Attached files
| file | filename |
|---|---|
| EX-5.1 - EXHIBIT 5.1 - AGA Medical Holdings, Inc. | a2194908zex-5_1.htm |
| EX-3.1 - EXHIBIT 3.1 - AGA Medical Holdings, Inc. | a2194736zex-3_1.htm |
| EX-1.1 - EXHIBIT 1.1 - AGA Medical Holdings, Inc. | a2194908zex-1_1.htm |
| EX-23.1 - EXHIBIT 23.1 - AGA Medical Holdings, Inc. | a2194413zex-23_1.htm |
| EX-23.2 - EXHIBIT 23.2 - AGA Medical Holdings, Inc. | a2194413zex-23_2.htm |
As filed with the Securities and Exchange Commission on October 13, 2009
Registration No. 333-151822
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT No. 11 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AGA MEDICAL HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) |
3845 (Primary Standard Industrial Classification Number) |
41-1815457 (I.R.S. Employer Identification No.) |
||
5050 Nathan Lane North Plymouth, MN 55442 Telephone: (763) 513-9227 (Address, including zip code, and telephone number, including area code, of Registrant's principal executive offices) |
||||
John R. Barr President and Chief Executive Officer AGA Medical Holdings, Inc. 5050 Nathan Lane North Plymouth, MN 55442 Telephone: (763) 513-9227 (Name, address, including zip code, and telephone number, including area code, of agent for service) |
||||
Copies to: |
||
| John B. Tehan, Esq. Kenneth B. Wallach, Esq. Simpson Thacher & Bartlett LLP 425 Lexington Avenue New York, NY 10017 Telephone: (212) 455-2000 Facsimile: (212) 455-2502 |
Alexander D. Lynch, Esq. Weil, Gotshal & Manges LLP 767 Fifth Avenue New York, NY 10153 Telephone: (212) 310-8000 Facsimile: (212) 310-8007 |
|
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
CALCULATION OF REGISTRATION FEE
|
||||||||
| Title of Each Class of Securities Registered |
Amount to Be Registered(1) |
Proposed Maximum Offering Price Per Share |
Proposed Maximum Aggregate Offering Price(2) |
Amount of Registration Fee(3) |
||||
|---|---|---|---|---|---|---|---|---|
Common stock, $0.01 par value |
15,812,500 | $21.00 | $332,062,500 | $15,229.09 | ||||
|
||||||||
- (1)
- Including shares of common stock which may be purchased by the underwriters to cover overallotments, if any. See "Underwriting."
- (2)
- Estimated
solely for the purpose of calculating the registration fee in accordance with Rule 457(a) promulgated under the Securities Act of 1933.
- (3)
- Previously paid.
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. Neither we nor the selling stockholders may sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus dated October 13, 2009
PROSPECTUS
13,750,000 Shares

AGA Medical Holdings, Inc.
Common Stock
This is the initial public offering of our common stock. We are selling 8,500,000 shares of our common stock, and the selling stockholders named in this prospectus, which are controlled by a member of our board of directors, are selling 5,250,000 shares. We will not receive any proceeds from the sale of the shares of our common stock by the selling stockholders.
We currently expect the initial public offering price to be between $19.00 and $21.00 per share. We have applied to have the common stock included for quotation on the Nasdaq Global Market under the symbol "AGAM."
Investing in our common stock involves risks. See "Risk Factors" beginning on page 12.
| |
Per Share
|
Total
|
||
|---|---|---|---|---|
| Public offering price | $ | $ | ||
| Underwriting discount | $ | $ | ||
| Proceeds, before expenses, to AGA Medical | $ | $ | ||
| Proceeds, before expenses, to the selling stockholders | $ | $ |
The underwriters may also purchase up to an additional 2,062,500 shares from us, at the public offering price, less the underwriting discount, within 30 days of the date of this prospectus to cover overallotments, if any.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The shares will be ready for delivery on or about , 2009.
| BofA Merrill Lynch | ||||||||
| Citi | ||||||||
| Deutsche Bank Securities | ||||||||
| Leerink Swann | ||||||||
| Wells Fargo Securities | ||||||||
Natixis Bleichroeder Inc.
The date of this prospectus is , 2009.
You should rely only on the information contained in this prospectus or in any free writing prospectus that we authorize to be delivered to you. We and the selling stockholders have not, and the underwriters have not, authorized anyone to provide you with additional or different information. If anyone provides you with additional, different or inconsistent information, you should not rely on it. We and the selling stockholders are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where an offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, prospects, financial condition and results of operations may have changed since that date.
Through and including (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
i
The following summary highlights certain significant aspects of our business and this offering, but you should read this entire prospectus, including the financial statements and related notes, before making an investment decision. Unless the context otherwise requires, references in this prospectus to (1) "AGA" refer only to AGA Medical Holdings, Inc., the issuer of the common stock offered hereby, (2) "AGA Medical" refer to AGA Medical Corporation, a wholly owned subsidiary of AGA and (3) "we," "us," "our," and the "company" refer collectively to AGA and its consolidated subsidiaries. For ease of understanding of our disclosure, a glossary of technical terms used in this prospectus can be found in Appendix A hereto.
Our Business
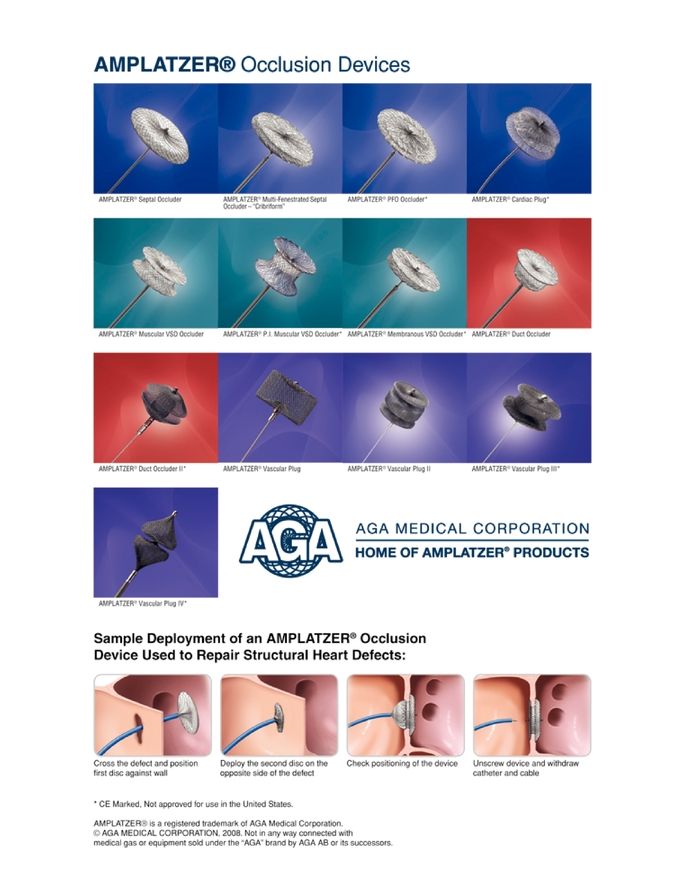
We are a leading innovator and manufacturer of medical devices for the treatment of structural heart defects and vascular diseases. Our AMPLATZER occlusion devices offer minimally invasive, transcatheter treatments that have been clinically shown to be highly effective in defect closure. Our devices and delivery systems use relatively small catheters and can be retrieved and repositioned prior to release from the delivery cable, enabling optimal placement without the need to repeat the procedure or use multiple devices. We are the only manufacturer with occlusion devices approved to close seven different structural heart defects, and we believe we have the leading market positions in the United States and Europe for each of our devices. In 2003, we launched our first vascular products, which are designed to occlude abnormal blood vessels. For the year ended December 31, 2008 and the six months ended June 30, 2009, respectively, we generated net sales of $166.9 million and $94.4 million, earnings before interest, taxes, depreciation and amortization, or EBITDA, of $46.5 million and $17.8 million and net income (loss) of $9.1 million and $(4.2) million.
Our AMPLATZER occlusion devices utilize our expertise in braiding nitinol, a metal alloy with superelastic and shape-memory characteristics, and designing transcatheter delivery systems. While our structural heart defect occlusion devices have historically been used primarily by pediatric cardiologists, we believe that there is a significant opportunity to expand the use of our devices by cardiologists focused on the adult population. We have also leveraged our core competencies to develop products for the treatment of certain vascular diseases, which we sell primarily to interventional radiologists and vascular surgeons. In addition, we are focused on further expanding our product portfolio by developing new products, product enhancements and new applications for our existing products to address:
- •
- Patent Foramen Ovale. There is a growing body of evidence that links the presence of a patent foramen ovale, or PFO, the most common structural heart defect, to certain types of strokes and migraines. By closing the PFO with an occlusion device, we may be able to reduce the incidence of certain types of strokes and migraines. We currently sell our AMPLATZER PFO Occluder outside the United States, representing approximately 12.0% and 15.1% of our net sales for the year ended December 31, 2008 and for the six months ended June 30, 2009, respectively. Our largest clinical trial, the RESPECT study, is being conducted at approximately 60 U.S. sites to assess the impact of our AMPLATZER PFO Occluder in reducing the occurrence of certain types of stroke. Based on statistical models, we believe 500 patients should be sufficient to support a successful outcome in the RESPECT study, and on June 30, 2009, we had enrolled 576 patients. According to decision rules, a successful outcome in the clinical trial is achieved once a claim of superiority of PFO closure versus drug therapy is supported. A successful outcome can be achieved at any time during the study. We intend to continue to enroll up to 900 patients unless a successful outcome is achieved earlier. If we can establish a successful outcome, the next step would be to prepare the submission of our pre-market approval, or PMA, to the U.S. Food and Drug Administration, or FDA, which we would expect to do within
1
- •
- Left Atrial Appendage. We are developing a device to occlude
the left atrial appendage, or LAA, which targets reducing the incidence of stroke in patients with atrial fibrillation, one of the most common cardiac abnormalities in older people. We received CE
Mark clearance in Europe in December 2008 and have initiated marketing of the device in Europe. We also applied to the FDA to begin a clinical trial to support U.S. approval of our AMPLATZER Cardiac
Plug, with an initial indication for LAA occlusion, in August 2008. We estimate that clinical trials in the United States could take
approximately two to three years, after which we would file a PMA application with the FDA. We estimate that the market opportunity for LAA closure with a transcatheter approach worldwide is greater
than $1 billion annually.
- •
- Vascular Grafts. We are developing vascular grafts made from multiple layers of braided nitinol for the transcatheter treatment of aneurysms in a variety of blood vessel sizes. Aneurysms are localized bulges of a blood vessel caused by disease or weakening of the vessel wall. Our initial focus has been on aneurysms that occur in smaller peripheral arteries, such as the iliac arteries, arteries that branch off from the aorta and lead to the legs. We have already designed a tubular graft with the appropriate diameter to be deployed in these arteries. We intend to refile for CE Mark clearance in Europe and to apply to the FDA for an Investigational Device Exemption, or IDE, in the United States in the first half of 2010. We had previously intended to apply for these clearances in the first half of 2009 and, in the case of Europe, filed for a CE Mark in June 2009. We, however, intend to refile in Europe and are delaying our expected filing of the IDE in the United States because we encountered some issues with the delivery system in connection with the final engineering validation of our graft. We estimate that CE Mark review could take 90 to 180 days from the time of submission, and after CE Mark is granted, we would be able to begin marketing the product in Europe. We also estimate that clinical trials in the United States could take approximately two to three years, after which we would file a PMA application with the FDA. We estimate that the market opportunity for the transcatheter treatment of aneurysms in the United States and Europe is greater than $1 billion annually.
three to six months after achieving a successful outcome. Following receipt of a PMA application, the FDA determines whether it is sufficiently complete and, therefore, may be accepted for review. On a statutory basis, the FDA is required to complete a preliminary review of a PMA application within six months. The FDA review process of a PMA application, however, may take up to several years. Once the FDA has approved the PMA application, we would be able to begin marketing the product in the United States. We estimate that the market opportunity for PFO closure in the prevention of certain types of stroke in the United States and Europe is greater than $1 billion annually.
We sell our devices in 112 countries through a combination of direct sales and the use of distributors. In the United States, we market our AMPLATZER family of devices through a direct sales force of 42 representatives who call on interventional cardiologists, vascular surgeons and interventional radiologists. International markets represented 59.2% of our net sales in 2008 and 61.1% of our net sales in the six months ended June 30, 2009, and we anticipate continued growth internationally as we expand our presence in underserved countries, such as China, expand our direct sales presence into additional European countries and selectively convert our distribution to direct sales in certain other countries.
Industry Overview
Structural Heart Defects
A structural heart defect is an abnormality in the structure of the heart and associated vessels, such as the aorta and pulmonary artery. Many structural heart defects result from problems in normal fetal heart development and are referred to as congenital heart defects. Structural heart defects can be
2
clinically significant and require immediate treatment early in life or can become clinically significant later in life when the child reaches adulthood. Two common categories of structural heart defects are (1) septal defects, which consist of a hole in the wall between the atria or ventricles that causes an errant flow of blood in the heart, and (2) failed closure of embryonic passageways, which reroute blood around the lungs in the prenatal heart. Another type of structural heart defect relates to the LAA, a small pouch on the left side of the heart, which is the remnant of the original embryonic left atrium that forms during early development. Atrial fibrillation, a condition that results in irregular electrical activity in the upper chambers of the heart, can cause blood to pool and stagnate in the LAA, increasing the chances of forming clots, which may travel to the brain and lead to stroke. Transcatheter devices can be used to close structural heart defects in lieu of other alternatives such as surgical closure.
Vascular Diseases
There are numerous vascular diseases characterized by defects in the blood vessel wall or abnormal or inappropriate blood flow. For example, aneurysms develop when the integrity and strength of the vessel wall is reduced, causing the vessel wall to progressively expand or balloon out. Peripheral embolization, a widely accepted treatment option for a large range of vascular conditions outside the heart, reduces or eliminates blood flow to an area of the body by blocking, or occluding, a blood vessel. Vascular occlusion can also be used to reroute blood away from inappropriately formed blood vessels to different blood vessels.
The AGA Solution
We develop products for the treatment of structural heart defects and vascular diseases. The performance or efficacy of our products has been documented in over 1,500 clinical publications. We have developed an expertise in the braiding of nitinol and in designing transcatheter delivery systems that enable simple and precise implantation of our AMPLATZER occlusion devices, while also providing the capability for physicians to retrieve and reposition the device during the procedure if they are not satisfied with its placement. Our AMPLATZER family of devices uses nitinol because its properties allow our devices to be compressed inside a delivery sheath and then return to their original shape once deployed at the implant site.
AMPLATZER Structural Heart Defect Occluders
We believe that our AMPLATZER structural heart defect occlusion devices offer the following advantages over our competitors' devices and open-heart surgery:
- •
- Easier to Implant, Retrieve and Reposition. We believe that
our AMPLATZER occlusion devices are easier to implant than our competitors' devices and are the only fully retrievable and repositionable occluders on
the market. The ability to retrieve and reposition the devices during the same procedure eliminates the need to remove the occlusion device and the catheter, which minimizes potential trauma to the
patient, potential complications with the procedure and disposal of damaged devices that could not be properly deployed.
- •
- Highly Effective Closure Rates. Our AMPLATZER occlusion devices have
consistently been shown to be highly effective in closing structural heart defects. Clinical publications have reported
closure rates of approximately 96% for our AMPLATZER ASD, or atrial septal defect, and PFO Occluders. Our occlusion devices have a long history of
durability as evidenced by some devices having been implanted in patients for over 13 years.
- •
- Minimally Invasive Procedures. All of our devices are inserted into the human body through a small catheter via the femoral artery in the patient's groin and then travel through the body's vasculature to the heart. This transcatheter approach minimizes blood loss, trauma and other
3
- •
- Cost Efficient. The minimally invasive nature of the procedures required to implant our AMPLATZER occlusion devices reduces costs by taking advantage of shorter hospital stays and reduced therapy and follow-up care requirements. In the United States, the average open-heart surgery procedure costs approximately $15,000 to $30,000, while the average total procedure cost to implant one of our AMPLATZER occlusion devices is generally less than $12,000, including device and hospital costs.
surgical complications associated with invasive open-heart surgery. Most patients have the procedure done on an outpatient or overnight basis and do not have to endure the lengthy two- to three-month recovery process required following open-heart surgery.
AMPLATZER Vascular Products
We are leveraging our expertise in nitinol braiding and our proficiency in the design of transcatheter delivery systems to develop products for the treatment of vascular diseases. We believe our existing vascular products and vascular products in our pipeline address a number of conditions characterized by large patient populations and existing therapies with significant shortcomings. Our initial vascular products seek to occlude abnormal blood vessels with our family of AMPLATZER Vascular Plugs and treat aneurysms in small arteries, such as the iliac arteries, and larger vessels, such as the thoracic and abdominal portions of the aorta, with our family of AMPLATZER Vascular Grafts.
Business Strategy
We seek to remain a leader in the innovation and manufacture of occlusion devices for the treatment of structural heart defects and to leverage our core competencies into leading positions in new markets. To accomplish this objective, we intend to:
- •
- grow our core business of structural heart defect occlusion and vascular devices;
- •
- capitalize on the PFO market opportunity;
- •
- expand and commercialize our research and development pipeline; and
- •
- continue to strengthen our global distribution.
Investment Risks
An investment in our common stock involves substantial risks and uncertainties. Some of the more significant risks include those associated with the following:
- •
- our ability to implement our business strategy;
- •
- regulatory approval and market acceptance of our new products, product enhancements or new applications for existing
products;
- •
- regulatory developments in key markets for our AMPLATZER occlusion
devices;
- •
- the success of our clinical trials; the success of existing and future research and development programs;
- •
- our ability to protect our intellectual property;
- •
- intellectual property claims exposure, related litigation expense, and any resultant damages, awarded royalties or other
remedies, in particular resulting from our Medtronic and Occlutech litigations;
- •
- demand for our products; product liability claims exposure;
4
- •
- failure to comply with laws and regulations, including the U.S. Foreign Corrupt Practices Act; changes in general economic
and business conditions; and
- •
- changes in currency exchange rates and interest rates.
You should carefully consider the matters described under "Risk Factors" before investing in our common stock. Any adverse effect on our business, financial condition, results of operations or cash flow could cause the trading price of our common stock to decline and could result in a partial or total loss of your investment.
Recent Developments
Effective January 1, 2009, we began direct distribution in Canada, Portugal, France, Belgium and the Netherlands as we purchased on such date the distribution rights, inventory and intangible assets from our distributors in these countries. The aggregate purchase price of these acquisitions totaled $10.8 million, consisting of cash payments of $6.1 million, the discounted value of $1.4 million in additional guaranteed payments and the discounted value of up to $3.3 million in additional contingent payments if certain revenue goals are achieved. On April 1, 2009, we paid our former French distributor $1.4 million in such additional guaranteed payments.
Effective January 1, 2009, we began direct distribution in Italy as a result of our purchase on January 8, 2009 of certain distribution rights, inventory, equipment, intangible assets and goodwill from our former Italian distributor. The aggregate purchase price was $41.0 million, consisting of cash payments of $26.6 million, the discounted value of $9.2 million in additional guaranteed payments and the discounted value of up to $5.2 million in additional contingent payments if certain revenue goals are achieved during the first three years following completion of the acquisition. On April 1, 2009, we paid our former Italian distributor $2.0 million in such additional contingent payments.
On January 5, 2009, in order to finance, in part, the acquisition of the assets of our former Italian distributor, AGA Medical issued to WCAS Capital Partners IV, L.P., one of our stockholders, for an aggregate purchase price of $15.0 million (1) $15.0 million in aggregate principal amount of 10% senior subordinated PIK notes due 2012, or the 2009 notes, and (2) 1,879 shares of Series B preferred stock. The 2009 notes are fully and unconditionally guaranteed by Amplatzer Medical Sales Corporation.
With respect to our patent litigation with Medtronic, on August 5, 2009, a jury returned a verdict that the subject AMPLATZER occluder and vascular plug products infringed two Medtronic patents and that the Medtronic patent claims at issue had not been proven by us to be invalid. The jury verdict awarded Medtronic damages of $57.8 million. This amount is equal to 11% of historical sales of the occluder and vascular plug products in question during the timeframe specified for each patent. Following the jury verdict, the court scheduled the non-jury phase of the trial for early December 2009, which will deal with other issues of invalidity and unenforceability. Upon conclusion of the non-jury phase of the trial, the court will consider post-trial motions and enter a judgment, which we expect will take place in early 2010. As part of its decision, the trial court could order a new trial on some or all of the issues in the case or amend or reaffirm the jury verdict based on one or more issues regarding infringement, validity, enforceability and damages. We also expect the trial court to decide whether any royalty payments relating to the patent that does not expire until 2018 are due for future periods and, if so, at what rate. Thereafter, the judgment will be subject to appeal by each party. See "Business—Legal Proceedings."
Notwithstanding the litigation proceedings, we are in the process of implementing changes to our business processes that will modify the final step in our manufacturing processes prior to inspection and the instructions for use of our products by physicians. These changes are intended to create an alternative process that we believe does not infringe Medtronic's patents. In addition, we intend to initiate a process to establish manufacturing operations in Europe for all of our devices that are sold in
5
international markets within 12 to 18 months. In addition to providing other benefits for our business, such as mitigating the risk inherent in relying on only one manufacturing facility, we believe the manufacture of our products outside of the United States would eliminate any claims for ongoing royalty payments on future sales with respect to such products sold outside of the United States.
Our executive offices are located at 5050 Nathan Lane North, Plymouth, MN 55442, and its telephone number is (763) 513-9227. We maintain a website at http://www.amplatzer.com. Information contained on our website or that can be accessed through our website does not constitute a part of this prospectus.
6
Common stock offered by AGA |
8,500,000 shares | |
Selling shareholders |
Gougeon Shares, LLC and Franck L. Gougeon Revocable Trust Under Agreement Dated June 28, 2008, which are beneficially owned by Franck L. Gougeon, a member of our board of directors. |
|
Common stock offered by the selling stockholders |
5,250,000 shares |
|
Overallotment option |
The underwriters may also purchase up to an additional 2,062,500 shares from us, at the public offering price, less the underwriting discount, within 30 days of the date of this prospectus to cover overallotments, if any. |
|
Common stock to be outstanding after this offering |
48,055,683 shares (or 50,118,183 if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any) |
|
Use of proceeds |
We estimate that the net proceeds to us from this offering, after deducting underwriting discounts and estimated offering expenses, will be approximately $154.2 million, assuming the shares are offered at $20.00 per share, which is the mid-point of the estimated offering price range set forth on the cover page of this prospectus (or approximately $192.7 million if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any). We will not receive any of the proceeds from the sale of shares by the selling stockholders. A member of our board of directors controls the selling stockholders. See "Principal and Selling Stockholders." |
|
|
We intend to use $65.0 million of our net proceeds from this offering to prepay the principal amount of our 10% senior subordinated PIK notes due 2012 that were issued in 2005, or the 2005 notes, and the 2009 notes. In addition, we will use a portion of our net proceeds from this offering to pay accrued and unpaid interest on the 2005 notes and the 2009 notes. We intend to use approximately $47.9 million of our net proceeds from this offering to pay all accrued and unpaid dividends on our Class A common stock and Series A and Series B preferred stock, which will be converted into our common stock immediately prior to completion of this offering. We intend to use $25.0 million of our net proceeds from this offering to repay indebtedness outstanding under our revolving credit facility. We intend to use any remaining proceeds for working capital and general corporate purposes. See "Use of Proceeds." |
|
Dividend policy |
We do not intend to declare or pay dividends on our common stock in the foreseeable future. See "Dividend Policy." |
|
Proposed Nasdaq Global Market symbol |
"AGAM" |
7
Conflicts of Interest |
Affiliates of certain of the underwriters will receive a portion of the net proceeds from this offering and may be deemed to have a "conflict of interest" with us and the selling stockholders. For more information, see "Conflicts of Interest." |
Unless we indicate otherwise or the context requires, all information in this prospectus:
- •
- assumes (1) no exercise of the underwriters' overallotment option to purchase additional shares of our common stock
and (2) an initial public offering price of $20.00 per share, the midpoint of the initial public offering price range indicated on the cover of this prospectus;
- •
- gives effect to (1) the conversion of our Class A and Class B common stock and all of our
Series A and Series B preferred stock into our common stock immediately prior to completion of this offering; and (2) the 7.15 for 1.00 reverse stock split of our common stock
that will occur immediately prior to completion of this offering; and
- •
- does not reflect (1) 2,897,637 shares of our common stock issuable upon the exercise of outstanding stock options at a weighted average exercise price of $10.18 per share as of June 30, 2009, 1,306,834 of which were then exercisable; (2) approximately 46,152 shares of our common stock issuable upon the exercise of stock options that we expect to grant to certain of our directors in connection with this offering at an exercise price equal to the offering price, none of which will then be exercisable; and (3) approximately 2,510,755 shares of our common stock reserved for future grants under our 2008 Equity Incentive Plan and our Employee Stock Purchase Plan, which is being adopted in connection with this offering.
8
Summary Consolidated Financial Data
The following table sets forth our summary consolidated financial data for the periods indicated. We derived the summary consolidated financial data presented below as of December 31, 2007 and 2008 and for the years ended December 31, 2006, 2007 and 2008 from our audited consolidated financial statements included elsewhere in this prospectus. We derived the consolidated summary financial data for the six months ended June 30, 2008 and 2009 from our unaudited consolidated interim financial statements included elsewhere in this prospectus. We have prepared the unaudited consolidated interim financial information set forth below on the same basis as our audited consolidated financial statements and have included all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair presentation of our financial position and operating results for such periods. The interim results set forth below are not necessarily indicative of results for the year ended December 31, 2009 or for any other period.
The pro forma basic and diluted weighted average shares and pro forma basic and diluted net loss per common share data in the summary consolidated financial data table presented below are unaudited and give effect to the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering.
The as adjusted balance sheet data as of June 30, 2009 and as adjusted basic and diluted net income per share data for the year ended December 31, 2008 and the six months ended June 30, 2008 are unaudited and give effect to (1) the conversion of our Class A and Class B common stock and all of our Series A and Series B preferred stock into our common stock immediately prior to consummation of this offering, (2) the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering, (3) the application of $68.2 million of our net proceeds from this offering to prepay the principal and accrued and unpaid interest on the 2005 notes and the 2009 notes, (4) the application of $47.9 million of our net proceeds from this offering to pay accrued and unpaid dividends on our Class A common stock and Series A and Series B preferred stock and (5) the application of $25.0 million of our net proceeds from this offering to repay indebtedness outstanding under our revolving credit facility, as if each had occurred as of June 30, 2009 in the case of the as adjusted balance sheet date, and December 31, 2008, in the case of the as adjusted basic and diluted net income per share. The as adjusted consolidated summary financial data is not necessarily indicative of what our financial position or results of operations would have been if this offering had been completed as of the date indicated, nor is such data necessarily indicative of our financial position or results of operations for any future date or period.
Our historical results are not necessarily indicative of future operating results. The following summary and as adjusted financial data should be read in conjunction with, and are qualified in their entirety by reference to, "Selected Consolidated Financial Data," "Use of Proceeds," "Capitalization," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and the related notes included elsewhere in this prospectus.
9
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands, except per share data) |
2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||
| |
|
|
|
(unaudited) |
|||||||||||||
Statement of Operations Data: |
|||||||||||||||||
Net sales |
$ | 127,529 | $ | 147,255 | $ | 166,896 | $ | 80,845 | $ | 94,381 | |||||||
Cost of goods sold |
24,985 | 22,819 | 26,635 | 12,456 | 17,004 | ||||||||||||
Gross profit |
102,544 | 124,436 | 140,261 | 68,389 | 77,377 | ||||||||||||
Operating expenses: |
|||||||||||||||||
Selling, general and administrative |
37,515 | 50,190 | 65,669 | 31,757 | 46,456 | ||||||||||||
Research and development |
12,096 | 26,556 | 32,760 | 16,210 | 16,477 | ||||||||||||
Amortization of intangible assets |
12,682 | 15,233 | 15,540 | 7,690 | 9,894 | ||||||||||||
Change in purchase consideration |
— | — | — | — | (698 | ) | |||||||||||
FCPA settlement |
— | 2,000 | — | — | — | ||||||||||||
Loss (gain) on disposal of property and equipment |
709 | (3 | ) | 68 | 14 | (26 | ) | ||||||||||
Operating income (loss) |
39,542 | 30,460 | 26,224 | 12,718 | 5,274 | ||||||||||||
Interest income and investment and other income (loss), net |
2,885 | 669 | (250 | ) | (222 | ) | (1,016 | ) | |||||||||
Interest income—related party |
— | 6 | — | — | — | ||||||||||||
Interest expense |
(22,893 | ) | (21,213 | ) | (16,492 | ) | (8,595 | ) | (8,149 | ) | |||||||
Income (loss) before income taxes |
19,534 | 9,922 | 9,482 | 3,901 | (3,891 | ) | |||||||||||
Provision for income taxes |
(6,909 | ) | (3,844 | ) | (386 | ) | (1,548 | ) | (306 | ) | |||||||
Net income (loss) |
12,625 | 6,078 | 9,096 | 2,353 | (4,197 | ) | |||||||||||
Series A and Series B preferred stock and Class A common stock dividends |
(59,410 | ) | (15,372 | ) | (17,067 | ) | (8,815 | ) | (8,471 | ) | |||||||
Net loss applicable to common stockholders |
$ | (46,785 | ) | $ | (9,294 | ) | $ | (7,971 | ) | $ | (6,462 | ) | $ | (12,668 | ) | ||
Net loss per common share—basic and diluted |
$ | (0.28 | ) | $ | (0.06 | ) | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.08 | ) | ||
Weighted average shares—basic and diluted |
167,000 | 161,236 | 153,600 | 153,600 | 153,600 | ||||||||||||
Pro forma net loss per common share—basic and diluted (unaudited)(1) |
$ | (2.00 | ) | $ | (0.41 | ) | $ | (0.37 | ) | $ | (0.30 | ) | $ | (0.59 | ) | ||
Pro forma weighted average shares—basic and diluted (unaudited)(1) |
23,356 |
22,550 |
21,482 |
21,482 |
21,482 |
||||||||||||
As adjusted net income per common share—basic and diluted (unaudited) |
0.27 | (0.02 | ) | ||||||||||||||
Weighted average shares used in computing as adjusted net income per common share—basic and diluted (unaudited) |
48,059 | 48,056 | |||||||||||||||
Other Data: |
|||||||||||||||||
Depreciation and amortization |
$ | 15,875 | $ | 19,953 | $ | 20,741 | $ | 10,433 | $ | 13,571 | |||||||
EBITDA(2) |
57,128 | 50,656 | 46,485 | 22,819 | 17,768 | ||||||||||||
| |
|
As of December 31, | As of June 30, 2009 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
2007 | 2008 | Actual | As Adjusted | |||||||||||
| |
|
|
|
(unaudited) |
||||||||||||
(in thousands) |
||||||||||||||||
Balance Sheet Data: |
||||||||||||||||
Cash and cash equivalents |
$ | 13,854 | $ | 22,808 | $ | 8,798 | $ | 25,096 | ||||||||
Working capital |
16,454 | 30,546 | 22,563 | 42,095 | ||||||||||||
Total assets |
256,015 | 272,328 | 326,282 | 339,280 | ||||||||||||
Long-term debt, less current portion |
242,600 | 253,442 | 291,420 | 206,036 | ||||||||||||
Redeemable convertible Series A and Series B preferred and Class A common stock |
158,701 | 174,571 | 184,922 | — | ||||||||||||
Total stockholders' deficit |
(217,769 | ) | (226,458 | ) | (237,058 | ) | 49,479 | |||||||||
(footnote on the following page)
10
- (1)
- Pro
forma presentation gives effect to the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this
offering.
- (2)
- EBITDA
represents net income (loss) before interest income, interest income—related party, interest expense, provision (benefit) for income tax,
and depreciation and amortization. We present EBITDA because we believe it is a useful indicator of our operating performance. Our management uses EBITDA principally as a measure of our operating
performance and believes that EBITDA is useful to investors because it is frequently used by securities analysts, investors and other interested parties in their evaluation of the operating
performance of companies in industries similar to ours. We also believe EBITDA is useful to our management and investors as a measure of comparative operating performance from period to period. Our
management also uses EBITDA for planning purposes, including the preparation of our annual operating budget and financial projections.
EBITDA, however, does not represent and should not be considered as an alternative to net income or cash flow from operations, as determined in accordance with GAAP, and our calculations thereof may not be comparable to similarly entitled measures reported by other companies. Although we use EBITDA as a measure to assess the operating performance of our business, EBITDA has significant limitations as an analytical tool because it excludes certain material costs. For example, it does not include interest expense, which has been a necessary element of our costs. Because we use capital assets, depreciation expense is a necessary element of our costs and ability to generate revenue. In addition, the omission of the substantial amortization expense associated with our intangible assets further limits the usefulness of this measure. EBITDA also does not include the payment of taxes, which is also a necessary element of our operations. Because EBITDA does not account for these expenses, its utility as a measure of our operating performance has material limitations. Because of these limitations management does not view EBITDA in isolation or as a primary performance measure and also uses other measures, such as net income, net sales, units sold and operating income, to measure operating performance.
The following is a reconciliation of EBITDA to net income for the periods presented:
| |
Year Ended December 31, | Six Months Ended June 30, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands) |
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
Net income (loss) |
$ | 12,625 | $ | 6,078 | $ | 9,096 | $ | 2,353 | $ | (4,197 | ) | |||||
Interest income |
(1,174 | ) | (426 | ) | (230 | ) | (110 | ) | (61 | ) | ||||||
Interest income—related party |
— | (6 | ) | — | — | — | ||||||||||
Interest expense |
22,893 | 21,213 | 16,492 | 8,595 | 8,149 | |||||||||||
Provision for income taxes |
6,909 | 3,844 | 386 | 1,548 | 306 | |||||||||||
Depreciation and amortization |
15,875 | 19,953 | 20,741 | 10,433 | 13,571 | |||||||||||
EBITDA |
$ | 57,128 | $ | 50,656 | $ | 46,485 | $ | 22,819 | $ | 17,768 | ||||||
11
Investing in our common stock involves risks. You should carefully consider the following risks as well as the other information included in this prospectus, including our financial statements and related notes, before investing in our common stock.
Risks Related to Our Business
If we do not successfully implement our business strategy, our business and results of operations will be adversely affected.
We may not be able to successfully implement our business strategy. Any such failure may adversely affect our business and results of operations. For example, to implement our business strategy we need to, among other things, develop and introduce new products, find new applications for our existing products, obtain regulatory approval for such new products and applications and educate physicians about the clinical and cost benefits of our products and thereby increase the number of hospitals and physicians that use our products. In addition, we are seeking to increase our international sales and will need to increase our worldwide direct sales force and enter into distribution agreements with third parties in order to do so, all of which may also result in additional or different foreign regulatory requirements, with which we may not be able to comply. Moreover, even if we successfully implement our business strategy, our operating results may not improve. We may decide to alter or discontinue aspects of our business strategy and may adopt different strategies due to business or competitive factors.
The market opportunities that we expect to develop for our products may not be as large as we expect or may not develop at all.
The growth of our business is dependent, in large part, upon the development of market opportunities for our new products, product enhancements and new applications for our existing products. The market opportunities that we expect to exist for our devices may not develop as expected, or at all. For example, clinical studies have shown linkages between the existence of PFOs and certain types of strokes and migraines. If the connection between PFO closure and the prevention or reduction of the occurrence of stroke and migraines is not as strong as we anticipate, the market opportunity for our AMPLATZER PFO Occluders will not develop as expected, if at all. Moreover, even if the market opportunities develop as expected, new technologies and products introduced by our competitors may significantly limit our ability to capitalize on any such market opportunity. Our failure to capitalize on our expected market opportunities would adversely effect our growth.
Our AMPLATZER Septal Occluders generate a large portion of our net sales. If sales of this family of products were to decline, our net sales and results of operations would be adversely affected.
Our lead family of products, the AMPLATZER Septal Occluders, represented approximately 55.4% of our net sales for the six months ended June 30, 2009, and we anticipate that this family of products will continue to account for a substantial portion of our net sales for the next few years. If sales of AMPLATZER Septal Occluders were to decline in any of our key markets because of decreased demand, adverse regulatory actions, patent infringement claims, failure to protect our intellectual property, manufacturing problems or delays, pricing pressures, competitive factors or any other reason, our net sales would decrease, which would negatively affect our business, financial condition and results of operations.
12
If we are unable to successfully develop and market new products or product enhancements or find new applications for our existing products, we will not remain competitive.
Our future success and our ability to increase net sales and earnings depend, in part, on our ability to develop and market new products, product enhancements and new applications for our existing products. However, we may not be able to, among other things:
- •
- successfully develop or market new products or enhance existing products;
- •
- find new applications for our existing products;
- •
- manufacture, market and distribute such products in a cost-effective manner; or
- •
- obtain required regulatory clearances and approvals.
Our failure to do any of the foregoing could have a material adverse effect on our business, financial condition and results of operations. In addition, if any of our new or enhanced products contain undetected errors or design defects or if new applications that we develop for existing products do not work as planned, our ability to market these products could be substantially impeded, resulting in lost net sales, potential damage to our reputation and delays in obtaining market acceptance of these products. We cannot assure you that we will continue to successfully develop and market new or enhanced products or new applications for our existing products.
We make our regulatory status forecasts, including determining expected dates of filings with, or submissions to, relevant authorities, based on the information currently available to us. The actual timing for any of these regulatory steps may vary, and we may revise any such forecasts as new information becomes available.
Moreover, most new or enhanced products or new applications for our existing products require that their safety and efficacy be proven by clinical trials before they receive regulatory approval. Our clinical trials may not prove the safety and efficacy of our products, and in such circumstances our products would not receive regulatory approval. In addition, these clinical trials typically last several years, and during that time competing products, procedures or therapies may be introduced that are less expensive and/or more effective than our products and thus render our products obsolete. If we do not continue to expand our product portfolio on a timely basis or if those products and applications do not receive regulatory and market acceptance or become obsolete, we will not grow our business as we currently expect.
If we fail to educate and train physicians as to the distinctive characteristics, benefits, safety, clinical efficacy and cost-effectiveness of our products, our sales will not grow.
Acceptance of our products depends, in large part, on our ability to (1) educate the medical community as to the distinctive characteristics, benefits, safety, clinical efficacy and cost-effectiveness of our products compared to alternative products, procedures and therapies and (2) train physicians in the proper use and implementation of our devices. Certain of the structural heart defects and vascular diseases that can be treated by our devices can also be treated by surgery, drugs or other medical devices, some of which have a longer history of use and are more widely used by the medical community. Physicians may be reluctant to change their medical treatment practices for a number of reasons, including:
- •
- lack of experience with new products;
- •
- lack of evidence supporting additional patient benefits;
- •
- perceived liability risks generally associated with the use of new products and procedures;
13
- •
- lack of availability of adequate reimbursement within healthcare payment systems; and
- •
- costs associated with the purchase of new products and equipment.
Convincing physicians to dedicate the time and energy necessary to properly train to use new devices is challenging, and we may not be successful in these efforts. If physicians are not properly trained, they may misuse or ineffectively use our products. Such misuse or ineffective use may result in unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us. Accordingly, even if our devices are superior to alternative treatments, our success will depend on our ability to gain and maintain market acceptance for our devices. If we fail to do so, our sales will not grow and our business, financial condition and results of operations will be adversely affected.
The expansion of our product portfolio is dependent upon the success of our clinical trials and receipt of regulatory approvals. If these trials are not completed on schedule or are unsuccessful, or if we fail to obtain or experience significant delays in obtaining the necessary regulatory approvals for our product pipeline, we will not be able to market the related products.
A number of our products are in the early stages of development. In the United States, before we can market a new medical device, or a new application of, claim for, or significant modification to, an existing device, we must first receive either approval of a PMA application from the FDA or clearance under section 510(k) of the U.S. Federal Food, Drug, and Cosmetic Act, or 510(k) clearance, unless an exemption applies. Clinical trials are always required to support a PMA application approval and may be required to support a 510(k) clearance. Currently, we have four studies underway designed to evaluate the safety and efficacy of our AMPLATZER PFO Occluder to treat migraine or recurrent stroke, as applicable, in patients with PFOs, as well as a number of post-approval studies.
Our current or future clinical trials contemplated in support of our PMA or 510(k) applications may not commence or conclude in a timely fashion, or at all, or may not produce the desired results. For example, several of our products under development do not yet have agreed-upon protocols or approved Investigational Device Exemptions, or IDEs. Agreeing on clinical trial designs and protocols may be time consuming and requires interaction with and advance approval from regulatory authorities. We cannot assure you that we will be able to agree on appropriate trial designs and protocols with the FDA and thus commence clinical trials or, if commenced, that our PMA applications will be approved or our 510(k) clearances will be granted, in a timely fashion or at all. If our trials for any reason do not commence, do not produce the intended results or are delayed or halted due to the occurrence of adverse events, or if we do not otherwise obtain FDA or other regulatory agency approval with respect to our products in a timely fashion, our future growth may be significantly hampered. Our failure to comply with the regulations relating to the PMA approval and 510(k) clearance processes could also lead to the issuance of warning letters, injunctions, consent decrees, manufacturing suspensions, loss of regulatory approvals, product recalls, termination of distribution arrangements or product seizures. In the most egregious cases, criminal sanctions or closure of our manufacturing facilities could be imposed.
Moreover, sales of our products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. Because a significant portion of our product sales are made in international markets, any failure to comply with directives and regulatory requirements imposed in foreign jurisdictions could also have a material adverse effect on our business, financial condition and results of operations.
Further, we continually evaluate the potential financial benefits and costs of our clinical trials and the products being evaluated in them. If we determine that the costs associated with attaining regulatory approval of a product exceed the potential financial benefits of that product or if the projected development timeline is inconsistent with our investment strategy, we may choose to stop a
14
clinical trial or the development of a particular product, enhancement or application, which could have a material adverse effect on the growth of our business and could result in a charge to our earnings.
We depend on clinical investigators and clinical sites to enroll patients in our clinical trials and on other third-party contract research organizations to manage our clinical trials and to perform related data collection and analysis, and as a result, we may face significant costs and delays that are outside of our control.
We rely on clinical investigators and clinical sites to enroll patients in our clinical trials and other third-party contract research organizations to manage our clinical trials and to perform related data collection and analysis. Our agreements with clinical investigators, clinical sites and other third parties for clinical testing place substantial responsibilities on these parties. If clinical investigators, clinical sites or other third parties do not carry out their contractual duties or fail to meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to our clinical protocols or the FDA's good clinical practice regulations, our clinical trials may be extended, delayed or terminated, we may face significant costs and we may be unable to obtain regulatory approval or clearance for, or successfully commercialize, new products, enhancements or applications, in a timely manner, or at all.
We also compete with other manufacturers of medical devices for investigators and clinical sites to conduct clinical trials. If we are unable to identify investigators and clinical sites on a timely and cost-effective basis, our ability to conduct trials of our products and, therefore, our ability to obtain required regulatory approval or clearance would be adversely affected.
We may be subject to compliance action, penalties or injunctions if we are determined to be promoting the use of our products for unapproved, or off-label, uses.
Our products are currently approved for the treatment of certain structural heart defects and vascular diseases. Pursuant to FDA regulations, we can only market our products in the United States for approved uses. Physicians may use our products for indications other than those cleared or approved by the FDA, even though we do not promote our products for such off-label uses. If the FDA, however, determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our training or promotional materials or could subject us to regulatory enforcement actions, including the issuance of warning letters, injunctions, consent decrees, seizures, civil fines or criminal penalties. Other federal, state or foreign enforcement authorities might also take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties from other statutory authorities.
We operate in a very competitive environment.
The medical device industry is characterized by strong competition. We have several competitors, including Boston Scientific Corporation, NMT Medical, Inc., W. L. Gore & Associates, Inc., St. Jude Medical Inc., Cook, Inc., Occlutech GmbH, Cardia, Inc. and Atritech, Inc. Certain of our competitors have substantially greater capital resources, larger customer bases, broader product lines, larger sales forces, greater marketing and management resources, larger research and development staffs and larger facilities than ours and have more established reputations with our target customers, as well as global distribution channels that may be more effective than ours.
Our competitors may develop and offer technologies and products that are safer or more effective, have better features, are easier to use, less expensive or more readily accepted by the marketplace than ours. Their products could make our technology and products obsolete or noncompetitive. Our competitors may also be able to achieve more efficient manufacturing and distribution operations than we may be able to and may offer lower prices than we could offer profitably. We may decide to alter or
15
discontinue aspects of our business and may adopt different strategies due to business or competitive factors or factors currently unforeseen, such as the introduction by our competitors of new products or new medical technologies that would make our products obsolete or uncompetitive.
In addition, consolidation in the medical device industry could make the competitive environment more difficult. The industry has recently experienced some consolidation, and there is a risk that larger companies will enter our markets.
We depend on third-party distributors to market and sell our products internationally in a number of markets. Our business, financial condition and results of operations may be adversely affected by both our distributors' performance and our ability to maintain these relationships on terms that are favorable to us.
We depend, in part, on third-party distributors to sell our medical devices outside the United States, including in China. In 2008 and the six months ended June 30, 2009, our net sales through third-party distributors were 34.9% and 19.0%, respectively, of our total net sales. Our international distributors operate independently of us, and we have limited control over their operations, which exposes us to significant risks. Distributors may not commit the necessary resources to market and sell our products and may also market and sell competitive products. In addition, our distributors may not comply with the laws and regulatory requirements in their local jurisdictions, which may limit their ability to market or sell our products. If current or future distributors do not perform adequately, or if we are unable to locate competent distributors in particular countries and secure their services on favorable terms, or at all, we may be unable to increase or maintain our level of net sales in these markets or enter new markets, and we may not realize our expected international growth.
The terms and effects of our Deferred Prosecution Agreement with the U.S. Department of Justice relating to potential violations of the U.S. Foreign Corrupt Practices Act may negatively affect our business, financial condition and results of operations.
On June 2, 2008, we entered into a Deferred Prosecution Agreement, or the DPA, with the Department of Justice concerning alleged improper payments that were made by our former independent distributor in China to (1) physicians in Chinese public hospitals in connection with the sale of our products and (2) an official in the Chinese patent office in connection with the approval of our patent applications, in each case, in potential violation of the Foreign Corrupt Practices Act, or the FCPA. The FCPA makes it unlawful for, among other persons, a U.S. company, acting directly or through an agent, to offer or to make improper payments to any "foreign official" in order to obtain or retain business or to induce such "foreign official" to use his or her influence with a foreign government or instrumentality thereof for such purpose.
As part of the DPA, we consented to the Department of Justice filing a two-count criminal statement of information against us in the U.S. District Court, District of Minnesota, which was filed on June 3, 2008. The two counts include a conspiracy to violate the FCPA and a substantive violation of the anti-bribery provisions of the FCPA related to the above-described activities in China. Although we did not plead guilty to that information, we accepted responsibility for the acts of our employees and agents as set forth in the DPA, and we face prosecution under that information, and possibly other charges as well, if we fail to comply with the terms of the DPA. Those terms require us to, for approximately three years, (1) continue to cooperate fully with the Department of Justice on any investigation relating to violations of the FCPA and any and all other matters relating to improper payments, (2) continue to implement a compliance and ethics program designed to detect and prevent violations of the FCPA and other applicable anti-corruption laws, (3) review existing, and if necessary, adopt new controls, policies and procedures designed to ensure that we make and keep fair and accurate books, records and accounts and maintain a rigorous anti-corruption compliance code designed to detect and deter violations of the FCPA and other applicable anti-corruption laws, and (4) retain and pay for an independent monitor to assess and oversee our compliance and ethics
16
program with respect to the FCPA and other applicable anti-corruption laws. The DPA also required us to pay a monetary penalty of $2.0 million. In the fourth quarter of 2007, we had recorded a financial charge of $2.0 million for this expected settlement, which was paid in June 2008. The terms of the DPA will remain binding on any successor or merger partner as long as the agreement is in effect.
The effects that compliance with any of the terms of the DPA will have on us are unknown and they may have a material impact on our business, financial condition and results of operations. The activities of the government-approved independent monitor, as well as the continued implementation of a compliance and ethics program and the adoption of internal controls, policies and procedures to detect and prevent future violations of the FCPA and other applicable anti-corruption laws, may result in increased costs to us and change the way in which we operate, the outcome of which we are unable to predict. For example, implementing and monitoring such compliance procedures in the large number of foreign jurisdictions where we operate can be expensive and time-consuming. As a result of our remediation measures, we may also encounter difficulties conducting business in certain foreign countries and retaining and attracting additional business with certain customers, and we cannot predict the extent of these difficulties.
In addition, entering into the DPA in the United States may adversely affect our operations or result in legal claims against us, which may include claims of special, indirect, derivative or consequential damages.
Our failure to comply with the terms of the deferred prosecution agreement with the Department of Justice would have a negative impact on our ongoing operations.
As described above, we are subject to a three-year DPA with the Department of Justice. If we comply with the DPA, the Department of Justice has agreed not to prosecute us with respect to the above-described activities in China and, following the term of the DPA, to permanently dismiss the criminal statement of information that is currently pending against us. Accordingly, the DPA could be substantially nullified, and we could be subject to severe sanctions and resumed civil and criminal prosecution, as well as severe fines, penalties and other regulatory sanctions, in the event of any additional violation of the FCPA or any other applicable anti-corruption laws by us or any of our officers, other employees or agents in any jurisdiction or of our failure to otherwise meet any of the terms of the DPA as determined by the Department of Justice in its sole discretion. The claims alleged in the DPA with the Department of Justice only relate to our actions in China as outlined above, and do not relate to any future violations or the discovery of past violations not expressly covered by the DPA. Any breach of the terms of the DPA would also cause damage to our business and reputation, as well as impair investor confidence in our company and result in adverse consequences on our ability to obtain or continue financing for current or future projects.
In addition, although we are not currently restricted by the U.S. Department of Health and Human Services, Office of the Inspector General, from participating in federal healthcare programs, any criminal conviction of our company under the FCPA in the future would result in our mandatory exclusion from such programs, and it may lead to debarment from U.S. and foreign government contracts. Any such exclusion or debarment would have a material adverse effect on our business, financial condition and results of operations.
Our ability to comply with the terms of the DPA is dependent, among other things, on the success of our ongoing compliance and ethics program, including our ability to continue to manage our distributors and agents and supervise, train and retain competent employees, as well as the efforts of our employees to adhere to our compliance and ethics program and the FCPA and other applicable anti-corruption laws. It is possible that, despite our best efforts, additional FCPA issues, or issues under anti-corruption laws of other jurisdictions, could arise in the future. Any failure by us to adopt appropriate compliance and ethics procedures, to ensure that our officers, other employees and agents
17
comply with the FCPA and other applicable anti-corruption laws and regulations in all jurisdictions in which we operate or to otherwise comply with any term of the DPA would have a material adverse effect on our business, financial condition and results of operations.
In certain international markets, we have converted, or are in the process of converting, to a direct sales force model from a distributor-based sales model. Our business, financial condition and operating results may be adversely affected by the transition to a new sales model.
In August 2006, we negotiated an early termination with one of our international distributors, and we have since then undertaken to distribute our products in such distributor's country through our direct sales force. We also gave notice of termination to a second distributor and began operations in April 2008 through our direct sales force in such distributor's country. We gave notice of termination to a third distributor and began operations in July 2008 through our direct sales force in such distributor's country. In addition, we gave notice of termination to six other distributors and began operations in January 2009 through our direct sales force in these distributors' countries. We may also assess the viability of distributing our products directly in other international markets. We have limited experience with direct sales of our products in international markets and, therefore, may not obtain the financial benefits that we expect. In addition, we may experience delays in implementing our direct sales force model due to the difficulty of hiring a sales force, establishing relationships with physicians, complying with local regulatory requirements, and other factors, which could have an adverse effect on our business, financial condition and results of operations.
Fluctuations in foreign exchange rates may adversely affect our consolidated results of operations.
Our foreign operations expose us to currency fluctuations and exchange rate risks. Approximately 24.3% and 42.0% of our net sales for 2008 and for the six months ended June 30, 2009, respectively, were in foreign currencies. Accordingly, our consolidated results of operations have been, and will continue to be, subject to fluctuations in foreign exchange rates. Although we have benefited from foreign currency exchange rate fluctuations in the past, we may not benefit from the effect of foreign currency exchange rate fluctuations in the future, which may adversely affect our consolidated results of operations. During a period in which the U.S. dollar appreciates against a given foreign currency, our consolidated net sales will be lower than they might otherwise have been because net sales earned in such foreign currency will translate into fewer U.S. dollars. At present, based on a foreign exchange rate exposure management policy adopted in December 2008, we have started to engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the U.S. dollar. As we grow our international direct sales, we expect our foreign currency-denominated net sales will increase, which would increase our risks related to fluctuations in foreign exchange rates. We cannot assure you that our monitoring of our net foreign currency exchange rate exposure, our foreign currency exchange rate exposure management policy or any foreign currency hedging activity that we implement will be effective or otherwise adequately protect us against fluctuations in foreign currency exchange rates.
Our ability to operate our company effectively could be impaired if we lose members of our senior management team or scientific personnel.
We depend on the continued service of key managerial, scientific and technical personnel, as well as our ability to continue to attract and retain highly qualified personnel. We compete for such personnel with other companies, academic institutions, government entities and other organizations. Any loss or interruption of the services of our key personnel could significantly reduce our ability to effectively manage our operations and meet our strategic objectives, because we may be unable to find an appropriate replacement, if necessary. For example, Dr. Amplatz plays a key role in the early stages of our research and development programs, which are crucial to expanding our product portfolio. We have a five-year research and development contract with Dr. Amplatz that expires in December 2010,
18
and we may not be able to renew it. The loss of Dr. Amplatz's services may negatively affect our ability to expand our product portfolio beyond our current pipeline. In addition, after termination of our contract with Dr. Amplatz, he is not allowed to compete with our company for 18 months in the United States. Any competition from Dr. Amplatz after that period or outside the United States may negatively affect our business.
Healthcare legislative or administrative changes resulting in restrictive third-party payor reimbursement practices or preferences for alternate treatment may decrease the demand for, or put downward pressure on the price of, our products.
Our products are purchased principally by hospitals, which typically receive reimbursement from various third-party payors, such as governmental programs (e.g., Medicare and Medicaid), private insurance plans and managed care plans, for the healthcare services provided to their patients. The ability of our customers to obtain appropriate reimbursement for their products and services from government and third-party payors is critical to our success. The availability of reimbursement affects which products customers purchase and the prices they are willing to pay. Reimbursement varies from country to country and can significantly impact the acceptance of new products. After we develop a promising new product, we may experience limited demand for the product unless reimbursement approval is obtained from private and governmental third-party payors.
Major third-party payors for hospital services in the United States and abroad continue to work to contain healthcare costs. The introduction of cost-containment incentives, combined with closer scrutiny of healthcare expenditures by both private health insurers and employers, has resulted in increased discounts and contractual adjustments to hospital charges for services performed. Initiatives to limit the growth of healthcare costs, including price regulation, are also underway in several countries in which we do business. Implementation of new legislative and administrative changes in the United States and in overseas markets, such as Germany and Japan, may limit the price of, or the level at which reimbursement is provided for, our products and, as a result, may adversely affect both our pricing flexibility and demand for our products. Hospitals or physicians may respond to such cost-containment pressures by substituting lower-cost products or other treatments for our products.
Further legislative or administrative changes to the U.S. or international reimbursement systems that significantly reduce reimbursement for procedures using our medical devices or deny coverage for such procedures, or adverse decisions relating to our products by administrators of such systems in coverage or reimbursement issues, would have an adverse impact on the number of products purchased by our customers and the prices our customers are willing to pay for them. This, in turn, would adversely affect our business, financial condition and results of operations.
Our business may be adversely affected if consolidation in the healthcare industry leads to demand for price concessions or if we are excluded from being a supplier by a group purchasing organization or similar entity.
Because healthcare costs have risen significantly over the past decade, numerous initiatives and reforms have been launched by legislators, regulators and third-party payors to curb these costs. As a result, there has been a consolidation trend in the healthcare industry to create larger companies, including hospitals, with greater market power. As the healthcare industry consolidates, competition to provide products and services to industry participants has become and will continue to become more intense. This has resulted and will likely continue to result in greater pricing pressures and the exclusion of certain suppliers from important markets as group purchasing organizations, independent delivery networks and large single accounts continue to use their market power to consolidate purchasing decisions. If a group purchasing organization excludes us from being one of their suppliers, our net sales will be adversely impacted. We expect that market demand, government regulation, third-party reimbursement policies and societal pressures will continue to change the worldwide healthcare industry, which may exert further downward pressure on the prices of our products.
19
We conduct substantially all of our operations at our corporate headquarters, and any fire, explosion, violent weather conditions or other unanticipated events affecting our corporate headquarters could adversely affect our business, financial condition and results of operations.
We conduct all of our manufacturing and research and development activities, as well as most of our sales, warehousing and administrative activities, at our corporate headquarters in Plymouth, Minnesota. Our corporate headquarters is subject to the risk of catastrophic loss due to unanticipated events, such as fires, explosions or violent weather conditions. This facility and the manufacturing equipment that we use to produce our products would be difficult to replace or repair and could require substantial lead-time to do so. For example, if we were unable to utilize our existing manufacturing facility, the use of any new facility would need to be approved by the FDA, which would result in significant production delays. We may also in the future experience plant shutdowns or periods of reduced production as a result of regulatory issues, equipment failure or delays in deliveries. Any disruption or other unanticipated events affecting our corporate headquarters and therefore our sales, manufacturing, warehousing, research and development and administrative activities would adversely affect our business, financial condition and results of operations. We currently carry $60.0 million of insurance coverage for damage to our property and the disruption of our business. Such insurance coverage, however, may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
We rely on a single supplier for nitinol, the key raw material in all of our products, which makes us susceptible to supply shortages of this material.
We rely on a single supplier for nitinol, the key raw material in all of our products, and have no written agreement with this supplier. If we are unable to obtain nitinol from this supplier, we may be unable to obtain nitinol through other sources, on acceptable terms, within a reasonable amount of time or at all. Further, even if we are able to find an alternative source for nitinol, we may not be able to prevent an interruption of production of our products. Our business would be adversely affected if such interruption was prolonged. For example, if a raw material or component is a critical element, an element that can have a significant effect on performance and safety of the related device, such as nitinol with respect to our devices, FDA and foreign regulations may require additional testing and prior approval of such raw material or component from new suppliers prior to our use of these materials or components. As a result, if we need to establish additional or replacement suppliers for nitinol or any other critical component, our access to these components may be delayed while we qualify such suppliers and obtain any necessary FDA and foreign regulatory approvals. Any disruption in the ongoing shipment of nitinol could interrupt production of our products, which could result in a decrease of our net sales, or could cause an increase in our cost of sales if we have to pay another supplier a higher price for nitinol.
Any failure of our management information systems could harm our business and results of operations.
Our business's rapid growth may continue to place a significant strain on our managerial, operational and financial resources and systems. We depend on our recently implemented management information systems to actively manage our controlled regulatory and manufacturing documents. We also depend on our enterprise resource planning system to actively manage our invoicing, production and inventory planning, clinical trial information and quality compliance. We must continually assess the necessity for any upgrades to our information systems. We are currently developing a plan to upgrade our enterprise resource planning system. We may not be able to successfully implement any such upgrade. The inability of our management information systems to operate as we anticipate could damage our reputation with our customers, disrupt our business or result in, among other things, decreased net sales and increased overhead costs. As a result, any such failure could harm our business, financial condition and results of operations.
20
As a result of our becoming a public company, we will become subject to additional reporting and corporate governance requirements that will require additional management time, resources and expense.
Since our inception in 1995, we have operated our business as a private company. In connection with this offering, we will become obligated to file with the U.S. Securities and Exchange Commission, or the SEC, annual and quarterly information and other reports that are specified in the U.S. Securities Exchange Act of 1934, or the Exchange Act, and we will also become subject to other new reporting and corporate governance requirements, including requirements of the Nasdaq Global Market and the U.S. Sarbanes-Oxley Act of 2002, or the Sarbanes Oxley Act, and the rules and regulations promulgated thereunder, all of which will impose significant compliance and reporting obligations upon us. These obligations will require a commitment of additional resources, and meeting these requirements may require the hiring of additional staff and result in increased operating expenses and the diversion of our senior management's time and attention from our day to day operations. In particular, we will be required to do the following:
- •
- create or expand the roles and duties of our board of directors, our board committees and management;
- •
- institute a more comprehensive compliance function;
- •
- prepare and distribute periodic public reports in compliance with our obligations under the federal securities laws;
- •
- evaluate, test and implement internal controls over financial reporting to enable management to report on, and our
independent registered public accounting firm to attest to, such internal controls as required by Section 404 of the Sarbanes-Oxley Act;
- •
- involve to a greater degree outside counsel and accountants in the activities listed above; and
- •
- establish new internal policies, such as those relating to disclosure controls and procedures and insider trading.
We may not be successful in complying with these obligations, and compliance with these obligations could be time consuming and expensive. Failure to comply with the additional reporting and corporate governance requirements could lead to fines imposed on us, suspension or delisting from the Nasdaq Global Market, deregistration under the Exchange Act and, in the most egregious cases, criminal sanctions could be imposed.
We may need to raise additional capital in the future, which may not be available to us on acceptable terms, or at all.
We may require significant additional debt and equity financing in order to implement our business strategy. In particular, our capital requirements depend on many factors, including the amount of expenditures on research and development and intellectual property, the number of clinical trials that we conduct, new product development and the cash required to service our debt. To the extent that our existing or future capital is insufficient to meet these requirements and cover any losses, we will need to refinance all or a portion of our existing debt, raise additional funds through financings or curtail our growth, reduce our costs or sell certain of our assets. For example, we raised additional capital from affiliates of Welsh, Carson, Anderson & Stowe IX, L.P., or Welsh Carson, to finance the acquisition of the assets of our Italian distributor. We cannot assure you that such investors will agree to provide us with additional financing in the future. Our ability to raise additional capital will likely depend on, among others, our performance, our prospects, our level of indebtedness and market conditions. Any additional equity or debt financing, if available at all, may be on terms that are not favorable to us. The recent global economic crisis and related tightening of credit markets has made it more difficult and more expensive to raise additional capital. If we are unable to access additional capital on terms acceptable to us, we may not be able to fully implement our business strategy, which may limit the future growth and development of our business. In addition, equity financings could
21
result in dilution to our stockholders, and equity or debt securities issued in future financings may have rights, preferences and privileges that are senior to those of our common stock. If our need for capital arises because of significant losses, the occurrence of these losses may make it more difficult for us to raise the necessary capital.
Product liability claims and uninsured or underinsured liabilities could have a material adverse effect on our business.
The manufacturing and marketing of medical devices involve an inherent risk of product liability claims. Our product development and production processes are extremely complex and could expose our products to defects. Any defects could harm our credibility, lead to product liability claims and litigation and decrease our products' market acceptance. Our current product liability policies provide $35.0 million of insurance coverage, with a $250,000 deductible per occurrence for new claims. We cannot assure you that such insurance will be available or adequate to satisfy future claims or that our insurers will be able to pay claims on insurance policies which they have issued to us. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, adversely affecting our financial condition and results of operations. In the event that we are held liable for a claim or for damages exceeding the limits of our insurance coverage, that claim could materially damage our reputation and business. We currently have seven outstanding products liability claims, none of which we expect will result in significant damage awards. However, defending a lawsuit, regardless of merit, could be costly, could divert management attention and might result in adverse publicity, and for these reasons, any product liability claims could result in significant costs and harm to our business, financial condition and results of operations.
We may not successfully make or integrate acquisitions or enter into strategic alliances.
We may pursue selected acquisitions and strategic alliances. We compete with other medical device companies for these opportunities, and we cannot assure you that we will be able to effect acquisitions or strategic alliances on commercially reasonable terms, or at all. Even if we enter into these transactions, we may experience the following, among other things:
- •
- difficulties in integrating any acquired companies and products into our existing business;
- •
- inability to realize the benefits we anticipate in a timely fashion, or at all;
- •
- attrition of key personnel from acquired businesses;
- •
- significant costs, charges or writedowns; or
- •
- unforeseen operating difficulties that require significant financial and managerial resources that would otherwise be available for the ongoing development and expansion of our existing operations.
Consummating these transactions could also result in the incurrence of additional debt and related interest expense, as well as unforeseen contingent liabilities, all of which could have a material adverse effect on our business, financial condition and results of operations. We may also issue additional equity in connection with these transactions which would dilute our existing stockholders.
Risks Related to Regulation
If we fail to comply with the U.S. Federal Anti-Kickback Statute and similar state and foreign laws, we could be subject to criminal and civil penalties and exclusion from Medicare, Medicaid and other governmental programs.
A provision of the U.S. Social Security Act, commonly referred to as the U.S. Federal Anti-Kickback Statute, prohibits the offer, payment, solicitation or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of items or services payable by Medicare, Medicaid or any other federal
22
healthcare program. The Federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states in which our products are sold in the United States have adopted laws similar to the Federal Anti-Kickback Statute, and some of these laws are even broader than the Federal Anti-Kickback Statute in that their prohibitions are not limited to items or services paid for by a federal healthcare program but, instead, apply regardless of the source of payment. Violations of the Federal Anti-Kickback Statute or such similar state laws may result in substantial civil or criminal penalties and exclusion from participation in federal or state healthcare programs. We derive a significant portion of our net sales from international operations, and many foreign governments have equivalent statutes with similar penalties.
All of our financial relationships with healthcare providers and others who provide products or services to federal healthcare program beneficiaries are potentially governed by the Federal Anti-Kickback Statute and similar state or foreign laws. We believe our operations are in material compliance with the Federal Anti-Kickback Statute and similar state or foreign laws. However, we cannot assure you that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us, could divert management's attention from operating our business and could prevent healthcare providers from purchasing our products, all of which could have a material adverse effect on our business. In addition, if our arrangements were found to violate the Federal Anti-Kickback Statute or similar state or foreign laws, it could have a material adverse effect on our business and results of operations.
The possibility of non-compliance with manufacturing regulations raises uncertainties with respect to our ability to manufacture our products. Our failure to meet strict regulatory requirements could require us to pay fines, incur other costs or even close our facilities.
The FDA and other federal, state and foreign regulatory authorities require that our products be manufactured according to rigorous standards, including, but not limited to, Quality System Regulations, Good Manufacturing Practices and International Standards Organization, or ISO, standards. These federal, state and foreign regulatory authorities may conduct periodic audits of our facilities or our processes to monitor our compliance with applicable regulatory standards. If a regulatory authority finds that we fail to comply with the appropriate regulatory standards, it may require product validation, new processes and procedures or shutdown of our manufacturing operations. It may impose fines on us or delay or withdraw clearances or other regulatory approvals. If a regulatory authority determines that our non-compliance is severe, it may impose other penalties including limiting our ability to secure approvals for new devices and accessories. In addition, many of the improvements we make to our manufacturing process must first be approved by the FDA and other federal, state and foreign regulatory authorities. Our failure to obtain the necessary approvals may limit our ability to improve the way in which we manufacture our products.
Our business will be harmed if we fail to obtain necessary clearances or approvals to market our medical devices.
Our products are classified as medical devices and are subject to extensive regulation in the United States by the FDA and other federal, state and local authorities. Similar regulatory review and approval processes also exist in foreign countries in which our products are marketed. These regulations relate to product design, development, testing, manufacturing, labeling, sale, promotion, distribution, import, export and shipping.
Before we can market a new medical device, or a new use of, or claim for, or significant modification to, an existing product in the United States, we must first receive either PMA approval or 510(k) clearance from the FDA unless an exemption applies. The PMA approval process, commonly used for riskier devices such as those which support or sustain life or are used invasively in the body, requires an applicant to demonstrate the safety and efficacy of the device based, in part, on data
23
obtained in clinical trials. The PMA approval process and clinical trials can be expensive and lengthy and entail significant user fees. In the 510(k) clearance process, the FDA must determine that the proposed device is "substantially equivalent" to a device legally on the market, known as a "predicate" device, with respect to intended use, technology and safety and efficacy, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. The PMA approval pathway is much more costly and uncertain than the 510(k) clearance process. It generally takes from one to three years, or even longer, from the time the PMA is submitted to the FDA until an approval is obtained. The 510(k) clearance process usually takes from three to 12 months, but it can take longer.
In many of the foreign regions in which we market our products, such as Europe, we are subject to regulations substantially similar to those of the FDA, although these foreign regulatory requirements may vary widely from country to country. In Europe, only medical devices which bear a CE Mark may be marketed. Japan has a regulatory process that generally accepts clinical data from either the United States or Europe supplemented by a small study in Japan to establish experience and confirm safety. In addition, as we selectively convert into direct sales forces in foreign regions, we will be subject to additional regulations in these markets.
Any failure to receive desired marketing clearances or approvals from the FDA or other federal, state or foreign regulatory authorities may adversely affect our ability to market our products and may have a significant adverse effect on our overall business. Moreover, the value of existing clearances or approvals can be eroded if safety or efficacy problems develop.
We may fail to comply with continuing post-market regulatory requirements of the FDA and other federal, state or foreign authorities and become subject to substantial penalties, or our products may subsequently prove to be unsafe, forcing us to recall or withdraw such products from the market.
Even after product clearance or approval, we and our contract manufacturers must comply with continuing regulation by the FDA and other federal, state or foreign authorities, including the FDA's Quality System Regulation requirements, which obligate manufacturers, including third-party contract manufacturers, to adhere to stringent design, testing, control, documentation and other quality assurance procedures during the design and manufacture of a device. We are also subject to medical device reporting regulations in the United States and abroad. For example, we are required to report to the FDA if our products may have caused or contributed to a death or serious injury or malfunction in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. We must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the U.S. Food, Drug, and Cosmetic Act caused by the device that may present a risk to health, and we must maintain records of other corrections or removals. The FDA closely regulates promotion and advertising, and our promotional and advertising activities may come under scrutiny. If any medical device reports we file with the FDA regarding death, serious injuries or malfunctions indicate or suggest that one of our products presents an unacceptable risk to patients, including when used off-label by physicians, we may be forced to recall our product or withdraw it from the market.
We have had several product recalls in the past. For example, in October 2006, we recalled catheter and delivery systems after internal testing revealed the potential for a tear to develop in the packaging under extreme shipping conditions. We immediately modified our shipping method and subsequently received approval from the FDA and our Notified Body in Europe to modify the packaging to prevent tears from developing. Approximately 15,871 devices were returned and replaced by us. On February 28, 2007, we submitted a letter to the FDA formally requesting the recall to be closed, and on October 9, 2008 the FDA confirmed that the recall has been completed. During the third quarter of 2005, we voluntarily recalled 80 of our AMPLATZER Vascular Plug devices over concerns that our operators failed to follow internal sterilization procedures. Of the 80 devices, only two had left our possession. After testing the recalled products, none of them were found to be
24
non-sterile. We submitted a letter to the FDA formally requesting closure of the recall, and the recall has been closed. In September 2005, we recalled our AMPLATZER Duct Occluder device after discovering through in-process testing during manufacturing that the device had the potential to rub against the catheter during the implant procedure. Approximately 2,800 devices were recalled, 92% of which had left our possession. We made the required changes to our AMPLATZER Duct Occluder, and these changes have been approved both internationally and by the FDA. We submitted letters to the FDA formally requesting closure of the recall, and the recall has been closed. Finally, on December 8, 2004, we initiated a voluntary recall of all catheters and delivery systems in the field because of non-toxic contaminated tubing produced by one of our suppliers. We received several toxicology tests that confirmed the level of contamination was negligible and posed no threat to patients. We submitted letters to the FDA formally requesting closure of the recall, and the recall has been closed.
We are currently conducting two post-approval studies that were required as a condition of approval by the FDA of the AMPLATZER Septal Occluder and the AMPLATZER Muscular VSD Occluder. The studies are designed to monitor, for a period of up to five years after the procedure, patients treated with a device in the clinical studies that supported approval of the product. The objective is to collect and report to the FDA additional data on the long-term safety and efficacy of the device. The majority of patients enrolled in these two studies were children at the time of receiving their implants. In some cases, it has been challenging to follow these patients for up to five years as they and their families move or otherwise stop seeing the physician who performed the treatment.
Any failure to comply with continuing regulation by the FDA or other federal, state or foreign authorities could result in enforcement action that may include regulatory letters requesting compliance action, suspension or withdrawal of regulatory clearances or approvals, product recall, modification or termination of product marketing, entering into a consent decree, seizure and detention of products, paying significant fines and penalties, criminal prosecution and similar actions that could limit product sales, delay product shipment and harm our profitability. Any of these actions could materially harm our business, financial condition and results of operations.
Modifications to our products may require new regulatory approvals or clearances or may require us to recall or cease marketing our modified products until approvals or clearances are obtained.
Modifications to our products may require new approvals or clearances in the United States and abroad, such as PMA approvals or 510(k) clearances in the United States and CE Marks in Europe. The FDA requires device manufacturers to initially make a determination of whether or not a modification requires a new approval, supplement or clearance. A manufacturer may determine that a modification does not significantly affect safety or efficacy or does not represent a major change in its intended use, so that no new U.S. or foreign approval or clearance is necessary. We have made modifications that we determined do not require approval or clearance. However, the FDA and foreign authorities can review a manufacturer's decision, including any of our decisions, and may disagree. If the FDA or other foreign authority disagrees and requires new approvals or clearances for the modifications, we may be required to recall and to stop marketing our products as modified, which could require us to redesign our products and harm our operating results. In these circumstances, we may also be subject to significant enforcement actions.
If we determine that a modification to an FDA-approved or cleared device could significantly affect its safety or efficacy, or would constitute a major change in its intended use, then we must obtain a new PMA or PMA supplement approval or 510(k) clearance. Where we determine that modifications to our products require a new PMA or PMA supplemental approval or 510(k) clearance, we may not be able to obtain those additional approvals or clearances for the modifications or additional indications in a timely manner, or at all. For those products sold in Europe, we must notify AMTAC, our European Union Notified Body, if significant changes are made to the products or if there are substantial changes to our quality assurance systems affecting those products. Delays in obtaining
25
required future approvals or clearances would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Risks Related to Our Intellectual Property and Potential Litigation
We have been and may in the future become subject to claims that our products violate the patent or intellectual property rights of others, which could be costly and disruptive to us.
We operate in an industry that is susceptible to significant patent litigation, and in recent years, it has been common for companies in the medical device industry to aggressively challenge the rights of other companies to prevent the marketing of new or existing devices. As a result, we or our products may become subject to patent infringement claims or litigation or interference proceedings declared by the U.S. Patent and Trademark Office, or USPTO, or the foreign equivalents thereto to determine the priority of claims to inventions. The defense of intellectual property suits, USPTO interference proceedings or the foreign equivalents thereto, as well as related legal and administrative proceedings, are both costly and time consuming and may divert management's attention from other business concerns. An adverse determination in litigation or interference proceedings to which we may become a party could, among other things:
- •
- subject us to significant liabilities to third parties, including treble damages;
- •
- require disputed rights to be licensed from a third party for royalties that may be substantial;
- •
- require us to cease using such technology; or
- •
- prohibit us from selling certain of our products.
Any of these outcomes could have a material adverse effect on our business, financial condition and results of operations.
On March 28, 2006, we settled a patent infringement suit with NMT Medical, Inc. in which we paid NMT Medical and a second patent holder a $30.0 million one-time payment. As part of the settlement, we received a fully paid, royalty-free license for the related patents.
In addition, we may have disputes with our licensors regarding the scope of their patents. We make royalty payments with respect to certain patents that were assigned to us by Mr. Curtis Amplatz. In 2008, Mr. Curtis Amplatz inquired regarding the scope of the royalty agreements we had with him and whether they applied to additional products of ours. In response, we had discussions in which we clarified the scope of the agreements and our payments under the agreements. We believe the inquiry of Mr. Curtis Amplatz to be concluded. However, any dispute relating to the products included in a portfolio subject to a royalty agreement or license could result in us being subject to additional royalty payments, although we do not believe any such dispute to limit our right to sell or market any of our devices.
We are currently subject to claims by Medtronic that substantially all of our occluder and vascular plug products infringe certain of Medtronic's patents. If we are not successful in our defense of these claims or in our counterclaims, we may be subject to damages, ongoing royalties and other remedies which could have a material adverse effect on our business, financial condition and results of operations.
In January 2007, Medtronic, Inc. filed a patent infringement action against us in the U.S. District Court for the Northern District of California, alleging that substantially all of our AMPLATZER occluder and vascular plug devices, which have historically accounted for substantially all of our net sales, infringe three of Medtronic's method and apparatus patents on shape memory alloy stents (U.S. Patent Nos. 5,190,546, 6,306,141 and 5,067,957). Medtronic is seeking compensatory damages with respect to our products manufactured or sold in the United States. Medtronic asserted but later withdrew its requests for injunctive relief and for damages based on willfulness.
We have asserted defenses and counterclaims for non-infringement and challenged the validity and enforceability of Medtronic's patents. On April 28, 2009, the court granted summary judgment in our
26
favor finding that we do not infringe Medtronic's '546 patent. Subsequently, Medtronic withdrew certain allegations with respect to the remaining two patents. The trial on the remaining issues in the case was divided into a jury trial phase and non-jury, bench trial phase.
The issues of infringement and certain issues of validity based on obviousness and anticipation of the asserted claims in the two remaining patents were the subject of a jury trial before the U.S. District Court for the Northern District of California that began on July 6, 2009. On August 5, 2009, the jury returned a verdict that the subject AMPLATZER occluder and vascular plug products infringed the claims at issue with respect to both of the two remaining Medtronic patents and that the Medtronic patent claims at issue had not been proven by us to be invalid. The jury verdict awarded Medtronic damages of $57.8 million. This amount is equal to 11% of historical sales of the occluder and vascular plug products in question during the timeframe specified for each patent. Any infringement of the '141 apparatus patent after March 31, 2009 to the date of a final, non-appealable judgment will be considered in calculating the final amount of damages, if any, to be paid. The '957 patent expired in May 2004. Because the issue was not before it, the jury made no determination regarding the payment of royalties on future sales of the company's products after the date of a final, non-appealable judgment. The verdict is not enforceable until the completion of the trial and entry of a final, non-appealable judgment. If we do not receive a favorable judgment, we expect we will appeal such judgment and expect that we will likely be required to post a bond in order to be allowed to appeal as set forth below.
On August 19, 2009, the United States Court of Appeal for the Federal Circuit, sitting en banc, issued a decision in Cardiac Pacemakers, Inc. v. St. Jude Medical, Inc. In Cardiac Pacemakers, the Federal Circuit established a new rule of law and held as a matter of law that the practice of a method claim of a patent outside of the United States cannot infringe a United States method patent. When a controlling new rule of law is announced that affects the parties to a patent litigation, the court will ordinarily apply the supervening change in law retroactively to a pending case. In our litigation with Medtronic, a portion of the jury's damage award was based on a finding of infringement of Medtronic's '957 method patent for sales of our products outside of the United States, and we believe it is therefore directly contrary to the holding as stated in the Cardiac Pacemakers decision. Applying the rate of damages established by the jury, 11% of historical sales, to the total sales of our products outside of the United States during the period for which we believe damages were awarded for infringement of the '957 patent would result in a reduction of approximately $14 million. Such $14 million of damages relates only to periods of time before the '141 patent was enforceable against us. As a result, we believe it is likely that the trial court in our case will reduce the jury's damage award by approximately such amount to reflect this recent decision, although there can be no assurance that Cardiac Pacemakers will not be reviewed and overturned by the United States Supreme Court or that the damage award will be reduced by this or any other amount. On September 8, 2009, we filed a motion seeking, at Medtronic's choice, either a new trial or the above-mentioned reduction of $14 million, which motion is publicly available. We do not expect a ruling on this motion until the conclusion of the non-jury phase of the trial in early 2010.
Following the jury verdict, the court scheduled the non-jury phase of the trial for early December 2009, which will deal with other issues of invalidity and unenforceability of one or both of the two remaining patents based on equitable claims of double patenting, equitable estoppel, inequitable conduct in patent prosecution and laches. Upon conclusion of the non-jury phase of the trial, the court will consider post-trial motions and enter a judgment, which we expect will take place in early 2010. As part of its decision, the trial court could order a new trial on some or all of the issues in the case or amend or reaffirm the jury verdict based on one or more issues regarding infringement, validity, enforceability and damages. We also expect the trial court to decide whether any royalty payments relating to the '141 patent, which does not expire until 2018, are due for future periods and, if so, at what rate. Any such royalties may not be on commercially reasonable terms, and may exceed the 11% rate of damages applied by the jury. If any damage amount is entered as a part of the judgment, we
27
will likely be required at that time to accrue a non-cash charge equal to the amount of such damages. Any such accrual will have an adverse effect on our results of operations for the applicable period.
Thereafter, the judgment will be subject to appeal which could result in the judgment being affirmed or amended, or in an order for a new trial on one or more of the same issues raised before the trial court. If we decide to appeal, such appeal may not be decided for several months and will likely require the posting of a bond in the face amount of 150% of the judgment amount, if any. We do not expect the cash cost associated with posting such a bond to be material, but we would likely be required to secure such bond with collateral. The amount of collateral required by the provider of the bond would be determined based on several factors, such as the amount of our debt and our financial condition.
If we are required to accrue a charge relating to, or post an appeal bond in connection with, the Medtronic litigation, we may not be able to comply with the covenants contained in our senior secured credit facility in future periods. While we do not believe that accruing a charge and/or posting an appeal bond in connection with the Medtronic litigation will cause us to breach any such covenant, doing so may adversely affect our ability to comply with our maintenance covenants in our senior secured credit facility. If we breached any such covenant, we would need to seek to amend or refinance our senior secured credit facility. We believe that such an amendment could require us to pay upfront fees and an increased interest rate on our borrowings thereunder, which could materially adversely affect our financial condition and results of operations. Alternatively, we could refinance our senior secured credit facility, but we may not be able to do so on reasonable terms or at all. If we fail to obtain such an amendment or refinancing, we would suffer an event of default under such facility and the lenders thereto would have the right to accelerate the indebtedness outstanding thereunder. In addition, the lenders' obligations to extend letters of credit or make loans under our senior secured credit facility are dependent upon our ability to make our representations and warranties thereunder at the time such letters of credit are extended or such loan is made. If we are unable to make the representations and warranties in our senior secured credit facility at such time, we will be unable to borrow additional amounts under our senior secured credit facility in the future.
These proceedings are costly and time consuming, and we cannot assure you that the outcome of any of these proceedings will be favorable to us. If we do not prevail before the trial or appellate courts in overturning the jury verdict or reducing or eliminating the damages award in the jury verdict, we will have to pay the awarded damages and may have to pay royalties on a substantial portion of our future sales, and our results of operations and financial condition may be materially adversely affected. Medtronic also could appeal from rulings adverse to it, which could result in an appellate decision with effects adverse to us on issues of liability and/or relief. In addition, Medtronic may attempt to request injunctive relief in the future, which, if granted, would have a material adverse effect on our business, financial condition and results of operations.
Notwithstanding the litigation proceedings, we are in the process of implementing changes to our business processes intended to create an alternative process that we believe does not infringe Medtronic's patents. We cannot assure you that any of these changes will avoid future litigation or will not result in a subsequent finding of infringement of Medtronic's patents and/or patents held by others. If any of our modified business processes were found to infringe any such patents, we may be required to pay additional damages and royalties. We cannot assure you that such damages and royalties would not exceed the 11% rate applied by the Medtronic case jury for past sales. Any such royalties may not be on commercially reasonable terms and could have a material adverse effect on our results of operations and financial condition. Also, if a court grants an injunction, we may be prevented from selling some or any infringing products. While we believe we have taken reasonable steps to inform third parties who use such products of the alternative process by changing the instructions for use that are referenced in the information that accompanies each such product, such third parties may not review such information and may not adopt the alternative process even if they do review the new
28
instructions for use. To the extent such third parties continue to use the prior process, we may be liable for such use whether or not the alternative process infringes Medtronic's patents.
We have filed and may in the future file patent litigation claims in the U.S. and foreign jurisdictions to protect our patent portfolio. If we are unsuccessful in these claims, our business, financial condition and results of operations could be adversely affected.
We may initiate litigation to assert claims of infringement, enforce our patents, protect our trade secrets or know-how, or determine the enforceability, scope and validity of the proprietary rights of others. Any lawsuits that we initiate could be expensive, time consuming and divert management's attention from other business concerns. Furthermore, litigation may provoke third parties to assert claims against us and may put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not being issued.
In August 2006, we brought a patent infringement action in Germany against Occlutech GmbH, an European manufacturer of cardiac occlusion devices, and DRABO Medizintechnik, based on the German part of one of our European patents, which was granted to us in October 2005 for intravascular occlusion devices and the method of manufacturing such devices. On July 31, 2007, the District Court in Düsseldorf entered a judgment in our favor finding that Occlutech and DRABO literally infringed the German part of our European patent. Under German practice, the court required us to post a bond in the amount of €1.0 million to secure our ability to respond to damages claimed by Occlutech in the event that the decision of the District Court is reversed on appeal or our patent is held invalid in related proceedings in the German patent court. The bond amount is not a limitation on such damages. On August 6, 2007, Occlutech filed an appeal against the District Court judgment before a German Court of Appeals contending that the District Court judgment was based on an overly broad interpretation of our European patent, and in addition, it initiated invalidation proceedings against the patent. On December 22, 2008, the German Court of Appeals dismissed Occlutech's appeal and entered a judgment in our favor finding that Occlutech infringed our patent. Occlutech has filed an appeal with the German Federal Court of Justice. A final decision on the appeal with the German Federal Court of Justice and a decision on the invalidation proceedings are not expected to be reached until 2010 or later. In addition, Occlutech initiated proceedings against our corresponding patents in Italy, the Netherlands, the United Kingdom, Spain and Sweden, seeking invalidity and non-infringement declarations. On October 29, 2008, the Patent Court in the Netherlands ruled in favor of Occlutech in the non-infringement declaration. The court did not rule on the invalidity claim. On November 3, 2008, we filed an appeal with the Dutch appellate court. On July 31, 2009, a United Kingdom patent court upheld the validity of our patent, but it ruled that the Occlutech products do not infringe on our patent. We intend to appeal the decision that Occlutech did not infringe on our patent. Final decisions in these actions are also not expected to be reached until 2010 or later. We cannot assure you that the outcome in any of these proceedings will be favorable to us, and if we do not prevail in one or more jurisdictions, we face the risk of increased competition and significant damages being awarded against us.
We have also been forced to defend our patent rights in China against various entities, including Shanghai Shape Memory Alloy Company Ltd., a medical device manufacturer based in Shanghai, China, and Beijing Starway Medical Devices Ltd., a medical device manufacturer based in Beijing, both of which in recent years have been manufacturing and exporting medical devices that we believe infringe our patent rights. We did not prevail in our lawsuits in China against these entities. Consequently, we are no longer able to assert rights under these patents within China and will need to rely primarily on foreign patents to prevent the importation of products from China into countries in which such importation would violate our local patent rights. In addition, their activities have resulted in litigation in India and could result in future and potentially costly litigation in other countries in which we have patent rights against importers and distributors of infringing products originating in China.
In addition, we may not prevail in lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially valuable. The occurrence of any of these events may have a material adverse effect on our business, financial condition and results of operations.
29
If our patents and other intellectual property rights do not adequately protect our products, we may lose market share to our competitors and be unable to operate our business profitably.
Patents and other proprietary rights are essential to our business, and our ability to compete effectively with other companies depends on the proprietary nature of our technologies. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop, maintain and strengthen our competitive position. We seek to protect these, in part, through confidentiality agreements with certain employees, consultants and other parties. We pursue a policy of generally obtaining patent protection in both the United States and key foreign countries for patentable subject matter in our proprietary devices and also attempt to review third-party patents and patent applications to the extent publicly available to develop an effective patent strategy, avoid infringement of third-party patents, identify licensing opportunities and monitor the patent claims of others. Our patent portfolio includes approximately 199 issued patents, the first of which expires in the United States in 2014 and in Europe in 2015, and approximately 110 pending patent applications. We cannot assure you that any pending or future patent applications will result in issued patents, that any current or future patents issued or licensed to us will not be challenged, invalidated or circumvented or that the rights granted thereunder will provide a competitive advantage to us or prevent competitors from entering markets which we currently serve. Any required license may not be available to us on acceptable terms, if at all. In addition, some licenses may be non-exclusive, and therefore our competitors may have access to the same technologies as we do. Furthermore, we may have to take legal action in the future to protect our trade secrets or know-how, or to defend them against claimed infringement of the rights of others. Any legal action of that type could be costly and time-consuming to us, and we cannot assure you that such actions will be successful. The invalidation of key patents or proprietary rights which we own or unsuccessful outcomes in lawsuits to protect our intellectual property may have a material adverse effect on our business, financial condition and results of operations.
The laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. For example, foreign countries generally do not allow patents to cover methods for performing surgical procedures. If we cannot adequately protect our intellectual property rights in these foreign countries, our competitors may be able to compete more directly with us, which could adversely affect our competitive position and, as a result, our business, financial condition and results of operations.
Risks Related to Our Debt
Our substantial debt may adversely affect our financial condition and operating activities.
We have a significant amount of indebtedness. As of June 30, 2009, after giving effect to this offering and the application of the net proceeds therefrom, we would have debt of $216.2 million outstanding. Based on that level of indebtedness and interest rates applicable as of June 30, 2009, our annual interest expense would have been $6.3 million. In addition, we expect to have $25.0 million of available borrowings under our revolving credit facility following completion of the offering, and we have the ability to increase the aggregate amount of our Tranche B term loan under our senior secured credit facility by up to $75.0 million without the consent of any person other than the institutions agreeing to provide all or any portion of such increase. Although we believe that our current cash flow is sufficient to cover our annual interest expense for the foreseeable future, any increase in the amount of debt or any decline in the amount of cash available to make interest payments may require us to divert funds identified for other purposes for debt service and impair our liquidity position.
Our substantial level of indebtedness has other significant consequences to you, including:
- •
- requiring us to use a substantial portion of our cash flow from operations to pay interest and principal on our debt, thereby reducing the availability of our cash flow to fund working capital,
30
- •
- limiting our ability to obtain additional financing in the future for working capital, research and development, including
clinical trials, acquisitions and other general corporate purposes;
- •
- subjecting us to the risk of interest rate increases on our indebtedness with variable interest rates;
- •
- subjecting us to the possibility of an event of default under the financial and operating covenants contained in the
agreements governing our indebtedness; and
- •
- limiting our ability to adjust to rapidly changing market conditions, reducing our ability to withstand competitive pressures and making us more vulnerable to a downturn in general economic conditions than our competitors with less debt.
research and development, including clinical trials, acquisitions and other general corporate purposes;
Our inability to generate sufficient cash flow may require us to seek additional financing.
If we are unable to generate sufficient cash flow from operations in the future to service our debt, we may be required to refinance all or a portion of our existing debt, sell assets, borrow more money or raise capital through sales of our equity securities. If these or other kinds of additional financing become necessary, we cannot assure you that we could arrange such financing on terms that are acceptable to us, or at all.
We may incur additional indebtedness from time to time to finance research and development, including clinical trials, acquisitions, investments or strategic alliances or for other purposes.
We may incur substantial additional indebtedness in the future. Although the agreements governing our senior secured credit facility contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of qualifications and exceptions, and the indebtedness incurred in compliance with these restrictions could be substantial. For example, we expect to have $25.0 million of available borrowings under our revolving credit facility following completion of the offering, and we have the ability to increase the aggregate amount of our Tranche B term loan under our senior secured credit facility by up to $75.0 million without the consent of any person other than the institutions agreeing to provide all or any portion of such increase. If we incur additional debt above the levels currently in effect, the risks associated with our leverage would increase.
We are subject to restrictive debt covenants, which may restrict our operational flexibility.
Our senior secured credit facility contains various financial and operating covenants, including, among other things, restrictions on our ability to incur additional indebtedness, pay dividends on and redeem capital stock, make other restricted payments, make investments, sell our assets or enter into consolidations, mergers and transfers of all or substantially all of our assets. Our senior secured credit facility also requires us to maintain specified financial ratios and satisfy financial condition tests. Our ability to meet those financial ratios and tests can be affected by events beyond our control, and we cannot assure you that we will continue to meet those ratios and tests. A breach of any of these covenants, ratios, tests or restrictions could result in an event of default under our senior secured credit facility. Agreements governing any additional indebtedness we incur in the future may contain similar or more stringent covenants. Covenants in our existing or future debt agreements could limit our ability to take actions that we believe are in our best interests. If an event of default exists under our senior secured credit facility or any additional indebtedness we incur in the future, the lenders under such agreements could elect to declare all amounts outstanding thereunder to be immediately due and payable. If any such lender accelerates the payment of one of our indebtednesses, we cannot assure you
31
that our assets would be sufficient to repay in full that indebtedness and our other indebtedness that would become due as a result of any acceleration.
Our obligations under our senior secured credit facility are secured by substantially all of our assets.
Our obligations under our senior secured credit facility are secured by liens on substantially all of our and our subsidiaries' assets. If we become insolvent or are liquidated, or if repayment under our senior secured credit facility is accelerated and we cannot repay such indebtedness, the lenders will be entitled to exercise the remedies available to a secured lender under applicable law and the applicable agreements and instruments, including the right to foreclose on all of our and our subsidiaries' assets.
Risks Related to Our Common Stock and this Offering
An active, liquid trading market for our common stock may not develop.
Prior to this offering, there has been no public market for shares of our common stock. We cannot predict the extent to which investor interest in our company will lead to the development of a trading market on the Nasdaq Global Market or how active and liquid that market may become. If an active and liquid trading market does not develop, you may have difficulty selling any of our common stock that you purchase. The initial public offering price of shares of our common stock is, or will be, determined by negotiation between us, the selling stockholders and the underwriters and may not be indicative of prices that will prevail following the completion of this offering. The market price of shares of our common stock may decline below the initial public offering price, and you may not be able to resell your shares of our common stock at or above the initial public offering price, or at all.
The market price of our common stock may be volatile, which could cause the value of your investment to decline.
Securities markets worldwide experience significant price and volume fluctuations. This market volatility, as well as general economic, market or political conditions, may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise adversely affect the liquidity of our common stock.
The trading market for our common stock will also be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we may lose visibility in the financial markets, which in turn may cause our stock price or trading volume to decline. Moreover, if one or more of the analysts who cover us downgrade our stock or if our results of operations do not meet their expectations, our stock price may decline.
Our controlling stockholders will continue to have substantial control over us after this offering and could limit your ability to influence the outcome of key transactions, including a change of control.
We are controlled by Welsh Carson, WCAS Capital Partners IV, L.P. and other individuals and entities affiliated with Welsh Carson, which we collectively refer to as the WCAS Stockholders, and Franck L. Gougeon, our director and co-founder, and other entities controlled by Mr. Gougeon, which we collectively refer to as the Gougeon Stockholders. The WCAS Stockholders and the Gougeon Stockholders will beneficially own or control approximately 46.7% and 24.7%, respectively (or 44.7% and 23.7%, respectively, if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any), of our common stock following completion of this offering, and they have entered into a stockholders agreement with us in relation to their stock ownership. Accordingly, we will continue to be a "controlled company" as set forth in Rules 5605 and 5615 of the Nasdaq Global Market because more than 50% of our voting power will be held by a group formed by the WCAS
32
Stockholders and the Gougeon Stockholders. As a result, the WCAS Stockholders and the Gougeon Stockholders, if acting together, would be able to influence or control matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other material corporate transactions. They may also have interests that differ from yours and may vote in a way with which you disagree and which may be adverse to your interests. Any conflict of interests between the WCAS Stockholders and the Gougeon Stockholders, on the one hand, and the other holders of our common stock, on the other, may result in an actual or perceived conflict of interest on the part of our directors affiliated with the WCAS Stockholders and the Gougeon Stockholders. The existence or perception of such a conflict of interest could materially limit the ability of these directors to participate in consideration of the matter. In addition, the concentration of ownership may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company and might ultimately affect the market price of our common stock.
Future sales of shares of our common stock by existing stockholders in the public market, or the possibility or perception of such future sales, may adversely affect the market price of our stock.
The market price of our common stock may decline as a result of sales of a large number of shares of our common stock in the market after this offering or the perception that these sales may occur. These sales, or the possibility that these sales may occur, also may make it more difficult for you to sell your shares of common stock at a time and at a price which you deem appropriate.
As of June 30, 2009 (as adjusted to give effect to the conversion of our Class A and Class B common stock and all of our Series A and Series B preferred stock into our common stock and as adjusted for this offering), there were 48,055,683 shares of our common stock outstanding (or 50,118,183 if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any). In addition, as of June 30, 2009, there were 2,897,637 shares of vested stock options outstanding. The 13,750,000 shares of common stock sold in this offering (15,812,500 shares if the underwriters exercise their overallotment option in full) will be freely tradeable without restriction or further registration under the U.S. Securities Act of 1933, as amended, or the Securities Act, by persons other than our affiliates within the meaning of Rule 144 under the Securities Act.
Following this offering, the WCAS Stockholders will beneficially own 22,421,141 shares of our common stock and the Gougeon stockholders will beneficially own 11,886,501 shares of our common stock. The WCAS Stockholders and the Gougeon Stockholders will be able to sell their shares in the public market from time to time, subject to certain limitations on the timing, amount and method of those sales imposed by SEC regulations. The WCAS Stockholders, the Gougeon Stockholders and the underwriters have agreed to a "lock-up" period, meaning that the WCAS Stockholders and the Gougeon Stockholders may not sell any of their shares without the prior consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated for 180 days after the date of this prospectus. The WCAS Stockholders and the Gougeon Stockholders each have the right to cause us to register the sale of shares of common stock owned by them and to include their shares in future registration statements relating to our securities. If the WCAS Stockholders or the Gougeon Stockholders were to sell a large number of their shares, the market price of our stock could decline significantly. In addition, the perception in the public markets that sales by the WCAS Stockholders or the Gougeon Stockholders might occur could also adversely affect the market price of our common stock.
In addition to the WCAS Stockholders' and the Gougeon Stockholders' lock-up period, sales of our common stock are also restricted by lock-up agreements that our directors and executive officers have entered into with the underwriters. The lock-up agreements restrict our directors and executive officers, subject to specified exceptions, from selling or otherwise disposing of any shares for a period of 180 days after the date of this prospectus without the prior consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated.
33
Although Merrill Lynch, Pierce, Fenner & Smith Incorporated has advised us that there is no present intention or arrangement to do so, under certain circumstances all or any portion of the shares may be released from the restrictions in the lock-up agreements described above, and those shares would then be available for resale in the market. Any release would be considered on a case-by-case basis.
After completion of this offering, 2,897,637 shares will be issuable upon the exercise of presently outstanding stock options, 46,152 shares will also be issuable upon the exercise of stock options that we expect to grant to certain of our directors in connection with this offering and approximately 2,510,755 shares will be reserved for future issuance under our 2008 Equity Incentive Plan and our Employee Stock Purchase Plan, which is being adopted in connection with this offering. Pursuant to Rule 701 of the Securities Act as currently in effect, and subject to the lock-up agreements described herein, any of our employees, consultants or advisors who purchases shares of our common stock from us pursuant to options granted prior to the completion of this offering under our existing stock option plans or other written agreement is eligible to resell those shares 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144. Additionally, following the consummation of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register the sale of shares issued or issuable upon the exercise of all these stock options. Subject to the exercise of issued and outstanding options and contractual restrictions, shares of our directors and executive officers to which Rule 701 is applicable or those shares that are registered under the registration statement on Form S-8 will be available for sale into the public market after the expiration of the 180-day lock-up agreements with the underwriters.
In the future, we may issue our securities in connection with acquisitions or other investments. The amount of our common stock issued in connection with any acquisition or other investment could constitute a material portion of our then outstanding common stock. Such issuances could result in dilution of our common stockholders.
You will incur immediate and substantial dilution.
The initial public offering price of our common stock is substantially higher than the net tangible book values per share of outstanding common stock prior to completion of the offering. Based on our net tangible book value as of June 30, 2009 and upon the issuance and sale of 8,500,000 shares of common stock by us at an assumed initial public offering price of $20.00 per share (the midpoint of the initial public offering price range indicated on the cover of this prospectus), if you purchase our common stock in this offering, you will pay more for your shares than the amounts paid by our existing stockholders for their shares and you will suffer immediate dilution of approximately $23.26 per share (or $22.36 per share assuming that the underwriters' option is exercised in full) in net tangible book value. We also have a large number of outstanding stock options to purchase common stock with exercise prices that are below the estimated initial public offering price of our common stock. To the extent that these options are exercised, you will experience further dilution.
Some provisions of Delaware law and our amended and restated certificate of incorporation and amended and restated bylaws may deter third parties from acquiring us.
Provisions contained in our amended and restated certificate of incorporation and amended and restated bylaws and the laws of Delaware, the state in which we are incorporated, could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our stockholders. Provisions of our amended and restated certificate of incorporation and bylaws impose various procedural and other requirements, which could make it more difficult for stockholders to effect certain corporate actions. For example, our amended and restated certificate of incorporation authorizes our board of directors to determine the rights, preferences, privileges and restrictions of unissued series of
34
preferred stock, without any vote or action by our stockholders. Thus, our board of directors can authorize and issue shares of preferred stock with voting or conversion rights that could adversely affect the voting or other rights of holders of our common stock.
These anti-takeover defenses could discourage, delay or prevent a transaction involving a change in control of our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing and cause us to take other corporate actions that you desire.
AGA does not anticipate paying any cash dividends in the foreseeable future.
AGA currently intends to retain our future earnings, if any, for the foreseeable future, to repay indebtedness and to fund the development and growth of our business. AGA does not intend to pay any dividends to holders of our common stock even if permitted to do so under our senior secured credit facility. As a result, capital appreciation in the price of our common stock, if any, may be your only source of gain on an investment in our common stock.
Even if we decide in the future to pay any dividends, AGA is a holding company with no independent operations of its own. As a result, AGA depends on its direct and indirect subsidiaries for cash to pay its obligations and make dividend payments. Deterioration in the financial conditions, earnings or cash flow of our subsidiaries for any reason could limit or impair their ability to pay cash dividends or other distributions to AGA. In addition, our ability to pay dividends in the future is dependent upon AGA's receipt of cash from its subsidiaries. Such subsidiaries may be restricted from sending cash to AGA by, among other things, existing law or certain provisions of our senior secured credit facility the documents governing our future indebtedness that restrict our ability to pay dividends or otherwise distribute cash or other assets.
Our results of operations may fluctuate significantly from period to period and cause the market price of our common stock to decline.
We have experienced significant fluctuations in our results of operations from period to period, and we cannot assure you that we will not do so in the future. Such fluctuations have occurred primarily due to non-recurring items. For example, we recorded for 2005 a $29.0 million charge related to our one-time payment in settlement of a patent infringement lawsuit and a $50.8 million charge related to in-process research and development that we determined would not reach technical feasibility or hold an alternative use. Our results of operations may fluctuate significantly in the future from period to period due to many factors, including current and potential patent infringement lawsuits and the timing of our research and development expenditures, as well as the other factors described in this section. Any such fluctuation may cause the market price of our common stock to decline.
We will qualify as a controlled company within the meaning of the Nasdaq Global Market rules and, as a result, will rely on exemptions from certain corporate governance requirements.
After completion of this offering, we will be a "controlled company" as set forth in Rules 5605 and 5615 of the Nasdaq Global Market, because more than 50% of our voting power will be held by a group formed by the WCAS Stockholders and the Gougeon Stockholders. Under the Nasdaq Global Market rules, a "controlled company" may elect not to comply with certain Nasdaq Global Market corporate governance requirements, including the requirement that a majority of the board of directors consist of independent directors and the requirement that directors nominations and executive compensation must be approved by a majority of independent directors or a nominating or compensation committee, as applicable, comprised solely of independent directors. Accordingly, you may not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq Global Market corporate governance requirements.
35
General
Unless the context otherwise requires, references in this prospectus to:
- •
- "AGA" or the "issuer" refer only to AGA Medical Holdings, Inc., the issuer of the common stock offered hereby;
- •
- "AGA Medical" refer to AGA Medical Corporation, a wholly owned subsidiary of AGA;
- •
- "we," "us," "our," and the "company" refer collectively to AGA and its consolidated subsidiaries; and
- •
- "Europe" refers to the countries in the European Union.
For ease of understanding of our disclosure, a glossary of technical terms used in this prospectus can be found in Appendix A hereto.
AGA Medical was formed on July 12, 1995. AGA was formed on July 25, 2005 and does not have any assets other than its ownership of AGA Medical. On July 28, 2005, the stockholders of AGA Medical contributed all of the outstanding shares of AGA Medical to AGA in exchange for shares of AGA. As a result, AGA Medical became a wholly owned subsidiary of AGA. All periods prior to July 28, 2005 are referred to as "Predecessor," and all periods on or after such date are referred to as "Successor." The financial information included in this prospectus for Predecessor periods represents consolidated financial information of AGA Medical, and the financial information for all Successor periods represents financial information of AGA. In addition, our consolidated financial statements for all Successor periods reflect the fair value adjustments made to our assets and liabilities in recording the July 2005 reorganization after taking into account the carryover basis. See note 2 to our consolidated financial statements included elsewhere in this prospectus.
Effective January 1, 2009, we began direct distribution in Italy as a result of our purchase of certain distribution rights, inventory, equipment, intangible assets and goodwill from our former Italian distributor. In connection with the acquisition, we established on that date a wholly owned subsidiary in Italy called AGA Medical Italia S.R.L., and the results of AGA Medical Italia S.R.L.'s operations have been included in our financial statements since such date. The results of sales to the distributor were included in our financial statements prior to the acquisition date.
Trademarks
We use the term AMPLATZER, one of our trademarks, in this prospectus. We believe that we have appropriate ownership or license rights to this trademark. Solely for convenience, we refer to this trademark in this prospectus without the ™ or ® symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to this trademark. All other trademarks appearing in this prospectus are the property of their holders.
Industry Data
Information contained in this prospectus concerning our industry, the domestic and international markets for our products and the historic growth rate of, and our position in, those markets is based on estimates that we prepared using data from various sources (including industry publications, surveys and forecasts and our internal research), on assumptions that we have made based on that data and other similar sources and our knowledge of the markets for our products. We have not independently verified market and industry data provided by third parties or by industry or general publications. Similarly, while we believe our internal estimates are reliable, our estimates have not been verified by any independent sources.
36
When we refer to the potential size of a market for our devices and the market opportunity it represents, this information is based on published third-party data on the prevalence and incidence in such market of the disease or defect that our devices are designed to repair, adjusted for relative purchasing power in such markets. In the case of congenital defects, we also use published, third-party data on the rates per birth and adjust for the birth rates in each major market. For example, to calculate the market for congenital defects, we begin by determining the number of potential new patients annually in a geographic area and then we multiply the total population of this area by the birth rate in such area and by the estimated incidence of the congenital defect. Using this method of calculation, we estimate the number of potential patients for the 12 major geographic markets for our products. These markets account for the majority of our sales and our estimates include the sum of these 12 geographic markets. However, not all geographic markets have equal economic conditions and purchasing power, which we believe affects access to medical treatment. We, therefore, adjust this potential number of new patients for this factor by using the relative purchasing power of such markets, based on data cited by the World Bank and sourced from the Population Reference Bureau. We use the United States as a reference point and assume a 100% factor for this country, and no other geographic market has a higher factor. For these other geographic markets, we adjust the number of potential new patients based on the percentage that the purchasing power of such geographic market represents in comparison to the purchasing power of the United States. The final step in calculating the market opportunity involves multiplying such adjusted number of potential new patients for such device by the average selling price for such device, which we establish based on our experience marketing and selling similar devices into the subject geographic areas.
In the case of the market opportunity for PFO products for stroke and migraine, we have relied on estimates developed by a third-party consultant, Easton Associates LLC, that was contracted by us to develop the specific market models in the United States and four countries in Europe.
37
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains certain forward-looking statements and information relating to us that are based on the beliefs of our management as well as assumptions made by, and information currently available to, us, including certain of the statements under "Prospectus Summary," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business." These statements include, but are not limited to, statements about our strategies, plans, objectives, expectations, intentions, expenditures, and assumptions and other statements contained in this prospectus that are not historical facts. When used in this document, words such as "anticipate," "believe," "continue," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should" and "will" and similar expressions are intended to identify forward-looking statements. These statements reflect our current views with respect to future events, are not guarantees of future performance and involve risks and uncertainties that are difficult to predict. Further, certain forward-looking statements are based upon assumptions as to future events that may not prove to be accurate.
Many factors could cause our actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements. These factors include, among other things:
- •
- failure to implement our business strategy;
- •
- failure to capitalize on our expected market opportunities;
- •
- lack of regulatory approval and market acceptance of our new products, product enhancements or new applications for
existing products;
- •
- regulatory developments in key markets for our AMPLATZER occlusion
devices;
- •
- failure to complete our clinical trials or failure to achieve the desired results in our clinical trials;
- •
- inability to successfully commercialize our existing and future research and development programs;
- •
- failure to protect our intellectual property;
- •
- intellectual property claims exposure, related litigation expense, and any resultant damages, awarded royalties or other
remedies, in particular resulting from our Medtronic and Occlutech litigations;
- •
- competition;
- •
- decreased demand for our products;
- •
- product liability claims exposure;
- •
- failure to otherwise comply with laws and regulations;
- •
- changes in general economic and business conditions; and
- •
- changes in currency exchange rates and interest rates.
You should not put undue reliance on any forward-looking statements. You should understand that many important factors, including those discussed under the headings "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" could cause our results to differ materially from those expressed or suggested in any forward-looking statement. All forward-looking statements included in this prospectus are based on information available to us on the date of this prospectus. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
38
Assuming an initial public offering price of $20.00 per share, the midpoint of the initial public offering price range indicated on the cover of this prospectus, we estimate that we will receive net proceeds from this offering of approximately $154.2 million, after deducting underwriting discounts and commissions and other estimated expenses (or approximately $192.7 million if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any). We will not receive any of the proceeds from the sale of shares by the selling stockholders. A member of our board of directors controls the selling stockholders. See "Principal and Selling Stockholders." Each $1.00 increase (decrease) in the assumed initial public offering price of $20.00 per share would increase (decrease) the net proceeds to us from this offering by approximately $7.9 million, assuming the number of shares that we offer, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and other estimated expenses.
We intend to use $50.0 million of our net proceeds from this offering to prepay the principal amount of our 10% senior subordinated PIK notes due 2012 that were issued in 2005, or the 2005 notes, and $15.0 million of our net proceeds from this offering to prepay the principal amount of our 10% senior subordinated PIK notes due 2012 that were issued in 2009, or the 2009 notes. In addition, we will use a portion of our net proceeds from this offering to pay accrued and unpaid interest on the 2005 notes and the 2009 notes. As of June 30, 2009 the amount of accrued and unpaid interest on the 2005 notes was $2.5 million, and the amount of accrued and unpaid interest on the 2009 notes was $0.7 million. Our 2005 notes and 2009 notes are held by one of the WCAS Stockholders. The 2005 notes were issued in connection with our July 2005 reorganization at a discounted issue value of $43.5 million, and the 2009 notes were issued in connection with our acquisition of our former Italian distributor at a discounted issue value of $13.1 million. Both series of notes are required to be prepaid at a price equal to 100% of their face principal amount plus accrued and unpaid interest upon occurrence of our initial public offering. The $6.5 million and $1.9 million of discount from the face value of the 2005 notes and 2009 notes, respectively, are being accreted on our balance sheet to the senior subordinated notes repayment amounts utilizing the respective effective interest rates. As of June 30, 2009, the accreted value of our outstanding 2005 notes and 2009 notes on our balance sheet was $60.4 million. In connection with the repayment of the principal amount of our outstanding 2005 notes and 2009 notes, we will write off unamortized financing costs of $4.6 million and record a charge in the same amount to our statement of operations. Our effective interest rates under the 2005 notes and the 2009 notes as of June 30, 2009 were 12.6% and 15.2%, respectively, compounded semiannually.
We intend to use approximately $47.9 million of our net proceeds from this offering to pay all accrued and unpaid dividends on our Class A common stock and Series A and Series B preferred stock, which are held by the WCAS Stockholders and the Gougeon Stockholders and which will be converted into our common stock immediately prior to completion of this offering.
We intend to use $25.0 million of our net proceeds from this offering to repay indebtedness under our revolving credit facility. Affiliates of Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citigroup Global Markets Inc., and Wells Fargo Securities, LLC are lenders of outstanding indebtedness under our revolving credit facility and will receive a portion of our net proceeds from this offering. As of June 30, 2009, we had $25.0 million outstanding under our revolving credit facility. The interest rate currently applicable to loans under our revolving credit facility is LIBOR plus 200 basis points. This rate is set periodically during the term of the loan. Our effective interest rate for the revolving credit facility as of June 30, 2009 was 2.32%. The revolving credit facility matures on July 20, 2011. In connection with the acquisition of the distribution rights from our former distributor in Italy in January 2009, we borrowed an aggregate amount of $15.5 million under our revolving credit facility. In addition, in March 2009, we borrowed an additional amount of $9.5 million under our revolving credit facility, which was used for general corporate purposes. As a result, we currently have no availability under our revolving credit facility. We will be permitted to reborrow any portion of the indebtedness outstanding under our revolving credit facility that is repaid with a portion of our net proceeds from this offering.
We intend to use any remaining proceeds for working capital and general corporate purposes.
39
In April 2008, in connection with certain transactions among our stockholders, we paid a total amount of $2.5 million in dividends to our stockholders. In connection with our April 2006 recapitalization, we paid a total amount of $99.9 million in dividends to our stockholders, which consisted of: (1) accrued dividends of $11.0 million paid to our Series A preferred and Class A common stockholders and (2) a $0.30 per share dividend to our Series A preferred, Class A common and other common stockholders. Previously, at the time of our July 2005 reorganization, we paid a dividend of $20.0 million, and following our July 2005 reorganization, we paid our remaining stockholder a dividend of $15.0 million. For information on our April 2006 recapitalization and July 2005 reorganization, see "Management's Discussion and Analysis of Financial Condition and Results of Operations." We also intend to use approximately $47.9 million of our net proceeds from this offering to pay all accrued and unpaid dividends on our Class A common stock and Series A and Series B preferred stock, which will be converted into our common stock immediately prior to completion of this offering. We do not, however, expect that any other dividends will be paid to our stockholders in the foreseeable future. Instead, we currently intend to retain all future earnings, if any, for use in the operation of our business and to fund future growth. Any future decision to declare and pay dividends will be at the discretion of our board of directors, after taking into account our financial results, capital requirements and other factors they may deem relevant. As we are a holding company with no independent operations, we depend on our direct and indirect subsidiaries for cash to pay our obligations and make dividend payments. In addition, our ability to pay dividends in the future is dependent upon AGA's receipt of cash from its subsidiaries. Such subsidiaries may be restricted from sending cash to AGA by, among other things, existing law or certain provisions of our senior secured credit facility or the documents governing our future indebtedness that restrict our ability to pay dividends or otherwise distribute cash or other assets. See "Description of Certain Indebtedness" and note 6 to the consolidated financial statements for restrictions on our ability to pay dividends.
40
The following table sets forth our consolidated capitalization as of June 30, 2009 on (1) an actual basis (without giving effect to the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering), (2) a pro forma basis to give effect to:
- •
- the conversion of our Class A and Class B common stock and all of our Series A and Series B
preferred stock into our common stock immediately prior to completion of this offering; and
- •
- the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering, as if each had occurred on June 30, 2009; and
- •
- the sale of 8,500,000 shares of common stock by us at an offering price of $20.00 per share, the midpoint of
the initial public offering price range indicated on the cover of this prospectus; and
- •
- the intended use of the net proceeds received by us in this offering, including to repay debt and pay dividends, as set forth in "Use of Proceeds," as if each had occurred on June 30, 2009.
(3) on a pro forma as further adjusted basis to give effect to:
You should read this table in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus, "Use of Proceeds," "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations."
| |
As of June 30, 2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands, except for number of shares)
|
Actual | Pro Forma(1) | Pro Forma as Adjusted |
|||||||||
Long-term debt, less current portion: |
||||||||||||
Term loan facility |
$ | 196,963 | $ | 196,963 | $ | 196,963 | ||||||
Revolving credit facility |
25,000 | 25,000 | — | |||||||||
Long-term obligations to former distributors, less discounts |
9,073 | 9,073 | 9,073 | |||||||||
Senior subordinated PIK notes payable, less discount |
60,384 | 60,384 | — | |||||||||
|
291,420 | 291,420 | 206,036 | |||||||||
Series A preferred stock, $0.001 par value; 148,524 shares authorized and 128,524 shares issued and outstanding actual, no shares authorized, issued and outstanding pro forma and pro forma as adjusted |
174,014 | 45,490 | — | |||||||||
Series B preferred stock, $0.001 par value; 1,879 shares authorized and 1,879 shares issued and outstanding actual, no shares authorized, issued and outstanding pro forma and pro forma as adjusted |
1,971 | 92 | — | |||||||||
Class A common stock, $0.01 par value; 20,000,000 shares authorized and 6,600,000 shares issued and outstanding actual, no shares authorized, issued and outstanding pro forma and pro forma as adjusted |
8,937 | 2,337 | — | |||||||||
|
184,922 | 47,919 | — | |||||||||
41
| |
As of June 30, 2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands, except for number of shares)
|
Actual | Pro Forma(1) | Pro Forma as Adjusted |
|||||||||
Stockholders' equity (deficit): |
||||||||||||
Common stock, $0.01 par value; 340,000,000 shares authorized, 147,000,000 shares issued and outstanding actual, 400,000,000 shares authorized and 39,555,683 issued and outstanding pro forma and 48,055,683 issued and outstanding pro forma as adjusted |
1,470 | 396 | 481 | |||||||||
Class B common stock, $0.01 par value; 35,000,000 shares authorized, 16,000 shares issued and outstanding actual, no shares authorized, issued and outstanding pro forma and pro forma as adjusted |
— | — | — | |||||||||
Additional paid-in capital |
— | 136,813 | 290,879 | |||||||||
Excess purchase price over predecessor basis |
(63,500 | ) | (63,500 | ) | (63,500 | ) | ||||||
Accumulated other comprehensive income |
(1,215 | ) | (1,215 | ) | (1,215 | ) | ||||||
Accumulated deficit |
(173,813 | ) | (172,549 | ) | (177,166 | ) | ||||||
Total stockholders' equity (deficit)(2) |
(237,058 | ) | (100,055 | ) | 49,479 | |||||||
Total capitalization(2)(3) |
$ | 239,284 | $ | 239,284 | $ | 255,515 | ||||||
- (1)
- Amounts
outstanding for Series A and Series B preferred stock and Class A common stock reflect amounts of accrued and unpaid dividends
that would be outstanding immediately after giving effect to the conversion of the securities into our common stock immediately prior to completion of this offering. We intend to use the net proceeds
of this offering to, among other things, repay such accrued and unpaid dividends. See "Use of Proceeds."
- (2)
- Each
$1.00 increase (decrease) in the assumed offering price per share would increase (decrease) our total capitalization and stockholders' equity (deficit)
by approximately $7.9 million.
- (3)
- Total capitalization consists of long-term debt, less current portion, redeemable equity (Series A and Series B preferred and Class A common stock) and stockholders' equity (deficit).
The table set forth above is based on the number of shares of our common stock outstanding as of June 30, 2009. This table assumes no exercise of the underwriters' option to purchase 2,062,500 additional shares of common stock to cover overallotments, if any, and does not reflect:
- •
- 2,897,637 shares of our common stock issuable upon the exercise of outstanding stock options at a weighted average
exercise price of $10.18 per share as of June 30, 2009, 1,306,834 of which were then exercisable;
- •
- approximately 46,152 shares of our common stock issuable upon the exercise of stock options that we expect to grant to
certain of our directors in connection with this offering at an exercise price equal to the offering price, none of which will then be exercisable; and
- •
- 2,510,755 shares of our common stock reserved for future grants under our 2008 Equity Incentive Plans and our Employee Stock Purchase Plan, which will be adopted in connection with this offering.
42
Dilution is the amount by which the offering price paid by the purchasers of the common stock to be sold in this offering exceeds the net tangible book value per share of common stock after this offering. Our pro forma net tangible book value per share presented below is determined by subtracting our total liabilities from the total book value of our tangible assets (total assets less intangible assets) as of June 30, 2009 and dividing the difference by the number of shares of our common stock outstanding immediately prior to completion of this offering after giving effect to the conversion of our Class A and Class B common stock and all of our Series A and Series B preferred stock into our common stock immediately prior to completion of this offering, and the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering.
Our pro forma net tangible book value as of June 30, 2009 would have been a deficit of $306.1 million, or $7.74 per share. After giving effect to the sale of 8,500,000 shares of common stock by us at an offering price of $20.00 per share, the midpoint of the initial public offering price range indicated on the cover of this prospectus, and the intended use of the proceeds received by us in this offering, including to repay debt and pay dividends, our pro forma as adjusted net tangible book value as of June 30, 2009 would have been a deficit of approximately $156.6 million, or $3.26 per share (assuming that the underwriters' overallotment option is not exercised), or a deficit of approximately $118.0 million, or $2.36 per share, (assuming that the underwriters' overallotment option is exercised in full). This represents an immediate increase in pro forma net tangible book value of $4.48 per share (or $5.38 per share assuming that the underwriters' overallotment option is exercised in full) to existing stockholders and an immediate dilution of $23.26 per share (or $22.36 per share assuming that the underwriters' overallotment option is exercised in full) to new investors purchasing shares of our common stock in this offering. We will not receive any of the proceeds from the sale of shares by the selling stockholders.
The following table illustrates this substantial and immediate per share dilution to new investors:
| |
Per Share | |||||||
|---|---|---|---|---|---|---|---|---|
Assumed initial public offering price |
$ | 20.00 | ||||||
Pro forma net tangible book value as of June 30, 2009 |
$ | (7.74 | ) | |||||
Increase in net tangible book value attributable to existing investors |
4.48 | |||||||
Pro forma as adjusted net tangible book value after this offering |
$ | (3.26 | ) | |||||
Dilution to new investors |
$ | 23.26 | ||||||
Each $1.00 increase or decrease in the offering price per share would increase or decrease the pro forma as adjusted net tangible book value by $0.17 per share and the dilution to new investors in the offering by $0.17 per share, assuming that the number of shares offered in this offering, as set forth on the cover page of this prospectus, remains the same. Our net tangible book value following completion of this offering is subject to adjustment based on the actual offering price of our common stock and other terms of this offering determined at pricing.
The table and discussion above assumes no exercise of any stock options after June 30, 2009. As of June 30, 2009, there were outstanding options to purchase a total of 2,897,637 shares of our common stock at an average exercise price of $10.18 per share. To the extent that all of these options are exercised, there will be dilution to new investors.
The following table summarizes, on the same pro forma as adjusted basis as of June 30, 2009, the total number of shares of common stock purchased from us, the total cash consideration paid to us and
43
the average price per share paid by the existing stockholders and by new investors purchasing common shares in this offering.
| |
Shares Purchased | Total Consideration | |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Average Price per Share |
||||||||||||||||
| |
Number | Percent | Amount | Percent | |||||||||||||
Existing stockholders |
39,555,683 | 82.31 | % | $ | 130,419,100 | 43.4 | % | $ | 3.30 | ||||||||
New investors |
8,500,000 | 17.69 | 170,000,000 | 56.6 | 20.00 | ||||||||||||
Total |
48,055,683 | 100 | % | $ | 300,419,100 | 100 | % | $ | 6.25 | ||||||||
Sales by the selling stockholders in this offering will reduce the number of shares held by existing stockholders to 34,305,683, or 71.4% of the total number of shares of our common stock to be outstanding after the offering, and will increase the number of shares held by new investors to 13,750,000, or 28.6% of the total number of shares of our common stock to be outstanding after the offering. If the underwriters exercise their overallotment option in full, the percentage of shares of common stock held by existing stockholders will decrease to 68.4% of the total number of shares of our common stock outstanding after the offering, and the number of shares of our common stock held by new public investors will increase to 15,812,500, or 31.6% of the total shares of our common stock outstanding after this offering.
44
SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected consolidated financial data for the periods indicated. We derived the selected consolidated financial data presented below as of and for the year ended December 31, 2004, as of and for the period from January 1, 2005 to July 27, 2005, and the period from July 28, 2005 to December 31, 2005 and as of December 31, 2006 from our audited consolidated financial statements not included in this prospectus. We derived the selected consolidated financial data presented below as of December 31, 2007 and 2008 and for the years ended December 31, 2006, 2007 and 2008 from our audited consolidated financial statements included elsewhere in this prospectus. We derived the consolidated selected financial data for the six months ended June 30, 2008 and 2009 from our unaudited consolidated interim financial statements included elsewhere in this prospectus. We have prepared the unaudited consolidated interim financial information set forth below on the same basis as our audited consolidated financial statements and have included all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair presentation of our financial position and operating results for such periods. The interim results set forth below are not necessarily indicative of results for the year ended December 31, 2009 or for any other period.
The pro forma basic and diluted weighted average shares and pro forma basic and diluted net loss per common share data in the selected consolidated financial data table presented below are unaudited and give effect to the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering for Successor periods.
On July 28, 2005, the stockholders of AGA Medical contributed all of the outstanding shares of AGA Medical to AGA in exchange for shares of AGA. As a result of that, AGA Medical became a wholly owned subsidiary of AGA. All periods prior to July 28, 2005 are referred to as "Predecessor", and all periods on or after such date are referred to as "Successor." The selected financial information for Predecessor periods represents consolidated financial information of AGA Medical and its consolidated subsidiaries and the selected financial information for all Successor periods represents financial information of AGA and its consolidated subsidiaries. The financial statements for all Successor periods are not comparable to those of Predecessor periods.
Our historical results are not necessarily indicative of future operating results. The following selected historical consolidated financial data should be read in conjunction with, and are qualified in their entirety by reference to, "Prospectus Summary—Summary Consolidated Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and the related notes included elsewhere in this prospectus.
45
| |
|
|
Successor | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Predecessor | |||||||||||||||||||||||||
| |
|
|
|
|
Six Months Ended June 30, |
|||||||||||||||||||||
| |
|
Period From January 1, to July 27, 2005 |
Period From July 28, to December 31, 2005 |
Year Ended December 31, | ||||||||||||||||||||||
| (in thousands, except per share data) |
2004 | 2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||||||||||
| |
|
|
|
|
|
|
(Unaudited) |
|||||||||||||||||||
Statement of Operations Data: |
||||||||||||||||||||||||||
Net sales |
$ | 87,631 | $ | 58,206 | $ | 39,917 | $ | 127,529 | $ | 147,255 | $ | 166,896 | $ | 80,845 | $ | 94,381 | ||||||||||
Cost of goods sold |
19,955 | 11,580 | 8,967 | 24,985 | 22,819 | 26,635 | 12,456 | 17,004 | ||||||||||||||||||
Gross profit |
67,676 | 46,626 | 30,950 | 102,544 | 124,436 | 140,261 | 68,389 | 77,377 | ||||||||||||||||||
Operating expenses: |
||||||||||||||||||||||||||
Selling, general and administrative |
26,131 | 14,145 | 15,035 | 37,515 | 50,190 | 65,669 | 31,757 | 46,456 | ||||||||||||||||||
Research and development |
5,098 | 4,012 | 3,084 | 12,096 | 26,556 | 32,760 | 16,210 | 16,477 | ||||||||||||||||||
Amortization of intangible assets |
— | — | 5,099 | 12,682 | 15,233 | 15,540 | 7,690 | 9,894 | ||||||||||||||||||
Change in purchase consideration |
— | — | — | — | — | — | — | (698 | ) | |||||||||||||||||
Litigation settlement |
— | — | 29,000 | — | — | — | — | — | ||||||||||||||||||
FCPA settlement |
— | — | — | — | 2,000 | — | — | — | ||||||||||||||||||
In-process research and development |
— | — | 50,800 | — | — | — | — | — | ||||||||||||||||||
Loss (gain) on disposal of property and equipment |
841 | — | 26 | 709 | (3 | ) | 68 | 14 | (26 | ) | ||||||||||||||||
Operating income (loss) |
35,606 | 28,469 | (72,094 | ) | 39,542 | 30,460 | 26,224 | 12,718 | 5,274 | |||||||||||||||||
Investment income (loss) |
145 | (166 | ) | 193 | 754 | (751 | ) | (1,202 | ) | (627 | ) | (2,352 | ) | |||||||||||||
Interest income |
989 | 777 | 423 | 1,174 | 426 | 230 | 110 | 61 | ||||||||||||||||||
Interest income—related party |
213 | 394 | — | — | 6 | — | — | — | ||||||||||||||||||
Interest expense |
(11 | ) | — | (6,418 | ) | (22,893 | ) | (21,213 | ) | (16,492 | ) | (8,595 | ) | (8,149 | ) | |||||||||||
Other income, (expense), net |
(384 | ) | 340 | (91 | ) | 957 | 994 | 722 | 295 | 1,275 | ||||||||||||||||
Income (loss) before income taxes |
36,558 | 29,814 | (77,987 | ) | 19,534 | 9,922 | 9,482 | 3,901 | (3,891 | ) | ||||||||||||||||
(Provision) benefit for income taxes |
(12,708 | ) | (10,565 | ) | 9,926 | (6,909 | ) | (3,844 | ) | (386 | ) | (1,548 | ) | (306 | ) | |||||||||||
Net income (loss) |
23,850 | 19,249 | (68,061 | ) | 12,625 | 6,078 | 9,096 | 2,353 | (4,197 | ) | ||||||||||||||||
Less Series A and Series B preferred stock and Class A common stock dividends |
— | — | (6,271 | ) | (59,410 | ) | (15,372 | ) | (17,067 | ) | (8,815 | ) | (8,471 | ) | ||||||||||||
Net income (loss) applicable to common stockholders |
$ | 23,850 | $ | 19,249 | $ | (74,332 | ) | $ | (46,785 | ) | $ | (9,294 | ) | $ | (7,971 | ) | $ | (6,462 | ) | $ | (12,668 | ) | ||||
Net income (loss) per common share—basic and diluted |
$ | 59.62 | $ | 48.12 | $ | (0.45 | ) | $ | (0.28 | ) | $ | (0.06 | ) | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.08 | ) | ||||
Cash dividends per Class A common stock |
— | — | $ | 0.00 | $ | 0.30 | $ | 0.00 | $ | 0.00 | $ | 0.00 | $ | 0.00 | ||||||||||||
Cash dividends per share of common stock |
$ | 0.00 | $ | 0.00 | $ | 0.10 | $ | 0.30 | $ | 0.00 | $ | 0.00 | $ | 0.00 | $ | 0.00 | ||||||||||
Weighted average shares—basic and diluted |
400 | 400 | 167,000 | 167,000 | 161,236 | 153,600 | 153,600 | 153,600 | ||||||||||||||||||
Pro forma net income (loss) per common share—basic and diluted (unaudited)(1) |
$ | (3.18 | ) | $ | (2.00 | ) | $ | (0.41 | ) | $ | (0.37 | ) | $ | (0.30 | ) | $ | (0.59 | ) | ||||||||
Pro forma cash dividends per Class A common stock (unaudited)(1) |
$ | 0.00 | $ | 2.15 | $ | 0.00 | $ | 0.00 | $ | 0.00 | $ | 0.00 | ||||||||||||||
Pro forma cash dividends per share of common stock (unaudited)(1) |
$ | 0.72 | $ | 2.15 | $ | 0.00 | $ | 0.00 | $ | 0.00 | $ | 0.00 | ||||||||||||||
Pro forma weighted average shares—basic and diluted (unaudited)(1) |
23,356 | 23,356 | 22,550 | 21,482 | 21,482 | 21,482 | ||||||||||||||||||||
| |
Predecessor | Successor | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
As of December 31, | |
|
|
|
|
|||||||||||||
| |
As of December 31, | |
|||||||||||||||||
| |
As of June 30, 2009 |
||||||||||||||||||
| (in thousands) |
2004 | 2005 | 2006 | 2007 | 2008 | ||||||||||||||
| |
|
|
|
|
|
(Unaudited) |
|||||||||||||
Balance Sheet Data: |
|||||||||||||||||||
Cash and cash equivalents |
$ | 18,250 | $ | 17,707 | $ | 8,190 | $ | 13,854 | $ | 22,808 | $ | 8,798 | |||||||
Working capital |
74,685 | 27,061 | 27,080 | 16,454 | 30,546 | 22,563 | |||||||||||||
Total assets |
116,513 | 282,372 | 258,794 | 256,015 | 272,328 | 326,282 | |||||||||||||
Long-term debt, less current portion |
— | 140,151 | 242,589 | 242,600 | 253,442 | 291,420 | |||||||||||||
Redeemable convertible Series A and Series B preferred stock and Class A common stock |
— | 154,795 | 158,425 | 158,701 | 174,571 | 184,922 | |||||||||||||
Total stockholders' equity (deficit) |
96,528 | (121,140 | ) | (210,568 | ) | (217,769 | ) | (226,458 | ) | (237,058 | ) | ||||||||
- (1)
- Pro forma presentation gives effect to the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering for Successor periods.
46
UNAUDITED PRO FORMA COMBINED FINANCIAL INFORMATION
The following unaudited pro forma combined financial information illustrates the estimated effects on our historical financial results of operations of (1) the acquisition, which was effective on January 1, 2009, of AB Medica/AGA Division (the "Italy Acquisition") and (2) the receipt of $22.7 million from (A) the issuance by AGA Medical on January 5, 2009 to one of the WCAS Stockholders for an aggregate purchase price of $15.0 million of (i) an aggregate principal amount of $15.0 million of 10% senior subordinated PIK notes due 2012 and (ii) 1,879 shares of Series B preferred stock at a purchase price of $1,000 per share and (B) borrowings in the aggregate amount of $15.5 million under our revolving credit facility to finance the Italy Acquisition (together with the Italy Acquisition, the "Italy Transactions"). The unaudited pro forma combined financial information gives effect to the Italy Transactions as if they had occurred on January 1, 2008.
The unaudited pro forma combined financial information has been derived by adding to our audited historical consolidated financial statements included elsewhere in this prospectus, the AB Medica/AGA Division financial information relating to the period preceding its inclusion in our consolidated financial statements, which was extracted from AB Medica/AGA Division's audited financial statements and notes to financial statements included elsewhere in this prospectus.
We then applied the above described adjustments relating to the Italy Transactions to such combined financial information.
The unaudited pro forma combined financial information is not necessarily indicative of our financial position or results of operations that would have occurred if the Italy Transactions had been consummated as of the dates indicated, nor should it be construed as being representative of our future financial position or results of operations. All pro forma adjustments and their underlying assumptions are described more fully in the notes to our unaudited pro forma combined financial statements.
The unaudited pro forma adjustments are based upon available information and certain assumptions that we believe are reasonable under the circumstances. These adjustments are more fully described in the notes to the unaudited pro forma combined financial information below. In the opinion of management, all adjustments have been made that are necessary to present fully the unaudited pro forma data. The unaudited pro forma combined financial information is presented for informational purposes only.
The pro forma basic and diluted weighted average shares and pro forma basic and diluted net loss per common share data presented below are unaudited and give effect to the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering.
The Transaction Adjustments in the unaudited pro forma combined financial information do not give effect to this offering, including (1) the conversion of our Class A and Class B common stock and all of our Series A and Series B preferred stock into our common stock immediately prior to the consummation of this offering, (2) the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering, (3) the application of our proceeds from this offering to prepay the principal and accrued and unpaid interest on our 2005 notes and 2009 notes, (4) the application of our net proceeds from this offering to pay accrued and unpaid dividends on our Class A common stock and Series A and Series B preferred stock and (5) the application of our net proceeds from this offering to repay indebtedness outstanding under our revolving credit facility.
The unaudited pro forma financial information should be read in conjunction with the accompanying notes and assumptions, "Summary—Recent Developments," "Capitalization," "Selected Consolidated Financial Data," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and both sets of consolidated financial statements and notes to consolidated financial statements and the other financial information included elsewhere in this prospectus.
47
Unaudited Pro Forma Combined Statement of Operations
For the Year Ended December 31, 2008
(in thousands, except per share amounts)
| |
AGA Medical Holdings, Inc. |
AB Medica/AGA Division(1) |
Combined | Transaction Adjustments |
Pro Forma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net sales |
$ | 166,896 | $ | 26,201 | $ | 193,097 | $ | (9,772 | )(2) | $ | 183,325 | ||||||
Cost of goods sold |
26,635 | 9,772 | 36,407 | (9,772 | )(2) | 26,635 | |||||||||||
Gross profit |
140,261 | 16,429 | 156,690 | — | 156,690 | ||||||||||||
Operating expenses: |
|||||||||||||||||
Selling, general and administrative |
65,669 | 7,218 | 72,887 | — | 72,887 | ||||||||||||
Research and development |
32,760 | — | 32,760 | — | 32,760 | ||||||||||||
Amortization of intangible assets |
15,540 | 116 | 15,656 | 4,615 | (3) | 20,271 | |||||||||||
Loss (gain) on disposal of property and equipment |
68 | — | 68 | — | 68 | ||||||||||||
Operating income |
26,224 | 9,095 | 35,319 | (4,615 | ) | 30,704 | |||||||||||
Investment income (loss) |
(1,202 | ) | — | (1,202 | ) | — | (1,202 | ) | |||||||||
Interest income |
230 | 50 | 280 | — | 280 | ||||||||||||
Interest expense |
(16,492 | ) | (322 | ) | (16,814 | ) | (1,384 | )(4) | (18,198 | ) | |||||||
Other income (expense), net |
722 | (31 | ) | 691 | — | 691 | |||||||||||
Income before income taxes |
9,482 | 8,792 | 18,274 | (5,999 | ) | 12,275 | |||||||||||
(Provision) benefit for income taxes |
(386 | ) | (3,017 | ) | (3,403 | ) | 2,040 | (5) | (1,363 | ) | |||||||
Net income |
9,096 | 5,775 | 14,871 | (3,959 | ) | 10,912 | |||||||||||
Less dividends |
(17,067 | ) | (7,504 | ) | (24,571 | ) | 7,504 | (6) | (17,067 | ) | |||||||
Net income (loss) applicable to common stockholders |
$ | (7,971 | ) | $ | (1,729 | ) | (9,700 | ) | 3,545 | (6,155 | ) | ||||||
Net income (loss) per common share—basic and diluted |
$ | (0.05 | ) | $ | (0.04 | ) | |||||||||||
Cash dividends per Class A common stock |
$ | 0.00 | $ | 0.00 | |||||||||||||
Cash dividends per share of common stock |
$ | 0.00 | $ | 0.00 | |||||||||||||
Weighted average shares—basic and diluted |
153,600 | 153,600 | |||||||||||||||
Pro forma net income (loss) per common share—basic and diluted (unaudited)(7) |
$ | (0.37 | ) | $ | (0.29 | ) | |||||||||||
Pro forma cash dividends per Class A common stock (unaudited)(7) |
$ | 0.00 | $ | 0.00 | |||||||||||||
Pro Forma cash dividends per share of common stock (unaudited)(7) |
$ | 0.00 | $ | 0.00 | |||||||||||||
Pro forma weighted average shares—basic and diluted (unaudited)(7) |
21,482 | 21,482 | |||||||||||||||
48
Notes to Unaudited Pro Forma Combined Financial Statements
(in thousands)
- (1)
- Results
for AB Medica/AGA Division have been converted from euros to dollars at the rate of 1.4639 dollars for each euro, representing the average exchange
rate for 2008.
- (2)
- Represents
the elimination of $9,772 in sales made by us to AB Medica/AGA Division during 2008.
- (3)
- Represents
the amortization by us resulting from identifiable intangibles of $4,615, as if the acquisition was made at the beginning of the year. The
purchase price was allocated between tangible assets and intangible assets (including goodwill). The excess purchase price over the fair value of underlying assets acquired and liabilities assumed was
allocated to goodwill. The acquired intangible assets, all of which are being amortized, have a weighted average useful life of approximately eight years. The intangible assets include a customer list
valued at $35,700 and a noncompete agreement valued at $3,500. The fair value of the identifiable intangible assets and inventory were determined by management. See note 4 to our consolidated
financial statements included elsewhere in this prospectus.
- (4)
- Represents
the adjustment to interest expense totaling $1,384 resulting from the financing of the Italy Acquisition from our incurrence of $22,700 of
indebtedness at an assumed 6.10% weighted-average interest rate. Substantially concurrently with the consummation of the Italy Acquisition, we borrowed $15,500 under our revolving credit facility and
issued $15,000 of our 2009 notes at assumed interest rates of 2.32% and 10%, respectively, which represent the actual rates at June 30, 2009. A change of one percentage point in the weighted
average interest rate would cause an increase or decrease in interest expense of approximately $227 on an annual basis. Such indebtedness was used to finance the Italy Acquisition ($22,700) and for
other corporate purposes ($7,800).
- (5)
- Represents
the estimated tax effect of the pro forma adjustments utilizing an assumed blended statutory tax rate of 34%.
- (6)
- Represents
the elimination of $7,504 in AB Medica/AGA Division dividends paid to its parent, AB Medica S.p.A., in 2008.
- (7)
- Pro forma presentation gives effect to the 7.15 for 1.00 reverse stock split of our common stock to occur immediately prior to the completion of this offering.
49
MANAGEMENT'S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read this discussion together with the consolidated financial statements, related notes and other financial information included in this prospectus. The following discussion may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed under "Risk Factors" and elsewhere in this prospectus. These risks could cause our actual results to differ materially from any future performance suggested below. Accordingly, you should read "Cautionary Notice Regarding Forward-Looking Statements" and "Risk Factors."
Overview
We are a leading innovator and manufacturer of medical devices for the minimally invasive treatment of structural heart defects and vascular diseases, which we market under the AMPLATZER brand. We were founded in 1995 to capitalize on the attributes of nitinol to make occlusion devices for the transcatheter treatment of structural heart defects, and our AMPLATZER occlusion devices initially focused on the treatment of these defects. We received a CE Mark in Europe for our occlusion devices and related delivery systems in 1998. In 2001, we received U.S. regulatory approval to commercialize our AMPLATZER Septal Occluder, which addresses one of the largest treatment areas of the structural heart defect market. We received U.S. regulatory approval to commercialize our AMPLATZER Duct Occluder device in 2003 and our AMPLATZER Muscular VSD Occluder device in 2007.
Our AMPLATZER occlusion devices utilize our expertise in braiding nitinol and designing transcatheter delivery systems. Historically, the majority of our sales were to interventional cardiologists to treat a range of structural heart defects. We have recently leveraged our core competencies in braiding nitinol and designing transcatheter delivery systems to develop products for the treatment of certain vascular diseases. Our vascular products are sold through a separate sales force targeted at interventional radiologists and vascular surgeons. Our first products in this area, which we launched in the United States in September 2003 and in Europe in January 2004, are vascular plugs for the closure of abnormal blood vessels that develop outside the heart. A second version of our vascular plug was approved and launched in the United States and Europe in August 2007, and a third version is under review by the regulatory authorities in the United States and Europe.
We are also focused on capitalizing on the growing body of evidence that links the presence of PFOs to certain types of strokes and migraines. We estimate that the market opportunity for PFO closure in the prevention of recurrent cryptogenic strokes in the United States and Europe is greater than $1 billion annually and potentially even greater for the treatment of migraines. Since 2001, we have initiated four PFO clinical trials, two focused on strokes and two focused on migraines. These clinical trials have significantly increased our research and development expenses.
Our other products in development include a new version of our AMPLATZER Duct Occluder, which received a CE Mark in February 2008 and will require a clinical trial to support U.S. approval, additional versions of our AMPLATZER Vascular Plugs, an occlusion device to close the Left Atrial Appendage, or LAA, and to prevent strokes, which device received a CE Mark in February 2008, and vascular grafts to treat aneurysms. Our AMPLATZER Vascular Plug III received a CE Mark in Europe in May 2008, and we applied for FDA approval in the United States in February 2008. The FDA has requested additional non-clinical testing of the device, and we are in the process of completing that testing and answering all remaining questions from the FDA. Our AMPLATZER Vascular Plug IV received a CE Mark in Europe in July 2009. We had previously intended to file for a CE Mark for the AMPLATZER Vascular Plug IV and an AMPLATZER Vascular Graft by the end of 2008. In the case of the AMPLATZER Vascular Plug IV, we decided to modify the design to improve the product's performance and subsequently filed for a CE Mark in March 2009. The CE Mark submission for the AMPLATZER Vascular Graft was delayed due to an issue with the delivery system in connection with
50
the final engineering validation of our graft. That issue was subsequently resolved, and we filed for CE Mark clearance in June 2009. We have applied to the FDA for U.S. approval to conduct a clinical trial for the new version of our AMPLATZER Duct Occluder and expect to apply to the FDA for approval to begin new clinical trials to support U.S. approval of other products in 2009. We believe that our new products for prevention of strokes related to the LAA and our family of vascular grafts will likely require clinical trials in order to receive U.S. regulatory approval.
On July 28, 2005, we implemented a corporate reorganization, which we refer to as our July 2005 reorganization. As part of our July 2005 reorganization, AGA Medical purchased and redeemed all of the outstanding shares of common stock owned by one of its two then existing stockholders. To finance our July 2005 reorganization, AGA Medical (1) issued an aggregate principal amount of $50.0 million of the 2005 notes, which were purchased by one of the WCAS Stockholders at a discount, (2) issued 128,524 shares of Series A preferred stock to the WCAS Stockholders at a purchase price of $1,000 per share and (3) borrowed $107.0 million under a $122.0 million senior credit facility, consisting of a $107.0 million senior term loan and a $15.0 million revolving credit facility. The remaining stockholder and new investors subsequently contributed all of their outstanding shares in AGA Medical to AGA in exchange for shares of AGA.
On April 28, 2006, we completed a $240.0 million recapitalization, which we refer to as our April 2006 recapitalization. As part of our April 2006 recapitalization, we entered into an amended and restated senior secured credit facility consisting of a $215.0 million seven-year Tranche B term loan facility and a $25.0 million revolving credit facility, which we refer to collectively as our senior secured credit facility. The Tranche B term loan was drawn in full in April 2006, and the proceeds were used to pay off existing senior debt, accrued dividends of $11.0 million to the Series A preferred and Class A common stockholders, a $0.30 per share dividend to all Series A preferred, Class A common and other common stockholders, and transaction-related expenses, as well as to fund general working capital needs. On October 5, 2008, Lehman Commercial Paper Inc. ("LCPI") filed for protection under Chapter 11 of the Federal Bankruptcy Code. LCPI had committed to provide $9.5 million under the $25 million revolving credit facility. In March 2009, Bank of America, N.A. assumed the commitment under our revolving credit facility previously held by LCPI. At June 30, 2009, we had outstanding borrowings of $25.0 million under our revolving credit facility. As a result, we currently have no additional availability under our revolving credit facility. We will be permitted to reborrow any portion of the indebtedness outstanding under our revolving credit facility that is repaid with a portion of our net proceeds from this offering.
In January 2007, Medtronic, Inc. filed a patent infringement action against us in the U.S. District Court for the Northern District of California, alleging that substantially all of our AMPLATZER occluder and vascular plug devices, which have historically accounted for substantially all of our net sales, infringe three of Medtronic's method and apparatus patents on shape memory alloy stents (U.S. Patent Nos. 5, 190,546, 6,306,141 and 5,067,957). Medtronic is seeking compensatory damages with respect to our products manufactured or sold in the United States. Medtronic asserted but later withdrew its requests for injunctive relief and for damages based on willfulness.
We have asserted defenses and counterclaims for non-infringement and challenged the validity and enforceability of Medtronic's patents. On April 28, 2009, the court granted summary judgment in our favor finding that we do not infringe Medtronic's '546 patent. Subsequently, Medtronic withdrew certain allegations with respect to the remaining two patents. The trial on the remaining issues in the case was divided into a jury trial phase and non-jury, bench trial phase.
The issues of infringement and certain issues of validity based on obviousness and anticipation of the asserted claims in the two remaining patents were the subject of a jury trial before the U.S. District Court for the Northern District of California that began on July 6, 2009. On August 5, 2009, the jury returned a verdict that the subject AMPLATZER occluder and vascular plug products infringed the
51
claims at issue with respect to both of the two remaining Medtronic patents and that the Medtronic patent claims at issue had not been proven by us to be invalid. The jury verdict awarded Medtronic damages of $57.8 million. This amount is equal to 11% of historical sales of the occluder and vascular plug products in question during the timeframe specified for each patent. Any infringement of the '141 apparatus patent after March 31, 2009 to the date of a final, non-appealable judgment will be considered in calculating the final amount of damages, if any, to be paid. The '957 patent expired in May 2004. Because the issue was not before it, the jury made no determination regarding the payment of royalties on future sales of the company's products after the date of a final, non-appealable judgment. The verdict is not enforceable until the completion of the trial and entry of a final, non-appealable judgment. If we do not receive a favorable judgment, we expect we will appeal such judgment and expect that we will likely be required to post a bond in order to be allowed to appeal as set forth below. The verdict is not enforceable because a judgment has not yet been entered, and as a matter of law only judgments are enforceable for purposes of execution against the non-prevailing party. A judgment has not yet been entered because all claims have not yet been adjudicated between the parties and the court has not yet ordered a judgment be entered. In addition, on August 5, 2009, Medtronic's counsel agreed with the judge on the record that a judgment would not be entered until after the non-jury trial phase is completed. Furthermore, upon approval of an appeal bond, the execution of any appealed judgment is stayed during the appeal.
On August 19, 2009, the United States Court of Appeal for the Federal Circuit, sitting en banc, issued a decision in Cardiac Pacemakers, Inc. v. St. Jude Medical, Inc.,. In Cardiac Pacemakers, the Federal Circuit established a new rule of law and held as a matter of law that the practice of a method claim of a patent outside of the United States cannot infringe a United States method patent. When a controlling new rule of law is announced that affects the parties to a patent litigation, the court will ordinarily apply the supervening change in law retroactively to a pending case. In our litigation with Medtronic, a portion of the jury's damage award was based on a finding of infringement of Medtronic's '957 method patent for sales of our products outside of the United States, and we believe it is therefore directly contrary to the holding as stated in the Cardiac Pacemakers decision. Applying the rate of damages established by the jury, 11% of historical sales, to the total sales of our products outside of the United States during the period for which we believe damages were awarded for infringement of the '957 patent would result in a reduction of approximately $14 million. Such $14 million of damages relates only to periods of time before the '141 patent was enforceable against us. As a result, we believe it is likely that the trial court in our case will reduce the jury's damage award by approximately such amount to reflect this recent decision, although there can be no assurance that Cardiac Pacemakers will not be reviewed and overturned by the United States Supreme Court or that the damage award will be reduced by this or any other amount. On September 8, 2009, we filed a motion seeking, at Medtronic's choice, either a new trial or the above-mentioned reduction of $14 million, which motion is publicly available. We do not expect a ruling on this motion until the conclusion of the non-jury phase of the trial in early 2010.
Following the jury verdict, the court scheduled the non-jury phase of the trial for early December 2009, which will deal with other issues of invalidity and unenforceability of one or both of the two remaining patents based on equitable claims of double patenting, equitable estoppel, inequitable conduct in patent prosecution and laches. The claims to be tried in the non-jury phase of the trial are claims "in equity" and for which there is no right to a trial by jury. Therefore, the trial was essentially bifurcated into a jury phase and non-jury phase subject to the same procedures. In the event the judge finds in our favor on one or more of our counterclaims in the non-jury phase of the trial, she can fashion an equitable remedy to compensate us for Medtronic's conduct, including barring all damages prior to filing of the lawsuit, barring past and future enforcement of the '141 patent alone, or eliminating all past and future damages on both of the patents in suit. Upon conclusion of the non-jury phase of the trial, the court will consider post-trial motions and enter a judgment, which we expect will take place in early 2010. As part of its decision, the trial court could order a new trial on some or all
52
of the issues in the case or amend or reaffirm the jury verdict based on one or more issues regarding infringement, validity, enforceability and damages. We also expect the trial court to decide whether any royalty payments relating to the '141 patent, which does not expire until 2018, are due for future periods and, if so, at what rate. Any such royalties may not be on commercially reasonable terms, and may exceed the 11% rate of damages applied by the jury. If any damage amount is entered as a part of the judgment, we will likely be required at that time to accrue a non-cash charge equal to the amount of such damages. Any such accrual will have an adverse effect on our results of operations for the applicable period.
Thereafter, the judgment will be subject to appeal which could result in the judgment being affirmed or amended, or in an order for a new trial on one or more of the same issues raised before the trial court. If we decide to appeal, such appeal may not be decided for several months and will likely require the posting of a bond in the face amount of 150% of the judgment amount, if any. We do not expect the cash cost associated with posting such a bond to be material, but we would likely be required to secure such bond with collateral. The amount of collateral required by the provider of the bond would be determined based on several factors, such as the amount of our debt and our financial condition.
These proceedings are costly and time consuming, and we cannot assure you that the outcome of any of these proceedings will be favorable to us. However, because Medtronic withdrew its request for injunctive relief from the trial court, we believe that it is likely that the final judgment will not prevent the continued marketing or sale of any our products. If we do not prevail before the trial or appellate courts in overturning the jury verdict or reducing or eliminating the damages award in the jury verdict, we will have to pay the awarded damages and may have to pay royalties on a substantial portion of our future sales, and our results of operations and financial condition may be materially adversely affected. Medtronic also could appeal from rulings adverse to it, which could result in an appellate decision with effects adverse to us on issues of liability and/or relief. In addition, Medtronic may attempt to request injunctive relief in the future, which, if granted, would have a material adverse effect on our business, financial condition and results of operations.
We have not recorded an expense related to damages in connection with this litigation matter because any potential loss is currently neither probable nor reasonably estimatable under SFAS No. 5, Accounting for Contingencies.
Notwithstanding the litigation proceedings, we are in the process of implementing changes to our business processes intended to create an alternative process that we believe does not infringe Medtronic's patents. These changes will not affect the product design, but will modify the final step prior to inspection in our manufacturing processes and the instructions for use of our products by physicians, which clarify what we believe to be standard practices. These changes will require that our products be cooled prior to use and therefore rely on a property of our nitinol material that makes use of temperature rather than stress for expansion. The use of temperature for expansion of nitinol was disclaimed during the prosecution of the Medtronic patents. We do not expect any of these changes to impact the future sales of our products. In addition, we intend to initiate a process to establish manufacturing operations in Europe for all of our devices that are sold in international markets within 12 to 18 months. In addition to providing other benefits for our business, such as mitigating the risks inherent in relying on only one manufacturing facility, the manufacture of our products outside of the United States would eliminate any claims for ongoing royalty payments on future sales with respect to such products sold outside of the United States. We cannot assure you that any of these changes will avoid future litigation or will not result in a subsequent finding of infringement of Medtronic's patents and/or patents held by others. If any of our modified business processes were found to infringe any such patents, we may be required to pay additional damages and royalties. We cannot assure you that such damages and royalties would not exceed the 11% rate applied by the Medtronic case jury for past sales. Any such royalties may not be on commercially reasonable terms and could have a material adverse effect on our results of operations and financial condition. Also, if a court grants an injunction,
53
we may be prevented from selling some or any infringing products. While we believe we have taken reasonable steps to inform third parties who use such products of the alternative process by changing the instructions for use that are referenced in the information that accompanies each such product, such third parties may not review such information and may not adopt the alternative process even if they do review the new instructions for use. To the extent such third parties continue to use the prior process, we may be liable for such use whether or not the alternative process infringes Medtronic's patents.
Recent Acquisitions
Effective January 1, 2009, we began direct distribution in Canada, Portugal, France, Belgium and the Netherlands as we purchased on such date the distribution rights, inventory and intangible assets from our distributors in these countries. The aggregate purchase price of these acquisitions totaled $10.8 million, consisting of cash payments of $6.1 million, the discounted value of $1.4 million in additional guaranteed payments and the discounted value of up to $3.3 million in additional contingent payments if certain revenue goals are achieved. On April 1, 2009, we paid our former French distributor $1.4 million in such additional guaranteed payments.
Effective January 1, 2009, we began direct distribution in Italy as a result of our purchase on January 8, 2009 of certain distribution rights, inventory, equipment, intangible assets and goodwill from our former Italian distributor. The aggregate purchase price was $41.0 million, consisting of cash payments of $26.6 million, the discounted value of $9.2 million in additional guaranteed payments and the discounted value of up to $5.2 million in additional contingent payments if certain revenue goals are achieved during the first three years following completion of the acquisition. On April 1, 2009, we paid our former Italian distributor $2.0 million in such additional contingent payments.
On January 5, 2009, in order to finance, in part, the acquisition of the assets of our former Italian distributor, AGA Medical issued to one of the WCAS Stockholders for an aggregate purchase price of $15.0 million (1) $15.0 million in aggregate principal amount of the 2009 notes, and (2) 1,879 shares of Series B preferred stock. The 2009 notes are fully and unconditionally guaranteed by Amplatzer Medical Sales Corporation.
Components of Results of Operations
Net Sales
Our net sales are derived primarily from the sales of our AMPLATZER occlusion devices for the repair of structural heart defects and, more recently, our AMPLATZER Vascular Plugs for the repair of abnormal blood vessels. In addition, we also sell accessories, such as delivery systems, sizing balloons and guidewires. Other components of net sales include freight revenue, restocking fees and net adjustments to sales return reserves. Since 2006, the geographic distribution of our net sales between U.S. and international net sales has remained relatively stable with U.S. net sales constituting approximately 40% of our net sales.
From 2006 to 2008, our net sales have presented a compound annual growth rate of 14.4%. Our net sales have grown during that time primarily due to:
- •
- the increasing awareness, acceptance and use of non-invasive devices for treatment of structural heart defects
and vascular diseases;
- •
- select conversion of our international distributors to direct sales and the expansion of our U.S. direct sales force in
2007;
- •
- higher average selling prices, primarily in the United States;
- •
- the expansion of our product portfolio; and
- •
- our geographic expansion into additional international markets.
54
We have been able to increase our average selling price despite cost containment and competitive pressures in the medical devices industry due to the fact that our products provide a more cost-effective alternative to competing procedures, devices and therapies.
A majority of our net sales are generated by our direct sales efforts for products that are shipped and billed to hospitals throughout the world. In countries where we do not have a direct sales force, sales are generated by shipments to distributors who, in turn, sell to hospitals. We have agreements in place with each of our distributors that provide them exclusive rights to sell our products in their specified territories. Substantially all of our net sales to our distributors are denominated in U.S. dollars, while international direct sales are typically denominated in the local currency.
Cost of Goods Sold
We manufacture a substantial majority of the products that we sell. Our cost of goods sold consists primarily of our costs associated with direct labor, raw materials and components, manufacturing overhead, salaries, other personnel-related expenses for our management team, quality control, royalties, freight, service warranty, insurance and depreciation.
Our cost of goods sold has decreased as a percentage of net sales over the past three years primarily as a result of higher average selling prices of our devices, lower royalty costs and improved manufacturing efficiencies. As we manufacture substantially all of our products at our headquarters in Plymouth, Minnesota, substantially all of our costs of goods sold are in U.S. dollars. We intend to initiate a process to establish manufacturing operations in Europe for all of our devices that are sold in international markets within 12 to 18 months.
Our management reviews and analyzes the components of cost of goods sold and utilizes cost of goods sold as a percentage of net sales as an indicator of our efficiency in manufacturing our products.
Selling, General and Administrative
Our selling and marketing expenses consist primarily of salaries, commissions and other personnel-related expenses for employees engaged in sales, marketing and support of our products, trade shows, promotions and physician training. General and administrative expenses consist of expenses for our executive, finance, legal, compliance, administrative, information technology and human resource departments.
Our selling, general and administrative expenses have increased as a percentage of net sales over the past three years primarily as a result of increased headcount resulting from the strengthening of our management and corporate infrastructure, particularly in the finance department, establishing and expanding our U.S. and European direct sales forces and increasing marketing and legal costs. Except for the costs associated with our European direct sales force and facilities, our selling, general and administrative expenses are primarily in U.S. dollars.
We expect our sales and marketing expenses to increase in absolute terms as we expand our direct sales force, both in the United States and internationally, and launch new products. However, we do not expect them to increase significantly as a percentage of net sales. We expect our general and administrative expenses will increase in absolute terms and as a percentage of net sales in the shorter term as a result of our becoming a public company and our intention to aggressively defend our intellectual property rights. We expect that these expenses will decrease as a percentage of net sales over the longer term.
Our management reviews and analyzes selling, general and administrative expenses in absolute terms and as a percentage of net sales as an indicator of our ability to manage our business to our annual operating plans.
55
Research and Development
Our research and development expenses consist primarily of those (1) associated with the development, design and testing of new products, product enhancements and new applications for our existing products, and (2) incurred to operate our clinical trials, including trial design, clinical-site reimbursement, data management and associated travel expenses. These expenses include engineering, pre-clinical studies and clinical personnel costs, cost of materials, supplies, services and an allocation of facility overhead costs. We expense all of our research and development costs as incurred.
Our research and development expenses have increased as a percentage of net sales over the past three years primarily as a result of increased clinical trial and research and development spending resulting from our strategy of focusing on commercializing our pipeline of new products, product enhancements and new applications for our existing products.
We expect to continue to invest in the development of new products and technologies. We, therefore, expect our total research and development expenses to increase in absolute terms but do not expect them to significantly increase as a percentage of net sales. We also expect period-to-period increases to be driven by research and development expenses related to clinical trials as these expenses are subject to periodic variation based on various factors, such as the number of clinical trials underway at any given time, the number of patients in each trial and the pace of enrollment. We expect research and development to decrease as a percentage of net sales over the longer term.
Amortization of Intangible Assets
We amortize intangible assets, such as developed technology, royalty rights, patents and customer relationships over their useful life, typically periods ranging from five to ten years. Our amortization of intangible assets has increased as a percentage of net sales over the past three years primarily as a result of our:
- •
- July 2005 reorganization. As a result of our
July 2005 reorganization, we recorded goodwill and intangibles in the amount of $178.0 million, resulting in amortization of intangible assets of $5.1 million in 2005,
$12.2 million in 2006, $12.2 million in 2007, $12.2 million in 2008 and $6.1 million for the six months ended June 30, 2009.
- •
- 2007 purchase of patent rights. Effective January 2007, we purchased certain patent rights relating to our products for a $14.5 million payment which resulted in the creation of an intangible asset to be amortized over 7.5 years. We recorded amortization expense of $1.9 million in each of 2007 and 2008 and $1.0 million for the six months ended June 30, 2009 related to this purchase of patent rights. The purchase reduced our cost of goods sold by approximately 2% per year. The royalty payments associated with these patents would have continued until 2014 and 2015 for U.S. and international sales, respectively, had the purchase not taken place.
Change in purchase consideration
Change in purchase consideration represents changes in the fair value of contingent payment obligations in accordance with SFAS No. 141(R) accounting for business combinations. See "—Recent Accounting Pronouncements." For the six months ended June 30, 2009, we accrued a charge of $0.7 million for a charge in purchase consideration derived from a reduction in the fair value of contingent payment obligations resulting from the acquisitions of distribution rights from former distributors in Canada, Italy and Portugal based on actual and forecasted revenue assumptions for 2009.
Litigation Settlement
Litigation settlement expense is exclusively related to our patent infringement lawsuit with NMT Medical, Inc. In March 2006, we settled a patent infringement suit with NMT Medical and a second patent holder in which we made a $30.0 million one-time payment, of which $29.0 million was recorded
56
in 2005 as a charge to our income statement because it was associated with the prior use of the patents allegedly infringed. The remaining $1.0 million was capitalized because such amount was deemed to be for the future benefit of the use of this patent and is being amortized over the remaining estimated five years of the useful life of the patent. There are no future royalties or additional liabilities related to this settlement.
Foreign Corrupt Practices Act Settlement
On June 2, 2008, we entered into a Deferred Prosecution Agreement, or the DPA, with the U.S. Department of Justice concerning alleged improper payments that were made by our former independent distributor in China. See "Business—Legal Proceedings—Foreign Corrupt Practices Act Settlement." As part of the DPA, we were required, among other things, to pay a monetary penalty of $2.0 million. In anticipation of the settlement, we recorded in 2007 a charge of $2.0 million.
Loss on disposal of property and equipment
Loss on disposal of property and equipment represents the remaining net book value of these assets at the time of their disposal.
Investment Income (Loss)
Investment income (loss) includes the difference between the proceeds received from the sale of an investment and its carrying value. In addition, investment income (loss) includes our portion of losses under the equity method of accounting that we recognize in connection with our investment in a private early-stage structural heart medical device company.
Interest Income
Interest income is comprised of interest income earned on our cash and cash equivalents and short-term investments, consisting primarily of certain investments that have contractual maturities no greater than three months at the time of purchase.
Interest Expense
Interest expense consists primarily of interest on borrowings under our senior secured credit facility entered into in connection with our July 2005 reorganization and amended and restated in connection with our April 2006 recapitalization, as well as the 2005 notes entered into in connection with our July 2005 reorganization. As a result of our April 2006 recapitalization, we wrote off $2.7 million of deferred financing fees relating to our July 2005 reorganization, which contributed to our decreased interest expense in 2007 compared to 2006.
Other Income (Expense), Net
Other income (expense), net primarily includes royalty income and foreign exchange gains and losses net of certain other expenses.
Income Taxes
Income taxes are comprised of federal, state, local and foreign taxes based on income.
Net Income
During the past three years, our net income has declined despite our significantly increased net sales and higher gross margins. These higher gross margins have been offset by increases in investments we have made in our business, such as increased expenses associated with selective conversion of our distributors to a direct sales force, research and development expenses, including increased clinical trial expenses related to the expansion of our product pipeline, increased selling, general and administrative expenses, including legal fees related to defending our intellectual property and expenses related to
57
strengthening our corporate infrastructure, and increased interest and amortization expense resulting primarily from our July 2005 reorganization.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, net sales and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates including those related to product returns, bad debts, inventories, income taxes, long-lived assets and intangibles. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
The accounting policies we believe to be most critical to understanding our financial results and condition and that require complex and subjective management judgments are discussed below.
Revenue Recognition
In the United States and certain European countries, where we primarily sell our products directly to hospitals, we recognize revenue when all of the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred; the sales price is fixed or determinable; and the collectibility is reasonably assured. These criteria are typically met at the time of shipment when the risk of loss and title passes to the customer.
In other international markets, we sell our products to international distributors which subsequently resell the products to hospitals. Sales to distributors are recognized at the time of shipment, provided that we have received an order, the sales price is fixed or determinable, collection of the resulting receivable is reasonably assured and we can reasonably estimate returns. In cases where our products are held on consignment at a customer's location, we recognize net sales at the time the product is used in the procedure rather than at shipment.
Product Returns Policy
We warrant that our products are free from manufacturing defects at the time of shipment. We allow for product returns in certain circumstances, such as damaged or faulty products or products that are incorrectly sized and subsequently unused during a procedure. Allowances are provided for estimated product returns at the time of sale based on historical returns experience and recorded as a reduction of sales. Allowances are provided for estimated warranty costs at the time of shipment. We reserve approximately 1% to 5% of sales for these various return categories.
In July 2006, we established a change to our product returns policy, effective January 1, 2007, whereby we would no longer accept the return of expired products from our customers. As a result of this change in policy, we recorded reductions to our product returns reserve balances of $7.7 million for the year ended December 31, 2006, $1.3 million for the year ended December 31, 2007 and none for the year ended December 31, 2008 and the six months ended June 30, 2009. These recorded reductions had the effect of increasing net sales in 2006 and 2007.
Accounts Receivable and Allowance for Doubtful Accounts
We have receivables from a diversified customer base. The creditworthiness of customers is analyzed and monitored before sales are approved. We record an allowance for doubtful accounts based on past history, current economic conditions and the composition of our accounts receivable aging, and in some cases, we make allowances for specific customers based on several factors, such as the creditworthiness of those customers, payment history and disputes with customers. We historically
58
have not had any material issues with respect to allowance for doubtful accounts as a result of collection.
Inventory Reserves
We calculate inventory reserves for estimated obsolescence or excess inventory based on historical turnover and assumptions about future demand for our products and market conditions. Our industry is characterized by regular new product development, and as such, our inventory is at risk of obsolescence following the introduction and development of new or enhanced products. Our estimates and assumptions for excess and obsolete inventory are reviewed and updated on a quarterly basis. The estimates we use for demand are also used for near-term capacity planning and inventory purchasing and are consistent with our sales forecasts. Future product introductions and related inventories may require additional reserves based upon changes in market demand or introduction of competing technologies. Increases in the reserve for excess and obsolete inventory result in a corresponding expense in cost of goods sold. Our reserve for excess and obsolete inventory was $1.5 million as of December 31, 2008 and $1.6 million as of June 30, 2009.
Goodwill and Indefinite Lived Intangibles
We periodically evaluate whether events and circumstances have occurred that may affect the estimated useful life or the recoverability of the remaining balance of our goodwill and indefinite lived intangible assets. If such events or circumstances were to indicate that the carrying amount of those assets would not be recoverable, we would estimate the future cash flows expected to result from the use of the assets and their eventual disposition. The process of evaluating the potential impairment is subjective and requires management to exercise judgment in making assumptions related to future cash flows and discount rates. If the sum of the expected future cash flows (undiscounted and without interest charges) were less than the carrying amount of goodwill and indefinite lived intangibles, we would recognize an impairment loss. Goodwill and indefinite lived intangibles is tested for impairment during the fourth quarter of each year or if events otherwise require. As of December 31, 2007 and 2008, we concluded that there was no impairment. Since that date, there has been no event or adverse business trend that would suggest that goodwill or our indefinite lived intangibles have been impaired or that an interim test should be performed.
Long-Lived Assets
We periodically review and evaluate long-lived assets, primarily property, plant and equipment and intangible assets with finite lives, when events and circumstances indicate that the carrying amount of these assets may not be recoverable. For long-lived assets, this evaluation is based on the expected future undiscounted operating cash flows of the related assets. Should such evaluation result in us concluding that the carrying amount of long-lived assets has been impaired, an appropriate write-down to their fair value is recorded.
Income Taxes
We account for income taxes in accordance with SFAS No. 109, Accounting for Income Taxes (SFAS No. 109). Under this method, we determine tax assets and liabilities based upon the differences between the financial statement carrying amounts and the tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. The tax consequences of most events recognized in the current year's financial statements are included in determining income taxes currently payable. However, because tax laws and financial accounting standards differ in their recognition and measurement of assets, liabilities and equity, revenues, expenses, gains and losses, differences arise between the amount of taxable income and pretax financial income for a year and between the tax basis of assets or liabilities and their reported amounts in the financial statements. Because we assume that the reported amounts of assets and liabilities will be
59
recovered and settled, respectively, a difference between the tax basis of an asset or liability and its reported amount in the balance sheet will result in a taxable or a deductible amount in some future years when the related liabilities are settled or the reported amounts of the assets are recovered, giving rise to a deferred tax asset or liability.
Effective January 1, 2007, we adopted FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes, or FIN 48, which prescribes detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in an enterprise's financial statements in accordance with SFAS No. 109. The interpretation prescribes a recognition threshold and measurement attribute for a tax position taken or expected to be taken in a tax return and also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. Tax positions must meet a more-likely-than-not recognition threshold at the effective date to be recognized upon the adoption of FIN 48 and in subsequent periods.
Stock-Based Compensation
We follow SFAS No. 123R, Share-Based Payment (SFAS 123R), in accounting for our stock-based awards. SFAS 123R establishes accounting for stock-based awards exchanged for employee services. Accordingly, stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the life of the grant. We calculate the fair value of the stock option grants using the Black-Scholes option pricing model. In addition, we use the straight-line (single option) method for expense attribution over the related vesting period, according to which we estimate forfeitures and only recognize expense for those shares expected to vest.
We account for equity instruments issued to non-employees in accordance with EITF Issue No. 96-18, Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling Goods, or Services (EITF 96-18), which requires that these equity instruments be recorded at their fair value on the measurement date. The measurement of stock-based compensation is subject to periodic adjustment as the underlying equity instruments vest. As a result, the non-cash charge to operations for non-employee options with vesting criteria is affected each reporting period by changes in the fair value of our common stock.
The fair value of each option is estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions used for grants:
| |
Year Ended December 31, | Six Months Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2008 | 2009 | ||||||
Risk-free interest rate |
5.04% | 4.96% – 5.04% | (1) | 1.87% – 3.57% | (1) | 1.97% – 3.57% | (1) | 1.83% – 3.18% | (1) | ||
Expected term |
6.5 years | 6.5 years | 6.5 years | 6.5 years | 6.5 years | ||||||
Estimated volatility |
45% | 64% | 54% | 55% | 57% | ||||||
Expected dividend yield |
0% | 0% | 0% | 0% | 0% | ||||||
- (1)
- Rates for options granted during this time period varied within this range.
We do not have information available which is indicative of future exercise and post-vesting behavior to estimate the expected term. As a result, we adopted the simplified method of estimating the expected term of a stock option, as permitted by the Staff Accounting Bulletin No. 107. Under this method, the expected term is presumed to be the mid-point between the vesting date and the contractual end of the term.
60
As a non-public entity, historic volatility is not available for our shares. As a result, we estimated volatility based on a peer group of companies, which collectively provides a reasonable basis for estimating volatility. We intend to continue to consistently use the same group of publicly traded peer companies to determine volatility in the future until sufficient information regarding volatility of our share price becomes available or the selected companies are no longer suitable for this purpose.
The risk-free interest rate is based on the implied yield available on U.S. Treasury zero-coupon issues with a remaining term approximately equal to the expected life of our stock options. The estimated pre-vesting forfeiture rate is based on our historical experience. We do not expect to declare dividends in the foreseeable future.
We recorded non-cash stock-based compensation expense under SFAS 123R and EITF 96-18 for employee and non-employee stock option grants of $2.6 million during the year ended December 31, 2008 and $1.7 million during the six months ended June 30, 2009. Based on stock options outstanding as of June 30, 2009, we had unrecognized stock-based compensation of $8.0 million, determined in accordance with SFAS 123R and EITF 96-18. We expect to continue to grant stock options in the future, and to the extent that we do, our actual stock-based compensation expense recognized in future periods will likely increase.
As of June 30, 2009, after giving effect to the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering, we had outstanding vested options to purchase 1,306,834 shares of our common stock and unvested options to purchase 1,590,803 shares of our common stock with an intrinsic value of approximately $14.8 million and $12.6 million, respectively, based on an assumed initial public offering price of $20.00 per share, the midpoint of the range on the cover of this prospectus.
Significant Factors Used in Determining Fair Value of Our Common Stock
The fair value of the shares of common stock that underlie the stock options we have granted has historically been determined by our board of directors based upon information available to it at the time of grant. Because, prior to this offering, there has been no public market for our common stock, our board of directors has determined the fair value of our common stock by utilizing, among other things, contemporaneous valuation studies conducted as of April 30, 2006 and June 30, 2007. The findings of these valuation studies were based on our business and general economic, market and other conditions that could be reasonably evaluated at that time. The analyses of the valuation studies incorporated extensive due diligence that included a review of our company, including its financial results, business agreements, intellectual property and capital structure. The valuation studies also included a thorough review of the conditions of the industry in which we operate and the markets that we serve. The methodologies of the valuation studies included an analysis of the fair market value of our company using three widely accepted valuation methodologies: (1) market multiple, (2) comparable transactions, and (3) discounted cash flow. These valuation methodologies were based on a number of assumptions, including our future revenues and industry, general economic, market and other conditions that could reasonably be evaluated at the time of the valuation.
The market multiple methodology involved the multiplication of revenues by risk-adjusted multiples. Multiples were determined through an analysis of certain publicly traded companies, which were selected on the basis of operational and economic similarity with our principal business operations. Revenue and cash flow multiples, when applicable, were calculated for the comparable companies based upon daily trading prices. A comparative risk analysis between our and the public companies formed the basis for the selection of appropriate risk-adjusted multiples for our company. The risk analysis incorporated factors that relate to, among other things, the nature of the industry in which we and other comparable companies are engaged. The comparable transaction methodology also involved multiples of earnings and cash flow. Multiples used in this approach were determined through
61
an analysis of transactions involving controlling interests in companies with operations similar to our principal business operations. The discounted cash flow methodology involved estimating the present value of the projected cash flows to be generated from the business and theoretically available to the capital providers of our company. A discount rate was applied to the projected future cash flows to reflect all risks of ownership and the associated risks of realizing the stream of projected cash flows. Since the cash flows were projected over a limited number of years, a terminal value was computed as of the end of the last period of projected cash flows. The terminal value was an estimate of the value of the enterprise on a going concern basis as of that future point in time. Discounting each of the projected future cash flows and the terminal value back to the present and summing the results yielded an indication of value for the enterprise. Our board of directors took these three approaches into consideration when establishing the fair value of our common stock.
In addition, we received input from the underwriters of this offering in October 2007 with respect to valuation of our company. Based on the foregoing factors, our board of directors determined on October 22, 2007 to increase the fair value of our common stock to $2.75 (without giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering, or $19.66 after giving effect to such reverse stock split). The valuation carried out by our board of directors in October 2007, which, as mentioned above, already took into consideration input from the underwriters, was based not only on our results for the nine months ended September 30, 2007 but also on our expected growth rates for certain periods of time, including for the fourth quarter of that year and for 2008. Results for the fourth quarter of 2007 and, subsequently, for the first quarter of 2008, reflected growth rates lower than the ones projected and used in the valuation carried out in October 2007. As a result, even though revenues increased in 2007 when compared to 2006, our board of directors determined that this increase was not sufficient to cause a change in the estimated fair market value of our common stock. Our valuation also did not increase, in part, due to the general decline in prices for public companies during this time period. In addition, until June 2, 2008, we were subject to an FCPA investigation that impaired our future prospects, both as a result of potential criminal charges and monetary fines and as a result of potential significant limitations on our business. Once the FCPA investigation was concluded as a result of the Deferred Prosecution Agreement we entered into with the Department of Justice, these potential impediments to the growth of our business were resolved. In addition, shortly thereafter, we became aware that our second-quarter results would achieve higher growth rates. Based on the foregoing factors, our board of directors determined to proceed with filing of the Registration Statement on June 20, 2008. In addition, our board of directors then determined that all commitments to grant stock options entered into between June 21, 2008 and December 31, 2008, would contain an exercise price equal to the actual initial public offering price (when determined), unless the initial public offering was not consummated by December 31, 2008, in which case the options would be granted on that date and would contain an exercise price that was based on the fair market value of our common stock determined by our board of directors for such date. The exercise price for all stock option grants was determined as of December 31, 2008, the date of grant, based on the estimated fair value of our common stock on that date, and not during the period in which we had solely a contractual commitment to grant such options upon occurrence of a pre-determined event that established the price. Accordingly, as of December 31, 2008, our board of directors confirmed that the fair market value of our common stock on that date remained at $2.75 (without giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering, or $19.66 after giving effect to such reverse stock split), based on the methodologies previously used by the board and additional informal input received from the underwriters of this offering with respect to their views of industry, general economic, market and other conditions. Even though we experienced an increase in revenue and other positive developments, such as receiving CE Mark clearance for the AMPLATZER Cardiac Plug in December 2008 and approval in Japan for the AMPLATZER Duct Occluder in December 2008, the recent economic recession and related substantial decrease in equity valuations of public companies in the United States offset these
62
positive developments in our business and operations. For these reasons, our board of directors determined the fair market value of the company's common stock had not changed. Stock options that have been granted from December 31, 2008 to June 30, 2009 have the same exercise price.
Although it is reasonable to expect that the completion of our initial public offering will increase the value of our common stock as a result of increased liquidity and marketability and the elimination of the liquidation preference of our Series A and Series B preferred stock, at this stage the amount of additional value cannot be measured with precision or certainty.
Common Stock Valuation
Information on stock options granted, without giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering, is summarized as follows:
Date of Issuance
|
Number of Options Granted |
Exercise Price | Grant Date Fair Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
April 27, 2006 |
11,553,109 | $ | 1.00 | $ | 1.00 | |||||
May 19, 2006 to May 29, 2007 |
4,945,000 | $ | 1.00 | $ | 1.00 | |||||
May 30, 2007 to October 22, 2007 |
2,090,000 | $ | 2.00 | $ | 1.99 | |||||
October 23, 2007 to June 30, 2009 |
4,335,000 | $ | 2.75 | $ | 2.75 | |||||
Information on stock options granted, after giving effect to the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering, is summarized as follows:
Date of Issuance
|
Number of Options Granted |
Exercise Price | Grant Date Fair Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
April 27, 2006 |
1,615,819 | $ | 7.15 | $ | 7.15 | |||||
May 19, 2006 to May 29, 2007 |
691,608 | $ | 7.15 | $ | 7.15 | |||||
May 30, 2007 to October 22, 2007 |
292,307 | $ | 14.30 | $ | 14.23 | |||||
October 23, 2007 to June 30, 2009 |
606,293 | $ | 19.66 | $ | 19.66 | |||||
63
Results of Operations
The following table sets forth, for the periods indicated, our results of operations expressed as a percentage of net sales.
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (% of net sales) |
2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||
Net sales |
100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | |||||||
Cost of goods sold |
19.6 | 15.5 | 16.0 | 15.4 | 18.0 | ||||||||||||
Gross profit |
80.4 | 84.5 | 84.0 | 84.6 | 82.0 | ||||||||||||
Operating expenses: |
|||||||||||||||||
Selling, general and administrative |
29.4 | 34.1 | 39.3 | 39.3 | 49.2 | ||||||||||||
Research and development |
9.5 | 18.0 | 19.6 | 20.0 | 17.4 | ||||||||||||
Amortization of intangible assets |
9.9 | 10.3 | 9.3 | 9.5 | 10.5 | ||||||||||||
Change in purchase consideration |
— | — | — | — | (0.7 | ) | |||||||||||
FCPA settlement |
— | 1.4 | — | — | — | ||||||||||||
Loss on disposal of property and equipment |
0.6 | — | — | — | — | ||||||||||||
Operating income (loss) |
31.0 | 20.7 | 15.8 | 15.8 | 5.6 | ||||||||||||
Investment income (loss) |
0.6 | (0.5 | ) | (0.7 | ) | (0.8 | ) | (2.5 | ) | ||||||||
Interest income |
0.9 | 0.3 | 0.1 | 0.1 | 0.1 | ||||||||||||
Interest expense |
(18.0 | ) | (14.4 | ) | (9.9 | ) | (10.6 | ) | (8.6 | ) | |||||||
Other income, net |
0.8 | 0.6 | 0.4 | 0.3 | 1.3 | ||||||||||||
Income (loss) before income taxes |
15.3 | 6.7 | 5.7 | 4.8 | (4.1 | ) | |||||||||||
Provision for income taxes |
(5.4 | ) | (2.6 | ) | (0.2 | ) | (1.9 | ) | (0.3 | ) | |||||||
Net income (loss) |
9.9 | 4.1 | 5.5 | 2.9 | (4.4 | ) | |||||||||||
Less Series A and Series B preferred stock and Class A common stock dividends |
(46.6 | ) | (10.4 | ) | (10.2 | ) | (10.9 | ) | (9.0 | ) | |||||||
Net loss applicable to common stockholders |
(36.7 | )% | (6.3 | )% | (4.7 | )% | (8.0 | )% | (13.4 | )% | |||||||
Six Months Ended June 30, 2009 Compared to Six Months Ended June 30, 2008
Net sales. Net sales for the six months ended June 30, 2009 increased 16.7% to $94.4 million from $80.8 million for the same period in 2008. Our family of AMPLATZER Septal Occluder devices represented 55.4% of net sales for the six months ended June 30, 2009 and 59.2% of net sales for the six months ended June 30, 2008, AMPLATZER PFO Occluder devices represented 15.1% and 12.7% of net sales for the six months ended June 30, 2009 and 2008, respectively, all other devices represented 17.7% and 17.0% of net sales for the six months ended June 30, 2009 and 2008, respectively, and accessories, including delivery systems, represented 11.8% and 11.2% of net sales for the six months ended June 30, 2009 and 2008, respectively. Of the total $13.6 million increase in net sales, $10.1 million was derived from increased international product sales, primarily as a result of the acquisitions of distribution rights from former distributors in January 2009 and the continued expansion of our direct sales force in several European countries in the six months ended June 30, 2009, which represented an increase of 21.1% compared to international product sales for the same period in 2008. An increase of $3.3 million was derived from increased U.S. product sales, which represented an increase of 10.1% compared to U.S. product sales for the same period in 2008. These increases were offset in part by decreases in freight revenue, restocking fees and adjustments to sales return reserves. The increases in international and U.S. product sales are primarily due to an increase of $4.5 million derived from higher sales volume of device and accessories units, which includes an increase of
64
$0.6 million derived from the launch of new products, and an additional $12.3 million derived from higher average selling prices, mainly as a result of the previously-mentioned conversion of distribution rights in January 2009, which allowed the company to sell directly to its customers and therefore increased its average selling prices, and, to a lesser extent, as a result of changes in product mix. These increases were partially offset by the effects on our international product sales of the appreciation of the U.S. dollar against foreign currencies. U.S. net sales and international net sales represented 38.9% and 61.1%, respectively, of our total net sales for the first six months of 2009, compared to 40.5% and 59.5%, respectively, for the same period in 2008. The increase in international net sales as a percentage of total net sales is mainly attributable to higher average selling prices internationally due to the acquisitions of distribution rights from former distributors in January 2009. International direct net sales represented 68.8% and 41.0% of total international net sales for the six months ended June 30, 2009 and 2008, respectively. This increased percentage of direct international sales is also mainly attributable to the January 2009 acquisitions of distribution rights from certain of our former distributors in Europe.
Cost of goods sold. Cost of goods sold for the six months ended June 30, 2009 increased 36.5% to $17.0 million from $12.5 million for the same period in 2008. This increase in cost of goods sold was attributable primarily to an increase of $3.4 million as a result of the repurchase of inventory from former distributors whose distribution rights were acquired in January 2009, and $1.2 million derived from higher volume of units sold. Excluding the $3.4 million attributable to higher cost of repurchased inventory, cost of goods sold as a percentage of net sales for the six months ended June 30, 2009 would have been 14.4% compared to 15.0% for the same period in 2008. Gross margins decreased to 82.0% for the six months ended June 30, 2009 from 84.6% for the six months ended June 30, 2008. Excluding the $3.4 million higher cost of repurchased inventory, gross margins for the six months ended June 30, 2009 improved to 85.6% from 85.0% during the same period in 2008.
Selling, general and administrative. Selling, general and administrative expenses for the six months ended June 30, 2009 increased 46.3% to $46.5 million from $31.8 million for the same period in 2008. This increase was primarily due to a $7.4 million increase in costs related to expanding our direct sales force in several European countries, a $4.3 million increase in ongoing investments in domestic and international corporate infrastructure and a $3.0 million increase in legal expenses associated with litigation and patent defense costs. Our investment in corporate infrastructure includes customer service, finance, information systems, human resources, regulatory and legal. As we continue to grow, we will be required to invest in corporate infrastructure to support our business. We have increased our international direct sales force over the past three years, which naturally caused an increase in the number of employees and infrastructure required to support our operations. In addition, we have added similar infrastructure at our U.S. headquarters to support larger U.S. and international operations. As a percentage of net sales, our selling, general and administrative expenses for the six months ended June 30, 2009 increased to 49.2% compared to 39.3% for the same period in 2008.
Research and development. Research and development expenses for the six months ended June 30, 2009 increased 1.6% to $16.5 million from $16.2 million for the same period in 2008. This increase was primarily attributable to a $0.5 million increase in payroll and related costs derived from an increase in headcount to support both our pre-clinical and development efforts. As a percentage of net sales, our research and development expenses for the six months ended June 30, 2009 decreased to 17.4% from 20.0% for the same period in 2008.
Amortization of intangible assets. Amortization expenses for the six months ended June 30, 2009 increased 28.7% to $9.9 million compared to $7.7 million for the same period in 2008. As a percentage of net sales, amortization of intangible assets for the six months ended June 30, 2009 increased to 10.5% from 9.5% for the same period in 2008. The increase is attributable to the intangible assets purchased as part of expanding our direct sales force in several European countries.
65
Change in purchase consideration. Change in purchase consideration for the six months ended June 30, 2009 included a benefit of $0.7 million derived from a reduction in the fair value of contingent payment obligations resulting from the acquisition of distribution rights from former distributors in Canada, Italy and Portugal based on actual and forecasted revenue assumptions for 2009.
Investment income (loss). Investment (loss) for the six months ended June 30, 2009 increased to $(2.4) million from $(0.6) million for the same period in 2008, reflecting our write-off in 2009 of our investment in a privately held early stage company that is focused on pre-clinical studies relating to the development of minimally invasive devices to treat structural heart defects.
Interest income. Interest income for the six months ended June 30, 2009 decreased to $61,000 from $110,000 for the same period in 2008, mainly as a result of reduced levels of cash and short-term investment balances.
Interest expense. Interest expense for the six months ended June 30, 2009 decreased 5.2% to $8.1 million from $8.6 million for the same period in 2008. This decrease in interest expense reflects our lower average interest rate for the six months ended June 30, 2009, which was partially offset by additional borrowings under our revolving credit agreement and the issuance of the 2009 notes, as well as the addition of accreted interest charges on future guaranteed obligations payable to the distributors acquired in January 2009 as part of expanding our direct sales force in several European countries.
Other income, net. Other income, net for the six months ended June 30, 2009 increased to $1.3 million from $0.3 million for the same period in 2008, mainly as a result of an increase of foreign exchange gains.
Income taxes. Income tax expense for the six months ended June 30, 2009 decreased to $0.3 million from $1.5 million for the same period in 2008, based on lower pre-tax income, purchase accounting related to the January 2009 acquisitions of distribution rights from former distributors, and the write-off in 2009 of our investment in a privately held early stage company that is focused on pre-clinical studies relating to the development of minimally invasive devices to treat structural heart defects.
Net income (loss). Net income (loss) for the six months ended June 30, 2009 of $(4.2) million decreased from net income of $2.4 million for the same period in 2008.
Year Ended December 31, 2008 Compared to Year Ended December 31, 2007
Net sales. Net sales for 2008 increased 13.3% to $166.9 million from $147.3 million for 2007. Excluding the favorable effect to net sales in 2007 of $1.3 million in reductions to our product returns reserve resulting from a change in our product returns policy, net sales would have increased $20.9 million, or 14.3%. Our family of AMPLATZER Septal Occluder devices represented 58.7% and 61.7% of net sales for 2008 and 2007, respectively, AMPLATZER PFO Occluder devices represented 12.0% and 13.2% of net sales for 2008 and 2007, respectively, all other devices represented 17.9% and 14.5% of net sales for 2008 and 2007, respectively, and accessories, including delivery systems, represented 11.4% and 10.6% of net sales for 2008 and 2007, respectively. Of the total $20.9 million increase in net sales (after excluding the effect to net sales resulting from our change in product returns policy), $14.0 million derived from increased international product sales, which represented an increase of 16.5%, and $6.9 million derived from increased U.S. product sales, which represented an increase of 11.4%, offset in part by decreases in freight revenue, restocking fees and adjustments to sales return reserves. The increases in international and U.S. product sales are primarily due to an increase of $13.3 million derived from higher sales volume of device and accessories units, which include an increase of $2.6 million derived from the launch of new products and an additional increase of $7.1 million derived from higher average selling prices (including as a result of changes to product
66
mix). U.S. net sales and international net sales represented 40.8% and 59.2%, respectively, of our net sales for 2008, compared to 42.1% and 57.9%, respectively, for 2007. International direct net sales represented 40.8% and 38.2% of total international net sales for 2008 and 2007, respectively, while international distributor net sales represented 59.2% and 61.8%, respectively, of total international net sales.
Cost of goods sold. Cost of goods sold for 2008 increased 16.7% to $26.6 million from $22.8 million for 2007. This increase in cost of goods sold was attributable primarily to an increase of $3.0 million derived from higher volume of units sold and an increase of $1.8 million derived from higher purchased inventory values as a result of the April and July 2008 acquisitions of the distribution rights from our former Spanish and Polish distributors, which were partially offset by a $1.1 million decrease due to increased manufacturing efficiencies. As a percentage of net sales, cost of goods sold in 2008 increased to 16.0% from 15.5% for 2007. As a result, gross margins decreased to 84.0% in 2008 from 84.5% for 2007.
Selling, general and administrative. Selling, general and administrative expenses for 2008 increased 30.8% to $65.7 million from $50.2 million for 2007. This increase was primarily due to a $4.9 million increase in costs related to expanding our direct sales force in several European countries, a $2.6 million increase in ongoing investments in corporate infrastructure and a $5.8 million increase in legal expenses associated with litigation, patent defense costs and the FCPA investigation. As a percentage of net sales, our selling, general and administrative expenses for 2008 increased to 39.3% compared to 34.1% for 2007.
Research and development. Research and development expenses for 2008 increased 23.4% to $32.8 million from $26.6 million for 2007. This increase was primarily attributable to a $3.7 million increase in payroll and related costs derived from an increase in headcount to support both our pre-clinical and development efforts and a $2.7 million increase derived from higher clinical trial expenses. As a percentage of net sales, our research and development expenses for 2008 increased to 19.6% from 18.0% for 2007.
Amortization of intangible assets. Amortization expenses for 2008 increased 2.0% to $15.5 million compared to $15.2 million for 2007. As a percentage of net sales, amortization of intangible assets for 2008 decreased slightly to 9.3% from 10.3% for 2007.
Investment income (loss). Investment (loss) for 2008 increased to $(1.2) million from $(0.8) million for 2007, reflecting our prorated share of the losses incurred in our investment in a privately held early stage company that is focused on pre-clinical studies relating to the development of minimally invasive devices to treat structural heart defects.
Interest income. Interest income for 2008 decreased to $0.2 million from $0.4 million for 2007, mainly as a result of reduced levels of cash and short-term investment balances.
Interest expense. Interest expense for 2008 decreased 22.3% to $16.5 million from $21.2 million for 2007. This decrease in interest expense reflects lower weighted-average effective interest rates on our Tranche B term loan facility for 2008 of 5.1% as compared to 7.4% for 2007. The decrease in the effective weighted-average interest rate is due to the decrease in LIBOR.
67
Other income net. Other income, net for 2008 decreased to $0.7 million from $1.0 million for 2007, mainly as a result of a decrease of foreign exchange gains.
Income taxes. Income tax expense for 2008 decreased to $0.4 million from $3.8 million for 2007. This decrease is primarily the result of a $2.4 million reduction in our FIN 48 tax liability as a result of the expiration of the applicable statutes of limitations, lower pre-tax income, and a decrease in the effective tax rate to 29.4% in 2008 from 38.7% in 2007, prior to this reduction in our FIN 48 tax liability. See note 8 to our consolidated financial statements included elsewhere in this prospectus.
Net income. Net income for 2008 increased 49.7% to $9.1 million from $6.1 million for 2007.
Year Ended December 31, 2007 Compared to Year Ended December 31, 2006
Net sales. Net sales for 2007 increased 15.5% to $147.3 million from $127.5 million for 2006. Excluding the favorable effect to net sales in 2006 of $7.7 million and in 2007 of $1.3 million in reductions to our product returns reserve resulting from a change in our product returns policy, net sales would have increased $26.1 million, or 21.8%. Our family of AMPLATZER Septal Occluder devices represented 61.7% and 58.3% of net sales for 2007 and 2006, respectively, AMPLATZER PFO Occluder devices represented 13.2% and 18.3% of net sales for 2007 and 2006, respectively, all other devices represented 14.5% and 10.9% of net sales for 2007 and 2006, respectively, and accessories, including delivery systems, represented 10.6% and 12.5% of net sales for 2007 and 2006, respectively. Of the total $26.1 million increase in net sales (after excluding the effect to net sales resulting from our change in product returns policy), $13.5 million derived from increased U.S. product sales, which represented an increase of 28.1%, $13.4 million derived from increased international product sales, which represented an increase of 19.0%, which was partially offset by adjustments to sales return reserves. The increases in U.S. and international product sales were primarily due to $14.5 million from higher average selling prices (including as a result of changes to product mix), $10.3 million from increased sales volume of device units, including a new version of our AMPLATZER Septal Occluder device in the United States in October 2006 and $1.5 million from the launch of new products. U.S. net sales and international net sales represented 42.1% and 57.9%, respectively, of our net sales for 2007, compared to 40.4% and 59.6%, respectively, for 2006. International direct net sales represented 38.2% and 34.7% of total international net sales for 2007 and 2006, respectively, while international distributor net sales represented 61.8% and 65.3%, respectively, of total international net sales.
Cost of goods sold. Cost of goods sold for 2007 decreased 8.7% to $22.8 million from $25.0 million for 2006. This decrease in cost of goods sold was attributable primarily to a $1.5 million recall reserve charge taken in September 2006 in connection with the voluntary recall of one of our delivery systems, a $1.4 million reduction in royalties resulting from our 2007 purchase of patent rights and $0.5 million in lower insurance costs in 2007, which were offset in part by a $1.5 million increase derived from higher volume of units sold. As a percentage of net sales, cost of goods sold for 2007 decreased to 15.5% from 19.6% for 2006. This decrease in cost of goods sold as a percentage of net sales resulted in improvements in gross margins, which increased to 84.5% for 2007 from 80.4% for 2006. This increase in gross margins was primarily attributable to improved manufacturing efficiencies, higher average selling prices and lower expenses noted above.
Selling, general and administrative. Selling, general and administrative expenses for 2007 increased 33.8% to $50.2 million from $37.5 million for 2006. This increase was primarily due to a $5.4 million increase in sales and marketing expenses resulting from additional hiring into the U.S. direct sales force and costs related to establishing a direct sales force in several European countries, $2.4 million of ongoing investments in corporate infrastructure and $4.9 million from higher legal expenses associated with litigation and our FCPA investigation. As a percentage of net sales, our selling, general and administrative expenses for 2007 increased to 34.1% from 29.4% for 2006.
68
Research and development. Research and development expenses for 2007 increased 119.5% to $26.6 million from $12.1 million for 2006. This increase was primarily attributable to a $5.0 million increase in payroll and related costs to support both our pre-clinical and our development efforts, a $2.6 million increase derived from higher clinical trial expenses and $1.5 million of increased pre-clinical testing costs related to the expansion of our product pipeline and the related regulatory approval processes. As a percentage of net sales, our research and development expenses for 2007 increased to 18.0% from 9.5% for 2006.
Amortization of intangible assets. Amortization expenses for 2007 increased 20.1% to $15.2 million from $12.7 million for 2006. This increase in amortization of intangible assets expenses was primarily attributable to the intangible assets resulting from our August 2006 purchase of distribution rights from a former distributor and our 2007 purchase of certain patent rights. As a percentage of net sales, amortization of intangible assets for 2007 increased to 10.3% from 9.9% for the same period in 2006.
FCPA settlement. In 2007, we recorded a $2.0 million charge for the recognition of the then estimated monetary penalty imposed in connection with settlement of our FCPA investigation. See "Business—Legal Proceedings—Foreign Corrupt Practices Act Settlement."
Investment income (loss). Investment (loss) for 2007 decreased to $(0.8) million from income of $0.8 million for 2006, reflecting the sale of short-term investments.
Interest income. Interest income for 2007 decreased to $0.4 million from $1.2 million for 2006, mainly as a result of reduced levels of cash and short-term investment balances.
Interest expense. Interest expense for 2007 decreased 7.3% to $21.2 million from $22.9 million for 2006. This decrease in interest expense reflects a $2.7 million write-off of deferred financing fees in 2006 relating to our July 2005 reorganization and lower effective interest rates following our April 2006 recapitalization, which was partially offset by an additional $108.0 million in debt following such April 2006 recapitalization.
Other income (expense), net. Other income, net for 2007 remained stable at $1.0 million.
Income taxes. Income tax expense for 2007 decreased 44.4% to $3.8 million from $6.9 million for 2006. This decrease is primarily the result of lower pre-tax income between periods, which was partially offset by an increase in our effective tax rate to 38.7% from 35.4%, mainly due to the $2.0 million FCPA charge recorded in 2007.
Net income (loss). Net income (loss) for 2007 decreased 51.9% to $6.1 million from $12.6 million for 2006.
Liquidity and Capital Resources
Our principal sources of liquidity are existing cash, including cash generated in this offering to the extent it is not used to repay our indebtedness and dividends, internally generated cash flow and borrowings under our senior secured credit facility. We believe that these sources will provide sufficient liquidity for us to meet our liquidity requirements for the next 12 months. Our principal liquidity requirements are to service our debt and to meet our working capital, research and development, including clinical trials, and capital expenditure needs. We may, however, require additional liquidity as we continue to execute our business strategy. We anticipate that to the extent that we require additional liquidity, it will be funded through the incurrence of indebtedness, additional equity financings or a combination of these potential sources of liquidity. We cannot assure you that we will be able to obtain this additional liquidity on reasonable terms, or at all. Additionally, our liquidity and our ability to fund our capital requirements is also dependent on our future financial performance, which is subject to general economic, financial and other factors that are beyond our control.
69
Accordingly, we cannot assure you that our business will generate sufficient cash flow from operations or that future borrowings will be available under our senior secured credit facility or otherwise to meet our liquidity needs.
Cash Flows
Cash Flows Provided By (Used in) Operating Activities
Net cash provided by (used in) operating activities for the six months ended June 30, 2009 decreased to a net cash used in operating activities of $(1.7) million from a net cash provided by operating activities of $5.2 million for the same period in 2008. This decrease compared to the prior period was primarily attributable to the following changes in cash flows for the six months ended June 30, 2009 compared to the same period in 2008: a $6.7 million decrease in net income, a $10.1 million increase in accounts receivable (mainly attributable to our acquisitions of distribution rights from former distributors in Italy, France, Portugal, Poland, Canada and the Netherlands in January 2009), a $2.2 million decrease in accrued expenses, a $0.7 million benefit in change in purchase consideration (resulting from a reduction in the fair value of contingent payment obligations derived from the acquisition of distribution rights from former distributors in Canada, Italy and Portugal), and a $0.1 million increase in prepaid and other expenses, which were partially offset by a change in $2.0 million payment in connection with the FCPA settlement during 2008, a $3.1 million increase in depreciation and amortization (resulting from increases in intangible assets resulting from our acquisitions of distribution rights from former distributors in Europe in January 2009), a $1.9 million decrease in inventory, a $1.1 million increase in trade accounts payable (mainly as a result of higher legal fees incurred during the period), a $1.9 million decrease in income tax receivable, a $1.7 million increase in losses on our equity investment in a privately held early-stage company that is focused on pre-clinical studies relating to the development of minimally invasive devices to treat structural heart defects (primarily the result of the impairment and write-off of this investment during March 2009), a $0.4 million increase in reserves for customer returns (primarily as a result of higher sales volumes including an increase of $4.5 million in net sales derived from higher sales volumes of devices and accessories units), a $0.3 million increase in litigation settlement reserves and a $0.5 million increase in stock-based compensation relating to issuances of stock options.
Net cash provided by operating activities for 2008 decreased to $18.8 million from $32.9 million for 2007. This decrease compared to the prior period was attributable mainly to the following changes in cash flows for 2008 compared to 2007: a $2.0 million payment in connection with the FCPA settlement, a $6.5 million decrease in deferred income taxes, a $0.3 million increase in inventory, a $8.0 million increase in accounts receivable (mainly as a result of increased sales volumes and our conversions to direct sales in Spain and Poland in mid-year 2008) and a $1.0 million increase in reserves for customer returns (primarily as a result of higher sales volumes, including an increase of $13.3 million in net sales derived from higher sales volumes of device and accessories units), which were partially offset by a $3.0 million increase in net income a $0.9 million increase in trade accounts payable, a $0.8 million increase in depreciation and amortization and a $0.8 million increase in stock-based compensation relating to issuances of stock option grants.
Net cash provided by operating activities for 2007 increased to $32.9 million from $13.2 million for 2006. This increase compared to the prior period was attributable mainly to the following changes in cash flows for 2007 compared to 2006: a $30.0 million payment for a litigation settlement in 2006, a $7.7 million decrease in reserves for customer returns, a $4.1 million increase in depreciation and amortization, a $4.0 million decrease in accounts receivable and a $2.0 million accrual for the FCPA settlement, which was partially offset by a $6.5 million decrease in net income, a $2.7 million write-off of deferred financing fees in 2006 relating to our April 2006 recapitalization, a $10.1 million decrease in trade accounts payable and accrued expenses, a $7.4 million decrease in accrued and deferred income taxes payable and a $1.8 million decrease in product-recall reserves.
70
Cash Flows Used In Investing Activities
Net cash used in investing activities for the six months ended June 30, 2009 increased to $41.2 million from $8.7 million for the same period in 2008. This increase compared to the prior period was primarily attributable to the following changes in cash flows for the six months ended June 30, 2009 compared to the same period in 2008: $31.2 million of increased acquisitions of distribution rights from former distributors and $2.8 million increase in purchases of property and equipment, which was partially offset by a $1.2 million incremental investment in the same period in 2008 in a privately held early-stage company that is focused on pre-clinical studies relating to the development of minimally invasive devices to treat structural heart defects and a $0.3 million decrease in restricted cash.
Net cash used in investing activities for 2008 increased to $16.8 million from $11.3 million for 2007. This increase compared to the prior period was primarily attributable to the following changes in cash flows for 2008 compared to 2007: a $20.0 million decrease in net proceeds from the sale of short-term investments, $5.0 million used in connection with our April 2008 purchase of distribution rights from a former Spanish distributor, a $2.8 million increase in purchases of property and equipment $1.0 million used in connection with our July 2008 purchase of distribution rights from a former Slovak Republic distributor and a $1.2 million incremental investment in a privately held early-stage company that is focused on pre-clinical studies relating to the development of minimally invasive devices to treat structural heart defects, which was partially offset by $14.5 million used to purchase patent rights in 2007.
Net cash used in investing activities for 2007 increased to $11.3 million from $10.5 million for 2006. This increase compared to the prior period was primarily attributable to the following changes in cash flows for 2007 compared to 2006: $14.5 million used to purchase patent rights in 2007 and a $6.0 million decrease in net proceeds from the purchase and sale of short-term investments, which were partially offset by a $13.7 million decrease in purchases of property and equipment and a $5.3 million decrease in cash used in our August 2006 purchase of distribution rights from a former distributor.
Cash Flows Provided By (Used In) Financing Activities
Net cash provided by (used in) financing activities for the six months ended June 30, 2009 increased to $28.4 million from $(2.5) million for the same period in 2008. This increase compared to the prior period was primarily attributable to the following changes in cash flows for the six months ended June 30, 2009 compared to the same period in 2008: $15.0 million resulting from the issuance of the 2009 notes, $15.1 million of amounts drawn under our revolving credit facility, and a decrease in dividends paid by $2.5 million, which was partially offset by a $1.6 million payment of deferred financing fees.
Net cash provided by financing activities increased to $6.4 million for 2008 from net cash used in financing activities of $16.2 million for 2007. This increase compared to the prior period was primarily attributable to the following changes in cash flows for 2008 compared to 2007: $9.9 million of proceeds from our line of credit and a redemption of 1.9 million shares of Class A common stock (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering) in July 2007, which resulted in a $15.1 million payment by us to one of our stockholders. This increase was partially offset by $2.5 million of dividends paid to our stockholders in April 2008.
Net cash used in financing activities increased to $16.2 million for 2007 from $9.9 million for 2006. This increase compared to the prior period was primarily attributable to the following changes in cash flows for 2007 compared to 2006: a redemption of 1.9 million shares of Class A common stock (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering) in July 2007, which resulted in a $15.1 million payment by us to one of our stockholders, and a $91.0 million decrease in proceeds from long-term debt, net of
71
payments, including a prepayment of principal of $17.5 million in September 2006, and payment of deferred financing fees associated with the 2006 senior secured credit facility, which were partially offset by a $98.2 million decrease in dividend payments.
Cash Position and Indebtedness
On April 28, 2006, we completed a $240.0 million recapitalization, in connection with which we entered into our senior secured credit facility, which consists of a $215.0 million seven-year Tranche B term loan facility and a $25.0 million revolving credit facility. The revolving credit facility matures on July 28, 2011, and the Tranche B term loan facility matures on April 28, 2013. As of June 30, 2009, we had $25.0 million of outstanding borrowings under our revolving credit facility and $197.0 million outstanding under our Tranche B term loan facility. We currently have no additional availability under our revolving credit facility. We will be permitted to reborrow any portion of the indebtedness outstanding under our revolving credit facility that is repaid with a portion of our net proceeds from this offering. On October 5, 2008, Lehman Commercial Paper Inc. ("LCPI") filed for protection under Chapter 11 of the Federal Bankruptcy Code. LCPI had committed to provide $9.5 million under the $25.0 million revolving credit facility. In March 2009 Bank of America, N.A. assumed the participation of this credit agreement previously held by LCI.
On July 28, 2005, in connection with our July 2005 reorganization, AGA Medical issued $50.0 million aggregate principal amount of the 2005 notes, which pay interest semiannually. The effective interest rate of the 2005 notes at June 30, 2009 was 12.6%, compounded semiannually. The 2005 notes were purchased by one of the WCAS Stockholders. Our 2005 notes are unsecured obligations of AGA Medical and are subordinated in right of payment to our senior secured credit facility. As part of the transaction agreement, AGA Medical issued 6,524 shares of Series A preferred stock valued at $6.5 million to the WCAS Stockholders. As a result, the discounted issue value of the 2005 note was $43.5 million. The $6.5 million of discount from the face value is being accreted on our balance sheet to the 2005 note repayment amount utilizing the effective interest rate. The original issue discount has been recognized as interest expense of $0.9 million, $0.9 million, $0.9 million and $0.5 million for the years ended December 31, 2006, 2007 and 2008, and the six months ended June 30, 2009, respectively. As of June 30, 2009, the accreted value of our outstanding 2005 notes on our balance sheet was $47.0 million. In compliance with the terms of the securities purchase agreement governing the 2005 notes, we will use a portion of our net proceeds from this offering to prepay these notes at a price equal to 100% of their face principal amount plus accrued and unpaid interest. See "Use of Proceeds."
On January 5, 2009, in order to finance, in part, the acquisition of the assets of our former Italian distributor, AGA Medical issued to one of the WCAS Stockholders for an aggregate purchase price of $15.0 million (1) $15.0 million in aggregate principal amount of the 2009 notes, and (2) 1,879 shares of Series B preferred stock valued at $1.9 million. The 2009 notes are fully and unconditionally guaranteed by Amplatzer Medical Sales Corporation. The discounted issue value of the subordinated note is $13.1 million. The $1.9 million of discount from the face value is being accreted on our balance sheet to the 2009 note repayment amount utilizing the effective interest rate. The original issue discount has been recognized as interest expense of $0.2 million for the six months ended June 30, 2009. As of June 30, 2009, the accreted value of our outstanding 2009 notes on our balance sheet was $13.4 milion. Interest on the senior subordinated note is payable on a semiannual basis in arrears on January 1 and July 1 of each year. The effective interest rate of the 2009 notes at June 30, 2009 was 15.2%, compounded semiannually. In compliance with the terms of the securities purchase agreement governing the senior subordinated notes, we will use a portion of our net proceeds from this offering to prepay our senior subordinated notes at a price equal to 100% of their face principal amount plus accrued and unpaid interest. See "Use of Proceeds."
72
Our total indebtedness was $243.5 million at December 31, 2006, $243.6 million at December 31, 2007, $253.4 million at December 31, 2008 and $291.4 million at June 30, 2009.
The following table sets forth the amounts outstanding under our Tranche B term loan facility and our revolving credit facility, the effective interest rates on such outstanding amounts and amounts available for additional borrowing thereunder as of June 30, 2009.
Senior Secured Credit Facility
|
Effective Interest Rate |
Amount Outstanding |
Amount Available for Additional Borrowing |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
(dollars in millions) |
|||||||||
Revolving Credit Facility |
2.32 | % | $ | 25.0 | $ | — | |||||
Tranche B Term Loan Facility |
3.01 | % | 197.0 | — | |||||||
Total |
$ | 222.0 | $ | — | |||||||
Our senior secured credit facility will not require us to use any of the net proceeds we receive from this offering to repay outstanding indebtedness under our senior secured credit facility but does allow for the optional repayment of the 2005 notes and the 2009 notes with a portion of the proceeds of this offering. We expect to voluntarily repay the indebtedness outstanding under our revolving credit facility with a portion of the proceeds of this offering. Following the end of each fiscal year, our Tranche B term loan facility requires us to make an annual prepayment of the term loan facility equal to 50% of any excess cash flow for any fiscal year for which the leverage ratio at the end of such fiscal year is greater than 4.50 to 1.00, 25% of excess cash flow for any fiscal year for which the leverage ratio at the end of such fiscal year is less than or equal to 4.50 to 1.00 and greater than 4.00 to 1.00, and none of excess cash flow for any fiscal year for which the leverage ratio at the end of such fiscal year is less than or equal to 4.00 to 1.00. The leverage ratio is defined as the ratio of total indebtedness on such date to Consolidated EBITDA (as defined in the credit agreement) for the four most recent consecutive fiscal quarters ended prior to such date. Consolidated EBITDA is defined under our senior secured credit facility as EBITDA further adjusted to give effect to unusual non-recurring items, non-cash items and certain other adjustments. These adjustments include items such as patent litigation defense costs and settlements, FCPA-related costs and product recalls.
Our senior secured credit facility also places certain restrictions on us, including restrictions on our ability to incur indebtedness, grant liens, pay dividends, sell all of our assets or any subsidiary, use funds for capital expenditures, make investments, make optional payments or modify debt instruments, or enter into sale and leaseback transactions. Our senior secured credit facility also requires us to maintain compliance with specified financial covenants. As of June 30, 2009, we were in compliance with all of our financial covenants specified in our senior secured credit facility. If we are required to accrue a charge relating to, or post an appeal bond in connection with, the Medtronic litigation, we may not be able to comply with the covenants contained in our senior secured credit facility in future periods. While we do not believe that accruing a charge and/or posting an appeal bond in connection with the Medtronic litigation will cause us to breach any such covenant, doing so may adversely affect our ability to comply with our maintenance covenants in our senior secured credit facility. If we breached any such covenant, we would need to seek to amend or refinance our senior secured credit facility. We believe that such an amendment could require us to pay upfront fees and an increased interest rate on our borrowings thereunder, which could materially adversely affect our financial condition and results of operations. Alternatively, we could refinance our senior secured credit facility, but we may not be able to do so on reasonable terms or at all. If we fail to obtain such an amendment or refinancing, we would suffer an event of default under such facility and the lenders thereto would have the right to accelerate the indebtedness outstanding thereunder. In addition, the lenders' obligations to extend letters of credit or make loans under our senior secured credit facility are dependent upon our ability to make our representations and warranties thereunder at the time such
73
letters of credit are extended or such loan is made. If we are unable to make the representations and warranties in our senior secured credit facility at such time, we will be unable to borrow additional amounts under our senior secured credit facility in the future.
The indebtedness under our senior secured credit facility is secured by a perfected first priority security interest in all of our tangible and intangible assets (including, without limitation, intellectual property, owned real property and all of our capital stock and each direct and indirect subsidiaries, provided that no assets of any foreign subsidiary is included as collateral and no more than 65% of the voting stock of any first-tier foreign subsidiary is required to be pledged).
Contractual Obligation and Commitments
The following table summarizes our outstanding contractual obligations as of December 31, 2008:
| |
Payments Due by Period | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in millions) |
Total | Less Than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years |
||||||||||||
Tranche B Term Loan Facility |
$ | 197.0 | $ | — | $ | — | $ | 197.0 | $ | — | |||||||
Revolving Credit Facility |
9.9 | — | 9.9 | — | — | ||||||||||||
Operating Leases |
4.7 | 1.2 | 1.8 | 0.7 | 1.0 | ||||||||||||
Royalty Obligations(1) |
1.9 | 1.9 | — | — | — | ||||||||||||
Former U.K. Distributor Obligations(2) |
1.5 | 1.5 | — | — | — | ||||||||||||
Former Slovakian Distributor Obligations(3) |
0.3 | 0.3 | |||||||||||||||
Senior Subordinated Notes(4) |
50.0 | — | — | 50.0 | — | ||||||||||||
Total Contract Obligation and Commitments |
$ | 265.3 | $ | 4.9 | $ | 11.7 | $ | 247.7 | $ | 1.0 | |||||||
- (1)
- We
have made and expect to continue making royalty payments under two royalty agreements relating to patented technology assigned to us, namely:
(1) a royalty-bearing research-related agreement with Dr. Kurt Amplatz, our founder, under which we pay Dr. Amplatz a fixed percentage of royalties on products of ours that
incorporate current and future patented technology developed by Dr. Amplatz and assigned to us under the agreement; and (2) royalty-payment agreements with Curtis Amplatz,
Dr. Amplatz's son, under which we pay Curtis Amplatz a fixed percentage of royalties throughout the life of certain of our patents for which Curtis Amplatz is the named inventor. These
agreements obligate us to pay royalties on specific product sales and are payable throughout the life of the patents. These payments will be variable because they depend on future product sales. In
2008, Mr. Curtis Amplatz inquired regarding the scope of the royalty agreements we had with him and whether they applied to additional products of ours. In response, we had discussions in which
we clarified the scope of the agreements and our payments under the agreements.
- (2)
- As
of December 31, 2008, we had a $1.5 million obligation due in August 2009.
- (3)
- As
of December 31, 2008, we had a $0.3 million obligation due if certain revenue goals are achieved.
- (4)
- We intend to use $50.0 million of our net proceeds from this offering to fully repay the 2005 notes. In addition, we will use a portion of our net proceeds from the offering to pay accrued and unpaid interest on these notes.
74
The following table summarizes our outstanding contractual obligations as of June 30, 2009, after giving effect to the intended use of net proceeds received by us from this offering, including to repay debt:
| |
|
Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in millions) |
Total | Less Than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years |
||||||||||||
Tranche B Term Loan Facility |
$ | 197.0 | $ | — | $ | — | $ | 197.0 | $ | — | |||||||
Revolving Credit Facility(1) |
— | — | — | — | — | ||||||||||||
Operating leases |
5.9 | 1.5 | 2.0 | 1.2 | 1.2 | ||||||||||||
Royalty obligations(2) |
2.0 | 2.0 | — | — | — | ||||||||||||
Former U.K. Distributor Obligations(3) |
1.5 | 1.5 | — | — | — | ||||||||||||
Former Slovakian Distribution Obligations(4) |
0.5 | 0.5 | — | — | — | ||||||||||||
Former France Distributor Obligations(5) |
1.4 | 1.4 | — | — | — | ||||||||||||
Former Portugal Distributor Obligations(6) |
0.6 | 0.6 | — | — | — | ||||||||||||
Former Netherlands Distributor Obligations(7) |
0.3 | 0.3 | — | — | — | ||||||||||||
Former Canada Distributor Obligations(8) |
0.9 | 0.9 | — | — | — | ||||||||||||
Former Italy Distributor Obligations(9) |
13.2 | 4.2 | 9.0 | — | — | ||||||||||||
Senior Subordinated Notes(10) |
— | — | — | — | — | ||||||||||||
Total contract obligation and commitments |
$ | 223.3 | $ | 12.9 | $ | 11.0 | $ | 198.2 | $ | 1.2 | |||||||
- (1)
- We
intend to use $25.0 million of our net proceeds from the offering to repay indebtedness outstanding under our revolving credit facility. Our total
indebtedness outstanding under our revolving credit facility as of June 30, 2009 was $25.0 million.
- (2)
- We
have made and expect to continue making royalty payments under two royalty agreements relating to patented technology assigned to us, namely:
(1) a royalty-bearing research-related agreement with Dr. Kurt Amplatz, our founder, under which we pay Dr. Amplatz a fixed percentage of royalties on products of ours that
incorporate current and future patented technology developed by Dr. Amplatz and assigned to us under the agreement; and (2) royalty-payment agreements with Curtis Amplatz,
Dr. Amplatz's son, under which we pay Curtis Amplatz a fixed percentage of royalties throughout the life of certain of our patents for which Curtis Amplatz is the named inventor. These
agreements obligate us to pay royalties on specific product sales and are payable throughout the life of the patents. These payments will be variable because they depend on future product sales. In
2008, Mr. Curtis Amplatz inquired regarding the scope of the royalty agreements we had with him and whether they applied to additional products of ours. In response, we had discussions in which
we clarified the scope of the agreements and our payments under the agreements.
- (3)
- As
of June 30, 2009, we had a $1.5 million obligation due in August 2009.
- (4)
- As
of June 30, 2009, we had a $0.5 million obligation due July 2009 to our former Slovakian distributor if certain revenue goals are achieved.
- (5)
- As
of June 30, 2009, we had a $1.4 million discounted contingent obligation ($1.5 million without giving effect to the discount) due
January 2010 to our former France distributor if certain revenue goals are achieved.
- (6)
- As of June 30, 2009, we had a $0.6 million discounted contingent obligation ($0.7 million without giving effect to the discount) due January 2010 to our former Portugal distributor if certain revenue goals are achieved.
75
- (7)
- As
of June 30, 2009, we had a $0.3 million discounted contingent obligation ($0.4 million without giving effect to the discount) due
January 2010 to our former Netherlands distributor if certain revenue goals are achieved.
- (8)
- As
of June 30, 2009, we had a $0.9 million discounted contingent obligation ($1.0 million without giving effect to the discount) due
January 2010 to our former Canada distributor if certain revenue goals are achieved.
- (9)
- As
of June 30, 2009, we had a $2.8 million obligation due January 2010, and a $1.4 million discounted contingent obligation
($1.5 million without giving effect to the discount) due January 2010, in each case, to our former Italy distributor if certain revenue goals are achieved. In addition, we had
$5.9 million in obligations due in each of 2011 and 2012, and $3.1 million in discounted contingent obligations ($3.9 million without giving effect to the discount) due in each of
2011 and 2012 if certain revenue goals are achieved.
- (10)
- We intend to use $65.0 million of our net proceeds from this offering to fully prepay the 2005 notes and the 2009 notes. In addition, we will use a portion of our net proceeds from the offering to pay accrued and unpaid interest on these notes.
In addition, we have agreements with clinical sites and contract research organizations for the conduct of our clinical trials. We make payments to these sites and organizations based upon the number of patients enrolled and the period of follow-up in the trials.
Off-Balance Sheet Arrangements
We do not currently have, nor have we ever had, any relationships with unconsolidated entities or financial partnerships, such as entities referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Seasonality
While our results of operations are not materially affected by seasonality, our net sales are affected by holiday and vacation periods, especially in the third quarter in Europe.
Related Party Transactions
For a description of our related party transactions, see "Certain Relationships and Related Transactions."
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements (SFAS 157), which defines fair value, establishes a framework for the measurement of fair value, and enhances disclosure about fair value measurement. The statement does not require any new fair value measures. The provisions under SFAS 157 are effective for all financial assets and liabilities and for nonfinancial assets and liabilities recognized or disclosed at fair value in our consolidated financial statements on a recurring basis beginning January 1, 2008, and are expected to be applied prospectively. We adopted the provisions of SFAS 157 for financial assets and liabilities that are measured at fair value for our fiscal year beginning January 1, 2008. For all other nonfinancial assets and liabilities, we adopted SFAS 157 effective beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities (SFAS 159). SFAS 159 expands opportunities to use fair value measurements in
76
financial reporting and permits entities to choose to measure many financial instruments and certain other items at fair value. SFAS 159 is effective for our fiscal year beginning January 1, 2008. The adoption of this statement did not have a material effect on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), Business Combinations (SFAS No. 141(R)). SFAS No. 141(R) expands the definition of a business combination and requires the fair value of the purchase price of an acquisition, including the issuance of equity securities, to be determined on the acquisition date. SFAS No. 141(R) also requires that all assets, liabilities, contingent considerations, and contingencies of an acquired business be recorded at fair value at the acquisition date with subsequent adjustments recorded to net earnings. In addition, SFAS No. 141(R) requires that acquisition costs generally be expensed as incurred, restructuring costs generally be expensed in periods subsequent to the acquisition date and changes in accounting for deferred tax asset valuation allowances and acquired income tax uncertainties after the measurement period impact income tax expense. We adopted SFAS No. 141(R) effective for the fiscal year beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements, an Amendment of ARB No. 51 (SFAS No. 160). SFAS No. 160 changes the accounting and reporting for minority interests such that minority interests will be recharacterized as noncontrolling interests and will be required to be reported as a component of equity, and requires that purchases or sales of equity interests that do not result in a change in control be accounted for as equity transactions and, upon a loss of control, requires the interest sold, as well as any interest retained, to be recorded at fair value with any gain or loss recognized in earnings. We adopted SFAS No. 160 effective for our fiscal year beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In March 2008, the FASB issued SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities, an amendment of FAS 133 ("SFAS 161"). SFAS 161 applies to all derivative instruments and non-derivative instruments that are designated and qualify as hedging instruments and related hedged items accounted for under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities ("SFAS 133"). The provisions of SFAS 161 require entities to provide greater transparency through additional disclosures about (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS 133 and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity's financial position, results of operations and cash flows. We adopted SFAS 161 effective for the company's fiscal year beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In April 2009, the Financial Accounting Standards Board (FASB) issued FASB Staff Position (FSP) 107-1 and Accounting Principles Board (APB) Opinion No. 28-1 (FSP 107-1 and APB 28-1), Interim Disclosures about Fair Value of Financial Instruments ("FSP 107-1"). FSP 107-1 amends SFAS 107, Disclosures about Fair Value of Financial Instruments, and APB No. 28, Interim Financial Reporting. FSP 107-1 requires publicly traded entities to disclose the fair value of all financial instruments for which it is practicable to estimate that value, whether recognized or not recognized in the statement of financial position, as required by SFAS 107. FSP 107-1 is effective for interim and annual reporting periods ending after June 15, 2009. We adopted FSP 107-1 and APB 28-1 for the period ending June 30, 2009, and it did not have a material impact on the results of operations or financial position.
In May 2009, the FASB issued Statement of Financial Accounting Standards (SFAS) No. 165, Subsequent Events (SFAS No. 165), which defines subsequent events as events or transactions that occur after the balance sheet date but before the financial statements are issued or available to be issued. SFAS No. 165 further categorizes subsequent events as either "recognized" or "nonrecognized."
77
Recognized subsequent events provide additional evidence about conditions that existed at the balance sheet date and are recognized at the balance sheet date even though the related event occurred subsequent to the balance sheet date. Nonrecognized subsequent events provide evidence about conditions that did not exist at the balance sheet date. Such events should only be disclosed in the financial statements to keep the financial statements from being misleading. We have evaluated the existence of both recognized and nonrecognized subsequent events through October 13, 2009.
Quantitative and Qualitative Disclosures about Market Risk
Foreign Exchange Risk Management
Fluctuations in the rate of exchange between the U.S. dollar and foreign currencies could adversely affect our financial results. In 2008 and for the six months ended June 30, 2009, approximately $40.6 million, or 24.3% and $39.7 million, or 42.0%, of our net sales were denominated in foreign currencies (37.2% in 2008 on a pro forma basis to give effect to the acquisition of certain assets from our former Italian distributor on January 1, 2009). We expect that the percentage of our net sales denominated in foreign currencies will increase in the foreseeable future as we selectively convert our distributions into direct sales and bill our clients in local currency. Selling, marketing and general costs related to these foreign currency sales are largely denominated in the same respective currency, thereby partially offsetting our foreign exchange risk exposure. For sales not denominated in U.S. dollars, if there is an increase in the rate at which a foreign currency is exchanged for U.S. dollars, it will require more of the foreign currency to equal a specified amount of U.S. dollars than before the rate increase. In such cases and if we price our products in the foreign currency, we will receive less in U.S. dollars than we did before the rate increase went into effect. If we price our products in U.S. dollars and competitors price their products in local currency, an increase in the relative strength of the U.S. dollar could result in our price not being competitive in a market where business is transacted in the local currency.
In 2005, we entered into foreign exchange forward contracts to cover our exposure of Euro-denominated accounts receivables. These agreements were executed at that time as a result of the appreciation of the U.S. dollar against the Euro. The amount per contract ranged from $2.0 million to $5.0 million, and the contract had generally a 90-day duration. The program was phased out at the end of 2005 as the U.S. dollar began to depreciate against the Euro. In 2005, we recorded as other income foreign currency gains on hedging contracts of $1.5 million, which was partially offset by foreign currency translation losses of $1.2 million recorded as other expense.
In the first quarter of 2009, we initiated a foreign currency hedging program. The objectives of the program are to reduce earnings volatility due to movements in foreign currency markets, limit loss in foreign currency-denominated cash flows, and preserve the operating margins of our foreign subsidiaries. We generally use foreign currency forward contracts to hedge transactions related to known inter-company sales and inter-company debt. We also may hedge firm commitments. These contracts generally relate to our European operations and are denominated primarily in euros and sterling. All of our foreign exchange contracts are recognized on the balance sheet at their fair value. We do not enter into foreign exchange contracts for speculative purposes. Amounts on our balance sheet at June 30, 2009 are immaterial.
Interest Rate Risk
We are exposed to interest rate risk in connection with our Tranche B term loan facility and any borrowings under our revolving credit facility, which bear interest at floating rates based on Eurodollar or the greater of prime rate or the federal funds rate plus an applicable borrowing margin. For variable rate debt, interest rate changes generally do not affect the fair value of the debt instrument, but do impact future earnings and cash flows, assuming other factors are held constant.
78
We entered into an amended and restated senior secured credit agreement in connection with our April 2006 recapitalization and repaid our prior senior term loan. The transaction added a $215.0 million Tranche B term loan facility and our revolving credit facility increased to $25.0 million from $15.0 million. At June 30, 2009, we had outstanding borrowings of $25.0 million under our revolving credit facility. As a result, we currently have no additional availability under our revolving credit facility. We will be permitted to reborrow any portion of the indebtedness outstanding under our revolving credit facility that is repaid with a portion of our net proceeds from this offering. Based on the amount currently outstanding under our Tranche B term loan facility and our revolving credit facility, a change of one percentage point in the applicable interest rate would cause an increase or decrease in interest expense of approximately $2.0 million on an annual basis.
79
We are a leading innovator and manufacturer of medical devices for the treatment of structural heart defects and vascular diseases. Our AMPLATZER occlusion devices offer minimally invasive, transcatheter treatments that have been clinically shown to be highly effective in defect closure. Our devices and delivery systems use relatively small catheters and can be retrieved and repositioned prior to release from the delivery cable, enabling optimal placement without the need to repeat the procedure or use multiple devices. We are the only manufacturer with occlusion devices approved to close seven different structural heart defects, and we believe we have the leading market positions in the United States and Europe for each of our devices. In 2003, we launched our first vascular products, which are designed to occlude abnormal blood vessels. We sell our devices in 112 countries through a combination of direct sales and the use of distributors, with international markets representing approximately 59.2% of our net sales in 2008 and 61.1% of our net sales in the six months ended June 30, 2009.
Our AMPLATZER occlusion devices utilize our expertise in braiding nitinol, a metal alloy with superelastic and shape-memory characteristics, and designing transcatheter delivery systems. While our structural heart defect occlusion devices have historically been used primarily by pediatric cardiologists, we believe that there is a significant opportunity to expand the use of our devices by cardiologists focused on the adult population. We have also leveraged our core competencies to develop products for the treatment of certain vascular diseases, which we sell primarily to interventional radiologists and vascular surgeons. In addition, we are focused on further expanding our product portfolio by developing new products, product enhancements and new applications for our existing products to address:
- •
- Patent Foramen Ovale. By closing the PFO with an occlusion
device, we may be able to reduce the incidence of certain types of strokes and migraines. We currently sell our AMPLATZER PFO Occluder outside the
United States, representing approximately 12.0% and 15.1% of our net sales for the year ended December 31, 2008 and for the six months ended June 30, 2009. Our largest clinical trial,
the RESPECT study, is being conducted at approximately 60 U.S. sites to assess the impact of our AMPLATZER PFO Occluder in reducing the occurrence of
certain types of stroke. Based on our statistical models, we believe 500 patients should be sufficient to support a successful outcome in the RESPECT study, and on June 30, 2009, we had
enrolled 576 patients. According to decision rules, a successful outcome in the clinical trial is achieved once a claim of superiority of PFO closure versus drug therapy is supported. A
successful outcome can be achieved at any time during the study. We intend to continue to enroll up to 900 patients unless a successful outcome is achieved earlier. If we can establish a successful
outcome, the next step would be to prepare the submission of our PMA to the FDA, which we would expect to do within three to six months after achieving a successful outcome. Following receipt of a PMA
application, the FDA determines whether it is sufficiently complete and, therefore, may be accepted for review. On a statutory basis, the FDA is required to complete a preliminary review of a PMA
application within six months. The FDA review process of a PMA application, however, may take up to several years. Once the FDA has approved the PMA application, we would be able to begin marketing
the product in the United States. We estimate that the market opportunity for PFO closure in the prevention of certain types of stroke in the United States and Europe is greater than $1 billion
annually.
- •
- Left Atrial Appendage. We are developing a device to occlude the LAA, which targets reducing the incidence of stroke in patients with atrial fibrillation, one of the most common cardiac abnormalities in older people. We received CE Mark clearance in Europe in December 2008 and have initiated marketing of the device in Europe. We also applied to the FDA to begin a clinical trial to support U.S. approval of our AMPLATZER Cardiac Plug, with an initial
80
- •
- Vascular Grafts. We are developing vascular grafts made from multiple layers of braided nitinol for the transcatheter treatment of aneurysms in a variety of blood vessel sizes. Aneurysms are localized bulges of a blood vessel caused by disease or weakening of the vessel wall. Our initial focus has been on aneurysms that occur in smaller peripheral arteries, such as the iliac arteries, arteries that branch off from the aorta and lead to the legs. We have already designed a tubular graft with the appropriate diameter to be deployed in these arteries. We intend to refile for a CE Mark in Europe and to apply to the FDA for an Investigational Device Exemption, or IDE, in the United States in the first half of 2010. We had previously intended to apply for these clearances in the first half of 2009 and, in the case of Europe, filed for a CE Mark in June 2009. We, however, intend to refile in Europe and are delaying our expected filing of the IDE in the United States because we encountered some issues with the delivery system in connection with the final engineering validation of our graft. We estimate that CE Mark review could take 90 to 180 days from the time of submission, and after a CE Mark is granted, we would be able to begin marketing the product in Europe. We also estimate that clinical trials in the United States could take approximately two to three years, after which we would file a PMA application with the FDA. We estimate that the market opportunity for the transcatheter treatment of aneurysms in the United States and Europe is greater than $1 billion annually.
indication for LAA occlusion, in August 2008. We estimate that clinical trials in the United States could take approximately two to three years, after which we would file a PMA application with the FDA. We estimate that the market opportunity for LAA closure with a transcatheter approach worldwide is greater than $1 billion annually.
Industry Overview
Structure of the Human Heart
The human heart is a muscular organ divided into four hollow parts called chambers. The two upper chambers are called the right and left atria, and the two bottom chambers are called the right and left ventricles. The heart also has four valves, or one-way openings, that let the blood go forward and keep it from going backward. In the heart, oxygen-poor blood flows in the right atrium, where it is pumped into the right ventricle on its way to the lungs through the pulmonary artery. Oxygen-rich blood then returns to the heart through the left atrium, where it is pumped into the left ventricle. From the left ventricle, the heart pumps the oxygen-rich blood through the body via the aorta.
81
Embryonic and Neonatal Development of the Human Heart
The heart and the circulatory system of a human embryo begin to form soon after conception. As the lungs develop, the mother supplies the embryo with oxygen-rich blood through the umbilical cord. Since the fetal lungs are not used until after birth, blood must bypass the lungs to circulate to the rest of the body. Inside the prenatal heart, four chambers and valves form to circulate the blood. Blood is routed around the lungs by two passageways in the prenatal heart:
- •
- Foramen Ovale: a tunnel that forms a small passageway
connecting the left atrium and right atrium; and
- •
- Ductus Arteriosus: a blood vessel that forms a small passageway connecting the aorta to the pulmonary artery.
In normal prenatal circulation, the right atrium receives and mixes both oxygen-rich blood from the mother and oxygen-poor blood from the fetus. From the right atrium, some of the mixed blood then passes through the foramen ovale into the left atrium, moves into the left ventricle, then into the aorta, which delivers the blood to the rest of the fetus. The rest of the mixed blood in the right atrium enters the right ventricle, which pumps it into the pulmonary artery, leading it to the lungs. Since the lungs are not yet functioning, however, the blood moves through the ductus arteriosus and into the aorta, which delivers the blood to the fetus.
Shortly after birth, the foramen ovale and ductus arteriosus typically close as the baby begins to breathe. The baby's first breath and the closures of the foramen ovale and the ductus arteriosus trigger the normal heart pumping process as there is no longer a need to bypass the lungs.
Structural Heart Defects
A structural heart defect is an abnormality in the structure of the heart and associated vessels, such as the aorta and pulmonary artery. Many structural heart defects result from problems in normal fetal heart development and are referred to as congenital heart defects. Structural heart defects can be clinically significant and require immediate treatment early in life or can become clinically significant later in life when the child reaches adulthood. Two common categories of structural heart defects are (1) septal defects, which consist of a hole in the wall between the atria or ventricles that causes an errant flow of blood in the heart, and (2) failed closure of embryonic passageways, which reroute blood around the lungs in the prenatal heart and occur when the foramen ovale or the ductus arteriosus remain open after birth.
Treatment of Structural Heart Defects
In many cases, structural heart defects that show symptoms during childhood or later in life require closure. Historically, open-heart surgery was the most accepted method of closure. Open-heart surgery is an invasive procedure that requires an incision in a patient's chest to gain access to the heart and close the defect using sutures and polyester patches. During the surgery, there is potential for blood loss, trauma and other surgical complications. Open-heart surgery is expensive, requiring long hospital stays and a recovery period of several weeks.
In response to the shortcomings of open-heart surgery, a number of attempts were made as early as the 1970s to develop a transcatheter device to close structural heart defects. These early devices were in some cases difficult to use or had designs that could not withstand the wear and tear of remaining in position in a continually beating heart. Our AMPLATZER occluders were one of the first of a number of devices to successfully address the shortcomings of earlier generations of devices and resulted in widespread adoption of a less invasive transcatheter approach to treat structural heart defects. In the transcatheter approach, an interventional cardiologist inserts a flexible catheter into the
82
patient's groin with a small puncture and maneuvers the catheter through the vasculature to the heart. A device is deployed through the catheter.
Commonly Diagnosed Types of Structural Heart Defects
According to the American Heart Association, approximately 36,000, or 1%, of newborn babies in the United States are diagnosed with congenital heart defects each year. The three heart defects that are most commonly diagnosed and treated during childhood or later in life are:
- •
- Atrial Septal Defect, or ASD. An atrial septal defect is an
abnormal opening in the wall between the left and right atria. Because the right side of the heart receives extra blood, it is forced to bear more than its normal workload. The potential complications
of ASDs include high blood pressure in the lung's vessels, which can lead to pulmonary hypertension, damage to blood vessel walls and heart failure. ASDs are increasingly being detected and treated in
adults.
- •
- Patent Ductus Arteriosus, or PDA. The failed closure of the
ductus arteriosus after birth is called a patent, or open, ductus arteriosus. In patients with a PDA, blood that should have traveled through the aorta to nourish the body goes instead back into the
lungs, which can lead to difficulty breathing, failure to grow normally and chronic respiratory failure.
- •
- Ventricular Septal Defect, or VSD. A ventricular septal defect is an abnormal opening in the wall between the left and right ventricles. Because the left side of the heart receives extra blood, it is forced to bear more than its normal workload. The potential complications of VSDs include heart failure, high blood pressure and failure of a child to grow at a normal rate. Ventricular septal defects can also occur following myocardial infarctions.
We estimate that the global market opportunity for treating these three structural defects is approximately $190 million annually, with ASD repair representing approximately 65% of that opportunity.
Patent Foramen Ovale
The patent foramen ovale, or PFO, is a common structural heart defect in which the foramen ovale does not seal completely. PFOs occur in approximately 20% to 25% of the overall population. While most people never experience any clinical problems related to a PFO, studies have suggested that blood clots that commonly develop outside the heart may pass directly through the PFO from the right atrium to the left atrium without passing through the lungs, where they are normally filtered out of the blood. These blood clots have been linked to serious neurological events such as types of stroke. In the case of migraine, it is speculated that very small blood clots or other unfiltered chemicals may pass through the PFO and help trigger the migraine attack.
- •
- Stroke. Stroke is the third leading cause of death in the United States, affecting approximately 700,000 people in the United States each year, according to the American Heart Association. Ischemic strokes, in which essential blood flow to the brain becomes obstructed, represent approximately 88% of all strokes. Such strokes can be caused by emboli, which are tiny blood clots, caught in the small vessels of the brain. Approximately 40% of all ischemic strokes are cryptogenic, meaning that the stroke occurs in a patient without the normal risk factors. In patients with a PFO, it is believed the foramen ovale opening allows blood unfiltered by the lungs to flow to the brain. The unfiltered blood may contain emboli and therefore block a blood vessel and cause the stroke. A number of articles have been published studying the prevalence of cryptogenic stroke in patients with a PFO. For example, in several studies, PFOs were detected
83
- •
- Migraines. Migraine headaches represent a large unmet clinical need, affecting approximately 29.5 million people in the United States. Approximately 10% to 20% of migraine patients suffer from migraines with aura, a severe migraine headache preceded by neurological symptoms such as flashing lights, temporary loss of sight, trouble speaking and numbness on one side of the body. Studies have indicated that as many as 50% of the patients that experience migraine attacks preceded by aura may have a PFO, far exceeding the average rate of individuals with PFO in the general population. It is believed that in patients with a PFO the foramen ovale opening allows blood unfiltered by the lungs to flow to the brain, where it acts as triggers for migraine.
in approximately 40% of cryptogenic stroke patients. Patients are not typically screened for a PFO unless they have actually had a stroke.
Historically, PFOs have not been routinely closed using surgery or transcatheter therapies. Instead, patients with stroke or migraine and a PFO have typically been treated with drug therapy to address the symptoms of the disorder. Drug therapy, however, is often ineffective and can cause serious complications, such as hemorrhagic strokes, which are caused by bleeding in the brain. We estimate that the addressable patient population for PFO closure in the prevention of recurrent cryptogenic strokes in the United States and Europe is approximately 200,000 patients annually, representing a market opportunity greater than $1 billion annually. We estimate that the addressable patient population for PFO closure in the treatment of people with severe migraines in the United States and Europe is approximately 1 million patients annually, representing an even larger potential market opportunity.
Left Atrial Appendage
The left atrial appendage, or LAA, is a small pouch on the left side of the heart, which is the remnant of the original embryonic left atrium that forms during early development. Atrial fibrillation, a condition that results in irregular electrical activity in the upper chambers of the heart, can cause blood to pool and stagnate in the LAA, increasing the chances of forming clots, which may travel to the brain and lead to stroke. Atrial fibrillation is the most commonly diagnosed heart rhythm disorder and affects over 2 million people in the United States and over 2.5 million people in Europe.
Patients with atrial fibrillation are typically treated by blood thinning drugs to reduce the risk of stroke. Common blood thinning medications, such as coumadin, may cause undesirable side effects such as bleeding and require frequent blood monitoring. Studies suggest only approximately 60% of patients can tolerate blood thinners, leaving less effective medications as the only readily available medical treatment. Surgical closure of the LAA is typically only performed when the patient is already undergoing open-heart surgery for another condition. We estimate that the addressable patient population for LAA closure with a transcatheter approach in the United States and Europe is approximately 300,000 patients annually, representing a market opportunity greater than $1 billion annually.
Vascular Diseases
There are numerous vascular diseases characterized by defects in the blood vessel wall or abnormal or inappropriate blood flow.
Aneurysms. Aneurysms develop when the integrity and strength of the vessel wall is reduced, causing the vessel wall to progressively expand or balloon out. Aneurysms are often caused by atherosclerosis, a disease characterized by the thickening and hardening of the arteries. This decreased arterial flexibility can result in weakening of the arterial wall and bulging at sites that are exposed to high blood flow and pressure. Aneurysms are commonly diagnosed in the aorta, the largest artery in
84
the human body, which stems from the heart and carries blood to the body's organs. The aorta is divided into four portions: (1) the ascending aorta, (2) the aortic arch, (3) the thoracic aorta and (4) the abdominal aorta. Aneurysms can also occur in other smaller arteries, such as the iliac arteries, which branch off from the aorta and lead to the legs.
In the United States alone, it is estimated that as many as 1.7 million people have an abdominal aortic aneurysm, or AAA, with only approximately 20%, or 360,000, presently diagnosed and approximately 10%, or 40,000, of those are treated by a procedure to correct the aneurysm. An estimated 21,000 people are diagnosed annually with a thoracic aortic aneurysm, or TAA. We believe that the market opportunity in Europe for technologies that address aneurysms is comparable in size to that in the United States. AAAs and TAAs are among the most serious cardiovascular diseases and, once diagnosed, currently require patients to either be treated through a combination of pharmacological therapy and non-invasive monitoring or undergo a major surgical procedure to repair the aneurysm. After an AAA or TAA develops, it continues to enlarge and, if left untreated, becomes increasingly susceptible to rupture. In a TAA of less than 6 cm, the rupture rate within five years is 16%. In a TAA greater than 6 cm, the rupture rate within five years increases to 31%. In AAAs, the rupture rate within five years for aneurysms in the range of 5–5.9 cm is 25%.
The conventional treatment for an aneurysm is a highly invasive surgical procedure requiring a large incision in the patient's abdomen, withdrawal of the patient's intestines to provide access to the aneurysm and the cross clamping of the aorta to stop blood flow. This surgery has an operative mortality rate of 3% to 5% in elective surgery and approximately 75% if the aneurysm ruptures. In addition, complication rates vary depending upon patient risk classification, ranging from 15% for low-risk patients to 40% for high-risk patients. The typical recovery period for conventional surgery includes a hospital stay of seven to ten days and post-hospital convalescence of 12 weeks. Given the high rate of complications of open surgery, many physicians choose medical management and "watchful waiting" until aneurysms grow to larger than 4–5 centimeters in size.
Due to the mortality rates, complications and lengthy recovery period described above, physicians have for years sought less invasive methods to treat AAAs and TAAs as alternatives to surgical repair. However, transcatheter devices currently used to repair an aneurysm are often too large to be considered a truly minimally invasive procedure. These devices require very large catheters that can only be introduced into a blood vessel by a procedure known as a cutdown, typically performed by a vascular surgeon. By eliminating the need for a cutdown, a broader range of physicians skilled in minimally invasive procedures would likely be able to perform the procedure with the potential for fewer complications. Currently, less than 15% of patients with AAA are treated for the disease. We estimate that the addressable patient population for a device that could address the shortcomings
85
described above is approximately 240,000 patients annually for AAA, representing a market opportunity of approximately $3 billion annually, and approximately 60,000 patients annually for TAA, representing a market opportunity of approximately $260 million annually.
Abnormal Blood Vessels. Peripheral embolization, a widely accepted treatment option for a large range of vascular conditions outside the heart, reduces or eliminates blood flow to an area of the body by blocking, or occluding, a blood vessel. Vascular occlusion can also be used to reroute blood away from inappropriately formed blood vessels to different blood vessels. Vascular occlusions can be performed by surgery. More commonly, however, occlusions have been performed by releasing small wire coils at the point of occlusion, causing a clot to form, and thus blocking the flow of blood. Rarely is a single coil sufficient to occlude a blood vessel. Six to ten and often times more coils are required to occlude the vessel, which results in a technically challenging, time-intensive and costly procedure with the potential for adverse events if the coils migrate away from the intended location. We believe that there is a significant opportunity for occlusion devices that can address existing shortcomings of coils by having a single device that can more quickly, safely and precisely occlude the vessel through a simple procedure. We estimate that the addressable patient population for peripheral embolization is approximately 300,000 patients annually, representing a market opportunity of approximately $260 million annually.
The AGA Solution
We develop products for the treatment of structural heart defects and vascular diseases. The performance or efficacy of our products has been documented in over 1,500 clinical publications. We have developed an expertise in the braiding of nitinol and in designing transcatheter delivery systems that enable simple and precise implantation of our AMPLATZER occlusion devices, while also providing the capability for physicians to retrieve and reposition the device during the procedure if they are not satisfied with the initial placement. Our AMPLATZER family of devices uses nitinol because its properties that allow our devices to be compressed inside a delivery sheath and then return to their original shape once deployed at the implant site. The combination of the use of nitinol and our manufacturing techniques allows us to create device shapes specific to each indication.
AMPLATZER Structural Heart Defect Occluders
We believe that our AMPLATZER structural heart defect occlusion devices offer the following advantages over our competitors' devices and open-heart surgery:
- •
- Easier to Implant, Retrieve and Reposition. We believe that
our AMPLATZER occlusion devices are easier to implant than our competitors' devices. Our occlusion devices are delivered through relatively smaller
catheters and incorporate a unique mechanism to attach, deliver and release the devices at the site of the defect to be closed. We believe that our occlusion devices are the only fully retrievable and
repositionable occluders on the market. The ability to retrieve and reposition the devices during the same procedure eliminates the need to remove the occlusion device and the catheter, which
minimizes potential trauma to the patient, potential complications with the procedure and disposal of damaged devices that could not be properly deployed.
- •
- Highly Effective Closure Rates. Our devices have consistently
been shown to be highly effective in closing structural heart defects. Clinical publications have reported closure rates of approximately 96% for our AMPLATZER ASD and PFO Occluders. Our occlusion
devices have a long history of durability as evidenced by some devices having been implanted in patients
for over 13 years.
- •
- Minimally Invasive Procedures. All of our devices are inserted into the human body through a small catheter via the femoral artery in the patient's groin and then travel through the body's
86
- •
- Cost Efficient. The minimally invasive nature of the procedures required to implant our AMPLATZER occlusion devices reduces costs by taking advantage of shorter hospital stays and reduced therapy and follow-up care requirements. The average open-heart surgery procedure costs approximately $15,000 to $30,000, while the average total procedure cost to implant one of our AMPLATZER occlusion devices is generally less than $12,000, including device and hospital costs.
vasculature to the heart. This transcatheter approach minimizes blood loss, trauma and other surgical complications associated with invasive open-heart surgery. Most patients have the procedure done on an outpatient or overnight basis and do not have to endure the lengthy two- to three-month recovery process required following open-heart surgery.
AMPLATZER Vascular Products
We are leveraging our expertise in nitinol braiding and our proficiency in the design of transcatheter delivery systems to develop products for the treatment of vascular diseases. We believe our existing vascular products and vascular products in our pipeline address a number of conditions characterized by large patient populations and existing therapies with significant shortcomings. Our initial vascular products seek to occlude abnormal blood vessels with our family of AMPLATZER Vascular Plugs and treat aneurysms in small arteries, such as the iliac arteries, and larger vessels, such as the thoracic and abdominal portions of the aorta, with our family of AMPLATZER Vascular Grafts.
Our Strategy
We seek to remain a leader in the innovation and manufacture of occlusion devices for the treatment of structural heart defects and to leverage our core competencies into leading positions in new markets. To accomplish this objective, we intend to:
- •
- Grow Our Core Business of Structural Heart Defect Occlusion and Vascular
Devices. We have a leading market position for occlusion devices to treat ASDs, PDAs and VSDs. We intend to build on our existing portfolio with a series of
product line extensions that will expand our addressable market opportunity. In 2007, we received approval to market two of these new products in the United States and one additional new product in
Europe. In February 2008, we filed for regulatory approval for one additional vascular device and received approval in Europe in May 2008. The device remains under review by the FDA in the United
States. In July 2009, we received regulatory approval in Europe for a second new device, and we plan to file for regulatory approval of the same device in the United States in the second half
of 2009.
- •
- Capitalize on the PFO Market Opportunity. We believe that the
performance of our devices, the AMPLATZER brand name and our global distribution network position us to take advantage of the large potential PFO market
opportunity. We believe that physicians prefer our AMPLATZER PFO Occluders over our competitors' devices because of their ease of use, highly effective
closure rates and its safety record. In Europe, we have the leading market position in PFO occlusion devices. In the U.S. market, we are conducting the RESPECT study to assess the impact of our PFO
closure device in reducing the occurrence of certain types of stroke. We believe that physicians outside the United States will rely on a successful outcome of the RESPECT trial, which may lead to an
acceleration of international PFO sales.
- •
- Expand and Commercialize Our Research and Development Pipeline. Our co-founder and former President, Dr. Amplatz, supported by a team of physicians, scientists and engineers in our research and development department, has leveraged our core competencies in nitinol braiding and transcatheter delivery systems to develop and expand our pipeline of products. We received CE Mark clearance in December 2008 for an occluder device with an initial indication to close
87
- •
- Continue to Strengthen Our Global Distribution. We currently market our products in 112 countries. We have established a direct U.S. field organization of 42 representatives, 26 of whom focus on our structural heart defect occlusion devices, with the other 16 focusing on vascular products. Over the same period, we have established significant direct sales presence in Germany, Austria, Switzerland, the United Kingdom, Spain, Slovakia and the Nordic countries. In January, 2009, we have established a direct sales presence in Canada, Portugal, France, Italy, Belgium and the Netherlands. We may continue to expand our direct sales force in other international markets to help us increase product sales in those countries and capture higher margins by recapturing the distributor's share of the margin. We also believe that there are significant opportunities to capture market share in developing markets, such as China, India and Latin America. In China, for example, we established a distributor relationship with the Abbott Vascular division of Abbott Laboratories, Inc. for the distribution of our products.
the LAA and have initiated marketing of the device in Europe. We are also developing uniquely designed vascular grafts made of multiple layers of braided nitinol that are delivered through small catheters for the treatment of aneurysms. We intend to refile for a CE Mark in Europe and to apply for an IDE in the United States for the first of these products in the first half of 2010. We had previously intended to apply for these clearances in the first half of 2009. See "—Our Product Portfolio—AMPLATZER Vascular Products—AMPLATZER Vascular Grafts."
Corporate History
We were founded as AGA Medical in Minnesota in 1995 by Dr. Kurt Amplatz, a professor and researcher at the University of Minnesota's Department of Radiology, Mr. Franck Gougeon and Mr. Michael Afremov to capitalize on the attributes of nitinol to make occlusion devices for the transcatheter treatment of structural heart defects. We received a CE Mark in Europe for our initial occlusion devices and related delivery systems in 1998. In 2001, we received U.S. regulatory approval to commercialize our AMPLATZER Septal Occluder, which addresses one of the largest treatment areas of the structural heart defect market. We received U.S. regulatory approval to commercialize our AMPLATZER Duct Occluder device in 2003 and our AMPLATZER Muscular VSD Occluder device in 2007.
We have recently leveraged our core competencies in braiding nitinol and designing transcatheter delivery systems to develop products for the treatment of certain vascular diseases. Our first products in this area, which we launched in the United States in September 2003 and in Europe in January 2004, are vascular plugs for the closure of abnormal blood vessels that develop outside the heart. A second version of our vascular plug was approved and launched in the United States and Europe in August 2007, and a third version was approved in May 2008 and is under review by the regulatory authorities in the United States. We received regulatory approval for a fourth device in Europe in July 2009 and plan to file for regulatory approval in the United States in the second half of 2009.
In June 2002, Dr. Amplatz ceased to be a stockholder of AGA Medical upon redemption of his stock ownership by the company. In October 2002, a dispute arose between the then existing shareholders of AGA Medical—Dr. Amplatz, Mr. Gougeon and Mr. Afremov—surrounding the termination of Mr. Afremov's employment by Dr. Amplatz and Mr. Gougeon. Thereafter, Mr. Afremov brought litigation against AGA Medical and the other shareholders asserting various statutory and common law shareholder claims and similar counterclaims were asserted against Mr. Afremov by AGA Medical and the other shareholders. In May 2003, AGA Medical became subject to a court-ordered receivership so as to preserve decision-making authority for the company during the litigation. In November 2003, the court ruled that as a result of the prior redemption agreement previously mentioned, Dr. Amplatz had ceased to be a stockholder in June 2002. Thereafter, the court ordered the remaining two shareholders to submit competing proposals to buy-out the entire interest of the
88
other shareholder. The remaining two shareholders subsequently negotiated a full and final settlement resolving all claims between the parties. In connection with the termination of this litigation, on July 28, 2005, we implemented our July 2005 reorganization. AGA was incorporated in connection with the July 2005 reorganization as the parent company of AGA Medical. As part of our July 2005 reorganization, AGA Medical purchased and redeemed all of the outstanding shares of common stock owned by Mr. Afremov, who has not been associated with us since such time. To finance our July 2005 reorganization, AGA Medical (1) issued an aggregate principal amount of $50.0 million of our 2005 notes, which was purchased by one of the WCAS Stockholders at a discount, (2) issued 128,524 shares of Series A preferred stock to the WCAS Stockholders at a purchase price of $1,000 per share and (3) borrowed $107.0 million under a $122.0 million senior credit facility, consisting of a $107.0 million senior term loan and a $15.0 million revolving credit facility. The remaining stockholder, Mr. Gougeon, and new investors subsequently contributed all of their outstanding shares in AGA Medical to AGA in exchange for shares of AGA. Since the July 2005 reorganization, our corporate control has been jointly held by the WCAS Stockholders and the Gougeon Stockholders. The receivership was discharged by the court in October 2005.
On April 28, 2006, we completed our $240.0 million April 2006 recapitalization. As part of our April 2006 recapitalization, we entered into an amended and restated senior secured credit facility consisting of a $215.0 million seven-year Tranche B term loan facility and a $25.0 million revolving credit facility, which we refer to collectively as our senior secured credit facility. The Tranche B term loan was drawn in full in April 2006, and the proceeds were used to repay existing senior debt and pay accrued dividends of $11.0 million to the Series A preferred and Class A common stockholders, a $0.30 per share dividend to all Series A preferred, Class A common and other common stockholders, and transaction-related expenses, as well as to fund general working capital needs. On October 5, 2008, Lehman Commercial Paper Inc. ("LCPI") filed for protection under Chapter 11 of the Federal Bankruptcy Code. LCPI had committed to provide $9.5 million under the $25 million revolving credit facility. In March 2009, Bank of America, N.A. assumed the commitment under our revolving credit facility previously held by LCPI. At June 30, 2009, we had outstanding borrowings of $25.0 million under our revolving credit facility. As a result, we currently have no additional availability under our revolving credit facility. We will be permitted to reborrow any portion of the indebtedness outstanding under our revolving credit facility that is repaid with a portion of our net proceeds from this offering.
As part of our strategy to grow internationally, we have converted our distribution to direct sales representation in the United Kingdom in 2006, in Spain in April 2008 and in Slovakia in July 2008, as well as developed more significant direct sales presence, such as in Germany, Austria, Switzerland and the Nordic countries. In addition, in January 2009, we converted from distribution to direct sales representation in Canada, France, Portugal, Italy, Belgium and the Netherlands. The aggregate purchase price for these conversions to direct sales was $51.8 million, of which $41.0 million related to Italy. In addition, we also believe that there are significant opportunities to capture market share in developing markets, such as China, India and Latin America. In China, for example, we recently established a distributor relationship with the Abbott Vascular division of Abbott Laboratories, Inc. for the distribution of our products in that country.
On January 5, 2009, in order to finance, in part, the acquisition of the assets of our former Italian distributor, AGA Medical issued to one of the WCAS Stockholders for an aggregate purchase price of $15 million (1) $15.0 million in aggregate principal amount of the 2009 notes, and (2) 1,879 shares of Series B preferred stock. The 2009 notes are fully and unconditionally guaranteed by Amplatzer Medical Sales Corporation.
In December 2006, we moved into our new headquarters in Plymouth, Minnesota, and consolidated our sales, manufacturing, warehousing, research and development and administrative activities into this one location.
89
Our Product Portfolio
We classify our product portfolio into two categories: structural heart defect products, which we market primarily to interventional cardiologists, and vascular products, which we market primarily to vascular surgeons and interventional radiologists.
A number of our products are in the early stages of development. In the United States, before we can market a new medical device, or a new use of, or claim for, or significant modification to, an existing product, we must first receive either approval of a PMA application from the FDA, or clearance under section 510(k) of the U.S. Federal Food, Drug, and Cosmetic Act, which we refer to as 510(k) clearance, unless an exemption applies. A clinical trial is always a required step to support the PMA application approval and may be required to support the 510(k) clearance. Moreover, sales of our products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. The approval process for occluders is generally more complex in the United States. The regulatory process in Europe generally requires the submission of pre-clinical processes. Our AMPLATZER Vascular Plug has been approved through the 510(k) clearance in the United States and with the submission of pre-clinical data only in Europe. The rest of the world, with the exception of Japan, generally accepts approval by the FDA or a CE Mark in Europe as a basis for approval to market. Japan has a regulatory process that generally accepts clinical data from either the United States or Europe supplemented by a small study in Japan to establish experience and confirm safety. See "—Government Regulation."
| |
|
Regulatory Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
Product
|
Indication | United States Status | European CE Mark Status |
||||||
Structural Heart Defects: |
|||||||||
AMPLATZER Septal Occluders |
|||||||||
Septal Occluder |
Atrial septal defect (ASD) repair for single hole |
PMA |
12/01–Approved |
2/98–Granted |
|||||
Multi-Fenestrated Septal Occluder (Cribriform) |
Atrial septal defect (ASD) repair for multiple holes |
PMA |
9/06–Approved |
9/02–Granted |
|||||
AMPLATZER Duct Occluders |
|||||||||
AMPLATZER Duct Occluder |
Closure of patent ductus arteriosus (PDA) |
PMA |
5/03–Approved |
2/98–Granted |
|||||
AMPLATZER Duct Occluder II |
Closure of patent ductus arteriosus (PDA) |
PMA |
Clinical Trials |
2/08–Granted |
|||||
AMPLATZER VSD Occluders |
|||||||||
Muscular VSD Occluder |
Closure of muscular ventricular septal defect (VSD) |
PMA |
9/07–Approved |
2/98–Granted |
|||||
P.I. Muscular VSD Occluder |
Closure of muscular ventricular septal defect (VSD) created as a result of a heart attack |
HDE |
6/07–Filed for HDE |
3/01–Granted |
|||||
Membraneous VSD Occluder |
Closure of membraneous ventricular septal defect (VSD) |
PMA |
Safety study completed |
2/98–Granted |
|||||
AMPLATZER Cardiac Plug |
Occlusion of the left atrial appendage (LAA) |
PMA |
8/08–Filed for IDE |
12/08–Granted |
|||||
AMPLATZER PFO Occluders |
|||||||||
Stroke |
Closure of patent foramen ovale (PFO) for stroke |
PMA |
Clinical Trials |
2/98–Granted |
|||||
Migraine |
Closure of patent foramen ovale (PFO) for migraine |
PMA |
Clinical Trials |
Clinical Trials |
|||||
90
| |
|
Regulatory Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
Product
|
Indication | United States Status | European CE Mark Status |
||||||
Vascular diseases: |
|||||||||
AMPLATZER Vascular Plugs |
|||||||||
Vascular Plug |
Closure of abnormal blood vessels |
510(k) |
9/03–Cleared |
2/04–Granted |
|||||
Vascular Plug II |
Closure of abnormal blood vessels |
510(k) |
8/07–Cleared |
8/07–Granted |
|||||
Vascular Plug III |
Closure of abnormal blood vessels |
510(k) |
2/08–Filed for clearance |
5/08–Granted |
|||||
Vascular Plug IV |
Closure of abnormal blood vessels (delivered through diagnostic catheter) |
510(k) |
To be filed in second half of 2009 |
7/09–Granted |
|||||
AMPLATZER Vascular Grafts |
|||||||||
Peripheral Graft |
Treatment of iliac artery aneurysms |
PMA |
IDE to be filed in first half of 2010 |
To be filed in first half of 2010 |
|||||
TAA Graft |
Treatment of thoracic aortic aneurysms (TAA) |
PMA |
In development |
In development |
|||||
AAA Graft |
Treatment of abdominal aortic aneurysms (AAA) |
PMA |
In development |
In development |
|||||
We make our regulatory status forecasts, including determining expected dates of filings with, or submissions to, relevant authorities, based on the information currently available to us. The actual timing for any of these regulatory steps may vary, and we may revise any such forecasts as new information becomes available.
Our AMPLATZER occlusion devices, vascular plugs and vascular grafts are composed of nitinol, a metal which contains a nearly equal mixture of nickel and titanium alloy. We use nitinol because its shape memory properties allow our AMPLATZER occlusion devices, vascular plugs and vascular grafts to be compressed into a small catheter and advanced to the site of interest and, upon deployment, to regain their original shape as the sheath is withdrawn.
All of our implants are sold with our proprietary delivery systems and accessories, which are designed to facilitate proper positioning when our devices are implanted. We believe that our delivery systems are the only systems that can fully retract and reposition an occlusion device during the procedure without having to remove the device from the patient. We also sell a number of complementary accessories including sizing balloons, sizing plates and guidewires. Our sizing balloons and plates enable accurate measurement of the size of the structural heart defect and enable selection of an appropriately sized device to close the defect. All of our delivery systems and accessories have FDA 510(k) clearance and a CE Mark.
The number of AMPLATZER occluders sold may differ from the number of delivery systems sold in any given period. We sell our products both directly to hospitals and to distributors. Distributors tend to place bulk orders and do not necessarily balance in any given order the number of devices and delivery sets ordered, which number is largely dependent on their current inventory levels of each type. Hospitals tend to stock more delivery sets than occluders for several reasons. Different delivery sets are used for different types of patients, and it is difficult to predict the mix of patients. For example, adult patients would typically require longer delivery sets. Larger devices require larger diameter catheters. Hospitals stock more delivery sets on average than devices, since often times the selection of the appropriate delivery set is not made until preliminary measurements are made of the patients during the clinical procedure. Delivery sets also have far lower selling prices than devices, which also contributes to the tendency by hospitals to stock more delivery sets than occlusion devices given the uncertainties described above.
91
AMPLATZER Occlusion Devices for Structural Heart Defects
Our AMPLATZER occlusion devices have the following common features:
- •
- Our occlusion devices are generally constructed using two discs made of braided nitinol wire, which come in varying shapes
and sizes depending on the defect that they are designed to occlude.
- •
- The two discs are linked together by a short connecting waist also made of nitinol wire. In certain defects, the waist
helps to center the device.
- •
- Each disc contains thin polyester fabric which aids in closure by promoting endothelialization, a process in which new tissue completely encloses the occlusion device in the septal wall of the heart, essentially becoming a part of the heart and permanently closing the defect. Certain of our next generation occlusion devices may not need to contain fabric as we are developing occluder designs using denser and multi-layer braiding that will replace the need for fabric. Occluders without fabric can be delivered through smaller catheters and can more readily conform to the anatomy near the defect.
Our AMPLATZER occlusion devices are delivered through small catheters, employ our unique screw attachment mechanism and are fully retrievable until released from the screw which allows for repositioning prior to release of the implant.
Our AMPLATZER occlusion devices are typically delivered through a standard percutaneous puncture of the femoral artery or vein, located near the patient's groin. Our delivery systems are used to facilitate attachment, loading, delivery and deployment of our AMPLATZER occlusion devices. The devices are connected to a proprietary delivery cable by a screw attachment. In a procedure generally lasting approximately one hour or less, the interventional cardiologist maneuvers our flexible catheter through the vasculature to the heart. For some procedures, we recommend that the interventional cardiologist use our sizing balloons to measure the exact size of the defect in order to select an appropriately sized AMPLATZER occlusion device. The physician positions the catheter across the defect and positions the first disc over the hole and against the wall separating the two chambers of the heart, using imaging technology to check for proper placement before deploying the second disc on the opposite side of the defect. The physician then checks one more time that the device is properly positioned and, if satisfied, unscrews the delivery cable used to position the device and withdraws both the catheter and the cable. A unique feature of the AMPLATZER occlusion device is the ability to retrieve and reposition the device multiple times, if necessary, prior to unscrewing the device from the wire. The procedure is typically performed on an overnight or outpatient basis, although in certain countries, it is common practice for patients to remain for one to two days in the hospital following the procedure.
AMPLATZER Septal Occluders
We market two occluders for ASDs: our AMPLATZER Septal Occluder for closure of single holes and our AMPLATZER Multi-Fenestrated Septal Occluder, also referred to as the Cribriform, for closure of multiple holes. The waist connecting the two discs comes in size ranging from 4 to 40 millimeters. The appropriate sized waist expands to the width of the hole helping to ensure appropriate positioning of the device.
The AMPLATZER Septal Occluder was granted a CE Mark in Europe in February 1998 and FDA PMA approval in December 2001. The AMPLATZER Multi-Fenestrated Septal Occluder was granted a CE Mark in Europe in September 2002 and FDA PMA approval in September 2006. In August 2005, our AMPLATZER Septal Occluder became the first approved occlusion device in Japan. Devices in Japan must also receive reimbursement approval prior to marketing. Following receipt of reimbursement approval in April 2006, we launched our AMPLATZER Septal Occluder in Japan in May 2006.
92
AMPLATZER Duct Occluder
Our AMPLATZER Duct Occluder is intended for the closure of PDAs larger than 4 millimeters, which we believe represent approximately 30% of total PDA defects. Smaller PDAs are treated using drug therapy or coils. Our AMPLATZER Duct Occluder products are uniquely shaped to achieve consistent, effective closure of PDAs. In addition to the typical features of our AMPLATZER occlusion devices, the AMPLATZER Duct Occluder employs what we call a retention "skirt," which allows the device to be positioned properly and remain in place at the entrance to the duct. The "skirt" is the flat top portion of the device that is connected to a conical body. The body is positioned into the duct.
The AMPLATZER Duct Occluder was granted a CE Mark in Europe in February 1998 and FDA PMA approval in May 2003 and approval in Japan in December 2008. Devices in Japan must also receive reimbursement approval prior to marketing. Our distributor has applied for reimbursement approval and expects to receive to it in the fourth quarter of 2009. Our AMPLATZER Duct Occluder and our AMPLATZER Septal Occluder are the only approved occlusion devices in Japan. Our second generation of AMPLATZER Duct Occluders, which has been granted a CE Mark in Europe, can be delivered in even smaller catheters and is appropriate for both smaller ducts and ducts with different geometries as we have eliminated the fabric, which is typically embedded in the disc, and instead rely solely on multi-layered braiding for rapid occlusion.
AMPLATZER VSD Occluders
We market and sell our AMPLATZER VSD Occluders to address membraneous VSDs and muscular VSDs. Membraneous VSDs are defects in the upper portion of the ventricular septum near the valve connecting the heart with the aorta and characterized by more flexible, membraneous tissue. Muscular VSDs are defects in the middle portion of the ventricular septum, which is characterized by thicker, more muscular tissue. We also market and sell a third version of the AMPLATZER VSD Occluders designed for VSDs that are created as a result of heart attacks. The different designs of our three AMPLATZER VSD Occluders are characterized by the shape and sizing of the discs and the length of the waist between the discs.
Our AMPLATZER Membraneous VSD Occluder device received a CE Mark in Europe in 1998. We have completed a safety study in the United States and are in the process of gathering data from the use of the device in Europe prior to proceeding with additional clinical studies in the United States. Our AMPLATZER Muscular VSD Occluder device received a CE Mark in 1998 and received PMA approval in the United States in September 2007. Our AMPLATZER P.I. Muscular VSD Occluder was approved in Europe in 2001. We filed for approval in the United States under a Humanitarian Device Exemption, or HDE, in 2007, and that application is under review by the FDA. An HDE exemption is typically granted for medical devices with a patient population of less than 4,000 patients per year. We believe the market for the AMPLATZER P.I. Muscular VSD Occluder is smaller than 4,000 patients per year, given the serious nature of the event (a tear in the ventricular septum) following a heart attack, as most potential patients do not survive long enough to have the tear in the ventricular septum repaired. The small population of potential users has made completion of the clinical trial challenging, and we have had extended discussions with the FDA on interpreting and clarifying the results of the trial as they would be presented in the instructions for use. We believe that we have resolved most of these issues. While the discussions were underway, the FDA also requested that we conduct additional pre-clinical testing. Specifically, the FDA requested additional testing designed to simulate (in the lab) the structural performance of the device when implanted for an extended period of time along with associated biocompatibility. This testing is routinely requested for devices designed to be implanted in the heart and associated major blood vessels. We are working to complete the additional pre-clinical testing and answer all remaining questions posed by the FDA.
93
AMPLATZER PFO Occluder
Our AMPLATZER PFO Occluder closes all types of PFOs and are shaped to achieve consistent, effective closure of PFOs. Unlike other structural heart defects that could be described as holes, PFOs are more like tunnels formed by two overlapping membranes that fail to seal closed at birth. Our AMPLATZER PFO Occluder is characterized by a thin waist that provides flexibility for the two discs to adjust dynamically to the unique anatomy of the patient's PFO. Our AMPLATZER PFO Occluder is available in four sizes of 18, 25, 30 and 35 millimeters, as measured by the diameter of the disk that is positioned on the right side of the atrium.
In Europe, we received a CE Mark in February 1998 for use in patients with PFO. From April 2002 to October 2006, we marketed the device in the United States under a HDE status granted by the FDA. On October 31, 2006, we agreed with the FDA to voluntarily withdraw the HDE designation of our AMPLATZER PFO Occluder. We currently can enroll patients in the United States who have had at least two strokes and do not otherwise qualify for our PFO stroke study. We can sell the device to hospitals that are approved to enroll patients in the PFO Access registry study. No more than 2,000 patients can be enrolled per year in the registry. There is a growing body of evidence that the presence of PFOs may be linked to stroke and migraine, and we are conducting two other clinical trials to support PMA approval in the United States for use of our AMPLATZER PFO Occluders in stroke or migraine patients with PFO. See "—Clinical Development Programs."
AMPLATZER Cardiac Plug
Our AMPLATZER Cardiac Plug, with an initial indication for LAA Occlusion, is constructed with braided nitinol wire, similar to our structural heart defect occluders. The device is implanted through a standard femoral artery procedure and uses a specially designed delivery system and catheter that includes the standard AMPLATZER screw attachment to permit retrieval and repositioning prior to release from the cable. Unless the patient has an open PFO permitting access by the catheter to the left atrium, implanting an AMPLATZER Cardiac Plug requires the puncture of a small hole in the wall of the atrium. This procedure, sometimes referred to as a transseptal puncture, is increasingly being used by cardiologists in other interventional procedures in the heart. Following the deployment of the device and similar to other AMPLATZER devices, tissue will grow over the device providing a permanent seal to the left atrial appendage. We believe that our AMPLATZER Cardiac Plug will be particularly appealing for those patients who cannot tolerate current blood thinners used in medical management or who do not wish to be subject to long term management on blood thinners with the corresponding frequent monitoring and risks of bleeding.
We received CE Mark clearance in December 2008 and commenced marketing the device in Europe. We also applied to the FDA in August 2008 to begin a clinical study to support U.S. approval of our AMPLATZER Cardiac Plug. We are in the process of completing additional non-clinical testing required by the FDA prior to initiation of the clinical trial. In addition, we received a request from the FDA in August 2009 for modifications to the clinical trial design, and we are in the process of preparing a response to their request.
AMPLATZER Vascular Products
AMPLATZER Vascular Plugs
Our AMPLATZER Vascular Plugs are expandable, cylindrical devices made from nitinol wire that reduce or eliminate blood flow to abnormal blood vessels. Vascular occlusion can be used to reroute blood away from inappropriately formed blood vessels to different blood vessels. Vascular occlusions were previously only accomplished by surgically closing the blood vessel. More commonly, occlusions have been performed by releasing small wire coils at the point of the occlusion, causing a clot to form, blocking the flow of blood. Typically six to ten coils are required to occlude the vessel, which results in a technically challenging, time-intensive, costly procedure with the potential for adverse events if the coils migrate away from the intended location. Our AMPLATZER Vascular Plug can be precisely
94
positioned in the vessel. The nitinol wire provides a cross sectional barrier that slows down the flow of the blood resulting in occlusion of the vessel. A single plug is generally sufficient even in a procedure that would have required many coils, which makes it a comparatively efficient and cost-effective alternative. Our AMPLATZER Vascular Plugs are designed for use in abnormal blood vessels outside the heart, below the neck and above the knee and utilize standard delivery systems commonly used by interventional radiologists and vascular surgeons in these procedures.
Two versions of the plug are approved for marketing in the United States and Europe, and two additional versions are under development:
Vascular Plug. Our original AMPLATZER Vascular Plug received a CE Mark in February 2004 and FDA 510(k) clearance in September 2003. Our AMPLATZER Vascular Plug provides occlusion of the vessel in an average of ten minutes.
Vascular Plug II. The AMPLATZER Vascular Plug II received a CE Mark and FDA 510(k) clearance in August 2007. Unlike the two surface areas of the AMPLATZER Vascular Plug, the AMPLATZER Vascular Plug II is designed to have six surface areas, with each surface area progressively slowing down the blood flow leading to formation of the clot within the device. In pre-clinical studies, the AMPLATZER Vascular Plug II's unique multi-segmented design significantly reduces the time to occlusion for transcatheter embolization procedures by 20% to 30% in comparable vessels when compared to the AMPLATZER Vascular Plug in pre-clinical studies. In many blood vessels, the typical occlusion time of the vessel in approximately six minutes. The AMPLATZER Vascular Plug II comes in a broader range of sizes than the AMPLATZER Vascular Plug, including smaller and larger sizes.
Vascular Plug III. We are developing the AMPLATZER Vascular Plug III. The combination of a denser braid and an oval shape will make the AMPLATZER Vascular Plug III an attractive alternative for irregularly shaped vessels requiring rapid occlusion. We received a CE Mark in Europe in May 2008 and applied for 510(K) clearance in the United States in February of 2008. The FDA has requested additional non-clinical testing of the device, and we are in the process of completing that testing and answering all remaining questions from the FDA.
Vascular Plug IV. We are developing the AMPLATZER Vascular Plug IV to be delivered through a standard diagnostic catheter. The advantage of this approach is that there will be no added cost or time required to exchange from a diagnostic catheter to a therapeutic delivery catheter. While we had previously intended to file for a CE Mark by the end of 2008, we decided to modify the design of the AMPLATZER Vascular Plug IV to improve the product's performance. We subsequently filed for a CE Mark in March 2009 and received a CE Mark in Europe in July 2009. We plan to file for 510(k) clearance in the United States in the second half of 2009. We had previously intended to apply for this clearance in the first half of 2009, but we are still in the process of completing certain non-clinical, functional tests requested by the FDA prior to submitting a 510(k) application with the expectation that the results of such tests will shorten the review cycle. We do not anticipate any technical challenges in conducting these tests; however, the conduct of these tests adds time to the development process and has postponed submission of the 510(k) application.
AMPLATZER Vascular Grafts
We are developing a family of AMPLATZER Vascular Grafts to treat aneurysms in a variety of blood vessels, including smaller arteries, such as the iliac arteries, and larger vessels, such as the thoracic and abdominal portions of the aorta. Our devices are composed of multiple layers of braided nitinol filaments woven into precise shapes and sizes. The unique design of our graft seals or excludes the aneurysm without the need for a fabric covering.
95
We believe our AMPLATZER Vascular Grafts will have the following advantages over traditional grafts:
Truly minimally invasive transcatheter procedure. Our AMPLATZER Vascular Grafts will be comprised of a highly flexible, multi-layer braided nitinol, eliminating the need for fabric covering the outside of the graft. Competitor devices typically use fabric coatings to reduce the size of the aneurysm. The occluder devices that we are developing use denser and multi-layer braiding without fabric and thus can be compressed to a much smaller size and delivered in a smaller catheter through a more superficial artery, eliminating the need for an arterial cut-down.
Unique graft design. We designed our grafts with nitinol, which quickly integrates with the arterial wall, strengthening the vessel from within. In contrast, commonly used grafts never integrate with the artery because they are covered by fabric, typically polyester, presenting a continued risk of leaks, migration or clots forming along the implant.
Ability to treat aneurysms at an earlier stage. Because our devices can be introduced through a smaller catheter and have the potential to avoid many of the safety concerns related to current products, we believe that we may be able to safely treat patients at an earlier stage.
May diminish concerns about obstructing collaterals. The multiple layers of nitinol quickly exclude or reduce the aneurysm by reducing pressure on the aneurysm sac, directing the flow of blood through the appropriate arterial channel. Unlike grafts currently in use, however, our device does not prevent blood flow to other healthy vessels, or collaterals, branching out away from the main artery. We believe this feature observed in pre-clinical studies should eliminate some common side-effects of TAA or AAA and other peripheral procedures.
Our initial focus will be on aneurysms that occur on smaller peripheral arteries, such as the iliac arteries, as we have already designed a tubular graft with the appropriate diameter to be deployed in this artery which is smaller than the aorta. We encountered some issues with the delivery system in connection with the final engineering validation of our graft. We believe these issues will require further engineering and design work on the delivery system. We had filed for a CE Mark in Europe in June 2009, but due to these issues, we now intend to complete this work and refile for CE Mark approval in the first half of 2010. We had previously intended to apply to the FDA for an IDE in the first half of 2009 and then in the second half of 2009, but due to the issues with the delivery system described above, we now expect to apply to the FDA for an IDE in the first half of 2010.
We are also developing vascular grafts to treat thoracic aortic aneurysms, or TAAs. Our vascular graft design incorporates features not currently available in other grafts. Key elements include smaller delivery systems and the ability to provide secure positioning without the use of barbs or hooks, which could damage the aorta. We are currently assessing whether we need a small feasibility or safety study in Europe. If we do not, we plan to file for a CE Mark in Europe of our AMPLATZER Vascular Grafts to treat TAA in the first half of 2010. We had previously intended to file for a CE Mark in Europe in the second half of 2009. The delay in filing is due to continuing engineering work and associated pre-clinical testing that we believe is required to ensure that we can meet our key design objectives. Our U.S. regulatory pathway is under review.
We are also developing vascular grafts to treat abdominal aortic aneurysms, or AAAs, which are in the product design phase.
Clinical Development Programs
We support many of our new product initiatives with scientific clinical studies in order to obtain regulatory approval and provide marketing data. The goal of a clinical trial is to meet the primary endpoint, which measures the clinical effectiveness and/or safety of a device and is the basis for FDA approval. Primary endpoints for clinical trials are selected based on the intended benefit of the medical device. Although clinical trial endpoints are measurements at an individual patient level, the results are
96
extrapolated to entire populations of patients based on clinical similarities to patients in the clinical trials.
RESPECT (U.S. Pivotal Trial for Recurrent Cryptogenic Stroke)
A number of studies have suggested a relationship between cryptogenic stroke and PFO. Cryptogenic stroke is a stroke that occurs in a patient who does not possess any of the known risk factors for stroke. A main cause of the stroke is believed to be an emboli that, ordinarily filtered out by the lungs, instead crosses the PFO and passes through the circulatory system to the brain where it blocks a blood vessel causing what is termed an ischemic stroke.
RESPECT is our U.S. trial to evaluate the safety and efficacy of our AMPLATZER PFO Occluder to prevent recurrent cryptogenic stroke. RESPECT is a randomized trial, meaning patients are randomly assigned to a treatment arm, which in this trial involves treatment with our AMPLATZER PFO Occluder, or a control arm, which involves treatment by one of the accepted drug therapies. The objective of the study is to determine whether PFO closure is superior to drug therapy in preventing recurrent cryptogenic stroke. RESPECT will enroll approximately 500 to 900 patients, with 50% randomly selected for the treatment arm and 50% for the control arm.
We are currently enrolling patients in approximately 60 centers. As of June 30, 2009, 576 patients were enrolled in the study. Patients must have recently experienced a cryptogenic stroke. The trial is designed as a comparison of the number of events, being either stroke or death, in each arm of the study, and is designed with a statistical method that allows it to be stopped when one of several decision rules are achieved. If a decision rule supporting superiority is reached, that is PFO closure is more successful than drug therapy, based on a comparison of the number of events between the two arms, then the trial can be stopped immediately, and we can begin preparation of a PMA for the FDA. This design is different from many FDA approved clinical trials that require the trial to wait one or more years following enrollment of the last patient to complete an analysis of the data. A decision rule can be met at any time during the study. We will continue to enroll up to 900 patients and monitor all patients until a decision rule is achieved. There are also decision rules that stop the study if it is determined that we will not be able to demonstrate superiority when compared to medical management by drug treatment.
PC (European Clinical Trial for Recurrent Cryptogenic Stroke)
PC is our European trial to evaluate the safety and efficacy of our AMPLATZER PFO Occluder to prevent recurrent cryptogenic stroke. PC is also a randomized trial that will involve data from at least 450 patients, randomly selected in equal numbers to participate in the treatment and control arms. As of June 30, 2009, 414 patients were enrolled in the study at approximately 30 centers.
PFO ACCESS Registry Study
Until October 31, 2006, our AMPLATZER PFO Occluder was approved in the United States under a humanitarian device exemption. An HDE designation permits the sale of devices in cases in which a small population of patients—defined as less than 4,000 eligible patients annually—are thought to be able to benefit from the procedure. After discussions with the FDA, we voluntarily withdrew our HDE for our AMPLATZER PFO Occluder because we agreed that the eligible patient population was greater than 4,000 patients. We subsequently received approval from the FDA for a registry of up to 2,000 patients annually who experience at least two cryptogenic strokes but would not otherwise qualify for the RESPECT study. The registry is open to centers that were approved to enroll patients under the HDE by each center's Institutional Review Board, being a committee of hospital and community experts that review and approve all clinical studies. Although we will collect and monitor data from the sites on product performance and safety, the data will not be used to support a regulatory filing. The study will terminate when a PFO occluder receives regulatory approval in the United States.
97
PREMIUM (U.S. Pivotal Trial for Migraine with and without Aura)
Historical research has noted a possible association between PFOs and migraine headaches. In particular, prevalence of a PFO is especially high in patients who have migraine with aura, which means visual, auditory, sensory and motor abnormalities preceding a migraine attack. Data from past studies have indicated that as many as 80% of migraine sufferers who had their PFO closed for other reasons have reported a resolution or significant reduction, i.e., a reduction of greater than 50% in the frequency, of migraine headaches. For example, two studies published in 2005 in the Journal of the American College of Cardiology reported elimination or a significant reduction in the frequency of migraine attacks in greater than 70% of patients one year after PFO closure.
PREMIUM is a U.S. trial to evaluate the safety and efficacy of our AMPLATZER PFO Occluder to treat migraine headaches in patients with a PFO. To be eligible for the study, prospective patients must have a PFO and recently experienced a number of migraine attacks per month within a specified range. The PREMIUM protocol was approved by the FDA in June 2006. At the time, the protocol specified enrolling a maximum of 470 patients, with 235 under a treatment arm and 235 under a control arm, in up to 40 centers. In April 2009, the FDA granted conditional approval to amend the protocol to modify some of the conditions for patient eligibility in the trial. The FDA also agreed to reduce the number of patients to be enrolled in the study to 230 patients, with 115 in the treatment arm and 115 in the control arm. The changes were granted based on newly published clinical literature used to develop key assumptions in migraine trials and based on our experience in enrolling patients in the study. Conditional approval allows the clinical trial to commence but requires the specified conditions to be satisfied before the completion of the clinical trial.
Patients in the treatment arm receive our AMPLATZER PFO Occluder to close their PFO, and patients in the control arm undergo a sham procedure whereby their PFO is not closed. Patients in each arm also continue on their approved medications. The primary efficacy endpoint for PREMIUM will be a 50% reduction in the number of migraine attacks in at least 50% of patients when compared to the control arm. The period from which the measurement will be calculated will be one year following the procedure being performed on the patient. We are currently enrolling patients in this trial.
PRIMA (International Trial for Migraine with Aura)
PRIMA is our international trial to evaluate the safety and efficacy of our AMPLATZER PFO Occluder to treat migraine headaches in patients with a PFO. PRIMA is also a randomized trial that will involve data from approximately 140 patients, with 70 under a treatment arm and 70 under a control arm.
The design of PRIMA is similar to PREMIUM with the same general eligibility, except that (1) PRIMA will only enroll patients who experience migraine attacks with aura, and (2) patients in the control arm will not undergo a procedure but will only receive conventional medical management by drug treatment for their migraines. Patients in the treatment arm will receive both the implant and conventional medical management.
PRIMA is currently enrolling in approximately 15 centers in Canada, the United Kingdom and Germany.
Other Studies
We are conducting, or plan to conduct, a number of other clinical studies in the United States and Europe. We are currently conducting two post-approval studies that were required as a condition of approval by the FDA of the AMPLATZER Septal Occluder and the AMPLATZER Muscular VSD Occluder. The studies are designed to monitor patients for a period of up to five years after the procedure. The objective is to collect and report to the FDA additional data on the long-term safety and efficacy of the device. The majority of patients enrolled in these two studies were children at the time of receiving their implant. In some cases, it can be challenging to follow these patients for up to five years as they and their families move or otherwise stop seeing the physician who performed the treatment. In these cases, we have requested and received approval from the FDA to enroll new patients and follow them for up to five years to satisfy the FDA's requirements.
98
We have conditional approval from the FDA to conduct a clinical study in the United States to support the approval of the AMPLATZER Duct Occluder II. The study is approved to enroll approximately 170 patients in up to 25 centers. The study is designed as a single arm study in which all eligible patients receive the device. We commenced enrolling patients in the second half of 2008. Conditional approval allows the clinical trial to commence but requires the specified conditions to be satisfied before the completion of the clinical trial. In this particular instance, the conditions require us to conduct additional laboratory testing designed to simulate the structural performance of the product when implanted for an extended period of time along with associated biocompatibility.
We filed for approval to conduct an IDE study in the United States, to support approval of the AMPLATZER Cardiac Plug with an initial indication to close the left atrial appendage. We expect to enroll the first patient in the trial in the second half of 2009. We are in the process of completing additional non-clinical testing required by the FDA prior to initiation of the clinical trial. In addition, we received a request from the FDA in August 2009 for modifications to the clinical trial design, and we are in the process of preparing a response to their request.
We are also planning other clinical studies for products in our development pipeline. We plan to develop these studies and, where appropriate and required, file for approval in the United States and Europe following the completion of the required pre-clinical studies.
Marketing and Sales
We market and sell our AMPLATZER family of devices to hospitals and physicians, including interventional cardiologists, vascular surgeons and interventional radiologists. Procedures that use our devices are generally recommended to patients by pediatric and adult interventional cardiologists, and physician referrals and peer-to-peer selling are critical elements of our sales strategy. Physicians are, in most cases, the decision makers on whether to use our products. In certain countries where the government administers the healthcare system, a tender or bidding process is often used in product selection. Even in these cases, and given the history and performance of our devices, we believe that physicians have a significant influence on product selection. As a relatively small percentage of patients treated with our devices today are over 65, we do not rely extensively on Medicare for reimbursement on the sale of our devices. However, this may change in the future as we commercialize products in our pipeline for the treatment of structural heart defects and vascular diseases.
We are not dependent on any single customer, and no single customer (including distributors) accounted for more than 10% of our net sales in any of the three years in the period ended December 31, 2008 and in the six months ended June 30, 2009.
International
We select our distributors outside the United States based on their familiarity with interventional structural heart and vascular procedures and devices. Typically, our products represent the majority of the sales of our distributors. We require all of our distributors to attend periodic sales and product training programs. Internationally, we have agreements with approximately 53 physician specialists to train new physicians in the use of our products. We have also placed computer simulation systems in both our United Kingdom and German offices for use in customer training.
As part of our strategy to grow internationally, we may continue to selectively convert our distribution to direct sales representation in certain countries, as we did in the United Kingdom in 2006, in Spain in April 2008 and in Slovakia in July 2008, and to develop more significant direct sales presence, as we have done in Germany, Austria, Switzerland and the Nordic countries. As of January 2009, we transitioned to direct sales force in France, Italy, Portugal, Belgium, the Netherlands, Luxembourg and Canada. We intend to also focus on expanding our presence in underserved countries, such as China, where we signed an agreement in 2008 with the Abbott Laboratories group for the distribution of our products.
99
United States
We market and sell our products in the United States through a direct sales force of 42 representatives, 26 of whom focus on our structural heart defect occlusion devices, with the other 16 focusing on vascular products. Marketing and selling of our products is largely accomplished by frequent sales calls to on-site locations, as well as our targeted marketing efforts through medical conferences, journals and various marketing materials. We use in the United States approximately 55 experienced physician specialists who are employed on a contract basis to provide training to new physicians in the use of our products.
Research and Development
Our research and development efforts are led by Dr. Kurt Amplatz, our co-founder, former President and inventor of our family of AMPLATZER occlusion devices and AMPLATZER vascular plugs. All of our research and development efforts take advantage of our core competency in the use of braided nitinol in the design and manufacture of occlusion devices. Since our first product sales in 1996, we have launched over nine different structural heart and vascular devices worldwide and have a successful track record of receiving product approvals and regulatory clearances. Introduced in the late 1990s, our family of AMPLATZER occlusion devices was the first of a number of devices that successfully resulted in widespread adoption worldwide of a less invasive transcatheter approach to treat structural heart defects. Our current research and development efforts are focused on both line extensions to our existing occluders and vascular devices and the development and commercialization of new structural heart devices such as the AMPLATZER Cardiac Plug, with an initial indication for LAA Occlusion, and our family of vascular grafts.
As of June 30, 2009, our research and development staff consisted of approximately 39 full-time physicians, scientists and engineers, most of whom have substantial experience in medical device development. Located at our corporate headquarters in Plymouth, Minnesota, our team has a demonstrated record of developing new products which receive the appropriate product approvals and regulatory clearances, with our products having been approved in 112 countries based largely on our internal product development. We expect to continue to increase the size of our research and development team during 2009 as part of our strategy of commercializing our research and development pipeline.
During the years ended December 31 2006, 2007 and 2008 and the six months ended June 30, 2009, we incurred research and development expenses of $12.1 million, $26.6 million, $32.8 million and $16.5 million, respectively. As a result of our expected investments in enhancing the capabilities of our devices and exploring new applications and devices, we expect research and development efforts and expenses to increase in absolute dollar terms but to decrease as a percentage of net sales.
We have contractual relationships with a number of outside laboratories to conduct preclinical studies. The normal process for designing a new occlusion device involves the development and refinement of device prototypes. We then validate these designs using preclinical models at outside laboratories. This is then followed by formal preclinical trials. In parallel with these trials, the design of the device and the components used in its manufacture are formally validated by our engineering staff. Once the testing and component validation has been completed, we may file directly for approval by the FDA or by our Notified Body in Europe or, in cases where clinical trials are required, permission by the FDA or similar agencies in other countries for the design and conduct of the trial. The clinical trials are conducted by physicians following approval of the study by independent monitoring groups at each hospital called Institutional Review Boards. Once the clinical trials are completed, we submit the results of the trials and all associated testing of the device for regulatory approval.
100
Manufacturing
We manufacture our AMPLATZER occlusion devices at our corporate headquarters in Plymouth, Minnesota. We intend to initiate a process to establish manufacturing operations in Europe for all of our devices that are sold in international markets within 12 to 18 months. The manufacturing process combines the use of advanced technology and manual labor. First, a technician uses large mechanical braiders to spin fine nitinol filaments into a braided tube, which composes the body of the occlusion devices. The tube is then secured into a metal mold and baked at a high temperature to set the shape of the occlusion device. For certain devices, the technicians then sew polyester fabric into the inside of each device. Lastly the devices are inspected, packaged, sent to a third party local subcontractor for sterilization and then returned to us ready for shipment.
We continue to invest in improvements to our manufacturing process. These investments include the automation and enhanced control of key processes, as well as the implementation of automated inspection to improve quality control. We plan to continue to invest in improvements to our manufacturing process. Many of these improvements, however, must first be approved by the FDA and foreign regulatory bodies before they can be implemented in routine production. We believe that continued improvements to our manufacturing process are important to our objective of remaining a leader in the innovation and manufacture of occlusion devices for the treatment of structural heart defects and leveraging our core competencies into leading positions in new markets.
Our devices undergo strict quality-control measures and manufacturing protocols. We are certified under ISO 13485 and had a qualification audit in January 2006 to qualify for this standard. Our quality system is compliant with both U.S. and European standards, including ISO 13485:2003, ISO 9001:2000, European 93/42/EEC for Medical Devices and Annex II (3). We use a combination of 100% inspection and statistical process control to ensure quality. The last stages of manufacturing of our products are completed using Class 10,000 clean rooms with sterilization for all products assured by a local subcontractor. Our quality system was audited by the FDA in July 2006 and in February 2009, when they made several findings, and no additional regulatory actions were required. In the February 2009 audit, the FDA's findings included a request to use alternate definitions when referring to certain product returns to require such returns to be characterized as complaints. We complied with this request and received a letter from the FDA in June 2009 indicating that the audit of our quality system was closed.
Most of the component parts and raw materials used in our manufacturing and assembly operations are purchased from outside suppliers and are, in some instances, manufactured on a custom basis. Also, most of these component parts and raw materials are available from more than one supplier. However, the primary component in our devices, nitinol, is provided by a single third-party supplier. We currently have no formal agreement with this supplier of nitinol. There are, however, additional suppliers of nitinol should our existing supplier fail to meet our requirements. In addition, this manufacturer has multiple facilities qualified to supply us with nitinol, and we maintain at least a year's supply of nitinol. We have never experienced supply interruptions during our ten-year relationship with our existing supplier. However, if we encounter a cessation, interruption or delay in the supply of nitinol, we may be unable to obtain nitinol through other sources, on acceptable terms, within a reasonable amount of time or at all. In addition, any change of supplier of nitinol would require FDA approval. Any such cessation, interruption or delay may impair our ability to meet scheduled product deliveries to our customers, hurt our reputation or cause customers to cancel orders. See "Risk Factors—Risks Related to Our Business."
Competition
The structural heart defect and the vascular disease and associated conditions device markets in which we compete are characterized as having relatively few competitors and high barriers to entry,
101
including intellectual property and the clinical and regulatory processes required for product approval. Competition in these product markets is primarily based on:
- •
- ability to treat defects and conditions safely and effectively;
- •
- ease of use;
- •
- predictable clinical performance;
- •
- brand name recognition; and
- •
- cost effectiveness.
We believe we compete favorably with respect to these factors, although there can be no assurance that we will be able to continue to do so in the future or that new products that perform better than those we offer will not be introduced. We believe that our continued success depends on our ability to:
- •
- continue to innovate and maintain scientifically advanced technology;
- •
- obtain and enforce patents or other protection for our products;
- •
- obtain and maintain regulatory approvals;
- •
- attract and retain skilled scientific and sales personnel; and
- •
- cost effectively manufacture and successfully market our products.
While our competitors include certain multi-product companies with significantly greater financial, marketing and other resources than we have, we compete with a limited number of companies across our product lines. In the United States, our primary competitor in the ASD occluder market is W.L. Gore & Associates, or W.L. Gore; our primary competitors in the PDA occluder market are Cook, Inc., or Cook, and Boston Scientific Corporation, or Boston Scientific; our primary competitor in the VSD occluder market is NMT Medical, Inc., or NMT Medical; and our primary competitors in the vascular plug market are Cook and Boston Scientific. In Europe, our primary competitors in the ASD occluder market are W.L. Gore and Cardia, Inc., or Cardia; our primary competitors in the PDA occluder market are Cook and Boston Scientific; our primary competitor in the VSD occluder market is NMT Medical; our primary competitors in the PFO occluder market are Cardia and NMT Medical; and our primary competitors in the vascular plug market are Cook and Boston Scientific.
Of these U.S. and European competitors, we are the only company with Japanese approval for ASD and PDA occluders, although others have received a CE Mark in Europe. Similar to us, NMT Medical and W.L. Gore are conducting clinical trials investigating PFO closure in the treatment of certain types of stroke.
Intellectual Property
We believe that to have a competitive advantage we must develop, maintain and protect the proprietary aspects of our technologies. We rely on a combination of patent, trademark, copyright, trade secret and other intellectual property laws, nondisclosure agreements, licenses and other measures to protect our intellectual property rights. We require our employees, consultants and advisors to execute confidentiality agreements. We also require our employees, consultants and advisors who develop intellectual property for us to assign their rights to all intellectual property conceived in connection with their relationship with us. We cannot provide assurance that employees and consultants will abide by the confidentiality or assignment terms of these agreements. In addition, despite measures we take to protect our intellectual property, unauthorized parties might obtain or use information that we regard as proprietary. Any patents or other intellectual property issued to us may be challenged by third parties as being invalid. For further details on these and other risks related to our intellectual property, see "Risk Factors—Risks Related to Our Intellectual Property and Potential Litigation."
102
Patents
Where appropriate, to protect our intellectual property rights related to our medical devices, we apply for U.S. and foreign patents. As of June 30, 2009, we had approximately 197 issued patents and a pipeline of approximately 126 pending patent applications. Our issued and pending patents cover many aspects of our devices' manufacture and usage. Our patent applications may not result in issued patents, and our patents that have been issued or might be issued may not adequately protect our intellectual property rights. The first U.S. patent owned by us expires in 2014, unless extended as may be allowed under applicable law in certain circumstances. As new patents are granted, the term for each U.S. patent granted will be 20 years from the date the application is filed. The actual protection afforded by a foreign patent may vary from country to country, depending upon the laws of such country.
We are currently a party to legal proceedings relating to certain of our patents. See "—Legal Proceedings."
We also make royalty payments with respect to certain patents that were assigned to us by Dr. Kurt Amplatz and Mr. Curtis Amplatz. See "Certain Relationships and Related Transactions—Agreements with Dr. Kurt Amplatz and Mr. Curtis Amplatz." In 2008, Mr. Curtis Amplatz inquired regarding the scope of the royalty agreements we had with him and whether they applied to additional products of ours. In response, we had discussions in which we clarified the scope of the agreements and our payments under the agreements. We believe the inquiry of Mr. Curtis Amplatz to be concluded. However, any dispute relating to the products included in a portfolio subject to a royalty agreement or license could result in us being subject to additional royalty payments, although we do not believe any such dispute to limit our right to sell or market any of our devices.
Trademarks
We have also registered, and filed applications to register, 57 trademarks with the U.S. Patent and Trademark Office and appropriate offices in foreign countries where we do business to distinguish our products from our competitors' products. We market and sell substantially all of our devices under the AMPLATZER trademark, which is recognized as a global leader in structural heart defect occlusion devices. U.S. trademark registrations are for an unlimited duration, provided the marks continue to be used in commerce.
Legal Proceedings
We are from time to time subject to, and are presently involved in, litigation or other legal proceedings.
Subject to the Medtronic litigation disclosed below, we believe that there are no pending lawsuits or claims, including those noted below, that, individually or in the aggregate, are likely to have a material adverse effect on our business, financial position or results of operations.
Foreign Corrupt Practices Act Settlement
On June 2, 2008, we entered into a Deferred Prosecution Agreement, or the DPA, with the Department of Justice concerning alleged improper payments that were made by our former independent distributor in China to (1) physicians in Chinese public hospitals in connection with the sale of our products and (2) an official in the Chinese patent office in connection with the approval of our patent applications, in each case, in potential violation of the Foreign Corrupt Practices Act, or the FCPA. The FCPA makes it unlawful for, among other persons, a U.S. company, acting directly or through an agent, to offer or to make improper payments to any "foreign official" in order to obtain or retain business or to induce such "foreign official" to use his or her influence with a foreign government or instrumentality thereof for such purpose.
103
We initiated the investigation that ultimately resulted in the DPA and initially disclosed such investigation to the Department of Justice in July 2005. Our investigation revealed that between December 1997 and February 2005, certain of our current and former employees were aware of and approved our former distributor's payment of kickbacks to physicians employed by government-owned hospitals in China in order to induce them to use our products. These physicians are deemed to be "foreign officials" under the FCPA, which would cause such kickbacks to be considered improper payments in violation of the FCPA. The investigation also revealed that in March 2001, our former distributor indicated to certain of our current and former employees that it would be necessary to "sponsor" a Chinese patent official in order to get approval for patent applications filed by us in China in a timely manner, and we agreed to cover the fee. Subsequently, our former distributor informed us that it had paid $20,000 to a Chinese patent official to help obtain approval of patents for our products. Our investigation included a review of our activities and those of our other foreign distributors, and this review revealed no other violations of the FCPA. During the period in question, our sales from China were approximately $13.5 million.
In July 2006, we voluntarily disclosed in writing to the Department of Justice the results of our internal investigation. Between July 2006 and March 2008, the Department of Justice conducted confirmatory interviews with certain of our employees and third parties, and we produced relevant documents to the Department of Justice obtained through our internal investigation and as requested by the Department of Justice. The Department of Justice has not filed individual charges against any of our officers or other employees, nor is there any indication that it will commence any investigation of any of our officers or other employees with respect to the conduct covered by the DPA.
As part of the DPA, we consented to the Department of Justice filing a two-count criminal statement of information against us in the U.S. District Court, District of Minnesota, which was filed on June 3, 2008. The two counts include a conspiracy to violate the FCPA and a substantive violation of the anti-bribery provisions of the FCPA related to the above-described activities in China. Although we did not plead guilty to that information, we accepted responsibility for the acts of our employees and agents as set forth in the DPA, and we face prosecution under that information, and possibly other charges as well, if we fail to comply with the terms of the DPA. Those terms require us to, for approximately three years, (1) continue to cooperate fully with the Department of Justice on any investigation relating to violations of the FCPA and any and all other matters relating to improper payments, (2) continue to implement a compliance and ethics program designed to detect and prevent violations of the FCPA and other applicable anti-corruption laws, (3) review existing and, if necessary, adopt new controls, policies and procedures designed to ensure that we make and keep fair and accurate books, records and accounts and maintain a rigorous anti-corruption compliance code designed to detect and deter violations of the FCPA and other applicable anti-corruption laws, and (4) retain and pay for an independent monitor to assess and oversee our compliance and ethics program with respect to the FCPA and other applicable anti-corruption laws. The DPA also required us to pay a monetary penalty of $2.0 million. In the fourth quarter of 2007, we had recorded a financial charge of $2.0 million for the potential settlement. The terms of the DPA will remain binding on any successor or merger partner as long as the agreement is in effect.
If we comply with the DPA, the Department of Justice has agreed not to prosecute us with respect to the activities in China that were disclosed, as described above, and, following the term of the DPA, to permanently dismiss the criminal statement of information that is currently pending against us. We have filed the DPA as exhibit 10.4 to the registration statement of which this prospectus is a part.
Furthermore, since July 2005, we have taken a number of steps to reinforce our commitment to conduct our business in compliance with all applicable anti-corruption laws by enhancing our compliance and ethics program, including (1) requiring the execution of revised contracts with foreign distributors containing comprehensive FCPA and other applicable anti-corruption provisions, (2) retaining an independent third party to perform background checks on all new foreign distributors and compliance audits for existing foreign distributors, (3) implementing FCPA and other applicable
104
anti-corruption training for all foreign distributors and our employees, (4) establishing and appointing the position of Chief Compliance Officer, (5) establishing a compliance committee, consisting of our entire executive management team, which must approve all requests to make any payments to foreign officials, healthcare providers and charities, (6) implementing an appropriate process for documenting and approving such payments, (7) establishing an audit committee of the board of directors with oversight over our compliance and ethics program, and (8) retaining an internal auditor to audit the compliance program against the terms of the DPA.
A criminal conviction of our company under the FCPA would lead to our mandatory exclusion from participation in federal healthcare programs, and it may lead to debarment from U.S. and foreign government contracts. We have discussed the DPA with the U.S. Department of Health and Human Services, Office of Inspector General, or the HHS/OIG, and the HHS/OIG confirmed to us in writing that the DPA and the activities related thereto will not result in exclusion from participation in federal healthcare programs.
Medtronic Litigation in the United States
In January 2007, Medtronic, Inc. filed a patent infringement action against us in the U.S. District Court for the Northern District of California, alleging that substantially all of our AMPLATZER occluder and vascular plug devices, which have historically accounted for substantially all of our net sales, infringe three of Medtronic's method and apparatus patents on shape memory alloy stents (U.S. Patent Nos. 5, 190,546, 6,306,141 and 5,067,957). Medtronic is seeking compensatory damages with respect to our products manufactured or sold in the United States. Medtronic asserted but later withdrew its requests for injunctive relief and for damages based on willfulness.
We have asserted defenses and counterclaims for non-infringement and challenged the validity and enforceability of Medtronic's patents. On April 28, 2009, the court granted summary judgment in our favor finding that we do not infringe Medtronic's '546 patent. Subsequently, Medtronic withdrew certain allegations with respect to the remaining two patents. The trial on the remaining issues in the case was divided into a jury trial phase and non-jury, bench trial phase.
The issues of infringement and certain issues of validity based on obviousness and anticipation of the asserted claims in the two remaining patents were the subject of a jury trial before the U.S. District Court for the Northern District of California that began on July 6, 2009. On August 5, 2009, the jury returned a verdict that the subject AMPLATZER occluder and vascular plug products infringed the claims at issue with respect to both of the two remaining Medtronic patents and that the Medtronic patent claims at issue had not been proven by us to be invalid. The jury verdict awarded Medtronic damages of $57.8 million. This amount is equal to 11% of historical sales of the occluder and vascular plug products in question during the timeframe specified for each patent. Any infringement of the '141 apparatus patent after March 31, 2009 to the date of a final, non-appealable judgment will be considered in calculating the final amount of damages, if any, to be paid. The '957 patent expired in May 2004. Because the issue was not before it, the jury made no determination regarding the payment of royalties on future sales of the company's products after the date of a final, non-appealable judgment. The verdict is not enforceable until the completion of the trial and entry of a final, non-appealable judgment. If we do not receive a favorable judgment, we expect we will appeal such judgment and expect that we will likely be required to post a bond in order to be allowed to appeal as set forth below. The verdict is not enforceable because a judgment has not yet been entered, and as a matter of law only judgments are enforceable for purposes of execution against the non-prevailing party. A judgment has not yet been entered because all claims have not yet been adjudicated between the parties and the court has not yet ordered a judgment be entered. In addition, on August 5, 2009, Medtronic's counsel agreed with the judge on the record that a judgment would not be entered until after the non-jury trial phase is completed. Furthermore, upon approval of an appeal bond, the execution of any appealed judgment is stayed during the appeal.
105
On August 19, 2009, the United States Court of Appeal for the Federal Circuit, sitting en banc, issued a decision in Cardiac Pacemakers, Inc. v. St. Jude Medical, Inc. In Cardiac Pacemakers, the Federal Circuit established a new rule of law and held as a matter of law that the practice of a method claim of a patent outside of the United States cannot infringe a United States method patent. When a controlling new rule of law is announced that affects the parties to a patent litigation, the court will ordinarily apply the supervening change in law retroactively to a pending case. In our litigation with Medtronic, a portion of the jury's damage award was based on a finding of infringement of Medtronic's '957 method patent for sales of our products outside of the United States, and we believe it is therefore directly contrary to the holding as stated in the Cardiac Pacemakers decision. Applying the rate of damages established by the jury, 11% of historical sales, to the total sales of our products outside of the United States during the period for which we believe damages were awarded for infringement of the '957 patent would result in a reduction of approximately $14 million. Such $14 million of damages relates only to periods of time before the '141 patent was enforceable against us. As a result, we believe it is likely that the trial court in our case will reduce the jury's damage award by approximately such amount to reflect this recent decision, although there can be no assurance that Cardiac Pacemakers will not be reviewed and overturned by the United States Supreme Court or that the damage award will be reduced by this or any other amount. On September 8, 2009, we filed a motion seeking, at Medtronic's choice, either a new trial or the above-mentioned reduction of $14 million, which motion is publicly available. We do not expect a ruling on this motion until the conclusion of the non-jury phase of the trial in early 2010.
Following the jury verdict, the court scheduled the non-jury phase of the trial for early December 2009, which will deal with other issues of invalidity and unenforceability of one or both of the two remaining patents based on equitable claims of double patenting, equitable estoppel, inequitable conduct in patent prosecution and laches. The claims to be tried in the non-jury phase of the trial are claims "in equity" and for which there is no right to a trial by jury. Therefore, the trial was essentially bifurcated into a jury phase and non-jury phase subject to the same procedures. In the event the judge finds in our favor on one or more of our counterclaims in the non-jury phase of the trial, she can fashion an equitable remedy to compensate us for Medtronic's conduct, including barring all damages prior to filing of the lawsuit, barring past and future enforcement of the '141 patent alone, or eliminating all past and future damages on both of the patents in suit. Upon conclusion of the non-jury phase of the trial, the court will consider post-trial motions and enter a judgment, which we expect will take place in early 2010. As part of its decision, the trial court could order a new trial on some or all of the issues in the case or amend or reaffirm the jury verdict based on one or more issues regarding infringement, validity, enforceability and damages. We also expect the trial court to decide whether any royalty payments relating to the '141 patent, which does not expire until 2018, are due for future periods and, if so, at what rate. Any such royalties may not be on commercially reasonable terms, and may exceed the 11% rate of damages applied by the jury. If any damage amount is entered as a part of the judgment, we will likely be required at that time to accrue a non-cash charge equal to the amount of such damages. Any such accrual will have an adverse effect on our results of operations for the applicable period.
Thereafter, the judgment will be subject to appeal which could result in the judgment being affirmed or amended, or in an order for a new trial on one or more of the same issues raised before the trial court. If we decide to appeal, such appeal may not be decided for several months and will likely require the posting of a bond in the face amount of 150% of the judgment amount, if any. We do not expect the cash cost associated with posting such a bond to be material, but we would likely be required to secure such bond with collateral. The amount of collateral required by the provider of the bond would be determined based on several factors, such as the amount of our debt and our financial condition.
These proceedings are costly and time consuming, and we cannot assure you that the outcome of any of these proceedings will be favorable to us. However, because Medtronic withdrew its request for injunctive relief from the trial court, we believe that it is likely that the final judgment will not prevent the continued marketing or sale of any our products. If we do not prevail before the trial or appellate
106
courts in overturning the jury verdict or reducing or eliminating the damages award in the jury verdict, we will have to pay the awarded damages and may have to pay royalties on a substantial portion of our future sales, and our results of operations and financial condition may be materially adversely affected. Medtronic also could appeal from rulings adverse to it, which could result in an appellate decision with effects adverse to us on issues of liability and/or relief. In addition, Medtronic may attempt to request injunctive relief in the future, which, if granted, would have a material adverse effect on our business, financial condition and results of operations.
Notwithstanding the litigation proceedings, we are in the process of implementing changes to our business processes intended to create an alternative process that we believe does not infringe Medtronic's patents. These changes will not affect the product design, but will modify the final step prior to inspection in our manufacturing processes and the instructions for use of our products by physicians, which clarify what we believe to be standard practices. These changes will require that our products be cooled prior to use and therefore rely on a property of our nitinol material that makes use of temperature rather than stress for expansion. The use of temperature for expansion of nitinol was disclaimed during the prosecution of the Medtronic patents. We do not expect any of these changes to impact the future sales of our products. In addition, we intend to initiate a process to establish manufacturing operations in Europe for all of our devices that are sold in international markets within 12 to 18 months. In addition to providing other benefits for our business, such as mitigating the risk inherent in relying on only one manufacturing facility the manufacture of our products outside of the United States would eliminate any claims for ongoing royalty payments on future sales with respect to such products sold outside of the United States. We cannot assure you that any of these changes will avoid future litigation or will not result in a subsequent finding of infringement of Medtronic's patents and/or patents held by others. If any of our modified business processes were found to infringe any such patents, we may be required to pay additional damages and royalties. We cannot assure you that such damages and royalties would not exceed the 11% rate applied by the Medtronic case jury for past sales. Any such royalties may not be on commercially reasonable terms and could have a material adverse effect on our results of operations and financial condition. Also, if a court grants an injunction, we may be prevented from selling some or any infringing products. While we believe we have taken reasonable steps to inform third parties who use such products of the alternative process by changing the instructions for use that are referenced in the information that accompanies each such product, such third parties may not review such information and may not adopt the alternative process even if they do review the new instructions for use. To the extent such third parties continue to use the prior process, we may be liable for such use whether or not the alternative process infringes Medtronic's patents.
Occlutech Litigation in Europe
In August 2006, we brought a patent infringement action in Germany against Occlutech GmbH, a European manufacturer of cardiac occlusion devices, and DRABO Medizintechnik based on the German part (DE No. 695 34 505) of one of our European patents (EP No. 08080 138), granted to us in October 2005, for intravascular occlusion devices and the method of manufacturing such devices.
On July 31, 2007, the District Court in Düsseldorf entered a judgment in our favor finding that Occlutech and DRABO literally infringed the German part of our European patent. The three-judge panel granted us the right to enforce an order prohibiting the defendants from possessing, manufacturing or selling the infringing products. The judgment also entitled us to enforce the immediate destruction of all infringing products in Occlutech's inventory. Consequently, Occlutech has destroyed over 2,300 occluders before a notary public and is currently prohibited from manufacturing or selling the infringing products in Germany. Under German practice, the District Court required us to post a bond in the amount of €1 million to secure our ability to respond to damages claimed by Occlutech in the event that the decision of the District Court is reversed on appeal or our patent is held invalid in related proceedings in the German patent court. The bond amount is not a limitation on damages.
107
On August 6, 2007, Occlutech filed an appeal against the District Court judgment before a German Court of Appeals, contending that the District Court judgment was based on an overly broad interpretation of our European patent, and, in addition, initiated invalidation proceedings against the patent. On January 6, 2009, the German Court of Appeals dismissed Occlutech's appeal and entered a judgment in favor of finding that Occlutech infringed our patent. Occlutech has filed an appeal with the German Federal Court of Justice. The German Federal Court of Justice has twice denied Occlutech's motions to stay enforcement of the judgment. A final decision on the appeal with the German Federal Court of Justice and a decision on the invalidation proceedings are not expected to be reached until 2010 or later. In the meantime, we expect to seek damages and other remedies in the enforcement proceedings based on the German Court of Appeals' decision. Neither the pending appeal nor the invalidation proceedings affect our ability to continue to enforce the District Court's judgment.
In addition, Occlutech initiated proceedings against our corresponding patents in Italy, the Netherlands, the United Kingdom, Spain and Sweden, seeking invalidity and/or non-infringement declarations. On October 29, 2008, the Patent Court in the Netherlands ruled in favor of Occlutech in the non-infringement declaration. The court did not rule on the invalidity claim. On November 3, 2008, we filed an appeal on the Dutch appellate court. On July 31, 2009, a United Kingdom patent court upheld the validity of our patent, but it ruled that the Occlutech products do not infringe on our patent. We intend to appeal the decision that Occlutech did not infringe upon our patent. Final decisions in these actions are not expected to be reached until 2010 or later. We intend to vigorously defend our patents and believe that we have a good basis for prevailing in both the appeals before the German and Dutch appellate courts, in the planned appeal before the United Kingdom appellate court and against Occlutech's various invalidation proceedings. However, the outcome in any of these proceedings may not be favorable to us.
Globe Bio-Medicals Litigation in India
In October 2007, we brought a patent infringement action in New Delhi, India, against Globe Bio-Medicals, an entity based in New Delhi, India, which distributes medical devices; Faisal M. Kapadi, a principal of Globe Bio-Medicals; and Shanghai Shape Memory Alloy Company Ltd., a medical device manufacturer based in Shanghai, China, with which we had patent litigation in China. See—"Patent Litigation in China." In this action we assert that the medical devices manufactured and sold by the defendants in India infringe our India Patent No. 192376 which covers our AMPLATZER occlusion devices imported, manufactured or sold in that country. Our complaint seeks, among other remedies, a permanent injunction, an accounting and damages. Shortly after bringing the action, the High Court of Delhi granted our motion and issued a preliminary injunction against the defendants. The injunction was enforced by local commissioners accompanied by police, which sealed all infringing goods located at the offices of Globe Bio-Medicals. The defendants have responded to the litigation and moved the Court to vacate the injunction. The Court has not yet decided on defendant's motion. Although we believe we have a strong case, we may not ultimately prevail in the action, and any litigation could take a number of years prior to entry of a final judgment.
Patent Litigation in China
We have sought patent protection for our products in the People's Republic of China. Our current patent portfolio in China comprises ten issued patents and three pending patent applications. Moreover, we have adopted an active policy of enforcement with respect to these patent rights. In March of 2004, we filed patent infringement actions against Shanghai Shape Memory Alloy Material Co. Ltd., a medical device manufacturer based in Shanghai, and Shenzhen Lifetech Scientific Co. Ltd., a medical device manufacturer based in Shenzhen, and Beijing Starway Medical Devices Ltd., a medical device manufacturer based in Beijing. In these actions, we were attempting to enforce our rights under one of our Chinese patents (No. ZL98808876.2, or the '876.2 patent). The defendants challenged the validity of the '876.2 patent and of another of our patents (No. ZL97194488.1, or the '488.1 patent) before the Patent Reexamination Board. As a result, the infringement actions were stayed and are
108
currently inactive. The defendants have prevailed in their challenge to the validity of the '876.2 and '488.1 patents. Consequently, we are no longer able to assert rights under these patents within China and will need to rely primarily on foreign patents to prevent the importation of products from China into countries in which such importation would violate our local patent rights.
University of Minnesota Litigation in the United States
On November 30, 2007, the University of Minnesota filed a patent infringement action against us in the United States District Court for Minnesota, alleging that our AMPLATZER occlusion devices infringe the University's method and apparatus patents on septal occlusion devices (U.S. Patent Nos. 6,077,291, or the '291 patent, and 6,077,281). The University is seeking injunctive relief as well as damages, including damages for alleged willful infringement, although no damage amount has been specified in the complaint. We have filed an answer and counterclaims seeking a declaration of noninfringement, invalidity, unenforceability, equitable estoppel, laches, and expiration of the '291 patent, among others. The litigation is currently in the discovery process. On April 18, 2008, the Court granted our motion for partial summary judgment declaring that the '291 patent expired for failure to pay the maintenance fees and has been unenforceable from and after June 21, 2004. Subsequently, the U.S. Patent and Trademark Office repeatedly rejected the University's petition to reinstate the '291 patent, which is, therefore, no longer enforceable. A trial date has not been set in the litigation. Although we presently believe that the lawsuit lacks merit, we cannot guarantee that the outcome of this litigation will be favorable to us.
Teirstein Litigation in the United States
On January 15, 2008, Dr. Paul Teirstein filed a patent infringement action against us in the United States District Court for the Eastern District of Texas, alleging that our AMPLATZER occlusion devices infringe Teirstein's patent for body passageway closure apparatus and method of use (U.S. Patent No. 5,499,995). In his complaint, Teirstein was seeking injunctive relief as well as damages, including damages for alleged willful infringement, although no damage amount was specified. We filed an answer and counterclaims seeking a declaration of noninfringement, invalidity and unenforceability of the patent. On September 23, 2009, we entered into a settlement agreement with Teirstein in which the parties agreed to the dismissal of all claims and counterclaims with prejudice in exchange for Teirstein's grant to us of a covenant not to sue on the '995 patent on all of our existing and future products, and the lump-sum payment by us to Teirstein in the amount of $375,000.
Lifetech Litigation in India
On April 23, 2008, we brought a patent infringement action in New Delhi, India, against Lifetech Scientific Co. Ltd., a Chinese medical device manufacturer, and its distributor in India. In this action we assert that the medical devices manufactured and sold by the defendants infringe our India Patent No. 192376 which covers occlusion devices imported, manufactured or sold in India. Our complaint seeks, among other remedies, a permanent injunction, an accounting and damages. We have applied to the High Court of Delhi for a preliminary injunction. The court has not yet decided on our motion. The litigation could take a number of years prior to entry of a final judgment. Although we believe we have a strong case, we cannot guarantee that we will ultimately prevail in the action.
Subject to the Medtronic litigation disclosed above, we believe that there are no pending lawsuits or claims, including those noted above, that, individually or in the aggregate, are likely to have a material adverse effect on our business, financial position or results of operations.
Government Regulation
Medical Device Regulation
United States. Our products and operations are subject to regulation by the Food and Drug Administration (FDA) and state authorities, as well as the comparable authorities in foreign
109
jurisdictions which are discussed below. The FDA regulates the design, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, promotion, and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses or substantially equivalent to marketed products. Medical device manufacturers are also inspected regularly by the FDA. In addition, the FDA regulates the export of medical devices manufactured in the United States to international markets and the import of components used in the manufacture of medical devices.
Under the Federal Food, Drug, and Cosmetic Act (FFDCA), medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device and the extent of manufacturer and regulator control needed to ensure safe and effective use. Classification of a device is important because the class to which a device is assigned determines, among other things, the type of application process required for FDA review and clearance or approval to market the device. Class I includes devices with the lowest risk to the patient (and subject to the least regulatory control), while Class III includes devices that pose the greatest risk to the patient (and strictest regulatory control).
The preponderance of our business involves products in Class III. FDA generally classifies devices that are surgically implanted into the heart as Class III. In contrast, some of our devices that are marketed for peripheral vascular use are designated by the FDA as Class II devices. A couple of our devices used to size the implant are classified as Class I.
Class I devices. Class I devices are considered low-risk devices subject to the least regulatory control. In general, a company can market a Class I device without premarket review by the FDA as long as it adheres to what the FFDCA calls General Controls, which sufficiently assure the safety and efficacy of the device. General Control requirements include compliance with the applicable portions of the FDA's manufacturing Quality System Regulation (QSR), facility registration and product listing, medical device reporting of adverse events, and truthful and non-misleading labeling, advertising, and promotional materials. Most Class I devices are exempt from the premarket notification process by the FDA which is discussed below. Some Class I devices are also exempt from the QSR. Examples of our Class I devices include the sizing plate and the sizing balloon used to determine the dimensions of the cardiac or vascular implants required by patients.
Class II devices. Class II devices are medium-risk devices subject to greater regulatory control than Class I devices. In addition to complying with the General Controls listed above, Class II devices are also subject to Special Controls, which may include performance standards, postmarket surveillance, patient registries, or guidelines. Most Class II devices are also required to obtain FDA clearance under Section 510(k) of the FFDCA (known as "premarket notification") before they can be marketed. When compliance with Section 510(k) is required, the company must submit to the FDA a premarket notification submission demonstrating that the device is "substantially equivalent" to a predicate device already on the market. A predicate device is either a device that was legally marketed prior to May 28, 1976 (the date upon which the Medical Device Amendments of 1976 were enacted) or another commercially available, similar device that was subsequently cleared through the 510(k) process. Data must support the safe and effective use of the device including bench testing, performance validation, and occasionally, but now more frequently, a limited amount of human clinical safety and efficacy data.
If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant clearance to commercially market the device. By regulation, the FDA is required to clear a completed 510(k) premarket notification application within 90 days of submission. As a practical matter, marketing clearance often takes longer in the event additional information is requested. Most device applications must now pay user fees under the Medical Device User Fee and Modernization Act which also contains FDA performance goals for faster and more predictable device product review. If the FDA determines that the device, or its intended use, is not "substantially equivalent" to a previously cleared device or use, the FDA may place the device, or the particular use
110
of the device, into Class III. The device sponsor must then fulfill more rigorous premarketing requirements or petition to down-classify the device to Class II under the process known as "de novo" or "risk-based classification" review. An example of a Class II device is our Vascular Plug for arterial and venous embolizations in the peripheral vasculature.
Class III devices. Class III devices are higher-risk devices such as those which support or sustain life or are used invasively in the body. Class III devices are subject to the greatest amount of regulatory control. In general, a Class III device cannot be marketed unless the FDA approves the device after submission of a premarket approval application, or PMA. The PMA process is more demanding than the 510(k) premarket notification process. A PMA application, which is intended to demonstrate that the device is safe and effective, must be supported by extensive data, including data from preclinical studies and human clinical trials. Human studies are conducted pursuant to a clinical protocol generally supervised by FDA and a patient Institutional Review Board (IRB) pursuant to an approved Investigational Device Exemption application (IDE). The PMA application must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing, and proposed labeling. Following receipt of a PMA application, once the FDA determines that the application is sufficiently complete to permit a substantive review, the FDA will accept the application for agency review. The FDA, by statute and by regulation, has 180 days to review a PMA application, although the review of an application more often occurs over a significantly longer period of time, and can take up to several years. The user fee act referenced above also contains performance goals in exchange for the application fees paid to make device product reviews more timely and predictable. In approving a PMA application, or clearing a 510(k) application, the FDA may also require some form of post-market surveillance when necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Examples of PMA devices are our Septal Occluder for transcatheter closure of atrial septal defects and our Duct Occluder for transcatheter non-surgical closure of patent ductus arteriosus (PDA).
Class III devices may also be marketed under a Humanitarian Device Exemption (HDE) for smaller patient populations. Such a designation can eliminate the need to file a full PMA supported by human clinical safety and efficacy trials. This is a two-step process. First one must request a Humanitarian Use Device (HUD) designation for a medical device. The applicant requests the designation for treatment of a rare disease or condition, or a medically plausible subset of a more common disease or condition. The applicant must demonstrate that that the disease or condition involves fewer that 4,000 diagnosed cases per year. Within 45 days the FDA must either approve or reject the request for designation. Once a HUD designation is approved, the sponsor must apply for the HDE. The applicant must show that the device would not be available unless the HDE were granted and that no comparable device, except another HUD, is available to treat the disease or condition. The FDA may request additional pre-clinical or other testing before approving or rejecting the application. Once the HUD device is available for marketing under an HDE, the amount charged for the device cannot exceed the costs of the device's research, development, fabrication, and distribution. We intend to seek an HDE for its device intended to treat Ventricular Septal Post Infarction.
Unlike a PMA, which is very difficult for the FDA to revoke, the HUD designation for an HDE may be revoked if circumstances change. For example, the FDA may revoke the HDE if the number of cases is shown to exceed 4,000 per year, another device becomes commercially available, or the disease or condition is no longer considered a medically plausible subset or indication. For example, we held an HDE for its PFO Occluder, that was subsequently converted into a conventional IDE, for the non-surgical closure of a PFO in patients with recurrent cryptogenic stroke due to presumed paradoxical embolism through a PFO and who have failed conventional drug therapy. This is an example of how the FDA may change its policies, adopt additional regulations, or revise existing regulations, any of which could impact our ability to market a device that was previously cleared or approved.
111
Medical devices can be marketed only for the indications for which they are cleared or approved. Modifications to a previously cleared or approved device that could significantly affect its safety or efficacy or that would constitute a major change in its intended use, design or manufacture require a 510(k) clearance, premarket approval supplement or new premarket approval. We cannot assure you that we will be successful in receiving approvals in the future or that the FDA will agree with our decisions not to seek approvals, supplements or clearances for particular device modifications. The FDA may require approval or clearances for past or any future modifications or new indications for our existing products. Such submissions may require the submission of additional clinical or preclinical data and may be time consuming and costly, and may not ultimately be cleared or approved by the FDA in a timely manner or at all.
Our manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and documentation of the design, testing, production, processes, controls, quality assurance, labeling, packaging and distribution of our products. The QSR also, among other things, requires maintenance of a device master record, device history record, and complaint files. Our manufacturing facility is subject to periodic scheduled or unscheduled inspections by the FDA. Based on internal audits and FDA inspections, we believe that our facility is in substantial compliance with the applicable QSR regulations. We are also required to report to the FDA if our products cause or contribute to a death or serious injury or malfunction in a way that would likely cause or contribute to death or serious injury were the malfunction to recur. The FDA and authorities in other countries can require the recall of products, or we can voluntary recall a product, in the event of material defects or deficiencies in design or manufacturing. The FDA can also withdraw or restrict our product approvals or clearances in the event of serious, unanticipated health or safety concerns.
The FDA has broad regulatory and enforcement powers. If the FDA determines that we failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions from public warning letters, fines, injunctions, consent decrees and civil penalties to suspension or delayed issuance of approvals, seizure or recall of our products, total or partial shutdown of production, withdrawal of approvals or clearances already granted, and criminal prosecution. The FDA can also require us to repair, replace or refund the cost of devices that we manufactured or distributed. If any of these events were to occur, it could materially adversely affect us.
Legal restrictions on the export from the United States of any medical device that is legally distributed in the United States are limited. However, there are restrictions under U.S. law on the export from the United States of medical devices that cannot be legally distributed in the United States. If a Class I or Class II device does not have 510(k) clearance, and the manufacturer reasonably believes that the device could obtain 510(k) clearance in the United States, then the device can be exported to a foreign country for commercial marketing without the submission of any type of export request or prior FDA approval, if it satisfies certain limited criteria relating primarily to specifications of the foreign purchaser and compliance with the laws of the country to which it is being exported (Importing Country Criteria). An unapproved Class III device can be exported if it complies with the criteria discussed above for a 510(k) device and the device has a marketing authorization in one of a list of countries listed in the FFDCA. If an unapproved Class II device is not cleared for marketing in one of the listed countries, a license from the FDA is required in order to export it. We believe that all of our current products which are exported to foreign countries currently comply with these restrictions.
International
In many of the foreign countries in which we market our products, we are subject to regulations essentially similar to those of the FDA. The regulation of our products in Europe falls primarily within the European Economic Area, which consists of the twenty-five member states of the European Union as well as Iceland, Liechtenstein and Norway. The legislative bodies of the European Union have adopted three directives in order to harmonize national provisions regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices: the Actives Implantables
112
Directive, the Medical Device Directive (MDD) and the In-Vitro-Diagnostics Directive. Our devices are registered under the MDD. The member states of the European Economic Area have implemented the directives into their respective national law. Medical devices that comply with the essential requirements of the national provisions and the directives will be entitled to bear a CE Mark. Unless an exemption applies, only medical devices which bear a CE Mark may be marketed within the European Economic Area. The European Commission has adopted numerous guidelines relating to the medical devices directives to ensure their uniform application. The method of assessing conformity varies depending on the class and type of the medical device and can involve a combination of self-assessment by the manufacturer and a third-party assessment by a Notified Body, which is an independent and neutral institution appointed by the member states to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer's quality system and specific testing of the manufacturer's devices. An assessment by a Notified Body in one country within the European Economic Area is generally required in order for a manufacturer to commercially distribute the product throughout the European Economic Area.
The European Standardization Committees have adopted numerous harmonized standards for specific types of medical devices. Compliance with relevant standards establishes the presumption of conformity with the essential requirements for a CE Mark. All of our products that we export or manufacture for sale in Europe bear a CE Mark.
Compliance activity is generally undertaken on a country-by-country basis under the control of the country's Competent Authority. A Competent Authority is the country's medical device regulatory agency, which is analogous to the U.S. FDA with regard to compliance matters. As discussed above, a Competent Authority has no role in facility inspection or product approval, which is done by the Notified Body. Adverse events relating to medical devices are reported to the Competent Authority under a system known as Vigilance, on a country-by-country basis. The Competent Authority also controls product recalls or any other compliance action within a country.
Third-Party Reimbursement
In the United States, as well as in foreign countries, government-funded or private insurance programs, commonly known as third-party payors, pay the cost of a significant portion of a patient's medical expenses. A uniform policy of reimbursement does not exist among these payors. Therefore, reimbursement can differ from payor to payor. These third-party payors may deny reimbursement if they determine that a device used in a procedure was not used in accordance with cost-effective treatment methods, as determined by the third-party payor. Also, third-party payors are increasingly challenging the prices charged for medical products and services. In international markets, reimbursement and healthcare payment systems vary significantly by country and many countries have instituted price ceilings on specific product lines. There can be no assurance that our products will be considered cost-effective by third-party payors, that reimbursement will be available or, if available, that the third-party payors' reimbursement policies will not adversely affect our ability to sell our products profitably. Reimbursement is also generally granted according to the surgical procedure for which the device is used, and not for the device itself. There can be no assurance that reimbursement for the procedure itself is sufficient to justify the use of any device deemed to be too expensive for the reimbursement available for a particular procedure code.
Reimbursement in the United States depends on our ability to obtain FDA clearances and approvals to market these products. Reimbursement also depends on our ability to demonstrate the short-term and long-term clinical and cost-effectiveness of our products from the results we obtain from clinical experience and formal clinical trials. We present these results at major scientific and medical meetings and publish them in respected, peer-reviewed medical journals.
The United States Center for Medicare and Medicaid Services, or CMS, sets reimbursement policy for the Medicare program in the United States. CMS policies may alter coverage and payment related to our product portfolio in the future. These changes may occur as the result of National Coverage
113
Decisions issued by CMS headquarters or as the result of local or regional coverage decisions by contractors under contract with CMS to review and make coverage and payment decisions. CMS maintains a national coverage policy, which provides for the utilization of our products in Medicare beneficiaries. Medicaid programs are funded by both federal and state governments. Medicaid programs are administered by the states and vary from state to state and from year to year. Commercial payor coverage for our products may vary across the United States.
All third-party reimbursement programs, whether government funded or insured commercially, whether inside the United States or outside, are developing increasingly sophisticated methods of controlling healthcare costs through prospective reimbursement and capitation programs, group purchasing, redesign of benefits, second opinions required prior to major surgery, careful review of bills, encouragement of healthier lifestyles and exploration of more cost-effective methods of delivering healthcare. These types of programs and legislative changes to reimbursement policies could potentially limit the amount which healthcare providers may be willing to pay for medical devices.
Fraud and Abuse
Our operations will be directly, or indirectly through our customers, subject to various state and federal fraud and abuse laws, including, without limitation, the FFDCA, federal Anti-Kickback Statute and False Claims Act. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, these laws require us to screen individuals and other companies, suppliers and vendors in order to ensure that they are not "debarred" by the federal government and therefore prohibited from doing business in the healthcare industry. The association or conduct of business with a "debarred" entity could be detrimental to our operations and result in a negative impact on our business.
The U.S. Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. This statute is normally used to insure that bribes or other illegal remuneration are not paid to physicians, or others, to induce their use of drugs or medical devices. Several courts have interpreted the statute's intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The U.S. False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Various states have also enacted laws modeled after the federal False Claims Act.
In addition to the laws described above, the U.S. Health Insurance Portability and Accountability Act of 1996 created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
Voluntary industry codes, federal guidance documents and a variety of state laws address the tracking and reporting of marketing practices relative to gifts given and other expenditures made to doctors and other healthcare professionals. In addition to impacting our marketing and educational programs, internal business processes will be affected by the numerous legal requirements and regulatory guidance at the state, federal and industry levels.
114
If our operations are found to be in violation of any of the laws described above or other applicable state and federal fraud and abuse laws, we, as well as our employees, may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations. Individual employees may need to defend such suits on behalf of us or themselves, which could lead to significant disruption in our present and future operations.
International Trade
The sale and shipment of our products and services across international borders, as well as the purchase of components and products from international sources, subject us to extensive governmental trade regulations. A variety of laws and regulations, both in the United States and in the countries in which we transact business, apply to the sale, shipment and provision of goods, services and technology across international borders. Because we are subject to extensive regulations in the countries in which we operate, we are subject to the risk that laws and regulations could change in a way that would expose us to additional costs, penalties or liabilities.
As a result of the varying legal and regulatory requirements to which our cross-border activities are subject, we cannot assure you that we are in compliance with these requirements in all respects. There are risks related to our international trading activities. You should read the information set forth under "Risk Factors—Risks Related to Our Business." The risks inherent in operating internationally and the risks of selling and shipping our products internationally may adversely impact our net sales, results of operations and financial condition.
Existing laws and regulations significantly affect cross-border transactions and shipments. These laws and regulations govern, among other things, the following activities:
Importing Activities: We engage in the importation of raw materials, components and finished products into the countries in which we transact business. We act as importer of record in many instances, but we also sell and ship goods to third parties who are themselves responsible for complying with applicable trade laws and regulations.
In our role as importer of record, we are directly responsible for complying with customs laws and regulations concerning the importation of our raw materials, components and finished products. If third parties violate FDA or customs laws and regulations when engaging in cross-border transactions involving our products, we may be subject to varying degrees of liability depending on our participation in the transaction. In addition, the activities of third parties may cause supply chain disruptions and delays in the distribution of our products that impact our business activities.
Exporting Activities: We are responsible for compliance with applicable export control and economic sanctions laws and regulations with respect to our export of goods, technology and services to customers and end-users located in countries in which we transact business. We also sell and provide goods, technology and services to agents, representatives and distributors who may export such items to customers and end-users.
If third parties violate applicable export control and economic sanctions laws and regulations when engaging in transactions involving our products, we may be subject to varying degrees of liability dependent upon our participation in the transaction. The activities of our third parties may cause disruption or delays in the distribution and sales of our products, or result in restrictions being placed upon our international distribution and sales of products may materially impact our business activities.
Many countries, including the United States, control the export and reexport of goods, technology and services for reasons including public health, national security, regional stability, antiterrorism policies and the nonproliferation of nuclear, chemical and biological weapons. In certain circumstances, approval from governmental authorities may be required before goods, technology or services are exported or reexported to certain destinations, to certain-end-users and for certain end-uses. In addition, governments, including the United States, may impose economic sanctions against certain countries, persons and entities. Because
115
export control and economic sanctions laws and regulations are complex and constantly changing, we cannot assure you that laws and regulations may not be enacted, amended, enforced or interpreted in a manner materially impacting our ability to sell or distribute products.
Antiboycott Laws: Under U.S. laws and regulations, U.S. companies and their controlled-in-fact foreign subsidiaries and affiliates are prohibited from participating or agreeing to participate in unsanctioned foreign boycotts in connection with certain business activities, including the sale purchase, transfer, shipping or financing of goods or services within the United States or between the United States and a foreign country. Currently, the United States considers the Arab League boycott of Israel to constitute an unsanctioned foreign boycott.
We are responsible for ensuring we comply with the requirements of U.S. antiboycott laws for all transactions in which we are involved. If we or third parties violate U.S. antiboycott laws and regulations when engaging in transactions involving our products, we may be subject to varying degrees of liability dependent upon the nature of the transaction and our participation in the transaction. Penalties for any violations of antiboycott laws and regulations could include criminal penalties and civil sanctions such as fines, imprisonment, debarment from government contracts, loss of export privileges and the denial of certain tax benefits, including foreign tax credits, foreign subsidiary deferrals, Foreign Sales Corporation benefits and Interest Charge-Domestic International Sales Corporation benefits.
Antibribery laws. Many of the countries in which we transact business have domestic laws that restrict the offer or payment of anything of value to government officials or other persons with the intent of gaining business or favorable government action. Moreover, some of the transactions in which we and our officers, directors, employees and agents engage may be governed by the legal obligations and standards set forth under the U.S. Foreign Corrupt Practices Act and, at times, other laws modeled on the OECD Convention for Combating Bribery of Foreign Public Officials in International Business Transactions. In addition to prohibiting certain bribery-related activity with foreign officials and other persons, these laws provide for recordkeeping and reporting obligations. Penalties for any violations of these anti-bribery laws could include criminal penalties and civil sanctions such as fines, imprisonment, debarment from government contracts and loss of export privileges.
Facilities
Our corporate headquarters and center of domestic operations in Plymouth, Minnesota, were purchased by us in 2003. In 2006, we refurbished the facility and consolidated all of our U.S. activities, including our sales, manufacturing, warehousing, research and development and administrative activities, into that location. The facility consists of approximately 205,000 square feet, of which manufacturing and distribution occupies approximately 24,000 square feet. Outside the United States, we lease a sales and distribution office in Birmingham, United Kingdom of approximately 5,800 square feet, a sales office in Frankfurt, Germany of approximately 5,000 square feet, a sales office in Madrid, Spain of approximately 5,700 square feet, a sales office in Paris, France of approximately 6,700 square feet, and a sales and distribution office in Milan, Italy of approximately 8,300 square feet.
Employees
As of June 30, 2009, we had 470 employees. From time to time, we also employ independent contractors to support our operations. We believe that our continued success will depend on our ability to continue to attract and retain skilled scientific and sales personnel. We have never had a work stoppage, and none of our worldwide employees is represented by a labor union. We believe our relationship with our employees is satisfactory.
116
Executive Officers, Directors and Other Significant Employee
The following table sets forth information concerning our directors, executive officers and significant employee as of June 30, 2009:
Name
|
Age | Position | Term Expires(1) | |||||
|---|---|---|---|---|---|---|---|---|
John R. Barr |
52 | President and Chief Executive Officer | ||||||
Brigid A. Makes |
53 | Chief Financial Officer | ||||||
Ronald E. Lund |
74 | General Counsel | ||||||
Dr. Kurt Amplatz |
85 | Research & Development Consultant, Co-Founder and Former President |
||||||
Tommy G. Thompson |
67 | Chairman | 2012 | |||||
Franck L. Gougeon |
43 | Director and Co-Founder | 2012 | |||||
Jack P. Helms |
56 | Director(2) | 2011 | |||||
Daniel A. Pelak |
57 | Director | 2010 | |||||
Paul B. Queally |
45 | Director | 2012 | |||||
Terry Allison Rappuhn |
53 | Director | 2011 | |||||
Darrell J. Tamosuinas |
55 | Director | 2010 | |||||
Sean M. Traynor |
40 | Director | 2011 | |||||
- (1)
- Our
board is divided into three classes of the same or nearly the same number of directors, each serving staggered three year terms expiring in the years
set forth in this table for each applicable director. See "Description of Capital Stock—Anti-takeover Effects of Our Certificate of Incorporation."
- (2)
- Nominee for director. Mr. Helms has consented to being named herein as a nominee for director, and is expected to join our board of directors upon completion of this offering.
The following is a biographical summary of the experience of our executive officers, significant employee and directors:
Executive Officers and Significant Employee
John R. Barr—President and Chief Executive Officer
John R. Barr has served as our President and Chief Executive Officer since June 2008 and, prior to that, served as our Chief Operating Officer since September 2005. Prior to joining our company, Mr. Barr served as President and Chief Executive Officer of V.I. Technologies prior to its merger with Panacos Pharmaceuticals in 2005. Prior to joining V.I. Technologies, Mr. Barr served from June 1990 to November 1997 as President of North American Operations for Haemonetics Corporation, a leading medical device manufacturer, and from July 1981 to April 1990 in both financial and operational roles for Baxter Healthcare. Mr. Barr holds a Master's Degree in management from the J.L. Kellogg Graduate School of Management and a Bachelor of Science Degree in bioengineering from the University of Pennsylvania.
Brigid A. Makes—Chief Financial Officer
Brigid A. Makes has served as our Chief Financial Officer since October 2006. Prior to joining our company, Ms. Makes served in various management positions at Nektar Therapeutics from 1999 to 2006, including Vice President—R&D Operations, Vice President—Operations Management, Vice President—Finance and Administration and Chief Financial Officer. Prior to joining Nektar Therapeutics, Ms. Makes served from 1998 to 1999 as Chief Financial Officer of Oravax, Inc. and from
117
1995 to 1998 as Chief Financial Officer at Haemonetics Corporation. Prior to that, Ms. Makes held various management positions at Lotus Development Corporation and General Electric Company. Ms. Makes holds an MBA from Bentley College in Waltham, Massachusetts, and a Bachelor Degree in finance and international business from McGill University in Montreal, Quebec.
Ronald E. Lund—General Counsel
Ronald E. Lund has served as our General Counsel since July 2007. Mr. Lund has over 17 years experience in the healthcare industry. Prior to joining our company, Mr. Lund served from 1988 to 2000, and from 2004 to 2005, as Senior Vice President, General Counsel and Secretary for Medtronic, Inc. From 2000 to 2001, Mr. Lund was the General Counsel for the American Red Cross in Washington, D.C. From 2002 to 2003, Mr. Lund was a partner of Briggs & Morgan, a Minneapolis law firm and from 2004 until joining AGA, Mr. Lund was "of counsel" at the Minneapolis law firm of Fredrikson & Byron.
Dr. Kurt Amplatz—Research and Development Consultant
Dr. Kurt Amplatz is a co-founder and former President of AGA Medical and, since 1995, has served as a research and development consultant to our company. From 1995 to September 1999, and again from August 2005 to April 2006, Dr. Amplatz also served as a director of our company. Dr. Amplatz retired in 1999 from the University of Minnesota after a distinguished career as the Malcolm B. Hanson Research Professor of Diagnostic Radiology. Dr. Amplatz's many inventions during his career include our first commercially available product, the AMPLATZER Septal Occluder. Dr. Amplatz received the Society of Interventional Radiology's Gold Medal in 1996 in recognition of his contributions to the field, and he is credited with more than 700 journal articles, books and abstracts in the field of radiology.
Directors
Tommy G. Thompson—Chairman
Tommy G. Thompson has served as chairman and a director of our board of directors since August 2005. Mr. Thompson has also been a member of our compensation committee since August 2005. Mr. Thompson has served as the independent chairman of the Deloitte Center for Health Solutions since March 2005, a partner at the law firm of Akin Gump Strauss Hauer & Feld in Washington, D.C. since March 2005, and on the board of directors of Centene Corporation, Pure Bioscience, and CR Bard since May 2005, January 2006, and October 2005, respectively. From 1987 to 2001, Mr. Thompson served four terms as the Governor of Wisconsin and was nominated in 2001 by President George W. Bush to serve as the secretary of the United States Department of Health & Human Services, a position which he held until 2005. At Deloitte and Akin Gump, and during his terms in office, Mr. Thompson focused on developing solutions to the healthcare challenges that face the nation by improving the use of information technology in hospitals and clinics and modernizing Medicare and Medicaid. Mr. Thompson holds both a Bachelor's Degree and Juris Doctor from the University of Wisconsin-Madison.
Franck L. Gougeon—Director and Co-Founder
Franck L. Gougeon is a director and co-founder of our company. Mr. Gougeon has served as a member of our Board since our company's inception in 1995. Mr. Gougeon also served as our Executive Vice President until October 2002, when he was promoted to President and Chief Executive Officer and served in that capacity until June 2008. Mr. Gougeon serves as chairman of our corporate development committee. Mr. Gougeon and the Gougeon Stockholders are controlling stockholders of AGA, and pursuant to the stockholders agreement with the WCAS Stockholders, Mr. Gougeon has
118
been elected to our board of directors. Prior to founding our company, Mr. Gougeon worked for Microvena Corporation (now ev3 Inc.) in various management positions in international sales and clinical and regulatory affairs. Mr. Gougeon holds a Bachelor's Degree in International Business from the École Nationale De Commerce in Paris, France.
Jack P. Helms—Director
Mr. Helms is expected to join our board of directors upon completion of this offering. He currently serves as the Chairman of the board of directors of Lazard Middle Market LLC. Lazard Middle Market, a subsidiary of Lazard Ltd., provides strategic advice on mergers and acquisitions, restructuring and capital raising to the middle market. Mr. Helms joined Goldsmith Agio Helms (GAH) (the predecessor firm to Lazard Middle Market) in 1987. He became president of GAH in 1992 and served in that capacity until 2007 when the firm was sold to Lazard. Prior to joining Lazard Middle Market, Mr. Helms was a partner at Fredrikson & Byron, an international law firm practicing in the area of mergers and acquisitions, limited partnerships, and tax and corporate law. Mr. Helms' former board positions include Applebee's International, Inc. and Luigino's, Inc. Mr. Helms holds a law degree from the University of Michigan Law School and a bachelor of science degree in industrial administration from Iowa State University. Mr. Helms is a member of the Minnesota bar, a certified public accountant, and a member of the Minnesota Society of CPAs.
Daniel A. Pelak—Director
Daniel A. Pelak has served as a member of our board of directors since 2006. Mr. Pelak served as the President and Chief Executive Officer of InnerPulse, Inc. from September 2005 until July 2008 and has served as Senior Advisor to Welsh, Carson, Anderson & Stowe, or WCAS, since November 2008. Entities and individuals affiliated with WCAS are controlling stockholders of AGA, and pursuant to the stockholders agreement with the Gougeon Stockholders, Mr. Pelak serves on our board of directors. Mr. Pelak currently serves as a member of the board of directors of InnerPulse, Inc., Mardil, Inc., Vertos, Inc. and Oncoscope, Inc. Additionally, he serves on the board of directors of Affinergy, Inc. From 2002 to 2005, Mr. Pelak was the President and Chief Executive Officer of Closure Medical Corporation, and from 1976 to 2002, Mr. Pelak was employed by Medtronic, Inc. where he served as Vice President from 1989 and was responsible for the general management of several operating divisions. Mr. Pelak holds a Bachelor of Science Degree from Pennsylvania State University.
Paul B. Queally—Director
Paul B. Queally has served as a member of our board of directors since July 2005, and as a member of our compensation committee since August 2005. Mr. Queally has been a general partner at WCAS since January 1996 and a member of its management committee since 2000. In October 2007, Mr. Queally became a Co-President of WCAS. WCAS is a private equity investment firm focused on investments in the healthcare and business/information services industries. Entities and individuals affiliated with WCAS are controlling stockholders of AGA, and pursuant to the stockholders agreement with the Gougeon Stockholders, Mr. Queally has been elected to our board of directors. Prior to joining WCAS, Mr. Queally was a general partner at the Sprout Group, which was the private equity arm of Donaldson, Lufkin & Jenrette Securities Corporation. Mr. Queally has been a board member of United Surgical Partners, Inc., Concentra Managed Care, Inc., MedCath Inc., Aptuit, Inc. and several other private companies. Mr. Queally holds a Bachelor of Arts Degree from the University of Richmond, where he is a member of the Board of Trustees, and an MBA from Columbia University.
Terry Allison Rappuhn—Director
Terry Allison Rappuhn has served as a member of our board of directors since May 2006, and as the chairman of our audit committee since July 2006. Ms. Rappuhn served from December 2003 until
119
July 2007 as a director and chair of the audit committee for Genesis HealthCare Corporation and also served on the Genesis compensation and compliance committees. Ms. Rappuhn has served since April 2002 as project leader and consultant for the Patient Friendly Billing® Project, a national initiative led by the Healthcare Financial Management Association. From 1999 to April 2001, Ms. Rappuhn served as Senior Vice President and Chief Financial Officer of Quorum Health Group, Inc., an owner and operator of acute care hospitals. From 1996 to 1999 and from 1993 to 1996, Ms. Rappuhn served as Quorum's Vice President, Controller and Assistant Treasurer and as Vice President, Internal Audit, respectively. Ms. Rappuhn holds a Bachelor of Business Administration from Middle Tennessee State University and is a Certified Public Accountant.
Darrell J. Tamosuinas—Director
Darrell J. Tamosuinas has served as a member of our board of directors since August 2005 and as a member of our audit committee since 2006. Mr. Tamosuinas has been elected to our board of directors pursuant to the stockholders agreement between the Gougeon Stockholders and the WCAS Stockholders as a nominee of the Gougeon Stockholders. Mr. Tamosuinas has over 30 years of financial and operational business experience in various industries including healthcare, manufacturing and distribution and energy. Mr. Tamosuinas is an independent consultant and most recently served as a Senior Vice President with GE Commercial Finance. From 1996 to 2007, Mr. Tamosuinas owned and operated a contract manufacturing company and provided financial and operational consulting services to companies in a broad variety of industries. From 1986 to 1996, Mr. Tamosuinas was with Ernst & Young LLP's Financial Advisory Services Group, most recently serving as partner in charge of the Midwest regional restructuring practice based in Chicago. Previously, Mr. Tamosuinas served as Vice President of Chase Manhattan Bank's Corporate Bank in New York and as a petroleum engineer with the Union Oil Company of California. Mr. Tamosuinas holds a Bachelor of Science Degree in Mechanical Engineering from the University of Southern California and an MBA from the California State Polytechnic University. Mr. Tamosuinas is also qualified as a Certified Public Accountant and a Certified Insolvency and Reorganization Advisor.
Sean M. Traynor—Director
Sean M. Traynor has served as a member of our board of directors and as a member of our audit committee since August 2005. Mr. Traynor currently serves as a member on the board of directors of Select Medical Corporation, Universal American Financial Corporation, Amerisafe, Inc., and certain other private companies. Since 1999, Mr. Traynor has been an investment professional at WCAS, and is currently a general partner, where he focuses on investments in the healthcare industry. WCAS is a private equity investment firm focused on investments in the healthcare and business/information service industries. Entities and individuals affiliated with WCAS are controlling stockholders of AGA, and pursuant to the stockholders agreement with the Gougeon Stockholders, Mr. Traynor has been elected to our board of directors. Prior to joining WCAS, Mr. Traynor worked from 1996 to 1999 in the healthcare and financial services investment banking groups at BT Alex Brown. From 1991 to 1994 Mr. Traynor served as an associate and senior associate with Coopers & Lybrand LLP (now PricewaterhouseCoopers). Mr. Traynor holds a Bachelor of Science Degree from Villanova University and an MBA with distinction from the Wharton School of Business.
Director Independence
Our board of directors has affirmatively determined that Ms. Rappuhn and Mr. Tamosuinas meet the definition of "independent director" under Rule 5605 of the Nasdaq Global Market listing standards.
After completion of this offering, we will continue to be a "controlled company" as set forth in Rules 5605 and 5615 of the Nasdaq Global Market because more than 50% of our voting power will be
120
held by a group formed by the WCAS Stockholders and the Gougeon Stockholders. Under the Nasdaq Global Market rules, a "controlled company" may elect not to comply with certain Nasdaq Global Market corporate governance requirements, including the requirement that a majority of the board of directors consist of independent directors and the requirement that directors nominations and executive compensation must be approved by a majority of independent directors or a nominating or compensation committee comprised solely of independent directors. Accordingly, you may not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq Global Market corporate governance requirements.
Family Relationships
There is no family relationship between any director, executive officer or person nominated to become a director or executive director.
Board of Directors
Composition of our Board of Directors upon Completion of this Offering
Our bylaws provide that our board of directors must consist of such number of directors as determined from time to time by resolution of our board. Upon the closing of this offering, we will have eight directors. We expect that we will appoint one additional director within one year of the completion of this offering. Our board of directors will be divided into three classes, each of which will be up for re-election every three years, as follows:
- •
- Class I, which will initially consist of Daniel A. Pelak and Darrell J. Tamousuinas, whose term will
expire at our annual meeting of stockholders to be held in 2010;
- •
- Class II, which will initially consist of Jack P. Helms, Terry Allison Rappuhn and Sean M. Traynor,
whose terms will expire at our annual meeting of stockholders to be held in 2011; and
- •
- Class III, which will initially consist of Franck L. Gougeon, Paul B. Queally and Tommy G. Thompson, whose terms will expire at our annual meeting of stockholders to be held in 2012.
Each director is elected for a term of three years and serves until a successor is duly elected and qualified or until his or her death, resignation or removal. Any additional directorships resulting from an increase in the number of directors or a vacancy may only be filled by the directors then in office. Directors may be removed with cause only and by the affirmative vote of the holders representing 75% of our voting power. Sean M. Traynor, Paul B. Queally and Daniel A. Pelak serve on our board of directors as WCAS Stockholders' nominees, and Franck L. Gougeon, Darrell J. Tamosuinas and Jack P. Helms serve on our board of directors as Gougeon Stockholders' nominees, in each case pursuant to an agreement among us, the WCAS Stockholders and the Gougeon Stockholders, described under "Certain Relationships and Related Transactions—Second Amended and Restated Stockholders Agreement."
Our current and future executive officers and significant employees serve at the discretion of our board of directors.
Committees of our Board of Directors
Our board of directors has three permanent committees: the audit committee, the compensation committee and the corporate development committee. The board of directors intends to review the current charter and adopt a revised written charter for our audit committee and our compensation committee. The board of directors has adopted a charter for our corporate development committee. In addition, from time to time, special committees may be established under the direction of our board of directors when necessary to address specific issues.
121
Audit committee
We have an audit committee consisting of Terry Allison Rappuhn, Darrell J. Tamosuinas and Sean M. Traynor. Upon completion of this offering, the audit committee will be responsible for, among other things:
- •
- appointment, termination, compensation and oversight of the work of any accounting firm engaged to prepare or issue an
audit report or other audit, review or attest services;
- •
- considering and approving, in advance, all audit and non-audit services to be performed by the independent
accountants;
- •
- reviewing and discussing the adequacy and effectiveness of our accounting and financial reporting processes and the audits
of our financial statements;
- •
- establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting,
internal accounting controls or auditing matters and the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters;
- •
- investigating any matter brought to its attention within the scope of its duties and engaging independent counsel and
other advisers as the audit committee deems necessary;
- •
- determining compensation of the independent auditors, compensation of advisors hired by the audit committee and ordinary
administrative expenses;
- •
- reviewing quarterly financial statements prior to their release;
- •
- reviewing and assessing the adequacy of a formal written charter on an annual basis;
- •
- reviewing and approving related party transactions for potential conflict of interest situations on an ongoing basis;
and
- •
- handling such other matters that are specifically delegated to the audit committee by our board of directors from time to time.
The SEC rules and the Nasdaq Global Market rules require us to have one independent audit committee member upon initial listing of our securities, a majority of independent audit committee members within 90 days of the initial listing of our securities and all independent audit committee members within one year of the initial listing of our securities. Our board of directors has affirmatively determined that Ms. Rappuhn and Mr. Tamosuinas meet the definition of "independent directors" for purposes of serving on an audit committee under Rules 5605 and 5615 of the Nasdaq Global Market listing standards. Mr. Traynor has been determined to be an "affiliated person" of our company in view of his affiliation with the WCAS Stockholders. See "Principal and Selling Stockholders." We intend to comply with these independence requirements within the time periods specified.
Compensation Committee
We have a compensation committee consisting of Tommy G. Thompson, Franck L. Gougeon and Paul B. Queally. Upon completion of this offering, the compensation committee will be responsible for, among other things:
- •
- reviewing and approving the compensation and benefits of all of our executive officers and key employees;
- •
- monitoring and reviewing our compensation and benefit plans;
- •
- overseeing the activities of the individuals responsible for administering cash incentive compensation plans and
equity-based plans; and
- •
- such other matters that are specifically delegated to the compensation committee by our board of directors from time to time.
122
Corporate Development Committee
We have a corporate development committee consisting of Franck L. Gougeon, Tommy G. Thompson and Sean M. Traynor. The corporate development committee is responsible for:
- •
- providing guidance and feedback to our Chief Executive Officer, and conducting, in the first instance, our Chief Executive
Officer's annual performance review and providing input to our compensation committee regarding the same;
- •
- evaluating attainment of personal goals by our Chief Executive Officer and reviewing our Chief Executive Officer's
evaluation of attainment of personal goals by other executives, and making recommendations in that regard to our compensation committee;
- •
- providing oversight of human resource functions, including strategic human resources and succession planning;
- •
- providing general oversight of research and development; and
- •
- reviewing, in the first instance, our annual strategic plan and making recommendations to the board of directors in regard to that.
Compensation committee interlocks and insider participation
None of our executive officers serves as a member of the board of directors or compensation committee (or other committee performing equivalent functions) of any entity that has one or more executive officers serving on our board of directors or compensation committee. No interlocking relationship exists between any member of the board of directors or any member of the compensation committee (or other committee performing equivalent functions) of any other company.
Code of Conduct
We expect to review our code of business conduct and ethics and adopt a revised code of business conduct and ethics upon completion of this offering relating to the conduct of our business by our employees, officers and directors, which will be posted on our website.
Director Compensation
We provide a combination of cash and non-cash compensation to our non-employee directors who are not affiliated with the WCAS Stockholders.
In January 2009, the compensation committee approved a new compensation plan for our non-employee directors who are not affiliated with the WCAS Stockholders (other than Mr. Thompson). Pursuant to this plan, these directors (namely, Mr. Gougeon, Mr. Pelak, Ms. Rappuhn and Mr. Tamosuinas) will receive from us an annual retainer of $25,000, $1,000 for each board meeting attended, $1,000 for each committee meeting attended in person and $500 for each committee meeting attended telephonically. In addition, the chair of the audit committee will receive $30,000 per year for serving as committee chair subsequent to an IPO. Mr. Thompson's compensation arrangement is not affected as a result of this new compensation plan for our directors.
Prior to the adoption of the new director compensation plan in January 2009, the cash compensation for Mr. Tamosuinas, Ms. Rappuhn and Mr. Pelak consisted of an annual retainer of $12,500, $2,500 for each board meeting attended in person and $500 for each board meeting attended telephonically. Mr. Thompson receives a monthly cash retainer of $8,333 for serving as the chairman of our board of directors and for providing other services to us pursuant to the independent contractor agreement described below.
123
Following the completion of this offering, the non-cash compensation for our non-employee directors who are not affiliated the WCAS Stockholders will consist of grants of options to purchase our common stock under our 2008 Equity Incentive Plan described under "Executive Compensation—Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table—2008 Non-Equity Incentive Plan." Prior to the completion of this offering, such options were granted under our 2006 Equity Incentive Plan described under "Executive Compensation—Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table—2006 Equity Incentive Plan." In 2006, we granted to each of Mr. Pelak, Mr. Tamosuinas and Ms. Rappuhn options to purchase 45,000 shares of our common stock (without giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering), all of which are currently fully vested.
On June 20, 2008, Mr. Gougeon resigned as our President and Chief Executive Officer, and until then he did not receive compensation to serve as a director. On that date, we entered into a transition agreement with Mr. Gougeon pursuant to which we agreed to pay him a special bonus of $500,000. Our board of directors decided to make this payment in order to recognize Mr. Gougeon's long-time service to our company, including his role as co-founder, and to provide for the orderly transition to a new President and Chief Executive Officer for our company. Our board of directors believed that $500,000 was an appropriate payment amount because it represents approximately one year's salary plus a pro rata portion of the bonus that Mr. Gougeon earned in 2007. We also entered into an engagement letter with Mr. Gougeon pursuant to which he is entitled to receive the same cash and non-cash compensation for serving as a director as Mr. Tamosuinas, Ms. Rappuhn and Mr. Pelak described above. In addition, on the same date we entered into a consulting agreement with Mr. Gougeon pursuant to which he is entitled to receive an annual retainer of $170,000 paid monthly for up to 30 days of consulting services to us per any 12-month period. In 2008, he received a retainer of $99,169 for consulting services to us pursuant to this consulting agreement. Mr. Gougeon did not receive any compensation in excess of his retainer for consulting services in 2008. See "Certain Relationships and Related Transactions—Consulting Agreements."
In July 2005, at the time of our reorganization, we entered into a five-year independent contractor agreement with Mr. Thompson pursuant to which he devotes up to 20% of his time to matters relating to our company that include assisting with FDA and regulatory affairs, customer relations, clinical trial awareness and media and investor communications, as well as serving as the chairman of our board of directors. For providing such services, Mr. Thompson receives a monthly cash payment of $8,333. In 2008, Mr. Thompson received $100,000 of cash compensation for his services to us pursuant to the terms of this agreement. In addition, pursuant to the terms of this agreement, Mr. Thompson was granted options to purchase 2,955,242 shares of our common stock (without giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering), equivalent to 1% of our then-outstanding shares, that vest 20% annually over a five-year period commencing upon the execution of the independent contractor agreement in July 2005. In April 2006, our stockholders and board of directors approved a special retention bonus of $255,000 for Mr. Thompson, subject to the successful completion of our April 2006 recapitalization, which is payable in three equal annual installments over a three-year period; provided that Mr. Thompson continues to serve as our chairman of the board of directors at each of the applicable times. In 2008, we paid Mr. Thompson the last installment of $85,000 of this $255,000 bonus. In accordance with our agreement with Mr. Thompson, we are also required to pay an annual retainer of $120,000 (in monthly installments of $10,000) to Akin Gump Strauss Hauer & Feld LLP, where Mr. Thompson serves as a partner.
We had not historically provided director compensation to Messrs. Paul Queally and Sean Traynor, who are non-employee directors affiliated with the WCAS Stockholders; however, in April 2009, our compensation committee awarded to each of Messrs. Queally and Traynor options to purchase 45,000 shares of our common stock, the same number of options that had been granted to each of our non-employee directors who are not affiliated with the WCAS Stockholders. These options have an
124
exercise price equal to $19.66 (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering). One third of these options vested immediately on the grant date, another one-third will vest on the first anniversary of the grant date and the remaining one-third will vest on the second anniversary of the grant date.
In addition, our board of directors has determined to grant to each of our directors (other than Mr. Thompson) options to purchase 55,000 shares of our common stock (prior to giving effect to the reverse stock-split) at the completing of this offering. These options will have an exercise price per share equal to the price per share offered in this offering, have a ten year term and vest annually over a three-year period commencing with the grant date. The board of directors determined not to make similar additional option grants to Mr. Thompson because he has already received substantial option grants relative to the other directors and thus no further option grants to Mr. Thompson were deemed necessary or appropriate.
We reimburse our directors for out-of-pocket expenses incurred in connection with attending our board and committee meetings.
The table below sets forth the compensation provided to our directors during the year ended December 31, 2008. Mr. Gougeon's compensation is set forth below in the "Executive Compensation—Summary Compensation Table" since he served as our President and Chief Executive officer during a portion of that year.
Director Compensation for 2008
Name
|
Fees Earned or Paid in Cash(1) ($) |
Option Awards (2)(3) ($) |
All Other Compensation(4) ($) |
Total ($) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Daniel A. Pelak |
$ | 29,500 | $ | 2,186 | $ | — | $ | 31,686 | |||||
Paul B. Queally |
$ | — | $ | — | $ | — | $ | — | |||||
Terry Allison Rappuhn |
$ | 29,500 | $ | 3,127 | $ | — | $ | 32,627 | |||||
Darrell J. Tamosuinas |
$ | 29,500 | $ | 3,149 | $ | — | $ | 32,649 | |||||
Tommy G. Thompson |
$ | 220,000 | $ | 311,405 | $ | 85,000 | $ | 616,405 | |||||
Sean M. Traynor |
$ | — | $ | — | $ | — | $ | — | |||||
- (1)
- For
Messrs. Pelak and Tamosuinas and Ms. Rappuhn, reflects an annual retainer of $12,500 for serving as a director plus a fee for each board
meeting attended.
For Mr. Thompson, reflects (i) the cash payment he received pursuant to the terms of his independent contractor agreement with us described above, which includes compensation paid for serving as the chairman of our board of directors and (ii) the $120,000 annual retainer paid under this agreement to Akin Gump Strauss Hauer & Feld LLP, where Mr. Thompson serves as a partner.
- (2)
- Reflects
the compensation expense we recognized in 2008 for financial statement reporting purposes under FAS 123R (excluding estimates of
forfeitures) with respect to the outstanding stock options we granted to certain of our directors under the 2006 Equity Incentive Plan. These values have been determined based on the assumptions set
forth in Note 12 to our consolidated financial statements for 2008.
- (3)
- None of the directors listed in the table above received any option awards during 2008, and none of them held any unvested option awards at December 31, 2008, except for Mr. Thompson, who held 1,182,098 unvested option awards (without giving effect to the 7.15 for 1.00 reverse stock split of our common stock that will occur immediately prior to completion of this offering).
125
- (4)
- For Mr. Thompson, reflects the third installment of a special retention bonus of $255,000 described above, which was paid to him in April 2008.
Executive Compensation
Compensation Discussion and Analysis
The following discussion and analysis of compensation arrangements of our named executive officers for 2008 should be read together with the compensation tables and related disclosures on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from currently planned programs summarized in this discussion.
Executive Summary
The primary objectives of our executive compensation program are to attract and retain talented executives to effectively manage and lead our company and create value for our stockholders. Through our executive compensation program, we seek to align the level of our executive compensation with the achievement of our corporate objectives, thereby aligning the interests of our management with those of our stockholders. We evaluate and reward our executive officers based upon the realization of our corporate objectives and the individual contributions of each executive officer to these results. We make decisions about the executive officers' salary increases and the amount of annual cash incentive payments, primarily based on individual performance as well as departmental performance, and we provide long-term equity awards, generally in the form of stock options, to encourage our executive officers to create long-term value for our stockholders.
The compensation packages for our named executive officers generally include a base salary, annual cash incentive payments, stock option awards, other benefits and perquisites. In addition, our named executive officers are eligible to receive severance or other benefits upon termination of their employment with us or a change in control of our company. In setting an individual executive officer's initial compensation package and the relative allocation among different types of compensation, we consider the nature of the position being filled, the scope of associated responsibilities, the individual's prior experience and skills and the individual's compensation expectations, as well as WCAS's general market knowledge with respect to executive compensation. In addition, with respect to our named executive officers, we consider the compensation of existing executive officers at our company.
The discussion below includes a review of our compensation decisions with respect to the 2008 performance year. Our named executive officers for 2008 were Franck L. Gougeon, John R. Barr, Brigid A. Makes and Ronald E. Lund. Mr. Gougeon is one of our directors and co-founders and resigned as our President and Chief Executive Officer in June 2008. Mr. Barr joined us in September 2005 as our Chief Operating Officer and started serving as our President and Chief Executive Officer in June 2008. Ms. Makes joined us in October 2006 as our Chief Financial Officer. Mr. Lund joined us in June 2007 as our General Counsel.
Role of Our Compensation Committee
Our compensation committee evaluates and determines the levels and forms of individual compensation for our named executive officers. Our compensation committee reviews and approves compensation for our named executive officers at least annually, generally at the beginning of each calendar year, consistent with the individual executive officers' employment agreements and based on the individual executive officer's performance and the company's overall performance during the prior year.
126
Our compensation committee solicits input from our Chief Executive Officer in determining compensation (particularly base salary and annual cash incentive payments) of our other named executive officers and from our corporate development committee since 2008 in determining compensation of our Chief Executive Officer. Our compensation committee does not delegate to others any of its authority to establish executive compensation but engaged outside consultants with respect to executive compensation matters for the first time in April 2008.
Compensation Determination Process
Our current compensation program for our named executive officers has been designed based upon WCAS's view that executive compensation should be set at levels that are necessary, within reasonable parameters, to successfully attract the desired executive candidates to us. In determining the appropriate levels of compensation, we considered the compensation expectations of the desired candidates communicated to us by an independent executive search firm that we had engaged and WCAS's general knowledge of market practices in related industries (for example, healthcare services). We designed compensation packages that we believed would facilitate recruiting our desired candidates but that were also fair and equitable in light of WCAS's view of market practices. We believe that these compensation packages have been critical in attracting and retaining executive officers of a caliber and level of experience necessary to effectively manage our business as a public company.
Prior to April 2008, we did not retain a compensation consultant to review our executive compensation policies and procedures and did not benchmark any element of our executive compensation against that of any specific companies. However, in April 2008, our compensation committee, for the first time, engaged a third-party compensation consultant to evaluate our executive compensation program and to benchmark the levels of base salaries, cash incentive payments and long-term equity incentives of our Chief Executive Officer, Mr. Barr, and our Chief Financial Officer, Ms. Makes, against those of executive officers of equivalent positions at a select group of fifteen publicly traded companies that we compete with for executive talent (the "Compensation Comparison Group"). The compensation of our General Counsel, Mr. Lund, was not included in the study because Mr. Lund was an independent contractor at the time the study was initiated, and the compensation of our former Chief Executive Officer, Mr. Gougeon, was not included in the final report of the consultant because Mr. Gougeon had resigned as our President and Chief Executive Officer prior to the time the study was completed. The Compensation Comparison Group included publicly traded companies in the medical devices and healthcare industries that have revenues comparable to our company. The Compensation Comparison Group consisted of the following companies: AngioDynamics, Inc.; ArthroCare Corp.; Edwards Lifesciences Corp.; LifeCell Corp.; Masimo Corp.; Mentor Corp.; Micrus Endovascular Corp.; Palomar Medical Technologies, Inc.; Possis Medical, Inc.; ResMed Inc.; Thoratec Corp.; and Volcano Corp.
The primary objective of the compensation consultant's evaluation was to determine how our named executive officers' base salaries, cash incentive payments and equity grants for performance year 2007 compared to the 50th percentile for equivalent positions in the Compensation Comparison Group. The compensation consultant's report revealed that our Chief Executive Officer and Chief Financial Officer's base salaries were approximately 10% lower than the median of the Compensation Comparison Group for equivalent positions. In addition, the compensation consultant's report revealed that the Chief Executive Officer's target cash incentive payment of 50% of base salary fell short of the 50th percentile of the Compensation Comparison Group, which was at 80% of base salary, whereas the Chief Financial Officer's target cash incentive payment of 50% of base salary was consistent with the 50th percentile of the Compensation Comparison Group. Our Chief Executive Officer's paid cash incentive payment was at approximately 32nd percentile of the Compensation Comparison Group, and our Chief Financial Officer's paid cash incentive payment was at approximately 64th percentile of the Compensation Comparison Group. The compensation consultant's report also revealed that equity
127
grants to our Chief Executive Officer and Chief Financial Officer were approximately at 27th and 7th percentile of the Compensation Comparison Group, respectively, based on the "at grant" values but were above the 50th percentile based on the total estimated in-the-money value of their outstanding options (assuming our company went public) as compared to the aggregate appreciated value of equity awards held by similarly situated executive officers within the Compensation Comparison Group. Overall, the compensation consultant's report determined that the differences in base salaries and target cash incentive payments from the median of the Compensation Comparison Group were in line with the differences in compensation practices of private companies as compared to publicly traded companies. In addition, the compensation consultant's report informed our compensation committee that the option grants made to our Chief Executive Officer and Chief Financial Officer in 2006 provided them with aggregate in-the-money option values at levels that the committee considered appropriate for their respective roles, taking into account the fact that these option grants were made to executive officers at a private company. Our compensation committee did not take any action with respect to the 2008 compensation based on the compensation consultant's report. However, in March 2009, our compensation committee approved an increase in the target cash incentive payment of Mr. Barr from 50% of base salary to 75% to provide a more competitive bonus opportunity as compared to the chief executive officers at companies in the Compensation Comparison Group.
Elements of Compensation
We generally deliver executive compensation through a combination of base salary, annual cash incentive payments, long-term equity incentives in the form of stock options, benefits and other perquisites. We believe that this mix of elements is useful in achieving the primary objectives of our compensation program.
Base Salary. Base salaries are designed to reward core competence in the executive officer's role relative to his or her skills, prior experience and contributions to our company. Our compensation committee established an initial base salary for our named executive officers based on the individual executive officer's experience prior to joining us, the scope of his or her responsibilities with us, WCAS's knowledge of market compensation practices, and estimates about the base salary that would be required to secure our candidate of choice based on input from the executive search firm. See "—Compensation Determination Process."
After our July 2005 recapitalization that resulted in certain WCAS Stockholders purchasing an ownership interest in us from one of our original founders, Mr. Gougeon was named our President and Chief Executive Officer. At that time, our board of directors established an annual base salary of $350,000 for Mr. Gougeon based upon the various factors described above.
As a condition of the recapitalization transaction, we agreed to hire a Chief Operating Officer. We also agreed that, once a Chief Operating Officer was hired, Mr. Gougeon's base salary would be adjusted to an amount that is 10% higher than the base salary of the Chief Operating Officer. In September 2005, we hired John R. Barr as our Chief Operating Officer at a starting annual base salary rate of $380,000 based upon the various factors described above. In accordance with the agreement we entered into as a condition of the recapitalization, we increased Mr. Gougeon's annual base salary rate to $418,000 when we hired Mr. Barr as our Chief Operating Officer.
In October 2006, we hired Brigid A. Makes as our Chief Financial Officer. Based upon the various factors described above, our compensation committee approved an annual base salary of $260,000 for Ms. Makes.
In June 2007, we entered into a consulting agreement with Mr. Lund pursuant to which Mr. Lund served as our General Counsel until July 1, 2008. Under this consulting agreement, we compensated Mr. Lund at a rate of $400.00 per hour, the same rate that he was charging in his private practice prior to entering into the consulting agreement with us. On July 1, 2008, we terminated Mr. Lund's
128
consulting agreement and entered into a three-year employment agreement with Mr. Lund pursuant to which he continues to serve as our General Counsel. Under this agreement, for the period from July 1, 2008 to December 31, 2008 we paid Mr. Lund at an annual base salary of $375,000.
We have adopted a single merit increase practice with respect to base salaries that applies to all employees, including our named executive officers. Each year our human resources department sets an average target merit increase for all employees based upon its view of the then current market conditions. Actual increases for individual employees, if any, may be more, or less, than this percentage based upon the recommendations of the employee's supervisors. Our company-wide target merit increases were 4.0% for 2007 and 3.8% for 2008. The specific percentage increases for the named executive officers were recommended to the compensation committee by our Chief Executive Officer based upon his subjective view of how the executive officers performed during the prior year in key qualitative areas. The areas that our former Chief Executive Officer, Mr. Gougeon, considered in 2008 were: delivering results, leadership, development of people, communications and acting in the best interest of our company. Our compensation committee then considered Mr. Gougeon's recommendations and approved adjustments to annual base salary, subject to the terms of the named executive officers' employment agreements, to appropriately reflect the performance of the named executive officer, any changes in the scope of his or her responsibilities and any changes in the competitive marketplace.
In January 2008, Mr. Gougeon recommended, and our compensation committee approved, a 4.92% increase in annual base salary for himself, Mr. Barr and Ms. Makes. This action increased the annual base salary for Mr. Gougeon to $438,566, Mr. Barr to $418,631 and Ms. Makes to $272,792. The increased annual base salaries took effect retroactively as of the anniversary date of hire in 2007 for Mr. Barr and Ms. Makes. In recommending this percentage increase, Mr. Gougeon considered, among others, the following achievements: for himself, his overall leadership skills in driving our company's performance against the Corporate Top Ten Objectives, establishing the research and development strategy for our product pipeline, strengthening his executive team with the addition of Mr. Lund as our General Counsel and leading initiatives for employee development in connection with our anticipated transition to a public company; for Mr. Barr, his overall leadership skills in driving our company's performance against the Corporate Top Ten Objectives, strengthening the overall organization with key hires across different functional areas and effecting changes that increased the organization's overall effectiveness, particularly in research and development and quality; and for Ms. Makes, strengthening the effectiveness of her department with the addition of key hires, enhancing key processes around financial controls and reporting and her overall leadership skills. He believed that this percentage increase appropriately reflected the applicable executive officer's performance in the key qualitative areas described above.
In March 2009, the compensation committee recommended and approved a 3.0% increase in annual base salary for Mr. Barr, Ms. Makes and Mr. Lund. The compensation committee generally assessed the performance of these executives but made a subjective determination without considering the executives' performances or using any objective criteria or formula that a 3% merit increase was appropriate for all executives in light of the current economic environment and low cost of living.
Annual Cash Incentive Payments.
In addition to base salaries, our compensation committee generally awards annual cash incentive payments to our named executive officers. The annual cash incentive payments are intended to compensate our named executive officers for achieving our short-term operating performance objectives and strategic initiatives that are important to our success. The annual cash incentive payments are paid during the first quarter following the completion of the performance year to which the payments relate.
129
Pursuant to the terms of his or her employment agreement, each named executive officer is eligible for a target annual cash incentive payment of an amount up to 50% of that executive officer's annual base salary. However, to reward exceptional performance, our compensation committee may discretionarily increase the actual payment above the target percentage. In March 2009, our compensation committee approved an increase in the target bonus of Mr. Barr to 75%. See "—Compensation Determination Process."
Prior to the start of each year, our executive officers identify key initiatives that are important to the success of our company for that year. Our annual "Corporate Top Ten Objectives" are derived from these initiatives. These objectives are specific goals that relate to what we believe are the most important value-creating deliverables at the time we set the goals. The company's performance against these objectives, together with other relevant information the board of directors considers, determines the amount of the overall bonus pool for all of our employees, including our named executive officers.
These objectives are reviewed with, and approved by, our board of directors at the beginning of each year, with the understanding that our business priorities may change over the course of the year, and such changes will be taken into account in evaluating our performance against these goals at the end of the year. These corporate goals and the proportional emphasis placed on each goal vary from year to year, depending on our overall strategic objectives, but they generally relate to factors such as:
- •
- achieving targeted financial results, including EBITDA performance;
- •
- progressing existing products and developing new products within our research and development pipeline;
- •
- delivering clinical and regulatory milestones, including enrolling patients in clinical studies, devising solutions to
medical problems and/or obtaining FDA approval for research protocols;
- •
- achieving sales and marketing targets (worldwide and for specified geographic areas or products);
- •
- establishing new collaborative arrangements, including with distributors or research partners;
and
- •
- meeting operational goals, including improving manufacturing efficiency, ensuring compliance with all regulatory obligations and implementing key business systems.
Because these objectives are set at extremely challenging levels, 100% achievement of these objectives constitutes superior performance.
Our Corporate Top Ten Objectives for 2008 were:
- •
- achieving annual sales, expenses and EBITDA targets of $176 million, $114 million and $56 million,
respectively. Quarterly sales, expenses and EBITDA targets, respectively, were as follows: first quarter—$41 million, $28 million and $12 million; second
quarter—$42 million, $20 million and $11 million; third quarter—$45 million, $28 million and $15 million; and fourth
quarter—$48 million, $28 million and $18 million;
- •
- identifying new products for our research and development pipeline;
- •
- meeting specific milestones for our AMPLATZER PFO RESPECT clinical trials
that included achieving certain levels of patient recruitment and enrollment of clinical sites;
- •
- achieving targeted sales volume of $5 million for specified new products;
- •
- gaining regulatory approval for certain products and developing a company-wide regulatory strategy;
- •
- converting Spanish distributor business to direct distribution and finalizing transition agreements by December 31, 2008 for France, the Netherlands, Belgium, Portugal and Canada;
130
- •
- meeting specific milestones for our migraine clinical trials that included achieving certain levels of patient recruitment
and enrollment of clinical sites;
- •
- completing necessary steps on schedule for future compliance with Section 404 of Sarbanes-Oxley;
- •
- completing the initial phase of implementing our new ERP system; and
- •
- meeting specific milestones for our other clinical trials that included achieving certain levels of patient recruitment and enrollment of clinical sites.
At the end of each year, each of our named executive officers assesses our performance against these objectives and rates our performance as "above target," "target" or "below target" for each objective. If an objective was not met, the rating would be "below target;" if an objective was achieved or marginally exceeded, the rating would be "target;" and if an objective was far exceeded or resulted in additional benefits, the rating would be "above target." Our named executive officers then recommend to the board of directors an overall level of achievement by our company against our Corporate Top Ten Objectives in numerical terms. In making the recommendation, appropriate consideration is given to the fact that these objectives are set at extremely challenging levels and 100% achievement of these objectives constitutes superior performance. Furthermore, appropriate consideration is given to qualitative factors that provide the context for our performance against various objectives, our other significant achievements that are not fully reflected in the Corporate Top Ten Objectives and changes in our business conditions, strategies and priorities that would assist the board in making a fair and comprehensive assessment of our performance. Based on the recommendations of our named executive officers, and after considering an array of information, our board of directors approves the overall level of achievement for our company against our Corporate Top Ten Objectives, which then determines the amount of aggregate bonus pool for all employees.
Taking into account the amount of the bonus pool, our compensation committee then makes individual determinations regarding the amount of the annual cash incentive payment for the named executive officers. The determinations of the amounts to be paid are not formulaic and are not based on specific performance targets. Rather, they are based on the Chief Executive Officer's subjective assessment of the contributions made by the executive officer to our company's performance and, in the case of our Chief Executive Officer, an evaluation of his performance from our corporate development committee.
With respect to the 2008 performance year, we performed above target with respect to one objective, namely the conversion of the Spanish distributor business, at "target" against two of the objectives and "below target" against the others. The objectives against which we performed "at target" were:
- •
- meeting specific milestones for our AMPLATZER PFO RESPECT clinical trials
that included achieving certain levels of patient recruitment and enrollment of clinical sites;
- •
- completing the initial phase of implementing our new ERP system.
In evaluating our performance against those objectives that were rated "below target," the following factors were considered:
- •
- we met some enrollment goals in our migraine trials but did not meet all of the elements of the objective;
- •
- although we did not meet all of our targeted financial results as a result of missing our sales target, we met our annual expense target;
131
- •
- we did not meet our new product sales goals partially due to delays in launch and approval of certain products, and the
business decision to execute a more focused launch for a certain product; and
- •
- we were delayed in gaining regulatory approval for certain products which resulted in missing revenues of $2.5 million against our target.
In addition, consideration was given to a number of significant achievements made by our company that were not fully reflected in the ratings for the Corporate Top Ten Objectives. These achievements included:
- •
- our strong progress in building a new product pipeline;
- •
- strong progress made by our manufacturing and quality teams in operational efficiencies;
- •
- our strengthened and focused management team.
Based on all of the factors described above, for 2008, our named executive officers recommended an overall level of achievement of 92% for our company against our Corporate Top Ten Objectives, and the board of directors approved this recommendation.
Our compensation committee approved total annual cash incentive payment amounts equal to 92% of the target percentage for Mr. Barr and Ms. Makes and 101.2% of the target percentage for Mr. Lund. These percentages reflect what Mr. Barr believed were the relative contributions of each such named executive officer based upon his subjective evaluation of the executive officer's individual contribution to our company's performance and, with respect to himself, an evaluation of his own performance by the corporate development committee. In recommending these percentages, Mr. Barr considered, among other things, the following achievements: for himself, his overall leadership skills, particularly in driving the support of research and development programs, negotiating the transition in key distribution relationships, and our strong year over year financial performance; for Ms. Makes, her overall leadership skills, particularly with respect to our strong year over year financial performance, advancing the implementation of strong business processes around our internal controls over financial reporting, keeping the ERP System implementation on plan and budget during the year, and executing against our distributor conversion objective; and for Mr. Lund, his overall leadership skills, particularly with respect to overseeing the various legal matters the company was handling, including the patent infringement matters and the distributor conversion. Mr. Barr believed that these percentages appropriately reflected the applicable executive officer's relative contribution to our company's performance during 2008.
Mr. Gougeon did not receive a cash incentive payment for the 2008 performance year because he resigned as our President and Chief Financial Officer in June 2008. He received a $500,000 special bonus pursuant to a transition agreement which our board of directors decided to pay in order to recognize his long-time service to our company, including his role as co-founder, and to provide for the orderly transition to a new President and Chief Executive Officer for our company. Our board of directors determined that $500,000 was an appropriate payment amount because it represents approximately one year's salary plus a pro rata portion of the bonus that Mr. Gougeon earned in 2007.
As part of achieving the 2006 recapitalization milestone, Mr. Barr was granted a $1.4 million retention bonus in April 2006 that would vest over a five-year period with the second year payment being accelerated in recognition of his contribution to our company during the eight months prior to the grant. Accordingly, he received 40% of his retention bonus in April 2006, no payment in 2007 and 20% in April 2008, and he is expected to receive a 20% payment in each of April 2009 and April 2010 if he continues to be employed by us at those times.
132
Our Corporate Top Ten Objectives for 2009 include:
- •
- achieving targeted financial results for sales, expenses and EBITDA;
- •
- meeting specific milestones for our AMPLATZER PFO Occluder clinical trials
that included recruitment and site enrollment;
- •
- progressing key pipeline projects, such as our vascular graft projects;
- •
- successful conversion of distributors to direct distribution in the Netherlands, Belgium, France, Portugal and Canada;
- •
- launching European centralized distribution center;
- •
- successful conversion to our new ERP system; and
- •
- achieving targeted sales growth for specific products and markets.
Long-Term Equity Incentives. We generally provide long-term equity incentive compensation to our named executive officers in the form of stock options granted under our 2006 Equity Incentive Plan, which has been our only long-term equity incentive program to date. We believe that our company's long-term financial success is achieved in part through an ownership culture that encourages our executive officers to focus on our company's long-term performance through the use of equity and equity-based awards.
In 2007, our General Counsel, Mr. Lund, was awarded 83,916 stock options (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering) under our 2006 Equity Incentive Plan in conjunction with entering into an independent contractor agreement to serve as our General Counsel. His stock options vest over a three-year period, consistent with the three-year term of his independent contractor agreement with us. In July 2008, Mr. Lund was awarded 41,958 additional stock options (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering) in conjunction with entering into a three-year employment agreement to continue to serve as our General Counsel. These stock options also vest over a three-year period, consistent with the three-year term of his employment agreement. In 2006, our then Chief Operating Officer, Mr. Barr, and our Chief Financial Officer, Ms. Makes, were awarded stock options under our 2006 Equity Incentive Plan, and shares underlying those stock options represented approximately 1.5% and 0.75% of the then-outstanding shares, respectively. The amounts of those stock options were determined as part of the terms of the executive officer's respective employment agreement at the time of his or her initial hire and reflected WCAS's practices with respect to the size of equity grants awarded to similarly situated executive officers at other private companies in its portfolio. Mr. Barr and Ms. Makes did not receive additional awards of stock options in 2007 or 2008 because our compensation committee believed that the awards they received in 2006 provided them with levels of equity ownership that the committee considered appropriate for their respective roles, taking into account the fact that the 2006 option grants were made to executive officers at a private company. As previously stated, Mr. Gougeon is one of our controlling stockholders and, therefore, did not receive any equity-based compensation while serving as our Chief Executive Officer.
All equity awards to our employees were granted at no less than the fair market value of our common stock as determined by independent valuations that were effective at the time of the grants. We do not have any program or plan obligation that requires us to grant equity compensation on specified dates, and we have not made equity grants in connection with the release or withholding of material non-public information.
Our board of directors and stockholders will adopt the 2008 AGA Medical Holdings, Inc. Equity Incentive Plan before the effective date of this offering. See "Narrative Disclosure to Summary
133
Compensation Table and Grants of Plan-Based Awards Table—2008 Equity Incentive Plan" for a description of the material terms of this plan. This new equity incentive plan replaces our existing 2006 Equity Incentive Plan and gives our compensation committee much greater flexibility to make a wide variety of equity awards. After we become a public company, we expect to award stock options to our named executive officers under our 2008 Equity Incentive Plan.
We have not adopted stock ownership guidelines for our named executive officers and other key employees.
Employee Stock Purchase Plan. Our board of directors and stockholders will adopt the 2008 AGA Holdings Inc. Employee Stock Purchase Plan, or the ESPP, before the effective date of this offering. The ESPP is designed to provide an incentive to attract, retain and reward eligible employees. We intend to qualify the ESPP as an "employee stock purchase plan" under Section 423 of the Internal Revenue Code. The ESPP will be generally available to all eligible employees, including our named executive officers under the same offering and eligibility terms, and will not be tied to any performance criteria.
A maximum of 559,440 shares of our common stock will be reserved for issuance under the ESPP. The number of shares reserved pursuant to the ESPP is subject to adjustment to prevent dilution or enlargement of participants' rights in the event of a stock split or other change in our capital structure. We describe below the material term of the ESPP.
- •
- Administration. The ESPP will be administered by our board
of directors or a committee of the board. The plan administrator will have the authority to construe and interpret the terms of the ESPP and the purchase rights granted under it, to determine
eligibility to participate, and to establish policies and procedures for administration of the ESPP.
- •
- Eligibility. Each of our employees and employees of any
subsidiary designated by the administrator will be eligible to participate in the ESPP if they are customarily employed by us for more than 20 hours per week and more than five months in any
calendar year, except that an employee may not be granted a right to purchase stock under the ESPP if, immediately after the grant, the employee would own stock possessing 5% or more of the total
combined voting power or value of all classes of our capital stock or of any parent or subsidiary entity.
- •
- Offerings. The ESPP will be implemented by offerings of
purchase rights to eligible employees. The initial offering period will begin on the day the underwriting agreement is executed in connection with this offering and will end on June 30, 2010,
unless sooner terminated.
- •
- Participation. Eligible employees who enroll in the ESPP
may elect to have up to 10% of their eligible compensation withheld and accumulated for the purchase of shares at the end of each offering period in which they participate. All eligible employees will
be automatically enrolled in the ESPP's initial offering period and may purchase shares by delivering an exercise notice and payment of the applicable purchase price prior to the initial purchase date
referred to above. Participation shall end automatically upon termination of employment for any reason.
- •
- Purchase of Shares. Amounts accumulated for each participant will be used to purchase shares of our common stock at the end of each offering period (each, a "purchase date") at a price generally equal to 85% of the lower of the fair market value of our common stock at the beginning of an offering period or at the end of the offering period. The maximum number of shares a participant may purchase in any calendar year is the lesser of: (1) the number of shares having a fair market value of $25,000 (determined on the purchase date); or (2) the number of shares having a fair market value equal to 10% of the participant's eligible compensation for such year (determined on the purchase date).
134
- •
- Corporate Transactions. In the event of certain corporate transactions, an acquiring or successor corporation may assume our rights and obligations under the ESPP. If the acquiring or successor corporation does not assume such rights and obligations or does not substitute them with similar rights and obligations, then the purchase date of the offering periods then in progress will be accelerated to a date prior to the effective time of the corporate transaction.
Other Benefits. We also provide certain other benefits to all of our named executive officers who are full-time employees that are not tied to any performance criteria and are intended to be part of a competitive compensation program. These benefits include:
- •
- health, vision and dental insurance;
- •
- flexible spending accounts;
- •
- paid time off;
- •
- basic and supplemental life insurance;
- •
- accidental death and dismemberment and short- and long-term disability insurance;
- •
- an employee stock purchase plan that is expected to be adopted in connection with this offering (see
"—Employee Stock Purchase Plan");
- •
- a 401(k) plan with a defined matching of contributions and a 3% "safe-harbor" contribution;
- •
- car allowances; and
- •
- an educational reimbursement program.
We believe that these benefits are comparable to those offered by other companies.
Severance and Change in Control Benefits
Our named executive officers are entitled to certain severance and change in control benefits, the terms of which are described below under "Potential Payments Upon Termination or Change in Control." We believe these severance and change in control benefits are an essential element of our compensation program for our executive officers and assist us in recruiting and retaining talented individuals. The compensation committee believes that these benefits are valuable as they address the valid concern that it may be difficult for our named executive officers to find comparable employment in a short period of time in the event of termination or change in control. Our compensation committee believes that consideration of a change in control could be a distraction to an executive officer and could cause an executive officer to consider alternative employment opportunities at a time when the executive's continued service may be crucial to our company. In order to ensure continued dedication, objectivity and stability of our named executive officers in the event of a change in control, the compensation committee has decided to provide our named executive officers with severance payments and acceleration of vesting of stock options in connection with termination of employment following a change in control and acceleration of vesting of stock options upon a change in control. These terms are incorporated into each named executive officer's employment agreement. These severance and change in control benefits may differ for named executive officers depending on the positions they hold and how difficult it might be or how long it might take for them to find comparable employment.
135
The following table sets forth summary compensation information for our named executive officers for 2008.
Name and Principal Position
|
Year | Salary(1) ($) |
Bonus ($) |
Option Awards(2) ($) |
Non-Equity Incentive Plan Compensation ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Franck Gougeon(3) |
2008 2007 |
$ |
233,673 418,000 |
$ |
500,000 — |
$ |
22,791 — |
— 172,738 |
$ |
157,271 20,635 |
$ |
913,735 611,373 |
||||||||||
John R. Barr(4) |
2008 2007 |
$ |
418,631 404,593 |
$ |
280,000 — |
$ |
466,922 466,196 |
192,513 211,400 |
$ |
71,294 76,405 |
$ |
1,429,360 1,158,594 |
||||||||||
Brigid A. Makes(5) |
2008 2007 |
$ |
272,792 263,198 |
— — |
$ |
230,708 231,392 |
$ |
125,458 131,270 |
$ |
22,567 41,062 |
$ |
651,525 666,922 |
||||||||||
Ronald E. Lund |
2008 2007 |
$ |
490,880 351,040 |
— — |
$ |
344,284 228,214 |
$ |
189,750 — |
$ |
12,118 — |
$ |
1,037,032 579,254 |
||||||||||
- (1)
- Reflects
base salary earned during the fiscal year covered.
- (2)
- Reflects
the compensation expense we recognized in 2008 and 2007 for financial statement reporting purposes under FAS 123R (excluding estimates of
forfeitures) with respect to the stock options granted to the named executive officer. These values have been determined based on the assumptions set forth in Note 12 to our consolidated
financial statements for 2008.
- (3)
- Mr. Gougeon's
"Salary" for 2008 reflects $209,162 of base salary earned while serving as our President and CEO through June 20, 2008 and
$24,511 of payment for accrued vacation. Mr. Gougeon's "Bonus" for 2008 reflects a special bonus of $500,000 paid at the time of his resignation as CEO pursuant to his transition agreement.
Mr. Gougeon's "All Other Compensation" for 2008 includes: $99,169 of retainer payments for consulting services rendered after his resignation as our President and Chief Executive Officer;
$20,000 of fees he received for his services as a director following his resignation as our President and Chief Executive Officer; a retirement gift for $22,288; car allowance of $4,846; company
401(k) matching contribution of $9,200; and health benefits of $1,768.
- (4)
- Mr. Barr's "Bonus" for 2008 reflects 20% of the $1.4 million retention bonus granted in 2006 as part of achieving the 2006 recapitalization milestone. Mr. Barr was paid $560,000 in April of 2006, which represented accelerated payments for years 2006 and 2007 in recognition of his contribution to our company during the eight months prior to the grant, and $280,000 in April of 2008. He was also paid $280,000 in April of 2009 and will be paid $280,000 in April of 2010 if he is still employed by us at that time. See "—Annual Cash Payments."
Mr. Barr's "All Other Compensation" for 2008 includes: relocation benefits of $49,622; car allowance of $9,689; company 401(k) matching contribution of $9,200; and life insurance premiums of $2,783. The incremental cost to us of Mr. Barr's relocation benefits is based on the amount billed to our company.
- (5)
- Ms. Makes' "All Other Compensation" for 2008 includes: car allowance of $10,071; air travel incentive (paid under our travel policy to employees who fly coach versus business class on
136
commercial international flights) of $1,250; company 401(k) matching contribution of $9,200; and life insurance premiums of $2,046.
- (6)
- Mr. Lund was engaged as an independent contractor through June 2008, after which he became employed by the Company as General Counsel pursuant to an employment agreement. See "Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table—Mr. Lund's Consulting Agreement and Employment Agreement."
Mr. Lund's "Salary" for 2008 reflects payments of $303,380 for contract services rendered through June 30, 2008 and $187,500 of base salary he earned for the period from July 1, 2008 through December 31, 2008 pursuant to his employment agreement. His salary for 2007 reflects payments for contract services rendered during 2007.
Mr. Lund's "All Other Compensation" for 2008 includes: a company 401(K) matching contribution of $7,048; car allowance of $4,119; and life insurance premiums of $951.
Grants of Plan-Based Awards in 2008
The following table lists grants of plan-based awards made to our named executive officers during 2008.
| |
|
Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) |
|
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
All Other Option Awards: Number of Securities Underlying Options (2)(3) |
|
|
||||||||||||
| |
|
Exercise or Base Price of Option Awards ($/Sh)(3)(4) |
|
|||||||||||||
| |
|
Grant Date Fair Value of Stock and Option Awards(5) |
||||||||||||||
| Name | Grant Date | Target ($) | ||||||||||||||
Franck Gougeon |
N.A. | $ | 219,283 | — | N.A. | N.A. | ||||||||||
John R. Barr |
N.A. |
$ |
209,253 |
— |
N.A. |
N.A. |
||||||||||
Brigid A. Makes |
N.A. |
$ |
136,368 |
— |
N.A. |
N.A. |
||||||||||
Ronald E. Lund |
N.A. |
$ |
187,500 |
N.A. |
N.A. |
|||||||||||
|
12/31/08 | 41,958 | $ | 19.66 | $ | 462,620 | ||||||||||
- (1)
- Reflects the target bonus amount payable under our annual non-equity incentive plan for each of the named executive officers for performance year 2008. The target bonus amount for each of our named executive officers for performance year 2008 was 50% of base salary. For Mr. Gougeon, the base salary used in calculating the target bonus amount was his 2008 annual base salary of $438,566. Mr. Gougeon did not receive a non-equity incentive payment for 2008 because he resigned as our President and Chief Executive Officer in June 2008. See "—Annual Cash Incentive Payments." For Mr. Barr and Ms. Makes, the base salary used in calculating the target bonus amount was his or her respective base salary paid in 2008, minus a portion of the retroactive salary adjustment that was paid in 2008 that related to a period in 2007. For Mr. Lund, the base salary used in calculating the target bonus amount was his 2008 annual base salary of $375,000.
137
- (2)
- Pursuant
to his employment agreement with the Company, Mr. Lund was granted 300,000 options on December 31, 2008. These options vest in
three equal annual increments over a three-year period beginning on July 1, 2009.
- (3)
- Amounts
give effect to the 7.15 for 1.00 reverse stock split to occur immediately prior to completion of this offering.
- (4)
- No
market for our common stock existed when options were granted in 2008. The exercise price of these options was the fair market value of our common stock
on the date of grant as determined by our board of directors.
- (5)
- The grant date fair values of the option awards shown in this column were calculated under FAS 123R (excluding estimates of forfeitures). See Note 7 to our consolidated financial statements included elsewhere in this prospectus for a description of the assumptions made in the valuation of our options under FAS 123R.
Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table
Employment Agreements
We have employment agreements with all of our named executive officers, and, other than with respect to Mr. Lund, the terms of employment are specified in offer letters extended to our named executive officers prior to their commencement of employment. Mr. Lund entered into a consulting agreement with us during 2007, which set forth the terms of his engagement as an independent contractor in the role of General Counsel. In July 2008, we terminated his consulting agreement and entered into an employment agreement with him.
Mr. Gougeon's Employment Agreement
We had an employment agreement with Mr. Gougeon, entered into on July 28, 2005, which was amended and restated by a four-year employment agreement dated as of April 21, 2008. The employment agreement provided that Mr. Gougeon would receive a base salary of at least $350,000 per year, payable in accordance with our payroll practices for our executives. Mr. Gougeon's base salary could not be decreased without Mr. Gougeon's consent. Mr. Gougeon was eligible for a cash bonus for each calendar year during the term of his agreement, in an amount determined by our compensation committee based upon goals and criteria determined by the compensation committee. Mr. Gougeon was also entitled to participate in any and all employee benefit plans, policies or perquisites from time to time in effect for our executives. We were required to reimburse Mr. Gougeon for all reasonable and necessary business expenses incurred or paid in the performance of his duties, subject to any maximum annual limit or other restrictions on such expenses set by our board of directors. The employment agreement was terminated on June 20, 2008, upon Mr. Gougeon's resignation as our President and Chief Executive Officer.
Mr. Barr's Employment Agreement
We have an employment agreement with Mr. Barr, entered into on September 19, 2005, with an initial term of three years but automatically renewable for successive one-year periods, unless terminated by either party upon 30 days' prior written notice. The employment agreement provides that Mr. Barr will receive a base salary of at least $380,000 per year, payable in accordance with our payroll practices for our executives. Mr. Barr's base salary may not be decreased without Mr. Barr's consent. So long as Mr. Barr is employed by us, he is entitled to a cash bonus for each calendar year during the term of the agreement, payable no later than March 15th following the close of such calendar year, in an amount determined by our compensation committee.
138
His employment agreement provided that he would receive, when an equity incentive plan was available, an equity grant equal to approximately 1.5% of our then outstanding shares, which resulted in a grant of options to purchase 619,980 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering) in 2006 upon the adoption of our 2006 Equity Incentive Plan. His employment agreement also provides that his options would vest 20% each year over a five-year period beginning on September 19, 2005, the date of commencement of his employment with us.
Mr. Barr is also entitled to participate in any and all employee benefit plans, policies or perquisites from time to time in effect for our executives. We are required to reimburse Mr. Barr for all reasonable and necessary business expenses incurred or paid in the performance of his duties, subject to any maximum annual limit or other restrictions on such expenses set by our board. Mr. Barr is entitled to receive an automobile allowance at the rate of $750 per month, payable as additional ordinary income, as well as reimbursement for his automobile insurance premiums incurred on his primary automobile used for business purposes, including any applicable taxes incurred as a result of such payments. We were also required to reimburse Mr. Barr for reasonable relocation expenses and temporary living costs incurred by him in connection with transferring his residence to the state of Minnesota, up to a maximum of $130,000.
Ms. Makes' Employment Agreement
We have an employment agreement with Ms. Makes, entered into on October 2, 2006, with an initial term of three years but automatically renewable for successive one-year periods, unless terminated by either party upon 30 days' prior written notice. The employment agreement provides that Ms. Makes will receive a base salary of at least $260,000 per year, payable in accordance with our payroll practices for our executives. Ms. Makes' base salary may not be decreased without Ms. Makes' consent. So long as Ms. Makes is employed by us, she is entitled to a cash bonus for each calendar year during the term of the agreement, payable no later than March 15th following the close of such calendar year, in an amount determined by our compensation committee.
At the time of Ms. Makes' hiring in October 2006, her employment agreement provided that she would receive an equity grant equal to approximately 0.75% of our then outstanding shares, which resulted in a grant of options under our 2006 Equity Incentive Plan to purchase 307,692 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering). Her employment agreement provided that her options would vest 20% each year over a five year period beginning on the date of commencement of her employment with us.
Ms. Makes is also entitled to participate in any and all employee benefit plans, policies or perquisites from time to time in effect for our executives. We are required to reimburse Ms. Makes for all reasonable and necessary business expenses incurred or paid in the performance of her duties, subject to any maximum annual limit or other restrictions on such expenses set by our board. Ms. Makes is entitled to receive an automobile allowance at the rate of $750 per month, payable as additional ordinary income, as well as reimbursement for her automobile insurance premiums incurred on her primary automobile used for business purposes, including any applicable taxes incurred as a result of such payments. We were also required to pay for or reimburse Ms. Makes for reasonable relocation expenses and temporary living costs incurred by her in connection with transferring her residence to the state of Minnesota, up to a maximum of $100,000.
Mr. Lund's Consulting Agreement and Employment Agreement
We had a three-year consulting agreement with Mr. Lund, entered into on June 1, 2007. The consulting agreement provided that we would pay Mr. Lund $400 per hour worked with a maximum compensation of $3,200 per day and $16,000 per week. The agreement also assured Mr. Lund at least
139
24 hours of pay per week. Mr. Lund was engaged as an independent contractor and as such did not participate in the non-equity incentive plan and was not eligible for any additional compensation or benefits offered by us to our employees. His independent contractor agreement also entitled him to receive an equity grant of options to purchase 83,916 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering). Mr. Lund's equity grant vests approximately 33% each year over the term of his three-year consulting agreement. We were required to reimburse Mr. Lund for all reasonable and necessary business expenses incurred or paid in the performance of his services, subject to any maximum annual limit or other restrictions on such expenses set by our board of directors.
On July 1, 2008, we entered into a three-year employment agreement with Mr. Lund pursuant to which he continues to serve as our General Counsel. This agreement is automatically renewable for successive one-year periods, unless terminated by either party upon 30 days' prior written notice. It provides that Mr. Lund will receive an initial base salary of $375,000 per year payable in accordance with our payroll practices for our executives. So long as Mr. Lund is employed by us, he is entitled to receive a cash bonus for each calendar year during the term of the agreement, payable no later than March 15th following the close of such calendar year, in an amount to be determined by our compensation committee. His agreement also entitled Mr. Lund to receive 41,958 additional stock options (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering) under our 2006 Equity Incentive Plan, which options vest approximately 33% each year over the three-year term of his employment agreement. As clarified by the reformation agreement, effective as of July 1, 2008, and filed as an exhibit to the registration statement of which this prospectus forms a part, such options were granted on December 31, 2008 and have an exercise price of $2.75 (without giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering), which is what our board of directors determined to be the fair value of our common stock on that date.
As described more fully in "Potential Payments to Named Executive Officers Upon Termination of Employment or Change in Control," each of our named executive officers is entitled to receive certain benefits upon a qualifying termination of his or her employment agreement.
2006 Equity Incentive Plan
The 2006 Equity Incentive Plan authorizes the compensation committee of the board of directors to grant stock options to directors, officers and key employees. The plan authorizes grants of options to purchase up to 4,895,104 shares of Class B common stock (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering). All outstanding shares of Class B common stock will be converted into shares of common stock upon completion of this offering. Although the plan allows for the granting of stock options, restricted stock units and stock appreciation rights as well as other awards, only stock options have been granted under the plan to date.
Options granted under the plan typically vest over a five-year period and are generally exercisable for ten years after the date of the grant. The exercise price of the options granted under the plan will not be less than 100% of the fair market value on the date of the grant. Upon termination of a participant's employment, the options granted under the plan are terminated, except that, in the case of termination other than for cause, they will remain exercisable for a certain period of time if they were vested at the time of termination.
Upon the occurrence of a "covered transaction" (as defined therein), which includes a change in control, each outstanding stock option and stock appreciation right will become fully exercisable, and the delivery of shares of stock under each outstanding award of stock units will be accelerated and such shares delivered prior to the covered transaction.
140
2008 Equity Incentive Plan
Our board of directors and stockholders will adopt the 2008 AGA Medical Holdings, Inc. Equity Incentive Plan, or the 2008 Plan before the effective date of this offering. The following description of the 2008 Plan is not complete and is qualified by reference to the full text of the stock incentive plan, which has been filed as an exhibit to the registration statement of which this prospectus forms a part. The 2008 Plan is designed to continue to give our company maximum flexibility in recruiting and retaining key employees and other service providers, and in motivating such professionals to exert their best efforts on behalf of our company.
The 2008 Plan permits the grant of the equity based compensation described below to our employees, directors or consultants or those of our affiliates. A maximum of approximately 1,951,315 shares of common stock (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering) may be subject to awards under the stock incentive plan. The maximum number of shares of common stock for which incentive stock options may be granted will be 839,160 (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering). Shares of common stock covered by awards that expire, terminate or lapse will again be available for grant under the stock incentive plan.
Administration. The 2008 Plan will be administered by our compensation committee, which may delegate its duties and powers to a subcommittee thereof or to our Chief Executive Officer. Our compensation committee has the sole discretion to determine the employees, directors and consultants to whom awards may be granted under the 2008 Plan and the manner in which such awards will vest.
Options. Our compensation committee will determine the exercise price for each option, provided that an option must have an exercise price that is at least equal to the fair market value of a share of common stock on the date the option is granted. Options granted under the plan are exercisable by the option holder at such time and upon such terms and conditions as determined by our compensation committee; however, in no event will an option be exercisable more than ten years after the date it is granted. All options granted under the plan are intended to be nonqualified stock options, unless the applicable award agreement expressly states that the option is intended to be an incentive stock option within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended (the "Code").
Stock Appreciation Rights. Our compensation committee may grant stock appreciation rights, or SARs, independent of or in connection with an option. The exercise price per share of a stock appreciation right will be an amount determined by our compensation committee, which generally will be the fair market value of a share on the date the stock appreciation right is granted; provided that if the stock appreciation right is granted in conjunction with an option, the exercise price of the SAR may not be less than the option price of the related option. Each SAR will entitle a participant upon exercise to an amount equal to the product of (1) the excess of (A) the fair market value on the exercise date of one share of common stock over (B) the exercise price per share, times (2) the number of shares of common stock covered by the stock appreciation right. Payment may be made in shares of common stock and/or in cash. Our compensation committee may impose, in its discretion, conditions upon the exercisability of SARs, but in no event will a SAR be exercisable more than ten years after the date it is granted.
Restricted Stock Units and Other Stock-Based Awards. Our compensation committee may grant awards of restricted stock units and other stock-based awards that are valued in whole or in part by reference to, or are otherwise based on the fair market value of shares of our common stock. Our compensation committee will determine all of the terms and conditions for such restricted stock unit awards and other stock based awards.
Performance-Based Awards. During any period when Section 162(m) of the Code is applicable to us and the stock incentive plan, certain other stock-based awards may be granted in a manner designed
141
to make them deductible by us under Section 162(m) of the Code ("Performance-Based Awards"). Such Performance-Based Awards will be determined based on the attainment of written objective performance goals approved by our compensation committee for a performance period established by our compensation committee. The performance goals will be based upon one or more of the performance criteria set forth in the 2008 Plan. The maximum amount of a Performance-Based Award denominated in shares and payable to any one participant under the 2008 Plan for a performance period is 167,832 shares of common stock (after giving effect to the reverse stock split that will occur immediately prior to completion of this offering); or, in the event the Performance-Based Award is paid in cash, the equivalent cash value thereof on the last day of the performance period to which such Performance-Based Award relates. With respect to a Performance-Based Award not denominated in shares, the maximum amount payable to any one participant under the plan is $3,000,000. Our compensation committee will determine whether, with respect to a performance period, the applicable performance goals have been met with respect to a given participant and, if they have, will so certify and ascertain the amount of the applicable performance-based award. The amount of the performance-based award actually paid to a participant may be reduced at the discretion of our compensation committee.
Change in Control. In the event of a change in control after the effective date of the 2008 Plan, our compensation committee will do one or more of the following: (A) accelerate the vesting of, or waive any restrictions with respect to, any outstanding award then held by a participant which is unexercisable or subject to lapse restrictions; (B) provide for the issuance of substitute awards that will substantially preserve the otherwise applicable terms of any affected award previously granted hereunder as determined by our compensation committee in its sole discretion, which (1) in the case of an option or stock appreciation right, will have an aggregate spread value (as defined below) that is identical to the aggregate spread value of the affected option or stock appreciation right previously granted hereunder as of the date of the change in control or (2) in the case of other stock-based awards, will have an equivalent fair market value to the affected other stock-based awards, which, in either case of clause (1) or (2), will either be fully vested or continue to vest on the same schedule as the affected award previously granted hereunder, and that have other terms as determined by our compensation committee in its sole discretion; and/or (C) if our company is the surviving entity in any change in control, continue to administer the plan and have any existing award remain outstanding in accordance with its terms following the change in control. In addition, in the event of a change in control after the effective date, our compensation committee may, in its sole discretion, cancel any portion of an award outstanding as of such change in control in exchange for the payment to the participant for fair value (as determined in the sole discretion of our compensation committee) which, in the case of an option or stock appreciation right, equals the excess, if any, of the fair market value as of the date of such change in control of the shares subject to the vested portion of the option or stock appreciation right over the aggregate exercise price (the "spread value").
142
Outstanding Equity Awards as of December 31, 2008
The following table lists the outstanding equity awards held by our named executive officers as of December 31, 2008 (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering).
| Option Awards | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name
|
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||
Franck Gougeon(1) |
2,097 | 4,195 | — | $ | 19.66 | 6/20/2017 | ||||||||||
John R. Barr(2) |
371,988 | 247,992 | — | $ | 7.15 | 4/27/2016 | ||||||||||
Brigid A. Makes(3) |
123,076 | 184,615 | — | $ | 7.15 | 10/1/2016 | ||||||||||
Ronald E. Lund(4) |
27,972 — |
55,944 41,958 |
— |
$ $ |
14.30 19.66 |
6/1/2017 7/1/2018 |
||||||||||
- (1)
- Mr. Gougeon's
option grant vests over a three-year period starting on June 20, 2008 at a rate of 33.3% per year. His grant will be fully
vested on June 20, 2010.
- (2)
- Mr. Barr's
option grant vests over a five-year period starting with September 19, 2005, the date of his hire, at a rate of 20% per year. His
grant will be fully vested as of September 19, 2010.
- (3)
- Ms. Makes'
option grant vests over a five-year period starting with October 2, 2006, the date of her hire, at a rate of 20% per
year. Her grant will be fully vested as of October 2, 2011.
- (4)
- Mr. Lund's initial option grant of 83,916 vests over a three-year period starting June 1, 2007, the date of his initial engagement with our company as an independent contractor, at a rate of one-third (33.3%) per year. His initial option grant will be fully vested as of June 1, 2010. On December 31, 2008, Mr. Lund was granted 41,958 additional options which vest over a 3 year period at a rate of 33.3% per year. These options will be fully vested on July 1, 2011.
Option Exercises and Stock Vested in 2008
None of our named executive officers had options that were exercised or restricted stock that vested during 2008.
We do not offer pension benefits to our named executive officers.
Non-Qualified Deferred Compensation for 2008
We do not offer non-qualified deferred compensation to our named executive officers.
143
Potential Payments Upon Termination or Change in Control
The following table describes the potential payments and benefits to which our named executive officers would be entitled upon termination of employment or a change in control of our company. All calculations are based on an assumed termination or change in control date of December 31, 2008.
The table below does not include payments and benefits to the extent they are provided generally to all salaried employees upon termination of employment and do not discriminate in scope, terms or operation in favor of the named executive officers.
Termination as of December 31, 2008
| |
Cash Severance Payments(1) |
Healthcare Premiums(2) |
Acceleration of Equity Awards |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
John R. Barr |
||||||||||||||
Involuntary termination without cause |
$ | 883,953 | $ | — | $ | — | $ | 883,953 | ||||||
Disability |
$ | 465,324 | $ | 22,768 | $ | — | $ | 488,091 | ||||||
Involuntary termination without cause upon a change in control |
$ | 883,953 | $ | — | $ | 3,103,006 | $ | 3,986,959 | ||||||
Involuntary termination for cause or voluntary termination |
$ | 46,693 | — | — | $ | 46,693 | ||||||||
Death |
$ | 1,506,009 | — | — | $ | 1,506,009 | ||||||||
Brigid A. Makes |
||||||||||||||
Involuntary termination without cause |
$ | 439,615 | $ | — | $ | — | $ | 439,615 | ||||||
Disability |
$ | 303,219 | $ | 6,753 | $ | — | $ | 309,972 | ||||||
Involuntary termination without cause upon a change in control |
$ | 439,615 | $ | — | $ | 2,310,000 | $ | 2,749,615 | ||||||
Involuntary termination for cause or voluntary termination |
$ | 30,427 | — | — | $ | 30,427 | ||||||||
Death |
$ | 1,416,823 | — | — | $ | 1,416,823 | ||||||||
Ronald E. Lund |
||||||||||||||
Involuntary termination without cause |
$ | 597,115 | $ | — | $ | — | $ | 597,115 | ||||||
Disability |
$ | 409,615 | $ | — | $ | — | $ | 409,615 | ||||||
Involuntary termination without cause upon a change in control |
$ | 597,115 | $ | — | $ | 300,000 | $ | 897,115 | ||||||
Involuntary termination for cause or voluntary termination |
$ | 34,615 | — | — | $ | 34,615 | ||||||||
Death |
$ | 222,115 | — | — | $ | 222,115 | ||||||||
- (1)
- Cash
severance payments include, in all instances, a payment equal to the balance of earned but unused vacation days as of the termination date. For
Mr. Barr and Ms. Makes, cash severance payments in the event of termination due to death also include a payment under a life insurance policy carried on behalf of the executive for the
benefit of his or her beneficiaries and executive's estate. In addition, cash severance payments in the event of involuntary termination without cause, termination due to disability and death include
a bonus payment for 2008 at 50% of base salary.
- (2)
- Mr. Lund would not be entitled to receive any health insurance benefits in the event of termination due to disability because he was not a participant in company provided healthcare benefit plans as of December 31, 2008.
On June 20, 2008, Mr. Gougeon resigned as our President and Chief Executive Officer. On that date, we terminated his employment agreement and entered into a transition agreement with Mr. Gougeon pursuant to which we paid to him a special bonus of $500,000. See "—Director
144
Compensation." As such, the Company would not have any severance obligations to Mr. Gougeon as of December 31, 2008.
Summary of Key Agreements
As described above, in 2008, each of our named executive officers (other than Mr. Gougeon) had an employment agreement with us. Mr. Lund had a consulting agreement with us through June, 2008. In July 2008, Mr. Lund entered into an employment agreement with us.
The following is a summary of severance benefits that would be provided to Mr. Barr, Ms. Makes and Mr. Lund in the event of termination of employment or change of control pursuant to the terms of the named executive officer's employment agreement.
Mr. Barr's Employment Agreement
Mr. Barr's employment agreement provides for the following payments and benefits upon certain termination events or change of control.
If we terminate Mr. Barr without "cause," we will pay Mr. Barr:
- •
- his base salary at the rate in effect on the termination date for another 18 months; and
- •
- a one-time lump sum payment equal to the balance of earned but unused vacation days as of the termination date.
In addition, if we terminate Mr. Barr without "cause," Mr. Barr's stock options that were vested at the time of termination will remain exercisable for a period of 90 days following termination after which time such stock options will be forfeited.
In the event of termination due to a disability, he will receive six months of base salary and a one-time lump sum payment equal to the balance of earned but unused vacation days as of the termination date, in addition to 18 months of continued company-paid health insurance benefits.
Upon a change of control, Mr. Barr's stock options will automatically vest, provided that he is still employed by us at the time of the change of control.
If Mr. Barr is terminated for cause, he will not receive any severance payments or benefits other than accrued vacation, and all vested and unvested options will be forfeited without further action. If Mr. Barr's employment is voluntarily terminated by him, he will not receive any severance payments or benefits other than accrued vacation.
In the event of termination due to death, we will pay Mr. Barr's designated beneficiary or his estate an amount equal to the balance of his earned but unused vacation days as of the termination date and any life insurance carried on behalf of Mr. Barr for the benefit of his beneficiaries and estate.
We also have an understanding that if we terminate Mr. Barr without "cause" or his employment is terminated due to a disability or death on the last day of the fiscal year, we would pay him a bonus for that calendar year in which the termination occurs.
Ms. Makes' Employment Agreement
Ms. Makes' employment agreement provides for the following payments and benefits upon certain termination events or change of control.
If we terminate Ms. Makes without "cause," we will pay Ms. Makes:
- •
- her base salary at the rate in effect on the termination date for another 12 months;
145
- •
- a one-time lump sum payment equal to the balance of earned but unused vacation days as of the termination
date; and
- •
- a bonus for the calendar year in which the termination date occurs on a pro-rata basis through the termination date to be determined in the sole discretion of the board; provided, however, that this bonus will not be less than 37.5% nor more than 50% of Ms. Makes' base salary through the termination date.
In addition, if we terminate Ms. Makes without "cause," Ms. Makes' stock options that were vested at the time of termination will remain exercisable for a period of 90 days following termination after which time such stock options will be forfeited.
In the event of termination due to a disability, she will receive six months of base salary and a one-time lump sum payment equal to the balance of earned but unused vacation days as of the termination date, in addition to 18 months of continued company-paid health insurance benefits.
Upon a change of control, Ms. Makes' stock options will automatically vest, provided that she is still employed by us at the time of the change of control.
If Ms. Makes is terminated for cause, she will not receive any severance payments or benefits other than accrued vacation, and all vested and unvested options will be forfeited without further action. If Ms. Makes' employment is voluntarily terminated by her, she will not receive any severance payments or benefits other than accrued vacation.
In the event of termination due to death, we will pay Ms. Makes's designated beneficiary or her estate an amount equal to the balance of her earned but unused vacation days as of the termination date and any life insurance carried on behalf of Ms. Makes for the benefit of her beneficiaries and estate.
We also have an understanding that if Ms. Makes' employment is terminated due to a disability or death on the last day of the fiscal year, we would pay her a bonus for that calendar year in which the termination occurs.
Mr. Lund's Employment Agreement
Mr. Lund's employment agreement provides for the following payments and benefits upon certain termination events or change of control.
If we terminate Mr. Lund without "cause" we will pay Mr. Lund:
- •
- his base salary at the rate in effect on the termination date for another 12 months; and
- •
- a one-time lump sum payment equal to the balance of earned but unused vacation days as of the termination date.
In addition, if we terminate Mr. Lund without "cause," Mr. Lund's stock options that are vested at the time of termination will remain exercisable for a period of 90 days following termination after which time such options will be forfeited.
In the event of termination due to a disability, he will receive six months of base salary and a one-time lump sum payment equal to the balance of earned but unused vacation days as of the termination date in addition to 18 months of continued company-paid health insurance benefits.
Upon a change of control, Mr. Lund's stock options will automatically vest; provided that he is still employed by us at the time of the change of control.
If Mr. Mr. Lund is terminated for cause, he will not receive any severance payments or benefits other than accrued vacation, and all vested and unvested options will be forfeited without further
146
action. If Mr. Lund's employment is voluntarily terminated by him, he will not receive any severance payments or benefits other than accrued vacation.
In the event of termination due to death, we will pay Mr. Lund's designated beneficiary or his estate an amount equal to the balance of his earned but unused vacation days as of the termination date and any life insurance carried on behalf of Mr. Lund for the benefit of his beneficiaries and estate.
We also have an understanding that if we terminate Mr. Lund without "cause" or his employment is terminated due to a disability or death on the last day of the fiscal year, we would pay him a bonus for that calendar year in which the termination occurs.
147
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
This section contains a summary of the material terms of all described agreements. You should read the agreements in their entirety, which have been filed with the SEC as exhibits to the registration statement of which this prospectus is a part.
2005 and 2009 Securities Purchase Agreements
On July 28, 2005, AGA Medical entered into a securities purchase agreement with WCAS Capital Partners IV, L.P., or WCAS CP IV. Pursuant to this agreement, AGA Medical sold to WCAS CP IV a $50.0 million aggregate principal amount of 10% senior subordinated PIK notes due 2012, or the 2005 notes at a $43.5 million purchase price. The 2005 notes pay interest semiannually, and the effective interest rate of the 2005 notes at June 30, 2009 was 12.6%. If (1) for certain reasons described in the securities purchase agreement we become prohibited from paying all or any portion of the accrued interest at a given interest payment date or (2) holders representing a majority of the aggregate principal amount outstanding at the time agree to defer all or a portion of the accrued interest, then such amount of accrued interest will be multiplied by 1.2 and the interest will be added to the principal amount of the notes on the interest payment date. The 2005 notes are unsecured obligations of AGA Medical and are subordinated in right of payment to our senior secured credit facility. As part of the securities purchase agreement, AGA Medical issued 6,524 shares of Series A preferred stock valued at $6.5 million to the WCAS Stockholders. As a result, the discounted issue value of the 2005 notes was $43.5 million. The $6.5 million of discount from the face value is being accreted on our balance sheet to the 2005 note repayment amount utilizing the effective interest rate and has been recognized as interest expense. The original issue discount has been recognized as interest expense of $0.9 million, $0.9 million, $0.9 million and $0.5 million for the years ended December 31, 2006, 2007, 2008 and the six months ended June 30, 2009, respectively. In compliance with the terms of the securities purchase agreement governing the 2005 notes, we will use a portion of our net proceeds from this offering to prepay the principal amount of 2005 notes at a price equal to 100% of their face principal amount plus accrued and unpaid interest.
On January 5, 2009, in order to finance, in part, the acquisition of the assets of our former Italian distributor, AGA Medical entered into a securities purchase agreement with WCAS CP IV, pursuant to which AGA Medical sold to WCAS CP IV an additional $15.0 million aggregate principal amount of 10% senior subordinated PIK notes due 2012, or the 2009 notes, at a $13.1 million purchase price. The 2009 notes pay interest semiannually and the effective interest rate of the 2009 notes at June 30, 2009 was 15.2%. If (1) for certain reasons described in the securities purchase agreement we become prohibited from paying all or any portion of the accrued interest at a given interest payment date or (2) holders representing a majority of the aggregate principal amount outstanding at the time agree to defer all or a portion of the accrued interest, then such amount of accrued interest will be multiplied by 1.2 and the interest will be added to the principal amount of the notes on the interest payment date. The 2009 notes are unsecured obligations of AGA Medical, fully and unconditionally guaranteed by Amplatzer Medical Sales Corporation and are subordinated in right of payment to our senior secured credit facility. As part of the transaction, we also issued 1,879 shares of Series B preferred stock valued at $1.9 million to the stockholder. As a result, the discounted issue value of the 2009 note was $13.1 million. The $1.9 million of discount from the face value is being accreted on our balance sheet to the 2009 note repayment amount utilizing the effective interest rate and has been recognized as interest expense. The original issue discount has been recognized as interest expense of $0.2 million for the six months ended June 30, 2009. In compliance with the terms of the securities purchase agreement governing the 2009 notes, we will use a portion of our net proceeds from this offering to prepay these notes at a price equal to 100% of their face principal amount plus accrued and unpaid interest.
As of June 30, 2009, the accreted value of our outstanding 2005 notes and 2009 notes on our balance sheet was $60.4 million.
148
Second Amended and Restated Stockholders Agreement
On October 2, 2009, we entered into an amended and restated stockholders agreement with the WCAS Stockholders and the Gougeon Stockholders. The stockholders agreement, upon completion of this offering:
- •
- imposes restrictions on transfer of our shares by the WCAS Stockholders and the Gougeon Stockholders;
- •
- provides the WCAS Stockholders and the Gougeon Stockholders with tag-along rights with respect to certain
transfers of our shares held by the other stockholder;
- •
- allows the parties thereto to each designate one nominee to our board of directors per each 7% they own of our common
stock up to a maximum of three directors each; following completion of this offering, three of our directors (Daniel A. Pelak, Paul B. Queally and Sean M. Traynor) will hold their
board positions as WCAS Stockholders designees and three of our directors (Franck L. Gougeon, Jack P. Helms and Darrell J. Tamosuinas) will hold their board positions as Gougeon
Stockholders designees;
- •
- provides that the parties thereto will agree to cause their board designees, to the extent permitted by law, to create and
maintain a compensation committee and, until Mr. Gougeon no longer serves as a directors of our board, a corporate development committee, each such committee consisting of three directors, one
of whom must be a WCAS Stockholder designee and one of whom must be a Gougeon Stockholder designee;
- •
- allows the WCAS Stockholders and the Gougeon Stockholders to appoint, subject to certain conditions, a board observer if
they own, in each case, at least five percent 5% (but less than 7%) of our common stock;
- •
- provides that if we propose to enter into a transaction that would result in the sale of all of our capital stock or
substantially all of our assets to one or more persons not affiliated with the WCAS Stockholders, and the WCAS Stockholders have voted in favor of such transaction, then, subject to certain
conditions, the Gougeon Stockholders also will be required to vote in favor of such transaction and, if applicable, to sell their shares of our capital stock; and
- •
- grants the WCAS Stockholders and the Gougeon Stockholders, subject to certain conditions, certain information rights if they own at least 5% of our common stock.
The amended and restated stockholders agreement will remain in effect following this offering. The agreement will terminate with respect to the WCAS Stockholders when they collectively hold less than 5% of the voting power of the outstanding shares our capital stock, with respect to the Gougeon Stockholders when they collectively hold less than 5% of the voting power of the outstanding shares of our capital stock and with respect to all stockholders party thereto upon any sale of all then issued and outstanding equity securities in exchange for equity securities of a person, as defined therein, registered under Section 12 of the Exchange Act.
Amended and Restated Registration Rights Agreement
On April 21, 2008, we entered into an amended and restated registration rights agreement with the WCAS Stockholders and the Gougeon Stockholders, which was subsequently amended on January 5, 2009. Pursuant to that agreement, the WCAS Stockholders and the Gougeon Stockholders are, in each case, entitled to registration rights. Following this offering, the WCAS Stockholders and the Gougeon Stockholders may require us to effect the registration of any or all of the shares of common stock held by the WCAS Stockholders or the Gougeon Stockholders, as the case may be. Such requirement is called a demand registration. Pursuant to the amended and restated registration rights agreement, we are required to pay all registration expenses in connection with a demand registration, unless subsequently withdrawn by the initiating stockholder. In addition, if we propose to register any of our
149
common stock or any other equity securities under the Securities Act, whether or not for our own account, the WCAS Stockholders and the Gougeon Stockholders are entitled to notice of registration and are entitled to include their shares of common stock in such registration with all registration expenses paid by us. Notwithstanding the foregoing, we will not be obligated to effect a demand registration prior to 180 days after the effective date of this offering.
Amended and Restated Stock Purchase Agreement
As part of our July 2005 reorganization, AGA Medical entered into an Amended and Restated Stock Purchase Agreement dated as of July 28, 2005, as amended on April 28, 2006 and June 20, 2008 with certain of the WCAS Stockholders and Franck L. Gougeon whereby 122,000 shares of Series A preferred stock were issued to such WCAS Stockholders at a purchase price of $1,000 per share. In accordance with the agreement, we agreed to indemnify the WCAS Stockholders for any breach or violation of the agreement by us or Mr. Gougeon.
Employment Agreements
We have employment agreements with certain of our named executive officers, the material terms of which are described in "Management—Executive Compensation."
Consulting Agreements
On June 20, 2008, we entered into a consulting agreement with Franck L. Gougeon, pursuant to which we engaged him as a consultant to (1) represent us with healthcare professionals and at medical industry conferences, (2) advise our Chief Executive Officer and (3) perform such other services as we may reasonably request from time to time. Mr. Gougeon will perform his services as a consultant up to a maximum of 30 days per any 12-month period and any additional day in excess of these 30 days to which Mr. Gougeon agrees. We pay Mr. Gougeon a retainer of $170,000 for each maximum of 30 days per any 12-month period commencing on the date of the agreement. If Mr. Gougeon serves in excess of 30 days during any 12-month period, we are required to compensate him at an hourly rate of $400. The agreement will terminate upon the earlier to occur of (1) five years or (2) the date upon which the Gougeon Stockholders beneficially owns less than 10% of all of the issued and outstanding shares of the fully diluted shares of our common stock.
We have also entered into an independent contractor agreement with Tommy G. Thompson. See "Management—Director Compensation."
Agreements with Dr. Kurt Amplatz and Mr. Curtis Amplatz
On December 23, 2005, we entered into a five-year research agreement with Dr. Amplatz. In accordance with this agreement, we pay Dr. Amplatz (1) $18,333.33 per month for the research, animal studies and related development services in the fields of our industry, and (2) a fixed percentage of royalties on products of ours that incorporate current and future patented technology developed by which Dr. Amplatz and assigned to us under the agreement. This agreement obligates us to pay royalties on specific product sales and are payable throughout the life of the patents. In 2006, 2007, 2008 and in the six months ended June 30, 2009, we made royalty payments to Dr. Amplatz under this agreements in amounts of $2.4 million, $2.9 million, $3.4 million and $1.9 million, respectively.
On April 22, 1996 and November 22, 2001, we entered into royalty-payment agreements with Mr. Curtis Amplatz, Dr. Amplatz's son, under which we pay Mr. Curtis Amplatz a fixed percentage of royalties throughout the life of certain of our patents for which Mr. Curtis Amplatz is a named inventor. These agreements also obligate us to pay royalties on specific product sales and are payable throughout the life of the patents, which currently expire no earlier than 2017. In 2006, 2007, 2008 and in the six months ended June 30, 2009, we made royalty payments earned by Mr. Curtis Amplatz under
150
these agreements in amounts of $2.5 million, $3.0 million, $3.2 million and $1.8 million, respectively. In 2008, Mr. Curtis Amplatz inquired regarding the scope of the royalty agreements we had with him and whether they applied to additional products of ours. In response, we had discussions in which we clarified the scope of the agreements and our payments under the agreements.
Dr. Amplatz served as a director of our company from August 2005 to April 2006 but received no compensation for serving in such capacity during that period.
Loans to Stockholders
In 2003, 2004 and 2005, we entered into loan agreements with Mr. Gougeon and Michael Afremov, both then beneficial owners of more than 5% of AGA Medical's capital stock, in the aggregate amount of $3.5 million and $16.5 million, respectively. Mr. Gougeon's loan bore interest at rates ranging from 4.6% to 5.0%, and he paid us a total of $196,234 in interest in 2005. Mr. Afremov's loan bore interest at rates ranging from 4.6% to 5.1%, and he paid us a total of $439,474 in interest in 2005. These loans were fully repaid, together with accrued and unpaid interest, by way of offset against amounts due to the stockholders from us in connection with our July 2005 reorganization. As discussed below, in connection with our July 2005 reorganization, AGA Medical purchased and redeemed all of AGA Medical's outstanding shares of common stock owned by Mr. Afremov, and AGA Medical paid dividends to both Mr. Afremov and Mr. Gougeon. These loans were made at the request of such then controlling stockholders.
Dividends
In April 2008, we paid a total amount of $2.5 million in dividends to our stockholders—the WCAS Stockholders and the Gougeon Stockholders. Our board of directors approved such dividend after our stockholders requested the payment of such dividend in connection with the structuring of transactions among our stockholders at such time. In connection with our April 2006 recapitalization, we paid a total amount of $99.9 million in dividends to our stockholders, which consisted of: (1) accrued dividends of $11.0 million paid to our Series A preferred and Class A common stockholders and (2) a $0.30 per share dividend to our Series A preferred, Class A common and other common stockholders. Previously, at the time of our July 2005 reorganization, we paid a dividend of $10.0 million to each of Mr. Afremov and Mr. Gougeon, and following our July 2005 reorganization, we paid Mr. Gougeon a dividend of $15.0 million. For information on our April 2006 recapitalization and July 2005 reorganization, see "Management's Discussion and Analysis of Financial Condition and Results of Operations—Overview."
Redemption
As part of our July 2005 reorganization, AGA Medical purchased and redeemed all of the outstanding shares of common stock owned by Mr. Afremov, which represented at that time 50.0% of AGA Medical's outstanding shares of common stock, for a total aggregate amount of $280.0 million, which was partially offset by amounts due to us by Mr. Afremov. To finance our July 2005 reorganization, AGA Medical (1) issued an aggregate principal amount of $50.0 million of 10% senior subordinated notes due 2012, which were purchased by one of the WCAS Stockholders at a discount, (2) issued 128,524 shares of Series A preferred stock to the WCAS Stockholders at a purchase price of $1,000 per share and (3) borrowed $107.0 million under a $122.0 million senior credit facility, consisting of a $107.0 million senior term loan and a $15.0 million revolving credit facility.
In addition, on July 27, 2007, we redeemed 1.9 million shares of Class A common stock (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering) held by Mr. Gougeon at a redemption price of $15.1 million,
151
including $1.7 million of accrued dividends. Such shares represented 4.5% of our total capital stock and voting power, and 8.0% of our outstanding shares of common stock.
Conversion of Shares of Common Stock and Series A and Series B Preferred Stock and Reverse Stock Split
Upon completion of this offering, all currently outstanding shares of our common stock and our Series A and Series B preferred stock will be converted into shares of a single class of common stock. Assuming full payment of accrued and unpaid dividends upon completion of this offering, each outstanding share of our Series A preferred stock will be converted into 1,000 shares of common stock, each outstanding share of our Series B preferred stock will be converted into 363.6364 shares of common stock, and each outstanding share of our designated and undesignated common stock will be converted into one share of common stock. In addition, a 7.15 for 1.00 reverse stock split of our common stock will occur immediately prior to completion of this offering, See "Capitalization."
Review, Approval or Ratification of Transactions with Related Parties
Prior to the completion of this offering, our board of directors will adopt a written statement of policy regarding transactions with related persons, which we refer to as our "related person policy." Our related person policy will require that a "related person" (as defined in Item 404(a) of Regulation S-K) must promptly disclose to our General Counsel or Chief Compliance Officer any "related person transaction" (defined as any transaction that is reportable by us under Item 404(a) of Regulation S-K) and all material facts with respect thereto. The General Counsel or Chief Compliance Officer will then promptly communicate that information to our audit committee. In reviewing a transaction, our audit committee will consider all relevant facts and circumstances, including (1) the commercial reasonableness of the terms, (2) the benefit and perceived benefits, or lack thereof, to us, (3) opportunity costs of alternate transactions, and (4) the materiality and character of the related person's interest, and the actual or apparent conflict of interest of the related person. Our audit committee will not approve or ratify a related person transaction unless it determines that, upon consideration of all relevant information, the transaction is in, or is not inconsistent with, the best interests of our company and stockholders. No related person transaction will be consummated without the approval or ratification of our audit committee. It will be our policy that directors interested in a related person transaction will recuse themselves from any vote relating to a related person transaction in which they have an interest.
152
PRINCIPAL AND SELLING STOCKHOLDERS
The following tables set forth information, as of the date of this prospectus, regarding the beneficial ownership of our equity securities: (1) immediately prior to the consummation of this offering and after giving effect to (x) the conversion of our Class A and Class B common stock and each share of our Series A preferred stock into 1,000 shares of our common stock and each share of our Series B preferred stock into 363.6364 shares of our common stock to be effected immediately prior to completion of this offering and (y) the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering and (2) as adjusted to reflect the sale of the shares of common stock in this offering, by:
- •
- each person that is a beneficial owner of more than 5% of our outstanding equity securities;
- •
- the selling stockholders;
- •
- each of our named executive officers;
- •
- each of our directors and nominees; and
- •
- all directors and executive officers as a group.
Beneficial ownership is determined under the SEC rules and generally includes voting or investment power over securities. Except in cases where community property laws apply or as indicated in the footnotes to this table, we believe that each stockholder identified in the table possesses sole voting and investment power over all shares of equity securities shown as beneficially owned by the stockholder. Percentage of beneficial ownership is based on 39,555,683 shares of our equity securities outstanding as of the date of this prospectus, 48,055,683 shares of our common stock outstanding after the completion of this offering (assuming the underwriters' option is not exercised) and 50,118,183 shares of our common stock outstanding after the completion of this offering (assuming the underwriters' option is exercised in full). Shares of common stock subject to options that are currently exercisable or exercisable within 60 days of the date of this prospectus are considered outstanding and beneficially owned by the person holding the options for the purposes of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless indicated otherwise, the address of each individual listed in the table is c/o AGA Medical Holdings, Inc., 5050 Nathan Lane North, Plymouth, MN 55442.
| |
Shares Beneficially Owned Prior to the Offering |
Shares Beneficially Owned After the Offering | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Assuming the Underwriters' Option is Not Exercised |
Assuming the Underwriters' Option is Exercised in Full |
|||||||||||||||
Name of Beneficial Owner
|
Number | Percentage | Number | Percentage | Number | Percentage | |||||||||||||
Welsh, Carson, Anderson & Stowe IX, L.P.(1) |
22,421,141 | 56.68 | % | 22,421,141 | 46.66 | % | 22,421,141 | 44.74 | % | ||||||||||
Franck L. Gougeon(2) |
17,136,501 | 43.32 | % | 11,886,501 | 24.73 | % | 11,886,501 | 23.72 | % | ||||||||||
Gougeon Shares, LLC(3)** |
4,694,638 | 11.87 | % | 1,952,703 | 4.06 | % | 1,952,703 | 3.90 | % | ||||||||||
Franck L. Gougeon Revocable Trust Under Agreement Dated June 28, 2006(3)** |
12,437,668 | 31.44 | % | 9,929,603 | 20.66 | % | 9,929,603 | 19.81 | % | ||||||||||
Executive Officers and Directors (not listed above) |
|||||||||||||||||||
John R. Barr |
495,984 | 1.24 | % | 495,984 | 1.03 | % | 495,984 | * | |||||||||||
Brigid A. Makes |
184,615 | * | 184,615 | * | 184,615 | * | |||||||||||||
Ronald E. Lund |
69,930 | * | 69,930 | * | 69,930 | * | |||||||||||||
Tommy G. Thompson |
330,656 | * | 330,656 | * | 330,656 | * | |||||||||||||
Jack P. Helms |
0 | * | 0 | * | 0 | * | |||||||||||||
153
| |
Shares Beneficially Owned Prior to the Offering |
Shares Beneficially Owned After the Offering | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Assuming the Underwriters' Option is Not Exercised |
Assuming the Underwriters' Option is Exercised in Full |
|||||||||||||||
Name of Beneficial Owner
|
Number | Percentage | Number | Percentage | Number | Percentage | |||||||||||||
Daniel A. Pelak |
6,293 | * | 6,293 | * | 6,293 | * | |||||||||||||
Terry Allison Rappuhn |
6,293 | * | 6,293 | * | 6,293 | * | |||||||||||||
Darrell J. Tamosuinas |
6,293 | * | 6,293 | * | 6,293 | * | |||||||||||||
Paul B. Queally(4)(5)(6) |
21,847,472 | 55.23 | % | 21,847,472 | 45.46 | % | 21,847,472 | 43.59 | % | ||||||||||
Sean Traynor(4)(7) |
21,778,618 | 55.06 | % | 21,778,617 | 45.32 | % | 21,778,618 | 43.45 | % | ||||||||||
All executive officers and directors and director nominees as a group (10 persons) |
40,101,243 | 99.48 | % | 34,851,243 | 72.52 | % | 34,851,243 | 69.54 | % | ||||||||||
- *
- Denotes
less than 1% beneficial ownership.
- **
- Selling
stockholders. The shares owned of record by the selling stockholders are beneficially owned by Franck L. Gougeon.
- (1)
- Includes
(A) 20,498,511 shares of common stock held by Welsh Carson over which it has sole voting and investment power, (B) 26,827 shares of
common stock held by WCAS Management Corporation, an affiliate of Welsh Carson, over which WCAS Management Corporation has sole voting and investment power, (C) 1,008,010 shares of common stock
held by WCAS Capital Partners IV, L.P., an affiliate of Welsh Carson, over which it has sole voting and investment power, (D) an aggregate 634,300 shares of common stock over
which individuals named in this table, who are managing members of the sole general partner of Welsh Carson and of WCAS Capital Partners IV, L.P. and/or employed by WCAS Management Corporation,
have voting and investment power, and (E) an aggregate 253,492 shares of common stock held by other co-investors named in this table over which Welsh Carson has sole voting
power. Voting and investment decisions over the shares held by Welsh Carson and WCAS Capital Partners IV, L.P. are made by the managing members of WCAS IX Associates LLC and WCAS CP IV
Associates LLC., their respective general partners. Messrs. Patrick J. Welsh, Russell L. Carson, Bruce K. Anderson, Thomas E. McInerney, Robert A. Minicucci,
Anthony J. De Nicola, Jonathan M. Rather, Paul B. Queally, Sanjay Swani, D. Scott Mackesy, John D. Clark, John Almeida, Jr., Sean M. Traynor and
Eric J. Lee are managing members of WCAS IX Associates LLC and WCAS CP IV Associates LLC. Additionally, Thomas A. Scully is a managing member of WCAS CP IV
Associates LLC. These individuals may be deemed to beneficially own the shares that are, or are deemed to be, beneficially owned by WCAS Capital Partners IV, L.P. and, except for
Thomas A. Scully, Welsh Carson. Such persons disclaim beneficial ownership of such shares. Voting and investment decisions over the shares held by WCAS Management Corporation are made by its
board of directors. The board of directors of WCAS Management Corporation consists of Messrs. Russell L. Carson, Anthony J. de Nicola, Paul B. Queally and Jonathan M. Rather. These individuals
may be deemed to beneficially own the shares that are, or are deemed to be, beneficially owned by WCAS Management Corporation. Such persons disclaim beneficial ownership of such shares. The address
for each of Welsh Carson, WCAS Capital Partners IV, L.P. and WCAS Management Corporation is 320 Park Avenue, Suite 2500, New York, New York 10022.
- (2)
- Includes
all shares owned by Gougeon Shares, LLC and all shares owned by the Franck L. Gougeon Revocable Trust Under Agreement Dated June 28,
2006 (the "Gougeon Trust"), all of which are beneficially owned by Frank L. Gougeon. It also includes 4,195 shares of common stock that are subject to options held by Mr. Gougeon which are
exercisable. Mr. Gougeon is the trustee and sole beneficiary of the Gougeon Trust, and the Gougeon Trust is the sole voting member of Gougeon Shares, LLC. Accordingly, Mr. Gougeon has
sole voting and dispositive power over these shares of our common stock.
- (3)
- The
shares owned of record by the selling stockholders are beneficially owned by Franck L. Gougeon. The selling stockholders will collectively sell
5,250,000 shares of common stock in this offering. Gougeon Shares, LLC will sell a number of shares sufficient to receive net proceeds of $51 million. Assuming an offering price of
$20.00 per share, which is the mid-point of the estimated offering price range set forth on the cover page of this prospectus, Gougeon Shares, LLC would sell 2,741,935 shares of our
common stock and the Franck L. Gougeon Revocable Trust Under Agreement Dated June 28, 2006 would sell 2,508,065 of our common stock.
- (4)
- Includes (A) 20,498,511 shares of common stock held by Welsh Carson over which it has sole voting and investment power, (B) 1,008,010 shares of common stock held by WCAS Capital Partners IV, L.P., over which it has sole voting and investment power, and (C) an aggregate 253,492 shares of common stock held by other co-investors named in this table over which Welsh Carson has sole voting power.
154
- (5)
- Includes
26,827 shares of common stock held by WCAS Management Corporation over which WCAS Management Corporation has sole voting and investment power.
- (6)
- 60,632
shares of common stock are held by Mr. Queally, P. Brian Queally Jr. Educational Trust U/ADTD 6/11/98, Erin F. Queally
Educational Trust U/ADTD 6/11/98, and Sean P. Queally Educational Trust U/ADTD 6/11/98. The trusts are established for the benefit of Mr. Queally's children for which, in each case,
Mr. Queally acts as a trustee and has voting and investment power over such shares. The address for Mr. Queally is c/o Welsh, Carson, Anderson & Stowe, 320 Park Avenue,
Suite 2500, New York, New York 10022.
- (7)
- 18,604 shares of common stock are held by Mr. Sean Traynor. The address for Mr. Traynor is c/o Welsh, Carson, Anderson & Stowe, 320 Park Avenue, Suite 2500, New York, New York 10022.
In April 2008, in connection with certain transactions among our stockholders, Franck L. Gougeon sold 31,104,000 shares of our common stock (or 4,350,209 shares of our common stock after giving effect to the 7.15 for 1.00 reverse stock split) to the WCAS Stockholders, and Gougeon Shares, LLC borrowed $49.5 million from Bank of America, N.A., an affiliate of Merrill Lynch, Pierce, Fenner & Smith Incorporated, one of the underwriters of this offering. This loan is secured by, among other things, 5,316,923 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split) owned by the Gougeon Stockholders prior to completion of this offering. The other Gougeon Stockholders and one of the WCAS Stockholders provided limited guarantees of this loan. Gougeon Shares, LLC intends to repay this loan in full with its proceeds from this offering. Upon such repayment, the pledge of the shares and the guarantees will be released.
155
The following is a description of the material provisions of our capital stock, as well as other material terms of our amended and restated certificate of incorporation and bylaws as they will be in effect as of the consummation of the offering. We refer you to the form of our amended and restated certificate of incorporation, as amended, and to the form of our amended and restated bylaws, copies of which have been filed as exhibits to the registration statement of which this prospectus forms a part.
Authorized Capital
Prior to the completion of this offering, our authorized capital stock consists of (without giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering): (1) 340,000,000 shares of common stock, par value $0.01 per share, including 20,000,000 shares of Class A common stock and 35,000,000 shares of Class B common stock, and (2) 150,403 shares of preferred stock, $0.001 par value per share, including 148,524 shares of Series A preferred stock and 1,879 shares of Series B Preferred stock. As of June 30, 2009, there were 35 holders of record of our common stock, one holder of record of our Class A common stock, who is also one of our directors, one holder of record of our Class B common stock, 33 holders of record of our Series A preferred stock and one holder of record of our Series B preferred stock.
Upon completion of this offering, we will amend and restate our certificate of incorporation to provide that our authorized capital stock will consist of (1) 400,000,000 shares of common stock, par value $0.01 per share, and (2) 100,000,000 shares of preferred stock, par value $0.01 per share. Upon completion of this offering, all currently outstanding shares of our designated and undesignated common stock and our Series A and Series B preferred stock will be converted into shares of a single class of common stock. Immediately following the completion of this offering, we expect to have 48,055,683 shares of common stock and no shares of preferred stock outstanding (or 50,118,183 shares of common stock and no shares of preferred stock outstanding if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any).
All of our existing stock is, and the shares of common stock being offered by us in this offering will be, upon payment therefore, validly issued, fully paid and non-assessable. The discussion set forth below describes the most important terms of our capital stock, amended and restated certificate of incorporation and amended and restated by-laws as will be in effect upon completion of this offering. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description you should refer to our amended and restated certificate of incorporation and amended and restated by-laws, copies of which have been filed as exhibits to the registration statement of which the prospectus is a part.
Common Stock
Voting. Except as otherwise required by Delaware law, at every annual or special meeting of stockholders, every holder of common stock is entitled to one vote per share. There is no cumulative voting in the election of directors.
Dividends Rights. Subject to dividend preferences that may be applicable to any outstanding preferred stock, holders of our common stock are entitled to receive ratably such dividends as may be declared from time to time by our board of directors out of funds legally available for that purpose. Our senior secured credit facility imposes restrictions on our ability to declare dividends with respect to our common stock. However, we do not intend to pay cash dividends on our common stock for the foreseeable future. See "Dividend Policy."
Liquidation and Preemptive Rights. In the event of our liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. The holders of our common stock have no preemptive or other subscription rights. There are no redemption or sinking fund provisions applicable to our common stock. The outstanding shares of our common stock are, and the shares offered in this offering, when issued and paid for, will be, fully paid and non-assessable.
156
Listing. We have applied to list our shares of common stock on the Nasdaq Global Market under the symbol "AGAM."
Transfer Agent and Registrar. American Stock Transfer & Trust Company, LLC has been appointed as the transfer agent and registrar for our common stock.
Preferred Stock
Our board of directors may, without further action by our stockholders, from time to time, direct the issuance of shares of preferred stock in series and may, at the time of issuance, determine the rights, preferences and limitations of each series. Satisfaction of any dividend preferences of outstanding shares of preferred stock would reduce the amount of funds available for the payment of dividends on shares of common stock. Holders of shares of preferred stock may be entitled to receive a preference payment in the event of our liquidation, dissolution or winding-up before any payment is made to the holders of shares of common stock. Under specified circumstances, the issuance of shares of preferred stock may render more difficult or tend to discourage a merger, tender offer or proxy contest, the assumption of control by a holder of a large block of our securities or the removal of incumbent management. Our board of directors, without stockholder approval, may issue shares of preferred stock with voting and conversion rights which could adversely affect the holders of shares of common stock. Upon consummation of the offering, there will be no shares of preferred stock outstanding, and we have no present intention to issue any shares of preferred stock.
Stock Options
As of June 30, 2009, there were 2,897,637 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split) issuable upon exercise of outstanding stock options and 1,997,467 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split) reserved for future grants under our existing stock option plan. After completion of this offering, 1,306,834 shares will be issuable upon the exercise of presently outstanding stock options, approximately 46,152 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split) will be issuable upon the exercise of stock options that we expect to grant to certain of our directors in connection with this offering, and approximately 2,510,755 shares will be reserved for future issuance under our 2008 Equity Incentive Plan and our Employee Stock Purchase Plan, which are being adopted in connection with this offering.
Certain Provisions of our Certificate Incorporation and Bylaws
Some provisions in our amended and restated certificate of incorporation and bylaws may be deemed to have an anti-takeover effect and may delay, defer, or prevent a tender offer or takeover attempt that a stockholder might deem to be in his or her best interest. The existence of these provisions could limit the price that investors might be willing to pay in the future for shares of our common stock. These provisions include:
Election and Removal of Directors. Our amended and restated certificate of incorporation provides for the division of our board of directors into three classes of the same or nearly the same number of directors, with staggered three-year terms. In addition, the holders of our outstanding shares of common stock will not be entitled to cumulative voting in connection with the election of our directors. Our directors will also not be subject to removal, except for cause and only by the affirmative vote of 75% of the voting power entitled to vote in the election of directors, prior to the expiration of their term. These provisions on the removal of directors could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from acquiring, control of us.
Vacancies. Under our amended and restated certificate of incorporation, any vacancy on our board, including a vacancy resulting from an enlargement of our board, may only be filled by vote of a majority of our remaining directors then in office. The filling of vacancies could make it more difficult for a third party to acquire, or discourage a third party from acquiring, control of us.
Stockholder Action by Written Consent; Annual and Special Meeting of Stockholders. Our amended and restated certificate of incorporation and bylaws provide that all stockholder actions must be effected at a duly called meeting and may not be taken by written action in lieu of a meeting. All
157
stockholder action must be properly brought before any stockholder meeting, which requires advance notice pursuant to the provisions of our bylaws. In addition, special stockholder meetings may only be called by the board of directors or the chairman of the board. These provisions could have the effect of delaying stockholder actions that are favored by the holders of a majority of our outstanding voting securities until a meeting is called or until the next annual meeting. These provisions could also discourage a potential acquiror from making a tender offer for our common stock, because even if it were able to acquire a majority of our outstanding voting securities, a potential acquiror would only be able to take actions such as electing new directors or approving a business combination or merger at a duly called stockholders' meeting, and not by written consent. In addition, only our board of directors can (1) schedule the date of the annual meeting and (2) provide written notice of the annual meeting. As a result, potential acquirors cannot accelerate the date of such meeting and must wait until the annual meeting of stockholders before they may propose taking certain actions to further their proposal.
Advance Notice Procedures. Advance notice is required for stockholders to nominate directors or to submit proposals for consideration at meetings of stockholders. This provision may have the effect of precluding the conduct of certain business at a meeting if the proper notice is not timely provided and may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer's own slate of directors or otherwise attempting to obtain control of our company.
Authorized but Unissued Shares. Authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the Nasdaq Global Market. These additional shares may be used for a variety of corporate acquisitions and employee benefit plans and could also be issued in order to deter or prevent an attempt to acquire us. The existence of authorized but unissued common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Amendments to Bylaws by Stockholders. Our board of directors is expressly authorized to amend or repeal our bylaws without the consent or vote of our stockholders. For our stockholders to amend our bylaws, however, the affirmative vote of the holders of at least 75% of the voting power of all of the then outstanding shares of our common stock entitled to vote generally in the election of directors, voting together as a single class, is required for our stockholders to amend or repeal our bylaws. This provision on amendments to our bylaws by our stockholders could have the effect of making it more difficult for a third-party to acquire, or discouraging a third-party from acquiring, control of us.
Limitations on Liability and Indemnification of Directors and Officers
Our amended and restated certificate of incorporation limits the liability of directors. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except liability for any of the following: any breach of their duty of loyalty to the corporation or its stockholders; acts or omissions not in good faith that involve intentional misconduct or a knowing violation of law; unlawful payments of dividends or unlawful stock repurchases or redemptions; or any transaction from which the director derived an improper personal benefit. These provisions do not limit or eliminate our rights or the rights of any stockholder to seek non-monetary relief, such as an injunction or rescission, in the event of a breach of a director's fiduciary duty. These provisions will not alter a director's liability under federal securities laws. Our amended and restated certificate of incorporation and bylaws also provide that we shall indemnify our directors and officers to the fullest extent permitted by law, and also permit us to obtain insurance on behalf of any officer, director, employee or other agent for any expense, liability or loss, whether or not we would have the power to indemnify such a person under Delaware law.
We currently maintain liability insurance for our directors and officers. In connection with this offering, we expect to obtain additional liability insurance for our directors and officers. Such insurance would be available to our directors and officers in accordance with its terms.
158
DESCRIPTION OF CERTAIN INDEBTEDNESS
On April 28, 2006, we entered into an amended and restated senior secured credit facility, which we refer to as our senior secured credit facility, with a syndicate of financial institutions and institutional lenders led by Lehman Commercial Paper Inc., as administrative agent, Citigroup Global Markets Inc., as syndication agent, and Lehman Brothers Inc. and Citigroup Global Markets Inc., as joint lead managers and joint bookrunners. Affiliates of Deutsche Bank Securities Inc. and Natixis Bleichroeder Inc. are lenders under our Tranche B term loan facility. Affiliates of Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citigroup Global Markets Inc. and Wells Fargo Securities, LLC are lenders under our revolving credit facility and will receive a portion of our net proceeds from this offering. Set forth below is a summary of the terms of our senior secured credit facility. This summary is not a complete description of all of the terms of the agreements. We have filed the senior secured credit agreement as exhibit 10.1 to the registration statement of which this prospectus is a part.
General
Our senior secured credit facility provides for borrowings of up to $240.0 million and consists of a $215.0 million Tranche B term loan facility drawn in full in April 2006 and with a maturity date of April 28, 2013 and a $25.0 million revolving credit facility with a maturity date of July 28, 2011, which was temporarily reduced to $15.5 million, as discussed below. As of June 30, 2009, there was approximately $197.0 million outstanding under our Tranche B term loan facility and $25.0 million outstanding under our revolving credit facility. We currently have no additional availability under the revolving credit facility. We will be permitted to reborrow any portion of the indebtedness outstanding under our revolving credit facility that is repaid with a portion of our net proceeds from this offering. All borrowings under our senior secured credit facility are subject to the satisfaction of customary conditions, including absence of a default and accuracy of representations and warranties.
On October 5, 2008, Lehman Commercial Paper Inc. ("LCPI") filed for protection under Chapter 11 of the Federal Bankruptcy Code. LCPI had committed to provide $9.5 million under the $25.0 million revolving credit facility. In March 2009, Bank of America, N.A. assumed the commitment under our revolving credit facility previously held by LCPI.
Interest and Fees
The interest rates per annum applicable to all alternate base rate borrowings are the Alternate Base Rate plus the revolving loan applicable rate or the Tranche B term loan applicable rate, as applicable. The Alternate Base Rate is a fluctuating interest rate equal to the greater of the prime rate in effect or the federal funds effective rate plus 0.50%. The revolving loan applicable rate for alternate base rate borrowings is 1.5% if our leverage ratio is greater than or equal to 4.5 to 1.0, 1.25% if our leverage ratio is less than 4.5 to 1.0 and greater than or equal to 4.0 to 1.0, 1.0% if our leverage ratio is less than 4.0 to 1.0 and greater than or equal to 3.0 to 1.0, and 0.75% if our leverage ratio is less than 3.0 to 1.0. The Tranche B term loan applicable rate for alternate base rate borrowings is 1.25% if our leverage ratio is greater than or equal to 4.5 to 1.0, 1.0% if our leverage ratio is less than 4.5 to 1.0 and greater than or equal to 3.0 to 1.0, and 0.75% if our leverage ratio is less than 3.0 to 1.0 and we satisfy the applicable ratings condition.
The interest rate per annum applicable to each Eurodollar borrowing bears interest at the Adjusted LIBOR Rate plus the revolving loan applicable rate or the Tranche B term loan applicable rate, as applicable. The Adjusted LIBOR Rate is an interest rate per annum equal to the LIBOR Rate for such interest period multiplied by the statutory reserve rate. The revolving loan applicable rate for Eurodollar borrowings is 2.5% if our leverage ratio is greater than or equal to 4.5 to 1.0, 2.25% if our leverage ratio is less than 4.5 to 1.0 and greater than or equal to 4.0 to 1.0, 2.0% if our leverage ratio is less than 4.0 to 1.0 and greater than or equal to 3.0 to 1.0, and 1.75% if our leverage ratio is less than 3.0 to 1.0. The Tranche B term loan applicable rate for Eurodollar borrowings is 2.25% if our leverage ratio is greater than or equal to 4.5 to 1.0, 2.0% if our leverage ratio is less than 4.5 to 1.0
159
and greater than or equal to 3.0 to 1.0, and 1.75% if our leverage ratio is less than 3.0 to 1.0 and we satisfy the applicable ratings condition.
Our effective interest rate as of June 30, 2009 was 2.32%. In addition, we are required to pay to the lenders under the revolving credit facility a commitment fee in respect of the unused commitments at a per annum rate of 0.5% if our leverage ratio is greater than or equal to 4.0 to 1.0 and 0.375% if our leverage ratio is less than 4.0 to 1.0.
Prepayments
Subject to certain exceptions, the Tranche B term loan facility must be prepaid with 50% of the net proceeds of any issuance of equity interests, as defined in the senior secured credit agreement, if our leverage ratio at the end of the preceding quarter is greater than 5.0 to 1.0, 25% of net proceeds if the leverage ratio at the end of the preceding quarter is greater than 4.25 to 1.0 and less than or equal to 5.0 to 1.0, none of such net proceeds if the leverage ratio of the preceding quarter is less than or equal to 4.25 to 1.00. Subject to certain exceptions, the Tranche B term loan facility must also be prepaid with 100% of the net proceeds of certain insurance proceeds, condemnation events, incurrance of indebtedness, sales, transfers or other disposition of assets.
In the event that the aggregate revolving exposures exceed the aggregate revolving commitments, we must prepay revolving borrowings or swingline borrowings, in an aggregate amount equal to such excess.
Voluntary prepayments of loans under our senior secured facility and voluntary reductions in the unused commitments under our revolving credit facility are permitted in whole or in part, in minimum amounts and subject to certain limitations set forth in our senior secured credit agreement.
Amortization of Principal
The Tranche B term loan facility amortizes in scheduled quarterly payments of $537,500 up to March 31, 2013 with the remaining $199,950,000 due upon the Tranche B loan facility maturity date in April 2013. We made a voluntary prepayment of $17.5 million that satisfied future scheduled quarterly principal payments through March 31, 2013, and the remaining $196,962,500 outstanding under our Tranche B term loan facility is due upon its maturity date.
Collateral and Guarantees
The indebtedness under our senior secured credit facility is secured by a perfected first priority security interest in substantially all of our tangible and intangible assets (including, without limitation, intellectual property, owned real property and all of our capital stock and each direct and indirect subsidiary provided that no assets of any foreign subsidiary shall be included as collateral and no more than 65% of the voting stock of any first-tier foreign subsidiary shall be required to be pledged). Our obligations under our senior secured credit facility are also guaranteed by substantially all of our domestic subsidiaries.
Covenants and Other Matters
Our senior secured credit facility requires us to comply with certain financial covenants, including maintaining a minimum interest expense coverage ratio and a maximum total leverage ratio.
Our senior secured credit facility also includes certain negative covenants restricting or limiting our ability to, among other things:
- •
- incur or assume additional indebtedness;
- •
- incur or create liens on any property or asset owned or hereafter acquired;
- •
- engage in mergers, consolidations, liquidations or dissolutions;
- •
- make loans, guarantees or investments;
160
- •
- sell assets;
- •
- engage in sale leaseback transactions;
- •
- enter into swap agreements;
- •
- declare dividends or other distributions;
- •
- engage in transactions with subsidiaries or affiliates; and
- •
- make capital expenditures.
Our senior secured credit facility also contains certain customary representations and warranties, affirmative covenants and events of default.
Following the end of each fiscal year, our Tranche B term loan facility requires us to make an annual prepayment of the term loan facility equal to 50% of any excess cash flow for any fiscal year for which the leverage ratio at the end of such fiscal year is greater than 4.50 to 1.00, 25% of excess cash flow for any fiscal year for which the leverage ratio at the end of such fiscal year is less than or equal to 4.50 to 1.00 and greater than 4.00 to 1.00, and none of excess cash flow for any fiscal year for which the leverage ratio at the end of such fiscal year is less than or equal to 4.00 to 1.00.
As of June 30, 2009, we were in compliance with all financial covenants and other requirements set forth in our senior secured credit facility.
If we are required to accrue a charge relating to, or post an appeal bond in connection with, the Medtronic litigation, we may not be able to comply with the covenants contained in our senior secured credit facility in future periods. While we do not believe that accruing a charge and/or posting an appeal bond in connection with the Medtronic litigation will cause us to breach any such covenant, doing so may adversely affect our ability to comply with our maintenance covenants in our senior secured credit facility. If we breached any such covenant, we would need to seek to amend or refinance our senior secured credit facility. We believe that such an amendment could require us to pay upfront fees and an increased interest rate on our borrowings thereunder, which could materially adversely affect our financial condition and results of operations. Alternatively, we could refinance our senior secured credit facility, but we may not be able to do so on reasonable terms or at all. If we fail to obtain such an amendment or refinancing, we would suffer an event of default under such facility and the lenders thereto would have the right to accelerate the indebtedness outstanding thereunder. In addition, the lenders' obligations to extend letters of credit or make loans under our senior secured credit facility are dependent upon our ability to make our representations and warranties thereunder at the time such letters of credit are extended or such loan is made. If we are unable to make the representations and warranties in our senior secured credit facility at such time, we will be unable to borrow additional amounts under our senior secured credit facility in the future.
2005 and 2009 Senior Subordinated PIK Notes
On July 28, 2005, AGA Medical entered into a Securities Purchase Agreement with WCAS Capital Partners IV, L.P., or WCAS CP IV, pursuant to which AGA Medical sold to WCAS CP IV a $50.0 million aggregate principal amount of 10% senior subordinated PIK notes due 2012 at a $43.5 million purchase price. See "Certain Relationships and Related Transactions—2005 Securities Purchase Agreement."
On January 5, 2009, AGA Medical entered into a Securities Purchase Agreement with WCAS CP IV, pursuant to which AGA Medical sold to WCAS CP IV an additional $15.0 million aggregate principal amount of 10% senior subordinated PIK notes due 2012 at a $13.1 million purchase price. These notes are fully and unconditionally guaranteed by Amplatzer Medical Sales Corporation. See "Certain Relationships and Related Transactions—2009 Securities Purchase Agreement."
161
UNITED STATES FEDERAL INCOME AND ESTATE TAX CONSEQUENCES
TO NON-U.S. HOLDERS
The following is a summary of the material United States federal income and estate tax consequences of the purchase, ownership and disposition of our common stock as of the date hereof. Except where noted, this summary deals only with common stock that is held as a capital asset by a non-U.S. holder.
A "non-U.S. holder" means a person (other than a partnership) that is not for United States federal income tax purposes any of the following:
- •
- an individual citizen or resident of the United States;
- •
- a corporation (or any other entity treated as a corporation for United States federal income tax purposes) created or
organized in or under the laws of the United States, any state thereof or the District of Columbia;
- •
- an estate the income of which is subject to United States federal income taxation regardless of its source; or
- •
- a trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable United States Treasury regulations to be treated as a United States person.
This summary is based upon provisions of the Internal Revenue Code of 1986, as amended (the "Code"), and regulations, rulings and judicial decisions as of the date hereof. Those authorities may be changed, perhaps retroactively, so as to result in United States federal income and estate tax consequences different from those summarized below. This summary does not address all aspects of United States federal income and estate taxes and does not deal with foreign, state, local or other tax considerations that may be relevant to non-U.S. holders in light of their personal circumstances. In addition, it does not represent a detailed description of the United States federal income tax consequences applicable to you if you are subject to special treatment under the United States federal income tax laws (including if you are a United States expatriate, "controlled foreign corporation," "passive foreign investment company" or a partnership or other pass-through entity for United States federal income tax purposes). We cannot assure you that a change in law will not alter significantly the tax considerations that we describe in this summary.
If a partnership holds our common stock, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. If you are a partner of a partnership holding our common stock, you should consult your tax advisors.
If you are considering the purchase of our common stock, you should consult your own tax advisors concerning the particular United States federal income and estate tax consequences to you of the ownership of the common stock, as well as the consequences to you arising under the laws of any other taxing jurisdiction.
Dividends
Dividends paid to a non-U.S. holder of our common stock generally will be subject to withholding of United States federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. However, dividends that are effectively connected with the conduct of a trade or business by the non-U.S. holder within the United States (and, if required by an applicable income tax treaty, are attributable to a United States permanent establishment) are not subject to the withholding tax, provided certain certification and disclosure requirements are satisfied. Instead, such dividends are subject to United States federal income tax on a net income basis in the same manner as
162
if the non-U.S. holder were a United States person as defined under the Code. Any such effectively connected dividends received by a foreign corporation may be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A non-U.S. holder of our common stock who wishes to claim the benefit of an applicable treaty rate and avoid backup withholding, as discussed below, for dividends will be required (a) to complete Internal Revenue Service Form W-8BEN (or other applicable form) and certify under penalty of perjury that such holder is not a United States person as defined under the Code and is eligible for treaty benefits or (b) if our common stock is held through certain foreign intermediaries, to satisfy the relevant certification requirements of applicable United States Treasury regulations. Special certification and other requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals.
A non-U.S. holder of our common stock eligible for a reduced rate of United States withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by filing an appropriate claim for refund with the Internal Revenue Service.
Gain on Disposition of Common Stock
Any gain realized on the disposition of our common stock generally will not be subject to United States federal income tax unless:
- •
- the gain is effectively connected with a trade or business of the non-U.S. holder in the United States (and,
if required by an applicable income tax treaty, is attributable to a United States permanent establishment of the non-U.S. holder);
- •
- the non-U.S. holder is an individual who is present in the United States for 183 days or more in the
taxable year of that disposition, and certain other conditions are met; or
- •
- we are or have been a "United States real property holding corporation" for United States federal income tax purposes.
An individual non-U.S. holder described in the first bullet point immediately above will be subject to tax on the net gain derived from the sale under regular graduated United States federal income tax rates. An individual non-U.S. holder described in the second bullet point immediately above will be subject to a flat 30% tax on the gain derived from the sale, which may be offset by United States source capital losses, even though the individual is not considered a resident of the United States. If a non-U.S. holder that is a foreign corporation falls under the first bullet point immediately above, it will be subject to tax on its net gain in the same manner as if it were a United States person as defined under the Code and, in addition, may be subject to the branch profits tax equal to 30% of its effectively connected earnings and profits or at such lower rate as may be specified by an applicable income tax treaty.
We believe we are not and do not anticipate becoming a "United States real property holding corporation" for United States federal income tax purposes.
Federal Estate Tax
Common stock held by an individual non-U.S. holder at the time of death will be included in such holder's gross estate for United States federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding
We must report annually to the Internal Revenue Service and to each non-U.S. holder the amount of dividends paid to such holder and the tax withheld with respect to such dividends, regardless of
163
whether withholding was required. Copies of the information returns reporting such dividends and withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty.
A non-U.S. holder will be subject to backup withholding for dividends paid to such holder unless such holder certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that such holder is a United States person as defined under the Code), or such holder otherwise establishes an exemption.
Information reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale of our common stock within the United States or conducted through certain United States-related financial intermediaries, unless the beneficial owner certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that the beneficial owner is a United States person as defined under the Code), or such owner otherwise establishes an exemption.
Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder's United States federal income tax liability provided the required information is furnished to the Internal Revenue Service.
164
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has not been any public market for our common stock, and we cannot predict what effect, if any, market sales of shares of common stock or the availability of shares of common stock for sale will have on the market price of our common stock. Nevertheless, sales of substantial amounts of common stock in the public market, or the perception that such sales could occur, could materially and adversely affect the market price of our common stock and could impair our future ability to raise capital through the sale of our equity or equity-related securities at a time and price that we deem appropriate.
Upon the closing of this offering, we will have an aggregate of 48,055,683 shares of common stock outstanding (or 50,118,183 if the underwriters exercise in full their option to purchase additional shares to cover overallotments, if any). Of the outstanding shares, the shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except that any shares held by our "affiliates," as that term is defined under Rule 144 of the Securities Act, may be sold only in compliance with the limitations described below. The remaining outstanding shares of common stock will be deemed "restricted securities" as that term is defined under Rule 144. Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144 under the Securities Act, which is summarized below.
The WCAS Stockholders, who will beneficially own 46.7% of our shares, or 44.7% if the underwriters exercise their overallotment in full, and the Gougeon Stockholders, who will beneficially own 24.7% of our shares, or 23.7% if the underwriters exercise their overallotment in full, upon the completion of this offering, each have the ability to cause us to register the resale of their shares beginning 180 days after completion of this offering. We do not have any other contractual obligations to register our common stock.
Subject to the lock-up agreements described below and the volume limitations and other conditions under Rule 144, 34,305,683 shares of common stock that were outstanding immediately prior to this offering (as adjusted to give effect to the conversion of our Class A and Class B common stock and all of our Series A and Series B preferred stock into our common stock and the 7.15 for 1.00 reverse stock split of our common stock, in each case, immediately prior to completion of the offering) will be available for sale in the public market after completion of this offering only if they are registered under the Securities Act or if they qualify for exemptions from registration requirements pursuant to Rule 144, which is summarized below.
Rule 144
The availability of Rule 144 will vary depending on whether shares of our common stock are restricted and whether they are held by an affiliate or a non-affiliate. For purposes of Rule 144, a non-affiliate is any person or entity who is not our affiliate at the time of sale and has not been our affiliate during the preceding three months.
In general, under Rule 144, once we have been a reporting company subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act for at least 90 days, an affiliate who has beneficially owned shares of our restricted common stock for at least six months would be entitled to sell within any three-month period a number of such shares that does not exceed the greater of:
- •
- 1% of the number of shares of our common stock then outstanding; or
- •
- the average weekly trading volume of our common stock on the open market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale.
In addition, any sales by our affiliates under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us. Our affiliates must comply with all the provisions of Rule 144 (other than the six month holding period requirement) in order to sell shares of our common stock that are not restricted securities, such as shares acquired by our affiliates either in this offering or through purchases in the open market following this offering.
165
Similarly, once we have been a reporting company for at least 90 days, a non-affiliate who has beneficially owned shares of our restricted common stock for at least six months would be entitled to sell those shares without complying with the volume limitation, manner of sale and notice provisions of Rule 144, provided that certain public information is available. Furthermore, a non-affiliate who has beneficially owned our shares of restricted common stock for at least one year will not be subject to any restrictions under Rule 144 with respect to such shares, regardless of how long we have been a reporting company.
We expect that a substantial amount of our common stock may be eligible for resale under Rule 144 upon completion of this offering.
Rule 701 and Registration of Shares under Stock Option Plans
After completion of this offering, 2,897,637 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering) will be issuable upon the exercise of presently outstanding stock options, 46,152 shares will also be issuable upon the exercise of stock options that we expect to grant to certain of our directors in connection with this offering and approximately 2,510,755 shares of our common stock (after giving effect to the 7.15 for 1.00 reverse stock split of our common stock to be effected immediately prior to completion of this offering) will be reserved for future issuance under our 2008 Equity Incentive Plan and Employee Stock Purchase Plan, each of which is being adopted in connection with this offering. In general, under Rule 701 of the Securities Act as currently in effect, any of our employees, consultants or advisors who purchases shares of our common stock from us pursuant to options granted prior to the completion of this offering under our existing stock option plan or other written agreement is eligible to resell those shares 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144. Additionally, following the consummation of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register the sale of shares issued or issuable upon the exercise of all these stock options. Subject to the exercise of issued and outstanding options and contractual restrictions, shares of our directors and executive officers to which Rule 701 is applicable or which are to be registered under the registration statement on Form S-8 will be available for sale into the public market after the expiration of the 180-day lock-up agreements with the underwriters.
Lock-Up Agreements
We, our directors and executive officers, the WCAS Stockholders and the Gougeon Stockholders have agreed, subject to certain exceptions, with the underwriters not to dispose of or hedge any of our and their common stock or securities convertible into or exchangeable for shares of common stock during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated. Merrill Lynch, Pierce, Fenner & Smith Incorporated has advised us that it has no current intent or arrangement to release any of the shares subject to the lock-up agreements prior to the expiration of the lock-up period. There are no contractually specified conditions for the waiver of lock-up restrictions and any waiver is at the sole discretion of Merrill Lynch, Pierce, Fenner & Smith Incorporated.
The 180-day restricted period described in the preceding paragraph will be extended if:
- •
- during the last 17 days of the 180-day restricted period we issue an earnings release or announce material news or
a material event; or
- •
- prior to the expiration of the 180-day restricted period, we announce that we will release earnings results during the 16-days period beginning on the last day of the 180-day period, in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the announcement of the material news or material event.
166
Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citigroup Global Markets Inc., Deutsche Bank Securities Inc., Leerink Swann LLC and Wells Fargo Securities, LLC are acting as joint bookrunning managers of the offering and as representatives of the underwriters named below. Subject to the terms and conditions stated in the underwriting agreement dated the date of this prospectus, each underwriter named below has agreed to purchase, and we and the selling stockholders have agreed to sell to that underwriter, the number of shares set forth opposite the underwriter's name.
| Underwriter |
Number of Shares |
|||
|---|---|---|---|---|
| Merrill Lynch, Pierce, Fenner & Smith Incorporated |
||||
| Citigroup Global Markets Inc. | ||||
| Deutsche Bank Securities Inc. | ||||
| Leerink Swann LLC | ||||
| Wells Fargo Securities, LLC | ||||
| Natixis Bleichroeder Inc. | ||||
| Total | 13,750,000 | |||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares (other than those covered by the overallotment option described below) are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated.
We and the selling stockholders have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer's certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount from the initial public offering price not to exceed $ per share. If all the shares are not sold at the initial offering price, the underwriters may change the offering price and the other selling terms. The representatives have advised us and the selling stockholders that the underwriters do not intend sales to discretionary accounts to exceed five percent of the total number of shares of our common stock offered by them.
167
The following table shows the underwriting discounts and commissions that we and the selling stockholders are to pay to the underwriters in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the underwriters' overallotment option.
| |
Per Share
|
Without Option
|
With Option
|
||||
|---|---|---|---|---|---|---|---|
Public offering price |
$ | $ | $ | ||||
Underwriting discount |
$ | $ | $ | ||||
Proceeds, before expenses, to AGA Medical |
$ | $ | $ | ||||
Proceeds, before expenses, to the selling stockholders |
$ | $ | $ |
The expenses of the offering, not including the underwriting discount, are estimated at $4.8 million and payable by us.
Overallotment Option
We have granted an option to the underwriters to purchase up to 2,062,500 additional shares from us at the public offering price less the underwriting discount. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover any overallotments. To the extent the option is exercised, each underwriter must purchase a number of additional shares approximately proportionate to that underwriter's initial purchase commitment. Any shares issued or sold under the option will be issued and sold on the same terms and conditions as the other shares that are the subject of this offering.
No Sales of Similar Securities
We, our officers and directors, the WCAS Stockholders and the Gougeon Stockholders have agreed that, for a period of 180 days from the date of this prospectus, we and they will not, without the prior written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated, dispose of or hedge any shares or any securities convertible into or exchangeable for our common stock. Merrill Lynch, Pierce, Fenner & Smith Incorporated in its sole discretion may release any of the securities subject to these lock-up agreements at any time without notice. Notwithstanding the foregoing, if (i) during the last 17 days of the 180-day restricted period, we issue an earnings release or material news or a material event relating to our company occurs; or (ii) prior to the expiration of the 180-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day restricted period, the restrictions described above shall continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event. See "Shares Eligible For Future Sale—Lock-Up Agreements."
Prior to this offering, there has been no public market for our common stock. Consequently, the initial public offering price for the shares was determined by negotiations among us, the selling stockholders and the representatives. Among the factors considered in determining the initial public offering price were our results of operations, our current financial conditions, our future prospects, our markets, the economic conditions in and future prospects for the industry in which we compete, our management, and currently prevailing general conditions in the equity securities markets, including current market valuations of publicly traded companies considered comparable to our company. We cannot assure you, however, that the price at which the shares will sell in the public market after this offering will not be lower than the initial public offering price or that an active trading market in our common stock will develop and continue after this offering.
Nasdaq Global Market Listing
We have applied to list our shares on the Nasdaq Global Market under the symbol "AGAM."
168
Price Stabilization and Short Positions
In connection with the offering, the underwriters may purchase and sell shares in the open market. Purchases and sales in the open market may include short sales, purchases to cover short positions, which may include purchases pursuant to the overallotment option, and stabilizing purchases.
- •
- Short sales involve secondary market sales by the underwriters of a greater number of shares than they are required to
purchase in the offering.
- •
- "Covered" short sales are sales of shares in an amount up to the number of shares represented by the underwriters'
overallotment option.
- •
- "Naked" short sales are sales of shares in an amount in excess of the number of shares represented by the underwriters'
overallotment option.
- •
- Covering transactions involve purchases of shares either pursuant to the overallotment option or in the open market after
the distribution has been completed in order to cover short positions.
- •
- To close a naked short position, the underwriters must purchase shares in the open market after the distribution has been
completed. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that
could adversely affect investors who purchase in the offering.
- •
- To close a covered short position, the underwriters must purchase shares in the open market after the distribution has
been completed or must exercise the overallotment option. In determining the source of shares to close the covered short position, the underwriters will consider, among other things, the price of
shares available for purchase in the open market as compared to the price at which they may purchase shares through the overallotment option.
- •
- Stabilizing transactions involve bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum.
Purchases to cover short positions and stabilizing purchases, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the shares. They may also cause the price of the shares to be higher than the price that would otherwise exist in the open market in the absence of these transactions. The underwriters may conduct these transactions on the Nasdaq Global Market, in the over-the-counter market or otherwise. If the underwriters commence any of these transactions, they may discontinue them at any time.
Other Relationships
Affiliates of Merrill Lynch, Pierce, Fenner & Smith Incorporated are lenders under a loan agreement with a limited liability company controlled by the Gougeon Stockholders. The Gougeon Stockholders intend to repay this loan in full with their proceeds from this offering.
The underwriters have performed investment banking and advisory services for us from time to time for which they have received customary fees and reimbursement of expenses. The underwriters may, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which they may receive customary fees and reimbursement of expenses. In addition, affiliates of Deutsche Bank Securities Inc. and Natixis Bleichroeder Inc. are lenders of outstanding indebtedness under our Tranche B term loan facility. Affiliates of Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citigroup Global Markets Inc., and Wells Fargo Securities, LLC are lenders under our revolving credit facility and will receive a portion of our net proceeds from this
169
offering. For a discussion of certain conflicts of interest involving the underwriters, see "Conflicts of Interest."
Reserved Share Program
At our request, the underwriters have reserved for sale, at the initial public offering price, up to 5% of the shares offered by this prospectus for sale to some of our directors, officers, employees, distributors, dealers, business associates and related persons. If these persons purchase reserved shares, it will reduce the number of shares available for sale to the general public. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same terms as the other shares offered by this prospectus.
Electronic Distribution
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters. The representatives may agree to allocate a number of shares to underwriters for sale to their online brokerage account holders. The representatives will allocate shares to underwriters that may make Internet distributions on the same basis as other allocations. In addition, shares may be sold by the underwriters to securities dealers who resell shares to online brokerage account holders.
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state), with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant implementation date), an offer of shares described in this prospectus may not be made to the public in that relevant member state prior to the publication of a prospectus in relation to the shares that has been approved by the competent authority in that relevant member state or, where appropriate, approved in another relevant member state and notified to the competent authority in that relevant member state, all in accordance with the Prospectus Directive, except that, with effect from and including the relevant implementation date, an offer of securities may be offered to the public in that relevant member state at any time:
- •
- to any legal entity that is authorized or regulated to operate in the financial markets or, if not so authorized or
regulated, whose corporate purpose is solely to invest in securities;
- •
- to any legal entity that has two or more of (1) an average of at least 250 employees during the last financial
year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or
consolidated accounts;
- •
- to fewer than 100 natural or legal persons (other than qualified investors as defined below) subject to obtaining the
prior consent of the representatives for any such offer; or
- •
- in any other circumstances that do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive.
Each purchaser of shares described in this prospectus located within a relevant member state will be deemed to have represented, acknowledged and agreed that it is a "qualified investor" within the meaning of Article 2(1)(e) of the Prospectus Directive.
For purposes of this provision, the expression an "offer to the public" in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the expression may be varied in that member state by any measure implementing the
170
Prospectus Directive in that member state, and the expression "Prospectus Directive" means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
The sellers of the shares have not authorized and do not authorize the making of any offer of shares through any financial intermediary on their behalf, other than offers made by the underwriters with a view to the final placement of the shares as contemplated in this prospectus. Accordingly, no purchaser of the shares, other than the underwriters, is authorized to make any further offer of the shares on behalf of the sellers or the underwriters.
Notice to Prospective Investors in the United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order") or (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (each such person being referred to as a "relevant person"). This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this document or any of its contents.
Notice to Prospective Investors in France
Neither this prospectus nor any other offering material relating to the shares described in this prospectus has been submitted to the clearance procedures of the Autorité des Marchés Financiers or of the competent authority of another member state of the European Economic Area and notified to the Autorité des Marchés Financiers. The shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France. Neither this prospectus nor any other offering material relating to the shares has been or will be:
- •
- released, issued, distributed or caused to be released, issued or distributed to the public in France;
or
- •
- used in connection with any offer for subscription or sale of the shares to the public in France.
Such offers, sales and distributions will be made in France only:
- •
- to qualified investors (investisseurs qualifiés) and/or to
a restricted circle of investors (cercle restreint d'investisseurs), in each case investing for their own account, all as defined in, and in accordance
with articles L.411-2, D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the French Code monétaire et financier;
- •
- to investment services providers authorized to engage in portfolio management on behalf of third parties;
or
- •
- in a transaction that, in accordance with article L.411-2-II-1°-or-2°-or 3° of the French Code monétaire et financier and article 211-2 of the General Regulations (Règlement Général) of the Autorité des Marchés Financiers, does not constitute a public offer (appel public à l'épargne).
The shares may be resold directly or indirectly, only in compliance with articles L.411-1, L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French Code monétaire et financier.
Notice to Prospective Investors in Hong Kong
The shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies
171
Ordinance (Cap. 32, Laws of Hong Kong), or (ii) to "professional investors" within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a "prospectus" within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in Japan
The shares offered in this prospectus have not been registered under the Securities and Exchange Law of Japan. The shares have not been offered or sold and will not be offered or sold, directly or indirectly, in Japan or to or for the account of any resident of Japan, except (i) pursuant to an exemption from the registration requirements of the Securities and Exchange Law and (ii) in compliance with any other applicable requirements of Japanese law.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA"), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
- •
- a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of
which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
- •
- a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
shares, debentures and units of shares and debentures of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
- •
- to an institutional investor (for corporations, under Section 274 of the SFA) or to a relevant person defined in Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares, debentures and units of shares and debentures of that corporation or such rights and interest in that trust are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid
172
- •
- where no consideration is or will be given for the transfer; or
- •
- where the transfer is by operation of law.
for in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions specified in Section 275 of the SFA;
Notice to Prospective Investors in Italy
This offering has not been registered with the Commissione Nationale per la Società e la Borsa (CONSOB) pursuant to Italian securities legislation. Our common stock offered by this prospectus may neither be offered or sold, nor may the prospectus or any other offering materials be distributed in the Republic of Italy unless such offer, sale or distribution is:
- •
- made by an investment firm, bank or financial intermediary permitted to conduct such activities in the Republic of Italy
in accordance with Legislative Decree No. 385 of September 1, 1993 (Decree No. 385), Legislative Decree No. 58 of February 24, 1998, CONSOB Regulation
No. 11971 of May 14, 1999 and any other applicable laws and regulations;
- •
- made (i) to professional investors (operatori qualificati) as defined in Article 31, second paragraph of
CONSOB Regulation No. 11422 of July 1, 1998, as amended, or Regulation No, 11522, (ii) in circumstances where an exemption from the rules governing solicitations to the public at
large applies pursuant to Article 100 of Legislative Decree No. 58 of February 24, 1998 and Article 33, first paragraph, of CONSOB Regulation No. 11971 of
May 14, 1999, as amended or (iii) to persons located in the Republic of Italy who submit an unsolicited request to purchase common stock; and
- •
- in compliance with all relevant Italian securities and tax laws and regulations.
Any investor purchasing our securities in the offer is solely responsible for ensuring that any offer or resale of securities it purchased in the offer occurs in compliance with applicable laws and regulations. This prospectus and the information contained herein are intended only for the use of its recipient and are not to be distributed to any third party resident or located in Italy for any reason. No person resident or located in Italy other than the original recipients of this document may rely on it or its content.
Notice to Prospective Investors in Switzerland
This prospectus as well as any other material relating to the shares which are the subject of the offering contemplated by this prospectus (the "Shares") do not constitute an issue prospectus pursuant to Article 652a of the Swiss Code of Obligations. The Shares will not be listed on the SWX Swiss Exchange and, therefore, the documents relating to the Shares, including, but not limited to, this prospectus , do not claim to comply with the disclosure standards of the listing rules of SWX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SWX Swiss Exchange.
The Shares are being offered in Switzerland by way of a private placement, i.e. to a small number of selected investors only, without any public offer and only to investors who do not purchase the Shares with the intention to distribute them to the public. The investors will be individually approached by the company from time to time.
This prospectus as well as any other material relating to the Shares is personal and confidential and do not constitute an offer to any other person. This prospectus may only be used by those investors to whom it has been handed out in connection with the offering described herein and may neither directly nor indirectly be distributed or made available to other persons without express consent
173
of the company. It may not be used in connection with any other offer and shall in particular not be copied and/or distributed to the public in (or from) Switzerland.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an exempt offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority. This prospectus is intended for distribution only to persons of a type specified in those rules. It must not be delivered to, or relied on by, any other person. The Dubai Financial Services Authority has no responsibility for reviewing or verifying any documents in connection with exempt offers. The Dubai Financial Services Authority has not approved this prospectus nor taken steps to verify the information set out in it, and has no responsibility for it. The shares which are the subject of the offering contemplated by this prospectus (the "Shares") may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the Shares offered should conduct their own due diligence on the Shares. If you do not understand the contents of this prospectus you should consult an authorised financial adviser.
Because at least five percent of the net proceeds, not including underwriting compensation, will be used to reduce or to retire the balance of a loan or credit facility extended by affiliates of each of Citigroup Global Markets Inc., Merrill Lynch, Pierce, Fenner & Smith Incorporated and Wells Fargo Securities, LLC, and an affiliate of Merrill Lynch, Pierce, Fenner & Smith Incorporated will receive a portion of the net proceeds from this offering from the repayment of the Gougeon Stockholders' loan, such underwriters may be deemed to have a "conflict of interest" with us and the selling stockholders under NASD Rule 2720 of the Financial Industry Regulatory Authority, Inc. In accordance with this rule, Leerink Swann LLC has assumed the responsibilities of acting as a qualified independent underwriter. In its role as a qualified independent underwriter, Leerink Swann LLC has performed a due diligence investigation and participated in the preparation of this prospectus and the registration statement of which this prospectus is a part. Leerink Swann LLC will not receive any additional fees for serving as qualified independent underwriter in connection with this offering. We have agreed to indemnify Leerink Swann LLC against liabilities incurred in connection with acting as a qualified independent underwriter, including liabilities under the Securities Act.
The validity of the shares of common stock offered hereby will be passed upon for us by Simpson Thacher & Bartlett LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriters by Weil, Gotshal & Manges LLP, New York, New York.
Ernst & Young LLP, independent registered public accounting firm, has audited our consolidated financial statements and schedule at December 31, 2007 and 2008, and for the three years ended December 31, 2008 as set forth in their report. We have included our financial statements and schedule in the prospectus and elsewhere in the registration statement in reliance on Ernst & Young LLP's report, given on their authority as experts in accounting and auditing.
The financial statements of AB Medica/AGA Division at December 31, 2007 and 2008, and for each of the three years in the period ended December 31, 2008, appearing in this prospectus and registration statement have been audited by Reconta Ernst & Young S.p.A, independent auditor, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
174
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus, filed as a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules thereto as permitted by the rules and regulations of the SEC. For further information about us and our common stock, you should refer to the registration statement and to its exhibits and schedules. This prospectus summarizes provisions that we consider material of certain contracts and other documents to which we refer you. Because the summaries may not contain all of the information that you may find important, you should review the full text of those documents. We have included copies of those documents as exhibits to the registration statement.
The registration statement and its exhibits and schedules filed with the SEC may be inspected, without charge, and copies may be obtained at prescribed rates, at the SEC's Public Reference Room at 100 F Street, N.E., Washington D.C., 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The registration statement and other information filed by us with the SEC are also available at the SEC's Internet site at http://www.sec.gov.
As a result of this offering, we and our stockholders will become subject to the proxy solicitation rules, annual and periodic reporting requirements, restrictions of stock purchases and sales by affiliates and other requirements of the Exchange Act, and we will file periodic reports and other information with the SEC. These periodic reports and other information will be available for inspection and copying at the SEC's Public Reference Room and the website of the SEC referenced above. We will furnish our stockholders with annual reports containing audited financial statements certified by independent auditors and quarterly reports containing unaudited financial statements for the first three quarters of each fiscal year.
175
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
AGA MEDICAL HOLDINGS, INC.
F-1
Report of Independent Registered Public Accounting Firm
The
Board of Directors
AGA Medical Holdings, Inc.
We have audited the accompanying consolidated balance sheets of AGA Medical Holdings, Inc. and subsidiaries as of December 31, 2008 and 2007, and the related consolidated statements of operations, stockholders' deficit, and cash flows for the three years in the period ended December 31, 2008. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of AGA Medical Holdings, Inc. and subsidiaries at December 31, 2008 and 2007, and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles.
| March 20, 2009 Minneapolis, Minnesota |
/s/ Ernst & Young LLP |
F-2
AGA Medical Holdings, Inc.
Consolidated Balance Sheets
(in thousands, except per share amounts)
| |
December 31, | June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2007 | 2008 | 2009 | ||||||||
| |
|
|
(Unaudited) |
||||||||
Assets |
|||||||||||
Current assets: |
|||||||||||
Cash and cash equivalents |
$ | 13,854 | $ | 22,808 | $ | 8,798 | |||||
Restricted cash |
129 | 59 | 44 | ||||||||
Accounts receivable, less allowance for doubtful accounts of $992, $933 and $946 at December 31, 2007 and 2008, and June 30, 2009, respectively |
19,697 | 26,851 | 42,173 | ||||||||
Inventory |
7,984 | 10,680 | 12,036 | ||||||||
Prepaid expenses |
1,078 | 1,019 | 1,571 | ||||||||
Deferred tax assets, net |
6,575 | 8,282 | 7,493 | ||||||||
Total current assets |
49,317 | 69,699 | 72,115 | ||||||||
Property and equipment, net |
31,295 |
35,103 |
39,004 |
||||||||
Goodwill |
61,111 | 63,009 | 85,067 | ||||||||
Intangible assets, net |
107,455 | 95,128 | 121,014 | ||||||||
Note receivable—related party |
706 | — | — | ||||||||
Other assets, net |
4,145 | 7,735 | 6,279 | ||||||||
Deferred financing costs, net |
1,986 | 1,654 | 2,803 | ||||||||
|
$ | 256,015 | $ | 272,328 | $ | 326,282 | |||||
F-3
AGA Medical Holdings, Inc.
Consolidated Balance Sheets
(in thousands, except per share amounts)
| |
December 31, | |
Pro Forma Stockholders' Deficit (Note 3) June 30, 2009 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
June 30, 2009 |
||||||||||||||
| |
2007 | 2008 | |||||||||||||
| |
|
|
(Unaudited) |
(Unaudited) |
|||||||||||
Liabilities and stockholders' deficit |
|||||||||||||||
Current liabilities: |
|||||||||||||||
Reserve for customer returns |
$ | 7,226 | $ | 8,025 | $ | 9,037 | |||||||||
Trade accounts payable |
7,373 | 9,693 | 9,891 | ||||||||||||
Accrued royalties |
1,758 | 1,933 | 2,247 | ||||||||||||
Accrued interest |
3,393 | 3,132 | 3,831 | ||||||||||||
Accrued wages |
6,827 | 7,373 | 7,018 | ||||||||||||
Short-term obligations to former distributors, less discount |
— | 1,500 | 10,129 | ||||||||||||
Accrued expenses |
2,927 | 6,642 | 5,981 | ||||||||||||
Accrued FCPA settlement |
2,000 | — | — | ||||||||||||
Income taxes payable |
389 | 855 | 1,418 | ||||||||||||
Current portion of long-term debt, less discount of $31 at December 31, 2007 |
969 | — | — | ||||||||||||
Total current liabilities |
32,862 | 39,153 | 49,552 | ||||||||||||
Long-term debt, less current portion and discount at December 31, 2007, |
196,963 | 206,883 | 221,963 | ||||||||||||
Senior subordinated note payable, less discount of $4,363, $3,441 and $4,616 at December 31, 2007 and 2008, and June 30, 2009, respectively |
45,637 | 46,559 | 60,384 | ||||||||||||
Long-term obligations to former distributors, less discount |
— | — | 9,073 | ||||||||||||
Deferred tax liabilities |
34,346 | 28,432 | 34,131 | ||||||||||||
Accrued income taxes |
5,275 | 3,188 | 3,315 | ||||||||||||
Series A preferred stock, $0.001 par value: |
|||||||||||||||
Authorized shares—149 |
|||||||||||||||
Issued and outstanding shares—129 at December 31, 2007, and 2008, and June 30, 2009; no shares pro forma |
150,949 | 166,044 | 174,014 | 45,490 | |||||||||||
Series B preferred stock, $0.001 par value: |
|||||||||||||||
Authorized shares—2 |
|||||||||||||||
Issued and outstanding shares—none at December 31, 2008, and 2 at June 30, 2009; no shares pro forma |
— | — | 1,971 | 92 | |||||||||||
Class A common stock, $0.01 par value: |
|||||||||||||||
Authorized shares—20,000 |
|||||||||||||||
Issued and outstanding shares—6,600 at December 31, 2007 and 2008, and June 30, 2009; no shares pro forma |
7,752 | 8,527 | 8,937 | 2,337 | |||||||||||
Stockholders' deficit: |
|||||||||||||||
Common stock, $0.01 par value: |
|||||||||||||||
Authorized shares—340,000 |
|||||||||||||||
Issued and outstanding shares—147,000 at December 31, 2007 and 2008, and June 30, 2009; 39,556 shares pro forma |
1,470 | 1,470 | 1,470 | 396 | |||||||||||
Class B common stock, $0.01 par value: |
|||||||||||||||
Authorized shares—35,000 |
|||||||||||||||
Issued and outstanding shares—none at December 31, 2007, 37 at December 31, 2008 and 16 at June 30, 2009; no shares pro forma |
— | — | — | — | |||||||||||
Additional paid-in capital |
— | — | — | 136,813 | |||||||||||
Excess purchase price over Predecessor basis |
(63,500 | ) | (63,500 | ) | (63,500 | ) | (63,500 | ) | |||||||
Accumulated other comprehensive income |
423 | (1,646 | ) | (1,215 | ) | (1,215 | ) | ||||||||
Accumulated deficit |
(156,162 | ) | (162,782 | ) | (173,813 | ) | (172,549 | ) | |||||||
Total stockholders' deficit |
(217,769 | ) | (226,458 | ) | (237,058 | ) | (100,055 | ) | |||||||
Total liabilities and stockholders' deficit |
$ | 256,015 | $ | 272,328 | $ | 326,282 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
AGA Medical Holdings, Inc.
Consolidated Statements of Operations
(in thousands, except per share amounts)
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||
| |
|
|
|
(Unaudited) |
|||||||||||||
Net sales |
$ | 127,529 | $ | 147,255 | $ | 166,896 | $ | 80,845 | $ | 94,381 | |||||||
Cost of goods sold |
24,985 | 22,819 | 26,635 | 12,456 | 17,004 | ||||||||||||
Gross profit |
102,544 | 124,436 | 140,261 | 68,389 | 77,377 | ||||||||||||
Operating expenses: |
|||||||||||||||||
Selling, general, and administrative |
37,515 | 50,190 | 65,669 | 31,757 | 46,456 | ||||||||||||
Research and development |
12,096 | 26,556 | 32,760 | 16,210 | 16,477 | ||||||||||||
Amortization of intangible assets |
12,682 | 15,233 | 15,540 | 7,690 | 9,894 | ||||||||||||
Change in purchase consideration |
— | — | — | — | (698 | ) | |||||||||||
FCPA settlement |
— | 2,000 | — | — | — | ||||||||||||
Loss (gain) on disposal of property and equipment |
709 | (3 | ) | 68 | 14 | (26 | ) | ||||||||||
Operating income (loss) |
39,542 | 30,460 | 26,224 | 12,718 | 5,274 | ||||||||||||
Investment income (loss) |
754 | (751 | ) | (1,202 | ) | (627 | ) | (2,352 | ) | ||||||||
Interest income |
1,174 | 426 | 230 | 110 | 61 | ||||||||||||
Interest income—related party |
— | 6 | — | — | — | ||||||||||||
Interest expense |
(22,893 | ) | (21,213 | ) | (16,492 | ) | (8,595 | ) | (8,149 | ) | |||||||
Other income, net |
957 | 994 | 722 | 295 | 1,275 | ||||||||||||
Income (loss) before income taxes |
19,534 | 9,922 | 9,482 | 3,901 | (3,891 | ) | |||||||||||
Provision for income taxes |
(6,909 | ) | (3,844 | ) | (386 | ) | (1,548 | ) | (306 | ) | |||||||
Net income (loss) |
12,625 | 6,078 | 9,096 | 2,353 | (4,197 | ) | |||||||||||
Less Series A and B preferred stock and Class A common stock dividends |
(59,410 | ) | (15,372 | ) | (17,067 | ) | (8,815 | ) | (8,471 | ) | |||||||
Net loss applicable to common stockholders |
$ | (46,785 | ) | $ | (9,294 | ) | $ | (7,971 | ) | $ | (6,462 | ) | $ | (12,668 | ) | ||
Net loss per common share—basic and diluted |
$ | (0.28 | ) | $ | (0.06 | ) | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.08 | ) | ||
Weighted average common shares—basic and diluted |
167,000 | 161,236 | 153,600 | 153,600 | 153,600 | ||||||||||||
Pro forma net loss per common share—basic and diluted (unaudited) |
$ | (2.00 | ) | $ | (0.41 | ) | $ | (0.37 | ) | $ | (0.30 | ) | $ | (0.59 | ) | ||
Pro forma weighted average common shares—basic and diluted (unaudited) |
23,356 | 22,550 | 21,482 | 21,482 | 21,482 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
AGA Medical Holdings, Inc
Consolidated Statements of Stockholders' Deficit
(in thousands)
| |
|
|
Class B Common Stock |
|
Excess Purchase Price Over Predecessor Basis |
|
|
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common Stock | |
Accumulated Other Comprehensive Income (Loss) |
|
|
|||||||||||||||||||||||||
| |
Additional Paid-In Capital |
Accumulated Earnings (Deficit) |
|
|||||||||||||||||||||||||||
| |
Shares | Stock | Shares | Stock | Total | |||||||||||||||||||||||||
Balance at January 1, 2006 |
147,000 | $ | 1,470 | — | $ | — | $ | 8,791 | $ | (63,500 | ) | $ | 160 | $ | (68,061 | ) | $ | (121,140 | ) | |||||||||||
Dividend paid to Series A preferred stockholders |
— | — | — | — | (1,670 | ) | — | — | (36,887 | ) | (38,557 | ) | ||||||||||||||||||
Dividend paid to Class A common stockholders |
— | — | — | — | (260 | ) | — | — | (5,740 | ) | (6,000 | ) | ||||||||||||||||||
Dividend paid to common stockholders |
— | — | — | — | (1,910 | ) | — | — | (42,190 | ) | (44,100 | ) | ||||||||||||||||||
Series A preferred stock dividends |
— | — | — | — | (5,536 | ) | — | — | (7,316 | ) | (12,852 | ) | ||||||||||||||||||
Class A common stock dividends |
— | — | — | — | (861 | ) | — | — | (1,139 | ) | (2,000 | ) | ||||||||||||||||||
Compensation expense related to stock option plan |
— | — | — | — | 1,446 | — | — | — | 1,446 | |||||||||||||||||||||
Other comprehensive income: |
||||||||||||||||||||||||||||||
Unrealized loss on short-term investments, net of tax of $99 |
— | — | — | — | — | — | (167 | ) | — | (167 | ) | |||||||||||||||||||
Translation adjustment, net of tax of $106 |
— | — | — | — | — | — | 178 | — | 178 | |||||||||||||||||||||
Net income for the year ended December 31, 2006 |
— | — | — | — | — | — | — | 12,624 | 12,624 | |||||||||||||||||||||
Total comprehensive income |
12,635 | |||||||||||||||||||||||||||||
Balance at December 31, 2006 |
147,000 | 1,470 | — | — | — | (63,500 | ) | 171 | (148,709 | ) | (210,568 | ) | ||||||||||||||||||
Series A preferred stock dividends |
— | — | — | — | (1,648 | ) | — | — | (12,209 | ) | (13,857 | ) | ||||||||||||||||||
Class A common stock dividends |
— | — | — | — | (193 | ) | — | — | (1,322 | ) | (1,515 | ) | ||||||||||||||||||
Issuance of Class B common stock |
— | — | 50 | 1 | 50 | — | — | — | 51 | |||||||||||||||||||||
Purchase of Class B common stock |
— | — | (50 | ) | (1 | ) | (130 | ) | — | — | — | (131 | ) | |||||||||||||||||
Tax benefit related to purchase of shares |
— | — | — | — | 30 | — | — | — | 30 | |||||||||||||||||||||
Compensation expense related to stock option plan |
— | — | — | — | 1,891 | — | — | — | 1,891 | |||||||||||||||||||||
Other comprehensive income: |
||||||||||||||||||||||||||||||
Unrealized gain on short-term investments, net of tax of $4 |
— | — | — | — | — | — | 7 | 7 | ||||||||||||||||||||||
Translation adjustment, net of tax of $243 |
— | — | — | — | — | — | 245 | — | 245 | |||||||||||||||||||||
Net income for the year ended December 31, 2007 |
— | — | — | — | — | — | — | 6,078 | 6,078 | |||||||||||||||||||||
Total comprehensive income |
6,330 | |||||||||||||||||||||||||||||
Balance at December 31, 2007 |
147,000 | 1,470 | — | — | — | (63,500 | ) | 423 | (156,162 | ) | (217,769 | ) | ||||||||||||||||||
Dividend paid to Series A preferred stockholders |
— | — | — | — | (62 | ) | — | — | (1,077 | ) | (1,139 | ) | ||||||||||||||||||
Dividend paid to Class A common stockholders |
— | — | — | — | (3 | ) | — | — | (56 | ) | (59 | ) | ||||||||||||||||||
Dividend paid to common stockholders |
— | — | — | — | (71 | ) | — | — | (1,232 | ) | (1,303 | ) | ||||||||||||||||||
Series A preferred stock dividends |
— | — | — | — | (2,396 | ) | — | — | (12,699 | ) | (15,095 | ) | ||||||||||||||||||
Class A common stock dividends |
— | — | — | — | (123 | ) | — | — | (652 | ) | (775 | ) | ||||||||||||||||||
Issuance of Class B common stock |
— | — | 47 | — | 47 | — | — | — | 47 | |||||||||||||||||||||
Purchase of Class B common stock |
— | — | (10 | ) | — | (27 | ) | — | — | — | (27 | ) | ||||||||||||||||||
Compensation expense related to stock option plan |
— | — | — | — | 2,635 | — | — | — | 2,635 | |||||||||||||||||||||
Other comprehensive income: |
||||||||||||||||||||||||||||||
Translation adjustment, net of tax of $(389) |
— | — | — | — | — | — | (2,069 | ) | — | (2,069 | ) | |||||||||||||||||||
Net income for the year ended December 31, 2008 |
— | — | — | — | — | — | — | 9,096 | 9,096 | |||||||||||||||||||||
Total comprehensive income |
7,027 | |||||||||||||||||||||||||||||
Balance at December 31, 2008 |
147,000 | 1,470 | 37 | — | — | (63,500 | ) | (1,646 | ) | (162,782 | ) | (226,458 | ) | |||||||||||||||||
Series A preferred stock dividends |
— | — | — | — | (1,540 | ) | — | — | (6,430 | ) | (7,970 | ) | ||||||||||||||||||
Series B preferred stock dividends |
— | — | — | — | (18 | ) | — | — | (74 | ) | (92 | ) | ||||||||||||||||||
Class A common stock dividends |
— | — | — | — | (79 | ) | — | — | (330 | ) | (409 | ) | ||||||||||||||||||
Issuance of Class B common stock |
— | — | 24 | — | 24 | — | — | — | 24 | |||||||||||||||||||||
Purchase of Class B common stock |
— | — | (45 | ) | — | (123 | ) | — | — | — | (123 | ) | ||||||||||||||||||
Compensation expense related to stock option plan |
— | — | — | — | 1,736 | — | — | — | 1,736 | |||||||||||||||||||||
Other comprehensive income (loss): |
||||||||||||||||||||||||||||||
Translation adjustment, net of tax of $(61) |
— | — | — | — | — | — | 431 | — | 431 | |||||||||||||||||||||
Net income (loss) for the six months ended June 30, 2009 |
— | — | — | — | — | — | — | (4,197 | ) | (4,197 | ) | |||||||||||||||||||
Total comprehensive income (loss) |
(3,766 | ) | ||||||||||||||||||||||||||||
Balance at June 30, 2009 (unaudited) |
147,000 | $ | 1,470 | 16 | $ | — | $ | — | $ | (63,500 | ) | $ | (1,215 | ) | $ | (173,813 | ) | $ | (237,058 | ) | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
AGA Medical Holdings, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| |
Year Ended December 31, |
Six Months Ended June 30, |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||||
| |
|
|
|
(Unaudited) |
||||||||||||||
Operating activities: |
||||||||||||||||||
Net income (loss) |
$ | 12,625 | $ | 6,078 | $ | 9,096 | $ | 2,353 | $ | (4,197 | ) | |||||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
||||||||||||||||||
Depreciation and amortization |
15,875 | 19,953 | 20,741 | 10,433 | 13,571 | |||||||||||||
Write-off of deferred financing fees |
2,700 | — | — | — | — | |||||||||||||
Provision for litigation settlement |
(30,000 | ) | — | — | — | 327 | ||||||||||||
Reserve for FCPA settlement |
— | 2,000 | (2,000 | ) | (2,000 | ) | — | |||||||||||
Stock-based compensation |
1,446 | 1,891 | 2,635 | 1,270 | 1,737 | |||||||||||||
Loss on equity investment |
101 | 751 | 1,202 | 627 | 2,352 | |||||||||||||
Foreign currency transaction gain/loss |
— | — | 93 | — | — | |||||||||||||
Change in deferred taxes |
8,994 | (6,087 | ) | (6,498 | ) | (2,364 | ) | (2,161 | ) | |||||||||
Change in purchase consideration |
— | — | — | — | (698 | ) | ||||||||||||
Loss (gain) on disposal of property and equipment |
710 | (3 | ) | 68 | 14 | (26 | ) | |||||||||||
Changes in operating assets and liabilities, net of acquisition: |
||||||||||||||||||
Accounts receivable |
(2,847 | ) | 1,180 | (8,033 | ) | (5,090 | ) | (15,214 | ) | |||||||||
Inventory |
17 | 914 | (312 | ) | 852 | 2,798 | ||||||||||||
Prepaid expenses and other assets |
(40 | ) | (887 | ) | (2,079 | ) | (1,000 | ) | (1,111 | ) | ||||||||
Income tax receivable |
(507 | ) | 507 | — | (1,904 | ) | — | |||||||||||
Reserve for customer returns |
(7,619 | ) | 87 | 957 | 520 | 911 | ||||||||||||
Reserve for product recall |
674 | (1,160 | ) | — | — | — | ||||||||||||
Trade accounts payable |
5,016 | (131 | ) | 864 | (835 | ) | 236 | |||||||||||
Income tax payable |
(1,033 | ) | 389 | 641 | 277 | 528 | ||||||||||||
Accrued income taxes |
— | 5,275 | (2,088 | ) | 686 | 127 | ||||||||||||
Accrued expenses |
7,142 | 2,162 | 3,505 | 1,385 | (850 | ) | ||||||||||||
Net cash provided by (used in) operating activities |
13,254 | 32,919 | 18,792 | 5,224 | (1,670 | ) | ||||||||||||
Investing activities: |
||||||||||||||||||
Acquisitions |
(6,330 | ) | (1,000 | ) | (7,138 | ) | (3,629 | ) | (34,805 | ) | ||||||||
Purchases of property and equipment |
(18,674 | ) | (4,942 | ) | (7,782 | ) | (3,284 | ) | (6,123 | ) | ||||||||
Purchases of short-term investments |
(12,002 | ) | (9,025 | ) | — | — | — | |||||||||||
Equity investment |
(2,500 | ) | (700 | ) | (1,200 | ) | (1,200 | ) | — | |||||||||
Purchase of patent rights |
— | (14,500 | ) | — | — | — | ||||||||||||
Decrease in restricted cash |
— | (1,100 | ) | (680 | ) | (625 | ) | (318 | ) | |||||||||
Proceeds from sale of short-term investments |
28,967 | 19,954 | — | — | — | |||||||||||||
Net cash used in investing activities |
(10,539 | ) | (11,313 | ) | (16,800 | ) | (8,738 | ) | (41,246 | ) | ||||||||
Financing activities: |
||||||||||||||||||
Proceeds from long-term debt |
$ | 215,000 | $ | — | $ | — | $ | — | $ | 15,000 | ||||||||
Payments on long-term debt |
(122,363 | ) | (1,000 | ) | (1,000 | ) | — | — | ||||||||||
Payment of deferred financing fees |
(2,635 | ) | — | (58 | ) | — | (1,625 | ) | ||||||||||
Proceeds from line of credit |
— | — | 9,920 | — | 15,080 | |||||||||||||
Redemption of Class A common stock |
— | (13,400 | ) | — | — | — | ||||||||||||
Proceeds from exercise of stock options |
— | 50 | 47 | 2 | 24 | |||||||||||||
Purchase of Class B common stock |
— | (131 | ) | (27 | ) | (5 | ) | (124 | ) | |||||||||
Dividends paid |
(99,879 | ) | (1,696 | ) | (2,501 | ) | (2,500 | ) | — | |||||||||
Net cash (used in) provided by financing activities |
(9,877 | ) | (16,177 | ) | 6,381 | (2,503 | ) | 28,355 | ||||||||||
Effect of exchange rate changes on cash |
178 | 235 | 581 | (183 | ) | 551 | ||||||||||||
Net change in cash and cash equivalents |
(6,984 | ) | 5,664 | 8,954 | (6,200 | ) | (14,010 | ) | ||||||||||
Cash and cash equivalents at beginning of period |
15,174 | 8,190 | 13,854 | 13,854 | 22,808 | |||||||||||||
Cash and cash equivalents at end of period |
$ | 8,190 | $ | 13,854 | $ | 22,808 | $ | 7,654 | $ | 8,798 | ||||||||
Supplemental disclosures of cash flow information: |
||||||||||||||||||
Interest paid |
$ | 16,822 | $ | 21,289 | $ | 15,363 | $ | 7,860 | $ | 6,046 | ||||||||
Taxes paid |
$ | 151 | $ | 5,976 | $ | 8,426 | $ | 5,116 | $ | 2,809 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements
1. Description of Business
AGA Medical Holdings, Inc. (AGA or the Company), a Delaware company, is a leading manufacturer of minimally invasive devices to treat structural heart defects and vascular diseases, which the Company markets under the AMPLATZER brand. The Company develops, manufactures, and markets a complete line of minimally invasive, transcatheter treatments to occlude, or close holes, relating to six different types of structural heart defects, as well as, to occlude abnormalities in blood vessels outside of the heart. AGA products are sold in 112 countries through a combination of direct sales and the use of distributors. The Company is investing in clinical trials to confirm new indications for existing devices and the development of both line extensions to existing devices and new devices to treat new therapeutic indications. All research and development programs take advantage of AGA's core competencies in using the alloy nitinol to develop innovative occlusion devices. The Company has a portfolio of patents to provide a barrier to entry for competitors.
2. Reorganization and Business Combination
On July 28, 2005, the two stockholders of AGA Medical Corporation (AGA Medical) entered into a settlement agreement pertaining to a pending lawsuit between them, in which AGA Medical purchased and redeemed all of the outstanding shares of common stock owned by one of the stockholders, resulting in the remaining stockholder owning all 200,000 of the remaining outstanding shares of common stock. Concurrent with this transaction, AGA Medical (1) issued and sold 128,254 shares of Series A preferred stock to new investors at a purchase price of $1,000 per share, (2) entered into a $122.0 million senior credit facility, consisting of a $107.0 million senior term loan and a $15.0 million revolving credit facility, and (3) issued and sold an aggregate principal amount of $50.0 million of 10% senior subordinated notes due 2012 at a discount.
Pursuant to a Contribution and Exchange Agreement dated July 28, 2005 (the Exchange Agreement), the remaining stockholder and the new investors contributed all of the above-referenced outstanding shares of AGA Medical to the Company, in exchange for shares of the Company as follows (which shares constituted all of the outstanding shares of the Company):
Holder
|
AGA Medical Common Stock Contributed |
AGA Medical Series A Preferred Stock Contributed |
Holdings Common Stock Received |
Holdings Class A Common Stock Received |
Holdings Series A Preferred Stock Received |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Remaining stockholder |
200,000 | — | 147,000,000 | 20,000,000 | — | |||||||||||
New investors |
— | 128,524 | — | — | 128,524 | |||||||||||
The Exchange Agreement provided for the cancellation of all shares of Series A preferred stock of AGA Medical contributed to the Company. As such, the 200,000 shares of common stock of AGA Medical contributed to the Company are the only shares of AGA Medical currently outstanding.
In addition to authorizing the issuance of the above-referenced shares of the Company, the Amended and Restated Certificate of Incorporation of the Company authorized the issuance of 20.0 million shares of Class B common stock of the Company, which are reserved for issuance pursuant to the Company's stock option plan.
The proceeds received from the Series A preferred stock financing, the senior credit facility, and the subordinated debt facility, along with existing cash, were used to pay the selling stockholder
F-8
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
2. Reorganization and Business Combination (Continued)
$280.0 million, each of the original stockholders of AGA Medical a $10.0 million dividend, and transaction expenses of $18.0 million, including deferred financing costs related to the senior credit facility of $3.0 million. Also, advances of $20.6 million to the two stockholders of AGA Medical were repaid by the stockholders in connection with the transaction. In addition, subsequent to the formation of the Company, the remaining stockholder received a dividend from the Company of $15.0 million.
The July 28, 2005, transactions were accounted for as a purchase in accordance with Statement of Financial Accounting Standards (SFAS) No. 141, Business Combinations, and Emerging Issues Task Force (EITF) No. 88-16, Basis in Leveraged Buyout Transactions. As a result of the reorganization transaction, the Company's remaining stockholder, along with the new investors, obtained unilateral control, as defined in EITF 88-16. As required by EITF 88-16, the ownership interest of the remaining stockholder was carried over at its historical basis and the 50% interest acquired by the new investors was recorded at fair value. As a result, the assets and liabilities were assigned new values, which are part carryover basis and part fair value basis. The excess of the purchase price over carryover basis of net assets acquired, the deemed dividend to continuing stockholders of $63.5 million, was recognized as an increase of stockholders' deficit.
The acquired assets and liabilities were recorded at their estimated fair values at July 28, 2005, as determined by management, after taking into account the carryover basis discussed above. The excess of the purchase price over the fair value of underlying assets acquired and liabilities assumed was allocated to goodwill in the amount of $59.9 million.
3. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Reclassification
The balance sheet reflects the reclassification of certain prior period amounts to conform to the current year presentation.
Unaudited Interim Financial Information
The accompanying balance sheet as of June 30, 2009, statements of operations and cash flows for the six months ended June 30, 2008 and 2009, statement of stockholders' deficit for the six months ended June 30, 2009 and related financial data and other information disclosed in these notes to the financial statements as of June 30, 2009 and for the six month periods ended June 30, 2008 and 2009 are unaudited. The unaudited interim financial statements have been prepared in accordance with generally accepted accounting principles in the United States. In the opinion of the Company's management, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, consisting of normal recurring accruals, necessary for the fair presentation of the Company's financial position, results of operations and cash flows for the six months ended June 30, 2008 and 2009. The results for the six months ended June 30, 2009 are not necessarily indicative of the results of operations to be expected for the year ending December 31, 2009.
F-9
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
Unaudited Pro Forma Stockholders' Deficit
The unaudited pro forma stockholder' deficit as of June 30, 2009 reflects the conversion of Series A and B preferred stock and Class A common stock, excluding accrued dividends, into 18,994,022 (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering) shares of common stock as though the completion of the initial public offering contemplated by the filing of the Company's registration statement with the Securities and Exchange Commission, had occurred on June 30, 2009. The remaining pro forma balances for Series A and B preferred stock and Class A common stock represent the accrued and unpaid dividends accumulated to date. These accumulated dividends do not convert into common stock and are intended to be paid in cash upon conversion.
Unaudited Pro Forma Net Loss per Common Share and Weighted Average Shares
The pro forma basic and diluted net loss per common share and pro forma basic and diluted weighted average shares are unaudited and give effect for all periods presented to the 7.15 for 1.00 reverse stock split of the Company's common stock to occur immediately prior to the completion of this offering.
Recently Issued Accounting Standards
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements (SFAS 157), which defines fair value, establishes a framework for the measurement of fair value, and enhances disclosure about fair value measurement. The statement does not require any new fair value measures. The provisions under SFAS 157 are effective for all financial assets and liabilities and for nonfinancial assets and liabilities recognized or disclosed at fair value in our consolidated financial statements on a recurring basis beginning January 1, 2008, and are expected to be applied prospectively. We adopted the provisions of SFAS 157 for financial assets and liabilities that are measured at fair value for our fiscal year beginning January 1, 2008. For all other nonfinancial assets and liabilities, we adopted SFAS 157 effective beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
SFAS 157 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (exit price). SFAS No. 157classifies the inputs used to measure fair value into the following hierarchy:
- •
- Level 1—Unadjusted quoted prices in active markets for identical assets or liability
- •
- Level 2—Unadjusted quoted prices in active markets for similar assets or liabilities, or unadjusted
quoted prices for identical or similar assets
- •
- Level 3—Unobservable inputs for the asset or liability
The Company utilizes the best available information in measuring fair value. Financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement.
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities. SFAS No. 159 expands opportunities to use fair value measurements in financial reporting and permits entities to choose to measure many financial instruments and certain other items
F-10
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
at fair value. SFAS No. 159 is effective for our fiscal year beginning January 1, 2008. The adoption of this statement did not have a material effect on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), Business Combinations (SFAS No. 141(R)). SFAS No. 141(R) expands the definition of a business combination and requires the fair value of the purchase price of an acquisition, including the issuance of equity securities, to be determined on the acquisition date. SFAS No. 141(R) also requires that all assets, liabilities, contingent considerations, and contingencies of an acquired business be recorded at fair value at the acquisition date with subsequent adjustments recorded to net earnings. In addition, SFAS No. 141(R) requires that acquisition costs generally be expensed as incurred, restructuring costs generally be expensed in periods subsequent to the acquisition date and changes in accounting for deferred tax asset valuation allowances and acquired income tax uncertainties after the measurement period impact income tax expense. We adopted SFAS No. 141(R) effective for the Company's fiscal year beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements, an Amendment of ARB No. 51 (SFAS No. 160). SFAS No. 160 changes the accounting and reporting for minority interests such that minority interests will be recharacterized as noncontrolling interests and will be required to be reported as a component of equity, and requires that purchases or sales of equity interests that do not result in a change in control be accounted for as equity transactions and, upon a loss of control, requires the interest sold, as well as any interest retained, to be recorded at fair value with any gain or loss recognized in earnings. We adopted SFAS No. 160 effective for the Company's fiscal year beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In March 2008, the FASB issued SFAS No. 161, Disclosures about Derivative Instruments and Hedging Activities, an amendment of FAS 133 ("SFAS 161"). SFAS 161 applies to all derivative instruments and non-derivative instruments that are designated and qualify as hedging instruments and related hedged items accounted for under SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities ("SFAS 133"). The provisions of SFAS 161 require entities to provide greater transparency through additional disclosures about (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS 133 and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity's financial position, results of operations and cash flows. We adopted SFAS 161 effective for the Company's fiscal year beginning January 1, 2009. The adoption of this statement did not have a material effect on our consolidated financial statements.
In April 2009, the Financial Accounting Standards Board (FASB) issued FASB Staff Position (FSP) 107-1 and Accounting Principles Board (APB) Opinion No. 28-1 (FSP 107-1 and APB 28-1), Interim Disclosures about Fair Value of Financial Instruments ("FSP 107-1"). FSP 107-1 amends SFAS 107, Disclosures about Fair Value of Financial Instruments, and APB No. 28, Interim Financial Reporting. FSP 107-1 requires publicly traded entities to disclose the fair value of all financial instruments for which it is practicable to estimate that value, whether recognized or not recognized in the statement of financial position, as required by SFAS 107. FSP 107-1 is effective for interim and annual reporting periods ending after June 15, 2009. We adopted FSP 107-1 and APB 28-1 for the
F-11
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
period ending June 30, 2009, and it did not have a material impact on the results of operations or financial position.
In May 2009, the FASB issued Statement of Financial Accounting Standards (SFAS) No. 165, Subsequent Events (SFAS No. 165), which defines subsequent events as events or transactions that occur after the balance sheet date but before the financial statements are issued or available to be issued. SFAS No. 165 further categorizes subsequent events as either "recognized" or "nonrecognized." Recognized subsequent events provide additional evidence about conditions that existed at the balance sheet date and are recognized at the balance sheet date even though the related event occurred subsequent to the balance sheet date. Nonrecognized subsequent events provide evidence about conditions that did not exist at the balance sheet date. Such events should only be disclosed in the financial statements to keep the financial statements from being misleading. The Company has evaluated the existence of both recognized and nonrecognized subsequent events through October 13, 2009.
Cash Equivalents
The Company considers all highly liquid investments with contractual maturities of three months or less when purchased to be cash equivalents. The carrying value of cash and cash equivalents approximates fair value for all periods presented. Cash and cash equivalents are classified as level 1 in the fair value hierarchy.
Restricted Cash
As part of the 2005 business reorganization, the Company entered into an agreement to act as an escrow agent for $2.5 million to be used for charity. At December 31, 2007 and 2008, and June 30, 2009, the balance remaining in the escrow account was $0.1 million, which is classified as restricted cash. Restricted cash is classified as level 1 in the fair value hierarchy.
Accounts Receivable and Allowance for Doubtful Accounts
The Company has receivables from a diversified customer base. The creditworthiness of customers is monitored before sales are approved. The Company records an allowance for doubtful accounts based on past history, current economic conditions, and the composition of its accounts receivable aging and, in some cases, makes allowances for specific customers based on several factors, such as the creditworthiness of those customers and payment history.
F-12
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
Inventory
Inventory is valued at the lower of cost or market with cost determined using the first-in, first-out method. Inventory consists of the following:
| |
December 31, | |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
June 30, 2009 |
|||||||||
| (in thousands) |
2007 | 2008 | ||||||||
| |
|
|
(Unaudited) |
|||||||
Raw materials |
$ | 4,289 | $ | 5,468 | $ | 6,441 | ||||
Work-in-process |
504 | 614 | 842 | |||||||
Finished goods |
5,075 | 6,105 | 6,330 | |||||||
Inventory reserve |
(1,884 | ) | (1,507 | ) | (1,577 | ) | ||||
|
$ | 7,984 | $ | 10,680 | $ | 12,036 | ||||
The Company makes adjustments to the value of inventory based on estimates of potentially excess and obsolete inventory after considering forecasted demand and forecasted average selling prices.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Additions and improvements that extend the lives of assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. Depreciation is provided using the straight-line method over the estimated useful lives of the individual assets and ranges from 3 to 40 years. Manufacturing equipment are depreciated over 5 years, office furniture and equipment are depreciated over 3 to 5 years, building costs are depreciated over 40 years, leasehold improvements are depreciated over the estimated lives of the related assets or the life of the lease, whichever is shorter, and building and land improvements are depreciated over 10 years. Construction in process is not depreciated until the related asset is put into use. Property and equipment consist of the following:
| |
December 31, | |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
June 30, 2009 |
|||||||||
| (in thousands) |
2007 | 2008 | ||||||||
| |
|
|
(Unaudited) |
|||||||
Manufacturing equipment |
$ | 2,730 | $ | 4,006 | $ | 6,031 | ||||
Land |
5,103 | 5,103 | 5,103 | |||||||
Office furniture and equipment |
8,903 | 10,659 | 15,916 | |||||||
Building |
16,123 | 16,123 | 16,123 | |||||||
Building improvements |
299 | 597 | 1,294 | |||||||
Leasehold improvements |
1,336 | 2,466 | 3,057 | |||||||
Land improvements |
1,478 | 1,478 | 1,494 | |||||||
Construction in progress |
1,097 | 4,212 | 1,841 | |||||||
|
37,069 | 44,644 | 50,859 | |||||||
Accumulated depreciation |
(5,774 | ) | (9,541 | ) | (11,855 | ) | ||||
|
$ | 31,295 | $ | 35,103 | $ | 39,004 | ||||
F-13
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
Total depreciation expense of property and equipment was $2.1 million, $3.3 million, $4.0 million, $2.1 million and $2.3 million, for the years ended December 31, 2006, 2007 and 2008 and the six months ended June 30, 2008 and 2009, respectively.
Goodwill and Intangible Assets
SFAS No. 142, Goodwill and Other Intangible Assets, requires that goodwill and other intangible assets with indefinite useful lives be evaluated for impairment on an annual basis or more frequently if certain events occur or circumstances exist. The Company completed its annual impairment tests of goodwill and indefinite lived intangible assets during the fourth quarters of 2007 and 2008 and identified no impairment associated with the carrying values of the indefinite lived intangibles or goodwill.
The Company evaluates goodwill for impairment based on a two-step process. The first step compares the fair value of a reporting unit with its carrying amount, including goodwill. The second step compares the implied fair value of reporting unit goodwill with the carrying amount of that goodwill. The measurement of possible impairment is based upon the comparison of the fair value of each reporting unit with the book value of its assets. The Company has one consolidated reporting unit. In reviewing intangible assets with indefinite useful lives for impairment, the Company compares the carrying amount of such asset to its fair value. The Company estimates the fair value using discounted cash flows expected from the use of the asset. When the estimated fair value is less than its carrying amount, an impairment loss is recognized equal to the difference between the asset's fair value and its carrying amount. In addition, intangible assets with indefinite useful lives are reviewed for impairment whenever events such as product discontinuance or other changes in circumstances indicate that the carrying amount may not be recoverable.
The performance of the goodwill and intangible asset impairment tests are subject to significant judgment in determining the estimation of future cash flows, the estimation of discount rates, and other assumptions. Changes in these estimates and assumptions could have a significant impact on the fair value and impairment of goodwill and intangible assets.
Impairment of Long-Lived Assets
Long-lived assets, primarily property, plant, and equipment and intangible assets with finite lives, are periodically reviewed and evaluated by the Company when events and circumstances indicate that the carrying amount of these assets may not be recoverable. For long-lived assets, this evaluation is based on the expected future undiscounted operating cash flows of the related assets. Should such evaluation result in the Company concluding that the carrying amount of long-lived assets has been impaired, an appropriate write-down to their fair value is recorded.
Revenue Recognition
In the United States and certain countries, the Company sells its products directly to hospitals and clinics. The Company recognizes revenue when all of the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred to a third-party shipper; the sales price is fixed or
F-14
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
determinable; and collectibility is reasonably assured. These criteria are met at the time of shipment when the risk of loss and title passes to the customer.
In other international markets, the Company sells its products to international distributors, which subsequently resell the products to hospitals. Sales to distributors are recognized at the time of shipment, provided that the Company has received an order, the price is fixed or determinable, collectibility of the resulting receivable is reasonably assured, and the Company can reasonably estimate returns. In cases where the Company's products are held in consignment at a customer's location, the Company recognizes net sales at the time the product is used in the procedure rather than at shipment.
The Company allows for product returns in certain circumstances, such as damaged or faulty products, products that are past their sterility dates, and physician mis-sizing. Allowances are provided for estimated product returns at the time of sale based on historical returns experience and recorded as a reduction of revenue. During 2006, the Company amended its product return policy to no longer accept returns for product past its sterility date after March 31, 2007. As a result, the Company reduced the required reserve by $7.7 million ($4.9 million, net of tax) in 2006 and $1.3 million ($0.8 million, net of tax) in 2007.
The Company warrants that its products are free from manufacturing defects at the time of shipment. Allowances are provided for estimated warranty costs at the time of shipment. To date, warranty costs have been insignificant.
Shipping Costs
Shipping costs are classified as cost of goods sold.
Income Taxes
Income taxes are accounted for under the liability method. Deferred income taxes are provided for temporary differences between financial reporting and tax bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
Research and Development
Research and development costs are charged to expense as incurred.
Advertising
The Company expenses advertising costs as incurred. Advertising costs for the years ended December 31, 2006, 2007 and 2008, and the six months ended June 30, 2008 and 2009 were $0.9 million, $0.9 million, $0.6 million, $0.3 million and $0.4 million, respectively.
F-15
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
Accounting for Stock-Based Compensation
At December 31, 2007 and 2008, and June 30, 2009, the Company had a stock- based employee compensation plan which is more fully described in Note 12. The Company follows the fair value recognition provisions of SFAS No. 123(R), Share-Based Payment, in accounting for its stock-based compensation.
Net Income (Loss) Per Share
Basic net income or loss per share is calculated in accordance with SFAS No. 128, Earnings Per Share, and EITF Issue No. 03-06, Participating Securities and the Two-Class Method Under SFAS No. 128. Basic earnings per share ("EPS") is calculated using the weighted-average common shares outstanding in each period under the two-class method. The two class method requires that the Company include in its basic EPS calculation when dilutive, the effect of the Company's convertible preferred stock as if that stock were converted into common shares. The convertible preferred shares are not included in the Company's basic EPS calculation when the effect of inclusion would be antidilutive.
Diluted EPS assumes the conversion, exercise or issuance of all potential common stock equivalents, unless the effect of inclusion would result in the reduction of a loss or the increase in income per share. For purposes of this calculation, the Company's stock options are considered to be potential common shares and are only included in the calculation of diluted EPS when the effect is dilutive. The shares used to calculate basic and diluted EPS represent the weighted-average common shares outstanding. The Company's preferred stockholders have the right to participate with common stockholders in the dividends and unallocated income. Net losses are not allocated to the preferred stockholders. Therefore, when applicable, basic and diluted EPS are calculated using the two-class method as the Company's convertible preferred stockholders have the right to participate, or share in the undistributed earnings with common stockholders. Diluted net loss per common share is the same as basic net loss per share for the years ended December 31, 2006, 2007 and 2008, since the effect of any potentially dilutive securities was excluded as they were anti-dilutive due to the net loss attributable to common stockholders.
The effect of the Company's participating convertible Series A and Series B preferred stock is excluded in basic EPS, under the two-class method in accordance with EITF Issue No. 03-06, Participating Securities and the Two-Class Method Under SFAS No. 128 because the effect is anti-dilutive as a result of the net loss attributable to common stockholders.
| |
Year Ended December 31, | Six Months Ended June 30, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands, except per share amounts) |
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| |
|
|
|
(Unaudited) |
||||||||||||
Numerator: |
||||||||||||||||
Net income (loss) |
$ | 12,625 | $ | 6,078 | $ | 9,096 | $ | 2,353 | $ | (4,197 | ) | |||||
Series A and Series B preferred stock and Class A common stock dividends |
(59,410 | ) | (15,372 | ) | (17,067 | ) | (8,815 | ) | (8,471 | ) | ||||||
Net loss applicable to common stockholders |
$ | (46,785 | ) | $ | (9,294 | ) | $ | (7,971 | ) | $ | (6,462 | ) | $ | (12,668 | ) | |
F-16
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
| |
Year Ended December 31, | Six Months Ended June 30, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in thousands, except per share amounts) |
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| |
|
|
|
(Unaudited) |
||||||||||||
Denominator: |
||||||||||||||||
Weighted average common shares outstanding |
147,000 | 147,000 | 147,000 | 147,000 | 147,000 | |||||||||||
Weighted average effect of the assumed conversion of Class A common stock from the date of issuance |
20,000 | 14,236 | 6,600 | 6,600 | 6,600 | |||||||||||
Weighted average shares of common stock outstanding—basic and diluted |
167,000 | 161,236 | 153,600 | 153,600 | 153,600 | |||||||||||
Net loss per share—basic and diluted |
$ | (0.28 | ) | $ | (0.06 | ) | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.08 | ) | |
Pro forma weighted average common shares outstanding (unaudited) |
20,559 | 20,559 | 20,559 | 20,559 | 20,559 | |||||||||||
Pro forma weighted average effect of the assumed conversion of Class A common stock from the date of issuance (unaudited) |
2,797 |
1,991 |
923 |
923 |
923 |
|||||||||||
Pro forma weighted average shares of common stock outstanding—basic and diluted (unaudited) |
23,356 | 22,550 | 21,482 | 21,482 | 21,482 | |||||||||||
Pro forma net loss per share—basic and diluted (unaudited) |
$ | (2.00 | ) | $ | (0.41 | ) | $ | (0.37 | ) | $ | (0.30 | ) | $ | (0.59 | ) | |
Foreign Currency Translation and Transaction Gains and Losses
The financial statements for operations outside the United States are maintained in their local currency. All assets and liabilities are translated to United States dollars at period-end exchange rates, while the statements of operations are translated at average exchange rates in effect during the year. Translation adjustments arising from the use of differing exchange rates are included in accumulated other comprehensive income in stockholders' deficit. Gains and losses on foreign currency transactions are also included in other income, net.
Sales originating in the United States denominated in a currency other than the U.S. dollar are generally fixed in terms of the amount of foreign currency that will be received or paid. A change in exchange rates between the U.S. dollar and the currency in which a transaction is denominated increases or decreases the expected amount of functional currency cash flows upon settlement of the transaction. That increase or decrease in expected functional currency cash flows is a foreign currency transaction gain or loss and is included in determining net income for the period in which the exchange rate changes. In the first quarter of 2009, we initiated a foreign currency hedging program. The objectives of the program are to reduce earnings volatility due to movements in foreign currency markets, limit loss in foreign currency-denominated cash flows, and preserve the operating margins of our foreign subsidiaries. We generally use foreign currency forward contracts to hedge transactions
F-17
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
related to known inter-company sales and inter-company debt. We also may hedge firm commitments. These contracts generally relate to our European operations and are denominated primarily in euros and sterling. All of our foreign exchange contracts are recognized on the balance sheet at their fair value. We do not enter into foreign exchange contracts for speculative purposes. Amounts on balance sheet at June 30, 2009 are immaterial.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Deferred Financing Costs
Debt financing costs are deferred and amortized to interest expense using the effective interest method over the term of the related debt instrument. In conjunction with the refinancing of the Company's debt arrangement on April 28, 2006 unamortized debt financing costs of $2.7 million, associated with the July 28, 2005 term loan obligation, were written off. Debt financing costs incurred in conjunction with the refinancing were $2.6 million and are being amortized using the effective interest method over the seven-year term of the related debt. In December 2008 and January 2009, the Company arranged for additional financing resulting in $0.1 million and $1.9 million of deferred financing costs, respectively. These costs will be amortized using the effective interest method over the 3.5 year term of the related debt.
Comprehensive Income
Other comprehensive income consists of net income, the effects of foreign currency translation, and unrealized gains (losses) on short-term investments.
Legal Proceedings
The Company is involved in a number of legal actions involving both product liability and intellectual property disputes. The outcomes of these legal actions are not within the Company's complete control and may not be known for prolonged periods of time. In some actions, the claimants seek damages, as well as other relief, that could require significant expenditures. In accordance with Financial Accounting Standards Board (FASB) Statement of Financial Accounting Standards (SFAS) No. 5, Accounting for Contingencies, (SFAS No. 5) the Company records a liability in its consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is possible, but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed in the notes to the consolidated financial statements. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded.
F-18
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Summary of Significant Accounting Policies (Continued)
The Company's significant legal proceedings are discussed in Note 11 to the consolidated financial statements. While it is not possible to predict the outcome for most of the matters discussed in Note 11 to the consolidated financial statements, the Company believes it is possible that costs associated with them could have a material adverse effect on the Company's consolidated earnings, financial position or cash flows.
4. Acquisitions
On August 18, 2006, the Company purchased the distribution rights, inventory, equipment, intangible assets and goodwill from its distributor located in the United Kingdom, which under SFAS No. 141 constitutes an acquired business. The Company established a wholly owned subsidiary in the United Kingdom called Amplatzer Medical UK Limited. The results of Amplatzer Medical UK's operations have been included in the financial statements since the date it was established. The results of sales to the distributor were included in the financial statements prior to the purchase date. The aggregate purchase price was $8.1 million.
The excess purchase price over the fair value of underlying assets acquired and liabilities assumed was allocated to goodwill. The following tables summarize the consideration paid and the estimated fair value of the assets acquired at the date of acquisition.
| (in thousands) |
|
||||
|---|---|---|---|---|---|
Consideration: |
|||||
Cash payment |
$ | 5,451 | |||
Accounts receivable forgiven |
774 | ||||
Discounted note |
1,788 | ||||
Liabilities incurred |
104 | ||||
Total consideration paid |
$ | 8,117 | |||
Purchase price allocation: |
|||||
Inventory |
$ | 1,414 | |||
Intangible assets, amortizable |
6,485 | ||||
Property and equipment |
19 | ||||
Goodwill |
199 | ||||
Total purchase price allocation |
$ | 8,117 | |||
The agreement calls for two additional payments of $1.0 million each, which are to be paid 30 days after the first anniversary of the agreement and 30 days after the second anniversary of the agreement. The required contractual payments were recorded on a discounted basis using a discount rate of 7.7%, the Company's effective borrowing rate. The unamortized discount at December 31, 2007 and 2008 was $31,000, and none, respectively. In September 2007 and 2008, the Company paid the first and second anniversary payments of $1.0 million each.
F-19
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
4. Acquisitions (Continued)
In addition, the Company has agreed to pay the former owners up to $3.5 million if certain revenue goals are achieved during the first three years of the agreement. The achievements are defined as follows:
Year 1—$1.0 million if gross revenues of Amplatzer Medical UK exceed 4.6 million pounds sterling
Year 2—$1.0 million if gross revenues of Amplatzer Medical UK exceed 5.0 million pounds sterling
Year 3—$1.5 million if gross revenues of Amplatzer Medical UK exceed 5.3 million pounds sterling
In September 2007, the Company paid the Year 1 contingent payment of $1.0 million. In September 2008, the Company paid the Year 2 contingent payment of $1.0 million. The Company did not accrue any of these contingent amounts as of December 31, 2006 or 2007. As of December 2008, the Company accrued $1.5 million related to contingent payments.
The acquired intangible assets, all of which are being amortized, have a weighted average useful life of approximately eight years. The intangible assets include a customer list valued at $5.3 million and a noncompete agreement valued at $1.2 million. The fair value of the identifiable intangible assets, inventory, and property and equipment were determined by management. The excess of the purchase price over the fair value of the assets acquired was recorded as goodwill.
On April 1, 2008, the Company purchased the distribution rights, inventory and intangible assets from its distributor located in Spain. The Company established a wholly owned subsidiary in Spain called Amplatzer Medical Espana, S.L. The results of Amplatzer Medical Spain's operations have been included in the financial statements since the date it was established. The results of sales to the distributor were included in the financial statements prior to the purchase date. The aggregate purchase price was $6.2 million.
The following tables summarize the consideration paid and the estimated fair value of the assets acquired at the date of acquisition.
| (in thousands) |
|
||||
|---|---|---|---|---|---|
Consideration: |
|||||
Cash payment |
$ | 3,528 | |||
Accounts receivable forgiven |
434 | ||||
Settlement payable |
761 | ||||
Accrued payable |
1,521 | ||||
Total consideration |
$ | 6,244 | |||
Purchase price allocation: |
|||||
Inventory |
$ | 2,328 | |||
Intangible assets, amortizable |
3,916 | ||||
Total purchase price allocation |
$ | 6,244 | |||
F-20
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
4. Acquisitions (Continued)
During July 2008, the Company paid the $761,000 settlement payable. In addition, the Company agreed to pay the former owners up to $1.5 million, in October 2008 and January 2009, if certain revenue goals are achieved during this period. These revenue goals were achieved and paid in accordance with the purchase agreement.
The acquired intangible assets, all of which are being amortized, have a weighted average useful life of approximately eight years. The intangible assets include a customer list valued at $3.8 million and a noncompete agreement valued at $0.1 million. The fair value of the identifiable intangible assets and inventory were determined by management.
On July 1, 2008, the Company purchased assets from its distributor located in Poland, consisting of distribution rights, inventory and intangible assets. The Company established a wholly owned subsidiary in Poland called AGA Medical Polska SP z.o.o. The results of AGA Medical Poland's operations have been included in the financial statements after the date it was established. The results of sales to the distributor were included in the financial statements prior to the purchase date. The $1.5 million aggregate purchase price included a $1.0 million payment made in July 2008 and a payment of $0.5 million to repurchase inventory. In addition, a contingent payment of up to $1.0 million payable is due in July 2009, if certain revenue goals are achieved during this period. No contingent payment amounts were accrued as of December 31, 2008.
The following tables summarize the consideration paid and the estimated fair value of the assets acquired at the date of acquisition.
(in thousands)
|
|
||||
|---|---|---|---|---|---|
Consideration: |
|||||
Cash payment |
$ | 1,000 | |||
Accrued payable |
500 | ||||
Total consideration |
$ | 1,500 | |||
Purchase price allocation: |
|||||
Inventory |
$ | 500 | |||
Intangible assets, amortizable |
1,000 | ||||
Total purchase price allocation |
$ | 1,500 | |||
The acquired intangible assets, all of which are being amortized, have a weighted average useful life of approximately eight years. The intangible assets include a customer list valued at $1.0 million. The fair value of the identifiable intangible assets and inventory were determined by management.
Effective January 1, 2009, the Company purchased the distribution rights, inventory and intangible assets from its distributor in France. The Company established a wholly owned subsidiary in France called Amplatzer Medical France SAS. The results of Amplatzer Medical France SAS's operations have been included in the financial statements since the date it was established. The results of sales to the distributor were included in the financial statements prior to the purchase date. The $3.5 million aggregate purchase price includes a payment on April 1, 2009 which as of the acquisition date had a net present value of $1.4 million, $0.8 million for inventory, and a contingent payment in April 2010 which as of the acquisition date had a net present value of $1.3 million payable if certain revenue goals are achieved during this period. On April 1, 2009, the Company made a payment in the amount of
F-21
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
4. Acquisitions (Continued)
$1.4 million. The Company recorded operating expense of approximately $126,000 representing the increase in fair value of the contingent obligation for the period ended June 30, 2009. As of June 30, 2009, the balance of the contingent obligation recorded was $1.4 million.
On January 1, 2009, the Company purchased the distribution rights, inventory and intangible assets from its two distributors in Portugal. The Company established a wholly owned subsidiary in Portugal called Amplatzer Medical Portugal, Unipessoal LDA. The results of Amplatzer Medical Portugal, Unipessoal LDA's operations have been included in the financial statements since the date it was established. The results of sales to the distributors were included in the financial statements prior to the purchase date. The $3.5 million aggregate purchase price includes payments of $2.5 million in January 2009, $0.2 million for inventory, and a contingent payment in January 2010 which as of the acquisition date had a net present value of $0.8 million payable if certain revenue goals are achieved during this period. The Company recorded as a reduction to operating expense approximately $118,000 representing the decrease in fair value of the contingent obligation for the period ended June 30, 2009. As of June 30, 2009, the balance of the contingent obligation recorded was $0.6 million.
On January 1, 2009, the Company purchased the distribution rights, inventory and intangible assets from its distributor in the Netherlands. The results of operations have been included in the financial statements since this date. The results of sales to the distributor were included in the financial statements prior to the purchase date. The $1.0 million aggregate purchase price includes payments of $0.4 million in January 2009, $0.3 million for inventory, and a contingent payment in January 2010 which as of the acquisition date had a net present value of $0.3 million payable if certain revenue goals are achieved during this period. The Company recorded operating expense of approximately $28,000 representing the increase in fair value of the contingent obligation for the period ended June 30, 2009. As of June 30, 2009, the balance of the contingent obligation recorded was $0.3 million.
On January 1, 2009, the Company purchased the distribution rights, inventory and intangible assets from its distributor in Canada. The Company established a wholly owned subsidiary in Canada called AGA Medical Canada Inc. The results of AGA Medical Canada Inc.'s operations have been included in the financial statements since the date it was established. The results of sales to the distributor were included in the financial statements prior to the purchase date. The $2.8 million aggregate purchase price includes payments of $1.1 million in January 2009, $0.8 million for inventory, and a contingent payment in January 2010 which as of the acquisition date had a net present value of $0.9 million payable if certain revenue goals are achieved during this period. The Company recorded as a reduction to operating expense approximately $25,000 representing the decrease in fair value of the contingent obligation for the period ended June 30, 2009. As of June 30, 2009, the balance of the contingent obligation recorded was $0.9 million.
On January 8, 2009 (and effective as of January 1, 2009), the Company purchased the distribution rights, inventory, equipment, intangible assets and goodwill from its distributor located in Italy, which under SFAS No. 141(R) constitutes an acquired business. The Company established a wholly owned subsidiary in Italy called AGA Medical Italia S.R.L. The results of AGA Medical Italia S.R.L.'s operations have been included in the financial statements since the date it was established. The results of sales to the distributor were included in the financial statements prior to the purchase date. The aggregate purchase price was $41.0 million.
The excess purchase price over the fair value of underlying assets acquired and liabilities assumed was allocated to goodwill. The goodwill recorded as a result of the acquisition is not deductible for
F-22
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
4. Acquisitions (Continued)
income tax purposes. The goodwill represents the strategic benefit of growing our business and the expected revenue growth from increased market penetration from future products and customers. The following tables summarize the consideration paid and the estimated fair value of the assets acquired at the date of acquisition.
| (in thousands) |
|
|||||
|---|---|---|---|---|---|---|
Consideration: |
||||||
Cash payment |
$ | 26,600 | ||||
Discounted guaranteed and contingent debt obligations |
14,400 | |||||
Total consideration |
$ | 41,000 | ||||
Purchase Price Allocation: |
||||||
Inventory |
$ | 1,900 | ||||
Goodwill |
21,606 | |||||
Other intangible assets |
26,398 | |||||
Total assets acquired |
$ | 49,904 | ||||
Current liabilities |
$ | 615 | ||||
Deferred income taxes, net |
8,289 | |||||
Net assets acquired |
$ | 41,000 | ||||
In addition, the Company has agreed to pay the former owners up to $6.7 million if certain revenue goals are achieved during the first three years following the date of the agreement. The achievements are defined as follows:
Year
2009—$3.1 million guaranteed payment
$2.5 million contingent payment if gross revenues of AB Medica-AGA Division S.R.L. exceed 20.0 million Euro
Year
2010—$3.4 million guaranteed payment
$2.2 million contingent payment if gross revenues of AB Medica-AGA Division S.R.L. exceed 22.0 million Euro
Year
2011—$3.7 million guaranteed payment
$2.0 million contingent payment if gross revenues of AB Medica-AGA Division S.R.L. exceed 24.0 million Euro
On April 1, 2009, the Company made a $2.0 million contingent payment as a result of certain revenue goals that were achieved.
The Company recorded as a reduction to operating expense approximately $709,000 representing the decrease in fair value of the contingent obligation for the period ended June 30, 2009. As of June 30, 2009, the balance of the contingent obligation recorded was $4.5 million.
The acquired intangible assets, all of which are being amortized, have a weighted average useful life of approximately eight years. The intangible assets include a customer list valued at $24.8 million
F-23
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
4. Acquisitions (Continued)
and a noncompete agreement valued at $1.6 million. The fair value of the identifiable intangible assets and inventory were determined by management.
5. Goodwill and Intangible Assets
The following table provides a reconciliation of goodwill:
| (in thousands) |
|
||||
|---|---|---|---|---|---|
Balance as of January 1, 2007 |
$ | 60,102 | |||
Goodwill acquired—U.K. distributor payment |
1,000 | ||||
Currency translation effect |
9 | ||||
Balance as of December 31, 2007 |
61,111 | ||||
Goodwill acquired—U.K. distributor payment |
2,500 | ||||
Currency translation effect |
(602 | ) | |||
Balance as of December 31, 2008 |
63,009 | ||||
Goodwill acquired—Italy distributor |
21,606 | ||||
Currency translation effect |
452 | ||||
Balance as of June 30, 2009 (unaudited) |
$ | 85,067 | |||
Intangible assets consist of the following:
| |
|
As of December 31, 2007 | As of December 31, 2008 | As of June 30, 2009 (Unaudited) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Weighted Average Useful Life (in Years) |
||||||||||||||||||||||||||||||
| (in thousands) |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||||||||||||||
Trade name |
Indefinite | $ | 10,650 | $ | — | $ | 10,650 | $ | 10,650 | $ | — | $ | 10,650 | $ | 10,650 | $ | — | $ | 10,650 | ||||||||||||
Developed technology |
6.0 | 86,650 | (23,976 | ) | 62,674 | 86,650 | (33,897 | ) | 52,753 | 86,650 | (38,857 | ) | 47,793 | ||||||||||||||||||
Customer relationships |
4.9 | 26,402 | (6,582 | ) | 19,820 | 29,429 | (9,497 | ) | 19,932 | 63,865 | (13,486 | ) | 50,379 | ||||||||||||||||||
Patent rights |
7.5 | 14,500 | (1,933 | ) | 12,567 | 14,500 | (3,867 | ) | 10,633 | 14,500 | (4,833 | ) | 9,667 | ||||||||||||||||||
Licensed patent |
2.3 | 1,000 | (355 | ) | 645 | 1,000 | (559 | ) | 441 | 1,000 | (661 | ) | 339 | ||||||||||||||||||
Noncompete agreement |
11.8 | 1,281 | (182 | ) | 1,099 | 992 | (273 | ) | 719 | 2,714 | (528 | ) | 2,186 | ||||||||||||||||||
|
$ | 140,483 | $ | (33,028 | ) | $ | 107,455 | $ | 143,221 | $ | (48,093 | ) | $ | 95,128 | $ | 179,379 | $ | (58,365 | ) | $ | 121,014 | ||||||||||
Intangible assets are amortized using methods that approximate the benefit provided by the utilization of the assets. Total amortization expense of intangible assets was $12.7 million, $15.2 million, $15.5 million, $7.7 million and $9.9 million for the years ended December 31, 2006, 2007 and 2008, and the six months ended June 30, 2008 and 2009, respectively. Based on the intangibles in service as of December 31, 2008, estimated annual amortization expense is $18.5 million for 2009, $15.3 million for 2010, $15.2 million for 2011, $11.7 million for 2012 and $8.0 for 2013.
In February 2007, the Company entered into an agreement to purchase the patent rights of an existing agreement with the Company. The purchase assigned the rights to receive royalties, the rights to certain inventions, and the rights for certain patent related filings to the Company. The agreement required the Company to make a onetime payment of $14.5 million. These patent rights are being amortized over 7.5 years.
F-24
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
6. Debt
On April 28, 2006, the Company entered into a $215.0 million term loan facility and a $25.0 million revolving credit facility. Proceeds were used to pay off existing senior debt; pay accrued dividends of $11.0 million to the Series A preferred and Class A common stockholders; pay a $0.30 per share dividend to all Series A preferred, Class A common, and common stockholders; pay transaction related expenses; and fund general working capital needs.
The term loan facility bears interest at either an alternate base rate, defined as the greater of the prime rate or the federal funds effective rate plus 0.50%, or the Eurodollar rate, plus an applicable spread based on the Company's leverage ratio. The interest rate in effect at December 31, 2007 and 2008, and June 30, 2009, 7.25%, 3.72%, and 3.01%, respectively. The Company is required to make payments on the term loan facility quarterly through the facility's termination date of April 28, 2013. Borrowings under our revolving credit facility bear interest at the alternate base rate or the Eurodollar rate, as defined above. At December 31, 2007, there were no borrowings outstanding under our revolving credit facility. On October 5, 2008, Lehman Commercial Paper Inc. ("LCP") filed for protection under Chapter 11 of the Federal Bankruptcy Code. LCP had committed to provide $9.5 million under the $25.0 million revolving credit facility. In March 2009 Bank of America, N.A. assumed the participation of this credit agreement previously held by LCP. At December 31, 2008, there was a borrowing of $9.9 million under our revolving credit facility, with subsequent borrowing on January 2, 2009 of $5.6 million, and March 20, 2009 of $9.5 million, respectively. Interest rates on these borrowings were 3.72%, 3.72%, and 2.75%, respectively. The revolving credit facility expires on July 28, 2011.
The Company has the ability to make prepayments on the outstanding borrowings at any time. In addition, the Company is required to make additional repayments on term loan borrowings of up to 75% of the excess cash flow, as defined in the agreement, in any fiscal year. The Company made a voluntary prepayment of $17.5 million which satisfied future scheduled principal payments through March 31, 2013; therefore, no additional principal payments were due at December 31, 2007 and 2008 and June 30, 2009, and accordingly, all of its senior debt is classified as long term. All of the Company's assets are pledged as collateral under this facility.
The financial covenants for these facilities include various restrictions with respect to the Company. In addition, there are restrictions on indebtedness, liens, guarantees, redemptions, mergers, acquisitions, and sale of assets over certain amounts. In addition, the covenants include maximum interest expense coverage, debt and leverage ratios, and restrictive covenants, including limitations on new debt, advances to subsidiaries and employees, capital expenditures, and transactions with stockholders and affiliates. The Company was in compliance with all covenants at December 31, 2007 and 2008, and June 30, 2009, respectively.
On July 28, 2005, AGA Medical entered into a $50.0 million, 10% senior subordinated note agreement with the new stockholder. As part of the agreement, AGA Medical issued 6,524 shares of Series A preferred stock (as discussed more fully in Note 7) valued at $6.5 million. The discounted issue value of the subordinated note was $43.5 million. Interest on the senior subordinated note is payable on a semiannual basis in arrears on January 1 and July 1 of each year.
The senior subordinated note has financial and restrictive covenants similar to the term loan facility covenants. The subordinated note agreement matures on July 28, 2012. The $6.5 million of value assigned to the Series A preferred stock represents a discount from the face value of the note, which will be accreted to its repayment amount utilizing the effective interest method. The original
F-25
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
6. Debt (Continued)
issue discount has been recognized as interest expense of $0.9 million for the years ended December 31, 2006, 2007 and 2008 and $0.5 million for the six months ended June 30, 2009. The accreted value of notes payable as of December 31 of the following years is:
| (in thousands) |
|
|||
|---|---|---|---|---|
2009 |
$ | 47,498 | ||
2010 |
48,455 | |||
2011 |
49,432 | |||
2012 |
50,000 | |||
As noted in Note 4, the Company incurred contractual payments as a result of the business transfer agreement with its UK distributor. The payments are paid within 30 days after the anniversary of the agreement. In September 2007 and 2008, the Company made its first and second $1.0 million payments under this business transfer agreement.
Contractual payments due on the term loan facility and senior subordinated note, and borrowings under the revolving credit facility during each of the five years subsequent to December 31, 2008, are as follows:
| (in thousands) |
|
|||
|---|---|---|---|---|
2009 |
$ | — | ||
2010 |
— | |||
2011 |
9,920 | |||
2012 |
50,000 | |||
2013 |
196,963 | |||
|
$ | 256,883 | ||
On January 5, 2009, the Company entered into a $15.0 million, 10% senior subordinated note agreement with a stockholder. As part of the agreement, the Company issued 1,879 shares of Series B preferred stock valued at $1.9 million to the stockholder. The discounted issue value of the subordinated note is $13.1 million. Interest on the senior subordinated note is payable on a semiannual basis in arrears on January 1 and July 1 of each year.
The senior subordinated note has financial and restrictive covenants similar to the term loan facility covenants. The subordinated note agreement matures on July 28, 2012. The $1.9 million of value assigned to the Series B preferred stock represents a discount from the face value of the note, which will be accreted to its repayment amount utilizing the effective interest method. The accreted value of notes payable as of December 31 of the following years is:
| (in thousands) |
|
|||
|---|---|---|---|---|
2009 |
$ | 13,617 | ||
2010 |
14,139 | |||
2011 |
14,681 | |||
2012 |
15,000 | |||
The carrying value of the Company's debt instruments approximates fair value for all periods presented.
F-26
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
7. Capital Stock
Shares of outstanding preferred and common stock as of June 30, 2009 were as follows:
Class or Series
|
Par Value | Authorized Shares |
Issued and Outstanding Shares |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Series A Preferred Stock |
$ | 0.001 | 148,524 | 128,524 | ||||||
Series B Preferred Stock |
$ | 0.001 | 1,879 | 1,879 | ||||||
Common Stock |
$ | 0.01 | 340,000,000 | 147,000,000 | ||||||
Class A Common Stock |
$ | 0.01 | 20,000,000 | 6,600,000 | ||||||
Class B Common Stock |
$ | 0.01 | 20,000,000 | 16,000 | ||||||
Series A and B Preferred Stock
Under the terms of the Series A and Series B preferred stock, the holders are entitled to receive cumulative dividends on each share of preferred stock at the rate of 10% per annum which shall accrue daily and, to the extent not paid, shall accumulate annually in arrears. Accrued dividends in arrears are $22.4 million, $37.5 million, and $45.6 million at December 31, 2007 and 2008, and June 30, 2009, respectively.
The shares of Series A and Series B preferred stock are convertible at any time, at the option of the holder, into shares of the Company's common stock at the then-applicable conversion price ($1.00 for Series A preferred stock and $2.75 for the Series B preferred stock, in each case, at June 30, 2009. The conversion price for each of the Series A and B preferred stock is subject to adjustment in the event of certain events, such as dilutive issuances of additional securities.
The number of shares of common stock the Series A and Series B preferred stock is convertible into is determined by taking the $1,000 per share face amount of the Series A or Series B, as applicable, preferred stock multiplied by the number of Series A or Series B, as applicable, preferred stock to be converted and then divided by the conversion price in effect at the time of the conversion. Notwithstanding any other adjustments, the conversion price is subject to adjustment as of the end of the Company's fiscal years for 2007, 2008, and 2009 if and to the extent the Company has satisfied or has been deemed to have satisfied one or more operating milestones. A milestone, as defined in the terms of the Series A and Series B preferred stock, means any one of the following: (1) the Company's EBITDA for its 2009 fiscal year being equal to or greater than $90,500,000, but less than $93,000,000, (2) the Company's EBITDA for its 2009 fiscal year being equal to or greater than $93,000,000, but less than $95,500,000, (3) the Company's EBITDA for its 2009 fiscal year being equal to or greater than $95,500,000, but less than $98,000,000, or (4) the Company's EBITDA for its 2009 fiscal year being equal to or greater than $98,000,000, provided, that, if and to the extent the Company satisfies a milestone set forth in clause (3), the Company shall be deemed to have satisfied three milestones for such fiscal year. In addition, if and to the extent the Company satisfies the milestone set forth in clause (4), the Company shall be deemed to have satisfied twelve milestones for such fiscal year, less the number of milestones satisfied or deemed to have been satisfied in all previous fiscal years. At each such time, the conversion price will be adjusted to be the conversion price that would have occurred at such time had the initial conversion price on the original issuance date been equal to the deemed conversion price which corresponds to the number of milestones the Company has satisfied or been deemed to have satisfied at such time and such deemed conversion price been thereafter subject to adjustment in accordance with the certificate of incorporation. The conversion price can be adjusted based on the number of milestones met during fiscal 2007, 2008, and 2009 between $1.01163 per share
F-27
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
7. Capital Stock (Continued)
if only one milestone is met to $1.16004 if all the milestones are met. The determination of whether or not the Company has satisfied or been deemed to have satisfied one or more milestones shall be made jointly by the Company and the holders of a majority of the outstanding Series A and B preferred stock. No milestones have been satisfied as of December 31, 2007 and 2008 and June 30, 2009, and the conversion price remains at $1.00 for the Series A preferred stock and $2.75 for the Series B preferred stock.
The shares of Series A and Series B preferred stock will be redeemed on the earlier of July 28, 2012, or the completion of an initial public offering that resulted in (i) a price per share in excess of either (a) two times the then-existing conversion price or (b) an amount that would cause the internal rate of return on the shares of Series A and Series B preferred stock to be at least 25% as of the date of consummation of such an initial public offering and (ii) gross proceeds to the Company in excess of $100.0 million. The redemption amount would be at a price per share equal to the current Series A and B Preferred Accrued Value (the Series A and B preferred stock face value plus any accrued but unpaid dividends).
In the event of the Company's liquidation, the holders of the Series A and Series B preferred stock will receive any distribution of corporate assets before any distributions are made to a junior class of equity. The amount to be distributed to the holders of Series A and Series B preferred stock will be the greater of (i) the Series A and Series B Preferred Accrued Value on such date and (ii) the amount that would have been payable in connection with such liquidation event on the number of shares of common stock into which a share of Series A and Series B preferred stock was convertible on such date.
Common Stock
The holders of shares of our common stock are each entitled to one vote for each share held with respect to all matters submitted to the stockholders of the Company for a vote or action by written consent.
In the event of the Company's liquidation, the holders of the common stock will receive any distribution of corporate assets together with the holders of the Class B Common Stock and any other series or class or classes of common stock but after the holders of the Series A and Series B preferred stock receive their liquidation preference and after any distributions have been made to the holders of the Class A Common Stock as described above.
Class A Common Stock
The holders of shares of Class A common stock are each entitled to one vote for each share held with respect to all matters submitted to the stockholders of the Company for a vote or action by written consent.
Under the terms of the Class A common stock, the holders are entitled to receive cumulative dividends on each share of Class A common stock at the rate of 10% per annum which shall accrue daily and, to the extent not paid, shall accumulate annually in arrears. Accrued dividends in arrears are $1.1 million, $1.9 million, and $2.3 million at December 31, 2007 and 2008, and June 30, 2009, respectively.
F-28
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
7. Capital Stock (Continued)
Each holder of shares of Class A common stock will have the right, at any time on or after July 28, 2007, at the holder's option, to convert each of its outstanding shares of Class A common stock, in whole or in part, into a fully paid and nonassessable share of common stock. The shares of Class A common stock will automatically be converted into shares of common stock upon the completion of an initial public offering that results in (i) a price per share in excess of either (a) two times the then-existing conversion price or (b) an amount that would cause the internal rate of return on the shares of Series A preferred stock to be at least 25% as of the date of consummation of such an initial public offering and (ii) gross proceeds to the Company in excess of $100.0 million; provided, however, that if the Series A preferred stock has been redeemed prior to such public offering, the shares of Class A common stock will be redeemed (rather than converted). The redemption amount would be at a price per share equal to the current Class A Common Accrued Value (the Class A common stock face value plus any accrued but unpaid dividends).
In the event of the Company's liquidation, the holders of the Class A common stock will receive any distribution of corporate assets before any distributions are made to holders of common stock but after the holders of the Series A and Series B preferred stock receive their liquidation preference. The amount to be distributed to the holders of the Class A common stock will be equal to the greater of (i) the Class A Common Accrued Value and (ii) the amount that would have been payable in connection with such liquidation event on the number of shares of common stock into which a share of Class A common stock was convertible on such date.
On July 27, 2007, the new investors caused the Company to redeem 13.4 million shares of Class A common stock under the terms of this agreement. A payment of $15.1 million represented the redemption of 13.4 million shares of Class A common stock at its face amount, plus $1.7 million of accrued dividends.
Class B Common Stock
The holders of shares of Class B common stock are not entitled to vote on any matters submitted to the stockholders of the Company for a vote or action by written consent.
The shares of Class B common stock will automatically be converted into shares of common stock upon the completion of an initial public offering that results in (i) a price per share in excess of either (a) two times the then-existing conversion price or (b) an amount that would cause the internal rate of return on the shares of Series A preferred stock to be at least 25% as of the date of consummation of such an initial public offering and (ii) gross proceeds to the Company in excess of $100.0 million.
In the event of the Company's liquidation, the holders of the Class B common stock will receive any distribution of corporate assets together with the holders of the Common Stock and any other series or class or classes of common stock but after the holders of the Series A and Series B preferred stock receive their liquidation preference and after any distributions have been made to the holders of the Class A Common Stock as described above.
F-29
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
8. Income Taxes
The provision for income taxes is based on earnings before income taxes reported for financial statement purposes. The components of earnings before income taxes are as follows:
| |
December 31, | June 30, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands)
|
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| |
|
|
|
(Unaudited) | ||||||||||||
United States |
$ | 19,071 | $ | 7,294 | $ | 4,712 | $ | 1,048 | $ | (5,036 | ) | |||||
International |
463 | 2,628 | 4,770 | 2,853 | 1,145 | |||||||||||
Total |
$ | 19,534 | $ | 9,922 | $ | 9,482 | $ | 3,901 | $ | (3,891 | ) | |||||
Significant components of the provision for income taxes for the following periods are as follows:
| |
Year Ended December 31, |
Six Months Ended June 30, |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands)
|
2006 | 2007 | 2008 | 2008 | 2009 | ||||||||||||
| |
|
|
|
(Unaudited) |
|||||||||||||
Current: |
|||||||||||||||||
Federal |
$ | (2,397 | ) | $ | 7,849 | $ | 7,030 | $ | 3,100 | $ | 614 | ||||||
State |
(129 | ) | 576 | 547 | 256 | 69 | |||||||||||
Foreign |
236 | 619 | 1,301 | 665 | 770 | ||||||||||||
Deferred: |
|||||||||||||||||
Federal |
8,269 | (5,128 | ) | (8,072 | ) | (2,275 | ) | (3,286 | ) | ||||||||
State |
719 | (446 | ) | (701 | ) | (198 | ) | (120 | ) | ||||||||
Foreign |
— | — | — | — | 586 | ||||||||||||
Valuation allowance against deferred tax assets |
211 | 374 | 281 | — | 1,673 | ||||||||||||
Total |
$ | 6,909 | $ | 3,844 | $ | 386 | $ | 1,548 | $ | 306 | |||||||
F-30
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
8. Income Taxes (Continued)
A reconciliation of the federal statutory income taxable to the effective tax rate is as follows:
| |
Year Ended December 31, |
Six Months Ended June 30, |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||
| |
|
|
|
(Unaudited) |
||||||||||||
Federal tax statutory rate |
35.0 | % | 35.0 | % | 35.0 | % | 35.0 | % | 35.0 | % | ||||||
State tax (net of federal benefit) |
2.3 | 2.1 | 1.5 | 0.6 | 1.5 | |||||||||||
FCPA resolution |
— | 7.0 | — | — | — | |||||||||||
Other permanent differences |
(0.2 | ) | (2.9 | ) | (1.3 | ) | 1.0 | (0.5 | ) | |||||||
Stock-based compensation expense |
0.7 | 2.3 | 4.5 | 2.7 | 1.4 | |||||||||||
Research and development credit |
(3.4 | ) | (10.0 | ) | (15.8 | ) | — | (4.6 | ) | |||||||
Tax reserves |
1.6 | 4.3 | (20.1 | ) | 2.9 | (2.1 | ) | |||||||||
Valuation allowance against deferred tax assets |
1.1 | 3.7 | 3.0 | — | (43.3 | ) | ||||||||||
State settlements |
(2.2 | ) | — | — | — | — | ||||||||||
Meals and entertainment |
0.5 | 1.0 | 1.0 | 1.0 | 0.5 | |||||||||||
APB 23 assertion |
— | — | — | — | 5.7 | |||||||||||
Domestic manufacturing deduction |
— | (3.8 | ) | (3.7 | ) | (3.5 | ) | (1.5 | ) | |||||||
Effective income tax rate |
35.4 | % | 38.7 | % | 4.1 | % | 39.7 | % | (7.9 | )% | ||||||
Significant components of deferred tax assets and liabilities are as follows:
| |
December 31, | June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands)
|
2007 | 2008 | 2009 | ||||||||
| |
|
|
(Unaudited) |
||||||||
Deferred tax assets: |
|||||||||||
Capital losses (including unrealized losses) |
$ | 589 | $ | 592 | $ | 2,232 | |||||
Allowance for doubtful accounts |
353 | 353 | 354 | ||||||||
Sales returns reserve |
2,460 | 2,728 | 2,728 | ||||||||
Inventory reserves |
655 | 668 | 1,163 | ||||||||
FAS 123R—nonqualified option expense |
854 | 1,385 | 1,959 | ||||||||
Foreign subsidiary profits |
1,905 | — | — | ||||||||
Other |
1,202 | 4,533 | 3,250 | ||||||||
Less valuation allowance |
(585 | ) | (866 | ) | (2,398 | ) | |||||
Total deferred tax assets |
7,433 | 9,393 | 9,288 | ||||||||
Deferred tax liabilities: |
|||||||||||
Depreciation |
(1,945 | ) | (1,809 | ) | (2,090 | ) | |||||
Intangibles |
(33,017 | ) | (28,455 | ) | (33,836 | ) | |||||
Other |
(242 | ) | 721 | — | |||||||
Total deferred tax liabilities |
(35,204 | ) | (29,543 | ) | (35,926 | ) | |||||
Net deferred tax liability |
$ | (27,771 | ) | $ | (20,150 | ) | $ | (26,638 | ) | ||
F-31
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
8. Income Taxes (Continued)
The Company has recorded no U.S. deferred taxes related to the undistributed earnings of its non-U.S. subsidiaries' as of June 30, 2009. Such amounts are intended to be reinvested outside of the United States indefinitely. The amount of unrecorded tax liability related to investments in foreign subsidiaries as of June 30, 2009 is approximately $0.5 million.
At December 31, 2007 and 2008, and June 30, 2009, the Company has capital loss carryforwards of $1.6 million, $1.6 million, and $6.0 million, respectively, which expire at various times beginning in 2009 through 2014. The Company has established a valuation allowance against these capital loss carryforwards, as it does not believe they will be realizable in future years.
The Company records all income tax contingency accruals in accordance with FASB Interpretation No. 48 Accounting for Uncertainty in Income Taxes, and SFAS No. 109, Accounting for Income Taxes. At December 31, 2007 and 2008, and June 30, 2009, the Company had $4.4 million, $2.3 million and $2.4 million of unrecognized tax benefits, including interest and penalties, that, if recognized would result in a reduction of the Company's effective tax rate. As of December 31, 2007 and 2008, and June 30, 2009, the Company had approximately $1.4 million, $1.2 million, and $1.3 million accrued for interest and penalties. Over the next 12 months, the Company expects its recorded FIN 48 liability to be reduced by approximately $0.7 million, as a result of statute closures. The Company recognizes interest and penalties related to income tax matters in income tax expense and reports the liability in current or long-term income taxes payable, as appropriate.
The following table summarizes the activity related to the Company's unrecognized tax benefits:
| |
December 31, | June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands)
|
2007 | 2008 | 2009 | |||||||
| |
|
|
(Unaudited) |
|||||||
Balance at beginning of period |
$ | 3,865 | $ | 3,865 | $ | 1,987 | ||||
Expiration of the statute of limitations for the assessment of taxes |
— | (1,878 | ) | — | ||||||
Balance at end of period |
$ | 3,865 | $ | 1,987 | $ | 1,987 | ||||
The Company's income tax returns are subject to examination for 2005 and subsequent years. The Company's 2007 federal income tax return is currently under examination. State and foreign income tax returns are generally subject to examination for a period of three to four years after filing of the respective return. The state impact of any federal changes remains subject to examination by various states for a period up to one year after formal notification to the states.
F-32
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
9. Commitments
The Company leases various pieces of equipment and offices under operating lease agreements. Future minimum operating lease obligations as of December 31, 2008, are as follows:
(in thousands)
|
|
|||
|---|---|---|---|---|
2009 |
$ | 1,201 | ||
2010 |
1,184 | |||
2011 |
571 | |||
2012 |
395 | |||
2013 |
279 | |||
Total |
$ | 3,630 | ||
Total rent expense under the operating leases for the years ended December 31, 2006, 2007 and 2008, and the six months ended June 30, 2008 and 2009, was $0.7 million, $0.8 million, $1.1 million, $0.5 million, and $1.0 million, respectively. One of the Company's operating lease agreements is noncancelable and renewable with an expiration date in the year 2012.
The Company has various royalty agreements with certain individuals which obligate the Company to pay royalties on net sales of certain products. These royalties are payable throughout the commercial life of the products. Royalties payable at December 31, 2007 and 2008, and June 30, 2009, totaled $1.8 million, $1.9 million, and $2.2 million, respectively. Royalty expense for the years ended December 31, 2006, 2007 and 2008, and the six months ended June 30, 2008 and 2009, was $6.7 million, $5.3 million, $6.0 million, $2.9 million, and $3.3 million, respectively.
10. Benefit Plan
The Company has a defined contribution salary deferral plan (the 401(k) Plan) covering substantially all employees under Section 401(k) of the Internal Revenue Code. Eligible employees may contribute a percentage of their annual compensation, subject to IRS limitations, with the Company matching a portion of the employees' contributions. Compensation expense of $85,000, $173,000, $939,000, $447,000 and $676,000, was recognized in the years ended December 31, 2006, 2007 and 2008, and the six months ended June 30, 2008 and 2009, respectively. The Company may also contribute an additional discretionary Safe Harbor amount to the Plan. The contribution for 2006 and 2007 was 3% of qualifying wages paid during the year. The Company paid discretionary contributions of $0.4 million for the year ended December 31, 2006 and $0.7 million for the year ended December 31, 2007. The Company did not make a Safe Harbor contribution for the year ended December 31, 2008.
11. Litigation
On July 25, 2006, the Company commenced the voluntary disclosure process to the Department of Justice under the Foreign Corrupt Practices Act for potentially impermissible payments by the Company's unaffiliated distributor in the People's Republic of China. As part of its process, the Company engaged in a comprehensive review of all of its international distributors and the Company's own internal practices. On June 2, 2008, the Company entered into a Deferred Prosecution Agreement with the Department of Justice concerning alleged improper payments that were made by the Company's former independent distributor in China. In accordance with the terms of the agreement,
F-33
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Litigation (Continued)
the Company paid a monetary penalty of $2.0 million in June 2008. In the fourth quarter of 2007, the Company had recorded a charge of $2.0 million for the potential settlement of this matter.
On January 29, 2007, Medtronic, Inc. filed a patent infringement action against the Company in the U.S. District Court for the Northern District of California, alleging that substantially all of the Company's AMPLATZER occluder and vascular plug devices, which have historically accounted for substantially all of the Company's net sales, infringe three of Medtronic's method and apparatus patents on shape memory alloy stents (U.S. Patent Nos. 5, 190,546, 6,306,141 and 5,067,957). Medtronic is seeking compensatory damages with respect to the Company's products manufactured or sold in the United States. Medtronic asserted but later withdrew its requests for injunctive relief and for damages based on willfulness.
The Company has asserted defenses and counterclaims for non-infringement and challenged the validity and enforceability of Medtronic's patents. On April 28, 2009, the court granted summary judgment in the Company's favor finding that the Company does not infringe Medtronic's '546 patent. Subsequently, Medtronic withdrew certain allegations with respect to the remaining two patents. The trial on the remaining issues in the case was divided into a jury trial phase and non-jury, bench trial phase.
The issues of infringement and certain issues of validity based on obviousness and anticipation of the asserted claims in the two remaining patents were the subject of a jury trial before the U.S. District Court for the Northern District of California that began on July 6, 2009. On August 5, 2009, the jury returned a verdict that the subject AMPLATZER occluder and vascular plug products infringed the claims at issue with respect to both of the two remaining Medtronic patents and that the Medtronic patent claims at issue had not been proven by us to be invalid. The jury verdict awarded Medtronic damages of $57.8 million. This amount is equal to 11% of historical sales of the occluder and vascular plug products in question during the timeframe specified for each patent. Any infringement of the '141 apparatus patent after March 31, 2009 to the date of a final, non-appealable judgment will be considered in calculating the final amount of damages, if any, to be paid. The '957 patent expired in May 2004. Because the issue was not before it, the jury made no determination regarding the payment of royalties on future sales of the Company's products after the date of a final, non-appealable judgment. The verdict is not enforceable until the completion of the trial and entry of a final, non-appealable judgment. If the Company does not receive a favorable judgment, the Company expects it will appeal such judgment and expects that it will likely be required to post in order to be allowed a bond to appeal as set forth below. The verdict is not enforceable because a judgment has not yet been entered, and as a matter of law only judgments are enforceable for purposes of execution against the non-prevailing party. A judgment has not yet been entered because all claims have not yet been adjudicated between the parties and the court has not yet ordered a judgment be entered. In addition, on August 5, 2009, Medtronic's counsel agreed with the judge on the record that a judgment would not be entered until after the non-jury trial phase is completed. Furthermore, upon approval of an appeal bond, the execution of any appealed judgment is stayed during the appeal.
On August 19, 2009, the United States Court of Appeal for the Federal Circuit, sitting en banc, issued a decision in Cardiac Pacemakers, Inc. v. St. Jude Medical, Inc.. In Cardiac Pacemakers, the Federal Circuit established a new rule of law and held as a matter of law that the practice of a method claim of a patent outside of the United States cannot infringe a United States method patent. When a controlling new rule of law is announced that affects the parties to a patent litigation, the court will ordinarily apply the supervening change in law retroactively to a pending case. In the Company's
F-34
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Litigation (Continued)
litigation with Medtronic, a portion of the jury's damage award was based on a finding of infringement of Medtronic's '957 method patent for sale of the Company's products outside of the United States, and the Company believes it is therefore directly contrary to the holding as stated in the Cardiac Pacemakers decision. Applying the rate of damages established by the jury, 11% of historical sales, to the total sales of our products outside of the United States during the period for which the Company believes damages were awarded for infringement of the '957 patent would result in a reduction of approximately $14 million. Such $14 million of damages relates only to periods of time before the '141 patent was enforceable against the Company. As a result, the Company believes it is likely that the trial court in the Company's case will reduce the jury's damage award by approximately such amount to reflect this recent decision, although there can be no assurance that Cardiac Pacemakers will not be reviewed and overturned by the United States Supreme Court or that the damage award will be reduced by this or any other amount. On September 8, 2009, the Company filed a motion seeking, at Medtronic's choice, either a new trial or the above-mentioned reduction of $14 million, which motion is publicly available. The Company does not expect a ruling on this motion until the conclusion of the non-jury phase of the trial in early 2010.
Following the jury verdict, the court scheduled the non-jury phase of the trial for early December 2009, which will deal with other issues of invalidity and unenforceability of one or both of the two remaining patents based on equitable claims of double patenting, equitable estoppel, inequitable conduct in patent prosecution and laches. The claims to be tried in the non-jury phase of the trial are claims "in equity" and for which there is no right to a trial by jury. Therefore, the trial was essentially bifurcated into a jury phase and non-jury phase subject to the same procedures. In the event the judge finds in the Company's favor on one or more of the Company's counterclaims in the non-jury phase of the trial, she can fashion an equitable remedy to compensate the Company for Medtronic's conduct, including barring all damages prior to filing of the lawsuit, barring past and future enforcement of the '141 patent alone, or eliminating all past and future damages on both of the patents in suit. Upon conclusion of the non-jury phase of the trial, the court will consider post-trial motions and enter a judgment, which the Company expects will take place in early 2010. As part of its decision, the trial court could order a new trial on some or all of the issues in the case or amend or reaffirm the jury verdict based on one or more issues regarding infringement, validity, enforceability and damages. The Company also expects the trial court to decide whether any royalty payments relating to the '141 patent, which does not expire until 2018, are due for future periods and, if so, at what rate. Any such royalties may not be on commercially reasonable terms, and may exceed the 11% rate of damages applied by the jury. If any damage amount is entered as a part of the judgment, the Company will likely be required at that time to accrue a non-cash charge equal to the amount of such damages. Any such accrual will have an adverse effect on the Company's results of operations for the applicable period.
Thereafter, the judgment will be subject to appeal which could result in the judgment being affirmed or amended, or in an order for a new trial on one or more of the same issues raised before the trial court. Medtronic also could appeal from rulings adverse to it, which could result in an appellate decision with effects adverse to us on issues of liability and/or relief. If the Company decides to appeal, such appeal may not be decided for several months and will likely require the posting of a bond in the face amount of 150% of the judgment amount, if any. The Company does not expect the cash cost associated with posting such a bond to be material, but the Company would likely be required to secure such bond with collateral. The amount of collateral required by the provider of the bond
F-35
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Litigation (Continued)
would be determined based on several factors, such as the amount of the Company's debt and the Company's financial condition.
The Company has not recorded an expense related to damages in connection with this litigation matter because any potential loss is currently neither probable nor reasonably estimatable under SFAS No. 5, Accounting for Contingencies.
On November 30, 2007, the University of Minnesota filed a patent infringement action alleging that the Company's AMPLATZER occlusion devices infringe their method and apparatus patents on septal devices. One of the two patents expired in 2004. The Company believes that it has significant defenses to the litigation, including unenforceability, invalidity and noninfringement. The Company believes this claim is without merit and will continue to vigorously defend its position. As the outcome is uncertain, the Company did not accrue any costs resulting from the claim at December 31, 2007 or 2008.
On January 15, 2008, Dr. Paul Teirstein filed a patent infringement action against the Company in the United States District Court for Minnesota, alleging that the Company's AMPLATZER® occlusion devices infringe Teirstein's patent for body passageway closure apparatus and method of use (U.S. Patent No. 5,499,995). The Company believes that it will have significant defenses to the litigation, which may include unenforceability, invalidity and noninfringement. On September 23, 2009, the Company entered into a settlement agreement with Teirstein in which the parties agreed to the dismissal of all claims and counterclaims with prejudice in exchange for Teirstein's grant to us of a covenant not to sue on the '995 patent on all of our existing and future products, and the lump-sum payment by us to Teirstein in the amount of $375,000.
The Company is subject to other various litigation claims in the normal course of business. Management does not believe that any of these claims will have a material impact on the financial statements.
12. Stock Option Plan
In 2006, the Company adopted a stock option plan (the Plan) pursuant to which the Company's Board of Directors may grant stock options to directors, officers, and key employees. The Plan authorizes grants of options of up to 20,000,000 shares of common stock. In 2008, the Company's Board of Directors increased the Plan by 15,000,000 shares of common stock, to approximate 10% of the Company's total outstanding shares.
Options granted under the Plan typically vest over a five-year period and are generally exercisable for ten years after the date of grant. The exercise price of options granted under the Plan will not be less than 100% of the fair market value on the date of grant.
At December 31, 2007 and 2008, and June 30, 2009, there were 1.7 million, 11.8 million, and 14.3 million shares remaining available for the Company to grant under the Plan, respectively. The fair value of each option award is estimated using the Black-Scholes option-pricing model that used the weighted average assumptions in the following table. The Company uses the shortcut method for estimating expected term as the midpoint between the vesting date and the end of the contractual term. Expected volatility is based on the historical volatilities of peer companies as the Company's stock is
F-36
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
12. Stock Option Plan (Continued)
not actively traded. The risk-free interest rate is based on U.S. Treasury yields in effect at time of grant.
| |
Year Ended December 31, | Six Months Ended June 30, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | 2008 | 2009 | |||||||
| |
|
|
|
(Unaudited) |
||||||||
Valuation assumptions: |
||||||||||||
Expected dividend yield |
—% | —% | —% | —% | —% | |||||||
Expected volatility |
45% | 64% | 54% | 55% | 57% | |||||||
Expected term (years) |
6.50 | 6.50 | 6.50 | 6.50 | 6.50 | |||||||
Risk-free interest rate |
5.04% | 4.96% - 5.04% | 1.87% - 3.57% | 1.97% - 3.57% | 1.83% - 3.18% | |||||||
The weighted average fair value of options granted was $0.52 per share in 2006, $1.24 in 2007, $1.52 in 2008, and $1.53 and $1.55 for the six months ended June 30, 2008 and 2009. The Company uses the straight-line method of expense attribution over the vesting period of the options. The Company recognized compensation expense related to its stock option plan of $1.4 million, $1.9 million and $2.6 million for the years ended December 31, 2006, 2007 and 2008, and $1.3 million and $1.7 million for the six months ended June 30, 2008 and 2009, respectively. The future income tax benefit to be realized by the Company related to this compensation expense is $0.4 million, $0.5 million, and $0.5 million, from the years ended December 31, 2006, 2007, and 2008, and $0.3 million and $0.3 million from the six months ended June 30, 2008 and 2009, respectively.
A summary of stock option activity is as follows:
| |
Options Outstanding |
Weighted Average Exercise Price |
Weighted Average Remaining Term |
Aggregate Intrinsic Value (in thousands) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at January 1, 2007 |
15,358,109 | $ | 1.00 | 8.9 years | $ | — | |||||||
Granted |
3,880,000 | 1.83 | |||||||||||
Exercised |
(50,000 | ) | 1.00 | ||||||||||
Cancelled and Forfeited |
(916,000 | ) | 1.00 | ||||||||||
Balance at December 31, 2007 |
18,272,109 | $ | 1.18 | 8.7 years | $ | 28,749 | |||||||
Granted |
1,795,000 | 2.75 | |||||||||||
Exercised |
(47,000 | ) | 1.00 | ||||||||||
Cancelled and Forfeited |
(477,000 | ) | 1.04 | ||||||||||
Balance at December 31, 2008 |
19,543,109 | $ | 1.33 | 8.0 years | $ | 27,847 | |||||||
Granted |
1,890,000 | 2.75 | |||||||||||
Exercised |
(24,000 | ) | 1.00 | ||||||||||
Cancelled and Forfeited |
(691,000 | ) | 2.43 | ||||||||||
Balance at June 30, 2009 (Unaudited) |
20,718,109 | $ | 1.42 | 8.0 years | $ | 27,490 | |||||||
Options exercisable at December 31, 2007 |
4,431,243 | $ | 1.00 | $ | 7,755 | ||||||||
Options exercisable at December 31, 2008 |
8,024,865 | $ | 1.09 | $ | 13,295 | ||||||||
Options exercisable at June 30, 2009 (Unaudited) |
9,343,865 | $ | 1.16 | $ | 14,844 | ||||||||
F-37
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
12. Stock Option Plan (Continued)
The aggregate intrinsic value in the table above represents the difference between the estimated fair value of common stock and the exercise price, multiplied by the number of in-the-money options that would have been received by the option holders had all option holders exercised their options on December 31, 2006, 2007 and 2008, and June 30, 2009, respectively.
The schedule below reflects the number and weighted average exercise price of outstanding and exercisable options segregated by exercise price ranges:
| |
December 31, 2007 | December 31, 2008 | June 30, 2009 (Unaudited) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Options Outstanding | Options Exercisable | Options Outstanding | Options Exercisable | Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Prices |
Number of Options |
Average Remaining Term |
Number of Options |
Number of Options |
Average Remaining Term | Number of Options |
Number of Options |
Average Remaining Term | Number of Options |
||||||||||||||||
| $1.00 | 15,532,109 | 8.5 years | 4,431,243 | 15,023,109 | 7.3 years | 7,384,865 | 14,888,109 | 7.0 years | 8,180,865 | ||||||||||||||||
| $2.00 | 2,090,000 | 9.6 years | — | 2,075,000 | 8.7 years | 495,000 | 1,915,000 | 8.2 years | 703,000 | ||||||||||||||||
| $2.75 | 650,000 | 9.9 years | — | 2,445,000 | 9.2 years | 145,000 | 3,915,000 | 9.3 years | 460,000 | ||||||||||||||||
| 18,272,109 | 8.7 years | 4,431,243 | 19,543,109 | 8.0 years | 8,024,865 | 20,718,109 | 8.0 years | 9,343,865 | |||||||||||||||||
Stock option activity for non-vested shares under the Plan is as follows:
| |
Options | Weighted Average Grant-Date Fair Value | ||||||
|---|---|---|---|---|---|---|---|---|
Balance at January 1, 2007 |
13,825,488 | $ | 0.52 | |||||
Granted |
3,880,000 | 1.24 | ||||||
Exercised |
(50,000 | ) | 0.52 | |||||
Vested |
(2,898,622 | ) | 0.52 | |||||
Cancelled and Forfeited |
(916,000 | ) | 0.52 | |||||
Balance at December 31, 2007 |
13,840,866 | $ | 0.72 | |||||
Granted |
1,780,000 | 1.53 | ||||||
Exercised |
(14,000 | ) | 0.61 | |||||
Vested |
(3,671,622 | ) | 0.65 | |||||
Cancelled and Forfeited |
(417,000 | ) | 0.57 | |||||
Balance December 31, 2008 |
11,518,244 | $ | 0.83 | |||||
Granted |
1,890,000 | 1.56 | ||||||
Vested |
(1,426,000 | ) | 0.93 | |||||
Cancelled and Forfeited |
(608,000 | ) | 1.56 | |||||
Balance June 30, 2009 (Unaudited) |
11,374,244 | $ | 0.92 | |||||
As of December 31, 2006, 2007 and 2008, and June 30, 2009, there was $6.7 million, $8.5 million, $7.4 million and $8.0 million, of unrecognized compensation cost related to non-vested, share-based compensation arrangements granted under the Plan. That cost is expected to be recognized over a weighted average period of 2.5 years.
Significant Factors Used in Determining Fair Value of the Company's Common Stock
The fair value of the shares of common stock that underlie the stock options the Company has granted has historically been determined by the board of directors of the Company based upon information available to it at the time of grant. Because there has been no public market for the common stock, the board of directors of the Company has determined the fair value of the common stock by utilizing, among
F-38
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
12. Stock Option Plan (Continued)
other things, contemporaneous valuation studies conducted as of April 30, 2006 and June 30, 2007. The findings of these valuation studies were based on the Company's business and general economic, market and other conditions that could be reasonably evaluated at that time. The analyses of the valuation studies incorporated extensive due diligence that included a review of the Company, including its financial results, business agreements, intellectual property and capital structure. The valuation studies also included a thorough review of the conditions of the industry in which the Company operates and the markets that it serves. The methodologies of the valuation studies included an analysis of the fair market value of the Company using three widely accepted valuation methodologies: (1) market multiple, (2) comparable transactions, and (3) discounted cash flow. The board of directors of the Company took these three approaches into consideration when establishing the fair value of the common stock of the Company. In addition, we received input from the underwriters of the offering in October 2007 with respect to valuation of the Company. Based on the foregoing factors, the Company's board of directors increased the fair value of the Company's common stock to $2.75 at October 22, 2007 and the value remained unchanged throughout 2008 and the first six months of 2009.
13. Investment
On November 2, 2006, the Company made an initial equity investment of $2.5 million in Ample Medical, Inc., a privately held company that is focused on the development of minimally invasive medical devices to treat structural heart diseases. The initial investment resulted in an approximate 30.0% ownership interest, accounted for under the equity method. In connection with the investment, the Company also entered into a stock purchase agreement, whereby the Company will have the option to make additional investments totaling $5.0 million that will be available based on the achievement of certain milestone objectives. The Company recorded losses on this investment of $0.1 million, $0.8 million, and $1.2 million based upon its prorated share of estimated losses during the period from November 2, 2006 to December 31, 2006, and the years ended December 31, 2007 and 2008, respectively.
On October 29, 2007, the Company entered into a $700,000 convertible promissory note with Ample Medical, Inc. The loan may be convertible into additional equity securities upon the achievement of certain milestone objectives. At December 31, 2007, the note balance of $700,000 along with accrued interest of $6,125 was recorded as a note receivable—related party.
On January 28, 2008, the Company made an additional $1.9 million equity investment in Ample Medical, Inc., which included the conversion of the above $700,000 promissory note, resulting in an ownership interest of approximately 35.6%. In connection with this investment, the Company entered into an amended and restated stock purchase agreement.
At March 31, 2009, the Company determined that its equity investment in Ample Medical, Inc. was impaired and wrote-off the remaining investment of $2.3 million.
14. Major Customer
A single customer's account balance of $2.3 million and $2.7 million represented approximately 11.2% and 10.0% of the Company's consolidated accounts receivable balances at December 31, 2007 and 2008, respectively.
F-39
AGA Medical Holdings, Inc.
Notes to Consolidated Financial Statements (Continued)
15. Geographic Information
Net sales to external customers and long-lived assets by geography are as follows:
| |
Year Ended December 31, | Six Months Ended June 30, | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands)
|
2006 | 2007 | 2008 | 2008 | 2009 | |||||||||||||
| |
|
|
|
(Unaudited) |
||||||||||||||
Net sales: |
||||||||||||||||||
United States |
$ | 51,880 | $ | 61,859 | $ | 68,034 | $ | 32,747 | $ | 36,748 | ||||||||
International: |
||||||||||||||||||
Europe |
44,875 | 58,162 | 64,436 | 33,464 | 40,275 | |||||||||||||
Other |
30,774 | 27,234 | 34,426 | 14,643 | 17,358 | |||||||||||||
Total International |
75,649 | 85,396 | 98,862 | 48,098 | 57,633 | |||||||||||||
Total net sales |
$ | 127,529 | $ | 147,255 | $ | 166,896 | $ | 80,845 | $ | 94,381 | ||||||||
| |
December 31, | June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands)
|
2007 | 2008 | 2009 | ||||||||
| |
|
|
(Unaudited) |
||||||||
Long-lived assets: |
|||||||||||
United States |
$ | 198,877 | $ | 189,552 | $ | 182,915 | |||||
International |
7,821 | 13,077 | 71,252 | ||||||||
Total long-lived assets |
$ | 206,698 | $ | 202,629 | $ | 254,167 | |||||
F-40
REPORT OF INDEPENDENT AUDITORS
The
Board of Directors of
AGA Medical Corporation
We have audited the accompanying balance sheets of The AGA Division of AB Medica S.p.A. (the "Division") as of December 31, 2006, 2007 and 2008, and the related statements of income, parent company investment, and cash flows for each of the three years in the period ended December 31, 2008. These financial statements are the responsibility of The Division's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of The AGA Division of AB Medica S.p.A. at December 31, 2006, 2007 and 2008, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with U.S. generally accepted accounting principles.
/s/ Reconta Ernst & Young S.p.A.
Milan,
Italy
June 5, 2009
F-41
The AGA Division of AB Medica S.p.A.
Balance Sheets
(in € thousands)
| |
December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | ||||||||
Assets |
|||||||||||
Current assets: |
|||||||||||
Cash and cash equivalents |
€ | 2,023 | € | 2,523 | € | 1,529 | |||||
Accounts receivables, third parties of which securitized financial assets €3,723, €4,819, €6,663 at December 31, 2006, 2007 and 2008 |
9,453 | 9,435 | 10,621 | ||||||||
Accounts receivables, related parties |
220 | 154 | 202 | ||||||||
Inventories, net |
993 | 1,085 | 1,408 | ||||||||
Prepaid expenses and other current receivables |
148 | 121 | 214 | ||||||||
Income tax receivable |
— | — | 91 | ||||||||
Deferred tax assets |
206 | 239 | 217 | ||||||||
Total current assets |
13,043 | 13,557 | 14,282 | ||||||||
Property and equipment, net |
1,240 | 1,087 | 948 | ||||||||
Intangible assets, net |
233 | 155 | 81 | ||||||||
Other assets |
29 | 29 | 41 | ||||||||
Total assets |
€ | 14,545 | € | 14,828 | € | 15,352 | |||||
Liabilities and Parent Company Investment |
|||||||||||
Current liabilities: |
|||||||||||
Advances from financial institutions |
€ | 3,740 | € | 5,042 | € | 7,115 | |||||
Obligations under capital lease, current portion |
51 | 54 | 57 | ||||||||
Trade accounts payable, third parties |
200 | 314 | 172 | ||||||||
Trade accounts payable, AGA Medical Corporation |
1,321 | 1,270 | 2,005 | ||||||||
Accrued expenses |
597 | 602 | 535 | ||||||||
Income taxes payable |
529 | 818 | — | ||||||||
Total current liabilities |
6,438 | 8,100 | 9,884 | ||||||||
Deferred tax liabilities |
40 | 50 | 59 | ||||||||
Obligations under capital lease, less current portion |
460 | 407 | 350 | ||||||||
Employees severance indemnities |
228 | 194 | 163 | ||||||||
Parent company investment |
7,379 | 6,077 | 4,896 | ||||||||
Total liabilities and parent company investment |
€ | 14,545 | € | 14,828 | € | 15,352 | |||||
The accompanying notes are an integral part of these financial statements.
F-42
The AGA Division of AB Medica S.p.A.
Statements of Income
(in € thousands)
| |
Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | ||||||||
Net sales, third parties |
€ | 14,358 | € | 16,338 | € | 17,471 | |||||
Net sales, related parties |
665 | 397 | 427 | ||||||||
Cost of goods sold, third parties |
87 | 63 | 75 | ||||||||
Cost of good sold, AGA Medical Corporation |
5,983 | 5,871 | 6,600 | ||||||||
Gross profit |
8,953 | 10,801 | 11,223 | ||||||||
Operating expenses: |
|||||||||||
Selling, general, and administrative |
3,207 | 4,271 | 4,931 | ||||||||
Amortization of intangible assets |
80 | 78 | 79 | ||||||||
Operating income |
5,666 | 6,452 | 6,213 | ||||||||
Financial income |
101 | 80 | 132 | ||||||||
Financial expense |
(376 | ) | (564 | ) | (426 | ) | |||||
Other income (expense), net |
11 | 28 | 87 | ||||||||
Income before income taxes |
5,402 | 5,996 | 6,006 | ||||||||
Provision for income taxes |
(2,095 | ) | (2,371 | ) | (2,061 | ) | |||||
Net income |
€ | 3,307 | € | 3,625 | € | 3,945 | |||||
The accompanying notes are an integral part of these financial statements.
F-43
The AGA Division of AB Medica S.p.A.
Statements of Parent Company Investment
(in € thousands)
| |
Parent Company Investment |
|||||
|---|---|---|---|---|---|---|
Balance as of December 31, 2005 |
€ | 4,737 | ||||
Net transfers to Parent Company |
(665 | ) | ||||
Net income for the year ended December 31, 2006 |
3,307 | |||||
Balance as of December 31, 2006 |
7,379 | |||||
Net transfers to Parent Company |
(4,927 | ) | ||||
Net income for the year ended December 31, 2007 |
3,625 | |||||
Balance as of December 31, 2007 |
6,077 | |||||
Net transfers to Parent Company |
(5,126 | ) | ||||
Net income for the year ended December 31, 2008 |
3,945 | |||||
Balance as of December 31, 2008 |
€ | 4,896 | ||||
The accompanying notes are an integral part of these financial statements.
F-44
The AGA Division of AB Medica S.p.A.
Statements of Cash Flows
(in € thousands)
| |
Years Ended December 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | |||||||||
Operating activities: |
||||||||||||
Net income |
€ | 3,307 | € | 3,625 | € | 3,945 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
Depreciation and amortization |
303 | 301 | 302 | |||||||||
Change in deferred taxes |
(40 | ) | (23 | ) | 31 | |||||||
Change in employee severance indemnities |
6 | (34 | ) | (31 | ) | |||||||
Changes in operating assets and liabilities: |
||||||||||||
Accounts receivable |
(2,054 | ) | 84 | (1,234 | ) | |||||||
Inventory |
(131 | ) | (92 | ) | (323 | ) | ||||||
Prepaid expenses and other assets |
(148 | ) | 27 | (93 | ) | |||||||
Trade accounts payable |
330 | 63 | 593 | |||||||||
Income taxes |
529 | 289 | (909 | ) | ||||||||
Accrued expenses |
597 | 5 | (67 | ) | ||||||||
Net cash provided by operating activities |
2,699 | 4,245 | 2,214 | |||||||||
Investing activities: |
||||||||||||
Purchases of property and equipment |
(187 | ) | (70 | ) | (84 | ) | ||||||
Purchases of intangibles |
(139 | ) | — | (5 | ) | |||||||
Purchase of other assets |
— | — | (12 | ) | ||||||||
Net cash used in investing activities |
(326 | ) | (70 | ) | (101 | ) | ||||||
Financing activities: |
||||||||||||
Net change in short-term borrowings |
€ | (1,450 | ) | € | 1,302 | € | 2,073 | |||||
Lease payments |
(48 | ) | (50 | ) | (54 | ) | ||||||
Net transfers to Parent Company |
(665 | ) | (4,927 | ) | (5,126 | ) | ||||||
Net cash used in financing activities |
(2,163 | ) | (3,675 | ) | (3,107 | ) | ||||||
Net change in cash and cash equivalents |
210 | 500 | (994 | ) | ||||||||
Cash and cash equivalents at beginning of period |
1,813 | 2,023 | 2,523 | |||||||||
Cash and cash equivalents at end of period |
€ | 2,023 | € | 2,523 | € | 1,529 | ||||||
Supplemental disclosures of cash flow information: |
||||||||||||
Interest paid |
€ | 318 | € | 386 | € | 220 | ||||||
Taxes paid |
€ | 1,607 | € | 2,105 | € | 2,939 | ||||||
The accompanying notes are an integral part of these financial statements.
F-45
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements
December 31, 2006, 2007 and 2008
(in € thousands)
1. Description of Business
AB Medica S.p.A. ("AB Medica" or the "Parent Company") started in 1997 to distribute on the Italian market minimally invasive devices to treat structural heart defects and vascular diseases, which AB Medica markets under the AGA brand. Such devices are acquired from AGA Medical Corporation (previously Amplazter Corporation). The Italian operations until December 10, 2008 were carried out by a business division within AB Medica ("AGA Business" or the "Company"). On December 10, 2008 the division was spun-off from AB Medica and contributed to a newly formed company, AB Medica-AGA Division S.r.l.. The Company markets a complete line of minimally invasive, transcatheter treatments to occlude, or close holes, in the septum of the heart, as well as, to occlude abnormalities in blood vessels outside of the heart. Products of the AGA Business are sold in Italy through a combination of direct sales and the use of local distributors. The Company has been the exclusive distributor of the AGA Business products in Italy during the three years ended December 31, 2008.
2. Summary of Significant Accounting Policies and Basis of Presentation
Basis of Preparation of Financial Statements
The accompanying financial statements consisting of balance sheets, statements of income and cash flows and of Parent Company investment, related financial data and the other information disclosed in these explanatory notes are derived from the financial statements prepared under Italian Generally Accepted Accounting Principles ("Italian GAAP") of AB Medica and, starting from December 10, 2008, of AB Medica-AGA Division S.r.l.. The Italian GAAP amounts related to the AGA Business were subsequently adjusted to apply accounting principles generally accepted in the United States (US GAAP).
The financial statements have been prepared on the basis of management's identification of the AGA Business, which formed part of AB Medica financial statements as of December 31, 2006, 2007 and 2008 and of AB Medica-AGA Division S.r.l. financial statements as of December 31, 2008 and for the period ended December 10, 2008 to December 31, 2008. The accompanying financial statements have been prepared on a going concern basis, and, in the opinion of the Company's management, have been prepared on a consistent basis and include all adjustments, consisting of normal recurring accruals, necessary for the fair presentation of the Company's financial position, results of operations and cash flows for the years ended December 31, 2006, 2007 and 2008.
The AGA Business activities were not previously separately reported within AB Medica financial statements in prior years. The spun-off business of the Company prior to December 10, 2008 was carried out on an integrated basis as part of AB Medica and, as such, the operations comprising the spun-off activities have been carved out from the financial statements of AB Medica. Accordingly, certain revenues, expenses, assets and liabilities have been allocated by management to reflect those attributable to the spun-off activities. In order to prepare these financial statements management has either specifically identified transactions (direct usage) or proportional cost methods based on specific drivers, computed on specifically identified ratios (such as amount of the respective revenues or number of the respective employees, as described below).
The asset, liability, revenue and expense allocated to the AGA Business were determined on bases that AB Medica management considered to be a reasonable reflection of the utilization of services
F-46
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
provided to or the benefit received by the AGA Business. Because of the allocations referred to above, the financial information presented for the years 2006, 2007 and 2008 is not indicative of the Company's financial position, results of operations or cash flows in subsequent periods nor is it necessarily indicative of what the Company's financial position, results of operations or cash flows would have been if the Company had been a separate, stand-alone entity in periods prior to December 10, 2008.
In particular these financial statements have been prepared on the basis set out below:
Directly attributable assets and liabilities
Certain of the assets and liabilities were directly attributable to the AGA Business. These include trade receivables, inventories, property and equipment and trade payables.
Indirectly attributable assets and liabilities
Where assets were obtained or liabilities were incurred for the benefit of AGA's activities, but are not attributable to specific business, for instance trade payables where goods and services were provided to both AGA and AB Medica, a reasonable method (based on proportionality ratios) of allocating common assets and liabilities has been identified and applied.
Revenues and related costs
Revenues of the AGA Business are specifically identifiable from the total revenues of AB Medica.
Certain costs were directly attributable to the AGA Business. These include mainly direct materials costs, transport expenses on purchases, commissions and agent's fees and the gain or loss realized on payments made in US Dollars.
Certain other costs have been allocated as follows:
- •
- staff and related costs have been allocated primarily based upon the proportion of average working hours dedicated to the
AGA Business over the total AB Medica's working hours available;
- •
- the portion of commercial, administrative expenses and costs related to the AB Medica structure not directly attributable to the AGA Division has been primarily allocated to such Division, based on the nature and destination of the costs, applying a driver linked to the incidence of the AGA Division's sales on AB Medica total sales. Commercial and administrative expenses and costs directly attributable to the AGA Division relate to sales commissions and fees, maintenance fees and rent expense for equipment used solely by the AGA Division. Costs allocated to the AGA Division based on the sales' driver, include other services, rentals, leases and depreciation (being related: (i) to the office building and warehouse, both not physically segregated between the two divisions of AB Medica, and (ii) rentals of the cars of the sales' managers working for both divisions). Certain other costs and expenses are allocated on the basis of different drivers, such as the Board of Directors' fees and expenses that have been
F-47
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
allocated to the AGA Division based on the estimated hours spent by the Chief Executive Officer for the AGA Division.
The management of AB Medica believes that the allocation of such costs have been calculated on reasonable bases and that the total amount of costs recognized to the AGA Business in the statements of income approximates what its actual costs would have been as a stand-alone entity.
Cash flow statement
Prior to December 10, 2008, the AGA Business did not maintain its own bank accounts, and therefore, all cash receipts and payments have been processed by AB Medica on its behalf. The cash flow statement has been presented as if such receipts and payments had been received or made by the Company.
Parent Company investment and net financial position
The Parent Company investment represents the carved-out net assets of the AGA Business of AB Medica as of January 1, 2006 and it is adjusted to reflect the net income for the year ended December 31, 2006, 2007 and 2008 of the AGA Business, net of the transfers to the Parent Company.
There were no borrowings specifically attributable to the AGA Business, apart from a financial lease. Accordingly, no borrowings are reflected in the accompanying balance sheets as of December 31, 2006, 2007 and 2008. The AGA Business needed no long-term borrowing but required short-term lines of credit to finance its working capital. AGA financed its working capital through factoring arrangements (See Note 4 and Note 7 below). Therefore, only interest expense related to the discounting of the AGA Business receivables under the factoring arrangements has been allocated to the AGA business.
The excess of cash generated by the AGA Business with respect to AB Medica available cash at year-end, has been considered as a transfer to the Parent Company. Such transfer to the Parent Company amounted to €665, €4,927 and €5,126 for the years ended December 31, 2006, 2007 and 2008, respectively.
Income taxes
The Company utilizes the liability method of accounting for income taxes in accordance with SFAS No. 109 Accounting for Income Taxes ("SFAS 109"). Under the liability method, deferred taxes assets and liabilities are determined based on the differences between the carrying value and tax bases of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets when it is not more likely than not that a tax benefit will be realized.
For the years ended December 31, 2006, 2007 and the period ended December 10, 2008, AGA was not a separate legal entity. The results of the business during these periods were included in the tax returns of AB Medica. However, taxes for the years presented have been specifically re-computed as if the Company were a stand alone entity.
F-48
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Recently Issued Accounting Standards
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurement. SFAS No. 157 defines fair value, establishes a framework for the measurement of fair value, and enhances disclosure about fair value measurement. The statement does not require any new fair value measures. The provisions under SFAS No. 157 are effective for all financial assets and liabilities and for nonfinancial assets and liabilities recognized or disclosed at fair value in our financial statements on a recurring basis for the Company beginning January 1, 2008, and are expected to be applied prospectively. For all other nonfinancial assets and liabilities, SFAS No. 157 is effective beginning January 1, 2009.
The Company has adopted the provisions of SFAS No. 157 for financial assets and liabilities that are measured at fair value for our fiscal year beginning January 1, 2008. The adoption did not have a material effect on the financial statements. The provisions of SFAS No. 157 have not been applied to nonfinancial assets and liabilities.
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities. SFAS No. 159 expands opportunities to use fair value measurements in financial reporting and permits entities to choose to measure many financial instruments and certain other items at fair value. SFAS No. 159 is effective for our fiscal year beginning January 1, 2008. The adoption of this statement did not have a material effect on the financial statements. The Company did not elect the option for any assets or liabilities not already recorded at fair value, but retains the option to elect fair value for new assets or liabilities acquired and or incurred.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), Business Combinations (SFAS No. 141(R)). SFAS No. 141(R) and SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements, an Amendment of ARB No. 51 (SFAS No. 160). SFAS No. 141(R) and is effective for the Company's fiscal year beginning January 1, 2009, with early adoption prohibited. The implementation of SFAS No. 141(R) and SFAS No. 160 is not expected to have a material effect on the financial statements.
In April 2009, the FASB issued three related Staff Positions (FSP): (i) FSP 157-4, Determining Fair Value When the Volume and Level of Activity for the Asset or Liability have Significantly Decreased and Identifying Transactions That Are Not Orderly (FSP 157-4), (ii) FSP Statement of Financial Accounting Standard (SFAS) 115-2 and SFAS 124-2, Recognition and Presentation of Other-Than-Temporary Impairments, (FSP SFAS 115-2 and SFAS 124-2), and (iii) FSP SFAS 107-1 and Accounting Principles Board (APB) 28-1, Interim Disclosures about Fair Value of Financial Instruments, (FSP SFAS 107 and APB 28-1), each of which will be effective for interim and annual periods ending after June 15, 2009. FSP 157-4 provides guidance on how to determine the fair value of
F-49
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
assets and liabilities under SFAS 157 Fair Value Measurements, in the current economic environment and reemphasizes that the objective of a fair value measurement remains the determination of an exit price. If we were to conclude that there has been a significant decrease in the volume and level of activity of the asset or liability in relation to normal market activities, quoted market values may not be representative of fair value and we may conclude that a change in valuation technique or the use of multiple valuation techniques may be appropriate. FSP SFAS 115-2 and SFAS 124-2 modify the requirements for recognizing other-than-temporarily impaired debt securities and revise the existing impairment model for such securities by modifying the current intent and ability indicator in determining whether a debt security is other-than-temporarily impaired. FSP SFAS 107 and APB 28-1 enhance the disclosure of instruments under the scope of SFAS 157 for both interim and annual periods. The Company does not expect that these Staff Positions will have a significant impact on the financial statements of the Company.
In April 2009, the FASB issued FSP No. 141R-1 Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies (FSP 141R-1). FSP 141R-1 amends the provisions in FASB Statement 141R for the initial recognition and measurement, subsequent measurement and accounting, and disclosures for assets and liabilities arising from contingencies in business combinations. FSP 141R-1 eliminates the distinction between contractual and non-contractual contingencies, including the initial recognition and measurement criteria in Statement 141R and instead carries forward most of the provisions in SFAS 141 for acquired contingencies. FSP 141R-1 is effective for contingent assets and contingent liabilities acquired in business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The Company does not expect that this will have a significant impact on the financial statements of the Company.
In March 2008, FASB issued Statement No. 161, Disclosures about Derivative Instruments and Hedging Activities. The new standard, commonly referred to as FAS 161, amends FAS 133, Accounting for Derivative Instruments and Hedging Activities. FAS 161 is effective for fiscal years beginning after November 15, 2008. Prior year disclosures are not required at initial adoption. The Company does not expect that this will have a significant impact on the financial statements of the Company.
In June 2006, the Financial Accounting Standards Board ("FASB") issued Interpretation No. 48, Accounting for Uncertainty in Income Taxes, an interpretation of FAS 109, Accounting for Income Taxes (FIN 48), to create a single model to address accounting for uncertainty in tax positions. FIN 48 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold to meet before a tax position is being recognized in the financial statements. FIN 48 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition. FIN 48 was effective for fiscal years beginning after December 15, 2006. According to the deferral of the effective date of FIN 48 for non public enterprises, the company elects not to adopt FIN 48.
F-50
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
Cash Equivalents
The Company considers all highly liquid financial instruments with contractual maturities of three months or less when purchased to be cash equivalents. The carrying value of cash and cash equivalents approximates fair value.
Accounts Receivable and Allowance for Doubtful Accounts
The Company has receivables from a diversified customer base. The creditworthiness of customers is monitored before sales are approved. The Company records an allowance for doubtful accounts based on past history, current economic conditions, and the composition of its accounts receivable aging and, in some cases, makes allowances for specific customers based on several factors, such as the creditworthiness of those customers and payment history.
Inventory
Inventory is valued at the lower of acquisition cost or market with cost determined using the first-in first-out method. The Company makes adjustments to the value of inventory based on estimates of potentially excess and obsolete inventory after considering forecasted demand and forecasted average selling prices.
Property and equipment
Property and equipment are stated at cost less accumulated depreciation. Additions and improvements that extend the lives of assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. Depreciation is provided using the straight-line method over the estimated useful lives of the individual assets and ranges from 3 to 33 years. Office furniture and equipment are depreciated over 4 to 6 years, building costs are depreciated over 33 years, leasehold improvements are depreciated over the life of the lease.
Intangible Assets
Intangible assets with finite useful lives are amortized either on a straight-line basis over the asset's estimated useful life or on a basis that reflects the pattern in which the economic benefits of the asset are realized. The estimated useful life for computer software costs is estimated as being 5 years. In no case is the useful life in excess of the legal or contractual period.
Impairment of Long-Lived Assets
Long-lived assets, primarily property, plant, and equipment and intangible assets with finite lives, are periodically reviewed and evaluated by the Company when events and circumstances indicate that the carrying amount of these assets may not be recoverable. For long-lived assets, this evaluation is based on the expected future undiscounted operating cash flows of the related assets. Should such evaluation result in the Company concluding that the carrying amount of long-lived assets has been impaired, an appropriate write-down to their fair value is recorded.
F-51
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
Revenue Recognition
The Company sells its products directly to state owned hospitals and private clinics. The Company recognizes revenue when all of the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred to a third-party shipper; the sales price is fixed or determinable; and collectability is reasonably assured. These criteria are met at the time of shipment when the risk of loss and title passes to the customer.
The Company allows for product returns in certain circumstances, such as damaged or faulty products, products that are past their sterility dates, and physician mis-sizing. Allowances are provided for estimated product returns at the time of sale based on historical returns experience and recorded as a reduction of revenue. Reserve for sales returns, classified as a reduction of Trade Receivables, were €494, €489 and €438, at December 31, 2006, 2007 and 2008, respectively.
Shipping Costs
Shipping costs are classified as cost of goods sold.
Income Taxes
Income taxes are accounted for under the liability method. Deferred income taxes are provided for temporary differences between financial reporting and tax bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established to reduce deferred tax assets to the amounts expected to be realized when it is not deemed more likely than not that a deferred tax asset will be realized.
Advertising
The Company expenses advertising costs as incurred. Total advertising expense is €320, €679 and €403, for the years ended December 31, 2006, 2007 and 2008, respectively.
Foreign Currency Translation and Transaction Gains and Losses
The Company's reporting currency is the euro ("euro", "Euro" or "€"). All assets and liabilities are translated into Euro at year-end exchange rates. Gains and losses on foreign currency transactions are determined using the exchange rates in effect at the date of the transaction, and related amounts are included in financial income (expense).
Derivative Financial Instruments
The Company monitors its exposure to various business risks including interest rates and foreign currency exchange rates and occasionally uses derivative financial instruments to manage the impact of certain of these risks. At the inception of a new derivative, the AGA Business designates the derivative as a cash flow or fair value hedge or determines the derivative to be undesignated as a hedging
F-52
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
instrument based on the underlying facts. The AGA Business does not enter into derivative instruments for trading purposes.
The derivatives instruments stipulated by the AGA Business during the three year period have not been designated as, nor qualified for hedge accounting treatment, and as such, all changes in fair value are treated as gains or losses for the period through the statement of income.
The Company's foreign exchange derivatives are initially recognized at fair value at the date a derivative contract is entered into and subsequently remeasured to their fair value at each balance sheet date. The resulting gain or loss is recognized in the income statement in financial income (expense).
The net effect recorded in the statement of income related to fair value of foreign exchange derivatives was a loss of €58 and €95 for the years ended December 31, 2006 and 2007, respectively, and a gain of €98 for the year ended December 31, 2008.
Total fair value of foreign exchange derivatives included in accrued expenses is €58, €153 and €55, respectively, at December 31, 2006, 2007 and 2008. The fair value of the foreign exchange derivatives is based on listed market prices if available. If a listed market price is not available then fair value is determined using other observable inputs. We believe the carrying values of these instruments determined using other observable inputs (Level 2) approximated their fair values at December 31, 2006, 2007 and 2008.
The fair value has been allocated to the Company based on the proportion of the net foreign exchange exposure position of the Company as compared to AB Medica's total exposure.
The total notional value of foreign exchange derivatives is $1,704, $1,837 and $2,655, respectively, at December 31, 2006, 2007 and 2008.
Provision for Employee Severance Indemnities
In accordance with the Italian severance pay statutes, an employee benefit is accrued for service to date and is payable immediately upon separation. The provision for staff leaving indemnity is calculated in accordance with local civil and labor laws based on each employee's or agent's length of service, employment category and remuneration. The termination liability is adjusted annually by a cost of living index provided by the Italian Government. There is no vesting period or funding requirement associated with the liability. The liability recorded in the balance sheet is the amount that the employee would be entitled to if the employee separates immediately. The provisions charged to earnings for each of the three years ended December 31, 2006, 2007 and 2008 are €62, €71 and €80, respectively.
Concentration of Credit Risk
A single customer's account balance of €1,174, €640 and €710 represented approximately 13%, 7.1% and 7.8% of the Company's accounts receivable balances at December 31, 2006, 2007 and 2008, respectively.
F-53
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
2. Summary of Significant Accounting Policies and Basis of Presentation (Continued)
The sales are almost exclusively performed towards Italian customers, consequently the AGA Business results and profitability are dependent on the economic conditions of the Italian market; in particular, all the customers are within the state and private health sector, which dynamics and development influence the sales of the AGA Business.
Foreign exchange risk
The AGA Business costs and results are affected by exchange rate fluctuations, particularly between Euro and US dollar. The Company enters into foreign exchange derivatives in order to reduce such risk.
Risk related to products
Any actual or even alleged defects or other quality issues in the AGA Business products, services and solutions could adversely affect the sales, results, reputation and the value of the AGA brand.
3. Inventories, Net
Inventories consist of the following:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (in € thousands) |
2006 | 2007 | 2008 | |||||||
Finished goods |
€ | 993 | € | 1,085 | € | 1,606 | ||||
Obsolescence reserve |
— | — | (198 | ) | ||||||
Inventory, net |
€ | 993 | € | 1,085 | € | 1,408 | ||||
Included in finished goods are amounts on consignment at third parties for €197, €245 and €257, respectively, at December 31, 2006, 2007 and 2008
4. Accounts Receivables, Third Parties Transferred to Factors (Securitized Financial Assets)
The Company's accounts receivables third parties transferred to factors include amounts sold under either recourse or without recourse agreements. As part of the transfer without recourse the Company continues to pay interest on outstanding receivables up to periods agreed in the agreements. Accordingly, the Company continues to recognize the full carrying amount of all receivables and has recognized the cash received on the transfers as a secured borrowing. The carrying amounts of all transferred receivables, which have been pledged as security for the borrowing, are as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (in € thousands) |
2006 | 2007 | 2008 | |||||||
Without recourse |
€ | 3,653 | € | 4,819 | € | 5,006 | ||||
With recourse |
70 | — | 1,657 | |||||||
Total |
€ | 3,723 | € | 4,819 | € | 6,663 | ||||
F-54
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
5. Property and Equipment, Net
Property and equipment consist of the following:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (in € thousands) |
2006 | 2007 | 2008 | |||||||
Manufacturing equipment |
€ | 98 | € | 94 | € | 91 | ||||
Office furniture and equipment |
457 | 466 | 454 | |||||||
Land |
219 | 219 | 219 | |||||||
Building |
510 | 510 | 510 | |||||||
Leasehold improvements |
767 | 802 | 854 | |||||||
|
2,051 | 2,091 | 2,128 | |||||||
Less accumulated depreciation |
(811 | ) | (1,004 | ) | (1,180 | ) | ||||
|
€ | 1,240 | € | 1,087 | € | 948 | ||||
Total depreciation expense of property and equipment was €223, €223, and €223, for the years ended December 31, 2006, 2007 and 2008, respectively. Land and buildings represent assets acquired under a capital lease allocated to the business based on the proportion of total AGA Business revenues to AB Medica revenues.
Included in the total depreciation expense is the depreciation expense for assets acquired under a capital lease of €15, €15, and €15 for the years ended December 31, 2006, 2007 and 2008, respectively.
6. Intangible Assets
Intangible assets consist of the following:
| |
|
As of December 31, 2006 | As of December 31, 2007 | As of December 31, 2008 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Weighted Average Useful Life (in Years) |
||||||||||||||||||||||||||||||
| (in € thousands) |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||||||||||||||
Software licenses |
5 | 313 | (80 | ) | 233 | 313 | (158 | ) | 155 | 319 | (238 | ) | 81 | ||||||||||||||||||
|
€ | 313 | € | (80 | ) | € | 233 | € | 313 | € | (158 | ) | € | 155 | € | 318 | € | (237 | ) | € | 81 | ||||||||||
Intangible assets are amortized using methods that approximate the benefit provided by the utilization of the assets. Total amortization expense of intangible assets was €80, €78 and €79, respectively, for the years ended December 31, 2006, 2007 and 2008.
7. Advances from Financial Institutions
AB Medica uses factoring agreements in order to ensure a constant cash flow in particular with regards to accounts receivables due from state hospital clients, to whom are granted extended payment terms.
F-55
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
7. Advances from Financial Institutions (Continued)
Up to the end of 2006 AB Medica used several factoring companies (Mediofactoring, ABF Factoring, GE Capital, Carige Factoring, Ifitalia, Centro Factoring) operating exclusively with transfers with recourse (the terms of the agreements provided for an immediate advance which varies from 80 to 90% of the amount of the receivable).
At the end of 2006 AB Medica reduced the number of factoring companies used and agreed with Ifitalia and Centro Factoring the terms and conditions for a transfer agreement without recourse (immediate advance of 100%, with commission on factoring and quarterly interest rates deferred).
Before the above change the conditions that were applied by the factor companies in 2006 were as follows:
Ifitalia—assignment
without recourse
Interest rate: Euribor 1 month + 3.5%
Flat commission: 1.5%
Handling expenses
Abf—assignment
with recourse
Interest rate: Euribor 3 months + 2%
Flat commission: 0.5%
Handling expenses
MCC—assignment
with recourse
Interest rate: Euribor 3 months letter + 1%
Flat commission 0.4%
Handling expenses
Centro
factoring contract—00/OLFT—assignment without recourse
Interest rate: Euribor 3 months + 1.8%
Flat commission 0.4%
Handling expenses
Centro
factoring contract—03/OLFT—assignment without recourse
Interest rate: Euribor 3 months + 0.9%
Flat commission 0.05%
Handling expenses
Centro
factoring contract—04/OLFM—assignment without recourse
Interest rate: Euribor 3 months + 1.8%
Flat commission 0.4%
Handling expenses
Centro
factoring contract—06/OLFM—assignment without recourse
Interest rate: Euribor 3 months + 0.9%
Flat commission 0.05%
Handling expenses
F-56
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
7. Advances from Financial Institutions (Continued)
At the end of 2007 AB Medica obtained from Ifitalia SpA new conditions that lead to the transfer without recourse of the receivables with an immediate advance of 100% deducting convened price with discount calculated on a DSO average of clientele). With regard to other transactions in 2007 the conditions that were applied by the factor companies were as follows:
Ifitalia—contract
48882/352—assignment without recourse
Interest rate: Euribor 1 month + 3.5%
Ifitalia—contract
56514/352—assignment with recourse
Interest rate: Euribor 1 month + 3%
Centro
Factoring—contract 00/OLFT—assignment without recourse
Interest rate: Euribor 3 Months + 3.5%
Flat commission 0.13%
Handling expenses
Centro
Factoring—contract 03/OLFT—assignment without recourse
Interest rate: Euribor 3 Months + 0.9%
Flat commission: 0.05%
Handling expenses
Centro
Factoring—contract 06/OLFT—assignment without recourse
Interest rate: Euribor 3 Months + 0.9%
Flat commission: 0.05%
Handling expenses
During 2008, AB Medica carried out a "spot transfer with recourse" operation with Ifitalia. The conditions which were applied during 2008 are the following:
Ifitalia—assignment
with recourse with a discount with definitive title
Factoring Commission: Euribor 3 months letter +1.60%
The usual conditions applied by factors are as follows:
Ifitalia—assignment
without recourse
Interest rate: Euribor 3 months letter + 1%
Flat Commission 0.05% per month
Handling expenses
Centro
Factoring—assignment without recourse having definite title
Interest rate: Euribor 3 months letter + 1.15%
Flat commission: 0.05% monthly
Plus factoring on payable accounts receivables due over 180 days: 0.1%
Due to the fact that the "legal isolation criteria" provided for by FAS 140 has not been met by the transfers of receivables performed by AB Medica, account receivables have not been derecognized from the balance sheet.
F-57
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
7. Advances from Financial Institutions (Continued)
Advances from financial institutions as of December 31, 2006, 2007 and 2008 consist of:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (in € thousands) |
2006 | 2007 | 2008 | |||||||
Secured borrowings |
€ | 3,723 | € | 4,819 | € | 6,663 | ||||
Unsecured borrowings |
17 | 223 | 452 | |||||||
|
€ | 3,740 | € | 5,042 | € | 7,115 | ||||
Secured borrowing relates to advance received relating to the sale of receivables with or without recourse.
8. Commitments and Contingencies
Commitments and contingencies as of December 31, 2006, 2007 and 2008 consist of obligations under capital leases.
| |
As of December 31, 2006 | As of December 31, 2007 | As of December 31, 2008 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (in € thousands) |
Minimum payments |
Present value of payments |
Minimum payments |
Present value of payments |
Minimum payments |
Present value of payments |
|||||||||||||
Within one year |
76 | 51 | 76 | 54 | 76 | 57 | |||||||||||||
Later than 1 and not later than five |
306 | 232 | 461 | 407 | 384 | 350 | |||||||||||||
Later than five years |
231 | 228 | |||||||||||||||||
Total minimum lease payments |
613 | 511 | 537 | 461 | 460 | 407 | |||||||||||||
Less amounts representing finance charges |
(102 | ) | (76 | ) | (53 | ) | |||||||||||||
Present value of minimum lease payments |
€ | 511 | € | 511 | € | 461 | € | 461 | € | 407 | € | 407 | |||||||
The capital lease relates to land and buildings under a 10 year lease contract which commenced on May 1, 2002. The agreement foresees a purchase option clause to be paid at the end of the lease equal to €219. Lease payments are linked to the fluctuation of the monthly average of the 6 months Euribor rate. The minimum lease payments are based on the rate existing at the inception of the lease; any increases or decreases in lease payments that result from subsequent changes in the index or rate are contingent rentals and thus affect the determination of income as accruable.
Contingent rent recognized as an expense was €2, €15 and €19, respectively, for the years ended December 31, 2006, 2007 and 2008.
F-58
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
9. Income Taxes
The provision for income taxes is based on earnings before income taxes reported for financial statement purposes.
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In € thousands |
2006 | 2007 | 2008 | |||||||
Current tax expense |
||||||||||
IRES (Corporate Tax) |
1,828 | 2,032 | 1,688 | |||||||
IRAP (Local Tax) |
308 | 362 | 342 | |||||||
Total current tax expense |
2,136 | 2,394 | 2,030 | |||||||
Deferred tax expense |
(41 | ) | (23 | ) | 31 | |||||
Total tax charge |
€ | 2,095 | € | 2,371 | € | 2,061 | ||||
A reconciliation of the statutory income tax rate to the effective tax rate is as follows:
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | |||||||
IRES/IRAP tax statutory rate |
37.25 | % | 37.25 | % | 31.40 | % | ||||
Entertaining expenses |
0.60 | 1.17 | 2.31 | |||||||
Lease rentals |
0.54 | 0.17 | 0.23 | |||||||
Other permanent differences |
0.39 | 0.95 | 0.38 | |||||||
Effective income tax rate |
38.78 | % | 39,54 | % | 34.32 | % | ||||
Significant components of deferred tax assets and liabilities are as follows:
| |
December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (in € thousands) |
2006 | 2007 | 2008 | ||||||||
Deferred tax assets: |
|||||||||||
Allowance for product returns |
€ | 184 | € | 182 | € | 138 | |||||
Obsolescence reserve |
— | — | 62 | ||||||||
Financial derivatives |
22 | 57 | 17 | ||||||||
Writeoff of start up expenses |
15 | 18 | 10 | ||||||||
Less valuation allowance |
(— | ) | (— | ) | (— | ) | |||||
Total deferred tax assets |
221 | 257 | 227 | ||||||||
Deferred tax liabilities: |
|||||||||||
Capital lease |
(55 | ) | (68 | ) | (69 | ) | |||||
Total deferred tax liabilities |
(55 | ) | (68 | ) | (69 | ) | |||||
Net deferred tax assets |
€ | 166 | € | 189 | € | 158 | |||||
F-59
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
9. Income Taxes (Continued)
Deferred tax assets (non current) on the write-off of start up expenses capitalized for tax purposes are compensated with deferred tax liabilities (non current) on capital lease and reported as deferred tax liabilities, net in the financial statements.
10. Geographic Information
Net sales to external customers by geographical area are:
| |
Years Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In € thousands |
2006 | 2007 | 2008 | ||||||||
Net sales |
|||||||||||
Italy |
€ | 14,721 | € | 16,627 | € | 17,751 | |||||
Switzerland |
€ | 231 | € | 67 | € | 64 | |||||
Malta |
€ | 71 | € | 41 | € | 83 | |||||
Total net sales |
€ | 15,023 | € | 16,735 | € | 17,898 | |||||
Long-lived assets are all in Italy.
11. Related Parties
Related parties consist of Studio Pacinotti S.r.l. and AB Medica S.a.g.l..
Studio Pacinotti S.r.l. is one of AB Medica's historical distributors and has been working with the Company for over 20 years, handling the distribution of products in central Italy. With reference to AGA products, Studio Pacinotti S.r.l. handles the distribution for the following regions: Marche, Abruzzo, Umbria and Molise. Studio Pacinotti S.r.l. is a company under common control of the shareholders of AB Medica and shares the same management.
AB Medica S.a.g.l., having its legal seat in Lugano (Switzerland), is a subsidiary of AB Medica whose main activity consists in the distribution of medical devices within the Swiss territory and the promotion and distribution of AGA devices at the Cardiocentro Ticino Foundation in Lugano. The promotion and sales are performed by employees duly prepared and supervised by the marketing management of AB Medica.
Starting from September 2008, AB Medica is the main supplier of AB Medica S.a.g.l. and the two companies have agreed particular payments conditions that allow AB Medica S.a.g.l. to pay supplies at 180 days. Starting from October 2008, payment terms were reduced to 90 days.
F-60
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
11. Related Parties (Continued)
Amounts of the transactions entered with related parties and amounts due to / from related parties as of and for the years ending December 31, 2006, 2007 and 2008 are as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | |||||||
Balance Sheet |
||||||||||
Receivables: |
||||||||||
Studio Pacinotti |
€ | 117 | € | 100 | € | 130 | ||||
AB Medica S.A.G.L. |
€ | 103 | € | 54 | € | 72 | ||||
Total receivables, related parties |
€ | 220 | € | 154 | € | 202 | ||||
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | |||||||
Income statement |
||||||||||
Sales: |
||||||||||
Studio Pacinotti |
€ | 434 | € | 330 | € | 363 | ||||
AB Medica S.A.G.L. |
€ | 231 | € | 67 | € | 64 | ||||
Total sales, related parties |
€ | 665 | € | 397 | € | 427 | ||||
12. Transactions with AGA Medical Corporation
The relationship between AB Medica and AGA Medical Corporation began at the beginning of 1997. From 1997 up to the end of 2008 AB Medica has taken care of the distribution of the AGA products on exclusive basis pincipally within the whole Italian territory. Certain sales are also made in Malta (for the sole client Technoline) and in Switzerland (for the sole client Cardiocentro Ticino Foundation in Lugano).
Purchases and payments by AB Medica to AGA Medical Corporation are carried out on the basis of contractual conditions agreed by the parties.
The amounts of transactions with AGA Medical Corporation and and the amounts due to / from related parties as of and for the years ending December 31, 2006, 2007 and 2008 are as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2006 | 2007 | 2008 | |||||||
Balance Sheet |
||||||||||
Trade accounts payable |
||||||||||
AGA Medical Corporation |
€ | 1,321 | € | 1,270 | € | 2,005 | ||||
F-61
The AGA Division of AB Medica S.p.A.
Notes to Financial Statements (Continued)
December 31, 2006, 2007 and 2008
(in € thousands)
12. Transactions with AGA Medical Corporation (Continued)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| In € thousands |
2006 | 2007 | 2008 | |||||||
Income statement |
||||||||||
Cost of good sold |
||||||||||
AGA Medical Corporation |
€ | 5,983 | € | 5,871 | € | 6,600 | ||||
13. Subsequent Events (unaudited)
On January 8, 2009, AB Medica and AGA Medical Italia S.r.l. (wholly owned by AGA Medical Corporation), entered into a purchase and sale agreement, that provides for the transfer of 100% of the capital quotas of AB Medica-AGA Division S.r.l. from AB Medica to AGA Medical Italia S.r.l..
The purchase price for the capital quotas was equal to Euro 24,700 (Base Purchase Price, of which Euro 17,500 paid by AGA Medical Italia S.r.l. before the signing of the agreement and the remaining portion to be paid based on further agreements between the parties), plus a contingent consideration (Additional Purchase Price) ranging from Euro 1,500 paid on March 31, 2009 to Euro 4,800 to be paid from 2009 to 2012 upon achievement of certain economic performances of AB Medica-AGA Division S.r.l.
F-62
510(k) Clearance: Clearance under Section 510(k) of the U.S. Federal Food, Drug, and Cosmetic Act. The FDA must determine that the proposed device is "substantially equivalent" to a device legally on the market, known as a "predicate" device, with respect to intended use, technology and safety and efficacy, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. The FDA's 510(k) clearance process usually takes from three to twelve months, but it can last longer.
AAA: Abdominal aortic aneurysm.
AMPLATZER Duct Occluder: Device intended for the closure of PDAs larger than 3 millimeters.
AMPLATZER Multi-Fenestrated Septal Occluder (Cribriform): Device for the closure of multiple hole ASDs.
AMPLATZER Septal Occluder: Device for the closure of single-hole ASDs.
Aneurysms: Localized bulges of a blood vessel caused by disease or weakening of the vessel wall.
Atrial Septal Defects (ASD): Abnormal opening in the wall between the left and right atria.
Atrial Fibrillation: Condition that results in irregular electrical activity in the upper chambers of the heart.
CE Mark: Product manufacturer's declaration that the product complies with all of the requirements of the relevant European health, safety and environmental protection legislation required in order to be sold in countries which are members of the European Free Trade Association or the European Union.
Cutdown: Procedure typically performed by a vascular surgeon involving an incision of a blood vessel in order to introduce a large catheter.
Ductus arteriosus: Blood vessel that forms a small passageway connecting the aorta to the pulmonary artery.
FDA: U.S. Food and Drug Administration.
Foramen ovale: Tunnel forms a small passageway connecting the left atrium and right atrium.
IDE: Investigational Device Exemption.
Iliac arteries: Arteries that branch off from the aorta and lead to the legs.
Left atrial appendage (LAA): Small pouch on the left side of the heart.
Muscular VSD: Defects in the middle portion of the ventricular septum, which is characterized by thicker, more muscular tissue.
Nitinol: Nickel and titanium alloy with superelastic and shape-memory characteristics.
Notified Body: The category of an organization certified by European regulatory authorities to review product applications.
Patent Ductus Arteriosus (PDA): Failed closure of the ductus arteriosus after birth that allows blood to mix between the aorta and the pulmonary artery.
Patent Foramen Ovale (PFO): Failed closure of the foramen ovale that allows blood to mix between the two atria.
A-1
Perimembraneous or Membraneous VSD: Defects in the upper portion of the ventricular septum near the valve connecting the heart with the aorta and characterized by more flexible, membraneous tissue.
PMA: Premarket approval granted by the FDA. A PMA application requires an applicant to demonstrate the safety and efficacy of the device based, in part, on data obtained in clinical trials. It generally takes from one to three years, or even longer, from the time the PMA application is submitted to the FDA until an approval is obtained.
RESPECT: Clinical trial being conducted at approximately 60 U.S. sites to assess the impact of our PFO closure device in reducing the occurrence of certain types of stroke.
TAA: Thoracic aorta aneurysm.
Thrombus: A clot inside a blood vessel, which potentially can migrate to the brain and cause complications such as stroke.
Transcatheter Approach: Procedure whereby an interventional cardiologist inserts a flexible catheter into the patient's groin with a small puncture and maneuvers the catheter through the vasculature to the heart.
Ventricular Septal Defect (VSD): Abnormal opening in the wall between the left and right ventricles.
A-2
Through and including (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
13,750,000 Shares

AGA Medical Holdings, Inc.
Common Stock
P R O S P E C T U S
BofA Merrill Lynch
Citi
Deutsche Bank Securities
Leerink Swann
Wells Fargo Securities
Natixis Bleichroeder Inc.
, 2009
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses to be paid by the Registrant in connection with the sale of the shares of common stock being registered hereby (other than underwriting discount). All amounts are estimates, except for the Securities and Exchange Commission registration fee, the Financial Industry Regulatory Authority filing fee and the Nasdaq Global Market filing fee:
Registration fee—Securities and Exchange Commission |
$ | 15,229 | |||
Filing fee—Financial Industry Regulatory Authority |
33,707 | ||||
Listing fee—Nasdaq Global Market |
130,000 | ||||
Printing and engraving expenses |
230,000 | ||||
Legal fees and expenses |
3,000,000 | ||||
Accounting fees and expenses |
860,000 | ||||
Transfer agent and registrar fees |
30,000 | ||||
Liability insurance for directors and officers |
400,000 | ||||
Miscellaneous |
101,064 | ||||
Total |
$ | 4,800,000 | |||
- *
- To be completed by amendments
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation's board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act of 1933, as amended (the "Securities Act").
As permitted by the Delaware General Corporation Law, the Registrant's Amended and Restated Certificate of Incorporation, which will become effective upon the closing of this offering, includes provisions that (i) eliminate, to the fullest extent permitted by the Delaware General Corporation Law, the personal liability of its directors for monetary damages for breach of fiduciary duty as a director, and (ii) require the Registrant to advance expenses, as incurred, to its directors and officers in connection with a legal proceeding to the fullest extent permitted by the Delaware General Corporation Law, subject to certain very limited exceptions.
As permitted by the Delaware General Corporation Law, the Amended and Restated Bylaws of the Registrant, which will become effective upon the closing of this offering, provide that (i) the Registrant is required to indemnify its directors and officers to the fullest extent permitted by the Delaware General Corporation Law, (ii) the Registrant may indemnify any other person as set forth in the Delaware General Corporation Law, and (iii) the rights conferred in the Amended and Restated Bylaws are not exclusive.
At present, there is no pending litigation or proceeding involving a director, officer or employee of the Registrant regarding which indemnification is sought, nor is the Registrant aware of any threatened litigation that may result in claims for indemnification.
Reference is also made to Section 8 of the Underwriting Agreement, which provides for the indemnification of officers, directors and controlling persons of the Registrant against certain liabilities. The indemnification provision in the Registrant's Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws may be sufficiently broad to permit indemnification of the Registrant's directors and officers for liabilities arising under the Securities Act.
We currently maintain liability insurance for our directors and officers. In connection with this offering, we expect to obtain additional liability insurance for our directors and officers. Such insurance would be available to our directors and officers in accordance with its terms.
II-1
See also the undertakings set out in response to Item 17.
Item 15. Recent Sales of Unregistered Securities
The following table sets forth the securities sold by us since January 1, 2006 (after giving effect to the reverse stock split or the conversion of our Class A and Class B common stock and all of our Series A and Series B preferred stock into our common stock to occur immediately prior to completion of this offering).
Individual or Group Name
|
Type of Securities |
Date of Sale | Preferred | Common | Debt | Total Consideration |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
David Jager |
Class B common stock |
June 19, 2007 | None | 1,559 | None | $ | 4,000 | |||||||
Jerilynn Rolbiecki |
Class B common stock |
July 6, 2007 | None | 839 | None | $ | 6,000 | |||||||
James Wiese |
Class B common stock |
October 17, 2007 | None | 5,594 | None | $ | 40,000 | |||||||
Wendy Pinor |
Class B common stock |
May 27, 2008 | None | 279 | None | $ | 2,000 | |||||||
Diane Hunt |
Class B common stock |
September 29, 2008 | None | 1,118 | None | $ | 8,000 | |||||||
Shanelle Zellinger Fernandez |
Class B common stock |
October 31, 2008 | None | 1,678 | None | 12,000 | ||||||||
Keith Hug |
Class B common stock |
December 1, 2008 | None | 3,496 | None | 25,000 | ||||||||
WCAS Capital Partners IV, L.P. |
10% Senior Subordinated PIK Note due 2012 | January 5, 2009 | None | None | 1 | $ | 13,121,000 | |||||||
WCAS Capital Partners IV, L.P. |
Series B preferred stock |
January 5, 2009 | 1,879 | None | None | $ | 1,879,000 | |||||||
Stephen Harrison |
Class B common stock |
January 16, 2009 | None | 1,118 | None | $ | 8,000 | |||||||
Debra Kwasek |
Class B common stock |
February 17, 2009 | None | 1,118 | None | $ | 8,000 | |||||||
Wendy Schweigert |
Class B common stock |
May 6, 2009 | None | 1,118 | None | $ | 8,000 | |||||||
George Bisker |
Class B common stock |
September 11, 2009 | None | 8,391 | None | $ | 60,000 | |||||||
The above-described sales to WCAS Capital Partners IV, L.P. were made in reliance upon the exemption from registration requirements of Securities Act available under Section 4(2) of the Securities Act. Such sales to WCAS Capital Partners IV, L.P. did not involve any underwriters, underwriting discounts or commissions, or any public offering. The recipient of the securities in such transactions represented it is a sophisticated persons and that it intended to acquire the securities for investment only and not with a view to, or for sale in connection with, any distribution thereof, and appropriate legends were affixed to the share certificates and instruments issued in such sales. We believe that WCAS Capital Partners IV, L.P. either received adequate information about us or had adequate access, through its relationships with us, to such information.
All other sales described above were made pursuant to the exercise of stock options granted under the Registrant's 2006 Equity Incentive Plan to Registrant's officers, directors, employees and consultants in reliance upon the available exemption from the registration requirements of the Securities Act, including those contained in Rule 701 promulgated under Section 3(b) of the Securities Act. Among other things, the Registrant relied on the fact that, under Rule 701, companies that are not subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act are
II-2
exempt from registration under the Securities Act with respect to certain offers and sales of securities pursuant to "compensatory benefit plans" as defined under that rule. The Registrant believes that its 2006 Equity Incentive Plan qualifies as a compensatory benefit plan.
The following table sets forth information on the stock options issued by us since January 1, 2006 (after giving effect to the 7.15 for 1.00 reverse stock split that will occur immediately prior to completion of this offering).
Date of Issuance
|
Number of Options Granted |
Exercise Price | Grant Date Fair Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
April 27, 2006 |
1,615,819 | $ | 7.15 | $ | 7.15 | |||||
May 19, 2006 to May 29, 2007 |
691,608 | $ | 7.15 | $ | 7.15 | |||||
May 30, 2007 to October 22, 2007 |
292,307 | $ | 14.30 | $ | 14.23 | |||||
October 23, 2007 to the date hereof |
628,671 | $ | 19.66 | $ | 19.66 | |||||
No consideration was paid to the Registrant by any recipient of any of the foregoing options for the grant of such options. All of the stock options described above were granted under the Registrant's 2006 Equity Incentive Plan to the Registrant's officers, directors, employees and consultants in reliance upon the available exemption from the registration requirements of the Securities Act, including those contained in Rule 701 promulgated under Section 3(b) of the Securities Act. Among other things, the Registrant relied on the fact that, under Rule 701, companies that are not subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act are exempt from registration under the Securities Act with respect to certain offers and sales of securities pursuant to "compensatory benefit plans" as defined under that rule. The Registrant believes that its 2006 Equity Incentive Plan qualifies as a compensatory benefit plan.
Item 16. Exhibits and Financial Statement Schedules
- (a)
- Exhibits
The attached Exhibit Index is incorporated herein by reference.
- (b)
- Financial Statement Schedules
II-3
Report of Independent Registered Public Accounting Firm
The
Board of Directors
AGA Medical Holdings, Inc.
We have audited the consolidated financial statements of AGA Medical Holdings, Inc. (the "Company") as of December 31 2007 and 2008 and for the years ended December 31, 2006, 2007 and 2008. We have issued our report thereon dated March 20, 2009, (included elsewhere in this Registration Statement). Our audits also included the financial statement schedule listed in Item 16(b) of this Registration Statement. This schedule is the responsibility of the Company's management. Our responsibility is to express an opinion based on our audits.
In our opinion, the financial statement schedule referred to above, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
/s/ Ernst & Young LLP
Minneapolis,
Minnesota
March 20, 2009
II-4
AGA Medical Holdings, Inc.
Schedule II
Valuation and Qualifying Accounts
Years Ended December 31, 2006, 2007 and 2008
(in thousands)
Description
|
Balance at Beginning of Period |
Additions | Deletions | Balance at End of Period |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year Ended December 31, 2008: |
|||||||||||||||
Allowance for doubtful accounts |
992 | 18 | (77 | ) | 933 | ||||||||||
Reserve for inventory |
1,883 | 1,265 | (1,331 | ) | 1,817 | ||||||||||
Reserve for sales returns |
7,226 | 1,312 | (513 | ) | 8,025 | ||||||||||
Total |
10,101 | 2,595 | (1,921 | ) | 10,775 | ||||||||||
Year Ended December 31, 2007: |
|||||||||||||||
Allowance for doubtful accounts |
588 | 754 | (350 | ) | 992 | ||||||||||
Reserve for inventory |
2,421 | 408 | (946 | ) | 1,883 | ||||||||||
Reserve for sales returns |
7,140 | 7,215 | (7,129 | ) | 7,226 | ||||||||||
Total |
10,149 | 8,377 | (8,425 | ) | 10,101 | ||||||||||
Year Ended December 31, 2006: |
|||||||||||||||
Allowance for doubtful accounts |
2,750 | 476 | (2,638 | ) | 588 | ||||||||||
Reserve for inventory |
2,692 | 2,298 | (2,569 | ) | 2,421 | ||||||||||
Reserve for sales returns |
14,759 | 7,977 | (15,596 | ) | 7,140 | ||||||||||
Total |
20,201 | 10,751 | (20,803 | ) | 10,149 | ||||||||||
All other schedules have been omitted because the information required to be shown in the schedules is not applicable or is included elsewhere in the financial statements and notes thereto.
Item 17. Undertakings
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the provisions described under Item 14 above, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-5
The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) For the purpose of determining liability under the Securities Act to any purchaser if the Registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(4) For the purpose of determining liability of the Registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser.
II-6
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this amendment no. 11 to the registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Plymouth, State of Minnesota on October 13, 2009.
AGA MEDICAL HOLDINGS, INC. |
||||
By: |
/s/ JOHN R. BARR Name: John R. Barr Title: President and Chief Executive Officer |
|||
Pursuant to the requirements of the Securities Act of 1933, this amendment no. 11 to the registration statement has been signed by the following persons in the capacities indicated on October 13, 2009.
Signature
|
Title
|
|||
|---|---|---|---|---|
| /s/ JOHN R. BARR John R. Barr |
President and Chief Executive Officer (Principal Executive Officer) |
|||
* Brigid A. Makes |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|||
* Tommy G. Thompson |
Chairman |
|||
* Franck L. Gougeon |
Director |
|||
* Daniel A. Pelak |
Director |
|||
* Paul B. Queally |
Director |
|||
II-7
Signature
|
Title
|
|||
|---|---|---|---|---|
| * Terry Allison Rappuhn |
Director | |||
* Darrell J. Tamosuinas |
Director |
|||
* Sean M. Traynor |
Director |
|||
*By: |
/s/ JOHN R. BARR John R. Barr Attorney- in- fact |
|||
II-8
Exhibit Number |
Description of Exhibit | |
|---|---|---|
| 1.1 | Form of Underwriting Agreement. | |
2.1 |
Sale and Purchase Agreement, dated December 9, 2008, between AB Medica S.p.A., AGA Medical Italia S.r.l. and AGA Medical Corporation. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 31, 2009.) |
|
3.1 |
Form of Amended and Restated Certificate of Incorporation of AGA Medical Holdings, Inc. |
|
3.2 |
Form of Amended and Restated Bylaws of AGA Medical Holdings, Inc. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on October 1, 2009.) |
|
4.1 |
Second Amended and Restated Stockholders Agreement, dated as of October 2, 2009, by and among AGA Medical Holdings, Inc., Welsh, Carson, Anderson & Stowe IX, L.P., WCAS Capital Partners IV, L.P., the WCAS Stockholders as defined therein, Franck L. Gougeon, Gougeon Shares, LLC and The Franck L. Gougeon Revocable Trust Under Agreement Dated June 28, 2006. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on October 5, 2009.) |
|
4.2 |
Amended and Restated Stock Purchase Agreement, dated as of July 28, 2005, by and among AGA Medical Corporation, Franck L. Gougeon and Welsh, Carson, Anderson & Stowe IX, L.P. and its co-investors listed therein. (Included as Exhibit 4.5 in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on July 20, 2009.) |
|
4.3 |
Omnibus Amendment Agreement by and among AGA Medical Holdings, Inc., AGA Medical Corporation, Welsh, Carson, Anderson & Stowe IX, L.P. and Franck L. Gougeon, dated July 28, 2005, which constitutes Amendment No. 1 to the Amended and Restated Stock Purchase Agreement dated as of July 28, 2005 and Amendment No. 1 to the Stockholders Agreement dated as of July 28, 2005. (Included as Exhibit 4.7 in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
4.4 |
Amendment No. 2 to Amended and Restated Stock Purchase Agreement, dated as of June 20, 2008, by and among AGA Medical Corporation, Welsh, Carson, Anderson & Stowe IX, L.P. and its co-investors therein and Franck L. Gougeon. (Included as Exhibit 4.8 in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
4.5 |
Amended and Restated Registration Rights Agreement, dated as of April 21, 2008, by and among AGA Medical Holdings, Inc., Welsh, Carson, Anderson & Stowe IX, L.P., WCAS Capital Partners IV, L.P., the WCAS Investors as defined therein, Franck L. Gougeon, Gougeon Shares, LLC and The Franck L. Gougeon Revocable Trust Under Agreement Dated June 28, 2006. (Included as Exhibit 4.8 in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on November 24, 2008.) |
|
4.6 |
First Amendment to Amended and Restated Registration Rights Agreement, dated as of January 5, 2009, by and between AGA Medical Holdings, Inc. and Welsh, Carson, Anderson & Stowe IX, L.P. (Included as Exhibit 4.9 in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on June 5, 2009.) |
|
5.1 |
Opinion of Simpson Thacher & Bartlett LLP. |
|
10.1 |
Amended and Restated Credit Agreement among AGA Medical Corporation, as borrower, AGA Medical Holdings, Inc., Lehman Commercial Paper Inc., Lehman Commercial Bank, Bank of America, N.A., Citicorp USA, Inc. and Wachovia Bank, National Association as lenders, Lehman Brothers Inc. and Citigroup Global Markets Inc., as joint lead arrangers and joint bookrunners, Citigroup Global Markets Inc., as syndication agent and Lehman Commercial Paper Inc., as administrative agent, dated April 28, 2006. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on July 20, 2009.) |
|
10.2 |
AGA Medical Holdings, Inc. 2006 Equity Incentive Plan. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
Exhibit Number |
Description of Exhibit | |
|---|---|---|
| 10.3 | Form of AGA Medical Holdings, Inc. 2008 Equity Incentive Plan. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on September 22, 2009.) | |
10.4 |
Deferred Prosecution Agreement, dated June 2, 2008, between AGA Medical Corporation and the U.S. Department of Justice. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.5 |
Consulting Agreement of Franck L. Gougeon, dated June 20, 2008. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.6 |
Transition Agreement dated as of June 20, 2008, between AGA Medical Corporation and Franck L. Gougeon. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.7 |
Amended and Restated Employment Agreement of Franck L. Gougeon, dated April 21, 2008. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.8 |
Engagement Letter of Franck L. Gougeon, dated June 20, 2008. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.9 |
Engagement Letter of Terry Allison Rappuhn, dated May 25, 2006. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.10 |
Engagement Letter of Daniel A. Pelak, dated June 1, 2006. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.11 |
Engagement Letter of Darrell J. Tamosuinas, dated May 6, 2006. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.12 |
Memorandum of Understanding between Secretary Tommy Thompson and AGA Medical Corporation. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.13 |
Summary of Proposed Terms of Employment between Secretary Tommy Thompson and AGA Medical Corporation, dated July 28, 2005. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.14 |
Employment Agreement of John R. Barr, dated September 19, 2005. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.15 |
Employment Agreement of Brigid A. Makes, dated October 2, 2006. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.16 |
Independent Contractor Agreement of Ronald E. Lund and Ronald E. Lund, LLC, dated June 1, 2007. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.17 |
Employment Agreement of Ronald Lund, dated July 1, 2008. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.18 |
Research Agreement with Dr. Kurt Amplatz, dated December 23, 2005. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on October 10, 2008.) |
|
10.19 |
Royalty Agreement with Mr. Curtis Amplatz, dated April 22, 1996. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on September 10, 2008.) |
|
10.20 |
Royalty Agreement with Mr. Curtis Amplatz, dated as of November 1, 2000. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on September 10, 2008.) |
|
10.21 |
Consent to Assignment of Royalty Agreements with Mr. Curtis Amplatz, dated January 5, 2007. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on September 10, 2008.) |
Exhibit Number |
Description of Exhibit | |
|---|---|---|
| 10.22 | Securities Purchase Agreement, dated as of July 28, 2005, between AGA Medical Corporation and WCAS Capital Partners IV, L.P. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on November 24, 2008.) | |
10.23 |
Amendment No. 1 to Securities Purchase Agreement, dated as of April 28, 2006, by and among AGA Medical Corporation and WCAS Capital Partners IV, L.P. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
10.24 |
Quit Claim Assignment from Mr. Curtis Amplatz, dated November 11, 2000. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on November 24, 2008.) |
|
10.25 |
Securities Purchase Agreement, dated as of January 5, 2009, among AGA Medical Corporation, AGA Medical Holdings, Inc., WCAS Capital Partners IV, L.P. and Amplatzer Medical Sales Corporation. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on June 5, 2009.) |
|
10.26 |
Reformation Agreement, dated as of December 30, 2008, between AGA Medical Corporation and Ronald Lund. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on June 5, 2009.) |
|
21.1 |
Subsidiaries of AGA Medical Holdings, Inc. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 31, 2009.) |
|
23.1 |
Consent of Ernst & Young LLP. |
|
23.2 |
Consent of Reconta Ernst & Young S.p.A. |
|
23.3 |
Consent of Simpson Thacher & Bartlett LLP. (Included in Exhibit 5.1.) |
|
23.4 |
Consent of Easton Associates, LLC. (Included as Exhibit 23.3 in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on August 8, 2008.) |
|
23.5 |
Consent of Mr. Jack P. Helms. (Included in Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on October 1, 2009.) |
|
24.1 |
Power of Attorney. (Included in signature pages to Registration Statement on Form S-1 of AGA Medical Holdings, Inc. filed on September 10, 2008.) |
