risks inherent in a growing business enterprise, including the likelihood of operating losses. As a smaller company with a limited operating history, our success will depend, among other factors, upon how we manage the problems, expenses, difficulties, complications and delays frequently encountered in connection with the growth of a new business, products and channels of distribution, and current and future development, as well as in the competitive emerging healthcare records management business. In addition, as a company with a limited operating history we have only a small management team and staff to grow our business and manage the risks inherent in a growing business enterprise. These factors could limit our ability to effectively seize market opportunities and grow our business.
Although we continue to develop improvements to our MyMedicalRecords PHR product and other uses for its technology, there can be no assurances that we will be able to develop these improvements in a timely manner. Further, the market for consumer health information management products is in an early development stage and there can be no guarantees that there will be marketplace acceptance for our MyMedicalRecords PHR product or our MyESafeDepositBox product, the failure of which could have a material adverse effect on our business prospects, results of operations and financial condition.
Fully functional versions of our MyMedicalRecords PHR product and our MyESafeDepositBox product are currently available in the market place. While we continue to design and develop additional features that we believe are necessary for the ongoing competitiveness of our products, there can be no assurances that we will be able to develop these features in a timely manner. Further, the marketplace for consumer health information and secure document management products is in an early stage and there can be no guarantee that there will be marketplace acceptance for the enhanced products once these additional improvements are completed or for our MyMedicalRecords Pro product once ready. If there is no marketplace acceptance of our products, it would have a material adverse affect on our business prospects, results of operations and financial condition.
We are dependent on outside suppliers and any disruptions in the supply of services could materially and adversely affect our business operations and financial condition.
We are totally reliant on outside telecommunications, data center and information technology service providers and developers to build and maintain our product offering. For example, we rely on third-party vendors for website development, communications and server space. Although these suppliers are not "sole" source suppliers, should any one of our current suppliers be unable to supply services for any reason, because of the time and expense involved in negotiating new supply arrangements, it could severely hamper our ability to keep our product competing in the market and to continue to improve and develop our products as planned.
Any significant limitation or failure of our technology systems that are critical to our operations could constrain our operations.
We are highly dependent upon the use of third-party technology systems to operate our business. Any failures in these systems could disrupt our business and subject us to losses. Moreover, although we have in place certain disaster recovery plans and backup servers, we may experience system delays and interruptions as a result of natural disasters, power failures, acts of war, and third-party failures. Potential system failures and the cost necessary to
10
address them could result in material financial loss or costs, regulatory actions, breach of client contracts, reputational harm or legal claims and liability, which in turn could negatively impact our business prospects, results of operations and financial condition.
We are highly dependent on our Chief Executive Officer and the loss of him could have a material adverse effect on our business and results of operations. Further, we may not be able to attract qualified officers to replace him or other key management personnel necessary to grow our business.
We are highly reliant on the services of our Chief Executive Officer, Robert H. Lorsch. If Mr. Lorsch left, it could have a material adverse effect on our business and results of operations. Further, we must continue to hire experienced managers to continue to grow our business. As a company with limited operating history and no proven track record, we may have difficulty attracting and retaining new individuals. If we are not successful in attracting management, it could have a material adverse effect on our ability to grow our business, which would adversely affect our results of operations and financial condition.
Our management will be required to devote substantial time to comply with public company regulations.
As a public company, we will incur significant legal, accounting and other expenses. The Sarbanes-Oxley Act of 2002 as well as rules implemented by the SEC impose various requirements on public companies, even those that are not listed. In light of the Merger and change in our management and business, our new management and other personnel will need to devote a substantial amount of time to these requirements, but do not have recent experience with these requirements. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time- consuming and costly.
The Sarbanes-Oxley Act of 2002 requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting when required by Section 404 of the Sarbanes-Oxley Act of 2002. Our compliance with Section 404 will require that we incur substantial accounting and related expense and expend significant management efforts. We will need to hire additional accounting and financial staff to satisfy the ongoing requirements of Section 404.
We will incur expenses to remediate significant deficiencies that together constitute a material weakness in our present internal control over financial reporting and in our ability to document, test and certify our system of internal control. The inability to do so in a timely manner may result in the inability to detect or prevent material misstatements in our consolidated financial statements and could also result in the loss of investor confidence.
Our independent auditors identified material weaknesses in MMR's internal control over financial reporting in connection with the audit for the years ended December 31, 2008 and 2007. These material weaknesses relate to an inadequate consolidated financial statement close- process and cut-off of expenses, lack of accounting staff with financial expertise, lack of supporting documentation and schedules for certain transactions and inadequate journal-entry review process. This is due in part to the small size of MMR prior to the Merger. Although we are developing a plan of action to correct these material weaknesses, there is no guarantee that our efforts will be successful.
If we are not able to remediate the existing material weaknesses in internal control over financial reporting, we may not have adequate, accurate or timely financial information and our disclosure controls and procedures may not be effective. Further, material misstatements in our consolidated financial statements may go undetected and we may be unable to meet our reporting obligations or comply with the requirements of the SEC, which may subject us to sanctions or investigation by regulatory authorities such as the SEC. If this occurs, there could be an adverse reaction in the financial markets due to a loss of confidence in the reliability of our consolidated financial statements.
In addition to remedying the material weaknesses in our internal control over financial reporting, we need to bring our financial reporting procedures up to public-company standards so as to allow management to report on, and, when required by Section 404 of the Sarbanes-Oxley Act of 2002, our independent registered public accountants to attest to, our internal control over financial reporting. As a result, we will need to incur additional expenses and
11
diversion of management's time in this area. We will be required to incur significant costs in documenting, evaluating, testing and remediating our internal control procedures and systems, including the hiring of consultants or additional personnel. Such actions, while necessary and beneficial, will adversely affect our financial results.
We face substantial competition.
While we believe that our MyMedicalRecords PHR product occupies a unique niche in the marketplace, other companies offer similar services that compete for subscriber enrollment from the same patient population, and could compete more directly with us by developing processes and technology functionally the same as those that form our MyMedicalRecords PHR product. Most of these competitors are larger, more deeply funded companies. To the extent they have more success in obtaining market share and customer acceptance of their products, it could have a negative effect on our ability to grow our business, which could have a material adverse effect on our results of operations and financial condition.
Our growth will depend on our ability to develop our brand and any failure to do so could limit our business prospects, which could have a material adverse effect on our results of operations and financial condition.
We believe that establishing a strong brand will be critical to achieving widespread acceptance and adoption of our products and services. Promoting and positioning our brand will depend largely on the success of our marketing efforts, distribution channels and ability to provide high quality service. Establishing a significant brand presence for an on-line company often requires substantial marketing investment, and many "dot.com" companies have failed to generate the necessary adoption rates even after such a process. Brand promotion activities may not yield increased revenues, and even if they do, any increased revenues may not offset the expenses we incur in building the MyMedicalRecords brand. If we are not successful in building the MyMedicalRecords brand, it could limit our business prospects, which could have a material adverse effect on our results of operations and financial condition.
The handling of medical records is highly regulated. Not only could we be subject to substantial liability for mishandling records if we fail to comply with applicable requirements or if third-parties gain unauthorized access to records or servers but our products may need constant revisions or modifications to comply with an increasingly complex regulatory regime.
The proper handling of medical records is subject to extensive state and federal regulations and legal requirements, and we anticipate incurring significant costs to keep informed of and in compliance with such regulations and requirements. The volume and complexity of the regulations is daunting and many have changed substantially in recent years and all are subject to the uncertainties of interpretation. Although we are not currently required to comply with the Health Insurance Portability and Accountability Act of 1996, commonly referred to as HIPAA, we are committed to implementing our products and services in accordance with the privacy and security standards and requirements of HIPAA. Any determination that we are in violation of the applicable regulations or requirements could create substantial liability. Further, our products and services may become subject to greater regulation, particularly at the federal level. We may have to reduce, enhance or remove certain offerings to comply with new regulatory standards. If we are not able to effectively modify our product and service offerings to adapt to stricter standards, it could have a material adverse effect on our ability to grow our business and our results of operations and financial condition. Further, there is a risk that third-parties could gain unauthorized access to our users' records or servers. While this risk of accessing user data is reduced because user records are stored in image form, we are still exposed to liability from unauthorized third-party access to user accounts.
Because of the types of agreements that we enter into with our customers for our products, there is a time lag between the date of the agreement or license and product launch and revenue generation, which may be significant.
In the ordinary course of our business, we enter into agreements with certain customers that typically provide for customization of our product. The degree of customization can vary from simple modifications required to co-brand to highly tailored product modifications, which require significant development efforts on our part. Because these customer arrangements typically do not provide for payment of revenues until product launch, there is often a time lag between the date we enter into an agreement or license with a particular customer and when we begin generating revenues. This time lag can be significant if there is a high degree of customization involved requiring extensive
12
product development efforts. Thus, even if we are successful in negotiating agreements and licenses with many customers to co-brand or private label our products for such customers, because of the nature of our arrangements with our customers, there is a risk that it may be some time before we generate revenues from such arrangements, if at all.
We may not be able to adequately protect or enforce our intellectual property rights.
In order to compete effectively in our developing industry, we must distinguish our product and service offerings from that of our competitors. Such distinction will depend partially on the strength of our enforcement efforts regarding our patents, trade secrets, trademarks, and similar intellectual property rights, which in turn depends on our ability to effectively assert our own intellectual property rights. If we are not able to effectively assert our intellectual property rights, we may not be able to distinguish our product and service offerings, which could have a material adverse effect on our business prospects. Further, our competitors may develop and use similar but distinct intellectual property, and we do not anticipate being able to prevent them from effectively competing with us despite our intention to invest substantially in the development and protection of our intellectual property.
Third parties claiming that we infringe their proprietary rights could cause us to incur significant legal expenses and prevent us from selling our products.
The software and Internet industries are characterized by the existence of a large number of patents, trademarks and copyrights and by frequent litigation based on allegations of patent infringement or other violations of intellectual property rights. As we expand our product offerings into areas where larger companies with large patent portfolios compete, the possibility of an intellectual property claim against us grows. We could receive claims that we have infringed the intellectual property rights of others, including claims regarding patents, copyrights and trademarks. Any such claim, with or without merit, could result in costly litigation and distract management from day-to-day operations. If we are not successful in defending such claims, we could be required to stop selling our products, pay monetary amounts as damages, enter into royalty or licensing arrangements, or satisfy indemnification obligations that we have with some of our customers.
Our executive officers and directors may have interests that are different from, or in addition to, those of our stockholders generally.
Our executive officers and directors may have interests that are different from, or are in addition to, those of our stockholders generally. These interests include having subordinated debt with a balance of $1,516,049 at June 30, 2009 secured by a security interest in substantially all of our assets, the provision and continuation of indemnification and insurance arrangements for our directors, as well as certain other interests described elsewhere in this prospectus. Notably, our current President, Chairman and Chief Executive Officer, Robert H. Lorsch, may be deemed to beneficially control a total of 29.75% of our voting capital stock, as of September 30, 2009, including shares issuable upon exercise of options and warrants held by Mr. Lorsch and The RHL Group. Because of his high percentage of beneficial ownership, Mr. Lorsch may be able to control matters requiring the vote of stockholders, including the election of our board of directors and certain other significant corporate actions. This control could delay, defer or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our other stockholders and us. This control could adversely affect the voting and other rights of our stockholders and could depress the market price of our common stock.
Risks Related to Our Common Stock
Our stock price is expected to continue to be volatile, and the market price of our common stock may drop further.
The market price of our common stock could continue to be subject to significant fluctuations. Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our common stock. In the past, following periods of volatility in the market price of a company's securities, stockholders have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our operating results, financial condition, profitability and/or reputation.
13
We do not expect to pay cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We anticipate that we will retain our earnings, if any, for future growth and therefore do not anticipate paying cash dividends in the future. As a result, only appreciation of the price of our common stock will provide a return to stockholders. Investors seeking cash dividends should not invest in our common stock.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws and applicable Delaware law may prevent or discourage third parties or our stockholders from attempting to replace our management or influencing significant decisions.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may have the effect of delaying or preventing a change in control of our company or our management, even if doing so would be beneficial to our stockholders. These provisions include:
- dividing our Board of Directors into three classes serving staggered three-year terms;
- authorizing our Board of Directors to issue preferred stock without stockholder approval;
- prohibiting cumulative voting in the election of directors;
- prohibiting stockholder actions by written consent;
- limiting the persons who may call special meetings of stockholders;
- prohibiting our stockholders from making certain changes to our certificate of incorporation or bylaws except with 66.7% stockholder approval; and
- requiring advance notice for raising business matters or nominating directors at stockholders' meetings.
We are also subject to provisions of the Delaware corporation law that, in general, prohibit any business combination with a beneficial owner of 15% or more of our common stock for three years unless the holder's acquisition of our stock was approved in advance by our Board of Directors. Together, these charter and statutory provisions could make the removal of management more difficult and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our common stock.
There is currently a limited trading market for our common stock, which may limit our stockholders' ability to sell shares of our stock.
Our common stock is currently quoted on the over-the-counter bulletin board, or OTC BB. Because of the limited trading volume on the OTC BB of our common stock and the penny stock regulations described below, our investors may not be able to sell their shares due to the absence of a trading market.
We may be subject to penny stock regulations and restrictions, which could make it difficult for stockholders to sell their shares of our stock.
SEC regulations generally define "penny stocks" as equity securities that have a market price of less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. As of September 30, 2009, the closing price for our common stock was $0.09 per share. If we do not fall within any exemptions from the "penny stock" definition, we will be subject to Rule 15g-9 under the Exchange Act, which regulations are commonly referred to as the "Penny Stock Rules." The Penny Stock Rules impose additional sales practice requirements on broker-dealers prior to selling penny stocks, which may make it burdensome to conduct transactions in our shares. If our shares are subject to the Penny Stock Rules, it may be difficult to sell shares of our stock, and because it may be difficult to find quotations for shares of our stock, it may be impossible to accurately price an investment in our shares. There can be no assurance that our common stock will qualify for an exemption from the Penny Stock Rules, or that if
14
an exemption currently exists, that we will continue to qualify for such exemption. In any event, we are subject to Section 15(b)(6) of the Exchange Act, which gives the SEC the authority to restrict any person from participating in a distribution of a penny stock if the SEC determines that such a restriction would be in the public interest.
The Financial Industry Regulatory Authority, or FINRA, sales practice requirements may also limit a stockholder's ability to buy and sell our stock.
In addition to the Penny Stock Rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer's financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
A majority of our issued and outstanding shares of common stock are restricted securities and may not be freely resold to the public. When the restriction on any or all of these shares is lifted, and the shares are sold in the open market, the price of our common stock could be adversely affected.
Currently, as a result of the Merger, a majority of our issued and outstanding shares of common stock are "restricted securities" as defined under Rule 144 promulgated under the Securities Act of 1933, as amended, and may only be sold pursuant to an effective registration statement or an exemption from registration, if available. Although Rule 144 may not be immediately available to permit resales of shares received in the Merger or issued upon the exercise of warrants issued to former creditors of ours because of our former shell company status, once available, and given the number of shares that would no longer be restricted, sales of shares by our shareholders, whether pursuant to Rule 144 or otherwise, could have an immediate negative effect upon the price of our common stock.
When we issue additional shares in the future, it will likely result in the dilution of our existing stockholders.
Our certificate of incorporation authorizes the issuance of up to 650,000,000 shares of common stock with a $0.001 par value and 5,000,000 preferred shares with a par value of $0.001. As of September 30, 2009, 151,625,775 common shares were issued and outstanding and no shares of preferred stock were issued and outstanding. If we issue any additional shares, such issuance will cause a reduction in the proportionate ownership and voting power of all current stockholders. Further, such issuance may result in a change of control of our corporation.
Moreover, in the past, we issued warrants and options to acquire shares of common stock. As of September 30, 2009, we had warrants, options and convertible notes to purchase an aggregate of 59,009,081 shares of our common stock. In addition, the issuance of any shares for acquisition, licensing or financing efforts, upon conversion of any preferred stock or exercise of warrants and options, pursuant to our equity compensation plans, or otherwise may result in a reduction of the book value and market price of the outstanding shares of our common stock.
Risks Related to this Offering
We are registering the resale of a maximum of 35,975,049 shares of common stock which may be issued to Dutchess under the Equity Line. The resale of such shares by Dutchess could depress the market price of our common stock.
We are registering the resale of a maximum of 35,975,049 shares of common stock under the registration statement of which this prospectus forms a part. The sale of these shares into the public market by Dutchess could depress the market price of our common stock. As of September 30, 2009, there were 151,625,775 shares of our common stock issued and outstanding. In total, we may issue up to 100,000,000 shares to Dutchess pursuant to the Equity Line, meaning that we are obligated to file one or more registration statements covering the 64,255,751 shares
15
not covered by the registration statement of which this prospectus forms a part. The sale of those additional shares into the public market by Dutchess could further depress the market price of our common stock.
Existing stockholders could experience substantial dilution upon the issuance of common stock pursuant to the Equity Line.
Our Equity Line with Dutchess contemplates our issuance of up to 100,000,000 shares of our common stock to Dutchess, subject to certain restrictions and obligations. If the terms and conditions of the Equity Line are satisfied, and we choose to exercise our put rights to the fullest extent permitted and sell 100,000,000 shares of our common stock to Dutchess, our existing stockholders' ownership will be diluted by such sales.
Dutchess will pay less than the then-prevailing market price for our common stock under the Equity Line.
The common stock to be issued to Dutchess pursuant to the Investment Agreement will be purchased at a 6% discount to the lowest closing best bid of our common stock during the five consecutive trading day period beginning on the trading day immediately following the date of delivery of a put notice by us to Dutchess, subject to certain exceptions. Therefore, Dutchess has a financial incentive to sell our common stock upon receiving the shares to realize the profit equal to the difference between the discounted price and the market price. If Dutchess sells the shares, the price of our common stock could decrease.
We may not be able to access sufficient funds under the Equity Line when needed.
Our ability to put shares to Dutchess and obtain funds under the Equity Line is limited by the terms and conditions in the Investment Agreement, including restrictions on when we may exercise our put rights, restrictions on the amount we may put to Dutchess at any one time, which is determined in part by the trading volume of our common stock, and a limitation on our ability to put shares to Dutchess to the extent that it would cause Dutchess to beneficial own more than 4.99% of our outstanding shares. In addition, we do not expect the Equity Line to satisfy all of our funding needs, even if we are able and choose to take full advantage of the Equity Line.
We will not receive any proceeds from the resale of our common stock offered by Dutchess. However, we will receive proceeds from the sale of our common stock to Dutchess pursuant to the Investment Agreement. The proceeds from our exercise of the put option pursuant to the Investment Agreement will be used to support the commercialization of our current and future product candidates, for general working capital needs, for the reduction of indebtedness and for other purposes that our board of directors, in its good faith, deems to be in our best interest.
All net proceeds from the sale of the common stock covered by this prospectus will go to the selling stockholder. See "Selling Stockholder" and "Plan of Distribution" described below.
16
The information provided in the table and discussions below has been obtained from the selling stockholder. The table below identifies the selling stockholder and shows the number of shares of common stock beneficially owned by it before and after this offering, and the numbers of shares offered for resale by the selling stockholder. Our registration of these shares does not necessarily mean that the selling stockholder will sell all or any of their shares of common stock. However, the "Shares Beneficially Owned After Offering" columns in the table assume that all shares covered by this prospectus will be sold by the selling stockholder and that no additional shares of common stock will be bought or sold by the selling stockholder. No estimate can be given as to the number of shares that will be held by the selling stockholder after completion of this offering because the selling stockholder may offer some or all of the shares and, to our knowledge, there are currently no agreements, arrangements or understanding with respect to the sale of any of the shares. In addition, the selling stockholder may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time or from time to time since the date on which it provided the information regarding the shares, all or a portion of the shares of common stock beneficially owned in transactions exempt from the registration requirements of the Securities Act.
The following table sets forth the name of the selling stockholder, an if applicable, the nature of any position, office, or other material relationship which the selling stockholder has had, within the past three years, with us or with any of our predecessors or affiliates, the amount of shares of our common stock beneficially owned by the stockholder prior to the offering, the amount being offered for the stockholder's account, the amount being offered for the stockholder's account and the amount to be owned by such stockholder after completion of the offering.
|
|
|
Shares Beneficially Owned |
|
|
|
Shares Beneficially Owned |
||||
|
Beneficial Owner |
|
Shares |
|
% |
|
Shares Being |
|
Shares |
|
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Dutchess Equity Fund, L.P.(2) |
|
230,800 |
|
* |
|
35,975,049 |
(3) |
- |
|
- |
_____________
* Connotes less than 1.0%.
(1) Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the Securities and Exchange Commission under the Exchange Act, and generally includes voting or investment power with respect to securities. The number and percentage of shares beneficially owned is determined in accordance with Rule 13d-3 of the Exchange Act and is not necessarily indicative of beneficial ownership for any other purpose. Applicable percentage ownership is based on 151,625,775 shares of common stock outstanding as of September 30, 2009. Except as otherwise noted, we believe that the stockholder named in the table has sole voting and investment power with respect to all shares of common stock shown as beneficially owned by it, subject to applicable community property laws.
(2) Dutchess is a Delaware limited partnership. Michael Novielli and Douglas H. Leighton are directors of Dutchess with voting and investment power over the shares.
(3) Represents (i) a portion of the 100,000,000 shares of common stock issuable by us and purchaseable by Dutchess under the Investment Agreement equal to one-third (1/3) of our public float as of September 30, 2009. As of that date, our public float was equal to 107,232,747 shares (where "public float" was derived by subtracting the number of shares of common stock held by our officers, directors and "affiliates" (as such term is defined in Rule 144(a)(1) of the 1933 Act) from the total number of shares of our common stock outstanding as of September 30, 2009), and (ii) 230,800 shares of our common stock that were issued to Dutchess as a document preparation fee for the Equity Line and are outstanding upon the effective date of the registration statement to which this prospectus relates.
17
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock traded on The Nasdaq Global Market under the symbol "FVRL" from our initial public offering through the time of our delisting on October 3, 2008. On October 3, 2008, our common stock began trading over-the-counter on the OTC Bulletin Board under the symbol "FVRL.OB". Following the Merger and our name change from Favrille, Inc. to MMR Information Systems, Inc., our common stock began trading on the OTC Bulletin Board under the symbol "MMRF.OB".
Holders
As of September 30, 2009, there were approximately 219 holders of record of our common stock.
Price Range
The market for our common stock is limited and volatile. The following table sets forth the range of high and low high and low closing prices for our common stock for each of the periods indicated as reported by the OTC Bulletin Board. The prices quoted on the OTC Bulletin Board reflect inter-dealer prices, without retail mark-up, mark-down or commissions. The OTC Bulletin Board prices listed below may not represent actual transaction prices.
|
|
High |
|
Low |
|
|
|
Fiscal year ended December 31, 2009: |
|
|
|
|
|
|
Third Quarter |
|
$0.15 |
|
$0.08 |
|
|
Second Quarter |
|
$0.35 |
|
$0.10 |
|
|
First Quarter |
|
$0.30 |
|
$0.05 |
|
|
Fiscal year ended December 31, 2008: |
|
|
|
|
|
|
Fourth Quarter |
|
$0.05 |
|
$0.02 |
|
|
Third Quarter |
|
$0.07 |
|
$0.02 |
|
|
Second Quarter |
|
$1.87 |
|
$0.05 |
|
|
First Quarter |
|
$1.97 |
|
$1.24 |
|
|
Fiscal year ended December 31, 2007: |
|
|
|
|
|
|
Fourth Quarter |
|
$3.10 |
|
$1.50 |
|
|
Third Quarter |
|
$3.70 |
|
$2.64 |
|
|
Second Quarter |
|
$4.30 |
|
$2.93 |
|
|
First Quarter |
|
$3.60 |
|
$2.45 |
|
Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our Board of Directors.
18
Organizational History
We were incorporated in Delaware in 2000 under the name "Favrille, Inc." and previously operated as a biopharmaceutical company focused on the development and commercialization of targeted immunotherapies for the treatment of cancer and other diseases of the immune system. In May 2008, our ongoing Phase 3 registration trial for our lead product candidate failed to show a statistically significant improvement in the treatment of patients with follicular B-cell non-Hodgkin's lymphoma, and accordingly, we determined to sell all of our equipment and other personal property in an auction. On September 9, 2008, this auction was consummated and we received $3.2 million in net proceeds from the sale of the assets. With the disposition of all of our equipment and other personal property, we ceased to engage in any operations and became a "shell company" as such term is defined in Rule 12b-2 of the Exchange Act.
Agreement and Plan of Merger and Reorganization
On January 27, 2009, we consummated a reverse subsidiary merger with what was then called MyMedicalRecords.com, Inc., a Delaware corporation, or MMRCI, pursuant to which the holders of MMRCI equity prior to the merger, on a fully diluted basis, owned or had the right to acquire approximately 60.3% of our equity, the holders of our equity prior to the merger, on a fully diluted basis, owned approximately 33.2% of our equity, and certain beneficiaries under a creditor plan (which beneficiaries consist of our former officers and former directors and their affiliates) had the right to own up to approximately 6.5% of our equity. We refer to this business combination as the "Merger" from time to time in this prospectus. In connection with the Merger, we changed our name from "Favrille, Inc." to "MMR Information Systems, Inc.", MMRCI changed its name to "MyMedicalRecords, Inc." and became our wholly owned subsidiary, and we ceased being a "shell company" as such term is defined in Rule 12b-2 of the Exchange Act. We refer to MyMedicalRecords, Inc. as "MMR" from time to time in this prospectus. MMR was incorporated in Delaware in 2005 and is headquartered in Los Angeles, California.
The Merger is being accounted for as a reverse acquisition in accordance with U.S. generally accepted accounting principles. Under this method of accounting, we are treated as the "acquired" company for financial reporting purposes. This determination is primarily based on the fact that MMR's former shareholders hold a majority of the equity of the consolidated company, MMR's operations comprise the ongoing operations of the consolidated entity and MMR's senior management and director designees have assumed control of the consolidated company.
Our Company and Business Subsequent to the Merger
As of the closing date of the Merger on January 27, 2009, we adopted MMR's business of empowering consumers to manage the important records in their life, whether paper-based or digital, and by doing so, to better control and organize their lives overall. We do this through web-based products that facilitate consumer access to medical records and vital documents (such as living wills, birth certificates, insurance policies and important financial records). The following description of our business relates to our current business and operations.
Business Overview
Our principal product, "MyMedicalRecords," is an easy-to-use, secure web-based Personal Health Record system, or PHR, which allows documents, images and voice mail messages to be transmitted in and out of our proprietary system using a variety of methods, including fax and file upload. Our platform converts documents it receives by fax into a Portable Digital Format, or PDF, file. These files and any other uploaded files are stored in the consumer's personal account, which is secured by a unique user ID and password combination. A notification is sent to up to three user e-mail addresses (including to e-mail enabled cell phones) whenever a new record is received. Users can then access their files via the Internet and take advantage of an easy-to-use, customized filing system that allows them to categorize, annotate and file their records for easy access, organization and retrieval. Users can then print, download or e-mail their records from their account, giving our customers greater control over their own personal
19
health and other information, which they can share with health care providers and others as needed. We are currently selling our MyMedicalRecords PHR product primarily through corporations as an employee benefit and to affinity groups as a "value-added" service for their members, as well as directly to retail customers, although we plan to sell through additional sales channels in the future.
In the second half of 2007, we launched our "MyESafeDepositBox" service, which is geared towards small businesses, the financial services, insurance and legal service industries. MyESafeDepositBox is based on the same technology and architecture as our MyMedicalRecords PHR product. However, rather than focusing on medical records, MyESafeDepositBox is designed to provide secure on-line storage for vital financial, legal and insurance documents. MyESafeDepositBox may be used as a virtual on-line "safe" and could serve as an essential part of any household's or business's disaster preparedness plan.
We are now in Beta launch for the MyMedicalRecords Pro (MMRPro) service, an integrated end to end solution that represents the first step towards a full deployment of EMR transition for a physician office. MMRPro is a turnkey system that for one low monthly fee enables a physician's office to scan their medical records on a day forward basis. The system gives 24/7 access to these records for both doctors and patients through separate secure web-based portals. We are offering an end to end solution which includes the deployment of a Kodak Scan Station 520 and Capture Pro software, plus e-Prescibe services in the bundle at no additional cost. We are also working with the Kodak Business Solutions Group to monitor the Beta so that we can create and deploy the best process management solutions to support next year's rollout of the product. We plan on distributing MMRPro as direct to health care professionals, as an OEM add on to traditional EMR solutions, and as an end to end system solution through Kodak Resellers and Distributors.
We recognize that the critical nature of personal health information requires that these product advances be implemented with the utmost care to protect the privacy and confidentiality of our customers' data. Although we are not a covered entity under the Health Insurance Portability and Accountability Act of 1996, commonly referred to as HIPAA, we consider it important to take into account privacy and security standards and requirements of HIPAA when implementing our products and services and we believe that we meet and/or exceed current HIPAA standards.
We plan to continue to take advantage of the burgeoning consumer health information market and leverage recent and anticipated federal legislation, including by adapting the technology supporting our products to be able to take advantage of government funds in the 2009 economic stimulus package. We believe that as medical costs and insurance costs increase, while benefits decrease, healthcare consumers will require greater control over their personal (and their family's) health and health information. Our MyMedicalRecords PHR product is designed to enable healthcare consumers to store their important medical records and data in one central and secure place where they can manage those records and control how they are accessed and shared. While every healthcare consumer in the U.S. is a potential user of our MyMedicalRecords PHR product, we believe that the product has most appeal to these particular market niches:
- the chronically ill with multiple disorders also known as comorbid patients, and their families who share information among many doctors;
- consumers with "senior" parents who, in their role as caregivers, want to be sure they have current medical information readily available to react quickly in case of parental illness;
- consumers with newborns who will be able to build a complete medical file for the newborn, which can last the newborn's entire life;
- travelers and businesspeople working overseas who want to ensure that they always have access to their medical records in the event of an emergency; and
- corporations seeking to offer their employees a valuable benefit (we believe those with expatriate employees would be particularly interested in our product).
20
Because of the similarity in functionality between our MyMedicalRecords PHR product and our MyESafeDepositBox product, we market these products through many of the same channels. Our marketing strategy for our MyMedicalRecords PHR product and MyESafeDepositBox product focuses primarily on the following channels:
- to corporate accounts, as an added employee benefit for their employees or bundled or co-branded with their product offerings to their customers;
- through affinity groups (such as alumni organizations and other membership organizations) and discount health benefit membership groups to their members; and
- direct to consumers as a retail subscription product, on-line and through hospitals and doctors.
Our MyMedicalRecords Pro product is designed to combine onsite scanning services with the storage and accessibility features of our MyMedicalRecords PHR product. We plan to market our MyMedicalRecords Pro product to doctors, particularly solo and small group practitioners, who are looking for an easy and inexpensive way to digitize patient medical files and give patients up-to-date access to those records.
Our Products
MyMedicalRecords - An On-line Personal Health Record (PHR)
Our MyMedicalRecords PHR product, principally accessible at www.mymedicalrecords.com, delivers an easy-to-use, web-based medical records and health information storage, retrieval and management system for use by both consumers and healthcare providers. Our MyMedicalRecords PHR product allows for paper-based medical records such as lab reports, radiology reports, MRI reports and progress notes to be transmitted via fax to a secure mailbox that is created when a user enrolls in the service. Our MyMedicalRecords PHR product is designed to allow a user to fax his or her health records or other vital documents into a personal MyMedicalRecords PHR account via a dedicated telephone number, which we refer to as the user's "Lifeline" telephone number, that is assigned to the user upon enrollment. We believe that providing each MyMedicalRecords PHR subscriber a personal telephone number, as opposed to a common gateway number with a secondary PIN, creates an immediate sense of personalization and privacy and distinguishes MyMedicalRecords PHR from competing services. A user can also upload digital files, such as x-rays, scans or other medical images, as well as other vital documents into his or her account from a personal computer through any Internet browser. Our MyMedicalRecords PHR product allows users to store and segregate information for up to ten family members in a single account.
Designed to allow healthcare providers to transmit documents to a patient's MyMedicalRecords PHR mailbox without making any changes to existing patient or practice management software, our PHR makes it possible for a provider to send a patient's medical records to his or her MyMedicalRecords PHR mailbox without incurring expenses for legacy systems conversions, scanners or other machines.
In addition to medical records, our MyMedicalRecords PHR product can be used to provide secure storage for a variety of important confidential records, including:
- patient charts and notes;
- patient medical histories;
- vaccination and immunization records;
- lab reports and test results, including any images (or, if offered by a laboratory, links that the user can use to access the laboratory's stored images);
- copies of prescription orders;
21
- x-ray results and images (either the actual images or, if offered by a radiology provider, links that the user can use to access the provider's stored images);
- EKG results and images (either the actual images or, if offered by an imaging provider, links that the user can use to access the provider's stored images); and
- birth certificates, living wills, healthcare powers of attorney and advance directives.
In addition, users can store important legal, insurance and financial documents, as well as copies of identification documents such as passports and driver licenses in their MyMedicalRecords PHR accounts.
How it Works
Most providers, regardless of size, have a fax machine in the office. We have based our service on the efficient and effective conversion of faxed documents into .pdf files, and the subsequent annotation and filing of these faxes using a simple, easy-to-use, on-line document management system.
- Upon enrollment, we send every user a Welcome Kit containing a set of stickers to be placed on their medical files at doctors' offices, as well as a wallet card and a sticker for the back of a driver license, both containing emergency access information.
- The user gives their doctor(s) a sticker to be applied on the face of their medical file. The sticker (see below) contains a standing instruction to the doctor's office to fax to the user's personal Lifeline number any documents being placed in the medical file.
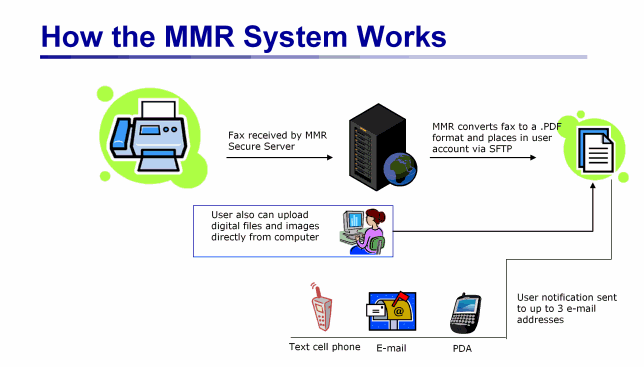
- When our MyMedicalRecords PHR system receives a document by fax transmission, it converts it into a PDF file and, using secure File Transfer Protocol transmission, or FTP, deposits it in the user's account on a secure server.
- When a faxed record or voice mail is received via the user's personal number, our MyMedicalRecords PHR system sends a notification message to up to three e-mail addresses (including to e-mail enabled cell phones) provided by the user to inform the user that a new document or voice message has arrived in his or her account.
22
- The user then accesses the web site, www.mymedicalrecords.com, from any Internet-connected computer, and logs in using their unique ID (Lifeline number) and password. Users are able to view their faxed records using Adobe Reader® software, which can be downloaded from the Internet free of charge.
- Our MyMedicalRecords PHR product allows users to organize and sort records by doctor, date, type of medical record, and, in the event multiple family members have enrolled in the service, by family member. This capability to index, file and sort medical records is intended to allow users to easily look up and retrieve their stored data, which we believe adds to the usability of our MyMedicalRecords PHR product.
- This document is now permanently filed, accessible 24/7 from any internet enabled computer.
- The user can also upload images, such as x-rays or EKG results directly into his or her MyMedicalRecords PHR account and can view these images using an Internet browser or other graphics software. Health information stored in the user's MyMedicalRecords PHR account can also be printed out, downloaded and e-mailed from the user's e-mail client so the user can easily take it to any provider who may be involved in the treatment of his or her medical condition.
We sell our MyMedicalRecords PHR product direct to consumers on a monthly and annual subscription basis. Current retail pricing for our MyMedicalRecords PHR product for a family that permits storing information for up to 10 people is $99.95 for an annual subscription or $9.95 for a monthly subscription. We also occasionally offer free trials and discounted pricing as a way to incentivize consumer acceptance of our MyMedicalRecords PHR product.
For special key account programs, such as healthcare providers that would like to make the service available to patients, corporations who want to offer the service as a benefit to employees, or affinity groups and other organizations who want to offer the service to their members, we provide different pricing plans. The pricing plans vary based on the number of people in the organization and the expected use of the product across the organization's members.
Additional Product Features
Our MyMedicalRecords PHR product offers users multiple ways to enter their personal medical information, including by fax, voice or digital file upload. We believe this capability makes our MyMedicalRecords PHR product easier to use than other products in the marketplace and makes our product's information storage features more accessible to potential customers. In addition to the core document conversion, storage and retrieval capabilities, our MyMedicalRecords PHR product provides a layer of useful, value-added interactive tools to help users better manage their personal and/or their families' medical records. These value-added interactive tools include:
- Health History . Users can enter their personal health history, including information about doctors (such as a doctor's name, address and specialty), vaccinations and immunizations, hospitalizations/surgeries, allergies and other medical conditions that may affect ongoing healthcare.
- Appointment Calendar Feature . Users are able to take advantage of a calendar feature to schedule and generate reminders about upcoming doctor and other health-related appointments. These reminders appear when a user logs into his or her MyMedicalRecords PHR account and also can be sent to the user's e-mail address (or e-mail enabled cell phone) and imported into Microsoft Outlook.
- Prescription History and Reminder Feature . Users are able to enter their prescriptions, pharmacies and refill dates into their MyMedicalRecords PHR accounts. The system generates reminders about refills, which are visible to users when they log into their accounts and are sent to their e-mail addresses (or e-mail enabled cell phone). In addition, when MyMedicalRecords PHR users enter a prescription into their personal MyMedicalRecords PHR accounts, they are able to access a third-party reference database that fully describes the drug, including possible contraindications with other drugs and over-the-counter medications the users may be taking.
23
- Voice Reminders and Messaging . We believe our MyMedicalRecords PHR product may create more efficient communication between doctors and patients. In addition to using a patient's personal MyMedicalRecords PHR telephone number to fax health information to a patient's secure on-line account, people can use the telephone number to leave a voice message, such as an appointment reminder, in a secure voice mailbox that is only accessible by the MyMedicalRecords PHR user. Users can also take advantage of this feature to leave themselves a reminder message such as for a doctor appointment or prescription refill reminder. Our MyMedicalRecords PHR product is designed to send a user a notification via e-mail when he or she receives a voice message. This gives users a helpful tool that they can access remotely.
- Secondary Passwords on Selected File Folders . Users can assign a second password to four of the file folders in their MyMedicalRecords PHR account. This feature creates an additional layer of security for personal or medical documents that a MyMedicalRecords PHR user does not want a doctor to have access to in the event that the user has given the doctor access to the account, or if the user does not want other family members to be able to see selected information.
- Emergency Log-In For Physicians and Other Emergency Response Personnel . Users can create a special password, discretely associated with each family member, which doctors and other emergency response personnel can use to gain access to the particular family member's medical records in the event of a medical emergency. This password grants access to an account but does not allow any additions, changes or deletions to be made to the account. In addition, users can decide which records a doctor will be able to see in an emergency situation. Users can write this password on an emergency sticker they receive when they enroll in our MyMedicalRecords PHR service, which can be affixed to a driver's license or personal ID.
- Health Information Library . Users have access to an interactive audio and visual encyclopedia, licensed from a third-party, of over 2,000 health information topics presented in both English and Spanish.
- Drug Information and Interaction Database . Users can check for potential interactions across multiple prescription and over the counter drugs in a single step. This database, licensed from Multum, includes over 20,000 drugs with potential interaction information across all of them.
- Ability to Attach Received Faxes to E-mail Notifications and Outbound Fax . Users can elect to have records attached to the notification e-mail they receive when a new medical record is received into their account. This capability is intended to save users the extra step of logging into their account to view new records, which we believe creates a higher level of convenience and usability. In addition, subscribers can also fax records or other documents stored in their PHR account to third parties using our integrated outbound fax service.
- Bilingual PHR . MyMedicalRecords PHR account holders are able to select between English and Spanish as the language of their PHR. We believe this bilingual capability makes our MyMedicalRecords PHR more accessible to Spanish speakers.
Other Applications for Our MyMedicalRecords PHR Product Technologies
We believe our MyMedicalRecords PHR product technology presents potential market opportunities beyond its core "personal health records" storage and management purpose. In the wake of recent hurricanes, fires and other disasters, a great deal of emphasis has been placed on families having a secure place to store their records and vital documents where they cannot be lost or damaged. Our MyMedicalRecords PHR product addresses this need because it allows for the fax transmission, upload and storage of insurance, financial and other personal documents, as well as a place to store digital files such as photographs. We believe this recent emphasis provides us with an opportunity to expand our core market and use our MyMedicalRecords PHR product technology to create the essential "safe deposit" box for all important documents and records of a family or small business. Accordingly, we have introduced a new service offering, MyESafeDepositBox, to accommodate this demand.
24
MyESafeDepositBox - An On-line Secure Document Storage System
Our MyESafeDepositBox product is designed to meet the needs of companies and individuals who are looking for a simple, efficient and economical way to securely store important legal, insurance and financial documents that they cannot afford to lose in a natural disaster such as a hurricane, flood or fire. We believe our MyESafeDepositBox product may also serve as a valuable tool for younger consumers who would not otherwise utilize a Personal Health Record storage system, but are looking for a secure way to organize their personal information to take better control over their financial affairs.
MyMedicalRecords Pro - Medical Records Digitization Service
We are now in Beta launch for the MMRPro service, an integrated end to end solution that represents the first step towards a full deployment of EMR transition for a physician office. MMRPro is a turnkey system that for one low monthly fee enables a physician's office to scan their medical records on a day forward basis. The system gives 24/7 access to these records for both doctors and patients through separate secure web-based portals. We are offering an end to end solution which includes the deployment of a Kodak Scan Station 520 and Capture Pro software, plus e-Prescibe services in the bundle at no additional cost. We are also working with the Kodak Business Solutions Group to monitor the Beta so that we can create and deploy the best process management solutions to support next year's rollout of the product. We plan on distributing MMRPro direct to healthcare professionals, as an OEM add-on to traditional EMR solutions, and as an end to end system solution through Kodak Resellers and Distributors.
MyMedicalRecords Pro is designed to allow physicians to efficiently scan and digitize their paper records with limited disruption to existing procedures, thus providing an alternative to the significant capital expenditures needed for implementing electronic medical records, or EMRs. MyMedicalRecords Pro is expected to provide physicians with 24/7 Internet-based access to all their scanned patient records through a doctor portal at www.mymedicalrecordsmd.com, while at the same time providing each patient with view-only access to his or her records from that doctor through a patient portal at www.mmrpatientview.com. We will offer the patient portal at no cost to patients, along with the opportunity to upgrade to our paid MyMedicalRecords PHR service, which is designed to allow them to store and manage records from all of their doctors. We expect to offer an administrative reimbursement to physicians who utilize our MyMedicalRecords Pro service and refer patients who decide to subscribe to the paid MyMedicalRecords PHR service.
The Market for Our Products
Demand for our medical records products is driven by the U.S. healthcare market and the health information management market. We believe demand for our MyESafeDepositBox product will be driven by consumer and small business focus on disaster preparedness and financial planning.
U.S. Healthcare Market
According to a report published by the Government Accountability Office in April 2008, healthcare spending per capita has grown on average about 2.5% faster than average annual per capita Gross Domestic Product, or GDP, over the past several decades. The report also finds that because this rapid growth in healthcare spending is expected to continue, it poses a fiscal challenge not just to the federal budget but to American business and the national economy. Figures released by the Centers for Medicare and Medicaid Services (CMS) of the Department of Health and Human Services (HHS) show that the U.S.is projected to spend over $2.5 trillion on healthcare in 2009, or $8,160 per U.S. resident, Health spending in 2009 is projected to account for 17.6% of GDP. By 2018, healthcare spending is projected to be over $4.3 trillion, or $13,100 per resident and account for 20.3% of GDP. HHS also determined that hospital spending grew 7% in 2006 to $648.2 billion, while spending on physician and clinical services grew 5.9% to $447.6 billion, representing about 22% of overall health care expenditures. HHS figures also show that prescription drug spending reached $216.7 billion in 2006, accelerating for the first time in six years, from a low of 5.8% in 2005 to 8.5% in 2006.
Today's healthcare consumers face a difficult set of factors. We believe that rising health insurance costs, combined with an aging population, makes it essential that people be more aware, and in greater control, of their health
25
status and their personal healthcare information than ever before. This is especially true for the 10% of the population that accounts for 63% of spending on health services (Kaiser Family Foundation, "Health Care Costs: A Primer, Key Information on Health Care Costs and Their Impact," March 2009) with comorbid patients, those having multiple chronic conditions, costing up to seven times as much as patients with only one chronic condition ("The High Concentration of U.S. Health Care Expenditures." Research in Action, Issue 19. AHRQ Publication No. 06-0060, June 2006). We believe our MyMedicalRecords PHR product addresses these challenges by giving users better access to and control of their own personal medical information. By having ready access to their medical records, healthcare consumers are better positioned to share such information with providers, which may avoid duplicative tests that may not be reimbursable. Our MyMedicalRecords PHR product is also designed to make accurate and complete medical information readily available to healthcare providers, which we believe will improve the consumer's treatment experience, if not the healthcare provider's ability to deliver it.
Recent legislative efforts demonstrate that healthcare reform, in particular health IT, is primed to be major national priority in the coming years. Notably, on February 17, 2009, President Barack Obama signed into law an economic stimulus package that includes $19 billion for health IT, and more specifically, electronic health records, or EHRs. We believe that we can benefit from the stimulus in two ways: first, from the heightened awareness regarding electronic medical records which ultimately translates into Personal Health Records, and second from the programs that need to be implemented under the Health Information Technology for Economic and Clinical Health Act, or HITECH Act, of 2009 (which was included in the stimulus package). We believe that the technology behind our products is capable of being adapted to meet current and future regulatory standards. We also believe that this climate of challenges and evolving provisions along with new emerging legislation will continue to create opportunities for us to meet an identified need in the U.S. healthcare market with our MyMedicalRecords PHR and MMRPro products.
The MyMedicalRecords PHR is designed to meet the particular needs of the comorbid health consumers - those who see multiple doctors. According to Dr. George Halverson, Chief Executive Officer of Kaiser Permanente, this segment represents approximately 20% of the health care population, but approximately 80% of health care costs in the United States.
Health Information Management Market
We believe the growing need to effectively manage health information is a significant marketplace opportunity that is attracting both start-up companies and well-funded technology companies given the recent U.S. government focus on better access to and management of medical and personal health information through EMRs.
Despite the significant momentum to adopt EMRs, many doctors and health care professionals, particularly small group practices, are still resisting conversion to a completely electronic medical records system because of the cost and significant operational changes associated with the conversion. MMRPro, is an alternative to EMR geared towards the small provider, whether a doctor's office, a small hospital or a clinic. MMRPro is designed to enable those still paper-based doctor offices to digitize patient records and place them on-line, without the need for the expensive hardware and software investment typically associated with EMR conversion. MMRPro is integrated with our MyMedicalRecords PHR product, allowing easier sharing of records between doctors and their patients through separate portals, which we believe will in turn further drive adoption of our MyMedicalRecords PHR product. In addition, MMRPro creates a revenue opportunity for doctors when patients upgrade from the free MMR Patient View to the full-featured MMR PHR. We believe this will give us the ability not only to exploit a growing consumer market, but also to have an active presence in the business market.
In addition, we believe that MMR helps solve the problem of lack of interoperability among systems. Interoperability is a requirement for providers, including hospitals, to receive some of the more than $19 billion in stimulus funding the government is offering to encourage adoption of electronic medical records. Lack of system interoperability is a challenge for providers. Both MMR and MMRPro are fax-based technologies, which means they are system agnostic and can integrate with any EMR that has a fax export component. Fax machines are ubiquitous in any doctor office and a doctor can send a fax anywhere without violating HIPAA.
26
As discussion about electronic health records and electronic medical records accelerates, consumers are increasingly concerned about privacy issues, such as how their records will be used and who will have access to them. The MyMedicalRecords PHR is 100% patient-centric, which means the individual controls who can view and share their health information.
Drivers for MyESafeDepositBox Market
The primary market driver for our MyESafeDepositBox product is the need for individuals and small business to be able to easily, efficiently and securely maintain backup copies of paper-based financial, insurance, legal and other vital business and personal documents.
External data storage is a rapidly growing market. According to IDC Worldwide Disk Storage Systems' Quarterly Data Tracker report published on September 5, 2008, there was nearly $7 billion spent on external disk storage in the second quarter of 2008, a 10.7% increase compared to the same period in 2007. Both companies and individuals are seeking solutions that will allow them to safely backup - and quickly restore - any lost data. We believe that our MyESafeDepositBox product meets the need of a sub-set of this vast data storage market, and we intend to target individuals and small businesses who want to find a secure, web-based solution for storing their most critical personal, financial, legal and insurance records, rather than those looking to backup the entire contents of their computer hard drive or corporate network.
In addition, the need for individuals to augment their personal plans for disaster preparedness is reflected in a July 2007 poll conducted by the Marist College Institute for Public Opinion in which 57% of respondents indicated that they did not have a family disaster emergency plan. The poll also reported that 57% of respondents indicated that their family disaster emergency plan did not include all basic essential items. Part of disaster planning is making sure that necessary and vital documents are available to be quickly retrieved in the event of evacuation and we believe our MyESafeDepositBox product meets this need by offering users a safe and easy way to store and access their vital records on-line.
We have spent the last two years in a development relationship with a large mortgage company and bank to distribute electronic safe deposit boxes. As more and more document delivery and storage is handled over the web, there will be a greater need for security and consumer privacy. We believe this creates a strong business opportunity to provide its MyESafeDepositBox product to financial and lending institutions.
Customers
To date, we have signed contracts and agreements for both our MyMedicalRecords PHR product and our MyESafeDepositBox product in the healthcare, corporate employee benefits and affinity and membership group markets. We have also sold these products directly to retail consumers.
In the healthcare market, we have an agreement with the Alexian Brothers Health Network, a Chicago area hospital chain that provides our MyMedicalRecords PHR product to its patients free of charge when they are discharged from inpatient facilities. We also private label our MyMedicalRecords PHR product for MedicAlert, which offers it as an enhancement to their existing PHR service. We have corporate clients who provide our MyMedicalRecords PHR product to their employees as a free benefit, and affinity group and membership clients who bundle the product as a "value added" service to their constituents. We receive compensation under these agreements either on a per enrolled account basis or on an access basis in which we are paid based on the number of persons who are eligible to sign up for our service, regardless of the number that actually enrolls. We are also an integrated service provider on the Google Health Network, which allows users to synchronize information between their Google Health account and their MyMedicalRecords PHR account.
27
Sales & Marketing Strategies
MyMedicalRecords PHR
Our marketing strategy for our MyMedicalRecords PHR product calls for continued focus on three main sales channels:
- Corporate Sales - Employee Benefit Offering . We are pursuing the human resources and benefits market to secure agreements or strategic arrangements providing for companies to offer our product to their employees and members. We believe the user-friendly nature of our MyMedicalRecords PHR product makes it readily acceptable to employees and gives companies a low cost way to demonstrate their employee-friendliness.
- Affinity Groups and Membership Organizations . Our MyMedicalRecords PHR product can be bundled with other health or travel-related services. For example, a travel accident insurance company can include our MyMedicalRecords PHR product in its suite of emergency medical and repatriation services for travelers going abroad. We believe giving users the ability to access their medical records in an emergency situation overseas may add considerable value to an insurance company's travel insurance policies.
- Expatriates and Multi-National Corporations . The product has significant benefit for Americans working overseas as well as companies with international offices. Users can access the MMR platform from anywhere in the world by dialing a Direct Inward Dial (DID) number or a local phone number that is then translated into a U.S. DID number to recive medical records or other vital documents into their account. Users also can upload files into their record directly from their computer. In addition, we believe that the fact many EMR systems can export records to MMR will make it easier for users to consolidate their records in one place. To serve the needs of the global market, we are expected to create relationships with Data Center providers in different countries as well as increase the number of languages in which the MyMedicalRecords PHR service is offered.
We utilize both our own direct sales organization and outside sales representatives who specialize in selling services to these market segments.
- Private Label Branding . Our MyMedicalRecords PHR product is designed to allow site pages to be customized with a corporate, affinity group or membership organization logo and content that is specific to that entity. The technology built into our MyMedicalRecords PHR product also is designed to allow companies, groups or organization to instantly communicate with hundreds or even thousands of employees in the event of an announcement or emergency situation.
- Direct to Consumer Marketing . We intend to continue to focus on marketing our MyMedicalRecords PHR product directly to consumers via both on-line and off-line advertising vehicles. Our MyMedicalRecords PHR product is currently available to individuals on a monthly or annual subscription basis.
MyESafeDepositBox
Because of the similarity in functionality between our MyESafeDepositBox product and our MyMedicalRecords PHR product, we market these products through many of the same channels. Both products are designed to offer users the ability to fax, upload and store important and private records or documents in a secure electronic environment, safe from fire or flood, and secure from the threat of identity theft. Thus, for example, we market our MyMedicalRecords PHR product to insurance companies as an additional benefit for health insurance policy holders, while at the same time marketing our MyESafeDepositBox product to insurance companies that may offer it to their risk and casualty policy customers. In addition, we market our MyESafeDepositBox to financial institutions and legal service providers as a safe and secure way for their customers to store important and private documents provided by these companies.
MyMedicalRecords Pro
We intend to target physician practices for our MMRPro product as a means to address their record storage and access requirements. We intend to position MMRPro as a simple and inexpensive way to scan and digitize patient medical records in anticipation of legislation that requires implementation of EMRs. We also plan to include features for advanced scheduling and e-prescription.
28
Additional On-Going Marketing Strategies
In addition to the three main marketing channels discussed above, we have also identified other potential markets for increasing sales of our products:
- Small Businesses . We believe our products could serve as an affordable and easy-to-implement tool for small companies that have not, or do not want to, invest in expensive IT data backup infrastructure.
- Government Agencies . We believe that federal, state and local governments would be interested in our products as they provide a powerful, yet easy to use addition to any family's disaster preparedness plan and we are interested in approaching agencies at all levels to explore the possibility of setting up pilot programs.
- Travel Companies and the Travel Industry . We believe that travelers represent a strong market for our products because these products are designed to enable travelers to access to their medical records, or other important documents (copies of passports, credit cards, etc.), in the event of an emergency when they are away from home. We plan to market our products to airlines, hotels, automobile clubs and other-travel related companies and organizations as well as to advertise on travel-related websites to reach this market.
International Licensing
We have licensed our intellectual property, technology and our MyMedicalRecords brand internationally. We currently have one exclusive license agreement with an unaffiliated company in Canada which runs through 2018. We recently terminated license agreements with MMR AU for Australia and New Zealand. We also recently terminated an agreement with an individual investor in Japan to fund and operate a joint venture which would have resulted in the formation of MMR Asia. To the best of our understanding, the joint venture in Asia was never formed.
We believe that the termination of these agreements will result in greater control of our products and the ability to better support existing international relationships that were not available to our licensees. We are considering terminating a license relationship in Canada, and we have recently entered into a Memorandum of Understanding for a non-exclusive distribution relationship in China with Unis-Thonge Technology (Zhengzhou) Co., Ltd., a subsidiary of the Unis Group of China.
Principal Suppliers and Supply Contracts
We contract with multiple third-party telecommunications, data center and information technology service providers and developers to develop and maintain our products.
We have a contract with an outside vendor to host one of our product websites and provide fax and voice messaging services and the toll-free numbers used by our customers to access their accounts. We pay our outside vendor a monthly website hosting fee and additional usage fees for fax, voice messaging and toll-free number services. Our contract is effective until September 2009, and automatically renews for successive one year periods under its current terms and conditions, unless terminated by either party by written notice no less than 90 days before expiration of the term. Neither party delivered a notice of termination, and thus this contract has renewed for an additional one-year period.
We contract with an outside vendor to house, manage and maintain the production servers that host our MyMedicalRecords PHR and MyESafeDepositBox web applications and store user related data. We pay a monthly fee for the management and technical support services required to maintain and operate our servers, which are housed in two separate data centers. Our agreement with this vendor is effective until terminated by either party upon fifteen days prior written notice.
We also contract with a third-party for the development and maintenance of the software applications necessary to run our MyMedicalRecords PHR, MyESafeDepositBox and MMRPro products. Our outside developer supports our software development needs through a team of software engineers, programmers, quality control personnel and testers, who work with our internal product development team on all aspects of
29
application development, design, integration and support of our products. Under our development contract, we own exclusive intellectual property rights over all software applications developed pursuant to the contract. We pay our developer a per project fee for the development services provided and a monthly fee for support and maintenance services. Our agreement with this developer was effective until June 30, 2009 at which time we exercised our option to extend the term of the agreement for another six months.
We are in the process of working with Kodak Corporation to negotiate long-term purchase agreements with designated Kodak resellers regarding hardware, software, warranty and maintenance services to support MMRPro. We have already agreed to purchase 50 Scan Station 520 scanners and associated Capture Pro software packages as well as software update and maintenance services to support our MMRPro Beta.
We have also contracted with H2H Solutions, Inc. for an ePrescription module that is offered at no additional cost as part of the MMRPro offering. The contract is effective until August 2012 and will automatically renew unless either party receives a notice of non-renewal 30 days prior to the renewal date.
On September 16, 2009, we entered into have a five year, exclusive agreement to license from an outside vendor the usage of that company's direct marketing database of street addresses, cellular phone numbers, e-mail addresses and other comprehensive data. In addition to an initial consulting fee, we pay our outside vendor a percentage of actual revenue received by us from successful sales made pursuant to use of the vendor's marketing database.
Disaster Recovery Plan
We have a disaster recovery plan in place that is designed to ensure the safekeeping of records stored in a user's MyMedicalRecords PHR or MyESafeDepositBox account, while maintaining continuity of our services should our main server site be affected by a natural or man-made disaster. We have a standby disaster recovery site over 100 miles away from the facility housing our main production servers. We backup the database holding our customers' records to servers housed in this "hot" standby disaster recovery site so that both systems contain identical information on a near-real-time basis. In addition, our main production site has redundancy measures built-in at all levels of the infrastructure and is designed to facilitate maximum availability of our product websites.
Competition
MyMedicalRecords PHR
Our MyMedicalRecords PHR product competes with a number of products and service providers in the consumer health information management marketplace today such as Microsoft HealthVault, SynChart, PersonalMD, Medikeeper, NoMoreClipBoard.com, Revolution Health, WebMD, and others. While Google may be considered a competitor with their Google Health product, we are an Integrated Service Provider on the Google Health platform. In addition HMOs, hospitals and insurance companies offer on-line portals for their subscribers and patients only. In contrast, the MMR PHR is meant to be used by anyone no matter what provider they use.
Each of our competitors offers varying PHR products and services for on-line storage and access to medical records at varying price points; however, we believe our MyMedicalRecords PHR product offers unique features that distinguish it from those of our competitors. In particular, we believe our MyMedicalRecords PHR product offers greater ease of use and accuracy than our competitors' products because copies of the actual medical records, such as laboratory test results and radiology reports can be faxed or uploaded directly into the user's MyMedicalRecords PHR account, rather than requiring users to input the data themselves, which may result in transcription errors. Our MyMedicalRecords PHR is designed to allow a user to send a medical record into their MyMedicalRecords PHR account from any fax machine, anywhere in the world. Many of our competitors require users to physically enter information on their medical data into a database. Likewise, while hospital patient and insurance policyholder Internet-portals allow users to see certain information regarding test results or claims data, these portals generally only provide the numeric values of a laboratory test, but do not offer users access to the complete information contained in the printed laboratory report reviewed by their physician. In addition, hospital, HMO and insurance company Internet-portals only allow users to view data from that specific provider and if a user changes his or her healthcare provider or insurance carrier, the information may not be available in the future. Our MyMedicalRecords PHR product is designed
30
to offer our customers a single secure on-line depository for all of their health information and records, from every provider, so that this information is available any time a MyMedicalRecords PHR user needs to access it.
We also believe the enhanced features offered with our MyMedicalRecords PHR product, such as appointment and prescription reminders and voice messaging features, offer consumers unique and attractive advantages that separate our MyMedicalRecords PHR product from the competition. All of these competing services, especially free services like Google and Revolution Health, raise consumer awareness about the need for access to personal health information. While this increased awareness may increase the marketability of our MyMedicalRecords PHR product, growth in the consumer health information management marketplace may also attract new entrants. While we believe that greater ease of use and certain enhanced features distinguish our MyMedicalRecords PHR product from those of our competitors, many of our competitors may have greater resources and more experience in this market, and can modify their product offerings to make them more competitive, including attempting to replicate some features of our MyMedicalRecords PHR product. We have also sought to protect our proprietary technology through patents in both the U.S. and overseas. See "- Intellectual Property - Patents" below.
MyESafeDepositBox
Our MyESafeDepositBox product competes with a number of on-line backup and electronic data storage services. The increasing use of external hard drives and flash drives to backup data also has the potential to compete with on-line data storage services such as our MyESafeDepositBox product. In addition, Wells Fargo Bank recently offered a VSafe service to its customers, which has many of the same features and functions as our MyESafeDepositBox product.
We believe that MyESafeDepositBox is a superior product when compared with Wells Fargo's VSafe in that we allow users to fax vital records directly into the MyESafeDepositBox account without having to first scan paper-based records and have access to a computer to upload information into their account. It also permits service providers, such as insurance agents or lenders, to fax documents directly into a user's account. We also believe the portability of MyESafeDepositBox creates a product advantage in the marketplace because users are not required to do business with any particular bank. Users also benefit from the ability to sort, store and manage their vital records, while also using a free form text search to find records stored in their account with specific annotations. Our ability to provide private label branding affords banks, insurance companies, escrow services and other financial and legal businesses to provide not only a useful product but also reinforces that business's identity.
MyMedicalRecords Pro
Our MyMedicalRecords Pro product, when complete, will compete with scanning services that market their services to doctors seeking to convert their historical paper records into electronic files. However, these services typically do not provide the doctor with an integrated system for scanning current patient records as they are produced on a daily basis. While many doctors may already have a scanner in their offices, those scanners merely digitize paper records, while MyMedicalRecords Pro is designed to be an integrated system that allows doctors to both manage their patient's records on-line, and more importantly, share these records with their patients via a secure on-line database. It is also designed to include modules for advanced scheduling and e-prescription.
MyMedicalRecords Pro also competes with EMR systems that offer doctors the opportunity to make their entire office paperless. MyMedicalRecords Pro provides an alternative to a full-blown EMR. MyMedicalRecords Pro is designed to allow a doctor to digitize and securely store his or her patient records on-line for a fraction of the cost of an EMR system.
Intellectual Property
Patents
In September 2005, we filed a utility patent application on key elements of our proprietary process with the United States Patent and Trademark Office. In March 2007, we filed a separate application for our Emergency "Read Only" Password service (see discussion above under "- Our Products - MyMedicalRecords - An On-line Personal Health Record (PHR) - Additional Product Features - Emergency Log-In For Physicians and Other Emergency
31
Response Personnel"). In May 2006, we filed a patent application with Australian authorities pursuant to the Patent Cooperation Treaty, which was awarded on May 29, 2008. In February 2006, we filed a patent application with Singapore authorities pursuant to the Patent Cooperation Treaty, which was awarded on January 30, 2009.
We intend to pursue patent protection in other countries where we may offer our products in the future. We also own the source code for our website. We consider patent protection an important element of a sound intellectual property portfolio, although we do not anticipate being able to prevent our competition from using other methods to achieve similar ends.
We acquired technology relating to anti-CD20 monoclonal antibodies through the reverse merger with Favrille, Inc. Anti-CD20 antibodies are used in treating B-Cell malignancies, including Non-Hodgkins Lymphoma (NHL) and additional B-Cell mediated conditions such as rheumatoid arthritis. In 2009, patent applications directed towards this technology were filed pursuant to the Patent Cooperation Treaty in the United States, Australia, Brazil, Canada, China, Europe, India, Japan, South Korea, and Mexico.
Other Intellectual Property and Trademarks
We own the URL and domain name for the web address www.mymedicalrecords.com. As we continue to develop our website, we intend to register our logo as a service mark and protect the copyright in the initial and any other proprietary content that we develop to support our MyMedicalRecords PHR product. We also own the domain names www.mymedicalrecordsMD.com and www.mmrpatientview.com for use with MyMedicalRecords Pro, and own the domain name www.myesafedepositbox.com for use with our MyESafeDepositBox product.
We also own the source code for a handheld software program, developed to operate on the Palm operating system, which allows Palm users to create a personal medical history on a personal data assistant, or PDA, so that they can have access to this information while traveling and in the event an Internet connection is not available. This product is currently available for purchase as a PDA download through the Palm website.
Research and Development
In 2007 and 2008, MMR spent $245,162 and $147,602, respectively, on research and development activities. From inception through December 31, 2008, MMR has spent a total of approximately $1.3 million relating to research and development activities, including approximately $644,000 in capitalized website development costs. In 2007 and 2008, Favrille spent $34,864,000 and $19,318,000, respectively, on research and development activities.
Properties
As a virtual Internet-based company we have minimal needs for office space to conduct our day-to-day business operations. Our corporate office is located at 468 N. Camden Drive in Beverly Hills, CA. Our mailing address is 2934 ½ Beverly Glen Cir., #702, Los Angeles CA 90077. The 468 Camden Drive property is leased on a month-to-month basis at rent of $4,600 per month. The 2934 ½ Beverly Glen Circle address is a post office box.
Employees
On May 29, 2008, Favrille provided a notice under the federal Worker Adjustment and Retraining Notification Act (the "WARN Act") to 132 of our then 144 employees, including six of our then executive officers, that their employment would end on June 6, 2008. Subsequently, we further reduced our operations and as of December 31, 2008 and prior to the Merger, we employed a total of two full-time employees.
Following the Merger and change in our business, as of the date hereof, we employ a total of six full-time employees and regularly use the services of an additional five consultants.
32
Legal Proceedings
From time to time, we may become involved in various legal proceedings generally incidental to our business. While the result of any litigation contains an element of uncertainty, management presently believes that the outcome of any unknown legal proceeding or claim, individually or combined, will not have a material adverse effect on our business, financial condition or future results of operations. At this time, we are not aware of any pending litigation.
Reports
We make available free of charge through our website, www.mmrinformationsystems.com, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or to be furnished pursuant to Section 13(a) of the Securities Exchange Act of 1934 as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Any information that is included on or linked to our Internet site is not a part of this report or any registration statement that incorporates this report by reference.
You may also read and copy any materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549, on official business days during the hours of 10:00 am to 3:00 pm. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at http://www.sec.gov.
33
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
You should read the following discussion of our financial condition and results of operations together with our financial statements and related notes included elsewhere in this prospectus. This discussion may contain forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements due to known and unknown risks, uncertainties and other factors, including those risks discussed under "Risk Factors" and elsewhere in this prospectus.
Business overview
Background and Basis of Presentation
As described above, on January 27, 2009, we consummated a business combination with MMR through a merger of our wholly-owned subsidiary with and into MMR pursuant to the terms of the Merger Agreement. In connection with the Merger, MMR became our wholly-owned subsidiary, with the former stockholders of MMR collectively owning (or having the right to acquire) shares of our common stock representing approximately 60.3% of the voting power of our capital stock on a fully diluted basis.
For accounting purposes, the Merger was treated as a reverse acquisition with MMR being the accounting acquirer. Accordingly, the historical financial results prior to the Merger are those of MMR and replace our historical financial results as we existed prior to the Merger. Our results of operations are included in MMR's financial results beginning on January 27, 2009. The following discussion of our financial condition and results of operations has been prepared, in part, using the financial statements of our wholly-owned subsidiary, MMR, for the fiscal years ended December 31, 2008 and 2007 included elsewhere in this prospectus. The results of operations and financial condition for those periods do not reflect our company on a consolidated basis.
MMR was incorporated in Delaware in 2005 and is headquartered in Los Angeles, CA. We provide users easy and ready access to medical records and other vital documents through our principal product, the MyMedicalRecords PHR, an easy-to-use, secure web-based personal health record system.
Source of Revenues
Our revenues are derived from the provision of services, which are comprised of facilitating electronic access to consumer medical records and other vital documents, as well as international licensing of our services. We offer our services to subscribers either on a direct subscription basis or an "access" basis through various types of organizations, and in both cases, we record these revenues under "Subscriber" in our income statement. On a direct subscription basis, which we use when we market our products direct to consumers or wholesale through corporations to their employees, or through affinity and membership organizations to their members, the subscriber pays us directly with a credit card or Paypal account either on a monthly or annual plan. On an access basis, which we currently use only with corporations, affinity and membership organizations, hospitals and other business to business customers, we charge a monthly fee to the organization based on the number of users who will have access to our services through such organization, whether or not such users actually enroll. Revenues from subscriptions accounted for (i) 62.2% and 56.7% of our total revenues in 2008 and 2007, respectively, (ii) 58.6% and 69.8% of our total revenues during the six months ended June 30, 2009 and 2008, respectively, and (iii) 54.3% and 72.0% of our total revenues during the three months ended June 30, 2009 and 2008, respectively.
We also generate revenues from licensing the sale and marketing of our services internationally and, to a lesser extent, from ancillary fee payments. We record these licensing and other ancillary revenues under "License and Other Fees" in our income statement. When we enter into a licensing arrangement, we are sometimes paid an up front license fee and typically receive ongoing royalty payments that are often based on a percentage of revenue earned by our licensee. These fees are recognized over the license period. When we receive ancillary one-time payments, we record them when services or products are delivered. Revenues from licensing and other fees accounted for (i) 37.8% and 43.3% of our total revenues in 2008 and 2007, respectively, (ii) 41.4% and 30.2% of our total revenues during the
34
six months ended June 30, 2009 and 2008, respectively, and (iii) 45.7% and 28.0% of our total revenues during the three months ended June 30, 2009 and 2008, respectively.
Cost of Revenue
Our cost of revenue includes the cost of maintaining our voice and fax mailboxes, long-distance call transport costs, fax and voice call processing costs, credit card transaction processing costs, web hosting and management fees, website maintenance and support costs, and costs associated with creating and mailing enrollment packages to our subscribers. Cost of revenue also includes customer service costs. We also charge to cost of revenue our direct selling costs, which include commissions paid to sales representatives who sell our wholesale and access based accounts.
Operating Expenses
The largest component of our operating expenses are our general and administrative expenses, which include personnel salaries and benefits, office rent and supplies, insurance costs, fees for legal and professional services, as well as our expenses for corporate telecommunications and internet access not associated with our products. Our operating expenses also include sales and marketing expenses (which include expenses associated with attending trade shows and travel costs, as well as a portion of personnel salaries allocated to sales and marketing activities), as well as technology development expenses (which includes expenses related to research and development as well as a portion of personnel salaries allocated to development activities).
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosures in the financial statements. Critical accounting estimates and assumptions are those that may be material due to the levels of subjectivity and judgment necessary to account for highly uncertain matters or the susceptibility of such matters to change, and that have a material impact on financial condition or operating performance. We base our estimates and judgments on our experience and on various other factors that we believe to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies used in the preparation of our financial statements require significant judgments and estimates. For additional information relating to these and other accounting policies, see note 2 to our financial statements appearing elsewhere in this prospectus.
Revenue Recognition
In general, we recognize revenue for the provision of such services only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service is performed and the collectability of the resulting receivable is reasonably assured.
We follow the provisions of Staff Accounting Bulletin ("SAB") No. 104, Revenue Recognition in Financial Statements ("SAB 104") and accordingly, we recognize annual recurring subscription fees paid in advance on a straight line basis over the subscription period. In addition, we follow the provisions of Emerging Issues Task Force ("EITF") 00-21, Revenue Arrangements with Multiple Deliverables, and accordingly, we defer recognition of upfront fees paid under our licensing agreement and recognize them over the period covered by the licensing agreement, and similarly, we defer recognition of minimum guaranteed royalty fees paid in advance under our licensing agreement and recognize them in the period to which they relate.
Accounting for Income Taxes and Uncertain Tax Positions
Deferred income taxes result primarily from temporary differences between financial and tax reporting. Deferred tax assets and liabilities are determined based on the difference between the financial statement basis and tax basis of assets and liabilities using enacted tax rates. Future tax benefits are subject to a valuation allowance when management is unable to conclude that deferred tax assets will more likely than not be realized from the results of operations. At each of the financial statement dates presented, we recorded a full valuation allowance against deferred
35
income taxes due to our limited operating history and net losses recorded since inception. Our estimate for the valuation allowance for deferred tax assets requires management to make significant estimates and judgments about projected future operating results. If actual results differ from these projections or if management's expectations of future results change, it may be necessary to adjust the valuation allowance.
We have generated losses for federal and state income tax reporting since inception. These tax losses are available for carryforward until their expiration. In addition to potential expiration, there are other factors that could limit our ability to use our federal and state tax loss carryforwards. For example, use of prior net operating loss carryforwards can be limited after an ownership change, such as the Merger. Accordingly, it is not certain how much of our existing net operating loss carryforwards will be available for use. In addition, we must generate taxable income in the future in order to use net operating loss carryforwards that have not expired. We are in the process of evaluating the effects of the Merger and change in control of our company under Internal Revenue Code Section 382, which may limit the availability of our net operating loss carryfowards.
Effective January 1, 2007, we began to measure and record uncertain tax positions in accordance with Financial Accounting Standards Board ("FASB") Interpretation Number ("FIN") 48 Accounting for Uncertainty in Income Taxes ("FIN 48") an Interpretation of Statement of Financial Accounting Standards ("SFAS") No. 109. FIN 48 prescribes a threshold for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. Only tax positions meeting the more-likely-than-not recognition threshold at the effective date may be recognized or continue to be recognized upon adoption of this Interpretation. FIN 48 also provides guidance on accounting for derecognition, interest and penalties, and classification and disclosure of matters related to uncertainty in income taxes. Accounting for uncertainties in income tax positions under FIN 48 involves significant judgments by management.
Intangible Assets
We account for website development costs in accordance with the provisions of EITF 00-2, Accounting for Website Development Costs, and with SFAS No. 86, Accounting for the Costs of Computer Software to Be Sold, Leased, or Otherwise Marketed. Pursuant to these provisions we capitalize internally developed website costs when the website under development has reached technological feasibility. These costs are amortized, typically over an estimated life of five years, using the larger of the amount calculated using the straight-line method or the amount calculated using the ratio between current period gross revenues and the total of current period gross revenues and estimated future gross revenues. At each balance sheet date, we evaluate the unamortized capitalized website costs compared to the net realizable value. The amount by which the unamortized capitalized website costs exceed its net realizable value is written off. The determination of estimated future gross revenues requires the exercise of judgment and assumptions by our management and actual results could vary significantly from such estimates.
Impairment of Long-Lived Assets and Intangibles
We evaluate long-lived assets and identifiable intangible assets with finite useful lives under the provisions of SFAS No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets, and accordingly, management reviews our long-lived assets and identifiable intangible assets with finite useful lives for impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. We recognize an impairment loss when the sum of the future undiscounted net cash flows expected to be realized from the asset is less than its carrying amount. If an asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. Considerable judgment is necessary to estimate the fair value of the assets and accordingly, actual results could vary significantly from such estimates. Our most significant estimates and judgments relating to the long-lived asset impairments include the timing and amount of projected future cash flows.
Share-Based Compensation
We recognize compensation expense, under the provisions of SFAS No. 123 (R), Share-Based Payment. As a result, we recognize compensation expense in an amount equal to the estimated fair value of stock-based awards and issuances, such as stock options granted to employees and non-employees and restricted stock awards. This estimation of the fair value of each stock-based grant or issuance on the date of grant involves numerous assumptions by
36
management. Although we calculate the fair value of each stock option under the Black Scholes option pricing model, which is a standard option pricing model, this model still requires the use of numerous assumptions. Assumptions used in this model include, among others, the expected life (turnover), volatility of the underlying equity security, and expected dividends. The model and assumptions also attempt to account for changing employee behavior as the stock price changes and capture the observed pattern of increasing rates of exercise as the stock price increases. Because we do not have adequate historic data regarding exercise rates and determined that MMR's options are "plain vanilla," in accordance with SAB No. 110, we assigned the expected life equal to the midpoint between the vesting period and the contractual option termination. Further, because there is no public market for MMR's shares, the expected volatility is based on ranges for similar companies. The use of different public company comparables in our assumptions and other assumptions by management in the Black Scholes option pricing model could produce substantially different results.
Recent Accounting Pronouncements
For a description of recent accounting pronouncements, please see note 2(o) to our financial statements appearing elsewhere in this prospectus.
Internal Control over Financial Reporting
In connection with the audit for the years ended December 31, 2008 and 2007, our independent registered public accounting firm identified significant deficiencies in our internal control over financial reporting that are material weaknesses. These included the following:
- Inadequate Financial Statement Close Process and Cut-Off. Because we did not perform a timely and efficient close of our financial records and did not require supporting documentation for all closing journal entries, we were required to make a significant amount of post-closing entries to adjust our financial statements to comply with generally accepted accounting principles.
- Lack of Financial Expertise and Accounting Staff. We have very limited accounting personnel and limited access to experts due to our limited cash situation. Because of these limitations, we misapplied accounting literature and authoritative guidance, which lead to errors in our financial statements.
- Lack of Supporting Documentation and Schedules. Because of the small size of our company prior to the Merger and the informal nature of our relationships with many of our consultants and directors, we did not always formally and promptly document equity-related transactions or maintain detailed accounting records supporting all cash transactions. This lack of supporting documentation and schedules led to errors in determining the impact on our financial statements of these transactions.
- Inadequate Journal Entry Review Process. Because of the small size of our company prior to the Merger, our journal entries were not reviewed and authorized by a person who has responsibility for oversight. This lack of oversight could lead to unauthorized entries, management override of controls or the concealment of errors and fraud.
In addition to the above, during the six months ended June 30, 2009, our independent registered public accounting firm identified additional material weaknesses regarding our inadequate approval process and documentation of equity grants because we did not formally document all equity related grants and formally approve them in Board meetings or formally document revenue arrangements with third parties.
To remedy these material weaknesses, we plan to hire experienced accounting staff with SEC public company experience and have already hired an outside consultant to develop and implement formal policies and procedures regarding the preparation of our financial statements.
37
Factors Affecting Future Results
In the first quarter of 2009, we entered into arrangements with The Latino Coalition, a non-profit organization with 600,000 business members nationwide, to market our product to its members (either on a direct to consumer basis or as an added benefit that its members can offer their employees), and with the United Marketing Group, a leading direct marketer of affinity membership programs and merchandise products, to market our products as a direct to consumer benefit. In addition, in May 2009 at its annual meeting, the National Rifle Association, or the NRA, announced the launch of NRA E-Safe, a private label version of our Electronic Safe Deposit product. The NRA is now gradually rolling out NRA E-Safe as an added benefit to its members. If members of these organizations take advantage of the introductory offers and subscribe for our services, these contracts could become important sources of revenue for our company over time, particularly after the initial introductory periods. The Company is also devoting its resources to the development of MMRPro which is expected to begin testing in Beta sites in September 2009. We are working with EMR companies and national scanning companies to create a national channel distribution for product rollout.
We have incurred losses since inception and our ability to achieve profitability in the near term is primarily dependent on our ability to invest capital in our business to increase revenues while controlling and limiting expenses at a rate slower than revenue growth. Some of the current conditions and factors that we expect could have a significant impact on our future results are:
- Our ability to raise capital in order to meet our financial obligations and invest in our business to grow revenues;
- The pace of market adoption of our current and future products;
- Our ability to launch new products;
- Managing costs while building up an effective sales and service delivery organization for our products; and
- Our ability to enter into marketing arrangements with large membership and affinity organizations for our products and maintain and grow subscribers from such arrangements, such as those noted above, particularly after the initial introductory period.
Going Concern
As more fully described in note 1 to the consolidated financial statements appearing elsewhere in this prospectus, our independent registered public accounting firm included an explanatory paragraph in their report on our financial statements for the year ended December 31, 2008 related to the uncertainty of our ability to continue as a going concern. At June 30, 2009, current liabilities of $5,856,404 exceeded cash and cash equivalents of $2,605.
Management's plan regarding this matter is to continue to increase its available line of credit with The RHL Group. If we are unable to increase its available line of credit with The RHL Group, or obtain suitable alternative debt financing, it may adversely affect our ability to execute our business plan and continue as a going concern. For further details regarding our indebtedness with The RHL Group, see "-Liquidity and Capital Resources- Description of Indebtedness-The RHL Group," below.
Additionally, we plan to sell additional equity securities, explore other debt financing arrangements and continue to increase our existing customer base to obtain additional cash flow over the next twelve months. There can be no assurance that funds from these sources will be available when needed or, if available, will be on terms favorable to us or to our stockholders. If additional funds are raised by issuing equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock.
38
Results of Operations
Three Months Ended June 30, 2009 as Compared to the Three Months Ended June 30, 2008
The following table sets forth items in our statements of operations for the periods indicated:
|
|
Three Months |
|
|||
|
|
|
2009 |
|
2008 |
|
|
Revenues |
|
|
|
|
|
|
Subscriber |
|
$ 87,972 |
|
$ 68,424 |
|
|
License fees and other |
|
74,126 |
|
26,625 |
|
|
Total revenues |
|
162,098 |
|
95,049 |
|
|
Cost of revenues |
|
102,285 |
|
94,580 |
|
|
Gross profit |
|
59,813 |
|
469 |
|
|
General and administrative expenses |
|
1,349,113 |
|
287,693 |
|
|
Sales and marketing expenses |
|
384,772 |
|
248,784 |
|
|
Technology development |
|
63,095 |
|
40,625 |
|
|
Loss from operations |
|
(1,737,167) |
|
(576,633) |
|
|
Gain on settlement of payables |
|
36,945 |
|
- |
|
|
Change in valuation of derivative liabilities |
|
1,495,569 |
|
- |
|
|
Interest and other expenses, net |
|
(116,404) |
|
(125,568) |
|
|
Net loss |
|
$ (321,057) |
|
$ (702,201) |
|
Revenues. Revenues increased by $67,049 for the three months ended June 30, 2009 from $95,049 for the three months ended June 30, 2008 primarily due to growth in both subscriber revenues, which resulted from growth in our corporate customer base and increased memberships with existing customers, as well as growth in license and other fees revenues, primarily due to higher royalty payments and platform access fees from our international licensees.
Cost of revenue. Our cost of revenue increased by $7,705, from $94,580 for the three months ended June 30, 2008 to $102,285 for the three months ended June 30, 2009. Cost of revenue increased in the second quarter of 2009 as compared to the second quarter of 2008 primarily due to an increase of approximately $12,000 related to dedicated individual dial-up fax numbers required to accommodate our additional subscribers and also to maintain an adequate inventory of numbers. This increase was offset by a decrease due to a reduction in personnel. During the three months ended June 30, 2008, we had an employee performing website maintenance resulting in increased costs of approximately $6,667. There were no such costs during the same period in 2009. We had a gross profit and positive gross margin of 36.9% for the second quarter of 2009 compared to a gross margin of 0.5% in the second quarter of 2008. The primary reasons for the improvement in our gross profit and margin in the second quarter of 2009 were because our revenues increased sufficiently to cover the fixed cost components of our costs of revenue and there was an increase in minimum guaranteed royalty payments with no corresponding cost increase.
Operating expenses. The following table sets forth the individual components of our operating expenses for the three months ended June 30, 2009 and 2008:
|
|
Three Months |
|
|||
|
|
|
2009 |
|
2008 |
|
|
General and administrative expenses |
|
$ 1,349,113 |
|
$ 287,693 |
|
|
Sales and marketing expenses |
|
384,772 |
|
248,784 |
|
|
Technology development |
|
63,095 |
|
40,625 |
|
|
Total |
|
1,796,980 |
|
577,102 |
|
39
Operating expenses increased by $1,219,878 for the three months ended June 30, 2009 from $577,102 for
the three months ended June 30, 2008. This significant increase is primarily due to the increase in our general and administrative expenses, which
increased 368.9% in large part because of increased legal and accounting fees of approximately $640,000 in 2009 as compared to 2008 associated
with the fees necessary to being a public company. A portion of the increase is due to increased share based compensation expenses associated
with stock option compensation, which increased by $102,873 compared to the second quarter of 2008, as well as $46,919 of expenses associated
with the issuance of warrants to consultants and vendors during the second quarter of 2009 compared to zero in the same period in 2008 and a
$100,000 write-off of advances due from a related party recorded during the second quarter of 2009 compared to no such write-off during the same
period in 2008. The increases in operating expenses were also driven by an increase in sales and marketing expenses, due to the timing of the
Healthcare Information and Management Systems Society Convention. In 2008 the convention was held in February while in 2009 the convention
was held in April which contributed to a $56,501 increase in the three months ended June 30, 2009 sales and marketing expense when compared to
the prior year same period. The increase was also due to increased salary expenses associated with consultants hired to support our growth in
revenues and development, along with our existing employees spending a higher proportion of their time dealing with sales and marketing related
activities. Gain on Settlement of Payables. During the three months ended June 30, 2009, we had a gain on the settlement of payables of
$36,945 in connection with settlement agreements entered into with certain vendors. There was no such gain during the three months ended June
30, 2008. Change in valuation of derivative liabilities. In November 2007, Favrille had issued warrants to purchase 4.4 million shares of
common stock in conjunction with a registered direct offering of common stock and warrants. We assumed these outstanding warrants as a result of
the Merger on January 27, 2009. The value associated with these warrants was recorded as a liability utilizing the Black-Scholes valuation model. As
of December 31, 2008, the warrants were valued at $0 and on March 31, 2009 and June 30, 2009 the warrants were valued at $355,993 and
$149,280, respectively. We recorded the change in valuation of $206,713 as a change in valuation of warrants during the three months ended June
30, 2009. There was no such change in valuation during the three months ended June 30, 2008. Also, we had certain non-employee options and warrants outstanding which were accounted for as derivatives as we had inadequate
authorized shares to settle 100% of these contracts. The event giving rise to this condition was the merger between Favrille, Inc. and
MyMedicalRecords.com, Inc. on January 27, 2009. On that date, the fair value of the option and warrant contracts subject to this analysis was
reclassified from equity to derivative liabilities. The condition was remedied on June 13, 2009 when certain of the option and warrant holders
indefinitely waived their rights under those contracts. On that date, the derivative liabilities were reclassified back into equity. On March 31, 2009 and
June 13, 2009, these contracts were again valued using the Black-Scholes option valuation model and the difference between the values at March
31, 2009 and June 13, 2009 of $2,665,889 and $1,377,033, respectively, was recorded as a gain on change in value of derivatives for the three
months ended June 30, 2009. The gain recorded for this matter was $1,288,856 for the three months ended June 30, 2009. There was no such
change in valuation during the three months ended June 30, 2008. Interest and Other Expenses, Net. We had interest and other expenses, net of $116,404 for the three months ended June 30, 2009,
a decrease of $9,164 from $125,568 for the three months ended June 30, 2008, primarily as a result of common stock issued as payment of interest
expense on the line of credit of $105,000 during the three months ended June 30, 2008, offset by increased interest expenses on the line of credit
payable to The RHL Group, a significant shareholder wholly-owned by Robert H. Lorsch, the Company's Chairman and Chief Executive Office. We
expect that our interest expense, net will increase given the increase in the outstanding balance of our line of credit payable to The RHL Group in
connection with the signing of the Third Amended and Restated Note on April 29, 2009 (see "-Liquidity and Capital Resources-Description of
Indebtedness-The RHL Group" below). Net loss. As a result of the foregoing, we had net income of $321,057 for the three months ended June 30, 2009 compared to a net
loss of $702,201 for the three months ended June 30, 2008. 40
Six Months Ended June 30, 2009 as Compared to the Six Months Ended June 30, 2008 The following table sets forth items in our statements of operations for the periods indicated: Six Months 2009 2008 Revenues Subscriber
$ 180,467 $ 121,435 License fees and other 127,418
52,625
Total revenues 307,885 174,060 Cost of revenues
209,137
211,047
Gross profit (loss) 98,748 (36,987) General and administrative expenses
2,643,518 707,410 Sales and marketing expenses
620,658 613,682 Technology development
106,843
85,625
Loss from operations
(3,272,271) (1,443,704) Gain on settlement of payables 89,170 - Change in valuation of derivative liabilities
(655,261) - Interest and other expenses, net
(359,084)
(242,421)
Net loss
$ (4,197,446)
$(1,686,125)
Revenues. Revenues increased to $307,885 for the six months ended June 30, 2009 from
$174,060 for the six months ended June 30, 2008 primarily due to growth in both subscriber revenues, which resulted from growth in our corporate
customer base and increased memberships with existing customers, as well as growth in license and other fees revenues, primarily due to higher
royalty payments and platform access fees from our international licensees. Cost of revenue. Our cost of revenue decreased to $209,137 for the six months ended June 30, 2009 from $211,047 for six months
ended June 30, 2008. Cost of revenue decreased in the six months ended June 30, 2009 as compared to2008, despite the increase in revenues,
primarily due to a reduction in personnel. During the six months ended June 30, 2008, we had a full time employee performing website maintenance
through April 30, 2008, resulting in increased costs in 2008 of approximately $27,000. There were no such costs during the same period in 2009.
These decreases in costs during 2009 were offset by an increase in costs of approximately $23,000 related to dedicated individual dial-up fax
numbers required to accommodate our additional subscribers and maintain an adequate inventory of numbers. We had a gross profit and positive
gross margin of 32.1% for the six months ended June 30, 2009 compared to a negative gross margin of 21.2% for the six months ended June 30,
2008. The primary reasons for the improvement in our gross profit and margin during the six months ended June 30, 2009 were because our
revenues increased sufficiently to cover the fixed cost components of our costs of revenue and there was an increase in minimum guaranteed royalty
payments with no corresponding cost increase. Operating expenses. The following table sets forth the individual components of our operating expenses for the six months ended
June 30, 2009 and 2008: Six Months 2009 2008 General and administrative expenses
$ 2,643,518 $ 707,410 Sales and marketing expenses
620,658 613,682 Technology development
106,843
85,625
Total
$ 3,371,019
$ 1,406,717
Ended
June 30,
Ended
June 30,
41
Operating expenses increased to $3,371,019 for the six months ended June 30, 2009 from $1,406,717 for the six months ended June 30, 2008. This significant increase is primarily due to the increase in our general and administrative expenses, which increased 273.7% in large part because of increased legal and accounting fees of approximately $1,500,000 in 2009 as compared to 2008 associated with the fees necessary to being a public company and the Merger which occurred on January 27, 2009. In addition, we had increased share based compensation expenses in 2009 associated with stock option compensation and expenses associated with the issuance of warrants to vendors as payment for services and a $100,000 write-off of advances due from a related party recorded during the second quarter of 2009 compared to no such write-off during the same period in 2008. We also incurred increased sales and marketing expenses, which increased by 1.1% in 2009 as compared to 2008, primarily due to increased sales and marketing salaries associated with additional consultants hired to support our growth in revenues, along with our existing employees spending a higher proportion of their time dealing with sales and marketing related activities. These increased sales and marketing expenses in 2009 were offset by decreases in expenses resulting from the completion of the initial phase of setting up a sales program with a customer during the first quarter of 2008. Our technology development expenses also increased by 24.8% in 2009 as compared to 2008 due to the hiring of a consultant focused on development activities.
Gain on Settlement of Payables. During the six months ended June 30, 2009, we had a gain on the settlement of payables of $89,170 in connection with settlement agreements entered into with certain vendors relating to liabilities we assumed in connection with the Merger. There was no such gain during the six months ended June 30, 2008.
Change in valuation of derivative liabilities. In November 2007, Favrille had issued warrants to purchase 4.4 million shares of common stock in conjunction with a registered direct offering of common stock and warrants. We assumed these outstanding warrants as a result of the Merger on January 27, 2009. The value associated with these warrants was recorded as a liability utilizing the Black-Scholes valuation model. During the six months ended June 30, 2009, the value of these warrants increased by $149,280. We recorded the change in valuation as a change in valuation of warrants during the six months ended June 30, 2009. There was no such change in valuation during the six months ended June 30, 2008.
Also, we had certain non-employee options and warrants outstanding which were accounted for as derivatives as we had inadequate authorized shares to settle 100% of these contracts. The event giving rise to this condition was the merger between Favrille, Inc. and MyMedicalRecords.com, Inc. on January 27, 2009. On that date, the fair value of the option and warrant contracts subject to this analysis was reclassified from equity to derivative liabilities. The condition was remedied on June 13, 2009 when certain of the option and warrant holders indefinitely waived their rights under those contracts. On that date, the derivative liabilities were reclassified back into equity. On June 13, 2009, these contracts were again valued using the Black-Scholes option valuation model and the difference between the original value at January 27, 2009 of $871,052 and the value at June 13, 2009 of $1,377,033 was recorded as a loss on change in value of derivatives for the six months ended June 30, 2009. The loss recorded for this matter was $505,981 for the six months ended June 30, 2009. There was no such change in valuation during the six months ended June 30, 2008.
Interest and Other Expenses, Net. We had interest and other expenses, net of $359,084 for the six months ended June 30, 2009, an increase of $116,663 from $242,421 for the six months ended June 30, 2008. The increase was primarily the result of increased interest expenses on the line of credit payable to The RHL Group, Inc. due to increased borrowings in 2009, interest expense for stock options issued as consideration for extending the line of credit, as well as additional interest expense from warrants issued to vendors as consideration for deferring payment of outstanding liabilities. The increased expenses in 2009 were offset by expenses associated with common stock issued as payment of interest expense on the line of credit of $210,000 during the six months ended June 30, 2008.
Net loss. As a result of the foregoing, we had a net loss of $4,197,446 for the six months ended June 30, 2009 compared to a net loss of $1,686,125 for the six months ended June 30, 2008.
42
Fiscal Year Ended December 31, 2008 as Compared to the Fiscal Year Ended December 31, 2007
The following table sets forth items in our statements of operations for the periods indicated:
|
|
Fiscal Years |
|
|||
|
|
|
2008 |
|
2007 |
|
|
Revenues |
|
|
|
|
|
|
Subscriber |
|
$ 280,572 |
|
$ 122,563 |
|
|
License fees and other |
|
170,783 |
|
93,527 |
|
|
Total revenues |
|
451,355 |
|
216,090 |
|
|
Cost of revenues |
|
409,900 |
|
319,447 |
|
|
Gross profit (loss) |
|
41,455 |
|
(103,357) |
|
|
General and administrative expenses |
|
2,108,063 |
|
1,588,911 |
|
|
Sales and marketing expenses |
|
914,162 |
|
1,080,457 |
|
|
Technology development |
|
143,031 |
|
245,162 |
|
|
Loss from operations |
|
(3,123,801) |
|
(3,017,887) |
|
|
Interest and other expenses, net |
|
(398,675) |
|
(108,519) |
|
|
Loss before income taxes |
|
(3,522,476) |
|
(3,126,406) |
|
|
Income tax expense |
|
- |
|
- |
|
|
Net loss |
|
$ (3,522,476) |
|
$ (3,126,406) |
|
Revenues. Revenues increased to $451,355 for the year ended December 31, 2008 from $216,090 for the year ended December 31, 2007 primarily due to significant growth in subscriber revenues, which resulted from growth in our corporate customer base, as well as growth in license and other fees revenues, primarily due to higher royalty payments and platform access fees from our international licensees.
Cost of revenue. Our cost of revenue increased to $409,900 for the year ended December 31, 2008 from $319,447 for the year ended December 31, 2007, primarily as a result of the added costs for telephone numbers associated with the larger number of subscribers, and the need to support two separate web platforms for two different customer groups. We had a gross profit and positive gross margin of 9.2% for the year ended 2008 compared to negative gross margin and a gross loss in 2007. The primary reasons for the improvement in our gross profit and margin in 2008 were because our revenues increased sufficiently to cover the fixed cost components of our costs of revenue and there was an increase in minimum guaranteed royalty payments with no corresponding cost increase.
Operating expenses. The following table sets forth the individual components of our operating expenses for the year ended December 31, 2008 and 2007:
|
|
Fiscal Years |
|
|||
|
|
|
2008 |
|
2007 |
|
|
General and administrative expenses |
|
$ 2,108,063 |
|
$ 1,588,911 |
|
|
Sales and marketing expenses |
|
914,162 |
|
1,080,457 |
|
|
Technology development |
|
143,031 |
|
245,162 |
|
|
Total |
|
$ 3,165,256 |
|
$ 2,914,530 |
|
43
Operating expenses increased to $3,165,256 for the year ended December 31, 2008, from $2,914,530 for the year ended December 31, 2007 primarily due to the increase in our general and administrative expenses, which increased 32.7%, in large part because of increased legal and accounting fees associated with the Merger, which more than offset the reduction in personnel and discretionary expenditures on account of a limited cash situation. In addition, we also incurred $197,000 in impairment charges associated with our capitalized website development costs in 2008, which we did not have in 2007. The increases in general and administrative expenses were offset by decreases in sales and marketing expenses and technology development expenses as we curtailed spending and investment in promoting and developing our products in 2008 because of our decreased liquidity in 2008 as compared to 2007.
Interest and Other Expenses, Net. We had interest and other expenses, net of $398,675 for the year ended December 31, 2008, an increase from $108,519 for the year ended December 31, 2007, primarily as a result of increased interest expenses on the line of credit payable to The RHL Group, Inc. The line of credit was entered into in July 2007 giving only a five month period during which interest costs were incurred in 2007 and a lower outstanding balance at period end ($283,098 at December 31, 2007), as compared to incurring interest expense for the entire period in 2008 and a higher outstanding balance ($822,520 at December 31, 2008) at period end. We expect that our interest expense, net will increase given the increase in the outstanding balance of our line of credit payable to The RHL Group, Inc. in connection with the signing of the Third Amended and Restated Note on April 29, 2009 (see "-Liquidity and Capital Resources-Description of Indebtedness-The RHL Group, Inc." below).
Net loss. As a result of the foregoing, we had a net loss of $3,522,476 for the year ended December 31, 2008 compared to a net loss of $3,126,406 for the year ended December 31, 2007.
Liquidity and Capital Resources
Historically, we have issued capital stock and received funds from The RHL Group to operate our business, and although we expect to continue to receive financing from The RHL Group, we also expect to continue to incur losses from operations and need additional sources of financing to fund our operations until we develop a profitable business. There can be no assurance that additional sources of financing will be available when needed or, if available, will be on terms favorable to us or to our stockholders. If additional funds are raised by issuing equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock.
Although we received approximately $692,000 in cash at the closing of the Merger, and additional funding from The RHL Group pursuant to the Third Amended and Restated Noted dated April 29, 2009, we nevertheless will still be required to obtain additional financing in order to meet the obligations for installment payments of $853,000 under the Creditor Plan and our obligations under the subordinated secured indebtedness to The RHL Group (which note payable had a balance of $1,516,049 at June 30, 2009), among other debt obligations.
Cash Flows for the Six Months Ended June 30, 2009 compared to Six Months Ended June 30, 2008
We incurred net losses of $4,197,446 and $1,686,125 for the six months ended June 30, 2009 and 2008, respectively. As of June 30, 2009, we had cash and cash equivalents of $2,605 compared to $0 at June 30, 2008.
Net cash used in operating activities for the six months ended June 30, 2009 was $1,319,458. The reconciliation of net loss of $4,197,446 to net cash used in operating activities for the six months ended June 30, 2009 included non-cash charges of $470,125 for share based compensation, $53,441 for depreciation and amortization, $66,668 for bad debt expense, $100,000 for a write-off of advances due from a related party and $655,261 for change in valuation of derivative liabilities, as well as increases of $1,361,577 in accounts payable and accrued expenses and $278,419 in related party payables, offset by a gain on the settlement of payables of $89,170.
Net cash used in operating activities for the six months ended June 30, 2008 was $725,660. The reconciliation of net loss of $1,686,125 to net cash used in operating activities for the six months ended June 30, 2008 included non-cash charges of $210,000 for common stock issued as payment of interest expense on the related party line of credit and $104,250 for restricted common stock issued for services and $74,719 for depreciation and amortization, as well as increases of $410,274 in accounts payable and accrued expenses and $115,532 in compensation payable, offset by a $74,611 decrease in related party payables.
44
Our investing activities resulted in a net cash inflow of $932,973 for the six months ended June 30, 2009 in connection with $1,050,506 in cash acquired from the Merger, offset by $107,087 in spending for international patent filing fee applications. During the second quarter of 2008, our net cash outflow from investing activities amounted to $3,566 due to the acquisition of property and equipment.
Our financing activities resulted in a net cash inflow of $313,311 for the six months ended June 30, 2009 in connection with $135,000 in proceeds from the line of credit with The RHL Group, $125,000 in proceeds from an unrelated third-party promissory note and $52,914 from stock option exercises. During the second quarter of 2008, our net cash inflow from financing activities consisted primarily of proceeds of $291,297 from the sale of stock, as well as $430,945 in proceeds from the line of credit with The RHL Group.
Cash Flows for the Year Ended December 31, 2008 compared to Year Ended December 31, 2007
We incurred net losses of $3,522,476 and $3,126,406 for the years ended December 31, 2008 and 2007, respectively. As of December 31, 2008, we had cash and cash equivalents of $75,779 compared to $11,450 at December 31, 2007.
Net cash used in operating activities for the year ended December 31, 2008 was $1,789,861. The reconciliation of net loss of $3,522,476 to net cash used in operating activities for the year ended December 31, 2008 included increases of $258,047 in a related party payable and $483,537 in accounts payable and accrued expenses, and non-cash charges of $397,939 for restricted common stock issued for services, $148,777 for depreciation and amortization, and $197,000 for impairment of intangible assets.
Net cash used in operating activities for the year ended December 31, 2007 was $2,347,195. The reconciliation of net loss of $3,126,406 to net cash used in operating activities for the year ended December 31, 2007 included an increase of $245,210 in related party payables and $191,584 in accounts payable and accrued expenses, as well as non-cash charges of $265,557 for restricted common stock issued for services and $137,330 for depreciation and amortization, which were offset by a $196,000 decrease in compensation payable to related parties and a $83,254 increase in prepaid expenses and advances.
Our investing activities resulted in a net cash outflow of $1,340 for the year ended December 31, 2008 in connection with the purchase of property and equipment. In 2007, investing activities resulted in a net cash outflow of $278,751 primarily for website development and our initial investment in a joint venture to market and sell our services in Japan, Korea, China, Taiwan and Thailand.
Cash provided from financing activities for the year ended December 31, 2008 and 2007 was $1,855,530 and $2,072,127, respectively. In 2008, financing activities primarily included proceeds generated from the sale of preferred stock and net proceeds from draw downs on our line of credit from The RHL Group, a significant stockholder wholly-owned by Robert H. Lorsch, our Chairman, Chief Executive Officer and President. In 2007, financing activities consisted primarily of the issuance of preferred stock, and to a lesser extent, proceeds from our line of credit from The RHL Group.
Description of Indebtedness
The RHL Group
In July 2007, we issued a promissory note to The RHL Group to borrow up to $100,000 under a revolving line of credit, which was subsequently increased to $1,000,000 in August 2007. In July 2007, we also entered into a Security Agreement, pursuant to which MMR granted a blanket security interest to The RHL Group over all of MMR's assets in order to secure the satisfaction of its obligations under the note. Interest on outstanding loan balances under the note accrued at a rate equal to the lesser of the Wall Street Journal Prime Lending Rate (7.5% on August 23, 2007) plus 3% or the maximum rate allowed by law under the California Constitution (5% per annum) plus the rate prevailing at the Federal Reserve Bank of San Francisco on November 25, 2007, for advances to member banks. In addition to interest, The RHL Group received one share of MMR common stock for each dollar drawn on the line of credit, in increments of 100,000, with a minimum grant of 400,000 shares, for each three month term of the line. In addition, we were required to maintain certain financial covenants.
45
In August 2008, we entered into the Second Amended and Restated Promissory Note Agreement (the "RHL Note") with The RHL Group for the renewal of the $1,000,000 secured line of credit on substantially the same terms, with the one change being the quarterly grant of shares under the earlier agreement was accelerated to a one time grant of 5,000,000 common shares (including 4,413,053 shares of treasury stock) for borrowings of up to $1,000,000 at any time during the twelve month term of the renewal ending on July 31, 2009. In addition, The RHL Group also received one share of common stock for each dollar of the line of credit, in increments of 100,000, for each 3 month term of the line for borrowings over $1,000,000. The entire unpaid principal and any unpaid interest under the note was originally due and payable on July 31, 2009.
As contemplated by the Merger Agreement and the Creditor Plan, as a condition to the Merger, at the effective time of the Merger, MMR and The RHL Group entered into an Allonge to the RHL Note and the Security Agreement pursuant to which The RHL Group agreed to suspend certain of its rights under the Security Agreement and the RHL Note until the earlier of (a) the date that we repaid all amounts outstanding under any promissory notes issued to our creditors under the Creditor Plan, (b) the date that we deposited into an escrow fund the maximum amount of cash payable in satisfaction of the promissory notes issued to our creditors under the Creditor Plan or (c) ten days after the two year anniversary of the closing date of the Merger. The suspended rights included any right of The RHL Group to (1) declare a default or event of default under the Security Agreement or the RHL Note (including for breach of the covenant for failure to maintain cash availability of at least $125,000), (2) accelerate the maturity date of the RHL Note, (3) exercise any of its principal remedies for a default or event of default under the Security Agreement, (4) assign the RHL Note, the proceeds of the RHL Note or to otherwise negotiate the RHL Note and (5) receive payment of the outstanding principal and interest owing under the RHL Note. We terminated the Allonge on April 29, 2009 as part of the credit restructuring described immediately below.
On April 29, 2009, we agreed to restructure our secured credit facility with The RHL Group and entered into a Secured Credit Restructuring Agreement with The RHL Group and Robert H. Lorsch, our Chairman, Chief Executive Officer and President (the "Restructuring Agreement"), issued The RHL Group a Third Amended and Restated Note (the "Third Amended Note"), and we agreed to guaranty MMR's obligations under the Third Amended Note (the "Guaranty").
The Third Amended Note amended and restated the RHL Note matures November 30, 2009, and bears interest at the lesser of 10% or the highest rate then permitted by law, and is secured (similar to the RHL Note) by the Security Agreement. Although the reserve credit line has been increased to $3,000,000, The RHL Group is only obligated to make a minimum of $100,000 of loans, advances and guarantees under the Third Amended Note within 30 days of execution pursuant to the Restructuring Agreement. The RHL Group was due to receive, as an origination fee, a note for $200,000, payable at our option in cash or warrants to acquire 2,800,000 shares of our common stock at an exercise price of $0.15 per share. If the term of the Third Amended Note is renewed, we would grant The RHL Group an additional origination fee on terms to be negotiated at such time.
As of June 30, 2009, we had an outstanding balance on the RHL Note of $1,516,049. The components of the $1,516,049 RHL Group Note payable and the related balance sheet presentation as of June 30, 2009 are: (1) $891,441 is included in the line of credit, related party payable; (2) $108,265 related to bank overdrafts, credit card charges and a vendor guaranty is included in accounts payable and accrued expenses; and (3) $516,343 related to deferred salary, consulting expenses and the $200,000 origination fee is included in related party payables. Interest expense on this note for the three months ended June 30, 2009 and 2008 amounted to $27,608 (unaudited) and $15,559 (unaudited), respectively. Interest expense on this note for the six months ended June 30, 2009 and 2008 amounted to $48,042 (unaudited) and $22,965 (unaudited), respectively. The unpaid interest balance was, as of June 30, 2009 and December 31, 2008, $72,104 (unaudited) and $24,963 (unaudited), respectively.
In conjunction with the loan agreement noted above, MMR was required to maintain certain financial covenants, including the requirement that MMR have at least $100,000 in cash in its bank accounts or such other amount as necessary to maintain operations through the subsequent thirty (30) days and timely pay any obligations due respecting payroll and all associated payroll taxes on and after June 1, 2009. As of June 30, 2009, MMR was in payment default under the terms of the Third Amended Note. However, the RHL Group waived MMR's default, which was subsequently memorialized in a Waiver Agreement, dated as of August 18, 2009. In consideration of The RHL Group's waiver of MMR's payment default under the Third Amended Note, we granted to The RHL Group a warrant to purchase an aggregate of 11,039,378 shares of our common stock, with an exercise price equal to $0.13 per share, the
46
closing price of our common stock on the date immediately preceding the date of grant. Under the Waiver Agreement, which was executed in order to facilitate the Company's pursuit of additional financing, The RHL Group agreed to waive MMR's payment default until August 31, 2009, which waiver period will automatically continue until The RHL Group notifies MMR otherwise. In addition, as repayment of the unpaid $200,000 origination fee owed to The RHL Group by MMR in connection with the credit line restructuring, we granted to The RHL Group 2,800,000 shares of common stock.
In addition, under the Restructuring Agreement, The RHL Group agreed to use commercially reasonable efforts to raise additional financing from third parties, and agreed to extend the maturity of the Third Amended Note for an additional six-month term if we are in full compliance with our covenants and other obligations under the Third Amended Note, on terms to be negotiated at such time. Additionally, Mr. Lorsch agreed to exercise all of his outstanding options prior to May 1, 2009 (which were exercised April 30, 2009), which resulted in an $113,220 reduction in principal owing The RHL Group. Finally, as a condition to agreeing to restructure our secured credit arrangement, we also terminated the Allonge. Thus, if at any time after June 1, 2009 we are not in compliance with our covenants under the Third Amended Note or Security Agreement, The RHL Group may, but is not obligated to, declare an event of default, subject to any continuing waiver period under the Waiver Agreement described above.
Creditor Plan
We are obligated to make installment payments of $853,000 under the Creditor Plan. Pursuant to the terms and conditions of the Creditor Plan, we issued interest-free promissory notes to the Favrille Creditors, which, as of June 30, 2009, had an aggregate outstanding balance of $853,000. The principal under such notes is payable in 18 equal installments beginning on August 2, 2009. We did not make the first installment payment that was due on August 2, 2009. There is no acceleration provision or other penalty upon an event of default, except as is otherwise provided for under applicable law. For additional information relating to the Creditor Plan see Exhibit 10.1 of our current report on Form 8-K filed on November 13, 2008.
Promissory Notes
On October 1, 2008, concurrent with the execution of the term sheet for the Merger, Favrille advanced MMR $100,000 pursuant to a promissory note issued by MMR to Favrille dated September 30, 2008 (the "First Bridge Note"), which was to be used solely for paying MMR's out of pocket expenses incurred in connection with the Merger. On November 10, 2008 (the first business day after execution of the Merger Agreement), Favrille advanced MMR an additional $500,000 pursuant to a promissory note issued by MMR to Favrille (the "Second Bridge Note,") and on December 22, 2008, January 8, 2009 and January 15, 2009, Favrille advanced MMR an additional $100,000, $35,000 and $50,000, respectively, pursuant to promissory notes issued by MMR to Favrille (the "Supplemental Bridge Notes" and together with the First Bridge Note and the Second Bridge Note, the "Promissory Notes") in each case, solely for MMR's use in paying its out of pocket expenses incurred in connection with the Merger and its operating expenses. Principal outstanding under the Promissory Notes accrued interest at the applicable federal rate of interest per annum, or the maximum rate permissible by the laws in California relating to permissible rates of interest on commercial loans, whichever is less. Because the Merger occurred, the principal and interest under the Promissory Notes has automatically been treated as a contribution to capital to MMR and the Promissory Notes have been fully discharged in accordance with their terms.
47
On June 11, 2009, we entered into a promissory note agreement to borrow $125,000 with PM Creative Corporation, an unrelated third-party. The promissory note is due and payable July 30, 2011, and bears interest of 12% per annum payable quarterly beginning on January 1, 2010. The proceeds of the loan will be used by us for the filing of international patent applications to expand and perfect the patent rights of the Company in the Anti-CD20 Antibodies outside the United States. PM Creative Corporation also received, as a commitment fee, $30,000. The commitment fee was payable at our option in cash or warrants to acquire 375,000 shares of our common stock at an exercise price per share equal to the lesser of (i) $0.15 per share and (ii) the weighted average trading price for a share of common stock for the ten consecutive trading days preceding the date on which the warrant is exercised. The warrants have a four year term. On July 29, 2009, we elected to pay the commitment fee through the issuance of warrants exercisable into 375,000 shares of our common stock at an exercise price of $0.14 per share. The warrants vested immediately. These warrants were valued using the Black-Scholes option pricing model. The total value of these warrants amounted to $32,441 (unaudited), all of which will be recorded as a reduction to the commitment fee payable in July 2009, with a loss on settlement of payables for $2,441.
Commitments and Contingencies
Under the terms of an agreement with an investor who purchased $500,000 of MMR's Series B Preferred shares in 2006, we were required to invest $250,000 in a joint venture with the investor to establish an entity to market and sell our services in Japan, China, Korea, Taiwan and Thailand. To date, we have paid $100,000 of this amount, but the joint venture has not been incorporated, has not commenced operations and there are no employees. We have expensed the $100,000 during the 3 months ended June 30, 2009 due to uncertainty surrounding the outcome of negotiations with the counter party. We are still involved in a relationship with the counter party. In September 2007, The RHL Group provided the investor with a guarantee that we would meet our remaining payment obligations under this agreement in exchange for 300,000 shares of restricted MMR common stock valued at $39,000. In January 2009, as consideration for renewing this guarantee, MMR issued The RHL Group 100,000 shares of MMR common stock (which converted into 328,174 shares of our common stock at the effective date of the Merger) valued at the date of issuance at approximately $5,000.
For additional information relating to this and other commitments and contingencies, please see the notes to our financial statements appearing elsewhere in this prospectus.
Off-Balance Sheet Arrangements
As of June 30, 2009 and December 31, 2008, we had no off-balance sheet arrangements.
48
Executive officers and directors
Our executive officers and directors and their respective ages as of September 30, 2009 are as set forth below:
|
Name |
|
Age |
|
Position(s) with the Company |
|
|
Robert H. Lorsch |
|
59 |
|
Chairman of the Board; President and CEO |
|
|
Hector V. Barreto, Jr. |
|
47 |
|
Director |
|
|
David A. Boyden |
|
48 |
|
Director |
|
|
Douglas H. Helm |
|
67 |
|
Director |
|
|
George Rebensdorf |
|
54 |
|
Director |
|
|
Bernard Stolar |
|
62 |
|
Director |
|
|
Jack Zwissig |
|
60 |
|
Director |
|
|
Naj Allana |
|
47 |
|
Senior Vice President and CFO |
|
Robert H. Lorsch, Chairman of the Board; President and Chief Executive Officer
. Since 2005, Mr. Lorsch has served as the Chairman, President and Chief Executive Officer of MMR. He is also Chairman and Chief Executive Officer of The RHL Group, Inc., a private equity and business management consulting firm Mr. Lorsch formed in April 1998. In 1994, he co-founded SmarTalk TeleServices, Inc., leading the company through a successful public offering in 1996 and building it into one of the largest providers of prepaid telecommunications products and services, serving as its Chairman and Chief Executive Officer until February 1998, following which SmarTalk moved its headquarters from Los Angeles to Dublin, Ohio with different management following its acquisition of ConQuest Telecommunication Services Corp. In March 1999, the combined company's assets subsequently were liquidated and sold to AT&T. In 1986, Mr. Lorsch founded the Lorsch Creative Network, a consulting company that developed marketing, advertising and interactive sales promotions campaigns for nationally and internationally recognized clients. Mr. Lorsch also served on the Personal Health Record Steering Committee of the Healthcare Information and Management Systems Society from 2006 to 2007. Mr. Lorsch is a Member of the Board of Trustees of the California Science Center; Member of the Board of Governors, Cedars-Sinai Medical Center; Member of the Board and of the Executive Committee of D.A.R.E. America; and Member of the Board of the Sheriff's Youth Foundation, and has received numerous honors and awards, including D.A.R.E. America's "Future of America Award"; the Muscular Dystrophy Association's "Humanitarian of the Year Award"; and the Starlight Children's Foundation's "Golden Wish Award." Mr. Lorsch was also awarded the Private Sector Initiatives Citation, or C-Flag, from the White House during the Reagan Administration for his commitment to raising millions of dollars for financing state and local earthquake preparedness education. Following the Merger, Mr. Lorsch continues to serve as Chairman of the Board of MMR and acts as its President and Chief Executive Officer.Hector V. Barreto, Jr., Director. Since July 2006, Mr. Barreto has been the owner and operator of the consulting company Barreto Associates. Mr. Barreto has also worked as a marketing and strategic planning advisor for our wholly-owned subsidiary MMR since August 2006. Prior to forming Barreto Associates, Mr. Barreto was unanimously approved by the U.S. Senate in July 2001 to serve as the Administrator of the Small Business Administration, a position he held until July 2006. From October 1995 until his appointment to the Small Business Administration in July 2001, Mr. Barreto worked as a broker dealer specializing in retirement plans for TELACU/Barreto Financial Services. Mr. Barreto currently serves on the California Commission for Economic Development and is the Chairman of The Latino Coalition, a non-profit, non-partisan organization based in Washington D.C. Prior to the Merger, Mr. Barreto served as a director of MMR. Mr. Barreto received a B.A. in Business Administration from Rockhurst University.
David A. Boyden, Director. Mr. Boyden has been in the healthcare and life science industry for 25 years. Mr. Boyden currently serves as President of Mirador Strategies Inc., a company he founded in January 2005 to provide consulting services focusing on commercialization strategy for life science companies. Prior to founding Mirador, Mr. Boyden was a Senior Brand Director at Amgen, Inc., a biotechnology company that develops, manufactures and markets human therapeutics, where he worked from April 1991 to November 2004 in a number of senior sales and marketing positions. Mr. Boyden received a B.A. in Biochemistry from U.C. San Diego and a M.B.A. in Marketing from the Anderson School of Management at UCLA.
49
Douglas H. Helm, Director. Mr. Helm is President and Chief Operating Officer of Plenary Insurance Services and has over 40 years of insurance experience. Mr. Helm also currently serves as Vice President of Business Development for Employers Direct Insurance Services, an affiliate of Plenary Insurance Services. From 2002 to September 2008, Mr. Helm served as Vice President and Chief Marketing & Sales Officer for Employers Direct Insurance Services. Mr. Helm majored in Labor Economics at the University of Washington and received a J.D. from Northwestern School of Law in 1973. Mr. Helm is a member of the Oregon State Bar.
George Rebensdorf, Director. Since 1996, Mr. Rebensdorf has served as Chief Executive Officer of The Rebensdorf Group, Inc., a company providing management consulting, investment banking and financial advisory services to the telecommunications and emerging technology industries. Mr. Rebensdorf is also currently a managing member of E/W Capital, LLC, a financial advisory and investment banking firm. Prior to forming The Rebensdorf Group, from 1995 to 1996, Mr. Rebensdorf served as Senior Vice President, External Affairs of MIDCOM Communications, Inc., where he managed mergers, acquisition, and corporate finance matters for the company. From 1987 to 1995, Mr. Rebensdorf was a general partner of Telenational Communications, Ltd., a diversified telecom services provider, offering services in the United States, Europe, South America and Asia. Following the Merger, Mr. Rebensdorf continues to serve as a director of MMR, a position he has held since 2005. Mr. Rebensdorf graduated magna cum laude from Arizona State University with a B.A. in English, and holds a J.D. from Creighton University School of Law.
Bernard Stolar, Director. Mr. Stolar currently serves as a consultant to the video games industry and is a marketing and strategic planning advisor for our wholly-owned subsidiary, MMR. From February 2007 to September 2008, Mr. Stolar served as Games Industry Evangelist for Google, Inc., where his responsibilities included building in-game advertising. From February 2006 until its purchase by Google, Inc. in February 2007, Mr. Stolar was the Chairman of the Board of Adscape Media. Prior to this, from January 2002 to November 2002, Mr. Stolar was President and Chief Operating Officer of BAM! Entertainment, where he helped transform the company from a content provider for hand-held electronics into a developer and marketer of interactive entertainment for next generation video game consoles. From January 2000 until the division was sold in April 2001, Mr. Stolar served as President of Mattel Interactive, where he was responsible for all of Mattel's software, on-line and computer-enhanced toys. Mr. Stolar also served as President and Chief Operating Officer of Sega of America, Inc. from June 1996 to October 1999 and as an Executive Vice President with Sony Computer Entertainment of America from 1994 to June 1996. Following the Merger, Mr. Stolar continues to serve as a director of MMR, a position he has held since 2005.
Jack Zwissig, Director. Since 1992, Mr. Zwissig has served as Chief Executive Officer of Zwissig and Associates, a consulting and executive leadership training firm that primarily concentrates on the designing and implementing of corporate culture change, including mergers and acquisitions. Throughout his tenure as head of Zwissig and Associates, Mr. Zwissig has offered consulting, marketing and advertising services to some of America's leading corporations and has led numerous corporate teambuilding workshops and seminars, both in the U.S. and abroad. Prior to the Merger, Mr. Zwissig served as a director of MMR. Mr. Zwissig received a B.S. in Marketing and Management and a M.B.A. from Santa Clara University.
Naj Allana, Senior Vice President and Chief Financial Officer. Mr. Allana has served as Chief Financial Officer and Chief Technical Officer of MMR since August 2005. Mr. Allana is also currently a partner with AMD Associates, LLC, a management consulting and temporary-staffing firm, where he has worked since February 2002. Prior to joining MMR, from April 2004 to May 2005, Mr. Allana was the Chief Financial Officer and Chief Operating Officer for Alterna, Inc., a manufacturer of high-end hair care products. Mr. Allana has 20 years of cross-industry experience with significant expertise in finance, accounting and information systems. Mr. Allana has a M.S. in Advanced Accounting and Costing from the University of Pune, India (formerly the University of Poona), and a M.B.A. from the UCLA Anderson School of Management. Prior to the Merger, Mr. Allana served as a director of MMR.
50
The following table summarizes the compensation earned by each "named executive officer" of MMR and Favrille for the past two fiscal years, determined on the basis of rules adopted by the SEC relating to "smaller reporting companies."
Summary Compensation Table
|
Name and Principal Position |
Year |
Salary ($) |
Stock Awards ($)(1) |
Option Awards ($) |
Non-Equity Incentive Plan Compensation ($) |
All Other Compensation ($) |
Total ($) |
|
MMR |
|||||||
|
Robert Lorsch |
2008 |
$120,000 |
- |
- |
- |
$380,082(2) |
$500,082 |
|
President & CEO |
2007 |
$140,000(3) |
- |
$20,970(4) |
- |
$358,918(2) |
$519,888 |
|
Naj Allana |
2008 |
$199,020(5) |
$17,324 |
$2,082(4) |
- |
$31,483(6) |
$249,909 |
|
Chief Financial Officer |
2007 |
$228,000(5) |
$6,500 |
$2,082(4) |
- |
$532(6) |
$237,114 |
|
Richard Teich |
2008 |
$101,625(7) |
$9,100 |
$2,082(4) |
- |
$19,981(8) |
$132,788 |
|
2007 |
$164,999(7) |
$6,500 |
$2,082(4) |
- |
$6,532(8) |
$180,113 |
|
|
Favrille |
|||||||
|
John P. Longenecker, Ph.D. |
2008 |
$401,272(9) |
- |
$717,579 (10) |
- |
- |
$1,118,851 |
|
Former President & CEO |
2007 |
$400,870 |
- |
$1,173,249 |
- |
- |
$1,574,19 |
|
Tamara A. Seymour |
2008 |
$275,797 |
- |
$176,971(10) |
$65,902(11) |
- |
$518,670 |
|
Former CFO |
2007 |
$275,797 |
- |
$291,750(10) |
- |
- |
$567,547 |
|
Daniel P. Gold, Ph.D. |
2008 |
$129,017(12) |
- |
$253,317(10) |
- |
$76,795(13) |
$459,129 |
|
Former Chief Scientific Officer |
2007 |
$295,855 |
- |
$449,928(10) |
- |
- |
$745,783 |
________________
1. Represents grants of restricted stock under MMR's Equity Incentive Plan based on the dollar amounts recognized for financial statement reporting purposes under SFAS No. 123R. The Company's determination of fair value is not binding upon individual grantees.
2. Includes $283,082 and $276,918 of consulting fees paid to The RHL Group, Inc., a California corporation wholly-owned and controlled by Mr. Lorsch for the years ended December 31, 2008 and 2007, respectively. In 2007, includes $42,000 of auto allowance ($21,000 of which was paid in 2007 and $21,000 of which was deferred). In 2008, includes $57,000 of auto allowance paid in 2008 (which includes $21,000 deferred in 2007). Includes approximately $40,000 of insurance premiums for each of 2007 and 2008, including finance charges on the financing of the premium payments, on The RHL Group, Inc.'s Directors' & Officers' insurance policy, which supplements coverage under MMR's Directors' & Officers' policy.
3. Mr. Lorsch received $70,000 in cash in salary in 2007 and we deferred payment of $70,000 until such payments could be made without jeopardizing our ability to continue as a going concern.
51
4. Amounts represent the dollar amounts recognized as share based compensation expense for financial statement reporting purposes during 2007 and 2008 under SFAS No. 123R. Assumptions made for the purpose of computing these amounts are discussed in note 5 to our consolidated financial statements appearing elsewhere in this prospectus.
5. Mr. Allana received $114,300 and $175,000 in cash as salary for the years ended December 31, 2008 and 2007, respectively. We deferred payment of $84,720 and $53,000 for the years ended December 31, 2008 and 2007, respectively, until such payments could be made without jeopardizing our ability to continue as a going concern.
6. In 2008, includes $15,000 in consulting fees for services rendered (paid on an hourly-fee basis) during the months of August through October 2008, when Mr. Allana's salary and time commitment were reduced to accommodate our cash situation. Of the $15,000 in fees, $3,750 were paid in cash and the balance of $11,250 remained unpaid as of December 31, 2008. Includes $6,574 and $532 of interest earned on deferred compensation for the years ended December 31, 2008 and 2007, respectively. In 2008, includes $10,000 of auto allowance (all of which was deferred).
7. Mr. Teich received $81,625 and $139,999 in cash as salary for the years ended December 31, 2008 and 2007, respectively. We deferred payment of $20,000 and $25,000 for the years ended December 31, 2008 and 2007, respectively, until such payments could be made without jeopardizing our ability to continue as a going concern.
8. In 2008, includes $12,825 in consulting fees for services rendered (paid on an hourly-fee basis) during the months of June through October 2008, when Mr. Teich either received a reduced salary or did not receive a salary to accommodate our cash situation. Includes $5,188 and $532 of interest earned on deferred compensation for the years ended December 31, 2008 and 2007, respectively. Includes $2,000 auto allowance in 2008 (all of which was deferred) and $6,000 auto allowance in 2007 (all of which was deferred).
9. Dr. Longenecker received $174,794 in cash from January 2008 through June 6, 2008. In accordance with a Salary Deferral Payment Agreement dated June 27, 2008 and filed as Exhibit 10.3 to our quarterly report on Form 10-Q for the quarter ended June 30 2008, we deferred payment of Dr. Longenecker's salary until such payments could be made without jeopardizing our ability to continue as a going concern. Following settlement with at least 85% of the our creditors, as required in the Creditor Plan, on January 15, 2009, Dr. Longenecker was paid $159,666 in cash, which represented salary from June 7, 2008 through October 31, 2008. The remaining two months of accrued salary was converted to a warrant to purchase 348,642 shares of our common stock. The warrant was granted on January 27, 2009 immediately prior to the Merger, has an exercise price equal to $0.12, and expires on January 26, 2014.
10. Amounts represent the dollar amounts recognized for financial statement reporting purposes during 2007 and 2008, excluding forfeitures, under SFAS No. 123R. Assumptions made for the purpose of computing these amounts are discussed in note 5 to the consolidated financial statements included elsewhere in this prospectus. Favrille used similar assumptions in fiscal year 2007.
11. Amount paid was in connection with the Retention Plan dated June 26, 2008 and extended on September 7, 2008, September 26, 2008 and October 23, 2008. A copy of the Retention Plan was filed as Exhibit 10.2 to our quarterly report on Form 10-Q for the quarter ended June 30, 2008 and the extensions were filed as Exhibits to our current reports on Form 8-K filed on September 11, 2008, October 10, 2008 and October 28, 2008.
12. Dr. Gold was terminated on June 6, 2008 in connection with Favrille's reduction in force following the announcement of the Phase 3 clinical trial failure, as reported on our current report on Form 8-K filed on September 15, 2008. Amounts represent salary paid through June 6, 2008. Dr. Gold's annual base salary was $295,855.
13. At termination, we had a liability to Dr. Gold for $39,712 and severance of $182,775, which represents 7 months of base salary. Amount represents the cash payment of approximately 85% of severance equal to two months of base salary and accrued flexible time off. In addition, Dr. Gold accepted a promissory note for $16,013 to be paid on August 31, 2009 and warrant to purchase 953.777 shares of our common stock pursuant to the Creditor Plan. The warrant was granted on January 27, 2009 immediately prior to the Merger, has an exercise price equal to $0.12, and expires on January 26, 2014.
52
Outstanding Equity Awards at Fiscal Year-End
The following table provides information on all outstanding equity awards held by our named executive officers as of December 31, 2008. Information for MMR named executive officers is provided on an as converted basis as if the Merger had occurred prior to that time.
|
|
Option Awards |
||||
|
Name |
Number of Securities Underlying Unexercised Options |
Number of Securities Underlying Unexercised Options1 |
Equity Incentive Plan Awards |
Option Exercise Price |
Option Expiration Date1 |
|
|
|||||
|
|
Number of Securities Underlying Unearned Options |
||||
|
|
|||||
|
|
(#) Exercisable |
(#) Unexercisable |
(#) |
($) |
|
|
MMR |
|||||
|
Robert Lorsch |
164,086 |
- |
- |
$0.046 |
10/1/2009 |
|
President and CEO |
2,461,298 |
- |
- |
$0.046 |
7/1/2010 |
|
|
|||||
|
Naj Allana |
164,086 |
- |
- |
$0.046 |
9/1/2011 |
|
Chief Financial Officer |
328,173 |
- |
- |
$0.046 |
8/1/2009 |
|
|
1,312,692 |
- |
- |
$0.046 |
1/1/2011 |
|
Richard Teich |
1,312,692 |
- |
- |
$0.046 |
1/1/2011 |
|
Favrille |
|
|
|
|
|
|
John P. Longenecker, Ph.D. |
130,187 |
140,508(1) |
- |
$2.57 |
3/9/2017 |
|
Former President and CEO |
194,873 |
72,382(1) |
- |
$7.40 |
3/23/2016 |
|
|
157,917 |
10,528(1) |
- |
$5.50 |
3/29/2015 |
|
|
6,379 |
-(1) |
- |
$0.73 |
12/31/2014 |
|
|
357,404 |
-(2) |
- |
$0.63 |
3/19/2014 |
|
11,934 |
-(1) |
- |
$0.63 |
11/17/2013 |
|
|
32,385 |
- |
- |
$0.63 |
7/25/2013 |
|
|
Tamara A. Seymour |
29,698 |
38,282(1) |
- |
$2.58 |
3/2/2017 |
|
Former CFO |
36,469 |
13,546(1) |
- |
$7.40 |
3/23/2016 |
|
|
29,797 |
1,987(1) |
- |
$5.50 |
3/29/2015 |
|
|
1,458 |
-(1) |
- |
$0.73 |
12/31/2014 |
|
|
67,507 |
-(2) |
- |
$0.63 |
3/19/2014 |
|
|
18,522 |
-(2) |
- |
$0.63 |
2/3/2014 |
|
|
|||||
|
Daniel P. Gold |
- |
-(3) |
- |
- |
- |
|
Former Chief Scientific Officer |
|||||
___________
(1) 25% of the shares subject to the option vest on the first anniversary of the vesting commencement date specified in the option grant notice; 1/48th of the shares subject to the option vest each month thereafter such that the stock options are fully vested on the fourth anniversary of the vesting commencement date.
53
(2) 1/48th of the shares subject to the option vest each month after the vesting commencement date specified in the option grant notice such that the stock options are fully vested on the fourth anniversary of the vesting commencement date.
(3) All unexercised options expired on September 6, 2008, 90 days following Dr. Gold's termination.
Employment Agreements
We have entered into an employment agreement with our Chairman, President and Chief Executive Officer, Robert H. Lorsch, with an initial term ending on December 31, 2011, subject to successive automatic extension unless we or Mr. Lorsch elect not to extend. Under the terms of his agreement, Mr. Lorsch shall serve as both our President and Chief Executive Officer and President and Chief Executive Officer of our wholly-owned subsidiary, MMR. The agreement provides for a base salary of $15,000 per month, subject to an upward increase and with an annual bonus and stock option grants in such amounts, if any, as the Board of Directors may determine in its sole discretion. Mr. Lorsch receives a monthly auto allowance, reimbursement of certain life insurance proceeds, and reimbursement for certain other insurance coverage, and is entitled to participate in benefits generally made to our senior executives.
Mr. Lorsch may terminate the agreement upon 30 days written notice without reason or for good reason (as defined in the agreements) if we fail to cure acts or omissions constituting good reason within 30 days. If Mr. Lorsch's employment is terminated by us for cause or voluntarily by Mr. Lorsch without good reason, he will not be entitled to receive any severance payments or benefits under the employment agreement. If Mr. Lorsch's employment is terminated by us without cause or voluntarily by Mr. Lorsch for good reason, Mr. Lorsch will be entitled to one year of salary at his then current rate of pay, including all monthly benefits, and the pro rata portion of the annual bonus otherwise due Mr. Lorsch. Mr. Lorsch is not entitled to any severance payments or benefits under the agreement if he is terminated for cause or voluntarily resigns without good reason. In the event of his disability, Mr. Lorsch would be entitled to receive compensation equal to 40% of his base salary as then in effect. Mr. Lorsch's employment agreement includes provisions that prohibit Mr. Lorsch from disclosing our confidential information and trade secrets and competing with us during the term of his employment agreement or soliciting our employees for 12 months following termination of employment.
We also have entered into a consulting agreement with The RHL Group, Inc., which is wholly-owned by Mr. Lorsch, that provides for a monthly fee of $25,000 plus reimbursement of expenses including medical insurance.
We also have approved an employment agreement with our Chief Financial Officer and Senior Vice President, Naj Allana. Under the terms of his agreement, Mr. Allana shall serve both as our Chief Financial Officer and Senior Vice President and as Chief Financial Officer, Senior Vice President and Chief Technical Officer of our wholly-owned subsidiary, MMR. The agreement provides for a base salary of $15,845 per month, subject to an upward increase. In addition to his base salary, Mr. Allana is entitled to receive a commission with respect to certain of our accounts.
Mr. Allana's employment agreement is effective until February 15, 2010 and would automatically renew for successive 12 month periods unless terminated at least 120 days prior to the end of the term. If we terminate Mr. Allana's employment, other than for misconduct (as defined in the employment agreement), we would continue to pay Mr. Allana his monthly salary for the remainder of the then current term, or if done 120 days preceding the end of the term, then through the end of the next 12 month term.
On January 27, 2009, immediately prior to the Merger, Favrille and MMR entered into an agreement with our former President, Chief Executive Officer and Director, John P. Longenecker, Ph.D. Under the terms of the agreement, we agreed to pay Dr. Longenecker $226,478.46 for seven months of deferred salary accrued from June 2008 through December 2008, $159,667 of which was paid in cash on January 27, 2009 and the remainder of which was paid in the form of a warrant to purchase 348,642 shares of our common stock. The warrant has an exercise price of $0.12 per share and expires on January 26, 2014. The agreement with Dr. Longenecker also provides that Dr. Longenecker will continue as our employee as a member of the merger transition team for an indefinite period of time under an "at will" employment arrangement. Dr. Longenecker also agreed to forego payment of $53,846 of
54
accrued flexible time off currently outstanding. In consideration for continuing as an employee and foregoing payment of accrued flexible time off, Dr. Longenecker will receive a base salary of $2,500 per month and a bonus totaling $66,424. Under the terms of the agreement, $55,572 of such bonus was paid in cash in January 2009 prior to the Merger, while the remaining $10,852 is to be paid on August 31, 2009 as evidenced by a promissory note issued by us to Dr. Longenecker. We also granted Dr. Longenecker a warrant to purchase 2,064,899 shares of our common stock at an exercise price of $0.12 per share that expires on January 26, 2014. The warrants granted immediately prior to the Merger and the amounts to be paid as a bonus will be deducted from cash and stock reserved for issuance under the Creditor Plan. For details regarding the Creditor Plan please see Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations."
On January 27, 2009, immediately prior to the Merger, Favrille and MMR entered into an agreement with our former Chief Financial Officer and Secretary, Tamara Seymour. Under the terms of the agreement, Ms. Seymour will continue as our employee as a member of the merger transition team for an indefinite period of time under an "at will" employment arrangement. Ms. Seymour also agreed to forego payment of $37,019 of accrued flexible time off currently outstanding. In consideration for continuing as an employee and foregoing payment of accrued flexible time off, we granted Ms. Seymour a warrant to purchase 855,514 shares of our common stock at an exercise price of $0.12 per share that expires on January 26, 2014 and will pay Ms. Seymour a base salary of $2,500 per month, plus an hourly rate of $150 and a bonus totaling $93,045. Under the terms of the agreement, $78,453 of such bonus was paid in cash in January 2009 prior to the Merger, while the remaining $14,592 is to be paid on August 31, 2009 as evidenced by a promissory note issued by us to Ms. Seymour. The warrant and the amounts to be paid as a bonus will be deducted from cash and stock reserved for issuance under the Creditor Plan.
Director Compensation
The following table sets forth certain information with respect to the compensation paid to our non-employee directors for the fiscal year ended December 31, 2008:
|
Name (a) |
Fees |
Option |
Other Compensation |
Total ($) |
|
Michael L. Eagle |
$43,000(4) |
$24,571(12) |
$12,000(4) |
$79,571 |
|
Cam L. Garner |
$30,000(5) |
$14,041(13) |
|
$44,041 |
|
Antonio J. Grillo-Lopez, MD |
$34,000(6) |
$14,041(14) |
|
$48,041 |
|
Peter Barton Hutt |
$17,250(7) |
$16,849(15) |
|
$34,099 |
|
Fred Middleton |
$40,500(8) |
$22,465(16) |
|
$62,965 |
|
David Molowa, Ph.D. |
$29,500(9) |
$48,103(17) |
|
$77,603 |
|
Wayne I. Roe |
$37,000(10) |
$14,041(18) |
|
$51,041 |
|
Ivor Royston, MD |
$34,000(11) |
$14,041(19) |
|
$48,041 |
_______________
1. All fees earned and accrued during 2008 were deferred in accordance with the Deferred Compensation Program approved by our Board of Directors during the November 29, 2007 meeting. A copy of the Director Fee Deferral Election Form is filed as Exhibit 10.32 to our annual report on Form 10-K for the fiscal year ended December 31, 2007. Under the Deferred Compensation Program, such fees were payable in cash during the period from September 1, 2008 through December 31, 2008. However, each director elected to receive a warrant to purchase our common stock in lieu of cash. The warrants were granted on January 27, 2009, immediately prior to the closing of the Merger, with an exercise price equal to $0.12, the closing market price on the date of the closing of the Merger and expire on January 26, 2014.
55
2. Amounts represent the dollar amounts recognized for financial statement reporting purposes during 2008, excluding forfeitures, under Financial Accounting Standards Board Statement of Financial Accounting Standard No. 123R, or SFAS No. 123R. Assumptions made for the purpose of computing these amounts are discussed in note 5 to the consolidated financial statements appearing elsewhere in this prospectus.
3. Each director waived their right to receive an option award under the 2005 Non-employee Director Stock Option Plan during 2008. Therefore, no options were granted to directors in 2008.
4. Mr. Eagle earned $43,000 of director fees and $12,000 of consulting fees during 2008. Total director fees of $45,000 deferred in accordance with the Deferred Compensation Program and $12,000 of consulting fees were paid in the form of a warrant to purchase 297,442 shares of our common stock. See footnote (1) above.
5. Amount was deferred in accordance with the Deferred Compensation Program and paid in the form of a warrant to purchase 156,548 shares of our common stock. See footnote (1) above.
6. Dr. Grillo-Lopez earned $34,000 of director fees during 2008. Total director fees of $36,000 deferred in accordance with the Deferred Compensation Program were paid in the form of a warrant to purchase 187,858 shares of our common stock. See footnote (1) above.
7. Mr. Hutt earned $17,250 of director fees during 2008. Total director fees of $18,750 deferred in accordance with the Deferred Compensation Program were paid in the form of a warrant to purchase 97,842 shares of our common stock. See footnote (1) above. Mr. Hutt resigned from our Board on June 24, 2008.
8. Mr. Middleton earned $40,500 of director fees during 2008. Total director fees of $42,500 deferred in accordance with the Deferred Compensation Program were paid in the form of a warrant to purchase 221,777 shares of our common stock. See footnote (1) above.
9. Dr. Molowa earned $29,500 of director fees during 2008. Total director fees of $31,500 deferred in accordance with the Deferred Compensation Program were paid in the form of a warrant to purchase 164,376 shares of our common stock. See footnote (1) above.
10. Mr. Roe earned $37,000 of director fees during 2008. Total director fees of $38,500 deferred in accordance with the Deferred Compensation Program were paid in the form of a warrant to purchase 200,904 shares of our common stock. See footnote (1) above.
11. Dr. Royston earned $34,000 of director fees during 2008. Total director fees of $36,000 deferred in accordance with the Deferred Compensation Program were paid in the form of a warrant to purchase 187,858 shares of our common stock. See footnote (1) above.
12. Reflects the compensation costs we recognized in fiscal year 2008 for stock option grants with the following fair values as of the grant date: (a) $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share; (b) $17,926.50 for a stock option grant to purchase 7,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share; (c) $4,481.63 for a stock option grant to purchase 1,875 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Mr. Eagle had options to purchase an aggregate of 42,375 shares of common stock outstanding at the end of fiscal year 2008.
13. Reflects the compensation costs we recognized in fiscal year 2008 for a stock option grant with the following fair value as of the grant date: $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Mr. Garner had options to purchase an aggregate of 45,500 shares of our common stock outstanding at the end of fiscal year 2008.
56
14. Reflects the compensation costs we recognized in fiscal year 2008 for a stock option grant with the following fair value as of the grant date: $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Dr. Grillo-Lopez had options to purchase an aggregate of 56,016 shares of common stock outstanding at the end of fiscal year 2008.
15. Reflects the compensation costs we recognized in fiscal year 2008 for stock option grants with the following fair values as of the grant date: (a) $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share; (b) $5,975.50 for a stock option grant to purchase 2,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Mr. Hutt's service as a Director ended on June 24, 2008. Mr. Hutt had no options to purchase common stock outstanding at the end of fiscal year 2008.
16. Reflects the compensation costs we recognized in fiscal year 2008 for stock option grants with the following fair values as of the grant date: (a) $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share; (b) $17,926.50 for a stock option grant to purchase 7,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Mr. Middleton had options to purchase an aggregate of 72,289 shares of common stock outstanding at the end of fiscal 2008.
17. Reflects the compensation costs we recognized in fiscal year 2008 for stock option grants with the following fair values as of the grant date: (a) $102,001 for a stock option grant to purchase 35,000 shares of common stock made on July 12, 2006, at an exercise price of $4.65 per share; (b) $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Dr. Molowa had options to purchase an aggregate of 47,500 shares of common stock outstanding at the end of fiscal year 2008.
18. Reflects the compensation costs we recognized in fiscal year 2008 for a stock option grant with the following fair value as of the grant date: $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Mr. Roe had options to purchase an aggregate of 59,099 shares of common stock outstanding at the end of fiscal year 2008.
19. Reflects the compensation costs we recognized in fiscal year 2008 for a stock option grant with the following fair value as of the grant date: $29,877.50 for a stock option grant to purchase 12,500 shares of common stock made on June 20, 2007, at an exercise price of $3.88 per share. Dr. Royston had options to purchase an aggregate of 57,289 shares of common stock outstanding at the end of fiscal year 2008.
For the year ended December 31, 2008, each of our non-employee directors was entitled to receive cash compensation in the form of an annual retainer of $16,000, a fee of $2,000 for each non-telephonic meeting of our Board of Directors that he attended in person, and a fee of $1,500 for attendance at each non-telephonic meeting of our Board of Directors in which he participated telephonically and each telephonic meeting of our Board of Directors in which he participated. In addition, the Chairman of the Board and the chairs of the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee were entitled to receive additional annual retainers of $5,000, $4,000, $2,500 and $2,500, respectively. Each non-employee director serving on a committee also was also entitled to receive a fee of $1,000 for each committee meeting he attended in person or in which he participated telephonically. However, such fees were deferred pursuant to a Deferred Compensation Program approved in November 2007, and subsequent to the Phase 3 clinical trial failure and the Merger, each of our directors serving at such time agreed to receive a warrant to purchase common stock as payment for all deferred director fees. The warrants were issued on January 27, 2009 pursuant to the Creditor Plan.
We reimbursed our non-employee directors for their reasonable expenses incurred in attending meetings of our Board of Directors and committees of our Board of Directors. All of these reimbursements were paid in cash, except for payments made to Mr. Middleton, who elected to receive payment in warrants pursuant to the Creditor Plan.
57
For the year ended December 31, 2008, each of our non-employee directors was also entitled to receive stock option grants under our 2005 Non-Employee Directors' Stock Option Plan, as amended, or the Directors' Plan. Only our non-employee directors or an affiliate of such directors (as defined in the Internal Revenue Code) were eligible to receive options under the Directors' Plan. Options granted under the Directors' Plan are intended by us not to qualify as incentive stock options under the Internal Revenue Code.
The Directors' Plan provides for the automatic grant of non-statutory options to purchase shares of common stock to non-employee directors. An aggregate of 600,000 shares of common stock have been reserved for future issuance under the Directors' Plan as of December 31, 2008 which includes a 90,000 share reserve increase approved by our Board of Directors in 2008. On the first day of each fiscal year, from 2005 to 2013, the share reserve under the Directors' Plan is automatically increased by the number of shares equal to the lesser of: (i) 90,000 shares of common stock; or (ii) an amount determined by our Board of Directors. Any person who for the first time becomes a non-employee director automatically receives an option to purchase 35,000 shares of our common stock upon his or her election. In addition, any person who is a non-employee director on the date of each annual meeting of our stockholders is automatically granted, on the annual meeting date, an option to purchase 12,500 shares of common stock. The size of an annual grant made to a non-employee director who has served for less than 12 months at the time of the annual meeting will be reduced by 25% for each full quarter prior to the date of grant during which such person did not serve as a non-employee director.
The Directors' Plan also provides for the automatic annual grant of options to purchase common stock on the annual meeting date in the following amounts to directors serving in the following positions:
Chairman of the Board - 10,000 shares
Chairman of the Audit Committee - 7,500 shares
Chairman of the Compensation Committee - 2,500 shares
Chairman of the Nominating and Corporate Governance Committee - 2,500 shares.
The exercise price of stock options granted under the Directors' Plan is equal to 100% of the fair market value of our common stock on the date of grant, which is deemed to be the closing trading price of our common stock on the trading day immediately preceding the date of grant. Unless the terms of an optionholder's stock option agreement provide for different vesting rates, initial grants under the Directors' Plan vest at the rate of 1/36th each month after the date of grant, and annual grants under the Directors' Plan vest at the rate of 1/12th each month after the date of grant. In general, the term of stock options granted under the Directors' Plan may not exceed ten years.
Unless the terms of an optionholder's stock option agreement provide for earlier termination, if an optionholder's service relationship with us, or any affiliate of ours, ceases due to disability or death, the optionholder, or his or her beneficiary, may exercise any vested options up to 12 months, or 18 months in the event of death, after the date such service relationship ends. If an optionholder's service relationship with us, or any affiliate of ours, ceases without cause for any reason other than disability or death, the optionholder may exercise any vested options up to three months from cessation of service, unless the terms of the stock option agreement provide for earlier or later termination.
Acceptable consideration for the purchase of common stock issued under the Directors' Plan may include cash or check, common stock previously owned by the optionholder, a "net exercise" of an option whereby we reduce the number of shares of our common stock issued upon the exercise of the option by the number of shares that has a fair market value that does not exceed the aggregate exercise price of the option, or a program developed under Regulation T as promulgated by the Federal Reserve Board. Generally, an optionholder may not transfer a stock option other than by will or the laws of descent and distribution. An optionholder may transfer an option with our written consent provided a Form S-8 registration statement is available for the exercise of the option and the subsequent resale of the shares. However, an optionholder may designate a beneficiary who may exercise the option following the optionholder's death.
In the event of certain corporate transactions, any or all outstanding options under the Directors' Plan may be assumed or substituted for by any surviving entity. If the surviving entity elects not to assume or substitute for all such options, vesting of the options not assumed or substituted for and held by participants whose continuous service has not terminated will be accelerated and such options will be terminated if not exercised prior to the effective date of the corporate transaction.
58
In addition, during the fiscal year ended December 31, 2008, all of our directors were eligible to participate in our Amended and Restated 2001 Equity Incentive Plan, and our employee directors were eligible to participate in our 2005 Employee Stock Purchase Plan.
During the fiscal year ended December 31, 2008, we did not grant any options to purchase our common stock under the Directors' Plan. Each director waived his right to receive the automatic option grant on the date of the 2008 Annual Meeting. As of April 10, 2009, none of the stock options granted to our non-employee directors under the Directors' Plan had been exercised.
Following the Merger, on January 27, 2009, our newly elected Board held a special meeting and decided to postpone the receipt of option grants under our currently existing Directors' Plan and requested that the Compensation Committee meet to consider and review our director compensation policies and program and the grant of equity compensation to our Board members. At the same special Board meeting, the Board agreed to immediately modify the fees paid to non-employee directors as follows: an annual fee of $12,000, plus an additional annual fee of $5,000 for the Chairman of the Audit Committee, and an additional annual fee of $4,000 for the Chairmen of our Compensation and Nominating and Corporate Governance Committees. Directors now will receive $1,000 for in-person attendance at non-telephonic meetings, and $500 for telephonic participation in both in-person and telephonic meetings. Our directors will continue to be reimbursed for reasonable out-of-pocket expenses incurred in attending meetings of our Board and for other expenses reasonably incurred in their capacity as directors. Our full Board will consider further changes upon the recommendation of the Compensation Committee when appropriate.
Our Board of Directors is currently comprised of seven directors and is divided into three classes, with each class serving a staggered three-year term. Effective upon the consummation of the Merger, the following individuals were appointed to the three classes of directors with terms expiring as follows:
Doug H. Helm - 2009 annual meeting
Jack Zwissig - 2009 annual meeting
David A. Boyden - 2010 annual meeting
George Rebensdorf - 2010 annual meeting
Hector V. Barreto, Jr. - 2011 annual meeting
Bernard Stolar - 2011 annual meeting
Robert H. Lorsch - 2011 annual meeting
Independence of Directors
Although our common stock is no longer listed on the NASDAQ Global Market, our Board of Directors determined that each of the following directors would be deemed "independent" under NASDAQ Stock Market LLC rules: Messrs. Barreto, Boyden, Helm, Rebensdorf, Stolar and Zwissig. These persons represent a majority of our Board of Directors. Mr. Lorsch, the Chairman of our Board and our President and Chief Executive Officer would not be deemed independent. Messrs Stolar, Barreto and Rebensdorf are members of our Compensation Committee and Mr. Barreto is the Chairman. Messrs. Helm, Zwissig and Rebensdorf are members of our Nominating and Corporate Governance Committee and Mr. Helm is the Chairman. Messrs. Stolar, Barreto and Rebensdorf are members of our Audit Committee but would not be deemed to be independent under the more stringent independence requirements for Audit Committee members set forth in NASDAQ Stock Market LLC Rule 5605(2). Mr. Stolar is Chairman of the Audit Committee.
59
Compensation Committee Interlocks and Insider Participation
No member of the Compensation Committee of our Board of Directors during fiscal year 2008 served as an officer, former officer or employee of us or any of our subsidiaries. During fiscal year 2008, no executive officer of us served as a member of the compensation committee of any other entity, one of whose executive officers served as a member of our Board of Directors or Compensation Committee, and no executive officer of us served as a member of the board of directors of any other entity, one of whose executive officers served as a member of our Compensation Committee.
60
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the beneficial ownership of our common stock as of September 30, 2009, by:
- each of our current directors;
- each of our current executive officers named in the Summary Compensation Table;
- all of our current directors and executive officers as a group; and
- each person or entity (or group of affiliated persons or entities) who is known by us to own beneficially more than 5% of the outstanding shares of common stock.
|
Name and Address of Beneficial Owner(1) |
|
Number of Shares of Common |
|
Percentage |
|
|
Directors and Named Executive Officers |
|
|
|
|
|
|
Robert H. Lorsch(3) |
|
49,616,726 |
|
29.75 |
% |
|
Hector V. Barreto, Jr.(4) |
|
2,192,207 |
|
1.44 |
% |
|
David A. Boyden (5) |
|
36,164 |
|
* |
|
|
Douglas H. Helm(6) |
|
715,685 |
|
* |
|
|
George Rebensdorf(7) |
|
2,119,121 |
|
1.39 |
% |
|
Bernard Stolar(8) |
|
1,368,156 |
|
* |
|
|
Jack Zwissig(9) |
|
1,845,109 |
|
1.21 |
% |
|
Naj Allana(10) |
|
3,767,507 |
|
2.46 |
% |
|
All Executive Officers and Directors as a group |
|
61,660,676 |
|
36.51 |
% |
|
|
|
|
|
|
|
|
5% Stockholders |
|
|
|
|
|
|
The RHL Group, Inc.(12) |
|
44,019,270 |
|
27.06 |
% |
__________
*
Less than 1%(1)
The business address of each director and executive officer listed is c/o MMR Information Systems, Inc., 2934½ Beverly Glen Circle, Suite 702, Los Angeles, CA 90077.(2)
This table is based upon information supplied by officers, directors, principal stockholders, and Schedules 13D and 13G filed with the SEC. Beneficial ownership is determined in accordance with the rules of the SEC. Applicable percentage ownership is based on 151,625,775 shares of common stock outstanding as of September 30, 2009. Shares of common stock subject to options, warrants and convertible notes exercisable or convertible within 60 days after September 30, 2009, are deemed outstanding for computing the ownership percentage of the person holding such options, warrants or notes, but are not deemed outstanding for computing the ownership percentage of any other person. Except as otherwise noted, we believe that each of the stockholders named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to applicable community property laws.(3)
Consists of (i) 2,190,851 shares of common stock held directly by Mr. Lorsch and 32,979,892 shares of common stock held directly by The RHL Group, which is wholly owned and controlled by Mr. Lorsch, (ii) a warrant held by Mr. Lorsch to purchase 706,605 shares of common stock and a warrant held by The RHL Group, to purchase 11,039,378 shares of common stock, and (iii) stock options held by Mr. Lorsch to purchase 2,700,000 shares of common stock that are vested and exercisable within 60 days after September 30, 2009.(4) Includes 46,110 shares subject to stock options that are fully vested and exercisable as of such date.
(5) Represents shares subject to options exercisable within 60 days after September 30, 2009.
61
(6) Includes 40,685 shares subject to options exercisable within 60 days after September 30, 2009.
(7) Includes 512,000 shares subject to options exercisable within 60 days after September 30, 2009.
(8) Includes 202,438 shares subject to options exercisable within 60 days after September 30, 2009.
(9) Includes 357,484 shares subject to options exercisable within 60 days after September 30, 2009.
(10) Includes 1,476,783 shares subject to options exercisable within 60 days after September 30, 2009.
(11) Includes 17,267,648 shares subject to options and warrants exercisable within 60 days after September 30, 2009.
(12) Includes a fully vested warrant to purchase 11,039,378 shares of common stock.
62
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation agreements and other arrangements with our executive officers and directors and the transactions described below, during our last three fiscal years, there has not been, and there is not currently proposed, any transaction or series of similar transactions to which we were or will be a party in which the amount involved exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years and in which any of our directors, nominees for director, executive officers, holders of more than five percent of any class of our voting securities or any member of the immediate family of the foregoing persons had or will have a direct or indirect material interest.
See "Management's Discussion and Analysis of Financial Condition and Results of Operations-Liquidity and Capital Resources-Description of Indebtedness" elsewhere in this prospectus for a description of certain of our debt financing transactions with The RHL Group. Our Chief Executive Officer, Robert H. Lorsch, is also the Chief Executive Officer of The RHL Group, and owns all of the capital stock of The RHL Group.
As disclosed above, we have also entered into a consulting agreement with The RHL Group, that provides for a monthly fee of $25,000 plus reimbursement of expenses including medical insurance.
During 2005 and 2006 we occupied space pursuant to a sublease from The RHL Group., pursuant to which we paid an aggregate $315,000 in rent in 2006. From August 2006 through August 2008, we leased space to The RHL Group at no cost at our former Santa Monica office space, which we have since vacated. Since August 2008, we have occupied space pursuant to a sublease from Robert H. Lorsch, for which we pay $3,000 per month plus utilities. The original term of the lease expired on April 15, 2009, and has been renewed on a month-to-month basis.
We have consulting agreements with our current directors Hector V. Barreto, Jr. and Bernard Stolar pursuant to which Mr. Barreto and Mr. Stolar provide marketing and strategic planning advice and actively seek strategic partnerships and alliances with other entities to market our products. Under the terms of these agreements, we pay Mr. Barreto and Mr. Stolar $50,000 a year payable monthly, and commissions equal to 1% of all revenue generated through their efforts. The agreement with Mr. Barreto is effective until August 2009 and the agreement with Mr. Stolar is effective until November 2009. In the past, both Mr. Barreto and Mr. Stolar have received shares of our common stock as consideration for deferral of payment. Each agreement will automatically renew each successive year until terminated by either party upon 30 days prior written notice.
MMR also has a consulting agreement, as amended, with The Rebensdorf Group, Inc., or TRGI, which is owned by our current director George Rebensdorf. Mr. Rebensdorf also serves as its Chief Executive Officer. Pursuant to the agreement, TRGI provides financial advisory services and assists in negotiations in connection with our efforts to raise funds through private placement transactions. In addition to the initial retainer fee of $30,000 we paid upon execution of the original letter agreement, we have agreed to pay TRGI (a) a $4,167 monthly retainer (commencing May 1, 2009), (b) for transactions with parties introduced by TRGI, a success fee equal to 8% of the value (payable 4% in cash, 4% in warrants), (c) for material assistance in closing transactions with parties not introduced by TRGI, an advisory fee equal to 2% of the value (payable in cash), and (d) an annual grant of 100,000 options, vesting monthly over two-years from the grant date, with an exercise price equal to fair market value on the grant date. Payment of the monthly retainer may be deferred, in which case it would be payable to TRGI pro rata upon payment of any other deferred management or consulting fees. The letter agreement, as amended, is effective until terminated by either party, with or without cause, upon 10 days prior written notice.
In connection with the execution of an addendum to the letter agreement with TRGI on May 21, 2009, we granted Mr. Rebensdorf stock options to acquire 100,000 shares of our common stock at an exercise price of $0.179 per share, which options vest monthly over 2 years and expire on May 21, 2014.
We also have an oral agreement with our current director Jack Zwissig to provide individual executive coaching services to our management team. Mr. Zwissig receives compensation in the form of stock as determined by our Board of Directors commensurate with the services performed. The agreement with Mr. Zwissig is on a month-to-month basis and continues until terminated by either party.
63
On August 6, 2009, we made the following equity issuances to the persons and for the reasons stated below:
- To Mr. Robert Lorsch, Chairman, CEO and President, an option to buy 9,000,000 shares of the Company's common stock at an exercise price of $0.125 per share, with the first 3,000,000 of such shares vesting on January 27, 2009, the second 3,000,000 vesting on January 27, 2010 and the final 3,000,000 vesting on January 27, 2011. These options were to be granted under Mr. Lorsch's employment agreement, but were in fact never granted.
- To George Rebensdorf, a member of our Board of Directors, in consideration of consulting and other services, (a) an option to purchase 2,000,000 shares of our common stock at an exercise price of $0.125 per share, vesting in eight equal quarterly installments with the first installment fully vesting on the date of grant and the remaining seven installments vesting on the last day of each quarter thereafter, and (b) a grant of 1,000,000 shares of our common stock at a price per share value of $0.125.
- To Jack Zwissig, a member of our Board of Directors, in consideration of consulting and other services, (a) an option to purchase 1,200,000 shares of our common stock at an exercise price of $0.125 per share, vesting in eight equal quarterly installments with the first installment fully vesting on the date of grant and the remaining seven installments vesting on the last day of each quarter thereafter, and (b) for an initial term of one year from the date hereof, which term may be extended for an additional year upon Board approval, a monthly grant of shares of our common stock, with the first such grant occurring at the end of August 2009 in an amount equal to $35,000 worth of our common stock (covering the period since February 2009) and each subsequent grant occurring at the end each following month in an amount equal to $5,000.00 worth of our common stock. With respect to (b) above, the price per share of our common stock shall be based on the daily volume weighted average price of such stock for the ten trading days preceding the end of the applicable month, and such stock shall be issued to Mr. Zwissig within five business days of the end of each month.
- To Bernard Stolar, a member of our Board of Directors, in consideration of consulting and other services, (a) an option to convert, at Mr. Stolar's election, all accrued and unpaid compensation owed to Mr. Stolar by the Company into shares of our common stock, at a per share value of $0.125 and (b) an option to purchase 1,200,000 million shares of our common stock, vesting in eight equal quarterly installments with the first installment fully vesting on the date of grant and the remaining seven installments vesting on the last day of each quarter thereafter.
- To Hector Barreto, a member of our Board of Directors, in consideration of consulting and other services, (a) an option to convert, at Mr. Barreto's election, all accrued and unpaid compensation owed to Mr. Stolar by the Company into shares of our common stock, at a per share value of $0.125 and (b) an option to purchase 1,200,000 million shares of our common stock, vesting in eight equal quarterly installments with the first installment fully vesting on the date of grant and the remaining seven installments vesting on the last day of each quarter thereafter.
- To Naj Allana, our Chief Financial Officer and Senior Vice President, a grant of 707,016 shares of our common stock at a price per share value of $0.125, in consideration of his agreement to defer compensation owed to him.
- To Richard Teich, an employee of the Company, a grant of up to 410,000 shares of our common stock at a price per share value of $0.125, in consideration of his agreement to defer compensation owed to him.
The securities above were issued to each of the foregoing in reliance upon exemptions from registration pursuant to Section 4(2) under the Securities Act of 1933, as amended, and the rules promulgated thereunder. At the time of their issuance, the securities granted above are restricted securities for purposes of the Securities Act and the certificates representing such securities shall bear legends to that effect. The exercise/conversion prices of the securities described above were equal to the closing price of our common stock as of the date of grant.
Creditor Plan Warrants
Pursuant to the terms and conditions of the Creditor Plan, on January 27, 2009, we issued warrants to acquire 9,999,992 shares of our common stock at an exercise price of $0.12 per share, which expire on January 26, 2014 to certain of our former officers, former directors and their affiliates who were willing to take such equity as partial or full payment for outstanding liabilities. This includes warrants to acquire an aggregate 3,269,055 shares of our common stock at an exercise price of $0.12 per share, which expire on January 26, 2014 to John Longenecker, Ph.D and Tamara Seymour our former Chief Executive Officer and former Chief Financial Officer, respectively.
64
This section summarizes certain of our authorized and outstanding securities and certain of the provisions of our amended and restated certificate of incorporation and our amended and restated bylaws, each as amended to date.
Description of capital stock
The following description of our capital stock and provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to our certificate of incorporation and bylaws, respectively, copies of which have been filed with the SEC and are incorporated by reference herein.
General background
Our certificate of incorporation authorizes the issuance of up to 650,000,000 shares of common stock with a $0.001 par value and 5,000,000 preferred shares with a par value of $0.001. As of September 30, 2009, we had issued and outstanding 151,625,775 shares of common stock, held by approximately 219 stockholders of record.
Common stock
Except as required by law, holders of our common stock are entitled to vote on all matters as a single class, and each holder of common stock is entitled to one vote for each share of common stock owned. Holders of common stock do not have cumulative voting rights.
Holders of our common stock are entitled to receive ratably any dividends that may be declared by the board of directors out of legally available funds, subject to any preferential dividend rights of any outstanding preferred stock. Upon any liquidation, dissolution, or winding up of the Company, holders of our common stock are entitled to share ratably in all assets remaining available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock.
Holders of our common stock have no preemptive, subscription, redemption or conversion rights. Our outstanding shares of common stock are validly issued, fully paid and nonassessable. The rights, preferences and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of holders of shares of any series of preferred stock which we may designate and issue in the future without further stockholder approval.
Our common stock is not currently traded on any securities exchange and instead is quoted on the OTC Bulletin Board under the symbol "MMRF.OB."
Preferred stock
Our board of directors is authorized without further stockholder approval to issue from time to time up to an aggregate of 5,000,000 shares of preferred stock in one or more series and to fix or alter the designations, preferences, rights, qualifications, limitations or restrictions of the shares of each series, including the dividend rights, dividend rates, conversion rights, voting rights, term of redemption including sinking fund provisions, redemption price or prices, liquidation preferences and the number of shares constituting any series or designations of such series without further vote or action by the stockholders. No shares of preferred stock have been issued or are outstanding.
The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of the Company without further action by the stockholders and may adversely affect the market price of and voting, economic and other rights of, holders of our common stock.
65
Anti-takeover effects of provisions of Delaware Law and our Charter and Bylaws
We are subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a "business combination" with an "interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder, unless either:
• prior to the date at which the person becomes an interested stockholder, the board of directors approves such transaction or business combination;
• the stockholder acquires more than 85% of the outstanding voting stock of the corporation (excluding shares held by directors who are officers or held in certain employee stock plans) upon consummation of such transaction; or
• the business combination is approved by the board of directors and by the holders of two-thirds of the outstanding voting stock of the corporation (excluding shares held by the interested stockholder) at a meeting of stockholders (and not by written consent).
For purposes of Section 203, "interested stockholder" is a person who, together with affiliates and associates, owns (or within three years prior, did own) 15% or more of the corporation's voting stock. A "business combination" includes a merger, asset sale or other transaction resulting in a financial benefit to such interested stockholder.
In addition, certain provisions of our certificate of incorporation and bylaws may be deemed to have an anti-takeover effect and may delay, defer or prevent a tender offer or takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares held by our stockholders. These provisions include:
- dividing our Board of Directors into three classes serving staggered three-year terms;
- authorizing our Board of Directors to issue preferred stock without stockholder approval;
- prohibiting cumulative voting in the election of directors;
- prohibiting stockholder actions by written consent;
- limiting the persons who may call special meetings of stockholders;
- prohibiting our stockholders from making certain changes to our certificate of incorporation or bylaws except with 66.7% stockholder approval; and
- requiring advance notice for raising business matters or nominating directors at stockholders' meetings.
The combination of the charter and bylaws provisions summarized above will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
These provisions may have the effect of deterring hostile takeovers or delaying changes in our control or management. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and in the policies they implement, and to discourage certain types of transactions that may involve an actual or threatened change of our control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in our management.
66
Transfer agent and registrar
The transfer agent and registrar for our common stock is BNY Mellon Shareowner Services LLC.
67
The purpose of this prospectus is to permit the selling stockholder to offer and resell up to 35,975,049 shares of our common stock at such times and at such places as it chooses. To the extent required, we may amend and supplement this prospectus from time to time to describe a specific plan of distribution. The decision to sell any shares offered pursuant to this prospectus is within the sole discretion of the selling stockholder.
The distribution of the common stock by the selling stockholder may be effected from time to time in one or more transactions. Any of the common stock may be offered for sale, from time to time, by the selling stockholder at prices and on terms then obtainable, at fixed prices, at prices then prevailing at the time of sale, at prices related to such prevailing prices, or in negotiated transactions at negotiated prices or otherwise. The common stock may be sold by one or more of the following:
• On the OTC Bulletin Board or any other national common stock exchange or automated quotation system on which our common stock is traded, which may involve transactions solely between a broker-dealer and its customers which are not traded across an open market and block trades.
• Through one or more dealers or agents (which may include one or more underwriters), including, but not limited to:
• Block trades in which the broker or dealer as principal and resale by such broker or dealer for its account pursuant to this prospectus.
• Purchases by a broker or dealer as principal and resale by such broker or dealer for its account pursuant to this prospectus.
• Ordinary brokerage transactions.
• Transactions in which the broker solicits purchasers.
• Directly to one or more purchasers.
• A combination of these methods.
Dutchess and any broker-dealers who act in connection with the sale of its shares are "underwriters" within the meaning of the Securities Act, and any discounts, concessions or commissions received by them and profit on any resale of the shares as principal may be deemed to be underwriting discounts, concessions and commissions under the Securities Act. Because the selling stockholder is an "underwriter" within the meaning of the Securities Act, it will be subject to the prospectus delivery requirements of the Securities Act, including Rule 172 thereunder.
The selling stockholder or its underwriters, dealers or agents may sell the common stock to or through underwriters, dealers or agents, and such underwriters, dealers or agents may receive compensation in the form of discounts or concessions allowed or reallowed. Underwriters, dealers, brokers or other agents engaged by the selling stockholder may arrange for other such persons to participate. Any fixed public offering price and any discounts and concessions may be changed from time to time. Underwriters, dealers and agents who participate in the distribution of the common stock may be deemed to be underwriters within the meaning of the Securities Act, and any discounts or commissions received by them or any profit on the resale of shares by them may be deemed to be underwriting discounts and commissions thereunder. The proposed amounts of the common stock, if any, to be purchased by underwriters and the compensation, if any, of underwriters, dealers or agents will be set forth in a prospectus supplement.
68
Unless granted an exemption by the SEC from Regulation M under the Exchange Act, or unless otherwise permitted under Regulation M, the selling stockholder will not engage in any stabilization activity in connection with our common stock, will furnish each broker or dealer engaged by the selling stockholder and each other participating broker or dealer the number of copies of this prospectus required by such broker or dealer, and will not bid for or purchase any common stock of our or attempt to induce any person to purchase any of the common stock other than as permitted under the Exchange Act.
We will not receive any proceeds from the sale of these shares of common stock offered by the selling stockholder. We shall use our reasonable efforts to prepare and file with the SEC such amendments and supplements to the registration statement and this prospectus as may be necessary to keep such registration statement effective and to comply with the provisions of the Securities Act with respect to the disposition of the common stock covered by the registration statement for the period required to effect the distribution of such common stock.
We are paying certain expenses (other than commissions and discounts of underwriters, dealers or agents) incidental to the offering and sale of the common stock to the public. If we are required to update this prospectus during such period, we may incur additional expenses in excess of the amount estimated above. We have agreed to indemnify the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act and the Exchange Act, subject to certain exceptions.
In order to comply with certain state securities laws, if applicable, the common stock will be sold in such jurisdictions only through registered or licensed brokers or dealers. In certain states the shares of common stock may not be sold unless they have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
69
CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS
None.
Selected legal matters with respect to the validity of the securities offered by this prospectus will be passed upon for us by Stradling Yocca Carlson & Rauth, a Professional Corporation, Newport Beach, California.
The financial statements as of and for the years ended December 31, 2008 and 2007, included in this prospectus have been audited by SingerLewak LLP, an independent registered public accounting firm, as stated in their report appearing herein. Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 with the SEC for the stock offered pursuant to this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules thereto. Statements contained in this prospectus as to the contents of any contract or other document referred to are not necessarily complete and in each instance reference is made to the copy of such contract or other document filed as an exhibit to the registration statement, each such statement being qualified in all respects by such reference. For further information with respect to us and the common stock offered hereby, please refer to the registration statement and its exhibits and schedules for further information relating to us and our common stock.
We are subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934 and in accordance therewith file reports, proxy statements and other information with the SEC. Such reports, proxy statements, other information and a copy of the registration statement may be inspected by anyone without charge and copies of these materials may be obtained upon the payment of the fees prescribed by the SEC, at the Public Reference Room maintained by the SEC at Room 1580, 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of this public reference room by calling 1-800-SEC-0330. The Registration Statement, including all exhibits and schedules and amendments, has been filed with the SEC through the Electronic Data Gathering Analysis and Retrieval system and is available to the public from the SEC's web site at http://www.sec.gov.
70
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Audited Annual Financial Statements of MyMedicalRecords, Inc. (formerly mymedicalrecords.com, Inc.) |
Page |
|
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
|
Balance Sheets at December 31, 2008 and 2007 |
F-3 |
|
|
Statements of Operations for the Years Ended December 31, 2008 and 2007 |
F-4 |
|
|
Statements of Stockholders' Deficit for the Years Ended December 31, 2008 and 2007 |
F-5 |
|
|
Statements of Cash Flows for the Years Ended December 31, 2008 and 2007 |
F-6 |
|
|
Notes to Financial Statements |
F-7 |
|
|
Pro Forma Financial Information |
F-27 |
|
|
Combined Pro Forma Balance Sheet as of December 31, 2008 (Unaudited) and Notes Thereto |
F-28 |
|
|
Combined Pro Forma Statement of Operations for the Year Ended December 31, 2008 (Unaudited) and Notes Thereto |
F-29 |
|
|
Unaudited Interim Consolidated Financial Statements of MMR Information Systems, Inc. |
|
|
|
Consolidated Balance Sheets at June 30, 2009 (unaudited) and December 31, 2008 |
F-30 |
|
|
Consolidated Statements of Operations — Six and Three Months Ended June 30, 2009 (unaudited) and June 30, 2008 (unaudited) |
F-31 |
|
|
Consolidated Statement of Stockholders' Deficit — Six Months Ended June 30, 2009 (unaudited) |
F-32 |
|
|
Consolidated Statements of Cash Flows — Six Months Ended June 30, 2009 (unaudited) and June 30, 2008 (unaudited) |
F-33 |
|
|
Notes to Consolidated Financial Statements (unaudited) |
F-34 |
|
F-1
REPORT OF INDEPENDENT REGISTERED ACCOUNTING FIRM
To the Board of Directors
MyMedicalRecords, Inc. (formerly mymedicalrecords.com, Inc)
We have audited the accompanying balance sheets of mymedicalrecords.com, Inc. ("the Company") as of December 31, 2008 and 2007, and the related statements of operations, stockholders deficit and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States of America). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2008 and 2007, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and its total liabilities exceed its total assets. This raises substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ SingerLewak LLP
Los Angeles, California
May 4, 2009
F-2
MYMEDICALRECORDS.COM, INC.
BALANCE SHEETS
| December 31, | December 31, | |||||
|
2008
|
2007
|
|||||
| ASSETS | ||||||
| Current assets: | ||||||
| Cash and cash equivalents | $ | 75,779 | $ | 11,450 | ||
| Accounts receivable | 6,928 | 24,356 | ||||
| Related party receivables | 22,057 | 1,000 | ||||
| Prepaid expenses and other current assets |
82,471
|
139,513
|
||||
| Total current assets | 187,235 | 176,319 | ||||
| Property and equipment, net | 47,050 | 66,641 | ||||
| Deposits | 1,885 | 53,337 | ||||
| Advances due from related party | 100,000 | 100,000 | ||||
| Intangible assets, net |
128,484
|
456,326
|
||||
| Total assets | $ |
464,654
|
$ |
852,623
|
||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||
| Current liabilities: | ||||||
| Line of credit, related party | $ | 822,520 | $ | 283,098 | ||
| Advances from affiliate | 701,322 | - | ||||
| Related party payables | 513,688 | 255,641 | ||||
| Compensation payable | 58,188 | 37,813 | ||||
| Accounts payable and accrued expenses | 944,438 | 460,901 | ||||
| Deferred revenue | 189,824 | 130,186 | ||||
| Deferred rent | - | 6,344 | ||||
| Capital leases payable, current portion |
6,929
|
6,262
|
||||
| Total current liabilities | 3,236,909 | 1,180,245 | ||||
| Capital leases payable, less current portion |
16,151
|
23,079
|
||||
| Total liabilities |
3,253,060
|
1,203,324
|
||||
| Commitments and contingencies (See Note 8) | ||||||
| Stockholders' deficit: | ||||||
| Preferred stock - $0.001 per value, 10,000,000 shares authorized; | ||||||
|
Series A - 3,332,694 shares issued and outstanding as of December 31, 2008 and 2007 with a liquidation preference of $1 per share |
3,333 | 3,333 | ||||
|
Series B - 1,263,750 shares issued and outstanding as of December 31, 2008 and 2007 with a liquidation preference of $2 per share |
1,264 | 1,264 | ||||
|
Series C - 1,580,082 and 1,111,213 shares issued and outstanding as of December 31, 2008 and 2007, respectively, with a liquidation preference of $1.33 per share |
1,580 | 1,111 | ||||
|
Common stock, $0.001 par value, 100,000,000 shares authorized, 18,392,575 and 12,622,819 shares issued and outstanding as of December 31, 2008 and 2007, respectively |
18,393 | 12,623 | ||||
| Treasury stock, at cost, 349,000 and 4,413,053 shares as of December 31, 2008 and 2007, respectively | (16,860) | - | ||||
| Additional paid-in capital | 8,356,519 | 7,225,507 | ||||
| Accumulated deficit |
(11,152,635)
|
(7,594,539)
|
||||
| Total stockholders' deficit |
(2,788,406)
|
(350,701)
|
||||
| Total liabilities and stockholders' deficit | $ |
464,654
|
$ |
852,623
|
||
| The accompanying notes are an integral part of these financial statements | ||||||
F-3
MYMEDICALRECORDS.COM, INC.
STATEMENTS OF OPERATIONS
|
Years Ended December 31,
|
|||||||
|
2008
|
2007
|
||||||
| Revenues | |||||||
| Subscriber | $ | 280,572 | $ | 122,563 | |||
| License fees and other |
170,783
|
93,527
|
|||||
| Total revenues | 451,355 | 216,090 | |||||
| Cost of revenues |
409,900
|
319,447
|
|||||
| Gross profit (loss) | 41,455 | (103,357) | |||||
| General and administrative expenses | 2,108,063 | 1,588,911 | |||||
| Sales and marketing expenses | 914,162 | 1,080,457 | |||||
| Technology development |
143,031
|
245,162
|
|||||
| Loss from operations | (3,123,801) | (3,017,887) | |||||
| Interest and other expenses, net |
(398,675)
|
(108,519)
|
|||||
| Net loss | $ | (3,522,476) | $ | (3,126,406) | |||
| Dividend to preferred shareholders |
(35,620)
|
(26,042)
|
|||||
| Net loss available to common shareholders | $ |
(3,558,096)
|
$ |
(3,152,448)
|
|||
| Net loss available to common shareholders per share: | |||||||
| Basic and Diluted | $ |
(0.28)
|
$ |
(0.31)
|
|||
| Weighted average common shares outstanding: | |||||||
| Basic and Diluted |
12,572,308
|
10,139,631
|
|||||
| The accompanying notes are an integral part of these financial statements | |||||||
F-4
MYMEDICALRECORDS.COM, INC.
STATEMENT OF STOCKHOLDERS' DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2007 AND 2008
|
Preferred Stock
|
|||||||||||||||||||||||||||||||||||||
|
Series A
|
Series B
|
Series C
|
Common Stock
|
Treasury Stock
|
Additional | Accumulated | |||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Paid-In Capital
|
Deficit
|
Total
|
|||||||||||||||||||||||||
| Balance at December 31, 2006 | 3,332,694 | $ | 3,333 | 1,041,250 | $ | 1,041 | - | $ | - | 10,329,999 | $ | 10,330 | - | $ | - | $ | 5,094,422 | $ | (4,442,091) | $ | 667,035 | ||||||||||||||||
| Exercise of stock options | 50,000 | 50 | 12,450 | 12,500 | |||||||||||||||||||||||||||||||||
| Shares returned to Treasury pursuant to a legal settlement | (4,413,053) | - | |||||||||||||||||||||||||||||||||||
| Issuance of Series B Preferred Stock for cash, net of | |||||||||||||||||||||||||||||||||||||
| expenses of $32,332 | 222,500 | 223 | 412,446 | 412,669 | |||||||||||||||||||||||||||||||||
| Issuance of Series C Preferred Stock for cash, net of | |||||||||||||||||||||||||||||||||||||
| expenses of $108,393 | 1,111,213 | 1,111 | 1,368,409 | 1,369,520 | |||||||||||||||||||||||||||||||||
| Issuance of Common Stock as an incentive to Preferred | |||||||||||||||||||||||||||||||||||||
| Series B and C holders | 200,320 | 200 | 25,842 | (26,042) | - | ||||||||||||||||||||||||||||||||
| Common shares issued for services | 2,042,500 | 2,043 | 263,514 | 265,557 | |||||||||||||||||||||||||||||||||
| Stock based compensation | 48,424 | 48,424 | |||||||||||||||||||||||||||||||||||
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
(3,126,406)
|
(3,126,406)
|
||||||||||||||||||||||||
| Balance at December 31, 2007 | 3,332,694 | 3,333 | 1,263,750 | 1,264 | 1,111,213 | 1,111 | 12,622,819 | 12,623 | (4,413,053) | - | 7,225,507 | (7,594,539) | (350,701) | ||||||||||||||||||||||||
| Issuance of Series C Preferred Stock for cash, net of | |||||||||||||||||||||||||||||||||||||
| expenses of $2,548 | 468,869 | 469 | 620,578 | 621,047 | |||||||||||||||||||||||||||||||||
| Issuance of Common Stock as an incentive to Preferred | |||||||||||||||||||||||||||||||||||||
| Series C holders | 468,869 | 469 | 35,151 | (35,620) | - | ||||||||||||||||||||||||||||||||
| Common shares issued for services | 4,713,940 | 4,714 | 393,225 | 397,939 | |||||||||||||||||||||||||||||||||
| Issuance of shares, including Treasury Stock, to related | |||||||||||||||||||||||||||||||||||||
| party as consideration for renewal of line of credit | 586,947 | 587 | 4,413,053 | - | 49,413 | 50,000 | |||||||||||||||||||||||||||||||
| Issuance of options replacing shares previously issued | (349,000) | (16,860) | 16,860 | - | |||||||||||||||||||||||||||||||||
| Stock based compensation | 15,785 | 15,785 | |||||||||||||||||||||||||||||||||||
| Net loss |
|
|
|
|
|
|
|
|
|
|
|
(3,522,476)
|
(3,522,476)
|
||||||||||||||||||||||||
| Balance at December 31, 2008 |
3,332,694
|
$ |
3,333
|
1,263,750
|
$ |
1,264
|
1,580,082
|
$ |
1,580
|
18,392,575
|
$ |
18,393
|
(349,000)
|
$ |
(16,860)
|
$ |
8,356,519
|
$ |
(11,152,635)
|
$ |
(2,788,406)
|
||||||||||||||||
| The accompanying notes are an integral part of these financial statements | |||||||||||||||||||||||||||||||||||||
F-5
MYMEDICALRECORDS.COM, INC.
STATEMENT OF CASH FLOWS
|
Year Ended December 31,
|
||||||||
|
2008
|
2007
|
|||||||
| Operating activities: | ||||||||
| Net loss | $ | (3,522,476) | $ | (3,126,406) | ||||
| Adjustments to reconcile net loss to net cash | ||||||||
| used in operating activities: | ||||||||
| Depreciation and amortization | 148,777 | 137,330 | ||||||
| Impairment of intangible assets | 197,000 | - | ||||||
| Change in allowance for doubtful accounts | - | 2,885 | ||||||
| Restricted common stock issued for services | 397,939 | 265,557 | ||||||
| Share based compensation | 15,785 | 48,424 | ||||||
| Common stock issued as interest expense on the line of credit | 50,000 | - | ||||||
| Loss from disposal of property and equipment | 2,996 | 875 | ||||||
| Effect of changes in: | ||||||||
| Accounts receivable | 17,428 | (25,763) | ||||||
| Related party receivables | (21,057) | 72,874 | ||||||
| Prepaid expenses and other current assets | 57,042 | (83,254) | ||||||
| Deposits | 51,452 | (9,000) | ||||||
| Related party payables | 258,047 | 245,210 | ||||||
| Compensation payable, related parties | - | (196,000) | ||||||
| Compensation payable | 20,375 | 37,813 | ||||||
| Accounts payable and accrued expenses | 483,537 | 191,584 | ||||||
| Deferred revenue | 59,638 | 84,332 | ||||||
| Deferred rent |
(6,344)
|
6,344
|
||||||
| Net cash used in operating activities |
(1,789,861)
|
(2,347,195)
|
||||||
| Investing activities: | ||||||||
| Purchase of property and equipment | (3,566) | (2,049) | ||||||
| Website development | - | (178,804) | ||||||
| Advances due from related party | - | (100,000) | ||||||
| Proceeds from sales of property and equipment |
2,226
|
2,102
|
||||||
| Net cash used in investing activities |
(1,340)
|
(278,751)
|
||||||
| Financing activities: | ||||||||
| Payments of capital lease payable | (6,261) | (5,659) | ||||||
| Proceeds from line of credit, related party | 539,422 | 283,098 | ||||||
| Proceeds from advances from affiliate | 701,322 | - | ||||||
| Proceeds from exercise of stock options | - | 12,500 | ||||||
| Issuance of preferred stock - Series B, net of issuance costs | - | 412,668 | ||||||
| Issuance of preferred stock - Series C, net of issuance costs |
621,047
|
1,369,520
|
||||||
| Net cash provided by financing activities |
1,855,530
|
2,072,127
|
||||||
| Net increase (decrease) in cash | 64,329 | (553,819) | ||||||
| Cash, beginning of period |
11,450
|
565,269
|
||||||
| Cash, end of period | $ |
75,779
|
$ |
11,450
|
||||
| Supplemental disclosures of cash flow information: | ||||||||
| Cash paid for interest | $ | 18,616 | $ | 8,435 | ||||
| Cash paid for income taxes | $ | 800 | $ | 800 | ||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Acquisition of equipment through capital lease | $ | - | $ | 35,000 | ||||
| The accompanying notes are an integral part of these financial statements | ||||||||
F-6
MYMEDICALRECORDS.COM, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2008
NOTE 1 - NATURE OF OPERATIONS
mymedicalrecords.com, Inc. ("the Company", "MMR", "our", "us" or "we"), incorporated in Delaware in 2005 and headquartered in Los Angeles, California, seeks to empower consumers and medical professionals by facilitating access to consumer medical records and associated vital documents (such as living wills, birth certificates and insurance policies). The Company offers consumers an easy-to-use, secure web-based product that allows documents, images and voice mail messages to be transmitted in and out of the MMR's proprietary system using a variety of methods, including fax, file upload and email.
Management's Plan
We have incurred net losses of approximately $3.5 million and $3.1 million for the years ended December 31, 2008 and 2007, respectively, and have an accumulated deficit of approximately $11.1 million as of December 31, 2008. Furthermore, our current liabilities exceed our current assets by approximately $3 million as of December 31, 2008.
Historically, we have issued capital stock and received funds from The RHL Group, Inc. (a significant shareholder wholly-owned by our Chairman and Chief Executive Officer) to operate our business. At December 31, 2008, we had $75,779 in cash and cash equivalents, and although we expect to receive additional funding from The RHL Group, Inc. pursuant to the Third Amended and Restated Note dated April 29, 2009, we nevertheless will still be required to obtain additional financing in order to meet the obligations for installment payments of $709,000 under the Creditor Plan (including payments under the transition employment agreements of our former Chief Executive Officer and former Chief Financial Officer) and the previously existing obligations under the subordinated secured indebtedness to The RHL Group, Inc. (which note payable had a balance of $822,520 at December 31, 2008), among other obligations.
As a result of the above, there is uncertainty about our ability to continue as a going concern.
Management's plan regarding this matter is to increase its available line of credit with The RHL Group (see Note 12). Additionally, we plan to sell additional equity securities, explore other debt financing arrangements and continue to increase our existing customer base to obtain additional cash flow over the next twelve months.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) MANAGEMENT'S USE OF ESTIMATES
The preparation of financial statements in accordance with accounting principles generally accepted in the United States ("GAAP") requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of net revenue and expenses during each reporting period. On an ongoing basis, management evaluates its estimates, including those related to revenue recognition, allowances for doubtful accounts, the valuation of deferred income taxes, tax contingencies, long-lived and intangible assets and stock-based compensation. These estimates are based on historical experience and on various other factors that we believe to be reasonable under the circumstances. Actual results could differ from those estimates.
F-7
(b) CASH AND CASH EQUIVALENTS
We consider cash equivalents to be only those investments that are highly liquid, readily convertible to cash and with maturities of 90 days or less at the purchase date. The Company maintains its cash in bank deposit accounts which, at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk on cash.
(c) TRADE AND OTHER RECEIVABLES
Receivables represent claims against third parties that will be settled in cash. The carrying value of receivables, net of an allowance for doubtful accounts, if any, represents their estimated net realizable value. The allowance for doubtful accounts, if any, is estimated based on historical collection trends, type of customer, the age of outstanding receivables and existing economic conditions. If events or changes in circumstances indicate that specific receivable balances may be impaired, further consideration is given to the collectibility of those balances and the allowance is adjusted accordingly. Past due receivable balances are written-off when collection efforts have been unsuccessful in collecting the amount due.
(d) FAIR VALUE OF FINANCIAL INSTRUMENTS
Statement of Financial Accounting Standards ("SFAS") No. 107, Disclosure about Fair Value of Financial Instruments requires entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet, for which it is practicable to estimate fair value. SFAS No. 107 defines the fair value of a financial instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties. As of December 31, 2008 and 2007, the carrying value of short-term and long-term investments, accounts receivable, interest receivable, accounts payable, accrued expenses, interest payable and customer deposits approximates fair value due to the short-term nature of such instruments. The carrying value of short-term debt approximates fair value as the related interest rates approximate rates currently available to the Company.
(e) PROPERTY AND EQUIPMENT
Property and equipment are stated at cost. Equipment under capital leases is stated at the present value of the minimum lease payments. Depreciation is calculated using the straight-line method, based upon the following estimated useful lives:
Furniture and Fixtures 5 Years
Computer Equipment 5 Years
When items are retired or disposed of, income is charged or credited for the difference between the net book value of the asset and the proceeds realized thereon.
Expenditures for maintenance and repairs are charged to operations as incurred while renewals and betterments are capitalized.
All property and equipment, along with all other assets of the Company, are pledged as collateral for a line of credit from a related party (see Note 5 - Line of Credit, Related party).
F-8
(f) INTANGIBLE ASSETS
Intangible assets are comprised of website development costs and domain names. We account for website development costs in accordance with the provisions of EITF 00-2, "Accounting for Website Development Costs" and with SFAS No. 86 "Accounting for the Costs of Computer Software to Be Sold, Leased, or Otherwise Marketed". Pursuant to the provisions of SFAS No. 86, "Accounting for the Costs of Computer Software to Be Sold, Leased or Otherwise Marketed," we capitalize internally developed website costs when the website under development has reached technological feasibility. These costs are amortized, typically over an estimated life of five years, using the larger of the amount calculated using the straight-line method or the amount calculated using the ratio between current period gross revenues and the total of current period gross revenues and estimated future gross revenues. At each balance sheet date, we evaluate the unamortized capitalized website costs compared to the net realizable value. The amount by which the unamortized capitalized website costs exceed its net realizable value is written off. During the year ended December 31, 2008, we concluded that our capitalized website development costs were impaired by $197,000, which have been included in general and administrative expenses in the accompanying statements of operations.
We account for domain names in accordance with the provisions of SFAS No. 142, "Goodwill and Other Intangible Assets" and amortize them on a straight-line basis. Identifiable intangible assets are amortized over their estimated useful lives as follows:
Website Development Costs 5 Years
Domain Name 5 Years
(g) IMPAIRMENT OF LONG-LIVED ASSETS AND INTANGIBLES
We account for long-lived assets, which include property and equipment and identifiable intangible assets with finite useful lives (subject to amortization), in accordance with the provisions of SFAS No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets" ("SFAS No. 144"). SFAS No. 144 requires that long-lived assets be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparing the carrying amount of an asset to the expected future net cash flows generated by the asset. If it is determined that the asset may not be recoverable, or if the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized to the extent of the difference.
We assessed whether events or changes in circumstances have occurred that potentially indicate the carrying amount of long-lived assets may not be recoverable. We had no impairment charges under SFAS No. 144 during the years ended December 31, 2008 and 2007.
(h) REVENUE RECOGNITION
The Company's revenues are derived from services, which are comprised of providing electronic access to consumer medical records and other vital documents and from the licensing of our service. Revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service is performed, and collectability of the resulting receivable is reasonably assured.
Our subscriber revenues consist of annual and monthly recurring retail subscriptions and usage-based fees, which are primarily paid in advance by credit card, and corporate accounts which are based on either on an access-fee or actual number of users, and in each case billed in advance at the beginning of each month of service. In accordance with Securities and Exchange Commission issued Staff Accounting Bulletin No. 104, Revenue Recognition, which clarifies certain existing accounting principles for the timing of revenue recognition and classification of revenues in the financial statements, we defer the portions of annual
F-9
recurring subscription fees collected in advance and recognize them on a straight line basis over the subscription period.
The Company grants exclusive licenses for the sale and marketing of its service in international territories in consideration of an up-front license fee and an ongoing royalty. The royalty fee is usually a percentage of revenue earned by the licensee and there usually are certain minimum guarantees. License fee revenues received in advance from international licensees for the grant of the license are deferred and recognized over the period covered by the agreement. Minimum guaranteed royalty payments received in advance are deferred and recognized over the period to which the royalty relates. All such revenues are included under License Fees and Other. In those cases where a license agreement contains multiple deliverables, the agreement is accounted for in accordance with the provisions of EITF 00-21, "Revenue Arrangements with Multiple Deliverables."
(i) INCOME TAXES AND UNCERTAIN TAX POSITIONS
The Company accounts for income taxes in accordance with SFAS No. 109, "Accounting for Income Taxes." ("SFAS No. 109") In accordance with SFAS No. 109, deferred tax assets and liabilities are recognized to reflect the estimated future tax effects, calculated at currently effective tax rates, of future deductible or taxable amounts attributable to events that have been recognized on a cumulative basis in the financial statements. A valuation allowance related to a deferred tax asset is recorded when it is more likely than not that some portion of the deferred tax asset will not be realized. Deferred tax assets and liabilities are adjusted for the effects of the changes in tax laws and rates of the date of enactment.
The Company adopted the provisions of FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes" ("FIN 48"), on January 1, 2007. FIN 48 prescribes a recognition threshold that a tax position is required to meet before being recognized in the financial statements and provides guidance on recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition issues. The adoption of FIN 48 did not have any impact on the Company. The Company classifies interest and penalties as a component of interest and other expenses. To date, there have been no interest or penalties assessed or paid.
(j) ADVERTISING
The Company expenses advertising costs as incurred. Advertising expense for the periods ended December 31, 2008 and 2007 was $22,960 and $32,058, respectively.
(k) SHARE-BASED COMPENSATION
The Company accounts for share-based compensation in accordance with SFAS No. 123(R), "Share Based Payment," ("SFAS123(R)"). The Company applies SFAS 123(R) in accounting for stock issued to employees under the recognition of compensation expense related to the fair value of employee share-based awards, including stock options and restricted stock. Determining the fair value of options at the grant date requires judgment, including estimating the expected term that stock options will be outstanding prior to exercise, the associated volatility and the expected dividends. Judgment is required in estimating the amount of share-based awards expected to be forfeited prior to vesting. If actual forfeitures differ significantly from these estimates, share-based compensation expense could be materially impacted.
F-10
The fair value of each stock option is estimated on the date of grant using the Black-Scholes option pricing model. Assumptions relative to volatility and anticipated forfeitures are determined at the time of grant with the following weighted average assumptions.
|
Years Ended December 31,
|
||||
|
2008
|
2007
|
|||
| Expected life in years | 0.38 | 3 | ||
| Stock price volatility | 65.93% | 70% | ||
| Risk free interest rate | 0.49% | 4.14% - 4.71% | ||
| Expected dividends | None | None | ||
| Forfeiture rate | 10% | 10% | ||
The assumptions used in the Black-Scholes models referred to above are based upon the following data: (1) As the Company does not have adequate historic data regarding option exercise rates, and it has determined these options to be "plain vanilla" options, then in accordance with SEC Staff Accounting Bulletin ("SAB") No. 110, it has adopted the simplified method of estimating the expected life of the options. This method prescribes assigning an expected life equal to the midpoint between the vesting period and the contractual option termination. (2) In the absence of a public market for the Company's shares, the expected stock price volatility of the underlying shares over the expected term of the option was taken at 65.93% and 70% for the years ended December 31, 2008 and 2007, respectively, which is approximately the mid-point of the range for similar companies. (3) The risk free interest rate is based on published U.S. Treasury Department interest rates for the expected terms of the underlying options. (4) Expected dividends are based on historical dividend data and expected future dividend activity. (5) The expected forfeiture rate is based on historical forfeiture activity and assumptions regarding future forfeitures based on the composition of current grantees.
Prior to the adoption of SFAS No. 123(R), the Company presented all tax benefits resulting from the exercise of stock options as operating cash inflows in the statements of cash flows, in accordance with the provisions of EITF Issue No. 00-15, "Classification in the Statement of Cash Flows of the Income Tax Benefit Received by a Company upon Exercise of a Nonqualified Employee Stock Option." SFAS No. 123(R) requires the benefits of tax deductions in excess of the compensation cost recognized for those options to be classified as financing cash inflows rather than operating cash inflows, on a prospective basis. The impact of this change was not material to the Company.
In accordance with SAB No. 107, options issued to non-employees are treated the same as those issued to employees with the exception of determination of the measurement date. Per EITF No. 96-18 "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services," as further clarified by EITF No. 00-18 "Accounting Recognition for Certain Transactions involving Equity Instruments Granted to Other Than Employees," the measurement date is the earlier of (1) The date at which a commitment for performance by the counterparty to earn the equity instruments is reached, or (2) The date at which the counterparty's performance is complete. Options granted to consultants are valued at their respective measurement dates, and recognized as expensed based on the portion of the total consulting services provided during the applicable period. As further consulting services are provided in future periods, the Company will revalue the associated options and recognize additional expense based on their then current values.
(l) NET LOSS PER SHARE
The Company uses SFAS No. 128, "Earnings Per Share" for calculating the basic and diluted loss per share. The basic loss per share is calculated by dividing the net loss attributable to common shareholders by the weighted average number of common shares outstanding. Diluted loss per share is computed similar to
F-11
basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential shares had been issued and if the additional shares were dilutive. Common equivalent shares are excluded from the computation of net loss per share if their effect is anti-dilutive.
For the year ended December 31, 2008 and 2007, 9,872,985 and 9,197,657 potentially dilutive shares, respectively, were excluded from the shares used to calculate diluted earnings per share as their effect was anti-dilutive.
(m) RESEARCH, DEVELOPMENT AND ENGINEERING
Research, development and engineering costs are expensed as incurred. Costs for software development relating to our website incurred subsequent to establishing technological feasibility, in the form of a working model, are capitalized and amortized over their estimated useful lives.
(n) CONCENTRATIONS
At December 31, 2008 and 2007, there were three and two customers, respectively, representing a combined 70.3% and 62% of the Company's revenue.
(o) RECENT ACCOUNTING PRONOUNCEMENTS
In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles". ("SFAS No. 162") SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements for nongovernmental entities that are presented in conformity with generally accepted accounting principles in the United States. The Company does not expect that this statement will result in a change in current practice.
In March 2008, the FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities, an amendment of FASB Statement No. 133. " ("SFAS No. 161") SFAS No. 161 requires enhanced disclosures about a company's derivative and hedging activities. These enhanced disclosures will discuss (a) how and why a company uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under FASB Statement No. 133 and its related interpretations and (c) how derivative instruments and related hedged items affect a company's financial position, results of operations and cash flows. SFAS No. 161 is effective for fiscal years beginning on or after November 15, 2008, with earlier adoption allowed. The Company does not anticipate that the adoption of this accounting pronouncement will have a material effect on its financial statements.
In February 2008, the FASB issued FASB Staff Position No. 157-2, "Effective Date of FASB Statement No. 157", ("SFAS No. 157") which delays the effective date of SFAS No. 157 for nonfinancial assets and nonfinancial liabilities to fiscal years beginning after November 15, 2008. Therefore, we will delay application of SFAS 157 to our nonfinancial assets and nonfinancial liabilities. The Company does not anticipate that the delayed adoption of this accounting pronouncement will have a material effect on its financial statements.
In September 2006, the FASB issued SFAS No. 157, "Fair Value Measurements." ("SFAS No. 157") SFAS No.157 defines fair value, establishes a framework for measuring fair value in accordance with GAAP, and expands disclosures about fair value measurements. The provisions of SFAS No. 157 are effective for the Company for fiscal years beginning after November 15, 2007. The Company implemented the provisions of SFAS No. 157 effective January 1, 2008, and it did not have a material impact on its financial statements.
F-12
In December 2007, the FASB issued SFAS No. 160, "Non-controlling Interests in Consolidated Financial Statements" - an amendment of ARB No. 51. ("SFAS No. 160") SFAS No. 160 establishes accounting and reporting standards for the non-controlling interest in a subsidiary and for the deconsolidation of a subsidiary. SFAS No. 160 clarifies that a non-controlling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements and requires retroactive adoption of the presentation and disclosure requirements for existing minority interests, of which the Company currently has none. All other requirements of SFAS No. 160 shall be applied prospectively. SFAS No. 160 is effective for fiscal years beginning after December 15, 2008. The Company does not anticipate that implementation of SFAS No. 160 will have any significant impact on its financial statements.
In December 2007, the FASB issued SFAS No. 141(revised 2007), "Business Combinations," ("SFAS No. 141R") which revises current purchase accounting guidance in SFAS No. 141, Business Combinations. SFAS No. 141R requires most assets acquired and liabilities assumed in a business combination to be measured at their fair values as of the date of acquisition. SFAS No. 141R also modifies the initial measurement and subsequent remeasurement of contingent consideration and acquired contingencies, and requires that acquisition related costs be recognized as expense as incurred rather than capitalized as part of the cost of the acquisition. SFAS No. 141R is effective for fiscal years beginning after December 15, 2008 and is to be applied prospectively to business combinations occurring after adoption. The Company is in the process of evaluating the impact of SFAS No. 141R as it relates to the Merger closing on January 27, 2009. The impact of SFAS No. 141R on the Company's financial statements will depend on the nature and extent of the Company's future acquisition activities.
In February 2007, the FASB issued SFAS No. 159, "The Fair Value Option of Financial Assets and Financial Liabilities". ("SFAS No. 159") SFAS No. 159 permits companies to choose to measure certain financial instruments and certain other items at fair value. The standard requires that unrealized gains and losses on items for which the fair value option has been elected be reported in earnings. SFAS No. 159 is effective as of the beginning of the entity's first fiscal year that begins after November 15, 2007. The adoption of SFAS No. 159 did not have any significant impact on the Company's financial statements.
In July 2008, the FASB issued EITF 07-5, "Determining Whether an Instrument (or Embedded Feature) is Indexed to an Entity's Own Stock". EITF 07-5 provides a two step approach for determining whether an equity-linked financial instrument (or an embedded feature) is indexed to an entity's own stock. The guidance in this Issue is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. The Company anticipates that EITF 07-5 will not have any significant impact on its financial statements.
In July 2008, the FASB issued EITF 08-4, "Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjusted Conversion Ratios". EITF 08-4 provides transition guidance for conforming changes made to EITF Issue No. 98-5, "Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjusted Conversion Ratios", that resulted from EITF Issue No. 00-27, "Application of Issue No. 98-5 to Certain Convertible Instruments" and SFAS No. 150, Accounting for Certain Financial Instruments with Characteristics of both Liability and Equity. The conforming changes are effective for financial statements issued for fiscal years ending after December 15, 2008, with earlier application permitted. The Company anticipates that EITF 08-4 will not have any significant impact on its financial statements.
In June 2008, the FASB issued FSP No. EITF 03-6-1, "Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities". FSP No. EITF 03-6-1 clarifies that instruments granted in share-based payment transactions can be participating securities prior to the requisite service having been rendered. The FSP is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those years. The Company anticipates that FSP No. EITF 03-6-1 will not have any significant impact on its financial statements.
F-13
NOTE 3 - PROPERTY AND EQUIPMENT
Property and equipment, stated at cost, at December 31, 2008 and 2007 consisted of the following:
|
Years Ended
|
||||||
| December 31, | December 31, | |||||
|
2008
|
2007
|
|||||
| Furnitures and fixtures | $ | 3,170 | $ | 5,639 | ||
| Computers and related equipment |
81,708
|
84,624
|
||||
| 84,878 | 90,263 | |||||
| Less: accumulated depreciation and amortization |
(37,828)
|
(23,622)
|
||||
| $ |
47,050
|
$ |
66,641
|
|||
Included in computers and related equipment at December 31, 2008 and 2007 are assets under capital leases of $28,565. Included in accumulated depreciation and amortization at December 31, 2008 and 2007 is $11,372 and $5,659, respectively, related to assets under capital leases. Amortization expense related to assets under capital leases for the years ended December 2008 and 2007 amounted to $5,713 and $5,659, respectively. Total depreciation expense was $17,935 and $18,081 for the years ended December 31, 2008 and 2007, respectively.
NOTE 4 - INTANGIBLE ASSETS
Intangible assets as of December 31, 2008 and 2007 consisted of the following.
|
Years Ended
|
||||||
| December 31, | December 31, | |||||
|
2008
|
2007
|
|||||
| Website development | $ | 644,212 | $ | 644,212 | ||
| Domain name |
10,000
|
10,000
|
||||
| 654,212 | 654,212 | |||||
| Less: accumulated amortization | (328,728) | (197,886) | ||||
| Reserve for impairment |
(197,000)
|
-
|
||||
| $ |
128,484
|
$ |
456,326
|
|||
F-14
Amortization expense for the years ended December 31, 2008 and 2007 amounted to $130,842 and $119,249, respectively. Estimated amortization expense for each of the next five succeeding years is as follows:
|
Year Ending December 31, |
|||
| 2009 | $ | 43,148 | |
| 2010 | 41,648 | ||
| 2011 | 35,344 | ||
| 2012 |
8,344
|
||
| Total | $ |
128,484
|
NOTE 5 - LINE OF CREDIT, RELATED PARTY
On July 31, 2007, the Company entered into a promissory note agreement and a security agreement with The RHL Group, Inc., a corporation wholly owned by the Chairman and CEO of the Company, to borrow up to $100,000 under a revolving line of credit. This agreement was subsequently superseded by an amended and restated promissory note on August 23, 2007 under the terms of which the line amount was increased to $1 million. Amounts borrowed under the terms of this agreement are secured by all the assets of the Company. Interest on outstanding loan balances accrues at a rate equal to the lesser of the Wall Street Journal Prime Lending Rate (7.5% on August 23, 2007) plus 3% or the maximum rate allowed by law under the California Constitution (5% per annum), plus the rate prevailing at the Federal Reserve Bank of San Francisco on November 25, 2007 (5%), for advances to member banks. Interest expense on this note for the years ended December 31, 2008 and 2007 was $62,442 and $9,960, respectively, and the unpaid balance of interest was $24,963 and $1,525, respectively.
Under the terms of the agreement, The RHL Group, Inc. receives, in addition to interest, one share of common stock for each dollar drawn on the line of credit, in increments of 100,000, with a minimum grant of 400,000 shares, for each three month term of the line. The Company issued the RHL Group, Inc. a total of 800,000 common shares valued at $104,000 under the terms of this agreement during the year ended December 31, 2007, and 1,400,000 common shares valued at $210,000 during the year ended December 31, 2008, which are included in interest expense.
In conjunction with the loan agreement noted above, the Company was required to maintain certain financial covenants. At December 31, 2007 and December 31, 2008, the Company was in compliance with all of its covenants, including the requirement that at any time after September 1, 2007, the Company had at least $125,000 in cash including availability under this Line of Credit or such other amount as necessary to maintain operations through the subsequent thirty (30) days. These covenants were amended subsequent to the year ended December 31, 2008 (see Note 12).
All outstanding principal and unpaid interest was due on July 31, 2008. On August 1, 2008, the Company entered into the Second Amended and Restated Promissory Note Agreement with the RHL Group, Inc., for the renewal of its $1 million secured line of credit.
The line was renewed on substantially the same terms, with the one change being the quarterly grant of shares under the earlier agreement was accelerated to a one time grant of 5 million common shares (including 4,413,053 shares of Treasury stock) for borrowings of up to $1 million at any time during the twelve month term of the renewal ending on July 31, 2009. In addition, The RHL Group, Inc. also receives one share of common stock for each dollar of the line of credit, in increments of 100,000, for each 3 month term of the line for borrowings over $1 million.
F-15
NOTE 6 - ADVANCES FROM AFFILIATE
On October 1, 2008, MMR Information Systems, Inc. (formerly Favrille, Inc.), ("Favrille") advanced MMR $100,000 pursuant to a promissory note dated September 30, 2008 (the "First Bridge Note"), which was to be used solely for paying MMR's out of pocket expenses incurred in connection with the proposed reverse merger transaction (see Note 12). On November 10, 2008 (the first business day after execution of the Merger Agreement), Favrille advanced MMR an additional $500,000 pursuant to a promissory note issued by MMR to Favrille (the "Second Bridge Note,") and on December 22, 2008 an additional $100,000 pursuant to promissory notes issued by MMR to Favrille (the "Supplemental Bridge Notes" and together with the First Bridge Note and the Second Bridge Note, the "Promissory Notes") in each case, solely for MMR's use in paying its out of pocket expenses incurred in connection with the Merger and its operating expenses. As of December 31, 2008, the total notes payable outstanding from Favrille amounted to $701,322, which included accrued interest of $1,322.
Subsequent to the year ended December 31, 2008, the Company borrowed an additional $35,000 and $50,000 on January 8, 2009 and January 15, 2009, respectively, under the same terms as the Promissory Notes.
Principal outstanding under the Promissory Notes accrued interest at the applicable federal rate of interest per annum, or the maximum rate permissible by the laws in California relating to permissible rates of interest on commercial loans, whichever is less. Pursuant to the terms of the Merger Agreement, upon occurrence of the Merger, the principal and interest under the Promissory Notes was automatically treated as a contribution to capital to MMR and the Promissory Notes were fully discharged.
NOTE 7 - INCOME TAXES
The items accounting for the difference between income taxes computed at the federal statutory rate and the provision for income taxes were as follows:
|
Years Ended December 31,
|
|||||||
|
2008
|
2007
|
||||||
| Federal statutory rate | -34.00% | -34.00% | |||||
| State tax, net of federal benefit | -5.71% | -5.70% | |||||
| Non-deductible items | 0.62% | 0.67% | |||||
| Valuation allowance |
39.12%
|
39.05%
|
|||||
| Effective income tax rate |
0.03%
|
0.02%
|
|||||
Significant components of deferred tax assets and (liabilities) are as follows:
|
Years Ended December 31,
|
|||||||
|
2008
|
2007
|
||||||
| Net operating loss carryforwards | $ | 4,565,404 | $ | 3,095,729 | |||
| Depreciation and amortization | (1,924) | (7,042) | |||||
| Share based compensation | 39,793 | 33,030 | |||||
| State tax and other |
(320,033)
|
(216,361)
|
|||||
| Deferred tax assets, net | 4,283,240 | 2,905,356 | |||||
| Less: valuation allowance |
(4,283,240)
|
(2,905,356)
|
|||||
| $ |
-
|
$ |
-
|
||||
F-16
The Company files income tax returns in the U.S. Federal and California State jurisdictions. The Company is subject to U.S. Federal, State and local income tax examinations by tax authorities for all years since inception in 2005.
At December 31, 2008, the Company had Federal and State net operating loss carry forwards available to offset future taxable income of $10,678,084 and $10,575,286, respectively. These carry forwards will begin to expire in the years ending December 31, 2025 and December 31, 2015, respectively. These net operating losses may be subject to various limitations on utilization based on ownership changes under Internal Revenue Code Section 382 as a result of the Merger, and the Company is in the process of evaluating the impact of this before any losses are used to offset future taxable income.
We periodically evaluate the likelihood of the realization of deferred tax assets, and adjust the carrying amount of the deferred tax assets by the valuation allowance to the extent the future realization of the deferred tax assets is not judged to be more likely than not. We consider many factors when assessing the likelihood of future realization of our deferred tax assets, including our recent cumulative earnings experience by taxing jurisdiction, expectations of future taxable income or loss, the carryforward periods available to us for tax reporting purposes, and other relevant factors.
At December 31, 2008, based on the weight of available evidence, including cumulative losses in recent years and expectations of future taxable income, we determined that it was more likely than not that our deferred tax assets would not be realized and have a $4,283,240 valuation allowance associated with our deferred tax assets.
As a result of the implementation of FIN 48, the Company performed an analysis of its previous tax filings and determined that there were no positions taken that it considered uncertain. Therefore, there were no unrecognized tax benefits as of December 31, 2008.
Future changes in the unrecognized tax benefit are not expected to have an impact on the effective tax rate due to the existence of the valuation allowance. The Company estimates that the unrecognized tax benefit will not change within the next twelve months. The Company will continue to classify income tax penalties and interest, if any, as part of interest and other expenses in its Statements of Operations. There is no interest or penalties accrued as of December 31, 2008.
The following table summarizes the open tax years for each major jurisdiction:
Jurisdiction Open Tax Years
Federal 2005 - 2008
California State 2005 - 2008
As the Company has significant net operating loss carryforwards, even if certain of the Company's tax positions were disallowed, it is not foreseen that the Company would have to pay any taxes in the near future. Consequently, the Company does not calculate the impact of interest or penalties on amounts that might be disallowed.
NOTE 8 - COMMITMENTS AND CONTINGENCIES
Leases
We lease certain facilities and equipment under non-cancelable capital and operating leases, which expire at various dates through 2011. Total rent expense for the years end December 2008 and 2007 was $179,335 and $150,955, respectively. Future minimum lease payments at December 31, 2008, under non-cancelable
F-17
operating leases (with initial or remaining lease terms in excess of one year) and future minimum capital lease payments are as follows:
| Year Ended | Operating | Capital | ||||
|
December 31,
|
Leases
|
Leases
|
||||
| 2009 | $ | 123,128 | $ | 8,959 | ||
| 2010 | - | 8,959 | ||||
| 2011 |
-
|
8,959
|
||||
| Total minimum lease payments | $ |
123,128
|
26,877 | |||
| Less interest portion |
(3,797)
|
|||||
| $ |
23,080
|
The amount shown under Operating Leases is the Company's obligation under a long term lease for office space. In August 2008, the Company vacated this space prior to the end of the lease term. In February 2009, the Company and the landlord approved a settlement agreement (see Note 12). The Company accrued for the amount of the settlement liability as of December 31, 2008, which amounted to $62,500 and is reflected in accounts payable and accrued expenses in the accompanying balance sheet.
Guarantee provided by The RHL Group, Inc.
Under the terms of an agreement with an investor who purchased $500,000 of Series B Preferred shares in 2006, the Company was required to invest $250,000 in a joint venture with the investor to establish an entity to market and sell the Company's services in the countries of Japan, China, Korea, Taiwan and Thailand. The Company has paid $100,000 to date of this amount, which is shown on the balance sheet as advances due from related party. To date, the joint venture has not been incorporated, has not commenced operations and there are no employees. The Company and the investor are still in the process of setting up the joint venture. In September 2007, The RHL Group, Inc., a shareholder, wholly owned by the Chairman and CEO of the Company, provided the investor with a guarantee that the Company would meet its obligations under this agreement. In consideration for this guarantee, The RHL Group, Inc. was granted 300,000 shares of restricted common stock valued at $39,000. This guarantee expired in September 2008 and was renewed for a year. As consideration for renewing the guarantee, on January 27, 2009 the Company issued The RHL Group, Inc. 100,000 shares valued at approximately $5,000 (see Note 12).
We have employment agreements with our Chairman, President and Chief Executive Officer, Robert H. Lorsch, and our Chief Financial Officer and Senior Vice President, Naj Allana. Under each employment agreement, our executive officers receive a base salary, subject to annual increases as determined by our board of directors, certain benefits as set forth in the employment agreements, and an annual bonus at the discretion of our board of directors.
Effective July 1, 2006, MMR entered into an employment agreement with our Chairman, President and Chief Executive Officer, Robert H. Lorsch. As part of Mr. Lorsch's employment agreement, he received the option to purchase 750,000 shares of MMR common stock at $.25 per share, which options were repriced on November 11, 2008 to $.15 per share, all of which are now vested
The current term of Mr. Lorsch's employment agreement is effective until June 30, 2009 unless earlier terminated by us without cause (as defined in the agreement) upon 30 days written notice or for cause if Mr. Lorsch fails to cure the acts or omissions constituting cause within 30 days. If Mr. Lorsch's employment is terminated by us for cause or voluntarily by Mr. Lorsch without good reason, he will not be entitled to receive any severance payments or benefits under the employment agreement. If Mr. Lorsch's employment is terminated by us without cause or voluntarily by Mr. Lorsch for good reason, Mr. Lorsch
F-18
will be entitled to one year of salary at his then current rate of pay, including all monthly benefits, and the pro rata portion of the annual bonus otherwise due Mr. Lorsch. Mr. Lorsch is not entitled to any severance payments or benefits under the agreement if he is terminated for cause or voluntarily resigns without good reason. Unless terminated by us or voluntarily terminated by Mr. Lorsch, his employment agreement will be extended automatically for successive one year periods at the expiration of the then current term, unless we provide written notice of non-extension at least 60 days prior to the expiration of the term.
Mr. Lorsch's employment agreement includes provisions that prohibit Mr. Lorsch from disclosing our confidential information and trade secrets and competing with us during the term of his employment agreement or soliciting our employees for 18 months following termination of employment.
Effective December 14, 2006, MMR entered into an employment agreement with our Chief Financial Officer and Senior Vice President, Naj Allana, which is subject to a memorandum of understanding entered between MMR and Mr. Allana on April 18, 2008, as amended by a subsequent memorandum of understanding on August 1, 2008. As part of Mr. Allana's employment agreement he received options to acquire 400,000 shares of MMR common stock at an exercise price of $0.25 per share, which options were subsequently repriced on November 11, 2008 to $0.15 per share. On the effective date of Mr. Allana's employment agreement, 100,000 options vested immediately, with the remainder to vest in equal monthly installments over a period of 36 months. Further, pursuant to the terms of the memorandum of understanding, as amended, in consideration for a loan from Mr. Allana to MMR in the form of deferred salary, MMR granted Mr. Allana 50,000 shares of MMR common stock in September 2007 and agreed to grant Mr. Allana one share of common stock for each outstanding dollar of deferred salary, plus interest, per 90 day period or part thereof, commencing April 18, 2008. Mr. Allana's employment agreement also provides for Mr. Allana to supply additional services for certain of MMR's client's accounts. In consideration for such additional services, Mr. Allana receives a percentage of the revenues generated on such accounts, if any, pursuant to a separate commission agreement.
Mr. Allana's employment agreement is effective until December 13, 2009, and will automatically renew for successive 12 month periods unless terminated at least 120 days prior to the end of the term. If we terminate Mr. Allana's employment, unless due to misconduct (as defined in the employment agreement), we must continue to pay Mr. Allana his monthly salary for the remainder of the then current term, or if done 120 days preceding the end of the term, then through the end of the next 12 month term.
Effective January 27, 2009, the Company entered into a new employment agreement with Mr. Lorsch, and approved a new employment agreement with Mr. Allana, which extended the terms of the original agreements (see Note 12).
Litigation Matters
In August 2008, 10100 Santa Monica, Inc. filed a lawsuit against the Company asserting claims under a lease dated July 20, 2006 between 10100 Santa Monica, Inc. and MMR. In February 2009, the Company approved a settlement relating to this lawsuit and has accrued for the amount as of December 31, 2008 (see Leases section above).
From time to time, the Company is involved in various legal proceedings generally incidental to its business. While the result of any litigation contains an element of uncertainty, management presently believes that the outcome of any other known, pending or threatened legal proceeding or claim, individually or combined, will not have a material adverse effect on the Company's financial statements.
F-19
NOTE 9 - STOCKHOLDERS' DEFICIT
Preferred Stock
The Company is authorized to issue 10,000,000 shares of Preferred Stock, par value $0.001 per share.
During the year ended December 31, 2007, 222,500 shares of Series B Preferred Stock were issued at $2.00 per share for proceeds of $412,669 (net of $32,332 incurred directly towards raising capital).
During the year ended December 31, 2007 1,111,213 shares of Series C Preferred Stock were issued to shareholders at $1.33 per share for proceeds of $1,369,520 (net of $108,393 incurred directly towards raising capital).
During the year ended December 31, 2008, 468,869 shares of Series C Preferred Stock were issued to shareholders at $1.33 per share for proceeds of $621,047 (net of $2,548 incurred directly towards raising capital).
From October 2007 to October 2008, the Company, as an incentive to investors, issued one share of Common Stock with each share of Series B or Series C Preferred stock purchased. The Company issued 200,320 and 468,869 shares of Common Stock for the years ending December 31, 2007 and 2008, valued at $26,042 and $35,620, respectively. Because the common shares were issued as an inducement to invest and were not issued to all purchasers of Series B and Series C Preferred Stock, the Common Stock has been accounted for as a distribution to the recipients of the common shares and has been reflected as a deemed dividend in the Company's financial statements.
In accordance with EITF 00-27, "Application of Issue No. 98-5 to Certain Convertible Instruments," and EITF 98-5, "Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios," convertible preferred stock must be analyzed to determine whether there is any intrinsic value relating to the embedded conversion option. Any intrinsic value of the embedded conversion option is considered to be a beneficial conversion feature and is recognized as a deemed dividend over the period from the date of issuance of the preferred stock to the date of earliest conversion. The Company has concluded that there is no beneficial conversion feature related to the Series A, Series B and Series C Preferred Stock as of December 31, 2008 and 2007, respectively.
The holders of the Series A, Series B and Series C Preferred Stock are entitled to dividends once declared by the Board of Directors. No dividends were declared for the years ended December 31, 2008 or 2007. Holders of Series A, B and C Preferred Stock are entitled to vote with the Common Stock and the other classes of Preferred Stock as a single class, as if converted to Common Stock. The Series A, B and C Preferred Stock shall not be redeemable by the holders or the Company.
Holders of Series A, B and C Preferred Stock have a preferential right in the event of a liquidation to receive, prior to any payment or distribution in respect of Common Stock, an amount equal to $1.00, $2.00 and $1.33 per share, respectively, whether in cash or in kind. Any partial liquidation shall be allocated on a pro rata basis to holders of Series A, Series B and Series C Preferred Stock. After the payment of the full liquidation preference of the Series A, Series B and Series C Preferred Stock, the remaining assets of the Company available for distribution to shareholders shall be distributed among the holders of Common Stock pro rata based on the number of shares of Common Stock held by each.
Series A, B and C Preferred Stock are each convertible into one share of Common Stock (subject to anti-dilution adjustment) at any time at the holder's option. Series A, B and C Preferred Stock automatically convert into Common Stock upon (i) the election of a majority of the outstanding shares of each respective
F-20
class of Preferred Stock or (ii) the consummation of an underwritten public offering with aggregate proceeds in excess of $10,000,000 (a "Qualified Public Offering").
Common Stock
The Company was authorized to issue 20,000,000 shares of Common Stock, par value $0.001 per share. In October 2008, the stockholders approved an increase in the authorized shares of Common Stock to 100,000,000.
During the years ended December 31, 2008 and 2007 the Company issued 4,713,940 and 2,042,500 shares of Common Stock, respectively, as consideration for goods and services from both employees and non-employees valued at $397,939 and $265,557, respectively.
In 2008, under the terms of the renewal of Line of Credit from The RHL Group, Inc. in August 2008, the Company issued 5,000,000 shares, consisting of 586,947 new shares of common stock and 4,413,053 shares of Treasury stock, valued at $50,000, as consideration for renewal of the line through July 31, 2009. The shares of Treasury stock were returned during 2007 by a director and shareholder (see Note 11). The value of the transaction was determined in accordance with EITF 96-18, "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services," as further clarified by EITF 00-18, "Accounting Recognition for Certain Transactions Including Equity Instruments Granted to Other Than Employees".
Options for Purchase of Preferred B shares
On April 1, 2007, the Company granted 200,000 options to purchase Series B Preferred Stock at an exercise price of $2.00. The options were issued to the largest distributor of employee assistance services in the US as an incentive to market and sell the Company's services into their customer base. The related services agreement was for five years, and the customer never bought any services from the Company.
In accordance with EITF 96-18, "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services", as further clarified by EITF 00-18, "Accounting Recognition for Certain Transactions involving Equity Instruments Granted to Other Than Employees", the fair value of the options issued in conjunction with selling services was calculated as $270 at the date of grant and charged to expense at that time as no further performance commitment was required on the part of the recipient. The value was determined using the Black-Scholes valuation model with input assumptions of (1) volatility of 70%, (2) expected life of 2.5 years, (3) risk free rate of 3.74%, (4) expected dividends of zero, and (5) a common stock value of $0.13.
The following summarizes the total options to purchase Series B Preferred Stock issued and outstanding as of December 31, 2008.
|
Options Outstanding
|
Options Exercisable
|
||||||||||||
| Weighted | Weighted | Weighted | |||||||||||
| Average | Average | Average | |||||||||||
| Exercise | Number | Remaining | Exercise | Number | Exercise | ||||||||
| Price | of Shares | Life (Years) | Price | of Shares | Price | ||||||||
| $ | 2.00 |
200,000
|
3.25 | $ | 2.00 |
200,000
|
$ | 2.00 | |||||
|
200,000
|
200,000
|
||||||||||||
F-21
NOTE 10 - EQUITY INCENTIVE PLAN
Stock Option Plans
In 2005, the Company adopted an Equity Incentive Plan ("the Plan") which allows the Company to grant up to 5,000,000 shares of common stock or options to employees, non-employee board members and consultants. The plan is divided into three separate incentive Programs, namely the Stock Option, Restricted Stock and Stock Bonus Programs.
Under the Stock Option Program, options are designated as non-qualified stock options (NSO's) and incentive stock options (ISO's). For NSO's, the exercise price of shares shall not be less than 85% of the fair market value of such shares on the grant date, with the provision that the exercise price for a 10% stockholder shall not be less than 110% of the fair market value of such shares on the grant date. ISO's may only be granted to employees and the exercise price of the shares shall not be less than 100% of the fair market value of such shares on the grant date. The fair market value of ISO's exercisable for the first time in a calendar year that exceeds $100,000 shall become NSO's. The term of the option cannot exceed ten years from the option grant date, with the provision that for a 10% stockholder, the option term cannot exceed five years.
In the event of a corporate transaction such as a merger, consolidation or reverse merger causing a change in control of the Company, as defined, the options' vesting is accelerated and any restrictions lapse, except under certain circumstances.
Stock Option Activity
A summary of option activity for the years ended December 31, 2008 and 2007 is presented below.
| Weighted- | |||||||||
| Average | |||||||||
| Weighted- | Remaining | Aggregate | |||||||
| Average | Contractual | Intrinsic | |||||||
| Exercise | Life | Value | |||||||
|
Options
|
Price
|
(Years)
|
|
||||||
| Outstanding at December 31, 2006 | 3,430,000 | $ | 0.15 | 3.68 | $ | - | |||
| Granted | 685,000 | 0.15 | |||||||
| Exercised | (50,000) | 0.15 | |||||||
| Forfeited |
(575,000)
|
0.15 | |||||||
| Outstanding at December 31, 2007 | 3,490,000 | 0.15 | 2.87 | - | |||||
| Granted | 406,450 | 0.02 | |||||||
| Forfeited |
(200,000)
|
0.15 | |||||||
| Outstanding at December 31, 2008 |
3,696,450
|
0.14 | 1.06 | - | |||||
| Vested and expected to vest | |||||||||
| at December 31, 2008 |
3,668,149
|
0.14 | 1.05 | 53,502 | |||||
| Exercisable at December 31, 2008 |
3,413,436
|
0.14 | 1.02 | 53,502 |
F-22
The aggregate intrinsic value in the table above is before applicable income taxes and is calculated based on the difference between the exercise price of the options and the quoted price of the common stock as of the reporting date.
A summary of the activity of the Company's nonvested options for the years ended December 31, 2008 and 2007 is presented below. Cash received from options exercised was $12,500 in 2007.
| Weighted | ||||||
| Average | ||||||
| Grant Date | ||||||
| Shares | Fair Value | |||||
| Nonvested at December 31, 2006 | 1,624,418 | $ | 0.03 | |||
| Granted | 685,000 | $ | 0.04 | |||
| Vested |
(1,500,274)
|
$ | 0.03 | |||
| Nonvested at December 31, 2007 | 809,144 | $ | 0.04 | |||
| Granted | 406,450 | $ | 0.01 | |||
| Forfeited | (100,000) | $ | 0.03 | |||
| Vested |
(832,580)
|
$ | 0.02 | |||
| Nonvested at December 31, 2008 |
283,014
|
$ | 0.04 |
As of December 31, 2008, total unrecognized stock-based compensation expense related to nonvested stock options was $8,134, which is expected to be recognized during the year ended December 31, 2009.
The weighted average grant date fair value of options granted during the years ended December 31, 2008 and 2007 was $0.01 and $0.04, respectively.
The following table summarizes information about stock options outstanding and exercisable at December 31, 2008.
|
Options Outstanding
|
Options Exercisable
|
||||||||||||
| Weighted | Weighted | Weighted | |||||||||||
| Average | Average | Average | |||||||||||
| Exercise | Number | Remaining | Exercise | Number | Exercise | ||||||||
| Price | of Shares | Life (Years) | Price | of Shares | Price | ||||||||
| $ | 0.004 | 366,450 | 0.20 | $ | 0.004 | 366,450 | $ | 0.004 | |||||
| 0.15 | 3,290,000 | 1.16 | 0.15 | 3,006,986 | 0.15 | ||||||||
| 0.19 |
40,000
|
0.85 | 0.19 |
40,000
|
0.19 | ||||||||
|
3,696,450
|
3,413,436
|
||||||||||||
Modification to Terms of Options Currently Outstanding
In light of the changed financial position of the Company, on November 11, 2008, the Board approved a repricing of all outstanding options from $0.25 per share to $0.15 per share. The Board also waived the current restriction requiring employees leaving the Company to exercise their vested options within 90 days of their departure date. The additional expense recognized during the year ended December 31, 2008 resulting from the modification of the option terms amounted to $4,391.
F-23
Restricted Stock Program
Under the Restricted Stock Program, a restricted stock award is an offer by the Company to sell to an eligible person shares that are subject to restrictions relating to the sale or transfer of the shares. A committee appointed by the Board to administer the program or the Board itself shall determine to whom an offer will be made, the number of shares the person may purchase, the price to be paid and the restriction to which the shares shall be subject. The offer shall be accepted by the Participant within thirty days from the date of the offer evidenced by the Restricted Stock Purchase Agreement. The purchase price of shares shall not be less than 85% of the fair market value of such shares on the issue date, with the provision that the purchase price for a 10% stockholder shall not be less than 110% of such fair market value. Shares are either fully and immediately vested upon issuance, or may vest in installments upon attainment of specified performance objectives.
During the years ended December 31, 2008 and 2007 the Company issued 4,713,940 and 2,042,500 shares of Common Stock in consideration of goods and services from both employees and non-employees valued at $397,939 and $265,557, respectively. For purposes of financial statement presentation, the issuances of shares during 2007 and for the period January 1, 2008 through June 29, 2008 were valued at $0.13 and $0.15 per share, respectively. These values were derived based on a `fair value' standard of value as defined by the AICPA. Issuances of shares for the period of June 29, 2008 through November 7, 2008 were valued at $0.01 per share. Issuances of shares after November 7, 2008 were valued at $0.05 per share.
Stock Bonus Program
Under the Stock Bonus Program, shares are issued as a bonus for past services rendered pursuant to the Stock Bonus Agreement. Stock bonuses may be awarded upon satisfaction of specified performance goals pursuant to the Performance Stock Bonus Agreement. No shares were issued under the Stock Bonus Program during the years ended December 31, 2008 or 2007.
NOTE 11 - RELATED PARTY TRANSACTIONS
In July 2007, the Company initiated legal action against a director and shareholder who was providing the Company with business development advisory services. A settlement agreement was reached in October 2007, under the terms of which the director paid the Company $243,000, resigned from the Board of Directors, had 300,000 options held by him for the purchase of common stock of the Company with an exercise price of $0.25 per share, cancelled and returned to the Company substantially his entire holding in the Company of 4,413,053 common shares, which were returned to Treasury at no cost. All of his unvested options were forfeited. The Company incurred $0 and $100,000 for the years ended December 31, 2008 and 2007, respectively, for business development advisory services from this director. These amounts are reflected in general and administrative expenses in the accompanying Statement of Operations.
Additionally, the Company incurred $51,025 and $50,000 in the years ended December 31, 2008 and 2007, respectively, towards marketing consulting services from a second director. Included in related party payables at December 31, 2008 and 2007 was $76,025 and $25,000, respectively, with respect to these services.
The Company also incurred $50,000 in each of the years ended December 31, 2008 and 2007, respectively, towards marketing consulting services from a third director. Included in related party payables at December 31, 2008 and 2007 was $75,000 and $25,000, respectively, with respect to these services.
The Company also incurred $20,833 in the year ended December 31, 2007 towards international sales consulting services from a fourth director.
F-24
In April 2007, 10,000 common stock options with an exercise price of $0.25 valued at $415 were issued to a fifth director for certain consulting work that was performed in conjunction with the Series C Preferred Stock offering. There were no outstanding payables at December 31, 2007 with respect to these services. During the year ended December 31, 2008, the Company incurred $50,000 in consulting services from this director, of which $10,000 is included in related party payables as of December 31 2008.
In January 2007, the Company incurred $13,500 in expenses for consulting services from a sixth director for sales training and implementation services for the launch of the Medicalert Gold product. In addition, the director was issued 25,000 common shares for these same services valued at $3,250. The same director was also issued 75,000 common shares for executive coaching and related consulting services during the year ended December 31, 2007 valued at $9,750. There were no such amounts in 2008.
In August 2007, the Company entered into a month-to-month lease with The RHL Goup, Inc. for the use of furniture and art for the Company's offices, for a total of $1,000 per month. The Company incurred expenses of $7,500 and $12,000 for the years ended December 31, 2008 and 2007, respectively. Included in related party payables at December 31, 2008 and 2007 was $0 and $2,000, respectively, with respect to these leases.
In August 2008, the Company entered into an eight month lease with its Chairman and Chief Executive Officer for the use of office space for a total of $3,000 per month plus a share of utilities. This lease was renewed in April 2009 on a month-to-month basis (see Note 12).
In February 2006, the Company entered into a letter agreement with MyMedicalRecords.com.au ("MMR AU") granting it a 10 year exclusive right to market and sell the Company's products and services in Australia and New Zealand. The controlling shareholder of MMR AU was at the time of the signing of the initial letter agreement, a shareholder of the Company. This letter agreement was subsequently formalized in the form of a ten year license agreement in October 2007, at which time the controlling shareholder of MMR AU was no longer a shareholder of the Company.
The Company received $50,000 from MMR AU upon the signing of the letter agreement towards set up costs of the platform to be used by customers of MMR AU. The Company completed the setup of the platform for MMR AU in 2007, at which time it started to recognize this amount into revenue. The Company also received $150,000 in 2007 from MMR AU towards the minimum guarantee payment for years 2 and 3 of the license term. This amount has been deferred and is being recognized into income based on the number of months elapsed. During the year ended December 31, 2008, the Company amortized $84,375 of these amounts into license fee and other revenues in the accompanying Statement of Operations. The Company has accounted for this transaction under EITF 00-21, "Revenue Arrangements with Multiple Deliverables."
NOTE 12 - SUBSEQUENT EVENTS
On November 8, 2008, the Company entered into a definitive agreement for a reverse merger with a wholly owned subsidiary of Favrille ("the Merger"), under which terms the current shareholders of the Company will own 60% of the equity of Favrille on a fully diluted basis, with current shareholders of Favrille holding 33% and certain officers, directors and creditors of Favrille owning the remaining 7%. The Merger was approved by the majority of shareholders of each class of the Company's shares in December 2008. Closing took place on January 27, 2009.
On January 27, 2009, the Company entered into an allonge agreement (the "Allonge") with The RHL Group, Inc. which modified the terms of the Company's outstanding line of credit (see Note 5). The Allonge agreement suspended certain rights (the "Suspended Rights") held by The RHL Group, Inc. from the closing date of the reverse merger transaction, January 27, 2009, until the earlier of: (1) the date that Favrille or the Company repays all amounts outstanding under the line of credit issued pursuant to the Creditor Plan (as defined in the Merger Agreement) in the aggregate amount of $709,000, (2) the date that
F-25
Favrille or Borrower deposits into the Escrow Fund (as defined in the Creditor Plan) cash of $709,000 payable in satisfaction of the outstanding line of credit notes issued pursuant to the Creditor Plan; or (3) ten days after the two year anniversary of January, 27, 2009 (the "New Due Date").
The Suspended Rights are as follows:
- Any right of The RHL Group, Inc. to declare a Default or an Event of Default (as defined in the Note and the Security Agreement) or deliver any notice thereof to the Company.
- Any right of The RHL Group, Inc. to accelerate the maturity date of the note.
- Any right of The RHL Group, Inc. to assign the note, or the proceeds of the note, or otherwise negotiate the note.
- Any right of the Lender to receive payment of the unpaid balance of the note prior to the dates described above.
On April 29, 2009, the Allonge was terminated as part of the credit restructuring described below.
Effective February 26, 2009, the Company and its landlord reached a final settlement agreement which has been approved by the managements of both parties regarding the vacated office space (see Note 8).
On January 27, 2009, the Company executed a new employment agreement with its Chairman, President and Chief Executive Officer, Robert H. Lorsch, and approved a new employment agreement with its Chief Financial Officer and Senior Vice President, Naj Allana. The new agreement for Mr. Lorsch extends the term of the existing employment agreement for through December 31, 2011. The proposed agreement for Mr. Allana extends the term of the existing employment agreement through February 15, 2010.
The Company also entered into an amended and restated consulting agreement with The RHL Group Inc. on January 27, 2009. The amended consulting agreement extends the term of the existing agreement through December 31, 2011.
In September 2008, The RHL Group, Inc. extended its guarantee with an investor through September 2009 that the Company would meet its obligations under the agreement. As consideration for renewing the guarantee, on January 27, 2009 the Company issued The RHL Group, Inc. 100,000 shares valued at approximately $5,000 (see Note 8).
In April 2009, the Company renewed its lease with its Chairman and Chief Executive Officer on a month-to-month basis for use of office space for a total of $3,000 per month.
Effective May 1, 2009, the Company entered into a lease agreement to lease additional office space in Beverly Hills, California. The lease is month-to-month and requires a monthly payment of $4,796 commencing in June 2009.
On April 29, 2009, the Company agreed to restructure its secured credit facility with The RHL Group, Inc. and entered into a Secured Credit Restructuring Agreement with MMR Information Systems, Inc. (the new legal entity name of Favrille), The RHL Group, Inc. and Robert H. Lorsch, our Chairman, Chief Executive Officer and President (the "Restructuring Agreement"), issued The RHL Group, Inc. a Third Amended and Restated Note (the "Third Amended Note"), and MMR Information Systems, Inc. agreed to guaranty our obligations under the Third Amended Note (the "Guaranty").
The Third Amended Note amends and restates the existing The RHL Group, Inc. note ("RHL Group Note"), matures November 30, 2009, and bears interest at the lesser of 10% or the highest rate then permitted by law, and is secured (similar to the RHL Group Note) by the Security Agreement. Although the reserve credit line has been increased to $3,000,000, The RHL Group, Inc. is only obligated to make a minimum of $100,000 of loans, advances and guarantees under the Third Amended Note within 30 days of execution pursuant to the Restructuring Agreement. The RHL Group, Inc. received, as an origination fee, a note for $200,000, payable at our option in cash or warrants to acquire 2,800,000 shares of MMR Information Systems, Inc. common stock at an exercise price of $0.15 per share. The warrants, if issued, would have a four year term and be nontransferable without our consent. If the term of the Third Amended Note is renewed, we would grant The RHL Group an additional origination fee on terms to be negotiated at such time.
In addition, under the Restructuring Agreement, The RHL Group, Inc. agreed to use commercially reasonable efforts to raise additional financing from third parties, and agreed to extend the maturity of the Third Amended Note for an additional six-month term if we are in full compliance with our covenants and other obligations under the Third Amended Note, on terms to be negotiated at such time. Additionally, Mr. Lorsch agreed to exercise all of his outstanding options prior to May 1, 2009 (which were exercised April 30, 2009), which resulted in an $113,220 reduction in principal owing The RHL Group, Inc. Finally, as a condition to agreeing to restructure our secured credit arrangement, we also terminated the Allonge. Thus, if at any time after June 1, 2009 we are not in compliance with our covenants under the Third Amended Note or Security Agreement, The RHL Group, Inc. may, but is not obligated to, declare an event of default.
F-26
Pro Forma Financial Information
Background Information Regarding Pro Forma Financial Statements
On November 8, 2008, mymedicalrecords.com, Inc. ("MMR") entered into a definitive agreement for a reverse merger with a wholly-owned subsidiary of MMR Information Systems, Inc. (formerly Favrille, Inc.), ("Favrille") under which terms the current shareholders of MMR will own 60% of the equity of Favrille on a fully diluted basis, with current shareholders of Favrille holding 33% and certain creditors under Favrille's creditor plan (which consist of its officers, directors and their affiliates) owning the remaining 7%. The Merger was approved by the majority of shareholders of each class of MMR's shares in December 2008. On January 27, 2009, the Merger was consummated as described elsewhere in this current report on Form 8-K/A.
The following unaudited pro forma combined balance sheet and statement of operations reflect the combination of MMR and Favrille as of December 31, 2008 and were prepared as if the Merger had occurred on December 31, 2007. The unaudited pro forma combined balance sheet and statement of operations have been derived from historical financial statements of both MMR and Favrille.
In the opinion of management, all adjustments necessary to present fairly the pro forma combined balance sheet and statement of operations have been made based on the terms and structure of the transaction.
The unaudited pro forma combined balance sheet and statement of operations are not necessarily indicative of what actual results would have been had the transaction occurred at the beginning of the period nor do they purport to indicate the results of future operations of MMR and Favrille. The unaudited pro forma combined financial statements should be read in conjunction with the accompanying notes and historical financial statements and notes to the financial statements of MMR and Favrille.
F-27
Combined Pro Forma Balance Sheet as of December 31, 2008 (Unaudited) and Notes Thereto
(in thousands, except share and per share data)
| mymedical | Pro Forma | Pro Forma | |||||||||||
| ASSETS |
records.com, Inc.
|
Favrille, Inc
|
Adjustments
|
Combined
|
|||||||||
| (unaudited) | (unaudited) | ||||||||||||
| A | |||||||||||||
| Current Assets: | |||||||||||||
| Cash and cash equivalents | $ | 76 | $ | 1,555 | $ | 138 | B | $ | 1,769 | ||||
| Accounts receivable, net | 7 | - | - | 7 | |||||||||
| Related party receivables | 22 | - | - | 22 | |||||||||
| Prepaid expenses and other current assets | 82 | 85 | 102 | B | 269 | ||||||||
| Advance to affiliate |
-
|
701
|
(701)
|
-
|
|||||||||
| Total current assets | 187 | 2,341 | (461) | 2,067 | |||||||||
| Property and equipment, net | 47 | - | - | 47 | |||||||||
| Other assets: | |||||||||||||
| Deposits | 2 | - | - | 2 | |||||||||
| Advances - MMR Asia | 100 | - | - | 100 | |||||||||
| Intangible assets, net |
128
|
-
|
-
|
128
|
|||||||||
| Total other assets |
230
|
-
|
-
|
230
|
|||||||||
| Total assets | $ |
464
|
$ |
2,341
|
$ |
(461)
|
$ |
2,344
|
|||||
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | |||||||||||||
| Current liabilities: | |||||||||||||
| Current portion of debt | $ | - | $ | 3 | $ | (3) | $ | - | |||||
| Line of credit, related party | 823 | - | - | 823 | |||||||||
| Advances from affiliate | 701 | - | 701 | ||||||||||
| Related party payable | 514 | - | - | 514 | |||||||||
| Compensation payable | 58 | - | - | 58 | |||||||||
| Accounts payable and accrued expenses | 944 | 2,957 | (1,576) | B | 2,325 | ||||||||
| Deferred revenue | 190 | - | - | 190 | |||||||||
| Current portion of capital lease |
7
|
-
|
-
|
7
|
|||||||||
| Total current liabilities | 3,237 | 2,960 | (1,579) | 4,618 | |||||||||
| Long term liabilities: | |||||||||||||
| Capital lease, net of current portion |
16
|
-
|
-
|
16
|
|||||||||
| Total liabilities |
3,253
|
2,960
|
(1,579)
|
4,634
|
|||||||||
| Stockholders' equity (deficit) | |||||||||||||
| Preferred Stock | 6 | - | - | 6 | |||||||||
| Common stock | 18 | 41 | (41) | 18 | |||||||||
| Treasury stock | (17) | - | - | (17) | |||||||||
| Additional paid-in capital | 8,357 | 236,756 | (236,756) | 8,357 | |||||||||
| Accumulated deficit |
(11,153)
|
(237,416)
|
237,915
|
C |
(10,654)
|
||||||||
| Total Stockholders' Equity (Deficit) |
(2,789)
|
(619)
|
1,118
|
(2,290)
|
|||||||||
| Total Liabilities and Stockholders' Equity (Deficit) | $ |
464
|
$ |
2,341
|
$ |
(461)
|
$ |
2,344
|
|||||
| A | This pro forma adjustment is to present Favrille, Inc. as a discontinued operation. | ||||||||||||
| B | At the closing of the merger on January 27, 2009, Favrille, Inc. had advanced or made available to mymedicalrecords.com, Inc. cash in the amount of $1,693,379. Furthermore, prepaid expenses in the amount of $186,575 and accounts payable and accrued expenses of $1,381,377 transferred to mymedicalrecords.com, Inc. on the date of closing. | ||||||||||||
| C | The pro forma adjustment to the accumulated deficit is a combination of the discontinuance of the Favrille, Inc. operations and the gain on disposal of the net assets of Favrille, Inc. as of the merger date of January 27, 2009 . |
F-28
Combined Pro Forma Statement of Operations For The Year Ended December 31, 2008 (Unaudited) and Notes
Thereto
(in thousands, except share and per share data)
| mymedical | Pro Forma | Pro Forma | ||||||||||
|
records.com, Inc.
|
Favrille, Inc
|
Adjustments
|
Combined
|
|||||||||
| (unaudited) | (unaudited) | |||||||||||
| A | ||||||||||||
| Revenues: | ||||||||||||
| Subscriber | $ | 280 | $ | - | $ | - | $ | 280 | ||||
| Other |
171
|
-
|
-
|
171
|
||||||||
| 451 | - | - | 451 | |||||||||
| Cost of Revenue |
410
|
-
|
-
|
410
|
||||||||
| Gross Profit |
41
|
-
|
-
|
41
|
||||||||
| Operating Expenses | ||||||||||||
| General, administrative, sales and marketing | 3,022 | 7,483 | (7,483) | 3,022 | ||||||||
| Research and development | - | 19,318 | (19,318) | - | ||||||||
| Loss on impairment of property and equipment | - | 28,478 | (28,478) | - | ||||||||
| Technology development |
143
|
-
|
-
|
143
|
||||||||
| Total Operating Expenses |
3,165
|
55,279
|
(55,279)
|
3,165
|
||||||||
| Loss from Operations |
(3,124)
|
(55,279)
|
55,279
|
(3,124)
|
||||||||
| Other Income (Expenses) | ||||||||||||
| Change in valuation of warrants | - | 2,492 | (2,492) | - | ||||||||
| Gain on assets held for sale | - | 680 | (680) | - | ||||||||
| Gain on lease termination | - | 12,645 | (12,645) | - | ||||||||
| Gain on settlement of debt | - | 1,124 | (1,124) | - | ||||||||
| Interest and other income (expenses) |
(398)
|
(103)
|
103
|
(398)
|
||||||||
| Net loss before discontinued operation | (3,522) | (38,441) | 38,441 | (3,522) | ||||||||
| Discontinued operation | ||||||||||||
| Net loss | - | - | (38,441) | (38,441) | ||||||||
| Gain on disposal |
-
|
-
|
499
|
499
|
||||||||
| Net loss | $ | (3,522) | $ | (38,441) | $ | 499 | $ | (41,464) | ||||
| Deemed dividend |
(36)
|
-
|
-
|
(36)
|
||||||||
| Net loss available to common stockholders | $ |
(3,558)
|
$ |
(38,441)
|
$ |
499
|
$ |
(41,500)
|
||||
| Basic and diluted loss before discontinued operation | $ | (0.28) | $ | (0.93) | $ | - | $ | (0.03) | ||||
| Discontinued operation |
-
|
-
|
-
|
(0.28)
|
||||||||
| Net loss per share | $ |
(0.28)
|
$ |
(0.93)
|
$ |
-
|
$ |
(0.31)
|
||||
| Weighted average shares outstanding: | ||||||||||||
| Basic and diluted |
12,572,308
|
41,272,723
|
92,599,196
|
B |
133,871,919
|
|||||||
| A | This pro forma adjustment is to present Favrille, Inc. as a discontinued operation. | |||||||||||
| B | These additional shares represent the Favrille, Inc. shares into which the historical mymedicalrecords.com, Inc. shares are convertible. This pro forma presentation assumes the shares were outstanding for the entire year. |
F-29
MMR INFORMATION SYSTEMS, INC.
CONSOLIDATED BALANCE SHEETS
| June 30, | December 31, | |||||
|
2009
|
2008
|
|||||
| (Unaudited) | ||||||
| ASSETS | ||||||
| Current assets: | ||||||
| Cash and cash equivalents | $ | 2,605 | $ | 75,779 | ||
| Restricted cash | 2,394 | - | ||||
|
Accounts receivable, net of allowance for doubtful accounts of $66,668 (unaudited) and $0 as of June 30, 2009 and December 31, 2008, respectively |
16,019 | 6,928 | ||||
| Related party receivables | - | 22,057 | ||||
| Deferred financing costs | 142,857 | - | ||||
| Prepaid expenses and other current assets |
335,465
|
82,471
|
||||
| Total current assets | 499,340 | 187,235 | ||||
| Property and equipment, net | 44,203 | 47,050 | ||||
| Deposits | 7,745 | 1,885 | ||||
| Advances due from related party | - | 100,000 | ||||
| Intangible assets, net |
190,792
|
128,484
|
||||
| Total assets | $ |
742,080
|
$ |
464,654
|
||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||
| Current liabilities: | ||||||
| Line of credit, related party | $ | 891,441 | $ | 822,520 | ||
| Advances from affiliate | - | 701,322 | ||||
| Related party payables | 944,966 | 513,688 | ||||
| Compensation payable | 69,315 | 58,188 | ||||
| Severance liability | 620,613 | - | ||||
| Accounts payable and accrued expenses | 2,754,056 | 944,438 | ||||
| Deferred revenue | 165,839 | 189,824 | ||||
| Warrant liability | 149,280 | - | ||||
| Notes payable, current portion | 250,971 | - | ||||
| Capital leases payable, current portion |
9,923
|
6,929
|
||||
| Total current liabilities | 5,856,404 | 3,236,909 | ||||
| Notes payable, less current portion | 199,372 | - | ||||
| Capital leases payable, less current portion |
12,415
|
16,151
|
||||
| Total liabilities |
6,068,191
|
3,253,060
|
||||
| Commitments and contingencies (See Note 5) | ||||||
| Stockholders' deficit: | ||||||
| Preferred stock - $0.001 per value, 5,000,000 shares authorized, none issued and outstanding | - | - | ||||
|
Common stock, $0.001 par value, 150,000,000 shares authorized, 126,098,201 (unaudited) and 80,629,266 shares issued and outstanding as of June 30, 2009 and December 31 2008, respectively |
126,098 | 80,629 | ||||
|
Treasury stock, at cost, 0 and 1,145,324 shares as of June 30, 2009 (unaudited) and December 31, 2008, respectively |
- | (16,860) | ||||
| Additional paid-in capital | 9,897,872 | 8,300,460 | ||||
| Accumulated deficit |
(15,350,081)
|
(11,152,635)
|
||||
| Total stockholders' deficit |
(5,326,111)
|
(2,788,406)
|
||||
| Total liabilities and stockholders' deficit | $ |
742,080
|
$ |
464,654
|
||
The accompanying notes are an integral part of these consolidated financial statements
F-30
MMR INFORMATION SYSTEMS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| Three Months Ended | Six Months Ended | ||||||||||||
|
June 30,
|
June 30,
|
||||||||||||
|
2009
|
2008
|
2009
|
2008
|
||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | ||||||||||
| Revenues | |||||||||||||
| Subscriber | $ | 87,972 | $ | 68,424 | $ | 180,467 | $ | 121,435 | |||||
| License fees and other |
74,126
|
26,625
|
127,418
|
52,625
|
|||||||||
| Total revenues | 162,098 | 95,049 | 307,885 | 174,060 | |||||||||
| Cost of revenues |
102,285
|
94,580
|
209,137
|
211,047
|
|||||||||
| Gross profit (loss) | 59,813 | 469 | 98,748 | (36,987) | |||||||||
| General and administrative expenses | 1,349,113 | 287,693 | 2,643,518 | 707,410 | |||||||||
| Sales and marketing expenses | 384,772 | 248,784 | 620,658 | 613,682 | |||||||||
| Technology development |
63,095
|
40,625
|
106,843
|
85,625
|
|||||||||
| Loss from operations | (1,737,167) | (576,633) | (3,272,271) | (1,443,704) | |||||||||
| Gain on settlement of payables | 36,945 | - | 89,170 | - | |||||||||
| Change in valuation of derivative liabilities | 1,495,569 | - | (655,261) | - | |||||||||
| Interest and other expenses, net |
(116,404)
|
(125,568)
|
(359,084)
|
(242,421)
|
|||||||||
| Net loss | $ |
(321,057)
|
$ |
(702,201)
|
$ |
(4,197,446)
|
$ |
(1,686,125)
|
|||||
| Net loss available to common shareholders per share: | |||||||||||||
| Basic and Diluted | $ |
(0.00)
|
$ |
(0.01)
|
$ |
(0.04)
|
$ |
(0.03)
|
|||||
| Weighted average common shares outstanding: | |||||||||||||
| Basic and Diluted |
124,881,831
|
67,666,718
|
117,357,033
|
65,683,009
|
|||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-31
MMR INFORMATION SYSTEMS, INC. The accompanying notes are an integral part of these consolidated financial statements F-32
MMR INFORMATION SYSTEMS, INC. The accompanying notes are an integral part of these consolidated financial statements F-33
MMR INFORMATION SYSTEMS, INC. NOTE 1 - NATURE OF OPERATIONS AND BASIS OF PRESENTATION On January 27, 2009, MMR Information Systems, Inc. ("MMRIS"), (formerly known as Favrille, Inc. ("Favrille"), a Delaware corporation
formed in January 2000, and hereinafter referred to as the "Company") consummated a business combination with MyMedicalRecords,
Inc. (formerly known as mymedicalrecords.com, Inc., a Delaware corporation formed in 2005) ("MMR") through a merger of a wholly-owned
subsidiary of MMRIS with and into MMR pursuant to the terms of the Agreement and Plan of Merger and Reorganization dated
November 8, 2008 (the "Merger"). Pursuant to the terms of the Merger, all of the outstanding common and preferred stock of MMR was
cancelled and the former stockholders of MMR received an aggregate of 79,812,116 shares of Favrille common stock. In addition,
MMRIS assumed the obligations of MMR under its outstanding stock options and warrants, which, pursuant to the terms of the Merger,
represented the right to receive an aggregate 12,787,080 shares of MMRIS common stock at the effective time of the Merger. In connection
with the Merger, MMR became a wholly-owned subsidiary of the Company, with the former stockholders of MMR collectively owning shares of
the Company's common and preferred stock representing approximately 60.3% of the voting power of the Company's outstanding capital
stock. For accounting purposes, the Merger was treated as a reverse acquisition with MMR being the accounting acquirer. Accordingly, the
historical financial results prior to the Merger are those of MMR. The results of operations for MMRIS are included in the Company's
consolidated financial results beginning on January 27, 2009. The presentation of consolidated statements of stockholders' equity (deficit) reflects the historical stockholders' equity (deficit) of MMR
through January 26, 2009. The effect of the issuance of shares of MMRIS common stock in connection with the Merger and the inclusion of
MMRIS's outstanding shares of common stock at the time of the Merger on January 27, 2009 is reflected in the six months ended
June 30, 2009. MMRIS, headquartered in Los Angeles, California, seeks to empower consumers and medical professionals by facilitating access to
consumer medical records and associated vital documents (such as living wills, birth certificates and insurance policies). MMRIS offers
consumers an easy-to-use, secure web-based product that allows documents, images and voice mail messages to be transmitted in and out of
MMRIS's proprietary system using a variety of methods, including fax, file upload and email. Principles of Consolidation The consolidated financial statements include the accounts of MMRIS, and its wholly-owned subsidiaries MMR and Montana Merger Sub,
Inc. All intercompany transactions have been eliminated upon consolidation. Basis of Presentation The accompanying consolidated unaudited financial statements of the Company have been prepared in accordance with accounting
principles generally accepted in the United States of America for interim financial statements and with instructions to Form 10-Q pursuant to
the rules and regulations of Securities and Exchange Act of 1934, as amended (the "Exchange Act") and Article 8-03 of Regulation S-X under
the Exchange Act. Accordingly, they do not include all of the information and footnotes required by U.S. Generally Accepted Accounting
Principles ("GAAP") for complete financial statements. In the opinion of management, all adjustments considered necessary (consisting of
normal recurring adjustments) for a fair presentation are included herein. Operating results for the three and six month periods ended June 30,
2009 are not indicative of the results that may be expected for the fiscal year ending December 31, 2009. These unaudited consolidated
financial statements should be read in conjunction with the audited financial statements and the notes thereto included in the Company's
annual report on Form 10-K for the year ended December 31, 2008, and in the Company's current report on Form 8-K/A filed on May 4,
2009. F-34
Management's Plan The Company has incurred net losses of approximately $3.5 million for the year ended December 31, 2008 and approximately $4.2
million (unaudited) for the six months ended June 30, 2009, and has an accumulated deficit of approximately $15.4 million (unaudited) as of
June 30, 2009. Furthermore, its current liabilities exceed its current assets by approximately $5.4 million (unaudited) as of June 30, 2009. Historically, the Company issued capital stock and received funds from The RHL Group, Inc. ("The RHL Group") (a significant
shareholder wholly-owned by the Company's Chairman and Chief Executive Officer) to operate its business. At December 31, 2008 and June
30, 2009, the Company had $75,779 and $2,605 (unaudited), respectively, in cash and cash equivalents, and although it received additional
funding from The RHL Group pursuant to the Third Amended and Restated Note dated April 29, 2009, it nevertheless will still be required to
obtain additional financing in order to meet installment payment obligations resulting from settlement payments with various creditors (the
"Creditor Plan") and the previously existing obligations under the subordinated secured indebtedness to The RHL Group, which
note payable had a balance of $1,516,049 (unaudited) at June 30, 2009. As a result of the above, there is uncertainty about the Company's ability to continue as a going concern. Management's plan regarding this matter is to continue to increase its available line of credit with The RHL Group (see Note 3). If the
Company is unable to increase its available line of credit with The RHL Group, or obtain suitable alternative debt financing, it may adversely
affect the Company's ability to execute its business plan and continue as a going concern. Additionally, the Company plans to sell additional equity securities, explore other debt financing arrangements and continue to increase its
existing customer base to obtain additional cash flow over the next twelve months. There can be no assurance that funds from these sources
will be available when needed or, if available, will be on terms favorable to us or to our stockholders. If additional funds are raised by issuing
equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution or such equity
securities may provide for rights, preferences or privileges senior to those of the holders of our common stock. NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (a) MANAGEMENT'S USE OF ESTIMATES The preparation of financial statements in accordance with GAAP requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of net revenue and expenses
during each reporting period. On an ongoing basis, management evaluates its estimates, including those related to revenue recognition,
allowances for doubtful accounts, the valuation of deferred income taxes, tax contingencies, long-lived and intangible assets and stock-based
compensation. These estimates are based on historical experience and on various other factors that it believes to be reasonable under the
circumstances. Actual results could differ from those estimates. (b) CASH AND CASH EQUIVALENTS The Company considers cash equivalents to be only those investments that are highly liquid, readily convertible to cash and with
maturities of 90 days or less at the purchase date. The Company maintains its cash in bank deposit accounts which, at times, may exceed
federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit
risk on cash. As of June 30, 2009, the Company had restricted cash amounts of $2,394 (unaudited). This represents balances maintained by the
Company for the payment of creditors under the Creditor Plan. Accordingly, the Company is not allowed to utilize these amounts in its
operations, and as a result, has reported the amounts separately from cash and cash equivalents on the accompanying consolidated balance
sheets. F-35
(c) TRADE AND OTHER RECEIVABLES Receivables represent claims against third parties that will be settled in cash. The carrying value of receivables, net of an allowance for
doubtful accounts, if any, represents their estimated net realizable value. The allowance for doubtful accounts, if any, is estimated based on
historical collection trends, type of customer, the age of outstanding receivables and existing economic conditions. If events or changes in
circumstances indicate that specific receivable balances may be impaired, further consideration is given to the collectibility of those balances
and the allowance is adjusted accordingly. Past due receivable balances are written-off when collection efforts have been unsuccessful in
collecting the amount due. (d) FAIR VALUE OF FINANCIAL INSTRUMENTS Statement of Financial Accounting Standards ("SFAS") No. 107, "Disclosure about Fair Value of Financial Instruments" requires
entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet, for
which it is practicable to estimate fair value. SFAS No. 107 defines the fair value of a financial instrument as the amount at which the
instrument could be exchanged in a current transaction between willing parties. As of December 31, 2008 and June 30, 2009 (unaudited), the
carrying value of accounts receivable, interest receivable, accounts payable, accrued expenses, interest
payable and customer deposits approximates fair value due to the short-term nature of such instruments. The carrying value of short-term debt
approximates fair value as the related interest rates approximate rates currently available to the Company. The Company utilizes SFAS No. 157 "Fair Value Measurements" ("SFAS 157"), for valuing financial assets and liabilities measured
on a recurring basis. SFAS 157 defines fair value, establishes a framework for measuring fair value and generally accepted accounting
principles and expands disclosures about fair value measurements. This standard applies in situations where other accounting
pronouncements either permit or require fair value measurements. SFAS 157 does not require any new fair value measurements. Fair value is defined in SFAS 157 as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction
between market participants at the measurement date. Fair value measurements are to be considered from the perspective of a market
participant that holds the asset or owes the liability. SFAS 157 also establishes a fair value hierarchy which requires an entity to maximize the
use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value: Level 1: Quoted prices in active markets for identical or similar assets and liabilities. Level 2: Quoted prices for identical or similar assets and liabilities in markets that are not active or observable inputs other than
quoted prices in active markets for identical or similar assets and liabilities. Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets
or liabilities. In determining the appropriate fair value of the option and
warrant liabilities, the Company used Level 2 inputs for its valuation
methodology. The Company applied the Black-Scholes model to value the option and
warrant liabilities. See Note 7 below for the inputs used in the Black-Scholes
model to value the option and warrant liabilities. (e) PROPERTY AND EQUIPMENT Property and equipment are stated at cost. Equipment under capital leases is stated at the present value of the minimum lease payments.
Depreciation is calculated using the straight-line method, based upon the following estimated useful lives: Furniture and Fixtures: 5 Years Computer Equipment: 5 Years When items are retired or disposed of, income is charged or credited for the difference between the net book value of the asset and the
proceeds realized thereon. F-36
Expenditures for maintenance and repairs are charged to operations as incurred while renewals and betterments are capitalized. All property and equipment, along with all other assets of the Company, are pledged as collateral for a line of credit from a related party
(see Note 3 - Line of Credit, Related Party). (f) INTANGIBLE ASSETS Intangible assets are comprised of website development costs, domain names and patents. The Company
accounts for website development costs in accordance with the provisions of Emerging Issues Task Force ("EITF") No. 00-2,
"Accounting for Website Development Costs" and with SFAS No. 86 "Accounting for the Costs of Computer
Software to Be Sold, Leased, or Otherwise Marketed". Pursuant to the provisions of SFAS No. 86, the Company capitalizes
internally developed website costs when the website under development has reached technological feasibility. These costs are
amortized, typically over an estimated life of five years, using the larger of the amount calculated using the straight-line method or the
amount calculated using the ratio between current period gross revenues and the total of current period gross revenues and estimated
future gross revenues. At each balance sheet date, the Company evaluates the unamortized capitalized website costs compared to the
net realizable value. The amount by which the unamortized capitalized website costs exceed its net realizable value is written off.
During the six months ended June 30, 2009 (unaudited), there were no amounts written off. The Company accounts for domain names and patents in accordance with the provisions of SFAS No. 142, "Goodwill and Other
Intangible Assets" and amortizes them on a straight-line basis. Patents costs represent legal fees associated with filing patent
applications. The Company is in the process of evaluating the patents' estimated useful life as the patent cost amortization begins on
July 1, 2009. Identifiable intangible assets are amortized over their estimated useful lives as follows: Website Development Costs: 5 Years Domain Name: 5 Years (g) IMPAIRMENT OF LONG-LIVED ASSETS AND INTANGIBLES The Company accounts for long-lived assets, which include property and equipment and identifiable intangible assets with finite useful
lives (subject to amortization), in accordance with the provisions of SFAS No. 144, " Accounting for the Impairment or Disposal of
Long-Lived Assets " ("SFAS No. 144"). SFAS No. 144 requires that long-lived assets be reviewed for impairment whenever events or changes in
circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparing the carrying
amount of an asset to the expected future undiscounted net cash flows generated by the asset. If it is determined that the asset may not be
recoverable, or if the carrying amount of an asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized to
the extent of the difference between the fair value and the asset's carrying amount. The Company assessed whether events or changes in circumstances have occurred that potentially indicate the carrying amount of
long-lived assets may not be recoverable. The Company had no impairment charges under SFAS No. 144 during the six months ended June 30,
2009 and 2008 (unaudited). (h) REVENUE RECOGNITION The Company's revenues are derived from services, which are comprised of providing electronic access to consumer medical records and
other vital documents and from the licensing of its services. Revenue is recognized only when the price is fixed or determinable, persuasive
evidence of an arrangement exists, the service is performed, and collectability of the resulting receivable is reasonably assured. F-37
The Company's subscriber revenues consist of annual and monthly recurring retail subscriptions and usage-based fees, which are
primarily paid in advance by credit card, and corporate accounts that are based on either an access-fee or actual number of users, and in each
case billed in advance at the beginning of each month of service. In accordance with Securities and Exchange Commission
("SEC") Staff Accounting Bulletin ("SAB") No. 104, "Revenue Recognition," which clarifies certain existing
accounting principles for the timing of revenue recognition and classification of revenues in the financial statements, the Company defers the
portions of annual recurring subscription fees collected in advance and recognizes them on a straight line basis over the subscription
period. The Company grants exclusive licenses for the sale and marketing of its services in international territories in consideration of an up-front
license fee and an ongoing royalty. The royalty fee is usually a percentage of revenue earned by the licensee and there usually are certain
minimum guarantees. License fee revenues received in advance from international licensees for the grant of the license are deferred and
recognized over the period covered by the agreement. Minimum guaranteed royalty payments received in advance are deferred and
recognized over the period to which the royalty relates. All such revenues are included under "License Fees and Other." In those
cases where a license agreement contains multiple deliverables, the agreement is accounted for in accordance with the provisions
of EITF 00-21, "Revenue Arrangements with Multiple Deliverables." (i) INCOME TAXES AND UNCERTAIN TAX POSITIONS The Company accounts for income taxes in accordance with SFAS No. 109, "Accounting for Income Taxes" ("SFAS No.
109"). In accordance with SFAS No. 109, deferred tax assets and liabilities are recognized to reflect the estimated future tax effects,
calculated at currently effective tax rates, of future deductible or taxable amounts attributable to events that have been recognized on a
cumulative basis in the financial statements. A valuation allowance related to a deferred tax asset is recorded when it is more likely than not
that some portion of the deferred tax asset will not be realized. Deferred tax assets and liabilities are adjusted for the effects of the changes in
tax laws and rates of the date of enactment. The Company adopted the provisions of FASB Interpretation No. 48, "Accounting for Uncertainty in Income Taxes" ("FIN 48"), on
January 1, 2007. FIN 48 prescribes a recognition threshold that a tax position is required to meet before being recognized in the financial
statements and provides guidance on recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure
and transition issues. The adoption of FIN 48 did not have any impact on the Company. The Company classifies interest and penalties as a
component of interest and other expenses. To date, there have been no interest or penalties assessed or paid. (j) ADVERTISING The Company expenses advertising costs as incurred. (k) SHARE-BASED COMPENSATION The Company accounts for share-based compensation in accordance with SFAS No. 123(R), "Share Based Payment,"
("SFAS123(R)"). The Company applies SFAS 123(R) in accounting for stock-based awards issued to employees under the recognition of compensation
expense related to the fair value of employee share-based awards, including stock options and restricted stock. Determining the fair value of
options at the grant date requires judgment, including estimating the expected term that stock options will be outstanding prior to exercise, the
associated volatility and the expected dividends. Judgment is required in estimating the amount of share-based awards expected to be
forfeited prior to vesting. If actual forfeitures differ significantly from these estimates, share-based compensation expense could be materially
impacted. F-38
The fair value of each stock option is estimated on the date of grant using the Black-Scholes option pricing model. Assumptions relative to
volatility and anticipated forfeitures are determined at the time of grant. During the six months ended June 30, 2008 (unaudited), there were no
stock option or warrant grants. Grants of stock options and warrants during the six months ended June 30, 2009 (unaudited) were valued using
the following assumptions: The assumptions used in the Black-Scholes models are based upon the following data: (1) The Company uses the contractual life of
the underlying option or warrant as the expected life. (2) In the absence of an extensive public market for the Company's shares, the
expected stock price volatility of the underlying shares over the expected term of the option or warrant was taken at
approximately the mid-point of the range for similar companies at the various grant dates. (3) The risk free interest rate is based on published
U.S. Treasury Department interest rates for the expected terms of the underlying options or warrants. (4) Expected dividends are based on historical
dividend data and expected future dividend activity. (5) The expected forfeiture rate is based on historical forfeiture activity and assumptions
regarding future forfeitures based on the composition of current grantees. In accordance with SAB No. 107, options issued to non-employees are treated the same as those issued to employees with the exception
of determination of the measurement date. Per EITF No. 96-18 "Accounting for Equity Instruments That Are Issued to Other Than
Employees for Acquiring, or in Conjunction with Selling, Goods or Services ," as further clarified by EITF No. 00-18 "Accounting
Recognition for Certain Transactions involving Equity Instruments Granted to Other Than Employees ," the measurement date is the earlier
of (1) the date at which a commitment for performance by the counterparty to earn the equity instruments is reached, or (2) the date at which
the counterparty's performance is complete. Options granted to consultants are valued at their respective measurement dates, and recognized
as expense based on the portion of the total consulting services provided during the applicable period. As further consulting services are
provided in future periods, the Company will revalue the associated options and recognize additional expense based on their then current
values. (l) NET INCOME/LOSS PER SHARE The Company uses SFAS No. 128, "Earnings Per Share" for
calculating the basic and diluted loss per share. Basic loss per share is calculated by
dividing the net loss attributable to common shareholders by the weighted average number
of common shares outstanding. Diluted loss per share is computed similar to basic loss
per share except that the denominator is increased to include the number of additional
common shares that would have been outstanding if the potential shares had been issued
and if the additional shares were dilutive. Common equivalent shares are excluded from
the computation of net loss per share if their effect is anti-dilutive. All potential common shares were excluded from the computation of diluted net loss
per common share for the three and six months ended June 30, 2009 and for the three and
six months ended June 30, 2008 because they were anti-dilutive due to the Company's net
loss position. Stock options and warrants excluded from the computation totaled
32,256,698 shares for the three and six months ended June 30, 2009 and 12,109,615 shares
for the three and six months ended June 30, 2008. Subsequent to June 30, 2009, the Company issued 6,464,642 shares of common stock, 12,120,983 warrants and 14,600,000 stock
options (see Note 11). F-39
(m) RESEARCH, DEVELOPMENT AND ENGINEERING Research, development and engineering costs are expensed as incurred. Costs for software development relating to our website incurred
subsequent to establishing technological feasibility, in the form of a working model, are capitalized and amortized over their estimated useful
lives. (n) CONCENTRATIONS For the three months ended June 30, 2009 (unaudited) and 2008 (unaudited), there were three customers representing a combined 74.3%
and 65.7%, respectively, of the Company's revenue. For the six months ended June 30, 2009 (unaudited) and 2008 (unaudited), these same
three customers represented a combined 75.3% and 68.8%, respectively, of the Company's revenue. (o) RECENT ACCOUNTING PRONOUNCEMENTS Effective April 1, 2009, the Company adopted Statement of Financial Accounting Standards ("SFAS") No. 165, Subsequent
Events. SFAS No. 165 establishes general standards for accounting for and disclosure of events that occur after the balance sheet date
but before financial statements are issued. SFAS No. 165 sets forth the period after the balance sheet date during which management of a
reporting entity should evaluate events or transactions that may occur for potential recognition in the financial statements, identifies the
circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements,
and the disclosures that should be made about events or transactions that occur after the balance sheet date. In preparing these financial
statements, the Company evaluated the events and transactions that
occurred between June 30, 2009 through August 26, 2009, the date these financials were issued and October 13, 2009, the date these financial statements were re-issued. In June 2009, the FASB issued SFAS No. 166, "Accounting for Transfers of Financial Assets - an amendment of FASB
Statement No. 140". SFAS No. 166 amends SFAS No. 140, "Accounting for the Transfers and Servicing of Financial Assets
and the Extinguishments of Liabilities," and seeks to improve the relevance and comparability of the information that a reporting
entity provides in its financial statements about transfers of financial assets; the effects of the transfer on its financial position, financial
performance, and cash flows; and a transferor's continuing involvement, if any, in transferred financial assets. SFAS No. 166 eliminates the
concept of a qualifying special-purpose entity, creates more stringent conditions for reporting a transfer of a portion of a financial asset as a
sale, clarifies other sale-accounting criteria, and changes the initial measurement of a transferor's interest in transferred financial assets. SFAS
No. 166 is effective for interim and annual reporting periods beginning after November 15, 2009. The Company does not expect the adoption
of SFAS No. 166 to have a material impact on its consolidated financial statements. In June 2009, the FASB issued SFAS No.167, Amendments to FASB Interpretation No. 46(R). SFAS No. 167 amends FASB
Interpretation No. ("FIN") 46, Consolidation of Variable Interest Entities (revised December 2003) - an interpretation of ARB
No. 51 , which requires an enterprise to determine whether its variable interest or interests give it a controlling financial interest in a
variable interest entity. The primary beneficiary of a variable interest entity is the enterprise that has both (1) the power to direct the activities of
a variable interest entity that most significantly impact the entity's economic performance, and (2) the obligation to absorb losses of the entity
that could potentially be significant to the variable interest entity or the right to receive benefits from the entity that could potentially be
significant to the variable interest entity. SFAS No. 167 also amends FIN 46(R) to require ongoing reassessments of whether an enterprise is
the primary beneficiary of a variable interest entity. SFAS No. 167 is effective for interim and annual reporting periods beginning after
November 15, 2009. The Company does not expect the adoption of SFAS No. 167 to have an impact on its consolidated financial
statements. In June 2009, the FASB issued SFAS No. 168, The FASB Accounting Standards Codification and Hierarchy of Generally Accepted
Accounting Principles, a replacement of FASB Statement No. 162. SFAS No. 168 replaces SFAS No. 162, The Hierarchy of Generally
Accepted Accounting Principles , to establish the FASB Accounting Standards Codification ("Codification") as the source of
authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in preparation of financial statements in
conformity with generally accepted accounting principles in the United States of America. SFAS No. 168 is effective for interim and annual
reporting periods ending after September 15, 2009. The Company does not expect the adoption of SFAS No. 168 to have a material impact on
its consolidated financial statements. F-40
NOTE 3 - RELATED PARTY NOTE PAYABLE On July 31, 2007, MMR entered into a promissory note agreement and a security agreement with The RHL Group, a corporation wholly
owned by Robert H. Lorsch, the Chairman and CEO of the Company, to borrow up to $100,000 under a revolving line of credit. This
agreement was subsequently superseded by an amended and restated promissory note on August 23, 2007 under the terms of which the line
amount was increased to $1 million. On April 29, 2009, the Company agreed to restructure MMR's secured credit facility with The RHL Group
and entered into a Secured Credit Restructuring Agreement with MMR, The RHL Group and Mr. Lorsch, (the "Restructuring Agreement").
MMR issued The RHL Group a Third Amended and Restated Note (the "Third Amended Note"), and the Company agreed to guaranty MMR's
obligations under the Third Amended Note (the "Guaranty"). The Third Amended Note amends and restates the previous The RHL Group note
("RHL Group Note"), matures November 30, 2009, and bears interest at the lesser of 10% or the highest
rate then permitted by law, and is secured (similar to the previous note) by the Security Agreement.
Although the reserve credit line has been increased to $3,000,000, The RHL Group is only obligated to
make a minimum of $100,000 of loans, advances and guarantees under the Third Amended Note within 30
days of execution pursuant to the Restructuring Agreement. The RHL Group Note payable had a balance of
$1,516,049 (unaudited) at June 30, 2009. The components of the $1,516,049 RHL Group Note payable and
the related balance sheet presentation as of June 30, 2009 are: (1) $891,441, which is included in the line of
credit, related party payable; (2) $108,265, which is related to bank overdrafts, credit card charges and a
vendor guaranty is included in accounts payable and accrued expenses; and (3) $516,343, which is related
to deferred salary, consulting expenses and the $200,000 origination fee in relation to the extension of The
RHL Group Note and is included in related party payables. The RHL Group was due to receive, as an
origination fee ("Fee"), a promissory note for $200,000, bearing interest at 10% per annum and
due on demand. The origination fee is payable at the Company's option in cash or warrants to acquire
2,800,000 shares of the Company's common stock at an exercise price of $0.15 per share. See Subsequent
Events (Note 11) below. Interest expense on this note for the three months ended June 30, 2009 and 2008 amounted to
$27,608 (unaudited) and $15,559 (unaudited), respectively. Interest expense on this note for the six
months ended June 30, 2009 and 2008 amounted to $48,042 (unaudited) and $22,965 (unaudited),
respectively. The unpaid interest balance as of June 30, 2009 and December 31, 2008 was $72,104
(unaudited) and $24,963 (unaudited), respectively. The Company considered the accounting for the origination Fee under SFAS No. 133,
"Accounting for Derivative Instruments and Hedging Activities", EITF 00-19
"Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a
Company's Own Stock," EITF 07-5, "Determining Whether an Instrument (or Embedded Feature)
Is Indexed to an Entity's Own Stock," APB No. 14, "Accounting for Convertible Debt and
Debt Issued with Stock Purchase Warrants," EITF No. 98-5, "Accounting for Convertible
Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios"
and FASB Staff Position ("FSP") No. APB 14-1 "Accounting for Convertible Debt
Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)"
("FSP APB 14-1"). Based on these assessments, it was determined that the Fee was not convertible debt as defined in
EITF 98-5 and APB No. 14. Secondly, SFAS No. 133 required bifurcation of the warrants from the Fee
for consideration of whether they would be considered derivatives. EITF No. 00-19 provides that when
the values of the two alternative settlements do not have the same economic value, then the
settlement assumption should be based on the economic substance of the transaction. In this case,
the economic substance of the transaction is that the Company owes the RHL Group, Inc. $200,000, and
settlement in warrants is a contingency entirely under the Company's control. Furthermore, the terms
of the warrant agreement are indeterminable at this time. Therefore, it cannot be assumed that the
Company will settle the liability for more than the $200,000 it owes. Furthermore, until and unless
the Company actually settles the liability, there is no basis for recording the liability at a value
less than its $200,000 face amount. Therefore, in accordance with FSP APB 14-1, the Company recorded
the Fee at its face amount of $200,000. When the Company finally settles the liability, a
determination of the accounting will be made based on the settlement method used. In conjunction with the loan agreement noted above, MMR was required to maintain certain financial covenants, including the
requirement that MMR have at least $100,000 in cash in its bank accounts or such other amount as necessary to maintain operations
through the subsequent thirty (30) days and timely pay any obligations due respecting payroll and all associated payroll taxes on and
after June 1, 2009. As of June 30, 2009, MMR was in payment default under the terms of the Third Amended Note. However, the RHL
Group waived MMR's default, which was subsequently memorialized in a Waiver Agreement, dated as of August 18, 2009. See
Subsequent Events (Note 11) below. F-41
NOTE 4 - INCOME TAXES Under Accounting Principles Board Opinion No. 28, "Interim Financial Reporting," the Company is required to adjust its effective tax
rate each quarter to be consistent with the estimated annual effective tax rate. The Company is also required to record the tax impact of certain
discrete items, unusual or infrequently occurring, including changes in judgment about valuation allowances and effects of changes in tax laws
or rates, in the interim period in which they occur. In addition, jurisdictions with a projected loss for the year or a year-to-date loss where no tax
benefit can be recognized are excluded from the estimated annual effective tax rate. The impact of such an exclusion could result in a higher
or lower effective tax rate during a particular quarter, based upon the mix and timing of actual earnings versus annual projections. MMR, in its capacity as the operating company taking over MMRIS's income tax positions in addition to its own positions after January 27,
2009 (see Note 1), has estimated its annual effective tax rate to be zero. This is based on an expectation that the combined entity will generate
net operating losses in 2009, and it is not more likely than not that those losses will be recovered using future taxable income. Therefore, no
provision for income tax has been recorded as of and for June 30, 2009. NOTE 5 - COMMITMENTS AND CONTINGENCIES Leases The Company leases certain facilities and equipment under non-cancelable capital and operating leases, which expire at various dates
through 2011. Total rent expense for the three months ended June 30, 2009 and 2008 was $10,600 (unaudited) and $39,237 (unaudited),
respectively. Total rent expense for the six months ended June 30, 2009 and 2008 was $19,600 (unaudited) and $78,474 (unaudited),
respectively. Future minimum lease payments as of June, 2009, under non-cancelable operating leases (with initial or remaining lease terms in
excess of one year) and future minimum capital lease payments are as follows: F-42
The amount shown under "Operating Leases" above includes the Company's obligation under a long term lease for office
space. In August 2008, MMR vacated this space prior to the end of the lease term. In February 2009, the Company and the landlord approved
a settlement agreement. The Company accrued for the amount of the settlement liability as of December 31, 2008, which amounted to
$62,500 and is reflected in accounts payable and accrued expenses in the accompanying consolidated balance sheet. The remaining balance
of the settlement liability as of June 30, 2009 amounts to $15,000 (unaudited). The remaining balance is being paid at a rate of $5,000 per
month. In April 2009, the Company renewed its lease with its Chairman and Chief Executive Officer on a month-to-month basis for use of office
space for a total of $3,000 per month. The Company terminated this lease in May 2009. Effective May 1, 2009, the Company entered into a lease agreement to lease additional office space in Beverly Hills, California. The lease
is month-to-month and requires a monthly payment of $4,600 commencing in June 2009. Guarantee provided by The RHL Group Under the terms of an agreement with an investor who purchased $500,000 of MMR's Series B Preferred shares
in 2006, the Company was required to invest $250,000 in a joint venture with the investor to establish an entity to market and sell the
Company's services in the countries of Japan, China, Korea, Taiwan and Thailand. The Company has paid $100,000 to date of this
amount which the Company has expensed during the 3 months ended June 30, 2009 due to uncertainty surrounding the outcome of
negotiations with the related party. The Company is still involved in a relationship with the related party. To date, the joint venture has
not been incorporated, has not commenced operations and there are no employees. The Company and the investor are still in the
process of setting up this operation. In September 2007, The RHL Group, provided the investor with a guarantee that the Company
would meet its obligations under this agreement in exchange for 300,000 shares of restricted MMR common stock valued at $39,000.
As consideration for renewing the guarantee when it expired in September 2008, in January 2009, MMR issued The RHL Group
100,000 shares of MMR common stock, which became 328,174 shares of MMRIS common stock upon the closing of the Merger,
valued at approximately $5,000 (unaudited). This expense has been reflected in general and administrative expenses in the
accompanying consolidated statement of operations for the six months ended June 30, 2009. Due to recent activities affecting the
launch of the Company's products in China and Japan, the Company is assessing its options to pursue opportunities in these markets
separate from the investor. Employment Agreements The Company has employment agreements with its Chairman, President and Chief Executive Officer, Robert H. Lorsch, and its Chief
Financial Officer and Senior Vice President, Naj Allana. Under each employment agreement, the executive officers receive a base salary,
subject to annual increases as determined by the board of directors, certain benefits as set forth in the employment agreements, and an
annual bonus at the discretion of the board of directors. The current term of Mr. Lorsch's employment agreement is effective until December 31, 2011 and will automatically renew for successive
12 month periods unless terminated at least 60 days prior to the end of the term. The employment agreement may be terminated by the
Company without cause (as defined in the agreement) upon 30 days written notice or for cause if Mr. Lorsch fails to cure the acts or omissions
constituting cause within 30 days. If Mr. Lorsch's employment is terminated by the Company for cause or voluntarily by Mr. Lorsch without
good reason, he will not be entitled to receive any severance payments or benefits under the employment agreement. If Mr. Lorsch's
employment is terminated by the Company without cause or voluntarily by Mr. Lorsch for good reason, Mr. Lorsch will be entitled to one year
of salary at his then current rate of pay, including all monthly benefits, and the pro rata portion of the annual bonus otherwise due Mr. Lorsch.
Mr. Lorsch is not entitled to any severance payments or benefits under the agreement if he is terminated for cause or voluntarily resigns
without good reason. F-43
Mr. Lorsch's employment agreement includes provisions that prohibit Mr. Lorsch from disclosing the Company's confidential information
and trade secrets and competing with the Company during the term of his employment agreement or soliciting the Company's employees for
18 months following termination of employment. Mr. Allana's employment agreement is effective until February 15, 2010, and will automatically renew for successive 12 month periods
unless terminated at least 120 days prior to the end of the term. If the Company terminates Mr. Allana's employment, unless due to misconduct
(as defined in the employment agreement), the Company must continue to pay Mr. Allana his monthly salary for the remainder of the then
current term, or if done 120 days preceding the end of the term, then through the end of the next 12 month term. Litigation Matters From time to time, the Company is involved in various legal proceedings generally incidental to its business. While the result of any
litigation contains an element of uncertainty, management presently believes that the outcome of any known, pending or threatened legal
proceeding or claim, individually or combined, will not have a material adverse effect on the Company's financial statements. NOTE 6 - STOCKHOLDERS' DEFICIT Preferred Stock As of December 31, 2008, there were 3,332,694; 1,263,750; and 1,580,082 shares of MMR's Series A, Series B and Series C Preferred
Stock, respectively, issued and outstanding. Immediately prior to the Merger, the outstanding MMR preferred stock was converted to MMR
common stock, representing an aggregate of 6,176,526 shares, which converted into MMRIS common stock in the Merger. As of June 30,
2009 (unaudited), there were no shares of MMR preferred stock issued and outstanding. The Company has 5,000,000 shares of MMRIS preferred stock authorized. As of June 30, 2009 (unaudited), there were no shares of
MMRIS preferred stock issued and outstanding. Common Stock As of June 30, 2009, the Company was authorized to issue 150,000,000 shares of common stock. Immediately prior to the Merger, there
were 41,254,550 shares of MMRIS common stock issued and outstanding. On July 10, 2009, the stockholders of the Company approved an increase to the
shares of common stock it is authorized to issue. As of July 10, 2009, the Company was authorized to issue 650,000,000 shares of common
stock. Pursuant to the Merger Agreement, MMRIS agreed to issue (or reserve for issuance) an aggregate 92,599,196 shares of MMRIS common
stock to the stockholders of MMR and the holders of options and warrants to acquire MMR common stock at the time of the Merger. Immediately prior to the Merger, and including the conversion of 6,176,526 shares of MMR preferred stock into MMR common stock, the
issuance of 100,000 shares of MMR common stock to The RHL Group for renewal of a guarantee (see Note 5), MMR had 24,320,100 shares
of common stock issued and outstanding. At the effective time of the Merger, and in accordance with the terms of the Merger Agreement,
these shares of outstanding MMR common stock automatically converted into 79,812,087 shares of MMRIS common stock. F-44
On March 13, 2009, the Company approved the issuance of 1,202,587 shares of MMRIS common stock including 1,145,324 shares of
treasury stock, as replacement of options to acquire 1,202,587 shares of common stock. This resulted in an additional expense of $1,466
(unaudited) recorded during the six months ended June 30, 2009. As of June 30, 2009, the total shares of MMRIS common stock issued and outstanding amounted to 126,098,201 (unaudited). Warrants for Purchase of Preferred B shares On April 1, 2007, MMR granted 200,000 warrants to purchase Series B Preferred Stock of MMR at an exercise price of $2.00. The
warrants were issued to the largest distributor of employee assistance services in the US as an incentive to market and sell MMR's services
into their customer base. The related services agreement was for five years, and the customer never bought any services from MMR. Upon closing of the Merger on January 27, 2009, these warrants converted into warrants to purchase 656,346 shares of MMRIS common
stock at an exercise price of $0.61 per share. There was no additional expense recorded during the six months ended June 30, 2009 resulting
from the modification of these warrants. NOTE 7 - EQUITY INCENTIVE PLAN Stock Option Activity A summary of option activity for the six months ended June 30, 2009 (unaudited) is presented below. Options granted by MMR prior to the
date of the Merger of January 27, 2009 have been retroactively restated as of December 31, 2008 to reflect the conversion ratio of MMR to
MMRIS shares. The aggregate intrinsic value in the table above is before applicable income taxes and is calculated based on the difference between the
exercise price of the options and the quoted price of the common stock as of the reporting date. F-45
A summary of the activity of the Company's nonvested options for the six months ended June 30, 2009 (unaudited) is presented
below. As of June 30, 2009, total unrecognized stock-based compensation expense related to nonvested stock options was $207,984
(unaudited), which is expected to be recognized by the year ended December 31, 2011. Total stock option expense recorded during the three
months ended June 30, 2009 and 2008 amounted to $104,893 (unaudited) and $2,020 (unaudited), respectively, and is reflected in general
and administrative expenses in the accompanying consolidated statements of operations. The total stock option expense recorded during the
six months ended June 30, 2009 and 2008 amounted to $217,602 (unaudited) and $5,965 (unaudited), respectively. The following table summarizes information about stock options outstanding and exercisable at June 30, 2009 (unaudited). Warrants On November 7, 2007, in conjunction with the registered direct offering of an aggregate of 7.4 million shares, MMRIS issued
warrants to purchase up to 4.4 million shares of MMRIS common stock at an exercise price of $2.77 per share. The warrants are exercisable
as of the date of grant through November 7, 2012. The Company valued the warrants using a Black-Scholes pricing model.
EITF 00-19, "Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company's Own Stock"
requires freestanding contracts that are settled in a Company's own stock, including common stock warrants to be designated as an equity
instrument, asset or liability. Under the provisions of EITF 00-19, a contract designated as an asset or a liability must be carried at fair value
until exercised or expired, with any changes in fair value recorded in the results of operations. SFAS No. 133, "Accounting for
Derivative Instruments and Hedging Activities" was applied to determine that the liability is accounted for as a derivative under SFAS
133 (see annual report on Form 10-K for the year ended December 31, 2008, filed on April 15, 2009 for a more detailed discussion). The
valuation of these warrants amounted to $0 and $149,280 (unaudited) as of December 31, 2008 and June 30, 2009, respectively. The increase
in fair value of $149,280 was presented as change in valuation of warrants in the accompanying consolidated statements of operations. F-46
Immediately prior to the Merger, MMRIS issued warrants to acquire 9,999,992 shares of MMRIS's common stock at an exercise price of
$0.12 per share, which expire on January 26, 2014, to certain former officers, former directors and their affiliates who were willing to take such
equity as partial or full payment for outstanding liabilities. These warrants vested immediately and were valued on the date of grant using the
Black-Scholes option pricing model using the assumptions as described in Note 2. As this transaction occurred immediately prior to the
Merger, the resulting gain on the issuance of warrants was reflected in the statement of operations of MMRIS. There is no impact of this
transaction recorded on the accompanying consolidated statement of operations for the six months ended June 30, 2009 (unaudited). On March 1, 2009, the Company granted to a consultant 500,000 warrants to purchase shares of the Company's common stock at an
exercise price of $0.15 per share. The warrants vest over a six month period and have a contractual life of five years. These warrants were
valued using the Black-Scholes option pricing model using the assumptions as described in Note 2. As of June 30, 2009, the total value of
these warrants amounted to $52,573 (unaudited), of which $34,762 (unaudited) was recorded as stock based compensation expense during
the six months ended June 30, 2009. On March 1, 2009, the Company granted an additional 1,250,000 warrants to this consultant with an
exercise price of $0.15 per share, conditional upon the consultant being hired as full time employee by November 1, 2009. If this condition is
met, the warrants vest over a 30 month period and expire on November 1, 2014. The Company has not recorded any expense for these
warrants as of June 30, 2009, as their vesting was not assessed as probable. On March 8, 2009, the Company granted to a group of consultants an aggregate total of 600,000 warrants to purchase shares of the
Company's common stock at an exercise price of $0.175 per share. Of the warrants granted, 100,000 vested immediately upon grant and
500,000 vest over a two year period. These warrants were valued using the Black-Scholes option pricing model using the assumptions as
described in Note 2. As of June 30, 2009, the total value of these warrants amounted to $64,380 (unaudited), of which $12,910 (unaudited)
related to the 100,000 warrants was recorded as a reduction of outstanding accounts payable during the six months ended June 30, 2009
and $6,345 related to the 500,000 warrants was recorded in sales and marketing expense for the six months ended June 30, 2009. On March 13, 2009, the Company granted to a group of vendors an aggregate total of 1,300,000 warrants to purchase shares of the
Company's common stock at an exercise price of $0.175 per share. These warrants all vested immediately. These warrants were used valued
using the Black-Scholes option pricing model using the assumptions as described in Note 2. The total value of these warrants amounted to
$173,636 (unaudited), which was recorded as interest expense during the six months ended June 30, 2009, as the Company granted these
warrants as consideration for the vendors agreeing to defer payment of their outstanding liabilities. On April 6, 2009, the Company granted to a customer 50,000 warrants to purchase shares of the Company's common stock at an exercise
price of $0.23 per share. The warrants vested immediately. These warrants were valued using the Black-Scholes option pricing model using
the assumptions as described in Note 2. The total value of these warrants amounted to $8,490 (unaudited), all of which was recorded as a
reduction to subscriber revenues during the six months ended June 30, 2009. On May 13, 2009, the Company granted to a group of consultants an aggregate total of 275,000 warrants to purchase shares of the
Company's common stock at an exercise price of $0.19 per share. Of the warrants granted, 75,000 vested immediately upon grant and
200,000 vest over a six month period. These warrants were valued using the Black-Scholes option pricing model using the assumptions as
described in Note 2. As of June 30, 2009, the total value of these warrants amounted to $31,588 (unaudited), of which $16,381 (unaudited)
was recorded as an operating expense during the six months ended June 30, 2009. The following summarizes the total warrants outstanding and exercisable as of June 30, 2009 (unaudited). The weighted average fair value of warrants granted during the six months ended June 30, 2009 amounted to $0.10 (unaudited). There
were no warrants exercised during the six months ended June 30, 2008 (unaudited). F-47
Restricted Stock Program Under the Restricted Stock Program, a restricted stock award is an offer by the Company to sell to an eligible person shares that are
subject to restrictions relating to the sale or transfer of the shares. A committee appointed by the Board to administer the program or the Board
itself shall determine to whom an offer will be made, the number of shares the person may purchase, the price to be paid and the restriction to
which the shares shall be subject. The offer must be accepted by the eligible person within thirty days from the date of the offer evidenced by
the Restricted Stock Purchase Agreement. The purchase price of shares shall not be less than 85% of the fair market value of such shares on
the issue date, with the provision that the purchase price for a 10% stockholder shall not be less than 110% of such fair market value. Shares
are either fully and immediately vested upon issuance, or may vest in installments upon attainment of specified performance objectives. There
were no shares issued under the restricted stock program for the six months ended June 30, 2009 (unaudited). During the six months ended
June 30, 2008, MMR issued 2,095,000 (unaudited) shares of restricted MMR common stock for services and interest expense on the line of
credit with The RHL Group. These shares of MMR became 6,875,226 (unaudited) shares of MMRIS subsequent to the merger. The total value
of these restricted shares amounted to $314,250, of which $104,250 is recorded in general administrative expenses and $210,000 is recorded
in interest and other expenses, net on the accompanying consolidated statement of operations for the six months ended June 30, 2008. Stock Bonus Program Under the Stock Bonus Program, shares are issued as a bonus for past services rendered pursuant to the Stock Bonus Agreement. Stock
bonuses may be awarded upon satisfaction of specified performance goals pursuant to the Performance Stock Bonus Agreement. No shares
were issued under the Stock Bonus Program during the six months ended June 30, 2009 or 2008 (unaudited). Derivative Liabilities In accordance with Emerging Issues Task Force Issue ("EITF") No. 00-19,
"Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a
Company's Own Stock," on January, 27, 2009, the Company performed an analysis as if all holders
of options and warrants exercised their rights under those contracts. This analysis resulted in the
conclusion that the Company had inadequate authorized shares to settle 100% of these contracts.
Therefore, as shareholder approval would be necessary to increase the number of authorized shares,
settlement of these obligations would not be within the control of the Company. Consequently certain
non-employee options and warrants were accounted for as derivative liabilities in accordance with
Statement of Financial Accounting Standards ("SFAS") No. 133, "Accounting for
Derivative Instruments and Hedging Activities." The event giving rise to this condition was the merger between Favrille, Inc. and MyMedicalRecords.com, Inc. on
January 27, 2009. On that date, the fair value of 3,436,458 vested non-employee option and 15,218,890 vested warrant contracts
subject to this analysis was reclassified from equity to derivative liabilities. The condition was remedied on June 13, 2009 when certain
of the option and warrant holders waived their rights under those contracts until such time that the Company had 250 million or more
shares authorized. On March 31, 2009 and June 13, 2009, these contracts were again valued using the Black-Scholes option valuation
model and the difference between the original value at January 27, 2009 of $871,052 and the values at March 31, 2009 and June 13,
2009 of $2,665,889 and $1,377,033, respectively, was recorded as a gain on change in value of derivatives for the three months ended
June 30, 2009 and a loss on change in value of derivatives for the six months ended June 30, 2009. On June 13, 2009, the derivative
liabilities were reclassified back into equity resulting in a gain of $1,288,856 for the three months ended June 30, 2009 and a loss of
$505,981 for the six months ended June 30, 2009. The inputs used for the Black-Scholes option valuation model were as follows: NOTE 8 - NOTES PAYABLE On June 11, 2009, the Company entered into a promissory note
agreement with PM Creative Corporation, an unrelated third-party, to borrow
$125,000. The promissory note is due and payable July 30, 2011, and bears
interest of 12% per annum payable quarterly beginning on January 1, 2011. The
proceeds of the loan will be used by the Company for the filing of international
patent applications to expand and perfect the patent rights of the Company in
the Anti-CD20 Antibodies outside the United States. PM Creative Corporation also
received, as a commitment fee ("Fee"), a payable for $30,000, due and
payable by August 15, 2009. The commitment fee is payable at the Company's
option in cash or warrants to acquire 375,000 shares of MMRIS common stock at an
exercise price per share equal to the lesser of (i) $0.15 per share and (ii) the
weighted average trading price for a share of common stock for the ten
consecutive trading days preceding the date on which the warrant is exercised.
The warrants have a four year term. On July 29, 2009, the Company elected to pay
the commitment fee through the issuance of warrants exercisable into 375,000
shares of the Company's common stock at an exercise price of $0.14 per share.
The warrants vested immediately. These warrants were valued using the
Black-Scholes option pricing model using the following inputs: (1) common stock value
of $0.12 (2) exercise price equal to $0.14 (3) expected price volatility of
109.39% (4) risk-free interest rate of 2.21%, and (5) expected life of 4 years.
The total value of these warrants amounted to $32,441 (unaudited), all of which
will be recorded as a reduction to the commitment fee payable in July 2009, with
a loss on settlement of payables for $2,441 (see Note 11). The Company considered the accounting for the commitment Fee under SFAS No. 133,
"Accounting for Derivative Instruments and Hedging Activities", EITF 00-19
"Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a
Company's Own Stock," EITF 07-5, "Determining Whether an Instrument (or Embedded Feature)
Is Indexed to an Entity's Own Stock," APB No. 14, "Accounting for Convertible Debt and
Debt Issued with Stock Purchase Warrants," EITF No. 98-5, "Accounting for Convertible
Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios"
and FASB Staff Position ("FSP") No. APB 14-1 "Accounting for Convertible Debt
Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)"
("FSP APB 14-1"). Based on these assessments, it was determined that the Fee was not convertible debt as defined in EITF 98-5
and APB No. 14. Secondly, SFAS No. 133 required bifurcation of the warrants from the Fee for consideration of whether they would be
considered derivatives. EITF No. 00-19 provides that when the values of the two alternative settlements do not have the same
economic value, then the settlement assumption should be based on the economic substance of the transaction. In this case, the
economic substance of the transaction is that the Company owes PM Creative Corporation $30,000, and settlement in warrants is a
contingency entirely under the Company's control. Furthermore, the terms of the warrant agreement are indeterminable at this time.
Therefore, it cannot be assumed that the Company will settle the liability for more than the $30,000 it owes. Furthermore, until and
unless the Company actually settles the liability, there is no basis for recording the liability at a value less than its $30,000 face amount.
Therefore, in accordance with FSP APB 14-1, the Company recorded the Fee at its face amount of $30,000. When the Company finally
settled the liability, a determination of the accounting will be made based on the settlement method used, which resulted in a loss on
settlement (see Note 11). Notes payable consisted of the following: Future maturities with respect to these notes payable as of June 30, 2009 were as follows: F-48
NOTE 9 - RESTRUCTURING ACTIVITIES From May 29, 2008 to November 7, 2008, MMRIS had provided notices under the federal Worker Adjustment and Retraining
Notification Act to 142 employees, including six members of senior management, that it planned to conduct a workforce reduction at its facility
in San Diego, California and that their employment was expected to end on various dates between June 6, 2008 to November 7, 2008.
Immediately prior to the date of the Merger on January 27, 2009, the total severance liability relating to former MMRIS employees amounted to
$1,682,416 (unaudited). On January 27, 2009, immediately prior to the Merger, as part of the 9,999,992 (unaudited) warrants issued to
creditors (see Note 7), the Company issued warrants as settlement of $985,020 (unaudited) of these amounts. In addition, the Company
signed promissory notes with certain former executives totaling $76,783 (unaudited), which notes are payable in full on August 31, 2009 (see
Note 8). As of June 30, 2009, the total remaining severance liabilities amounted to $620,613 (unaudited), which is reflected as severance liability
on the accompanying consolidated balance sheets. This consists of $571,362 payable to former non-executive employees in 18 monthly
installments starting on July 27, 2009, as well as $49,251 in estimated payroll tax. During the period from January 27, 2009 through June 30, 2009, the Company entered into a series of settlement agreements with certain
vendors of MMRIS pursuant to the Creditor Plan, in which the Company settled approximately $302,982 (unaudited) of its outstanding
accounts payable for an aggregate settlement amount of $214,402 (unaudited), including promissory notes of
$139,355 (unaudited) payable in 18 monthly installments starting on July 27, 2009 (see Note 8). This resulted in a gain of $36,945 (unaudited)
and $89,170 (unaudited), which has been recorded as a gain on settlement of payables in the accompanying consolidated statement of
operations for the three and six months ended June 30, 2009, respectively. NOTE 10 - RELATED PARTY TRANSACTIONS The Company incurred $30,000 (unaudited) and $26,025 (unaudited) during the six months ended June 30, 2009 and 2008, respectively,
towards marketing consulting services from a director. Included in related party payables at June 30, 2009 and December 31, 2008 was
$96,025 (unaudited) and $76,025, respectively, with respect to these services. The Company also incurred $25,000 (unaudited) during each of the six months ended June 30, 2009 and 2008, respectively, towards
marketing consulting services from a second director. Included in related party payables at June 30, 2009 and December 31, 2008 was
$100,000 (unaudited) and $75,000, respectively, with respect to these services. In August 2006, the Company entered into a month-to-month lease with The RHL Group for the use of furniture and art for the Company's
offices, for a total of $1,000 per month which terminated on August 15, 2008. The Company incurred expenses of $0 (unaudited) and $6,000
(unaudited) during the six months ended June 30, 2009 and 2008, respectively. In August 2008, the Company entered into an eight month lease with its Chairman and Chief Executive Officer for the use of office space
for a total of $3,000 per month plus a share of utilities. This lease was renewed in April 2009 on a month-to-month basis and terminated in May
2009. In February 2006, the Company entered into a letter agreement with MyMedicalRecords.com.au ("MMR AU") granting it a 10 year
exclusive right to market and sell the Company's products and services in Australia and New Zealand. The controlling shareholder of MMR AU
was, at the time of the signing of the initial letter agreement, a shareholder of the Company. This letter agreement was subsequently
formalized in the form of a ten year license agreement in October 2007, at which time the controlling shareholder of MMR AU was no longer a
shareholder of the Company. F-49
The Company received $50,000 from MMR AU upon the signing of the letter agreement towards set up costs of the platform to be used by
customers of MMR AU. The Company completed the setup of the platform for MMR AU in 2007, at which time it started to recognize this
amount into revenue. The Company also received $150,000 in 2007 from MMR AU towards the minimum guarantee payment for years 2 and
3 of the license term. This amount has been deferred and is being recognized as income based on the number of months elapsed. During the
three months ended June 30, 2009 and 2008, the Company amortized $3,125 (unaudited) and $20,000 (unaudited), respectively, of these
amounts as license fee and other revenues in the accompanying statement of operations. During the six months ended June 30, 2009 and
2008, the Company amortized $18,750 (unaudited) and $40,000 (unaudited), respectively, of these amounts as license fee and other revenues
in the accompanying statement of operations. The Company has accounted for this transaction under EITF 00-21, "Revenue
Arrangements with Multiple Deliverables." NOTE 11 - SUBSEQUENT EVENTS Effective April 1, 2009, the Company adopted Statement of Financial Accounting Standards ("SFAS") No. 165, Subsequent
Events. SFAS No. 165 establishes general standards for accounting for and disclosure of events that occur after the balance sheet date
but before financial statements are issued. SFAS No. 165 sets forth the period after the balance sheet date during which management of a
reporting entity should evaluate events or transactions that may occur for potential recognition in the financial statements, identifies the
circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements,
and the disclosures that should be made about events or transactions that occur after the balance sheet date. In preparing these financial
statements, the Company evaluated the events and transactions that
occurred between June 30, 2009 through August 26, 2009, the date these financials were issued and October 13, 2009, the date these financial statements were re-issued. On August 18, 2009, the Company and The RHL Group entered into a
Waiver Agreement, pursuant to which, in consideration of The RHL Group's waiver
of MMR's payment default under the Third Amended Note (see Note 3 above), the
Company granted to The RHL Group a warrant to purchase an aggregate of
11,039,378 shares of the Company's common stock, with an exercise price equal to
$0.13 per share, the closing price of the Company's common stock on the date
immediately preceding the date of grant. Under the Waiver Agreement, The RHL
Group agreed to waive MMR's payment default until August 31, 2009, which waiver
period will automatically continue until The RHL Group notifies MMR otherwise.
In addition, as a repayment of the unpaid $200,000 origination fee owed to The
RHL Group by MMR in connection with the credit line restructuring, the Company
granted to The RHL Group 2,800,000 shares of common stock. On August 17, 2009, in consideration of a personal guaranty given by Robert
H. Lorsch, the Company's Chairman, Chief Executive Officer and President, to a
vendor, to guarantee payments due to the vendor from the Company for services
rendered, the Company granted Mr. Lorsch (i) a warrant to purchase 706,605
shares of Company common stock, at an exercise price of $0.13 per share, the
closing price of the Company's common stock on the date immediately preceding
the date of grant, and (ii) 141,321 shares of Company common stock. On August 6, 2009, the Company made the following equity issuances to the persons and for the reasons stated below: The Shares were issued to each of the foregoing in reliance upon exemptions from registration pursuant to Section 4(2) under the
Securities Act of 1933, as amended (the "Securities Act"), and the rules promulgated thereunder. At the time of their issuance, the securities
granted above are restricted securities for purposes of the Securities Act and the certificates representing such securities shall bear legends to
that effect. The exercise/conversion prices of the securities described above were equal to the closing price of the Company's common stock
as of the date of grant. On July 29, 2009, the Company elected to pay the $30,000 commitment fee payable to PM Creative Corporation
through the issuance of warrants exercisable into 375,000 shares of the Company's common stock at an exercise price of $0.14 per
share. The warrants vested immediately. These warrants were valued using the Black-Scholes option pricing model using the following
inputs: (1) common stock value of $0.12 (2) exercise price equal of $0.14 (3) expected price volatility of 109.39% (4) risk-free interest
rate of 2.21%, and (5) expected life of 4 years. The total value of these warrants amounted to $32,441 (unaudited), all of which will be
recorded as a reduction to the commitment fee payable in July 2009, with a loss on settlement payables for $2,441. In July and August 2009, the Company entered into a 12% Convertible Promissory Note ("the Convertible Notes") with
three unrelated third-parties (the "Holders") for a principal amount totaling $160,000. The Convertible Notes matures on
December 31 2009, and the Company may, in its sole discretion, extend the maturity date to June 30, 2010. The Convertible Notes
bears an interest of 12% per annum payable in cash or shares of common stock, or a combination of cash and shares of common
stock. The decision whether to pay in cash, shares of common stock or combination of both shall be at the sole discretion of the
Company. Each Convertible Note is convertible into shares of common stock by dividing (i) the then outstanding balance of such note
by (ii) the product of eighty percent (80%) multiplied by the arithmetic average of the volume weighted average price of the common
stock for the ten (10) consecutive trading days ending on the day that is three (3) trading days prior to the applicable conversion date. On July 10, 2009, the stockholders of the Company approved an increase to the shares of common stock it is authorized to issue. As of
July 10, 2009, the Company is authorized to issue 650,000,000 shares of common stock. F-50
35,975,049 SHARES COMMON STOCK PROSPECTUS Through and including , 2009 (the 25th day after the date of this prospectus), all
dealers effecting transaction in these securities, whether or not
participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting
as underwriters and with respect to their unsold allotments or subscriptions.
PART II INFORMATION NOT REQUIRED IN PROSPECTUS Item 13. Other Expenses of Issuance and Distribution The following table sets forth expenses in connection with the issuance and distribution of the securities being registered. All amounts
shown are estimated, except the SEC registration fee. SEC registration fee
$ 160.60 Legal fees and expenses
20,000 Accountants' fees and expenses
5,000 Miscellaneous fees
5,000
Total
$ 30,160.60
Item 14. Indemnification of Directors and Officers Section 145 of the Delaware General Corporation Law, or DGCL, empowers a Delaware corporation to indemnify any persons
who are, or are threatened to be made, parties to any threatened, pending or completed legal action, suit or proceeding, whether civil, criminal,
administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer or
director of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or
enterprise. The indemnity may include expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and
reasonably incurred by such person in connection with such action, suit or proceeding, provided that such officer or director acted in good faith and
in a manner he reasonably believed to be in or not opposed to the corporation's best interests, and, for criminal proceedings, had no reasonable
cause to believe his conduct was illegal. A Delaware corporation may indemnify officers and directors in an action by or in the right of the corporation
under the same conditions, except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the
corporation in the performance of his duty. Where an officer or director is successful on the merits or otherwise in the defense of any action referred
to above, the corporation must indemnify him against the expenses which such officer or director actually and reasonably incurred. Our amended and restated certificate of incorporation and amended and restated bylaws provide that we will indemnify our directors and officers
to the fullest extent not prohibited by Delaware law, except that that we shall not be required to indemnify any director or executive officer in
connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law, (ii)
the proceeding was authorized by the Board of Directors of the corporation, (iii) such indemnification is provided by us, in our sole discretion,
pursuant to the powers vested in the corporation under the DGCL or any other applicable law or (iv) such indemnification is required to be made
under separate contract. The bylaws also provide that the right of directors and officers to indemnification shall be a contract right and shall not be
exclusive of any other right now possessed or hereafter acquired under any statute, provision of our certificate of incorporation, bylaws, agreements,
vote of stockholders or disinterested directors or otherwise. The bylaws also permit us to secure insurance on behalf of any officer, director,
employee or other agent for any liability arising out of his or her actions in such capacity. In accordance with Section 102(b)(7) of the DGCL, our amended and restated certificate of incorporation provides that directors
shall not be personally liable for monetary damages for breaches of their fiduciary duty as directors to the fullest extent permitted under applicable
law. The effect of this provision is to eliminate the personal liability of directors for monetary damages for actions involving a breach of their fiduciary
duty of care, including any actions involving gross negligence. Notwithstanding this provision the DGCL does not permit us to eliminate personal
liability for (i) breaches of their duty of loyalty to us or our stockholders, (ii) acts or omissions not in good faith or which involve
intentional misconduct or knowing violations of law, (iii) certain transactions under Section 174 of the DGCL (unlawful payment of
dividends or unlawful stock purchases or redemptions) or (iv) transactions from which a director derives an improper personal benefit. II-1
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant
to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such
indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. Item 15. Recent Sales of Unregistered Securities The following is a summary of transactions by us within the past three years involving sales of our securities that were not
registered under the Securities Act. Each offer and sale was exempt from registration under either Section 4(2) of the Securities Act or Rule 506
under Regulation D of the Securities Act because (i) the securities were offered and sold only to accredited investors; (ii) there was no general
solicitation or general advertising related to the offerings; (iii) each investor was given the opportunity to ask questions and receive answers
concerning the terms of and conditions of the offering and to obtain additional information; (iv) the investors represented that they were acquiring the
securities for their own account and for investment; and (v) the securities were issued with restrictive legends. 1. On September 18, 2009, we issued to Hal J. Meyer, a grant of 100,000 shares of common stock at a price per share value of $0.09,
in consideration for services rendered to us. 2. On September 16, 2009, we entered into a Note and Warrant Subscription Agreement (the "Note Agreement") with David T. Loftus
(the "Purchaser"). Pursuant to the terms of the Note Agreement, the Purchaser purchased a convertible note in the amount of $200,000 (the "Note").
The Note is convertible at the option of the Purchaser into a number of shares of our common stock, equal to the product of eighty percent (80%)
multiplied by the arithmetic average of the dollar volume-weighted average price ("VWAP") of the shares for the ten (10) consecutive trading days
ending on the day that is three (3) trading days prior to the applicable conversion date. In addition to the Note, the Purchaser received a warrant to purchase initially up to 1,200,000 shares of our common stock at a per share
price equal to the lesser of (i) the product of fifty percent (50%) multiplied by the arithmetic average of the VWAP of the shares for the ten (10)
consecutive trading days ending on the day that is three (3) trading days prior to the applicable exercise date, or (ii) $0.15, whichever is less (the
"Warrant"). The Warrant expires on September 15, 2012. 3. On September 16, 2009, we paid a consulting fee to David T. Loftus, in consideration of his services in assisting us with our use of a
marketing database, in an amount equal to $250,000 in the form of 2,777,778 shares of common stock at a price per share value of $0.09, which
was the closing price of our common stock on September 15, 2009. 4. On September 15, 2009, in connection with the preparation of the Investment Agreement and the Registration Rights Agreement with
Dutchess Equity Fund, L.P. ("Dutchess"), we paid Dutchess a document preparation fee in the amount of $5,000, and issued Dutchess 230,800
shares of common stock, which equaled $30,000 worth of common stock based on a $0.13 price per share value, which was the closing sales price
of our common stock on August 13, 2009, the execution date of the non-binding term sheet with Dutchess. 5. On September 4, 2009, we issued to Michael Psomas a grant of 770,000 shares of common stock at a price per share value of
$0.09, in consideration of services rendered to us. 6. On September 2, 2009, we made the following equity issuances to the persons and for the reasons stated below. Unless otherwise
stated, the prices of the shares of common stock described below were equal to $0.10 per share, the closing price of such hares as of the date of
grant. II-2
7. On August 31, 2009, we issued to Mr. Psomas a grant of 500,000 shares of common stock at a price per share value of $0.11, in
consideration of services rendered to us. 8. On August 18, 2009, we entered into a Waiver Agreement with The RHL Group, Inc., pursuant to which, in consideration of The RHL
Group's waiver of MMR's payment default under the Third Amended Note, we granted to The RHL Group a warrant to purchase an aggregate of
11,039,378 shares of our common stock, with an exercise price equal to $0.13 per share, the closing price of our common stock on the date
immediately preceding the date of grant. In addition, as repayment of the unpaid origination fee owed to The RHL Group by MMR in connection with
the credit line restructuring, we granted to The RHL Group an aggregate of 2,800,000 shares of common stock. 9. On August 17, 2009, in consideration of a personal guaranty given by Robert H. Lorsch, our Chairman, Chief Executive Officer and
President, to a vendor, to guarantee payments due to the vendor from the Company for services rendered, we granted Mr. Lorsch (i) a warrant to
purchase 706,605 shares of our common stock at an exercise price of $0.13 per share, the closing price of our common stock on the date
immediately preceding the date of grant, and (ii) 141,321 shares of our common stock. 10. On August 6, 2009, our Board of Directors, upon recommendation of the Compensation Committee, approved the issuance of an
option grant (the "Option") to Robert H. Lorsch, our President, Chairman and Chief Executive Officer, pursuant to the terms of Mr. Lorsch's
employment agreement with us, dated January 27, 2009. Mr. Lorsch's employment agreement provided that, each year during the term of Mr.
Lorsch's employment with the Company, Mr. Lorsch is entitled to a grant or grants of stock options as determined by the Board of Directors.
However the option grant was not considered until the August 6, 2009 meeting. The Option entitles Mr. Lorsch to purchase up to 3,000,000 shares of
Company common stock per year for the three year term of the employment agreement, at an exercise price of $0.125 per share, which was the
closing price of our common stock as reported on the Over the Counter Bulletin Board on August 6, 2009. 3,000,000 shares are being vested as of
the date of grant for the period from January 27, 2009 through January 27, 2009, 3,000,000 shares vest on January 27, 2010, and the remaining
3,000,000 shares will vest on January 27, 2011. The Option agreement has a five year term. 11. On August 6, 2009, our Board of Directors approved the issuance of 707, 016 shares of common stock to Naj Allana, our Chief
Financial Officer and Senior Vice President. The shares were issued to Mr. Allana in consideration of Mr. Allana agreeing to allow us to defer
payments of salaries under the terms of Mr. Allana's employment agreement with us. 12. On April 6, 2009 and May 13, 2009, we granted warrants to certain unrelated third-party creditors and a customer to acquire an
aggregate 325,000 shares of our common stock. The warrants, 125,000 of which are immediately exercisable, have exercise prices ranging from
$0.19 to $0.23 and all expire on May 13, 2014 except 50,000 which expire on April 6, 2010. The warrants were granted in exchange for consulting
services rendered or in exchange for a prepayment for subscriber fees. 13. On May 21, 2009, in connection with the execution of an addendum to a letter agreement with The Rebensdorf Group, Inc., we
granted George Rebensdorf stock options to acquire 100,000 shares of our common stock at an exercise price of $0.179 per share, which options
vest monthly over 2 years and expire on May 21, 2014. The Rebensdorf Group, Inc. is wholly-owned and controlled by George Rebensdorf, a
member of our Board of Directors, and Mr. Rebensdorf serves as The Rebensdorf Group, Inc.'s President. 14. On March 1, 2009, March 8, 2009 and March 13, 2009, we granted warrants to certain unrelated third-party creditors and service
providers to acquire an aggregate 3,650,000 shares of our common stock. The warrants, 1,400,000 of which are immediately exercisable, have
exercise prices ranging from $0.15 to $0.175 and all expire on March 1, 2014 except 1,250,000 which expire on November 1, 2014. The warrants
were granted in exchange for the agreement of such unrelated third parties to agree to defer payment by our company for services rendered or in
exchange for agreement to pay our company for services in advance of contractual deadlines. II-3
15. Reference is made to the disclosure set forth under Item 3.02 of the current report on Form 8-K, filed with the SEC on February 2,
2009, which disclosure is incorporated herein by reference. 16. Reference is made to the disclosures set forth under Part II - Item 2 of our quarterly reports on Form 10-Q for the quarterly periods
ended March 31, 2008, September 30, 2007 and September 30, 2006 under the heading "Unregistered Sales of Equity Securities and Use of
Proceeds," which disclosures are incorporated herein by reference. 17. In the past three years through the date of this registration statement, we granted stock options and stock purchase rights to acquire
an aggregate of 55,527,795 shares of our common stock at prices ranging from $0.046 to $5.26 per share to employees, consultants and directors.
We generally used the proceeds of the foregoing sales of securities for repayment of indebtedness, working capital and other general
corporate purposes. Item 16. Exhibits and Financial Statement Schedules (a) The exhibits set forth under the caption "Exhibit Index" below are included herein and incorporated by reference. (b) Financial Statement Schedules. The financial statement schedules have been omitted because they are not applicable, not required, or
the information is included in the consolidated financial statements or notes thereto. II-4
Item 17. Undertakings The undersigned registrant hereby undertakes: 1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would
not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no
more than 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration
statement. iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any
material change to such information in the registration statement; 2. That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the
initial bona fide offering thereof. 3. To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. 4. That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to
Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or
other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date
it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration
statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the
registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made
in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date
of first use. Insofar as indemnification for liabilities arising under the Securities Act of 1933, may be permitted to directors, officers and controlling persons of
the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that, in the opinion of the SEC, such indemnification
is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such
liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the
successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being
registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of
appropriate jurisdiction the question whether such indemnification by them is against public policy as expressed in the Securities Act and will be
governed by the final adjudication of such issue. II-5
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement on Form S-1 to
be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Los Angeles, State of California, on October 13,
2009. MMR INFORMATION SYSTEMS, INC. By: /s/ Robert H. Lorsch Name: Robert H. Lorsch Title: Chief Executive Officer POWER OF ATTORNEY We, the undersigned officers and directors of MMR Information Systems, Inc., do hereby constitute and appoint Robert H. Lorsch and
Naj Allana, and each of them individually, our true and lawful attorney-in-fact and agent with full power of substitution and re-substitution, for him and
in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration
Statement, or any related registration statement that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of
1933, as amended, and to file the same, with exhibits thereto, and other documents in connection therewith, with the SEC, granting unto said
attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about
the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and
agent, or his substitute, may lawfully do or cause to be done by virtue hereof. Pursuant to the requirements of the Securities Act of 1933, this Registration Statement on Form S-1 has been signed below by the
following persons in the capacities and on the dates indicated. Name Title Date /s/ Robert H. Lorsch Chief Executive Officer and Director October 13, 2009 Robert H. Lorsch (Principal Executive Officer) /s/ Naj Allana Chief Financial Officer, Chief Accounting Officer October 13, 2009 Naj Allana (Principal Financial and Accounting Officer) /s/ Hector V. Barreto, Jr. Director October 13, 2009 Hector V. Barreto, Jr. /s/ David A. Boyden Director October 13, 2009 David A. Boyden /s/ Douglas H. Helm Director October 13, 2009 Douglas H. Helm /s/ George Rebensdorf Director October 13, 2009 George Rebensdorf /s/ Bernard Stolar Director October 13, 2009 Bernard Stolar /s/ Jack Zwissig Director October 13, 2009 Jack Zwissig
EXHIBIT INDEX Exhibit Description of Exhibit 2.1 Agreement and Plan of Merger and Reorganization, dated as of November 8, 2008, by and among Favrille, Inc., Montana
Merger Sub, Inc. and mymedicalrecords.com, Inc. (incorporated by reference to Exhibit 2.1 of the registrant's current report on Form 8-K filed on
November 13, 2008) 2.2 Form of Voting Agreement, dated as of November 8, 2008, by and among Favrille, Inc. and certain stockholders of
mymedicalrecords.com, Inc. (incorporated by reference to Exhibit 2.2 of the registrant's current report on Form 8-K filed on November 13, 2008)
3.1 Amended and Restated Certificate of Incorporation, as amended by a Certificate of Amendment of Amended and Restated
Certificate of Incorporation (incorporated by reference to Exhibit 3.1 of the registrant's current report on Form 8-K filed on February 2,
2009) 3.2 Certificate of Amendment of Certificate of Incorporation of MMR Information Systems, Inc., dated as of July 10, 2009
(incorporated by reference to Exhibit 3.2 of the registrant's current report on Form 8-K filed on July 13, 2009) 3.3 Certificate of Ownership and Merger (incorporated by reference to Exhibit 3.2 of the registrant's current report on Form 8-K filed
on February 2, 2009) 3.4 Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 of the registrant's current report on Form 8-K filed on
October 9, 2007) 4.1 Form of Common Stock Certificate (incorporated by reference to Exhibit 4.1 to registrant's registration statement on Form S-1
(File No. 333-114299) filed on May 11, 2004) 4.2 Amended and Restated Investor Rights Agreement dated March 26, 2004 among the registrant and certain of its stockholders
(incorporated by reference to Exhibit 4.2 to registrant's registration statement on Form S-1 (File No. 333-114299) filed on April 8,
2004) 4.3 Amendment No. 1 to Amended and Restated Investor Rights Agreement dated April 6, 2004 among the registrant and certain
of its stockholders (incorporated by reference to Exhibit 4.3 to registrant's registration statement on Form S-1 (File No. 333-114299) filed on April 8,
2004) 4.4 Securities Purchase Agreement dated March 6, 2006, by and among registrant and the individuals and entities identified on
Exhibit A thereto (incorporated by reference to Exhibit 4.4 of the registrant's current report on Form 8-K filed on March 10, 2006) 4.5 Form of Warrant issued pursuant to the Securities Purchase Agreement dated March 6, 2006, by and among registrant and the
individuals and entities identified on Exhibit A thereto (incorporated by reference to Exhibit 4.5 of the registrant's current report on Form 8-K filed on
March 10, 2006) 4.6 Securities Purchase Agreement dated February 12, 2007, by and among registrant and certain investors (incorporated by
reference to Exhibit 10.1 of the registrant's current report on Form 8-K filed on February 13, 2007) 4.7 Warrant to purchase 250,000 shares of common stock dated December 19, 2006 issued to Kingsbridge Capital Limited
(incorporated by reference to Exhibit 4.1 of the registrant's current report on Form 8-K filed on December 20, 2006) 4.8 Registration Rights Agreement dated December 19, 2006, by and between registrant and Kingsbridge Capital Limited
(incorporated by reference to Exhibit 4.2 of the registrant's current report on Form 8-K filed on December 20, 2006) 4.9 Amendment No. 1 to Registration Rights Agreement dated December 19, 2006, by and between registrant and Kingsbridge
Capital Limited dated August 10, 2007 (incorporated by reference to Exhibit 4.11 of the registrant's quarterly report on Form 10-Q for the quarter
ended June 30, 2007) 4.10 Warrant to purchase 48,834 shares of common stock dated December 30, 2005 issued to General Electric Capital Corporation
(incorporated by reference to Exhibit 4.6 of the registrant's annual report on Form 10-K for the year ended December 31, 2005) 4.11 Warrant to purchase 48,834 shares of common stock dated December 30, 2005 issued to Oxford Finance Corporation
(incorporated by reference to Exhibit 4.7 of the registrant's annual report on Form 10-K for the year ended December 31, 2005) 4.12 Form of Warrant issued to investors in November 2007 registered direct offering (incorporated by reference to Exhibit 4.1 of the
registrant's current report on Form 8-K filed on November 5, 2007) 4.13 Placement Agent Agreement dated November 2, 2007, by and between registrant and Lazard Capital Markets, LLC
(incorporated by reference to Exhibit 1.1 of the registrant's current report on Form 8-K filed on November 5, 2007) 4.14 Warrant to purchase 10,000 shares of common stock dated April 8, 2008 issued to Porter Novelli Life Sciences, LLC
(incorporated by reference to Exhibit 4.13 of the registrant's quarterly report on Form 10-Q for the quarter ended March 31, 2008) 4.15 Form of Warrant issued pursuant to the Creditor Plan dated as of November 8, 2008 by and among registrant,
mymedicalrecords.com, Inc. and Kershaw, Mackie & Co. as the administrative agent (incorporated by reference to Exhibit 4.15 of the
registrant's current report on Form 8-K filed on February 2, 2009) 5.1 Opinion of Stradling Yocca Carlson & Rauth, a Professional Corporation.* 10.1 Amended and Restated 2001 Equity Incentive Plan and Form of Stock Option Agreement thereunder (incorporated by
reference to Exhibit 10.2 to registrant's registration statement on Form S-1 (File No. 333-114299) filed on April 8, 2004) 10.2 2005 Non-Employee Directors' Stock Option Plan, as amended (incorporated by reference to Exhibit 10.4A of the registrant's
annual report on Form 10-K for the year ended December 31, 2006) 10.3 Form of Stock Option Agreement to the 2005 Non-Employee Directors' Stock Option Plan, as amended (incorporated by
reference to Exhibit 10.3 to registrant's registration statement on Form S-1 (File No. 333-114299) filed on January 7, 2005) 10.4 2005 Employee Stock Purchase Plan and Form of Offering Document thereunder (incorporated by reference to Exhibit 10.4 to
registrant's registration statement on Form S-1 (File No. 333-114299) filed on January 7, 2005) 10.5 Creditor Plan dated as of November 8, 2008 by and among registrant, mymedicalrecords.com, Inc. and Kershaw, Mackie
& Co. as the administrative agent. (incorporated by reference to Exhibit 10.1 of the registrant's current report on Form 8-K filed on November
13, 2008) 10.6 Security Agreement dated July 31, 2007 by and between MMR and The RHL Group, Inc. (incorporated by reference to
Exhibit10.6 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.7 Second Amended and Restated Secured Promissory Note dated August 1, 2008 by and between MMR and The RHL Group,
Inc. (incorporated by reference to Exhibit 10.7 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.8 Allonge to RHL Group Promissory Note and Security Agreement dated January 27, 2009 by and between
mymedicalrecords.com, Inc. and The RHL Group, Inc. (incorporated by reference to Exhibit 10.8 of the registrant's current report on Form 8-K filed
on February 2, 2009) 10.9 Form of Indemnity Agreement for the registrant's directors and executive officers (incorporated by reference to Exhibit 10.9 of
the registrant's current report on Form 8-K filed on February 2, 2009) 10.10 Employment Agreement dated as of January 27, 2009 by and among the registrant, MMR and Robert H. Lorsch (incorporated
by reference to Exhibit 10.10 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.11 Form of Employment Agreement dated as of January 27, 2009 by and among the registrant, MMR and Naj Allana (incorporated
by reference to Exhibit10.11 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.12 Amended and Restated Consulting Agreement dated as of January 27, 2009 by and between MMR and The RHL Group, Inc.
(incorporated by reference to Exhibit 10.12 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.13 Marketing and Strategic Planning Agreement dated August 24, 2006 by and between MMR and Hector V. Barreto, Jr.
(incorporated by reference to Exhibit 10.13 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.14 Marketing and Strategic Planning Agreement dated November 23, 2005 by and between MMR and Bernard Stolar
(incorporated by reference to Exhibit 10.14 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.15 Letter Agreement dated December 28, 2007 by and between MMR and The Rebensdorf Group, Inc. (incorporated by reference
to Exhibit 10.15 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.16 Letter Agreement dated as of December 30, 2008 by and between MMR and Richard Teich (incorporated by reference to
Exhibit 10.16 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.17 Letter Agreement dated January 27, 2009 by and among the registrant, MMR and John P. Longenecker (incorporated by
reference to Exhibit 10.17 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.18 Letter Agreement dated January 27, 2009 by and among the registrant, MMR and Tamara A. Seymour (incorporated by
reference to Exhibit 10.18 of the registrant's current report on Form 8-K filed on February 2, 2009) 10.19 Third Amended and Restated Secured Promissory Note dated April 29, 2009 by and between MMR and The RHL Group, Inc.
(incorporated by reference to Exhibit 10.1 of the registrant's current report on Form 8-K filed on May 4, 2009) 10.20 Secured Credit Restructuring Agreement dated April 29, 2009, by and between the registrant, MMR, The RHL Group, Inc. and
Robert H. Lorsch (incorporated by reference to Exhibit 10.2 of the registrant's current report on Form 8-K filed on May 4, 2009) 10.21 Guaranty dated April 29, 2009, made by the registrant in favor of The RHL Group, Inc. (incorporated by reference to Exhibit
10.3 of the registrant's current report on Form 8-K filed on May 4, 2009) 10.22 Addendum dated May 21, 2009 to the Letter Agreement with The Rebensdorf Group, Inc. dated December 27, 2007
(incorporated by reference to Exhibit 10.1 of the registrant's current report on Form 8-K filed on May 27, 2009) 10.23 Stock Option Agreement dated August 6, 2009, by and between MMR Information Systems, Inc. and Robert H. Lorsch
(incorporated by reference to Exhibit 10.1 of the registrant's quarterly report on Form 10-Q filed on August 27, 2009) 10.24 Waiver Agreement, dated August 18, 2009, by and among MMR Information Systems, Inc., MyMedicalRecords, Inc., and The
RHL Group, Inc. (incorporated by reference to Exhibit 10.2 of the registrant's quarterly report on Form 10-Q filed on August 27, 2009) 10.25 Warrant dated August 18, 2009, issued by MMR Information Systems, Inc. in favor of Robert H. Lorsch (incorporated by
reference to Exhibit 10.3 of the registrant's quarterly report on Form 10-Q filed on August 27, 2009) 10.26 Warrant dated August 18, 2009, issued by MMR Information Systems, Inc. in favor of The RHL Group, Inc. (incorporated by
reference to Exhibit 10.4 of the registrant's quarterly report on Form 10-Q filed on August 27, 2009) 10.27 Investment Agreement, dated September 15, 2009, by and between the registrant and Dutchess Equity Fund, L.P.
(incorporated by reference to Exhibit 10.1 of the registrant's current report on Form 8-K filed on September 15, 2009) 10.28 Registration Rights Agreement, dated September 15, 2009, by and between MMR Information Systems, Inc. and Dutchess
Equity Fund, LP. (incorporated by reference to Exhibit 10.2 of the registrant's current report on Form 8-K filed on September 15,
2009) 21.1 Schedule of Subsidiaries (incorporated by reference to Exhibit 21.1 of the registrant's current report on Form 8-K filed on
February 2, 2009) 23.1 Consent of SingerLewak LLP, Independent Registered Public Accounting Firm* 23.2 Consent of Stradling Yocca Carlson & Rauth, a Professional Corporation (see Exhibit 5.1)* 24.1 Power of Attorney (included in the signature pages hereof)* *
CONSOLIDATED STATEMENT OF STOCKHOLDERS' DEFICIT (UNAUDITED)
FOR THE SIX MONTHS ENDED JUNE 30, 2009
Preferred Stock
Common Stock
Treasury Stock
Additional
Accumulated
Shares
Amount
Shares
Amount
Shares
Amount
Paid-In Capital
Deficit
Total
Balance at December 31, 2008, as restated in terms of
shares of Favrille, Inc.
-
$
-
80,629,266
$
80,629
(1,145,324)
$
(16,860)
$
8,300,460
$
(11,152,635)
$
(2,788,406)
Shares issued to RHL Group for extension of guarantee
-
-
328,174
328
-
-
4,672
-
5,000
Reverse merger with Favrille, Inc.
-
-
41,254,550
41,255
-
-
441,346
-
482,601
Reclassification of options and warrants to derivative liabilities
-
-
-
-
-
-
505,981
-
505,981
Shares issued to replace options previously issued
-
-
57,263
57
1,145,324
16,860
(15,451)
-
1,466
Stock based compensation
-
-
-
-
-
-
217,602
-
217,602
Warrants issued for services
-
-
-
-
-
-
252,523
-
252,523
Stock options exercised by RHL Group as a reduction to
related party note payable
-
-
2,461,298
2,461
-
-
110,759
-
113,220
Shares sold
-
-
82,044
82
-
-
3,692
-
3,774
Shares issued for one year rent
-
-
137,000
137
-
-
24,523
-
24,660
Stock option exercises
-
-
1,148,606
1,149
-
-
51,765
-
52,914
Net loss
-
-
-
-
-
-
-
(4,197,446)
(4,197,446)
Balance as of June 30, 2009 (unaudited)
-
$
-
126,098,201
$
126,098
-
$
-
$
9,897,872
$
(15,350,081)
$
(5,326,111)
CONSOLIDATED STATEMENT OF CASH FLOWS
Six Months Ended
June 30,
2009
2008
(Unaudited)
(Unaudited)
Operating activities:
Net loss
$
(4,197,446)
$
(1,686,125)
Adjustments to reconcile net loss to net cash
used in operating activities:
Depreciation and amortization
53,441
74,719
Change in allowance for doubtful accounts
66,668
-
Common stock issued as consideration for extension of guarantee
5,000
-
Common stock issued as replacement of options previously issued
1,466
-
Loss on disposal of property and equipment
2,237
-
Change in valuation of derivative liabilities
655,261
-
Warrants issued for services
252,523
-
Stock based compensation
217,602
5,965
Restricted common stock issued for services
-
104,250
Common stock issued as interest expense on line of credit
-
210,000
Gain on settlement of payables
(89,170)
-
Loss on sale of shares
6,260
-
Loss on write-off of advances due from related party
100,000
-
Loan commitment fee amortization
58,393
-
Effect of changes in:
Accounts receivable
(75,759)
12,825
Related party receivables
22,057
-
Prepaid expenses and other current assets
(19,269)
21,648
Deposits
(5,860)
26,718
Related party payables
278,419
(74,611)
Compensation payable
11,127
115,532
Accounts payable and accrued expenses
1,361,577
410,274
Deferred revenue
(23,985)
56,980
Deferred rent
-
(3,835)
Net cash used in operating activities
(1,319,458)
(725,660)
Investing activities:
Purchase of property and equipment
(8,052)
(3,566)
Patents
(107,087)
-
Restricted cash
(2,394)
-
Cash acquired from reverse merger with Favrille, Inc.
1,050,506
-
Net cash provided by (used in) investing activities
932,973
(3,566)
Financing activities:
Payments of capital lease payable
(3,377)
(4,466)
Proceeds from line of credit, related party
135,000
430,945
Proceeds from issuance of note payable
125,000
-
Proceeds from stock option exercises
52,914
Issuance of common stock
3,774
291,297
Net cash provided by financing activities
313,311
717,776
Net (decrease) in cash
(73,174)
(11,450)
Cash, beginning of period
75,779
11,450
Cash, end of period
$
2,605
$
-
Supplemental disclosures of cash flow information:
Cash paid for interest
$
901
$
9,082
Cash paid for income taxes
$
1,989
$
-
Supplemental disclosure of non-cash investing and financing activities:
Payment of payables through issuance of notes payable
$
139,355
$
-
Capitalized loan commitment fees payable
$
230,000
$
-
Payment of related party line of credit and office rent
through issuance of common stock
$
137,880
$
-
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
JUNE 30, 2009
Six Months Ended June 30, 2009
Stock Options
Warrants
Expected life in years
5 years
1 - 5 years
Stock price volatility
104.72%
102.18% - 179.12%
Risk free interest rate
2.16%
0.60% - 2.54%
Expected dividends
None
None
Forfeiture rate
0%
0%
Year Ended
Operating
Capital
December 31,
Leases
Leases
2009 (remainder of)
$
24,200
$
7,114
2010
-
8,959
2011
-
8,959
Total minimum lease payments
$
24,200
25,032
Less interest portion
(2,693)
$
22,338
Weighted-
Average
Weighted-
Remaining
Average
Contractual
Aggregate
Exercise
Life
Intrinsic
Options
Price
(Years)
Value
Outstanding at December 31, 2008
13,862,228
$
0.47
1.76
$
-
Granted
1,570,000
$
0.18
Exercised
(3,609,913)
$
0.05
Forfeited
(826,065)
$
2.51
Cancelled
(1,202,587)
$
0.00
Outstanding at June 30, 2009 (unaudited)
9,793,663
$
0.46
2.11
$
665,251
Vested and expected to vest
at June 30, 2009 (unaudited)
9,605,631
$
0.47
2.08
$
661,514
Exercisable at June 30, 2009 (unaudited)
7,913,403
$
0.54
1.66
$
627,884
Weighted
Average
Grant Date
Shares
Fair Value
Nonvested at December 31, 2008
1,207,822
$
0.57
Granted
1,570,000
$
0.14
Vested
(696,474)
$
0.50
Forfeited
(201,088)
$
2.28
Nonvested at June 30, 2009 (unaudited)
1,880,260
$
0.11
Options Outstanding
Options Exercisable
Weighted
Weighted
Weighted
Average
Average
Average
Exercise
Number
Remaining
Exercise
Number
Exercise
Price
of Shares
Life (Years)
Price
of Shares
Price
$
0.05 - 0.06
7,072,153
0.89
$
0.05
6,675,867
$
0.05
0.18 - 2.58
2,249,678
5.08
0.47
765,705
1.05
3.88 - 7.40
471,832
6.31
6.59
471,831
6.59
9,793,663
7,913,403
Warrants Outstanding
Warrants Exercisable
Weighted
Weighted
Weighted
Average
Average
Average
Exercise
Number
Remaining
Exercise
Number
Exercise
Price
of Shares
Life (Years)
Price
of Shares
Price
$
0.12 - 0.61
14,631,338
4.59
$
0.15
12,629,774
$
0.15
1.80 - 2.77
4,459,910
3.36
2.77
4,459,910
2.77
3.98 - 5.19
355,378
2.52
4.04
355,378
4.04
5.26 - 6.33
3,016,409
1.68
5.27
3,016,409
5.27
22,463,035
$
1.42
20,461,471
$
1.54
January 27, 2009
March 31, 2009
June 13, 2009
Expected life in years
0.25 - 5.0 yrs
0.07 - 5.59 yrs
0.13 - 5.4 yrs
Stock price volatility
102.42% - 183.93%
103.45% - 178.61%
104.54% - 152.74%
Risk free interest rate
0.13% - 1.59%
0.17% - 1.67%
0.19% - 2.81%
Expected dividends
None
None
None
Forfeiture rate
0%
0%
0%
June 30,
December 31,
2009
2008
Promissory notes payable due to the former officers of MMRIS as part of
severance packages, due in full on August 31, 2009 with no stated interest
$
76,783
$
-
Promissory notes payable due to the two remaining officers of MMRIS pursuant
to the Resignation and Post-Merger Employment Arrangement, due in full on
August 31, 2009 with no stated interest
25,444
-
Promissory notes payable due to vendors relating to settlement of certain
outstanding accounts payable, payable in 18 equal monthly installments
commencing on July 27, 2009 and ending on January 27, 2011, with no stated interest
223,116
-
Promissory notes payable due to PM Creative Corporation, due in full on July
30, 2011, with a 12% per annum stated interest.
125,000
-
450,343
-
Less: current portion
250,971
-
Notes payable, less current portion
$
199,372
$
-
Year Ended
December 31,
2009 (remainder of)
$
176,599
2010
148,744
2011
125,000
Total
$
450,343

![]()
![]()
Number
